Published online Mar 21, 2020. doi: 10.3748/wjg.v26.i11.1156
Peer-review started: November 12, 2019
First decision: December 23, 2019
Revised: December 27, 2019
Accepted: February 21, 2020
Article in press: February 21, 2020
Published online: March 21, 2020
Intestinal inflammation is a common digestive tract disease, which is usually treated with hormone medicines. Hormone medicines are effective to some extent, but long-term use of them may bring about many complications.
To explore the protective effects of panax notoginseng saponin (PNS) against dextran sulfate sodium (DSS)-induced intestinal inflammatory injury through phosphoinositide-3-kinase protein kinase B (PI3K/AKT) signaling pathway inhibition in rats.
Colitis rat models were generated via DSS induction, and rats were divided into control (no modeling), DSS, DSS + PNS 50 mg/k, and DSS + PNS 100 mg/kg groups. Then, the intestinal injury, oxidative stress parameters, inflammatory indices, tight junction proteins, apoptosis, macrophage polarization, and TLR4/AKT signaling pathway in colon tissues from rats in each of the groups were detected. The PI3K/AKT signaling pathway in the colon tissue of rats was blocked using the PI3K/AKT signaling pathway inhibitor, LY294002.
Compared with rats in the control group, rats in the DSS group showed significantly shortened colon lengths, and significantly increased disease activity indices, oxidative stress reactions and inflammatory indices, as well as significantly decreased expression of tight junction-associated proteins. In addition, the DSS group showed significantly increased apoptotic cell numbers, and showed significantly increased M1 macrophages in spleen and colon tissues. They also showed significantly decreased M2 macrophages in colon tissues, as well as activation of the PI3K/AKT signaling pathway (all P < 0.05). Compared with rats in the DSS group, rats in the DSS + PNS group showed significantly lengthened colon lengths, decreased disease activity indices, and significantly alleviated oxidative stress reactions and inflammatory responses. In addition, this group showed significantly increased expression of tight junction-associated proteins, significantly decreased apoptotic cell numbers, and significantly decreased M1 macrophages in spleen and colon tissues. This group further showed significantly increased M2 macrophages in colon tissues, and significantly suppressed activation of the PI3K/AKT signaling pathway, as well as a dose dependency (all P < 0.05). When the PI3K/AKT signaling pathway was inhibited, the apoptosis rate of colon tissue cells in the DSS + LY294002 group was significantly lower than that of the DSS group (P < 0.05).
PNS can protect rats against DSS-induced intestinal inflammatory injury by inhibiting the PI3K/AKT signaling pathway, and therefore may be potentially used in the future as a drug for colitis.
Core tip: Panax notoginseng saponin is a drug widely used for cardiovascular diseases and diabetes, with good proven inhibitory effects on inflammation. Our study also found that panax notoginseng saponin exerted good inhibitory effects on inflammation in dextran sulfate sodium-induced colitis.
- Citation: Lu QG, Zeng L, Li XH, Liu Y, Du XF, Bai GM, Yan X. Protective effects of panax notoginseng saponin on dextran sulfate sodium-induced colitis in rats through phosphoinositide-3-kinase protein kinase B signaling pathway inhibition. World J Gastroenterol 2020; 26(11): 1156-1171
- URL: https://www.wjgnet.com/1007-9327/full/v26/i11/1156.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i11.1156
The gastrointestinal tract is an organ with many complex functions in terms of endocrine, immunity, nutrition, and others. These play an important role in the normal operation of the human body[1,2]. Intestinal inflammation is primarily caused by immune dysfunction due to intestinal barrier damage by invading pathogens or microbial toxins in the body[3]. Once intestinal injury occurs in the body, intestinal mucosa will release oxygen free radicals, leading to lipid peroxidation and complete loss of the intestinal barrier. This will cause the also release of inflammatory factors, which may have a more severe consequence - death or systemic inflammatory response syndromes[4,5]. At present, intestinal inflammatory injury is mainly treated with corticoid drugs for intestinal inflammation. Although these drugs are effective to some extent, long-term use of them may cause various hormone medicine-related adverse reactions, including other gastrointestinal complications and neurogenic obesity[6]. Therefore, finding a new drug that is effective for intestinal inflammatory injury is a current clinical challenge. Panax notoginseng, a traditional Chinese medicine, has been widely used for cardiovascular diseases[7], and panax notoginseng saponin (PNS) is one of the most effective ingredients of panax notoginseng. A previous study pointed out that PNS had a variety of pharmacological functions, including anti-inflammatory and anti-apoptosis effects[8]. Another study found that PNS could alleviate oxidative stress reactions and eliminate free radicals[9]. Both oxidative stress reactions and free radicals are pathological features of intestinal inflammation[10]. Although there have been discussions on the role of notoginsenoside R1, an effective component in PNS, in intestinal ischemia-reperfusion[11], there was no elaboration on PNS in enteritis. The dextran sulfate sodium (DSS)-induced colitis model has been widely used, because it can mimics human inflammatory bowel diseases[11]. Therefore, we constructed colitis rat models based on DSS induction, and explored the role of PNS in intestinal inflammatory injury and its mechanism in rats.
A total of 80 Sprague-Dawley rats aged 6-7 wk with a body mass of about 235-290 g were raised at a temperature of 20-25 °C and a relative humidity of 45%-65%, and allowed to eat and drink freely under normal circadian rhythm alternation after being purchased from the Laboratory Animal Centre of Sun Yat-Sen University. In addition to the rats, there were other materials as follows: Escherichia coli DSS (Sigma, United States, L2880); PNS (Chengdu Must Biotechnology Co., Ltd., A0760); fluorescein isothiocyanate (FITC)-labeled anti-mouse CD11b antibody, phycoerythrin (PE)-labeled anti-mouse F4/80 antibody, PerCp/Cy5.5-labeled anti-mouse CD16/32 antibody, APC-labeled anti-mouse CD206 antibody (BioLegend Company, United States); interleukin-6 (IL-6), IL-1β, tumor necrosis factor-α (TNF-α), IL-10, and enzyme-linked immunosorbent assay kit (Shanghai Mlbio Co., Ltd.); p-PI3K, p-AKT, claudin-1, occludin, ZO-1, Bax, Bcl-2, and caspase-3 monoclonal antibodies (Cell Signaling Company, United States); rabbit anti-human β-actin monoclonal antibody (Proteintech Group, Inc); multiple factor flow cytometry kit (BioLegend Company, United States); in situ cell apoptosis determination kit (Roche Diagnostics, Basel, Switzerland), and fetal bovine serum (Hyclone company), and red blood cell lysate (Miltenyi Company, Germany).
The rats were randomly assigned to a control group (no treatment), a DSS group, a DSS + PNS 50 mg/kg group and a DSS + PNS 100 mg/kg group, 20 rats in each group. The rats were raised normally for 3 d. After 3 d, rats in the DSS group, DSS + PNS 50 mg/kg group and DSS + PNS 100 mg/kg group were made to drink water containing 50 g/LDSS instead of their previous drinking water for 7 consecutive days. During the 7 d, rats in the DSS+PNS 50 mg/kg group were given 50 mg/kg PNS at 7 mg/mL by gavage, and rats in the DSS + PNS 100 mg/kg group were given 100 mg/kg PNS at 7 mg/mL by gavage from the 2nd day. Rats in the two groups were weighted each day, and the disease of those rats was evaluated using the disease activity index[12]. After the 7 d, the rats were killed by cervical dislocation, and their spleen and colons were collected for subsequent analysis.
Colon tissues were fixed with 4% paraformaldehyde for one night, and then paraffin embedding and serial sections (3.5 μm) were performed. Subsequently, tissues were stained with hematoxylin & eosin, and images of the tissues were obtained using the Image-Pro Plus 5.0 system. Villar heights and crypt depths were measured and analyzed, and the intestinal injury of the tissues was scored[13]. The score spanned between 0 and 4, and indicated no epithelium injury and inflammatory infiltration with 0 points, slight goblet cell reduction and inflammatory infiltration into crypts with 1 point, relatively extensive goblet cell reduction and inflammatory infiltration into the mucosal muscularis with 2 points, extensive goblet cell reduction, slight crypt decrease, and extensive inflammatory infiltration into the mucosal muscularis with 3 points, and extensive decrease in crypts and inflammatory infiltration into the submucosa with 4 points.
Inflammation-related factors and oxidative stress parameters: Enzyme-linked immunosorbent assay was employed to determine serum pro-inflammatory factors (IL-6, IL-1β, and TNF-α), and an anti-inflammatory factor (IL-10) in strict accordance with kit instructions. The electrochemiluminescence immunoassay was used to determine serum oxidative stress parameters including malondialdehyde (MDA), myeloperoxidase (MPO), catalase (CAT), and superoxide dismutase (SOD) in strict accordance with kit instructions.
Western blot assay: The total protein of the sampled colon tissues was extracted using the radio immunoprecipitation assay lysis method, and the concentration of the total protein was determined using the bicinchoninic acid method, and adjusted to 4 μg/μL. The total protein was separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred to a polyvinylidene fluoride membrane, stained with Ponceau’s stain liquid, and soaked in phosphate buffer saline with Tween for 5 min for washing. Then, the total protein was blocked with 5% skim milk powder for 2 h, and added with p-PI3K (1:500), p-AKT (1:500), claudin-1 (1:500), occludin (1:500), ZO-1 (1:500), Bax (1:500), Bcl-2 (1:500), Caspase-3 (1:500) and β-Actin primary antibody (1:500), and blocked at 4 °C for one night. The membrane was washed to remove the primary antibody, added with horseradish peroxidase-labeled goat anti-rabbit secondary antibody (1:1000), incubated at 37 °C for 1 h, and rinsed with phosphate buffer saline (PBS) three times, 5 min each time. Filter paper was used for the membrane to remove excess liquid, and the membrane was then made to be luminescent with ECL and developed. The protein band was scanned, and the gray value was analyzed using Quantity One software. The relative expression of protein = gray value of the target protein band/gray value of the β-Actin protein band.
TUNEL assay for cell apoptosis determination: The mesenteric lymph node tissues were fixed with 4% formaldehyde at room temperature, and then washed, dehydrated, paraffin embedded, and cut into 4 μm sections. Cell apoptosis in the sections were determined in strict accordance with instructions of the in situ cell apoptosis determination kit. Cells with brown nuclei after staining were apoptotic cells, and these cells in five microscopic fields were counted under a fluorescence microscope. Cells staining positive for TUNEL were also counted.
Flow cytometry for macrophage and M1/M2 macrophage determination: Colon and spleen of rats were sampled to prepare a single-cell suspension. The spleens were placed in 0.9% sodium chloride solution, and then ground, filtered and lysed using red blood cell lysate. Subsequently, the tissues were centrifuged at 300 g for 5 min. After centrifugation, the tissues were washed with PBS, and resuspended. A total of 1 × 105 cells were taken from the tissues for determination. After being sheared, colon tissues were added into PBS containing 5% fetal bovine serum, and placed into a 37 °C water bath for 20 min. Then, epithelial cells were removed from the tissues, and the tissues were fully sheared again and placed into a solution containing 1mg/ml collagenase IV, and then placed in a 37 °C water bath for 30 min for digestion. Subsequently, the tissue suspension was filtered, centrifuged at 300 g for 10 min, and resuspended. A total of 1 × 105 cells in the middle layer were taken for detection. Then, the cells were added to human surface antibodies (FITC-CD11b, PE-F4/80, and Per Cp/Cy5.5-CD16/32), incubated at a normal temperature in the dark for 20 min, and then washed with PBS to remove excess antibodies. Subsequently, the cells were added with 2 mL of stationary liquid/membrane permeabilization buffer solution, and washed two times. Then, the cells were added to APC-CD206 antibody, incubated in the dark at 4 °C for 30 min, and added to 1 mL of stationary liquid/membrane permeabilization buffer solution, and then washed one time. Finally, the cells were isolated using flow cytometry after being resuspended in 200 μL PBS. The surface markers of macrophages, M1 and M2 macrophages were CD11b and F4/80, CD16/32 and CD206, respectively.
In this study, the collected data were statistically analyzed using the SPSS20.0 software package, and organized into figures using GraphPad 7 software. Comparison between groups was analyzed using independent t-tests, and comparison among multiple groups was analyzed using one-way ANOVA. Post hoc pairwise comparisons were subject to LSD-t tests. P < 0.05 indicated a significant difference.
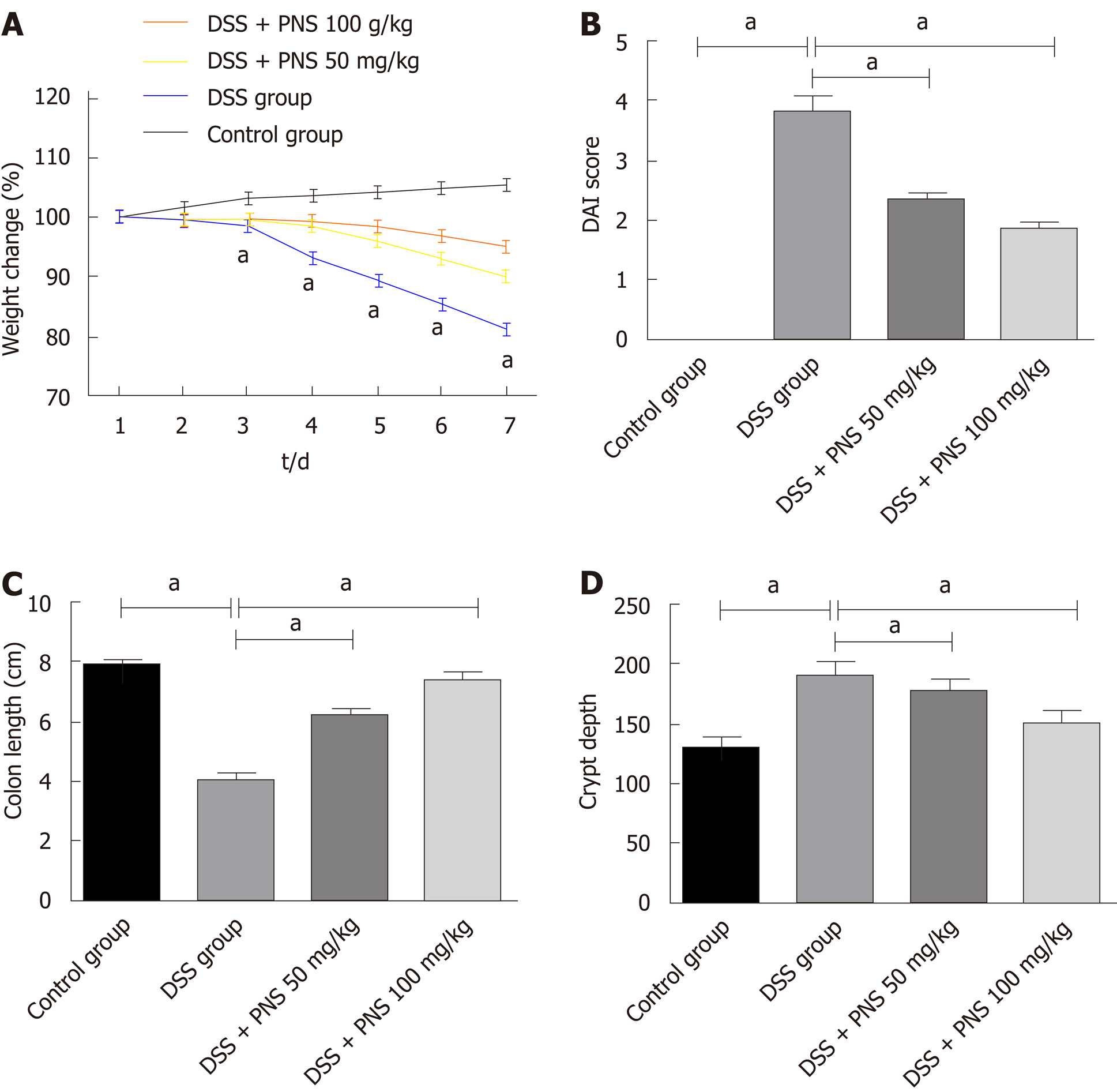
Compared with rats in the control group, after being injected with DSS, rats in the DSS group showed significantly shortened colon lengths, significantly increased crypt depths, and significant weight loss since the first 3rd day (all P < 0.05). Compared with rats in the DSS group, rats in the DSS + PNS group showed significantly lengthened colons, significantly decreased crypt depths, and significantly improved disease activity indices (all P < 0.05; Figure 1).
Compared with rats in the control group, rats in the DSS group showed obvious intestinal pneumatosis in the small intestine, mucosa edema in the intestinal wall, and severe mesenteric venous congestion. In addition, rates in the DSS + PNS group showed mild mucosa edema in the intestinal wall, and partial mesenteric venous congestion without obvious congestion points. In addition, compared with rats in the control group, rats in the DSS group had significantly higher histopathological scores, but rats undergoing PNS intervention had significantly improved histopathological scores, and showed a dose-dependency (Figure 2).
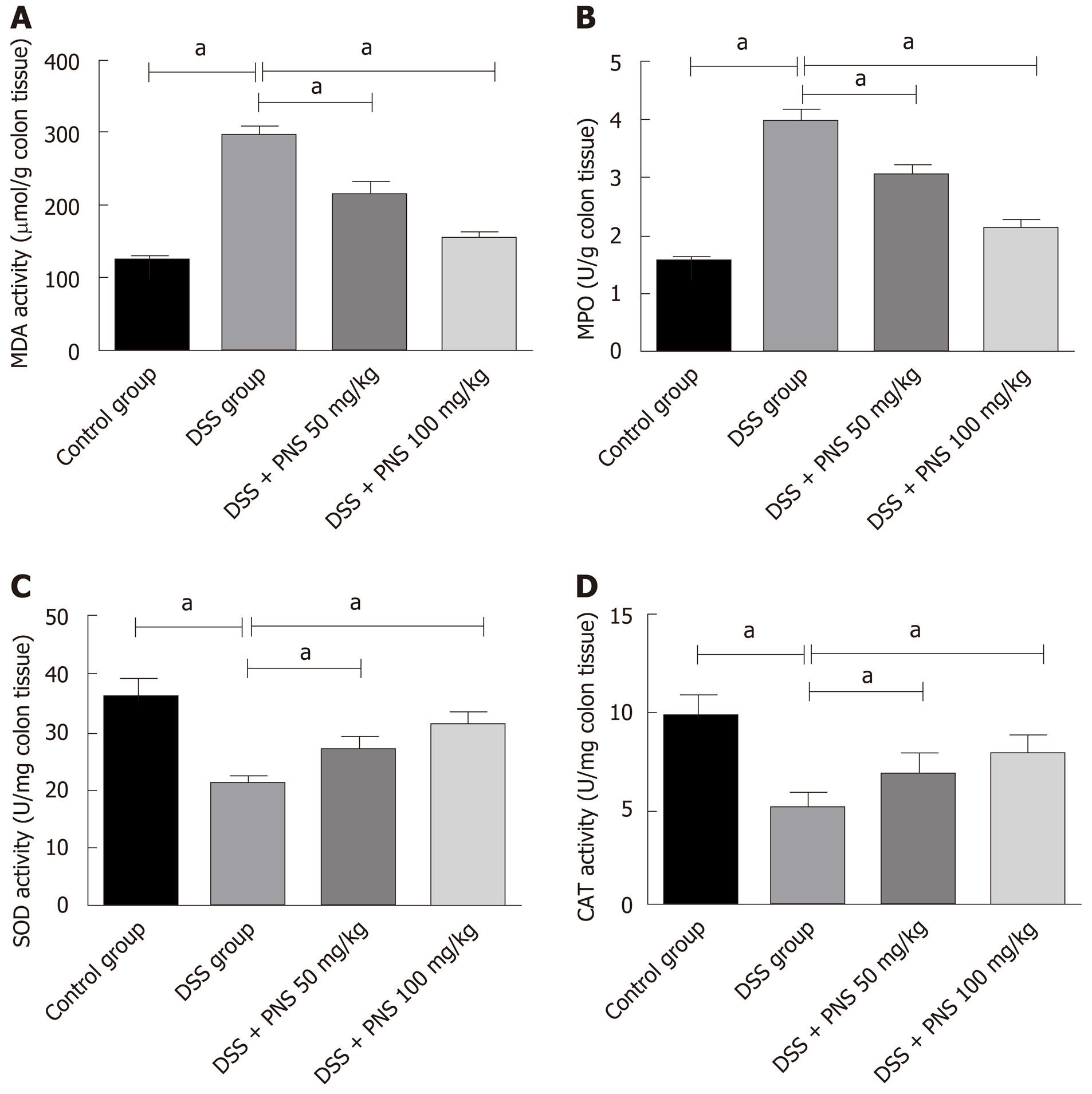
Compared with rats in the control group, rats in the DSS group showed significantly increased MDA and MPO activities and significantly decreased CAT and SOD activities in intestinal tissues (all P < 0.05). Compared with rats in the DSS group, rats in the DSS + PNS group showed significantly decreased MDA and MPO activities, and significantly improved SOD and CAT activities in intestinal tissues (all P < 0.05; Figure 3).
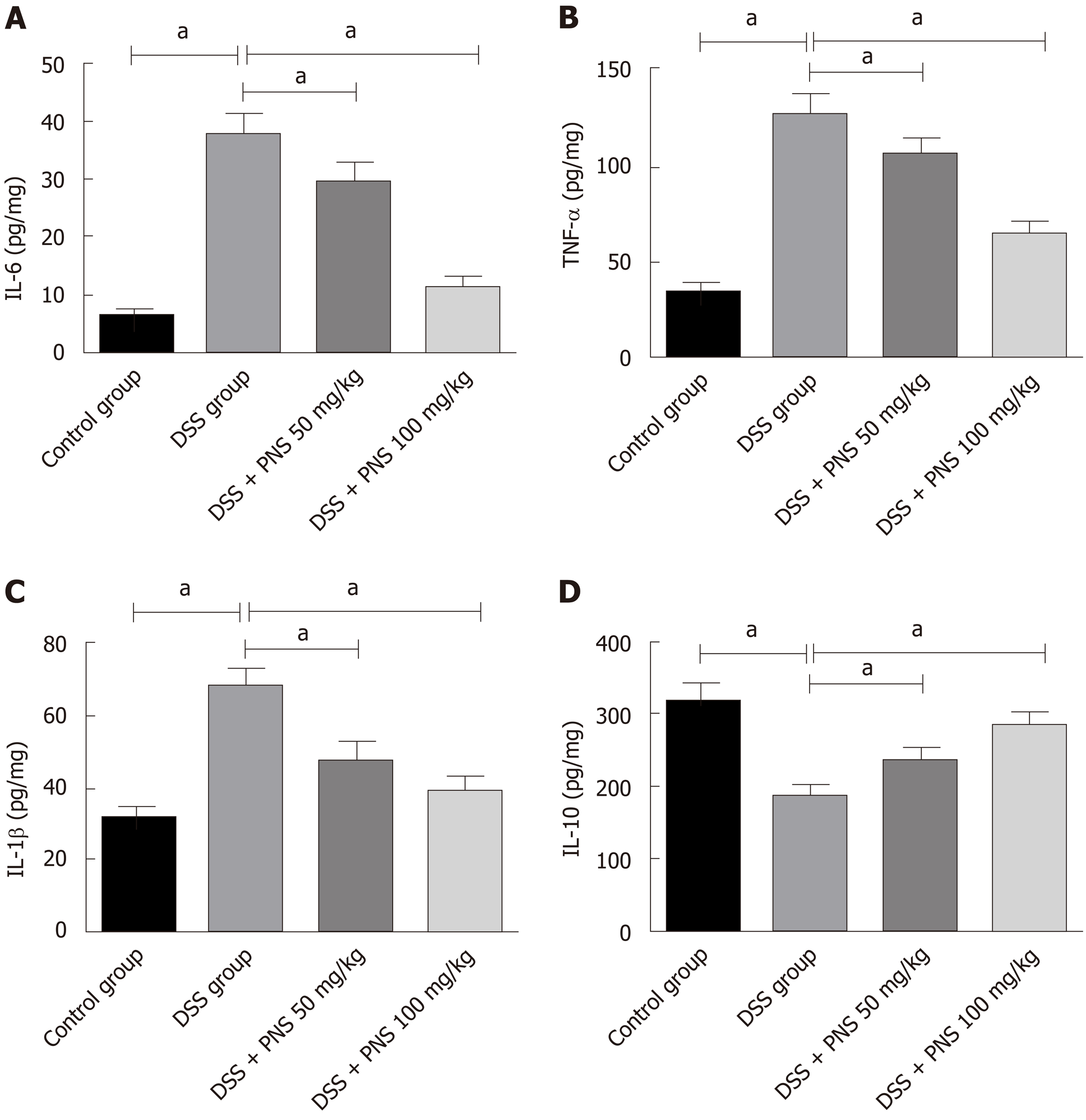
Compared with rats in the control group, rats in the DSS groups showed significantly increased expression of IL-6, IL-1β and TNF-α, and significantly decreased expression of IL-10 in intestinal tissues (all P < 0.05). Compared with rats in the DSS group, rats in the DSS + PNS group showed significantly decreased expression of IL-6 and TNF-α, and significantly increased expression of IL-10 (P < 0.05; Figure 4).
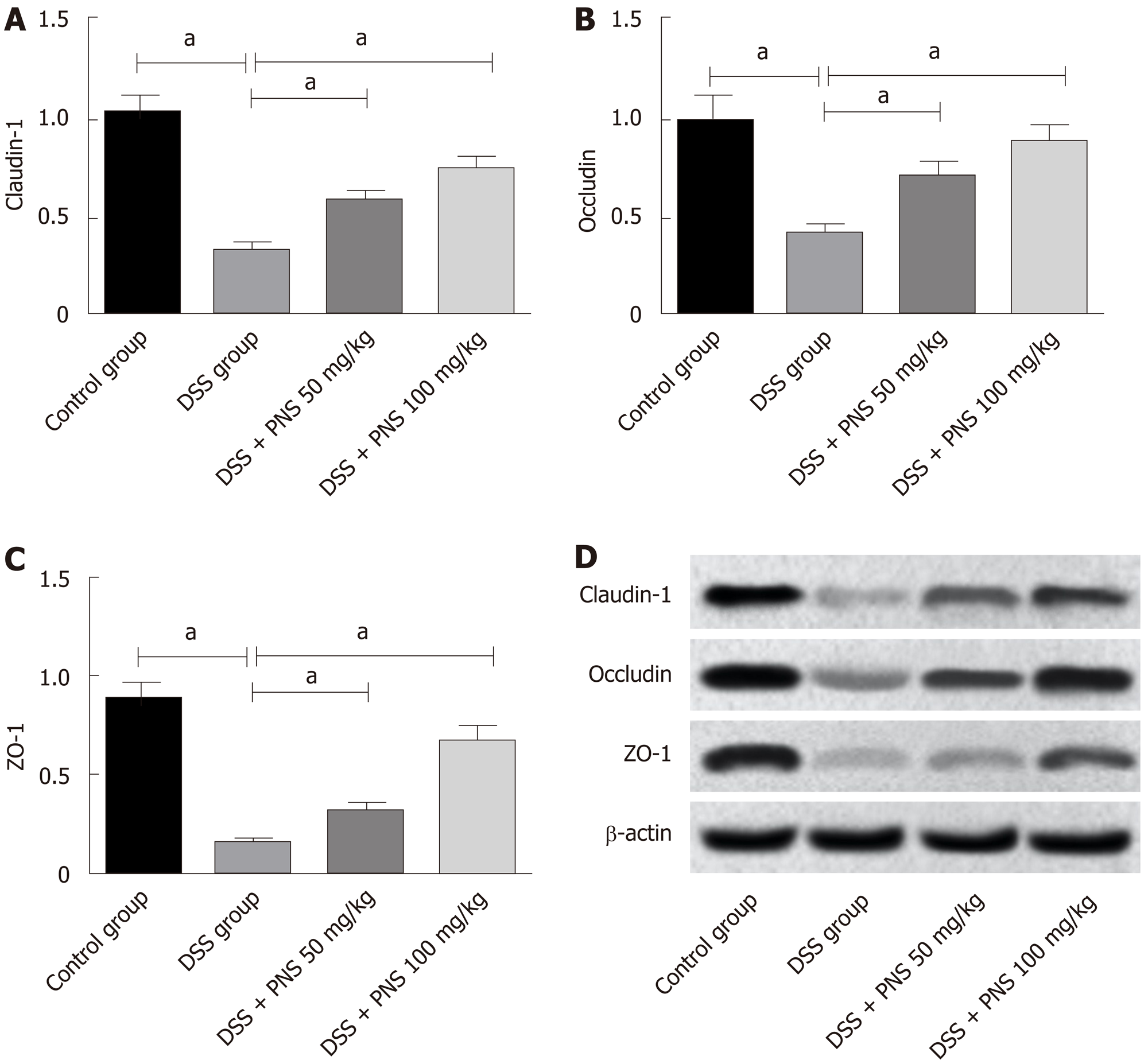
Compared with rats in the control group, rats in the DSS group showed significantly decreased expression of tight junction proteins including claudin-1, occludin, and ZO-1 in intestinal tissues (all P < 0.05). Compared with rats in the DSS group, rats in the DSS + PNS group showed significantly increased expression of claudin-1, occludin, and ZO-1, and showed a dose-dependency (all P < 0.05; Figure 5).
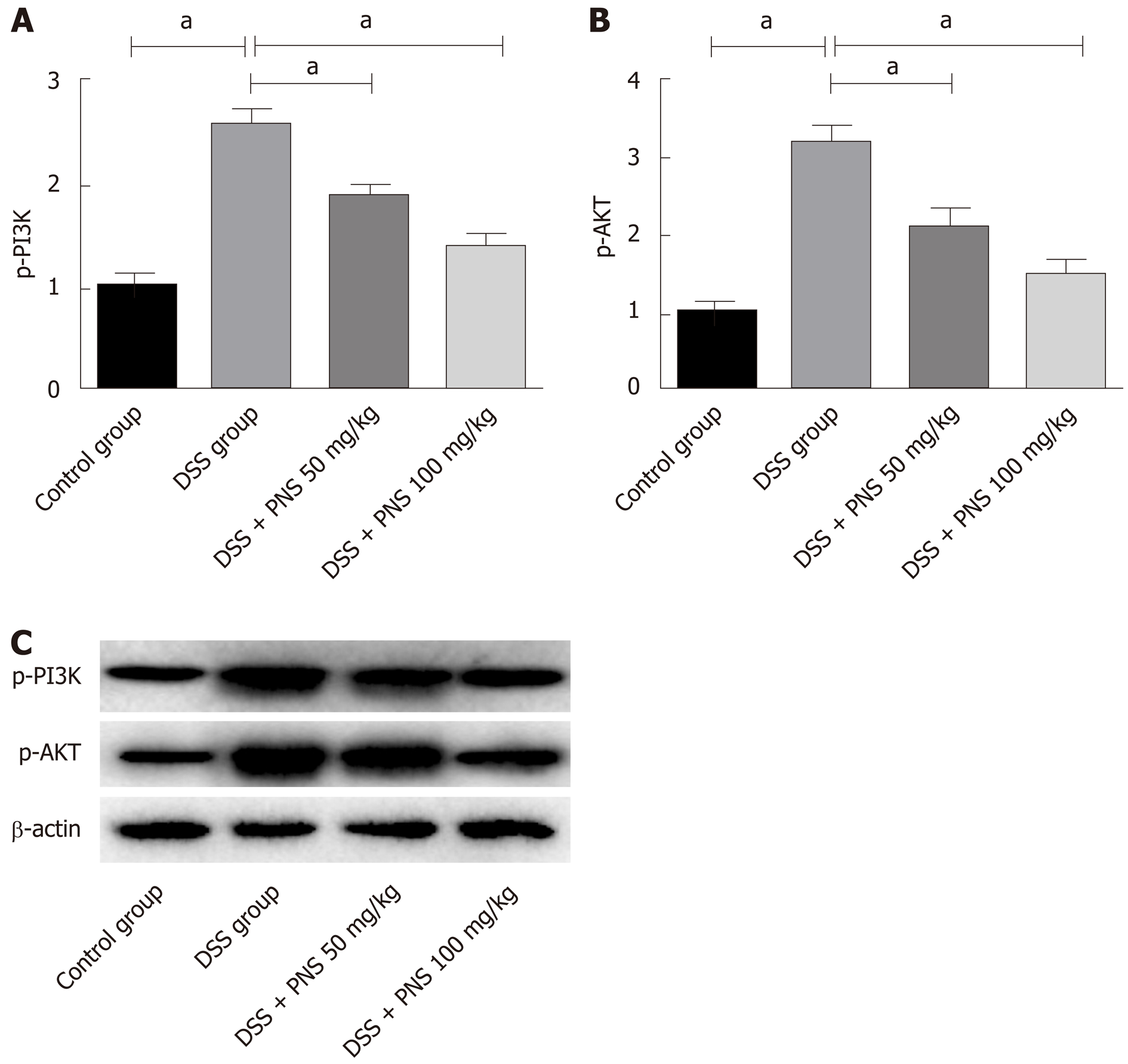
Compared with rats in the control group, rats in the DSS group showed significantly increased expression of p-PI3K and p-AKT (both P < 0.05). Compared with rats in the DSS group, rats in the DSS+PNS group showed significantly decreased expression of p-PI3K and p-AKT and showed a dose-dependency (all P < 0.05; Figure 6).
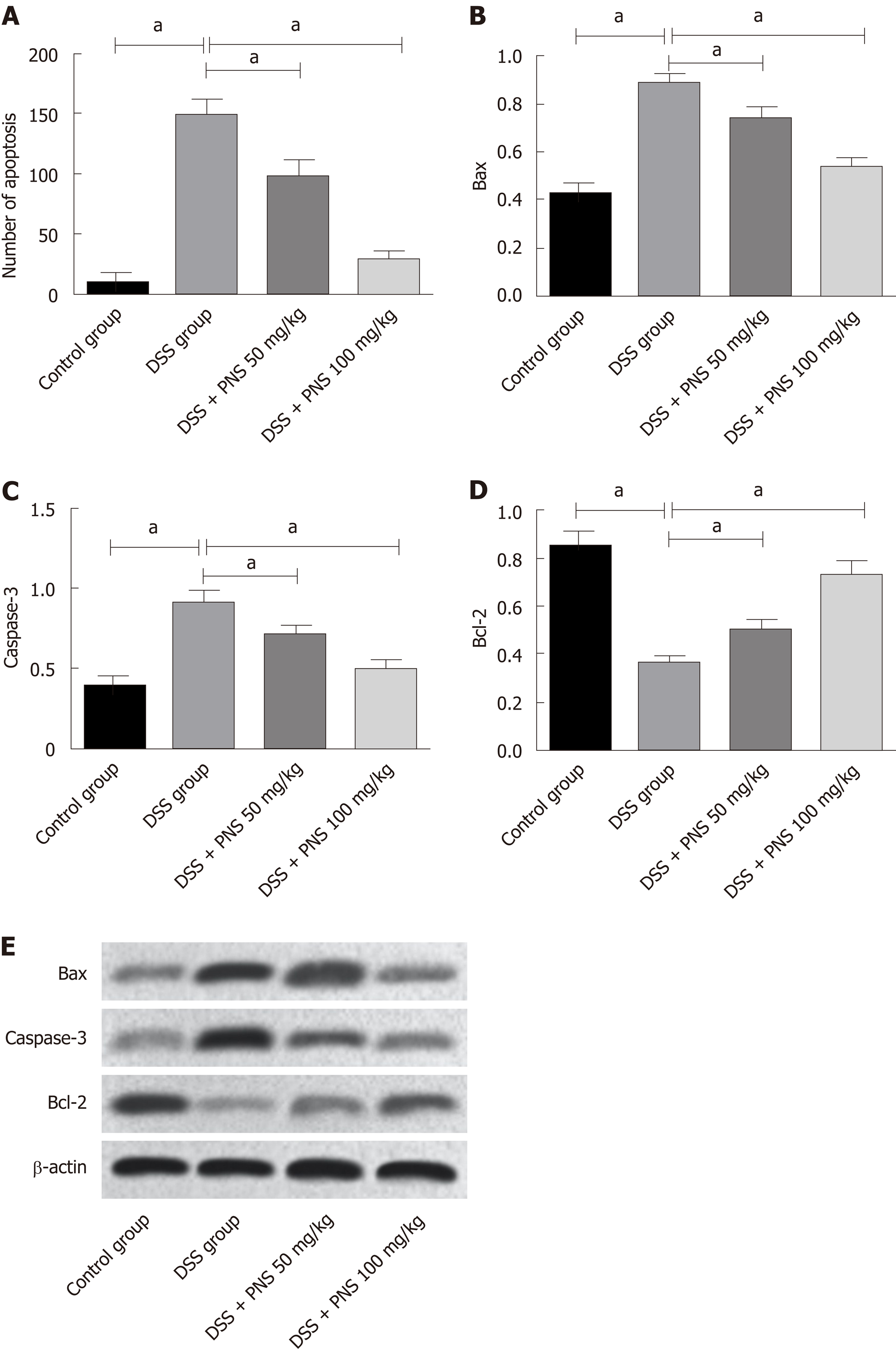
TUNEL assay revealed that compared with the situation in the control group, DSS significantly increased the number of apoptotic TUNEL-positive cells; while compared with the use of DSS alone, PNS intervention decreased the number of apoptotic TUNEL-positive cells, and showed a dose-dependency (P < 0.05). Western blotting analysis consistently revealed that PNS treatment significantly up-regulated the expression of anti-apoptotic factor Bcl-2 and down-regulated the expression of pro-apoptotic factors Bax and caspase-3 (all P < 0.05; Figure 7).
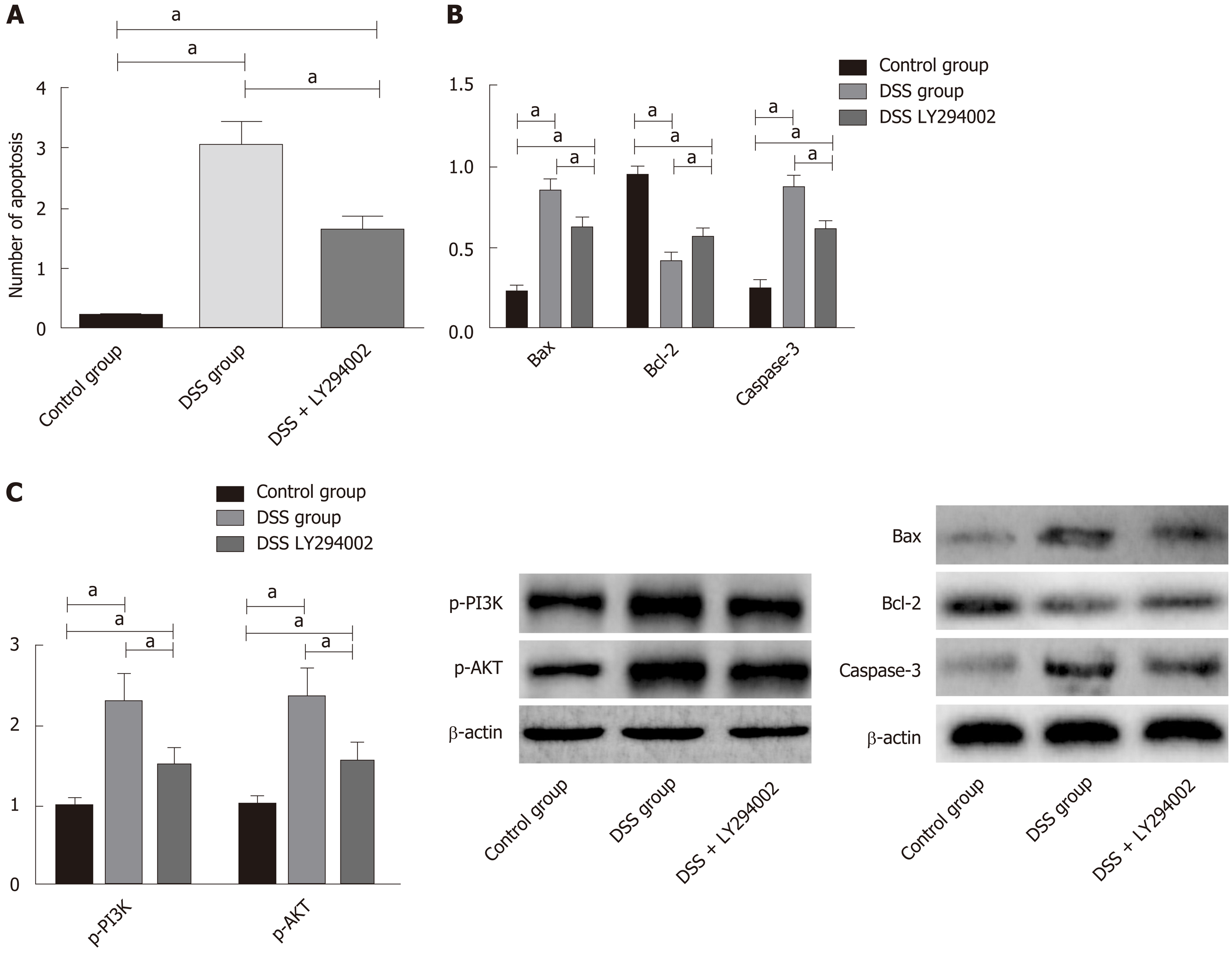
In order to further confirm that PNS has protective effects in rats against intestinal injury through PI3K/AKT signaling, we additionally selected 30 rats, and divided them into a control group, a DSS group, and a DSS+LY294002 group. We intraperitoneally injected 20 mg/kg PI3K/AKT signal pathway inhibitor, LY294002, into each rat in the DSS + LY294002 group. We found that, compared with the DSS group, the DSS + LY294002 group showed significantly decreased phosphorylation levels of PI3K and AKT in colon tissues, significantly reduced apoptosis of colon tissue cells, dramatically down-regulated expression of Bax and Caspase-3, and dramatically up-regulated expression of Bcl-3 (Figure 8).
Compared with rats in the control group, rats in the DSS group showed significantly decreased percentages of CD11b+F4/80-labeled macrophages and significantly increased percentages of M1 macrophages in the spleen (both P < 0.05). Compared with rats in the DSS group, rats in the DSS + PNS group showed significantly increased percentages of CD11b+F4/80-labeled macrophages and significantly decreased percentages of M1 macrophages in the spleen, and they also showed a dose-dependency (all P < 0.05). There were no significant differences among the three groups in the percentage of M2 macrophages. Compared with rats in the control group, rats in the DSS group showed significantly increased percentages of CD11b+F4/80-labeled macrophages and M1 macrophages, and significantly decreased percentages of M2 macrophages in colon tissues. Compared with rats in the DSS group, rats in the DSS + PNS group showed significantly decreased percentages of CD11b+F4/80-labeled macrophages and M1 macrophages, and significantly increased percentages of M2 macrophages, and they also showed a dose-dependency (all P < 0.05; Figure 9).
Inflammatory bowel disease is a chronic inflammatory disease caused by dysfunction of the gastrointestinal mucosal immune system, which shows an increasing incidence with a change in living habits[14]. DSS, a water-soluble sulfated polysaccharide, can destroy the integrity of the intestinal mucosal barrier and lead to enteritis. This is mainly characterized by ulcers, diarrhea, or inflammatory cell infiltration, so it is an ideal choice to induce enteritis in an animal model[15].
In our study, PNS has been proven for the first time to alleviate DSS-induced colitis injury in rats. PNS, an active ingredient in panax notoginseng, is typically been used in the past to treat cardiovascular diseases or diabetes, and it has been proven to alleviate organ inflammatory damage induced by oxidative stress[16]. However, there are few studies and discussions on its role in enteritis. In this study, we found that PNS could alleviate intestinal inflammatory injury in DSS-induced colitis rats. For example, colitis rats undergoing PNS intervention showed significantly increased colon lengths, significantly improved disease activity indices and alleviated pathological damage, which all indicated that PNS could relieve intestinal injury in DSS-induced colitis rats. Enteritis is caused by a very complicated pathological process. A study pointed out that the large number of inflammatory mediators due to enteritis was an important reason for further aggravation of enteritis[17]. To verify this, we determined the inflammatory factors in intestinal tissues of DSS-induced enteritis rats, revealing that rats in the DSS group showed significantly increased expression of IL-6, IL-1β and TNF-α, significantly decreased expression of IL-10, significantly increased MDA and MPO activities, and significantly decreased CAT and SOD activities. This suggested that inflammatory and oxidative stress reactions in rats in the DSS group intensified, which was consistent with previous research results[18]. However, after PNS intervention, the rats showed significantly decreased expression of IL-6, IL-1β, TNF-α, and MDA and MPO activities, and significantly increased expression of IL-10 and CAT and SOD activities, and they also showed a dose-dependency. This suggested that PNS could alleviate intestinal inflammatory and oxidative stress reactions in enteritis rats. IL-6, IL-1β and TNF-α are all typical pro-inflammatory factors, and their up-regulated expression indicates the aggravation of the body’s inflammatory reaction. IL-10 is an anti-inflammatory factor, with down-regulated expression indicating that body inflammation cannot be effectively suppressed[19,20]. MPO activity is closely related to inflammation, because MPO contains abundant neutrophils[21].
A previous study showed that the large number of inflammatory mediators, due to the polarization of macrophages to M1 macrophages caused by external stimulation, is one of the reasons for the acute inflammatory response of enteritis[22]. Macrophages can polarize into M1 macrophages or M2 macrophages. M1 macrophages can secrete inflammatory factors including IL-6 and TNF-α, and M2 macrophages can secrete anti-inflammatory factors including IL-10[23]. In our study, we detected macrophages and their polarization in the spleen and intestinal tissues of DSS-induced colitis rats. We found that the rats showed significantly decreased percentages of CD11b+F4/80-labeled macrophages in the spleen, but significantly increased percentages of these macrophages in colon tissues, which may be due to the rapid migration of macrophages in the spleen to the intestinal inflammatory site during inflammatory reactions. In addition, we also found that the percentages of M1 macrophages in the spleen and colon significantly increased, and the percentages of M2 macrophages in the colon significantly decreased, but the spleen showed no difference. Furthermore, after PNS intervention, the colitis rats showed significantly increased percentages of CD11b+F4/80-labeled macrophages in the spleen, significantly decreased percentages of CD11b+F4/80-labeled macrophages and M1 macrophages in colon tissues, and significantly increased percentages of M2 macrophages in the intestine, and they also showed dose-dependency. It suggested that PNS could suppress the polarization of macrophages into M1 macrophages and induce the polarization of macrophages into M2 macrophages, thus suppressing the intestinal inflammatory response. This is also consistent with previous results for inflammatory factors. In order to explore the mechanism of PNS in relieving intestinal inflammatory injury, we determined PI3K/AKT signaling, revealing that after PNS intervention, colitis rats have significantly decreased phosphorylation levels of PI3K and AKT in intestinal tissues and showed a dose-dependency. This indicated that PNS could inhibit the activation of PI3K and AKT in colitis rats. In order to further confirm that PNS protects colitis rats against intestinal injury through PI3K/AKT signaling, we also intervened with the PI3K/AKT signaling pathway through its inhibitor, LY294002. It turned out that when PI3K/AKT signaling in the colon of colitis rats was inhibited, the apoptosis rate of colon cells significantly decreased, which proved that PNS protected colitis rats against intestinal injury through the PI3K/AKT signaling pathway. PI3K/AKT has long been considered as the primary way of promoting cell proliferation and preventing cell apoptosis, because AKT phosphorylation can initiate the expression of proteins involved in cell proliferation and apoptosis regulation[24,25]. The results of our study suggest that the protective effects of PNS against intestinal inflammatory injury in rats may be achieved by inhibiting the activation of the TLR4/NFκB signaling pathway. Previous studies found that PNS contributes to hepatocyte proliferation after liver regeneration by regulating the PI3K/AKT signaling pathway, which is similar to our results[26].
Tight junction proteins play a very important role in enteritis. When the tight junction is damaged, intestinal barrier function declines, which further causes migration of intestinal antigen substances to the lamina propria of the intestinal mucosa. This stimulates immune cells to produce a large number of inflammatory factors and further aggravates intestinal injury[27,28]. Based on detection, we found that the intestinal tissues of DSS-induced colitis rats showed significantly decreased expression of claudin-1, occludin, and ZO-1, but showed significantly increased expression of them after PNS intervention, which indicated that PNS could also alleviate intestinal mucosal barrier damage in colitis rats. Based on TUNEL experiments, we also found that DSS-induced colitis rats showed increased apoptotic intestinal epithelial cells, significantly increased expression of Bax and Caspase-3, and significantly decreased expression of Bcl-2, which was consistent with the TUNEL results. In addition, compared with rats in the DSS group, colitis rats undergoing PNS intervention showed significantly decreased apoptotic intestinal epithelial cells, significantly down-regulated expressions of Bax and Caspase-3, and significantly up-regulated expression of Bcl-2, which suggested that PNS could also inhibit the apoptosis of intestinal epithelial cells in colitis rats, thus playing a role in intestinal protection.
However, there are still some deficiencies in this study. For example, we did not carry out other in vitro cell experiments to explore the effects of PNS on intestinal epithelial cells, and we also did not explore other possible regulatory pathways through which PNS plays a role in colitis. In the future, we will carry out subsequent basic experiments to address these problems. Secondly, there are some differences in the pathogenesis of the DSS-induced rat enteritis model and human enteritis, which requires subsequent human experiments to prove the role of PNS in human enteritis. In summary, PNS can suppress the activation of the PI3K/AKT signaling pathway to protect DSS-induced rats against intestinal inflammatory injury, and thus it may be effective as a potential future drug for colitis.
Intestinal inflammation is a common digestive tract disease at present, which is usually treated with hormone medications. Hormone medications are effective to some extent, but long-term use of them may bring about many complications. Therefore, it is very important to find new drugs to treat intestinal inflammation.
Panax notoginseng saponins (PNS) are a class of drugs widely used in cardiovascular diseases and diabetes, which have been proven to have good inflammatory inhibition effects. However, there are few studies on the role and mechanism of PNS in rat models of intestinal inflammation. PNS may be an effective drug for intestinal inflammation.
This study aimed to explore the effects of PNS on dextran sulfate sodium (DSS)-induced intestinal inflammatory injury in rats, and its possible mechanism.
The colitis rat models were constructed by inducing DSS, and treating with different concentrations of PNS to inhibit the phosphoinositide-3-kinase protein kinase B (PI3K/AKT) signaling pathway in colon tissues. Then the intestinal injury, oxidative stress parameters, inflammatory indices, tight junction proteins, apoptosis, macrophage polarization, and PI3K/AKT signaling were detected in the tissues.
Compared with colitis rats, rats intervened with PNS showed significantly lengthened colons, decreased disease activity index, as well as significantly alleviated oxidative stress reactions and inflammatory responses. Furthermore, they showed significantly increased expression of tight junction-associated proteins, significantly decreased apoptotic cells, significantly decreased M1 macrophages in spleen and colon tissues, and significantly increased M2 macrophages in colon tissues. They also showed significantly suppressed activation of the PI3K /AKT signaling pathway, and dose-dependency. When the PI3K/AKT signaling pathway was inhibited, compared with colitis rats, the apoptosis rate of colon tissue treated with LY294002 decreased significantly.
This study confirmed that PNS can protect rats against DSS-induced intestinal inflammatory injury by inhibiting the PI3K/AKT signaling pathway, and revealed that it may have potential to be used in the future as a drug for colitis.
It has been proven that PNS can play a protective role against intestinal injury in colitis rats by inhibiting the PI3K/AKT signaling pathway, and PNS may be a potential effective drug for treating colitis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maric I, M'Koma A S-Editor: Wang JL L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Blevins LK, Crawford RB, Bach A, Rizzo MD, Zhou J, Henriquez JE, Khan DMIO, Sermet S, Arnold LL, Pennington KL, Souza NP, Cohen SM, Kaminski NE. Evaluation of immunologic and intestinal effects in rats administered an E 171-containing diet, a food grade titanium dioxide (TiO2). Food Chem Toxicol. 2019;133:110793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Wang S, Martins R, Sullivan MC, Friedman ES, Misic AM, El-Fahmawi A, De Martinis ECP, O'Brien K, Chen Y, Bradley C, Zhang G, Berry ASF, Hunter CA, Baldassano RN, Rondeau MP, Beiting DP. Diet-induced remission in chronic enteropathy is associated with altered microbial community structure and synthesis of secondary bile acids. Microbiome. 2019;7:126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Ito H, Sadatomo A, Inoue Y, Yamada N, Aizawa E, Hishida E, Kamata R, Karasawa T, Kimura H, Watanabe S, Komada T, Horie H, Kitayama J, Sata N, Takahashi M. Role of TLR5 in inflammation and tissue damage after intestinal ischemia-reperfusion injury. Biochem Biophys Res Commun. 2019;519:15-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Noval Rivas M, Wakita D, Franklin MK, Carvalho TT, Abolhesn A, Gomez AC, Fishbein MC, Chen S, Lehman TJ, Sato K, Shibuya A, Fasano A, Kiyono H, Abe M, Tatsumoto N, Yamashita M, Crother TR, Shimada K, Arditi M. Intestinal Permeability and IgA Provoke Immune Vasculitis Linked to Cardiovascular Inflammation. Immunity. 2019;51:508-521.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Silva MC, Sales-Campos H, Oliveira CJF, Silva TL, França FBF, Oliveira F, Mineo TWP, Mineo JR. Treatment with a Zinc Metalloprotease Purified from Bothrops moojeni Snake Venom (BmooMP-Alpha-I) Reduces the Inflammation in an Experimental Model of Dextran Sulfate Sodium-Induced Colitis. Mediators Inflamm. 2019;2019:5195134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Yao B, He J, Yin X, Shi Y, Wan J, Tian Z. The protective effect of lithocholic acid on the intestinal epithelial barrier is mediated by the vitamin D receptor via a SIRT1/Nrf2 and NF-κB dependent mechanism in Caco-2 cells. Toxicol Lett. 2019;316:109-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Yang X, Xiong X, Wang H, Wang J. Protective effects of panax notoginseng saponins on cardiovascular diseases: a comprehensive overview of experimental studies. Evid Based Complement Alternat Med. 2014;2014:204840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Xu D, Huang P, Yu Z, Xing DH, Ouyang S, Xing G. Efficacy and Safety of Panax notoginseng Saponin Therapy for Acute Intracerebral Hemorrhage, Meta-Analysis, and Mini Review of Potential Mechanisms of Action. Front Neurol. 2014;5:274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Hu S, Liu T, Wu Y, Yang W, Hu S, Sun Z, Li P, Du S. Panax notoginseng saponins suppress lipopolysaccharide-induced barrier disruption and monocyte adhesion on bEnd.3 cells via the opposite modulation of Nrf2 antioxidant and NF-κB inflammatory pathways. Phytother Res. 2019;33:3163-3176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Schiapaccassa A, Maranhão PA, de Souza MDGC, Panazzolo DG, Nogueira Neto JF, Bouskela E, Kraemer-Aguiar LG. 30-days effects of vildagliptin on vascular function, plasma viscosity, inflammation, oxidative stress, and intestinal peptides on drug-naïve women with diabetes and obesity: a randomized head-to-head metformin-controlled study. Diabetol Metab Syndr. 2019;11:70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Li C, Li Q, Liu YY, Wang MX, Pan CS, Yan L, Chen YY, Fan JY, Han JY. Protective effects of Notoginsenoside R1 on intestinal ischemia-reperfusion injury in rats. Am J Physiol Gastrointest Liver Physiol. 2014;306:G111-G122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Zhai Z, Zhang F, Cao R, Ni X, Xin Z, Deng J, Wu G, Ren W, Yin Y, Deng B. Cecropin A Alleviates Inflammation Through Modulating the Gut Microbiota of C57BL/6 Mice With DSS-Induced IBD. Front Microbiol. 2019;10:1595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Li HM, Wang YY, Wang HD, Cao WJ, Yu XH, Lu DX, Qi RB, Hu CF, Yan YX. Berberine protects against lipopolysaccharide-induced intestinal injury in mice via alpha 2 adrenoceptor-independent mechanisms. Acta Pharmacol Sin. 2011;32:1364-1372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Choi YI, Kim TJ, Park DK, Chung JW, Kim KO, Kwon KA, Kim YJ. Comparison of outcomes of continuation/discontinuation of 5-aminosalicylic acid after initiation of anti-tumor necrosis factor-alpha therapy in patients with inflammatory bowel disease. Int J Colorectal Dis. 2019;34:1713-1721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Wang J, Tian M, Li W, Hao F. Preventative delivery of IL-35 by Lactococcus lactis ameliorates DSS-induced colitis in mice. Appl Microbiol Biotechnol. 2019;103:7931-7941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Dong Y, Duan L, Chen HW, Liu YM, Zhang Y, Wang J. Network Pharmacology-Based Prediction and Verification of the Targets and Mechanism for Panax Notoginseng Saponins against Coronary Heart Disease. Evid Based Complement Alternat Med. 2019;2019:6503752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Pham TT, Ban J, Lee K, Hong Y, Lee J, Truong AD, Lillehoj HS, Hong YH. MicroRNA gga-miR-10a-mediated transcriptional regulation of the immune genes in necrotic enteritis afflicted chickens. Dev Comp Immunol. 2020;102:103472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Wang R, Wu G, Du L, Shao J, Liu F, Yang Z, Liu D, Wei Y. Semi-bionic extraction of compound turmeric protects against dextran sulfate sodium-induced acute enteritis in rats. J Ethnopharmacol. 2016;190:288-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, Maes M. The Role of Aberrations in the Immune-Inflammatory Response System (IRS) and the Compensatory Immune-Regulatory Reflex System (CIRS) in Different Phenotypes of Schizophrenia: the IRS-CIRS Theory of Schizophrenia. Mol Neurobiol. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Zhang T, Hu C, Wu Y, Wang S, Liu X, Zhang D, Huang F, Gao H, Wang Z. Carbon Disulfide Induces Embryo Implantation Disorder by Disturbing the Polarization of Macrophages in Mice Uteri. Chem Res Toxicol. 2019;32:1989-1996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Pêgo B, Martinusso CA, Bernardazzi C, Ribeiro BE, de Araujo Cunha AF, de Souza Mesquita J, Nanini HF, Machado MP, Castelo-Branco MTL, Cavalcanti MG, de Souza HSP. Schistosoma mansoni Coinfection Attenuates Murine Toxoplasma gondii-Induced Crohn's-Like Ileitis by Preserving the Epithelial Barrier and Downregulating the Inflammatory Response. Front Immunol. 2019;10:442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Kang J, Zhang Z, Wang J, Wang G, Yan Y, Qian H, Zhang X, Xu W, Mao F. hucMSCs Attenuate IBD through Releasing miR148b-5p to Inhibit the Expression of 15-lox-1 in Macrophages. Mediators Inflamm. 2019;2019:6953963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Shahbazi MA, Sedighi M, Bauleth-Ramos T, Kant K, Correia A, Poursina N, Sarmento B, Hirvonen J, Santos HA. Targeted Reinforcement of Macrophage Reprogramming Toward M2 Polarization by IL-4-Loaded Hyaluronic Acid Particles. ACS Omega. 2018;3:18444-18455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Ming M, Feng L, Shea CR, Soltani K, Zhao B, Han W, Smart RC, Trempus CS, He YY. PTEN positively regulates UVB-induced DNA damage repair. Cancer Res. 2011;71:5287-5295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 880] [Cited by in F6Publishing: 933] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 26. | Zhong H, Wu H, Bai H, Wang M, Wen J, Gong J, Miao M, Yuan F. Panax notoginseng saponins promote liver regeneration through activation of the PI3K/AKT/mTOR cell proliferation pathway and upregulation of the AKT/Bad cell survival pathway in mice. BMC Complement Altern Med. 2019;19:122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Shimizu Y, Suzuki T. Brazilian propolis extract reduces intestinal barrier defects and inflammation in a colitic mouse model. Nutr Res. 2019;69:30-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Lopetuso LR, Jia R, Wang XM, Jia LG, Petito V, Goodman WA, Meddings JB, Cominelli F, Reuter BK, Pizarro TT. Epithelial-specific Toll-like Receptor (TLR)5 Activation Mediates Barrier Dysfunction in Experimental Ileitis. Inflamm Bowel Dis. 2017;23:392-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |