Published online Nov 21, 2020. doi: 10.3748/wjg.v26.i43.6837
Peer-review started: June 29, 2020
First decision: August 8, 2020
Revised: August 21, 2020
Accepted: September 18, 2020
Article in press: September 18, 2020
Published online: November 21, 2020
Laparoscopic assisted total gastrectomy (LaTG) is associated with reduced nutritional status, and the procedure is not easily carried out without extensive expertise. A small remnant stomach after near-total gastrectomy confers no significant nutritional benefits over total gastrectomy. In this study, we developed a modified laparoscopic subtotal gastrectomy procedure, termed laparoscopic-assisted tailored subtotal gastrectomy (LaTSG).
To evaluate the feasibility and nutritional impact of LaTSG compared to those of LaTG in patients with advanced middle-third gastric cancer (GC).
We retrospectively analyzed surgical and oncological outcomes and postoperative nutritional status in 92 consecutive patients with middle-third GC who underwent radical laparoscopic gastrectomy at Department of Pancreatic Stomach Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College between 2013 and 2017. Of these 92 patients, 47 underwent LaTSG (LaTSG group), and the remaining underwent LaTG (LaTG group).
Operation time (210 ± 49.8 min vs 208 ± 50.0 min, P > 0.05) and intraoperative blood loss (152.3 ± 166.1 mL vs 188.9 ± 167.8 mL, P > 0.05) were similar between the groups. The incidence of postoperative morbidities was lower in the LaTSG group than in the LaTG group (4.2% vs 17.8%, P < 0.05). Postoperatively, nutritional indices did not significantly differ, until postoperative 12 mo. Albumin, prealbumin, total protein, hemoglobin levels, and red blood cell counts were significantly higher in the LaTSG group than in the LaTG group (P < 0.05). No significant differences in Fe or C-reaction protein levels were found between the two groups. Endoscopic examination demonstrated that reflux oesophagitis was more common in the LaTG group (0% vs 11.1%, P < 0.05). Kaplan–Meier analysis showed a significant improvement in the overall survival (OS) and disease free survival (DFS) in the LaTSG group. Multivariate analysis showed that LaTSG was an independent prognostic factor for OS (P = 0.048) but not for DFS (P = 0.054). Subgroup analysis showed that compared to LaTG, LaTSG improved the survival of patients with stage III cancers, but not for other stages.
For advanced GC involving the middle third stomach, LaTSG can be a good option with reduced morbidity and favorable nutritional status and oncological outcomes.
Core Tip: We developed a modified the laparoscopic subtotal gastrectomy procedure termed laparoscopic-assisted tailored subtotal gastrectomy (LaTSG) to treat advanced middle-third gastric cancer. Compared with laparoscopic assisted total gastrectomy (LaTG), LaTSG is a safer procedure in terms of both short and long-term outcomes. The long-term survival of patients who underwent LaTSG was better than that of patients who underwent LaTG. Furthermore, LaTSG may have an advantage over LaTG by improving the postoperative nutritional status and preventing reflux oesophagitis.
- Citation: Liu H, Jin P, Ma FH, Ma S, Xie YB, Li Y, Li WK, Kang WZ, Tian YT. Feasibility and nutritional impact of laparoscopic assisted tailored subtotal gastrectomy for middle-third gastric cancer. World J Gastroenterol 2020; 26(43): 6837-6852
- URL: https://www.wjgnet.com/1007-9327/full/v26/i43/6837.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i43.6837
In recent decades, the incidence of gastric cancer (GC) has declined worldwide, however, it remains the second most common cancer among men and the third most common among women. According to data from the National Central Cancer Registry of China, in 2015, there were 677000 new cases of GC in China, which accounted for half of all incident cases globally[1].
In resectable advanced GC, the range of resection is determined by tumor characteristics, including its size, location, clinical stage, and distance from the proximal resection margin. According to the latest Japanese GC treatment guidelines[2], tumors located in the upper or low-third of the stomach have a definite range of gastric resection. However, consensus on the optimal surgical treatment strategy for advanced GC located in the middle of the stomach is yet to be established. Such cancer is not easily cured by distal or proximal gastrectomy, and most patients ultimately undergo total gastrectomy (TG). However, numerous studies have demonstrated that TG was more traumatic than partial gastrectomy, and it is further suggested that TG could result in higher rates of postoperative complications[3-5]. Moreover, patients who underwent TG showed significant nutritional deficits, including a higher incidence of anemia and low serum vitamin E levels[6,7].
A recently reported procedure[8], laparoscopic subtotal gastrectomy with a very small remnant stomach, has demonstrated advantages over laparoscopic-assisted total gastrectomy (LaTG), showing reduced postoperative morbidity and improved nutritional status, when used to treat patients with early GC located in the upper-third of the stomach. Recently, several large clinical studies have demonstrated that laparoscopy can be used for advanced GC[9,10]. We developed a modified laparoscopic subtotal gastrectomy procedure termed laparoscopic-assisted tailored subtotal gastrectomy (LaTSG) to treat advanced middle-third GC. In the present study, we evaluated short-term postoperative patient outcomes, nutritional status, and long-term oncological outcomes to assess the safety and efficacy of LaTSG compared to those of LaTG.
This retrospective study assessed patient outcomes in 47 patients who received LaTSG and 45 patients who received LaTG for preoperatively diagnosed GC. Patients (aged 18-75 years) were enrolled and treated at the Department of Pancreatic Stomach Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College from February 2013 to November 2017. Preoperative contrast-enhanced computed tomography (CT) and endoscopic ultrasonography were performed to assess the tumor.
LaTSG was performed in patients who fulfilled the following criteria: (1) Tumor identified by clinical staging as T2-4bN0-3M0 based on the 8th American Joint Committee on Cancer staging system; (2) Non-Bormman type IV tumor located in the middle-third of the stomach, or non-Bormman type IV lesion in the lower-third of the stomach that extends into the middle-third of the stomach, with no distant metastasis. In view of the susceptibility to No. 2 lymph node (LN) metastasis, TG was performed for the tumors located in the greater curvature of the stomach; (3) The proximal margin was at least 3 cm from the tumor with a non-infiltrative growth pattern; and (4) Intraoperative peritoneal washing cytology was negative. In patients selected to receive LaTSG, an additional intraoperative frozen pathology was performed. If the margin of the biopsy was positive for tumor cells, LaTG instead of LaTSG was performed. Standard D2 lymph node dissection was performed in all cases. All methods were carried out in accordance with Japanese GC treatment guidelines2014 (ver. 4)[2].
Patients were excluded from the study if they met any of the following criteria: (1) Contemporaneous existence of other malignancies; (2) Gastric stump cancer; (3) Received preoperative radiotherapy; (4) Existence of distant metastasis including No. 16 LN, left supraclavicular lymph node, liver, lung, or bone metastasis; (5) Peritoneal dissemination or positive intraoperative peritoneal washing cytology; (6) Bormman type IV tumor; and (7) Existence of enlarged or bulky regional lymph nodes, larger than 3 cm in the long diameter according to preoperative imaging.
Early postoperative complications (occurring on postoperative days 0–30) were graded using the Clavien–Dindo classification. To evaluate the postoperative nutritional status, body mass index (BMI) and serum concentrations of hemoglobin (HGB), ferrous iron (Fe), albumin (ALB), prealbumin (PALB), total protein (TP), and red blood cell counts (RBC) were measured 1 d, 1 mo, and 12 mo after the procedure was conducted. Gastro-esophageal reflux was evaluated via endoscopy based on the Los Angeles classification system[11] 12 mo after the surgery. For patients with pathologic stage II or higher tumors, adjuvant chemotherapy with 6 mo of a fluorouracil-based chemotherapy was mandated. All patients were followed regularly by clinic visits and telephone. Chest, abdominal, and pelvic contrast-enhanced CT was performed every 3 mo for the first 3 years and 6 mo thereafter.
Endotracheal intubation was conducted under general anesthesia, and the laparoscopic surgery was performed with the patient placed in the split-leg reverse Trendelenburg position. A 10-mm flexible laparoscope was used, and CO2 pressure was maintained at 13-15 mmHg. During surgery, the operator was positioned on the left side of the patient, the first assistant was on the right side, and the cameraman stood between the legs of the patient.
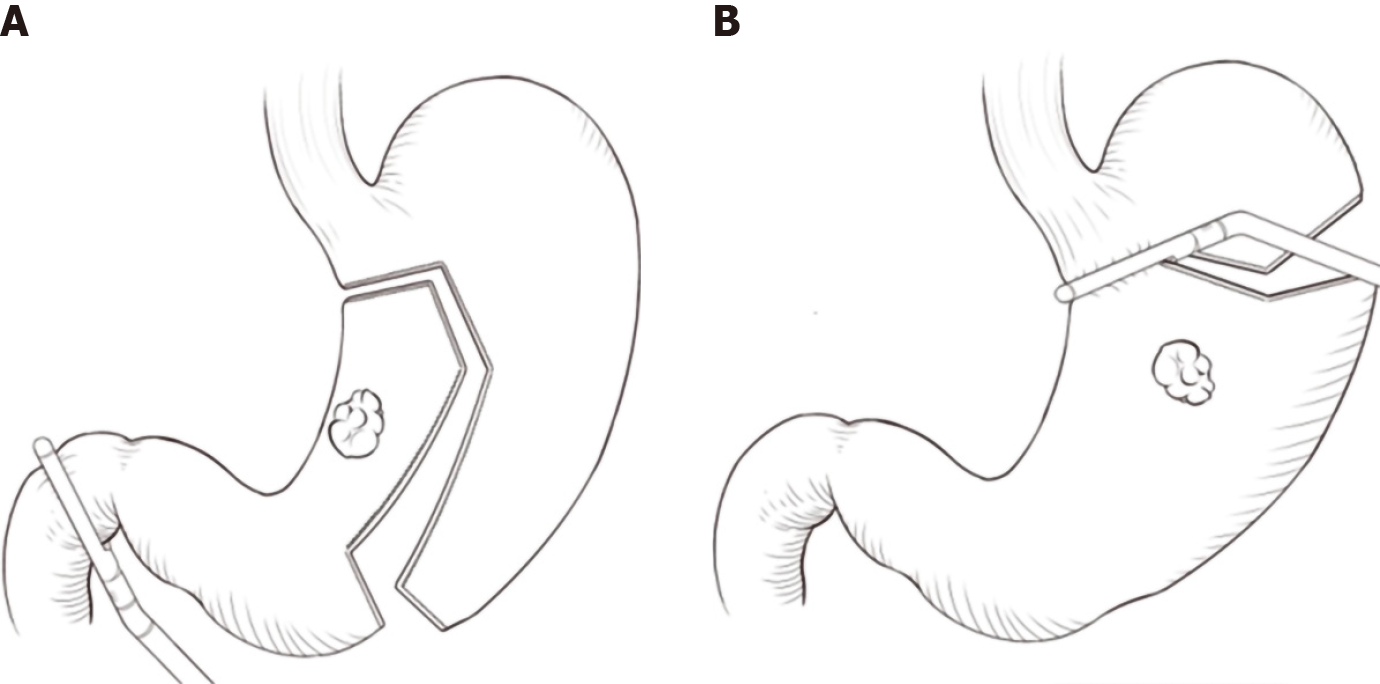
A five-port system (two 5-mm ports and three 12-mm ports) was used for the laparoscopic assisted gastrectomy (Figure 1). The location of the observation port was determined based upon the distance between the patient's xiphoid and umbilicus.
A 12-mm trocar could be inserted into the umbilicus with sufficient distance from the xiphoid. Our additional ports (two ports with 12-mm diameter and two with 5-mm diameter) were inserted under direct visualization into the upper abdomen. Trocars were separated by a width of at least four fingers which helped prevent each trocar from impacting the others via the “chopstick effect”.
After dissociating the distal stomach and completing D2 LN dissection (D1+7+8a+9+11p+12a), at least two branches of the short gastric artery near the gastric cardia were preserved. An endoscopic liner stapler was used to cut and close the duodenum.
Different resection lines were selected based on different locations of the tumors (Figure 1). In tumors located within the lesser curvature of the stomach, a liner stapler was used to make a curved transaction line 3 cm from the lateral edge of the tumor, thereby retaining the fundus and cardia, which were used to form a proximal tube-shaped stomach (Figure 1A, Figure 2 and Figure 3). In tumors located in the middle of the stomach, a liner stapler was used to make a curved transection line 3 cm from the upper edge of the tumor (Figure 1B). After removing the specimen and ensuring that a negative frozen margin was obtained, conventional Billroth II reconstruction, alongside either Braun’s or Roux-en-Y anastomosis, was performed with a circular stapler.
Patients with positive tumor indications in their proximal margin frozen sections were transitioned to undergo LaTG. After dissecting the LNs, the duodenum was transected using a linear stapler. The jejunum was subsequently extracorporeally transected 20 cm distal to the ligament of Treitz using another linear stapler. Jejunojejunostomy was performed using a liner stapler, 44 cm from the cutting end. The ensuing mesenteric hole was closed using a 3-0 absorbable suture. Oesophagojejunostomy was performed with a circular stapler. The reverse piercing method was used to put the nail base into the oesophagus and to perform esophagojejunal end-to-side anastomosis.
Clinical data were obtained from patients’ records. Statistical analyses were performed using SPSS.20 (SPSS Inc., Armonk, NY, United States). All values are expressed as the mean ± SD. We compared categorical and continuous variables using the χ2 test and Student’s t test, respectively. Kaplan–Meier estimation and log-rank test were performed to compare survival. A Cox proportional hazards regression model was used to verify independent prognostic factors through univariate and multivariate analyses. A P value < 0.05 was considered statistically significant.
Table 1 details the clinical and nutritional characteristics of patients undergoing LaTSG and LaTG. Between the LaTSG and LaTG groups, no significant differences were observed in patients’ BMI, ASA-PS, the number of patients with a previous abdominal operation, or the number of patients receiving neoadjuvant chemotherapy. Although the pT stage was significantly different between the two groups (P = 0.027), there were no significant intergroup differences in the pTNM stage (P = 0.217).
| Characteristic | LaTSG (n = 47) | LaTG (n = 45) | P value |
| Sex (M/F) | 11/36 | 17/28 | 0.175 |
| Age | 57.0 ± 11.1 | 58.0 ± 9.9 | 0.571 |
| BMI (kg/m2) | 23.6 ± 3.4 | 23.7 ± 3.0 | 0.559 |
| ASA-PS (1/2/3) | 2/44/1 | 2/42/1 | 0.998 |
| pT stage | 0.027 | ||
| pT1 | 18 (38.3) | 10 (22.2) | |
| pT2 | 5 (10.6) | 4 (8.9) | |
| pT3 | 8 (17.0) | 22 (48.9) | |
| pT4a | 16 (34.0) | 9 (20.0) | |
| pN stage | 0.371 | ||
| pN0 | 23 (48.9) | 14 (31.2) | |
| pN1 | 10 (21.3) | 14 (31.2) | |
| pN2 | 6 (12.8) | 7 (15.6) | |
| pN3 | 8 (17.0) | 10 (22.2) | |
| pTNM stage | 0.217 | ||
| I | 18 (38.3) | 11 (24.4) | |
| II | 10 (21.3) | 16 (35.6) | |
| III | 19 (40.4) | 18 (40.0) | |
| Previous abdominal operation | 4 (8.5) | 1 (2.2) | 0.362 |
| Neoadjuvant chemotherapy | 9 (19.1) | 6 (13.3) | 0.575 |
| Histological type | 0.237 | ||
| Signet-ring cell carcinoma | 20 (42.6) | 12 (26.7) | 0.187 |
| Blood vessel invasion | 30 (63.9) | 30 (66.6) | 0.829 |
| Neural infiltration | 26 (55.4) | 27 (60.0) | 0.532 |
| Tumor location | 0.604 | ||
| Lesser curvature | 25 (53.2) | 24 (53.4) | |
| Middle | 3 (6.4) | 5 (11.1) | |
| Middle and low | 19 (40.4) | 16 (35.6) |
Operative outcomes are summarized in Table 2. Mean operation time and mean estimated blood loss were similar between the groups. There were no significant differences between the two groups in the levels of intraoperative blood transfusion. Proportionately, more LNs were retrieved in the LaTG group than in the LaTSG group (P < 0.05). Tumor size was larger in the LaTG group than in the LaTSG group, while there were no significant differences between the two groups in the proximal and distal margins. Neural infiltration and lymph vessel invasion were more common in the LaTG group than in the LaTSG group, however, these differences were not significant (P > 0.05).
| Variable | LaTSG (n = 44) | LaTG (n = 58) | P value |
| Operation time (min) | 210.0 ± 49.8 | 208.0 ± 50.0 | 0.684 |
| Estimated blood loss (mL) | 152.3 ± 166.1 | 188.9 ± 167.8 | 0.141 |
| Intraoperative blood transfusion | 6/47 | 8/45 | 0.570 |
| Tumor size | 3.4 ± 1.2 | 4.5 ± 1.8 | 0.020 |
| No. of retrieved lymph nodes | 31.8 ± 10.1 | 44.0 ± 16.5 | 0.008 |
| Proximal margin | 3.7 ± 1.6 | 3.9 ± 2.0 | 0.233 |
| Distal margin | 5.0 ± 2.6 | 6.2 ± 3.2 | 0.149 |
Postoperative patient complications are listed in Table 3. The mean postoperative hospital stay was significantly shorter in the LaTSG group (8.9 ± 5.0 d) than in the LaTG group (12.0 ± 7.3 d) (P < 0.05). No mortality was recorded for the LaTSG group; however, one patient in the LaTG group died from acute kidney failure due to intraabdominal bleeding. The overall postoperative complication rate was 4.25% in the LaTSG group, and 17.8% in the LaTG group (P < 0.05). The frequency of anastomotic complications was significantly higher in the LaTG group (8.9%) than in the LaTSG group (0%) (P < 0.05). Los Angeles Grade B or more severe reflux oesophagitis was observed in ten (17.2%) patients in the LaTG group, while such severe reflux oesophagitis was observed in only two (4.5%) patients in the LaTSG group (P = 0.002).
| Complication | LaTSG (n = 47) | LaTG (n = 45) | P value |
| Anastomotic leakage | 0 (0) | 3 (6.7) | |
| Anastomotic stricture | 0 (0) | 1 (2.2) | |
| Anastomotic bleeding | 0 (0) | 0 (0) | |
| Duodenal stump leakage | 1 (2.1) | 0 (0) | |
| Intra-abdominal bleeding | 0 (0) | 0 (0) | |
| Intra-abdominal abscess | 1 (2.1) | 3 (6.7) | |
| Lymph leak | 0 (0) | 1 (2.2) | |
| Ileus | 0 (0) | 0 (0) | |
| Incision | 0 (0) | 1 (2.2) | |
| Total | 2 (4.2) | 8 (17.8) | 0.048 |
| Mortality | 0 (0) | 1 (2.2) | |
| Postoperative hospital stay | 8.9 ± 5.0 | 12.0 ± 7.3 | 0.025 |
| Reflux esophagitis (LA) | 0.002 | ||
| LA –B | 2 (4.2) | 5 (11.1) | |
| LA-C | 0 (0) | 5 (11.1) |
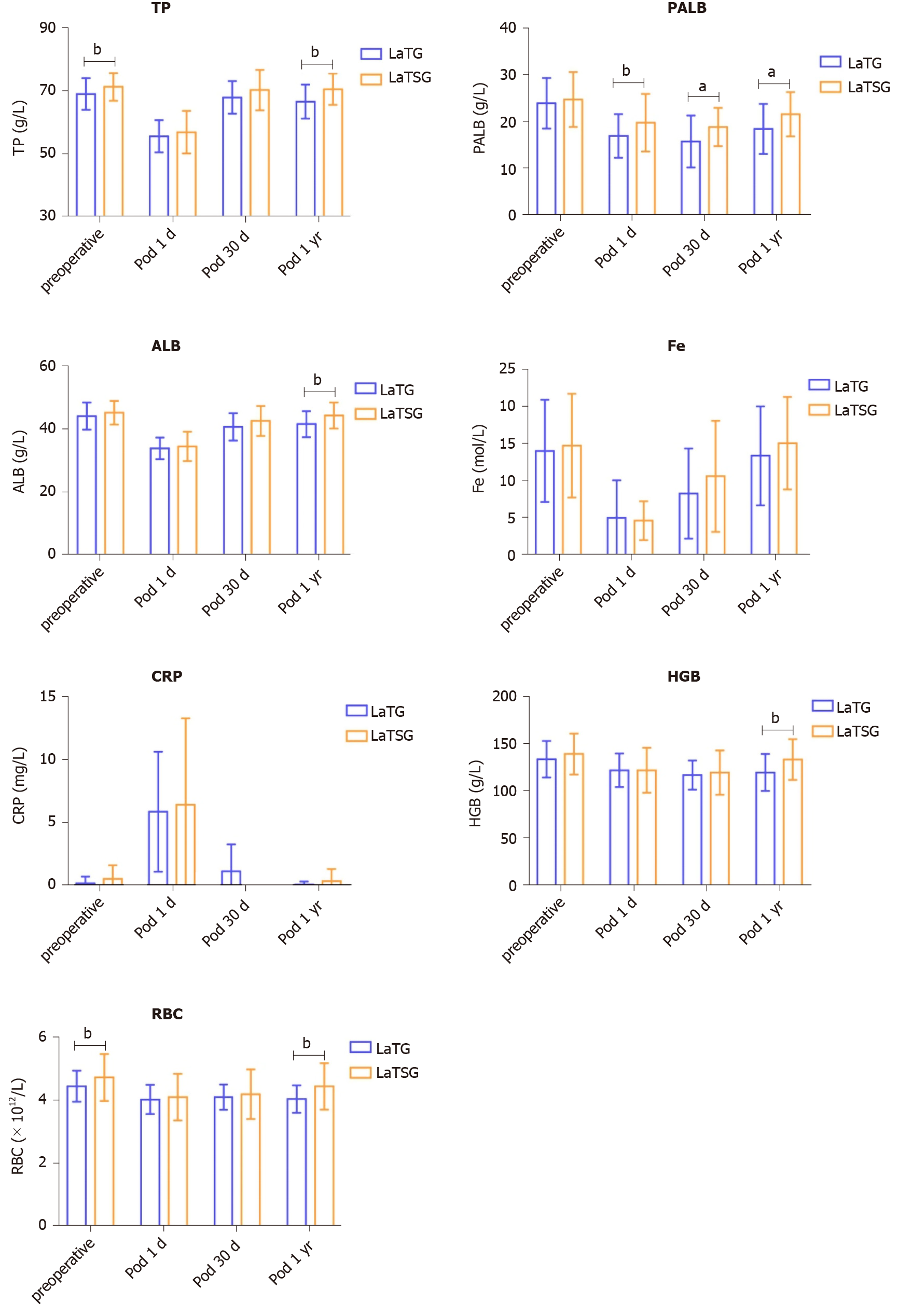
Figure 4 shows the pre- and postoperative mean levels of ALB, TP, PALB, RBC, HGB, C-reactive protein (CRP), and Fe in patients who underwent LaTSG and LaTG. On the first day postoperatively, these nutritional indices did not significantly differ between the groups. However, at 12 mo post-surgery, ALB, TP, HGB, and RBC levels were significantly higher in the LaTSG group than in the LaTG group (P < 0.05). No significant differences in Fe, PALB, or CRP levels were found between the groups (P > 0.05) (Table 4).
| Variable | LaTSG (n = 47) | LaTG (n = 45) | P value |
| ALB (g/L) | 45.2 ± 3.7 | 45.1 ± 7.2 | 0.005 |
| PALB (g/L) | 21.6 ± 4.7 | 18.5 ± 5.3 | 0.015 |
| TP (g/L) | 71.0 ± 4.2 | 67.6 ± 4.6 | 0.002 |
| Fe | 15.6 ± 5.7 | 13.4 ± 6.9 | 0.194 |
| HGB (g/L) | 133 ± 20.7 | 120.0 ± 20.1 | 0.007 |
| RBC(1012/L) | 4.5 ± 0.7 | 4.0 ± 0.4 | 0.003 |
| CRP | 0.37 ± 0.97 | 0.05 ± 0.25 | 0.187 |
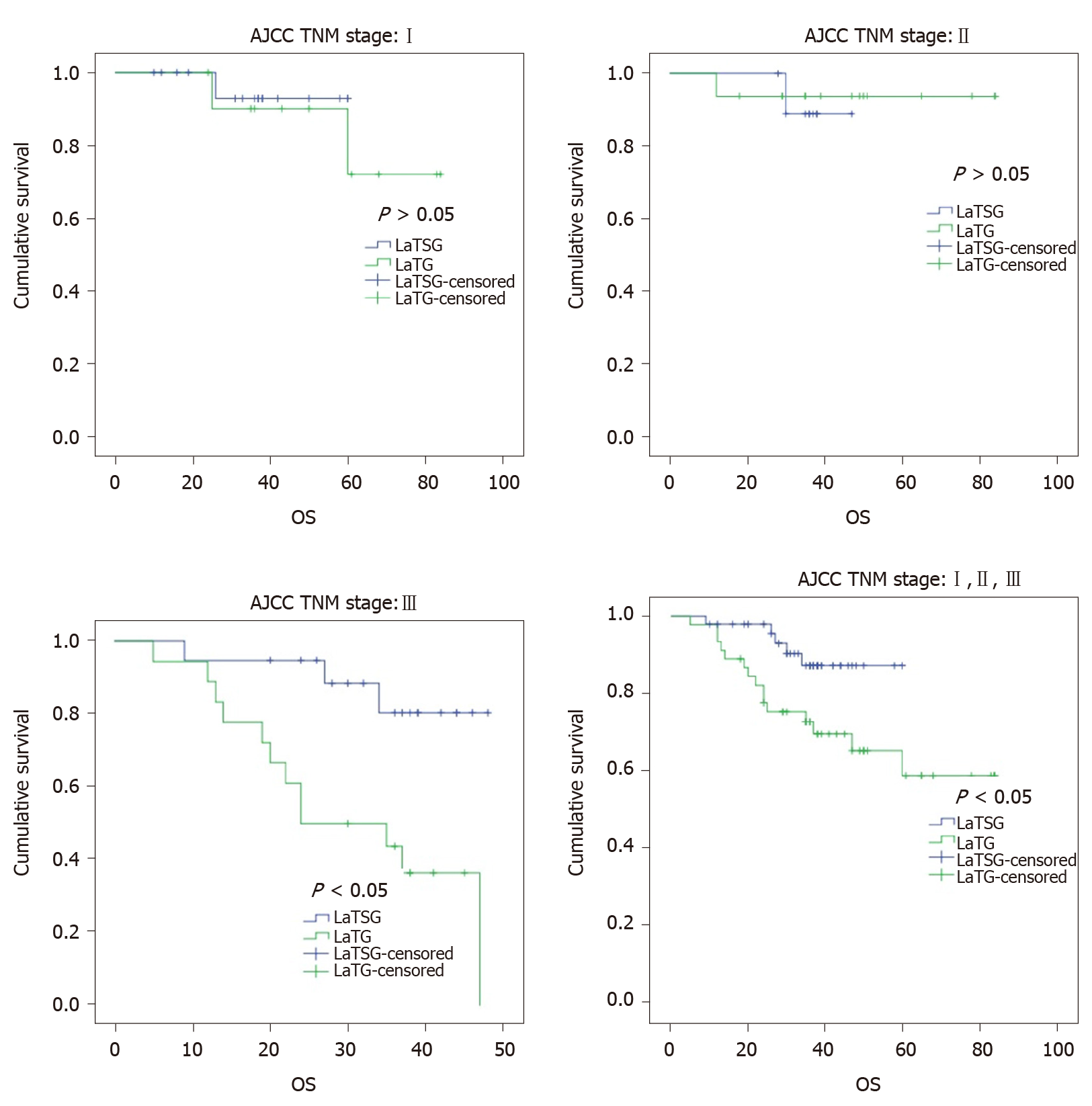
The median follow-up time was 41 mo (range, 37-46 mo) in this study. Kaplan–Meier analysis showed a significant difference in overall survival between the two groups (P = 0.020) (Figure 5). The 3-year overall survival rates in the LaTSG and LaTG groups were 85.6% and 67.4%, respectively (P < 0.05). Subgroup analysis showed that compared to LaTG, LaTSG improved the survival of patients with stage III cancer, but not for other stages. Univariate analysis identified tumor size (P = 0.036), TNM stage (P = 0.002), and surgical type (P = 0.036) to be prognostic factors for overall survival. Multivariate analysis revealed that pTNM stage (P = 0.004) and surgical type (P = 0.048) were independent prognostic factors for the overall survival (Table 5).
| Variable | Univariate | P value | Multivariate | P value |
| HR (95%CI) | HR (95%CI) | |||
| Age, yr (≤ 65) | 0.831 (0.301-2.293) | 0.721 | - | - |
| Sex (female) | 0.493 (0.204-1.192) | 0.117 | - | - |
| BMI (≤ 25 kg/m2) | 0.703 (0.270-1.832) | 0.471 | - | - |
| ASA (I/II/III) | 1.440 (0.256-8.086) | 0.679 | - | - |
| Previous abdominal operation | 0.046 (0.000-250.286) | 0.482 | - | - |
| Primary site (L/M/LM) | 0.981 (0.707-1.360) | 0.908 | - | - |
| Tumor size (> 4 cm) | 2.607 (1.064-6.390) | 0.036 | 1.180 (0.906-1.538) | 0.219 |
| Neoadjuvant chemotherapy (Yes) | 1.369 (0.454-4,129) | 0.577 | - | - |
| Tumor grade (W/M/P/U) | 0.851 (0.578-1.253) | 0.415 | - | - |
| Lauren classification (I/D/M) | 0.949 (0.513-1.758) | 0.869 | - | - |
| pTNM stage (I/II/III) | 3.195 (1.518-6.724) | 0.002 | 3.170 (1.461-6.878) | 0.004 |
| Lymphovascular invasion (Yes) | 2.203 (0.906-5.358) | 0.081 | - | - |
| Neural infiltration (Yes) | 1.755 (0.715-4.304) | 0.219 | - | - |
| Surgical type (LaTSG/LaTG) | 2.977 (1.073-8.263) | 0.036 | 2.876 (1.009-8.195) | 0.048 |
| Operation time (> 210 min) | 1.130 (0.456-2.803) | 0.791 | - | - |
| Estimated blood loss (> 200 mL) | 1.261 (0.522-3.048) | 0.606 | - | - |
| Intraoperative blood transfusion (Yes) | 0.933 (0.273-3.192) | 0.912 | - | - |
| No. of retrieved lymph nodes (> 30) | 1.631 (0.590-4.511) | 0.346 | - | - |
| Proximal margin (> 3 cm) | 0.876 (0.362-2.120) | 0.770 | - | - |
| Distal margin (> 3 cm) | 0.500 (0.203-1.121) | 0.132 | - | - |
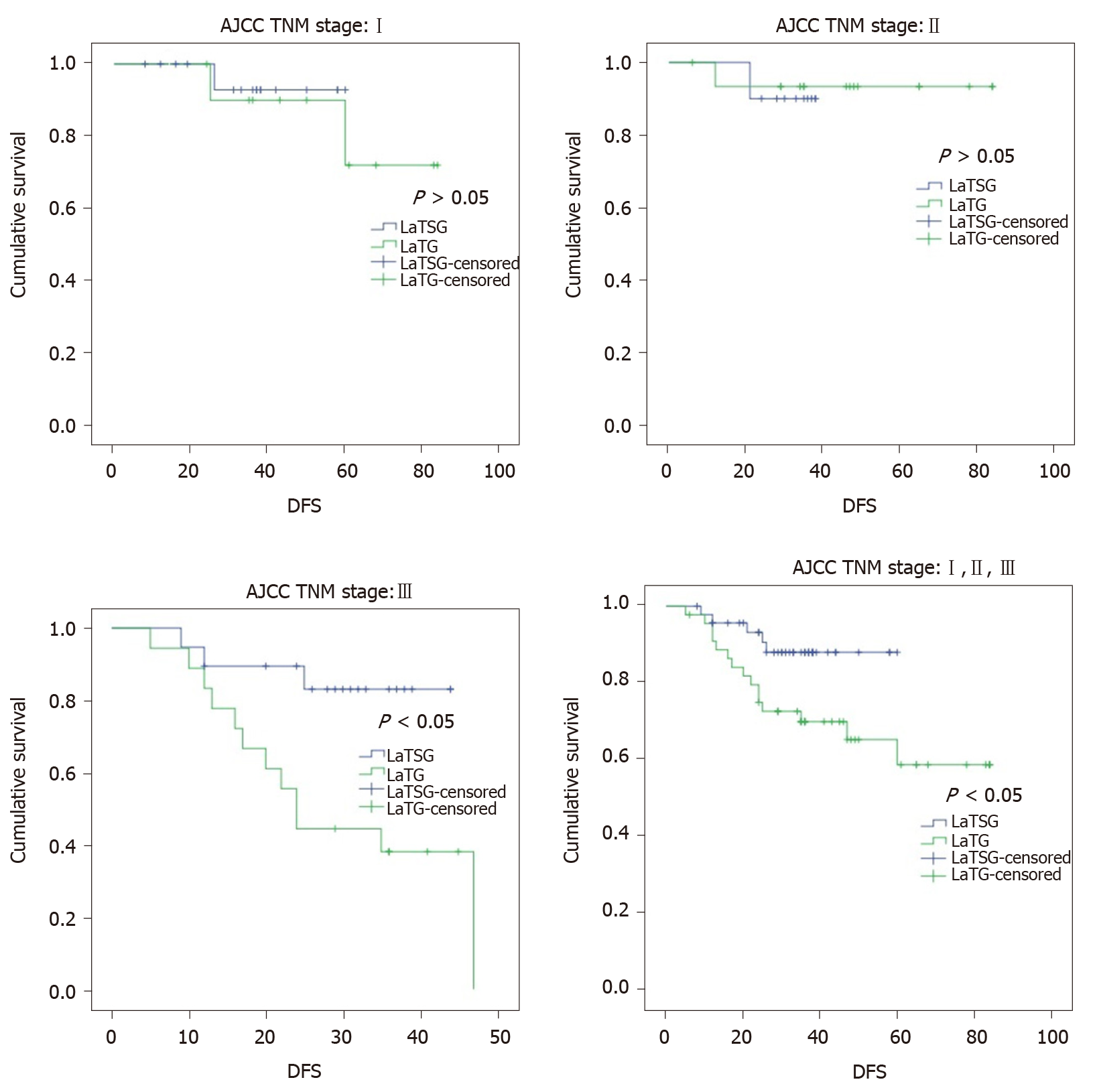
Kaplan–Meier analysis also showed a significant difference in disease-free survival between the two groups (P = 0.022) (Figure 6). The 3-year disease-free survival rates in the LaTSG and LaTG groups were 86.7% and 57.3%, respectively (P < 0.05). Multivariate analysis shows that pTNM stage (P = 0.046) was the only independent prognostic factor for disease-free survival (Table 6). Subgroup analysis showed that compared to LaTG, LaTSG improved the disease free survival of patients with stage III cancer, but not for other stages.
| Variable | Univariate | P value | Multivariate | P value |
| HR (95%CI) | HR (95%CI) | |||
| Age, yr (≤ 65) | 0.505 (0.209-1.221) | 0.129 | - | - |
| Sex (female) | 0.795 (0.288-2.192) | 0.658 | - | - |
| BMI (≤ 25 kg/m2) | 0.675 (0.259-1.761) | 0.422 | - | |
| ASA (I/II/III) | 1.864 (0.277-12.523) | 0.522 | - | - |
| Previous abdominal operation | 0.045 (0.000-188.621) | 0.467 | - | - |
| Primary Site (L/M/LM) | 0.993 (0.715-1.377) | 0.964 | - | - |
| Tumor size, (> 4 cm) | 2.535 (1.035-6.208) | 0.042 | 1.740 (0.706- 4.294) | 0.229 |
| Neoadjuvant chemotherapy (Yes) | 1.338 (0.442-4.051) | 0.606 | - | - |
| Tumor grade (W/M/P/U) | 0.852 (0.580-1.252) | 0.416 | - | - |
| Lauren classification (I/D/M) | 0.970 (0.523-1.798) | 0.923 | - | - |
| pTNM stage (I/II/III) | 3.317 (1.566-7.023) | 0.002 | 3.353 (1.558-7.214) | 0.002 |
| Lymphovascular invasion (Yes) | 2.209 (0.906-5.383) | 0.081 | - | - |
| Neural infiltration (Yes) | 1.729 (0.703-4.253) | 0.233 | - | - |
| Surgical type (LaTSG/LaTG) | 2.859 (1.027-7.958) | 0.044 | 2.776 (0.981-7.854) | 0.054 |
| Operation time (> 210 min) | 1.115 (0.450-2.761) | 0.814 | - | - |
| Estimated blood loss (> 200 mL) | 1.273 (0.526-3.080) | 0.592 | - | - |
| Intraoperative blood transfusion (Yes) | 0.938 (0.274-3.208) | 0.919 | - | - |
| No. of retrieved lymph nodes (> 30) | 1.643 (0.593-4.553) | 0.339 | - | - |
| Proximal margin (> 3 cm) | 0.904 (0.373-2.192) | 0.823 | - | - |
| Distal margin (> 3 cm) | 0.537 (0.219-1.319) | 0.175 | - | - |
In the present study, we evaluated the efficacy of LaTSG compared to LaTG in patients with advanced middle-third GC, and the findings demonstrated that long-term survival was better for those who underwent LaTSG than for those who underwent LaTG.
In this study, our inclusion criteria included non-Bormman type IV tumors, in which the lateral resection margin was at least 3 cm from the tumor. Bormman type IV tumors were excluded because of difficulty in obtaining a negative margin. It is advisable to utilize frozen pathology to obtain complementary diagnostic information, despite the risk for false-negatives. Preoperative markers or intraoperative guidance is necessary so that the tumor does not penetrate any serous membranes. If the margin biopsy was positive for tumor, LaTG, instead of LaTSG, was performed. In this study, all patients received R0 resection, and whether proximal or distal margin was greater than 3 cm was not a prognostic factor for overall survival, as has also been reported in previous reports[12-14].
With respect to the number of retrieved LNs, the LaTG group had a significantly larger number than the LaTSG group (P < 0.05). The main reason for this difference is that the LN dissection extent differed between the two groups. In the LaTSG group, we did not resect the No.2, No.4sa, and No.11d LNs, which were dissected in the LaTG group, in accordance with the Japanese GC treatment guidelines[2]. With respect to No. 4sa, it is possible to remove most of the lymph nodes along the short gastric vessels. However, a complete dissection, which also includes the nodes around the superior pole of the spleen, should be avoided, so as to preserve blood supply to the remnant stomach. This is especially important when the tumor is located within the lesser curvature of the stomach.
Many patients undergoing TG suffer from iron and/or vitamin B12 deficiencies due to malabsorption. The absence of gastric acid secretion and a lack of intrinsic factor have been reported to cause poor absorption of these nutrients, resulting in clinically evident anemia or neuropathy. Previous retrospective studies have documented that distal gastrectomy has advantages over TG in postoperative nutritional status and quality of life of patients[15,16]. Moreover, previous studies have indicated that the gastrectomy itself may induce iron deficiency, because less oral intake of food, and thereby of dietary Fe, occurs due to the small stomach volume.
Patients who underwent LaTSG showed significantly higher HGB and RBC levels at 12 mo post-surgery than those who underwent LaTG. Furthermore, significant differences in ALB, TP, and PLAB levels were found between the two groups. In contrast, we did not find a significant difference between the LaTG and LaTSG groups with respect to Fe absorption. Nevertheless, in aggregate, our data suggested that partial preservation of the remnant stomach increased the absorption of some nutrients compared to total resection.
LaTSG is a feasible and safe technique in terms of the operation time, estimated blood loss, and intraoperative blood transfusion. In our analysis, the frequency of anastomotic complications was significantly lower in the LaTSG group than in the LaTG group, suggesting that LaTSG has advantages over LaTG by reducing the incidence of postoperative anastomotic complications. Our results are supported by the study of Lee et al[17], who reported a higher rate of postoperative complications with LaTG than with laparoscopic-assisted distal gastrectomy, especially with respect to the incidence of anastomotic stricture. This reduced incidence of postoperative complications could be related to the fact that LaTG is more complicated than LaTSG. In particular, when using a tubular stapler, sophisticated purse-string suture and anvil placement were severely limited by the narrow space.
Studies largely agree that subtotal gastrectomy for distal gastric adenocarcinoma improves the quality of life without causing any adverse effects on long-term survival[15,18,19]. Likewise, in this study, 1 year postoperatively, the anti-reflux effect was much better in patients who underwent LaTSG than in those who underwent LaTG. Indeed, LaTSG retains the complete cardia structure and further preserves a lager remnant stomach than subtotal gastrectomy, which collectively greatly reduces the incidence of postoperative reflux oesophagitis, without any anastomotic stricture.
For the treatment of malignant tumors, the focus has always been on survival. Other studies have showed a survival advantage for subtotal procedures[20]. In our study, the overall survival was better for patients in the LaTSG group than those in the LaTG group, and the 3-year survival rates in the LaTSG and TG groups were 85.6% and 67.2%, respectively (P < 0.05).
Specifically, in previous studies as well as in our study, subgroup analysis showed that compared to TG, distal gastrectomy was shown to improve the survival of patients with stage III cancer, but not for other stages[16,18]. According to the above analyses, compared with LaTG, LaTSG did not increase local recurrence of advanced middle-third stomach carcinoma, but prolonged the overall survival.
There is some bias in this study. This study was a retrospective study and involved only a few patients. Further randomized control trials and more enrolled patients are needed to help validate our findings. We have yet to assess the patients’ quality of life with a long-term follow-up duration. Furthermore, we did not test vitamin B12 and vitamin E levels, which could better reflect the nutritional status of patients after surgery.
Our results suggest that LaTSG is a safer procedure than LaTG in both short and long-term outcomes. The long-term survival of patients who undergo LaTSG is better than that of patients who undergo LaTG. Furthermore, LaTSG may have an advantage over LaTG in improving the postoperative nutritional status and preventing reflux oesophagitis.
Consensus on the optimal surgical treatment strategy for advanced gastric cancer located in the middle of the stomach is yet to be established. Most patients ultimately undergo total gastrectomy (TG).
TG is associated with reduced nutritional status, and higher rates of postoperative complications. We modified the laparoscopic subtotal gastrectomy procedure to treat advanced middle-third gastric cancer.
This study aimed to evaluate short-term postoperative patient outcomes, nutritional status, and long-term oncological outcomes to assess the safety and efficacy of laparoscopic-assisted tailored subtotal gastrectomy (LaTSG) compared to those of laparoscopic-assisted total gastrectomy (LaTG).
This study retrospectively analyzed surgical and oncological outcomes and postoperative nutritional status in 92 consecutive patients with middle-third gastric cancer who underwent LaTSG (47 cases) or LaTG (45 cases) at Department of Pancreatic Stomach Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College between 2013 and 2017.
The incidence of postoperative morbidities was lower in the LaTSG group than in the LaTG group (4.2% vs 17.8%, P < 0.05). At postoperative 12 mo, albumin, prealbumin, total protein, hemoglobin levels, and red blood cell counts were significantly higher in the LaTSG group than in the LaTG group (P < 0.05). Endoscopic examination demonstrated that reflux oesophagitis was more common in the LaTG group (0% vs 11.1%, P < 0.05). Kaplan–Meier analysis showed a significant improvement in the overall survival (OS) and disease free survival (DFS) in the LaTSG group.
LaTSG is a safer procedure than LaTG in terms of both short and long-term outcomes. The long-term survival of patients who undergo LaTSG is better than that of patients who undergo LaTG.
Further randomized control trials and more enrolled patients are needed to help validate our findings.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ehrhardt M, Shih B, Tsikas D S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11444] [Cited by in F6Publishing: 12500] [Article Influence: 1562.5] [Reference Citation Analysis (0)] |
| 2. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1575] [Cited by in F6Publishing: 1800] [Article Influence: 257.1] [Reference Citation Analysis (0)] |
| 3. | Jang YJ, Park MS, Kim JH, Park SS, Park SH, Kim SJ, Kim CS, Mok YJ. Advanced gastric cancer in the middle one-third of the stomach: Should surgeons perform total gastrectomy? J Surg Oncol. 2010;101:451-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Kawamura Y, Satoh S, Suda K, Ishida Y, Kanaya S, Uyama I. Critical factors that influence the early outcome of laparoscopic total gastrectomy. Gastric Cancer. 2015;18:662-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Suda K, Nakauchi M, Inaba K, Ishida Y, Uyama I. Minimally invasive surgery for upper gastrointestinal cancer: Our experience and review of the literature. World J Gastroenterol. 2016;22:4626-4637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Rino Y, Yukawa N, Sato T, Yamamoto N, Tamagawa H, Hasegawa S, Hayashi T, Atsumi Y, Oshima T, Yoshikawa T, Masuda M, Imada T. Vitamin E deficiency begins within 6 mo after gastrectomy for gastric cancer. World J Surg. 2014;38:2065-2069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Lim CH, Kim SW, Kim WC, Kim JS, Cho YK, Park JM, Lee IS, Choi MG, Song KY, Jeon HM, Park CH. Anemia after gastrectomy for early gastric cancer: long-term follow-up observational study. World J Gastroenterol. 2012;18:6114-6119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Jiang X, Hiki N, Nunobe S, Nohara K, Kumagai K, Sano T, Yamaguchi T. Laparoscopy-assisted subtotal gastrectomy with very small remnant stomach: a novel surgical procedure for selected early gastric cancer in the upper stomach. Gastric Cancer. 2011;14:194-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34:1350-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 470] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 10. | Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Kim MC; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg. 2019;270:983-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 276] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 11. | Sami SS, Ragunath K. The Los Angeles Classification of Gastroesophageal Reflux Disease. Video J Encyclopedia GI Endosc. 2013;1:103-104. [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Lee JH, Kim YI. Which Is the Optimal Extent of Resection in Middle Third Gastric Cancer between Total Gastrectomy and Subtotal Gastrectomy? J Gastric Cancer. 2010;10:226-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Kim MG, Lee JH, Ha TK, Kwon SJ. The distance of proximal resection margin dose not significantly influence on the prognosis of gastric cancer patients after curative resection. Ann Surg Treat Res. 2014;87:223-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Ji X, Yan Y, Bu ZD, Li ZY, Wu AW, Zhang LH, Wu XJ, Zong XL, Li SX, Shan F, Jia ZY, Ji JF. The optimal extent of gastrectomy for middle-third gastric cancer: distal subtotal gastrectomy is superior to total gastrectomy in short-term effect without sacrificing long-term survival. BMC Cancer. 2017;17:345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal vs total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 260] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Liu Z, Feng F, Guo M, Liu S, Zheng G, Xu G, Lian X, Fan D, Zhang H. Distal gastrectomy vs total gastrectomy for distal gastric cancer. Medicine (Baltimore). 2017;96:e6003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, Cho SJ, Lee JY, Kim CG, Choi IJ, Kook MC, Park SR, Kim MJ, Lee JS. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol. 2009;100:392-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Mocan L, Tomus C, Bartos D, Zaharie F, Ioana I, Bartos A, Puia C, Necula A, Mocan T, Iancu C. Long term outcome following surgical treatment for distal gastric cancer. J Gastrointestin Liver Dis. 2013;22:53-58. [PubMed] [Cited in This Article: ] |
| 19. | Angelov KG, Vasileva MB, Grozdev KS, Toshev SY, Sokolov MB, Todorov GT. The impact of the extent of surgical resection on survival of gastric cancer patients. Onco Targets Ther. 2016;9:4687-4694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol. 2015;22:2965-2971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |