Published online Sep 7, 2019. doi: 10.3748/wjg.v25.i33.4892
Peer-review started: April 11, 2019
First decision: May 17, 2019
Revised: May 31, 2019
Accepted: June 8, 2019
Article in press: June 8, 2019
Published online: September 7, 2019
Mesenchymal stromal cell (MSC)-based therapy is currently under study to treat inflammatory bowel diseases. MSC bioactive products could represent a valid alternative to overcome issues associated with systemic whole-cell therapies. However, MSC anti-inflammatory mechanisms differ between rodents and humans, impairing the reliability of preclinical models.
To evaluate the effect of conditioned medium (CM) derived from porcine vascular wall MSCs (pVW-MSCs) on survival and differentiation of porcine and guinea pig enteric ganglia exposed to lipopolysaccharide (LPS).
Primary cultures of enteric ganglia were obtained by mechanic and enzymatic digestion of ileum resections from guinea pigs (Cavia porcellus) (GPEG) and pigs (Suus scrofa) (PEG). pVW-MSCs were derived by enzymatic digestion from vascular wall resections of porcine aorta and tested by immunoflowcytometry for MSC immune profile. Enteric ganglia were treated with increasing concentrations of LPS, CM derived by pVW-MSCs or a combination of CM and LPS 1 µg/mL. Cell count and morphometric analysis of HuD positive neurons and glial fibrillary acidic protein positive glial cells were performed by immunofluorecent staining of cultured ganglia.
PEG showed a higher number of neurons compared to GPEG. Overall, CM exerted a protective role on LPS-treated enteric ganglia. CM in combination with LPS increased the number of glial cells per ganglion in both cultures evoking glial cells differentiation in porcine cultures.
These findings suggest an immunomodulating activity of pVW-MSCs mediators on the enteric nervous system in inflammatory conditions.
Core tip: Secretome of porcine vascular wall mesenchymal stromal cells (pVW-MSCs) induced an increase of glial cell number in swine and guinea pig-derived enteric ganglia. Co-treatment of enteric ganglia with lipopolysaccharide and conditioned medium promoted glial cell differentiation only in pigs. These data indicate an immune activation promoted by pVW-MSCs which could be more specific in higher mammals, suggesting a careful consideration of the animal models used in research studies on cell-based therapies.
- Citation: Dothel G, Bernardini C, Zannoni A, Spirito MR, Salaroli R, Bacci ML, Forni M, Ponti FD. Ex vivo effect of vascular wall stromal cells secretome on enteric ganglia. World J Gastroenterol 2019; 25(33): 4892-4903
- URL: https://www.wjgnet.com/1007-9327/full/v25/i33/4892.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i33.4892
Inflammatory bowel diseases (IBDs), encompassing the two major forms Crohn’s disease (CD) and ulcerative colitis (UC), are characterized by an overactive immune response to unknown environmental triggers associated with specific genetic traits[1]. Genome-wide association studies advanced previous knowledge of genetic variants associated with innate and adaptive immunity (e.g., NOD2, IL23R) revealing novel pathophysiological mechanisms linked to autophagy and loss of epithelial barrier function[2,3]. In IBD, chronic intestinal inflammation induces several morpho-functional changes of the enteric nervous system (ENS), including swallowing of enteric nerve bundles and higher expression of several neurotransmitters[4].
Mesenchymal stromal cells (MSCs) are currently under study as a therapeutic option in regenerative medicine and as a novel treatment for autoimmune and chronic inflammatory disorders including IBDs[5]. Although the mechanism underlying the immunoregulatory effect of MSCs is still to be clarified, their role in balancing immune homeostasis has been acknowledged[6]. Notably, pro- or anti-inflammatory activity[7] along with other MSC biomolecules is settled by toll-like receptors 3 and 4, the latter being one of the main sensors of bacterial lipopolysaccharide (LPS)[8,9].
MSCs respond to an inflammatory environment releasing CC chemokine ligand 2 and IL-10, which inhibits CD4 Th17 cells proliferation and IL-17 production[10] and polarizes naïve T-cells to the regulatory Foxp3-positive phenotype (T-reg)[6]. These pleiotropic, anti-inflammatory properties justify a proof of concept study for a possible application of MSCs in IBDs, where Th-17 and Th-4/5 lymphocytes drive the aberrant immune reaction of CD and UC, respectively. A phase III clinical trial of CD with a systemic infusion of MSCs is currently ongoing[11], while local treatment of the severe fistulizing form of CD was recently approved by EMA[12].
One of the main drawbacks of cell-based therapy regards uncertainty about biodistribution and homing of cells to the target site of action. In particular, MSCs tend to remain trapped in the microcirculation of pulmonary alveoli, allegedly for an increased diameter acquired during in vitro expansion[13]. For these reasons, MSC-derived exosomes as well as MSC secretome are gaining attention in current research[14-17]. Furthermore, a recent study showed that a vascular wall mesenchymal stem cells isolated by porcine aortic tissue (pVW-MSCs) showed mesenchymal features[18] and the ability to differentiate in all the cellular components of a mature vessel[19]. A deeper characterization demonstrated their metabolic properties[20] and their intrinsic attitude to promote angiogenesis also by paracrine action[21].
Interestingly, the key factor responsible for MSC anti-inflammatory action varies among species and is related to a specific phylogenetic tree[22]. On this basis, this study aims at investigating a possible gap between rodent and swine neuro-immune response to MSC-derived bioactive products assuming pVW-MSC secretome as a closer model from a translational point of view. To this purpose, we first compared the effect of LPS on cell survival and differentiation in primary enteric ganglia derived from guinea pig and pig myenteric plexus (MP) (GPEG and PEG, respectively); thereafter, we evaluated the effect of pVW-MSC secretome in these two ex-vivo models of ENS.
Animals were used after approval of the protocol by the local ethics committee and following the guidelines of 3Rs implied in the EU directive 2010/63/EU for the use of animal for experimental purposes and in accordance with the national legislation (Decree 116/1992). In accordance with the 3Rs principle of Reduction[23] the animals used in the present study served as controls in other experimental protocols carried out in our facility.
Swine (Protocol number n.43-IX/9 all.37; 20/11/2012): Young commercial hybrids of Sus scrofa (4 males–aged 4-5 wk, 7 ± 0.5 Kg live weight), born at the ASA Unit (DIMEVET, University of Bologna), were enrolled in the study. Piglets were bred under the lactating sow till 28 d, then weaned and kept in a multiple box for young piglets, temperature was kept at 28 ± 1 °C with adequate ventilation and humidity in relation to the young age. Surgical procedures were carried out during the morning in the surgical theatre of the DIMEVET facilities. Animal received an i.m. bolus of tiletamine-zolazepam (5 mg/kg) 10 min before induction; general anesthesia was achieved using sevoflurane with an induction mask[24]. Animals were then sacrificed with a single bolus (0.3 mL/kg) of Tanax (embutramide/mebezonium iodide/tetracaine hydrochloride; Msd Animal Health Srl) and the abdomen was opened to remove the small intestine.
Guinea pigs (Protocol number 18/79/14): Male Dunkin-Hartley guinea pigs (Cavia porcellus, 8 males–aged 3-5 wk, weight 200-280 g, Harlan Italy, Udine, IT) were kept in home cages with a controlled environment (12 h dark/light cycle, 20-24 °C temperature, 40%-70% humidity) with unlimited access to water and chow. The day of the experiment, animals were sacrificed through isoflurane inhalation followed by exsanguination through jugular excision. All the procedures were carried out in the operating room of Medical and Surgical Department.
Isolation of MP from 8 guinea pigs (3-5 wk) and 4 pigs (4-5 wk) was performed as previously described[25,26]. Briefly, the small intestine was washed with sterile, oxygenated Krebs solution containing (mM) NaCl 120.9, KCl 5, MgCl2 1.2, CaCl2 2.5, glucose 11.5, NaHCO3 14.4, NaH2PO4 1.2 additioned with fungizone and penicillin-streptomycin 10 ml/L (Sigma Aldrich-Merck, Darmstadt, Germany). MP was peeled by 2-cm traits of small intestine cut in 1 mm × 1 mm fragments and digested in T25 plastic flasks with an enzymatic solution containing 1.25 mg/mL collagenase IV from Clostridium histolyticum, 1 mg/mL dispase II from Bacillus polymyxa and 1 mg/mL bovine serum albumin (Sigma Aldrich-Merck) in gentle agitation 30 min (guinea pig tissues) or 45 min (pig tissues) at 37 °C. Reaction was stopped by placing flasks in ice for 3 min. Digested tissues were washed with cold Krebs solution and collected in DMEM. Fragmented neuronal fibers were selected over muscle bundles with a stereomicroscope (Nikon C-PSCN - Nikon, Tokyo, Japan) and seeded on polyornithine-covered coverslips in 24-well plates with M199 medium enriched with 5% fetal bovine serum, 10 mL/L penicillin-streptomycin and 5% glucose (complete M199-cM199). Plates were kept 24 h in a humidified chamber at 37 °C with 5% CO2.
pVW-MSCs were isolated, characterized and maintained as previously described[27]. In order to confirm the mesenchymal immunophenotype after cryopreservation, flow cytometry analysis was performed before media collection. Briefly, 2 × 105 cells were resuspended in 100 µL of phosphate buffered saline (PBS) and incubated for 1 h at 4 °C in the dark with appropriate fluorochrome-conjugated antibodies at the titers reported in Table 1. Unstained controls to evaluate inherent background or autofluorescence were obtained omitting primary antibodies. After incubation, cells were washed twice and resuspended in 200 µl of PBS then analyzed with MacsQuant Analyzer10 (Miltenyi Biotec, Bergisch Gladbach, Germany). For CD34 staining, after the first incubation with the primary antibody, cells were washed and incubated with PE-conjugated secondary antibody (Table 1) for 40 min at 4 °C in the dark. Data were analyzed using the Flowlogic™ software (Miltenyi Biotec).
| Name | Target | Clonality | Conjugation | Research resource identifiers | Species | Supplier | Catalog number | Application | Concentrationused |
| Anti-HuD | Hu N-terminus of human HuD | Poly | - | AB_2101223 | Gt | Santa Cruz Biotechnologies | sc-5977 | IC | 5 µg/mL |
| Anti-GFAP | Hu Glial Fibrillary Acidic Protein | Mono | - | AB_10689630 | Ms | BD Biosciences | 561483 | IC | 1 µg/mL |
| Alexa 488 | Gt IgM heavy and light chains | Poly | Alexa Fluor® 488 | AB_2535792 | Dk | Thermo Fisher Scientific | A-21206 | IC | 0.5 µg/mL |
| Alexa 555 | Ms IgM heavy and light chains | Poly | Alexa Fluor® 555 | AB_2535853 | Dk | Thermo Fisher Scientific | A-21432 | IC | 0.5 µg/mL |
| Anti-CD 105 | Hu CD105 (L-isoform) cell surface antigen | Mono | FITC | AB_868768 | Ms | Abcam | Ab53318 | FC | 2 µL/105 cells/100 µL |
| Anti-CD90 | Hu CD90/Thy-1 cell surface antigen | Mono | APC | AB_10677422 | Ms | Abcam | Ab139364 | FC | 1 µL/105 cells/100 µL |
| PE anti-human CD56 | Hu CD56 cell surface antigen | Mono | PE | AB_314448 | Ms | Biolegend | 304606 | FC | 2 µL/105 cells/100 µL |
| Human CD44 antibody | Hu CD44 isoforms, 80-95 Kd cell surface antigen | Mono | PerCP | AB_10645506 | Rt | Biolegend | 103036 | FC | 0.5 µL/105 cells/100 µL |
| CD34 antibody [EP373Y] | Hu CD34 cell surface antigen | Mono | - | AB_1640331 | Rb | Abcam | Ab81289 | FC | 0.8 µL/105 cells/100 µL |
| Rabbit-PE | Rb IgG heavy and light chains | Poly | PE | AB_10680576 | Gt | Abcam | Ab97070 | FC | 0.5 µL/105 cells/100 µL |
After thawing cellular suspensions were plated in a 24-multi well plate at a concentration of 3 × 104 cells/well in PGM medium (Promocell, Heidelberg, Germany), the day after, cells were washed with PBS and cultured for additional 24 h in PGM, then media were collected, centrifuged at 800 × g for 10 min, filtered through a 0.20-μm syringe filter, immediately frozen in liquid nitrogen and stored at -80 °C until use.
Enteric ganglia derived from each animal were seeded in 24 wells plates, a pool of 35 ganglia per well from 3 wells (triplicates) were considered for the analysis. After 2 d, ganglia were incubated for 24 h with cM199 (CRTL) or one of the followings: cM199 + 0-0.1-1-10 µg/mL LPS (LPS from Escherichia coli O111:B4, Sigma Aldrich-Merck); conditioned medium (CM) derived by culture flasks containing adherent pVW-MSCs (10% in M199) or CM combined with LPS 1 µg/mL. Treatments were coded arbitrary so that a second operator could carry on the operation blindly.
At the end of 24-h treatment, cells were washed twice in cold PBS and fixed in 4% paraformaldehyde for 1 h. After three washes with cold PBS, unspecific epitopes were blocked by incubating fixed ganglia with a blocking solution of 0.5% Triton and donkey serum 5% for 1 h. Ganglia were double-stained by overnight incubation at 4°C with a mix containing antibodies directed to the pan-neuronal marker HuD and to the glial fibrillary acidic protein (GFAP). The following day, cells were washed three times with PBS and incubated 2 h at room temperature with appropriate fluorescent anti-antibodies (Table 1). Negative controls included a pre-adsorption step for 2 h with the specific blocking peptides in the preliminary tests and the omission of the primary antibody in every run experiment. At the end of the procedure, coverslips were mounted on slides with an anti-fade solution (10% Mowiol 4-88, Sigma Aldrich-Merck) containing 0.1 µg/mL DAPI. Photomicrographs of single ganglion were obtained with a Zeiss Imager M1 microscope with dedicated software (AxioVision, Carl Zeiss, Jena, Germany).
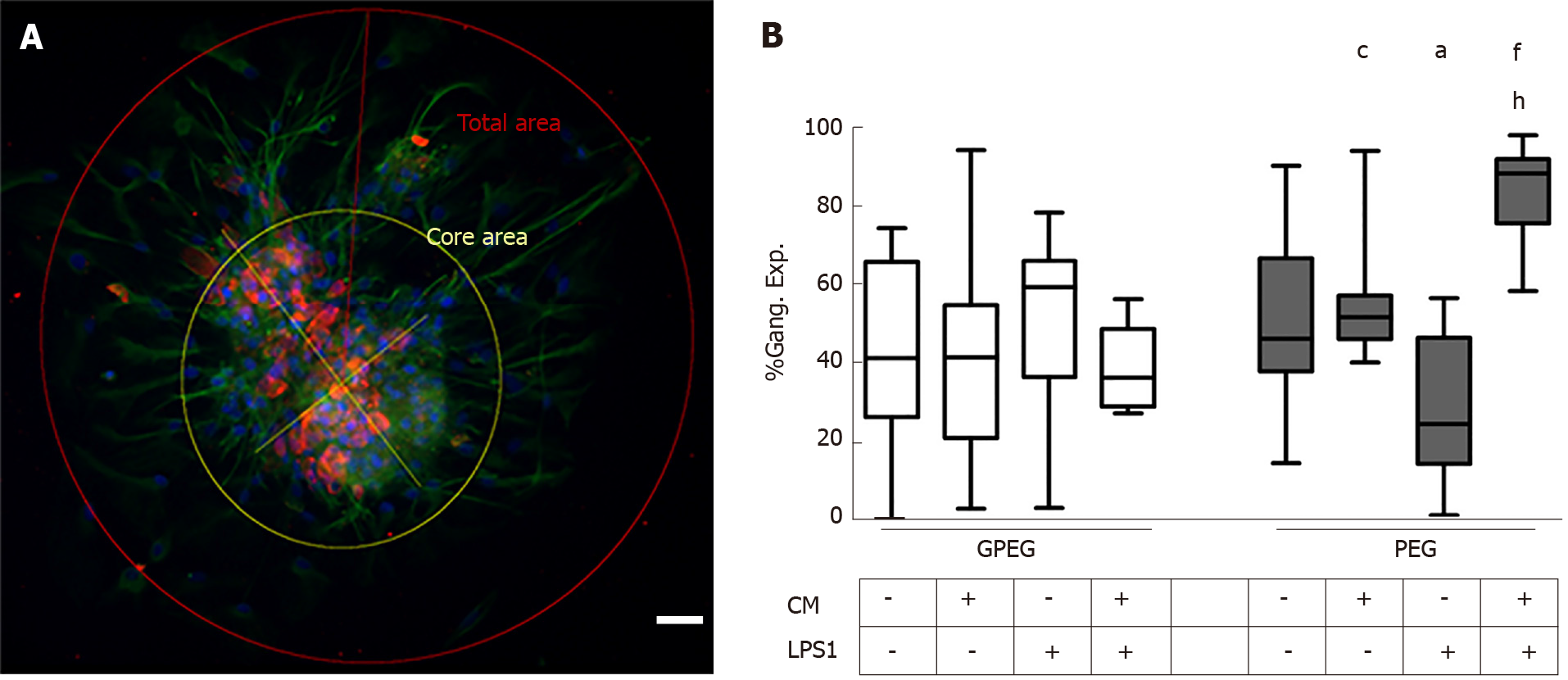
Cell count and morphometric analysis of photomicrographs were carried out blindly with Image J software on the basis of a previously applied method[28]. Briefly, two axis intersecting at a 90° angle were traced from the furthest ends of the cluster of cell bodies. A first circle representing the core area was traced considering the intersection of the two axis as the center and the longest axis as the diameter. Likewise, an outer circle, having the same center as the former and the diameter extending to the furthest fillopodium, was considered as the total area. The percentage of ganglion expansion (Gang. Exp. %) on total area was calculated as follows: Gang.Exp.% = [(total area-core area)]%100/(total area).
Results are reported as Tukey box-plots (middle lines-median values; lower and upper sides of the rectangles - 1st and 3rd percentile, whiskers - confidence intervals; black dots - outliers). Statistical analysis was performed through GraphPad Prism software (GraphPad, La Jolla, CA, United States) on data retrieved from 35 ganglia/well analyzed in triplicates for each experimental group. Normal distribution was confirmed by Shapiro-Wilk test and Student t test was used to determine statistical significance of the differences observed. Data significance was considered when P < 0.05 or as reported in text.
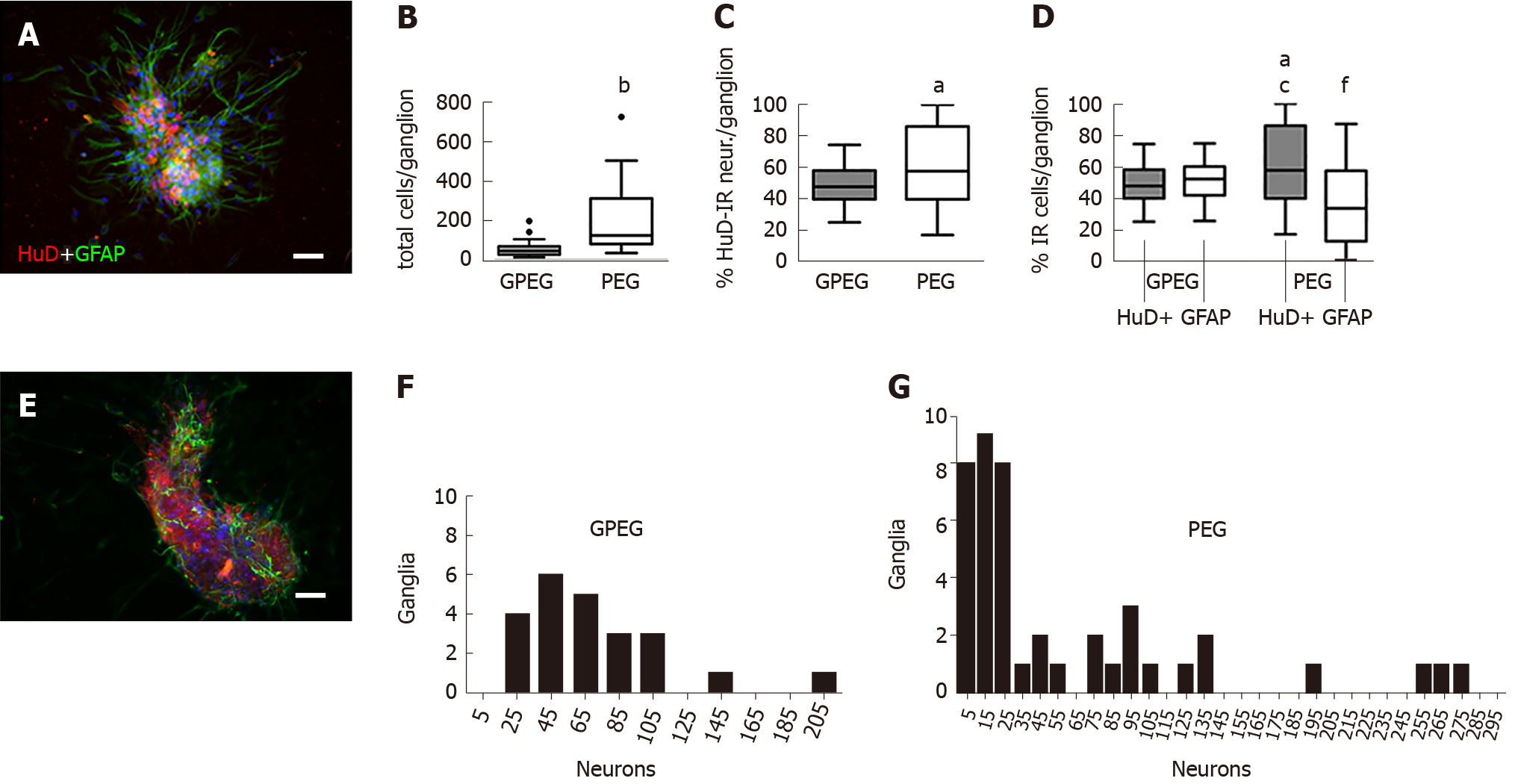
After 2 d of culture in vitro, GPEG showed a more consistent morphology and cell composition in comparison with PEG. GPEG showed a globular or bean-like shapes with a core of cell bodies and glial cells radially protruding outward (Figure 1A). Conversely, PEG were characterized by larger globular, bi- or tri-lobed shapes (Figure 1E) with a number of total cells per ganglion about 4-fold higher when compared to GPEG (213.7 ± 50.4/PEG vs 53.3 ± 5.2 cells/GPEG, P < 0.001, Figure 1B) and a higher number of HuD-immunoreactive (HuD-IR) neurons per ganglion (+13.7%, Figure 1C). Frequency analysis in Figure 1F and Figure 1G describes differences between GPEG and PEG in terms of number of ganglia presenting 5 to 205 neurons. Moreover, PEG showed a different proportion of HuD-IR neurons and GFAP-immunoreactive (GFAP-IR) glial cells (+12.7%, P < 0.05), whereas GPEG presented a more homogenous distribution of both cell types. Notably, a higher number of neurons/ganglion (+12.7%, P < 0.05) and a lower number of glial cells/ganglion (-15.7%, P < 0.05) were detected in PEG compared to GPEG (Figure 1D).
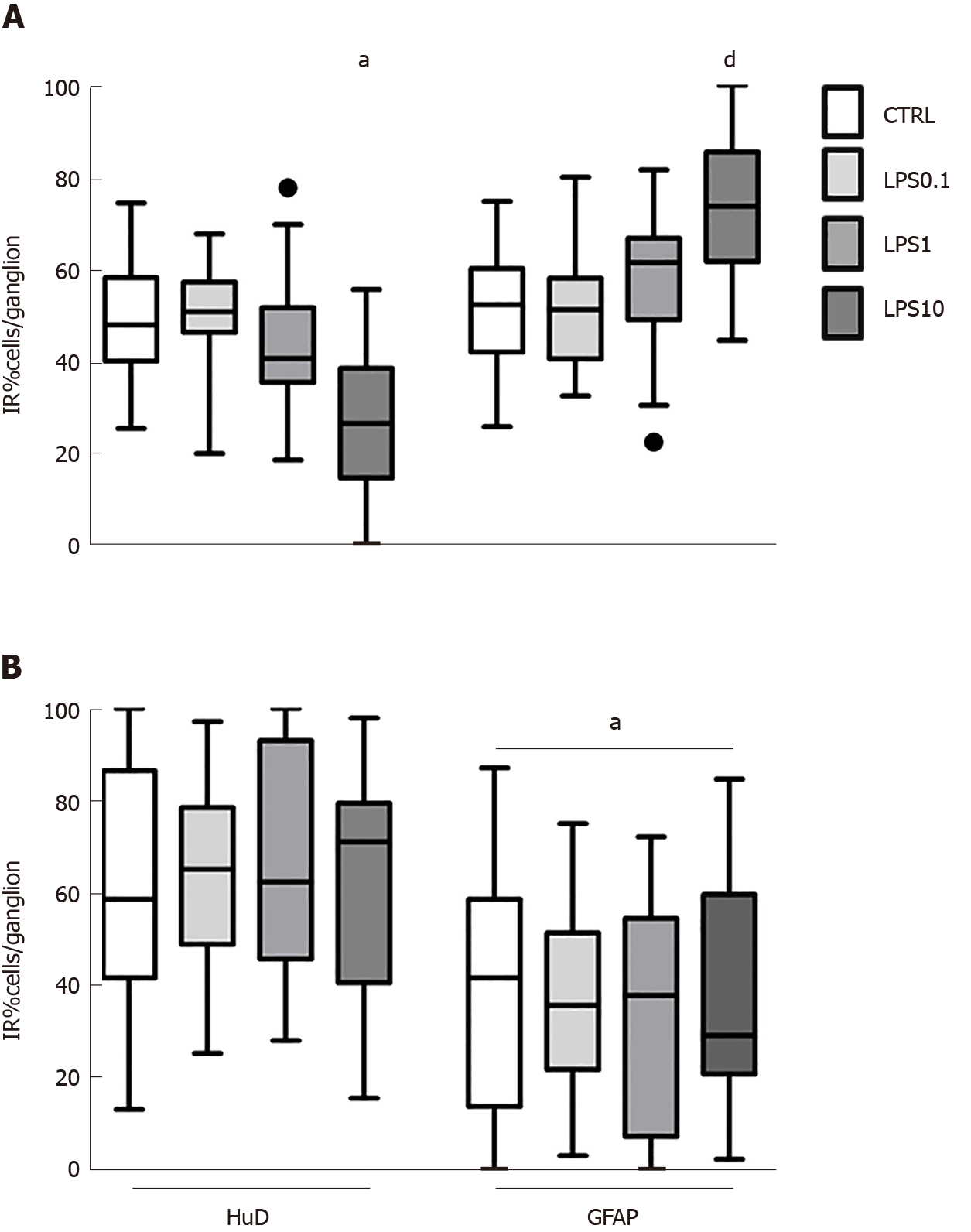
GPEG exposed to increasing concentrations of LPS displayed a trend towards a decreased number of neurons/ganglion, which was statistically significant only at the concentration of 10 µg/ml (-22.3%, P < 0.05, Figure 2A). This effect was paralleled by an increased number of glial cells/ganglion (+22.2%, P < 0.05, Figure 2A). Conversely, no effect of LPS was detected on cell number in PEG cultures at any of the concentrations tested. Notably, the observed lower number of GFAP-IR glial cells compared with HuD-IR neurons was similar in all the experimental groups (P < 0.05, Figure 2B).
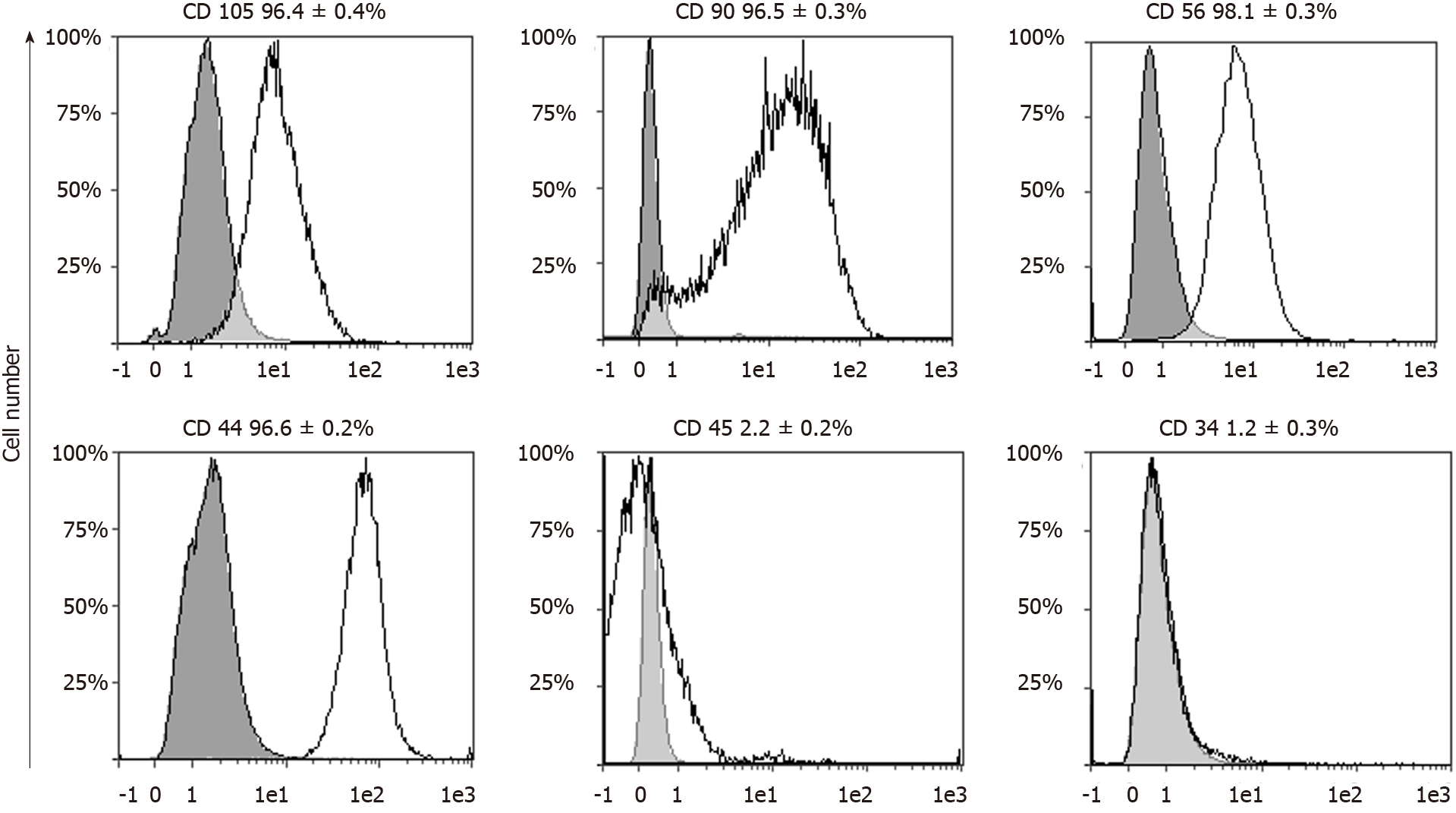
Flowcytometric analysis confirmed an unvaried immunophenotype of pVW-MSCs at the third passage after cryopreservation, displaying MSC profile. In line with the criteria for MSC characterization[18] more than 96% of the cell population analyzed was positive for the markers of mesenchymal stemness, CD105, CD90, CD56, CD44, and less than 2.5% was positive for the hematopoietic markers CD45 and CD34 (Figure 3).
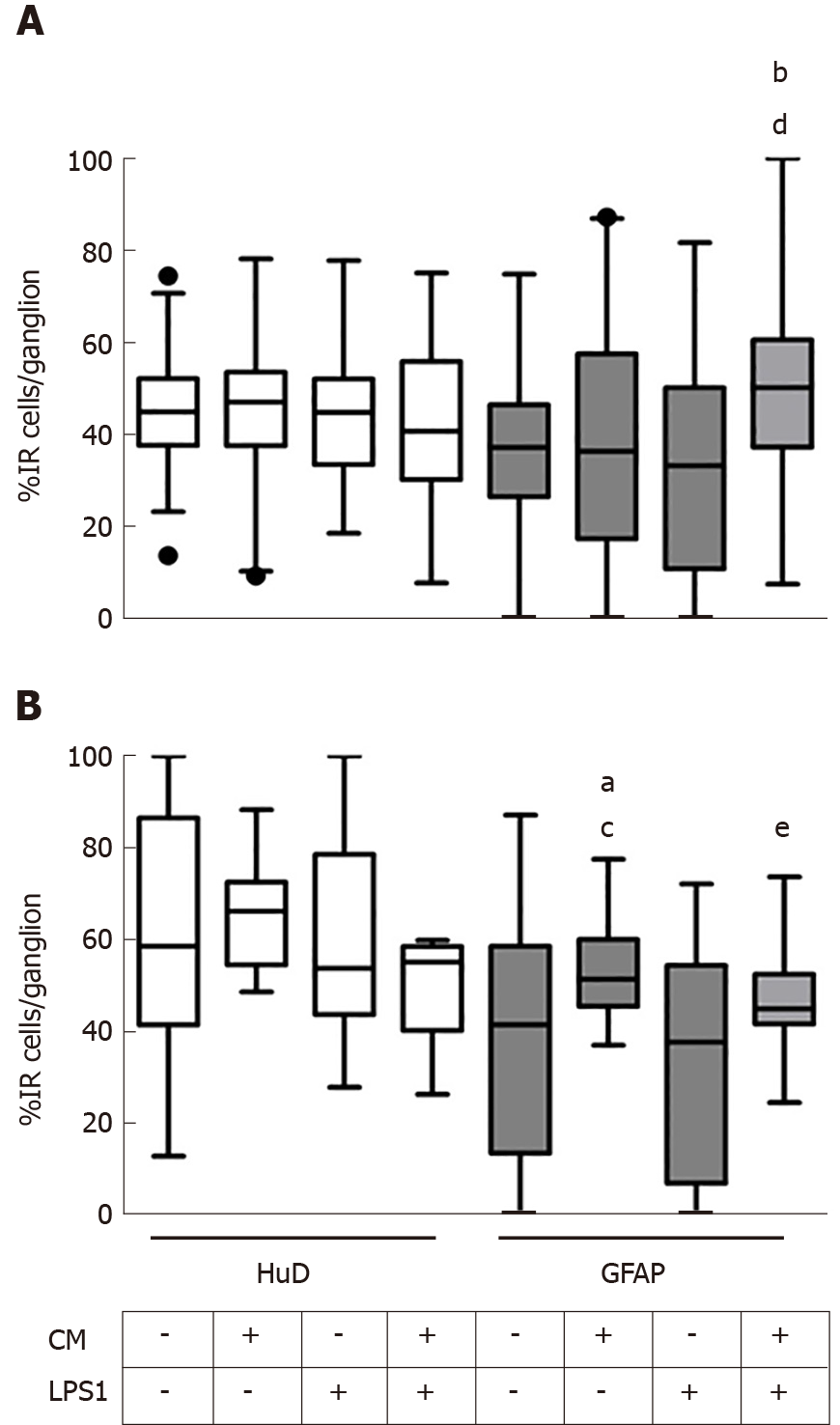
Thereafter, we tested the effect of medium conditioned by pVW-MSCs (CM) on GPEG and PEG cultured with LPS 1 µg/mL (LPS1). The concentration of 1 µg/mL was chosen in order to resemble a plausible pathophysiological condition of a high bacterial overload. Both guinea pig and pig cultures did not show any significant change in the number of HuD+ neurons after treatments (Figure 4A and B, white columns), whereas glial cell number varied significantly (Figure 4A and B, gray columns). In particular, GPEG cultures showed a higher number of glial cells as a result of co-treatment with CM+LPS1, compared to control and LPS1 groups (+13.9%, P < 0.001; +16.5%, P < 0.01, respectively). As for PEG cultures an increased number of GFAP+ glial cells was observed in CM group compared to control (+13.6%, P < 0.05) and LPS1 groups (+20.2%, P < 0.05). In addition, number of glial cells was higher in GPEG treated with CM+LPS1 compared to LPS1 (+14.2%, P < 0.05). The main interspecies difference was the variation of number of glial cells exposed to CM, which increased in PEG but not in GPEG cultures compared to the relative control (Figure 4A and B, third gray columns).
As most of the observed differences regarded glial rather than neuronal cells, we proceeded with a morphometric analysis of glial processes protruding outward the ganglion center area measuring the extent of the ganglion expanded area (Gang. Exp.%, Figure 5A). PEG morphology underwent more substantial changes in comparison to GPEG cultures which did not show any significant change following treatments showing a trend towards decreased Neur.Exp. (not statistically significant) after LPS1 treatment compared to control and CM groups. Furthermore, CM+LPS1 induced a marked increase of Gang.Exp. which was approximately 2 fold higher compared to both LPS1 and control groups (+43.2% vs CTRL, P < 0.01, Figure 5B).
The present study shows higher reactivity to MSC mediators of glial cells in pig compared to guinea pig myenteric ganglia. In particular, we tested the effect of CM derived by pVW-MSCs cultures on myenteric ganglia isolated from ileal tissue of GPEG and PEG. These primary cultures exposed to LPS combined with pVW-MSCs medium showed a more pronounced proliferation and differentiation in PEG compared to GPEG. This finding suggests a different and higher response of neuroimmune cells in higher mammals, which could impact on translational aspects of current research on cell-based therapies.
In the present study, we reported interspecies differences in the cellular composition of GPEG and PEG, with a higher neuronal/glial cells ratio in the latter, which is in line with previous findings[29]. Furthermore, we described a slight decrease in the number of neurons with a correspondent increase of glial cells as a result of increasing micromolar concentrations of LPS in GPEG, but not in PEG. Finally, we detected a marked modification of glial cell number and morphological modifications of PEG in response to CM derived by pVW-MSCs cultures.
The higher number of neurons detected in PEG is in line with previous findings describing an anatomical correlation in the size of myenteric ganglia and number of cells per ganglion in large mammals[30]. Moreover, PEG size and number of cells were more variable compared to GPEG, partially reflecting ganglia composition observed in larger mammals, including humans[31]. Our findings show a low glial cells/neurons ratio, particularly in PEG, which is in line with previous published data[29]. This disproportion is easily filled within 48 h of culture, due to the rapid proliferation of glial cells. In order to avoid a possible confounder, we chose a shorter time (24 h) to limit the proliferation of glial cells, so as to detect small variations in number and morphology of ganglia resulting after treatment.
Notably, our data of cell count analysis correspond to micromolar LPS concentrations as a result of previous tests performed with nanomolar concentrations. This analysis did not provide any measurable difference between groups (10-100 nM, data not shown). Moreover, the scarce decrease of cell number in GPEG and the absence of any effect in PEG cultures even with LPS at the highest concentrations (10 µM) reflects a remarkable resilience of myenteric neurons, already reported in previous works[32]. The slight decrease of neuronal cells at 10 µM of LPS in GPEG could be ascribed to a lower sensitivity of guinea pigs to LPS compared to pigs, which was tested in previous studies on LPS-induced endotoxic shock[33,34]. However, Schuster and colleagues described a counterintuitive effect of LPS promoting neuronal viability and stemness in myenteric ganglia derived by MP of newborn mice[32]. Differently from this work, our data did not show a higher neuron number as a result of LPS treatment. Rather, most of the variations observed, as probably due to age-related features of the animals used (young animals rather than newborns), regarded glial cell number, which markedly varied upon treatment with pVW-MSCs supernatants, while it did not evoke any measurable change on the neuronal component in either pig or guinea pig cultures. Indeed, CM derived by pVW-MSCs alone or combined with 1 µg/mL LPS induced a higher number of glial cells in PEG, while in GPEG-treated samples an akin effect was found only after the co-treatment, suggesting a synergic activity of pVW-MSC-secreted molecules and LPS in promoting glial cells mitosis in both models. This observation is in accordance with the properties showed by brain vascular pericytes which favor glial cells’ phenotype, being also spatially in close relation with this cell type in brain vessels[35,36]. Indeed, pVW-MSCs, along with a MSC-like immune profile, exhibited an intrinsic pro-angiogenic features in previous studies[19,27]. In addition, both LPS and MSCs promote the activation of glial cells in brain-derived ganglia. In particular, a recent study in vitro described the induction of glia proliferation induced by Wharton-jelly-derived MSCs[37], while in vivo injection of LPS induced an overexpression of the glial marker GFAP in brain tissue[38]. Interestingly, we observed a substantial variation of this cell population in swine but not in guinea pig primary cultures. Allegedly, this might be due to the species correspondence of porcine enteric glia with pVW-MSCs, which would reflect the phylogenetic differences previously reported in signaling modalities for MSC immunomodulation[39]. Indeed, in humans, non-human primates and pigs, immunomodulation is a mechanism dependent by indoleamine 2,3-dioxygenase secretion whereas in rodents the same mechanism is associated with inducible nitric oxide synthase expression and nitric oxide production[21,39]. Whether the observed increase in number and shape of glial cells should be associated with a compensative/therapeutic rather than a noxious stimulus should be addressed by further investigations on cytokine expression patterns. In this sense, an exhaustive characterization of molecular mechanisms activated by MSC-derived bioactive molecules was beyond the scope of our analysis.
Taken together, these lines of evidence suggest an effect of pVW-MSCs mediators on glial cells promoting neuronal remodeling and confirm the paramount role of this cell type in modulating immune-mediated changes of the ENS. A further characterization of the type of glial cells involved in these changes is warranted. Moreover, the observed interspecies differences should be taken into consideration in future investigations of immune-mediated response to MSCs secretome in rodents models.
There is growing interest on mesenchymal stromal cells (MSC) as a novel therapeutic strategy to treat auto-immune and inflammatory diseases. However, identifying optimal MSC sources and limited reliability of current experimental models still represent a challenge in this field. Pigs represent more closely human physiology and an accessible resource for ex vivo procedures. Recently, our group isolated a population of pericytes from porcine aortic wall with an MSC profile, currently cited as porcine vascular wall-MSC (pVW-MSC).
Inflammatory bowel diseases (IBDs), comprising the two major forms ulcerative colitis and Crohn’s disease, are characterized by an aberrant immune response leading to severe damage of the intestinal wall and functioning. Current trials are evaluating the application of cell-based therapies for the treatment of IBDs. The present study describes the effect of pVW-MSC-conditioned medium (CM) on enteric ganglia in two ex vivo models of IBDs in order to investigate a potential development of MSC-based treatment of IBDs.
To evaluate the effect of pVW-MSC secretome on survival and differentiation of enteric ganglionic cells isolated by guinea pigs (GPEG) and pigs (PEG) and exposed to lipopolysaccharide (LPS).
The expression of standard MSC markers in pVW-MSC were assessed by flow cytometry. Increasing concentration of LPS were tested in both GPEG and PEG cultures. CM derived by pVW-MSC cultures were added alone or in combination with 1µg of LPS in GPEG and PEG cultures. Ganglionic cells were double-stained with antibodies directed to the pan-neuronal marker, HuD and the glial fibrillary acidic protein, GFAP. Cell count and morphometric analysis were performed to determine changes of neuronal and glial population.
Guinea-pig neurons and glial cells decreased and increased respectively in response to high concentrations of LPS. These changes were not observed in pig primary cultures. pVW-MSC secretome increased the number and differentiation of glial cells compared to neurons with a more pronounced effect in PEG and in combination with LPS.
These data showed a higher resilience of pig enteric ganglia to the main bacterial product LPS compared to guinea pig and a higher responsiveness of glial cells to pVW-MSC secreted mediators.
Neuro-immune changes induced by pVW-MSC represent an essential aspect in the development of cell-based therapies. Further studies are warranted to investigate inter-species differences of pVW-MSC secretome.
Professor Patrizia Hrelia (Department of Pharmacology and Biotechnology, University of Bologna) kindly provided access to the imaging facility (Zeiss Imager M1 microscope and AxioVision software). Doctor Alberto Elmi kindly gave his support for the statistical analysis. Doctor Giulio Bassi gave his excellent support for flowcytometry analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bagyánszki M, Krishnan T, Tang ST, Xu WX S-Editor: Yan JP L-Editor: A E-Editor: Li X
| 1. | Baumgart DC, Carding SR. Inflammatory bowel disease: Cause and immunobiology. Lancet. 2007;369:1627-1640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1299] [Cited by in F6Publishing: 1358] [Article Influence: 79.9] [Reference Citation Analysis (1)] |
| 2. | Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 3. | Cleynen I, Vermeire S. The genetic architecture of inflammatory bowel disease: Past, present and future. Curr Opin Gastroenterol. 2015;31:456-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, De Ponti F, De Giorgio R. Enteric neuroplasticity evoked by inflammation. Auton Neurosci. 2006;126-127:264-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Sherman LS, Shaker M, Mariotti V, Rameshwar P. Mesenchymal stromal/stem cells in drug therapy: New perspective. Cytotherapy. 2017;19:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 532] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 7. | Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 798] [Cited by in F6Publishing: 887] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 8. | Rashedi I, Gómez-Aristizábal A, Wang XH, Viswanathan S, Keating A. TLR3 or TLR4 Activation Enhances Mesenchymal Stromal Cell-Mediated Treg Induction via Notch Signaling. Stem Cells. 2017;35:265-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Najar M, Krayem M, Meuleman N, Bron D, Lagneaux L. Mesenchymal Stromal Cells and Toll-Like Receptor Priming: A Critical Review. Immune Netw. 2017;17:89-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E, Yuan S, Young YK, Boivin MN, Forner K, Basik M, Galipeau J. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994-6002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | UCSF Clinical Trials. Evaluation of PROCHYMAL® for Treatment-refractory Moderate-to-severe Crohn’s Disease [accessed 2019 Feb 28]. Available from: https://clinicaltrials.ucsf.edu/trial/NCT01233960. [Cited in This Article: ] |
| 12. | European Medicines Agency. Summary of Opinion (initial authorisation) -Alofisel [Internet], 2017 [cited 2018 Jan 17]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/004258/WC500240363.pdf. [Cited in This Article: ] |
| 13. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell. 2009;4:206-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1012] [Cited by in F6Publishing: 1025] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 14. | Watanabe S, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nasuno M, Yamashita K, Idogawa M, Naishiro Y, Murata M, Adachi Y, Fujimiya M, Imai K, Shinomura Y. Conditioned mesenchymal stem cells produce pleiotropic gut trophic factors. J Gastroenterol. 2014;49:270-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, Vykoukal JV, Ruppert KA, Cox CS, Olson SD. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells. 2018;36:79-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 16. | Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92:114-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 225] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 17. | Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 256] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 18. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11055] [Cited by in F6Publishing: 11772] [Article Influence: 692.5] [Reference Citation Analysis (1)] |
| 19. | Zaniboni A, Bernardini C, Alessandri M, Mangano C, Zannoni A, Bianchi F, Sarli G, Calzà L, Bacci ML, Forni M. Cells derived from porcine aorta tunica media show mesenchymal stromal-like cell properties in in vitro culture. Am J Physiol Cell Physiol. 2014;306:C322-C333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Nesci S, Bernardini C, Salaroli R, Zannoni A, Trombetti F, Ventrella V, Pagliarani A, Forni M. Characterization of metabolic profiles and lipopolysaccharide effects on porcine vascular wall mesenchymal stem cells. J Cell Physiol. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Bernardini C, Bertocchi M, Zannoni A, Salaroli R, Tubon I, Dothel G, Fernandez M, Bacci ML, Calzà L, Forni M. Constitutive and LPS-stimulated secretome of porcine Vascular Wall-Mesenchymal Stem Cells exerts effects on in vitro endothelial angiogenesis. Bmc Vet Res. 2019;15:123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Su J, Chen X, Huang Y, Li W, Li J, Cao K, Cao G, Zhang L, Li F, Roberts AI, Kang H, Yu P, Ren G, Ji W, Wang Y, Shi Y. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21:388-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 23. | Russell WMS, Burch RL. The Principles of Humane Experimental Technique, 1959. Available from: http://altweb.jhsph.edu/pubs/books/humane_exp/het-toc. [Cited in This Article: ] |
| 24. | Romagnoli N, Ventrella D, Giunti M, Dondi F, Sorrentino NC, Fraldi A, Surace EM, Bacci ML. Access to cerebrospinal fluid in piglets via the cisterna magna: Optimization and description of the technique. Lab Anim. 2014;48:345-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Kugler EM, Mazzuoli G, Demir IE, Ceyhan GO, Zeller F, Schemann M. Activity of protease-activated receptors in primary cultured human myenteric neurons. Front Neurosci. 2012;6:133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Schäfer KH, Saffrey MJ, Burnstock G, Mestres-Ventura P. A new method for the isolation of myenteric plexus from the newborn rat gastrointestinal tract. Brain Res Brain Res Protoc. 1997;1:109-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Zaniboni A, Bernardini C, Bertocchi M, Zannoni A, Bianchi F, Avallone G, Mangano C, Sarli G, Calzà L, Bacci ML, Forni M. In vitro differentiation of porcine aortic vascular precursor cells to endothelial and vascular smooth muscle cells. Am J Physiol Cell Physiol. 2015;309:C320-C331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Kim HJ, Shaker MR, Cho B, Cho HM, Kim H, Kim JY, Sun W. Dynamin-related protein 1 controls the migration and neuronal differentiation of subventricular zone-derived neural progenitor cells. Sci Rep. 2015;5:15962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | di Giancamillo A, Vitari F, Bosi G, Savoini G, Domeneghini C. The chemical code of porcine enteric neurons and the number of enteric glial cells are altered by dietary probiotics. Neurogastroenterol Motil. 2010;22:e271-e278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Gabella G, Trigg P. Size of neurons and glial cells in the enteric ganglia of mice, guinea-pigs, rabbits and sheep. J Neurocytol. 1984;13:49-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 82] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Ippolito C, Segnani C, De Giorgio R, Blandizzi C, Mattii L, Castagna M, Moscato S, Dolfi A, Bernardini N. Quantitative evaluation of myenteric ganglion cells in normal human left colon: Implications for histopathological analysis. Cell Tissue Res. 2009;336:191-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Schuster A, Klotz M, Schwab T, Di Liddo R, Bertalot T, Schrenk S, Martin M, Nguyen TD, Nguyen TN, Gries M, Faßbender K, Conconi MT, Parnigotto PP, Schäfer KH. Maintenance of the enteric stem cell niche by bacterial lipopolysaccharides? Evidence and perspectives. J Cell Mol Med. 2014;18:1429-1443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Chiang CE, Luk HN, Wang TM. Swelling-activated chloride current is activated in guinea pig cardiomyocytes from endotoxic shock. Cardiovasc Res. 2004;62:96-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Forni M, Mazzola S, Ribeiro LA, Pirrone F, Zannoni A, Bernardini C, Bacci ML, Albertini M. Expression of endothelin-1 system in a pig model of endotoxic shock. Regul Pept. 2005;131:89-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Sakuma R, Kawahara M, Nakano-Doi A, Takahashi A, Tanaka Y, Narita A, Kuwahara-Otani S, Hayakawa T, Yagi H, Matsuyama T, Nakagomi T. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflammation. 2016;13:57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 36. | Barón M, Gallego A. The relation of the microglia with the pericytes in the cat cerebral cortex. Z Zellforsch Mikrosk Anat. 1972;128:42-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Oppliger B, Joerger-Messerli MS, Simillion C, Mueller M, Surbek DV, Schoeberlein A. Mesenchymal stromal cells from umbilical cord Wharton's jelly trigger oligodendroglial differentiation in neural progenitor cells through cell-to-cell contact. Cytotherapy. 2017;19:829-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Drommelschmidt K, Serdar M, Bendix I, Herz J, Bertling F, Prager S, Keller M, Ludwig AK, Duhan V, Radtke S, de Miroschedji K, Horn PA, van de Looij Y, Giebel B, Felderhoff-Müser U. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. 2017;60:220-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 39. | Ren G, Su J, Zhang L, Zhao X, Ling W, L'huillie A, Zhang J, Lu Y, Roberts AI, Ji W, Zhang H, Rabson AB, Shi Y. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954-1962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 452] [Article Influence: 32.3] [Reference Citation Analysis (0)] |