Published online Apr 14, 2020. doi: 10.3748/wjg.v26.i14.1628
Peer-review started: December 26, 2019
First decision: February 18, 2020
Revised: March 25, 2020
Accepted: April 1, 2020
Article in press: April 1, 2020
Published online: April 14, 2020
Hepatic portal venous gas (HPVG) generally indicates poor prognoses in patients with serious intestinal damage. Although surgical removal of the damaged portion is effective, some patients can recover with conservative treatments.
To establish an optimal treatment strategy for HPVG, we attempted to generate computed tomography (CT)-based criteria for determining surgical indication, and explored reliable prognostic factors in non-surgical cases.
Thirty-four cases of HPVG (patients aged 34-99 years) were included. Necessity for surgery had been determined mainly by CT findings (i.e. free-air, embolism, lack of contrast enhancement of the intestinal wall, and intestinal pneumatosis). The clinical data, including treatment outcomes, were analyzed separately for the surgical cases and non-surgical cases.
Laparotomy was performed in eight cases (surgical cases). Seven patients (87.5%) survived but one (12.5%) died. In each case, severe intestinal damage was confirmed during surgery, and the necrotic portion, if present, was removed. Non-occlusive mesenteric ischemia was the most common cause (n = 4). Twenty-six cases were treated conservatively (non-surgical cases). Surgical treatments had been required for twelve but were abandoned because of the patients’ poor general conditions. Surprisingly, however, three (25%) of the twelve inoperable patients survived. The remaining 14 of the 26 cases were diagnosed originally as being sufficiently cured by conservative treatments, and only one patient (7%) died. Comparative analyses of the fatal (n = 10) and recovery (n = 16) cases revealed that ascites, peritoneal irritation signs, and shock were significantly more frequent in the fatal cases. The mortality was 90% if two or all of these three clinical findings were detected.
HPVG related to intestinal necrosis requires surgery, and our CT-based criteria are probably useful to determine the surgical indication. In non-surgical cases, ascites, peritoneal irritation signs and shock were closely associated with poor prognoses, and are applicable as predictors of patients’ prognoses.
Core tip: Hepatic portal venous gas caused by intestinal necrosis is a life-threatening condition and requires surgery. Computed tomography findings of free-air, embolism, lack of contrast enhancement of the intestinal wall, and intestinal pneumatosis are useful criteria to determine the surgical indication. In non-surgical cases, ascites, peritoneal irritation signs and shock were closely associated with poor prognoses, and are valuable as predictors of patients’ prognoses.
- Citation: Gonda M, Osuga T, Ikura Y, Hasegawa K, Kawasaki K, Nakashima T. Optimal treatment strategies for hepatic portal venous gas: A retrospective assessment. World J Gastroenterol 2020; 26(14): 1628-1637
- URL: https://www.wjgnet.com/1007-9327/full/v26/i14/1628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i14.1628
Hepatic portal venous gas (HPVG) was first presented by Wolfe and Evans[1] in 1955 as a pediatric case, followed in 1960 by the report of Susman and Senturia[2] of an adult case, and was recognized as a fatal condition in patients with serious intestinal damage, including severe intestinal ischemia, enterocolitis, etc.[3,4]. Although surgical removal of the damaged portion has generally been considered the sole effective therapy, it has been shown by advanced imaging modalities, including computed tomography (CT), that some of the patients can recover with non-surgical, conservative treatments[3,5-9]. HPVG in the patients who recovered was mostly not associated with intestinal necrosis, suggesting that not all HPVG patients require surgery[9,10]. Conversely, unnecessary laparotomy might have been performed in such mild cases. A robust criterion of surgical indication is necessary to prevent unpredictable under- and/or over-treatments.
The challenging surgery conducted in emergency settings is not applicable to every patient with acute intestinal damage. Some pre-existing conditions, such as poor performance status (PS), severe frailty and extreme exhaustion, may rule out surgery as a therapeutic option. In those cases, to make the best management plan for each non-surgical patient and to explain anticipated outcomes clearly to their families, physicians require reliable prediction indices for estimating the curative potential of non-surgical, conservative treatments.
In this retrospective study, we attempt to determine novel CT-based criteria for deciding surgical indication, and to define prognostic factors in non-surgical conservative treatments of HPVG. A goal of the study is the establishment of optimal treatment strategies against HPVG especially in non-surgical cases.
From April 2012 to February 2019, 34 patients (35 cases; one patient was treated twice conservatively) were diagnosed as HPVG and treated at Takatsuki General Hospital. One patient was excluded due to insufficient clinical data, and the remaining 33 patients (19 women and 14 men; aged 34-99 years) were included in this retrospective study. Their chief complaints were abdominal pain (n = 28), nausea/vomiting (n = 12), melena (n = 9) and abdominal fullness (n = 28). Their comorbidities were diabetes (n = 8), cerebral infarction (n = 6), ischemic heart disease (n = 3), pancreatic cancer (n = 1), cerebral palsy (n = 1) and chronic subdural hematoma (n = 1). All the patients presented with acute and serious illnesses, and ten of them were in shock (systolic blood pressure ≤ 90 mmHg) at the initial consultations. This study was reviewed and approved by the ethical committee of Takatsuki General Hospital (Approval No: 2018-1).
The necessity for surgical treatments of these patients was determined mainly based on presence of one or more of the following CT findings: (1) Abdominal free-air; (2) Mesenteric artery embolism; (3) Lack of contrast enhancement of the intestinal wall; and (4) Intestinal pneumatosis. However, for some of the patients who required surgery, laparotomy was abandoned because of poor physical status and socio-medical conditions (inoperable patients). Consequently, the patients were divided into a surgical treatment group and a non-surgical conservative treatment group (including inoperable patients and patients who did not require surgery). Three typical cases are presented below.
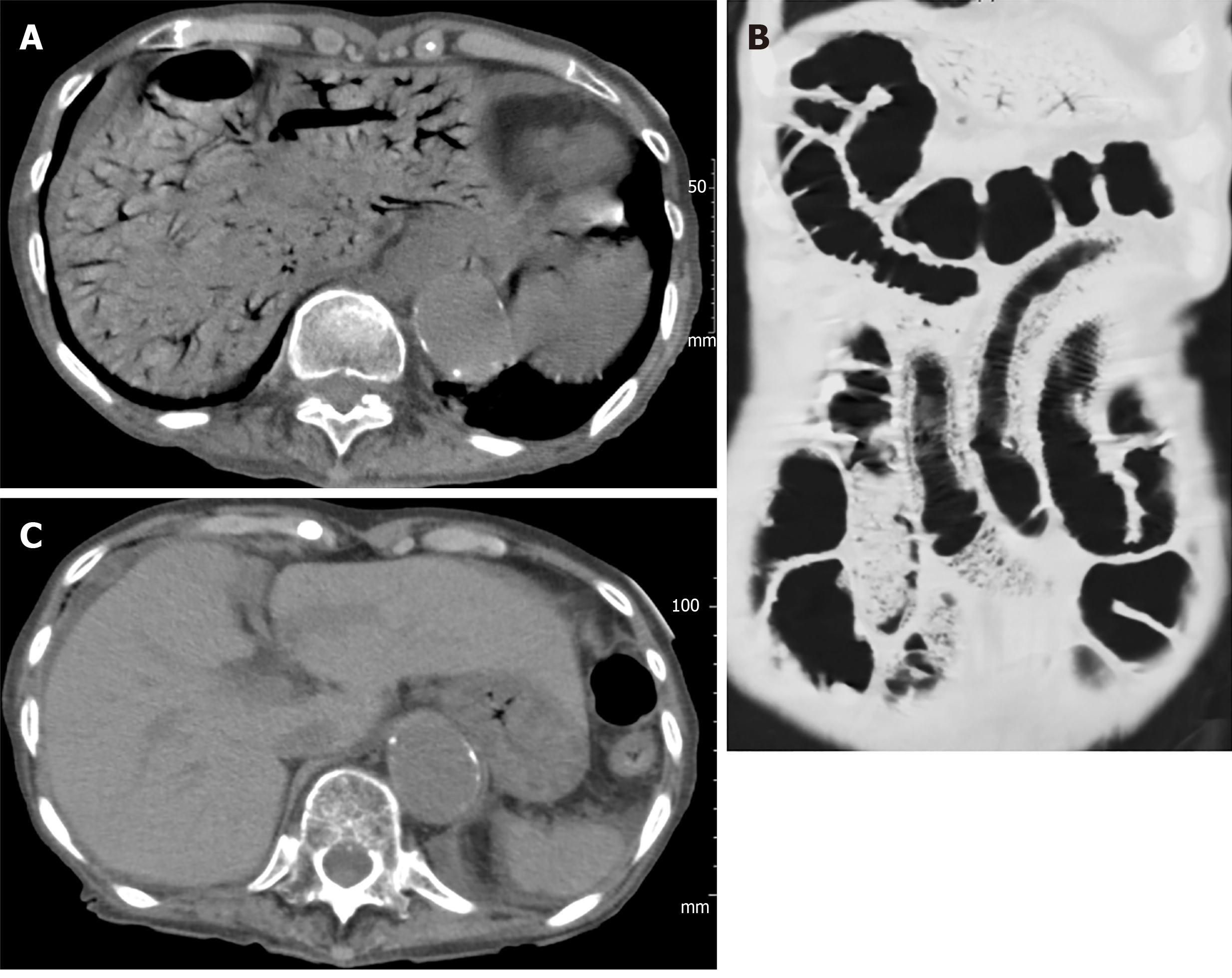
Case 1 (surgical case): A 72-year-old female patient, hospitalized for treatment of ischemic heart disease and cerebral infarction complained suddenly of abdominal pain, vomiting and melena. She fell into shock and an abdominal CT was performed immediately, providing a diagnosis of HPVG with intestinal ischemia (Figure 1A). Partial intestinal resection was carried out to save the patient and the pathologic specimen obtained revealed hemorrhagic necrosis associated with pneumatosis (Figure 1B and C), which was concordant with a clinical diagnosis of non-occlusive mesenteric ischemia (NOMI).
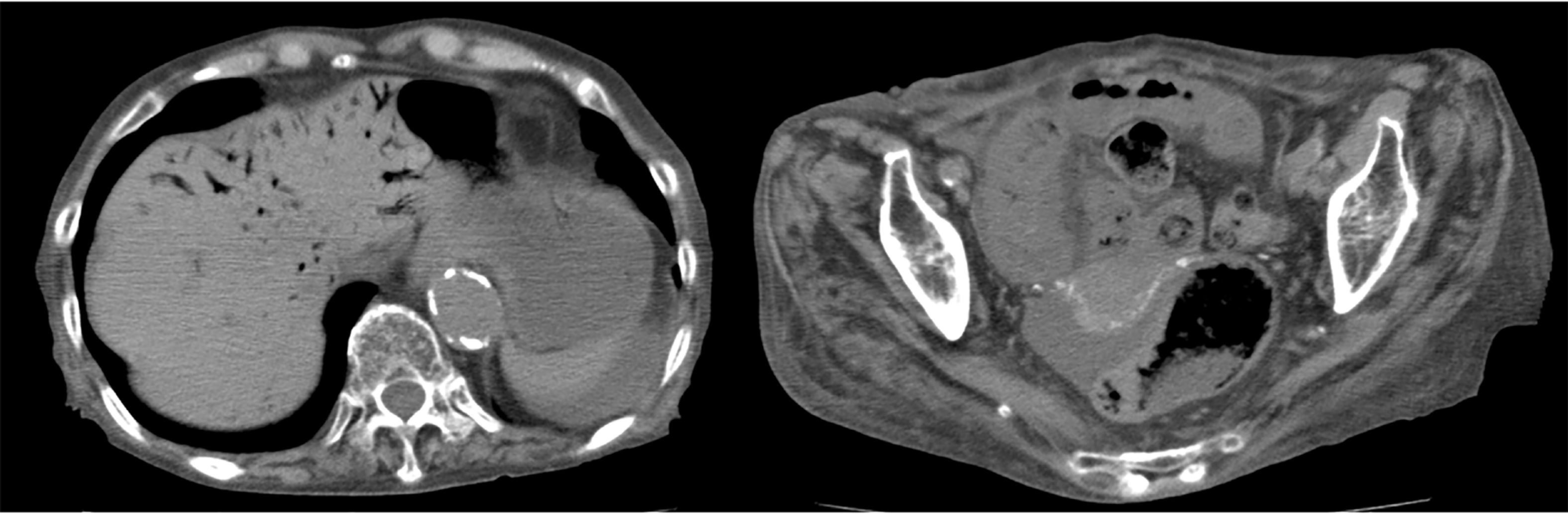
Case 2 (inoperable case with recovery): An 86-year-old female patient with dementia and a history of aortic dissection complained of vomiting and melena after her evening meal. She was transferred to our hospital by ambulance. An emergency abdominal CT revealed HPVG and intestinal pneumatosis (Figure 2A). Laparotomy was considered but not performed because of her poor general condition. However, HPVG was alleviated (Figure 2B) by conservative treatment (rehydration and antibiotics), and she survived and recovered.
Case 3 (inoperable fatal case): A 91-year-old female patient with dementia and extremely poor PS [Eastern Cooperative Oncology Group (ECOG) PS 4] complained of vomiting and melena. On admission, abdominal CT showed HPVG and intestinal pneumatosis (Figure 3). Surgical treatment was abandoned because of the expected poor postoperative prognosis. She died the day after admission.
The patients’ data including clinical backgrounds, physical examination findings, laboratory test results, CT images, and treatment outcomes were analyzed separately for the surgical patients and non-surgical patients. The primary purposes of the analyses were validation of the appropriateness of CT findings-based decision criteria for surgery and development of a prediction index to estimate the mortality of non-surgical patients. Fisher’s exact test and Mann-Whitney U-test were used for statistical analysis, and P < 0.05 was considered to be significant.
As shown in Figure 4, 20 patients were considered to be suitable for surgical treatments, eight were treated with operations (surgical cases) but 12 were determined to be inoperable cases because of their poor general conditions, e.g. ECOG PS 4 (n = 9). Of the eight surgical cases, five patients were originally in shock status, which was resolved preoperatively by rapid rehydration. In 14 cases, CT findings on admission suggested that surgery was not necessary. Consequently, a total of 26 cases (25 patients) were managed conservatively as non-surgical cases, of which 16 cases (15 patients) survived/recovered and 10 died. The overall mortality was 32% (11 of 34 cases).
Clinical and pathological details of the eight surgically-treated patients are shown in Table 1. Necrotic portions of the intestine were resected in seven patients, and one patient could be rescued only by separation of intestinal adhesions. Final diagnoses were NOMI (n = 4), clostridium difficile enteritis (n = 1), strangulation ileus (n = 1), superior mesenteric artery thrombosis (n = 1) and gastric perforation (n = 1, a fatal case).
| Case | Age | Gender | Final diagnosis | Outcome | Chief complaints | Shock1 | Peritoneal irritation signs | Ascites2 | Intestinal pneumatosis | LOCE in intestinal wall | Free air | WBC (μL) | CRP (mg/dL) | BE (mmol/L) | Lactate (mg/dL) |
| 13 | 72 | F | NOMI | Recovery | Abdominal pain, nausea | + | + | 2+ | - | + | - | 26000 | 1.57 | -4.9 | 18 |
| 2 | 74 | M | Clostridium enteritis | Recovery | Nausea, vomiting | - | + | 2+ | - | + | - | 15400 | 16 | 5.3 | 7 |
| 3 | 65 | F | NOMI | Recovery | Abdominal pain | - | - | - | - | + | + | 31500 | 7.39 | -7 | 13 |
| 4 | 86 | M | Gastric perforation | Death | Abdominal pain, vomiting | + | + | 3+ | + | + | + | 7800 | 0.17 | -11.3 | 73 |
| 5 | 69 | M | NOMI | Recovery | Abdominal pain | + | + | 2+ | + | - | - | 13200 | 10.8 | 2.3 | 14 |
| 6 | 71 | M | Mesenteric artery thrombosis | Recovery | Abdominal pain, vomiting | + | + | 1+ | + | + | + | 22500 | 1.81 | -6.3 | 46 |
| 7 | 84 | M | NOMI | Recovery | Abdominal fullness | + | + | 2+ | + | NE | - | 36700 | 15.54 | -5.5 | 47 |
| 8 | 34 | M | Strangulation ileus | Recovery | Abdominal fullness | - | NE | - | + | + | - | 10000 | 4.67 | -3.1 | 42 |
Seven (87.5%) of the eight surgically treated patients survived, and one (12.5%) died (three days post-operation due to sepsis). In contrast, only three (25%) of 12 inoperable patients survived, and nine (75%) died. The difference in the survival rate was statistically significant (P = 0.02), indicating that the decision for operation/laparotomy was very appropriate. This was confirmed by the very low mortality rate (n = 1, 7%) of 14 cases not requiring surgery. In the fatal case, the HPVG had disappeared, and the general condition had also improved, but the patient died of recurrent illness.
Of the 26 non-surgical cases, 16 (61.5%) were cured by conservative treatments (rehydration, antibiotics, ileus tube insertion, etc.), but 10 (38.5%) died. Nine of the 10 fatal cases had been defined as inoperable. Detailed clinical and laboratory data of the patients who died or recovered are shown in Table 2.
| Recovery (n = 16) | Death (n = 10) | P value | |
| Age [median (range)] | 86 (56-92) | 84 (72-99) | P > 0.9991 |
| Gender (M:F) | 4:12 | 4:6 | P = 0.6652 |
| Shock (≤ systolic BP 90 mmHg) (%) | 0 (0%) | 6 (60%) | P = 0.0012 |
| Peritoneal irritation (%) | 2 (13%) | 8 (80%) | P = 0.0012 |
| Ascites (%) | 5 (31%) | 8 (80%) | P = 0.0412 |
| Intestinal pneumatosis (%) | 8 (50%) | 7 (70%) | P = 0.4282 |
| WBC (/μL) [median (range)] | 9050 (4200-31800) | 13400 (9900-19000) | P = 0.0251 |
| CRP (mg/dL) [median (range)] | 2.39 (0.11-28.41) | 12.84 (0.1-33.26) | P = 0.3551 |
| BE (mmol/L) [median (range)] | 1.8 (-8.4 – 14.6) | -6.2 (-18.2 – 6.8) | P = 0.0711 |
| Lactate (mg/dL) [median (range)] | 26 (9-63) | 36 (11-120) | P = 0.2311 |
To determine the critical prognostic factors in the non-surgical cases, comparative analyses were performed between the fatal and recovery cases (Table 2). Rates of ascites (80% vs 31%), peritoneal irritation sign (80% vs 12.5%) and shock (60% vs 0%) were significantly higher in the fatal cases. Of the laboratory test results, leukocyte counts were significantly higher in the fatal cases than the recovery cases (median 13400 vs 9050 /µL; P = 0.025). Base excess (median -6.2 vs 1.8 mEq/L) tended to be lower, and plasma levels of CRP (median 12.84 vs 2.39 mg/dL) and lactic acid (median 36 vs 26 mg/dL) tended to be higher in the fatal cases, but the differences were not significant.
A predictive index was developed using the three statistically significant clinical/non-laboratory factors, i.e. ascites, peritoneal irritation sign and shock. Most of the fatal cases (90%) presented two or three of the factors, while none of the recovery cases presented two or three of the factors (Table 3). This indicates that prediction of mortality with detection of two or all of the three factors is a superior index of sensitivity 90% and specificity 100%. Notably, the three inoperable patients who recovered, without exception, had none or only one of the three factors. The predictive accuracy was not improved by adding the leukocyte counts as a fourth factor (data not shown).
| Recovery | Death | |
| 0-1 Factor | 16 | 1 |
| 2-3 Factors | 0 | 9 |
HPVG has been recognized as a serious condition that is associated with poor prognosis and requires urgent surgical treatment. Formerly its mortality was reported to be 75%-90%[3,11] but this has improved recently to 29%-56%[6,10,12] with an increase in the detection rate and advances in therapy. At the same time, non-surgical cases have become more common[6-9] but reliable guidelines to select the optimal treatment for each patient have not yet been established.
The pathologic mechanisms of HPVG are summarized as (1) intramural gas-producing bacterial proliferation; (2) elevated intraluminal pressure because of bowel obstruction, endoscopic procedures, etc.; and (3) air-translocation through damaged/necrotic mucosa[3,7]. Kinoshita et al[10] reported that the etiologies /underlying conditions of HPVG were mesenteric ischemia (43%), digestive tract dilation (12%), intraperitoneal abscess (11%), ulcerative colitis (4%), gastric ulcer (4%), complications from endoscopic procedures (4%), intraperitoneal tumors (3%), and others (15%). HPVG of our patients were related to NOMI (n = 12, including eight suspicious cases), ischemic enterocolitis (n = 5), superior mesenteric arterial thrombosis (n = 3) , ileus [strangulation (n = 1) or non-strangulation (n = 4)], constipation (n = 4), postoperative intestinal necrosis (n = 1), clostridium difficile enteritis (n = 1), acute pancreatitis (n = 1), gastric perforation (n = 1), and bladder cancer invading the rectum (n = 1). The proportion of these background conditions and the overall mortality in our cases (32%) were almost identical with those of other recent reports, indicating that our HPVG group was standard and not at all unusual.
Through the retrospective observation of this standard HPVG group, we validated the appropriateness of our original CT findings-based determination to select subjects for surgical treatment. The four CT findings, i.e. abdominal free-air, mesenteric artery embolism, lack of contrast enhancement of the intestinal wall, and intestinal pneumatosis, which are hallmarks of intestinal perforation and/or severe ischemia/necrosis[13], seem to be appropriate as convenient decision criteria for laparotomy. A similar preoperative assessment was previously reported from a group of Japanese surgeons[14]. Our CT-based simple method provides not only comparable accuracy but also superior convenience.
In addition, we found reliable clinical indices for predicting the mortality of non-surgical patients with HPVG, including inoperable cases. Non-surgical patients who have more than two of three clinically obtainable factors, ascites (by CT), peritoneal irritation (by physical examination), and shock (by checking vital signs), are thought to be in life-threatening conditions. Critical intestinal ischemia is associated frequently with perforation, sepsis and peritonitis, and hence, ascites, peritoneal irritation and shock are thought to be its typical manifestations. An analogues prediction system using Acute Physiology and Chronic Health Evaluation (APACHE) II was proposed by Yoo et al[15]. However, because APACHE II requires several items of laboratory data[16,17], this predictive algorithm may be difficult to disseminate as a general procedure. Very recently, similar emergency medicine scorings also have been suggested for use for the identical purpose[18,19]. Same as APACHE II, they are not specific to abdominal illnesses, and seem not to be perfect as predictors of HPVG.
The importance of laboratory test results for determination of surgical indication and for prediction of non-surgical patients’ prognoses was also examined, as had been done in previous analogous studies. Although some of these tended to show greater degrees of abnormal values in patients who died than in those who survived, the differences were not statistically significant. Only leukocyte counts were significantly higher in the fatal cases than in the recovery cases, but were not a contributory factor to the mortality prediction. As a result, we were able to develop a quite simple diagnostic algorithm composed of characteristic CT findings and physical examination findings, to provide the optimal treatment for each HPVG patient. With the progression of an aging society, the incidence of HPVG, especially of inoperable cases, is inevitably increasing. Our two-step decision and prediction process may be useful not only for selection of surgical cases but also for considering non-surgical but intensive treatments for such inoperable patients. In other words, a strategic non-surgical management may be recommended in the future to HPVG patients who have 0-1 of the high-mortality factors (ascites, peritoneal irritation sign and shock). Alternatively, a challenging surgery may be considered in patients who have 2-3 of the factors, regardless of their background conditions. A further validation study, such as a prospective study, may be required to generalize our novel treatment strategy for HPVG.
Hepatic portal venous gas (HPVG) is generally recognized as a life-threatening sign in patients with serious intestinal damage. While most of such patients require surgical treatments, some patients can recover without surgery.
We aimed to establish an optimal treatment strategy for HPVG, i.e., how to select surgical or conservative treatments.
We tested accuracy of our original computed tomography (CT)-based selection criteria. Additionally, we found if there were reliable prognostic factors in non-surgical cases.
Thirty-four cases of HPVG were included. Surgical indication had been decided by CT findings, including free-air, embolism, lack of contrast enhancement of the intestinal wall, and intestinal pneumatosis. Their clinical findings and treatment outcomes were analyzed separately in the surgical cases and non-surgical cases.
Of eight surgical cases, seven patients (87.5%) survived but one (12.5%) died. All the surgical patients had severe intestinal damage and the necrotic portions were resected. In addition to 14 cases without surgical indication, 12 inoperable cases were defined as non-surgical cases (total 26 cases). Three (25%) of the 12 inoperable patients survived. Only one patient (7%) died among the 14 patients diagnosed as being surgery unnecessary. Comparative analyses of the fatal (n = 10) and recovery (n = 16) cases revealed that ascites, peritoneal irritation signs, and shock were significantly more frequent in the fatal cases. The mortality was 90% if two or all of these three clinical findings were detected.
Our CT-based criteria were useful to determine the surgical indication for HPVG patients. In non-surgical cases, ascites, peritoneal irritation signs and shock were closely associated with poor prognoses, and are applicable as predictors of patients’ prognoses.
Our two-step decision and prediction process may be applicable not only for selection of surgical cases but also for considering non-surgical but intensive treatments for such inoperable patients.
The authors thank Dr. Masashi Shimizu (Department of Radiology, Takatsuki General Hospital) who helped us by reviewing the CT findings of all the HPVG patients.
Manuscript source: Invited Conference Manuscripts
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tsoulfas G S-Editor: Zhang L L-Editor: A E-Editor: Ma YJ
| 1. | Wolfe JN, Evans WA. Gas in the portal veins of the liver in infants; a roentgenographic demonstration with postmortem anatomical correlation. Am J Roentgenol Radium Ther Nucl Med. 1955;74:486-488. [PubMed] [Cited in This Article: ] |
| 2. | Susman N, Senturia HR. Gas embolization of the portal venous system. Am J Roentgenol Radium Ther Nucl Med. 1960;83:847-850. [PubMed] [Cited in This Article: ] |
| 3. | Liebman PR, Patten MT, Manny J, Benfield JR, Hechtman HB. Hepatic--portal venous gas in adults: etiology, pathophysiology and clinical significance. Ann Surg. 1978;187:281-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 296] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Hussain A, Mahmood H, El-Hasani S. Portal vein gas in emergency surgery. World J Emerg Surg. 2008;3:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Chan SC, Wan YL, Cheung YC, Ng SH, Wong AM, Ng KK. Computed tomography findings in fatal cases of enormous hepatic portal venous gas. World J Gastroenterol. 2005;11:2953-2955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Faberman RS, Mayo-Smith WW. Outcome of 17 patients with portal venous gas detected by CT. AJR Am J Roentgenol. 1997;169:1535-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Nelson AL, Millington TM, Sahani D, Chung RT, Bauer C, Hertl M, Warshaw AL, Conrad C. Hepatic portal venous gas: the ABCs of management. Arch Surg. 2009;144:575-581; discussion 581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Gorospe EC. Benign hepatic portal venous gas in a critically ill patient. ScientificWorldJournal. 2008;8:951-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Abboud B, El Hachem J, Yazbeck T, Doumit C. Hepatic portal venous gas: physiopathology, etiology, prognosis and treatment. World J Gastroenterol. 2009;15:3585-3590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 154] [Cited by in F6Publishing: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Kinoshita H, Shinozaki M, Tanimura H, Umemoto Y, Sakaguchi S, Takifuji K, Kawasaki S, Hayashi H, Yamaue H. Clinical features and management of hepatic portal venous gas: four case reports and cumulative review of the literature. Arch Surg. 2001;136:1410-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Fred HL, Mayhall CG, Harle TS. Hepatic portal venous gas. A review and report on six new cases. Am J Med. 1968;44:557-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Seak CJ, Hsu KH, Wong YC, Ng CJ, Yen DH, Seak JC, Seak CK. The prognostic factors of adult patients with hepatic portal venous gas in the ED. Am J Emerg Med. 2014;32:972-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212:160-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 149] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Koami H, Isa T, Ishimine T, Kameyama S, Matsumura T, Yamada KC, Sakamoto Y. Risk factors for bowel necrosis in patients with hepatic portal venous gas. Surg Today. 2015;45:156-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Yoo SK, Park JH, Kwon SH. Clinical outcomes in surgical and non-surgical management of hepatic portal venous gas. Korean J Hepatobiliary Pancreat Surg. 2015;19:181-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Wu JM, Tsai MS, Lin MT, Tien YW, Lin TH. High APACHE II score and long length of bowel resection impair the outcomes in patients with necrotic bowel induced hepatic portal venous gas. BMC Gastroenterol. 2011;11:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [PubMed] [Cited in This Article: ] |
| 18. | Seak CJ, Yen DH, Ng CJ, Wong YC, Hsu KH, Seak JC, Chen HY, Seak CK. Rapid Emergency Medicine Score: A novel prognostic tool for predicting the outcomes of adult patients with hepatic portal venous gas in the emergency department. PLoS One. 2017;12:e0184813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Olsson T, Terent A, Lind L. Rapid Emergency Medicine score: a new prognostic tool for in-hospital mortality in nonsurgical emergency department patients. J Intern Med. 2004;255:579-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |