Published online Nov 21, 2019. doi: 10.3748/wjg.v25.i43.6465
Peer-review started: July 25, 2019
First decision: August 27, 2019
Revised: October 24, 2019
Accepted: November 7, 2019
Article in press: November 7, 2019
Published online: November 21, 2019
Ulcerative colitis (UC) is a chronic disease characterized by inflammation of intestinal epithelium, primarily of the colon. An increasing prevalence of metabolic syndrome (MetS) in patients with UC has been documented recently. Still, there is no evidence that MetS alters the course of the UC.
To test the influence of the MetS on the severity of UC and the local and systemic immune status.
Eighty nine patients with de novo histologically confirmed UC were divided in two groups, according to ATP III criteria: Group without MetS (no MetS) and group with MetS.
Clinically and histologically milder disease with higher serum level of immunosuppressive cytokine interleukin-10 (IL-10) and fecal content of Galectin-3 (Gal-3) was observed in subjects with UC and MetS, compared to subjects suffering from UC only. This was accompanied with predomination of IL-10 over pro-inflammatory cytokines tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), and interleukin-17 (IL-17) in the sera as well as Gal-3 over TNF-α and IL-17 in feces of UC patients with MetS. Further, the patients with both conditions (UC and MetS) had higher percentage of IL-10 producing and Gal-3 expressing innate and acquired immune cells in lamina propria.
Local dominance of Gal-3 and IL-10 over pro-inflammatory mediators in patients with MetS may present a mechanism for limiting the inflammatory process and subsequent tissue damage in UC.
Core tip: Metabolic syndrome (MetS) is among most common ulcerative colitis (UC) comorbidity. Still, there is no data considering whether the comorbidity of UC and MetS affects the pathology of UC. The aim of this study was to investigate the effects of MetS on severity and immunopathology of UC. Our results revealed that patients with MetS have milder form of UC accompanied with higher level of Galectin-3 and interleukin-10 and altered functional phenotype and intracellular content of lymphocytes infiltrating affected tissue.
- Citation: Jovanovic M, Simovic Markovic B, Gajovic N, Jurisevic M, Djukic A, Jovanovic I, Arsenijevic N, Lukic A, Zdravkovic N. Metabolic syndrome attenuates ulcerative colitis: Correlation with interleukin-10 and galectin-3 expression. World J Gastroenterol 2019; 25(43): 6465-6482
- URL: https://www.wjgnet.com/1007-9327/full/v25/i43/6465.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i43.6465
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD), characterized by inflammation of the intestinal lamina propria, starting from the rectum and potentially involving the whole colonic mucosa. The course of UC is unpredictable, characterized by spontaneous remission and relapses[1,2]. There is evidence suggesting that the disease occurs in genetically susceptible subjects, triggered by environmental factors, which lead to an exaggerated and uncontrolled immune response to the intestinal flora[1].
It is established that many other diseases are associated with UC such as rheumatoid arthritis, multiple sclerosis, lupus, psoriasis, hypothyroidism, and metabolic syndrome (MetS)[3-6]. Among these diseases, the MetS is the most common comorbidity with pathogenic, clinical and therapeutic implications[3]. MetS represents significant public health concern for its high global prevalence and association with an increased risk for developing chronic diseases[7]. In addition, MetS has been found to have suppressive effect on the immune response, which is confirmed by the higher incidence of unsuccessful vaccinations and complications in infections registered in patients with MetS[8,9].
There is no data considering whether the comorbidity of UC and MetS affects the pathology of UC[3]. Although specific studies dealing with the very mechanism of this aspect have not yet been implemented, this phenomenon deserves attention.
Despite the sustained interest of the researchers in Galectin-3 (Gal-3) and the pronounced and constitutive expression of this molecule in the epithelium of the digestive tract of mice and humans, only a few studies have addressed the possible role of this member of β-galactoside binding proteins in IBDs[10,11]. Gal-3 is produced mainly by monocytes/macrophages and in UC is expressed on CD68+ colon-infiltrating macrophages[12,13].
The aim of our study was to investigate the effects of MetS on severity and immunopathology of UC.
Herewith, we provide the evidence that patients with MetS have milder form of UC accompanied with higher fecal and serum level of Gal-3 and altered functional phenotype and intracellular content of lymphocytes infiltrating affected tissue.
Eighty nine patients (52 male and 37 female), between 21 and 80 years of age, with de novo histologically confirmed UC, were included in observational cross-sectional study. Recruited UC patients were divided in two groups, using ATP III criteria for the diagnosis of MetS: Group without MetS (no MetS) and group with MetS[25]. According to these criteria, for the diagnosis of the MetS, it is necessary that patients have at least 3 of 5 disorders: Disglycemia (fasting plasma glucose higher than 5.5 mmol/L and/or 2 h-post load plasma glucose higher than 7.8 mmol/L or active treatment of disglycemia), arterial hypertension (arterial tension higher than 130/85 mmHg or active treatment), central type of obesity, and high-density lipoprotein cholesterol below and triglycerides higher than reference values. All patients within UC + MetS group fulfill all of these criteria.
In each individual case, the diagnosis and assessment of the severity of UC was determined by histological and clinical scores[14-18]. All endoscopies were performed by the same experienced endoscopist (NZ) thus ensuring uniformity in mucosal assessment. The severity of endoscopic lesions was defined using the Mayo endoscopic sub-score that includes erosions/ulcerations, mucosal erythema, visibility of vascular pattern and bleeding provoked/spontaneous, with scores ranging from 0 to 3[17,18]. The clinical score was determined using the Truelove and Witts clinical activity index and the Mayo clinical index[15,19,20]. Histological score was determined based on Geboes grading[16] and histological sections were examined in a blinded manner by two pathologists, independently. Sections were analyzed for architectural changes, crypt destruction, erosion of the mucous membranes, eosinophilic infiltration, neutrophilic infiltration and chronic inflammatory infiltration. Patients with UC were classified according to Montreal classification of the localization of the UC lesions, as E1 (proctitis), E2 (left-sided colitis), or E3 (pancolitis)[21]. Thirty-four patients had detectable extraintestinal manifestations (fatty liver metamorphosis, primary sclerosing cholangitis, cholelithiasis, bone-joint changes, hematopoietic changes, changes in the reproductive system, eye changes, and dermatologic manifestations- pyoderma gangrenosum or erythema nodosum).
For all study participants, demographic and clinical data were entered in SPSS database. Patients with previously diagnosed colorectal cancer, as well as patients with Crohn’s disease or UC who were previously treated with antibiotics, aminosalicylates, corticosteroids, immunosuppressive agents, statins, and biological therapy were not included in the study. All patients had complete medical history, including physical examination, routine laboratory tests and diagnostic imaging (chest X-ray, abdominal ultrasound, abdominal computed tomography scan, and endoscopy). The study was conducted at Center for Gastroenterology, Clinical Center of Kragujevac and Center for Molecular Medicine and Stem Cell Research, Faculty of Medical Sciences, University of Kragujevac, Serbia and was approved by Ethics Committees of these institutions. Additionally, adherence was made to the Principle of Good Clinical Practice and the Declaration of Helsinki at all times. All patients gave their informed consent for blood and tissue analysis. Patients were under continuous medical supervision at the Clinical Center Kragujevac.
Fecal samples were prepared as previously described[22]. Briefly, 1g of fecal samples was diluted, mixed, and homogenized in 5mL of protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO, United States; P83401)[22]. Blood was obtained from patients and healthy control subjects at 8 am and serums were separated, collected and stored at ﹣80 °C before use. Concentrations of tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-17 (IL-17), and Gal-3 were measured in serum and fecal supernatants of UC patients by using commercially available ELISA tests, according to the manufacturer’s instructions.
Immune cells were isolated from colons of patients with UC, as previously described[23,24]. Briefly, after biopsy, tissue samples were washed and incubate in medium with 1 mmol/L EDTA for 10 min at 37 °C with gentle shaking to remove intestinal epithelial cells. Further, specimens were incubated for 20-30 min in 2 mL Dulbecco's Modified Eagle Medium (Lonza, Basel, Swiss) with 1 mg/mL collagenase type I, 0.1 mg/mL DNase and 1 mg/mL hyaluronidase (Sigma-Aldrich, St-Louis, MO, United States, respectively) without fetal bovine serum at 37 °C. Cells were washed and finally submitted to Ficoll density gradient centrifugation for 20 min at 690 g. The interphase was carefully removed. Single-cell suspensions were obtained and the cells were washed twice with FACS medium.
For flow cytometry, 1 × 106 cells per sample were incubated with anti-human CD4, CD8, CD56, and Gal-3 antibodies conjugated with fluorescein isothiocyanate (FITC; BD Biosciences, Franklin Lakes, NJ, United States), phycoerythrin (PE; BD Biosciences), Peridinin Chlorophyll A Protein (PerCP; BD Biosciences), or allophycocyanin (APC; BD Biosciences). For the intracellular staining, cells were stimulated with phorbol myristate acetate and ionomycin for 4 h at 37 °C with the addition of 1 μg/mL Golgi plug. Intracellular staining for IL-10 and Foxp3 was performed using the BD Bioscience fixation/ permeabilization buffer kit. Flow cytometry was conducted on a BD Biosciences FACSCalibur and the data were analyzed using FlowJo (Tree Star).
Data were analyzed using commercially available software (SPSS version 22). Results were analyzed using Student’s t test, Mann-Whitney U test, Chi-squared test or Kruskal-Wallis test where appropriate. Data are presented as mean ± standard error of the mean and the difference was considered significant when P < 0.05.
Laboratory findings and clinical features of all patients are presented in Table 1. We have compared laboratory findings. Significantly lower white blood cells count (WBC) and increased blood cholesterol, triglycerides, low-density lipoprotein, aspartate aminotransferase, alanine aminotransferase, as well as urea and creatinine were detected in UC patients with MetS, compared to UC patients without MetS (Table 1). There was no significant difference in platelet count and in the concentration of hemoglobin, albumin and globulin between patients with MetS and without MetS (Table 1).
| Characteristics | All (n = 89) | Without MetS (n = 17) | With MetS (n = 72) | P value |
| Age (yr) | ||||
| Median (range) | 50 (21-80) | 34 (23-54) | 53 (21-80) | 0.001 |
| Gender, n (%) | ||||
| Male | 52 (58.43) | 11 (64.71) | 41 (56.95) | > 0.05 |
| Female | 37 (41.57) | 6 (35.29) | 31 (43.05) | |
| Smoking status, n (%) | ||||
| Yes | 42 (47.19) | 7 (41.18) | 35 (48.61) | > 0.05 |
| No | 47 (52.81) | 10 (58.82) | 37 (51.39) | |
| Localization (PT/LC/PC) | 13/51/25 | 3/7/7 | 10/44/18 | > 0.05 |
| Extraintestinal manifestations (+/-) | 34/55 | 7/10 | 27/45 | > 0.05 |
| WBC (109/L) | 8.18 ± 0.48 | 10.27 ± 1.04 | 7.69 ± 0.49 | 0.025 |
| Platelet (109/L) | 377.2 ± 11.4 | 400.80 ± 28.10 | 371.60 ± 11.60 | > 0.05 |
| Hb (g/L) | 125.7 ± 1.97 | 120.90 ± 4.10 | 126.80 ± 2.10 | > 0.05 |
| Cholesterol (mmol/L) | 4.91 ± 0.18 | 3.71 ± 0.21 | 5.19 ± 0.19 | 0.001 |
| Triglycerides (mmol/L) | 1.63 ± 0.11 | 0.89 ± 0.07 | 1.81 ± 0.11 | 0.001 |
| HDL (mmol/L) | 1.54 ± 0.07 | 1.73 ± 0.14 | 1.50 ± 0.07 | > 0.05 |
| LDL (mmol/L) | 2.62 ± 0.18 | 1.49 ± 0.28 | 2.89 ± 0.19 | 0.001 |
| AST (U/L) | 30.08 ± 1.87 | 20.65 ± 2.27 | 32.31 ± 2.07 | 0.002 |
| ALT (U/L) | 33.19 ± 4.50 | 23.29 ± 2.24 | 35.53 ± 5.28 | 0.039 |
| GGT (U/L) | 36.00 ± 6.70 | 20.94 ± 2.51 | 39.56 ± 7.88 | > 0.05 |
| Urea (mmol/L) | 5.13 ± 0.30 | 3.69 ± 0.43 | 5.47 ± 0.33 | 0.014 |
| Creatinine (µmol/L) | 81.89 ± 2.79 | 68.71 ± 3.31 | 85.00 ± 3.09 | 0.014 |
| Albumin (g/L) | 41.33 ± 0.88 | 41.88 ± 0.88 | 41.19 ± 0.40 | > 0.05 |
| Globulin (g/L) | 27.44 ± 0.37 | 27.82 ± 0.59 | 27.35 ± 0.42 | > 0.05 |
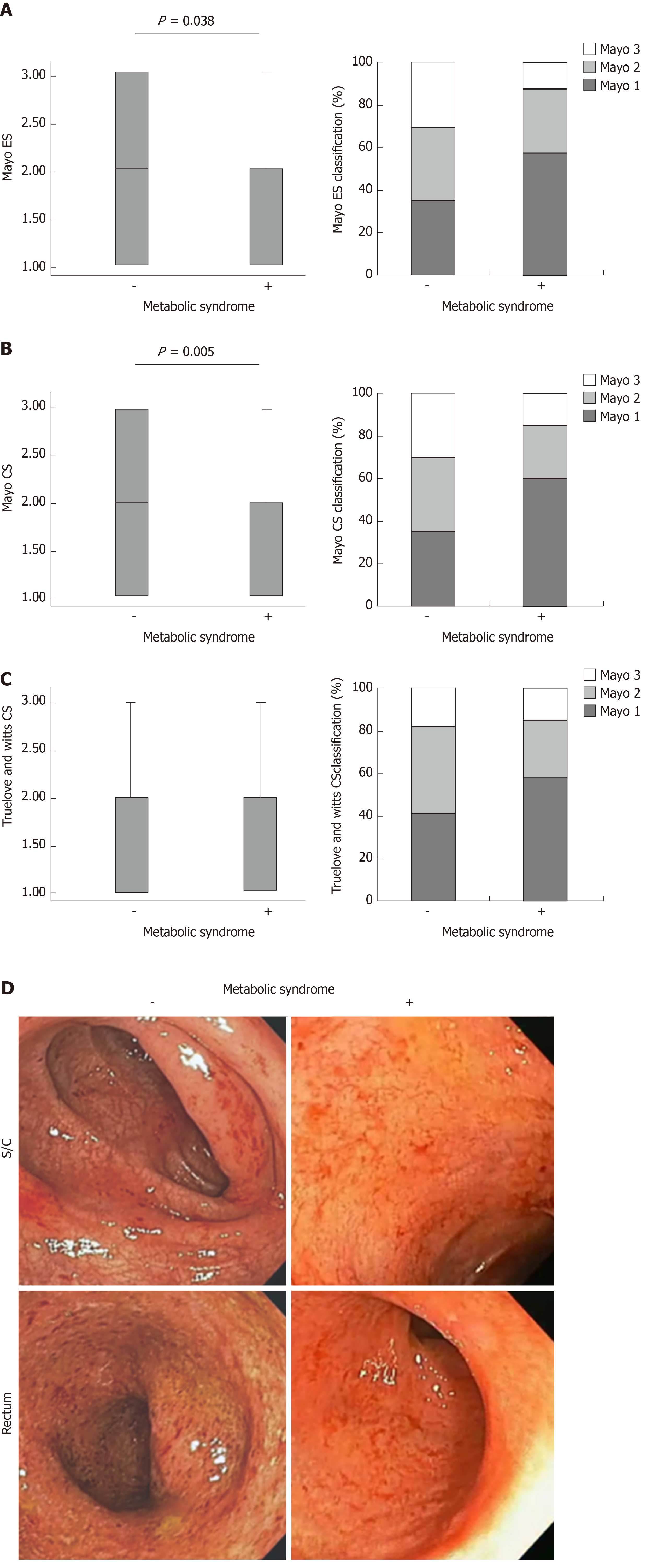
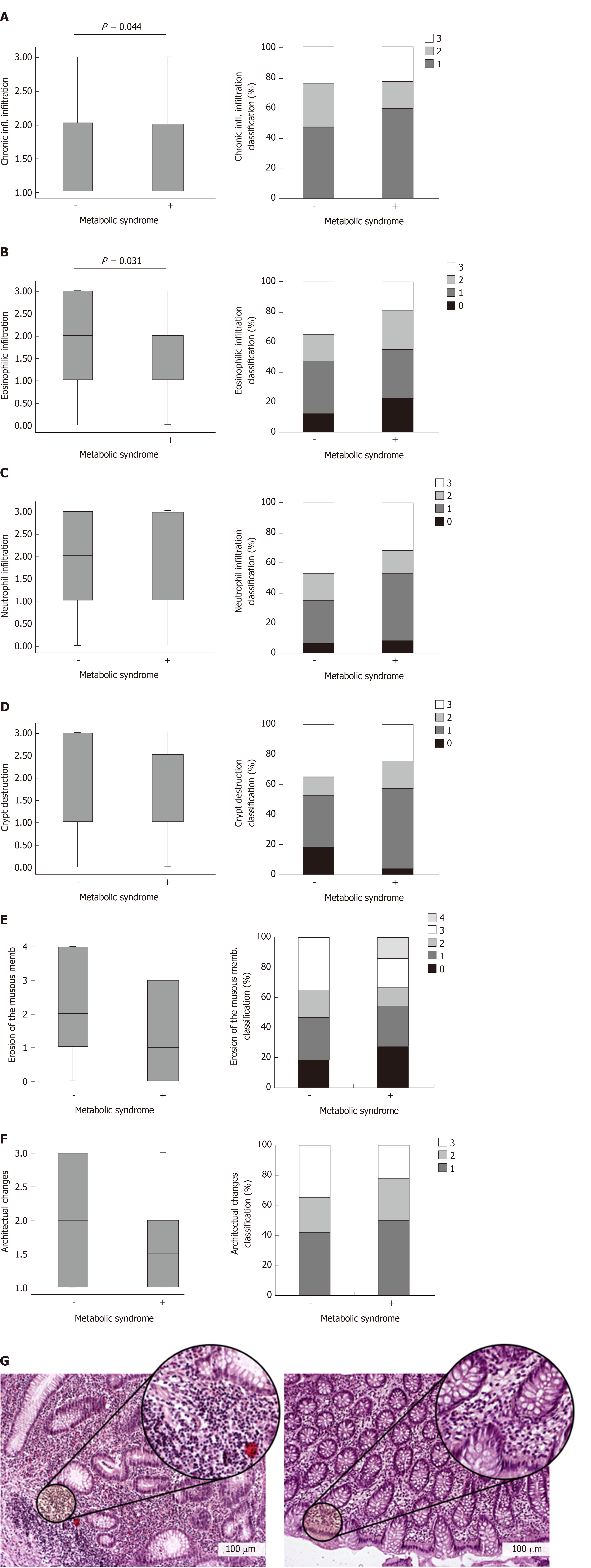
Despite the fact that cholesterol, triglycerides and liver enzymes were higher in MetS, the local findings of UC were milder in MetS + UC patients. Patients with UC and MetS had significantly lower Mayo endoscopic subscore (P = 0.038; Figure 1A) and Mayo clinical score (P = 0.005; Figure 1B). Within these group of patients (UC + MetS), Mayo ES 1 was recorded in 56.95% patients, while Mayo CS 1 was recorded in 59.72% patients. Therefore, the Mayo ESs and CSs classified the majority of the patients with MetS as having mild UC. We have also registered lower Truelove and Witts clinical score of disease in patients with MetS, but the difference did not reach statistical significance (Figure 1C). Endoscopic data are illustrated in Figure 1D. Endoscopic findings in patients with UC + Mets revealed normal mucosa or slight mucosal erythema, decreased vascular pattern, mild friability, comparing to frank friability, marked erythema, absent vascular pattern and erosions that are characteristic for UC patients without MetS (Figure 1D). Clinical and endoscopic data are supported by pathohistological findings (Figure 2): Chronic inflammatory infiltration (P = 0.044; Figure 2A) and eosinophilic infiltration (P = 0.031; Figure 2B) in affected tissue of UC patients with MetS were milder than in UC patients without MetS. Neutrophil infiltration, crypt destruction, erosion of the mucous membranes, architectural changes were also milder in UC patients with MetS, but these differences did not reach statistical significance (Figure 2C-F). Representative pathohistological characteristics are shown in Figure 2G.
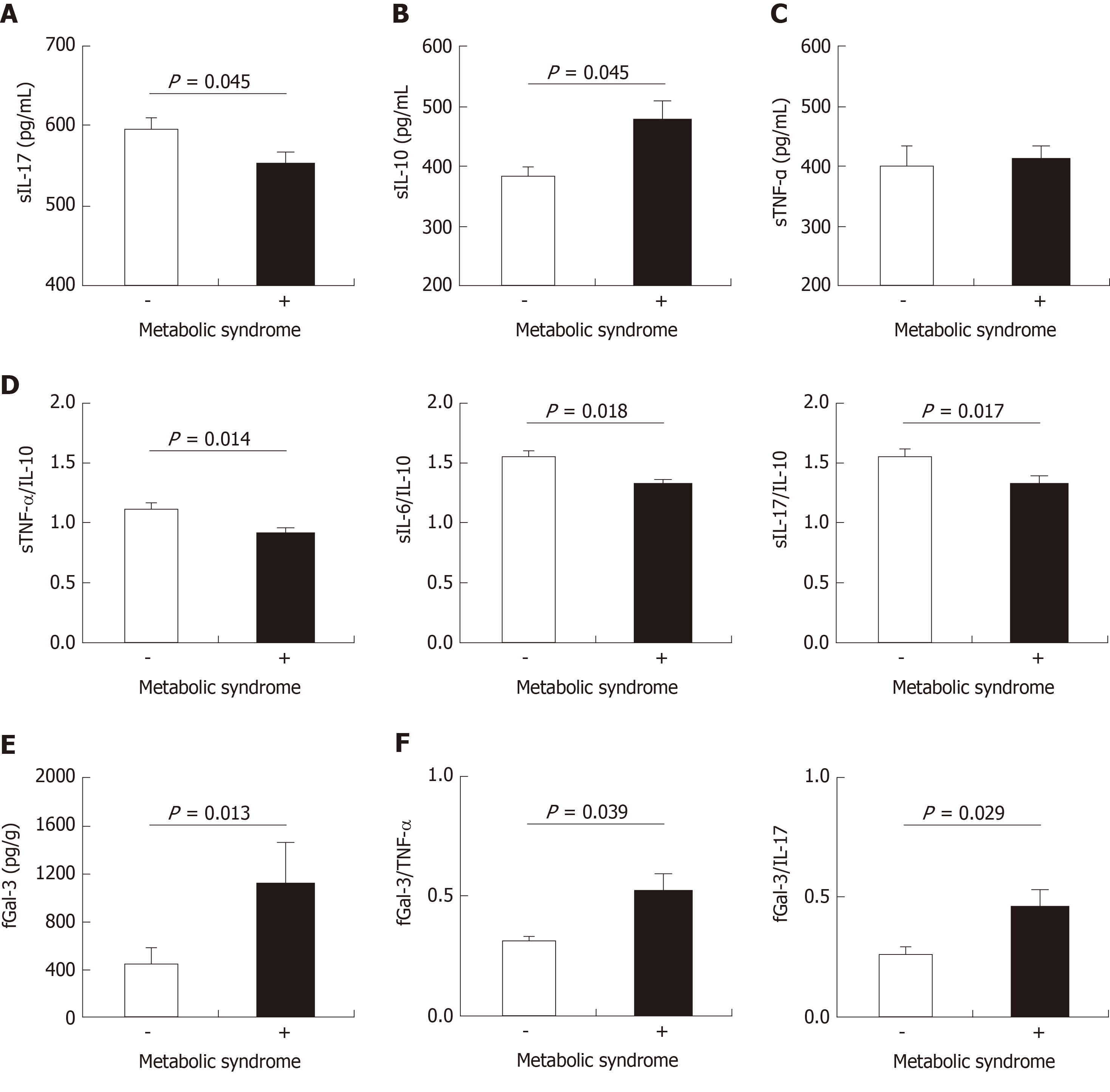
We have assessed concentration of pro and anti-inflammatory cytokines in sera and fecal liquid fraction of all UC patients (Figure 3). Patients with MetS had significantly lower serum level of pro-inflammatory cytokine IL-17 (P = 0.045; Figure 3A), while immunosuppressive IL-10 was significantly higher (P = 0.045; Figure 3B). There was no significant difference in systemic concentration of proinflammatory TNF-α between patients with and without MetS (P = 0.542; Figure 3C).
It is considered that the ratio of counter-regulatory cytokines can be relevant indicator of disease activity[22]. Ratios of TNF-α/IL-10, IL-6/IL-10, and IL-17/IL-10 were significantly lower in the group of patients with MetS (P = 0.014; P = 0.018; P = 0.017; respectively; Figure 3D).
In feces samples, there was no significant difference in the concentration of any tested cytokine. However, there was strikingly higher level of Gal-3 in UC + MetS patients, compared to UC patients (Figure 3E). Accordingly, ratios of Gal-3 and the two inflammatory cytokines (TNF-α and IL-17, were significantly higher in UC + MetS patients in comparison to UC patients (P = 0.039; P = 0.029; respectively; Figure 3F).
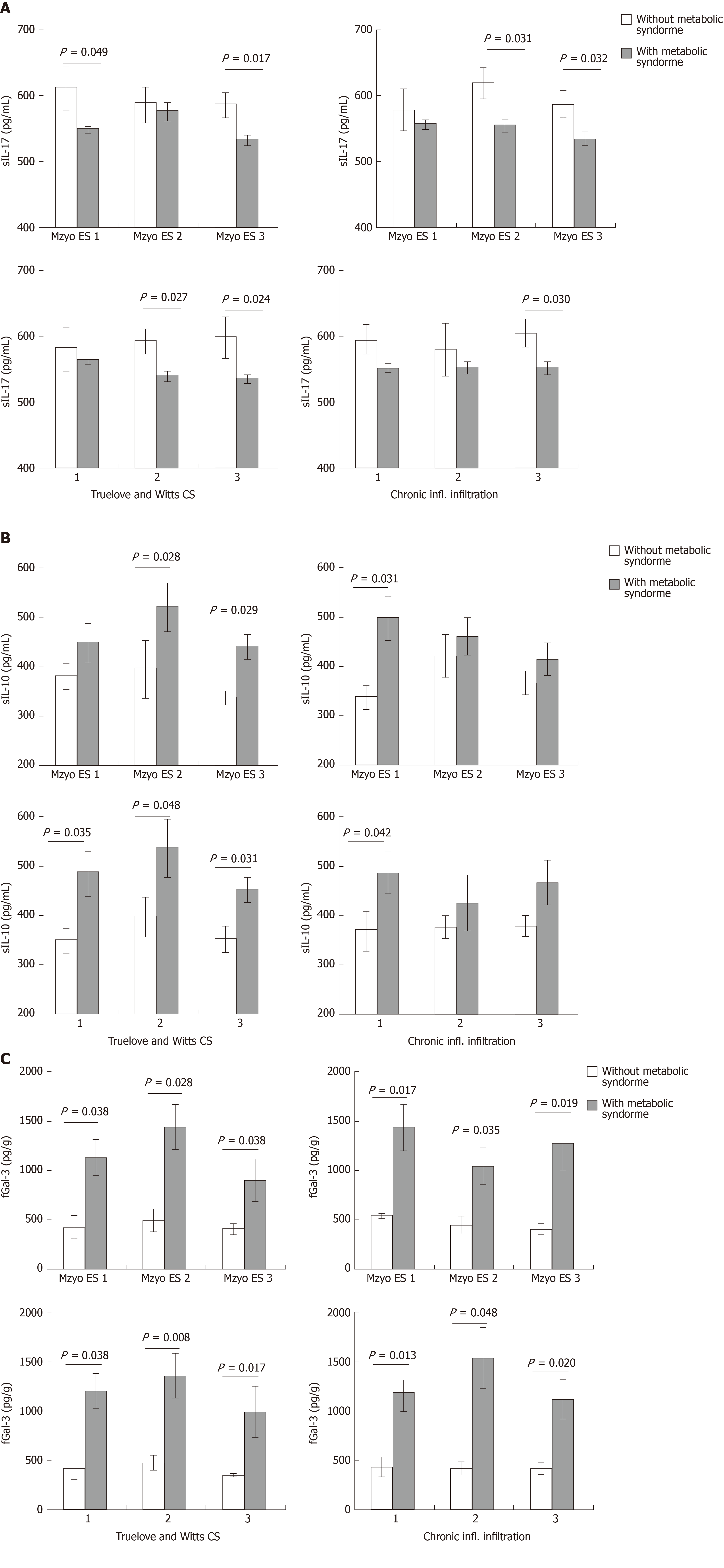
We further analyzed serum level of cytokines of interest and fecal level of Gal-3 in groups with and without MetS in especially same clinical, endoscopic and histopathological stage of UC, respectively. We detected significantly lower serum level of IL-17 in MetS patients with Mayo endoscopic subscore 1 (P = 0.049) and 3 (P = 0.017), Mayo clinical score 2 (P = 0.031) and 3 (P = 0.032), Truelove and Witts clinical score 2 (P = 0.027) and 3 (P = 0.024) as well as chronic inflammatory infiltration score 3 (P = 0.030), in comparison to UC patients without MetS but in exactly the same scores (Figure 4A). There was no significant difference in systemic concentration of TNF-α between patients with and without MetS in same endoscopic, clinical and histopathological scores (data not shown). Higher serum level of IL-10 was detected in MetS patients with Mayo endoscopic subscore 2 (P = 0.028) and 3 (P = 0.029), Mayo clinical score 1 (P = 0.031), all 3 Truelove and Witts clinical scores (P = 0.035; P = 0.048; P = 0.031, respectively) and chronic inflammatory infiltration score 1 (P = 0.042) (Figure 4B). Higher level of Gal-3 in feces was observed in MetS patients with all Mayo endoscopic subscores (P = 0.038; P = 0.028; P = 0.038, respectively), Mayo clinical scores (P = 0.017; P = 0.035; P = 0.019, respectively) Truelove and Witts clinical scores (P = 0.038; P = 0.008; P = 0.017, respectively) and chronic inflammatory infiltration scores (P = 0.013; P =0.048; P = 0.020, respectively) (Figure 4C). The same trend was observed for cytokines of interest in all other histopathological scores (eosinophilic infiltration, neutrophil infiltration, crypt destruction, erosion of the mucous membranes, architectural changes), but the difference did not reached statistical significance (data not shown).
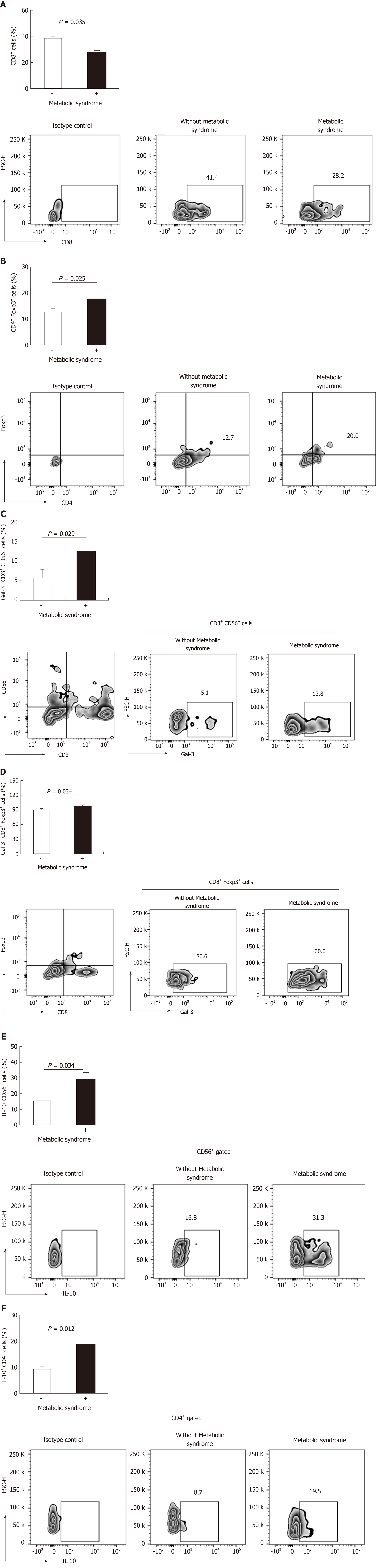
Flow cytometric analysis of colon infiltrating lymphocytes showed no significant differences in the percentage of CD56+ NK cells, CD3+CD56+ NKT cells, CD4+Th cells and CD19+ B cells (data not shown). Significantly lower percentage of CD8+ T cells (P = 0.035) and higher percentage of CD4+Foxp3+ regulatory T cells (P = 0.025) were detected in UC patients with MetS (Figure 5A and B). Interestingly, percentage of both, CD3+CD56+ NKT cells and CD8+Foxp3+ regulatory T cells expressing Gal-3 was significantly higher in UC + MetS patients (P = 0.029; P = 0.034; respectively; Figure 5C and D). Finally, we analyzed cytokine content in infiltrating immune cells. We have not found the difference in the percentage of infiltrating Th cells, CD8+ T cells and NK cells producing pro-inflammatory cytokines IFN-γ and IL-17 (data not shown). There was higher percentage of regulatory innate CD56+ NK cells and CD4+Th cells producing IL-10 in lamina propria of patients with UC + MetS vs UC only (P = 0.034; P = 0.012; respectively; Figure 5E and F).
We analyzed the effect of MetS as comorbidity in patients with UC. In this study we included de novo histologically confirmed UC patients without previous treatment with antibiotics, aminosalicylates for at least two mo, without corticosteroids, statins, immunosuppressive agents as well as any kind of biological therapy previously. The limitation of our work is that this was cross-sectional study with only one time point evaluation. Patients with MetS are significantly older than patients without MetS (Table 1). As all patients were de novo diagnosed with UC and MetS, we could not elucidate the influence of MetS durability on UC severity. Our findings suggest that in general MetS attenuates inflammatory and immunopathogenic correlates of UC. Protective effect of MetS is reflected by clinical and endoscopic score (Figure 1) as well as on histopathology (Figure 2), phenotype of inflammatory cells (Figure 4) and cytokine levels in liquid fraction of feces (Figure 3).
It is believed that immunopathology is the main mechanism in the genesis and progression of UC[1]. The destruction of the intestinal epithelium, which is directly related to the severity of the disease, is due to an intense immune response[26]. There are ample evidences that both, cells of innate and acquired immunity participate in immunopathogenesis of UC[1,2]. This is clearly showed in human[11-13] and experimental studies in animal models by us[10] and others[27-29]. It appears that MetS favors immunosuppressive environment in diseased colon, as evidenced by increased percentage of Foxp3+ regulatory T cells (Figure 5) and IL-10 production (Figures 3 and 5). The lower systemic values of pro-inflammatory IL-17, with higher IL-10 values, and lower ratios of TNF-α/IL-10, IL-6/IL-10, and IL-17/IL-10 (Figure 3A-C) support the prevalence of immunosuppressive over pro-inflammatory mediators in the serum of subjects with MetS. In order to clarify the correlation between UC and cytokine levels in patients with or without MetS, we analyzed serum cytokines and fecal Gal-3 in the same endoscopic, clinical or histopathological score. Significantly lower serum level of IL-17 with higher IL-10 values in sera and Gal-3 values in feces in MetS patients with almost all Mayo endoscopic subscores, Mayo clinical scores, Truelove and Witts clinical scores and chronic inflammatory infiltration scores (Figure 4) implicate that disease severity does not affect difference in the concentration of systemic and fecal proinflammatory and immunosuppressive cytokines between UC patients with and without MetS.
Analysis of functional phenotype of lymphoid cells revealed increased accumulation of IL-10 producing NK cells and Th lymphocytes (Figures 3B, 5E and F), in agreement with higher IL-10 level in the serum of the patients with MetS. In line with our finding, study on UC patients, by Acovic et al[24], revealed that mucosal healing was accompanied by decreased serum and fecal levels of pro-inflammatory cytokines and elevation of anti-inflammatory IL-10 as well as significantly higher percentage of immunosuppressive regulatory T cells- Tregs, IL-10- producing Th lymphocytes and NK cells, indicating that the milder form of UC in subjects with MetS is most likely due to altered local immune response. Other study[30] showed two fold increase of the number of peripheral Th lymphocytes in patients with MetS, compared to healthy controls, with the prevalence of Th2 cells. Increased percentage of Th lymphocytes and reduced percentage of CD8+ T lymphocytes in peripheral blood of patients with MetS which is in line with our results was also recorded[31].
Recent studies have suggested the association of MetSs with immune system dysfunction[32,33]. MetS induces the activation of the immune system in some tissues, which is often manifested by slightly elevated markers of chronic inflammation[32,33], but also negatively affects the immune response, which is confirmed by the higher incidence of unsuccessful vaccinations and complications in infections[8,9].
Gal-3 concentration is significantly increased in feces of UC + MetS patients, as well as Gal-3/TNF-α and Gal-3/IL-17 ratios (Figure 3E and F). Recently, Li et al[34] have shown that Gal-3 causes cellular and systemic insulin resistance. It is also interesting that Gal-3 appears to be involved in protective role of MetS in UC (Figures 3-5). Despite that Gal-3 has been found to promote inflammation in some experimental models[11,35], there is also evidence that it may attenuate pathologic condition in the others[36,37]. Our recent research has shown higher systemic concentration of Gal-3 in end stage renal disease patients infected with hepatitis C virus, suggesting on hepatoprotective role of Gal-3 from virus destruction[38]. Our other study revealed higher fecal concentration of Gal-3 and higher Gal-3/TNF-α ratio in patients with more severe form of colorectal cancer, thus suggesting immunosuppressive effect of Gal-3 on antitumor immune response[39]. Moreover, Tsai et al[40] showed that Gal-3 favors accumulation of regulatory T cells in the colon mucosa which suppresses inflammation and decreases the severity of dextran sulfate sodium-induced colitis. In line with these studies are our results showing significantly higher number of NKT cells and CD8+ regulatory T cells expressing Gal-3 in affected lamina propria derived from UC + MetS patients (Figure 5). Moreover, significantly higher fecal values of Gal-3 and Gal-3/TNF -α and Gal-3/IL-17 ratios (Figure 3D) indicate pronounced local Gal-3 predominance over pro-inflammatory mediators in patients with MetS.
In summary, our data shows for the first time clinically and endoscopically milder disease in UC patients with MetS. The presence of MetS may attenuate colon inflammation, possibly by deviating local inflammatory response toward enhanced participation of immunosuppressive cells and molecules. The increase in systemic IL-10 and local Gal-3 production as well as expression on Tregs and immunocompetent cells accumulating in affected colon tissue implicate on IL-10 and Gal-3 dependent immunomodulation. The precise mechanism of Gal-3 effect in MetS and UC comorbidity is still to be clarified.
Ulcerative colitis (UC) is a chronic disease associated with many other diseases such as rheumatoid arthritis, multiple sclerosis, lupus, psoriasis, hypothyroidism, and metabolic syndrome (MetS). Among these diseases, the MetS is the most common comorbidity. There is no evidence considering whether the comorbidity with MetS alters the course of the UC.
We hope to offer reliable evidence that MetS affects the outcome of the UC, given the increasingly common comorbidity.
Test the impact of the MetS on the severity of UC and the local and systemic immune response.
A total of 89 patients with de novo confirmed UC were enrolled in this cross-sectional study, and they were further divided in two groups, according to ATP III criteria: group without MetS (no MetS) and group with MetS. Severity of UC was determined by histological and clinical scores, fecal and serum cytokines levels were determined using an enzyme-linked immunosorbent assay, while cellular makeup of colon infiltrations was determined by flow cytometry.
Research resultsWhen compared to UC patients without MetS, clinically and histologically milder disease with higher serum level of immunosuppressive cytokine interleukin- 10 (IL-10) and fecal content of Galectin-3 (Gal-3) was observed in subjects with UC and MetS. This was accompanied with predomination of IL-10 over pro-inflammatory cytokines tumor necrosis factor α (TNF-α), interleukin-6, and interleukin-17 (IL-17) in the sera as well as Gal-3 over TNF-α and IL-17 in feces of UC patients with MetS. Significantly lower systemic values of IL-17, higher values of IL-10 and Gal-3 values in feces were determined in MetS patients in especially same clinical, endoscopic and histopathological stage of UC as patients without MetS. In addition, UC + MetS patients had higher percentage of IL-10 producing and Gal-3 expressing innate and acquired immune cells in lamina propria of affected colon tissue.
UC patients with MetS have clinically and histologically milder disease. Predominance of Gal-3 and IL-10 over pro-inflammatory mediators in patients with MetS may present a mechanism for limiting the inflammatory process and subsequent tissue damage in UC.
Future studies are needed to investigate the exact mechanism underlying the protective effect of MetS in biology of UC. And it is necessary to determinate the influence of developmental stages of MetS on the severity of UC. Large sample size studies are also required to confirm the current findings.
We particularly want to thank Milomir Simovic MD, PhD from United States. Army Institute of Surgical Research, Department of Pathology for verifying the language of the manuscript. Also the authors would like to thank Aleksandar Ilic and Milan Milojevic for excellent technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Serbia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiba T, Blanco JR S-Editor: Tang JZ L-Editor: A E-Editor: Ma YJ
| 1. | Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1309] [Cited by in F6Publishing: 1307] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 2. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2894] [Cited by in F6Publishing: 3114] [Article Influence: 183.2] [Reference Citation Analysis (8)] |
| 3. | Maconi G, Furfaro F, Sciurti R, Bezzio C, Ardizzone S, de Franchis R. Glucose intolerance and diabetes mellitus in ulcerative colitis: pathogenetic and therapeutic implications. World J Gastroenterol. 2014;20:3507-3515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 386] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 5. | Cohen R, Robinson D, Paramore C, Fraeman K, Renahan K, Bala M. Autoimmune disease concomitance among inflammatory bowel disease patients in the United States, 2001-2002. Inflamm Bowel Dis. 2008;14:738-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Bardella MT, Elli L, De Matteis S, Floriani I, Torri V, Piodi L. Autoimmune disorders in patients affected by celiac sprue and inflammatory bowel disease. Ann Med. 2009;41:139-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769-1778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1211] [Cited by in F6Publishing: 1192] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 8. | Bandaru P, Rajkumar H, Nappanveettil G. The impact of obesity on immune response to infection and vaccine: an insight into plausible mechanisms. Endocrinol Metab Syndr. 2013;2:113. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, Holland LA, Weir S, Noah TL, Beck MA. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (. Lond). 2012;36:1072-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 10. | Simovic Markovic B, Nikolic A, Gazdic M, Bojic S, Vucicevic L, Kosic M, Mitrovic S, Milosavljevic M, Besra G, Trajkovic V, Arsenijevic N, Lukic ML, Volarevic V. Galectin-3 Plays an Important Pro-inflammatory Role in the Induction Phase of Acute Colitis by Promoting Activation of NLRP3 Inflammasome and Production of IL-1β in Macrophages. J Crohns Colitis. 2016;10:593-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Frol'ová L, Smetana K, Borovská D, Kitanovicová A, Klimesová K, Janatková I, Malícková K, Lukás M, Drastich P, Benes Z, Tucková L, Manning JC, André S, Gabius HJ, Tlaskalová-Hogenová H. Detection of galectin-3 in patients with inflammatory bowel diseases: new serum marker of active forms of IBD? Inflamm Res. 2009;58:503-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Müller S, Schaffer T, Flogerzi B, Fleetwood A, Weimann R, Schoepfer AM, Seibold F. Galectin-3 modulates T cell activity and is reduced in the inflamed intestinal epithelium in IBD. Inflamm Bowel Dis. 2006;12:588-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Brazowski E, Dotan I, Tulchinsky H, Filip I, Eisenthal A. Galectin-3 expression in pouchitis in patients with ulcerative colitis who underwent ileal pouch-anal anastomosis (IPAA). Pathol Res Pract. 2009;205:551-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 15. | TRUELOVE SC, WITTS LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1832] [Cited by in F6Publishing: 1777] [Article Influence: 25.8] [Reference Citation Analysis (1)] |
| 16. | Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 625] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 17. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F. European Crohn´s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1024] [Cited by in F6Publishing: 1094] [Article Influence: 156.3] [Reference Citation Analysis (0)] |
| 18. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2744] [Cited by in F6Publishing: 2693] [Article Influence: 141.7] [Reference Citation Analysis (2)] |
| 19. | Gomollón F, García-López S, Sicilia B, Gisbert JP, Hinojosa J; Grupo Espa˜nol de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa. Therapeutic guidelines on ulcerative colitis: a GRADE methodology based effort of GETECCU. Gastroenterol Hepatol. 2013;36:104-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Walsh AJ, Ghosh A, Brain AO, Buchel O, Burger D, Thomas S, White L, Collins GS, Keshav S, Travis SP. Comparing disease activity indices in ulcerative colitis. J Crohns Colitis. 2014;8:318-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1970] [Cited by in F6Publishing: 2080] [Article Influence: 115.6] [Reference Citation Analysis (2)] |
| 22. | Jovanovic M, Gajovic N, Jurisevic M, Simovic-Markovic B, Maric V, Jovanovic M, Arsenijevic N, Zdravkovic N. Fecal sST2 correlates with disease severity of ulcerative colitis. Vojnosanit pregl. 2018;In press. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Rogler G, Hausmann M, Vogl D, Aschenbrenner E, Andus T, Falk W, Andreesen R, Schölmerich J, Gross V. Isolation and phenotypic characterization of colonic macrophages. Clin Exp Immunol. 1998;112:205-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 142] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Acovic A, Simovic Markovic B, Gazdic M, Arsenijevic A, Jovicic N, Gajovic N, Jovanovic M, Zdravkovic N, Kanjevac T, Harrell CR, Fellabaum C, Dolicanin Z, Djonov V, Arsenijevic N, Lukic ML, Volarevic V. Indoleamine 2,3-dioxygenase-dependent expansion of T-regulatory cells maintains mucosal healing in ulcerative colitis. Therap Adv Gastroenterol. 2018;11:1756284818793558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1297] [Cited by in F6Publishing: 1149] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 26. | Krausgruber T, Schiering C, Adelmann K, Harrison OJ, Chomka A, Pearson C, Ahern PP, Shale M, Oukka M, Powrie F. T-bet is a key modulator of IL-23-driven pathogenic CD4(+) T cell responses in the intestine. Nat Commun. 2016;7:11627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Knosp CA, Schiering C, Spence S, Carroll HP, Nel HJ, Osbourn M, Jackson R, Lyubomska O, Malissen B, Ingram R, Fitzgerald DC, Powrie F, Fallon PG, Johnston JA, Kissenpfennig A. Regulation of Foxp3+ inducible regulatory T cell stability by SOCS2. J Immunol. 2013;190:3235-3245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol. 2010;40:1877-1883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Hall LJ, Murphy CT, Quinlan A, Hurley G, Shanahan F, Nally K, Melgar S. Natural killer cells protect mice from DSS-induced colitis by regulating neutrophil function via the NKG2A receptor. Mucosal Immunol. 2013;6:1016-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | van der Weerd K, Dik WA, Schrijver B, Schweitzer DH, Langerak AW, Drexhage HA, Kiewiet RM, van Aken MO, van Huisstede A, van Dongen JJ, van der Lelij AJ, Staal FJ, van Hagen PM. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes. 2012;61:401-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 31. | O'Rourke RW, Kay T, Scholz MH, Diggs B, Jobe BA, Lewinsohn DM, Bakke AC. Alterations in T-cell subset frequency in peripheral blood in obesity. Obes Surg. 2005;15:1463-1468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13:707-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 322] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 33. | Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1468] [Cited by in F6Publishing: 1558] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 34. | Li P, Liu S, Lu M, Bandyopadhyay G, Oh D, Imamura T, Johnson AMF, Sears D, Shen Z, Cui B, Kong L, Hou S, Liang X, Iovino S, Watkins SM, Ying W, Osborn O, Wollam J, Brenner M, Olefsky JM. Hematopoietic-Derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell. 2016;167:973-984.e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 35. | Volarevic V, Milovanovic M, Ljujic B, Pejnovic N, Arsenijevic N, Nilsson U, Leffler H, Lukic ML. Galectin-3 deficiency prevents concanavalin A-induced hepatitis in mice. Hepatology. 2012;55:1954-1964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Arsenijevic A, Milovanovic M, Milovanovic J, Stojanovic B, Zdravkovic N, Leung PS, Liu FT, Gershwin ME, Lukic ML. Deletion of Galectin-3 Enhances Xenobiotic Induced Murine Primary Biliary Cholangitis by Facilitating Apoptosis of BECs and Release of Autoantigens. Sci Rep. 2016;6:23348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Pejnovic NN, Pantic JM, Jovanovic IP, Radosavljevic GD, Milovanovic MZ, Nikolic IG, Zdravkovic NS, Djukic AL, Arsenijevic NN, Lukic ML. Galectin-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets. Diabetes. 2013;62:1932-1944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Lukic R, Gajovic N, Jovanovic I, Jurisevic M, Mijailovic Z, Maric V, Popovska Jovicic B, Arsenijevic N. Potential Hepatoprotective Role of Galectin-3 during HCV Infection in End-Stage Renal Disease Patients. Dis Markers. 2017;2017:6275987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Jovanovic M, Gajovic N, Zdravkovic N, Jovanovic M, Jurisevic M, Vojvodic D, Maric V, Arsenijevic A, Jovanovic I. Fecal Galectin-3: A New Promising Biomarker for Severity and Progression of Colorectal Carcinoma. Mediators Inflamm. 2018;2018:8031328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Tsai HF, Wu CS, Chen YL, Liao HJ, Chyuan IT, Hsu PN. Galectin-3 suppresses mucosal inflammation and reduces disease severity in experimental colitis. J Mol Med (Berl). 2016;94:545-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |