Published online Jun 28, 2020. doi: 10.3748/wjg.v26.i24.3472
Peer-review started: January 22, 2020
First decision: March 6, 2020
Revised: April 30, 2020
Accepted: May 15, 2020
Article in press: May 15, 2020
Published online: June 28, 2020
Treatments for hepatic sinusoidal obstruction syndrome (HSOS) are limited.
To evaluate transjugular intrahepatic portosystemic shunting (TIPS) as a treatment for pyrrolidine alkaloid-related HSOS (PA-HSOS).
This retrospective analysis included patients with PA-HSOS admitted to the First Affiliated Hospital of the University of Science and Technology of China (June 2015 to January 2019). Baseline clinical characteristics and follow-up data were extracted from the medical records. All patients included in this study experienced failure of initial therapy. Patients were divided into the TIPS and conservative treatment groups according to the therapy they received. Liver function, maximal ascites depth, imaging characteristics, pathology findings, and survival were compared between groups.
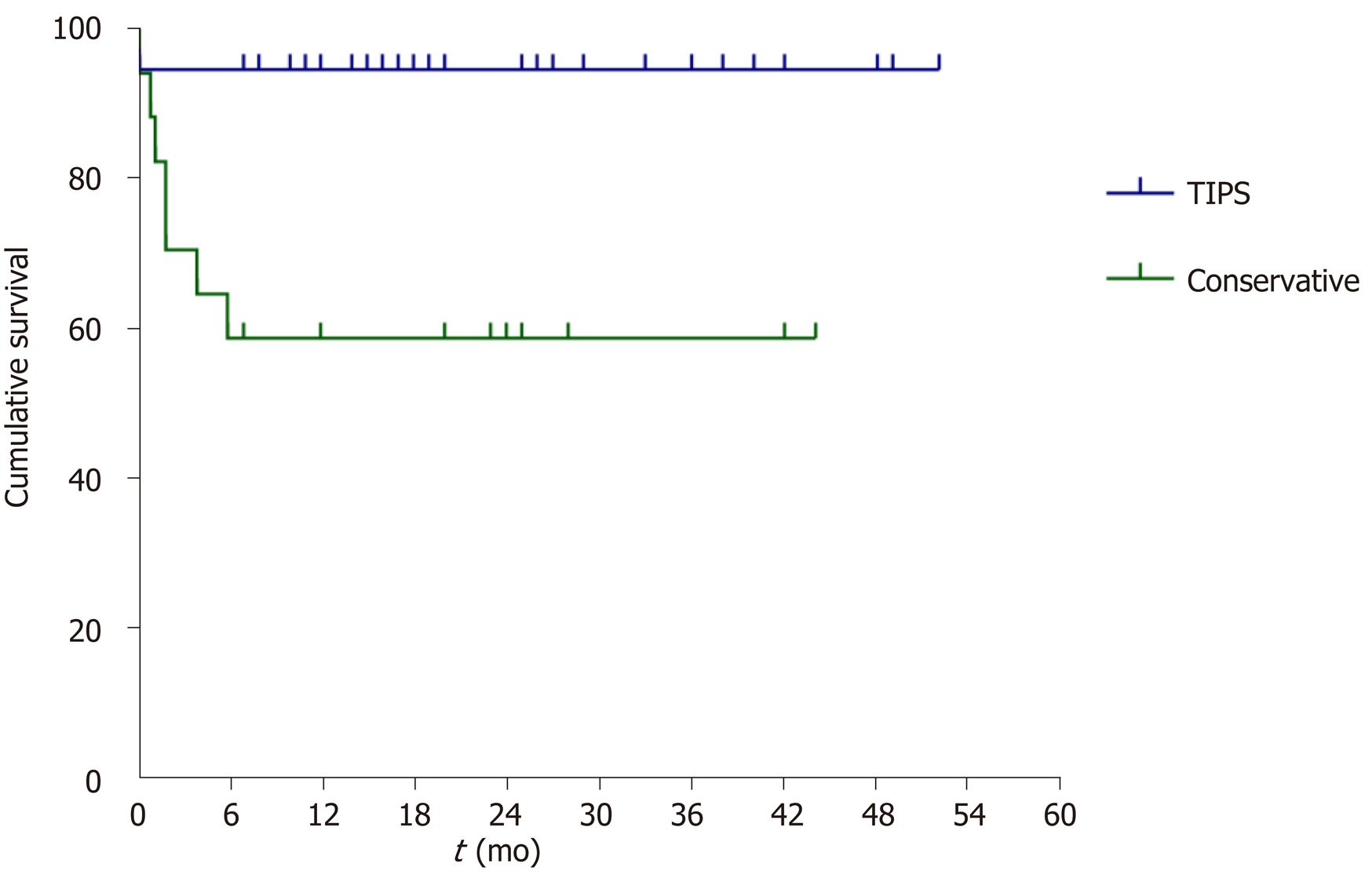
The TIPS group included 37 patients (28 males), and the conservative treatment group included 17 patients (11 males). Baseline characteristics were similar between groups. There were two deaths in the TIPS group and seven deaths in the conservative treatment group during follow-up (3-48 mo). The 3-, 6-, 12- and 24-mo survival rates were 94.6%, 94.6%, 94.6% and 94.6%, respectively, in the TIPS group and 70.6%, 57.8%, 57.8% and 57.8%, respectively, in the conservative treatment group. Kaplan-Meier analysis revealed significantly longer survival for the TIPS group than for the conservative treatment group (P = 0.001). Compared with the pre-treatment value, maximal ascites depth was significantly lower at 1 wk, 2 wk, 1 mo, and 3 mo for the TIPS group (all P < 0.05) but not in the conservative treatment group. Contrast-enhanced computed tomography demonstrated the disappearance of patchy liver enhancement after TIPS. Pathology showed that liver congestion and hepatocyte swelling improved with time after TIPS placement.
TIPS may achieve better outcomes than conventional symptomatic treatment in patients with PA-HSOS.
Core tip: Kaplan-Meier analysis revealed significantly longer survival for the transjugular intrahepatic portosystemic shunting (TIPS) group than for the conservative treatment group in this retrospective study. Compared with the pre-treatment value, maximal ascites depth was significantly lower at 1 wk, 2 wk, 1 mo, and 3 mo for the TIPS group (all P < 0.05) but not the conservative treatment group. Contrast-enhanced computed tomography demonstrated the disappearance of patchy liver enhancement after TIPS.
- Citation: Zhou CZ, Wang RF, Lv WF, Fu YQ, Cheng DL, Zhu YJ, Hou CL, Ye XJ. Transjugular intrahepatic portosystemic shunt for pyrrolizidine alkaloid-related hepatic sinusoidal obstruction syndrome. World J Gastroenterol 2020; 26(24): 3472-3483
- URL: https://www.wjgnet.com/1007-9327/full/v26/i24/3472.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i24.3472
Hepatic sinusoidal obstruction syndrome (HSOS) is a hepatic vascular disease in which edema, necrosis, and shedding of endothelial cells and formation of microthrombi occur in the hepatic sinusoids, hepatic venules, and interlobular vein, leading to intrahepatic congestion, liver dysfunction and portal hypertension[1,2]. In China, the predominant cause of HSOS is the administration of plants containing pyrrolizidine alkaloids (PAs), particularly Gynura segetum[3-5].
The clinical manifestations of hematopoietic stem cell transplantation-induced HSOS (HSCT-HSOS) and PA-induced HSOS (PA-HSOS) differ in several aspects. First, HSCT-HSOS typically presents with hepatomegaly, hepatalgia, ascites, and jaundice[6]. By contrast, PA-HSOS mainly manifests as abdominal distension and ascites[7], with only around half of all patients exhibiting hepatomegaly or jaundice and few having hepatalgia[8]. Furthermore, the serum levels of alanine transaminase, aspartate transaminase, alkaline phosphatase, gamma-glutamyl transferase and total bilirubin are not severely elevated in most patients with PA-HSOS. Second, the speed of onset differs, with HSCT-HSOS usually occurring within 21 d after bone marrow transplantation[9] but PA-HSOS developing after a variable latent period that is generally around 30 d after drug ingestion but can be as long as several years. Third, the proportion of patients with severe disease is higher for HSCT-HSOS than for PA-HSOS[9-12]. Fourth, HSCT-HSOS is associated with higher mortality than PA-HSOS. The mortality rate can exceed 80% in patients with severe HSCT-HSOS[13], with most dying from multiple organ dysfunction syndrome and pyemia. However, the mortality rate is generally around 40% in patients with PA-HSOS[4,7], with most deaths due to progressive liver failure and infection.
Currently, there are no standardized management protocols for HSOS, and the available therapeutic options are limited. Symptomatic and supportive treatments for HSOS include strategies for liver protection, diuresis, and improvement of the microcirculation[14]. Glucocorticoids and anticoagulant therapy may be effective in some patients, but this remains controversial[7,15], while defibrotide has shown promise[16,17]. During the past two decades, attempts have been made to use transjugular intrahepatic portosystemic shunting (TIPS) for the management of HSCT-HSOS[18,19]. However, TIPS is not a recommended treatment in current guidelines because the overall efficacy is not satisfactory and the postoperative survival rate is only about 20%[20-22], but those European guidelines are based mostly on HSCT-HSOS, which is more common in Europe, while PA-HSOS is more common in China and there might be some differences between the two conditions. Postoperative death in these patients is predominantly associated with multiple organ failure, sepsis and hemorrhage largely caused by underlying hematologic disease. However, patients with PA-HSOS generally have no major underlying hematologic disease, less severe disease, and a slower rate of progression than those with HSCT-HSOS.
The aim of this retrospective analysis was to evaluate whether TIPS might have clinical benefit in the management of PA-HSOS.
This was a retrospective analysis of patients with PA-HSOS who were admitted to the Department of Interventional Radiology, Gastroenterology, and Infectious Diseases of the First Affiliated Hospital of USTC (Hefei, Anhui Province, China) between June 2015 and January 2019. The inclusion criteria were: (1) Diagnosis of PA-HSOS[23]; (2) Age 18-80 years; (3) < 60 d since symptom onset; and (4) Failure of conservative therapy (including medications for liver protection, diuresis, and anticoagulation) given for at least 2 wk. The exclusion criteria were: (1) Multiple organ failure at admission; (2) Other liver diseases; and (3) Incomplete clinical and follow-up data at the required time points (such as the results of liver and renal function tests and radiological examinations). The patients included in the final analysis were assigned to a TIPS group and conservative treatment group according to the treatment method. The study was approved by the Medical Research Ethics Committee of the First Affiliated Hospital of USTC. The study was exempted from informed consent because it was retrospective and anonymized.
After admission, all patients received reduced glutathione (1.8 ivgtt qd) and ademetionine (1.0 ivgtt qd) for liver protection and treatment of cholestasis, low molecular weight heparin (LMWH 4000-5000 U q12h ih) for anticoagulation and diuretic drugs (20-120 mg/d furosemide and 40-240 mg/d spironolactone). The initial treatment was considered effective if there was a decrease in ascitic fluid, improved liver function and discontinuation of diuretic drugs after 2 wk; the initial therapy was considered ineffective if there was no improvement or even progression of abdominal distension, ascites and/or liver dysfunction or if diuretics were still needed to maintain treatment after 2 wk. All patients included in this study experienced failure of initial therapy.
Although TIPS was recommended after clinical assessment for all patients who failed the initial 2 wk of therapy, some patients refused TIPS given the controversy regarding the long-term efficacy of this technique in the treatment of HSOS. The patients who declined TIPS (conservative treatment group) were given symptomatic and supportive treatments, including reduced glutathione (1.8 ivgtt qd), ademetionine (1.0 ivgtt qd), LMWH (4000-5000 U ih Q12h) and diuretic drugs (20-120 mg/d furosemide and 40-240 mg/d spironolactone). The use of antibacterial drugs was determined according to the presence or absence of infection.
Patients in the TIPS group underwent TIPS under local anesthesia. After successful puncture of the internal jugular vein, the guidewire was maneuvered into the right hepatic vein, and a RUPS-100 puncture needle was introduced. Then a balloon catheter (generally 6-7 mm in diameter) was delivered along the guidewire to dilate the puncture channel, and a double stent was implanted for shunting (8 mm in diameter; a bare stent measuring 8-10 cm in length and a covered stent measuring 6-8 cm in length). The distal end of the covered stent was at the junction of the portal vein and liver parenchyma, and the proximal end reached the entrance of the inferior vena cava of the hepatic vein. The pressures of the portal vein, splenic vein, and inferior vena cava were measured separately before and after placement of the shunt. The target portal pressure gradient after shunting was ≤ 12 mmHg. The main differences between the present technique and conventional TIPS were a smaller balloon diameter and no requirement for combined embolization of the esophageal and gastric varicose vessels. Anticoagulation with warfarin or rivaroxaban was administered for at least 3 mo postoperatively.
Baseline clinical characteristics and follow-up data were extracted from the medical records. Data were recorded at 7 d, 14 d, 30 d, 90 d, 6 mo, 1 year, and 2 years after surgery (TIPS group) or at the same time points after completion of the initial 2-wk conservative therapy (conservative treatment group).
Statistical analyses were performed using SPSS 17.0 (SPPS Inc., Chicago, IL, United States). All measurement data were tested for normality using the Kolmogorov-Smirnov method. Data conforming to a normal distribution are presented as the mean ± SD and were compared between groups using the independent-samples t-test or one-way analysis of variance (ANOVA) and the least significant difference post hoc test. Data not conforming to a normal distribution are presented as the median (interquartile range) and were compared between groups using the Kruskal-Wallis test and Mann-Whitney U test. Count data were compared using the chi-squared test, and ranked data were compared using the rank-sum test. Survival time was compared between the two groups using Kaplan-Meier survival analysis and the log-rank test. P < 0.05 was considered significant.
A total of 54 patients with PA-HSOS were included, with 37 in the TIPS group and 17 in the conservative treatment group. The baseline clinical characteristics of the patients in the two groups are shown in Table 1. Portal pressure gradient in the TIPS group was 29 (18-39) mmHg before shunting and reduced to 7 (2-17) mmHg after shunting.
| Characteristic | TIPS group (n = 37) | Conservative treatment group (n = 17) | P value |
| Gender (male/female) | 28/9 | 11/6 | 0.403 |
| Age (yr) | 62.30 ± 10.91 | 60.18 ± 15.37 | 0.563 |
| Onset-to-treatment time (d) | 28.57 ± 15.26 | 26.18 ± 12.44 | 0.535 |
| MELD score | 12.13 ± 5.32 | 12.76 ± 4.96 | 0.683 |
| Early therapeutic regimen | |||
| Reduced glutathione | 37 (100) | 17 (100) | NA |
| Ademetionine | 37 (100) | 17 (100) | NA |
| Diuretics | 37 (100) | 17 (100) | NA |
| Low molecular weight heparin | 37 (100) | 17 (100) | NA |
| Glucocorticoids | 11 (29.73) | 6 (35.29) | 0.757 |
| Antibacterial drugs, | 23 (62.16) | 12 (70.60) | 0.549 |
| Clinical symptoms on admission | |||
| Ascites (yes/no) | 37/0 | 17/0 | NA |
| Hepatomegaly (yes/no) | 26/11 | 10/7 | 0.407 |
| Hepatalgia (yes/no) | 15/22 | 7/10 | 0.965 |
| Peritonitis (yes/no) | 7/30 | 2/15 | 0.512 |
| Loss of appetite (yes/no) | 34/3 | 15/2 | 0.667 |
| Gastrointestinal bleeding (yes/no) | 1/36 | 0/17 | 0.494 |
| Weight gain (yes/no) | 12/25 | 6/11 | 0.836 |
| Laboratory indicators at 2 wk | |||
| Total bilirubin (μmol/L) | 53.3 (39.8) | 53.6 (45.0) | 0.904 |
| Direct bilirubin (μmol/L) | 32.4 (32.6) | 37.4 (47.0) | 0.520 |
| Alanine transaminase (U/L) | 45.0 (69.0) | 55.0 (86.0) | 0.342 |
| Aspartate transaminase (U/L) | 65.0 (48.0) | 62.0 (89.0) | 0.628 |
| Gamma-glutamyl transferase (U/L) | 125.0 (90.0) | 91.0 (94.0) | 0.099 |
| Alkaline phosphatase (U/L) | 112.6 (60.0) | 95.0 (91.0) | 0.087 |
| Albumin (g/L) | 31.32 ± 3.38 | 31.95 ± 4.92 | 0.588 |
| Prothrombin time (s) | 15.81 ± 3.64 | 16.75 ± 2.28 | 0.342 |
| White blood cell count (109/L) | 6.75 ± 3.05 | 6.66 ± 2.20 | 0.913 |
| Neutrophil proportion (%) | 64.19 ± 12.16 | 64.39 ± 10.70 | 0.955 |
| Red blood cell count (1012/L) | 4.91 ± 0.81 | 4.49 ± 0.63 | 0.064 |
| Platelet count (109/L) | 108.0 (49.0) | 90.0 (68.0) | 0.165 |
| Blood urea nitrogen (mmol/L) | 6.8 (3.4) | 5.6 (4.3) | 0.252 |
| Creatinine (μmol/L) | 67.6 (32.0) | 75.0 (35.0) | 0.780 |
| Maximum depth of ascites (mm) | 95.22 ± 22.58 | 81.65 ± 26.29 | 0.057 |
The follow-up period was 18 (0-52) mo. A total of 9 patients died by the end of follow-up, including 2 in the TIPS group and 7 in the conservative treatment group. The 3-, 6-, 12- and 24-month survival rates were 94.6%, 94.6%, 94.6% and 94.6%, respectively, in the TIPS group and 70.6%, 57.8%, 57.8% and 57.8%, respectively, in the conservative treatment group. Kaplan-Meier survival analysis (Figure 1) revealed that patients in the TIPS group had a significantly longer survival time than those in the conservative treatment group (P = 0.001, log-rank test).
The differences in liver function at the same time point between the two groups were compared. There was no statistically significant difference in changes in liver function between the two groups at each time point, and the box plot was shown in Supplementary Figure 1. The results showed that there were significant differences (all P < 0.05) in total bilirubin, direct bilirubin, alanine transaminase, and aspartate transaminase before and after treatment in the TIPS group, but there were no significant changes in these indicators in the conservative treatment group, as shown in Table 2.
| Group and time point | TBil (μmol/L) | DBil (μmol/L) | ALT (U/L) | AST (U/L) |
| TIPS group | ||||
| Before treatment (n = 37) | 53.30 (39.80) | 32.40 (32.60) | 45.00 (69.00) | 65.00 (48.00) |
| 1 wk after treatment (n = 36) | 60.20 (70.60) | 44.85 (49.43) | 40.50 (53.00) | 59.00 (65.00) |
| 2 wk after treatment (n = 35) | 56.40 (30.40) | 35.20 (28.40) | 36.00 (26.00) | 47.00 (17.00)a |
| 1 mo after treatment (n = 35) | 47.20 (13.70)c | 29.20 (14.10)c | 26.00 (20.00)ace | 40.00 (16.00)ac |
| 3 mo after treatment (n = 35) | 44.20 (9.70)c | 27.40 (13.10)c | 31.00 (11.00) | 41.00 (12.00)ac |
| P value | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Conservative treatment group | ||||
| Before treatment (n = 17) | 53.60 (45.00) | 37.40 (47.00) | 55.00 (86.0) | 62.00 (89.00) |
| 1 wk after treatment (n = 17) | 67.80 (41.60) | 43.60 (35.95) | 56.00 (99.00) | 67.00 (109.00) |
| 2 wk after treatment (n = 16) | 52.25 (71.30) | 37.60 (60.30) | 44.00 (42.00) | 53.50 (44.00) |
| 1 mo after treatment (n = 16) | 44.40 (39.24) | 31.20 (31.80) | 34.50 (44.00) | 41.50 (50.00) |
| 3 mo after treatment (n = 12) | 38.15 (37.95) | 26.00 (35.80) | 25.00 (55.00) | 39.00 (64.00) |
| P value | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
The maximum depth of ascitic fluid in the TIPS group was significantly smaller at all post-treatment time points than before treatment (P < 0.001; Table 3, Supplementary Figure 2). Indeed, the majority of patients in the TIPS group showed complete resolution of ascites by 2 wk after treatment, and the effect was maintained at 1 month and 2 mo after treatment (Table 3, Supplementary Figure 2). By contrast, there were no changes in maximal ascites depth in the conservative treatment group (Table 3, Supplementary Figure 2). Furthermore, the maximum depth of ascitic fluid was significantly smaller in the TIPS group than in the conservative treatment group at all post-treatment time points (all P < 0.05). Ascites drainage was only considered if the patient has obvious symptoms of abdominal distension. In the TIPS group, after the operation, the ascites subsided, and no patients were treated with ascites drainage. In the conservative treatment group, even if ascites drainage were performed, it is only for symptom relief without managing the cause of ascites.
| Group and time point | Ascites depth (mm) |
| TIPS group | |
| Before treatment (n = 37) | 95 (30) |
| 1 wk after treatment (n = 36) | 35 (35)c |
| 2 wk after treatment (n = 35) | 0 (13)ac |
| 1 mo after treatment (n = 35) | 0 (0)ac |
| 3 mo after treatment (n = 35) | 0 (0)ac |
| P value | < 0.001 |
| Conservative treatment group | |
| Before treatment (n = 17) | 85 (20) |
| 1 wk after treatment (n = 17) | 71 (34) |
| 2 wk after treatment (n = 16) | 69.5 (37) |
| 1 mo after treatment (n = 16) | 56.5 (53) |
| 3 mo after treatment (n = 12) | 31 (74) |
| P value | 0.158 |
Preoperative imaging in both groups suggested a diffuse enlargement of the liver, and plain scans showed an uneven decrease in the density of the liver parenchyma and a large amount of ascites. Enhanced computed tomography scanning demonstrated that the liver parenchyma exhibited characteristic confluent patchy enhancement during the venous and equilibrium phases as well as a high degree of enhancement around the hepatic vein with the characteristic "clover sign"[24]; the lumen of the hepatic vein was stenosed or unclear, and the hepatic segment of the inferior vena cava was compressed and thinned (Figure 2). Imaging investigations performed after TIPS revealed a smaller liver volume than that before surgery, a reduction in ascites, and the disappearance of signs of uneven enhancement (Figure 2). In addition, atrophy of the liver lobe was present on the shunt side, and compensatory enlargement of the liver was noted on the non-shunt side.
Eight patients in the TIPS group underwent percutaneous transhepatic biopsy (n = 5) or transjugular liver biopsy (n = 3) before surgery. Of these eight patients, 6 underwent biopsy again at 1 month after surgery, five underwent repeat biopsy at 6 months after surgery, and two underwent biopsy at 2 years after surgery. Hematoxylin/eosin-stained sections taken before surgery (Supplementary Figure 3) demonstrated hepatocyte swelling with focal degeneration and necrosis, localized abnormalities in the arrangement of hepatic cells, mild vacuolar degeneration, hepatic sinusoidal dilatation, congestion, focal hemorrhage, and a prominent central vein. Scattered and small focal lymphatic infiltration was present in the portal area, and mild proliferation of the small bile duct was seen. Liver congestion and hepatocyte swelling notably improved with time after surgery (Supplementary Figure 3).
Two patients in the conservative treatment group received remedial TIPS 2 wk and 3 wk after ineffective conservative treatment. Table 4 compares the treatment outcomes between the two groups. The TIPS group had a significantly lower incidence of spontaneous peritonitis (P < 0.001), pyemia (P = 0.014), liver failure (P = 0.013), multiple organ failure (P = 0.033) and death (P = 0.001). Two patients in the TIPS group died, and none of them had any complications like iatrogenic hemorrhage or hepatic encephalopathy. One patient with preoperative bilirubin of 380 μmol/L died of liver failure 1 wk after surgery. Another patient with bilirubin at 84 μmol/L before surgery developed a severe pulmonary infection on the third day after surgery and died of respiratory failure.
| Outcome | TIPS group (n = 37) | Conservative treatment group (n = 17) | P value |
| Spontaneous peritonitis (yes/no) | 3/34 | 9/8 | < 0.001 |
| Pyemia (yes/no) | 1/36 | 4/13 | 0.014 |
| Liver failure (yes/no) | 3/34 | 6/11 | 0.013 |
| Gastrointestinal bleeding (yes/no) | 0/37 | 1/16 | 0.136 |
| Multiple organ failure (yes/no) | 0/37 | 2/15 | 0.033 |
| Hepatic encephalopathy (yes/no) | 8/29 | 1/16 | 0.149 |
| Main portal vein thrombosis (yes/no) | 0/37 | 1/16 | 0.136 |
| Intraoperative bleeding (yes/no) | 1/36 | 0/2 | 0.814 |
| Stenosis of shunt channel (yes/no) | 0/37 | 0/2 | NA |
| Attributable deaths (death/survival) | 2/35 | 7/10 | 0.001 |
| Cause of death | |||
| Pyemia (yes/no) | 1/36 | 3/14 | 0.051 |
| Liver failure (yes/no) | 1/36 | 2/15 | 0.177 |
| Gastrointestinal bleeding (yes/no) | 0/37 | 1/16 | 0.136 |
| Multiple organ failure (yes/no) | 0/37 | 2/15 | 0.033 |
Our data provide evidence that TIPS may result in better outcomes than conventional symptomatic therapies in patients with PA-HSOS. However, prospective randomized controlled trials will be needed to verify our findings.
In this study, patients with PA-HSOS who were treated conservatively had a 1-year and 2-year mortality rate of 42.2%, which is similar to the value of approximately 40% reported in the literature for patients receiving conventional symptomatic therapy[7]. By contrast, patients in the TIPS group had a significantly higher survival rate and a significantly longer survival time than those in the conservative treatment group. The preoperative baseline characteristics of the two groups were similar, and the conservative group at baseline did not show more severe conditions. In addition, most of the patients in the conservative group died by 1-6 mo after the conservative treatment, and the main causes of death were infection, liver failure, and multiple organ failure. We consider that this was due to the aggravation of the disease rather than the severity of the underlying disease. Limited evidence is available regarding the use of TIPS in the treatment of HSCT-HSOS, and it might not be appropriate to compare the mortality of PA-HSOS to that of HSCT-HSOS. Nevertheless, a small series of case reports, which mainly included patients with severe disease, described a 1-mo mortality rate of 33.3%-83.3% and a 6-mo mortality rate of 90%-100% after TIPS[18,25,26]. The clinical cure rate reached 64.3%-95.2% in patients with PA-HSOS[7,27]. Although two patients in the present study died within 2 wk after surgery, the remaining patients showed clinical improvement and survived for at least 2 years. Therefore, we believe that patients with PA-HSOS can benefit from decompression of the portal vein. However, it should be noted that the timing of surgery is critical. Careful monitoring of ascites and jaundice should be performed for patients during the conservative treatment stage, and a gradual worsening of ascites and jaundice should prompt timely intervention with TIPS to avoid progression to severe SOS since severe disease is associated with a significant increase in the risk of death (one patient with severe SOS in the TIPS group died of acute liver failure 1 d after surgery). Two of 37 patients in the TIPS group died within 2 wk after surgery (due to liver failure and pyemia, respectively), but all the other patients achieved long-term survival (i.e., at least 2 years). By contrast, 7 of 17 patients in the conservative treatment group died within 6 months after initiation of therapy, and the main causes of death were pyemia and chronic liver failure. Therefore, it is clear that TIPS is beneficial in terms of patient survival. Nevertheless, attention should be given to reducing the risks during the 2-wk postoperative period to further improve patient survival after TIPS.
The effect of TIPS on liver function in patients with PA-HSOS remains unclear. According to previous experience, TIPS can exacerbate liver dysfunction in patients with cirrhotic portal hypertension[28,29]. Therefore, many clinicians are concerned that shunting will further damage the hepatic function of patients with PA-HSOS. However, the results of this study suggest that there was a short-term exacerbation of liver dysfunction at 7-14 d after TIPS, followed by a gradual improvement. Indeed, liver function was better at 1 month after surgery than before TIPS, although the bilirubin level did not decrease to within the normal range. The reasons for these biphasic alterations in liver function remain unclear, but there are several possibilities. During the initial stage (7-14 d after surgery), portal vein blood flow would have been rapidly reduced after shunting. Given that the portal vein would have been the main nutrient vessel of the liver, short-term compensation by the hepatic artery may not have been sufficient to prevent a reduction in blood supply that worsened liver function after TIPS. The trauma of surgery may also have impacted negatively on hepatic function in the short term. During the next stage, three mechanisms may have contributed to a gradual improvement in liver function. First, patients with PA-HSOS have hepatic veno-occlusive disease and hepatic sinusoidal obstruction that lead to obstruction of hepatic arterial flow and reversal of portal vein flow, and this results in hypoxia and dysfunction of hepatocytes. The reduction of hepatic sinusoidal resistance after TIPS would improve hepatic arterial flow and perfusion (which has been demonstrated by ultrasonography) and thereby relieve hepatocyte hypoxia. Second, portal hypertension damages the intestinal mucosal barrier, leading to endotoxemia and aggravation of liver damage[30,31]. The TIPS-induced decrease in portal hypertension would help to restore the normal intestinal microecology, reduce endotoxin levels in the portal vein, and protect liver function. Third, TIPS increases glomerular filtration rate[32] and the clearance of metabolic products; this would reduce the damaging effects of toxic substances on hepatic cells, thus promoting recovery of liver function.
The changes in liver pathology observed after TIPS were consistent with those detected by imaging studies. Preoperative pathological characteristics included remarkable dilatation and congestion of hepatic sinusoids with hepatic acinar zone III predominating, various degrees of hepatocyte swelling and focal hemorrhage, localized disordering of hepatic cell arrangement, focal degeneration and necrosis with a prominent central vein, scattered and small focal lymphatic infiltration of the portal area, and proliferation of the small bile ducts[3,21]. After TIPS, there were improvements in hepatic swelling and congestion over time. However, although TIPS reduced the extent of the liver damage, it did not completely reverse it. In our study, biopsy after 2 years of follow-up suggested that the histopathological structure of the liver had not completely returned to normal, and the imaging findings concurred with the pathology results. Although the characteristic signs of SOS, such as hepatomegaly, perfusion disorder, and uneven enhancement, disappeared after shunting[24], manifestations similar to those after portal vein embolization appeared over time, including a reduction in liver volume on the shunt side and compensatory liver enlargement on the non-shunt side.
This study may have some limitations. First, the study may have been prone to selection bias and information bias. Second, the sample size is small, so the generalizability of the findings is not known. Third, since the methods of taking Gynura segetum varied between patients, the effects of dosage on prognosis could not be accurately evaluated. Fourth, in this study, conservative treatment was undertaken in patients who had failed conservative treatment for 2 wk or who had refused TIPS treatment for ascites and jaundice during treatment. Since the patients with effective conservative treatment were not included in this study, the sample size of the conservative treatment group was small, and there was indeed a bias. Nevertheless, the preoperative baseline level of the two groups was basically the same, which reduced the bias to some extent. Finally, although this study attempted to conduct a complete follow-up, the reexamination times were not completely consistent for all patients, and this may have influenced the results.
In conclusion, this study suggests that TIPS may have advantages over conventional symptomatic therapy in the treatment of PA-HSOS, including better resolution of ascites and longer survival time. Large-scale, multicenter, randomized controlled trials are needed to confirm the findings.
Hepatic sinusoidal obstruction syndrome (HSOS) is a hepatic vascular disease in which edema, necrosis, and shedding of endothelial cells and formation of microthrombi occur in the hepatic sinusoids, hepatic venules, and interlobular vein, leading to intrahepatic congestion, liver dysfunction and portal hypertension
Treatments for HSOS are limited.
This study was to evaluate transjugular intrahepatic portosystemic shunting (TIPS) as a treatment for pyrrolidine alkaloid-related HSOS (PA-HSOS).
This retrospective analysis included patients with PA-HSOS and they were divided into the TIPS and conservative treatment groups.
The TIPS group included 37 patients (28 males), and the conservative treatment group included 17 patients (11 males). Baseline characteristics were similar between groups. There were two deaths in the TIPS group and seven deaths in the conservative treatment group during follow-up (3-48 mo). The 3-, 6-, 12- and 24-mo survival rates were 94.6%, 94.6%, 94.6% and 94.6%, respectively, in the TIPS group and 70.6%, 57.8%, 57.8% and 57.8%, respectively, in the conservative treatment group. Kaplan-Meier analysis revealed significantly longer survival for the TIPS group than for the conservative treatment group (P = 0.001). Compared with the pre-treatment value, maximal ascites depth was significantly lower at 1 wk, 2 wk, 1 mo, and 3 mo for the TIPS group (all P < 0.05) but not in the conservative treatment group. Contrast-enhanced computed tomography demonstrated the disappearance of patchy liver enhancement after TIPS. Pathology showed that liver congestion and hepatocyte swelling improved with time after TIPS placement.
TIPS may achieve better outcomes than conventional symptomatic treatment in patients with PA-HSOS.
Large-scale, multicenter, randomized controlled trials are needed to confirm the findings.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sempokuya T S-Editor: Yan JP L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol. 2014;4:332-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis. 2002;22:27-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 501] [Cited by in F6Publishing: 416] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 3. | Lin G, Wang JY, Li N, Li M, Gao H, Ji Y, Zhang F, Wang H, Zhou Y, Ye Y, Xu HX, Zheng J. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J Hepatol. 2011;54:666-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Wang JY, Gao H. Tusanqi and hepatic sinusoidal obstruction syndrome. J Dig Dis. 2014;15:105-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Gao H, Li N, Wang JY, Zhang SC, Lin G. Definitive diagnosis of hepatic sinusoidal obstruction syndrome induced by pyrrolizidine alkaloids. J Dig Dis. 2012;13:33-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Dalle JH, Giralt SA. Hepatic Veno-Occlusive Disease after Hematopoietic Stem Cell Transplantation: Risk Factors and Stratification, Prophylaxis, and Treatment. Biol Blood Marrow Transplant. 2016;22:400-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Qiao D, Li Y, Xu F. Risk factors for hepatic veno-occlusive disease caused by Gynura segetum: a retrospective study. BMC Gastroenterol. 2018;18:156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Ren XF, Zhuge YZ, Chen SY, Yang L, Jiang HX, Zhang XL, Ma X, Xie WF, Liu YL, Xu JM; Hepatobiliary Cooperative Group of Chinese Society of Gastroenterology. Gynura segetum-related hepatic sinusoidal obstruction syndrome: a national multicenter clinical study. Zhonghua Xiaohua Zazhi. 2017;37:523-529. [Cited in This Article: ] |
| 9. | McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 891] [Cited by in F6Publishing: 800] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 10. | Chao N. How I treat sinusoidal obstruction syndrome. Blood. 2014;123:4023-4026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Kalayoglu-Besisik S, Yenerel MN, Caliskan Y, Ozturk S, Besisik F, Sargin D. Time-related changes in the incidence, severity, and clinical outcome of hepatic veno-occlusive disease in hematopoietic stem cell transplantation patients during the past 10 years. Transplant Proc. 2005;37:2285-2289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Zhu CK, Zhang F, Zhuge YZ, Zhang M, Zhang W, Wang Y, He QB, He J, Yang J, Chen J, Zou XP. Clinical characteristics of 115 cases of gynura segetum induced hepatic sinusoidal obstruction syndrome. Zhonghua Xiaohua Zazhi. 2017;37:448-452. [Cited in This Article: ] |
| 13. | Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, Guinan E, Vogelsang G, Krishnan A, Giralt S, Revta C, Carreau NA, Iacobelli M, Carreras E, Ruutu T, Barbui T, Antin JH, Niederwieser D. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 379] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 14. | Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. Br J Haematol. 2015;168:481-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Imran H, Tleyjeh IM, Zirakzadeh A, Rodriguez V, Khan SP. Use of prophylactic anticoagulation and the risk of hepatic veno-occlusive disease in patients undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. Bone Marrow Transplant. 2006;37:677-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, Veys P, Potter MN; Haemato-oncology Task Force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163:444-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 17. | Richardson PG, Soiffer RJ, Antin JH, Uno H, Jin Z, Kurtzberg J, Martin PL, Steinbach G, Murray KF, Vogelsang GB, Chen AR, Krishnan A, Kernan NA, Avigan DE, Spitzer TR, Shulman HM, Di Salvo DN, Revta C, Warren D, Momtaz P, Bradwin G, Wei LJ, Iacobelli M, McDonald GB, Guinan EC. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant. 2010;16:1005-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Azoulay D, Castaing D, Lemoine A, Hargreaves GM, Bismuth H. Transjugular intrahepatic portosystemic shunt (TIPS) for severe veno-occlusive disease of the liver following bone marrow transplantation. Bone Marrow Transplant. 2000;25:987-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Annaloro C, Robbiolo L, Pozzoli E, Ibatici A, Nicolini A, Salerno F, Deliliers GL. Four-year survival after trans-jugular intrahepatic porto-systemic shunt for veno-occlusive disease following autologous bone marrow transplantation. Leuk Lymphoma. 2004;45:1485-1487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Senzolo M, Riggio O, Primignani M; Italian Association for the Study of the Liver (AISF) ad hoc. Vascular disorders of the liver: recommendations from the Italian Association for the Study of the Liver (AISF) ad hoc committee. Dig Liver Dis. 2011;43:503-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, Arat M, Bader P, Baron F, Bazarbachi A, Blaise D, Ciceri F, Corbacioglu S, Dalle JH, Duarte RF, Fukuda T, Huynh A, Masszi T, Michallet M, Nagler A, NiChonghaile M, Pagluica T, Peters C, Petersen FB, Richardson PG, Ruutu T, Savani BN, Wallhult E, Yakoub-Agha I, Carreras E. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015;50:781-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 22. | Fagiuoli S, Bruno R, Debernardi Venon W, Schepis F, Vizzutti F, Toniutto P, Senzolo M, Caraceni P, Salerno F, Angeli P, Cioni R, Vitale A, Grosso M, De Gasperi A, D'Amico G, Marzano A; AISF TIPS Special Conference. Consensus conference on TIPS management: Techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Zhuge Y, Liu Y, Xie W, Zou X, Xu J, Wang J; Chinese Society of Gastroenterology Committee of Hepatobiliary Disease. Expert consensus on the clinical management of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome. J Gastroenterol Hepatol. 2019;34:634-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 24. | Zhou H, Wang YX, Lou HY, Xu XJ, Zhang MM. Hepatic sinusoidal obstruction syndrome caused by herbal medicine: CT and MRI features. Korean J Radiol. 2014;15:218-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Fried MW, Connaghan DG, Sharma S, Martin LG, Devine S, Holland K, Zuckerman A, Kaufman S, Wingard J, Boyer TD. Transjugular intrahepatic portosystemic shunt for the management of severe venoocclusive disease following bone marrow transplantation. Hepatology. 1996;24:588-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Zenz T, Rössle M, Bertz H, Siegerstetter V, Ochs A, Finke J. Severe veno-occlusive disease after allogeneic bone marrow or peripheral stem cell transplantation--role of transjugular intrahepatic portosystemic shunt (TIPS). Liver. 2001;21:31-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Zhuge YZ, Zhang F, Zhang M, Zhang W, He QB, Zou XP. Treatment for Gynura segetum caused hepatic vein occlusive disease: a single-center retrospective study. Zhonghua Xiaohua Zazhi. 2016;36:811-815. [Cited in This Article: ] |
| 28. | Ginès P, Uriz J, Calahorra B, Garcia-Tsao G, Kamath PS, Del Arbol LR, Planas R, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839-1847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 343] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 29. | Itkin M, Trerotola SO, Stavropoulos SW, Patel A, Mondschein JI, Soulen MC, Tuite CM, Shlansky-Goldberg RD, Faust TW, Reddy KR, Solomon JA, Clark TW. Portal flow and arterioportal shunting after transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol. 2006;17:55-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Fukui H. Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World J Hepatol. 2015;7:425-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 31. | Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005;54:556-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 32. | Rössle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59:988-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |