Published online Jul 7, 2020. doi: 10.3748/wjg.v26.i25.3603
Peer-review started: March 3, 2020
First decision: March 24, 2020
Revised: April 30, 2020
Accepted: May 27, 2020
Article in press: May 27, 2020
Published online: July 7, 2020
Endoscopic ultrasound-guided gastroenterostomy (EUS-GE) is an alternative method for the surgical treatment of gastric outlet obstruction, but it is regarded as a challenging technique for endoscopists as the bowel is highly mobile and can tent away. Thus, the technique requires superb skill. In order to improve EUS-GE, we have developed a retrievable puncture anchor traction (RPAT) device for EUS-GE to address the issue of bowel tenting.
To evaluate the feasibility of RPAT-assisted EUS-GE using an animal model.
Six Bama mini pigs each weighing between 15 and 20 kg underwent the RPAT-assisted EUS-GE procedure. Care was taken to ensure that the animals experienced minimal pain and discomfort. Two days prior to the procedure the animals were limited to a liquid diet. No oral intake was allowed on the day before the procedure. A fully covered metal stent was placed between the stomach and the intestine using the RPAT-assisted EUS-GE method. Infection in the animals was determined. Four weeks after the procedure, a standard gastroscope was inserted into the pig’s intestine through a previously created fistula in order to check the status of the stents under anesthesia. The pig was euthanized after examination.
The RPAT-assisted EUS-GE method allowed placement of the stents with no complications in all six animals. All the pigs tolerated a regular diet within hours of the procedure. The animals were monitored for four weeks after the RPAT-assisted EUS-GE, during which time all of the animals exhibited normal eating behavior and no signs of infection were observed. Endoscopic imaging performed four weeks after the RPAT-assisted EUS-GE showed that the stents remained patent and stable in all the animals. No tissue overgrowth or ingrowth was observed in any case. Each animal had a mature fistula, and the stents were removed without significant bleeding. Autopsies of all six pigs revealed complete adhesion between the intestine and the stomach wall.
The RPAT method helps reduce mobility of the bowel. Therefore, the RPAT-assisted EUS-GE method is a minimally invasive treatment modality.
Core tip: To evaluate the feasibility of retrievable puncture anchor traction (RPAT)-assisted endoscopic ultrasound-guided gastroenterostomy (EUS-GE), six Bama mini pigs underwent the RPAT-assisted EUS-GE procedure. Four weeks later, a standard gastroscope was inserted into the pig’s intestine through a previously created fistula in order to check the status of the stents under anesthesia. The pigs were euthanized after examination. The results showed that the RPAT-assisted EUS-GE method allowed placement of the stents with no complications in all six animals. This study proved that the RPAT method helps reduce mobility of the bowel. Therefore, the RPAT-assisted EUS-GE method is a minimally invasive treatment modality.
- Citation: Wang GX, Zhang K, Sun SY. Retrievable puncture anchor traction method for endoscopic ultrasound-guided gastroenterostomy: A porcine study. World J Gastroenterol 2020; 26(25): 3603-3610
- URL: https://www.wjgnet.com/1007-9327/full/v26/i25/3603.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i25.3603
Endoscopic ultrasound-guided gastroenterostomy (EUS-GE) has become an alternative to the surgical or standard endoscopic treatment methods for gastric outlet obstruction (GOO) when endoscopic stents cannot be placed. However, EUS-GE presents certain challenges and the current procedural methods have limitations. One source of difficulty during EUS-GE is the high mobility of the intestine, which can tent away during puncture with a needle or electrocautery-enhanced delivery of lumen-apposing metal stents (ECE-LAMS). In order to improve EUS-GE, we developed a retrieval puncture anchor traction (RPAT) method for EUS-GE. We evaluated the feasibility of the RPAT method using a pig model.
Six Bama mini pigs (15-20 kg) were selected to undergo the RPAT method of EUS-GE, which consisted of placing fully covered metal stents between the animals’ stomach and bowel walls. This pig model study was approved by the Ethics Review Committee and Ethics Committee of Shengjing Hospital of China Medical University (No. 2018PS482K).
Each pig received a full liquid diet two days before the operation, and drinking water was forbidden the day before surgery. Based on the weight of each animal, propofol was intramuscularly injected for the induction of anesthesia. The pigs were placed on their left side. Venous access was established through the ear vein. The animals’ breathing was maintained by the insertion of a tracheal tube. The animals were monitored with continuous cardiopulmonary monitoring.
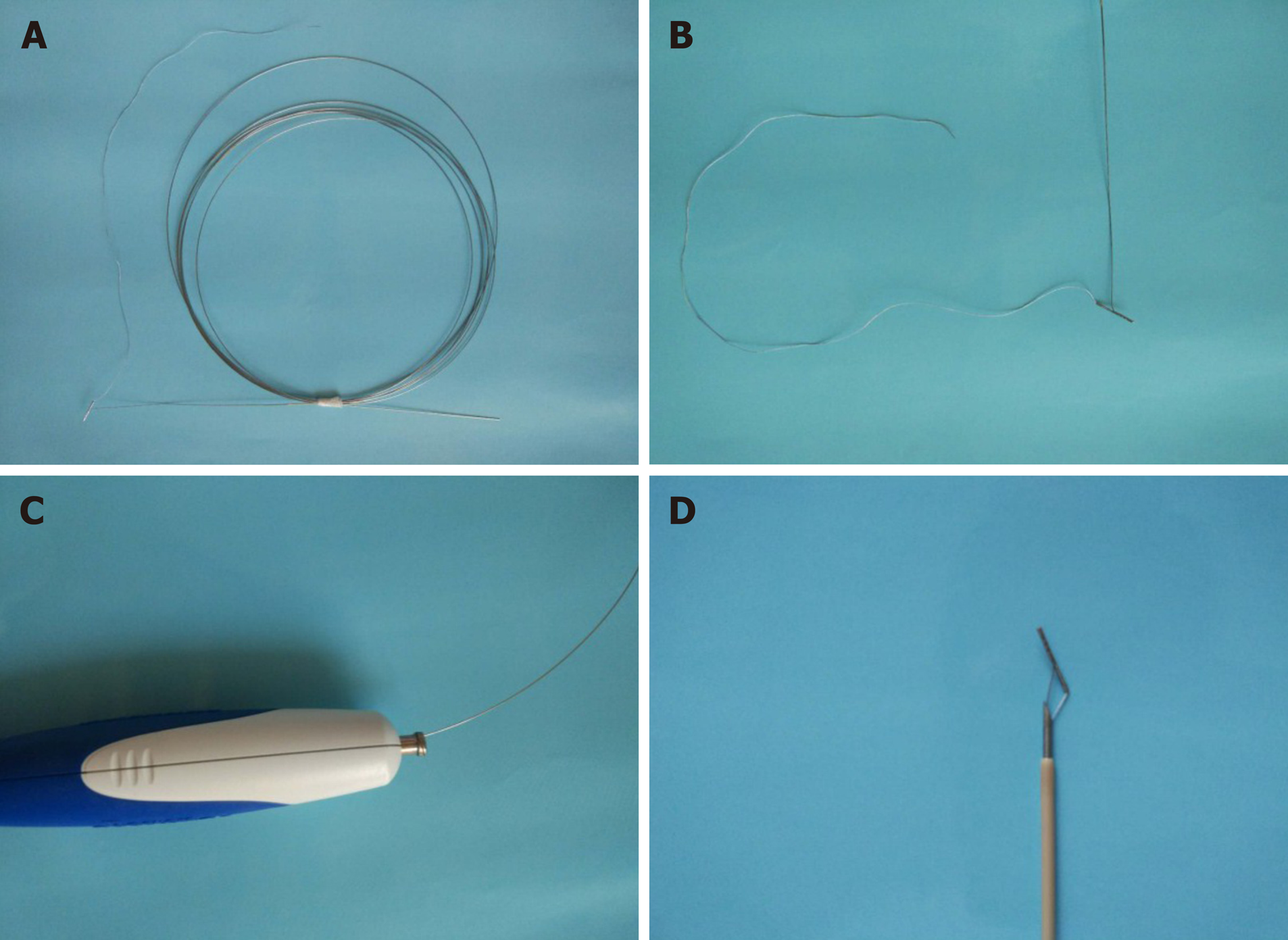
The RPA (Vedkang Inc., Changzhou, Jiangsu, China) is made up of two wires (Figure 1A). The direction of the anchor head can be altered by pulling the retrieval wire (Figure 1B). The anchor can be sent through the needle (Figure 1C and D)[1].
RPAT method of EUS-GE: First, a gastroscope was advanced into the duodenum. A guidewire (0.035 inch/480 mm; Cook Medical Inc., Bloomington, IN, United States) was sent through the working channel of the gastroscope. A nasal biliary drainage catheter (NBDC) (7Fr; Cook Medical Inc., Limerick, Ireland) was then inserted along the guidewire. Next, the gastroscope and guidewire were removed, leaving the NBDC in place. Approximately 200 mL of saline solution containing methylene blue was injected into the bowel through the NBDC to optimally dilate the bowel and facilitate the subsequent EUS-guided puncture. Methylene blue was added to confirm bowel placement by aspirating the blue saline solution with a needle (described below) before the ECE-LAMS insertion. A longitudinal (linear) ultrasound endoscope (EG-3830-UT; Pentax Japan) was inserted into the stomach and the puncture area (i.e., the closest area between the intestine and the stomach) was marked. We used color Doppler to prevent vascular damage during the puncture. A needle (19-G, Boston Scientific Corp., Massachusetts, United States) was then passed through the working channel, and the intestine was punctured under EUS guidance (Figure 2A and B). After removal of the stylet, saline solution with methylene blue was drawn out and a contrast agent was injected for endoscopy. The RPAT was then passed through the needle (Figure 2C and D) and inserted into the small intestine. The needle and the echoendoscope were removed over the wire of the RPAT. The small bowel was pulled with the anchor (Figure 2E and F). RPAT only pulls the intestinal canal during stent implantation to prevent free action, so the pulling action is gentle rather than violent. During the procedure, we did not observe the angle of the digestive tract wall due to stretching. The echoendoscope was reinserted into the stomach and the 19-G needle was advanced through the working channel. Additional saline solution with methylene blue was injected through the NBDC using multiple 50-mL syringes if the bowel expansion was insufficient. The identified small-bowel loop was punctured under EUS guidance followed by aspiration of the methylene blue solution to confirm the correct puncture site when the small bowel was pulled with the anchor. The guidewire was inserted through the needle. Next, the needle was removed, and ECE-LAMS (16 mm/30 mm; Micro-Tech/Nan Jing Co., Ltd. Nanjing, Jiangsu, China) was inserted over the guidewire and released into the small intestine until the distal flares were fully open under EUS guidance (Figure 2G and Figure H). Through endoscope monitoring, while keeping the proximal section in the field of vision, the rest of the stent was released. Finally, the stent was confirmed and leakages were excluded by EUS (Figure 3A and B). After the operation, the retrieval cord was pulled using a pair of forceps (Figure 2I and J). This changed the direction of the anchor and made it easy to remove (Figure 2K and L).
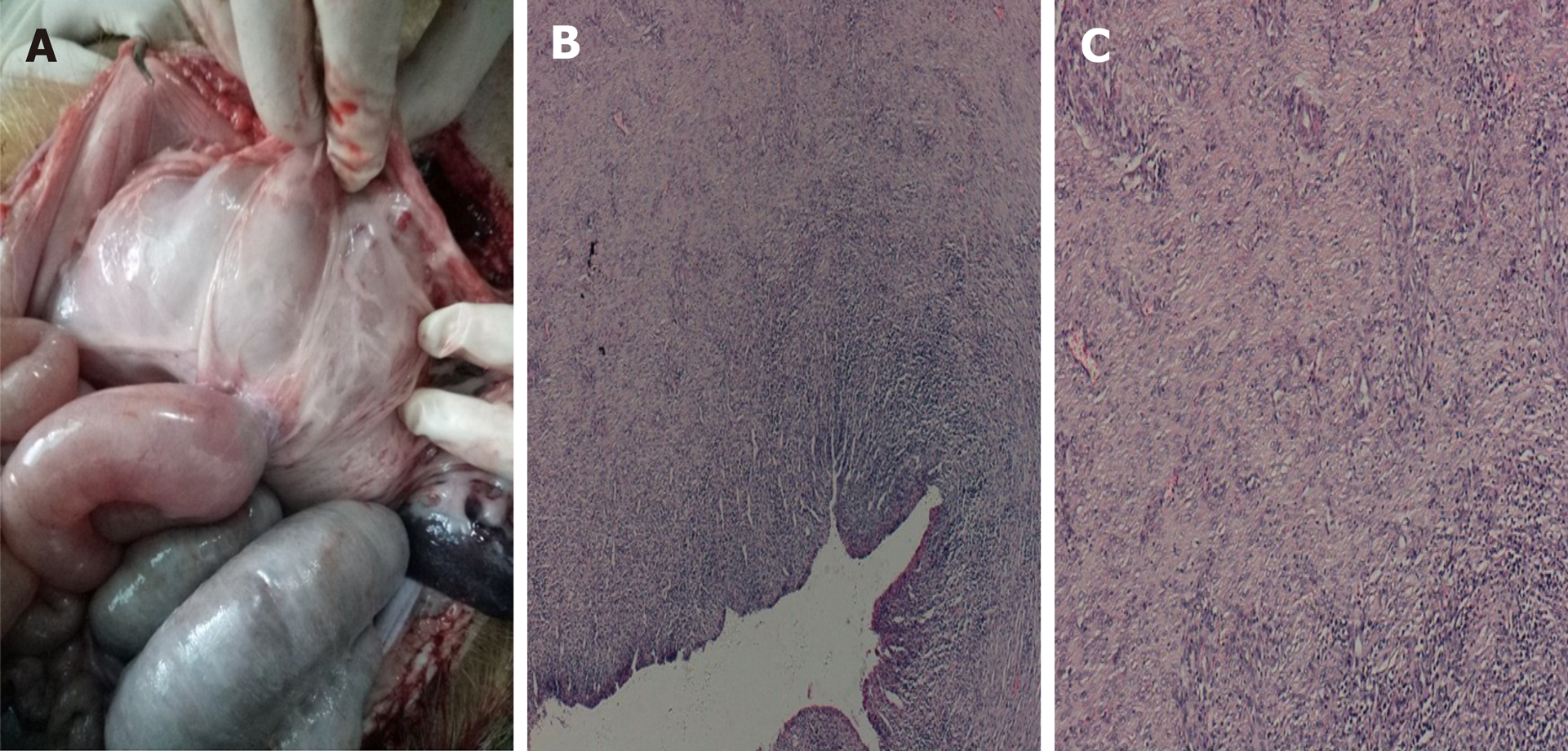
Postoperative care: Postoperatively, the pigs were observed for signs of peritonitis. Four weeks after surgery, the pigs underwent anesthesia, and a standard gastroscope was used to examine the pigs’ intestines. After removing the metal stent using a pair forceps, the gastroscope was advanced into the afferent and efferent bowel loops (Figure 3C and D). X-ray examination was performed to check for the presence of free gas. All pigs were then euthanized (Figure 4).
All six operations were successfully performed. Each metal stent was placed successfully, and the EUS-GEs selected the body of the stomach as the puncture site in each case. All RPAs were able to be removed with no complications. No animal showed signs of peritonitis or any other complications after the procedure. Within a few hours after anesthesia, the pigs were able to tolerate a regular diet. No clinically significant adverse reactions were observed in the following weeks. Four weeks after EUS-GE, it was confirmed that the stents remained intact, in their original positions, with no hyperplastic tissue overgrowth or ingrowth in any animal. The fistulas were mature in all six pigs and all the stents were easily removed using forceps without significant bleeding. On autopsy, there were no signs of bleeding, organ damage, or peritonitis, and complete adhesions of the intestines to the stomach wall were seen.
Surgical GE is a traditional choice for patients with malignant GOO. Intraluminal self-expanding metal stents (SEMS) placed through the endoscope is another method to relieve GOO[2-4]. Surgical GE is superior to endoscopic SEMS placement in terms of lumen patency[4]; however, adverse events including delayed gastric emptying, extended hospital stay, increased cost of hospitalization, and delayed treatment of cancer are not uncommon[4,5]. The main limitation of placing SEMS endoscopically is repeated occlusion of the lumen, which is mainly due to tumor ingrowth, overgrowth, or both[4]. In general, EUS-GE can provide durable lumen patency, avoid the risk of tumor overgrowth and ingrowth, and avoids the need for surgery in patients with end-stage disease[6]. In 2002, Fritscher-Ravens et al[7] reported the results of EUS-GE using special compression buttons in pigs. In 2012, Binmoeller et al[8] described the use of a new intraluminal metal stent in a porcine model that was used to invent an EUS-GE anastomosis. Recently, Itoi et al[9] used another endoluminal stent for EUS-GE.
The emergence of stents allows easier creation of anastomoses between the small intestine and the stomach wall. These stents are fully covered with flanges at either end to prevent leakage and migration. The EUS-GE technique using an echoendoscope is an excellent means of providing endoscopic access to the duodenal or jejunal lumen distal to obstructed intestinal segments. Various techniques have been used with EUS-GE[10], including antegrade EUS-GE (the “traditional/downstream” method and the “rendezvous method), retrograde EUS-GE, EUS-guided double-balloon-occluded GE, and direct EUS-GE[9,11-13].
One of the challenges associated with direct EUS-GE is the inability to puncture the small bowel, despite the use of an ECE-LAMS, resulting in it being pushed away rather than punctured[11]. Inadequate expansion of the small bowel or rapid dissipation of the infused contrast and methylene blue (due to peristalsis) can also be limiting factors[11]. The RPAT method for EUS-GE did not use glucagon or other antispasmodic drugs to reduce the mobility of the bowel. The RPA can pull the intestine closer to the stomach wall to reduce intestinal mobility. Compared with traditional EUS-GE, RPAT reduces the distance between the stomach and the bowel, and provides sufficient reverse tension during ECE-LAMS implantation. With sufficient opposite pulling force, the ECE-LAMS is relatively easy to implant. The stomach wall and intestine of six experimental animals were clearly visible in the ultrasound field of view during the entire surgical procedure. We used an NBDC in the RPAT method to reduce the procedure time for EUS-GE, while avoiding the infusion of large amounts of saline into the intestine to prevent colon swelling. Prior to puncturing and guidewire insertion, a saline solution containing methylene blue was added in order to maintain the expansion of the bowel, provide a better field of view, and improve the safety of the operation.
In contrast to other techniques used with EUS-GE, the RPAT method for EUS-GE does not use a balloon, which reduces the difficulty of performing the operation as the advancement of the dilated balloon over the guidewire under fluoroscopic guidance can be quite challenging. Moreover, the RPAT method for EUS-GE is suitable for cases where the digestive tract is severely stenotic, allowing only the passage of liquid solution. The RPAT method for EUS-GE can help pull the intestine by pulling the anchor and can inflate the intestine by allowing the injection of saline solution through the NBDC at any time.
Although advanced EUS techniques and the availability of the proper equipment allow EUS-GE to be performed, the procedure is still in its early stages of development. This new technique is accompanied by technical issues requiring instrument modification that must be addressed prior to its clinical application[10,14-21]. Interventional EUS treatment is a newly developed method for the treatment of many diseases[9,19-22]. The small bowel has variable motility and thus tents away from the puncturing needle or the cautery tip of the ECE-LAMS. Hence, we have developed the RPAT method to improve the current EUS-GE method.
The success rate of EUS-GE using the RPAT method was 100% in our study. Remarkably, the retrievable puncture devices anchor to the bowel without causing tissue injury, and the RPA can also be removed without complications. Four weeks after the EUS-GE, it was found that all the stents remained in their original positions. Necropsy revealed complete adhesion between the intestinal wall and the stomach wall.
There are a few limitations in this study. First, the study consisted of a small number of animals. Second, the follow-up period was four weeks, limiting the evaluation of long-term observations and results. Lastly, the procedure outcomes and findings were not compared to those of other non-EUS-guided gastrojejunostomy techniques[8]. A larger study with a longer follow-up period that compares several gastrojejunostomy techniques is needed.
In general, we proved the technical success of the RPAT method used with EUS-GE in a pig model. The RPAT method for EUS-GE is promising as a minimally invasive treatment. Clinical prospective trials are warranted to verify the efficacy of this treatment method. We are convinced that this treatment method can be widely used in EUS-GE in the future.
Endoscopic ultrasound-guided gastroenterostomy (EUS-GE) is an alternative method for the surgical treatment of gastric outlet obstruction (GOO), but it is regarded as a challenging technique for endoscopists as the bowel is highly mobile and can tent away. Thus, the technique requires superb skill. In order to improve EUS-GE, we have developed a retrievable puncture anchor traction (RPAT) device for EUS-GE to address the issue of bowel tenting.
One source of difficulty in EUS-GE is the high mobility of the intestine, which can tent away during puncture with a needle or electrocautery-enhanced delivery of lumen-apposing metal stents. In order to improve EUS-GE, we developed a RPAT method for EUS-GE. We evaluated the feasibility of the RPAT method using a pig model.
The present study aimed to use an animal model to evaluate the feasibility of RPAT-assisted EUS-GE.
Six Bama mini pigs each weighing between 15 and 20 kg underwent the RPAT-assisted EUS-GE procedure. Care was taken to ensure that the animals experienced minimal pain and discomfort. Two days prior to the procedure the animals were limited to a liquid diet. No oral intake was allowed on the day before the procedure. A fully covered metal stent was placed between the stomach and the intestine using the RPAT-assisted EUS-GE method. Infection in the animals was determined. Four weeks after the procedure, a standard gastroscope was inserted into the pig’s intestine through a previously created fistula in order to check the status of the stents under anesthesia. The pigs were euthanized after examination.
The RPAT-assisted EUS-GE method allowed placement of the stents with no complications in all six animals. All the pigs tolerated a regular diet within hours of the procedure. The animals were monitored for four weeks after the RPAT-assisted EUS-GE, during which time all of the animals exhibited normal eating behavior and no signs of infection were observed. Endoscopic imaging performed four weeks after RPAT-assisted EUS-GE showed that the stents remained patent and stable in all the animals. No tissue overgrowth or ingrowth was observed. Each animal had a mature fistula, and the stents were removed without significant bleeding. Autopsies of all six pigs revealed complete adhesion between the intestine and the stomach wall.
The RPAT method helps reduce mobility of the bowel. Therefore, the RPAT-assisted EUS-GE method is a minimally invasive treatment modality.
We proved the technical success of the RPAT method used with EUS-GE in a pig model. The RPAT method for EUS-GE is promising as a minimally invasive treatment. Clinical prospective trials are warranted to verify the efficacy of this treatment method. We are convinced that this treatment method can be widely used in EUS-GE in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Altonbary Y, Kanno Y, Kozarek RA, Minaga K, Yoshinaga S S-Editor: Dou Y L-Editor: Webster JR E-Editor: Zhang YL
| 1. | Zhang K, Sun S, Guo J, Wang S, Ge N, Liu X, Wang G. Retrievable puncture anchor traction method for EUS-guided gallbladder drainage: a porcine study. Gastrointest Endosc. 2018;88:957-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | van Hooft JE, Uitdehaag MJ, Bruno MJ, Timmer R, Siersema PD, Dijkgraaf MG, Fockens P. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc. 2009;69:1059-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Telford JJ, Carr-Locke DL, Baron TH, Tringali A, Parsons WG, Gabbrielli A, Costamagna G. Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: outcomes from a multicenter study. Gastrointest Endosc. 2004;60:916-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Khashab M, Alawad AS, Shin EJ, Kim K, Bourdel N, Singh VK, Lennon AM, Hutfless S, Sharaiha RZ, Amateau S, Okolo PI, Makary MA, Wolfgang C, Canto MI, Kalloo AN. Enteral stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Surg Endosc. 2013;27:2068-2075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 111] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 5. | Doberneck RC, Berndt GA. Delayed gastric emptying after palliative gastrojejunostomy for carcinoma of the pancreas. Arch Surg. 1987;122:827-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Itoi T, Ishii K, Tanaka R, Umeda J, Tonozuka R. Current status and perspective of endoscopic ultrasonography-guided gastrojejunostomy: endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy (with videos). J Hepatobiliary Pancreat Sci. 2015;22:3-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Fritscher-Ravens A, Mosse CA, Mills TN, Mukherjee D, Park PO, Swain P. A through-the-scope device for suturing and tissue approximation under EUS control. Gastrointest Endosc. 2002;56:737-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Binmoeller KF, Shah JN. Endoscopic ultrasound-guided gastroenterostomy using novel tools designed for transluminal therapy: a porcine study. Endoscopy. 2012;44:499-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Itoi T, Itokawa F, Uraoka T, Gotoda T, Horii J, Goto O, Moriyasu F, Moon JH, Kitagawa Y, Yahagi N. Novel EUS-guided gastrojejunostomy technique using a new double-balloon enteric tube and lumen-apposing metal stent (with videos). Gastrointest Endosc. 2013;78:934-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Rimbas M, Larghi A, Costamagna G. Endoscopic ultrasound-guided gastroenterostomy: Are we ready for prime time? Endosc Ultrasound. 2017;6:235-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Irani S, Baron TH, Itoi T, Khashab MA. Endoscopic gastroenterostomy: techniques and review. Curr Opin Gastroenterol. 2017;33:320-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Chen YI, James TW, Agarwal A, Baron TH, Itoi T, Kunda R, Nieto J, Bukhari M, Gutierrez OB, Sanaei O, Moran R, Fayad L, Khashab MA. EUS-guided gastroenterostomy in management of benign gastric outlet obstruction. Endosc Int Open. 2018;6:E363-E368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 13. | Itoi T, Ishii K, Ikeuchi N, Sofuni A, Gotoda T, Moriyasu F, Dhir V, Teoh AY, Binmoeller KF. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut. 2016;65:193-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 14. | Manvar A, Karia K, Ho S. Endoscopic ultrasound-guided drainage of pelvic abscesses with lumen-apposing metal stents. Endosc Ultrasound. 2017;6:217-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Adler DG, Taylor LJ, Hasan R, Siddiqui AA. A retrospective study evaluating endoscopic ultrasound-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery enhanced delivery system. Endosc Ultrasound. 2017;6:389-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | De Cobelli F, Marra P, Diana P, Brembilla G, Venturini M. Therapeutic EUS: Biliary drainage - The interventional radiologist's perspective. Endosc Ultrasound. 2017;6:S127-S131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Rimbaş M, Larghi A. Endoscopic Ultrasonography-Guided Techniques for Accessing and Draining the Biliary System and the Pancreatic Duct. Gastrointest Endosc Clin N Am. 2017;27:681-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Saumoy M, Arvanitakis M, Kahaleh M. Pancreatic fluid collections and necrosectomy with plastic stents versus lumen-apposing stents. Endosc Ultrasound. 2017;6:S132-S137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Giovannini M. Endoscopic ultrasound-guided pancreatic duct drainage: Ready for the prime time? Endosc Ultrasound. 2017;6:281-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc. 2012;75:870-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 293] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 21. | Teoh AY, Binmoeller KF, Lau JY. Single-step EUS-guided puncture and delivery of a lumen-apposing stent for gallbladder drainage using a novel cautery-tipped stent delivery system. Gastrointest Endosc. 2014;80:1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Binmoeller KF, Shah J. A novel lumen-apposing stent for transluminal drainage of nonadherent extraintestinal fluid collections. Endoscopy. 2011;43:337-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |