Published online Nov 21, 2020. doi: 10.3748/wjg.v26.i43.6782
Peer-review started: May 7, 2020
First decision: May 15, 2020
Revised: May 28, 2020
Accepted: October 1, 2020
Article in press: October 1, 2020
Published online: November 21, 2020
Colitis-associated cancer (CAC) accounts for 2%-3% of colorectal cancer (CRC) cases preceded by inflammatory bowel diseases (IBD) such as Crohn's disease and ulcerative colitis. Intestinal microbiota has been reported to play a central role in the pathogenesis of IBD and CAC. Recently, numerous prebiotics and probiotics have being investigated as antitumor agents due to their capacity to modulate inflammatory responses. Previous studies have indicated that lactic acid bacteria could be successfully used in managing sporadic CRC, however little is known about their role in CAC.
To investigate the effect of the probiotic Lactobacillus bulgaricus (L. bulgaricus) during the development of an experimental model of colitis associated colon cancer (CAC).
C57BL/6 mice received an intraperitoneal injection of azoxymethane (10 mg/kg), followed by three cycles of sodium dextran sulphate diluted in water (5% w/v). Probiotic group received daily L. bulgaricus. Intestinal inflammation was determined by scoring clinical signs. Cytokines levels were determined from colon and/or tumor samples by ELISA BD OptEIATM kits. The level of significance was set at P < 0.05. Graphs were generated and statistical analysis performed using the software GraphPad Prism 6.0.
L. bulgaricus treatment inhibited of total tumor volume and mean size of tumors. In addition, the probiotic also attenuated the clinical signs of intestinal inflammation inducing a decrease in intestinal and tumor levels of IL-6, TNF-α, IL-17, IL-23 and IL-1β.
Our results suggest a potential chemopreventive effect of probiotic on CAC. L. bulgaricus regulates the inflammatory response and preventing CAC.
Core Tip: Recent studies suggested that consideration of the intestinal microbiota has an essential role in carcinogenesis. Probiotic supplementation is an alternative means of favourably modulating the intestinal microbiota. In this study, we investigate the effect of Lactobacillus bulgaricus (L. bulgaricus) during the development of an experimental model of colitis-associated colon cancer. Our results evidence an anti-inflammatory role and consequent antitumor effect of L. bulgaricus on colitis-associated cancer that may be used as a promising tool for the prevention and treatment of colitis-associated cancer.
- Citation: Silveira DSC, Veronez LC, Lopes-Júnior LC, Anatriello E, Brunaldi MO, Pereira-da-Silva G. Lactobacillus bulgaricus inhibits colitis-associated cancer via a negative regulation of intestinal inflammation in azoxymethane/dextran sodium sulfate model. World J Gastroenterol 2020; 26(43): 6782-6794
- URL: https://www.wjgnet.com/1007-9327/full/v26/i43/6782.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i43.6782
Colorectal cancer (CRC) remains one of the most incident type of cancer worldwide, being the third and second most frequently cancer diagnosed in men and women, respectively[1]. Colitis-associated cancer (CAC) specifically accounts for 2%-3% of CRC cases preceded by inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis[2]. The link between inflammation and cancer was firstly recognized in 1863 and has been recently exemplified by CAC. Patients with IBD have a higher risk for developing CRC and are affected by the disease earlier than patients with sporadic CRC[3,4].
Currently, it has become increasingly evident that intestinal microbiota plays a crucial role in the pathogenesis of IBD and CRC. Changes in intestinal microbiota have been reported in patients with colon cancer, supporting this idea[5]. Among the gut microorganisms, probiotic bacteria may be defined as live microbial food supplements that confer benefits to the health of the consumer (WHO), including reduction of pathogen colonization by competition[6], improvement in vitamin synthesis and nutrients absorption, stimulation of epithelial cell proliferation and differentiation, fortification of intestinal barrier and optimization of intestinal transit[7].
In addition to the direct benefit of probiotics on the improvement of the host gut microbiota, probiotics have received considerable attention due to their anti-carcinogenic activities, mainly in CRC[8]. The underlying mechanisms for their anti-tumor effects are versatile and include: Modulation of host immune responses, such as proliferation of regulatory T cells, activation of macrophages and dendritic cells, and production of immunoglobulins and cytokines[9]; alteration of intestinal microbiota metabolism[10]; regulation of cell death, apoptosis, cell cycle, proliferation, invasion and metastasis[11]; competition with pathogenic bacteria[11]; and inactivation of carcinogenic compounds[12].
The most common microorganisms used as probiotics comprise a group of bacteria named lactic acid bacteria (LAB) that produces lactic acid as the primary metabolite of sugar metabolism, such as Lactobacillus and Bifidobacterium[13,14]. Although previous studies have indicated that LAB could be successfully used in managing food allergies, diarrhea, IBDs and sporadic CRC[15-17], little is known about its role in CAC. In this study, we sought to investigate the effects of the probiotic Lactobacillus bulgaricus (L. bulgaricus) in colitis-associated carcinogenesis.
In the present study, we used male C57BL/6 wild type (WT) mice, between 4-6 wk old and weighing between 20-25 g. The animals were purchased from the Animal Facility of the University of São Paulo (USP) and housed at the facility of Ribeirão Preto College of Nursing - EERP/USP (Ribeirão Preto, SP, Brazil) under controlled temperature conditions (25 ± 2 °C) with 12/12 photoperiod hours. Water and food were available ad libitum. All experiments were handled in accordance with institutional ethical guidelines, and the study was approved by the Ethics Committee on Animal Research from the University of São Paulo (CEUA PUSP-RP: No. 14.1.418.53.1).
Lactobacillus delbrueckii ssp bulgaricus, LOT No. FK0201, identification LB-G040, Chinese origin, was purchased from Liane Drugstore, Ribeirão Preto, SP, Brazil and stored in at 4 °C. For mice treatment, 1 × 109 CFU were diluted in 200 μL of PBS and orally given to each mouse, 3 times a week during all experimental period. Prior to tumor induction, mice were randomly distributed in 2 groups (n = 10) and treated with PBS (control group) or L. bulgaricus (Lb group) by gavage (0.2 mL/mouse) for one week.
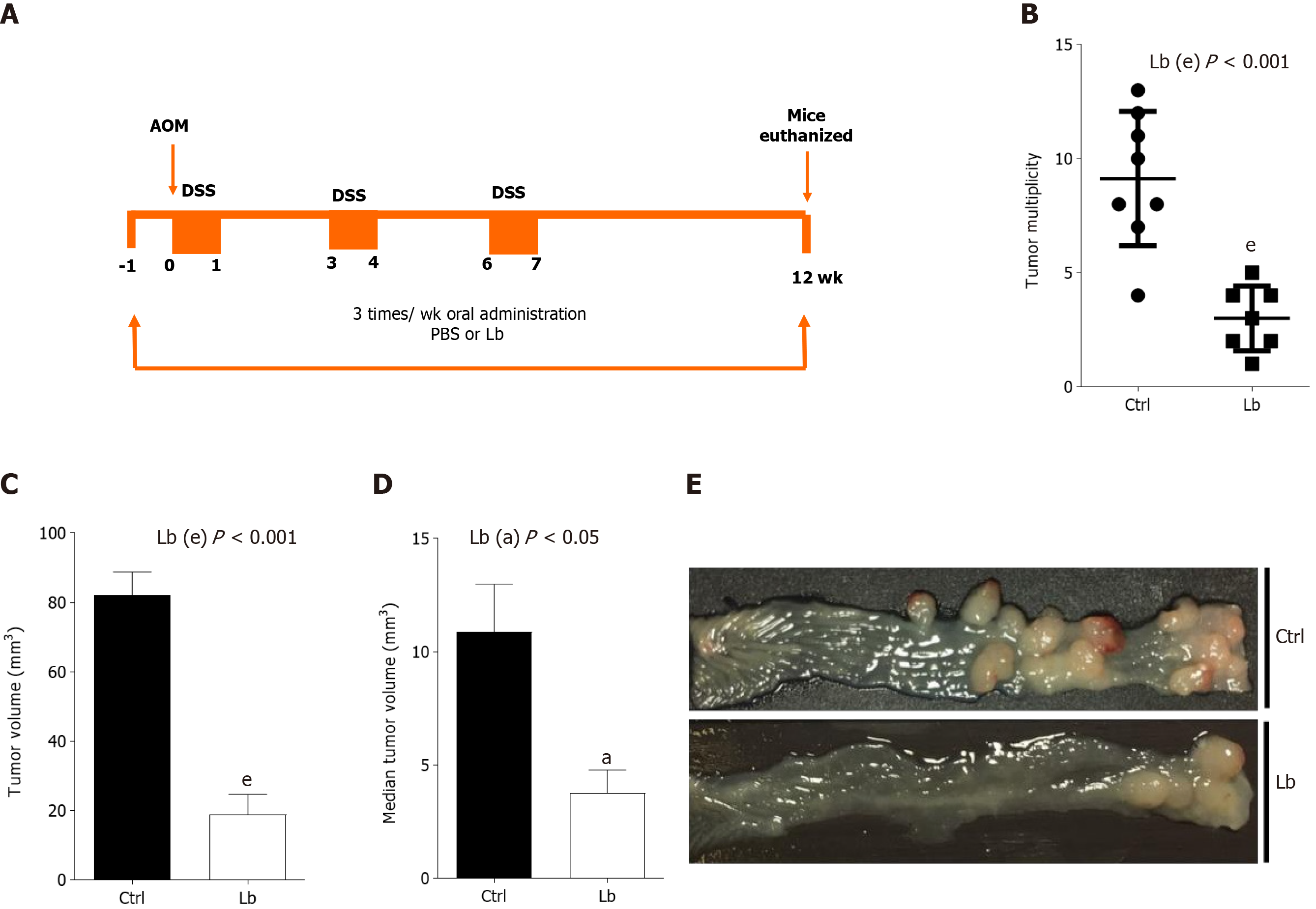
For CAC induction, mice were intraperitoneally (i.p.) injected with a single dose (10 mg/kg in 300 μL solution) of azoxymethene (AOM, Sigma-Aldrich), followed by 3 cycles of one week of 2.5% dextran sulfate sodium (DSS) diluted in drinking water intercalated for 2 wk of normal water[18]. Mice were euthanized 12th week after CAC induction (Figure 1A).
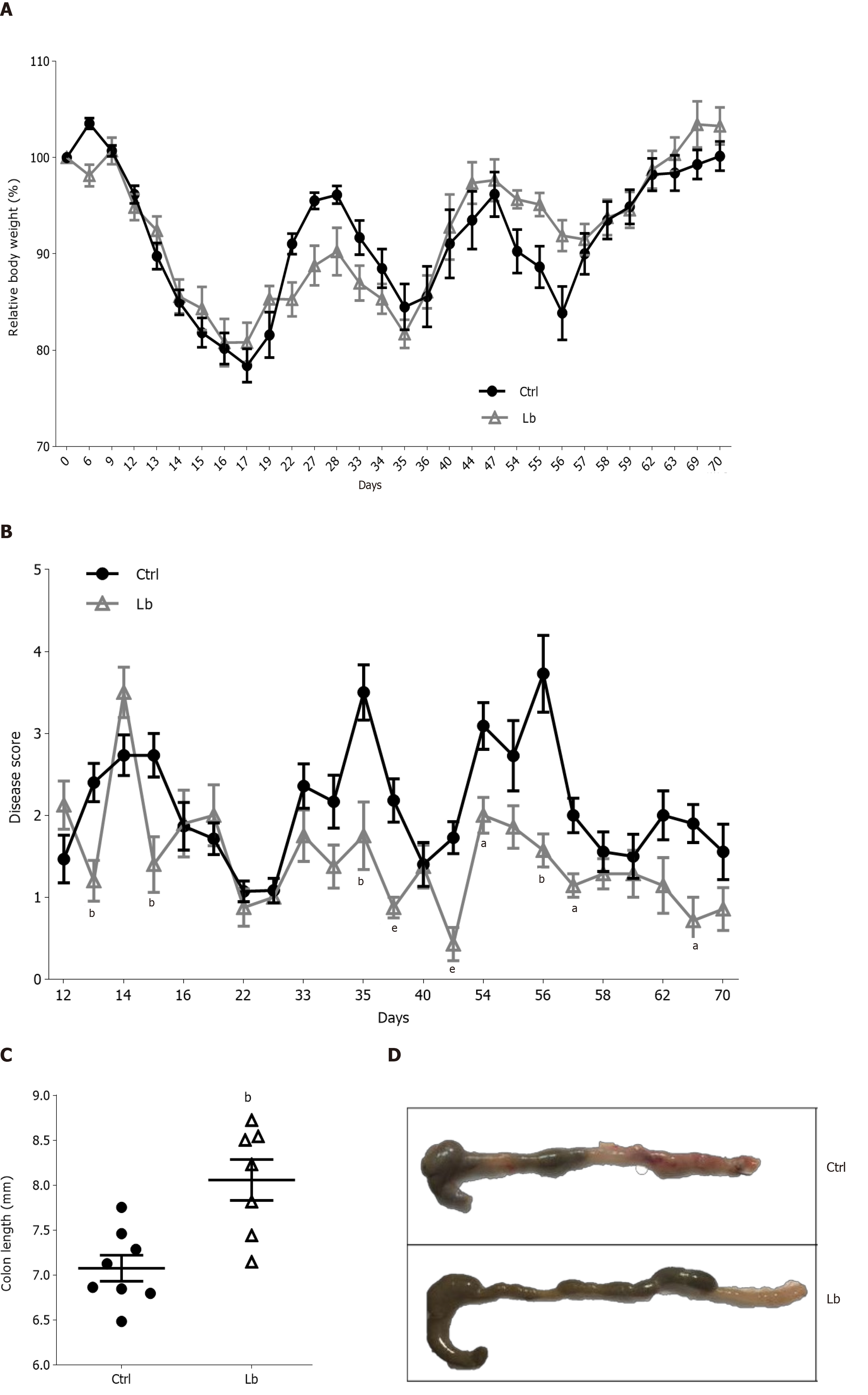
Intestinal inflammation in vivo, or disease score, was determined by scoring clinical signs as previously described[19]. Briefly, we used a scoring system in which one point (1.0) was attributed to each signal presented by the mouse, including: Weight loss ≥ 5% and < 10% of body weight compared to the previous day; presence of humid perianal region; presence of diarrhea; blood in the stool or perianal region; hyporativity and piloerection. When weight loss was ≥ 10% 2 points were attributed to the "weight loss" signal. Finally, the final sum of these points determined the clinical score of the disease.
The severity of intestinal inflammation was also assessed by measuring the length of the entire large intestine. After euthanasia, colons were collected, carefully placed on a clean surface and photographed. The images were calibrated by the presence of a graduated ruler that served as a scale for the analyzes. Subsequently, images of the large intestine were evaluated using ImageJ software.
After euthanasia, the colons were longitudinally opened, washed and examined with regards to presence of tumors. The multiplicity of tumors was verified for each animal in the experimental groups. The dimensions of the colorectal tumors were measured with pachymeter and the volumes were calculated by the formula: (Width)2 × length / 2[20]. Total tumor volume, indicates the sum of the volumes of all tumors found in each colon. Mean tumor volume refers to the mean tumor size, i.e., total tumor volume divided by the number of tumors of each colon.
Distal colon parts were fixed in 4% p-formaldehyde in phosphate-buffered formalin and unblocked in paraffin. Tissue sections (4.0 μm) were prepared from the paraffin-embedded tissue blocks, stained with hematoxylin and eosin and evaluated in a blinded fashion by an experienced pathologist (MOB). Normal colon, polyp without dysplasia, adenoma with low-grade dysplasia, adenoma with high-grade dysplasia, and invasive adenocarcinoma were identified in the different groups.
Cytokines levels were determined from colon and/or tumor samples. Tissues were collected, weighed and immediately homogenized in PBS in the presence of protease inhibitors (Roche) using a tissue homogenizer (Polytron System PT 1200E). The material was then centrifuged at 6000 rpm for 15 min at 4 °C and the supernatant collected, aliquoted and stored at -80 °C until the time of use. The concentrations of TNF-α, IL-6, IL-12 (p70), IL-17, IFN-γ, IL-1 β, IL-10, TGF-β, IL-23 were determined by ELISA BD OptEIATM kits (BD Biosciences Pharmingen) or DuoSet (R&D Systems). The protocol was performed according to the manufacturers’ instructions. Cytokine concentrations were determined with reference to the linear regression line obtained with the serial dilution data of each recombinant mouse cytokine.
Data were analyzed using the statistical program GraphPad Prism version 6. Parametric and non-parametric samples were analyzed by one-way Analysis of Variance (ANOVA test) and Kruskal-Wallis test followed by Dunn’s test, respectively. The probability was considered statistically significant if P < 0.05. Results were expressed as mean ± SEM.
To investigate whether L. bulgaricus is able to inhibit the progression of CAC, we compared tumor development in AOM/DSS-induced mice treated or not with the probiotic (Figure 1A). As shown in Figure 1, animals from both groups developed tumors at the end of the experimental protocol, whereas those treated with the probiotic developed fewer and smaller tumors. Control mice developed between 4-13 colorectal tumors, whereas animals treated with L. bulgaricus developed only 1-5 (Figure 1B). L. bulgaricus-treated animals showed a total tumor volume (Figure 1C) and a mean tumor volume (Figure 1D and E) about 4.4-fold and 3-fold lower, respectively. However, no difference was observed in the incidence of tumors (data not shown).
Once inflammation plays a critical role in CAC carcinogenesis, we evaluated intestinal inflammation in AOM/DSS-induced mice treated with L. bulgaricus by three different parameters: Body weight, disease score and colon length. Although we did not observe differences in body weight loss between control and L. bulgaricus-treated (Figure 2A), we found differences in clinical signals in L. bulgaricus-treated mice, which showed a lower clinical score on the 13th and 15th days after tumor initiation (Figure 2B).
In addition to the attenuation of intestinal inflammation score, we observed that the treatment with L. bulgaricus reduced the DSS-induced shortening of the colon (Figure 2C and D) so that the control group had a shorter colon extension when compared to Lb group (Figure 2C and D). After histopathological evaluation of the tumor sections, we observed that, regardless of treatment, both groups of mice presented morphologically similar neoplastic lesions. In general, colorectal tumors were lesions of the polypoid adenoma type with variation between low and high degrees of dysplasia and mixed inflammation (Supplementary Figure 1).
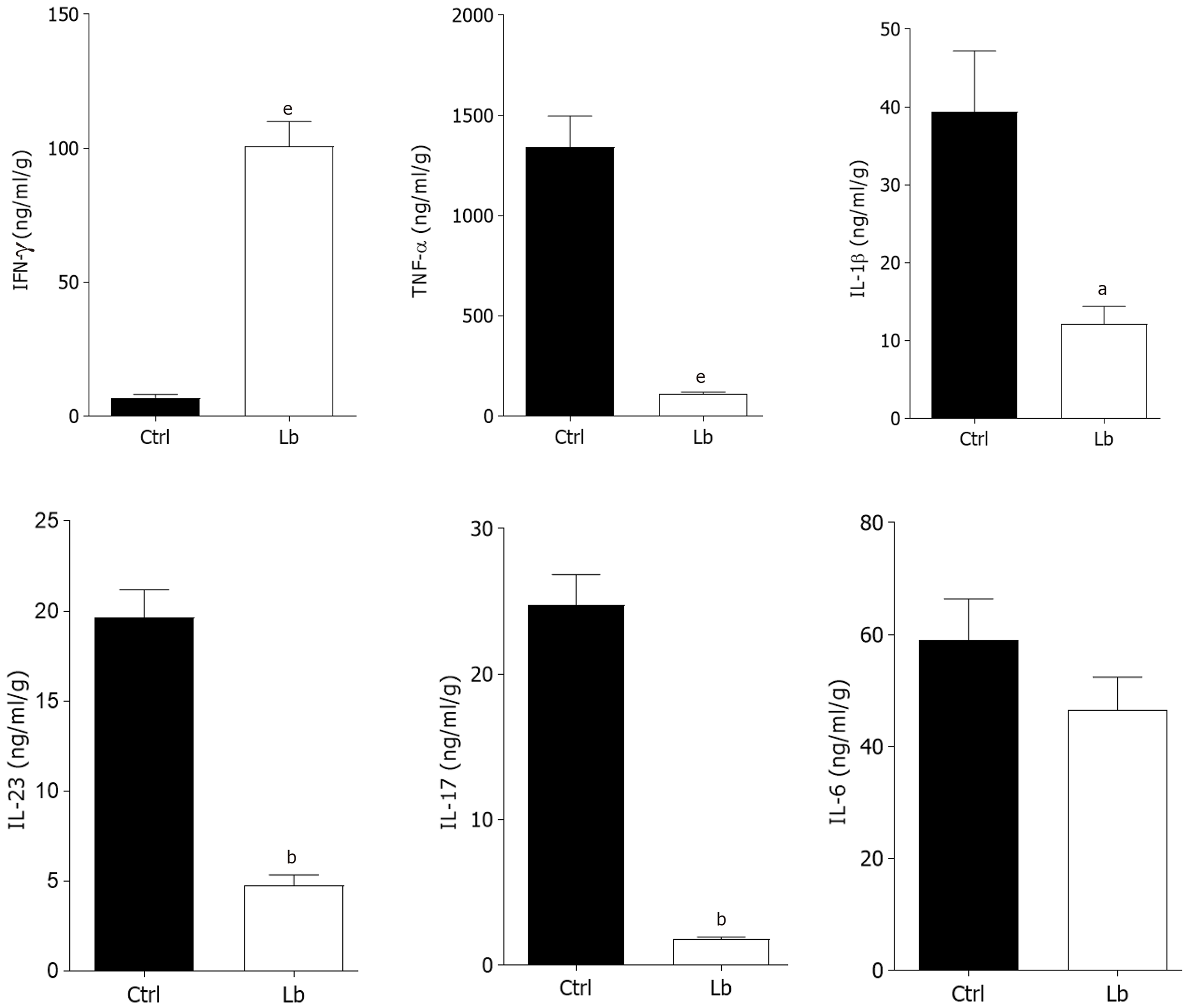
Once we observed that L. bulgaricus regulates gut inflammation, we also measured the intestinal concentration of inflammatory mediators involved in CAC pathogenesis. In segments of the large intestine that did not present tumors (inflamed colon) we observed a reduction of at least 2-fold in the levels of the cytokines TNF-α, IL-1 β, IL-23 and IL-17 in L. bulgaricus-treated mice in comparison to controls (Figure 3). In contrast, increased concentrations of IFN-γ were also observed in Lb group (Figure 3). We did not observe differences in IL-6 levels (Figure 3).
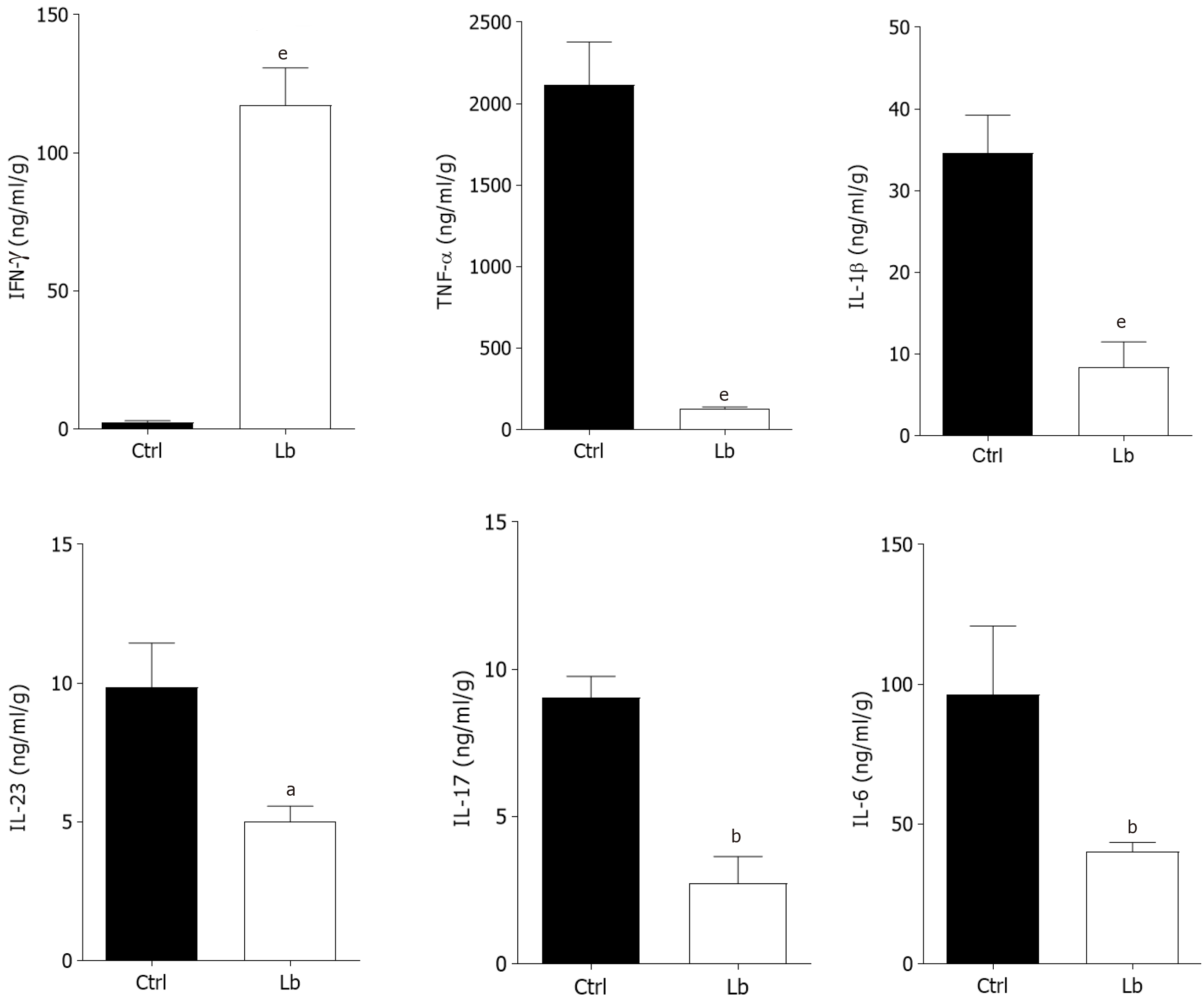
Regarding the cytokines measured in tumor tissues, we observed a pattern similar to that found in the inflamed colon (Figures 3 and 4). We observed a negative regulation of all analyzed cytokines, including IL-6, in mice treated with the probiotic (Figure 4), and an increase in IFN-γ levels in this group (Figure 4).
Recently, prebiotics and probiotics are being investigated as antitumor agents due to their capacity to modulate inflammatory responses. Studies have shown that probiotics may exert positive effects at different stages of colorectal carcinogenesis: Antimutagenic activity; inactivation of mutagens or carcinogens; reduction of intestinal pH; immunomodulatory effects; intestinal microbiota modulation; regulation of apoptosis and cell differentiation; and tyrosine kinase signaling pathway inhibition[21]. In addition, among probiotics, the genera Lactobacillus has been reported to exert immuno-regulatory effects, including modulation of innate immune responses and promotion of humoral and cellular immunity[22], suppression of pathogens and restoration of gut microbiota homeostasis[23] and improvement of IBD[24]. In the present study, we used an experimental model of CAC to investigate the effects of the probiotic L. bulgaricus on colon carcinogenesis. We showed that L. bulgaricus negatively regulated tumor progression, resulting in an expressive reduction of total tumor volume and mean size of tumors. Furthermore, the probiotic also attenuated the clinical signs of intestinal inflammation inducing a decrease in intestinal and tumor levels of IL-6, TNF-α, IL-17, IL-23 and IL-1β.
Similarly, it has been recently observed that L. salivary and L. fermentum reduced the proliferation of colon cells in sporadic CRC[25,26]. Given that cell proliferation defines the speed of cancer development[27], probiotics capable of modulating cell proliferation are of great interest to prevent tumor growth and/or metastasis.
Our results also demonstrated that the probiotic L. bulgaricus attenuated intestinal inflammation by decreasing intestinal and tumor levels of IL-6, TNF-α, IL-17, IL-23 and IL-1β. Finally, we also demonstrated an increase in IFN-γ levels in animals treated with L. bulgaricus. Due to their involvement in the pathogenesis of IBDs and CAC, the development of strategies that target the inflammatory cytokines IL-6, TNF-α, IL-17, IL-23 and IL-1β is of potential interest in the therapeutic field. In a clinical trial with CRC patients a significant reduction in the blood levels of the proinflammatory cytokines TNF-α, IL-12, IL-1β, IL-6, IL-17 and IL-22, was observed after six months of a mix of probiotics consumption[28].
The cytokine IL-1β is found at high levels in several types of cancers and in CRC its expression is increased throughout the tumor progression[29]. IL-1β activates Wnt pathway in colon cancer cells promoting their growth and invasion[30]. TNF-α is an important inflammatory mediator whose effects have been implicated in several cellular events, such as cell proliferation, differentiation and cell death[31]. Anti-TNF therapies have been successfully used in IBD patients which confirms the crucial role of this cytokine in IBD and CAC development[32]. Increased expression of TNF-α promotes cancer development through both leukocytic and nonhematopoietic cell TNFR1 expression in colonic tissue has been reported in studies using CAC model induced by AOM and DSS[33,34].
Up-regulation of IL-17 has also been reported in colitis and colorectal tumors[35]. The differentiation of Th17 cells may occur in the presence of different combinations of the cytokines TGF-β, IL-6, IL-1β and/or IL-23, while its maintenance requires only IL-23 and/or IL-1β[36]. Although we have not attempted to elucidate the molecular mechanisms that mediate the inhibitory role of L. bulgaricus in the regulation of IL-17 and IL-23 in our study, we hypothesize that the reduced expression of TNF-α is involved. In fact, previous studies have clearly shown that NF-κB, a critical mediator of TNF-α signaling, regulates the transcription of the IL-23p19 gene[37]. A recent finding showed that the probiotics Bifidobacterium breve and Lactobacillus rhamnosus GG inhibit LPS-induced expression of IL-23 in intestinal cells cultured in a condition of histone acetylation inhibition and increased DNA methylation[38]. This finding might provide another potential mechanism for the L. bulgaricus-mediated negative regulation of IL-23.
Only a few trials in IBD patients have examined the composition of intestinal microbiota before and after supplementation therapy, so the effect (if any) of administering probiotics to the resident microbiota is not fully understood. However, it has been suggested that probiotics can change the intestinal ecosystem by generating an ecological environment that is unfavorable to the growth of noxious species, increasing the number of Lactobacillus and Bifidobacteria and stabilizing the intestinal microbiota[39,40].
Dysregulation of gut microbiota has been associated with increased inflammation and the administration of probiotics have been reported to prevent chronic inflammatory diseases[41]. In recent years there has been growing interest in the possible application of probiotics as a part of combination therapy with conventional treatment of cancer[41-43]. However, studies investigating probiotics effects in patients with CRC are still very limited. For clinical application in humans many other studies, mainly randomized controlled trials would be needed to better evaluate the dosage, duration of the intervention and host physiology for confirm these findings[41].
Several researches have indicated that the use of probiotics might improve beneficial microbiota, induce the release of antimicrobials and anticarcinogenic agents that help to remove carcinogens, and modulate immune responses that decrease intestinal inflammation in CRC patients[41,44,45]. Here, we have shown that L. bulgaricus inhibited CAC via a negative regulation of intestinal inflammation. Although a deeper characterization of the molecular mechanisms underlying L. bulgaricus anti-inflammatory activity, the strength of our findings indicates a relevant and evidenced phenotypic pattern, which may be important in IBD field to prevent inflammation-associated tumorigenesis. To the best of our knowledge this is the first study to investigate and provide promising evidences of a preventive effect of the probiotic L. bulgaricus in cancer development in an experimental model of CAC.
In conclusion, our results show an anti-inflammatory and antitumor role of L. bulgaricus in colitis-associated carcinogenesis which may play an important role in prevention and treatment of CAC in the future. However, although the antitumor effects of this probiotic are promising, the mechanisms by which they occur still need to be better elucidated. Nevertheless, our study is extremely important as regards to the potential use of L. bulgaricus as a new therapeutic agent that can mediate and/or regulate the progression of CAC.
Intestinal inflammatory disorders are associated with the infiltration of immune cells and the proinflammatory release of cytokines that play a critical role in the onset and progression of colitis-associated cancer (CAC). Recent studies suggested that the intestinal microbiota has an essential role in carcinogenesis. Probiotic supplementation is an alternative means of favorably modulating the intestinal microbiota. Currently, it has become increasingly evident that intestinal microbiota plays a crucial role in the pathogenesis of inflammatory bowel diseases (IBD) and colorectal cancer. Moreover, increasing evidence suggests that probiotics prevent inflammation and carcinogenesis and several bacteria strains have been used for the prevention and treatment of the infectious colitis, IBD. Thus, probiotic modulation of intestinal microbiota has emerged as a potential chemo-preventive agent.
Although supplementation with probiotics have been reported to prevent CAC, little is known about the administration of strains of Lactobacillus bulgaricus (L. bulgaricus), as well as their impact on neoplastic changes in the intestinal mucosa. Our study may contribute to address the gaps in the literature of how this probiotic, dose and supplementation time used for this experimental model impact on colitis, serum cytokines and neoplastic development.
The purpose of this study is to investigate the effect of the probiotic L. bulgaricus during the development of an experimental model of CAC. Overall, this study intents to strengthen data from preclinical studies, encouraging clinical trials to investigate their role in preventing colitis and CAC in humans.
We used an experimental model of CAC. For mice treatment, 1 × 109 CFU were diluted in 200 μL of PBS and orally given to each mouse, 3 times a week during all experimental period. Prior to tumor induction, C57BL/6 mice were randomly distributed in 2 groups (n = 10) and treated with PBS (control group) or L. bulgaricus (Lb group) by gavage (0.2 mL/mouse) for one week. For CAC induction, mice were intraperitoneally (i.p.) injected with a single dose (10 mg/kg in 300 μL solution) of azoxymethene (Sigma-Aldrich), followed by 3 cycles of one week of 2.5% dextran sulfate sodium (DSS) diluted in drinking water intercalated for 2 wk of normal water. Mice were euthanized 12th week after CAC induction. Intestinal inflammation in vivo, or disease score, was determined by scoring clinical signs. The severity of intestinal inflammation was assessed by measuring the length of the entire large intestine. Also, the dimensions of the colorectal tumors were measured with pachymeter and the volumes were calculated by the formula: (width)2 × length/2. For histological analysis, distal colon parts were fixed in 4% p-formaldehyde in phosphate-buffered formalin and unblocked in paraffin. Tissue sections (4.0 μm) were prepared from the paraffin-embedded tissue blocks, stained with hematoxylin and eosin and evaluated in a blinded fashion by an experienced pathologist. Cytokines levels were determined from colon and/or tumor samples by ELISA. Statistical analyses were performed using GraphPad Prism version 6.0. A 2-tailed P value < 0.05 was considered to be statistically significant.
We have shown that L. bulgaricus treatment inhibited the total tumor volume and mean size of tumors. Although we did not observe differences in body weight loss between control and L. bulgaricus-treated, we found differences in clinical signals in L. bulgaricus-treated mice, which showed a lower clinical score on the 13th and 15th days after tumor initiation. In addition to the attenuation of intestinal inflammation score, we observed that the treatment with L. bulgaricus reduced the DSS-induced shortening of the colon. In segments of the large intestine that did not present tumors (inflamed colon) we also observed a reduction of at least 2-fold in the levels of the cytokines TNF-α, IL-1β, IL-23 and IL-17 in L. bulgaricus-treated mice in comparison to controls. In contrast, increased concentrations of IFN-γ were also observed in Lb group. Regarding the cytokines measured in tumor tissues, we observed a pattern similar to that found in the inflamed colon with a negative regulation of proinflammatory cytokines in mice treated with the probiotic and an increase in IFN-γ levels in this group. Overall, these findings highlight the protective effect of L. bulgaricus in the regulation of gut inflammation and preventing CAC development. Thus, further clinical trials are needed to confirm these preclinical insights.
We found an anti-inflammatory role and consequent antitumor effect of L. bulgaricus on CAC that may be used as a promising tool for the prevention and treatment of CAC. In summary, L. bulgaricus treatment during colitis-associated colorectal carcinogenesis model may be responsible for anti-inflammatory and antitumor role by lowering proinflammatory cytokine expression.
The present study has shown that L. bulgaricus inhibited CAC via a negative regulation of intestinal inflammation. Hence, has demonstrates promising evidence on L. bulgaricus probiotic has a preventive potential in CAC development. Therefore, clinical trials are needed to confirm this hypothesis and increase the therapeutic arsenal against CAC.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kameyama H, Otsuka M S-Editor: Huang P L-Editor: A P-Editor: Wang LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 50871] [Article Influence: 8478.5] [Reference Citation Analysis (44)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11573] [Cited by in F6Publishing: 12481] [Article Influence: 2080.2] [Reference Citation Analysis (3)] |
| 3. | Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17:230-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 4. | Zenlea T, Peppercorn MA. Immunosuppressive therapies for inflammatory bowel disease. World J Gastroenterol. 2014;20:3146-3152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 71] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 5. | Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, Corthier G, Tran Van Nhieu J, Furet JP. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One. 2011;6:e16393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 606] [Cited by in F6Publishing: 566] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 6. | Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1596] [Cited by in F6Publishing: 1664] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 7. | Hooper L, Summerbell CD, Higgins JP, Thompson RL, Clements G, Capps N, Davey S, Riemersma RA, Ebrahim S. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev. 2001;(3):CD002137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Chong ES. A potential role of probiotics in colorectal cancer prevention: review of possible mechanisms of action. World J Microbiol Biotechnol. 2014;30:351-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Marinelli L, Tenore GC, Novellino E. Probiotic species in the modulation of the anticancer immune response. Semin Cancer Biol. 2017;46:182-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Nouri Z, Karami F, Neyazi N, Modarressi MH, Karimi R, Khorramizadeh MR, Taheri B, Motevaseli E. Dual Anti-Metastatic and Anti-Proliferative Activity Assessment of Two Probiotics on HeLa and HT-29 Cell Lines. Cell J. 2016;18:127-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 22] [Reference Citation Analysis (0)] |
| 11. | Lebeer S, Vanderleyden J, De Keersmaecker SC. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev. 2008;72:728-764, Table of Contents. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 618] [Cited by in F6Publishing: 613] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 12. | Sreekumar O, Hosono A. The antimutagenic properties of a polysaccharide produced by Bifidobacterium longum and its cultured milk against some heterocyclic amines. Can J Microbiol. 1998;44:1029-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Masood MI, Qadir MI, Shirazi JH, Khan IU. Beneficial effects of lactic acid bacteria on human beings. Crit Rev Microbiol. 2011;37:91-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Zhong L, Zhang X, Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J Gastroenterol. 2014;20:7878-7886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 159] [Cited by in F6Publishing: 139] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 15. | Chouraqui JP, Van Egroo LD, Fichot MC. Acidified milk formula supplemented with bifidobacterium lactis: impact on infant diarrhea in residential care settings. J Pediatr Gastroenterol Nutr. 2004;38:288-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Pohjavuori E, Viljanen M, Korpela R, Kuitunen M, Tiittanen M, Vaarala O, Savilahti E. Lactobacillus GG effect in increasing IFN-gamma production in infants with cow's milk allergy. J Allergy Clin Immunol. 2004;114:131-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Azcárate-Peril MA, Sikes M, Bruno-Bárcena JM. The intestinal microbiota, gastrointestinal environment and colorectal cancer: a putative role for probiotics in prevention of colorectal cancer? Am J Physiol Gastrointest Liver Physiol. 2011;301:G401-G424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30:183-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 19. | Sales-Campos H, Basso PJ, Alves VB, Fonseca MT, Bonfá G, Nardini V, Cardoso CR. Classical and recent advances in the treatment of inflammatory bowel diseases. Braz J Med Biol Res. 2015;48:96-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Dougherty U, Mustafi R, Wang Y, Musch MW, Wang CZ, Konda VJ, Kulkarni A, Hart J, Dawson G, Kim KE, Yuan CS, Chang EB, Bissonnette M. American ginseng suppresses Western diet-promoted tumorigenesis in model of inflammation-associated colon cancer: role of EGFR. BMC Complement Altern Med. 2011;11:111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Ambalam P, Raman M, Purama RK, Doble M. Probiotics, prebiotics and colorectal cancer prevention. Best Pract Res Clin Gastroenterol. 2016;30:119-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 22. | Cunningham-Rundles S, Ahrné S, Bengmark S, Johann-Liang R, Marshall F, Metakis L, Califano C, Dunn AM, Grassey C, Hinds G, Cervia J. Probiotics and immune response. Am J Gastroenterol. 2000;95:S22-S25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69:1052S-1057S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 430] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 24. | Leung JM, Davenport M, Wolff MJ, Wiens KE, Abidi WM, Poles MA, Cho I, Ullman T, Mayer L, Loke P. IL-22-producing CD4+ cells are depleted in actively inflamed colitis tissue. Mucosal Immunol. 2014;7:124-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Zhu J, Zhu C, Ge S, Zhang M, Jiang L, Cui J, Ren F. Lactobacillus salivarius Ren prevent the early colorectal carcinogenesis in 1, 2-dimethylhydrazine-induced rat model. J Appl Microbiol. 2014;117:208-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Kahouli I, Malhotra M, Westfall S, Alaoui-Jamali MA, Prakash S. Design and validation of an orally administrated active L. fermentum-L. acidophilus probiotic formulation using colorectal cancer Apc Min/+ mouse model. Appl Microbiol Biotechnol. 2017;101:1999-2019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1163] [Cited by in F6Publishing: 1200] [Article Influence: 92.3] [Reference Citation Analysis (1)] |
| 28. | Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645-2650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 729] [Cited by in F6Publishing: 744] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 29. | Zaharuddin L, Mokhtar NM, Muhammad Nawawi KN, Raja Ali RA. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019;19:131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 30. | Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene. 2009;28:3892-3902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 31. | Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005;15:24-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 32. | Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1178] [Cited by in F6Publishing: 1259] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 33. | Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 34. | Stillie RM, Sapp HL, Stadnyk AW. TNFR1 Deficiency Protects Mice from Colitis-Associated Colorectal Cancer Coupled with a Decreased Level of Oxidative Damage in the Colon: Implications for Anti-TNF Therapy of Unremitting Colitis. J Cancer Ther. 2012;3:926-940. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 888] [Cited by in F6Publishing: 957] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 36. | Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 862] [Cited by in F6Publishing: 925] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 37. | Fiorucci S, Distrutti E, Mencarelli A, Barbanti M, Palazzini E, Morelli A. Inhibition of intestinal bacterial translocation with rifaximin modulates lamina propria monocytic cells reactivity and protects against inflammation in a rodent model of colitis. Digestion. 2002;66:246-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Miyauchi E, Ogita T, Miyamoto J, Kawamoto S, Morita H, Ohno H, Suzuki T, Tanabe S. Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: involvement of intestinal epithelial costimulatory molecules. PLoS One. 2013;8:e79735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 40. | Liévin V, Peiffer I, Hudault S, Rochat F, Brassart D, Neeser JR, Servin AL. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut. 2000;47:646-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Sivamaruthi BS, Kesika P, Chaiyasut C. The Role of Probiotics in Colorectal Cancer Management. Evid Based Complement Alternat Med. 2020;2020:3535982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 42. | Gianotti L, Morelli L, Galbiati F, Rocchetti S, Coppola S, Beneduce A, Gilardini C, Zonenschain D, Nespoli A, Braga M. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J Gastroenterol. 2010;16:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 131] [Cited by in F6Publishing: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 43. | Górska A, Przystupski D, Niemczura MJ, Kulbacka J. Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy. Curr Microbiol. 2019;76:939-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 44. | Molska M, Reguła J. Potential Mechanisms of Probiotics Action in the Prevention and Treatment of Colorectal Cancer. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 45. | Raman M, Ambalam P, Kondepudi KK, Pithva S, Kothari C, Patel AT, Purama RK, Dave JM, Vyas BR. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes. 2013;4:181-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |