Published online Sep 14, 2019. doi: 10.3748/wjg.v25.i34.5105
Peer-review started: April 12, 2019
First decision: May 24, 2019
Revised: June 14, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: September 14, 2019
Non-alcoholic fatty liver disease (NAFLD) has become a major cause of chronic liver disease. The Chinese herbal medicine (CHM) Dachaihu decoction (DCHD) has been proved to treat NAFLD with good efficacy in previous studies. Based on the TCM principle of formula formation, we divided DCHD into soothing liver part, invigorating spleen part, and dredging intestine part. Marshall officially proposed the concept of “intestinal-hepatic axis”, which systematically explains the interactions between the intestine and liver. We hypothesized that the effect of CHM on NAFLD is achieved by regulating the liver and intestine. Thus, we aimed to investigate the possible effect of a CHM formula on NAFLD in a rat model.
To investigate the effects of a CHM formula (a decoction of Chinese thorowax root, scutellaria root, and white peony root) on NAFLD and its regulatory effect on the “intestinal-liver” axis.
Sixty rats were randomly divided into control, model, pioglitazone hydrochloride (PH), and CHM (a decoction of Chinese thorowax root, scutellaria root, and white peony root) groups. An NAFLD rat model was established using a high-fat high-fructose diet for 16 wk. From the 13th week, rats were administered with PH or a decoction of Chinese thorowax, scutellaria, and white peony root (CHM group) for 4 wk. Rats in the control group and model group were administered with an equal volume of distilled water. At the end of the study, blood was collected via the abdominal aorta. Liver tissues were harvested and any morphological changes were observed by hematoxylin-eosin (HE) staining, Oil red O staining, and Masson staining. In addition, blood lipids, liver function markers, and triglyceride (TG) in liver tissues were analyzed. The levels of transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNF-α), Toll-like receptor-4 (TLR4), and nuclear factor-kappa B (NF-кB) in liver tissues and secreted immunoglobulin A (sIgA) in intestinal tissues were analyzed by ELISA, and protein and mRNA expression of occludin and zonula occludens-1 (ZO-1) in the intestine were measured using Western blot and reverse transcription-quantitative polymerase chain reaction, respectively. The endotoxin level in plasma was detected by endpoint chromogenic assay.
Compared to the normal control group, the liver coefficient, serum TG, total cholesterol (TC), low density lipoprotein (LDL), aspartate aminotransferase (AST), and alanine aminotransferase (ALT), blood glucose, plasma endotoxin, and the levels of TG, TNF-α, TGF-β, NF-kB, and TLR4 in liver tissues increased significantly in the model group, while serum high density lipoprotein (HDL), intestinal sIgA, and protein and mRNA expression of occludin and ZO-1 decreased significantly in the model group (P < 0.01). PH and CHM attenuated the elevated liver coefficient, serum TG, TC, LDL, AST, and ALT, blood glucose, plasma endotoxin, and the levels of TG, TNF-α, TGF-β, NF-kB, and TLR4 in liver tissues and increased serum HDL levels compared to the model group (P < 0.01). Intestinal sIgA and the protein and mRNA expression of intestinal occludin and ZO-1 were significantly increased in the PH group compared to the model and CHM groups (P < 0.01).
The decoction of Chinese thorowax root, scutellaria root, and white peony root is beneficial in regulating lipid metabolism and liver function, which indicates that it has a good effect on the liver. To a certain extent, this CHM formula can affect both the liver and intestine, while its effect on the liver is superior to that on the intestine.
Core tip: Based on the previous study about the “intestinal-hepatic axis”, we hypothesized that the effect of Chinese herbal medicine (CHM) on non-alcoholic fatty liver disease is achieved by regulating the liver and intestine. In this study, we found that a CHM formula (a decoction of thorowax root, scutellaria root, and white peony root) had a good effect in regulating lipid metabolism and liver function, which suggested its ability to regulate the liver. To a certain extent, it also had a regulatory effect on the intestinal mucosal barrier. Our study demonstrated that the CHM formula can affect both the liver and intestine, while its effect on the liver is superior to that on the intestine.
- Citation: Yang JM, Sun Y, Wang M, Zhang XL, Zhang SJ, Gao YS, Chen L, Wu MY, Zhou L, Zhou YM, Wang Y, Zheng FJ, Li YH. Regulatory effect of a Chinese herbal medicine formula on non-alcoholic fatty liver disease. World J Gastroenterol 2019; 25(34): 5105-5119
- URL: https://www.wjgnet.com/1007-9327/full/v25/i34/5105.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i34.5105
Non-alcoholic fatty liver disease (NAFLD) is one of the manifestations of fatty liver and occurs when fat is deposited (steatosis) in the liver due to causes other than excessive alcohol use[1]. The incidence of NAFLD has increased over the years with a prevalence of 25%-30% in the general population. This makes it one of the most major causes of chronic liver disease and affects both adults and children. NAFLD is more common in patients with severe diabetes and obesity and has gained more attention worldwide[2,3]. Hepatic morphology and functionality in NAFLD patients are adversely affected. These adverse effects are categorized into four different stages, starting from simple steatosis (liver fat deposition and mild inflammation), development of non-alcoholic steatohepatitis (NASH) that includes steatosis plus inflammation and hepatocyte “ballooning”, the appearance of fibrosis and, in some cases, leading to late stage hepatocellular carcinoma (HCC). Although the exact mechanisms leading to NAFLD are not fully deciphered, insulin resistance, hormones secreted from adipose tissues, nutritional factors, gut microbiota, and genetic/epigenetic factors have been suggested to play a major role[4]. Approximately 90% of patients with NAFLD show a close association with one or more of the following risk factors: Hypertension, dyslipidemia, elevated triglyceride (TG) levels, obesity, insulin resistance, metabolic syndrome, type 2 diabetes mellitus, and cardiovascular disease[1]. There are currently no effective treatment options for NAFLD, with the exemption of lifestyle changes[5].
The pathogenesis of NAFLD remains unclear; however, the “two-hit” hypothesis is the most widely accepted theory, which states that fat deposition in the liver represents the “first hit”, and increased levels of oxidative stress, insulin resistance, and inflammatory cytokines induced by fat deposition represents the “second hit”[6]. In addition, the “multi-hit” theory has been proposed, which states that several extrahepatic factors, such as adipose tissue and the intestinal tract participate in accelerating the occurrence of liver inflammation and the development of NAFLD[7]. In 1998, Marshall officially proposed the concept of “intestinal-hepatic axis”, which systematically explains the interactions between the intestine and liver and how they interact and regulate substances, cells, and cytokines and each other.
Chinese herbal medicine (CHM) has been traditionally used in China and other Asian countries for thousands of years. A specific and basic feature of Chinese medicine is the use of formulas containing several herbs (herbal cocktail) to ameliorate abnormal symptoms associated with a particular disease[8]. Dachaihu decoction (DCHD), a classical formula from the Treatise on Febrile Disease, is one of the well-known traditional Chinese medicines and consists of: Chinese thorowax root (Bupleurum chinensis DC.), scutellaria root (Scutellaria baicalensis Georgi), white peony root (Paeonia tacti lora Pall), prepared pinellia tuber (Pinellia ternate), fresh ginger (Zingiber officinale Roscoe), Chinese date (Ziziphus jujube MILL.), rhubarb root and rhizome (Rheum palmatum L.), and immature bitter orange (Citrus aurantium L.)[9]. Previous studies have demonstrated these TCM formulas had good efficacy in treating NAFLD[10,11]. Based on the TCM principle of formula formation, we divided DCHD into three parts; part 1 is “soothing liver” (Chinese thorowax root, scutellaria root, and white peony root), which has a good effect in regulating the liver; part 2 is “invigorating spleen” (prepared pinellia tuber, fresh ginger, and Chinese date), which has a good effect in regulating the spleen, and part 3 is “dredging intestine” (rhubarb root and rhizome and immature bitter orange), which has an effect in dredging the turbidity and stool.
The aim of the present study was to investigate the therapeutic effects of the soothing liver herbs of DCHD on NAFLD in a rat model. We hypothesized that the effect of CHM on NAFLD may be achieved by regulating the intestinal-hepatic axis. Whether the regulatory effect is mainly on the liver or the intestine needs experimental observation. To test our hypothesis, we measured the liver weight/body weight ratio, liver histopathology, serum liver enzymes, cholesterol lipoproteins, cytokines, and gene and protein expression in the intestine in a rate model of NAFLD.
A total of 60 male Sprague-Dawley (SD) rats weighing 180 ± 20 g were purchased from SPF (Beijing) Biotechnology [License No: SCXK (2016-0002; Beijing, China]. Rats were housed at a constant temperature of 23 °C ± 1 °C, a constant humidity of 45p.100 ± 5p.100, with 12 h light/dark cycles. Water and laboratory rodent chow were provided ad libitum. This study was approved by the Medical and Experimental Animal Ethics Committee of Beijing University of Chinese Medicine (BUCM-1-2017051030-2030).
Pioglitazone hydrochloride (PH) tablets were used as the positive control and was purchased from Jingdong Pharmacy (Huadong Medicine, product lot number: 170102). Based on a previous study[12], pioglitazone was administered at a dosage of 10 mg/kg per day. Tablets were ground into a powder and dissolved in distilled water. The prepared suspension was refrigerated and dispensed when required.
TCM herbs bupleurum, scutellaria roots, and white peony roots were purchased from Beijing Tong Ren Tang Pharmacy (Beijing, China). Their dosages were as follows: Chinese thorowax root (Bupleurum chinense DC = 15 g), scutellaria root (Scutellaria baicalensis georgi = 9 g), and white peony root (Paeonia lactiflora PALL = 9 g). Based on body surface area and human-rat dose conversion, the dosage of the crude drugs for rats was set at 8.64 g/kg[13].
Initially the ingredients were placed in an earthenware pot and distilled water was added until all the ingredients were covered. The ingredients were soaked for 30 min and then the solution was boiled and simmered. The decoction was filtered, and more water was added to the remaining ingredients and then boiled again as described previously. The two decoctions were mixed and the final concentration of the herbal medicinal extracts was 1 g/mL. The decoction was stored in a refrigerator until used.
Sixty SD rats were randomly and equally divided into four groups: Control, model, PH, and CHM groups. The control group was fed a standard diet (n = 15), while the other three groups (model group, PH group, and CHM group) were fed a high-fat high-fructose diet containing 52.5% basic food, 2% cholesterol, 10% lard, 5% egg yolk powder, 0.5% sodium cholate, and 30% sucrose. The calories percent of the fat in the diet was 11.6%, and the calories percent of carbohydrates in the diet was 65.4%. The rats were housed in a temperature-controlled environment with 50% humidity and fed for 12 wk. From the 13th week, rats in the PH group were administered intragastrically with PH at a dose of 10 mg/kg/d (n = 8) for 4 wk and fed the high-fat high-fructose diet. Rats in the CHM group were administered intragastrically with thorowax root, scutellaria root, and white peony root decoction for 4 wk and fed the high-fat high-fructose diet. The normal group and model group were provided with an equal volume of distilled water and fed the standard diet and high-fat high-fructose diet, respectively.
After 16 wk of feeding, all rats were fasted for 12 h and eight of them from each group were randomly selected for blood and liver tissue collection. Blood samples from the tail tip were obtained for blood sugar detection. At the end of the study, the animals were anesthetized and abdominal blood samples from all rats were collected for serum biochemical assays. Liver tissues from the same region for all rats were fixed in 4% paraformaldehyde, paraffin-embedded, and sectioned. The remaining liver sections were stored at -80°C until needed for other assays.
At the end of the experiment, body weight and liver weight of all rats for the four groups were measured and recorded. Liver coefficients were calculated as absolute liver weight (g)/body weight of rat on sacrifice day (g) × 100%.
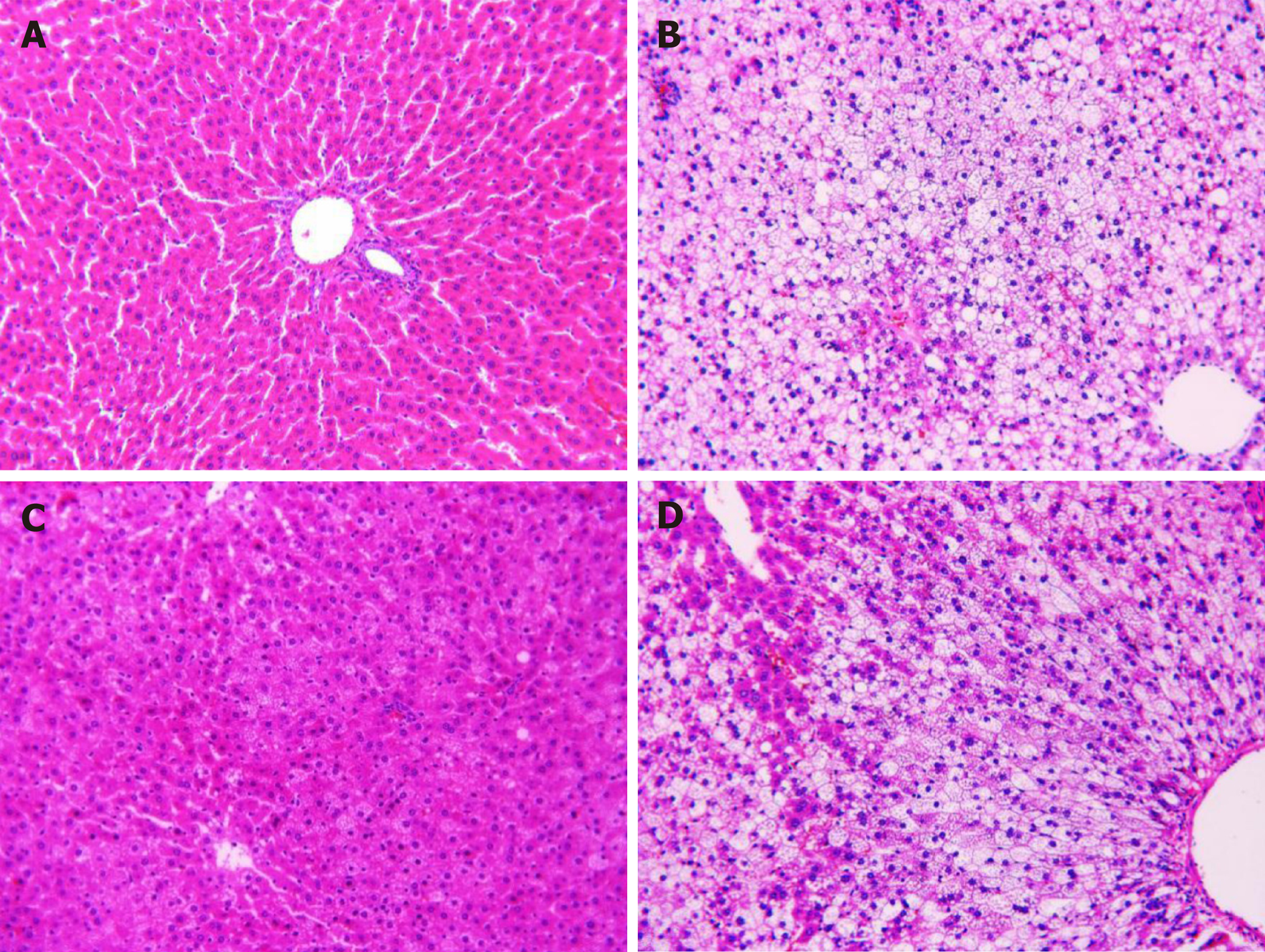
Liver tissues were fixed in 4% paraformaldehyde and then embedded in paraffin and sliced into 5 μm thick sections. The sections were then stained with HE for morphological examination using a light microscope (BX-53; Olympus) at 200× magnification.
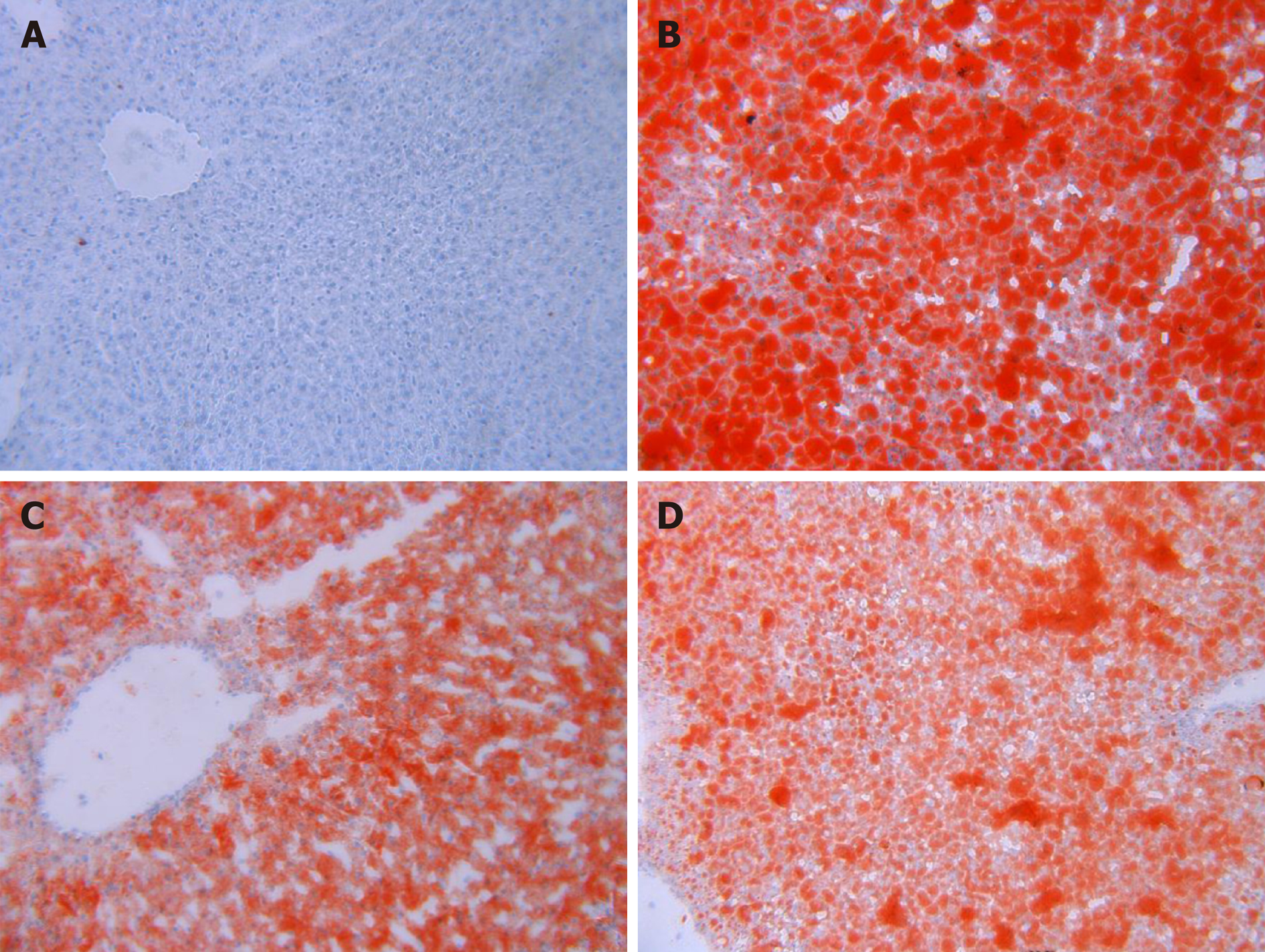
Frozen hepatic tissues were cut at 6 μm and mounted on slides, air-dried, and then fixed in ice-cold 10% formaldehyde solution for 10 min. Slides were rinsed immediately in distilled water for several seconds and then placed in isopropanol solution for 20-30 s. Slides were then stained in ORO solution for 15-20 min, and then rinsed in distilled water prior to staining with hematoxylin for 40 s. After washing thoroughly in running tap water for 5 min, the slides were placed in distilled water and then mounted with glycerin jelly. The HE- and ORO-stained slides were visualized using a light microscope to visualize the architecture of the liver and hepatic lipid droplets. Image processing software pro plus 6.0 (Media Cybernetics, Maryland, United States) was used for data quantification. Liver lipid droplet vacuolar area ratio was calculated as lipid droplet area/total area of the picture × 100%.
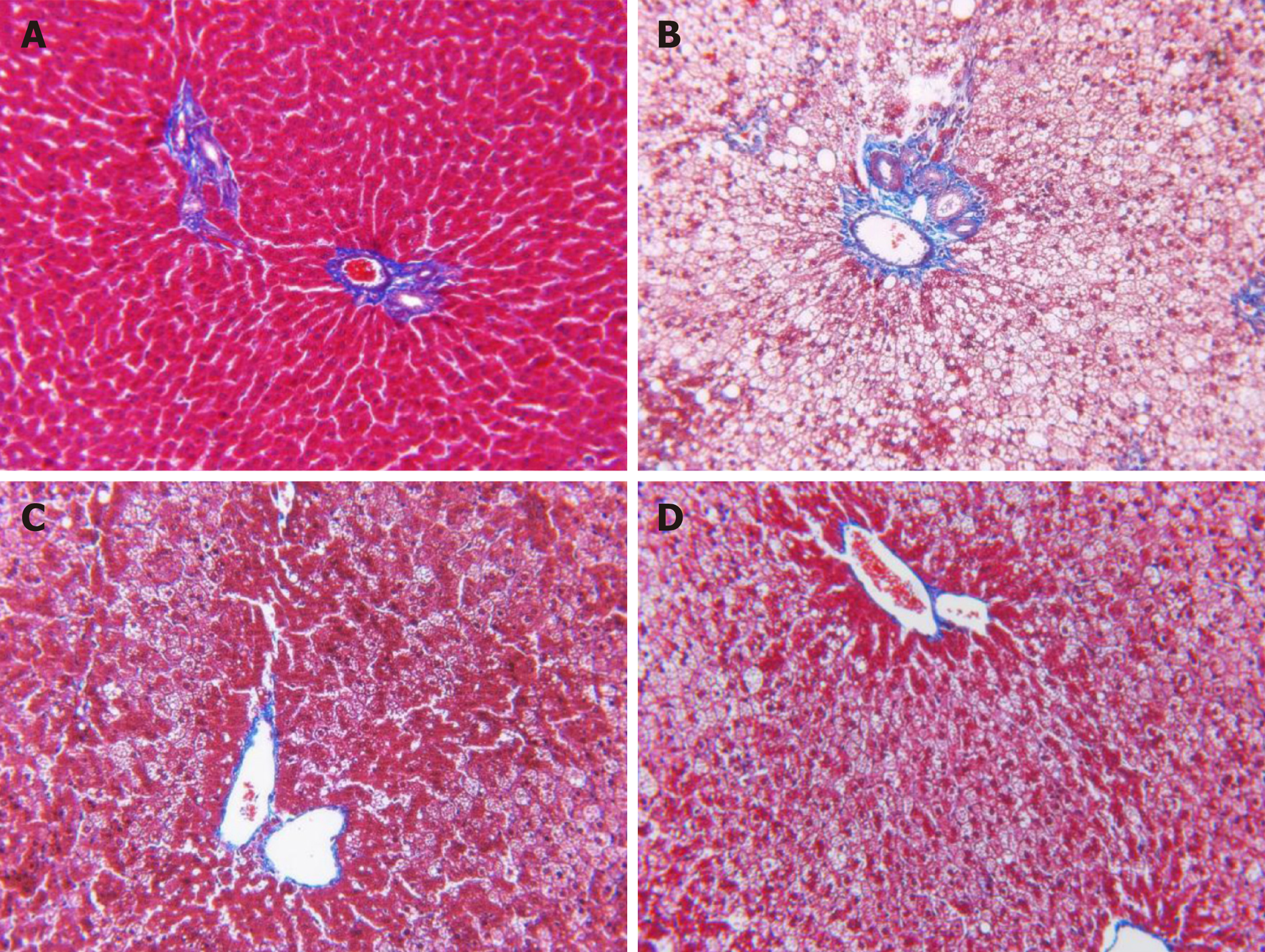
Liver tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and then sectioned. Slides were then hydrated through a series of graded alcohols (100, 95, 90, 80, and 70%) for 5 min each. The slides were then stained with Masson’s trichrome dye.
Abdominal aortic blood was collected and serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), TG, high density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were measured using an automatic biochemical detector from Beckman Coulter Inc. (California, United States).
A 100 mg liver tissue piece and intestine tissue piece from the same portion were placed into a 1.5 mL tube, 1 mL of 0.9% saline was added, and the piece was cut into smaller pieces. After grinding, the supernatant was absorbed after centrifugation for 15 min (4 °C, 1500 rpm). Enzyme-linked immunosorbent assay (ELISA) kits were used to test the levels of each protein according to the instructions provided. The levels of tumor necrosis factor (TNF-α) and transforming growth factor (TGF-β1) in liver tissues and sIgA in intestinal tissues were measured using ELISA kits (NeoBioscience, Shenzhen, China). The levels of nuclear factor-kappa B (NF-кB) and Toll-like receptor (TLR4) in liver tissues were measured using a commercial kit (USCN Life Science, Wuhan, China). The TG level in liver tissue was measured using an automatic biochemical detector from Beckman Coulter Inc. (California, United States).
Total RNA was extracted using TRIZOL reagent according to the manufacturer’s instructions. The concentration and purity of the RNA samples were measured. cDNA was synthesized for RT-PCR amplification according to the instructions in the First Strand cDNA Synthesis Kit (Invitrogen). The sequences of the primers used for the RT-PCR assay are shown in Table 1. The reverse transcription conditions were as follows: 37 °C for 15 min, followed by 5 s at 85 °C for RT inactivation. qPCR was performed using the following conditions: 30s at 95 °C for denaturation; 5 s at 95 °C for annealing, and 40 s at 60 °C for extension.
| Gene | Forward | Reverse |
| ZO-1 | 5’- CTGATGGTGTTCTGCCAAATTC – 3’ | 5' - GTCGCAAACCCACACTATCT - 3' |
| Occludin | 5’- CCATCTGACTATGCGGAAAGAG – 3’ | 5' - TACCAGAGGCGGTGACTTAT - 3' |
| β-actin | 5’ - ACCGTGAAAAGATGACCCAGAT – 3’ | 5' - CCAGAGGCATACAGGGACAA - 3' |
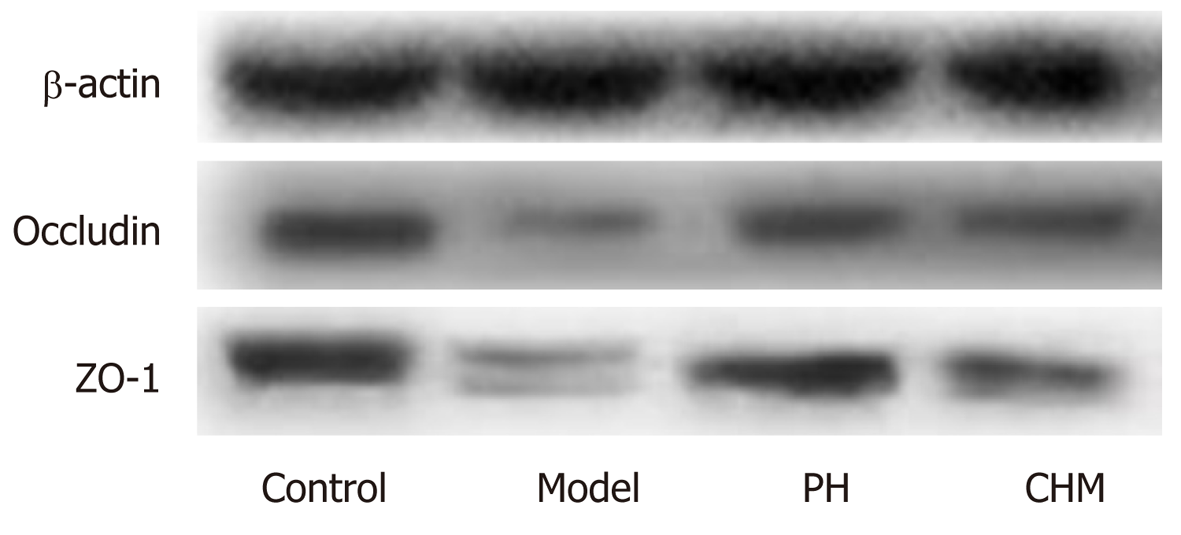
Frozen intestine tissues were homogenized in ice-cold PIPA lysis buffer and then centrifuged at 12000 rpm for 15 min. The supernatants were collected and used for Western blot analysis. Total protein concentration was determined using the BCA protein assay kit [Beijing Pulilai Gene Technology Co., Ltd. (Beijing, China)]. Equal amounts of protein samples were resolved by 12% SDS-PAGE and transferred to polyvinylidene difluoride (PVDV) membranes (Millipore, United States). The membranes were blocked at room temperature with 5% dried skimmed milk for 1 h and then incubated at 4 °C overnight with different primary antibodies: ZO-1 (Proteintech), occludin (Abcam), and β-actin (Proteintech). Afterwards, the membranes were washed and incubated for 1 h with secondary antibodies at room temperature. Protein bands were visualized using a LAS-4000MINI Biomolecular imager (BIO-RAD, United States). Protein amounts were quantified using Quantity One (BIO-RAD, United States).
Blood was collected from the abdominal aorta and centrifuged at 2500 rpm at 4 °C for 5 min. The supernatant was transferred to a non-pyrogenic centrifuge tube. The endotoxin level in plasma was detected with an endpoint chromogenic assay from Chinese Horseshoe Crab Reagent Manufactory Co., Ltd. (Xiamen, China).
All data are expressed as the mean ± SD. SPSS17.0 software package (IBM, Armonk, NY, United States) was used for statistical analyses. Homogeneity of variance test was performed. Then, the data were analyzed using a one-way ANOVA test or Welch test and a post hoc test. P < 0.05 was considered statistically significant.
After feeding a high-fat high-fructose diet for 16 wk, the liver coefficient increased significantly in the model group compared to the control group (P < 0.01). Compared to the model group, the liver coefficient was significantly reduced in the PH and CHM groups (P < 0.01). The liver coefficient in the PH group was lower compared to that of the CHM group (P < 0.01) (Table 2).
HE-stained sections are shown in Figure 1. Liver sections from the normal control group had intact and clear hepatic lobule structures, the boundary of the portal area was clear, normal hepatocytes were radially distributed around the central vein, and the hepatic sinus was clearly visible. Liver sections from the model group had hepatocyte swelling, ballooning degeneration, different sizes of lipid droplets, and hepatic cord changes. Liver sections from the PH group had mild steatosis, small lipid droplets that were visible in some cells, and disorderly arranged hepatic cords. The liver sections from the CHM group had diffused hepatocyte steatosis with different degrees of steatosis that were lower compared to that of the model group (Figure 1).
The liver tissues from control animals were histopathologically normal. Diffused and granular lipid droplets were observed in the liver sections from the model group by ORO staining. Compared to rats in the model group, rats in the PH and CHM group had markedly reduced lipid droplet accumulation in their hepatocytes (Table 3 and Figure 2).
There was no increase in collagen tissue fibers in the model, PH, and CHM groups when compared to the control group. Masson’s trichrome staining of liver sections demonstrated the absence of liver fibrosis in NAFLD rats (Figure 3).
Compared to the normal control group, there was a significant increase in TC, TG, and LDL levels and a significant decrease in HDL levels in the model group (P < 0.01). However, compared to the model group, the TC, TG, and LDL levels were si-gnificantly decreased, while HDL levels were significantly increased in both the PH and CHM groups (P < 0.01, P < 0.05) (Table 4).
Compared to the normal control group, there was a significant increase in TG level in liver tissue in the model, PH, and CHM groups (P < 0.01). Compared to the model group, the TG level in liver tissue was significantly decreased in both the PH and CHM groups (P < 0.01) (Table 5).
Compared to the normal control group, blood glucose levels in the model group increased significantly (P < 0.01). However, compared to the model group, blood glucose levels were significantly decreased in the PH and CHM groups (P < 0.01) (Table 6).
Compared to the normal control group, serum AST and ALT levels were significantly increased in the model group (P < 0.01). However, compared to the model group, serum AST and ALT levels were significantly decreased in the PH and CHM groups (P < 0.01, P < 0.05) (Table 7).
Compared to the normal control group, TNF-α, TGF-β1, NF-kB, and TLR4 levels were significantly increased in the model group (P < 0.01). However, compared to the model group, TNF-α, TGF-β1, NF-kB, and TLR4 levels were significantly decreased in the PH and CHM groups (P < 0.01, P < 0.05) (Table 8).
Compared to the control group, intestinal sIgA levels were significantly decreased in the model group, PH group, and CHM group (P < 0.01, P < 0.05). However, compared to the control group, intestinal sIgA levels were significantly increased in the PH group (P < 0.01). Compared to the PH group, intestinal sIgA levels were significantly decreased in the CHM group (P < 0.01) (Table 9).
Compared to the control group, occludin and ZO-1 protein expression was significantly decreased in the model and CHM groups (P < 0.01). Compared to the model group, occludin and ZO-1 protein expression was significantly increased in the PH group (P < 0.01). Compared to the PH group, occludin and ZO-1 protein levels were significantly decreased in the CHM group (P < 0.01) (Table 10 and Figure 4).
Compared to the control group, occluding and ZO-1 mRNA expression was significantly decreased in the model and CHM groups (P < 0.01). Compared to the model group, occludin and ZO-1 mRNA expression levels were significantly increased in the PH group (P < 0.01). Compared to the PH group, occludin and ZO-1 mRNA expression levels were significantly decreased in the CHM group (P < 0.01, P < 0.05) (Table 11).
Compared to the control group, plasma endotoxin level was significantly increased in the model group (P < 0.01). Compared to the model group, plasma endotoxin levels were significantly decreased in the PH and CHM groups (P < 0.01) (Table 12).
Epidemiological studies have demonstrated that liver disease remains a major health concern worldwide. NAFLD and its subtype non-alcoholic steatohepatitis affect approximately 35% of the worldwide population. Half of all NAFLD deaths are due to cardiovascular disease and malignancy. Despite recent medical breakthroughs, modern medicine still lacks an efficacious hepato-protective therapy with few side effects[14]. Based on the characteristics of traditional Chinese medicine and good efficacy of CHM, we aimed to investigate the regulatory effect of a CHM formula (Chinese thorowax root, scutellaria root, and white peony root) on NAFLD.
Previous studies have found that saikosaponin contained in Chinese thorowax root is effective in reducing experimental liver damage, reducing transaminase levels, and lowering cholesterol, TG, and phospholipid levels. Scutellaria root contains baicalein, flavonoid II, and baicalin. Experimental studies have demonstrated that oral flavonoid II could significantly reduce serum cholesterol levels in experimental hyperlipidemic rats fed a high fat diet. In addition, baicalein and baicalin are effective in reducing TG levels. White peony has also been demonstrated to have a protective role on the liver and detoxification functions.
Dyslipidemia is one of the hallmarks of metabolic syndrome and has been shown to be strongly associated with NAFLD. Dyslipidemia associated with NAFLD is typically characterized by elevated levels of circulating TG and LDL-C, as well as decreased HDL-C levels. In addition, previous studies have demonstrated that dietary fructose significantly increases TG plasma levels within a relatively short period of time in both rats and mice[15]. In the present study, compared to rats in the normal group, the liver coefficient and levels of serum TG, TC, LDL, fasting blood glucose, AST and ALT, and TG in liver tissue were significantly increased, while the levels of HDL were markedly decreased in NAFLD model rats. Administration of the CHM formula (Chinese thorowax root, scutellaria root, and white peony root) was able to decrease the levels of serum TG, TC, LDL, fasting blood sugar, AST, ALT, and TG in liver tissue while increasing serum HDL levels. Morphological changes in the liver correlated with liver function. Hepatocyte swelling, balloon degeneration, lipid droplet size, and hepatic cord disorders were observed in rats in the NAFLD model group. Treatment with CHM decoction (Chinese thorowax root, scutellaria root, and white peony root) reduced these histopathological changes in rats induced with a high-fat high-fructose diet. These results suggested that the CHM decoction (Chinese thorowax root, scutellaria root, and white peony root) is efficacious in protecting the liver and lowering lipid levels. CHM may function by “soothing the liver and gallbladder”.
Previous studies have shown that high-fat diet-induced NAFLD in rats had higher levels of oxidative stress factors, lipid peroxidation products, and pro-inflammatory cytokines[14]. TNF-α is a cell signaling protein that is involved in systemic in-flammation and has been associated with insulin resistance. TNF-α is produced predominantly by Kupffer cells (monocyte macrophage lineage) in the liver. Kupffer cells produce several cytokines that modulate TNF-α levels[1,16]. Rats in the Chinese herbal formula treatment group had significantly lower levels of TNF-α compared to rats in the NAFLD model group. These results showed that the CHM decoction (Chinese thorowax root, scutellaria root, and white peony root) is efficacious in reducing TNF-α levels and inflammation in rats fed a high-fat-high-fructose diet.
TGF-β is a proinflammatory cytokine whose role in the kidney, liver, and lungs, and in cardiac fibrosis is well documented[17]. During liver fibrosis, TGF-β1 levels are markedly increased in stellate cells. TGF-β2 is primarily expressed in Kupffer cells, followed by stellate and endothelial cells. A previous study using a murine steatohepatitis model observed that severe necro-inflammation and fibrosis were accompanied by an increase in TGF-β expression[18]. Results from our study demonstrated that TGF-β was significantly increased in rats in the NAFLD model group compared to the control group. Treatment with PH and CHM decoction (Chinese thorowax root, scutellaria root, and white peony root) significantly attenuated TGF-β levels.
In murine models of high-fat diet-induced steatosis, increased NF-kB activity has been associated with elevated levels of hepatic inflammatory cytokines, including TNF-α and activation of Kupffer cells. NF-kB modulates DNA transcription involved in inflammatory processes, infection, and apoptotic processes. NF-kB dysfunction may lead to inflammatory and autoimmune diseases. A number of studies have demonstrated that NF-kB activation contributes to the pathogenesis of NAFLD/NASH and the development of HCC[8]. Results from our study showed that NF-kB levels were significantly increased in rats in the NAFLD model group compared to the control group, while the CHM decoction significantly reduced NF-kB levels in rats in the NAFLD model group.
Lipopolysaccharide-binding protein (LBP) is an acute-phase protein that is derived from the liver. LBP binds to the lipid A portion of lipopolysaccharide and interacts with TLR-4 to induce the downstream signaling pathways of the innate immune system, such as NF-kB and activator protein 1, the major transcription factors involved in inflammation. Activation of the TLR-4 signaling pathway by li-popolysaccharide and LBP complex has been shown to lead to NAFLD progression in animal models from simple fatty liver to steatohepatitis[19]. In this study, compared to the normal group, TLR4 levels were increased significantly in the NAFLD model group, while treatment with the Chinese herbal formula decreased TLR4 levels compared to the model group.
The “intestinal-liver” axis is closely associated with the occurrence of NAFLD. During NAFLD, the intestinal mucosal mechanical barrier is compromised, leading to increased barrier permeability[20]. The important structures that make up the intestinal mechanical barrier is the tight junctions around the apical side of the mucosal epithelial cells and are mainly composed of occludin, claudin, junction adhesion molecules (JAMs), and ZOs. The main function of the intestinal mucosal immune barrier is to produce a local immune response after antigenic stimulation and protect the body from damage by neutralizing the antigen. These involve the immune organs, immune cells and secretion of sIgA induced by cytokines[21]. When the mechanical barrier of the intestinal mucosa is severely damaged, the bacteria are translocated, endotoxin is circulated into the liver through the portal system, and liver Kupffer cells are activated, thereby releasing a series of inflammatory factors and leading to hepatocyte inflammatory reaction and fibrosis[22]. In this study, we investigated the effects of traditional Chinese medicine on liver tissue-associated inflammatory factors and tight junction proteins on the intestinal mucosa and investigated whether the CHM decoction could play a role in the treatment of NAFLD by regulating the "intestinal-liver" axis. Our results demonstrated that compared to the control group, intestinal sIgA levels were significantly decreased in the model, PH, and CHM groups, while intestinal sIgA levels in the PH group were significantly higher compared to the model and CHM groups. Compared with the control group, the gene and protein expression of occludin and ZO-1 decreased significantly in the model group and CHM group, while it was significantly higher in the PH group compared to the model and CHM groups. Compared to the control group, plasma endotoxin level was significantly increased in the model group, while it was significantly decreased in the PH and CHM groups compared to the model group. Our results indicated that the intestinal barrier and endotoxin affect the occurrence of NAFLD, and there is a certain correlation between the intestine and liver. The CHM decoction can affect both the liver and intestine, while the effect of the CHM decoction on the liver is superior to that on the intestine.
In conclusion, the CHM decoction (thorowax root, scutellaria root, and white peony root) has a good effect in regulating lipid metabolism and liver function, which suggests its ability to affect the liver. To a certain extent, it also has a regulatory effect on the intestinal mucosal barrier. Our study demonstrates that the mechanism of “soothing the liver” by DCHD for the treatment of NAFLD is mainly through the effect on the liver.
Non-alcoholic fatty liver disease (NAFLD) has become one of the most major causes of chronic liver disease and affects both adults and children. There are currently no effective treatment options for NAFLD, with the exemption of lifestyle changes. The Chinese herbal medicine (CHM) Dachaihu decoction (DCHD) has been proved to treat NAFLD with good efficacy in previous studies.
Based on the TCM principle of formula formation and good efficacy of CHM, we divided DCHD into soothing liver part, invigorating spleen part, and dredging intestine part. Marshall officially proposed the concept of “intestinal-hepatic axis”, which systematically explains the interactions between intestine and liver. We hypothesized that the effect of CHM on NAFLD is achieved by regulating the liver and intestine.
We aimed to investigate the possible effect of a CHM formula on NAFLD in a rat model, which will provide more evidence for the therapeutic effect of CHM on NAFLD in the future.
Sixty rats were randomly divided into four groups: Control, model, PH, and CHM (Chinese thorowax root, scutellaria root, and white peony root) groups. An NAFLD rat model was established using a high-fat high-fructose diet for 16 wk. From the 13th week, rats in PH group and CHM group were administered with PH solution and a decoction of Chinese thorowax, scutellaria, and white peony root, respectively. Rats in the control group and model group were administered with an equal volume of distilled water. At the end of the study, blood was collected via the abdominal aorta. Liver tissues were harvested and any morphological changes were observed by hematoxylin-eosin (HE) staining, Oil red O staining, and Masson staining. In addition, blood lipids, liver function markers, and TG in liver tissues were analyzed. The levels of transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNF-α), Toll-like receptor-4 (TLR4), and nuclear factor-kappa B (NF-кB) in liver tissues and sIgA in intestinal tissues were analyzed by ELISA, and protein and mRNA expression of occludin and ZO-1 in the intestine were measured using Western blot and reverse transcription-quantitative polymerase chain reaction, respectively. The endotoxin level in plasma was detected by endpoint chromogenic assay. SPSS17.0 software package (IBM, Armonk, NY, United States) was used for statistical analysis.
Compared to the normal control group, the liver coefficient, serum TG, TC, LDL, AST, and ALT, blood glucose, the levels of TG, TNF-α, TGF-β, NF-kB, and TLR4 in liver tissues, and plasma endotoxin increased significantly in the model group, while serum HDL, intestinal sIgA, and protein and mRNA expression of occludin and ZO-1 decreased significantly in the model group (P < 0.01). PH and CHM attenuated the elevated liver coefficient, serum TG, TC, LDL, AST, and ALT, blood glucose, the levels of TG, TNF-α, TGF-β, NF-kB, and TLR4 in liver tissues, and plasma endotoxin, and increased serum HDL levels compared to the model group (P < 0.01). Intestinal sIgA and the protein and mRNA expression of intestinal occludin and ZO-1 were significantly increased in the PH group compared to the model and CHM groups (P < 0.01).
In this study, the CHM decoction (Chinese thorowax root, scutellaria root, and white peony root) is beneficial in regulating lipid metabolism and liver function, which indicates that they have a good effect on the liver. To a certain extent, the CHM decoction can affect both the liver and intestine, while the effect of the CHM decoction on the liver is superior to that on the intestine.
This study demonstrated that the mechanism of the soothing liver part of DCHD for the treatment of NAFLD was mainly via the regulation of the liver. The Chinese formula does have its special formation principle. Different CHMs in the same formula are effective on different syndromes. This study proposed formula division and tried to find the relationship between Chinses herbs and internal organs (liver and intestine), which will provide more experimental evidence for the therapeutic effect of CHM on NAFLD in the future. This study preliminary applied the liver-intestine axis to explain the relationship between Chinese formula and NAFLD.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
The ARRIVE Guidelines: The ARRIVE guidelines have been adopted in this manuscript.
P-Reviewer: Ikura Y, Kharbanda KK, Sherif Z, Zhu HF S-Editor: Cui LJ L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Jiang W, Guo MH, Hai X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J Gastroenterol. 2016;22:10180-10188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 87] [Cited by in F6Publishing: 83] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 2. | Tan DY, Shi HY, Li CP, Zhong XL, Kang M. Effect of nuclear factor-κB and angiotensin II receptor type 1 on the pathogenesis of rat non-alcoholic fatty liver disease. World J Gastroenterol. 2015;21:5877-5883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Vespasiani-Gentilucci U, Gallo P, Dell'Unto C, Volpentesta M, Antonelli-Incalzi R, Picardi A. Promoting genetics in non-alcoholic fatty liver disease: Combined risk score through polymorphisms and clinical variables. World J Gastroenterol. 2018;24:4835-4845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Kapravelou G, Martínez R, Nebot E, López-Jurado M, Aranda P, Arrebola F, Cantarero S, Galisteo M, Porres JM. The Combined Intervention with Germinated Vigna radiata and Aerobic Interval Training Protocol Is an Effective Strategy for the Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD) and Other Alterations Related to the Metabolic Syndrome in Zucker Rats. Nutrients. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Christensen CU, Glavind E, Thomsen KL, Kim YO, Heebøll S, Schuppan D, Hamilton-Dutoit S, Würtz Heegaard C, Grønbæk H. Niemann-Pick type C2 protein supplementation in experimental non-alcoholic fatty liver disease. PLoS One. 2018;13:e0192728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Nie J, Li CP, Li JH, Chen X, Zhong X. Analysis of nonalcoholic fatty liver disease microRNA expression spectra in rat liver tissues. Mol Med Rep. 2018;18:2669-2680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Veena J, Muragundla A, Sidgiddi S, Subramaniam S. Non-alcoholic fatty liver disease: need for a balanced nutritional source. Br J Nutr. 2014;112:1858-1872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Guo Y, Li JX, Mao TY, Zhao WH, Liu LJ, Wang YL. Targeting Sirt1 in a rat model of high-fat diet-induced non-alcoholic fatty liver disease: Comparison of Gegen Qinlian decoction and resveratrol. Exp Ther Med. 2017;14:4279-4287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Gao XM. “Chinese herbal medicinal,” People’s Medical Publishing House, Beijing, China, 2nd edition, 2013. [Cited in This Article: ] |
| 10. | Fan YH, Miao BQ. Treatment of 63 Cases of Nonalcoholic Fatty Liver disease with Dachaihu Decoction. Mongol J Traditional Chinese Medicine. 2014;33:9. [Cited in This Article: ] |
| 11. | Lin LL, Li LL. Clinical Observation on Treatment of Nonalcoholic Fatty Liver with Combination of Traditional Chinese Medicine and Western Medicine. J Practical Traditional Chinese Medicine. 2017;33:1069-1070. [Cited in This Article: ] |
| 12. | Huang ZL, Huang XJ, Huang XL, Liu FZ, Hu NN, Huang GX. Effects of diacerein on inflammatory cytokines and adipose metabolism as well as the expression of chemerin in adipose tissue of type 2 diabetic rats. J Xi’an Jiaotong University (Medical Sciences). 2017;38:693-698. [Cited in This Article: ] |
| 13. | Chen Q. “Research methods in pharmacology of Chinese materia medica” Beijing, People’s Medical Publishing House, Beijing, China, 2nd edition, 2006. [Cited in This Article: ] |
| 14. | Ou-Yang Q, Xuan CX, Wang X, Luo HQ, Liu JE, Wang LL, Li TT, Chen YP, Liu J. 3-Acetyl-oleanolic acid ameliorates non-alcoholic fatty liver disease in high fat diet-treated rats by activating AMPK-related pathways. Acta Pharmacol Sin. 2018;39:1284-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Jensen VS, Hvid H, Damgaard J, Nygaard H, Ingvorsen C, Wulff EM, Lykkesfeldt J, Fledelius C. Dietary fat stimulates development of NAFLD more potently than dietary fructose in Sprague-Dawley rats. Diabetol Metab Syndr. 2018;10:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Tsai CC, Lin YJ, Yu HR, Sheen JM, Tain YL, Huang LT, Tiao MM. Melatonin alleviates liver steatosis induced by prenatal dexamethasone exposure and postnatal high-fat diet. Exp Ther Med. 2018;16:917-924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Soofi A, Wolf KI, Emont MP, Qi N, Martinez-Santibanez G, Grimley E, Ostwani W, Dressler GR. The kielin/chordin-like protein (KCP) attenuates high-fat diet-induced obesity and metabolic syndrome in mice. J Biol Chem. 2017;292:9051-9062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Song L, Qu D, Zhang Q, Jiang J, Zhou H, Jiang R, Li Y, Zhang Y, Yan H. Phytosterol esters attenuate hepatic steatosis in rats with non-alcoholic fatty liver disease rats fed a high-fat diet. Sci Rep. 2017;7:41604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Nien HC, Sheu JC, Chi YC, Chen CL, Kao JH, Yang WS. One-year weight management lowers lipopolysaccharide-binding protein and its implication in metainflammation and liver fibrosis. PLoS One. 2018;13:e0207882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Xin D, Zong-Shun L, Bang-Mao W, Lu Z. Expression of intestinal tight junction proteins in patients with non-alcoholic fatty liver disease. Hepatogastroenterology. 2014;61:136-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 21. | Ito H, Takemura N, Sonoyama K, Kawagishi H, Topping DL, Conlon MA, Morita T. Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune functions, and immunoglobulin A secretion in the rat cecum. J Agric Food Chem. 2011;59:5771-5778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Miele L, Marrone G, Lauritano C, Cefalo C, Gasbarrini A, Day C, Grieco A. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. 2013;19:5314-5324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |