Published online Jul 7, 2020. doi: 10.3748/wjg.v26.i25.3562
Peer-review started: March 15, 2020
First decision: March 21, 2020
Revised: May 29, 2020
Accepted: June 18, 2020
Article in press: June 18, 2020
Published online: July 7, 2020
The gastrointestinal tract is the key interface between the ingesta and the human body. There is wide recognition that the gastrointestinal response to nutrients or bioactive compounds, particularly the secretion of numerous hormones, is critical to the regulation of appetite, body weight and blood glucose. This concept has led to an increasing focus on “gut-based” strategies for the management of metabolic disorders, including type 2 diabetes and obesity. Understanding the underlying mechanisms and downstream effects of nutrient-gut interactions is fundamental to effective translation of this knowledge to clinical practice. To this end, an array of research tools and platforms have been developed to better understand the mechanisms of gut hormone secretion from enteroendocrine cells. This review discusses the evolution of in vitro and in vivo models and the integration of innovative techniques that will ultimately enable the development of novel therapies for metabolic diseases.
Core tip: The development of platforms for investigating nutrient-gut interactions is critical to understanding how nutrients trigger the release of gut hormones and has the potential to yield novel targets for improved management of metabolic disorders. In addition to the use of endoscopic or surgical gut tissues or primary enteroendocrine cells, in vitro models now include enteroendocrine cell lines originating from rodent (STC-1 and GLUTag) or human (NCI-H716 and HuTo-80) intestinal tumours, and intestinal organoids differentiated from intestinal stem cells. The physiological relevance of these models has been challenged, but may be improved substantially by incorporating advanced biomedical techniques (e.g., microfluidic devices) into the culture system. These approaches have complemented clinical studies utilising intestinal intubation, often with integrated manometry and impedance recording, which have revealed gut region-specific responses to intraluminal contents. Newer clinical developments include the use of novel ingestible sensors.
- Citation: Huang WK, Xie C, Young RL, Zhao JB, Ebendorff-Heidepriem H, Jones KL, Rayner CK, Wu TZ. Development of innovative tools for investigation of nutrient-gut interaction. World J Gastroenterol 2020; 26(25): 3562-3576
- URL: https://www.wjgnet.com/1007-9327/full/v26/i25/3562.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i25.3562
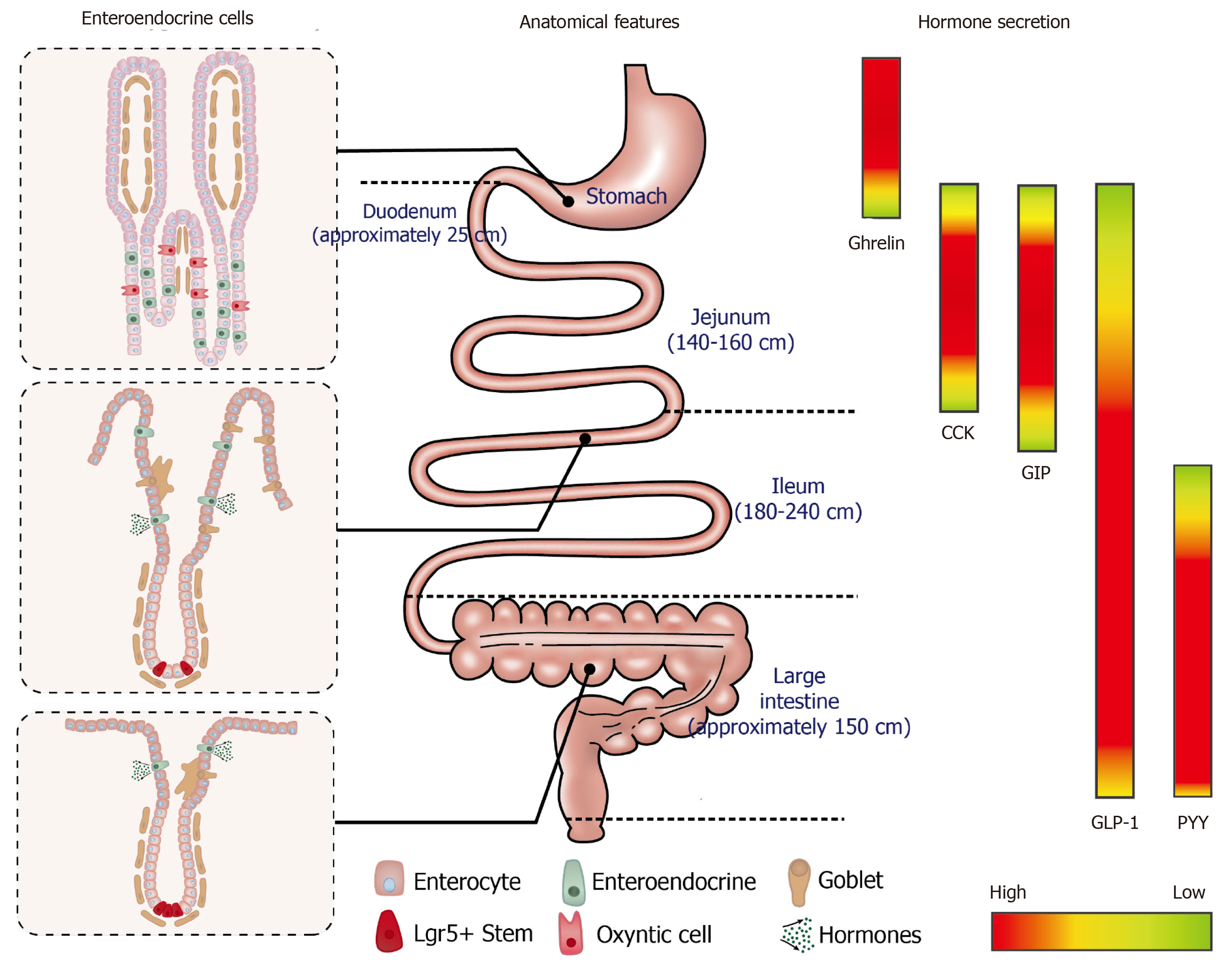
It is now widely appreciated that the gastrointestinal (GI) tract not only serves to process food, but also represents the largest endocrine organ in the body, releasing a wide array of peptide hormones to orchestrate metabolic homeostasis[1]. Ghrelin, for example, is released from gastric Gr-cells into the circulation during fasting or periods of negative energy balance and triggers hunger to drive food intake[2]; ghrelin levels in circulation are subsequently suppressed upon feeding[3]. The interaction of nutrients and digestive juices with the intestinal mucosa triggers the secretion of a number of postprandial hormones, including cholecystokinin (CCK) from enteroendocrine (EE) I-cells[4] and glucose-dependent insulinotropic polypeptide (GIP) from K-cells in the upper small intestine, and glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) from L-cells located predominantly in the distal small and large intestine[5,6]. A subset of EE cells in the proximal small intestine have also been shown to secrete both GLP-1 and GIP[7]. GLP-1 and GIP are known as the incretin hormones; both stimulate insulin secretion in a glucose-dependent manner[8,9]. GLP-1 also suppresses glucagon and acts with CCK and PYY to inhibit appetite, slow the delivery of nutrients from the stomach into the small intestine and retard their subsequent absorption[10]. Accordingly, the integrated responses of GI hormones to meal ingestion is a critical determinant of energy balance and postprandial glycaemia.
That plasma concentrations of GI hormones are typically increased after enteral, but not intravenous, nutrient administration attests to the importance of nutrient-gut interactions to the release of these hormones[11]. Accordingly, improved understanding of the sensor and actuator mechanisms through which nutrients or bioactive compounds interact with EE cells, has the potential to yield novel “gut-based” approaches for the management of metabolic diseases. In the last few decades, a broad range of preclinical and clinical models have been developed to study nutrient-gut interactions, with increasing efforts to achieve clinically relevant outcomes. To this end, ex vivo studies have extended from the use of EE cell lines towards primary intestinal tissues and organoids, and have increasingly incorporated sophisticated culture conditions to mimic normal physiology. Clinical studies employing customised intestinal perfusion catheters for targeted delivery of nutrients or therapeutic compounds, or novel ingestible sensors, have attempted to better characterise the regional specificity of GI responses. In this review, we summarise the research tools and models used to investigate nutrient-gut interactions, and discuss their advantages and limitations for clinical translation of findings (Table 1).
| Tools | Advantages | Disadvantages/challenges | |
| Cellular models | EE cell lines | Established secretion profiles; genetically modifiable; readily accessible | Limited resemblance to native l-cells; lack of inter-organ interaction; limited success in clinical translation |
| Tissue-based approaches | Intestinal organoids | Preserved native architecture; region-specific functions; high plasticity for oriented differentiation | Undefined secretion profiles; lack of integrated nervous or immune systems; inconsistent culture outcomes |
| Isolated intestinal tissues | Preserved native intestinal structure; access to luminal and basolateral surface; high physiological relevance | Short viable period; lack of inter-organ interaction; limited access to human tissue; low EE cell density | |
| Intestinal intubation | In vivo infusion in gut | Region-specific delivery; direct insights into human (patho-)physiology | Technically demanding; restricted to specialised research centre |
| Novel techniques | 3D culture | Enhanced anatomical complexity; compatibility with co-culture system | Limited cellular variety; static culture environment |
| Intestine-on-a-chip | Dynamic culture environment; recapitulation of luminal events | Sophisticated validation of the system; partial resemblance to luminal physiology | |
| Ingestible sensors | A broad range of application; high potential for multi-purposed in vivo investigation | Difficulty in signal interpretation; lack of stability; High cost | |
The GI mucosa incorporates a monolayer of columnar epithelium with region-specific architecture and EE cell composition that is uniquely tuned to secrete specific gut hormones and absorb nutrients to fulfil specific metabolic functions. EE cells account for less than 1% of all epithelial cells, and their distribution varies substantially along the GI tract (Figure 1)[12]. Immortalised cell lines derived from murine and human intestinal tumours have been developed for in vitro studies, and retain the capacity to secrete GI hormones in response to nutrient stimuli (Table 2).
| Species | Model | Origin | Hormones | Features |
| Mouse | STC-1 | Duodenal secretin tumour cells | GLP-1, GLP-2, CCK, GIP, PYY, | Heterogeneous cell population; respond to glucose, amino acids, fatty acids and neural stimuli; poor expression of CaSR |
| GLUTag | Colonic tumour | GLP-1, GLP-2, CCK | Subcloned homogenous cells; respond to glucose, bile acids, fatty acids, amino acids | |
| Human | NCI-H716 | Colorectal carcinoma | GLP-1, GLP-2 | Heterogeneous cell population; poorly differentiated; respond to glucose, fatty acids, protein hydrolysates |
| HuTu-80 | Duodenal carcinoma | GLP-1, PYY, GIP, CCK | Respond to antioxidant compounds, sweet and bitter substances |
STC-1 cells are a heterogeneous and poorly differentiated EE cell line derived from intestinal secretin-producing tumours in mice. They have a high immunoreactivity to anti-proglucagon sera and are capable of releasing glucagon-like immuno-reactants[13]. STC-1 cells were subsequently shown to secrete multiple gut hormones, including CCK[14], GLP-1[15,16], GIP[17], and PYY[18,19], in a similar manner to native murine EE cells, when stimulated by glucose[20], amino acids and fatty acids. As a result, STC-1 cells have been a popular model to screen for gut hormone-releasing stimuli. However, the clinical relevance of this model has been frequently questioned. For example, treatment with potato protease inhibitor concentrate (PPIC) or whey protein does not induce CCK secretion from STC-1 cells[21,22]. By contrast, oral administration of PPIC (100 mg/kg per day) stimulates CCK secretion in rodents[21], while ingestion of whey protein (55 g) increases plasma CCK levels in humans[23].
GLUTag cell line is a subcloned homogeneous EE cell model developed by the Drucker group from an endocrine carcinoma of the large bowel in transgenic mice[24]. These cells express both proglucagon and CCK genes[25] but produce primarily GLP1(7-36)-amide. GLUTag cells are equipped with a wide repertoire of nutrient sensors and transporters, including G-protein coupled receptors (GPCRs)[26], glucokinase[27] and the sodium-glucose linked transporter 1 (SGLT1)[28] involved in nutrient-induced GLP-1 secretion. In agreement with in vivo findings, GLUTag cells exhibit robust release of GLP-1 in response to glucose[29], bile acids[30], fatty acids[31] and amino acids[32]. These observations have promoted GLUTag cells as a frontline model of L cells, leading to a wide application for studying the mechanisms underlying GLP-1 secretion and for screening potential GLP-1 secretagogues. However, clinical studies are still required to validate in vitro findings. For example, the treatment of glutamine (10 mmol/L) was shown to markedly increase GLP-1 secretion (7-fold) from GLUTag cells[32]. However, oral administration of encapsulated ileal-release glutamine (6 g) or intra-duodenal glutamine infusion (7.5-15 g) evoked only modest increases in plasma GLP-1 levels in healthy subjects and patients with type 2 diabetes[33,34].
The human cell lines NCI-H716 and HuTu-80 have also been used widely to characterise nutrient-evoked GLP-1 release. The NCI-H716 cell line was first reported by Park et al[35] from human colorectal carcinoma. It contains dense-core granules, expresses chromogranin A, and secretes GLP-1 in response to glucose, fatty acids and protein hydrolysates[36]. Studies incorporating the NCI-H716 cell line have revealed critical roles of amino acid transporters[37], type 1 taste receptors[38] and monoacylglycerol-sensing GPCR[31] in GLP-1 secretion. However, the secretory profile of NCI-H716 cells is more limited compared to murine STC-1 or GLUTag cells. For example, NCI-H716 cells secrete GLP-1 and GLP-2 but not GIP, PYY or CCK in response to 50 mmol/L KCl, or combined glucose (10 mmol/L), forskolin and phosphodiesterase inhibitor (10 µmol/L)[39]. That NCI-H716 cells do not secrete PYY reflects their limited resemblance to native L-cells.
The HuTu-80 cell line is an alternative EE cell model of human origin that secretes GLP-1, GIP, PYY and CCK[40] and was developed initially to study the biology of GI cancers[41]. Sweet and bitter taste receptors are abundantly expressed in HuTo-80 cells as in native human L-cells, making them a potential model to investigate tastant-induced gut hormone secretion[42,43]. However, unlike native L-cells, bitter tastants, including quinine, denatonium benzoate and phenylthiocarbamide fail to trigger GLP-1 secretion from HuTu-80 cells[44]. Relative to the three aforementioned cell lines, HuTu-80 cells have been less frequently employed to study nutrient-gut interactions.
The major functional differences between immortalised intestinal cell lines and primary EE cells have led to an increased research focus on primary intestinal models to study the endocrine function of the gut. These have included the isolation and use of primary EE cells[45-47] and use of ex vivo intestinal tissue preparations from animals[48-51] and humans[52,53]. These tissue-based approaches maintain native cell-cell connections and polarity, and have hitherto yielded a deep understanding of the mechanisms governing nutrient and drug-evoked 5-hydroxytryptamine and GLP-1 release[54,55]. However, clinical access to gut endoscopic, colonoscopic or surgical tissues, tissue viability and potentially low EE cell density can limit these primary models. The purification of primary EE cells is also technically demanding. The recent development of intestinal organoids holds the promise to overcome some of these limitations.
Intestinal organoids, also known as “mini-guts”, are miniaturised intestinal units that display many features of gut tissue architecture and function. In 2007, Barker and colleagues identified leucine-rich repeat-containing GPCRG-5 (Lgr5) -positive cells as stem cells in the small intestine and colon via genetic lineage tracing experiments[56]. Subsequently, a single Lgr5-positive stem cell was shown to differentiate into crypt-villus organoids, namely enteroids, that are inclusive of all cell types present in the native intestinal epithelium[57]. Of note, enteroids can be developed from Lgr5-positive cells originating from any section of the gut. Ex vivo characterisation has shown that these enteroids display the basal-apical polarity of mature epithelial cells[58,59]. Moreover, they retain many region-specific functions of the original location from which the stem cells were taken[60].
Intestinal organoids can also be developed from human pluripotent stem cells, which are referred to as human intestinal organoids (HIOs)[61,62]. HIOs have similar morphology as enteroids and display crypt-villus structures inclusive of all intestinal cell types. By contrast, HIOs contain a mesenchyme layer that is composed of myofibroblasts, endothelial cells and smooth muscle[63]. Moreover, HIOs do not show region-specific features and eventually grow into an unselective population of EE cells[61]. The differentiation process has been shown to be enhanced by the Happy Cell Advanced Suspension Medium[64] and by activation of the bone morphogenetic protein signalling pathway[65].
In contrast to primary intestinal epithelium, intestinal organoids remain viable for over 1-year ex vivo and show plasticity in cellular composition in response to changes in the culture environment or modified gene expression. Accumulating evidence suggests that the density of EE cells in organoids is subject to the expression of several translational factors, including Neurogenin 3 and Aristaless-related homobox[61,66,67], raising the prospect that EE cells can be customised in an organoid. Indeed, exposure of mouse or human enteroids to short-chained fatty acids (SCFAs) increases the number of L cells, and hence GLP-1 secretion, over 48 h of SCFA treatment[68]. Similar trends in differentiation have also been observed with enteroids treated with dibenzazepine or bile acids[69,70]. However, the secretory profile of intestinal organoids in response to nutrients or non-nutritive compounds has not been well characterised. It should also be noted that delivery of stimuli to the lumen of the organoids requires individual microinjection, which is both labour-intensive and technically demanding due to their small size[71]. Moreover, the culture of organoids in conventional platforms makes it difficult to mimic the continuous movement of luminal contents and constantly changing nature of the extracellular fluid. Finally, it is not yet possible to recreate the architectural complexity of the GI tract, including its vascular, nervous, immune, mucous elements and the microbiome, in any organoid preparation.
The development of intestinal perfusion techniques and analytic methods capable of measuring GI hormones released into the peripheral circulation has allowed the evaluation of nutrient-gut interactions in vivo. In rodents, dietary effects on gut hormone secretion have been investigated in models of isolated intestinal perfusion[72,73]. In humans, it is also possible to characterise the responses of various regions of the gut to intraluminal stimuli, and to examine the underlying mechanisms.
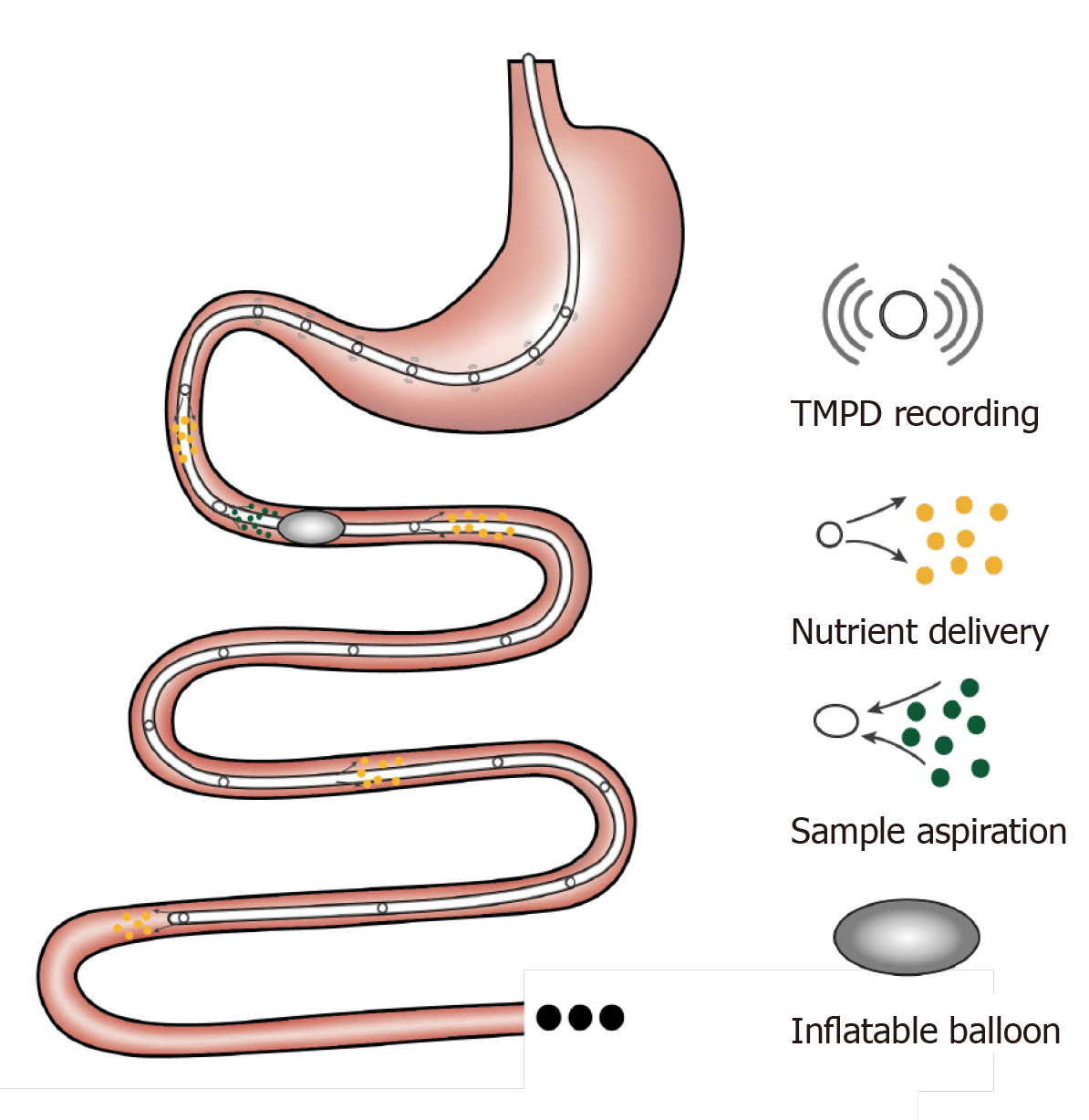
A rubber feeding tube was initially designed to deliver medication to the intestine and to examine luminal contents in paediatric patients[74]. This early design incorporated 1-2 cm wide lateral window(s) for infusion/aspiration of liquids and a weighted terminal bulb to facilitate passage of the catheter by peristalsis. Subsequently, intestinal catheters have been increasingly customised to study gut function. For example, the integration of an inflatable balloon at the distal end of the catheter was employed to evaluate the perception of distension or control the position of the catheter[75]. Use of a multi-lumen catheter has allowed for multiple inflatable balloons, making it possible to isolate segments of the lumen[76], within which nutrient absorption can be carefully characterised[77-79]. Incorporation of manometry and impedance sensors into the catheter design has further facilitated concurrent recording of gut motility[34] and flow events[80]. Positioning these catheters has relied on fluoroscopy, for which radiation exposure represents a major limitation. To overcome this, Andersson and Grossman established an alternative method of monitoring catheter position by measuring transmucosal potential difference (TMPD) between skin or blood and the intestinal lumen[81]. Corresponding to the differences in pH between the stomach and the duodenum, TMPD in the distal antral channel and the proximal duodenal channel record around -40 mV and 0 mV, respectively[82,83]. Accordingly, a change of TMPD from -40 mV to 0 mV reflects passage of channels through the transpyloric area (Figure 2).
Relative to oral administration, intestinal perfusion of nutrients or investigational compounds circumvents the impact of inter-individual variations in the rate of gastric emptying – which can be substantial[84-86] – such that the exposure of the small intestine to nutrients can be standardised. Studies employing intraduodenal infusion of nutrients spanning the normal range of gastric emptying (1-4 kcal/min) have established that the stimulation of gut hormones, including CCK, GIP, GLP-1 and PYY, is dependent on the rate of nutrient entry into the small intestine. In line with the biological distribution of respective EE cells, the secretion of CCK and GIP appears to be proportional to the load of glucose, lipid or protein, whereas GLP-1 and PPY responses are non-linear, being modest at 1-2 kcal/min and substantially greater at 3-4 kcal/min[87]. Moreover, when glucose and fat are infused intraduodenally at an identical rate of 2 kcal/min, it is observed that fat is significantly more potent than glucose at stimulating GLP-1 and GIP secretion[88].
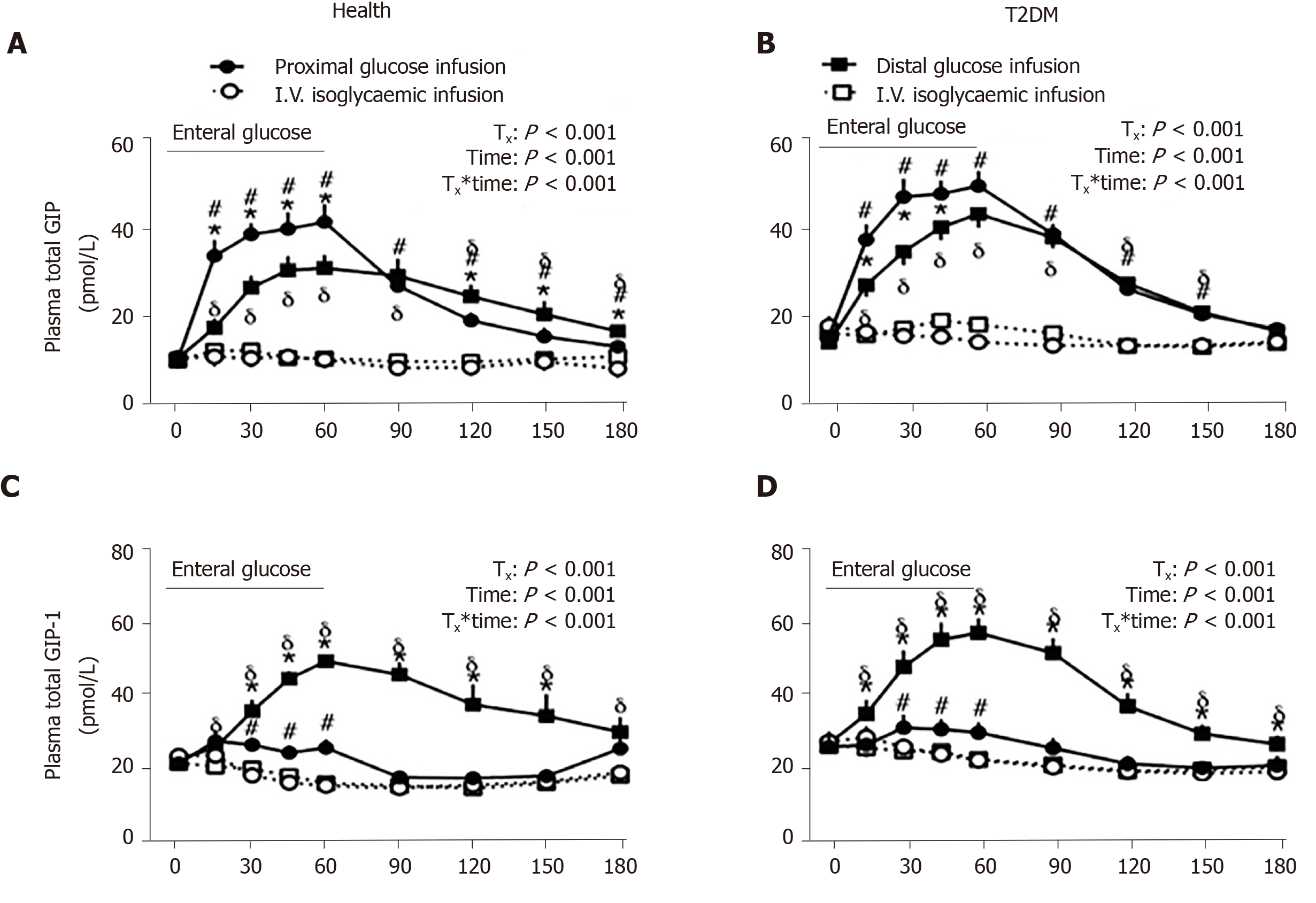
A multi-lumen catheter of adequate length can also be positioned over a long length of small intestine to allow targeted delivery of nutrients or investigational compounds into proximal or distal sites, to determine the regional specificity of nutrient-gut interactions. In this way, infusion of glucose (2 kcal/min) into jejunum (50 cm distal to pylorus) was shown to elicit more GLP-1 and GIP release compared to equivalent duodenal infusion (12 cm distal to pylorus) in healthy men[89]. Furthermore, ileal glucose infusion (2 kcal/min, 190 cm distal to pylorus) resulted in markedly greater GLP-1 and lower (but more sustained) GIP responses compared to intraduodenal infusion, and was associated with a greater incretin effect and GI-mediated glucose disposal in both healthy subjects and patients with type 2 diabetes (Figure 3)[90]. Administration of compounds into the rectum can similarly be undertaken using a soft tube with minimal discomfort[91,92]. Characterisation of the region-specific profile of gut hormone release has shed light on the mechanisms by which Roux-en-Y gastric bypass surgery improves blood glucose control in type 2 diabetes[93]. In addition, this knowledge has directed the precise delivery of stimuli to optimise gut hormone response for therapeutic gain. For example, enteric coating of a small dose of lauric acid to allow targeted release in the ileum and colon was shown to be effective at stimulating GLP-1 secretion and lowering blood glucose in patients with type 2 diabetes[94].
Access to the intestines via endoscopy and colonoscopy has provided an additional means for targeting intestinal perfusion to a specific region, while also allowing for the collection of mucosal biopsies to study anatomical and molecular mechanisms underlying nutrient-gut interactions (discussed in earlier section). In this way, sweet taste receptors (STRs) (heterodimeric T1R2 and T1R3) were found to be involved in intestinal glucose sensing and linked to regulation of glucose absorption in both health and type 2 diabetes; in patients with type 2 diabetes, a defect in the downregulation of STRs in the face of hyperglycaemia was shown to contribute to excessive postprandial glycaemic excursions[95]. Moreover, ex vivo studies using human intestinal biopsies have revealed a critical role for both SGLT1 and the facilitative glucose transporter 2 in mediating glucose-induced GLP-1 secretion[55].
Several novel techniques are emerging to evaluate nutrient-gut interactions with improved physiological or therapeutic relevance, while overcoming limitations of clinical studies.
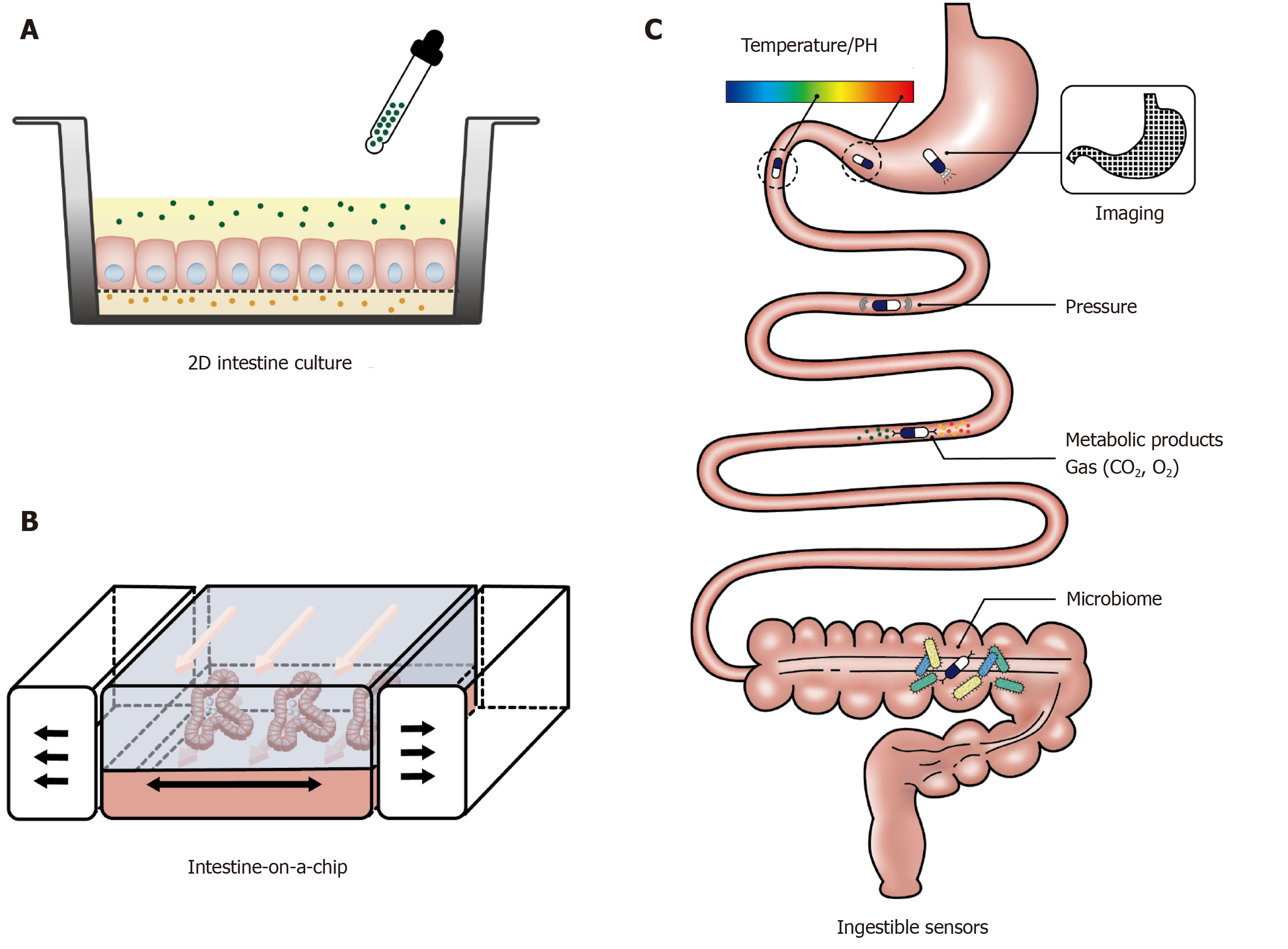
Recent development of culture engineering techniques has allowed integration of advanced culture interfaces into the conventional 2D culture platforms of intestinal organoids and primary epithelial cells. This has enabled the provision of culture frameworks that support the growth of intestinal cells and facilitate the assessment of tissue function in a more physiologically relevant environment[96,97]. For example, culturing intestinal cells on a porous polyester membrane provides access to both basolateral and apical sides of the polarised epithelial cells, which is of particular importance for the investigation of the intestinal function in response to luminal stimuli (Figure 4A). In addition, the membrane can be coated with an extracellular matrix containing growth factors to induce growth and differentiation of organoids. This experimental platform is being increasingly used to study intestinal barrier function[98,99], immune responses[100], and drug metabolism[101,102], with a handful of studies focusing on nutrient-gut interactions. Kozuka et al[103] developed an intestinal monolayer culture platform utilising Transwell (a culture plate with an inserted membrane) with a 0.4 µm or 1 µm pore membrane and successfully cultured murine and human intestinal enteroids. Treatment with forskolin (100 µM) in the apical chamber stimulated GLP-1 release into the basolateral chamber, consistent with the presence of functional L-cells and GLP-1 deployment mechanisms. With this compartmental culture system, it is possible to model the interaction between the intestinal epithelium and luminal content and monitor the hormonal response in the downstream chamber.
More advanced and complex intestine models have been achieved by applying microfluidic devices in gut function studies, also known as “intestine-on-a-chip”. These microfluidic devices have the capacity to provide a dynamic culture environment, including continuously refreshed culture media and biomimetic mechanical strain, to more accurately resemble physiological conditions (Figure 4B). Current in vitro gut models on microfluidic devices have mainly been used to investigate drug metabolism[104] and gut-liver interactions[105]. The application of the “intestine-on-a-chip” model for gut hormone secretion study is in its infancy. In 2016, Hsiao et al[106] developed a high-throughput automated microfluidic platform to assess the response of NCL-H716 cells to sweet and bitter stimuli. Although gut hormones were not measured in the study, the microfluidic system recorded the dynamic changes in intracellular Ca2+ in over 500 single NCI-H716 cells trapped in each micro-well. In another study, Park and his colleagues established a co-culture of GLUTag cells and the β cell line INS-1 to screen compounds of anti-diabetic potential[107]. Relative to the use of intestinal cancer cell line, intestinal organoids cultured on a microfluidic device display a high resemblance to the native intestine transcriptome, including the expression of genes related to cell proliferation, digestion and responses to nutrients[108], and may prove to be a useful ex vivo model for studying GI hormone secretion.
Ingestible sensors are under rapid development in clinical settings. These are typically capsule devices of up to 11 mm in diameter and 28 mm in length, to allow easy transit through the gut while measuring biomedical parameters (Figure 4C). To date, ingestible sensors have been developed for imaging[109-112] and measurements of gases[113], pH, temperature[114-117], pressure[118] and luminal contents[119-121]. The pH sensors have been used to assess gastric emptying and small intestinal transit, marked by abrupt pH changes between the stomach and duodenum (> 3 units) and between the ileum and colon (> 1 unit)[122,123]. The wide application of ingestible sensors will require further technical development to improve stability, signal interpretation and reduce costs, but offer an exciting glimpse into the future of GI surveillance.
A better understanding of the mechanisms underlying nutrient-gut interactions is fundamental to the development of gut-based therapies for major metabolic disorders. For this purpose, the development of in vitro EE cell models, and techniques suitable for in vivo studies, particularly in humans, is of critical importance. EE cell lines of both murine (STC-1 and GLUTag) and human (NCI-H716 and HuTo-80) origin are useful for early studies on gut hormone secretion, but have had limited translational success. This necessitates the development of more physiologically relevant in vitro gut models. The emergence of intestinal organoids and novel co-culture systems represents a major advance in this area. In particular, the combination of intestinal organoids and microfluidics will provide an unprecedented opportunity to study the dynamic hormonal response to stimuli under various conditions. In vivo validation of research outcomes derived from these models remains critical. In clinical studies, intestinal intubation and the application of novel ingestible sensors, have provided deep knowledge of the region-specific nature of nutrient-gut interactions, and ensuing hormonal and metabolic responses. Further development of non-invasive techniques suitable for use in humans will expand opportunities to translate research findings from the bench to bedside.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): o
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jamali R, Kung WM, Qin R, Wada R S-Editor: Yan JP L-Editor: A E-Editor: Wang LL
| 1. | Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol Rev. 2017;97:411-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 353] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 2. | Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 594] [Cited by in F6Publishing: 613] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 3. | Spiegel K, Tasali E, Leproult R, Scherberg N, Van Cauter E. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 2011;96:486-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Polak JM, Bloom SR, Rayford PL, Pearse AG, Buchan AM, Thompson JC. Identification of cholecystokinin-secreting cells. Lancet. 1975;2:1016-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 95] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Buffa R, Capella C, Fontana P, Usellini L, Solcia E. Types of endocrine cells in the human colon and rectum. Cell Tissue Res. 1978;192:227-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Svendsen B, Pedersen J, Albrechtsen NJ, Hartmann B, Toräng S, Rehfeld JF, Poulsen SS, Holst JJ. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology. 2015;156:847-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 7. | Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290:E550-E559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79:616-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 575] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300-1304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1228] [Cited by in F6Publishing: 1225] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 10. | Näslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, Rössner S, Hellström PM. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord. 1999;23:304-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 352] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest. 1967;46:1954-1962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 636] [Cited by in F6Publishing: 610] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | McLaughlin JT, Lomax RB, Hall L, Dockray GJ, Thompson DG, Warhurst G. Fatty acids stimulate cholecystokinin secretion via an acyl chain length-specific, Ca2+-dependent mechanism in the enteroendocrine cell line STC-1. J Physiol. 1998;513:11-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 112] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Rindi G, Grant SG, Yiangou Y, Ghatei MA, Bloom SR, Bautch VL, Solcia E, Polak JM. Development of neuroendocrine tumors in the gastrointestinal tract of transgenic mice. Heterogeneity of hormone expression. Am J Pathol. 1990;136:1349-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Wang Y, Prpic V, Green GM, Reeve JR, Liddle RA. Luminal CCK-releasing factor stimulates CCK release from human intestinal endocrine and STC-1 cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G16-G22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Cordier-Bussat M, Bernard C, Levenez F, Klages N, Laser-Ritz B, Philippe J, Chayvialle JA, Cuber JC. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes. 1998;47:1038-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1108] [Cited by in F6Publishing: 1093] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 17. | Hand KV, Giblin L, Green BD. Hormone profiling in a novel enteroendocrine cell line pGIP/neo: STC-1. Metabolism. 2012;61:1683-1686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Geraedts MC, Troost FJ, Saris WH. Peptide-YY is released by the intestinal cell line STC-1. J Food Sci. 2009;74:H79-H82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Hand KV, Bruen CM, O'Halloran F, Panwar H, Calderwood D, Giblin L, Green BD. Examining acute and chronic effects of short- and long-chain fatty acids on peptide YY (PYY) gene expression, cellular storage and secretion in STC-1 cells. Eur J Nutr. 2013;52:1303-1313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Mangel AW, Prpic V, Scott L, Liddle RA. Inhibitors of ATP-sensitive potassium channels stimulate intestinal cholecystokinin secretion. Peptides. 1994;15:1565-1566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Komarnytsky S, Cook A, Raskin I. Potato protease inhibitors inhibit food intake and increase circulating cholecystokinin levels by a trypsin-dependent mechanism. Int J Obes (Lond). 2011;35:236-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Foltz M, Ansems P, Schwarz J, Tasker MC, Lourbakos A, Gerhardt CC. Protein hydrolysates induce CCK release from enteroendocrine cells and act as partial agonists of the CCK1 receptor. J Agric Food Chem. 2008;56:837-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Ma J, Stevens JE, Cukier K, Maddox AF, Wishart JM, Jones KL, Clifton PM, Horowitz M, Rayner CK. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care. 2009;32:1600-1602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 275] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 24. | Lee YC, Asa SL, Drucker DJ. Glucagon gene 5'-flanking sequences direct expression of simian virus 40 large T antigen to the intestine, producing carcinoma of the large bowel in transgenic mice. J Biol Chem. 1992;267:10705-10708. [PubMed] [Cited in This Article: ] |
| 25. | Drucker DJ, Jin T, Asa SL, Young TA, Brubaker PL. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol Endocrinol. 1994;8:1646-1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 91] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Oya M, Kitaguchi T, Pais R, Reimann F, Gribble F, Tsuboi T. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J Biol Chem. 2013;288:4513-4521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Parker HE, Adriaenssens A, Rogers G, Richards P, Koepsell H, Reimann F, Gribble FM. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55:2445-2455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 29. | Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes. 2002;51:2757-2763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Parker HE, Wallis K, le Roux CW, Wong KY, Reimann F, Gribble FM. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br J Pharmacol. 2012;165:414-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 31. | Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58:1058-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 32. | Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47:1592-1601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Meek CL, Lewis HB, Vergese B, Park A, Reimann F, Gribble F. The effect of encapsulated glutamine on gut peptide secretion in human volunteers. Peptides. 2016;77:38-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Chang J, Wu T, Greenfield JR, Samocha-Bonet D, Horowitz M, Rayner CK. Effects of intraduodenal glutamine on incretin hormone and insulin release, the glycemic response to an intraduodenal glucose infusion, and antropyloroduodenal motility in health and type 2 diabetes. Diabetes Care. 2013;36:2262-2265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Park JG, Oie HK, Sugarbaker PH, Henslee JG, Chen TR, Johnson BE, Gazdar A. Characteristics of cell lines established from human colorectal carcinoma. Cancer Res. 1987;47:6710-6718. [PubMed] [Cited in This Article: ] |
| 36. | Reimer RA, Darimont C, Gremlich S, Nicolas-Métral V, Rüegg UT, Macé K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology. 2001;142:4522-4528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Reimer RA. Meat hydrolysate and essential amino acid-induced glucagon-like peptide-1 secretion, in the human NCI-H716 enteroendocrine cell line, is regulated by extracellular signal-regulated kinase1/2 and p38 mitogen-activated protein kinases. J Endocrinol. 2006;191:159-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 2007;104:15069-15074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 732] [Cited by in F6Publishing: 739] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 39. | Kuhre RE, Wewer Albrechtsen NJ, Deacon CF, Balk-Møller E, Rehfeld JF, Reimann F, Gribble FM, Holst JJ. Peptide production and secretion in GLUTag, NCI-H716, and STC-1 cells: a comparison to native L-cells. J Mol Endocrinol. 2016;56:201-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792-G802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 212] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 41. | Schmidt M, Deschner EE, Thaler HT, Clements L, Good RA. Gastrointestinal cancer studies in the human to nude mouse heterotransplant system. Gastroenterology. 1977;72:829-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Ohtsu Y, Nakagawa Y, Nagasawa M, Takeda S, Arakawa H, Kojima I. Diverse signaling systems activated by the sweet taste receptor in human GLP-1-secreting cells. Mol Cell Endocrinol. 2014;394:70-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Pham H, Hui H, Morvaridi S, Cai J, Zhang S, Tan J, Wu V, Levin N, Knudsen B, Goddard WA, Pandol SJ, Abrol R. A bitter pill for type 2 diabetes? The activation of bitter taste receptor TAS2R38 can stimulate GLP-1 release from enteroendocrine L-cells. Biochem Biophys Res Commun. 2016;475:295-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Le Nevé B, Foltz M, Daniel H, Gouka R. The steroid glycoside H.g.-12 from Hoodia gordonii activates the human bitter receptor TAS2R14 and induces CCK release from HuTu-80 cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1368-G1375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Buchan AM, Barber DL, Gregor M, Soll AH. Morphologic and physiologic studies of canine ileal enteroglucagon-containing cells in short-term culture. Gastroenterology. 1987;93:791-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Koop I, Buchan AM. Cholecystokinin release from isolated canine epithelial cells in short-term culture. Gastroenterology. 1992;102:28-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Brubaker PL, Vranic M. Fetal rat intestinal cells in monolayer culture: a new in vitro system to study the glucagon-like immunoreactive peptides. Endocrinology. 1987;120:1976-1985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Drucker DJ, Brubaker PL. Proglucagon gene expression is regulated by a cyclic AMP-dependent pathway in rat intestine. Proc Natl Acad Sci USA. 1989;86:3953-3957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 81] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Shima K, Suda T, Nishimoto K, Yoshimoto S. Relationship between molecular structures of sugars and their ability to stimulate the release of glucagon-like peptide-1 from canine ileal loops. Acta Endocrinol (Copenh). 1990;123:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Orskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986;119:1467-1475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 341] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC. Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology. 1998;139:3780-3786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Sun EW, Martin AM, Wattchow DA, de Fontgalland D, Rabbitt P, Hollington P, Young RL, Keating DJ. Metformin Triggers PYY Secretion in Human Gut Mucosa. J Clin Endocrinol Metab. 2019;104:2668-2674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Young RL, Sutherland K, Pezos N, Brierley SM, Horowitz M, Rayner CK, Blackshaw LA. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2009;58:337-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 54. | Lumsden AL, Martin AM, Sun EW, Schober G, Isaacs NJ, Pezos N, Wattchow DA, de Fontgalland D, Rabbitt P, Hollington P, Sposato L, Due SL, Rayner CK, Nguyen NQ, Liou AP, Jackson VM, Young RL, Keating DJ. Sugar Responses of Human Enterochromaffin Cells Depend on Gut Region, Sex, and Body Mass. Nutrients. 2019;11:234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Sun EW, de Fontgalland D, Rabbitt P, Hollington P, Sposato L, Due SL, Wattchow DA, Rayner CK, Deane AM, Young RL, Keating DJ. Mechanisms Controlling Glucose-Induced GLP-1 Secretion in Human Small Intestine. Diabetes. 2017;66:2144-2149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 56. | Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3854] [Cited by in F6Publishing: 4011] [Article Influence: 235.9] [Reference Citation Analysis (0)] |
| 57. | Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4394] [Cited by in F6Publishing: 4474] [Article Influence: 298.3] [Reference Citation Analysis (0)] |
| 58. | Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, Watanabe T, Kanai T, Sato T. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med. 2015;21:256-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 692] [Cited by in F6Publishing: 745] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 59. | Sugimoto S, Sato T. Establishment of 3D Intestinal Organoid Cultures from Intestinal Stem Cells. Methods Mol Biol. 2017;1612:97-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Middendorp S, Schneeberger K, Wiegerinck CL, Mokry M, Akkerman RD, van Wijngaarden S, Clevers H, Nieuwenhuis EE. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells. 2014;32:1083-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 61. | Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1455] [Cited by in F6Publishing: 1295] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 62. | McCracken KW, Howell JC, Wells JM, Spence JR. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc. 2011;6:1920-1928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 63. | Watson CL, Mahe MM, Múnera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y, Grabowski G, Finkbeiner SR, Spence JR, Shroyer NF, Wells JM, Helmrath MA. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20:1310-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 383] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 64. | Takahashi Y, Sato S, Kurashima Y, Yamamoto T, Kurokawa S, Yuki Y, Takemura N, Uematsu S, Lai CY, Otsu M, Matsuno H, Osawa H, Mizushima T, Nishimura J, Hayashi M, Yamaguchi T, Kiyono H. A Refined Culture System for Human Induced Pluripotent Stem Cell-Derived Intestinal Epithelial Organoids. Stem Cell Reports. 2018;10:314-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 65. | Múnera JO, Sundaram N, Rankin SA, Hill D, Watson C, Mahe M, Vallance JE, Shroyer NF, Sinagoga KL, Zarzoso-Lacoste A, Hudson JR, Howell JC, Chatuvedi P, Spence JR, Shannon JM, Zorn AM, Helmrath MA, Wells JM. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling. Cell Stem Cell. 2017;21:51-64.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 66. | Chang-Graham AL, Danhof HA, Engevik MA, Tomaro-Duchesneau C, Karandikar UC, Estes MK, Versalovic J, Britton RA, Hyser JM. Human Intestinal Enteroids With Inducible Neurogenin-3 Expression as a Novel Model of Gut Hormone Secretion. Cell Mol Gastroenterol Hepatol. 2019;8:209-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 67. | Du A, McCracken KW, Walp ER, Terry NA, Klein TJ, Han A, Wells JM, May CL. Arx is required for normal enteroendocrine cell development in mice and humans. Dev Biol. 2012;365:175-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Petersen N, Reimann F, Bartfeld S, Farin HF, Ringnalda FC, Vries RG, van den Brink S, Clevers H, Gribble FM, de Koning EJ. Generation of L cells in mouse and human small intestine organoids. Diabetes. 2014;63:410-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 69. | Petersen N, Reimann F, van Es JH, van den Berg BM, Kroone C, Pais R, Jansen E, Clevers H, Gribble FM, de Koning EJ. Targeting development of incretin-producing cells increases insulin secretion. J Clin Invest. 2015;125:379-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Lund ML, Sorrentino G, Egerod KL, Kroone C, Mortensen B, Knop FK, Reimann F, Gribble FM, Drucker DJ, de Koning EJP, Schoonjans K, Bäckhed F, Schwartz TW, Petersen N. L-Cell Differentiation Is Induced by Bile Acids Through GPBAR1 and Paracrine GLP-1 and Serotonin Signaling. Diabetes. 2020;69:614-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 71. | Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ, Clevers H. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148:126-136.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 524] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 72. | Svendsen B, Holst JJ. Regulation of gut hormone secretion. Studies using isolated perfused intestines. Peptides. 2016;77:47-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 74. | Scheltema G. Permeation in the examination and treatment of the stomach and intestines. Arch Roentg Ray. 1908;13:144-149. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 75. | Miller G, Abbott W. Intestinal intubation: a practical technique. Am J Med Sci. 1934;187:595-598. [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 77] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Abbott WO, Miller TG. Intubation Studies of the human small intestine: III. A technic for the collection of pure intestinal secretion and for the study of intestinal absorption. JAMA. 1936;106:16-18. [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 77. | Abbott WO, Karr WG, Miller TG. Intubation studies of the human small intestine: VII. Factors concerned in absorption of glucose from the jejunum and ileum. Am J Dig Dis. 1937;4:742-752. [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 78. | Groen J. THE ABSORPTION OF HEXOSES FROM THE UPPER PART OF THE SMALL INTESTINE IN MAN. J Clin Invest. 1937;16:245-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 79. | Shay H, Gershon-Cohen J, Fels SS, Munro FL, Siplet H. The absorption and dilution of glucose solutions in the human stomach and duodenum. Am J Dig Dis. 1939;6:535-544. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 80. | Silny J. Intraluminal multiple electric impedance procedure for measurement of gastrointestinal motility. Neurogastroenterol Motil. 1991;3:151-162. [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 81. | Andersson S, Grossman MI. Profile of pH, pressure, and potential difference at gastroduodenal junction in man. Gastroenterology. 1965;49:364-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 105] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Veedfald S, Wu T, Bound M, Grivell J, Hartmann B, Rehfeld JF, Deacon CF, Horowitz M, Holst JJ, Rayner CK. Hyperosmolar Duodenal Saline Infusion Lowers Circulating Ghrelin and Stimulates Intestinal Hormone Release in Young Men. J Clin Endocrinol Metab. 2018;103:4409-4418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Wu T, Trahair LG, Little TJ, Bound MJ, Zhang X, Wu H, Sun Z, Horowitz M, Rayner CK, Jones KL. Effects of Vildagliptin and Metformin on Blood Pressure and Heart Rate Responses to Small Intestinal Glucose in Type 2 Diabetes. Diabetes Care. 2017;40:702-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96:11-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 187] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 85. | Watson LE, Xie C, Wang X, Li Z, Phillips LK, Sun Z, Jones KL, Horowitz M, Rayner CK, Wu T. Gastric Emptying in Patients With Well-Controlled Type 2 Diabetes Compared With Young and Older Control Subjects Without Diabetes. J Clin Endocrinol Metab. 2019;104:3311-3319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 86. | Cecil JE, Francis J, Read NW. Comparison of the effects of a high-fat and high-carbohydrate soup delivered orally and intragastrically on gastric emptying, appetite, and eating behaviour. Physiol Behav. 1999;67:299-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Wu T, Rayner CK, Young RL, Horowitz M. Gut motility and enteroendocrine secretion. Curr Opin Pharmacol. 2013;13:928-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Wu T, Rayner CK, Watson LE, Jones KL, Horowitz M, Little TJ. Comparative effects of intraduodenal fat and glucose on the gut-incretin axis in healthy males. Peptides. 2017;95:124-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Wu T, Thazhath SS, Marathe CS, Bound MJ, Jones KL, Horowitz M, Rayner CK. Comparative effect of intraduodenal and intrajejunal glucose infusion on the gut-incretin axis response in healthy males. Nutr Diabetes. 2015;5:e156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Zhang X, Young RL, Bound M, Hu S, Jones KL, Horowitz M, Rayner CK, Wu T. Comparative Effects of Proximal and Distal Small Intestinal Glucose Exposure on Glycemia, Incretin Hormone Secretion, and the Incretin Effect in Health and Type 2 Diabetes. Diabetes Care. 2019;42:520-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 91. | Wu T, Bound MJ, Standfield SD, Gedulin B, Jones KL, Horowitz M, Rayner CK. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes Metab. 2013;15:474-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 92. | Lennernäs H, Fagerholm U, Raab Y, Gerdin B, Hällgren R. Regional rectal perfusion: a new in vivo approach to study rectal drug absorption in man. Pharm Res. 1995;12:426-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 93. | Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709-1716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 382] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 94. | Ma J, Checklin HL, Wishart JM, Stevens JE, Jones KL, Horowitz M, Meyer JH, Rayner CK. A randomised trial of enteric-coated nutrient pellets to stimulate gastrointestinal peptide release and lower glycaemia in type 2 diabetes. Diabetologia. 2013;56:1236-1242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 95. | Young RL, Chia B, Isaacs NJ, Ma J, Khoo J, Wu T, Horowitz M, Rayner CK. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes. 2013;62:3532-3541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 96. | Wang Y, Gunasekara DB, Reed MI, DiSalvo M, Bultman SJ, Sims CE, Magness ST, Allbritton NL. A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials. 2017;128:44-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 97. | Chen Y, Zhou W, Roh T, Estes MK, Kaplan DL. In vitro enteroid-derived three-dimensional tissue model of human small intestinal epithelium with innate immune responses. PLoS One. 2017;12:e0187880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 98. | Eaton AD, Zimmermann C, Delaney B, Hurley BP. Primary human polarized small intestinal epithelial barriers respond differently to a hazardous and an innocuous protein. Food Chem Toxicol. 2017;106:70-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Bhatt AP, Gunasekara DB, Speer J, Reed MI, Peña AN, Midkiff BR, Magness ST, Bultman SJ, Allbritton NL, Redinbo MR. Nonsteroidal Anti-Inflammatory Drug-Induced Leaky Gut Modeled Using Polarized Monolayers of Primary Human Intestinal Epithelial Cells. ACS Infect Dis. 2018;4:46-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 100. | Graves CL, Harden SW, LaPato M, Nelson M, Amador B, Sorenson H, Frazier CJ, Wallet SM. A method for high purity intestinal epithelial cell culture from adult human and murine tissues for the investigation of innate immune function. J Immunol Methods. 2014;414:20-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | Speer JE, Wang Y, Fallon JK, Smith PC, Allbritton NL. Evaluation of human primary intestinal monolayers for drug metabolizing capabilities. J Biol Eng. 2019;13:82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Takayama K, Negoro R, Yamashita T, Kawai K, Ichikawa M, Mori T, Nakatsu N, Harada K, Ito S, Yamada H, Yamaura Y, Hirata K, Ishida S, Mizuguchi H. Generation of Human iPSC-Derived Intestinal Epithelial Cell Monolayers by CDX2 Transduction. Cell Mol Gastroenterol Hepatol. 2019;8:513-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 103. | Kozuka K, He Y, Koo-McCoy S, Kumaraswamy P, Nie B, Shaw K, Chan P, Leadbetter M, He L, Lewis JG, Zhong Z, Charmot D, Balaa M, King AJ, Caldwell JS, Siegel M. Development and Characterization of a Human and Mouse Intestinal Epithelial Cell Monolayer Platform. Stem Cell Reports. 2017;9:1976-1990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 104. | Kimura H, Sakai Y, Fujii T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacokinet. 2018;33:43-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 234] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 105. | Choe A, Ha SK, Choi I, Choi N, Sung JH. Microfluidic Gut-liver chip for reproducing the first pass metabolism. Biomed Microdevices. 2017;19:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 106. | Hsiao YH, Hsu CH, Chen C. A High-Throughput Automated Microfluidic Platform for Calcium Imaging of Taste Sensing. Molecules. 2016;21:896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 107. | Nguyen DT, van Noort D, Jeong IK, Park S. Endocrine system on chip for a diabetes treatment model. Biofabrication. 2017;9:015021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 108. | Kasendra M, Tovaglieri A, Sontheimer-Phelps A, Jalili-Firoozinezhad S, Bein A, Chalkiadaki A, Scholl W, Zhang C, Rickner H, Richmond CA, Li H, Breault DT, Ingber DE. Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids. Sci Rep. 2018;8:2871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 506] [Cited by in F6Publishing: 421] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 109. | Gluck N, Shpak B, Brun R, Rösch T, Arber N, Moshkowitz M. A novel prepless X-ray imaging capsule for colon cancer screening. Gut. 2016;65:371-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 110. | Becker D, Zhang J, Heimbach T, Penland RC, Wanke C, Shimizu J, Kulmatycki K. Novel orally swallowable IntelliCap(®) device to quantify regional drug absorption in human GI tract using diltiazem as model drug. AAPS PharmSciTech. 2014;15:1490-1497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 111. | Gora MJ, Sauk JS, Carruth RW, Gallagher KA, Suter MJ, Nishioka NS, Kava LE, Rosenberg M, Bouma BE, Tearney GJ. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat Med. 2013;19:238-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 112. | Abramson A, Caffarel-Salvador E, Khang M, Dellal D, Silverstein D, Gao Y, Frederiksen MR, Vegge A, Hubálek F, Water JJ, Friderichsen AV, Fels J, Kirk RK, Cleveland C, Collins J, Tamang S, Hayward A, Landh T, Buckley ST, Roxhed N, Rahbek U, Langer R, Traverso G. An ingestible self-orienting system for oral delivery of macromolecules. Science. 2019;363:611-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 113. | Kalantar-Zadeh K, Berean KJ, Ha N, Chrimes AF, Xu K, Grando D, Ou JZ, Pillai N, Campbell JL, Brkljača R, Taylor KM, Burgell RE, Yao CK, Ward SA, McSweeney CS, Muir JG, Gibson PR. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat Electron. 2018;1:79-87. [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 114. | van der Schaar PJ, Dijksman JF, Broekhuizen-de Gast H, Shimizu J, van Lelyveld N, Zou H, Iordanov V, Wanke C, Siersema PD. A novel ingestible electronic drug delivery and monitoring device. Gastrointest Endosc. 2013;78:520-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 115. | Sarosiek I, Selover KH, Katz LA, Semler JR, Wilding GE, Lackner JM, Sitrin MD, Kuo B, Chey WD, Hasler WL, Koch KL, Parkman HP, Sarosiek J, McCallum RW. The assessment of regional gut transit times in healthy controls and patients with gastroparesis using wireless motility technology. Aliment Pharmacol Ther. 2010;31:313-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 116. | Koziolek M, Grimm M, Becker D, Iordanov V, Zou H, Shimizu J, Wanke C, Garbacz G, Weitschies W. Investigation of pH and Temperature Profiles in the GI Tract of Fasted Human Subjects Using the Intellicap(®) System. J Pharm Sci. 2015;104:2855-2863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 268] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 117. | Maurer JM, Schellekens RC, van Rieke HM, Wanke C, Iordanov V, Stellaard F, Wutzke KD, Dijkstra G, van der Zee M, Woerdenbag HJ, Frijlink HW, Kosterink JG. Gastrointestinal pH and Transit Time Profiling in Healthy Volunteers Using the IntelliCap System Confirms Ileo-Colonic Release of ColoPulse Tablets. PLoS One. 2015;10:e0129076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 118. | Rao SS, Kuo B, McCallum RW, Chey WD, DiBaise JK, Hasler WL, Koch KL, Lackner JM, Miller C, Saad R, Semler JR, Sitrin MD, Wilding GE, Parkman HP. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 119. | Demosthenous P, Pitris C, Georgiou J. Infrared Fluorescence-Based Cancer Screening Capsule for the Small Intestine. IEEE Trans Biomed Circuits Syst. 2016;10:467-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 120. | Qiao P, Liu H, Yan X, Jia Z, Pi X. A Smart Capsule System for Automated Detection of Intestinal Bleeding Using HSL Color Recognition. PLoS One. 2016;11:e0166488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 121. | Mimee M, Nadeau P, Hayward A, Carim S, Flanagan S, Jerger L, Collins J, McDonnell S, Swartwout R, Citorik RJ, Bulović V, Langer R, Traverso G, Chandrakasan AP, Lu TK. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science. 2018;360:915-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 234] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 122. | Kuo B, Maneerattanaporn M, Lee AA, Baker JR, Wiener SM, Chey WD, Wilding GE, Hasler WL. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Dig Dis Sci. 2011;56:2928-2938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 123. | Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: comparison of the SmartPill GI monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci. 2009;54:2167-2174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |