Published online May 28, 2020. doi: 10.3748/wjg.v26.i20.2669
Peer-review started: January 27, 2020
First decision: March 6, 2020
Revised: March 26, 2020
Accepted: May 15, 2020
Article in press: May 15, 2020
Published online: May 28, 2020
Non-alcoholic fatty liver disease (NAFLD) is an emerging liver disease and currently the most common cause of incidental abnormal liver tests. The pathogenesis of NAFLD is multifactorial and many mechanisms that cause fatty liver infiltration, inflammation, oxidative stress and progressive fibrosis have been proposed. Obstructive sleep apnea (OSA) may be linked with the pathogenesis and the severity of NAFLD.
To study the association between NAFLD and OSA considering also the efficacy of continuous positive airway pressure (CPAP) treatment.
A PubMed search was conducted using the terms “non-alcoholic fatty liver disease AND (obstructive sleep apnea OR obstructive sleep disorders OR sleep apnea)”. Research was limited to title/abstract of articles published in English in the last 5 years; animal and child studies, case reports, commentaries, letters, editorials and meeting abstracts were not considered. Data were extracted on a standardized data collection table which included: First author, publication year, country, study design, number of patients involved, diagnosis and severity of OSA, diagnosis of NAFLD, patient characteristics, results of the study.
In total, 132 articles were initially retrieved on PubMed search and 77 in the last five years. After removal of irrelevant studies, 13 articles were included in the qualitative analysis. There was a total of 2753 participants across all the studies with a mean age between 42 and 58 years. The proportion of males ranged from 21% to 87.9% and the mean body mass index ranged from 24.0 to 49.9 kg/m2. The results of this review showed an increased prevalence of NAFLD in patients with diagnosis of OSA, even in the absence of coexisting comorbidities such as obesity or metabolic syndrome. Furthermore, the severity of NAFLD is associated with the increase in OSA severity. Effective CPAP treatment, although not always decisive, may stabilize or slow NAFLD progression with benefits on metabolic and cardiovascular functions.
In NAFLD patients, although asymptomatic, it is recommended to systematically perform polysomnography in order to early and better treat them before the development of potentially life threatening systemic dysfunctions.
Core tip: The development of non-alcoholic fatty liver disease (NAFLD) seems to be closely associated with obstructive sleep apnea (OSA) even in the absence of coexisting comorbidities such as obesity or metabolic syndrome. Furthermore, the severity of NAFLD is associated with the increase in OSA severity. Effective continuous positive airway pressure therapy for OSA may improve serum aminotransferase levels and liver steatosis. As clinicians, our aim should be to screen OSA patients for NAFLD and vice versa those with NAFLD for OSA in order to early and better treat them before the development of potentially life threatening systemic dysfunctions.
- Citation: Umbro I, Fabiani V, Fabiani M, Angelico F, Del Ben M. Association between non-alcoholic fatty liver disease and obstructive sleep apnea. World J Gastroenterol 2020; 26(20): 2669-2681
- URL: https://www.wjgnet.com/1007-9327/full/v26/i20/2669.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i20.2669
Non-alcoholic fatty liver disease (NAFLD) is an emerging liver disease in Western countries[1,2] and currently the most common cause of incidental abnormal liver tests. Fatty liver includes a wide spectrum of histologic alterations. Simple steatosis generally represents a benign condition following a non-progressive clinical course. On the contrary, a subset of patients with non-alcoholic steatohepatitis (NASH), in particular those with a more severe fibrosis, are at higher risk for progressing to liver disease complications such as decompensated cirrhosis, liver cancer, and liver mortality[3]. NASH is projected to eventually overtake the hepatitis C virus and alcoholic liver disease as the leading cause of liver transplant[4].
However, the association of liver steatosis with a number of common metabolic conditions and cardiovascular risk factors has been also extensively reported. Indeed, it appears that in NAFLD the increased mortality of patients is primarily a result of cardiovascular diseases and, to a lesser extent, to liver related diseases[5,6]. In fact, patients with NAFLD show early signs of atherosclerosis, such as increased carotid artery intima-media thickness[7], coronary artery calcification[8] and endothelial dysfunction[9].
The pathogenesis of NAFLD is multifactorial and many mechanisms that cause fatty liver infiltration, inflammation, oxidative stress and progressive fibrosis have been proposed. Insulin resistance, the key feature of the metabolic syndrome (MetS), is considered to play a central role in the first stages of fatty liver infiltration[10,11]. However, whether insulin resistance and hyperinsulinemia are components of MetS promoting fatty liver or whether NAFLD itself induces chronic hyperinsulinemia by impaired insulin degradation is still under debate. Chronic oxidative stress is a major player triggering the progression of simple steatosis to NASH as the result of an imbalance between pro-oxidant and anti-oxidant chemicals that lead to liver cell damage[12,13].
Finally, several lines of evidence clearly indicated that also genetic factors may predispose to NAFLD and among the others a variant located at the PNPLA3 gene (I148M) appears to show the strongest effect[14,15].
Obstructive sleep apnea (OSA) is a breathing disorder characterized by narrowing of the upper airway during sleep which compromise the normal ventilation[16]. The most common symptoms of OSA are excessive daytime sleepiness, fragmented sleep, snoring, fatigue and impairments in cognitive functions[17,18].
The prevalence of OSA is estimated to be 4% in the general population increasing up to 40% in some disease-specific populations, such as in patients suffering from metabolic syndrome[19], obesity[20], diabetes mellitus[21], arterial hypertension[22], cardiovascular disease[23], chronic kidney disease[24] and non-alcoholic fatty liver disease[25]. Furthermore, the prevalence of OSA increases with age, race and world region[26].
In these clinical settings, OSA is still underdiagnosed; probably the atypical presentation, the lack of data on the criteria for identifying the disorder and the lack of awareness of this entity among clinicians are important reasons.
The polysomnography (PSG) is the gold standard for the diagnosis of OSA[16]. The severity of OSA is defined by an apnea-hypopnea index (AHI) ≥ 5 and < 15 events/h as mild, ≥ 15 and < 30 events/h as moderate, and ≥ 30 events/h as severe[27].
Since continuous positive airway pressure (CPAP) can eliminate upper airway narrowing during sleep improving sleep fragmentation, daytime symptoms and quality of life[28,29], it remains the gold standard treatment for the clinical management of OSA.
OSA and chronic intermittent hypoxia may be linked with the pathogenesis and the severity of NAFLD[30]. Several studies indicate that OSA is a well-established independent factor of insulin resistance, which may predispose to the development and the progression of liver steatosis[31-33]. However, to clarify the independent effects of OSA on the development and progression of NAFLD in literature data is challenging due to the numerous cardiovascular and metabolic comorbidities which often coexist.
The aim of this review is to provide a more comprehensive overview of the association between NAFLD and OSA considering also the efficacy of CPAP treatment.
A PubMed search was conducted using the terms “non-alcoholic fatty liver disease AND (obstructive sleep apnea OR obstructive sleep disorders OR sleep apnea)”. Research was limited to title/abstract of articles published in English in the last 5 years; animal and child studies, case reports, commentaries, letters, editorials and meeting abstracts were not considered. Review articles were examined to identify studies that were potentially eligible for inclusion.
Only potentially relevant studies underwent full-text review. Data were extracted on a standardized data collection table which included: First author, publication year, country, study design, number of patients involved, diagnosis and severity of OSA, diagnosis of NAFLD, patient characteristics, results of the study.
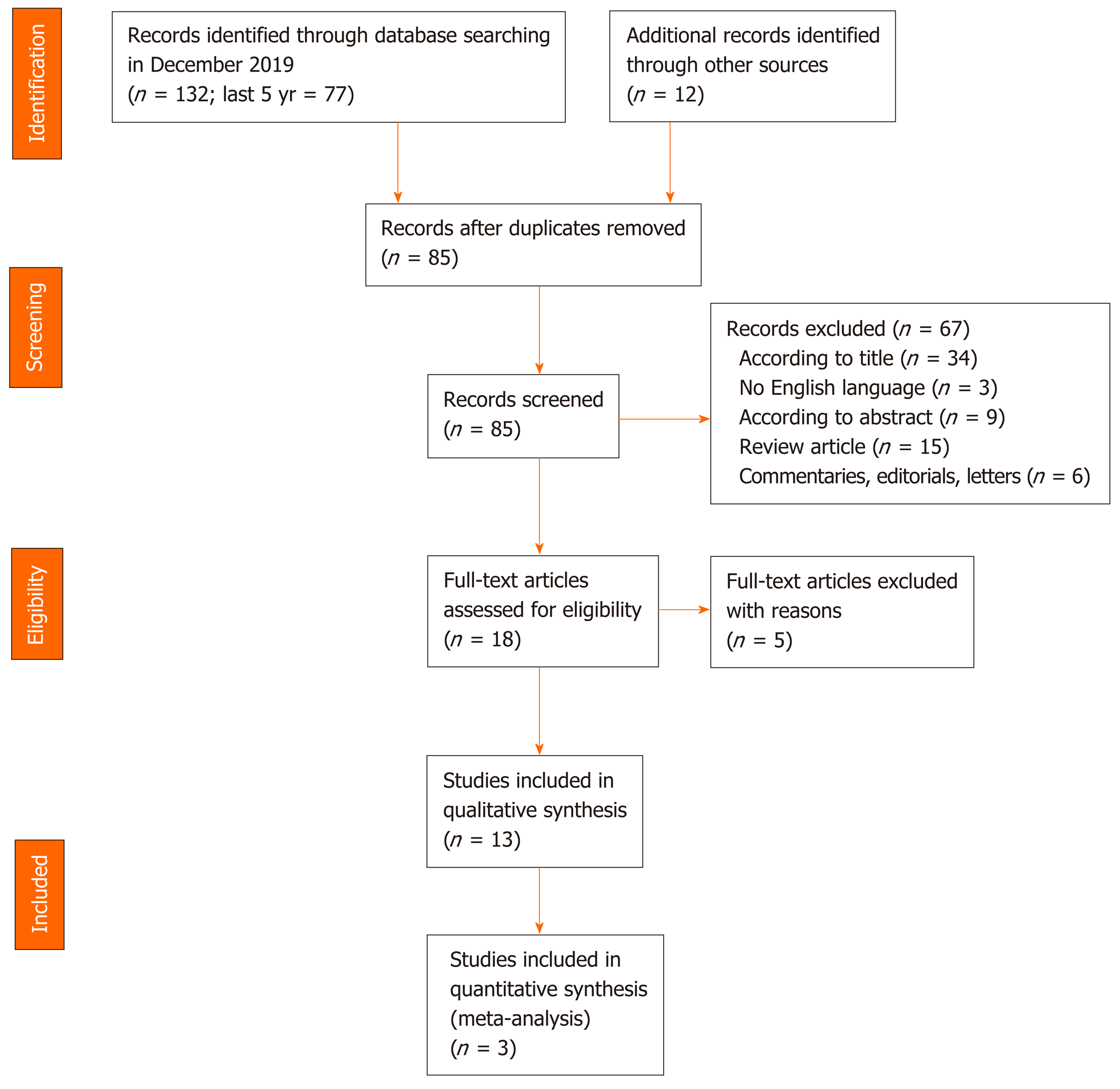
A flow chart of the search for relevant studies is presented in Figure 1. In total, 132 articles were initially retrieved on PubMed search and 77 in the last five years. After removal of irrelevant studies and exclusion according to title, language and abstract (n = 67), 18 articles were selected for full-text review. A further 5 articles were excluded for the following reasons: 1 did not have a clear description of OSA diagnosis, 2 had an inaccurate diagnosis of OSA and 2 did not have a clear description of patients enrollment and evaluation. Finally, 13 articles were included in the qualitative analysis.
A summary of the 13 relevant studies is reported in Table 1. There was a total of 2753 participants across all the studies with a mean age between 42 and 58 years. The proportion of males ranged from 21% to 87.9% and the mean body mass index (BMI) ranged from 24.0 to 49.9 kg/m2.
| Ref. | Study design | Number of patients | Diagnosis and severity of OSA | Diagnosis of NAFLD | Patient characteristics | Results |
| Agrawal[49], 2015 (India) | Prospective | 23 (3 mild OSA, 5 mode rate OSA, 15 severe OSA) | - No OSA, AHI < 5; | Abdominal ultrasound | Consecutive patients with diagnosis of OSA and abdominal obesity | - The prevalence of NAFLD in patients with OSA was 91.3% |
| - AHI was an independent predictor of significant fibrosis | ||||||
| - Mild OSA, 5-14.9; | ||||||
| - No differences in the prevalence of NAFLD, raised transaminase levels and fibrosis according to the severity of OSA | ||||||
| - Moderate OSA, 15-30; | Mean age: 46; Mean BMI: 32.2; Males: 78% | |||||
| - Severe OSA, > 30 | ||||||
| Cakmak[51], 2015 (Turkey) | Retrospective | 137 (118 OSA: -19 mild, - 39 moderate, - 60 severe, 19 no OSA) | - No OSA, AHI < 5; | Abdominal ultrasound | All consecutive patients referred to a sleep laboratory due to sleep apnea symptoms | - Severity of NAFLD increased as AHI increased and lowest SpO2, mean nocturnal SpO2 levels decreased |
| - There was a strong association between NAFLD severity and a decrease in lowest SpO2 levels | ||||||
| - Mild OSA, 5-14; | ||||||
| - Moderate OSA, 15-29; | Mean age: 55.7; Mean BMI: 34.5 (OSA), 33.2 (no OSA); Males: 44.5% | |||||
| - Strong association between elevated liver enzymes and increase in nocturnal hypoxia severity in OSA patients | ||||||
| - Severe OSA, ≥ 30 | ||||||
| Petta[34], 2015 (Italy) | Cross-sectional | 50 (25 OSA, 25 no OSA) | - No OSA, AHI < 5; | Liver biopsy | Consecutive patients with biopsy-proven NAFLD who underwent cardio-respiratory polygraphy | - Significant fibrosis was independently associated with mean nocturnal oxygen saturation < 95% in patients with NAFLD and OSA |
| - OSA, AHI ≥ 5 | ||||||
| Mean age: 53; Mean BMI: 33.5 (OSA), 29.0 (no OSA); Males: 58% | ||||||
| Yu[36], 2015 (South Korea) | Cross-sectional | 621 (286 OSA, 335 no OSA) | - No OSA, AHI < 5; | Abdominal CT scan | Subjects who examined the PSG and abdominal CT | - Patients with OSA were significantly older and had significantly higher BMI than those without OSA |
| - The prevalence of NAFLD was 34% among patients with OSA and 21% among patients without OSA | ||||||
| Mean age: 56.6; Mean BMI: 24.7; Males: 57.2% | ||||||
| - OSA, AHI ≥ 5 | ||||||
| - Association between OSA and NAFLD independent of the visceral fat level in relatively lean individuals | ||||||
| - This association was particularly strong in participants with excessive daytime sleepiness or short sleep duration regardless of visceral fat level | ||||||
| Arısoy[41], 2016 (Turkey) | Case-control | 176 (52 mild, 34 moderate, 48 severe, 42 no OSA) | - No OSA, AHI < 5; | Abdominal ultrasound | Subjects referred to a sleep center with clinical suspicion of OSA | - Hepatosteatosis grade, ALT and AST levels, BMI differed significantly among the groups |
| - Mild OSA, 5-14; | - BMI and hepatosteatosis grade increased progressively and significantly from no OSA to severe OSA | |||||
| Mean age: 45.1 (no OSA), 42.9 (mild), 47.6 (moderate), 47.0 (severe); Mean BMI: 28.3 (no OSA), 30.1 (mild), 34.1 (moderate), 32.7 (severe); Males: 73.9% | ||||||
| - Moderate OSA, 15-29; | - Average desaturation and BMI were the parameters with the greatest independent effects on hepatosteatosis in the subjects with OSA | |||||
| - Severe OSA, ≥ 30 | ||||||
| Benotti[40], 2016 (United States) | Retrospective | 362 (115 mild, 80 moderate, 74 severe, 93 no OSA) | - No OSA, AHI < 5; | Liver biopsy | Bariatric surgery candidates with clinical suspicion of OSA | - OSA severity was associated with NAFLD liver histology only in patients without metabolic syndrome |
| - Mild OSA, 5-14; | ||||||
| Mean age: 46.2; Mean BMI: 49.9; Males: 21% | ||||||
| - Moderate OSA, 15-29; | ||||||
| - Severe OSA, ≥ 30 | ||||||
| Buttacavoli[35], 2016 (Italy) | Observational | 15 | - Severe OSA, AHI ≥ 30 | Abdominal ultrasound and elastography | Consecutive severe OSA patients at baseline and after 6-12 mo of CPAP treatment | - Most patients at diagnosis had severe liver steatosis (87%) |
| - During follow-up, steatosis significantly improved in six patients without concurrent changes in the BMI range in the entire sample | ||||||
| - No correlation was found between steatosis score and BMI at baseline, although a positive relationship between these variables was evident during CPAP treatment | ||||||
| Mean age: 49.3; Mean BMI: 35.4; Males: 86.7% | ||||||
| Chen[37], 2016 (China) | Cross-sectional | 319 (Group 1: 187 OSA with FLI < 60; Group 2: 132 OSA with FLI ≥ 60) | - No OSA, AHI < 5; | Fatty liver index (FLI) ≥ 60 | All consecutive patients referred to a sleep center and diagnosed with OSA | - Participants with a FLI ≥ 60 tended to be significantly fatter and had higher transaminase levels and severe PSG parameters of sleep apnea |
| - Mild OSA, 5-14.9; | ||||||
| Mean age: 46.8 (Group 1), 42.3 (Group 2); Mean BMI: 24.5(Group 1), 28.5 (Group 2); Males: 79% | ||||||
| - Moderate OSA, 15-30; | ||||||
| - Severity of OSA was independently associated with prevalence of NAFLD (52.1% in patients with AHI ≥ 15 vs. 20.4% in patients with AHI < 15) | ||||||
| - Severe OSA, > 30 | ||||||
| Qi[50], 2016 (China) | Cross-sectional | 175 (149 OSA: - 96 NAFLD, - 53 no NAFLD, 26 no OSA: - 10 NAFLD, - 16 no NAFLD) | - No OSA, AHI < 5; | Abdominal ultrasound | All consecutive non-obese patients referred to a sleep laboratory with clinical suspicion of OSA | - Prevalence of NAFLD in OSA patients was 64% |
| - BMI, lowest SpO2, and triglycerides may be risk factors for promoting NAFLD in OSA patients | ||||||
| - Mild OSA, 5-14.9; | ||||||
| Mean age: 52.9 (OSA and NAFLD); Mean BMI: 24.0; Males: 87.9% (OSA), 77.3% (no OSA) | ||||||
| - Moderate OSA, 15-29.9; | ||||||
| - Severe OSA, > 30 | ||||||
| Chen[52], 2018 (China) | Observational | 160 (42 moderate OSA, 88 severe OSA, 30 controls) | - No OSA, AHI < 5; | Abdominal ultrasound | All consecutive patients referred to a sleep laboratory with clinical suspicion of OSA | - Prevalence of liver steatosis was 64% among the groups; 59.5% and 81.8% in patients with moderate and severe OSA respectively |
| - Moderate OSA, 5-30; | ||||||
| - Increasing OSA severity was associated with higher BMI, waist circumference and neck circumference | ||||||
| Mean age: 42.6; Mean BMI: 28.0; Males: 86.9% | ||||||
| - Severe OSA, ≥ 30 | ||||||
| - ALT, AST and liver steatosis score increased significantly with an increase in OSA severity | ||||||
| - OSA severity was independently associated with liver steatosis and elevation of serum aminotransferases, but not with liver fibrosis | ||||||
| - Serum aminotransferase, as a biomarker of liver injury, decreased in OSA patients after 3 months of CPAP treatment | ||||||
| Kim[38], 2018 (United States) | Retrospective | 351 (73 mild OSA, 102 moderate OSA, 176 severe OSA) | - No OSA, AHI < 5; | Suspected NAFLD was diagnosed if serum ALT > 30 U/L for men and > 19 U/L for women; Advanced fibrosis was identified by the AST to platelet ratio index (APRI) score | CPAP-treated OSA adult patients who had available serum ALT data before (within 3 months) and after (within 6 months) CPAP treatment | - The prevalence of suspected NAFLD was higher (90.3%) among patients with moderate to severe OSA versus among those with mild OSA (86.3%) |
| - Mild OSA, 5-14.9; | ||||||
| - Fibrosis was correlated with OSA severity (7.6% for mild OSA versus 12.0% moderate OSA versus 19.7% for severe OSA) | ||||||
| - Moderate OSA, 15-30; | ||||||
| Mean age: 57.6; Mean BMI: 32.2; Males: 59.3% | ||||||
| - There was a dose-response relationship between OSA severity and improvement in ALT and AST levels and APRI score after CPAP treatment, correlating with adherence status and without differences in the obesity severity status | ||||||
| - Severe OSA, > 30 | ||||||
| Trzepizur[39], 2018 (France) | Cross-sectional | 124 (34 mild, 38 moderate, 52 severe) | - No OSA, AHI < 5; | Elastography | Patients with at least one criterion for metabolic syndrome with diagnosis of OSA | - Prevalence of advanced liver fibrosis was 12% |
| - Increasing OSA severity was associated with BMI, waist circumference, ODI, percentage of sleep time with SpO2 < 90% and higher proportions of male patients with metabolic syndrome | ||||||
| - Mild OSA, 5-14.9; | ||||||
| Mean age: 52.4; Mean BMI: 29.9; Males: 65.6% | ||||||
| - Moderate OSA, 15-29.9; | ||||||
| - Increasing OSA severity was also associated with higher LSM values with a marked increase between mild-to-moderate OSA and severe OSA | ||||||
| - Severe OSA, ≥ 30 | ||||||
| - Patients with severe OSA and metabolic comorbidities are at higher risk of significant liver disease (LSM ≥ 7.3 kPa) and advanced liver fibrosis (LSM ≥ 9.6 kPa) | ||||||
| - AHI and ODI were the factors with the strongest independent association with LSM | ||||||
| Bhatt[42], 2019 (India) | Case-control | 240 (124 OSA and NAFLD, 47 OSA without NAFLD, 44 NAFLD without OSA, 25 no OSA and no NAFLD) | - No OSA, AHI < 5; | Abdominal ultrasound | Overweight/obese subjects (BMI > 23 kg/m2) | - Mean values of AST, ALT and BMI were significantly higher in OSA with NAFLD group as compared to the other groups |
| - Inflammatory markers showed a significant correlation in the OSA and NAFLD group | ||||||
| Mean age: 44.8 (OSA and NAFLD); Mean BMI: 33.3 (OSA and NAFLD); Males: 55.0% | ||||||
| - OSA, AHI ≥ 5 | ||||||
| - OSA and NAFLD operate as an independent contributors to the increased systemic inflammation that occurs in overweight/obese subjects |
All the studies used PSG and AHI to diagnose OSA, according to the American Academy of Sleep Medicine (AASM) Clinical Practice Guideline[16], except for two that used cardio-respiratory polygraphy[34,35]. In 7 studies NAFLD was diagnosed by abdominal ultrasound, in 4 studies by abdominal computed tomography[36], fatty liver index (FLI)[37], aspartate aminotransferase (AST) to platelet ratio index (APRI)[38] and elastography[39], whereas only two studies used the gold standard liver biopsy[34,40].
The exclusion criteria considered in the relevant studies were as follows: Patients who had been previously diagnosed with or treated for OSA, patients with other sleep disorders or other chronic liver disease besides NAFLD; patients who were infected with hepatitis B and/or C virus; patients with excessive alcohol consumption; patients with current use of hepatotoxic drugs; patients who had any acute or chronic inflammatory disease, coronary heart disease, chronic obstructive pulmonary disease, and/or any solid organ failure or transplantation. Furthermore, diabetes mellitus represented an exclusion criterion in three studies[39,41,42].
The results of this review showed an increased prevalence of NAFLD in patients with diagnosis of OSA. Therefore, hypoxia should be considered to have a key role in the pathogenesis of NAFLD.
The pathogenesis of NAFLD is commonly described as a two-hit model. The first hit is characterized by an increased intrahepatocytes triglyceride accumulation from adipose tissue lipolysis due to obesity and insulin resistance. The second hit is characterized by lipotoxic metabolite production, liver inflammation and steatosis progression due to oxidative stress, lipid peroxidation, mitochondrial dysfunction and some gene polymorphisms[43,44]. In OSA hypoxic environment, there is an increased adipose tissue lipolysis, oxidative stress, inflammation and liver fibrosis[45].
OSA is a well-established risk factor for hypertension, renal failure, obesity, insulin resistance, diabetes mellitus, MetS, liver steatosis and cardiovascular diseases[31-33,46,47]. However, to clarify the effects of OSA on the development and progression of NAFLD is challenging due to the several comorbidities which common coexist and are independently associated with systemic inflammation[48].
Bhatt et al[42] reported significantly higher levels of interleukin-6, leptin, macrophage migration inhibitory factor, high-sensitive C-reactive protein and tumor necrosis factor alpha, and significantly lower serum adiponectin levels in obese patients with OSA and NAFLD compared to the other groups, as a consequence of nocturnal hypoxia. All these inflammatory biomarkers seem to have an important pathophysiological role in the development of early metabolic and cardiovascular dysfunctions. Therefore, NAFLD represents an additional risk for systemic inflammation in patients with OSA. Furthermore, Agrawal et al[49] described a prevalence of 91.3% of NAFLD in a small group of patients with OSA and abdominal obesity whereas Qi et al[50] found a prevalence of 64% in 149 non-obese OSA patients.
Furthermore, the results of this review showed that the association between OSA and NAFLD seems to be independent of coexisting comorbidities such as visceral fat or MetS. Yu et al[36] showed an association between OSA and NAFLD independently from visceral fat level in subjects with mean BMI of 24.7 kg/m2, particularly in those with short sleep duration or excessive daytime sleepiness. Benotti et al[40] reported that, in patients with OSA without MetS, as the severity of AHI and hypoxia increased, the prevalence of more severe NAFLD significantly increased as well. However, the exact mechanisms involved in this association in the absence of visceral fat and MetS is still unclear. Certainly the effects of chronic intermittent hypoxia on liver may involve increased lipogenesis, formation of reactive oxygen species and proinflammatory cytokines which cause lipid peroxidation and hepatocyte injury[45]. Therefore, lipid metabolism, inflammation and OSA hypoxic environment may be of key importance in reducing the risk of NAFLD in OSA patients.
Another important result of this review is that the severity of NAFLD is associated with the increase in OSA severity. Cakmak et al[51] found a significant association between the increase in NAFLD development and severity and the lowest oxygen saturation. Similarly, Petta et al[34] showed an association between the severity of liver damage with high risk of OSA and lower oxygen saturation. Arisoy et al[41] observed that BMI and hepatosteatosis grade progressively and significantly increased from patients without OSA to those with severe OSA. Chen et al[37] found a positive association between the severity of OSA and NAFLD. In particular, the prevalence of NAFLD was 20.4% in patients with AHI < 15 whereas it reached 52.1% in patients with AHI ≥ 15. Trzepizur et al[39] demonstrated an association between increasing OSA severity and liver fibrosis; patients with severe OSA and metabolic comorbidities are at higher risk of significant liver disease and advanced liver fibrosis.
The gold standard for the clinical management of OSA is CPAP treatment. Effective CPAP therapy for OSA may improve AST/alanine aminotransferase (ALT) levels[38,52] and liver steatosis[35]. Chen et al[52] showed a statistically significant increase in liver steatosis and serum aminotransferases with increasing OSA severity, and a significant decrease in both ALT and AST levels just after 3 mo of CPAP treatment. Kim et al[38] showed a favorable dose-response association between the severity of OSA and the improvement in serum aminotransferase levels and the regression of hepatic fibrosis after 6 mo of CPAP treatment; these findings correlated with the degree of adherence and were independent from the severity of obesity. Buttacavoli et al[35] described a significant improvement in hepatic steatosis after 6-12 mo of therapy with CPAP. Since in these studies the treatment with CPAP was relatively short, it was difficult to state definite and clear conclusions. However, some other longitudinal studies showed that 1 to 3 years CPAP therapy improved and reversed liver steatosis[35,53].
In conclusion, the development of NAFLD seems to be closely associated with OSA even in the absence of coexisting comorbidities such as obesity or MetS. These findings suggest that even relatively lean patients with OSA should be referred to hepatologists for specific management. As clinicians, our aim should be to screen OSA patients for NAFLD and vice versa those with NAFLD for OSA. Therefore, it is of great importance to set up a strong collaboration between gastroenterology and sleep medicine, in which internal medicine, cardiology and nephrology should have a key role. Furthermore, in NAFLD patients, although asymptomatic, it is recommended to systematically perform PSG in order to early and better treat them before the development of potentially life threatening systemic dysfunctions. Effective CPAP treatment, although not always decisive, may stabilize or slow NAFLD progression with benefits on metabolic and cardiovascular functions.
The pathogenesis of non-alcoholic fatty liver disease (NAFLD) is multifactorial and is commonly described as a two-hit model. The first hit is characterized by an increased triglyceride accumulation in the hepatocytes due to obesity and insulin resistance. The second hit is characterized by lipotoxic metabolite production, liver inflammation and steatosis progression due to oxidative stress, lipid peroxidation, mitochondrial dysfunction and some gene polymorphisms. In obstructive sleep apnea (OSA) hypoxic environment, there is an increased adipose tissue lipolysis, oxidative stress, inflammation and liver fibrosis. OSA is a well-established independent factor of insulin resistance, which may predispose to the development and the progression of liver steatosis. However, to clarify the effects of OSA on the development and progression of NAFLD is challenging due to the several comorbidities which common coexist and are independently associated with systemic inflammation.
NAFLD is an emerging liver disease. The increased mortality of patients with NAFLD is primarily a result of cardiovascular diseases and, to a lesser extent, to liver related diseases. OSA is still underdiagnosed; its prevalence is estimated to be 4% in the general population increasing up to 40% in some disease-specific populations, such as in patients suffering from cardiovascular disease or metabolic syndrome. Probably the atypical presentation, the lack of data on the criteria for identifying OSA and the lack of awareness of this entity among clinicians are important reasons. Since OSA may be linked with the pathogenesis and the severity of NAFLD, it is very important to early and better diagnose and treat OSA in NAFLD patients, in which numerous cardiovascular and metabolic comorbidities often coexist.
The aim of this systematic review is to provide a more comprehensive overview of the association between NAFLD and OSA considering also the efficacy of the gold standard treatment for the clinical management of OSA, the continuous positive airway pressure (CPAP) treatment.
A PubMed search limited to the last 5 years was conducted using the terms “non-alcoholic fatty liver disease AND (obstructive sleep apnea OR obstructive sleep disorders OR sleep apnea)”. We did not consider animal and child studies, case reports, commentaries, letters, editorials and meeting abstracts.
Initially, a total of 132 articles were retrieved on PubMed search and 77 in the last 5 years. After removal of irrelevant studies, 13 articles were included in the qualitative analysis. 2753 participants with a mean age between 42 and 58 years were included across all the studies. The proportion of males ranged from 21% to 87.9% and the mean body mass index ranged from 24.0 to 49.9 kg/m2. The results of this systematic review showed an increased prevalence of NAFLD in patients with OSA, even in the absence of coexisting comorbidities such as obesity or metabolic syndrome. Furthermore, the severity of NAFLD is associated with the increase in OSA severity. Effective CPAP treatment may stabilize or slow NAFLD progression with benefits on metabolic and cardiovascular functions.
NAFLD seems to be closely associated with OSA even in the absence of coexisting comorbidities such as obesity or MetS. Hypoxia should be considered to have a key role in the pathogenesis of NAFLD. Therefore, all OSA patients, even relatively lean, should be referred to hepatologists for specific management and all NAFLD patients, even if asymptomatic, should be screened for OSA. Effective CPAP treatment, although not always decisive, may stabilize or slow NAFLD progression with benefits on metabolic and cardiovascular functions. The systematic use of polysomnography in NAFLD patients, although asymptomatic, will help clinicians to early diagnose OSA and better treat it before the development of potentially life threatening systemic dysfunctions.
The association between NAFLD and OSA has been reviewed. A strong collaboration between gastroenterology and sleep medicine will have a key role in the management of these two conditions. Future research is needed to validate the efficacy of CPAP treatment on liver steatosis with longer longitudinal studies.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Corresponding Author's Membership in Professional Societies: European Kidney Transplant Association; European Liver and Intestine Transplant Association; Ethical Legal and Psychosocial Aspects of organ Transplantation; European Renal Association – European Dialysis and Transplant Association; European Society for Organ Transplantation; Italian Society of Internal Medicine; Italian Society of Nephrology; Italian Society of Organ Transplantation; and Young Professionals in Transplantation.
P-Reviewer: Athyros V, Tanaka N, Yoshioka K S-Editor: Zhang H L-Editor: A E-Editor: Zhang YL
| 1. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3655] [Cited by in F6Publishing: 3609] [Article Influence: 164.0] [Reference Citation Analysis (2)] |
| 2. | Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063-2072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 671] [Cited by in F6Publishing: 760] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 3. | Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis. 2010;28:162-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1211] [Cited by in F6Publishing: 1259] [Article Influence: 139.9] [Reference Citation Analysis (1)] |
| 5. | Del Ben M, Baratta F, Polimeni L, Angelico F. Non-alcoholic fatty liver disease and cardiovascular disease: epidemiological, clinical and pathophysiological evidences. Intern Emerg Med. 2012;7 Suppl 3:S291-S296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Baratta F, Pastori D, Angelico F, Balla A, Paganini AM, Cocomello N, Ferro D, Violi F, Sanyal AJ, Del Ben M. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol. 2019; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 7. | Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 289] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 8. | Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, Kim YJ, Yoon JH, Jeong SH, Lee DH, Lee HS, Larson J, Therneau TM, Kim WR. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | Pastori D, Loffredo L, Perri L, Baratta F, Scardella L, Polimeni L, Pani A, Brancorsini M, Albanese F, Catasca E, Del Ben M, Violi F, Angelico F. Relation of nonalcoholic fatty liver disease and Framingham Risk Score to flow-mediated dilation in patients with cardiometabolic risk factors. Am J Cardiol. 2015;115:1402-1406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Angelico F, Del Ben M, Conti R, Francioso S, Feole K, Maccioni D, Antonini TM, Alessandri C. Non-alcoholic fatty liver syndrome: a hepatic consequence of common metabolic diseases. J Gastroenterol Hepatol. 2003;18:588-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Angelico F, Del Ben M, Conti R, Francioso S, Feole K, Fiorello S, Cavallo MG, Zalunardo B, Lirussi F, Alessandri C, Violi F. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2005;90:1578-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Polimeni L, Del Ben M, Baratta F, Perri L, Albanese F, Pastori D, Violi F, Angelico F. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J Hepatol. 2015;7:1325-1336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 13. | Del Ben M, Polimeni L, Carnevale R, Bartimoccia S, Nocella C, Baratta F, Loffredo L, Pignatelli P, Violi F, Angelico F. NOX2-generated oxidative stress is associated with severity of ultrasound liver steatosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2014;14:81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Carpino G, Pastori D, Baratta F, Overi D, Labbadia G, Polimeni L, Di Costanzo A, Pannitteri G, Carnevale R, Del Ben M, Arca M, Violi F, Angelico F, Gaudio E. PNPLA3 variant and portal/periportal histological pattern in patients with biopsy-proven non-alcoholic fatty liver disease: a possible role for oxidative stress. Sci Rep. 2017;7:15756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Del Ben M, Polimeni L, Brancorsini M, Di Costanzo A, D'Erasmo L, Baratta F, Loffredo L, Pastori D, Pignatelli P, Violi F, Arca M, Angelico F. Non-alcoholic fatty liver disease, metabolic syndrome and patatin-like phospholipase domain-containing protein3 gene variants. Eur J Intern Med. 2014;25:566-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1074] [Cited by in F6Publishing: 1379] [Article Influence: 197.0] [Reference Citation Analysis (0)] |
| 17. | Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6643] [Cited by in F6Publishing: 6179] [Article Influence: 199.3] [Reference Citation Analysis (0)] |
| 18. |
Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research.
The Report of an American Academy of Sleep MedicineTask Force. |
| 19. | Calvin AD, Albuquerque FN, Lopez-Jimenez F, Somers VK. Obstructive sleep apnea, inflammation, and the metabolic syndrome. Metab Syndr Relat Disord. 2009;7:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62:569-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 476] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 21. | Nagayoshi M, Punjabi NM, Selvin E, Pankow JS, Shahar E, Iso H, Folsom AR, Lutsey PL. Obstructive sleep apnea and incident type 2 diabetes. Sleep Med. 2016;25:156-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 22. | Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 426] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 23. | Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. 2020;22:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 24. | Umbro I, Fabiani V, Fabiani M, Angelico F, Del Ben M. A systematic review on the association between obstructive sleep apnea and chronic kidney disease. Sleep Med Rev. 2020;. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep Breath. 2018;22:841-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, Jackson CL, Williams MA, Redline S. Racial/Ethnic differences in sleep disturbances: The multi-ethnic study of atherosclerosis (MESA). Sleep. 2015;38:877-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 317] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 27. | Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events. Version 2.5. Darien, IL: American Academy of Sleep Medicine; 2018. [Cited in This Article: ] |
| 28. | Morrone E, Giordano A, Carli S, Visca D, Rossato F, Godio M, Paracchini E, Rossi S, Balbi B, Sacco C, Braghiroli A. Something is changing in adherence to CPAP therapy: real world data after 1 year of treatment in patients with obstructive sleep apnoea. Eur Respir J. 2020;55:1901419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Wimms AJ, Kelly JL, Turnbull CD, McMillan A, Craig SE, O'Reilly JF, Nickol AH, Hedley EL, Decker MD, Willes LA, Calverley PMA, Benjafield AV, Stradling JR, Morrell MJ; MERGE trial investigators. Continuous positive airway pressure versus standard care for the treatment of people with mild obstructive sleep apnoea (MERGE): a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:349-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Savransky V, Nanayakkara A, Vivero A, Li J, Bevans S, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia predisposes to liver injury. Hepatology. 2007;45:1007-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Musso G, Olivetti C, Cassader M, Gambino R. Obstructive sleep apnea-hypopnea syndrome and nonalcoholic fatty liver disease: emerging evidence and mechanisms. Semin Liver Dis. 2012;32:49-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 822] [Cited by in F6Publishing: 776] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 33. | Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 697] [Cited by in F6Publishing: 693] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 34. | Petta S, Marrone O, Torres D, Buttacavoli M, Cammà C, Di Marco V, Licata A, Lo Bue A, Parrinello G, Pinto A, Salvaggio A, Tuttolomondo A, Craxì A, Bonsignore MR. Obstructive sleep apnea is associated with liver damage and atherosclerosis in patients with non-alcoholic fatty liver disease. PLoS One. 2015;10:e0142210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Buttacavoli M, Gruttad'Auria CI, Olivo M, Virdone R, Castrogiovanni A, Mazzuca E, Marotta AM, Marrone O, Madonia S, Bonsignore MR. Liver steatosis and fibrosis in OSA patients after long-term CPAP treatment: A preliminary ultrasound study. Ultrasound Med Biol. 2016;42:104-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Yu JH, Ahn JH, Yoo HJ, Seo JA, Kim SG, Choi KM, Baik SH, Choi DS, Shin C, Kim NH. Obstructive sleep apnea with excessive daytime sleepiness is associated with non-alcoholic fatty liver disease regardless of visceral fat. Korean J Intern Med. 2015;30:846-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Chen X, Lin X, Chen LD, Lin QC, Chen GP, Yu YH, Huang JC, Zhao JM. Obstructive sleep apnea is associated with fatty liver index, the index of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2016;28:650-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Kim D, Ahmed A, Kushida C. Continuous positive airway pressure therapy on nonalcoholic fatty liver disease in patients with obstructive sleep apnea. J Clin Sleep Med. 2018;14:1315-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Trzepizur W, Boursier J, Le Vaillant M, Ducluzeau PH, Dubois S, Henni S, Abraham P, Aubé C, Calès P, Gagnadoux F; on the behalf of the METABOL group. Increased liver stiffness in patients with severe sleep apnoea and metabolic comorbidities. Eur Respir J. 2018;51:1800601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Benotti P, Wood GC, Argyropoulos G, Pack A, Keenan BT, Gao X, Gerhard G, Still C. The impact of obstructive sleep apnea on nonalcoholic fatty liver disease in patients with severe obesity. Obesity (Silver Spring). 2016;24:871-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Arısoy A, Sertoğullarından B, Ekin S, Özgökçe M, Bulut MD, Huyut MT, Ölmez Ş, Turan M. Sleep apnea and fatty liver are coupled via energy metabolism. Med Sci Monit. 2016;22:908-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Bhatt SP, Guleria R, Vikram NK, Gupta AK. Non-alcoholic fatty liver disease is an independent risk factor for inflammation in obstructive sleep apnea syndrome in obese Asian Indians. Sleep Breath. 2019;23:171-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2953] [Cited by in F6Publishing: 2955] [Article Influence: 113.7] [Reference Citation Analysis (1)] |
| 44. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003;37:1202-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1488] [Cited by in F6Publishing: 1434] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 45. | Parathath S, Mick SL, Feig JE, Joaquin V, Grauer L, Habiel DM, Gassmann M, Gardner LB, Fisher EA. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ Res. 2011;109:1141-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 46. | Hou H, Zhao Y, Yu W, Dong H, Xue X, Ding J, Xing W, Wang W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J Glob Health. 2018;8:010405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 47. | Li M, Li X, Lu Y. Obstructive sleep apnea syndrome and metabolic diseases. Endocrinology. 2018;159:2670-2675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 504] [Cited by in F6Publishing: 550] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 49. | Agrawal S, Duseja A, Aggarwal A, Das A, Mehta M, Dhiman RK, Chawla Y. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9:283-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 50. | Qi JC, Huang JC, Lin QC, Zhao JM, Lin X, Chen LD, Huang JF, Chen X. Relationship between obstructive sleep apnea and nonalcoholic fatty liver disease in nonobese adults. Sleep Breath. 2016;20:529-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Cakmak E, Duksal F, Altinkaya E, Acibucu F, Dogan OT, Yonem O, Yilmaz A. Association between the severity of nocturnal hypoxia in obstructive sleep apnea and non-alcoholic fatty liver damage. Hepat Mon. 2015;15:e32655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Chen LD, Zhang LJ, Lin XJ, Qi JC, Li H, Wu Z, Xu QZ, Huang YP, Lin L. Association between continuous positive airway pressure and serum aminotransferases in patients with obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2018;275:587-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Shpirer I, Copel L, Broide E, Elizur A. Continuous positive airway pressure improves sleep apnea associated fatty liver. Lung. 2010;188:301-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |