Published online Sep 28, 2019. doi: 10.3748/wjg.v25.i36.5403
Peer-review started: April 26, 2019
First decision: May 30, 2019
Revised: June 7, 2019
Accepted: August 24, 2019
Article in press: August 24, 2019
Published online: September 28, 2019
The Chinese Society of Hepatology developed the current guidelines on the management of hepatic encephalopathy in cirrhosis based on the published evidence and the panelists’ consensus. The guidelines provided recommendations for the diagnosis and management of hepatic encephalopathy (HE) including minimal hepatic encephalopathy (MHE) and overt hepatic encephalopathy, emphasizing the importance on screening MHE in patients with end-stage liver diseases. The guidelines emphasized that early identification and timely treatment are the key to improve the prognosis of HE. The principles of treatment include prompt removal of the cause, recovery of acute neuropsychiatric abnormalities to baseline status, primary prevention, and secondary prevention as soon as possible.
Core tip: The guidelines provided recommendations for the diagnosis and management of hepatic encephalopathy (HE) including minimal hepatic encephalopathy (MHE) and overt hepatic encephalopathy, emphasizing the importance on screening MHE in patients with end-stage liver diseases. The guidelines emphasized that early identification and timely treatment are the key to improve the prognosis of HE. The principles of treatment include prompt removal of the cause, recovery of acute neuropsychiatric abnormalities to baseline status, primary prevention, and secondary prevention as soon as possible.
- Citation: Xu XY, Ding HG, Li WG, Jia JD, Wei L, Duan ZP, Liu YL, Ling-Hu EQ, Zhuang H, Hepatology CSO, Association CM. Chinese guidelines on management of hepatic encephalopathy in cirrhosis. World J Gastroenterol 2019; 25(36): 5403-5422
- URL: https://www.wjgnet.com/1007-9327/full/v25/i36/5403.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i36.5403
Hepatic encephalopathy (HE) is a serious neuropsychiatric complication of cirrhosis and/or porto-systemic shunt. The clinical symptoms are widely variable, extending from subtle impairment in mental state to coma[1].
To promote the standardization of HE clinical diagnosis and treatment, some international gastrointestinal and hepatic disease associations have continued to issue HE guidelines or consensus statements and to recommend HE definitions and treatments. In 1998, the 11th World Congress of Gastroenterology established the HE Working Party, and in 2002, “hepatic encephalopathy-definition, nomenclature, diagnosis, and quantification” was formulated. The Practice Standards Committee of the American Gastroenterological Association, the International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN), the American Association for the Study of Liver Diseases, and the European Association for the Study of the Liver have continued to develop numerous guidelines or consensus statements with recommendations regarding the pathogenesis, natural history, epidemiology, diagnostic evaluation, and treatment of HE. Experimental HE models, neuro-physiological studies, neurophysiological testing, radiology evaluation, clinical trial designs, etc. have also been described[2-4].
In 2013, the Chinese Society of Digestive Diseases and the Chinese Society of Hepatology of the Chinese Medical Association formulated the “consensus on the diagnosis and treatment of hepatic encephalopathy in China (Chongqing, 2013)”[5]. Recently, due to progress in basic and clinical research, people's understanding of HE, especially minimal hepatic encephalopathy (MHE), has deepened. The Chinese Society of Hepatology of the Chinese Medical Association organized specialists in fields such as liver disease, infectious diseases, digestion, surgery, Chinese medicine, intervention, oncology, pharmacology, nursing, and clinical research methodology to coauthor these guidelines and to provide guidance regarding the clinical diagnosis and treatment. However, these guidelines are not a mandatory standard. It is impossible to include or solve all the problems with HE diagnosis and treatment. Therefore, when managing a given patient, clinicians should follow the principles of these guidelines, fully understand the patient's illness, and carefully consider the patient's point of view and wishes; additionally, they should have an understanding of local medical resources and practical experience to develop a comprehensive and rational individualized treatment plan.
Classification (Table 1) is carried out in accordance with these guidelines, which are based on the evidence level and recommendation strength determined by the Grading of Recommendations, Assessment, Development, and Evaluation system.
| Level | Detailed description |
| Evidence Level | |
| A | High quality: Further research cannot change the reliability of these treatment assessment results. |
| B | Moderate quality: Further research may influence the reliability of these treatment assessment results, and may change the treatment assessment results. |
| C | Low or very low quality: Further research will very likely influence the reliability of these treatment assessment results, and will very likely change the treatment assessment results. |
| Recommendation strength | |
| 1 | Strong recommendation: It is clearly shown that either the benefits of intervention clearly outweigh the disadvantages, or that the disadvantages outweigh the benefits. |
| 2 | Weak recommendation: The benefits and disadvantages are unclear, or, regardless of the quality of the evidence, the benefits and disadvantages are comparable. |
Depending on the type of underlying liver disease, HE is classified into types A, B, and C. Type A HE occurs due to underlying acute liver failure and progresses rapidly. One of its important pathophysiological features is cerebral edema and intracranial hypertension. Type B HE is caused by portosystemic shunt; there is no marked liver dysfunction, and liver biopsy suggests normal liver histology. Type C refers to HE that occurs due to underlying chronic liver damage, such as cirrhosis (Table 2)
| Type of hepatic encephalopathy | Definition | Subcategory | Subdivision |
| Type A | Hepatic encephalopathy associated with acute liver failure | None | None |
| Type B | Hepatic encephalopathy associated with portosystemic shunt and no liver cell injury-associated liver disease | None | None |
| Type C | Hepatic encephalopathy associated with cirrhosis with portal hypertension or portosystemic shunt | Episodic hepatic encephalopathy | Accompanying predisposition |
These guidelines are mainly for type C HE, that is, HE caused by cirrhosis, in which type A or B HE associated with acute liver failure and portosystemic shunt has been excluded.
At present, the main causes of liver cirrhosis in China are chronic hepatitis B and chronic hepatitis C, followed by alcohol- or drug-induced liver disease. Autoimmune liver disease, especially primary biliary cirrhosis, is gradually increasing in clinical practice. In the Yangtze River basin, schistosomiasis is also an important cause of cirrhosis. There is no marked correlation between the occurrence of MHE and the etiology, but the incidence increases with the degree of cirrhosis decompensation. Even in patients with Child-Pugh grade A cirrhosis, the incidence of MHE can be as high as 24.8%[6].
Reports of HE associated with cirrhosis in China and other countries are not uniform, likely because clinicians use different diagnostic criteria for HE and have different perceptions of MHE. Among symptoms of decompensated cirrhosis, HE developed at an annual rate of 8% in one Japanese cohort[7]. Most patients with cirrhosis develop a certain degree of MHE during a certain period of the disease, and MHE has an incidence of 30% to 84% over the course of cirrhosis[8].
In recent years, Chinese researchers have conducted a multicenter study on the epidemiology of HE, including MHE. They found that approximately 40% of hospitalized cirrhosis patients have MHE. Moreover, 30% to 45% of cirrhosis patients and 10% to 50% of posttransjugular intrahepatic portosystemic shunt (TIPS) patients presented with overt hepatic encephalopathy (OHE)[9]. According to data from other countries, the HE incidence in cirrhosis patients is 30% to 45%, and the incidence may be higher during the progression of the disease. The North American Consortium for the Study of End-stage Liver Disease confirmed that HE has an independent correlation with death in cirrhosis patients[10].
In cirrhotic portal hypertension, hepatocyte dysfunction and portosystemic shunt (i.e., the formation of collateral circulation between the portal vein and the vena cava) reduce the detoxification function, which causes the intestinal tract to absorb large amounts of toxic substances such as ammonia. Toxic substances bypassing the liver via the portal vein and directly entering systemic circulation and brain tissue are the main pathophysiological features of HE associated with cirrhosis.
The pathogenesis of HE has not yet been fully elucidated. Currently, the ammonia poisoning theory is still the core; however, the roles of inflammatory mediators and other toxic substances are receiving increasing attention[11].
Hyperammonemia: Ammonia poisoning theory is an important explanation for HE pathogenesis. Protein in the diet is decomposed by intestinal bacteria, producing ammonia, and increased permeability of the intestinal wall can lead to increased ammonia in the portal vein. Liver dysfunction prevents effective detoxification of blood ammonia through the ornithine cycle[12]; at the same time, the portosystemic shunt causes blood containing ammonia to directly enter systemic circulation. The entry of blood ammonia into the brain tissue increases the synthesis of glutamine by astrocytes, leading to cellular degeneration, edema, and tissue degeneration that result in acute neurocognitive dysfunction. Ammonia can also directly lead to an imbalance in the ratio of excitatory and inhibitory neurotransmitters, produce clinical symptoms, and impair the autoregulation of intracranial blood flow.
Neuroinflammation: It is currently believed that hyperammonemia interacts with inflammatory mediators to promote HE development. Inflammation can lead to the destruction of the blood-brain barrier, which causes toxic substances such as ammonia and inflammatory cytokines to enter the brain tissue, resulting in brain parenchymal change and brain dysfunction. At the same time, high blood ammonia can induce neutrophil dysfunction, release reactive oxygen species, and promote bodily oxidative stress production and inflammatory response, resulting in a vicious circle. Moreover, cytokines produced by the inflammatory process aggravate liver damage and increase HE incidence. In addition, HE is related to the presence of infection in the body. Studies have shown that peritonitis, urinary tract infections, pneumonia, etc. are the most common infections in cirrhosis patients[13,14].
Other theories: Neurotransmitter dysfunction: When cirrhotic liver dysfunction occurs, the ability to degrade aromatic amino acids is reduced, and blood phenylalanine and tyrosine increase, thereby inhibiting normal neurotransmitter production. Increased phenylalanine and tyrosine produce the false transmitters phenylethanolamine and hydroxyphenylethanolamine, and a large number of false neurotransmitters replace normal neurotransmitters, resulting in HE[15].
γ-aminobutyric acid is a unique neurotransmitter that is the most important inhibitory neurotransmitter in the central nervous system. It exists in the brain in the form of a complex receptor with benzodiazepine receptors. The γ-aminobutyric acid content in the blood increases in HE, as does the amount that passes the blood-brain barrier, resulting in an increase in the endogenous benzodiazepine level in the brain. Experimental studies have confirmed that drugs that activate γ-aminobutyric acid/benzodiazepine complex receptors, such as phenobarbital and diazepam, can induce or aggravate HE in cirrhotic animals and that benzodiazepine receptor antagonists such as flumazenil can be administered to reduce the onset of HE[16].
Manganese toxicity: Some studies have found that the blood or brain manganese content of some cirrhosis patients is two to seven times higher than that of healthy people. When manganese enters human nerve cells, low-value manganese ions are oxidized into high-value manganese ions, which accumulate in the mitochondria due to the unique affinity of the mitochondria for manganese. At the same time, manganese ions can produce a large number of free radicals during valence transition, which leads to a further decrease in key enzyme activity in the mitochondrial respiratory chain of the brain cells in the substantia nigra and striatum, thus affecting the function of these brain cells[17].
Brainstem reticular system dysfunction: The brainstem reticular systems and their substantia nigra-striatum system neuronal activity are damaged to varying degrees in severe cirrhosis patients, resulting in HE, asterixis production, and muscle tone change. The degree of brainstem reticular system damage corresponds to HE severity[18].
The most common predisposing factor for HE is infection (especially abdominal infections, including those of the intestines, urinary tract, and respiratory tract). The next most common factors are gastrointestinal bleeding, electrolyte and acid-base balance disorders, large amounts of ascites, high-protein diets, hypovolemia, diuresis, diarrhea, vomiting, constipation, and the use of benzodiazepine drugs and anesthetics. HE incidence is increased after TIPS in association with preoperative liver function reserve status, the presence or absence of HE history, and stent type and diameter[19]. Studies have found that proton pump inhibitors (PPIs) may cause excessive growth of intestinal bacteria, thereby increasing the risk of HE in cirrhosis patients; this risk increases with increased drug dose and treatment course[20].
In the presence of high blood ammonia in patients with cirrhosis, the above factors may further aggravate brain edema and oxidative stress, leading to the rapid deterioration of cognitive function.
HE manifests as a continuum from unimpaired cognitive function with intact consciousness through coma. Presently, the West-Haven criteria, which grade HE on a scale from 0 to 4, are still the most widely used standard for grading HE in China and other countries[21]. The main drawback of the West-Haven criteria is that grading is very subjective for discriminating grade 0 (probably MHE) and grade 1. MHE is an abnormal change that is not detectable based on personality or behavior; it is characterized by normal nervous system signs but abnormal neuropsychological test results. In the clinical manifestations of grade 1 HE, signs such as euphoria, depression, or a reduced attention span are difficult to identify. Only close relatives familiar with a patient’s personality notice mild abnormal changes in cognitive function, and repeatability in clinical practice and multicenter studies is poor.
Recently, the ISHEN proposed the Spectrum of Neurocognitive Impairment in Cirrhosis (SONIC) grading standard, in which MHE and West-Haven grade 0 or 1 HE are labeled covert hepatic encephalopathy. If there are abnormalities falling under West-Haven classifications of HE grades 2 to 4, such as personality or behavior changes, mental abnormalities, coma, and other neurological abnormalities, the disorder is labeled OHE[3,4]. It should be noted that HE grade 1 patients will have mild cognitive dysfunction and that a small number of patients who are positive for asterixis are classified as having OHE under the SONIC standard.
In the past, cirrhosis patients with grade 0 HE were described as having “subclinical hepatic encephalopathy” or “early hepatic encephalopathy” or simply as patients without mental and neurological abnormalities. In 1998, the 11th World Congress of Gastroenterology unanimously adopted the term MHE[2]. MHE is an insidious stage in the pathogenesis of HE and is defined as neuropsy-chological/neurophysiological abnormalities in cirrhosis patients without directional dysfunction or asterixis, in other words, with normal cognitive function[4,22]. The incidence is as high as 25% to 39.9%[6,23] and is unrelated to age, gender, tobacco use, or education level but has a clear relationship with the Child-Pugh grade. Although MHE has no marked clinical symptoms or signs, the clinical prognosis and quality of life are worse than those of cirrhosis patients with normal neuropsychological test results[24]. During clinical follow-up, 56% of MHE patients develop OHE within three years, and other complications and mortality also increase markedly. After recovery from OHE, MHE may persist[25]. Furthermore, these patients’ general health-related quality of life, driving safety, work efficiency, and socioeconomic status are all significantly reduced. Some patients’ MHE may progress to OHE if they are not treated effectively. Therefore, the clinical focus is on screening patients with end-stage liver diseases, such as cirrhosis, for MHE. Consequently, these guidelines use the revised grading standards for MHE and HE grades 1 to 4 (Tables 3 and 4). Patients with marked changes in consciousness can be further evaluated and classified using the Glasgow Coma Scale score (Supplementary Annex 1).
| Traditional West-Haven criteria | Grade 0 | HE grade 1 | HE grade 2 | HE grade 3 | HE grade 4 | |
| Proposed revision of the HE grading criteria | No HE | MHE | HE grade 1 | HE grade 2 | HE grade 3 | HE grade 4 |
| Revised HE grading criteria | Neuropsychiatric symptoms (that is, cognitive function) | Nervous system signs |
| No HE | Normal | Normal nervous system signs, normal neuropsychological test results |
| MHE | Potential HE, no noticeable personality or behavioral changes | Normal nervous system signs, but abnormal neuropsychological test results |
| HE grade 1 | Trivial and mild clinical signs, such as mild cognitive impairment, decreased attention, sleep disorders (insomnia and sleep inversion), euphoria, or depression | Asterixis can be elicited and neuropsychological tests are abnormal |
| HE Grade 2 | Marked personality or behavioral changes, lethargy or apathy, slight orientation abnormality (time and orientation), decreased mathematical ability, dyskinesia, or unclear speech | Asterixis is easily elicited, and neurophysiological testing is unnecessary |
| HE Grade 3 | Marked dysfunction (time and spatial orientation), abnormal behavior, semi-coma to coma, but responsive | Asterixis usually cannot be elicited. There is ankle clonus, increased muscle tone, and hyperreflexia. Neurophysiological testing is unnecessary |
| HE Grade 4 | Coma (no response to speech and external stimuli) | Increased muscle tone or positive signs of the central nervous system. Neurophysiological testing is unnecessary |
Biochemical indicators: Patients’ liver biochemical indicators, such as bilirubin, alanine aminotransferase, aspartate aminotransferase, albumin, prothrombin time activity, and the like should be tested for marked abnormalities. Renal function and routine blood tests are used as routine tests for suspected HE.
Blood ammonia: Elevated blood ammonia has relatively high value for HE diagnosis. Multiple studies have shown that HE patients, especially those with portosystemic HE, often have elevated blood ammonia, but the level of elevation does not completely correlate with disease severity[26,27]. The presence of normal blood ammonia cannot exclude HE. If a tourniquet is maintained in place for too long, if testing is carried out too long after blood collection, or if blood is transported at high temperatures, a false elevation in blood ammonia may result. Venous blood should be collected at room temperature and immediately sent for testing, and the blood should be kept at low temperatures. Testing should be completed within 30 minutes, or if blood is kept at 4 °C after centrifuging, testing should be completed within two hours.
Other: Chitinase-3-like protein 1 (CHI3L1) is a member of the glycosyl hydrolase family. It can bind to chitin, but without the activity of chitinase. It plays an important role in inflammation and tissue remodeling. It is a protein secreted by the liver to the extracellular matrix. It is significantly increased in liver cirrhosis and liver fibrosis. The expression level of CHI3L1 reflects the degree of cirrhosis and liver fibrosis[28].
Golgi protein 73 (GP73) is a type of transmembrane glycoprotein located in the Golgi apparatus. GP73 is primarily expressed in biliary epithelial cells and is rarely expressed in hepatocytes. However, in various types of advanced liver disease due to various causes, the GP73 expression level in hepatocytes is increased[29]. Recent studies have found that elevated levels of GP73 in patients with hepatocellular carcinoma (HCC) are primarily associated with cirrhosis but not with HCC itself.
Neuropsychological testing is the easiest method for clinical screening and early diagnosis of MHE and grade 1 HE. Neuropsychological testing methods are recommended in many national HE guidelines as an important method for MHE screening or early diagnosis. Each test needs to be combined with other tests (Table 5).
| Testing methods | Testing purposes | Time | Remarks |
| Psychological tests | |||
| Psychometric hepatic encephalopathy score (PHES) | PHES is an important method for determining cognitive dysfunction and diagnosing MHE in cirrhosis patients | Includes five subtests, namely the number connection test A and B, digit symbol test, line tracing test, and serial dotting test | Pen and paper |
| Positives on at least two tests are required for clinical diagnosis | |||
| Number connection test A | Ability to concentrate, mental activity speed, can be used for rapid outpatient screening for MHE | 30 to 120 s | Correction for age and education level improves accuracy |
| Number connection test B | Ability to concentrate, mental activity speed, distributed attention ability, can be used for rapid outpatient screening for MHE | 1 to 3 min | Psychologist is required |
| More complicated than number connection test A | |||
| Digit symbol test | Ability to concentrate, mental activity speed, can be used for rapid outpatient screening for MHE | 2 min | Psychologist is required |
| Stroop Smartphone app (Encephal App) | Attention, can be used for rapid outpatient screening for MHE | 3 to 5 min | Reliable and easy to use |
| Repeatable battery for the assessment of neuropsychological status | Compliance and working memory, visual spatial ability, language, cognitive processing speed | 25 min | Pen and paper |
| Psychologist is required | |||
| ISHEN recommends HE psychometric scores as substitute indicators | |||
| Inhibition control test | Attention, reaction inhibition, working memory | 15 min | Computer processing |
| Patient cooperation is required, and patients must learn before testing | |||
| Neurophysiological testing | |||
| Flicker fusion frequency | Visual identification, can be used on outpatient basis for HE scores of 2 or lower, value of supplemental diagnosis is low | 10 min | Patients must learn before testing |
| EEG | Generalized brain activity. Suitable for children | Variation | Psychologist and specialized tools are required |
| Evoked potential | Tests the time difference between electrical stimulation and response | Variation | P300 hearing has been used for the diagnosis of MHE |
Traditional pen-and-paper neuropsychological tests: The Psychometric Hepatic Encephalopathy Score (PHES) includes five subtests, namely, number connection tests (NCTs) A and B, a digit symbol test (DST), a line tracing test, and a serial dotting test (Annex 2). At present, if both the NCT-A and DST are positive or there are abnormalities in any two of the five subtests, an MHE diagnosis can be made. Although the sensitivity and specificity of the PHES are high, the results can be affected by various factors, such as age, education level, cooperation level, and the patient’s learning effectiveness[30,31].
Some scholars in China have adopted age- and education-corrected NCT and DST tests, which show higher accuracy and applicability[32,33]. In short, the NCT and DST are simple and easily carried out and have high operability and suitability for epidemiological investigations of MHE. In recent years, computer software-assisted tools such as the electronic number connection test (eNCT) have been developed to monitor and screen for cognitive dysfunction in cirrhosis patients; these tools offer enhanced repeatability and reliability[34].
Repeatable battery for the assessment of neuropsychological status: The repeatable battery for the assessment of neuropsychological status is one of two neuropsychological testing tools recommended by the ISHEN guidelines. Its content examines immediate memory; delayed memory; attention; and visual, spatial, and linguistic abilities. It has been used in Alzheimer's disease, schizophrenia, and traumatic brain injury and in some research on patients waiting for liver transplants, but not specifically as an HE detection tool.
Stroop and EncephalApp tests: The Stroop test (Annex 3) evaluates mental activity speed and cognitive flexibility by recording the interference response time between color fields and written color names and is considered the most effective and direct tool for examining cognitive regulation and interference control. Recently, EncephalApp, a mobile application software tool based on this test, was developed. It has better discrimination ability, is better in distinguishing known cirrhosis-related cognitive dysfunctions, and has great prospects for applicability[35]. It should be noted that this test tool is not available for patients with color blindness.
Inhibitory control test: Among cirrhosis-related neurological dysfunctions, low-level cognitive dysfunctions, such as changes in vigilance and attention, are the most sensitive indicators. The inhibitory control test (ICT) uses computer technology to display letters over a 50-ms period to test patient response inhibition, attention, and working memory, which can be useful for MHE detection. Studies have shown that the ICT’s sensitivity for diagnosing MHE is as high as 88%. It is an easy way to diagnose MHE.
Critical flicker frequency (CFF) test: This test detects the minimum stimulation frequency that can cause a flicker fusion sensation. The CFF can assess cerebral nerve conduction dysfunction. The findings of this test are sensitive and specific for MHE diagnosis and easily interpreted, making it useful as an auxiliary testing method[36,37]. When the threshold is 39 Hz, there is no difference between MHE patients and healthy individuals, but the difference between grade 2 HE and grade 1 is larger, making it more suitable for distinguishing grade 2 HE[38]. Cirrhosis patients with a CFF threshold < 39 Hz have a five-year survival rate that is significantly lower than that of patients with CFF ≥ 39 Hz. Older age, CFF < 39 Hz, and Model for End-stage Liver Disease (MELD) scores are independently associated with survival during follow-up[39].
Scan test: This is a computerized test that measures speed and accuracy when performing increasingly complex digital recognition memory tasks. The scan test was found to have predictive value for prognosis, but its clinical application is heavily influenced by educational background.
New neuropsychological testing methods: This category of tools includes the animal naming test[40], the posture control and stability test[41] , and multisensory integration testing[42].
Electroencephalography (EEG): EEG can show cerebral cortex function without patient cooperation and without learning effects risks. Although EEG has been widely studied and applied in clinical practice, typical EEG changes can only be detected in patients with severe HE. Therefore, this tool is not clinically useful for early HE diagnosis and is only used for auxiliary HE diagnosis. The primary EEG abnormality is a slowing rhythm, but this change is not specific to HE and is also observed in other metabolic brain diseases, such as hyponatremia and uremic encephalopathy[43].
Evoked potential detection: Evoked potentials include visual evoked potentials, auditory evoked potentials, and somatosensory evoked potentials. Of the endogenous time-related evoked potentials, P300 has the best diagnostic sensitivity. Patients with MHE can show an increase in latency and a decrease in amplitude.
The advantages of neurophysiological testing are that the results are relatively specific and have no learning effects, but the disadvantages are poor sensitivity, the need for specialized equipment and personnel, and poor consistency of results.
Liver and brain CT: Liver-enhanced CT revascularization can indicate whether there is obvious portosystemic shunt. CT scans of the brain itself cannot be used for the diagnosis or grading of HE, but they can determine the presence of cerebral edema and exclude cerebrovascular accidents and intracranial tumors[44,45].
Magnetic resonance imaging (MRI): (1) Damage or alteration of brain structure: Diffusion tensor imaging (DTI) is a new method for describing brain structure. It can show the degree and scope of damage to the white matter structure. Research shows that cirrhosis and HE patients have normal MRI findings in the white matter area, but mean diffusivity (MD) can still show a marked increase related to HE stage, blood ammonia levels, neurophysiological status, and neuropsychological changes[46].
(2) Blood perfusion changes: Arterial spin labeling (ASL) using magnetically labeled water protons as a tracer allows the noninvasive detection of cerebral blood perfusion changes through the acquisition of cerebral blood volume, cerebral blood flow, oxygen metabolism rate, and other perfusion parameters. Studies have shown that MHE patients have a greater increase in cerebral blood flow perfusion than non-MHE patients, and this change is associated with neuropsychological scores[47]. However, large-scale clinical verification is necessary to determine whether blood perfusion changes can be used as an MHE diagnostic marker.
Functional MRI (fMRI): In recent years, great progress has been made in the use of fMRI technology to study the functional localization and pathophysiological mechanisms of brain functions, such as cognition and sensory perception. A number of scholars[48-50] using resting-state fMRI studies have shown that basal-thalamo-cortical loops in HE patients are impaired and that changes in functional connectivity are associated with altered cognitive function in HE patients. Resting state fMRI using ReHo analysis can be used as a noninvasive method for detecting cognitive changes in patients with cirrhosis.
Due to the poor prognosis of patients with MHE as well as the risk of OHE, safety risks, and high risks for other complications of cirrhotic portal hypertension, clinicians should make appropriate use of current detection techniques and methods and attach great importance to MHE screening and early diagnosis.
OHE: OHE diagnosis based on clinical manifestations and signs in accordance with the West-Haven criteria is not difficult[51,52]. Neuropsychological, neurophysiological, and radiological evaluations are generally unnecessary. The diagnostic points are: (1) Underlying diseases are present that cause HE, severe liver disease, and/or extensive portosystemic shunt; (2) Clinically identifiable neuropsychiatric symptoms and signs are present; (3) Other diseases that cause neuropsychiatric disorders, such as metabolic encephalopathy, toxic encephalopathy, neurological diseases (such as intracranial hemorrhage, intracranial infection and intracranial space occupation), and mental illness, are excluded; (4) Special attention should be paid to determining the cause of HE (type C or type B), such as infection, upper gastrointestinal bleeding, or a large amount of ascites; and (5) Blood ammonia is elevated.
MHE: Patients have no obvious manifestations of cognitive dysfunction, so it is often necessary to use special examinations to confirm this diagnosis; these special examinations are the focus of clinical attention[53-55]. MHE can be diagnosed based on any one or more of the following primary diagnostic points: (1), (2), or (3) through (6). These primary diagnostic points are as follows: (1) Underlying disease causing HE, severe liver disease and/or extensive portosystemic shunt; (2) At least two abnormal traditional neuropsychological test indicators; (3) At least one abnormal result of new neuropsychological test methods (ANT, posture control and stability test, and multisensory integration test); (4) Abnormal CFF; (5) Abnormal EEG, visual evoked potential (VEP), or brainstem auditory evoked potential (BAEP); and (6) Abnormal fMRI.
Differential diagnosis points: HE needs to be differentiated from the following diseases: (1) Mental disorders: When the only prominent manifestations of HE are mental symptoms, such as personality changes, abnormal behavior, or insomnia, it is easily misdiagnosed as a mental disorder. Therefore, when patients with severe liver disease or a history of portosystemic shunt present with neurological and mental abnormalities, physicians should be alert to the possibility of HE; (2) Intracranial lesions: Subarachnoid, epidural, or intracerebral hemorrhage, cerebral infarction, brain tumors, intracranial infections, epilepsy, and the like should be diagnosed using testing such as physical examinations of the nervous system or meningeal stimulation combined with CT, lumbar puncture, angiography, EEG, virological detection, and the like; (3) Other metabolic encephalopathy: Ketoacidosis, hypoglycemia, hyponatremia, renal encephalopathy, and pulmonary encephalopathy can be differentially diagnosed by performing blood biochemical analyses for characteristics corresponding to the underlying disease; (4) Wernicke encephalopathy: Patients with severe alcoholic liver disease often suffer from vitamin B1 deficiency, and symptoms can be significantly improved after vitamin B1 supplementation[56]; (5) Toxic encephalopathy: Alcoholic encephalopathy, acute poisoning, withdrawal syndrome, heavy metal (mercury, manganese, etc.) encephalopathy, and psychotropic or salicylate drug toxicity can be differentially diagnosed by reviewing the corre-sponding medical history and/or corresponding toxicology tests; (6) Liver cirrhosis associated with Parkinson's disease; (7) Hepatic myelopathy: This disorder often occurs due to cirrhosis. Sympathetic pathological changes are characterized by laterally symmetrical demyelination of the cortical spinal cord. The clinical manifestation is slow, progressive, symmetrical paralysis of the limbs, including decreased muscle strength, increased muscle tone, spasticity, hyperreflexia, often pathological positive reflexes, and, in some patients, elevated blood ammonia; and (8) Acquired hepatic degeneration: This diagnosis is a rare but generally irreversible extrapyramidal syndrome caused by chronic liver disease. The manifestations include Parkinson's syndrome, ataxia, intentional tremor, chorea, dyskinesia, other mental and behavioral disorders, impaired intellect, and neuropsychological changes. fMRI has good discriminating value.
Recommendation 1: HE comprises neuropsychiatric abnormalities with a wide range and scope. Using a combination of clinical manifestations, neuropsychological testing methods, and differential diagnosis, HE can be divided into MHE and HE grades 1 through 4 (C1).
Recommendation 2: HE is a continuous clinical process. Based on the presence of severe liver disease, HE grades 1 through 4 can be diagnosed based on clinical manifestations. Neuropsychology, neurophysiology, and radiology evaluations are not recommended (B1).
Recommendation 3: MHE is an undetectable cognitive dysfunction with normal neurological signs but abnormal neuropsychological test results. The diagnosis of MHE requires specialized neuropsychological tests or brain function imaging (B1).
Recommendation 4: Currently, the traditional pen-and-paper PHES and computer-aided PHES are widely used for MHE screening and diagnosis (A1). Correcting the PHES for age and education level can improve the accuracy of the MHE diagnosis (B1).
Recommendation 5: MHE is common in patients with cirrhosis, especially those with Child-Pugh grade C cirrhosis and TIPS, which may affect the prognosis of patients; thus, these patients require focused screening (A1). Cirrhosis patients with high safety requirements, such as those who must drive, should be routinely screened for MHE (B1).
Recommendation 6: Attention must be paid to quality control in blood ammonia testing. If a tourniquet is maintained for too long, if testing is carried out too long after blood collection, or if blood is transported at high temperatures, a false elevation in blood ammonia may result. Venous blood should be collected at room temperature and immediately sent for testing. Testing should be completed within 30 min, or if blood is kept at 4°C after centrifuging, testing should be completed within two hours (B1).
Recommendation 7: Elevated blood ammonia is not an indicator of HE severity, prognosis, or grade (C1).
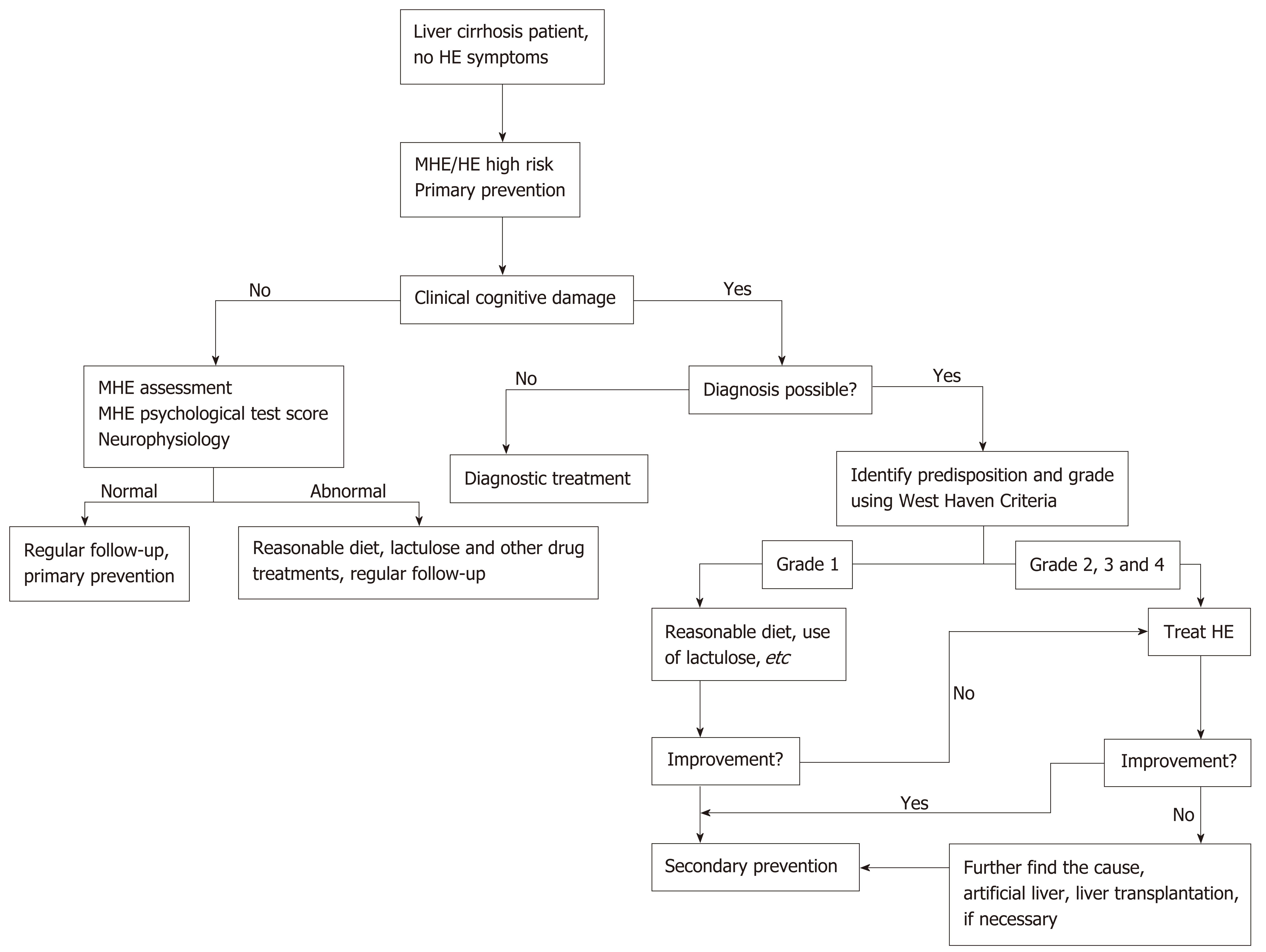
HE is one of the primary causes of death in end-stage liver disease patients. Early HE identification and timely treatment are keys to improving prognosis. The treatment of HE relies on the hierarchical management of its severity (Figure 1). Treatment principles include the timely elimination of risk factors, recovery of acute neuropsychiatric abnormalities to baseline status, primary prevention, and secondary prevention as soon as possible[57-59].
Clinically, more than 90% of MHE/HE cases have predisposing factors, and the elimination of MHE/HE predisposing factors is an important treatment measure.
For HE patients with cirrhosis, infection is the most common predisposing factor. Actively searching for the source of infection is necessary. Even if there is no obvious infection, there is potential for an inflammatory state due to increased intestinal bacterial translocation and endotoxin levels. Antibiotic treatment can reduce this inflammatory state. Therefore, empirical antibiotic treatment should be started as soon as possible.
Gastrointestinal hemorrhage is also a common predisposing factor for HE. HE is easily induced on the day of or the day after bleeding. Moreover, occult gas-trointestinal hemorrhage can also induce HE. Bleeding should be stopped as soon as possible, and blood should be removed from the gastrointestinal tract.
Insufficient alkalosis and electrolyte disturbances caused by excessive diuresis induce HE. When this occurs, diuretics use should be suspended, fluids and albumin should be replenished, and electrolyte imbalances (hypokalemia or hyperkalemia, hyponatremia or hypernatremia) should be corrected. For hypovolemic hyponatremia (especially in cases of blood sodium less than 110 mmol/L), intravenous saline should be administered. For patients with hypervolemic or isovolumic hyponatremia, a selective vasopressin type 2 receptor (V2) antagonist can be administered. For patients with HE grades 3 to 4, the active control of cerebral edema with 20% mannitol (250 to 1000 mL/d, 2 to 6 times/d) alone or combined with compound furosemide (40 to 80 mg/d) is recommended[60,61].
Ammonia reduction treatment: High blood ammonia is an important factor in HE; therefore, it is very important to reduce ammonia production and absorption. The primary drugs for lowering blood ammonia are as follows:
Lactulose: Lactulose is a disaccharide composed of galactose and fructose that does not exist in nature. It has few adverse reactions and can be administered to patients with diabetes or lactose intolerance. Lactulose is converted into a low molecular weight organic acid by the digestive flora in the colon, which causes the intestinal pH to decrease. Lactulose also increases stool volume by retaining water, which stimulates colonic peristalsis, keeping the stool smooth, relieving constipation, and exerting a cathartic effect that restores the circadian rhythm of the colon. In HE, lactulose promotes the growth of intestinal acidophilic bacteria (such as lactobacilli), inhibits proteolytic bacteria, and converts ammonia into an ionic state. Lactulose also reduces intestinal bacterial translocation and prevents spontaneous bacterial peritonitis. A number of randomized controlled clinical trials have shown that lactulose not only can improve the neuropsychological test results of MHE patients but can also improve their quality of life, prevent MHE progression, and prevent HE recurrence. The usual dose is 15 to 30 mL per oral administration, 2 to 3 times/d (dose adjusted according to patient response), and two to three soft stools per day are considered appropriate. If necessary, lactulose can be combined with retention enema treatment. Lactitol or other antihypertensive drugs can be used in lactulose intolerant patients. The effects of lactitol and lactulose with enema are similar[62-65].
Lactitol: Lactitol is a disaccharide that is not absorbed by the intestines and that can cleanse and acidify the intestinal tract, reduce ammonia absorption, regulate intestinal microecology, and effectively reduce endotoxins[66]. The efficacy of lactitol for treating HE is similar to that of lactulose. It has a rapid onset, a low incidence of abdominal distension, and low sweetness. It can be administered normally in diabetic patients[67]. A randomized controlled clinical trial of cirrhosis patients who underwent TIPS found no statistically significant difference between the lactitol group and the lactulose group in terms of the incidence of HE and related parameters (mental state, EEG, asterixis, number connection test results, and blood ammonia) during treatment, suggesting that lactitol can effectively prevent HE onset over the long term in TIPS cirrhosis patients. The recommended initial dose is 0.6 g/kg, divided into three administrations and taken with meals. The dose can be increased or decreased to achieve the standard of two soft stools per day[68].
L-ornithine L-aspartate (LOLA): LOLA can be administered as an alternative treatment or given to patients who do not respond to conventional therapy. The dose is 10 to 40 g/d intravenous infusion. LOLA has a therapeutic effect on OHE and MHE; it can be administered alone or combined with lactulose, and oral preparations are available. LOLA can reduce the level of ammonia by promoting the liver ornithine cycle and glutamine synthesis, which can markedly reduce fasting blood ammonia and postprandial blood ammonia, improve the HE grade and neuropsychological test results, shorten hospital stays, and improve quality of life[69].
Rifaximin-α: Rifaximin-α is a synthetic derivative of rifamycin with a low absorption rate. In theory, orally administered antibiotics that are not absorbed by the intestinal tract can inhibit intestinal bacterial overgrowth, reduce the number of ammonia-producing bacteria, reduce the production and absorption of intestinal NH3, and thereby reduce HE symptoms and prevent HE occurrence. In fact, however, they have no marked effect on type B HE. Routine dose is 800 to 1200 mg/d, divided into three to four oral doses. The course of treatment is still under investigation.
Other antibacterial drugs: Neomycin, metronidazole, vancomycin, paromomycin, and the like have been administered in the past but are rarely used due to side effects and poor efficacy.
Microbial ecological agents: This category includes probiotics, prebiotics, and synbiotics, which can promote the growth of bacterial strains beneficial to the host and inhibit the reproduction of harmful bacteria (such as urease-producing bacteria). Moreover, these preparations can improve the nutritional status of intestinal epithelial cells and reduce intestinal mucosal permeability, thereby reducing bacterial translocation and endotoxemia and improving hyperdynamic circulation. They can also reduce hepatocyte inflammation and oxidative stress, thereby increasing ammonia clearance in the liver. A number of randomized controlled clinical trials have shown that probiotics and lactulose have similar efficacy for improving MHE test results[70,71].
Other therapeutic drugs: (1) Arginine: Arginine hydrochloride contains hydrochloric acid and is acidic; consequently, it can be used to treat metabolic alkalosis with HE. During the administration process, it is important to carefully monitor blood gas testing and analysis and be alert to excessive acidosis. The efficacy of arginine hydrochloride in HE treatment is limited, and clinically, it is seldom used; (2) Glutamine: Recently, we have come to believe that glutamate can only temporarily reduce blood ammonia; it cannot pass the blood-brain barrier or reduce ammonia in brain tissue. Moreover, it can induce metabolic alkalosis and even aggravate HE. Furthermore, excessive brain glutamine produces a hyperosmotic effect and contributes to the formation of cerebral edema, which is not conducive to HE recovery. Currently, it is seldom used clinically; (3) Acarbose: Originally used to treat diabetes, the exact mechanism of acarbose in HE is unknown, but it may be related to the inhibition of α-glucosidase in the small intestine brush border. Acarbose 300 mg/d can reduce the clinical symptoms of type 2 diabetes and grades 1 to 2 HE. Side effects include abdominal pain, flatulence, and diarrhea; and (4) Elimination of Helicobacter pylori (Hp): Research shows that the incidence of Hp infection is statistically significantly higher for patients with HE or MHE than for cirrhosis patients without HE. There may be a relationship between Hp infection and HE in cirrhosis. Eradicating Hp may be beneficial for the clinical prevention and treatment of HE in liver cirrhosis[72-74].
Sedative drugs: HE is associated with the upregulation of gamma-aminobutyric acid neuroinhibitory receptors and N-methyl-D-aspartate-glutamate excitatory receptors, causing imbalances between inhibitory and excitatory signals. Theoretically, the use of flumazenil, bromocriptine, levodopa, and acetylcholine esterase inhibitors is feasible. For comatose HE patients taking benzodiazepines or opioid factors, flumazenil or naloxone can be tried. There is less evidence for the efficacy of bromocriptine or levodopa in HE treatment. A recent double-blind randomized controlled trial showed that bromocriptine is effective in the treatment of cirrhosis with mild to moderate Parkinson's syndrome and is safe. However, it lacks evidence and experience in evidence-based medicine for patients in China. It is recommended to carefully evaluate before use[75].
Naloxone: Plasma β-endorphin (β-EP) is closely related to HE occurrence. On the one hand, β-EP interferes with the ATP metabolic process in brain cells, resulting in decreased cell membrane stability and dysfunction. β-EP binds to the opioid receptors in the brain and inhibits blood circulation in the cerebral cortex; the resulting insufficient blood supply to brain tissues further aggravates brain cell dysfunction. A meta-analysis found that after HE patients were treated with LOLA combined with naloxone, their blood ammonia and total bilirubin levels were lower than those of the control group; additionally, their time to awareness upon awakening was shortened, and their NCT and DST test results improved markedly with no obvious adverse reactions. Some research shows that naloxone alone or in combination with drugs such as lactulose promotes patient awakening, but these studies had small sample sizes and some design defects[76,77].
Propofol: A study comparing the clinical efficacy and adverse reactions of propofol with those of diazepam in 40 HE patients who had manic episodes showed that propofol was safer and more effective for controlling HE symptoms[78]. Compared with midazolam, the propofol group had a shorter recovery time and faster recovery of cognitive function.
Benzodiazepine sedatives: Due to high incidences of anxiety, depression, pain, and sleep cycle disturbance in cirrhosis patients, these patients often have a history of sedative-hypnotic drug or pain killer use, and these drugs can induce HE. Flumazenil is a benzodiazepine antagonist. A randomized, double-blind, controlled trial showed that flumazenil was superior to placebo, and no subjects who used flumazenil died[78]. Serious mental disorders, such as mania, endanger the safety of others and make patients unable to cooperate with doctors. Benzodiazepine sedatives can be used, after informing patients' families of the risks, to gain initial symptomatic control. These drugs should be administered intravenously at reduced doses.
Traditional Chinese medicine: Chinese medicine holds that HE is caused by liver and kidney deficiency, unhealthy influences of damp heat and pestilent toxin, combined with factors such as internal injuries, eating disorders, and alcoholism; these factors result in illness from blazing heat toxin, pericardium heat attack, excessive phlegm turbidity, and phlegm confounding the heart orifices. The urgent need is to cure the symptoms, and treatment entails refreshing the brain and clearing the phlegm. Chinese patent medicines or decoctions, such as the Angong Niuhuang pills, can be used to clear phlegm, refresh the brain, remove heat, and detoxify[79]. Moreover, in the prevention and treatment of HE, the traditional Chinese medicine theory of "orifice opening" is widely used in accordance with the ammonia poisoning theory and the endogenous endotoxin theory of HE[80-82]. The most representative treatment is a retention enema using traditional Chinese medicine decoctions such as Chengqi soup, rhubarb decoctions, rehmannia preparations, and the like. A number of clinical studies have shown that the use of rhubarb decoction retention enemas to treat HE achieves good results; they act as a laxative to promote the excretion of toxic substances from the intestines, reduce blood ammonia levels, and shorten coma time.
Radical treatment of chronic disease: Fuzheng Huayu tablets (capsules), Anluo Huaxian pills and compound Biejia soft liver tablets are tonics with actions that activate the blood and reduce stasis; they have an anti-hepatic fibrosis and cirrhosis effect, improve liver function and immune function, reduce liver blood circulation disorders, and attenuate portal vein hypertension[83-86]. Consequently, they may have certain value in the prevention of cirrhosis HE.
Traditional Chinese medicine has certain preventive measures for HE/MHE. Many studies have shown that Chinese herbal formula has a higher effect on the cognitive and neurophysiological functions of cirrhosis patients with MHE compared with single lactulose alone. When used together, synergistic effects have been shown, but the mechanism of action of the drug is still unclear and under investigation. Some experts believe that metabolomics may help explain the mechanism of Chinese medicine for the treatment of HE patients.
The traditional view is that protein should be strictly limited in the diets of HE patients. In recent years, however, it has been found that 80.3% of cirrhosis patients are enterally malnourished; additionally, diets that excessively restrict protein for a long time can cause muscle group reduction, which makes HE more likely to occur. Correct assessment of the patient's nutritional status and early nutritional interventions can improve quality of life, reduce the incidence of complications, and prolong patient survival.
Energy intake and pattern: Reduced liver glycogen synthesis and storage leads to increased resting energy expenditure, causing the body to produce a fasting response similar to that of a healthy body experiencing extreme hunger. Currently, the ideal daily energy intake is believed to be 35 to 40 kcal/kg (1 kcal = 4.184 kJ). Patients should be encouraged to eat more frequent, smaller meals; distribute these small meals evenly throughout each day; and add a meal before bedtime that includes at least 50 g of complex carbohydrates so that fasting time does not exceed 3 to 6 h during the day. Chronic hyperammonemia may negatively affect attention. Cirrhotic patients had significantly lower total scores and significantly lower subscores in 4 of 7 cognitive categories, which is indicative of MHE. Patients' scores improved after breakfast consumption. Therefore, experts believe that eating breakfast can improve MHE patients’ attention and function[87].
Protein: The European Society of Parenteral and Enteral Nutrition's recommended daily protein intake is 1.2-1.5 g/kg to maintain nitrogen balance. The daily dietary protein intake for obese or overweight cirrhosis patients should be maintained at 2 g/kg, which is safe for HE patients. Because vegetable proteins contain less methionine and cysteine, they do not easily induce HE; additionally, vegetable proteins contain more ornithine and arginine, which can promote ammonia removal through the urea cycle. Therefore, patients with recurrent or persistent HE can consume 30 to 40 g of plant protein each day. The following principles should be applied when supplementing protein in HE patients: Patients with grade 3-4 HE should be prevented from supplementing protein intestinally. Grade 1 to 2 MHE patients and HE patients should limit protein for several days, control it at 20 g/d, and then increase it by 10-20 g every two to three days as symptoms. Plant protein is better than animal protein; intravenous albumin supplementation is safe. For chronic HE patients, measures including less food spread over more meals, gradually increasing the total protein, and individualizing the protein intake to the patient should be adopted.
Branched chain amino acids (BCAA): Patients with grade 3-4 HE can be provided with parenteral nutrition supplements rich in BCAA (valine, leucine, and isoleucine). Although several studies have shown that BCAA do not reduce mortality in HE patients, those who can tolerate normal protein diets or long-term BCAA supplementation can benefit from long-term improvement in nutritional status. Moreover, in addition to supporting glutamine synthesis in the brain and muscles, BCAA also promote ammonia detoxification and reduce the entry of excessive aromatic amino acids into the brain[88,89].
Other micronutrients: Mental symptoms caused by HE may be related to insufficient trace elements and water-soluble vitamins, especially thiamine, which can lead to elevated ammonia levels. Patients with decompensated cirrhosis or malnutrition risk can be treated with multivitamins or zinc supplements[90].
When liver failure is complicated with HE, some artificial liver models can be used to improve HE, depending on the medical treatment, and some inflammatory factors, endotoxins, blood ammonia, bilirubin, etc. can be eliminated to an extent. The artificial liver models commonly used to improve HE include blood perfusion, hemofiltration, plasma filtration dialysis, the molecular adsorbents recirculating system (MARS), the dual plasma molecular adsorption system (DPMAS), and plasma exchange combined with blood perfusion[91,92].
The therapeutic efficacy of internal medicine treatment is not ideal, and recurrent refractory HE accompanied by liver failure is an indication for liver transplantation[93].
A system of three preventions and three guards should be implemented. The “three preventions" are preventing the patient from wandering and getting lost, preventing injuries, and preventing self-harm. The “three guards” refers to bed guard rails, restraint belts (after family members sign informed consent), and table tennis gloves. It is important to closely observe HE patients' personality and behavior, mental state and awareness, and neuropsychiatric symptoms and signs for changes; monitor patients' diets, especially their daily protein intake, and carefully record their intake and excretion; observe the color, characteristics, and frequency of urine and stools; monitor vital signs, changes in coma patients' pupil size and light reflex, and sputum condition; and ensure that the intravenous infusion channel is unobstructed, and check surrounding skin conditions for extravasation, puncture points, etc.
Recommendation 8: HE factors (such as infection, gastrointestinal bleeding, and electrolyte imbalance) should be actively identified and eliminated (A1).
Recommendation 9: Lactulose can effectively improve the quality of life and survival rate of cirrhosis patients with HE/MHE. The recommended dose is 15 to 30 mL, 2 to 3 times/d, and two to three soft stools per day is considered appropriate (A1).
Recommendation 10: Lactitol can acidify the intestinal tract, regulate intestinal microecology, reduce ammonia absorption, effectively reduce endotoxins, and improve HE/MHE clinical symptoms/indicators. The recommended initial dose is 0.6 g/kg, divided into three administrations and taken with meals (B1).
Recommendation 11: Aspartate ornithine can reduce HE patients’ blood ammonia levels, shorten hospital stays, and have a therapeutic effect on HE (B1).
Recommendation 12: BCAA can be used as an alternative treatment or a long-term nutritional intervention (B2). Routine dose is 800 to 1200 mg/d taken orally two to four times a day (B2). Rifaximin is not recommended for type B HE (A1).
Recommendation 13: Serious mental disorders, such as mania, endanger the safety of others and make patients unable to cooperate with doctors. Benzodiazepine sedatives can be used for symptom control after patients' families are informed of the risks; these sedatives should be administered intravenously at a slow rate (B1).
Recommendation 14: HE patients with liver cirrhosis complicated by metabolic alkalosis can be treated with arginine hydrochloride, glutamine, and other drugs (C2).
Recommendation 15: A reasonable diet and nutritional supplements (daily breakfast and moderate amounts of protein) can help improve patient quality of life and prevent MHE/HE recurrence (B1).
Recommendation 16: Blood perfusion, hemofiltration, MARS, etc. can reduce blood ammonia, inflammatory factors, bilirubin, and other factors and improve clinical HE symptoms in liver failure patients (B1).
Recommendation 17: Patients with recalcitrant, recurrent HE accompanied by liver failure should be prioritized for liver transplantation (B1).
Recommendation 18: Chinese medicine has certain preventive actions for HE/MHE (B2).
HE primary prevention refers to reducing the risk of developing HE when HE has not yet occurred. The goal is to prevent MHE/OHE, reduce OHE-related hospitalization, improve quality of life, and improve survival rates. In addition to closely observing the conditions of liver cirrhosis, liver failure, and post-TIPS patients for changes, regular screening for MHE should be performed using neurophysiology and neuropsychology tests, radiology evaluation, etc. Once MHE is diagnosed, immediate treatment is necessary to avoid progression to OHE.
The focus of primary prevention is primary liver disease treatment and nutritional intervention. Etiological treatment can reduce liver inflammation and liver fibrosis, reduce portal vein blood pressure, and prevent or reverse cirrhosis progression, all of which are important for the prevention and control of HE and other complications. Infection, gastrointestinal bleeding, electrolyte imbalance, acid-base balance disorders, constipation, and other HE predisposing factors should be actively treated and prevented. Additional recommendations for patients include avoiding excessive ascites or diuresis, eating less and spreading energy intake over more meals, and avoiding the excessive intake of high amounts of protein.
After the first OHE episode, patients have a high risk of HE recurrence, and secondary prevention is recommended to improve quality of life and survival rates. The focus of secondary prevention is health education for patients and their families, control of elevated blood ammonia, and regulation of intestinal microecology. It is important to strengthen health education for patients and their families; inform them of the potential hazards of HE, especially MHE; and make them aware of the causes of HE. Under the guidance of a doctor, patients should rationally adjust their diet according to their degree of liver function damage and avoid high-protein diets and large one-time intakes of protein while suffering from HE. Lactulose, lactitol, and the like can be used as prophylactic drugs. Patients should be gradually guided toward self-managing their health, and family members should be instructed to carefully monitor the patients’ behavior and personality for changes. Patients should be checked for declines in attention, memory, and orientation, and efforts should be made to detect HE as soon as possible to ensure early diagnosis and treatment.
Recommendation 19: If there is a high risk of MHE or OHE, primary prevention is required (B1). The focus of primary prevention is targeting disease-causing factors and providing nutritional intervention (C1).
Recommendation 20: After OHE is controlled, secondary prevention must be carried out (A1), and lactulose and lactitol can be used as first-line drugs (A1).
Recommendation 21: The focus of secondary prevention is to provide relevant health education to patients and their families and to strengthen appropriate nutritional support, which can markedly reduce the recurrence of OHE episodes (B1). Sleep disorders and decreased attention are the earliest manifestations of OHE. Instruct family members to watch for them closely (C1).
The problems that need to be resolved include use of neuroimaging, genomics biomarkers, and fMRI in HE diagnosis research and application; the study and application of serum biomarkers and new neuropsychological testing methods for the early diagnosis of MHE; and research into new HE treatment methods, including fecal transplantation for HE prevention and treatment, HE stem cell therapy, and new HE therapeutic targets.
We thank all members of the Chinese Society of Hepatology, Chinese Medical Association. The Expert Group members (in order by the Pinyin Romanization of the individual’s last name) include: Ji-Hong An, Da-Chuan Cai, Guo-Feng Chen, Hong-Song Chen, Jing-Long Chen, Yu Chen, Jun Cheng, Hui-Guo Ding, Xiao-Guang Dou, Zhong-Ping Duan, Zhi-Jie Feng, Hui Gao, Yan-Hang Gao, Jia-Wei Geng, Hui-Min Guo, Wu-Hua Guo, Tao Han, Ying Han, Yuan Huang, Ji-Dong Jia, Jian-Ning Jiang, Ying-An Jiang, Yuan-Yuan Kong, Cang-You Li, Guang-Ming Li, Jie Li, Shu-Chen Li, Tai-Sheng Li, Wen-Gang Li, Yu-Fang Li, En-Qiang Ling-Hu, Jing-Feng Liu, Xiao-Qing Liu, Ying-Di Liu, Yu-Lan Liu, Lun-Gen Lu, Xin-Hua Luo, An-Lin Ma, Xiong Ma, Qing Mao, Yi-Min Mao, Yue-Min Nan, Yu-Qiang Nie, Jun-Qi Niu, Hong Ren, Wan-Hua Ren, Jia Shang, Lei Wang, Yu-Ming Wang, Xiao-Jun Wang, Lai Wei, Jing Wu, Wei-Fen Xie, Wen Xie, Shao-Jie Xin, Hui-Chun Xing, Jian-Ming Xu, Jie Xu, Jing-Hang Xu, Xiao-Yuan Xu, You-Qing Xu, Ming Yan, Dong-Liang Yang, Ji-Ming Yang, Jin-Hui Yang, Yong-Feng Yang, Yong-Ping Yang, Hong You, Yan-Yan Yu, Suo-Di Zhai, Chun-Qing Zhang, Da-Zhi Zhang, Ling-Yi Zhang, Lun-Li Zhang, Wen-Hong Zhang, Jing-Min Zhao, Ping Zhao, Shou-Song Zhao, Xuan Zhu, Hui Zhuang, and Wei-Ze Zuo. Academic secretaries are: Ying Han, Qian Kang, Hao Luo, and Ning Tan.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manenti A, Muengtaweepongsa S S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Hadjihambi A, Arias N, Sheikh M, Jalan R. Hepatic encephalopathy a critical current review. Hepatol Int. 2018;12:135-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 2. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1468] [Cited by in F6Publishing: 1364] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 3. | Dhiman RK, Saraswat VA, Sharma BK, Sarin SK, Chawla YK, Butterworth R, Duseja A, Aggarwal R, Amarapurkar D, Sharma P, Madan K, Shah S, Seth AK, Gupta RK, Koshy A, Rai RR, Dilawari JB, Mishra SP, Acharya SK; Indian National Association for Study of the Liver. Minimal hepatic encephalopathy: consensus statement of a working party of the Indian National Association for Study of the Liver. J Gastroenterol Hepatol. 2010;25:1029-1041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | American Association for the Study of Liver Diseases; European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61:642-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 281] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 5. | Chinese Society of Gastroenterology; Chinese Society of Hepatology, Chinese Medical Association. [Consensus on the diagnosis and treatment of hepatic encephalopathy]. Zhonghua Gan Zang Bing Za Zhi. 2013;21:641-651. [PubMed] [Cited in This Article: ] |
| 6. | Wang JY, Zhang NP, Chi BR, Mi YQ, Meng LN, Liu YD, Wang JB, Jiang HX, Yang JH, Xu Y, Li X, Xu JM, Zhang G, Zhou XM, Zhuge YZ, Tian DA, Ye J, Liu YL. Prevalence of minimal hepatic encephalopathy and quality of life evaluations in hospitalized cirrhotic patients in China. World J Gastroenterol. 2013;19:4984-4991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 7. | Moriwaki H, Shiraki M, Iwasa J, Terakura Y. Hepatic encephalopathy as a complication of liver cirrhosis: an Asian perspective. J Gastroenterol Hepatol. 2010;25:858-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Lin Y, Fan YP. [The neuropsychologic tests and the minimal hepatic encephalopathy investigations in liver cirrhotic patients]. Zhonghua Gan Zang Bing Za Zhi. 2011;19:65-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 9. | Guo JS. Epidemiology, diagnosis and treatment of minimal hepatic encephalopathy. Chin J Hepatol. 2014;22:92-93. [DOI] [Cited in This Article: ] |
| 10. | Bajaj JS, O'Leary JG, Tandon P, Wong F, Garcia-Tsao G, Kamath PS, Maliakkal B, Biggins SW, Thuluvath PJ, Fallon MB, Subramanian RM, Vargas HE, Lai J, Thacker LR, Reddy KR. Hepatic Encephalopathy Is Associated With Mortality in Patients With Cirrhosis Independent of Other Extrahepatic Organ Failures. Clin Gastroenterol Hepatol. 2017;15:565-574.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 88] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 11. | Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5:S7-S20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 12. | Lu LG. Hepatic encephalopathy: not so far away from us. Chin J Dig. 2017;37:508-512. [DOI] [Cited in This Article: ] |
| 13. | Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. 2014;5:397-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Wijarnpreecha K, Chesdachai S, Thongprayoon C, Jaruvongvanich V, Ungprasert P, Cheungpasitporn W. Association of Helicobacter pylori with the Risk of Hepatic Encephalopathy. Dig Dis Sci. 2017;62:3614-3621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168-G175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 16. | Janve VS, Hernandez CC, Verdier KM, Hu N, Macdonald RL. Epileptic encephalopathy de novo GABRB mutations impair γ-aminobutyric acid type A receptor function. Ann Neurol. 2016;79:806-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Kobtan AA, El-Kalla FS, Soliman HH, Zakaria SS, Goda MA. Higher Grades and Repeated Recurrence of Hepatic Encephalopathy May Be Related to High Serum Manganese Levels. Biol Trace Elem Res. 2016;169:153-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Butterworth RF. Neurosteroids in hepatic encephalopathy: Novel insights and new therapeutic opportunities. J Steroid Biochem Mol Biol. 2016;160:94-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Pereira K, Carrion AF, Martin P, Vaheesan K, Salsamendi J, Doshi M, Yrizarry JM. Current diagnosis and management of post-transjugular intrahepatic portosystemic shunt refractory hepatic encephalopathy. Liver Int. 2015;35:2487-2494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Tsai CF, Chen MH, Wang YP, Chu CJ, Huang YH, Lin HC, Hou MC, Lee FY, Su TP, Lu CL. Proton Pump Inhibitors Increase Risk for Hepatic Encephalopathy in Patients With Cirrhosis in A Population Study. Gastroenterology. 2017;152:134-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Blei AT, Córdoba J; Practice Parameters Committee of the American College of Gastroenterology. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968-1976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 409] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 22. | Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, Morgan MY; International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN). Review article: the design of clinical trials in hepatic encephalopathy--an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 23. | Sharma P, Sharma BC, Puri V, Sarin SK. Critical flicker frequency: diagnostic tool for minimal hepatic encephalopathy. J Hepatol. 2007;47:67-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Agrawal S, Umapathy S, Dhiman RK. Minimal hepatic encephalopathy impairs quality of life. J Clin Exp Hepatol. 2015;5:S42-S48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Wang AJ, Peng AP, Li BM, Gan N, Pei L, Zheng XL, Hong JB, Xiao HY, Zhong JW, Zhu X. Natural history of covert hepatic encephalopathy: An observational study of 366 cirrhotic patients. World J Gastroenterol. 2017;23:6321-6329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Iwasa M, Sugimoto R, Mifuji-Moroka R, Hara N, Yoshikawa K, Tanaka H, Eguchi A, Yamamoto N, Sugimoto K, Kobayashi Y, Hasegawa H, Takei Y. Factors contributing to the development of overt encephalopathy in liver cirrhosis patients. Metab Brain Dis. 2016;31:1151-1156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Lockwood AH. Blood ammonia levels and hepatic encephalopathy. Metab Brain Dis. 2004;19:345-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Huang H, Wu T, Mao J, Fang Y, Zhang J, Wu L, Zheng S, Lin B, Pan H. CHI3L1 Is a Liver-Enriched, Noninvasive Biomarker That Can Be Used to Stage and Diagnose Substantial Hepatic Fibrosis. OMICS. 2015;19:339-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Yao M, Wang L, Leung PSC, Li Y, Liu S, Wang L, Guo X, Zhou G, Yan Y, Guan G, Chen X, Bowlus CL, Liu T, Jia J, Gershwin ME, Ma X, Zhao J, Lu F. The Clinical Significance of GP73 in Immunologically Mediated Chronic Liver Diseases: Experimental Data and Literature Review. Clin Rev Allergy Immunol. 2018;54:282-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Jeong JY, Jun DW, Bai D, Kim JY, Sohn JH, Ahn SB, Kim SG, Kim TY, Kim HS, Jeong SW, Cho YK, Song DS, Kim HY, Jung YK, Yoon EL. Validation of a Paper and Pencil Test Battery for the Diagnosis of Minimal Hepatic Encephalopathy in Korea. J Korean Med Sci. 2017;32:1484-1490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Thomsen KL, Macnaughtan J, Tritto G, Mookerjee RP, Jalan R. Clinical and Pathophysiological Characteristics of Cirrhotic Patients with Grade 1 and Minimal Hepatic Encephalopathy. PLoS One. 2016;11:e0146076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Giménez-Garzó C, Garcés JJ, Urios A, Mangas-Losada A, García-García R, González-López O, Giner-Durán R, Escudero-García D, Serra MA, Soria E, Felipo V, Montoliu C. The PHES battery does not detect all cirrhotic patients with early neurological deficits, which are different in different patients. PLoS One. 2017;12:e0171211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Hao L, Hu Y, Hou X. [Age-and, education-corrected number connection test and digit symbol test in diagnosis of minimal hepatic encephalopathy]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:533-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 34. | Wuensch T, Ruether DF, Zöllner C, Mueller T, Jung T, Kaffarnik M, Kassner U, Schott E, Kiefer S, Pratschke J, Stockmann M, Jara M. Performance characterization of a novel electronic number connection test to detect minimal hepatic encephalopathy in cirrhotic patients. Eur J Gastroenterol Hepatol. 2017;29:456-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Bajaj JS, Heuman DM, Sterling RK, Sanyal AJ, Siddiqui M, Matherly S, Luketic V, Stravitz RT, Fuchs M, Thacker LR, Gilles H, White MB, Unser A, Hovermale J, Gavis E, Noble NA, Wade JB. Validation of EncephalApp, Smartphone-Based Stroop Test, for the Diagnosis of Covert Hepatic Encephalopathy. Clin Gastroenterol Hepatol. 2015;13:1828-1835.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Shi XJ, Abuduheilili X, Fan XT, Ma HL, Feng J, Sun J, A LY, He FP. [Critical flicker frequency for the diagnosis of minimal hepatic encephalopathy]. Zhonghua Gan Zang Bing Za Zhi. 2013;21:546-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 37. | Ma QY, Zhu-Ge ZY. Diagnostic value of critical flicker frequency in minimal hepatic encephalopathy. J Clin Hepatol. 2012;28:559-561. [DOI] [Cited in This Article: ] |
| 38. | Kircheis G, Hilger N, Häussinger D. Value of critical flicker frequency and psychometric hepatic encephalopathy score in diagnosis of low-grade hepatic encephalopathy. Gastroenterology. 2014;146:961-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Ampuero J, Simón M, Montoliú C, Jover R, Serra MÁ, Córdoba J, Romero-Gómez M. Minimal hepatic encephalopathy and critical flicker frequency are associated with survival of patients with cirrhosis. Gastroenterology. 2015;149:1483-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Campagna F, Montagnese S, Ridola L, Senzolo M, Schiff S, De Rui M, Pasquale C, Nardelli S, Pentassuglio I, Merkel C, Angeli P, Riggio O, Amodio P. The animal naming test: An easy tool for the assessment of hepatic encephalopathy. Hepatology. 2017;66:198-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 41. | Urios A, Mangas-Losada A, Gimenez-Garzó C, González-López O, Giner-Durán R, Serra MA, Noe E, Felipo V, Montoliu C. Altered postural control and stability in cirrhotic patients with minimal hepatic encephalopathy correlate with cognitive deficits. Liver Int. 2017;37:1013-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Seo K, Jun DW, Kim JK, Ryu H. Multi-Sensory Integration Impairment in Patients with Minimal Hepatic Encephalopathy. Sci Rep. 2017;7:14947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Guerit JM, Amantini A, Fischer C, Kaplan PW, Mecarelli O, Schnitzler A, Ubiali E, Amodio P; members of the ISHEN commission on Neurophysiological Investigations. Neurophysiological investigations of hepatic encephalopathy: ISHEN practice guidelines. Liver Int. 2009;29:789-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Shi YB, Seng SJ, Guo M, Zhao HL. Analysis of magnetic resonance and CT imaging findings in hepatic encephalopathy. Chin J Pract Nerv Dis. 2016;19:127-127, 128. [DOI] [Cited in This Article: ] |
| 45. | Qi R, Zhang LJ, Zhong J, Zhu T, Zhang Z, Xu C, Zheng G, Lu GM. Grey and white matter abnormalities in minimal hepatic encephalopathy: a study combining voxel-based morphometry and tract-based spatial statistics. Eur Radiol. 2013;23:3370-3378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Kale RA, Gupta RK, Saraswat VA, Hasan KM, Trivedi R, Mishra AM, Ranjan P, Pandey CM, Narayana PA. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology. 2006;43:698-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 47. | Zheng G, Zhang LJ, Zhong J, Wang Z, Qi R, Shi D, Lu GM. Cerebral blood flow measured by arterial-spin labeling MRI: a useful biomarker for characterization of minimal hepatic encephalopathy in patients with cirrhosis. Eur J Radiol. 2013;82:1981-1988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Chen HJ, Chen QF, Yang ZT, Shi HB. Aberrant topological organization of the functional brain network associated with prior overt hepatic encephalopathy in cirrhotic patients. Brain Imaging Behav. 2019;13:771-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Chen HJ, Chen QF, Liu J, Shi HB. Aberrant salience network and its functional coupling with default and executive networks in minimal hepatic encephalopathy: a resting-state fMRI study. Sci Rep. 2016;6:27092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Jiao Y, Tang T, Wang X, Zhu X, Teng G. [Anterior and posterior default mode networks impairments in minimal hepatic encephalopathy]. Zhonghua Yi Xue Za Zhi. 2016;96:334-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 51. | Shawcross DL, Dunk AA, Jalan R, Kircheis G, de Knegt RJ, Laleman W, Ramage JK, Wedemeyer H, Morgan IE; New Insights Steering Committee. How to diagnose and manage hepatic encephalopathy: a consensus statement on roles and responsibilities beyond the liver specialist. Eur J Gastroenterol Hepatol. 2016;28:146-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Suraweera D, Sundaram V, Saab S. Evaluation and Management of Hepatic Encephalopathy: Current Status and Future Directions. Gut Liver. 2016;10:509-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Hanai T, Shiraki M, Watanabe S, Kochi T, Imai K, Suetsugu A, Takai K, Moriwaki H, Shimizu M. Sarcopenia predicts minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatol Res. 2017;47:1359-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 54. | NeSmith M, Ahn J, Flamm SL. Contemporary Understanding and Management of Overt and Covert Hepatic Encephalopathy. Gastroenterol Hepatol (N Y). 2016;12:91-100. [PubMed] [Cited in This Article: ] |
| 55. | Yan M. Selection of the diagnostic and therapeutic methods for minimal hepatic encephalopathy. J Clin Hepatol. 2016;32:1092-1099. [DOI] [Cited in This Article: ] |
| 56. | Scalzo SJ, Bowden SC, Ambrose ML, Whelan G, Cook MJ. Wernicke-Korsakoff syndrome not related to alcohol use: a systematic review. J Neurol Neurosurg Psychiatry. 2015;86:1362-1368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Phan AQ, Pacifici M, Esko JD. Advances in the pathogenesis and possible treatments for multiple hereditary exostoses from the 2016 international MHE conference. Connect Tissue Res. 2018;59:85-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Liu A, Yoo ER, Siddique O, Perumpail RB, Cholankeril G, Ahmed A. Hepatic encephalopathy: what the multidisciplinary team can do. J Multidiscip Healthc. 2017;10:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Goyal O, Sidhu SS, Kishore H. Minimal hepatic encephalopathy in cirrhosis- how long to treat? Ann Hepatol. 2017;16:115-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Ding HG, Xu XY, Linghu EQ, Jia JD. Guildlines for prevention and treatment of esophageal variceal bleeding in cirrhotic portal hypertension. J Clin Hepatol. 2016;32:220-222. [DOI] [Cited in This Article: ] |
| 61. | Chinese Society of Hepatology; Chinese Medical Association. [Guidelines on the management of ascites and complications in cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2017;25:664-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 62. | de Lorenzo-Pinto A, García-Sánchez R, Lorenzo-Salinas A. Lactulose enemas in the treatment of hepatic encephalopathy. Do we help or harm? Rev Esp Enferm Dig. 2017;109:736-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Lauridsen MM, Mikkelsen S, Svensson T, Holm J, Klüver C, Gram J, Vilstrup H, Schaffalitzky de Muckadell OB. The continuous reaction time test for minimal hepatic encephalopathy validated by a randomized controlled multi-modal intervention-A pilot study. PLoS One. 2017;12:e0185412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Moratalla A, Ampuero J, Bellot P, Gallego-Durán R, Zapater P, Roger M, Figueruela B, Martínez-Moreno B, González-Navajas JM, Such J, Romero-Gómez M, Francés R. Lactulose reduces bacterial DNA translocation, which worsens neurocognitive shape in cirrhotic patients with minimal hepatic encephalopathy. Liver Int. 2017;37:212-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Singh J, Sharma BC, Puri V, Sachdeva S, Srivastava S. Sleep disturbances in patients of liver cirrhosis with minimal hepatic encephalopathy before and after lactulose therapy. Metab Brain Dis. 2017;32:595-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Li LJ, Chen CL, Wu ZW, Chen HG, Fu SZ, Sheng JF. Effects of Lactitol on intestinal flora and plasma endotoxin in patients with chronic viral hepatitis. Chin J Infect Dis. 2005;23:395-397. [Cited in This Article: ] |
| 67. | Gong JS, Zhang YX, Chen H. Safety and efficacy of rifaximin versus nonabsorbable disaccharides in treatment of hepatic encepha: a meta analysis. Clini Focus. 2015;30:191-195. [DOI] [Cited in This Article: ] |
| 68. | Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016;CD003044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Bai M, Yang Z, Qi X, Fan D, Han G. l-ornithine-l-aspartate for hepatic encephalopathy in patients with cirrhosis: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2013;28:783-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Pratap Mouli V, Benjamin J, Bhushan Singh M, Mani K, Garg SK, Saraya A, Joshi YK. Effect of probiotic VSL#3 in the treatment of minimal hepatic encephalopathy: A non-inferiority randomized controlled trial. Hepatol Res. 2015;45:880-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | Saab S, Suraweera D, Au J, Saab EG, Alper TS, Tong MJ. Probiotics are helpful in hepatic encephalopathy: a meta-analysis of randomized trials. Liver Int. 2016;36:986-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 72. | Hu BL, Wang HY, Yang GY. Association of Helicobacter pylori infection with hepatic encephalopathy risk: a systematic review. Clin Res Hepatol Gastroenterol. 2013;37:619-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Schulz C, Schütte K, Malfertheiner P. Does H. pylori eradication therapy benefit patients with hepatic encephalopathy?: systematic review. J Clin Gastroenterol. 2014;48:491-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Schulz C, Schütte K, Reisener N, Voss J, Malfertheiner P. Prevalence of Helicobacter pylori Infection in Patients with Minimal Hepatic Encephalopathy. J Gastrointestin Liver Dis. 2016;25:191-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Sahney A, Sharma BC, Jindal A, Anand L, Arora V, Vijayaraghavan R, Dhamija RM, Kumar G, Bhardwaj A, Sarin SK. A double-blind randomized controlled trial to assess efficacy of bromocriptine in cirrhotic patients with hepatic parkinsonism. Liver Int. 2019;39:684-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Jiang Q, Jiang G, Welty TE, Zheng M. Naloxone in the management of hepatic encephalopathy. J Clin Pharm Ther. 2010;35:333-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Zhou ZW, Zhong XN, Zhou BY, Xiang JF, Wang RH, Yi J. [Ornithine aspartate and naloxone combined therapy for hepatic encephalopathy affects cognitive function, prognosis, and neuropeptide levels]. Zhonghua Gan Zang Bing Za Zhi. 2013;21:385-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 78. | Khamaysi I, William N, Olga A, Alex I, Vladimir M, Kamal D, Nimer A. Sub-clinical hepatic encephalopathy in cirrhotic patients is not aggravated by sedation with propofol compared to midazolam: a randomized controlled study. J Hepatol. 2011;54:72-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Zhao M, Ye DN. Clinical Observation on Treatment of Hepatic Cirrhosis Complicated with Hepatic Encephalopathy by Integrative Chinese and Western Medicine Therapy. Res Integr Trad Chin West Med. 2017;9:6-8. [Cited in This Article: ] |
| 80. | Zhou Y, Ma YL. Clinical observation of “Yangyin Huatan Xifeng Decoction” in the treatment of recurrent hepatic encephalopathy. Shanghai J Trad Chin Med. 2016;50:42-44. [Cited in This Article: ] |
| 81. | Gan DN, Ye YA, Jiang F. Analysis on the Preponderant Efficacy of Traditional Chinese Medicine to Treat Minimal Hepatic Encephalopathy. World Chin Med. 2014;4:504-506, 509. [Cited in This Article: ] |
| 82. | Yao C, Huang G, Wang M, Xia M, Yao F, Niu D, Zhang Y, Li S, Zhong Y. Chinese herbal medicine formula Jieduhuayu granules improves cognitive and neurophysiological functions in patients with cirrhosis who have minimal hepatic encephalopathy: a randomized controlled trial. Complement Ther Med. 2014;22:977-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Ge XJ, Zhao CQ, Xu LM. [Effect of Fuzheng Huayu capsules on survival rate of patients with liver cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2017;25:834-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 84. | Chi XL, Xiao HM. Traditional Chinese medicine prevention and treatment of liver fibrosis under the new situation of prevention and treatment of viral hepatitis. J Clin Hepatol. 2018;34:694-697. [DOI] [Cited in This Article: ] |
| 85. | Lu W, Gao YH, Wang ZZ, Cai YS, Yang YQ, Miao YQ, Pei F, Liu XE, Zhuang H. [Effects of Anluohuaxianwan on transforming growth factor-β1 and related signaling pathways in rats with carbon tetrachloride-induced liver fibrosis]. Zhonghua Gan Zang Bing Za Zhi. 2017;25:257-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 86. | Wu G, He H, Li H, Chen W. [Clinical effect of combination therapy with Fufang Biejia Ruangan tablet and entecavir in patients with hepatitis B virus-related cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2014;22:604-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 87. | Vaisman N, Katzman H, Carmiel-Haggai M, Lusthaus M, Niv E. Breakfast improves cognitive function in cirrhotic patients with cognitive impairment. Am J Clin Nutr. 2010;92:137-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 89. | Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr Clin Pract. 2013;28:580-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Mousa N, Abdel-Razik A, Zaher A, Hamed M, Shiha G, Effat N, Elbaz S, Elhelaly R, Hafez M, El-Wakeel N, Eldars W. The role of antioxidants and zinc in minimal hepatic encephalopathy: a randomized trial. Therap Adv Gastroenterol. 2016;9:684-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Hanish SI, Stein DM, Scalea JR, Essien EO, Thurman P, Hutson WR, Bartlett ST, Barth RN, Scalea TM. Molecular Adsorbent Recirculating System Effectively Replaces Hepatic Function in Severe Acute Liver Failure. Ann Surg. 2017;266:677-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 92. | Saliba F, Camus C, Durand F, Mathurin P, Letierce A, Delafosse B, Barange K, Perrigault PF, Belnard M, Ichaï P, Samuel D. Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: a randomized, controlled trial. Ann Intern Med. 2013;159:522-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 93. | Osman MA, Sayed MM, Mansour KA, Saleh SA, Ibrahim WA, Abdelhakam SM, Bahaa M, Yousry WA, Elbaz HS, Mikhail RN, Hassan AM, Elsayed EH, Mahmoud DA. Reversibility of minimal hepatic encephalopathy following liver transplantation in Egyptian cirrhotic patients. World J Hepatol. 2016;8:1279-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |