Published online Sep 14, 2020. doi: 10.3748/wjg.v26.i34.5181
Peer-review started: May 22, 2020
First decision: June 13, 2020
Revised: June 18, 2020
Accepted: August 20, 2020
Article in press: August 20, 2020
Published online: September 14, 2020
Mixed tumors of the colon and rectum, composed of a combination of epithelial and endocrine elements of benign and malignant potential are rare neoplasms. These can occur anywhere in the gastrointestinal tract and are often diagnosed incidentally. Though they have been a well-documented entity in the pancreas, where the exocrine-endocrine mixed tumors have been known for a while, recognition and accurate diagnosis of these tumors in the colon and rectum, to date, remains a challenge. This is further compounded by the different terminologies that have been attributed to these lesions over the years adding to increased confusion and misclassification. Therefore, dedicated literature reviews of these lesions in the colon and rectum are inconsistent and are predominantly limited to case reports and case series of limited case numbers. Though, most of these tumors are high grade and of advanced stage, intermediate and low grade lesions of these mixed tumors are also increasingly been reported. There are no established independent consensus based guidelines for the therapeutic patient management of these unique lesions.
To provide a comprehensive targeted literature review of these complex mixed tumors in the colon and rectum that chronicles the evolution over time with summarization of historical perspectives of terminology and to further our understanding regarding their pathogenesis including genomic landscape, clinicoradiological features, pathology, treatment, prognosis, the current status of the management of the primary lesions, their recurrences and metastases.
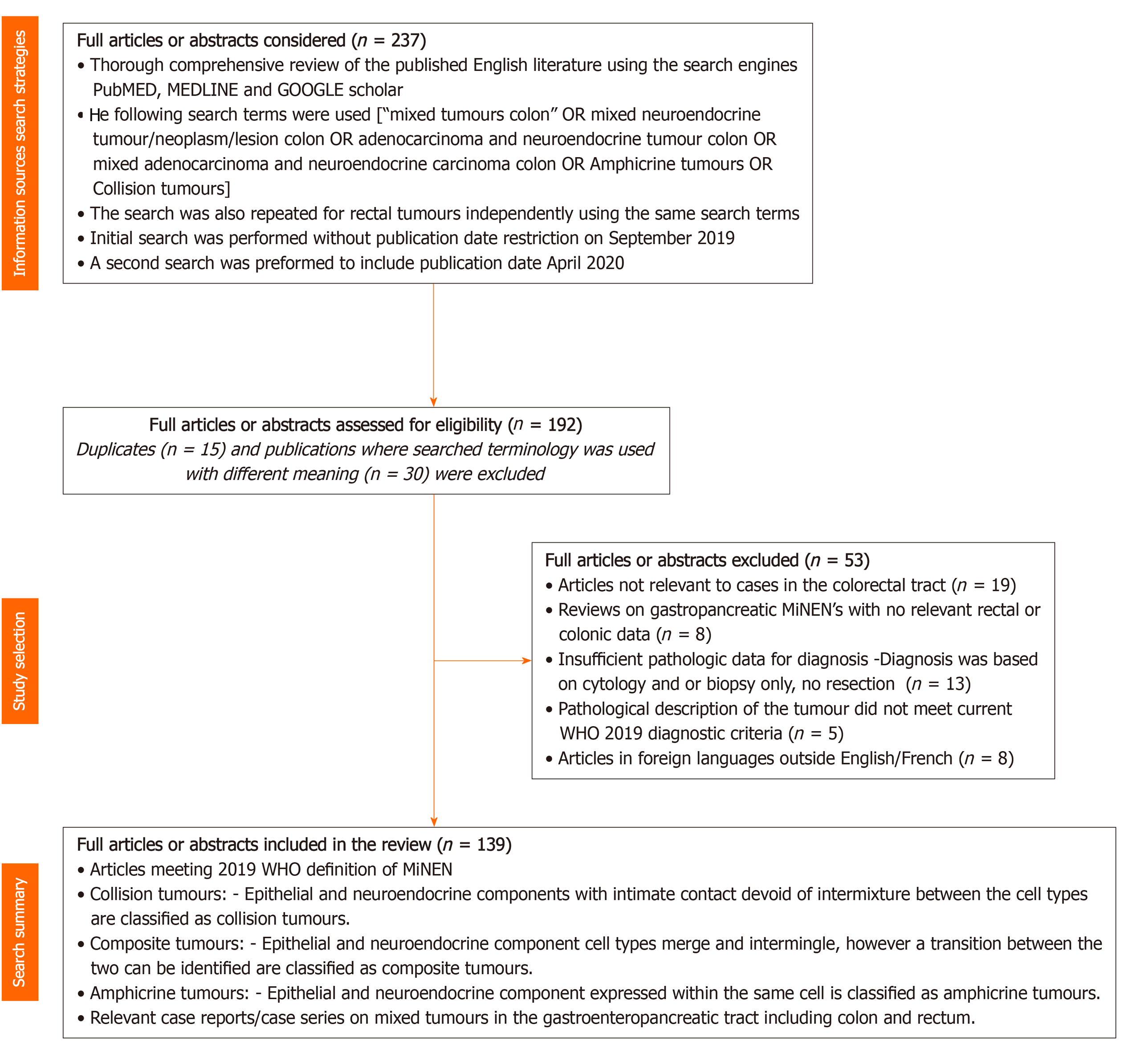
A comprehensive review of the published English literature was conducted using the search engines PubMed, MEDLINE and GOOGLE scholar. The following search terms [“mixed tumors colon” OR mixed endocrine/neuroendocrine tumor/neoplasm/lesion colon OR adenocarcinoma and endocrine/neuroendocrine tumor colon OR mixed adenocarcinoma and endocrine/neuroendocrine carcinoma colon OR Amphicrine tumors OR Collision tumors] were used. Eligibility criteria were defined and all potential relevant items, including full articles and/or abstracts were independently reviewed, assessed and agreed upon items were selected for in-depth analysis.
In total 237 full articles/abstracts documents were considered for eligibility of which 45 articles were illegible resulting in a total of 192 articles that were assessed for eligibility of which 139 have been selected for reference in this current review. This seminal manuscript is a one stop article that provides a detailed outlook on the evolution over time with summarization of historical perspectives, nomenclature, clinicoradiological features, pathology, treatment, prognosis and the current status of the management of both the primary lesions, their recurrences and metastases. Gaps in knowledge have also been identified and discussed. An important outcome of this manuscript is the justified proposal for a new, simple, clinically relevant, non-ambiguous terminology for these lesions to be referred to as mixed epithelial endocrine neoplasms (MEENs).
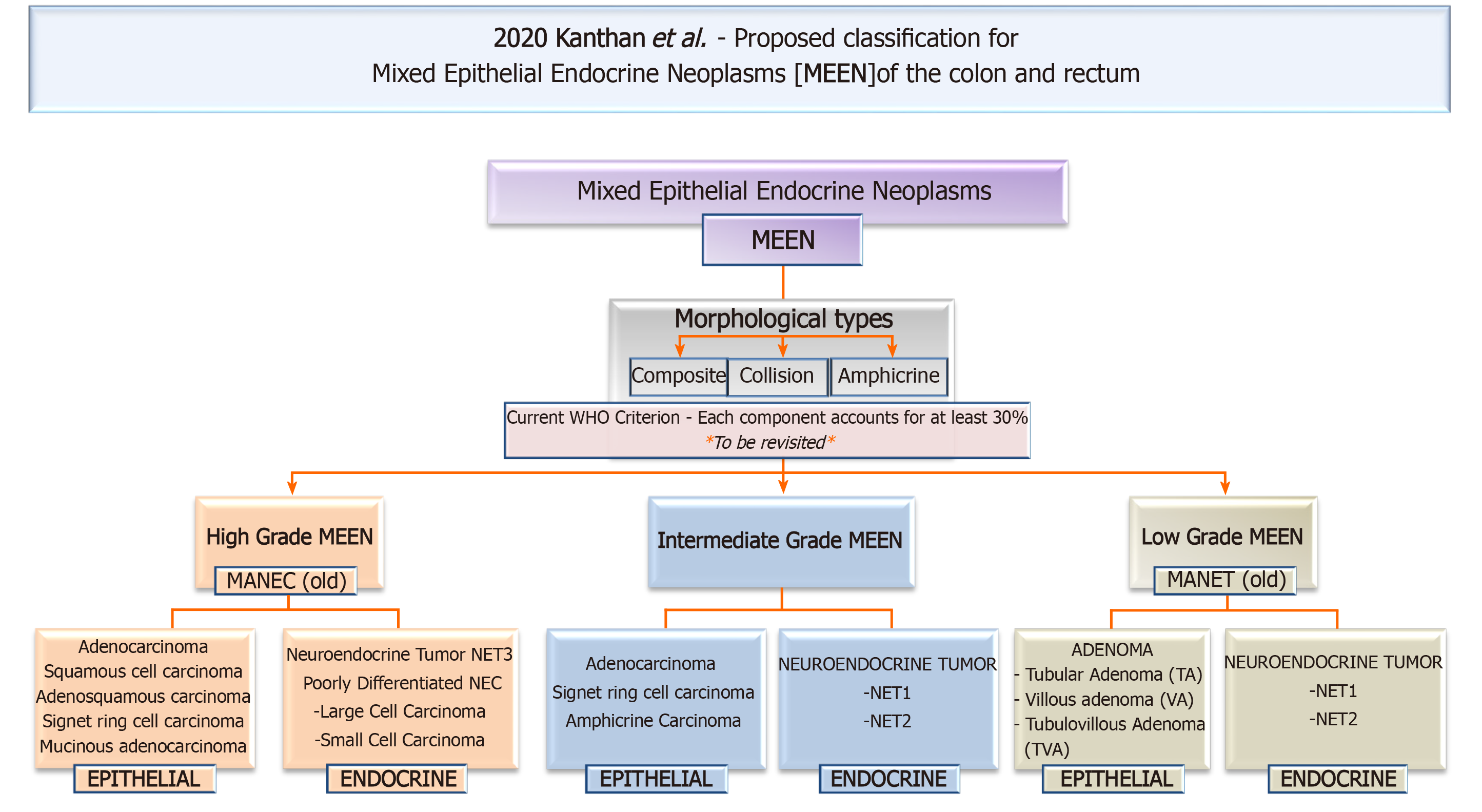
MEEN of the colon and rectum are poorly understood rare entities that encompass an extensive range of heterogeneous tumors with a wide variety of combinations leading to tumors of high, intermediate or low grade malignant potential. This proposed new revised terminology of MEEN will solve the biggest hurdle of confusion and misclassification that plagues these rare unique colorectal neoplasms thus facilitating the future design of multi institutional prospective randomized controlled clinical trials to develop and evaluate newer therapeutic strategies that are recommended for continued improved understanding and personal optimization of clinical management of these unique colorectal neoplasms.
Core Tip: This seminal manuscript provides a targeted review of these complex, rare neoplasms of the colon and rectum that should interest colorectal pathologists, surgeons, oncologists and gastroenterologists. This targeted review chronicles the evolution over time with summarization of historical perspectives of terminology with a proposal for a new-innovative, clinically-relevant, simple, non-ambiguous terminology for these lesions as mixed epithelial endocrine neoplasms. The pathogenesis including genomic landscape, clinico-radio-pathological features, treatment and prognosis, with gaps in knowledge are discussed. In keeping with our philosophy of a multidisciplinary management of these tumors, this manuscript has a multidisciplinary team (pathologists, oncologist and surgeon) of authorship.
- Citation: Kanthan R, Tharmaradinam S, Asif T, Ahmed S, Kanthan SC. Mixed epithelial endocrine neoplasms of the colon and rectum – An evolution over time: A systematic review. World J Gastroenterol 2020; 26(34): 5181-5206
- URL: https://www.wjgnet.com/1007-9327/full/v26/i34/5181.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i34.5181
Benign and malignant epithelial neoplasms of the colon and rectum are common with colorectal cancer being the second commonest cancer in Canada[1]. Mixed tumors of the colon and rectum include a broad category of neoplasms composed of the coexistence of epithelial-adenocarcinoma and non-neuroendocrine elements such as ovarian granulosa cell tumor[2] malignant lymphoma[3-9], malignant melanoma[10] and mesenchymal elements such as carcinosarcoma[11] and leiomyosarcoma[12]. However, the presence of a gastrointestinal tumor with a neuroendocrine and an exocrine component in the same neoplasm was first reported by Cordier[13] in 1924. Such tumors of mixed epithelial and neuroendocrine elements are infrequent and accounts for approximately 1%-2% of all colorectal malignancies in the majority of the reported literature[14,15]. Capella et al[16] however report the prevalence of colorectal adenocarcinomas with endocrine differentiation to range from 16% to 32.5%. These however include all carcinoma cells that express endocrine markers including amphicrine cancers and do not follow the current World Health Organization (WHO) guidelines of each component being represented to at least 30%[17]. The exact true incidence and prevalence of these neoplasms continues to be fraught with difficulties, mainly attributed to diagnostic limitation of biopsy samples, classification with different names due to the varied terminologies used over the years, and continued controversy regarding the validity of 30% threshold used as a discriminatory criterion for inclusion as mixed tumors[15,18-21].
This comprehensive literature review was conducted utilizing published reports predominantly written in the English language (at least the abstract) outlining both epithelial and endocrine/neuroendocrine tumors in the colorectal region. The majority of the research was focused between 2010 to the present with targeted review of older manuscripts as deemed useful to establish historical perspectives of continuity regarding terminology. Majority of the studies pertain to studies reporting on the colorectal region with focused excerpts from few articles on the gastroenteropancreatic tract as an entirety.
A comprehensive review of the published English literature was conducted using the search engines PubMed, MEDLINE and GOOGLE scholar. The following search terms (“mixed tumors colon” OR mixed endocrine/neuroendocrine tumor/neoplasm/lesion colon OR adenocarcinoma and endocrine/neuroendocrine tumor colon OR mixed adenocarcinoma and endocrine/neuroendocrine carcinoma colon OR Amphicrine tumors OR Collision tumors) were used. This was repeated for rectal tumors independently using the same search terms. The initial search was conducted in September of 2019. In addition an updated search was conducted in April 2020.
This search was conducted independently by ST and RK. Duplicates were excluded. Selection criteria primarily included articles restricted to the colon and/or rectum with exclusion of those articles describing this tumor arising elsewhere in the gastrointestinal tract.
Collision tumors included for this study were defined as “neoplasms involving epithelial and endocrine/neuroendocrine components with intimate contact devoid of intermixture between the cell types”, therefore all collision tumors with mesenchymal or non-gastrointestinal components were excluded from detailed analysis.
Composite tumors included for this review were defined as “neoplasms with epithelial and endocrine/neuroendocrine component cell types that merge and intermingle retaining a transition between the two that can be identified”.
Amphicrine tumors included for this review were defined neoplasms with “Epithelial and endocrine/neuroendocrine component expressed within the same cell”.
All titles of the references were initially independently screened by (ST) and (RK). Potentially relevant items, including full articles and/or abstracts were independently reviewed, assessed and agreed upon items were selected for in-depth analysis. Relevant secondary references were retrieved and reviewed. These selected potentially relevant items were reviewed thoroughly, including detailed analysis to determine eligibility for inclusion according to the eligibility criteria as outlined above.
In total 237 full articles/abstracts documents were considered for eligibility of which 45 articles were illegible resulting in a total of 192 articles that were assessed for eligibility of which 139 have been selected for reference in this current review and are summarized as a flow chart in Figure 1 adapted on the principles recommended by the Preferred Reporting for Systematic Reviews and Meta-Analyses report[22].
The design of this manuscript is to review the current status of these entities with an outlook on the evolution over time with summarization of historical perspectives, nomenclature, clinicoradiological features, pathology, treatment, prognosis and the current status of the management of both the primary lesions, their recurrences and metastases. Guidelines for therapeutic strategies, patient management and the continued lack of independent consensus guidelines were also explored. An additional feature of this manuscript is the proposal for a new revised terminology for these unique tumors that encompasses all the relevant criteria and is simple, clinically relevant and free of semantic ambiguity.
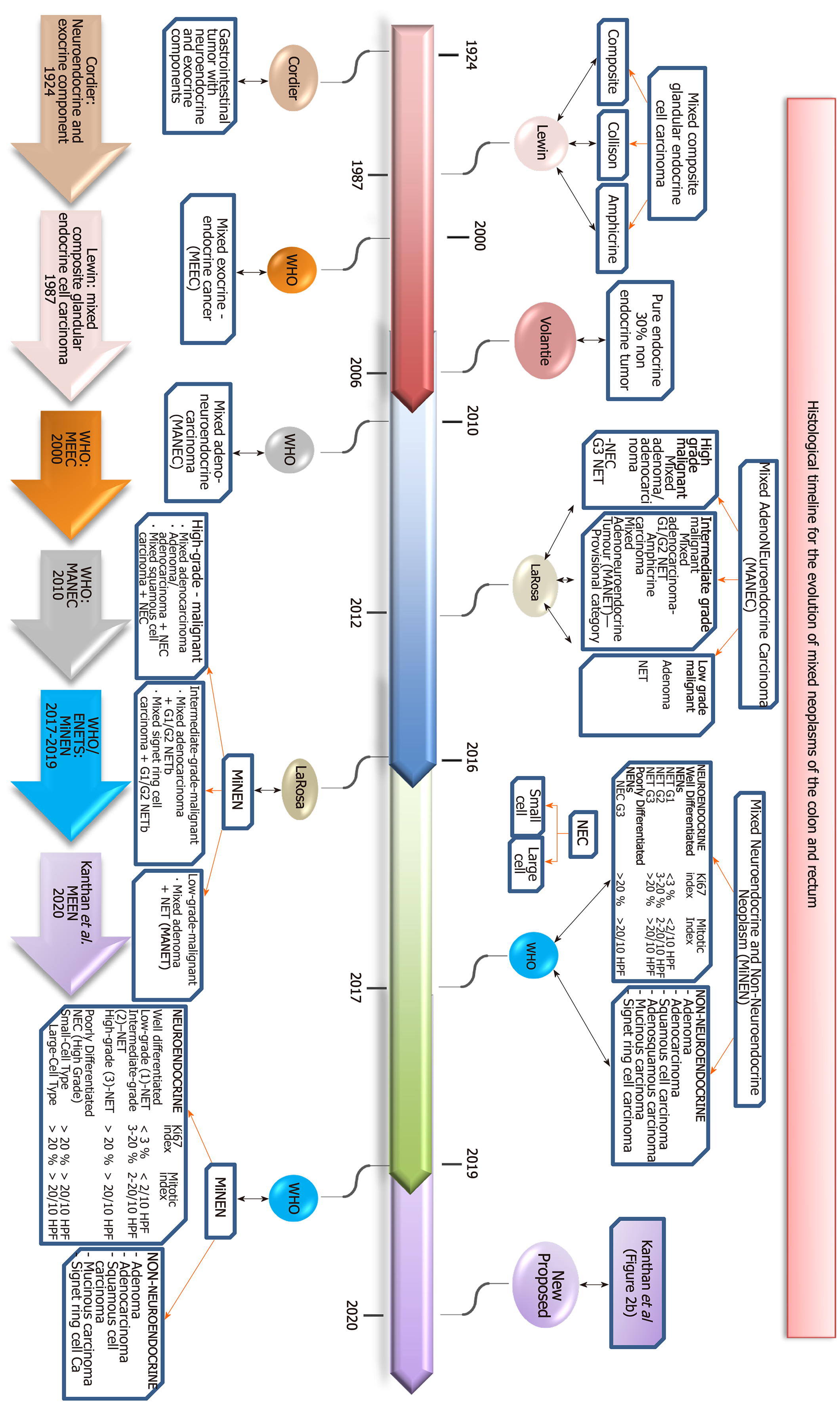
Though colorectal cancer is the second commonest cancer, mixed tumors composed of epithelial and endocrine elements are rare accounting for approximately 1%-2% of all colorectal malignancies in the reported literature[14,15]. The first report recognizing the presence of a gastrointestinal tumor with a neuroendocrine and an exocrine component in the same neoplasm is attributed to Cordier[13] in 1924. Initial reports of such cases were identified by vast descriptions with several names often leading to confusion[23,24]. In 1987, Lewin recognized that carcinoid tumors included a wider histopathological spectrum of mixed glandular-endocrine composite tumors. He was the first to propose a methodical classification of “mixed” or composite tumors of glandular-endocrine cell carcinoma-dividing this entity into three broad categories of composite, collision and amphicrine tumors[25]. The literature reports for the next ten years shows a huge inconsistency in published data of these neoplasms with a wide variety of lesions being called composite/mixed tumors as there were no clear definitions of each component nor established criteria for inclusion for this designation. Capella et al[16] tried to group the different clinicopathological entities into prognostic classes to include benign, low grade, intermediate grade and high grade malignant endocrine-exocrine tumors of the gastrointestinal tract. In 2000, the WHO expanded the classification of endocrine neoplasms to include mixed exocrine-endocrine carcinomas, with definition of each component representing at least 30% of the tumor[26]. The grey zone between pure neuroendocrine and non-neuroendocrine tumors continued to be explored by Volante et al[27] with comments on the concepts and proposed classification. In 2010, mixed neoplasms from the gastroenteropancreatic tract containing neuroendocrine and exocrine component each present in at least 30% of the tumor mass and being malignant was classified as a separate entity “mixed adeno-neuroendocrine carcinomas (MANECs)” by the WHO[28]. Following this, in 2012 La Rosa updated the classification of these entities into (1) MANEC as high-grade and intermediate grade malignant tumors and (2) Mixed adenoma-well differentiated neuroendocrine tumors (MANET) as low grade malignant tumor for lesions composed of low grade neuroendocrine tumors in adenomas without neoplastic transformation. This introduced the new terminology of mixed adenoneuroendocrine tumors as a provisional category, as MANET was not specifically categorized in the 2010 WHO[29,30]. In 2016, La Rosa et al[31] additionally proposed a unifying concept of mixed neuroendocrine-non neuroendocrine neoplasms for these heterogeneous group of neoplasms and grouped them according to the grade of malignancy of each tumor component resulting in a three tiered classification of high, intermediate and low grade malignant tumors[31]. This was adopted in 2017 by the WHO with reclassification of MANEC as, mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN) to include histological variants such as squamous or sarcomatoid phenotypes. The term ’carcinoma’ was substituted by the term ‘neoplasm’ to allow for inclusion of the well-recognized low grade malignant tumors. The classification of the neuroendocrine component relies on histological grade and proliferation index into neuroendocrine tumor neuroendocrine tumor NET1, NET2, NET3 and neuroendocrine carcinoma (NEC) based on morphology, mitoses and Ki67 proliferation[32]. The WHO describes MiNEN as mostly made of a poorly differentiated NEC component, together with an adenocarcinoma component. Further it stated MiNENs composed of low grade NET component such as the MANETs are exceedingly rare[15,33,34]. These mixed tumors can display various percentages of each component, theoretically ranging from 1%-99%. With the utilization of immunohistochemical stains[15]. Similarly as in MANEC, current recommendations continue to state that MiNEN also must consist at least 30% of both neuroendocrine and the epithelial component. However, it is increasingly being recognized that all high grade malignant components should be reported even if it represents less than 30%[15,18]. The term MiNEN has also been adopted by the French ENTS group[15]. Very recently, the International Agency for Research on Cancer and the WHO expert consensus panel have proposed a common classification framework for all neuroendocrine neoplasms of the gastropancreatic tract and have extended the usage of the term MiNEN to all neoplasms meeting the diagnostic criteria of possible combinations between neuroendocrine and non-neuroendocrine elements for any site within the gastroenteropancreatic tract[17,18,35].
The non-neuroendocrine elements in MiNENs are always epithelial (glandular, squamous, mucinous and/or sarcomatoid) and do not include non-epithelial components such as lymphomas[3-9] or mesenchymal components such as smooth muscle tumors[12] as discussed earlier. In this context, we therefore propose that to be devoid of ambiguity and for simplicity and semantic accuracy, these unique mixed tumors should be referred to as mixed epithelial endocrine neoplasms (MEEN). The term endocrine is appropriate and accurate as it is now well established for over 30 years that enteroendocrine cells are derived from common precursor gastrointestinal stem cells within the intestinal crypts and do not originate from the neural crest cells for the misnomer term of “neuroendocrine”[36-38].
The historical timeline of the evolution of this nomenclature from its inception in 1924 to its current status are summarized in Figure 2 and our proposed all-inclusive terminology of these MEEN are outlined in Figure 3.
Mixed epithelial endocrine neoplasms of the colon and rectum are a rare, under documented entity with unique histology and clinical behavior. The exact pathogenesis of these neoplasms remains unclear as they are often characterized by significant histological heterogeneity that preclude accurate diagnosis[14,20,31,39]. Though, there are no unique identifiable risk factors, association with long standing intestinal inflammatory disease and MiNEN have been reported[24]. The exact pathogenesis of these tumors continues to be debated and multiple hypotheses have been proposed as to the origin and development of mixed tumors. Three main theories have been proposed: (1) The first theory proposes that the epithelial and the endocrine components arise independently from distinct precursor cells in a synchronous or metachronous manner; (2) The second theory proposes that the two components are derived from a common pluripotent stem cell progenitor that acquires biphenotypic bidirectional differentiation during carcinogenesis; and (3) The third theory though similar to the second in its origin from a common monoclonal origin, believes in a stepwise process-that the neuroendocrine trans/de differentiation develops from a non-neuroendocrine epithelial phenotype due to the progressive accumulation of molecular and genetic aberrations and/or stromal/tumor micro environmental changes such as field cancerization[15,18,39-42]. The last two theories are gaining popularity as it is now agreed that multipotent gastrointestinal stems cells gives rise to the endocrine cells of the gastrointestinal tract, contrary to previous belief of migrating from the neural crest cells[36-38]. Arguments supporting this assertion are amphicrine cells, which express both exocrine and neuroendocrine components in the same cell[24].
Genomic/Molecular landscape: In an effort to understand the molecular pathogenesis of these mixed tumors many reports including an extensive review of 33 retrospective studies and eight case reports indicate that the most common molecular feature were mutations in tumor associated genes including TP53 gene, RB1, PTEN, adenomatous polyposis coli (APC), P13KCA, BRAF, Kristen rat sarcoma viral oncogene homolog (KRAS), MYC, aberrations in the p16/Rb/cyclin D1 signaling pathway, and microsatellite instability as potential drivers of MiNEN[15,18,20,43-57]. Though, HER2 overexpression has been reported in a rare case of mixed adenoneuroendocrine carcinoma of the gastroesophageal junction, there are no such reports in colorectal MINENs[58]. Abnormalities of chromosome 5q, 11q, 17q, and 18q are shown to be closely related to the formation of MANEC. Presence of transcription factors (TTF1, ASH1) has been reported, however this does not correlate with the patient’s prognosis and is not specific for gastrointestinal carcinomas[24]. Specific transcription factors such as the family of basic helix-loop-helix-bHLH factors, including the human achaete-scute homologue(l-hASH1) that are known to play a pivotal role in the development and differentiation of neuronal and endocrine cells of the foregut and midgut are also found to be expressed in Neuroendocrine carcinomas. Such transcriptional molecules may be a key tool for the identification of functional NE differentiation in mixed exocrine-endocrine carcinomas[27]. The genome wide copy number alterations of 14 MANEC and 5 NEC reveals recurrent amplification of PTGER4 and MYC genes as the pathway for neuroendocrine differentiation[49]. Based on targeted next generation panel and whole exome sequencing, the neuroendocrine component compared to the epithelial component usually carries a higher number of aberrations and higher allele balance, suggesting a more aggressive biology[56]. Comparative exome sequencing of germline deoxyribonucleic acid from the two separate components of colonic MANECs identified six somatic modifications in the cancer consensus genes. Both components of the tumor (glandular and neuroendocrine) shared somatic mutations in APC, KRAS, B-cell CLL/lymphoma 9 and Forkhead Box P1 genes[43]. Mutations in the SMARC4 gene (SWI/SNF) related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4 was only identified in the neuroendocrine carcinoma component. The Belgian researchers believe that there is a relationship between the two components as inactivation of the SMARC4A gene due to an underlying mutation is responsible for the transformation/of the exocrine cell into the neuroendocrine phenotype or the transdifferentiation to a more aggressive phenotype[24,43]. KRAS mutation (G12D) is reported to be associated with large cell neuroendocrine carcinoma[43]. The presence of identical KRAS mutations in both components of the tumor supports a common clonal relationship between the two components which is also supported by several shared mutations in both components by exome sequencing[43,59]. In comparison to colorectal carcinoma BRAF mutations are more frequently seen with less KRAS mutations[44,48]. TP53 seems to be the cornerstone gene in the carcinogenetic process of mixed tumors. Analysis of p53 and loss of heterozygosity also point to the common cell of origin differentiation at an early stage of tumorigenesis[45,46]. Genetic analysis of the p53 gene mutations suggests that neuroendocrine carcinoma may have developed from a phenotypic change of adenocarcinomas cells to endocrine cells during tumor progression though the exact mechanisms of such differentiation are still unknown[60]. Massive genomic rearrangement “chromothripsis”, acquired as a single cellular catastrophic event resulting in altered genomic landscape with genomic remodeling and temporal emergence of cancer is proposed as one of the mechanisms of the acquisition of the neuroendocrine phenotype from the epithelial cell[24,61]. The mutational profile of colorectal neuroendocrine neoplasms, including MANECs, show frequent single or combined inactivation with co-mutations of APC, KRAS and TP53 genes[50]. Interestingly, in a case report of a collision tumor of poorly differentiated cecal adenocarcinoma with signet ring cell and mucinous features admixed with a well differentiated neuroendocrine tumor, next generation sequencing showed BRCA2 mutation in both the adenocarcinoma and NET components, while a TP53 mutation was only found in the adenocarcinoma component, with no mutations of KRAS, NRAS or BRAF in either component[47]. Immunohistochemical studies in a case a mixed adenoneuroendocrine carcinoma of the pancreas suggests NEC as the precursor giving rise to the adenocarcinoma component, with both of them diverging from a single cancer stem cell[62,63]. Immunohistochemistry study of seven colorectal MANEC as part of a study of gastrointestinal MANECs by Gurzu et al[64] in 2019 reports that gastrointestinal MANEC is a microsatellite stable tumor with strong positivity to CD44 and no expression of maspin suggesting an origin from a CD44-positive stem like precursor cell. In depth mutational analyses by Woischke et al[56] supports the concept of early separation of epithelial and neuroendocrine components during malignant transformation followed by subsequent independent mutational evolution. Both non-neuroendocrine and neuroendocrine components display loss of heterozygosity at multiple loci and mutations in key oncogenes, (such as APC, TP53), or retinoblastoma genes, while poorly differentiated neuroendocrine carcinomas (PDNEC) component contain additional mutations. PDNEC of various origins showed cyclin-dependent kinase Inhibitor 2A/B and APC mutations. These neoplasms show increased methylation profile and prolonged survival, similar to elderly patients with sporadic colorectal adenocarcinoma and mismatch repair deficiency[15]. Aberrant gene methylation appears to be a specific epigenetic characterization of NEC/MANEC providing clues for early diagnostic markers and for tumor-specific methylation profiles[65]. The recognition of microsatellite unstable NEC and MANECs as a new clinicopathological entity that has relatively better prognosis than microsatellite stable tumors is a novel new finding with therapeutic implications. The pathway leading to MSI high NEC/MANECs is the widespread methylation induced silencing of the MLH1 gene as seen in sporadic colorectal carcinomas. This seems to occur early in the tumorigenic pathway of these mixed tumors[54]. In summary, intermediate to high grade colorectal MiNENs exhibit a genetic/molecular profile of identical copy number aberrations, similar to pure colorectal adenocarcinomas though they lack structural chromosomal alterations seen in pure colorectal NEC[48,49]. The lack of mutations in ATRX, DAXX, MEN1, and TSC2 also supports a distinctive molecular evolution for the NEC component in comparison to NET tumors[45].
In contrast to high grade mixed tumors there is limited data regarding the pathogenesis of low grade MANETs i.e., low grade endocrine tumors arising in intestinal adenomas as they are uncommon lesions. Approximately, 5% of colorectal MiNEN are low grade mixed tumors, presenting as polyps < 3 cm in diameter and being composed of tubulovillous or villous adenoma with G1-G2 NET[15]. Estrella et al[51] support evidence of alterations in adenomatous APC/beta catenin pathway in 25 mixed tumors of adenomas with low grade NET. The presence of nuclear B-catenin expression in both the adenoma and the NET components supports a clonal origin of the two components from a common multipotent stem cell[31,33]. Well differentiated NET components of MiNEN appear to harbor genetic alterations that are different from their counterparts. Adenoma/Adenocarcinoma showed loss of heterozygosity of APC, KRAS, and TP53 while the NET showed loss of heterozygosity of Von Hippel–Lindau[15]. In the study by La Rosa et al[33] on 12 cases of MANET composed of adenoma and well differentiated NET G1/G2, no KRAS, BRAF or P13KCA or MSI were identified in both tumor components[33]. This is corroborated by a similar study by Bongiovanni et al[55] that confirm no alterations are seen of KRAS, BRAF and Pik3CA genes in either tumor component of colonic MANETS. This suggests that intestinal MANET may develop through a KRAS-independent pathway in contrast to sporadic colorectal adenomas[33]. However, P53 was negative in the WDNET component and focally expressed in the adenomatous one suggesting a common origin from a single progenitor cell for the two components of MANET[55]. In a rare case of rectal villous adenoma admixed with poorly differentiated NEC component hypothesize a high grade evolution of a MANET[66]. However, the lack of mutations in ATRX, DAXX, MEN1, and TSC2 supports a distinctive molecular evolution for the NEC component in comparison to NET tumors[45]. Additionally, primary high grade NEC rarely occurs in patients with FAP in contrast to neuroendocrine hyperplasia and well differentiated NET supporting the theory that the evolution of NEC is distinct from NET in these mixed tumors[52].
Amphicrine carcinomas are a unique entity with distinct biological and histological features. Though recognized as a distinct clinicopathological entity in the stomach and intestine and described in the colon as part of MINEN, dedicated studies of amphicrine carcinomas are not available in the colorectal literature[15,20,29]. Despite a clinicopathological and pan-cancer transcriptome analysis of 10 cases in the stomach and small intestine the genetic background of these rare tumors and their molecular relationship to adenocarcinoma/NEC remains largely unknown[67].
Benign and malignant epithelial neoplasms of the colon and rectum are common with colorectal cancer being the second commonest cancer in Canada[1]. Gastroenteropancreatic neuroendocrine neoplasms have been studied in detail[68,69] with few dedicated reports of endocrine/ neuroendocrine cell carcinomas of the colon and rectum[70,71]. Reviews on mixed neuroendocrine and non-neuroendocrine neoplasms of the digestive system that include colon and rectum are limited[15,18,72-75]. Clinico-radiological and pathological data that have been obtained from the dedicated publications of mixed neuroendocrine and non-neuroendocrine tumors of the colon and rectum are limited due to a large body of this literature being isolated case reports or as part of a larger series[14-16,18-20,23-25,29-31,33-35,45,47,66,75-107].
Mixed tumors of the colon and rectum primarily affect the adult population with a mean age of 61.9 years[20]. Slightly higher values with median age of 72 years (range 41-87) are reported in a case matched study by Watanabe et al[77]. Racial predilections of MiNEN are not reported in the literature. The true incidence of these lesions may be underestimated, as some cases might have been unrecognized or inappropriately reported[18,20]. Although the true incidence and prevalence of MiNENs are unknown, some reports indicate an incidence of MiNENs as 0.2% of all colorectal cancers[70,80] while a large retrospective case matched study by Watanabe et al[77] reported a prevalence of 3.2% of MANECs[14]. In comparison to colorectal cancers, mixed tumors account for 2.4% of surgically resected colorectal neoplasms: With colonic MiNENs accounting for 14%-20% of colonic NEN and rectal MiNEN only accounting for 1%-3% of all rectal NEN[15,18]. Extrapolated data on gender in a systematic review on MiNENs by Frizziero et al[18] revealed that most gastroenteropancreatic MiNENs occurred in males 65.6% to 34.4% in females, and sub-group analysis per primary tumor site showed male gender prevalence in the colon 63.2% and in the rectum 63.1%. Endocrine cell carcinomas of the colon and rectum usually arise in the right colon or the rectosigmoid[70]. MANEC can involve any part of the colorectal from the cecum to the rectum[24,77] with 78.6% involvement of colon while 21.4% presented with rectal lesions[77]. In contrast, in the Asian literature, gastrointestinal-NEN mostly occurred in the rectum[68]. In the colon, mixed tumor seems to be commonly occur in the rectosigmoid/sigmoid colon, and is rare in the ascending/transverse colon, cecum and ileocecal valve[77,80,81,83-85,88,92,95,97,99,103-105]. In a review of sixty one cases of colorectal MANEC by Qiu et al[100] 58% were located in the right colon, 8.1% in the transverse, 16.1% in the left colon and 16.1% in the rectum.
Location of the tumor, behaviors, type and extent of the disease dictates the presenting symptoms with great variations. There are no specific clinical presentations of mixed tumors as many present at an advanced stage with local and/or distant spread. As this is a pathological diagnosis, many a time, biopsy samples obtained are small and accurate recognition of the second component is difficult[39]. Symptoms of MANECs are nonspecific and similar to colorectal carcinomas. These include routine symptoms of a mass lesion, gastrointestinal bleeding, bowel obstruction, intussusception, or paraneoplastic syndromes[79,82]. Rarely a functioning NET component complicated by carcinoid syndrome with elevated serum serotonin and serum chromogranin A levels and 24-h urine 5-hydroxyindoleacetic acid level has been reported[30]. Expression of appropriate or inappropriate hormonal peptides such as somatostatin, adrenocorticotropic hormone or vasoactive peptide have been reported in few cases of MANEC[24,29]. Depending on the location, increase in lactate dehydrogenase level and decrease in prothrombin time may be observed[89]. The signs and symptoms of most mixed tumors are nonspecific and often present at an advanced stage.
Although patients can present without any clinical features, in 69% of cases, patients reported pain and signs of obstruction (nausea, vomiting and abdominal bloating), stomach cramp, malaise, diarrhea and weight loss[14,30,41,82]. Some may present with positivity for fecal occult blood, intussusception, bleeding per rectum and/or anemia[14,38,82]. If unusual patterns of metastases are encountered, in an otherwise routine colorectal carcinoma-example-groin metastases in a rectal cancer, or distant spread to meninges orbit or thyroid gland, a mixed tumor should be suspected and in this context it would be worthwhile to reexamine the resected tumor or the biopsy material to identify the presence of the second component of the mixed tumor[58,78,96,106]. Occasionally, rapid progression of liver metastases following resection of the primary tumor can present with hepatic rupture[89].
Clinical examination may reveal a distended abdomen with diffuse pain and guarding, regional lymphadenopathy or a tender palpable mass may be felt[24,80]. Tumoral markers such as carcinoembryonic antigen, carbohydrate antigen 15-3 and 19.9 (CA15-3, CA19.9) can be used clinically to identify and follow up for disease progression and remission[81,85]. Serum CGA levels along with an epithelial marker may be used for the surveillance of metastatic or recurrent disease[72].
Imaging features are generally nonspecific, necessitating histopathology for confirmation of MiNENs[62,80]. Rectal magnetic resonance imaging and ultrasonography are modalities of choice for determining metastatic disease to regional lymph nodes[79]. Colonoscopy is the most reliable modality for definite diagnosis of colorectal neoplasms, although computer tomography (CT) remains as the most useful imaging method[24,79]. Distinguishing between MANEC and adenocarcinoma using endoscopic imaging is difficult[79]. Although CT may be normal in some patients, positron emission tomography-computer tomography (PET-CT) usually will show 18F-fludeoxyglucose-avid lesion as it associates with high sensitivity and proliferation of the tumor (NEC component) and is therefore recommended when mixed epithelial/endocrine tumors are suspected or to detect metastatic disease[30,38,71]. However as its sensitivity in colorectal adenocarcinoma and neuroendocrine tumors is low (42.0% and 33%, respectively), it is therefore deemed as inapplicable in routine clinical practice: Thus evaluating the stage of MANEC using CT images, lacks efficacy[79]. It is worth mentioning however that the sensitivity is proportional to the size of the tumor[24]. CT may additionally show pneumoperitoneum, free intraperitoneal fluid, particularly around the caecum and in the pelvis[80]. Contrast enhanced CT may reveal intraluminal heterogeneous enhancing mass, irregular wall thickening and mesenteric haziness[84]. CT/MRI can also be a useful tool in identifying mass lesions, metastatic disease and obstructive findings and in demonstrating the extent of the disease, as in serosal spread and into the surrounding fatty tissue with possible metastatic in the iliac bone and liver[81]. As reported by others to date, CT remains as the first-intention method in identifying and staging MANEC as they readily identify submucosal lesions, liver metastases, lymph node metastases and osteosclerotic or mixed osteolytic-osteosclerotic metastatic lesions. Liver metastasis were the most commonly recognized element, their size may well exceed that of the primary tumor[24]. Additionally CT can also identify calcifications and stellate/speculated masses. In cases involving intussusception, a characteristic bowel within bowel appearance along with inflammatory changes in the adjacent pericolonic fat can be appreciated. MANECs should be considered as one of the differential diagnosis in adults presenting with intussusception[84,105]. Pathological diagnosis is confirmed by tissue examination obtained either by colonoscopy, laparoscopy, and sigmoidoscopy, core biopsy of metastases or by examination of resected surgical specimens.
Most high and intermediate grade tumors are large and bulky with an average tumor size of 52.2 mm with 88.1% presenting as advanced tumors with increased depth of invasion as T3 -T4 lesions[77]. The gross appearance of the neoplastic lesions are similar to colorectal adenocarcinoma[79] with varied macroscopic appearances including polypoid or ulcerated tumor with raised edges or large fungating mass with tendency to bleed easily[23], serosal and invasion of adjacent structures[81,85], stenosing, ulceroproliferative, encircling stricture with surface epithelial atrophy and/or plaque-like with diameter variation ranging from 0.5 to 14 cm. Diffuse necrosis and constriction of the intestinal lumen are often appreciated[24,103]. Generally these tumors form a semicircular mass with deep ulceration, occupying the lumen though stenotic lesions or prominent polypoid mass occupying the lumen can also be seen[79,81]. Large tumors can occasionally cause colonic intussusception[79]. Macroscopic tumor perforation, with adherence to surrounding organs/structures and infiltration of peritoneum, pericolic fat maybe observed[84]. The cut surface of the tumor often shows white tan, poorly circumscribed lesions with an infiltrating border. It is not uncommon to see focal necrosis and foci of hemorrhage. However it is important to note that there are no unique gross features to distinguish a mixed epithelial endocrine neoplasm from a pure epithelial -adeno/squamous/mucinous carcinoma[20].
In contrast, the gross appearances of low grade tumors are usually of an adenomatous polyp: Tubular/villous/Tubulovillous with an incidental endocrine tumor or rarely neuroendocrine carcinoma discovered only on microscopic examination[42,66,99].
Microscopically, the two components of the mixed tumor are an epithelial and endocrine of which each component should be at least 30% according to the current WHO guidelines[17,32]. In a recent systematic review by Frizziero et al[18] the two components were present in equal proportion in 27.9 cases, whereas in the remaining 72.1% one of the components was predominant; the neuroendocrine component in 42.2% and the non-neuroendocrine component/epithelial in 29.9%. The Neuroendocrine component encountered in these mixed lesions was high grade NEC or NETG3 in 4.3% and low grade component of NET1 or NET2 in 3.1%. Many recent studies question the validity of this 30% inclusion criterion as mixed tumors with a lesser percentage may still be of significant prognosis[15,18,19-21]. This is particularly so in the identification of NEC within a poorly differentiated carcinoma wherein recognition of this component has significant prognostic value as these cases will require more complex management as discussed subsequently[77]. WHO suggests, evaluating and grading each histologic component separately. The definition of MANEC includes presence of at least 30% of each components (neuroendocrine and adenocarcinoma) and both components must be malignant[14,17,32,62]. This threshold was arbitrarily initially proposed in 1987[25], with the hypotheses that prognosis was influenced by the predominant histological component. One must keep in mind this cut off value only applies to resected specimens after histological examination of the entire neoplasm within the specimen[15]. We recommend like other authors that the percentage of all components of the mixed tumors be reported and all these neoplasms even with less than 30% be classified as mixed epithelial endocrine tumors. Alternative thresholds of 10% or 20% as discriminatory cut of criterion need to be explored as even the presence of small focus of NEC can be associated with aggressive behavior and metastases[18,20]. Most neuroendocrine neoplasms (NEN) in high grade cancers are PDNEC either large (82.2%) or small cell (17.8%) admixed with a non-neuroendocrine component-epithelial tumor usually adenocarcinoma NOS or mucinous/signet ring cell adenocarcinoma (92.2%), though squamous cell carcinoma (2.5%) and other variants such as adenosquamous (< 1%), AFP negative hepatoid carcinoma (< 1%)have also been reported[18,20,84,86,90,91,94,102,103]. Histologically, the poorly differentiated neuroendocrine component resembles small or large cell NEC tumors. The small cell subtype has cells arranged in nests or a diffuse manner, with small amount of cytoplasm, fusiform nuclei with granular chromatin and inconspicuous nucleoli. The mitotic index is high usually between 20-80 mitotic figures/ten high-power fields. While the high grade non-small cell morphology consists of cells with abundant cytoplasm, vesicular nuclei, and prominent nucleoli[24,31,79]. Prominent oncocytic differentiation represented by large cells with markedly eosinophilia has also been reported in a mixed tumor of the transverse colon[108]. Amphicrine tumors are distinguished by a divergent immunophenotype in which both exocrine and neuroendocrine traits are expressed in the same cell[24,31,67]. All neuroendocrine components are classified according to the WHO criterion-based on mitotic index and KI67 proliferation into well differentiated neuroendocrine tumors G1, G2 and G3 and poorly differentiated neuroendocrine carcinomas of small or large cell type as seen in Figure 2. Microscopically vascular and perineural tumor invasion associated with geographic necrosis, vascular thrombosis, infarction and ischemia may also be present in the adjacent sampled bowel[24,29,81].
The most important factor for improving outcome of MANEC remains early and accurate diagnosis with adequate histopathological examination to establish the presence of two components within the same neoplasm[39,71]. This often requires a diligent search for morphologically different areas on the haematoxylin and eosin stained sections and liberal use of immunohistochemical markers[20]. Immunohistochemical tests are a key cornerstone that have identified a large number of these mixed tumors ranging from adenomas or adenocarcinomas with several neuroendocrine cells to classical neuroendocrine tumors with focal exocrine/epithelial elements[24,46,62]. Pathologists must pay attention to morphology and actively preform Immunohistochemical staining[14,19,76]. Appropriate selections of immunohistochemical stains are essential to establishing accurate diagnosis in a timely manner[2]. Above all, high pathological suspicion and clinical awareness is vital for the accurate diagnosis of these mixed tumors. Although small cell type neuroendocrine carcinoma can be distinguished easily from adenocarcinoma by hematoxylin-eosin staining, it is often difficult to distinguish large cell type neuroendocrine carcinoma from adenocarcinoma on hematoxylin-eosin slides leading to the danger of pathologists overlooking these entities[14,77]. Neuroendocrine portion of MANEC display high proliferative activity with an average Ki-67 labeling index of 78.0% (30%-99%) in the neuroendocrine component, while lower expression of 25% in adenocarcinoma[39,46,77], with positivity for synaptophysin, while the adenocarcinoma is positive for pancytokeratin AE1/AE3, cytokeratin 20 and CDX2[39,80]. Two of three commonly used immunohistochemical neuroendocrine markers, chromogranin A, synaptophysin, CD56, and neuron specific enolase should be positive in the neuroendocrine portion to be deemed as MANEC[14,80]. Synaptophysin and Chromogranin A are reported as the most reliable marker to identify neuroendocrine differentiation[31]. Neuroendocrine component of amphicrine cells are additionally reported to harbor somatostatin, serotonin and glucagon[24]. Immunohistochemical examinations have shown positivity for programmed cell death receptor ligand 1 in 5%-10% of the tumor cells with a case report of targeted immunotherapy with pembrolizumab (programmed cell death receptor ligand 1 antibody) being used in treatment of metastatic mixed adenoneuroendocrine carcinoma of the colon[109].
Reports of both exocrine/epithelial and endocrine components showing positivity for cyclin D1, p53, and beta-catenin are also documented. Interestingly beta-catenin showed very high positivity rate in the neuroendocrine component[20]. MANECs positive for E-cadherin, villin, and CgA have also been reported in the literature[85]. Secretion of mucin can be demonstrated with periodic acid Schiff reagent[41]. In MANECs involved by squamous cell carcinoma, the squamous cell component can be highlighted by positivity for CK5/6 and CDX2[91]. Recently CD133, the marker of cancer stem cells has been reported to be positive in 64% of MANEC of digestive tract, and have been associated with tumor aggressiveness[95].
Adenocarcinoma, adenoma or squamous cell carcinomas are known to arise from the mucosa, while the neuroendocrine component frequently develops from deeper layer of the colon wall. In this context, endoscopy-guided biopsies may not sample the deep seated neuroendocrine component thus potentially leading to under-diagnosis in biopsy samples prone to sampling bias[15]. In cases where more than one diagnostic sample was available, the suspicion or diagnosis of MiNEN was observed in 36.1%[18]. Depending on the stage of the disease, the tumor may involve the entire thickness of the bowel wall or even further to involve adjacent organs. Histologically a gland forming lesion along with solid nests or sheets of tumor cells with large vesicular nuclei with prominent nucleoli may be seen[43].
In the case of low grade MANETs composed of mixed glandular-carcinoid tumors, the dysplastic glandular component usually occupies the periphery of the polyp extending to involve the stalk, while the carcinoid/neuroendocrine component is usually found in the center of the polyp[30]. Well differentiated NET displays insular or trabecular growth pattern, with cuboidal or polygonal with uniform round nuclei with finely stippled chromatin and eosinophilic granular cytoplasm. The glandular component consists of abnormal epithelial cells with enlarged nuclei, coarse chromatin, forming irregular glands[38]. Rare cases of adenomatous polyps with neuroendocrine carcinoma have been reported that progresses rapidly and often displays similar morphology to that of an advanced adenocarcinoma raising the question of high grade evolution of a MANET[66,79].
Up-to-date evidence suggests that treatment should be based on the most aggressive histological component[62] even if less than 30% especially in the context of NEC wherein this is the most important determinant of aggressive behavior. MANETs that consist of adenomas and well differentiated neuroendocrine tumor, although less aggressive, are still known to metastasize[24]. The histology of synchronous or metachronous distant metastases usually have similar histologic appearance to the primary tumor though it may be composed entirely of only one component or may represent a mixed epithelial/endocrine metastatic foci usually confirmed with the aid of ancillary immunohistochemical studies. Usually a single or predominant PDNEC is seen in 60.8% of cases with a mixed endocrine carcinoma and adenocarcinoma in 33.3% and a single predominant adenocarcinoma component in 5.9%[18].
Given the rarity and marked heterogeneity of these mixed tumors of the colon and rectum with varied histological phenotypes: The histogenesis, classification and management remain ill-defined. These tumors continue to present challenges, with respect to its true nature, behavior and establishment of guidelines for optimal management strategies[14,15,18,39,44,65,69,74,77,79,80,110].
Many cases present in advanced stage locally, or may present with synchronous metastases. Recurrent disease also behaves aggressively, growing at an accelerated phase with metastasis to multiple sites. Although both components can metastasize, often it’s the poorly differentiated component that does[20]. Lymph node metastases were detected in 72% of the patients, 20% presented with distant metastasizes while all cases presented with lymphovascular invasion[14,77]. Most commonly reported distant metastatic sites were liver, paraaortic lymph node, bone and lung followed by peritoneum[14,77,92]. The histologic subtype of the primary tumor is not an accurate predictor for the pattern of metastases[20]. As discussed previously, unusual patterns of metastases from colorectal MEENs to brain, meninges or groin nodes can be encountered occasionally. In cases of metastatic MEENs the treatment should target the components present within the metastases, depending on their respective grades of malignancy[15].
The optimal management strategy for patients with these MEEN is not defined due to their rarity[23]. Majority of the cases-upto 92.5%, undergo surgical resection with curative intent-i.e. Surgery with R0 resection with appropriate negative margins is the goal for localized with or without loco-regional nodal involvement or locally advanced disease. The surgical approach is tailored depending on the location and extent of the disease. Often surgery may be performed, targeting both the primary and metastatic lesions. Patients who present with non-metastatic disease, where surgical resection is not feasible without unacceptable morbidity, and those with metastatic disease are assessed on case by case bases for the best therapeutic approach[15] with the recognition that surgery (performed 13.8% in the palliative setting) may not be the sole option for most patients with metastatic disease[18]. In such patients, preoperative or neoadjuvant chemotherapy with or without radiation for conversion surgery for liver metastases[19,79] and or surgical debulking of primary tumor and ablative therapy or chemotherapy targeting the metastatic disease is utilized[15]. Increased physician awareness of the terminology and the spectrum of MiNENs/MEENs with better understanding of the prognosis, plays a vital part in optimal treatment decisions[23]. Although the presentation and surgical management of MiNENs/MEENs are similar to that of pure adenocarcinomas, the recommendation is for each case to be discussed in multidisciplinary rounds, both preoperatively and postoperatively for multidisciplinary, oncologic and surgical management as soon as the diagnosis is confirmed[18,62].
Radiotherapy in combination with chemotherapy has a place in locally advanced or node positive rectal lesions. These cases are treated with well-established preoperative protocols in a multidisciplinary tumor board setting with consensus discussion of each case. Preoperative neoadjuvant chemoradiation protocol has a special place for rectal lesions where the primary aim is to downstage large bulky tumors for a more effective oncological surgical resection in keeping with the gold standard for surgical rectal cancer management that aims to achieve surgical removal of the tumor together with all its draining lymph nodes as one intact mesorectal package in order to minimize local recurrence. There are no special protocols in place exclusive for tumors with mixed histology of epithelial and endocrine components. Pathological composition of these tumors does not alter their management[111].
Role of chemotherapy–emphasis on targeted modalities: Almost 40% of cases of gastroenteropancreatic (GEP) NECs contain non-neuroendocrine components, including adenocarcinoma, signet ring cell carcinoma, and more rarely, squamous cell cancer with the WHO 30% cut off criterion as discussed earlier[17,31]. In most MiNENs, both the neuroendocrine and non-neuroendocrine components are poorly differentiated, and the neuroendocrine component has proliferation indices in the same range as NECs, but rarely one or both components may be well differentiated.
The clinical behavior of the disease mostly depends on neuroendocrine component in high-grade neuroendocrine tumor. The adenocarcinoma component only influences the outcomes in cases where there is a well-differentiated neuroendocrine counterpart. MiNEN with well-differentiated neuroendocrine components should be treated as conventional colorectal adenocarcinomas, while MiNEN with poorly differentiated neuroendocrine component, the more common entity, should be treated as neuroendocrine carcinomas[111]. Recently, the role of somatostatin analogs for low grade well differentiated tumors G1, G2 has been proposed in MANETs as expression of SSTR-5 is 81.8% and 60% in G1 tumors and G2 tumors respectively. Their role if any in PDNEC remains unexplored[79].
Watanabe et al[77] reported that the immunohistochemical staining of metastatic lesions in 9 patients with recurrent MiNEN demonstrated a neuroendocrine carcinomatous component in 5 patients and an adenocarcinomatous component in 4 patients. These results indicate that in most patients with MiNEN, only one of the two components metastasizes and that the histology of distant metastases should be confirmed by biopsy before planning a systemic strategy for managing metastatic disease.
At present, most MiNENs are treated similarly to pure NECs, as the neuroendocrine component that is mostly poorly differentiated and high-grade, dictates prognosis[65,68,87,98,112,113], in keeping with the origin of these mixed tumors that is thought to be a totipotent stem cell present in the submucosa that can differentiate into various cell lines[31,33,39,114-116].
Systemic treatment: Currently, the prospective data is lacking to guide us in making treatment decisions for high-grade GEP NEC or MiNEN. If the neuroendocrine component is high-grade and poorly differentiated then current treatment suggestions are based primarily on retrospective data and extrapolation of data from small cell lung cancer (SCLC), given similarities in biologic behavior.
GEP NEC patients generally have a poor prognosis with rapid disease progression. Multimodality treatment is recommended for most patients with early stage/localized disease. These are chemotherapy-responsive neoplasms, and platinum-based chemotherapy represents the backbone of treatment for both early and advanced-stage GEP NEC[117].
(1) Systemic treatment for localized disease: For early stage, potentially resectable disease, surgical resection followed by four to six cycles of adjuvant systemic treatment with a platinum drug (cisplatin/carboplatin) plus etoposide is generally used similar to the SCLC protocol.
Recommendations from the north american neuroendocrine tumor society (NANETS)[118] suggest administration of four to six cycles of adjuvant chemotherapy with a platinum drug (cisplatin/carboplatin) plus etoposide. The available data in limited stage SCLC suggest that carboplatin and cisplatin are equivalent in terms of overall survival, progression free survival, objective response rate but different toxicity profiles[119]. Carboplatin causes more grade 3–4 hematologic toxicities whereas Cisplatin mainly causes neurotoxicity and renal toxicity.
Neoadjuvant chemoradiation is reasonable, if the risk of local recurrence is high, depending upon the anatomic location of the tumor (e.g, rectum). However, distant recurrences are more frequent than local recurrences. A neoadjuvant approach to treatment with chemotherapy alone followed by surgery is an option for patients with localized rectal tumor, especially if it is estimated that postoperative morbidity will be high and adjuvant treatment might be delayed[120].
(2) Systemic treatment for metastatic disease: For metastatic disease, early institution of palliative chemotherapy is necessary, given the aggressive nature of the high-grade neuroendocrine components. Standard chemotherapy consists of platinum plus etoposide[121-127].
In a retrospective study of Nordic consortium of GI NEC patients, platinum plus etoposide was prescribed to 252 patients (82/252 CR). Median survival was 11 mo in those who received palliative chemotherapy versus 1 mo in 53 (16/53 CR) patients receiving best supportive care. The response rate to first-line chemotherapy was 31% and 33% had stable disease. Those with Ki-67 < 55% had a lower response rate (15% vs 42 %, P < 0.001) but better survival than patients with Ki-67 > 55 % (14 vs 10 mo P < 0.001). The most important negative prognostic factors for survival were poor performance status, primary colorectal tumors and elevated platelets or LDH levels[128].
Irinotecan/cisplatin doublet is an acceptable alternative initial chemotherapy regimen in patients with metastatic disease. A multicenter Japanese retrospective study of gastrointestinal NEC[129] demonstrated the benefit of Irinotecan plus cisplatin (IP) over etoposide plus (EP) cisplatin. Of 258 patients, (31/258 were colorectal, CR) 160/258 (62%) received IP and 46/258 (18%) EP and 37/258 (14%) received 5 fluorouracil based FOLFOX/S-1. Of note, 15/31 of colorectal patient has received IP whereas 2/31 received EP and 13/31 received 5 FU based chemotherapy. Response rates were 50% for IP and 28% for EP and median overall survival was 13.0 and 7.3 mo for IP and EP respectively. The optimal duration of treatment is not established. Usually 4-6 cycles of therapy are administered, but with a continuous response and minimal toxicities, continuation of chemotherapy to at least maximal response is appropriate.
Extrapolating data from small cell lung cancer literature, those with platinum-sensitive disease may be re-challenged with a platinum and etoposide combination if relapse occurs at least three to six months after discontinuation of first-line treatment. In the Nordic study retreatment with the same regimen yielded a response rate of 15%, with another 27% achieving stable disease[128]. These tumors are usually responsive to chemotherapy but the duration of response is usually short and the relapse rate is very high.
There is minimal data on survival benefit of second-line therapy and to our knowledge no study has compared active chemotherapy to best supportive care. Currently for high-grade mixed currently there is no standard regimen has been established. Second-line treatment options include temozolomide, 5 FU, irinotecan/oxaliplatin based treatment[130-132], or topotecan[133].
Temozolomide is commonly used in the second-line setting. Temozolomide was given with or without capecitabine and bevacizumab as second-line treatment after cisplatin-based chemotherapy in a cohort of 25 patients with GEP NEC[134]. The response rate was 33%, another 9 patients had stable disease, and median overall survival was 22 mo from time of diagnosis.
Another study of temozolomide monotherapy in 28 patients with NEC, there were no responses and median survival was just 3.5 mo[135]. It was noted that patients with Ki-67 < 50% did better than those with Ki-67 > 50% (median survival 10.9 vs 2.7 mo) suggesting temozolomide-based chemotherapy may be more effective in patients with Ki-67 indices in the 20%-55% range and platinum-refractory disease.
Evidence available so far with single agent immune checkpoint inhibitors targeting programmed cell death 1 (PD-1) has shown very low response rates[109,136,137].
A prospective, open level, multicenter, PhaseII basket trial of dual immune checkpoint blockade using ipilimumab (an inhibitor of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (1 mg/kg IV Q 6 wk) plus nivolumab (a PD-1 inhibitor) (240 mg IV Q 2 wk) across 37 cohorts of rare tumor included two cohorts that permitted accrual of NETs of various grades and primary sites. In a preliminary report of an unplanned subset analysis of one of the cohorts (which included lung NECs), objective responses were seen in 8 out of 19 patients (42%) with high-grade NENs[138].
In rare cases of MiNEN, the neuroendocrine component is well-differentiated, low-grade along with adenocarcinoma component. In those cases, the treatments administered are usually those of colorectal adenocarcinoma protocol in neoadjuvant, adjuvant and metastatic setting.
In summary, curative surgical resection, chemotherapy, radiotherapy, somatostatin analogs, embolization of liver secondaries and radionuclide therapy are some of the therapeutic modalities used in MANEC with surgery being the primary first line of therapy. Though multimodal treatment has better treatment outcomes; yet, the optimal strategy for such patients with primary colorectal disease still remains unclear[75,110].
In studies that have investigated the prognosis of MiNENs/MEENs in comparison to other neoplasm it is well recognized that patients with MiNENs/MEENs diagnosis carry a worse prognosis than patients with pure well differentiated NET1, NET2. There is however, continued controversy whether MiNENs/MEENs have a better or worse prognosis than pure NEC[18]. The biological behavior of MiNENs/MEENs is predominantly driven by the endocrine component, which in more than 90% of cases is poorly differentiated and once metastasized, high grade neuroendocrine carcinoma carries a very poor prognosis with no definitive curative treatment options[18,43,71,73]. Compared to survival rate of patients with adenocarcinoma, survival rate of MANEC patients is substantially worse[14]. However, it is reported that the MANEC version of colonic NEC seems to be less aggressive with a better prognosis that pure colorectal NEC[107]. Overall the prognosis of MANEC remains poor with a median survival of 7-18 mo from initial diagnosis, largely influenced by disease stage and tumor type[18,29,39]. Better survival was registered in patients with loco-regional disease compared with those with distant metastases. In another study patients with colorectal MANECs and pure NECs, showed similar survival. An Italian multicenter study on 51 patients with a diagnosis of advanced MANECs of the gastroenteropancreatic tract concluded that the prognoses of these neoplasms are mostly driven by the NEC component regardless of treatments[139]. Most MANECs with a NET component particularly in the large bowel presented as advanced disease, with deep wall invasion, lymph node and distant metastases. However, recent recognition of microsatellite unstable MANECS with a better prognosis to pure colorectal NEC is emerging as a unique clinicopathological subset of MANECs. Thus the prognosis of patients with MANECs consisting of NEC component needs to be better defined[29]. Multiple factors contribute to the overall prognosis, led by the type of tumor size, histology, degree of differentiation followed by extent of disease[24]. The endocrine component is known for rapid progression with diagnosis in an advanced stage, leading to the poor survival rates as it appears to be the driver of the clinical course of the disease[14,39]. Most studies have led to the conclusion that the degree of component differentiation influences the prognosis greater than the volume of tumor occupied[24,27]. Slowly, yet surely, understanding the molecular mutations harboring prognostic significances are emerging with promise of targeted therapies. In this context, in addition to somatostatin analogs, depending on the KRAS status, a molecular predictor, the efficacy of anti-EGFR drugs in mixed tumors unlike colorectal adenocarcinomas is still unknown[79]. Five year disease free survival and 5 year overall survival were significantly lower in the MANEC cases compared to those diagnosed with adenocarcinoma (60.5% vs 76.2% and 69% vs 82%, respectively)[14]. Specific prognostic factors or parameters are not reported in the literature concerning survival of MANEC patients[14]. However, MANECs with vascular invasion and CD117 expression in colorectal locations are reported as independent prognostic factors, correlating with shorter survival[14,24]. Conversely, large cell type, lymphatic involvement around the tumor, CD117 (-), absence of vascular invasion, and unstable microsatellite owing to methylation have shown to improve prognoses[89]. In cases of MiNENs/MEENs no long-term survivors have been reported in stage IV disease with median progression-free survival reported at 4.5 mo while median overall survival was 9.5 mo and no patients survived beyond 17 mo[24,43]. Higher volume of high grade neuroendocrine component (> 50%) is of the total volume of tumor is shown to be an independent poor prognostic indicator exhibiting worse survival rates. Adverse outcome correlated with lack of staining for chromogranin and intense staining for somatostatin[62]. Overall, the most important prognostic factor is reported as the overall Ki67 profiling index in both components[24]. The prognosis of intermediate grade MiNENs/MEENs is generally determined by the epithelial/non-neuroendocrine/component, while in contrast should a more aggressive neuroendocrine component such as poorly differentiated neuroendocrine carcinomas be present, this would automatically default as the dominant prognostic predictor. It should be noted however, that, both components can metastasize in a poor unpredictable fashion[15]. True low grade MiNENs/MEENs mixed tumors composed of adenoma with well differentiated low grade neuroendocrine tumors are however associated with a relatively good prognosis and behaves as an indolent disease[33].
Continued research to outline the pathogenic pathways are yet to be identified, including recognizing the critical carcinogenic “turning point” of this mixed neoplasm in the colon and rectum and answers to the? Why or? How two different neoplastic cells, with different phenotype and different behaviors coexist as one neoplasm. More accessible genomic materials such as circulating tumor cells or deoxyribonucleic acid deoxyribonucleic acid may prove useful in the future. Such liquid biopsies with genomic profiling of tumor may shed light to the complex genomic landscape of MiNENs/MEENs. Knowledge of unique molecular signatures may serve as the stepping stone to the development of targeted therapeutic options and may facilitate detection of precancerous and early stage lesions which is central to improving patient prognosis. Thus important questions remain that have not yet been addressed by research.
Another area of continued debate is the WHO discriminatory criterion cut off 30% rule for each component for inclusion as a mixed tumor. Most studies have led to the conclusion that it is the degree of component differentiation that influences the prognosis rather than the percentage volume of tumor occupied in the neoplasm. We strongly recommend that there should be continued reporting of any second component especially with poorly differentiated histology or evidence of invasion, regardless of its percentage volume. Further exploration of the minimum percentage of each neoplastic component and their prognostic impact must be revisited.
Finally, recommendations regarding best management strategies for patients with these complex tumors of mixed components are difficult to formulate due to the lack of evidence based guidelines. Clinicopathological risk factors regarding metastatic disease to lymph nodes and distant disease are yet to be explored in detail. The reasons underlying the presence of only one component in the most distant metastases need to be explored. Information regarding synchronous, metachronous metastases, local recurrence, metastases to unusual sites, development of new rapidly growing metastatic lesions while on treatment in otherwise stable disease are stories that needs to be retold and revisited for a deeper understanding of the true biology of this disease.
MEEN of the colon and rectum are poorly understood rare entities that encompass an extensive range of heterogeneous tumors with a wide variety of combinations leading to tumors of high, intermediate or low grade malignant potential. Morphologically, the two components may present as composite mixed, collision or as amphicrine tumors. Unfortunately, those most commonly encountered in clinical practice are high grade tumors with an epithelial carcinomatous component admixed with a poorly differentiated NEC carrying a poor prognosis. These tumors continue to present great histopathological diagnostic challenges on limited biopsy material as in upto one third of the cases only one of the tumor components is identified. Although published cases of these entities are limited, careful dedicated pathological examination of bulky, or locally advanced, poorly differentiated tumors may reveal the true prevalence of this neoplasm. Continued vigilance with methodical detailed examination of the definitive resected specimen with ancillary immunohistochemical studies is required for accurate diagnosis. It is postulated that most pure endocrine cell carcinomas of colon and rectum if examined thoroughly will be associated with a carcinomatous element thus being a MANECs. Variations in morphological characteristics and degrees of cellular differentiation significantly influence the evolution of these cases. Clinical vigilance and histological identification of such tumors is the cornerstone in determining appropriate treatment strategies for these complex lesions. Continued molecular and genetic analysis is crucial for understanding the genetic drivers of this neoplasm as potential for future targeted therapy. Acknowledging, the vital role of the endocrine component in dictating the clinical behavior of this tumor, has led to continued exploration for the use of somatostatin receptors scintigraphy for the diagnosis and follow-up of MANECs. Early referral to a high-volume center with potential multidisciplinary expertise in pathological diagnosis with surgical and medical oncologist multidisciplinary teams in the management of these tumors is an important step in the expectation of improved overall outcomes. Steps to increase clinical and pathological awareness of these rare and complex entities is a key step to better optimize treatment options and lead to the establishment of evidence based guidelines for management.
We strongly recommend a multicentric approach with multi institutional collaborative trials of treatment protocols including novel multimodality approach of surgery, chemotherapy, radiotherapy (rectal lesions) and potentially evolving systemic therapy with targeted antineoplastic pharmacological interventions for these unique rare lesions.
Mixed tumors of the colon and rectum, composed of a combination of epithelial and endocrine elements of benign and malignant potential are rare neoplasms. These can occur anywhere in the gastrointestinal tract and are often diagnosed incidentally. Though they have been a well-documented entity in the pancreas, recognition and accurate diagnosis of these tumors in the colon and rectum, to date, remains a challenge. This is further compounded by the different terminologies that have been attributed to these lesions over the years adding to increased confusion and misclassification. Therefore, dedicated literature reviews of these lesions in the colon and rectum are inconsistent and are predominantly limited to case reports and case series of limited case numbers.
Though, most of these tumors are high grade and of advanced stage, intermediate and low grade lesions of these mixed tumors are also increasingly been reported. Currently therefore, there are no established independent consensus based guidelines for the therapeutic patient management of these unique lesions: However, as most lesions of clinical significance typically present as high grade, and at an advanced stage, they are usually treated by default as high stage adenocarcinomas of the colon and rectum; though additional specialized targeted therapies for the endocrine elements are emerging with improved understanding.
This manuscript provides a targeted comprehensive literature review of such mixed tumors in the colon and rectum that chronicles the evolution over time with summarization of historical perspectives of terminology and advocates a rationale for the proposal for a new, simple, clinically relevant, non-ambiguous terminology for these lesions to be referred to as mixed epithelial endocrine neoplasms. Discussion of the pathogenesis including genomic landscape, clinicoradiological features, pathology, treatment, prognosis, the current status of the management of the primary lesions, their recurrences and metastases in the context of evidence provides a comprehensive upto date review of these unique colorectal neoplasms.
The key topic of interest is to unravel the varied terminologies that have been attached to these neoplasms ever since its first recognition in 1924 to its current state of World Health Organization 2019. Unfortunately, due to the different terminologies used there is continued lack of clarity and misclassification of these lesions. Therefore, dedicated literature reviews of these lesions in the colon and rectum are inconsistent and are predominantly limited to case reports and case series of limited case numbers.
The detailed review with understanding has resulted in the new proposed terminology that is simple, clinically relevant, non-ambiguous and free of semantic error. Acceptance and utilization of this terminology will facilitate study of identical tumors in larger numbers for improved understanding on their pathogenesis resulting in finding better targeted treatment options and for accurate evaluation of predictive and prognostic factors of these rare and unusual neoplasms.
The main objective of this study was to conduct a targeted review of these complex, rare colorectal neoplasms to further our understanding regarding their pathogenesis, genomic landscape, clinico-radio-pathological features, treatment and prognosis and realize the gaps in our knowledge. Additionally getting the terminology right for these tumors will facilitate future studies of well-designed multi institutional prospective randomized controlled clinical trials to develop and evaluate newer therapeutic strategies for continued improved understanding and personal optimization of clinical management of these rare types of colorectal neoplasms.
A comprehensive review of the published English literature was conducted using the search engines PubMed, MEDLINE and GOOGLE scholar. The following search terms (“mixed tumors colon” OR mixed endocrine/neuroendocrine tumor/neoplasm/lesion colon OR adenocarcinoma and endocrine/neuroendocrine tumor colon OR mixed adenocarcinoma and endocrine/neuroendocrine carcinoma colon OR Amphicrine tumors OR Collision tumors) were used. This was repeated for rectal tumors independently using the same search terms. The initial search was conducted in September of 2019. In addition an updated search was conducted in April 2020. Eligibility criteria were defined and all potential relevant items, including full articles and/or abstracts were independently reviewed, assessed and agreed upon items were selected for in-depth analysis. Relevant secondary references were retrieved and reviewed by two of the authors.
In total 237 full articles/abstracts documents were considered for eligibility of which 45 articles were illegible resulting in a total of 192 articles that were assessed for eligibility of which 139 have been selected for reference in this current review. Therefore this seminal manuscript is a one stop article that provides a detailed outlook on the evolution over time with summarization of historical perspectives, nomenclature, clinicoradiological features, pathology, treatment, prognosis and the current status of the management of both the primary lesions, their recurrences and metastases. Guidelines for therapeutic strategies, patient management and the continued lack of independent consensus guidelines were also explored. An additional feature of this manuscript is the proposal for a new revised terminology for these unique tumors that encompasses all the relevant criteria and is simple, clinically relevant and free of semantic ambiguity. This will solve the biggest hurdle of confusion and misclassification that plagues these rare unique colorectal neoplasms.
The unique outcome of this targeted review is the proposal for a new revised terminology for these unique tumors that encompasses all the relevant criteria and is simple, clinically relevant and free of semantic ambiguity.
One of the new theories proposed is to report the two components of these tumors-epithelial and endocrine irrespective of their percentages and on their clinical relevance as high grade, intermediate grade and low grade neoplasms.
This manuscript has reviewed the current status of these entities providing a detailed outlook on the evolution over time with summarization of historical perspectives, nomenclature, clinicoradiological features, pathology, treatment, prognosis and the current status of the management of both the primary lesions, their recurrences and metastases. Guidelines for therapeutic strategies, patient management and the continued lack of independent consensus guidelines were also explored. An additional feature of this manuscript is the proposal for a new revised terminology for these unique tumors that encompasses all the relevant criteria and is simple, clinically relevant and free of semantic ambiguity.
Gaps in knowledge that have been identified are threefold that include (1) the pathogenetic pathway are yet to be identified including recognition of the critical carcinogenic “turning point” of this mixed neoplasm in the colon and rectum and answers to the? Why or? How two different neoplastic cells, with different phenotype and different behaviors coexist as one neoplasm. More accessible genomic materials such as circulating tumor cells or deoxyribonucleic acid may prove useful in the future. Such liquid biopsies with genomic profiling of tumor may shed light to the complex genomic landscape of MiNENs/MEENs. Knowledge of unique molecular signatures may serve as the stepping stone to the development of targeted therapeutic options and may facilitate detection of precancerous and early stage lesions which is central to improving patient prognosis. Thus important questions remain that have not yet been addressed by research.
(2) Another area of continued debate is the World Health Organization discriminatory criterion cut off 30% rule for each component for inclusion as a mixed tumor. Most studies have led to the conclusion that it is the degree of component differentiation that influences the prognosis rather than the percentage volume of tumor occupied in the neoplasm. We strongly recommend that there should be continued reporting of any second component especially with poorly differentiated histology or evidence of invasion, regardless of its percentage volume. Further exploration of the minimum percentage of each neoplastic component and their prognostic impact must be revisited.
(3) Finally, recommendations regarding best management strategies for patients with these complex tumors of mixed components are difficult to formulate due to the lack of evidence based guidelines. Clinicopathological risk factors regarding metastatic disease to lymph nodes and distant disease are yet to be explored in detail. The reasons underlying the presence of only one component in the most distant metastases need to be explored. Information regarding synchronous, metachronous metastases, local recurrence, metastases to unusual sites, development of new rapidly growing metastatic lesions while on treatment in otherwise stable disease are stories that needs to be retold and revisited for a deeper understanding of the true biology of this disease.
MEEN of the colon and rectum are poorly understood rare entities that encompass an extensive range of heterogeneous tumors with a wide variety of combinations leading to tumors of high, intermediate or low grade malignant potential. Morphologically, the two components may present as composite mixed, collision or as amphicrine tumors. Unfortunately, those most commonly encountered in clinical practice are high grade tumors with an epithelial carcinomatous component admixed with a poorly differentiated neuroendocrine component (MANEC) carrying a poor prognosis. These tumors continue to present great histopathological diagnostic challenges on limited biopsy material as in upto one third of the cases only one of the tumor components is identified. Although published cases of these entities are limited, careful dedicated pathological examination of bulky, or locally advanced, poorly differentiated tumors may reveal the true prevalence of this neoplasm. Continued vigilance with methodical detailed examination of the definitive resected specimen with ancillary immunohistochemical studies is required for accurate diagnosis. It is postulated that most pure endocrine cell carcinomas of colon and rectum if examined thoroughly will be associated with a carcinomatous element thus being a MANEC. Variations in morphological characteristics and degrees of cellular differentiation significantly influence the evolution of these cases. Clinical vigilance and histological identification of such tumors is the cornerstone in determining appropriate treatment strategies for these complex lesions. Continued molecular and genetic analysis is crucial for understanding the genetic drivers of this neoplasm as potential for future targeted therapy. Acknowledging, the vital role of the endocrine component in dictating the clinical behavior of this tumor, has led to continued exploration for the use of somatostatin receptors scintigraphy for the diagnosis and follow-up of MANECs. Early referral to a high-volume center with potential multidisciplinary expertise in pathological diagnosis with surgical and medical oncologist multidisciplinary teams in the management of these tumors is an important step in the expectation of improved overall outcomes. Steps to increase clinical and pathological awareness of these rare and complex entities is a key step to better optimize treatment options and lead to the establishment of evidence based guidelines for management. We strongly recommend a multicentric approach with multi institutional collaborative trials of treatment protocols including novel multimodality approach of surgery, chemotherapy, radiotherapy [rectal lesions] and potentially evolving systemic therapy with targeted antineoplastic pharmacological interventions for these unique rare lesions.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shamseddine A S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Canadian Cancer Statistics 2019; Statistics Canada, Canadian Cancer Registry CANSIM Table 103-0554; Canadian Partnership against Cancer. Cancerview. ca - 2017 Cancer System Performance Report Her Majesty the Queen in Right of Canada, as represented by the Minister of Health, 2019. Cat.: HP35-83/2-2019E-PDF | ISBN: 978-0-660-31888-2 | Pub: 190222. [Cited in This Article: ] |
| 2. | Brahmania M, Kanthan CS, Kanthan R. Collision tumor of the colon--colonic adenocarcinoma and ovarian granulosa cell tumor. World J Surg Oncol. 2007;5:118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Lin HH, Jiang JK, Lin JK. Collision tumor of low-grade B-cell lymphoma and adenocarcinoma with tuberculosis in the colon: a case report and literature review. World J Surg Oncol. 2014;12:147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Kus T, Aktas G, Kalender ME, Sari I, Ulker E, Camci C. Collision tumor consisting of primary follicular lymphoma and adenocarcinoma in the cecum: A case report and literature review. Oncol Lett. 2016;11:2801-2805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Genovese F, Becchina G, Nagar C, Ottoveggio G, Giacalone B, Scaglione G, Varriale E, Tralongo V. Primary diffuse large B-cell lymphoma developing within a rectal tubular adenoma with low-grade dysplasia: a case report. J Med Case Rep. 2014;8:103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Bhattacharya A, Saha R, Biswas J, Biswas J, Ghosh B. Collision tumors in the gastrointestinal tract: a rare case series. Int Med Case Rep J. 2012;5:73-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Mannweiler S, Dinges HP, Beham-Schmid C, Hauser H, Starlinger M, Regauer S. Colliding / concomitant tumors of the intestine: report of 3 cases. Pathol Oncol Res. 2003;9:188-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Sasaki S, Hatanaka K, Sahara N, Uekusa T, Hirayama K, Shirahata A, Ishimaru M. Collision tumor of primary malignant lymphoma and adenocarcinoma in the colon: report of a case. Surg Today. 2010;40:975-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Soto AR, Vazquez EG, Grigg-Gutierrez NM, Magno-Pagatzaurtundua P, Cáceres W, Toro DH. Conundrum of a Large Bowel Neoplasm: Collision Tumor. ACG Case Rep J. 2018;5:e13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Dias AR, Pinto RA, Mory E, Silva IC, Siqueira SA, Nahas SC, Cecconello I, Wexner SD. Synchronous collision malignant melanoma and adenocarcinoma of the rectum. Tech Coloproctol. 2010;14:181-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Sudlow A, Liu MH, Waters G, Velchuru VR. Carcinosarcoma of the Rectum: Report of a Rare Colorectal Malignancy and Review of the Literature. Case Rep Surg. 2015;2015:412918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Kim VM, Goicochea L, Fang SH. Case Report: Collision Tumour of Colon Leiomyosarcoma and Adenocarcinoma. J Clin Diagn Res. 2016;10:PD03-PD04. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Cordier R. Les Cellules argentaffines dans les tumeurs intestinales. Arch Int Med Exp. 1924;1:5. [Cited in This Article: ] |
| 14. | Paspala A, Machairas N, Prodromidou A, Spartalis E, Ioannidis A, Kostakis ID, Papaconstantinou D, Nikiteas N. Management of MANEC of the colon and rectum: A comprehensive review of the literature. Mol Clin Oncol. 2018;9:219-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | de Mestier L, Cros J. Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN). Ann Endocrinol (Paris). 2019;80:172-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Capella C, La Rosa S, Uccella S, Billo P, Cornaggia M. Mixed endocrine-exocrine tumors of the gastrointestinal tract. Semin Diagn Pathol. 2000;17:91-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 17. | WHO. Classification of Tumours Editorial Board. WHO Classification of Tumors. Digestive System Tumours, 5th ed; International Agency for Research on Cancer (IARC): Lyon, France, 2019. [Cited in This Article: ] |
| 18. | Frizziero M, Chakrabarty B, Nagy B, Lamarca A, Hubner RA, Valle JW, McNamara MG. Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: A Systematic Review of a Controversial and Underestimated Diagnosis. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 19. | Sato O, Tsuchikawa T, Yamada T, Sato D, Nakanishi Y, Asano T, Noji T, Yo K, Ebihara Y, Murakami S, Nakamura T, Okamura K, Shichinohe T, Mitsuhashi T, Hirano S. Metastatic mixed adenoneuroendocrine carcinoma of the liver successfully resected by hepatic trisectionectomy following chemotherapy: A case report. Clin Case Rep. 2019;7:491-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Li Y, Yau A, Schaeffer D, Magliocco A, Gui X, Urbanski S, Waghray R, Owen D, Gao ZH. Colorectal glandular-neuroendocrine mixed tumor: pathologic spectrum and clinical implications. Am J Surg Pathol. 2011;35:413-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Desai GS, Pande P, Shah RC, Jagannath P. Dilemmas in Diagnosis and Management of Gastroenteropancreatic Mixed Neuroendocrine Non-neuroendocrine Neoplasms: First Single-Centre Report from India. J Gastrointest Cancer. 2020;51:102-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 800] [Reference Citation Analysis (0)] |
| 23. | Shin SH, Kim SH, Jung SH, Jang JW, Kang MS, Kim SI, Kim JH, Lee JH. High-Grade Mixed Adenoneuroendocrine Carcinoma in the Cecum: A Case Report. Ann Coloproctol. 2017;33:39-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Alexandra Bolocan, Dan Nicolae Pä‚ Duraru, Cornelia Nièšipir, Rä‚ Zvan Hainä‚ Roè˜ie, Silviu Mirel PIÈšuru, Camelia Diaconu, Andra Suceveanu, Anca Pantea Stoian. Mixed adenoneuroendocrine carcinoma of the gastrointestinal tract-features, diagnosis, management and pognostics. Romanian Biotechnological Letters. 2018;23. [DOI] [Cited in This Article: ] |
| 25. | Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987;11 Suppl 1:71-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 168] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Solcia E, Kloppel G, Sobin LH. Histological typing of endocrine tumors (WHO International Histological Classification of Tumors) WHO 2000. 2nd edition, Berlin Springer 2000. [DOI] [Cited in This Article: ] |
| 27. | Volante M, Rindi G, Papotti M. The grey zone between pure (neuro) endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006;449:499-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System, 4th ed. International Agency for Research on Cancer (IARC): Lyon, France, 2010. [Cited in This Article: ] |
| 29. | La Rosa S, Marando A, Sessa F, Capella C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers (Basel). 2012;4:11-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 30. | Hui CK. Collision adenoma-carcinoid tumour of the colon complicated by carcinoid syndrome. Singapore Med J. 2012;53:e195-e197. [PubMed] [Cited in This Article: ] |
| 31. | La Rosa S, Sessa F, Uccella S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr Pathol. 2016;27:284-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 119] [Article Influence: 14.9] [Reference Citation Analysis (2)] |
| 32. | Lloyd RV, Osamura, RY, Klöppel G, Rosai J. WHO Classification of Tumors of Endocrine Organs, 4th ed. International Agency for Research on Cancer (IARC): Lyon, France, 2017. [Cited in This Article: ] |
| 33. | La Rosa S, Uccella S, Molinari F, Savio A, Mete O, Vanoli A, Maragliano R, Frattini M, Mazzucchelli L, Sessa F, Bongiovanni M. Mixed Adenoma Well-differentiated Neuroendocrine Tumor (MANET) of the Digestive System: An Indolent Subtype of Mixed Neuroendocrine-NonNeuroendocrine Neoplasm (MiNEN). Am J Surg Pathol. 2018;42:1503-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Hu JC, Chen XC, Lin DZ, Yu ZL, Guo XF. Neuroendocrine tumor mixed with adenoma in rectum: a case report and literature review. International Journal of Surgery Oncology. 2019;4:e79. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 35. | Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Klöppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770-1786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 546] [Cited by in F6Publishing: 583] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 36. | Brittan M, Wright NA. Gastrointestinal stem cells. J Pathol. 2002;197:492-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639-2644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Kanno-Okada H, Mitsuhashi T, Mabe K, Shimoda T, Matsuno Y. Composite neuroendocrine tumor and adenocarcinoma of the rectum. Diagn Pathol. 2017;12:85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Gül-Klein S, Sinn M, Jurmeister PS, Biebl M, Weiß S, Rau B, Bläker H, Pratschke J, Aigner F. Two patients with rare mixed adenoneuroendocrine carcinomas of the rectum. SAGE Open Med Case Rep. 2018;6:2050313X18758816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Bazerbachi F, Kermanshahi TR, Monteiro C. Early precursor of mixed endocrine-exocrine tumors of the gastrointestinal tract: histologic and molecular correlations. Ochsner J. 2015;15:97-101. [PubMed] [Cited in This Article: ] |
| 41. | Pecorella I, Memeo L, Ciardi A, Rotterdam H. An unusual case of colonic mixed adenoendocrine carcinoma: collision versus composite tumor. A case report and review of the literature. Ann Diagn Pathol. 2007;11:285-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Soliman ML, Tiwari A, Zhao Q. Coexisting tubular adenoma with a neuroendocrine carcinoma of colon allowing early surgical intervention and implicating a shared stem cell origin. World J Gastroenterol. 2017;23:1106-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Vanacker L, Smeets D, Hoorens A, Teugels E, Algaba R, Dehou MF, De Becker A, Lambrechts D, De Greve J. Mixed adenoneuroendocrine carcinoma of the colon: molecular pathogenesis and treatment. Anticancer Res. 2014;34:5517-5521. [PubMed] [Cited in This Article: ] |
| 44. | Olevian D, Harris B, Nikiforova M, Kuan, SF, Pai R. 718 Clinicopathologic analysis of colorectal carcinoma with high-grade neuroendocrine differentiation: Identification of a unique subtype with mixed large cell neuroendocrine carcinoma/signet ring cell adenocarcinoma with frequent BRAF mutation and poor overall survival. Lab Investig. 2015;95:182A. [Cited in This Article: ] |
| 45. | Scardoni M, Vittoria E, Volante M, Rusev B, Bersani S, Mafficini A, Gottardi M, Giandomenico V, Malleo G, Butturini G, Cingarlini S, Fassan M, Scarpa A. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology. 2014;100:310-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | Schizas D, Katsaros I, Michalinos A, Damaskos C, Garmpis N, Ntomi V, Agrogiannis G, Stergiopoulos S, Tsaroucha AK. Collision Tumors of the Gastrointestinal Tract: A Systematic Review of the Literature. Anticancer Res. 2018;38:6047-6057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Li L, Lu Y, Mackovick L, Zhou J. A case report: Collision tumor of colon: High-grade adenocarcinoma and low-grade neuroendocrine tumor. AJCP. 2016;146:S109-S110. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Jesinghaus M, Konukiewitz B, Keller G, Kloor M, Steiger K, Reiche M, Penzel R, Endris V, Arsenic R, Hermann G, Stenzinger A, Weichert W, Pfarr N, Klöppel G. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod Pathol. 2017;30:610-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 49. | Sinha N, Gaston D, Manders D, Goudie M, Matsuoka M, Xie T, Huang WY. Characterization of genome-wide copy number aberrations in colonic mixed adenoneuroendocrine carcinoma and neuroendocrine carcinoma reveals recurrent amplification of PTGER4 and MYC genes. Hum Pathol. 2018;73:16-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Lee SM, Broaddus R, Singh R, Luthra R, Chen H. Mutation profile of colorectal neuroendocrine neoplasm. Annual Meeting Abstracts Lab. Investig. 2016;182A. [Cited in This Article: ] |
| 51. | Estrella JS, Taggart MW, Rashid A, Abraham SC. Low-grade neuroendocrine tumors arising in intestinal adenomas: evidence for alterations in the adenomatous polyposis coli/β-catenin pathway. Hum Pathol. 2014;45:2051-2058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Detweiler CJ, Cardona DM, Hsu DS, McCall SJ. Primary high-grade neuroendocrine carcinoma emerging from an adenomatous polyp in the setting of familial adenomatous polyposis. BMJ Case Rep. 2016;2016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Girardi DM, Silva ACB, Rêgo JFM, Coudry RA, Riechelmann RP. Unraveling molecular pathways of poorly differentiated neuroendocrine carcinomas of the gastroenteropancreatic system: A systematic review. Cancer Treat Rev. 2017;56:28-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 54. | Sahnane N, Furlan D, Monti M, Romualdi C, Vanoli A, Vicari E, Solcia E, Capella C, Sessa F, La Rosa S. Microsatellite unstable gastrointestinal neuroendocrine carcinomas: a new clinicopathologic entity. Endocr Relat Cancer. 2015;22:35-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 55. | Bongiovanni M, Molinari F, Uccella S, Savio A, Sessa F, La Rosa S. Mixed Adenoma-Well Differentiated Neuroendocrine Tumors (MANETs) of the Colon. Clinico-Pathologic and Molecular Analysis of 6 Cases of a Rare and Recently Recognized Entity. Annual Meeting Abstracts Lab. Investig. 2017;97:145A. [Cited in This Article: ] |
| 56. | Woischke C, Schaaf CW, Yang HM, Vieth M, Veits L, Geddert H, Märkl B, Stömmer P, Schaeffer DF, Frölich M, Blum H, Vosberg S, Greif PA, Jung A, Kirchner T, Horst D. In-depth mutational analyses of colorectal neuroendocrine carcinomas with adenoma or adenocarcinoma components. Mod Pathol. 2017;30:95-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 57. | Iannuccelli M, Micarelli E, Surdo PL, Palma A, Perfetto L, Rozzo I, Castagnoli L, Licata L, Cesareni G. CancerGeneNet: linking driver genes to cancer hallmarks. Nucleic Acids Res. 2020;48:D416-D421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Golombek T, Henker R, Rehak M, Quäschling U, Lordick F, Knödler M. A Rare Case of Mixed Adenoneuroendocrine Carcinoma (MANEC) of the Gastroesophageal Junction with HER2/neu Overexpression and Distinct Orbital and Optic Nerve Toxicity after Intravenous Administration of Cisplatin. Oncol Res Treat. 2019;42:123-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Yang HM, Schaaf C, Schaeffer D, Vieth M, Veits L, Geddert H, Jung A, Kirchner T, Horst D. Molecular analysis of mixed adenoneuroendocrine carcinomas (MANEC) signifies a common clonal origin of adeno and neuroendocrine components. Meeting Abstract Lab. Investig. 2015;95:199A. [Cited in This Article: ] |
| 60. | Olinici CD, Domşa I, Crişan D. Collision adenocarcinoma--carcinoid tumor of the colon. Case report and review of the literature. Rom J Morphol Embryol. 2007;48:75-78. [PubMed] [Cited in This Article: ] |
| 61. | Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, Pleasance ED, Lau KW, Beare D, Stebbings LA, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal S, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Quail MA, Burton J, Swerdlow H, Carter NP, Morsberger LA, Iacobuzio-Donahue C, Follows GA, Green AR, Flanagan AM, Stratton MR, Futreal PA, Campbell PJ. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1675] [Cited by in F6Publishing: 1656] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 62. | Düzköylü Y, Aras O, Bostancı EB, Keklik Temuçin T, Ulaş M. Mixed Adeno-Neuroendocrine Carcinoma; Case Series of Ten Patients with Review of the Literature. Balkan Med J. 2018;35:263-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Murata M, Takahashi H, Yamada M, Song M, Hiratsuka M. A case of mixed adenoneuroendocrine carcinoma of the pancreas: Immunohistochemical analysis for histogenesis. Medicine (Baltimore). 2017;96:e6225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Gurzu S, Fetyko A, Bara T, Banias L, Butiurca VO, Bara T, Tudorache V, Jung I. Gastrointestinal mixed adenoneuroendocrine carcinoma (MANEC): An immunohistochemistry study of 13 microsatellite stable cases. Pathol Res Pract. 2019;215:152697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | La Rosa S, Marando A, Furlan D, Sahnane N, Capella C. Colorectal poorly differentiated neuroendocrine carcinomas and mixed adenoneuroendocrine carcinomas: insights into the diagnostic immunophenotype, assessment of methylation profile, and search for prognostic markers. Am J Surg Pathol. 2012;36:601-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 66. | Grillo F, Valle L, Sammito G, Scabini S, Albertelli M, Mastracci L. Rectal mixed neuroendocrine non-neuroendocrine neoplasm (MiNEN): High grade evolution of a MANET? Pathol Res Pract. 2020;216:152869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Huang D, Ren F, Ni S, Tan C, Weng W, Zhang M, Xu M, Wang L, Xu Q, Sheng W. Amphicrine carcinoma of the stomach and intestine: a clinicopathologic and pan-cancer transcriptome analysis of a distinct entity. Cancer Cell Int. 2019;19:310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 68. | Bu S, Ding D, Wang X, Fan R, Xu J, Chen Y, Liu H. The clinical characteristics and prognosis of 73 patients with Nonfunctional Gastroenteropancreatic neuroendocrine neoplasm: A 10-year retrospective study of a single center. Clin Pract. 2017;14:198–203. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C, Anlauf M, Cwikla JB, Caplin M, O'Toole D, Perren A; Vienna Consensus Conference participants. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology. 2016;103:186-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 375] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 70. | Komatsubara T, Koinuma K, Miyakura Y, Horie H, Morimoto M, Ito H, Lefor AK, Sata N, Fukushima N. Endocrine cell carcinomas of the colon and rectum: a clinicopathological evaluation. Clin J Gastroenterol. 2016;9:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 71. | Yoshida T, Kamimura K, Hosaka K, Doumori K, Oka H, Sato A, Fukuhara Y, Watanabe S, Sato T, Yoshikawa A, Tomidokoro T, Terai S. Colorectal neuroendocrine carcinoma: A case report and review of the literature. World J Clin Cases. 2019;7:1865-1875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Hanumappa HK, D’Souza D, Rao JS, Naduvathkundil FR, Chowdappa RG, Joseph EP. Gastrointestinal mixed neuroendocrine carcinoma: Case reports and review of literature. Indian J Case Reports. 2018;4:340-343. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 73. | Frizziero, Wang, Chakrabarty, Childs, Luong, Walter, Elshafie, Shah, Fulford, Minicozzi, Mansoor, Meyer, Hubner, Valle, and McNamara. "Mixed Adeno-neuroendocrine Carcinoma (MANEC) of the Gastroenteropancreatic (GEP) Tract: A Multicentre Retrospective Study.". Annals of Oncology. 2017;. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Frizziero M, Wang X, Chakrabarty B, Childs A, Luong TV, Walter T, Khan MS, Morgan M, Christian A, Elshafie M, Shah T, Minicozzi A, Mansoor W, Meyer T, Lamarca A, Hubner RA, Valle JW, McNamara MG. Retrospective study on mixed neuroendocrine non-neuroendocrine neoplasms from five European centres. World J Gastroenterol. 2019;25:5991-6005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 33] [Cited by in F6Publishing: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Vijayakumar, Saravanakumari, Sajini Jacob, Bhawana Badhe, and Bheemanathi Srinivas. "Mixed Adenoneuroendocrine Carcinoma: A Report of Four Cases from South India". Journal of Health Research and Reviews. 2019;6:31-35. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 76. | Asmita Dhurve, Varsha. S.Barai and Aniket.B. Payagude. Mixed adeno-neuroendocrine carcinoma [manec] of ascending colon a rare occurrence: a case report. International Journal of Current Medical and Pharmaceutical Research. 2017;1854-1856. [DOI] [Cited in This Article: ] |
| 77. | Watanabe J, Suwa Y, Ota M, Ishibe A, Masui H, Nagahori K, Tsuura Y, Endo I. Clinicopathological and Prognostic Evaluations of Mixed Adenoneuroendocrine Carcinoma of the Colon and Rectum: A Case-Matched Study. Dis Colon Rectum. 2016;59:1160-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Gluszak M, FÄfara A, Rymarz E, Kawecki A. Mixed adenoneuroendocrine carcinoma: case report and review of lliterature. Nowotwory J Oncol. 2017;67:59–64. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 79. | Tanaka T, Kaneko M, Nozawa H, Emoto S, Murono K, Otani K, Sasaki K, Nishikawa T, Kiyomatsu T, Hata K, Morikawa T, Kawai K, Watanabe T. Diagnosis, Assessment, and Therapeutic Strategy for Colorectal Mixed Adenoneuroendocrine Carcinoma. Neuroendocrinology. 2017;105:426-434. [PubMed] [DOI] [Cited in This Article: ] |
| 80. | Cherbanyk F, Gassend JL, Dimitrief M, Andrejevic-Blant S, Martinet O, Pezzetta E. A Rare Type of Colorectal Cancer: Mixed Adeno-Neuroendocrine Carcinoma (MANEC). Chirurgia (Bucur). 2017;112:152-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 81. | Bernardica Juric, Josip Šojat, Ivana Brnadic, Bozo Krušlin. Mixed adenoneuroendocrine carcinoma (collision tumor) of the gastrointestinal tract: A case report. Endocrine Oncology and Metabolism. 2018;4. [DOI] [Cited in This Article: ] |
| 82. | Colonic Collision Tumour as a cause of Intussusception. Case 13943. Eurorad, 27.12.2016. [DOI] [Cited in This Article: ] |
| 83. | Gonzalez HH, Skrove JL, Sharma R, Sobrado J. A Rare Case of Mixed Adenoneuroendocrine Carcinoma of the Ileocecal Valve. Cureus. 2019;11:e3942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Jain A, Singla S, Jagdeesh KS, Vishnumurthy HY. Mixed adenoneuroendocrine carcinoma of cecum: a rare entity. J Clin Imaging Sci. 2013;3:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | Liu XJ, Feng JS, Xiang WY, Kong B, Wang LM, Zeng JC, Liang YF. Clinicopathological features of an ascending colon mixed adenoneuroendocrine carcinoma with clinical serosal invasion. Int J Clin Exp Pathol. 2014;7:6395-6398. [PubMed] [Cited in This Article: ] |
| 86. | Xin Z, Yao GW, Kong D. AFP-negative mixed hepatoid adenocarcinoma of the colon: a case report with review of literature. Int J Clin Exp Pathol. 2018;11:3324-3327. [PubMed] [Cited in This Article: ] |
| 87. | Song LJ, Yuan L. Comparative analysis of colorectal mixed adenoneuroendocrine carcinoma and adenocarcinoma with neuroendocrine differentiation: a population-based study. Int J Clin Exp Pathol. 2019;12:922-932. [PubMed] [Cited in This Article: ] |
| 88. | Yamauchi H, Sakurai S, Tsukagoshi R, Suzuki M, Tabe Y, Fukasawa T, Kiriyama S, Fukuchi M, Naitoh H, Kuwano H. A case of very well-differentiated adenocarcinoma with carcinoid tumor in the ascending colon. Int Surg. 2014;99:132-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Ito H, Kudo A, Matsumura S, Ban D, Irie T, Ochiai T, Nakamura N, Tanaka S, Tanabe M. Mixed adenoneuroendocrine carcinoma of the colon progressed rapidly after hepatic rupture: report of a case. Int Surg. 2014;99:40-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Minaya-Bravo AM, Garcia Mahillo JC, Mendoza Moreno F, Noguelares Fraguas F, Granell J. Large cell neuroendocrine - Adenocarcinona mixed tumour of colon: Collision tumour with peculiar behaviour. What do we know about these tumours? Ann Med Surg (Lond). 2015;4:399-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 91. | Vardas K, Papadimitriou G, Chantziara M, Papakonstantinou A, Drakopoulos S. Mixed large cell neuroendocrine carcinoma with squamous cell carcinoma of the rectum: Report of a rare case and review of the literature. Int J Surg Case Rep. 2013;4:1076-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Cendra CS, Pascual BA, Vila BL, Gonzalez MCP, Maestu JR, Martel IJ, Rocabado RSC. Mixed Adenoneuroendocrine Carcinoma in the Transverse Colon: An Infrequent Neoplasm in an Unusual Location. Journal of Tumor Research. 2017;3:131. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 93. | Khudiakov A, Al-Sadawi M, Qin J, Haddadin M, Soroka S, Hartt A, Soni L, Arora S, McFarlane SI. Rare Case of Rectal Mixed Adeno-Neuroendocrine Carcinoma. Am J Med Case Rep. 2019;7:117-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Choi PW. Synchronous small cell carcinoma and adenocarcinoma of the rectum. J Surg Case Rep. 2017;2017:rjx069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 95. | Gurzu S, Kadar Z, Bara T, Bara T, Tamasi A, Azamfirei L, Jung I. Mixed adenoneuroendocrine carcinoma of gastrointestinal tract: report of two cases. World J Gastroenterol. 2015;21:1329-1333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 51] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 96. | Kanazawa Y, Kikuchi M, Imai Y, Katakami N, Kaihara S, Shinohara S. Successful Treatment of a Mixed Neuroendocrine-Nonneuroendocrine Neoplasm of the Colon with Metastases to the Thyroid Gland and Liver. Case Rep Otolaryngol. 2020;2020:5927610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Dulskas A, Pilvelis A. Oncologic outcome of mixed adenoneuroendocrine carcinoma (MANEC): A single center case series. Eur J Surg Oncol. 2020;46:105-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Smith JD, Reidy DL, Goodman KA, Shia J, Nash GM. A retrospective review of 126 high-grade neuroendocrine carcinomas of the colon and rectum. Ann Surg Oncol. 2014;21:2956-2962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 99. | Silva JRD, Pinho RT, Furtado A. A case of a mixed adenoneuroendocrine tumor of the colon. Rev Esp Enferm Dig. 2017;109:673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Qiu S, Pellino G, Warren OJ, Mills S, Goldin R, Kontovounisios C, Tekkis PP. Mixed adenoneuroendocrine carcinoma of the colon and rectum. Acta Chir Belg. 2018;118:273-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Liu M, Zhang H, Yang Y, Fu X, Nan K, Tian T. Colorectal neuroendocrine carcinoma and colorectal mixed adeno-neuroendocrine carcinoma: A population-based study of the surveillance, epidemiology, and end results registry. Annals of Oncology. 2018;29:v61. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 102. | Elkbuli A, Dowd B, McKenney M, Boneva D. Mixed neuroendocrine and squamous cell carcinoma of the colon: A case report and literature review. Int J Surg Case Rep. 2019;60:309-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Bhattacharjee PK, Halder S. Combined adenocarcinoma-carcinoid tumor of transverse colon. J Cancer Res Ther. 2013;9:746-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 104. | Carboni F, Valle M, Russo A. Mixed adenoneuroendocrine carcinoma of the cecum. Clin Res Hepatol Gastroenterol. 2019;43:627-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 105. | Morais M, Pinho AC, Marques A, Lopes J, Duarte A, Correia da Silva P, Lopes JM, Maia JC. Mixed Adenoneuroendocrine Carcinoma causing colonic intussusception. Case Reports in Surgery. 2016;7684364. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 106. | Constantinou P, Karagkounis G, Kazamias G, Taliadoros A, Poullou C, Vourlakou C. Metastatic Mixed Mdenoneuroendocrine Carcinomas (MANECs) of the colorectum: Two cases. Virchows Arch. 2015;467:S184–S185. [Cited in This Article: ] |
| 107. | Kolasinska-Cwikla AD, Lewczuk A, Cichocki A, Maciejkiewicz K, Nowicka E, Roszkowska-Purska K, Jodkiewicz Z, Tenderenda M, C´wikła J. B. Neuroendocrine carcinomas of the colorectal origin—Polish experience. Ann Oncol. 2016;27. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 108. | Marando A, Dainese E, La Rosa S, Capella C. Images in endocrine pathology: oncocytic differentiation in a mixed adenoneuroendocrine carcinoma of the colon. Endocr Pathol. 2013;24:54-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 109. | Stueger A, Winder T, Tinguely M, Petrausch U, Helbling D. Metastatic Mixed Adenoneuroendocrine Carcinoma of the Colon with Response to Immunotherapy with Pembrolizumab: A Case Report. J Immunother. 2019;42:274-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Apostolidis L, Bergmann F, Haag GM, Jager D, Winkler EC. Treatment outcomes of patients with mixed neuroendocrine non-neuroendocrine neoplasms (MiNEN). Journal of Clinical Oncology. 2018;36:15. [DOI] [Cited in This Article: ] |
| 111. | Feeney G, Sehgal R, Sheehan M, Hogan A, Regan M, Joyce M, Kerin M. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25:4850-4869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 107] [Cited by in F6Publishing: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 112. | Hervieu V, Scoazec JY. [Mixed endocrine tumors]. Ann Pathol. 2005;25:511-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | Brathwaite S, Rock J, Yearsley MM, Bekaii-Saab T, Wei L, Frankel WL, Hays J, Wu C, Abdel-Misih S. Mixed Adeno-neuroendocrine Carcinoma: An Aggressive Clinical Entity. Ann Surg Oncol. 2016;23:2281-2286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 114. | Casas F, Ferrer F, Farrús B, Casals J, Biete A. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer. 1997;80:1366-1372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 115. | Ho KJ, Herrera GA, Jones JM, Alexander CB. Small cell carcinoma of the esophagus: evidence for a unified histogenesis. Hum Pathol. 1984;15:460-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 108] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 116. | Sidhu GS. The endodermal origin of digestive and respiratory tract APUD cells. Histopathologic evidence and a review of the literature. Am J Pathol. 1979;96:5-20. [PubMed] [Cited in This Article: ] |
| 117. | Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120:2814-2823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 118. | Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, Kvols LK; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39:799-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 119. | Rossi A, Di Maio M, Chiodini P, Rudd RM, Okamoto H, Skarlos DV, Früh M, Qian W, Tamura T, Samantas E, Shibata T, Perrone F, Gallo C, Gridelli C, Martelli O, Lee SM. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012;30:1692-1698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 120. | Brenner B, Tang LH, Shia J, Klimstra DS, Kelsen DP. Small cell carcinomas of the gastrointestinal tract: clinicopathological features and treatment approach. Semin Oncol. 2007;34:43-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Walenkamp AM, Sonke GS, Sleijfer DT. Clinical and therapeutic aspects of extrapulmonary small cell carcinoma. Cancer Treat Rev. 2009;35:228-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 122. | Janson ET, Sorbye H, Welin S, Federspiel B, Grønbæk H, Hellman P, Ladekarl M, Langer SW, Mortensen J, Schalin-Jäntti C, Sundin A, Sundlöv A, Thiis-Evensen E, Knigge U. Nordic guidelines 2014 for diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. Acta Oncol. 2014;53:1284-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 123. | Mitry E, Baudin E, Ducreux M, Sabourin JC, Rufié P, Aparicio T, Aparicio T, Lasser P, Elias D, Duvillard P, Schlumberger M, Rougier P. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81:1351-1355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 379] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 124. | Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, Kulke MH, Liu EH, Metz DC, Phan AT, Sippel RS, Strosberg JR, Yao JC; North American Neuroendocrine Tumor Society. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 407] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 125. | Eriksson B, Annibale B, Bajetta E, Mitry E, Pavel M, Platania M, Salazar R, Plöckinger U; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: chemotherapy in patients with neuroendocrine tumors. Neuroendocrinology. 2009;90:214-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 126. | Fazio N, Spada F, Giovannini M. Chemotherapy in gastroenteropancreatic (GEP) neuroendocrine carcinomas (NEC): a critical view. Cancer Treat Rev. 2013;39:270-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 127. | Moertel CG, Kvols LK, O'Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 128. | Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 641] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 129. | Yamaguchi T, Machida N, Morizane C, Kasuga A, Takahashi H, Sudo K, Nishina T, Tobimatsu K, Ishido K, Furuse J, Boku N, Okusaka T. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014;105:1176-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 130. | Hentic O, Hammel P, Couvelard A, Rebours V, Zappa M, Palazzo M, Maire F, Goujon G, Gillet A, Lévy P, Ruszniewski P. FOLFIRI regimen: an effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr Relat Cancer. 2012;19:751-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 131. | Walter T, Tougeron D, Baudin E, Le Malicot K, Lecomte T, Malka D, Hentic O, Manfredi S, Bonnet I, Guimbaud R, Coriat R, Lepère C, Desauw C, Thirot-Bidault A, Dahan L, Roquin G, Aparicio T, Legoux JL, Lombard-Bohas C, Scoazec JY, Lepage C, Cadiot G; CEPD investigators. Poorly differentiated gastro-entero-pancreatic neuroendocrine carcinomas: Are they really heterogeneous? Insights from the FFCD-GTE national cohort. Eur J Cancer. 2017;79:158-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 132. | Hadoux J, Malka D, Planchard D, Scoazec JY, Caramella C, Guigay J, Boige V, Leboulleux S, Burtin P, Berdelou A, Loriot Y, Duvillard P, Chougnet CN, Déandréis D, Schlumberger M, Borget I, Ducreux M, Baudin E. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr Relat Cancer. 2015;22:289-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 133. | Olsen IH, Knigge U, Federspiel B, Hansen CP, Skov A, Kjær A, Langer SW. Topotecan monotherapy in heavily pretreated patients with progressive advanced stage neuroendocrine carcinomas. J Cancer. 2014;5:628-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 134. | Welin S, Sorbye H, Sebjornsen S, Knappskog S, Busch C, Oberg K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer. 2011;117:4617-4622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 135. | Olsen IH, Sørensen JB, Federspiel B, Kjaer A, Hansen CP, Knigge U, Langer SW. Temozolomide as second or third line treatment of patients with neuroendocrine carcinomas. ScientificWorldJournal. 2012;2012:170496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 136. | Vijayvergia N, Dasari A, Deng M, Litwin S, Al-Toubah T, Alpaugh RK, Dotan E, Hall MJ, Ross NM, Runyen MM, Denlinger CS, Halperin DM, Cohen SJ, Engstrom PF, Strosberg JR. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer. 2020;122:1309-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 137. | Yao JC, Strosberg J, Fazio N, Pavel ME, Ruszniewski P, Bergsland E, Li BD, Tafuto S, Raj N, Campana D, Hijioka S, Raderer M, Guimbaud R, Gajate P, Pusceddu S, Reising A, Degtyarev E, Mookerjee B, Aimone P, Singh S. Activity Safety of Spartalizumab (PDR001) in Patients (pts) With Advanced Neuroendocrine Tumors (NET) of Pancreatic (Pan), Gastrointestinal (GI), or thoracic (T) origin, gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx) (abstract, ESMO 2018). Annals of Oncology. Available from: https://academic.oup.com/annonc/article/29/suppl_8/mdy293.001/5140835. [Cited in This Article: ] |
| 138. | Patel SP, Othus M, Chae YK, Giles FJ, Hansel DE, Singh PP, Fontaine A, Shah MH, Kasi A, Baghdadi TA, Matrana M, Gatalica Z, Korn WM, Hayward J, McLeod C, Chen HX, Sharon E, Mayerson E, Ryan CW, Plets M, Blanke CD, Kurzrock R. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin Cancer Res. 2020;26:2290-2296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 139. | Spada F, Milione M, Maisonneuve P, Prinzi N, Smiroldo V, Bolzacchini E, Carnaghi C, La Rosa S, Cappella C, Sessa F. An Italian Multicenter Study in Patients with Advanced Mixed AdenoNeuroendocrine Carcinomas (MANECs) of the Gastro-Entero-Pancreatic Tract Treated with Chemotherapy. Neuroendocrinology. 2019;108:190. [Cited in This Article: ] |