Published online Jan 28, 2019. doi: 10.3748/wjg.v25.i4.398
Peer-review started: November 30, 2018
First decision: January 11, 2019
Revised: January 15, 2019
Accepted: January 18, 2019
Article in press: January 18, 2019
Published online: January 28, 2019
Hepatotropic viruses induced hepatitis progresses much faster and causes more liver- related health problems in people co-infected with human immunodeficiency virus (HIV). Although treatment with antiretroviral therapy has extended the life expectancy of people with HIV, liver disease induced by hepatitis B virus (HBV) and hepatitis C virus (HCV) causes significant numbers of non-acquired immune deficiency syndrome (AIDS)-related deaths in co-infected patients. In recent years, new insights into the mechanisms of accelerated fibrosis and liver disease progression in HIV/HCV and HIV/HBV co-infections have been reported. In this paper, we review recent studies examining the natural history and pathogenesis of liver disease in HIV-HCV/HBV co-infection in the era of direct acting antivirals (DAA) and antiretroviral therapy (ART). We also review the novel therapeutics for management of HIV/HCV and HIV/HBV co-infected individuals.
Core tip: In this review, we summarized the literature and our recent findings on liver damage associated with co-infection with human immunodeficiency virus (HIV) and hepatotropic viruses [hepatitis C virus (HCV), hepatitis B virus (HBV)]. The combination of HIV with HCV or HBV causes progressive liver injury and chronic liver inflammation ultimately leading to end-stage liver disease, such as cirrhosis and hepatocellular carcinoma. These outcomes are related to many events including apoptosis-mediated cross-talk between liver parenchymal and non-parenchymal cells, accumulation of inflammatory cells in the liver, microbial translocation and impaired immune responses. The treatment of these co-infections requires the combination of direct acting antiviral (DAA) and antiretroviral therapy (ART) for HCV + HIV and comparatively high doses of DAA for HBV, which should be controlled for drug-drug interactions to avoid hepatotoxicity.
- Citation: Ganesan M, Poluektova LY, Kharbanda KK, Osna NA. Human immunodeficiency virus and hepatotropic viruses co-morbidities as the inducers of liver injury progression. World J Gastroenterol 2019; 25(4): 398-410
- URL: https://www.wjgnet.com/1007-9327/full/v25/i4/398.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i4.398
Human immunodeficiency virus (HIV)-related liver disease has become one of the most common causes of increased non-acquired immune deficiency syndrome (AIDS) mortality in HIV-infected people[1]. Major co-morbidities that drive this liver disease progression are co-infections with hepatotropic viruses as well as alcohol and drug abuse. Recently, we published a review article on the mechanisms of liver cells targeting by HIV and the role of alcohol in potentiation of hepatocyte permissiveness to HIV-infection[2]. Here, we overview another crucial factor i.e., co-infection with hepatotropic viruses, such as hepatitis B virus (HBV) and hepatitis C virus (HCV) that promotes liver injury progression. We will also address the therapeutic strategies to reduce HIV-mediated liver damage in the presence of these co-morbidities that could ultimately mitigate/prevent the development of end-stage liver disease.
It has been reported that of 170 million people chronically infected with HCV, 4 to 5 million persons are co-infected with HIV worldwide[3-7]. As estimated, up to 25% of HIV- infected population in the United States are also co-infected with HCV[8]. Co-infection with HCV and HIV has been associated with a faster progression of hepatitis and a higher liver-related mortality even in the era of antiretroviral therapy (ART)[9,10]. The exact role of HCV-infection in the natural history of HIV-infection is not clear. As reported, while HCV infection is not associated with an increase in AIDS-related events or deaths, co-infected individuals may have lower CD4+ T cell counts as compared to HIV-mono-infected patients[11,12]. In contrast, HIV co-infection produces numerous adverse effects by increasing replication of HCV[13], slowing down the HCV clearance, enhancing fibrogenesis[14] and decreasing the response to direct acting antiviral (DAA) treatment[10], ultimately increasing liver dysfunction and incidence of death. Eyster et al[15] compared the levels of HCV RNA before and after HIV-seroconversion in HCV-infected patients and reported that HCV RNA levels were enhanced 8-fold in patients co-infected with HIV compared to HCV mono-infected. Furthermore, CD4+ T cell count in these patients correlated with the levels of HCV RNA. In contrast to the 20% HCV RNA clearance because of acute HCV mono-infection, only 5%-10% of HIV/HCV co-infected patients were able to successfully clear the virus[16,17]. Although ART does not directly inhibit HCV replication, the progression of liver disease is reduced in the settings of co-infection by HIV replication inhibition and by CD4+ cell count increase[18,19]. Furthermore, ART enhances HCV-specific T cell responses concurrently with a significant decrease in HCV-RNA levels[20]. In addition, liver-related mortality and hepatic decompensation were shown to be reduced in ART-treated HIV-positive patients with chronic viral hepatitis. Low CD4+ cell counts and a failure to achieve the complete suppression of HIV replication are the risk factors of liver-related death[21,22]. Importantly, the progression to fibrosis in HIV/HCV-co-infected patients is reduced by ART. However, acute and long-term ART-associated hepatotoxicity occurs more frequently in HIV/HCV-co-infected persons since simultaneous ART-DAA treatment is toxic than either treatment with each kinds of drugs alone and is generally not well-tolerated by patients[23,24]. Thus, the opposing effects of ART in mitigating or worsening liver disease in HIV/HCV-co-infected patients have been reported.
The mechanisms by which HIV/HCV co-infection accelerate the liver disease are not well understood. In our recent in vitro study, we observed that hepatocytes co-infected with HIV and HCV expressed higher levels of HCV RNA and HIV RNA than mono-infected cells. Importantly, an enhanced viral RNA expression in co-infection was even more potent in cells exposed to pan-caspase inhibitor since double-infections caused significant hepatocyte apoptosis[25]. In the same study, plating hepatocytes on 2D gels that recapitulated varying liver stiffness revealed that the increased stiffness was accompanied by a higher HCV/HIV RNA and up-regulation of hepatocyte apoptosis in co-infected cells. Our in vitro data suggests that co-infections potentially may enhance hepatocyte apoptosis in patients with liver fibrosis. These events can further perpetuate disease progression based on our studies demonstrating that engulfment of these apoptotic hepatocytes by hepatic stellate cells promotes fibrogenesis[25]. Aforementioned in vitro results were supported by a recent report on increased liver disease severity in HIV/HCV co-infected patients, particularly in patients with elevated liver stiffness[9]. For diagnosing of liver fibrosis/cirrhosis, there is a tendency now to reduce the number of liver biopsies (especially, in United States). Thus, liver stiffness is determined by Fibroscan, and the distribution of liver stiffness by cutoff is reported as as follows: < 7.1 kPa (absent or mild liver fibrosis), 58.0%; > 9.5 kPa (advanced fibrosis), 23.3%; and > 12.5 kPa (cirrhosis)[26]. The search for non-invasive parameters to predict the fibrosis progression becomes an important goal for monitoring HIV/HCV co-infected patients. As indicated, co-infection with HIV and HCV demonstrated an immunosuppressive profile compared to HIV-mono-infection, and in advanced cirrhosis patients, (stiffness > 25 kPa), was associated with the lowest plasma values of T-helper 1 and T-helper 17 response[27]. Furthermore, transient elastography evaluated in a prospective study on a cohort of 154 HCV/HIV co-infected patients identified that elevated alanine amino aspartate (AST) level and liver stiffness at the baseline were increased in individuals under the risk of fibrosis progression[28].
Both HIV and HCV cause inflammation, playing an important role in the development of liver diseases in co-infected patients[29-31]. This inflammation is linked to endothelial dysfunction[32] since there is evidence that cytokine and chemokine production increased the expression of cell adhesion molecules and induce cellular infiltration to the sites of hepatic infection, which finally contributes to tissue damage and fibrosis in HIV/HCV co-infected patients[33]. These studies were confirmed by the researchers from two independent groups who demonstrated that the pro-inflammatory effects on HCV replication were amplified by HIV/HCV co-infection[34] by causing an increase in the levels of cell adhesion molecules, soluble vascular cell adhesion molecule-1 (sVCAM1) and soluble intercellular adhesion molecule-1 (sICAM1), to induce endothelial dysfunction and develop decompensated cirrhosis and death[9]. There is a link between liver inflammation and fibrosis development. Thus, transforming growth factor beta1 (TGF-β1), a central mediator of liver fibrogenesis, is a regulatory cytokine released by numerous cell types during inflammation[35]. In this regard, HIV/HCV co-infection induces a significant increase in TGF-β1 in patients’ liver and serum[7,36,37]. In addition, HIV-gp120 may directly influence hepatic necro-inflammation and fibrosis in HIV/HCV co-infection.
Liver infiltration with immune cells plays a key role in fibrosis progression. Thus, CD4+ T- regulatory cells and activated HIV-specific CD8+ T cells detected in the livers of HIV-1/HCV co-infected patients may promote liver fibrosis via the secretion of tumor necrosis factor alpha (TNFα)[38] or by direct induction of TNFα-related apoptosis-inducing ligand (TRAIL)[39]. In fact, immune activation at HIV-1 infection is associated with increased circulating levels of TRAIL and with TRAIL-induced apoptosis in CD4+ T-cell[40,41]. Hence, the combination of chronic HIV-1 infection with HCV-associated inflammatory changes may result in increased intrahepatic TRAIL levels followed by increased susceptibility of hepatocytes, lymphocytes, Kupffer cells and hepatic stellate cells (HSCs) to TRAIL-mediated apoptosis[42-44].
In HIV/HCV co-infection, oxidative stress is one of the key factors to cause liver injury. It has been demonstrated that the magnitude of oxidative stress and liver injury were more pronounced in HIV/HCV co-infection than in HIV mono-infection[45,46]. Huang et al[47] reported that HIV/HCV co-infection aggravated liver damages compared with HCV mono-infection, and a linkage between HIV-induced oxidative stress and higher incidence rate of advanced liver disease was found in co-infected patients. The same authors indicated that the worsening of liver fibrosis status in HIV/HCV-co-infected patients may be associated with HIV- infection and related, in part, to higher levels of oxidant markers, such as oxidized glutathione and malondialdehyde. In agreement with this report, previous studies also confirmed that the increased levels of oxidative stress markers, including oxidized glutathione, malondialdehyde, 8-hydroxy-2'-deoxyguanosine, activities of electron transport chain enzymes, complex I and complex IV, decreased antioxidants, and impaired mitochondrial transmembrane potential have been observed in HIV/HCV co-infection[48-53]. Moreover, oxidative stress-elicited acceleration of fibrosis and cirrhosis may be a consequence of a long-term exposure to circulating pro-oxidant components induced by HIV/HCV co-infection, which further influences the hepatic microenvironment in HCV-infected patients[47].
Another major cause of systemic immune activation in HIV and HCV mono-infection is the bacterial translocation, which accelerates liver damage in HIV/HCV co-infected patients[31]. The mechanism of liver fibrosis promotion by microbial translocation in HIV/HCV co-infection is associated with activation of liver macrophages and HSC: (1) directly by microbial products, resulting in the secretion of pro-fibrotic cytokines; or (2) indirectly via induction of systemic immune responses and promotion of local hepatocyte activation-induced apoptotic death resulting in collagen deposition[54]. Normally, bacterial translocation products are cleared by liver macrophages, resident Kupffer cells, but, the clearance of these products is compromised by HIV-infection[55], which may cause phenotypic/functional changes or depletion of Kupffer cells.
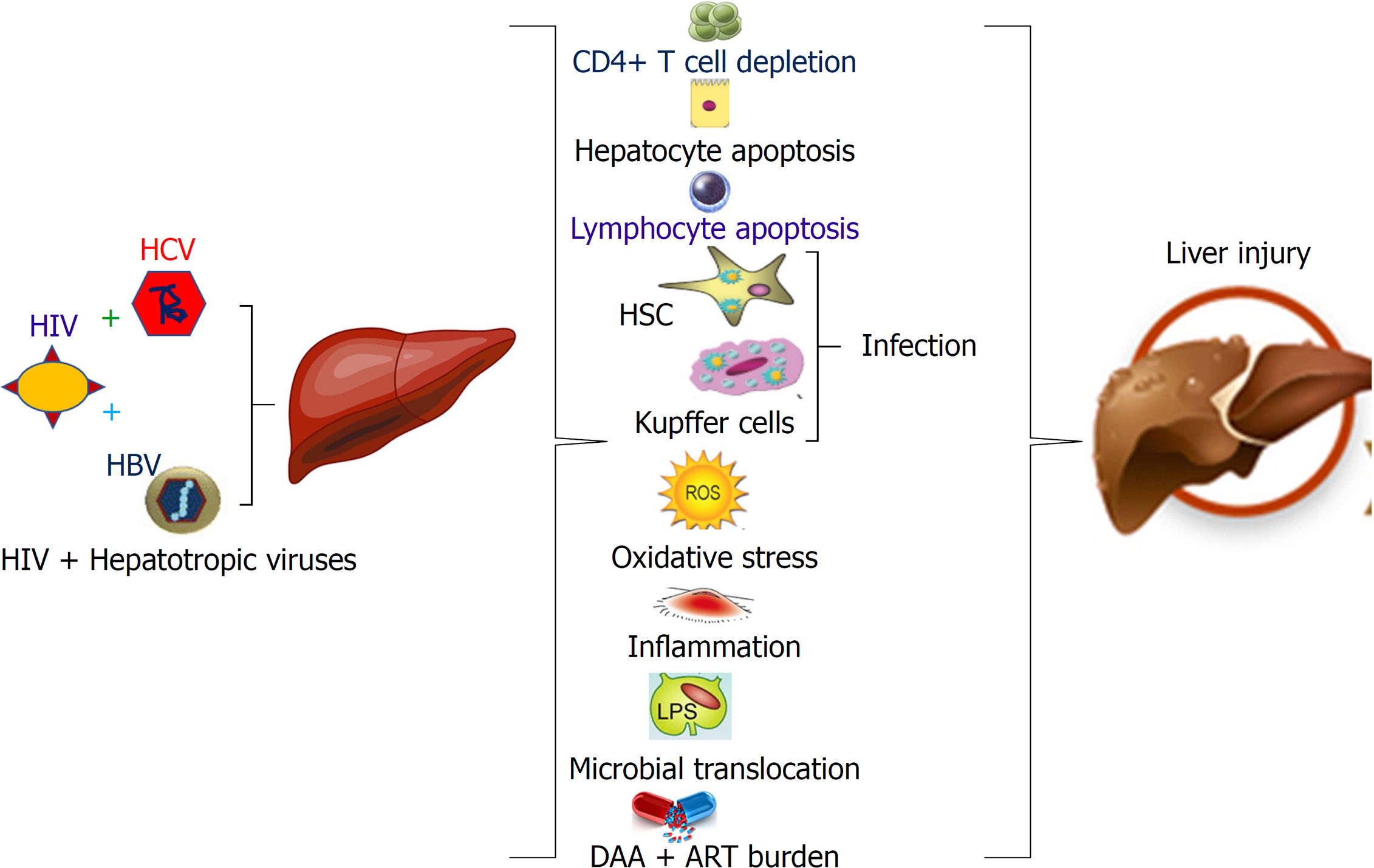
Collectively, the mechanisms to drive the progressive liver disease in HCV/HIV co-infection are multi-component and include HIV-1-induced immune suppression due to CD4+ T-cell depletion, systemic immune activation, increased lymphocyte and hepatocyte apoptosis, oxidative stress, impaired anti-HCV immune responses, promotion of retroviral infection of HSCs and Kupffer cells and microbial translocation. Altogether, it contributes to the increased rates of liver fibrosis[5-7].
Worldwide, about 10% of all people infected with HIV are also chronically co-infected with HBV[4,56]. For two large European and North American HIV treatment cohorts, the prevalence of HBV co-infection was reported as 8.7% and 7.6%, respectively[57]. HBV and HIV have common routes of transmission in endemic areas, though HBV is about 100 times more infectious than HIV[58-60]. Morbidity and mortality in HIV/HBV co-infections is higher than in mono-infections[61,62]. The development of chronic HBV infection in HIV-infected patients is age-dependent: while only < 5% of adults negative for HIV acquire chronic HBV-infection, HBV infection chronically persists in 25% of those co-infected with HIV in adulthood and in 50%-90% of those who were exposed to HIV at birth or in early childhood[63].
Co-infection with HIV completely changes the natural history of HBV-infection since higher serum HBV DNA levels were accompanied by higher rates of cirrhosis, especially in HIV patients with low CD4+ T cell counts[64,65]. Lipopolysaccharide (LPS) serum levels are elevated in HIV/HBV co-infected patients, likely predisposing to similar intrahepatic inflammation and fibrosis[66]. Interestingly, HBV has been shown to suppress TLR-mediated innate immune responses leading to activation of pro-inflammatory cytokines[67]. This could have implications for the development of liver fibrosis during HIV co-infection, but there are no current studies to clarify this issue. TGF-β expression has also been associated with liver fibrosis in HBV-infected patients, but its role in HIV-co-infected patients has not been addressed[68]. In contrast to HIV/HCV co-infection, liver fibrosis mediated through apoptosis-related receptors do not play a key role in HIV/HBV co-infection. It has been hypothesized that HIV alters HBV-specific immune response by affecting the hepatic cytokine environment, thereby promoting liver fibrosis and disease progression; however, this hypothesis has not been thoroughly tested[69,70]. In HIV/HBV co-infection, it is difficult to determine the stage of liver disease[71]. Liver biopsy remains the best method for disease staging because non-invasive measures of hepatic fibrosis have not been broadly used in these patients[72]. The incidence of liver fibrosis in HIV/HBV co-infected patients exceeds by 3-fold when compared to HIV-monoinfected individuals[73]. Liver fibrosis progression and regression were evaluated as endpoints with respect to an extensive list of determinants: host characteristics (age, alcohol consumption etc.), HIV and HBV viral suppression, immunosuppression, antiretroviral and antiviral therapy, biomarkers of HIV/HBV-related liver disease and surrogates of metabolic disorders[74].
In general, HIV infection worsens the course and progression of hepatitis B more than vice versa[75]. The main HIV proteins responsible for activation of cell metabolism trans-activator of transcription (TAT), viral turnover (Vpr), and attachment of virus to cell surface/induction of cell death (gp120), are released from HIV-infected cells and are detectable in the plasma of AIDS patients[76]. These proteins are captured by many cell types, including hepatocytes, thereby accelerating HBV-induced fibrosis and HCC development[77,78]. It has been reported that HIV-induced immunosuppression increases HBV antigen titers, including HBsAg, HBeAg and HbxAg, finally leading to the aggravation of hepatitis[79]. It appears paradoxical that HBV related liver damage, which is an immune‐mediated process, is exacerbated by the HIV-mediated immunodeficient state[69]. However, there are several possible reasons for this paradoxical relationship. In HIV‐infected persons, a rapidly progressive form of liver disease known as fibrosing cholestatic hepatitis, has been attributed to viral cytopathic effect rather than to the immune response[80]. Hence, it is possible that HBV-induced chronic active hepatitis develops not due to anti-HBV-specific immune response, but due to cytotoxic effects of HIV or HIV-induced mutations in HBV, which, in turn, increase HBV cytotoxic properties[81,82]. Studies by Revill et al[81] supported the hypothesis about a novel mutation in the pre-core/core region of the HBV genome and found it to be more common among HIV/HBV co-infected than HBV mono-infected individuals. In fact, co-infected persons with this mutation have higher HBV DNA levels than those without the mutation[69].
As shown previously, there are elevated levels of circulating LPS in HIV/HBV co-infected individuals compared with uninfected controls and HBV monoinfected individuals, but no direct correlation was determined between elevated circulating LPS and liver fibrosis[66]. These observations are consistent with the results of similar studies in HIV/HCV co-infection[83,84]. It is possible that for driving liver disease progression, the concentration of LPS in the portal vein and/or in the liver may be more important than LPS levels in peripheral blood. Recent studies in simian immunodeficiency virus (SIV)-infected rhesus macaques suggested that an increased microbial load in the liver can also trigger chemokine production and upregulate infiltration with CXCR6+-activated natural killer cells, which may contribute to liver fibrosis[85]. It has been reported that the chemokine CXCL10, a chemokine and a ligand for CXCR3 expressed on activated T cells, is associated with elevations in liver enzymes in HIV/HBV co-infection and may contribute to liver disease via migration of activated T-cells to the liver[61,66]. Inhibition of these chemokines may potentially reduce liver disease in HIV/HBV co-infection and should be further explored[61]. It has been reported that HIV- infects productively Kupffer cells, alters their response to translocated microbial products, which breaks their tolerance[86] and may promote hepatic inflammation and fibrosis in HIV-HBV co-infected patients.
The pathogenesis of HIV/HBV co-infection is based on the properties of both viruses and is influenced by differential human cell populations in the liver, which are permissive for each infection. In this regard, HBV, being by itself not a cytopathogenic virus, requires activation of immune response for elimination of HBV-infected hepatocytes. Concordantly, immune cells and namely, CD4+ T-lymphocytes and macrophages, are HIV-permissive and cause disruptions in both innate and adaptive immunity. This disbalance between high infectivity of HBV in hepatocytes and HIV-weaken immune response in the liver is a key feature of HBV/HIV co-infection pathogenesis and a suggested target for co-infection treatment. Thus, the most suitable in vivo model for the studies of co-infection pathobiology and therapeutic applications should combine human hepatocytes and human immune cells, including liver resident macrophages (Kupffer cells) and lymphocytes, which can cross-talk in the settings of both infections.
In HIV/HCV co-infection, the progression to liver cirrhosis and HCC may take about 10-20 years and has been demonstrated even in case of HCV eradication by successful DAA treatment. Thus, a large multi-center study from Spain has reported HCV-related cirrhosis in 7.6% of HIV-infected individuals, and out of them 15% of patients had active HCV, while 31.5% of were those who cleared HCV after anti-HCV therapy[26]. The conclusion from this study was that the burden of HCV-related cirrhosis in HIV-infected patients was very significant even if overall morbidity and mortality was reduced by HCV elimination in this patient cohort. The mechanisms why cirrhosis is progressing in some patients or can be stopped in some individuals currently are not clear. While HCC development also reduced by DAA, HCV/HIV co-infected patients with advanced cirrhosis is still represent a risk group for this outcome, with the highest risk of HCC progression in DAA non-responders. Thus, the heterogenicity of these cirrhotic patients based on various co-morbidities, genetic polymorphism[87-89], late treatment initiation and altered response to treatment may affect the end-stage liver disease progression in coinfected patients.
As reported, ART delays the progression to cirrhosis in HIV/HCV co-infection[90,91]. Patients with undetectable or low levels of HIV RNA tend to develop liver disease gradually compared to patients with detectable viremia[92,93]. Furthermore, HIV treatment reduces the complications of the end-stage liver diseases, such as hepatocellular carcinoma (HCC) and death[94-96]. Hence, as per European and United States guidelines, ART is recommended for co-infected patients regardless of CD4+ T cell count[94,97]. Nevertheless, co-infected patients with advanced liver disease experience a higher pill burden as well as an increased risk of drug-drug interactions and drug-induced liver injury regardless of sustained virologic response (SVR) during simultaneous HCV and HIV therapy[98-100]. Therefore, treatment-of naïve HIV/HCV co-infected patients with CD4+ T cell counts > 500 cells/μL requires treating HCV first, and delaying HIV treatment to reduce the potential for drug toxicity and interactions[94]. For those patients on ART who are likely to be subsequently treated with DAA for HCV, the treatment regimens should be chosen with an assessment of potential drug-drug interactions with the planned HCV therapy in the future. For example, HIV integrase inhibitors, such as dolutegravir and raltegravir, have relatively few drug-drug interactions, while use of HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors might preclude the use of some DAA for HCV treatment, particularly those targeting the HCV protease.
In most of co-infected patients, DAA-based therapy was shown as safe and effective. However, according to the results of the large real-world study, the predictors of failure included male gender, HIV-related immunosuppression, high HIV RNA load, severity of liver disease and suboptimal DAA-based regimens[101]. Baseline NS5A resistance (NS5A RAS) is associated with suppressed response to DAA in HCV-infected patients[102,103]. While low rates of HCV recurrence in HCV/HIV patients after DAA treatment was reported, this outcome cannot be totally excluded[104]. Reinfection by HCV (with other virus genotypes) following successful DAA treatment is also possible[105]. Eradication of HCV by anti-viral therapy does not guarantee prevention of continuous liver injury and fibrosis development and often depends on the stage of fibrosis at the time when the treatment started: it is more successful at early stage of fibrosis and less successful at the later stages[26,106]. In United States, the Veterans Affairs (VA) National Health Care System has the best experience in treating of co-infected patients with DAA since DAA treatment is available for all HCV-infected patients, thereby revolutionizing the treatment outcomes. As the negative predictors of sustained viral response at week 12 (SVR 12), VA studies have reported African-American race/ethnicity and advanced liver disease[107]. At VA national trials, they found high SVR in patients treated with ledispavir/sofosbuvir and paritaprevir/ritonavir/ombitasvir and dasabuvir (PrOD)[108]. In this study, SVR was achieved in 92.8% of genotype 1 HCV patients, 86.2% genotype 2 patients, 74.8% genotype 3 patients and 89.8% genotype 4 patients. Dependence of sensitivity to DAA on HCV genotype was confirmed by other studies: in 1150 co-infected individuals treated with sofosbuvir plus ribavirin, sofosbuvir plus ledipasvir and sofosbuvir plus daclatasvir, SVR was achieved in 91-100% patients; the lowest response was in genotype 3 patients that have cirrhosis (88%) (reviewed by[109]). DAA-based therapy (in combination with ribavirin in half of patients) was generally well tolerated in a Spanish cohort of HIV/HCV co-infected individuals with compensated cirrhosis, with only 1.2% stopping due to adverse events, and the SVR12 rate was the highest among those without cirrhosis (93.5%) compared with those with compensated (91.2%) or decompensated cirrhosis (80.8%) (reviewed by[109].
In HIV/HBV-co-infected patients, early initiation of ART, including the drugs active against both HIV and HBV, is recommended. Among the antiretroviral drugs with dual activity, tenofovir is the preferred choice due to its high potency and high genetic barrier to resistance[110,111]. Wit et al[23] showed that previously, every licensed antiretroviral drug has been associated with liver enzyme elevation. The panel of drugs used for the co-infection treatment includes tenofovir, lamivudine, emtricitabine, entecavir, and adefovir at the doses > 10 mg[112]. However, the combination of drugs efficient for both viruses is very toxic since hepatocytes are not only infected by viruses, but also are the sites of drug metabolism. The role of hepatocytes in handling toxic drug substances makes them vulnerable to drug-induced liver injury (hepatotoxicity) and consequently, to cell death[113,114]. Powderly et al[115] analyzed many drugs used for therapy of HIV/HBV co-infection, including, lamivudine and tenofovir. The results of this study indicated that since HIV/HBV co-infected patients received the combined therapy rather than individual drugs, considerable attention should be paid to hepatotoxic properties of these drugs.
A recent prospective multicenter cohort study found no difference in virologic response between patients with and without lamivudine resistance at baseline throughout 5 years of therapy with tenofovir, emphasizing tenofovir’s suitable resistance profile[90]. It is not clear if hepatitis B combination therapy with tenofovir and lamivudine is more potent than monotherapy with tenofovir. A small randomized clinical trial on 36 patients and a large retrospective observational study failed to show any significant difference in HBV suppression between monotherapy and combination therapy[116,117]. Conversely, another retrospective cohort analysis revealed a superior HBV suppression by lamivudine-tenofovir combination compared with tenofovir monotherapy[118]. In addition, a recent report provides evidence of the benefits of effective ART on virologic and clinical outcomes in HIV and HIV/HBV coinfection, especially regimens that contain potent HBV activity, such as tenofovir disoproxil fumarate (TDF)[119]. However, Boyd et al[74] reported that liver fibrosis decreases only in a small number of HIV-HBV co-infected patients during TDF.
According to Guidelines for the Use of Antiviral Agents in Adults and Adolescents Living with HIV[120], all people with HIV should be screened for HCV. ART slows down the progression of liver disease, and its benefits outweigh concerns regarding the drug-drug interaction. However, drug-drug interactions should be taken into consideration. All HCV/HIV-infected patients should be evaluated for HCV therapy. These patients also need to be screened for HBV since HBV reactivation may happen during HCV treatment with DAA. Persons with HCV/HIV co-infection and active HBV infection should receive ART that includes two agents with anti-HBV activity prior to initiation of HCV therapy.
Significant accomplishments were made in HIV and HCV research during the last 20 years. ART introduction contributed to substantial decrease in HIV-related disease and death. Likewise, better understanding of the hepatotropic virus life cycle and virus-host interactions opened the avenues for the advancement of antiviral drugs accompanied with improved rates of cure among HCV or HBV mono-infected as well as HIV and HCV/HBV co-infected patients. Furthermore, new insight has been made into the multiple mechanisms of HIV and HCV/HBV pathogenesis leading to enhanced disease progression. Despite these developments, HCV or HBV and HIV co-infection continue to cause accelerated liver disease and death in these patients[90].
The mechanisms that drives the progressive liver disease in HIV/HCV and HIV/HBV co-infections are multi-factorial, which includes HIV induced immune suppression due to CD4+ T-cell depletion, systemic immune activation, increased lymphocyte and hepatocyte apoptosis, oxidative stress, inflammation, impaired immune responses, promotion of retroviral infection of HSC and Kupffer cells and microbial translocation (Figure 1). Co-infection can complicate the treatment and management of HIV infection. Therefore, HIV infected patients co-infected with HBV or HCV should seek care from providers with expertise in the management of both HIV and hepatotropic viruses. In this regard, HIV and viral hepatitis testing should be followed by lifesaving care and treatment, which ensures the best possible health outcomes and prevents deaths among persons living with these co-infections.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aghakhani A, Manesis EK, Sagnelli E S- Editor: Ma RY L- Editor: A E- Editor: Yin SY
| 1. | Palella FJ, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD; HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1002] [Cited by in F6Publishing: 1053] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 2. | Ganesan M, Poluektova LY, Kharbanda KK, Osna NA. Liver as a target of human immunodeficiency virus infection. World J Gastroenterol. 2018;24:4728-4737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Shmagel KV, Saidakova EV, Shmagel NG, Korolevskaya LB, Chereshnev VA, Robinson J, Grivel JC, Douek DC, Margolis L, Anthony DD, Lederman MM. Systemic inflammation and liver damage in HIV/hepatitis C virus coinfection. HIV Med. 2016;17:581-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Utsumi T, Lusida MI. Viral hepatitis and human immunodeficiency virus co-infections in Asia. World J Virol. 2015;4:96-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 5. | Kim AY, Chung RT. Coinfection with HIV-1 and HCV-a one-two punch. Gastroenterology. 2009;137:795-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Operskalski EA, Kovacs A. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep. 2011;8:12-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Rotman Y, Liang TJ. Coinfection with hepatitis C virus and human immunodeficiency virus: virological, immunological, and clinical outcomes. J Virol. 2009;83:7366-7374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356:1445-1454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Medrano LM, Garcia-Broncano P, Berenguer J, González-García J, Jiménez-Sousa MÁ, Guardiola JM, Crespo M, Quereda C, Sanz J, Canorea I, Carrero A, Hontañón V, Muñoz-Fernández MÁ, Resino S; GESIDA 3603b Study Group. Elevated liver stiffness is linked to increased biomarkers of inflammation and immune activation in HIV/hepatitis C virus-coinfected patients. AIDS. 2018;32:1095-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Portocarrero Nuñez JA, Gonzalez-Garcia J, Berenguer J, Gallego MJV, Loyarte JAI, Metola L, Bernal E, Navarro G, Del Amo J, Jarrín I; and the Cohort of the Spanish HIV Research Network (CoRIS). Impact of co-infection by hepatitis C virus on immunological and virological response to antiretroviral therapy in HIV-positive patients. Medicine (Baltimore). 2018;97:e12238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Hernando V, Perez-Cachafeiro S, Lewden C, Gonzalez J, Segura F, Oteo JA, Rubio R, Dalmau D, Moreno S, Amo JD; CoRIS. All-cause and liver-related mortality in HIV positive subjects compared to the general population: differences by HCV co-infection. J Hepatol. 2012;57:743-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Chen TY, Ding EL, Seage Iii GR, Kim AY. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis. 2009;49:1605-1615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, Yanny I, Razavi H, Vickerman P. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16:797-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 455] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 14. | Kovari H, Ledergerber B, Cavassini M, Ambrosioni J, Bregenzer A, Stöckle M, Bernasconi E, Kouyos R, Weber R, Rauch A; Swiss HIV Cohort Study. High hepatic and extrahepatic mortality and low treatment uptake in HCV-coinfected persons in the Swiss HIV cohort study between 2001 and 2013. J Hepatol. 2015;63:573-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Eyster ME, Fried MW, Di Bisceglie AM, Goedert JJ. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood. 1994;84:1020-1023. [PubMed] [Cited in This Article: ] |
| 16. | Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, Hu PY, Miller JK, Gerber MA, Sampliner RE. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992;327:1899-1905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1137] [Cited by in F6Publishing: 1157] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 17. | Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, Nolt K, Nelson KE, Strathdee SA, Johnson L, Laeyendecker O, Boitnott J, Wilson LE, Vlahov D. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450-456. [PubMed] [Cited in This Article: ] |
| 18. | Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C, Poynard T. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 942] [Cited by in F6Publishing: 895] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 19. | Pineda JA, González J, Ortega E, Tural C, Macías J, Griffa L, Burgos A; Grafihco Study Team. Prevalence and factors associated with significant liver fibrosis assessed by transient elastometry in HIV/hepatitis C virus-coinfected patients. J Viral Hepat. 2010;17:714-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Rohrbach J, Robinson N, Harcourt G, Hammond E, Gaudieri S, Gorgievski M, Telenti A, Keiser O, Günthard HF, Hirschel B, Hoffmann M, Bernasconi E, Battegay M, Furrer H, Klenerman P, Rauch A; Swiss HIV Cohort Study. Cellular immune responses to HCV core increase and HCV RNA levels decrease during successful antiretroviral therapy. Gut. 2010;59:1252-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Qurishi N, Kreuzberg C, Lüchters G, Effenberger W, Kupfer B, Sauerbruch T, Rockstroh JK, Spengler U. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708-1713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 422] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 22. | Pineda JA, García-García JA, Aguilar-Guisado M, Ríos-Villegas MJ, Ruiz-Morales J, Rivero A, del Valle J, Luque R, Rodríguez-Baño J, González-Serrano M, Camacho A, Macías J, Grilo I, Gómez-Mateos JM; Grupo para el Estudio de las Hepatitis Víricas de la Sociedad Andaluza de Enfermedades Infecciosas (SAEI). Clinical progression of hepatitis C virus-related chronic liver disease in human immunodeficiency virus-infected patients undergoing highly active antiretroviral therapy. Hepatology. 2007;46:622-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JM. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis. 2002;186:23-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 24. | Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, Akerlund B, Calvo G, Monforte Ad, Rickenbach M, Ledergerber B, Phillips AN, Lundgren JD. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 859] [Cited by in F6Publishing: 900] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 25. | Ganesan M, Dagur RS, Makarov E, Poluektova LI, Kidambi S, Osna NA. Matrix stiffness regulate apoptotic cell death in HIV-HCV co-infected hepatocytes: Importance for liver fibrosis progression. Biochem Biophys Res Commun. 2018;500:717-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Berenguer J, Jarrín I, Pérez-Latorre L, Hontañón V, Vivancos MJ, Navarro J, Téllez MJ, Guardiola JM, Iribarren JA, Rivero-Juárez A, Márquez M, Artero A, Morano L, Santos I, Moreno J, Fariñas MC, Galindo MJ, Hernando MA, Montero M, Cifuentes C, Domingo P, Sanz J, Domíngez L, Ferrero OL, De la Fuente B, Rodríguez C, Reus S, Hernández-Quero J, Gaspar G, Pérez-Martínez L, García C, Force L, Veloso S, Losa JE, Vilaró J, Bernal E, Arponen S, Ortí AJ, Chocarro Á, Teira R, Silvariño R, Vegas A, Geijo P, Bisbe J, Esteban H, González-García J; GeSIDA 8514 Study Group. Human Immunodeficiency Virus/Hepatits C Virus Coinfection in Spain: Elimination Is Feasible, but the Burden of Residual Cirrhosis Will Be Significant. Open Forum Infect Dis. 2018;5:ofx258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Garcia-Broncano P, Medrano LM, Berenguer J, González-García J, Jiménez-Sousa MÁ, Carrero A, Hontañón V, Guardiola JM, Crespo M, Quereda C, Sanz J, García-Gómez AB, Jimenez JL, Resino S; GESIDA 3603b Study Group. Dysregulation of the Immune System in HIV/HCV-Coinfected Patients According to Liver Stiffness Status. Cells. 2018;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Gonzalez FA, Van den Eynde E, Perez-Hoyos S, Navarro J, Curran A, Burgos J, Falcó V, Ocaña I, Ribera E, Crespo M. Liver stiffness and aspartate aminotransferase levels predict the risk for liver fibrosis progression in hepatitis C virus/HIV-coinfected patients. HIV Med. 2015;16:211-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Younas M, Psomas C, Reynes J, Corbeau P. Immune activation in the course of HIV-1 infection: Causes, phenotypes and persistence under therapy. HIV Med. 2016;17:89-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 30. | Márquez M, Fernández Gutiérrez del Álamo C, Girón-González JA. Gut epithelial barrier dysfunction in human immunodeficiency virus-hepatitis C virus coinfected patients: Influence on innate and acquired immunity. World J Gastroenterol. 2016;22:1433-1448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Lin W, Weinberg EM, Chung RT. Pathogenesis of accelerated fibrosis in HIV/HCV co-infection. J Infect Dis. 2013;207 Suppl 1:S13-S18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Pasarín M, Abraldes JG, Liguori E, Kok B, La Mura V. Intrahepatic vascular changes in non-alcoholic fatty liver disease: Potential role of insulin-resistance and endothelial dysfunction. World J Gastroenterol. 2017;23:6777-6787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 33. | Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, Jacobson IM, Dimova R, Markatou M, Talal AH. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440-1450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 34. | Keating SM, Dodge JL, Norris PJ, Heitman J, Gange SJ, French AL, Glesby MJ, Edlin BR, Latham PS, Villacres MC, Greenblatt RM, Peters MG; Women’s Interagency HIV Study. The effect of HIV infection and HCV viremia on inflammatory mediators and hepatic injury-The Women's Interagency HIV Study. PLoS One. 2017;12:e0181004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Bissell DM, Roulot D, George J. Transforming growth factor beta and the liver. Hepatology. 2001;34:859-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 270] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 36. | Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, Kim KA, Kim SS, Borges CB, Shao RX, Chung RT. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology. 2008;134:803-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 37. | Blackard JT, Kang M, Sherman KE, Koziel MJ, Peters MG, Chung RT. Effects of HCV treatment on cytokine expression during HCV/HIV coinfection. J Interferon Cytokine Res. 2006;26:834-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Vali B, Yue FY, Jones RB, Sheth PM, Kaul R, Betts MR, Wong D, Kovacs C, Loutfy M, Common A, Halpenny R, Ostrowski MA. HIV-specific T-cells accumulate in the liver in HCV/HIV co-infection. PLoS One. 2008;3:e3454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, Strom SC. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 625] [Cited by in F6Publishing: 606] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 40. | Herbeuval JP, Boasso A, Grivel JC, Hardy AW, Anderson SA, Dolan MJ, Chougnet C, Lifson JD, Shearer GM. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood. 2005;105:2458-2464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Herbeuval JP, Grivel JC, Boasso A, Hardy AW, Chougnet C, Dolan MJ, Yagita H, Lifson JD, Shearer GM. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood. 2005;106:3524-3531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 42. | Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Babu CK, Suwansrinon K, Bren GD, Badley AD, Rizza SA. HIV induces TRAIL sensitivity in hepatocytes. PLoS One. 2009;4:e4623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Jang JY, Shao RX, Lin W, Weinberg E, Chung WJ, Tsai WL, Zhao H, Goto K, Zhang L, Mendez-Navarro J, Jilg N, Peng LF, Brockman MA, Chung RT. HIV infection increases HCV-induced hepatocyte apoptosis. J Hepatol. 2011;54:612-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Shin DH, Martinez SS, Parsons M, Jayaweera DT, Campa A, Baum MK. Relationship of Oxidative Stress with HIV Disease Progression in HIV/HCV Co-infected and HIV Mono-infected Adults in Miami. Int J Biosci Biochem Bioinforma. 2012;2:217-223. [PubMed] [Cited in This Article: ] |
| 46. | Baum MK, Sales S, Jayaweera DT, Lai S, Bradwin G, Rafie C, Page JB, Campa A. Coinfection with hepatitis C virus, oxidative stress and antioxidant status in HIV-positive drug users in Miami. HIV Med. 2011;12:78-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Huang X, Liang H, Fan X, Zhu L, Shen T. Liver Damage in Patients with HCV/HIV Coinfection Is Linked to HIV-Related Oxidative Stress. Oxid Med Cell Longev. 2016;2016:8142431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Baum MK. Role of micronutrients in HIV-infected intravenous drug users. J Acquir Immune Defic Syndr. 2000;25 Suppl 1:S49-S52. [PubMed] [Cited in This Article: ] |
| 49. | Gil L, Martínez G, González I, Tarinas A, Alvarez A, Giuliani A, Molina R, Tápanes R, Pérez J, León OS. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res. 2003;47:217-224. [PubMed] [Cited in This Article: ] |
| 50. | Morris D, Guerra C, Donohue C, Oh H, Khurasany M, Venketaraman V. Unveiling the mechanisms for decreased glutathione in individuals with HIV infection. Clin Dev Immunol. 2012;2012:734125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Parsons M, Campa A, Lai S, Li Y, Martinez JD, Murillo J, Greer P, Martinez SS, Baum MK. Effect of GSTM1-Polymorphism on Disease Progression and Oxidative Stress in HIV Infection: Modulation by HIV/HCV Co-Infection and Alcohol Consumption. J AIDS Clin Res. 2013;4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Perl A, Banki K. Genetic and metabolic control of the mitochondrial transmembrane potential and reactive oxygen intermediate production in HIV disease. Antioxid Redox Signal. 2000;2:551-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Repetto M, Reides C, Gomez Carretero ML, Costa M, Griemberg G, Llesuy S. Oxidative stress in blood of HIV infected patients. Clin Chim Acta. 1996;255:107-117. [PubMed] [Cited in This Article: ] |
| 54. | Page EE, Nelson M, Kelleher P. HIV and hepatitis C coinfection: pathogenesis and microbial translocation. Curr Opin HIV AIDS. 2011;6:472-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Balagopal A, Ray SC, De Oca RM, Sutcliffe CG, Vivekanandan P, Higgins Y, Mehta SH, Moore RD, Sulkowski MS, Thomas DL, Torbenson MS. Kupffer cells are depleted with HIV immunodeficiency and partially recovered with antiretroviral immune reconstitution. AIDS. 2009;23:2397-2404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Kaspar MB, Sterling RK. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol. 2017;4:e000166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 57. | Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188:571-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 207] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 58. | Tarantola A, Abiteboul D, Rachline A. Infection risks following accidental exposure to blood or body fluids in health care workers: a review of pathogens transmitted in published cases. Am J Infect Control. 2006;34:367-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 59. | World Health Organization. Management of hepatitis B and HIV coinfection; Clinical Protocol for the WHO European Region. 2011; Available from: URL: http://www.euro.who.int/en/health-topics/communicable-diseases/hepatitis/publications/pre2009/protocol-7.-management-of-hepatitis-b-and-hiv-coinfection. [Cited in This Article: ] |
| 60. | Puoti M, Manno D, Nasta P, Carosi G. Hepatitis B virus and HIV coinfection in low-income countries: unmet needs. Clin Infect Dis. 2008;46:367-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS. 2017;31:2035-2052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 62. | Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection-a global challenge. N Engl J Med. 2012;366:1749-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 63. | Lacombe K, Bottero J, Lemoine M, Boyd A, Girard PM. HIV/hepatitis B virus co-infection: current challenges and new strategies. J Antimicrob Chemother. 2010;65:10-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Parvez MK. HBV and HIV co-infection: Impact on liver pathobiology and therapeutic approaches. World J Hepatol. 2015;7:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 65. | Lewin SR, Ribeiro RM, Avihingsanon A, Bowden S, Matthews G, Marks P, Locarnini SA, Ruxrungtham K, Perelson AS, Dore GJ. Viral dynamics of hepatitis B virus DNA in human immunodeficiency virus-1-hepatitis B virus coinfected individuals: similar effectiveness of lamivudine, tenofovir, or combination therapy. Hepatology. 2009;49:1113-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Crane M, Avihingsanon A, Rajasuriar R, Velayudham P, Iser D, Solomon A, Sebolao B, Tran A, Spelman T, Matthews G, Cameron P, Tangkijvanich P, Dore GJ, Ruxrungtham K, Lewin SR. Lipopolysaccharide, immune activation, and liver abnormalities in HIV/hepatitis B virus (HBV)-coinfected individuals receiving HBV-active combination antiretroviral therapy. J Infect Dis. 2014;210:745-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D, Roggendorf M, Gerken G, Lu M, Schlaak JF. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49:1132-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 268] [Article Influence: 17.9] [Reference Citation Analysis (3)] |
| 68. | Ming D, Yu X, Guo R, Deng Y, Li J, Lin C, Su M, Lin Z, Su Z. Elevated TGF-β1/IL-31 Pathway Is Associated with the Disease Severity of Hepatitis B Virus-Related Liver Cirrhosis. Viral Immunol. 2015;28:209-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49:S138-S145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 70. | Debes JD, Bohjanen PR, Boonstra A. Mechanisms of Accelerated Liver Fibrosis Progression during HIV Infection. J Clin Transl Hepatol. 2016;4:328-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, Auperin A, Degott C, Benhamou JP, Erlinger S, Valla D, Marcellin P. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 349] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 72. | Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8:1002-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 73. | Wandeler G, Mulenga L, Vinikoor MJ, Kovari H, Battegay M, Calmy A, Cavassini M, Bernasconi E, Schmid P, Bolton-Moore C, Sinkala E, Chi BH, Egger M, Rauch A; for IeDEA-Southern Africa and the Swiss HIV Cohort Study. Liver fibrosis in treatment-naïve HIV-infected and HIV/HBV co-infected patients: Zambia and Switzerland compared. Int J Infect Dis. 2016;51:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Boyd A, Bottero J, Miailhes P, Lascoux-Combe C, Rougier H, Girard PM, Serfaty L, Lacombe K. Liver fibrosis regression and progression during controlled hepatitis B virus infection among HIV-HBV patients treated with tenofovir disoproxil fumarate in France: a prospective cohort study. J Int AIDS Soc. 2017;20:21426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Mallet V, Vallet-Pichard A, Pol S. The impact of human immunodeficiency virus on viral hepatitis. Liver Int. 2011;31 Suppl 1:135-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Herbein G, Gras G, Khan KA, Abbas W. Macrophage signaling in HIV-1 infection. Retrovirology. 2010;7:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 77. | Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 599] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 78. | Yu Y, Gong R, Mu Y, Chen Y, Zhu C, Sun Z, Chen M, Liu Y, Zhu Y, Wu J. Hepatitis B virus induces a novel inflammation network involving three inflammatory factors, IL-29, IL-8, and cyclooxygenase-2. J Immunol. 2011;187:4844-4860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Gürtler LG. Effect of antiretroviral HIV therapy on hepatitis B virus replication and pathogenicity. Intervirology. 2014;57:212-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Fang JW, Wright TL, Lau JY. Fibrosing cholestatic hepatitis in patient with HIV and hepatitis B. Lancet. 1993;342:1175. [PubMed] [Cited in This Article: ] |
| 81. | Revill PA, Littlejohn M, Ayres A, Yuen L, Colledge D, Bartholomeusz A, Sasaduesz J, Lewin SR, Dore GJ, Matthews GV, Thio CL, Locarnini SA. Identification of a novel hepatitis B virus precore/core deletion mutant in HIV/hepatitis B virus co-infected individuals. AIDS. 2007;21:1701-1710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Warner N, Locarnini S. The antiviral drug selected hepatitis B virus rtA181T/sW172* mutant has a dominant negative secretion defect and alters the typical profile of viral rebound. Hepatology. 2008;48:88-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 83. | Kooij KW, Wit FW, van Zoest RA, Schouten J, Kootstra NA, van Vugt M, Prins M, Reiss P, van der Valk M; AGEhIV Cohort Study Group. Liver fibrosis in HIV-infected individuals on long-term antiretroviral therapy: associated with immune activation, immunodeficiency and prior use of didanosine. AIDS. 2016;30:1771-1780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Márquez M, Romero-Cores P, Montes-Oca M, Martín-Aspas A, Soto-Cárdenas MJ, Guerrero F, Fernández-Gutiérrez C, Girón-González JA. Immune activation response in chronic HIV-infected patients: influence of Hepatitis C virus coinfection. PLoS One. 2015;10:e0119568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 85. | Evans TI, Li H, Schafer JL, Klatt NR, Hao XP, Traslavina RP, Estes JD, Brenchley JM, Reeves RK. SIV-induced Translocation of Bacterial Products in the Liver Mobilizes Myeloid Dendritic and Natural Killer Cells Associated With Liver Damage. J Infect Dis. 2016;213:361-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Mosoian A, Zhang L, Hong F, Cunyat F, Rahman A, Bhalla R, Panchal A, Saiman Y, Fiel MI, Florman S, Roayaie S, Schwartz M, Branch A, Stevenson M, Bansal MB. Frontline Science: HIV infection of Kupffer cells results in an amplified proinflammatory response to LPS. J Leukoc Biol. 2017;101:1083-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 87. | García-Álvarez M, Berenguer J, Jiménez-Sousa MA, Pineda-Tenor D, Aldámiz-Echevarria T, Tejerina F, Diez C, Vázquez-Morón S, Resino S. Mx1, OAS1 and OAS2 polymorphisms are associated with the severity of liver disease in HIV/HCV-coinfected patients: A cross-sectional study. Sci Rep. 2017;7:41516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Medrano LM, Berenguer J, Jiménez-Sousa MA, Aldámiz-Echevarria T, Tejerina F, Diez C, Vigón L, Fernández-Rodríguez A, Resino S. ADAR1 polymorphisms are related to severity of liver fibrosis in HIV/HCV-coinfected patients. Sci Rep. 2017;7:12918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Sherman KE, Rouster SD, Kang M, Umbleja T, Sterling R, Butt AA; ACTG 5294 BIRTH Study Team. PNPLA3 Gene Polymorphisms in HCV/HIV-Coinfected Individuals. Dig Dis Sci. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Chen JY, Feeney ER, Chung RT. HCV and HIV co-infection: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2014;11:362-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 91. | Llewellyn A, Simmonds M, Irving WL, Brunton G, Sowden AJ. Antiretroviral therapy and liver disease progression in HIV and hepatitis C co-infected patients: a systematic review and meta-analysis. Hepatol Med Policy. 2016;1:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 92. | Helou E, Shenoi S, Kyriakides T, Landry ML, Kozal M, Barakat LA. Characterizing Patients with Very-Low-Level HIV Viremia: A Community-Based Study. J Int Assoc Provid AIDS Care. 2017;16:261-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Macías J, Berenguer J, Japón MA, Girón JA, Rivero A, López-Cortés LF, Moreno A, González-Serrano M, Iribarren JA, Ortega E, Miralles P, Mira JA, Pineda JA. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50:1056-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 94. | U.S. Department of Health and Human Services AI. Considerations for Antiretroviral Use in Patients with Coinfections Hepatitis C Virus/HIV Coinfection. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. 2018;. [Cited in This Article: ] |
| 95. | Mandorfer M, Schwabl P, Steiner S, Reiberger T, Peck-Radosavljevic M. Advances in the management of HIV/HCV coinfection. Hepatol Int. 2016;10:424-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 96. | Limketkai BN, Mehta SH, Sutcliffe CG, Higgins YM, Torbenson MS, Brinkley SC, Moore RD, Thomas DL, Sulkowski MS. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308:370-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 97. | Society EEAC. EACS Guidelines 9.0. 2017; Available from: URL: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. [Cited in This Article: ] |
| 98. | Bischoff J, Rockstroh JK. Are there any challenges left in hepatitis C virus therapy of HIV-infected patients? Int J Antimicrob Agents. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, Peters MG, Koziel MJ, Bhan AK, Alston B, Colquhoun D, Nevin T, Harb G, van der Horst C; AIDS Clinical Trials Group A5071 Study Team. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 716] [Cited by in F6Publishing: 751] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 100. | Aranzabal L, Casado JL, Moya J, Quereda C, Diz S, Moreno A, Moreno L, Antela A, Perez-Elias MJ, Dronda F, Marín A, Hernandez-Ranz F, Moreno A, Moreno S. Influence of liver fibrosis on highly active antiretroviral therapy-associated hepatotoxicity in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis. 2005;40:588-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 101. | Berenguer J, Gil-Martin Á, Jarrin I, Moreno A, Dominguez L, Montes M, Aldámiz-Echevarría T, Téllez MJ, Santos I, Benitez L, Sanz J, Ryan P, Gaspar G, Alvarez B, Losa JE, Torres-Perea R, Barros C, Martin JVS, Arponen S, de Guzmán MT, Monsalvo R, Vegas A, Garcia-Benayas MT, Serrano R, Gotuzzo L, Menendez MA, Belda LM, Malmierca E, Calvo MJ, Cruz-Martos E, González-García JJ. All-oral direct-acting antiviral therapy against hepatitis C virus (HCV) in human immunodeficiency virus/HCV-coinfected subjects in real-world practice: Madrid coinfection registry findings. Hepatology. 2018;68:32-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 102. | Carrasco I, Arias A, Benítez-Gutiérrez L, Lledó G, Requena S, Cuesta M, Cuervas-Mons V, de Mendoza C. Baseline NS5A resistance associated substitutions may impair DAA response in real-world hepatitis C patients. J Med Virol. 2018;90:532-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 103. | Wyles DL. Resistance to DAAs: When to Look and When It Matters. Curr HIV/AIDS Rep. 2017;14:229-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | Wyles DL, Kang M, Matining RM, Murphy RL, Peters MG; VHICS Study Team. Similar Low Rates of HCV Recurrence in HCV/HIV- and HCV-Infected Participants who Achieved SVR After DAA Treatment: Interim Results From the ACTG A5320 Viral Hepatitis C Infection Long-term Cohort Study (V-HICS). Open Forum Infect Dis. 2018;5:ofy103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 105. | Berenguer J, Gil-Martin Á, Jarrin I, Montes ML, Domínguez L, Aldámiz-Echevarría T, Téllez MJ, Santos I, Troya J, Losa JE, Serrano R, De Guzmán MT, Calvo MJ, González-García JJ; Madrid-CoRe Study Group. Reinfection by HCV following effective all-oral DAA therapy in HIV/HCV-coinfected individuals. AIDS. 2018; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 106. | Sanmartín R, Tor J, Sanvisens A, López JJ, Jou A, Muga R, Ojanguren I, Barluenga E, Videla S, Planas R, Clotet B, Tural C. Progression of liver fibrosis in HIV/hepatitis C virus-coinfected individuals on antiretroviral therapy with early stages of liver fibrosis at baseline. HIV Med. 2014;15:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Benhammou JN, Dong TS, May FP, Kawamoto J, Dixit R, Jackson S, Dixit V, Bhattacharya D, Han SB, Pisegna JR. Race affects SVR12 in a large and ethnically diverse hepatitis C-infected patient population following treatment with direct-acting antivirals: Analysis of a single-center Department of Veterans Affairs cohort. Pharmacol Res Perspect. 2018;6:e00379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 108. | Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, Su F, Berry K. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151:457-471.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 109. | Luetkemeyer AF, Wyles DL. CROI 2017: Highlights of Advances in Viral Hepatitis and Liver Fibrosis. Top Antivir Med. 2017;25:84-92. [PubMed] [Cited in This Article: ] |
| 110. | Maponga TG, Andersson MI, van Rensburg CJ, Arends JE, Taljaard J, Preiser W, Glashoff RH. HBV and HIV viral load but not microbial translocation or immune activation are associated with liver fibrosis among patients in South Africa. BMC Infect Dis. 2018;18:214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 111. | U.S. Department of Health and Human Services AI. Considerations for Antiretroviral Use in Patients with Coinfections Hepatitis B Virus/HIV Coinfection. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. 2017;. [Cited in This Article: ] |
| 112. | Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315-41; quiz 1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 362] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 113. | Hewitt NJ, Lechón MJ, Houston JB, Hallifax D, Brown HS, Maurel P, Kenna JG, Gustavsson L, Lohmann C, Skonberg C, Guillouzo A, Tuschl G, Li AP, LeCluyse E, Groothuis GM, Hengstler JG. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39:159-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 523] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 114. | Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 115. | Powderly WG. Antiretroviral therapy in patients with hepatitis and HIV: weighing risks and benefits. Clin Infect Dis. 2004;38 Suppl 2:S109-S113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | de Vries-Sluijs TE, Reijnders JG, Hansen BE, Zaaijer HL, Prins JM, Pas SD, Schutten M, Hoepelman AI, Richter C, Mulder JW, de Man RA, Janssen HL, van der Ende ME. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus. Gastroenterology. 2010;139:1934-1941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 117. | Matthews GV, Avihingsanon A, Lewin SR, Amin J, Rerknimitr R, Petcharapirat P, Marks P, Sasadeusz J, Cooper DA, Bowden S, Locarnini S, Ruxrungtham K, Dore GJ. A randomized trial of combination hepatitis B therapy in HIV/HBV coinfected antiretroviral naïve individuals in Thailand. Hepatology. 2008;48:1062-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 118. | Matthews GV, Seaberg E, Dore GJ, Bowden S, Lewin SR, Sasadeusz J, Marks P, Goodman Z, Philp FH, Tang Y, Locarnini S, Thio CL. Combination HBV therapy is linked to greater HBV DNA suppression in a cohort of lamivudine-experienced HIV/HBV coinfected individuals. AIDS. 2009;23:1707-1715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 119. | Vinikoor MJ, Sinkala E, Chilengi R, Mulenga LB, Chi BH, Zyambo Z, Hoffmann CJ, Saag MS, Davies MA, Egger M, Wandeler G; IeDEA- Southern Africa. Impact of Antiretroviral Therapy on Liver Fibrosis Among Human Immunodeficiency Virus-Infected Adults With and Without HBV Coinfection in Zambia. Clin Infect Dis. 2017;64:1343-1349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 120. | Info A. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. 2018; Available from: URL: https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL003510.pdf. [Cited in This Article: ] |