Published online Sep 7, 2019. doi: 10.3748/wjg.v25.i33.4814
Peer-review started: May 21, 2019
First decision: June 9, 2019
Revised: July 4, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: September 7, 2019
The intimate connection and the strict mutual cooperation between the gut and the liver realizes a functional entity called gut-liver axis. The integrity of intestinal barrier is crucial for the maintenance of liver homeostasis. In this mutual relationship, the liver acts as a second firewall towards potentially harmful substances translocated from the gut, and is, in turn, is implicated in the regulation of the barrier. Increasing evidence has highlighted the relevance of increased intestinal permeability and consequent bacterial translocation in the development of liver damage. In particular, in patients with non-alcoholic fatty liver disease recent hypotheses are considering intestinal permeability impairment, diet and gut dysbiosis as the primary pathogenic trigger. In advanced liver disease, intestinal permeability is enhanced by portal hypertension. The clinical consequence is an increased bacterial translocation that further worsens liver damage. Furthermore, this pathogenic mechanism is implicated in most of liver cirrhosis complications, such as spontaneous bacterial peritonitis, hepatorenal syndrome, portal vein thrombosis, hepatic encephalopathy, and hepatocellular carcinoma. After liver transplantation, the decrease in portal pressure should determine beneficial effects on the gut-liver axis, although are incompletely understood data on the modifications of the intestinal permeability and gut microbiota composition are still lacking. How the modulation of the intestinal permeability could prevent the initiation and progression of liver disease is still an uncovered area, which deserves further attention.
Core tip: The integrity of the gut-liver axis is crucial for the maintenance of the homestasis of the organism. The disruption of the intestinal barrier and consequent increased intestinal permeability has been recently associated with the development of liver damage. This review summarizes present evidence on the relevance of the derangement of the gut-liver axis in the pathogenesis of liver damage and non-alcoholic fatty liver disease, the development of the complications of liver cirrhosis and its modifications after liver transplantation.
- Citation: Nicoletti A, Ponziani FR, Biolato M, Valenza V, Marrone G, Sganga G, Gasbarrini A, Miele L, Grieco A. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J Gastroenterol 2019; 25(33): 4814-4834
- URL: https://www.wjgnet.com/1007-9327/full/v25/i33/4814.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i33.4814
The gut is one of the largest mucosal surfaces of the human body. Besides being involved in the absorption of nutrients and water introduced with ingested food, it acts as a barrier that guarantees protection against pathogenic microorganisms and potentially harmful substances, such as toxins and pollutants[1]. In addition, the interaction that occurs between the gut microbiota and immunological cells at this level is crucial for the development and maintenance of the immune system[2,3].
The gut and the liver are anatomically connected by portal circulation, and their functional unit realizes the gut-liver axis[4]. Thus, any type of substance that goes beyond the gut barrier can reach the liver where is processed into metabolic pathways or interacts with the immune system cells or resident cells.
Liver disease affects gut homeostasis, altering intestinal permeability (IP) and the gut microbiota composition, proportionally to the degree of liver function impairment. Indeed, once portal hypertension (PHT) is established, the intestinal barrier functions are altered, causing the passage of substances that are normally kept in the intestinal lumen[5]. In particular, the translocation of bacterial fragments or products into the bloodstream activates the immune system, stimulating inflammation. This process not only could further worsen liver function, but it is implicated in a series of chain reactions involving the whole organism, realizing a systemic inflammatory condition typical of advanced liver cirrhosis[5].
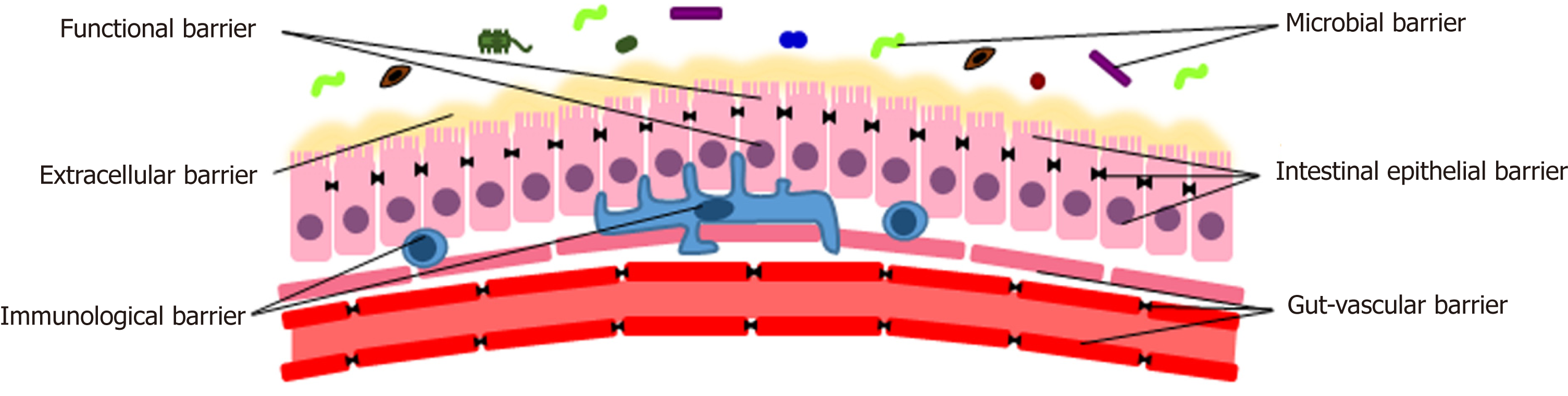
Normally, the gut constitutes a complex physical, chemical, functional and immunological barrier. In order to perform its tasks, different components are necessary[1,6]. Proceeding from the lumen inwards, they can be classified into the following levels: The microbiota, the extracellular elements, the epithelial cells, the immune system, the vascular structure (Figure 1).
The human gut microbiota harbors one hundred trillions of microorganisms, about ten times the number of eukaryotic cells. It has about ten times the genes of the human genome and has a mass of about 1-2 kg[7].
Several factors, such as birth mode, age, diet and lifestyle, influence the human gut microbiota. In physiological conditions, its compositional and functional armony is quite stable over time. However, the onset of disease and/or the use of certain drugs (e.g., antibiotics) can break this balance, resulting in dysbiosis with significant consequences on human homeostasis. Indeed, the gut microbiota integrates the metabolism of the organism providing crucial pathways to process nutrients, vitamins and endogenous substances[8]. Microorganisms host in the lumen interact with the intestinal mucosa, shaping the mucus[9], exerting a trophic and protective function towards enterocytes. Moreover, it plays a pivotal role in the development, maturation and maintenance of the immune system[10-15] and induces local production of antimicrobial peptides and immunoglobulins[8,12].
Intestinal mucus is a gel formed by glycosylated proteins secreted by intestinal goblet cells called mucins[16]. It covers the whole gut and its thickness depends on the location, being almost absent in the stomach and maximum in the colon[17]. Mucus prevents harmful substances and bacteria from directly contacting cell surface, causing inflammation[18-20]. Thus, a proper structure of mucins is crucial for the maintenance of the gut barrier, and alterations could facilitate the absorption of harmful substances, leading to inflammation[20]. Indeed, quantitative or qualitative alterations of the mucus layer has been documented in several diseases, such as cystic fibrosis[21] and inflammatory bowel disease (IBD)[22]. In addition, it has been demonstrated in mice models that a high MUC2 mucin production increases the susceptibility of goblet cells to apoptosis and endoplasmic reticulum stress[23]. An increased mucus thickness has been related to alcohol intake and cirrhosis[24]. Conversely, an incorrect assembly of MUC2 inside the epithelial cells leads to the development of an inflammatory disease resembling ulcerative colitis in mice[23,25]. This process may be responsible of the depletion of goblet cells documented in IBD[16].
The inner side of the intestinal mucus is made of a fluid, which is not reached by the mixing forces of the luminal flow and peristalsis, called unstirred layer. The inner face of the mucin layer is devoid of bacteria[18] and directly contacts the intestinal epithelial cells, modulating the absorption of water and nutrients due to its static nature. A thicker unstirred layer has been observed in patients with coeliac disease and has been related to malabsorption[26].
To make the picture more complex, it has to be considered that this system is dynamic and subject to regulation by gastrointestinal motility and secretions. The outer part of the mucus layer is continuously moved forward by peristalsis. The luminal flow prevents the proliferations of microorganism and a prompt clearance of detrimental elements. This is crucial in the protection against pathogens[1,27]. Gastric acid decreases microbial colonization of the small intestine. Only acid resistant microorganism, such as Helicobacter pylori and Lactobacilli are able to survive at low pH[28]. Bile acids, the main constituents of bile, have direct antimicrobial properties interfering with membrane and protein production and integrity[29-32]. Thus, alterations of the bile and gastric fluid and impairment of the peristalsis cause both qualitative and quantitative modifications of the gut microbiota composition up to the derangement of intestinal homeostasis and the development of pathology[28,33].
Underneath the intestinal mucus, there is a continuous monocellular layer of enterocytes. Goblet cells, responsible for the production of the mucus, and Paneth cells, which produce antimicrobial peptides, provide additional functions and support to the homeostasis of the gut barrier. Enterocytes plasma membrane represents the main mechanical element of the mucosal barrier. Because of its lipidic structure, it is impermeable to most solutes that need a specific transporter to cross the barrier (transcellular pathway)[1]. In order to limit the gut permeability, intercellular spaces are sealed by the presence of a specific apical junctional complex, which is composed by a tight junction (TJ) and an adherens junction. Overall, over 40 proteins form a TJ, being claudins, peripheral membrane proteins, such as zonula occludens (ZO) 1 and 2, and occludin the main components[34,35]. Both tight and adherens junctions are connected to the cytoskeleton[36]. TJ are important elements for both active and passive transport through the gut barrier[37]. They regulate the passive flow of the solutes and water through the paracellular pathway, operating both as a size- and charge-selective filter[38]. The passive movement of substances across TJ occurs through two different routes: The leak pathway, that allows the transport of larger substances (e.g., proteins, bacterial components), and a second pathway mediated by claudin proteins, that is charge selective and limits the flow to molecules smaller than 4 Å[1,38-40].
As for active transport, an intact intestinal epithelial barrier, formed by TJ and the plasma membrane of intestinal cell, realizes a gradient between the lumen and the inner interstice. This condition prevents an uncontrolled translocation of substances and allows an active transcellular transport through the enterocytes[1]. Moreover, the complex system of TJ is finely regulated by the influence of cytokines, particularly tumor necrosis factor-alfa (TNFα)[41] and interferon gamma (IFNγ)[42], and by signaling kinases and cytoskeleton, like myosin light chain kinases (MLCK)[43,44]. Both qualitative and quantitative alterations of TJ have been described in the context of liver disease[45,46]. Finally, intestinal cells own another defensive element. In fact, apical brush border microvilli are negatively charged, owing to the presence of polar carbohydrates and charged transmembrane proteins, and cause an electrostatic repulsive force towards bacterial cell wall, that is negatively charged as well[47].
In response to the exposure to bacteria and to their components, Paneth cells produce antimicrobial peptides, such as defensins, cathelicidines, resistin-like molecules, bactericidial-permeability-inducing proteins and lectins, and immunoglobulins, particularly secretory IgA[5]. These elements are secreted into the gut lumen and are host in the inner face of the mucin layer hosts[48]. Whenever microbial and pathogen-associated molecular patterns cross the intestinal barrier, they are identified through the interaction between pattern-recognition receptors, such as Toll-like receptors (TLRs) and nucleotide binding oligomerization domain-like receptors on the intestinal epithelial cells. Then, recruited dendritic cells are responsible for the transport of the captured antigens to the mesenteric lymph nodes (MLNs) for antigen presentation. This mechanism allows the priming and maturation of B and T lymphocytes, that become part of the adaptive immune response in the gut associated lymphoid tissue[49-51]. Hence, immune response is compartmentalized in mucosal lymphatics in healthy individuals.
Since 2015, the knowledge about barrier mechanisms for the modulation of IP stopped to the basocellular membrane of the enterocytes. Recent studies have successively revealed that the intestinal defense mechanisms actually go further, and also include a gut-vascular barrier[52]. Observing functional similarities between blood-brain barrier and intestinal barrier, Spadoni et al[52,53] hypothesized that a parallel structure in the gut could be responsible for the prevention of the translocation of bacteria and/or microbial components passed through the extracellular and the intestinal epithelial barrier.
The fundamental structure of this entity is the gut-vascular unit. It is composed by the intestinal endothelium, which is anatomically and functionally associated with pericytes and enteric glial cells that surround it. The barrier is completed by TJ and adherens junctions, which are permeable to most of the small nutrients. Endothelial plasma membrane provides isolation and is equipped with active and passive transporters[53,54]. Glial cells play an important role in the homeostasis of the gut and in the regulation of IP[52,53]. In fact, in murine models, it has been demonstrated that either genetical or autoimmune targeting of glial cells determines the development of fulminant enteritis with increased translocation of microbes and evidence of bacteremia[55,56]. When the endothelium is intact, it allows the free diffusion of 4 kD dextran, whereas 70 kD dextran is blocked. Infection with Salmonella enterica serovar Typhimurium disrupts the gut-vascular barrier, allowing the translocation of larger substances, and this happens independently of the increase in the blood flow provoked by inflammation[52,53]. Furthermore, 70 kD dextran was only found in the liver and not in the spleen, demonstrating that dissemination occurs through the portal circulation rather than the lymphatic vessels. The increase in plasmalemma vesicle-associated protein-1 (PV1), a marker of endothelial permeability, during Salmonella infection confirms this evidence. Finally, the authors demonstrated that bacteria with the ability to cross the intestinal epithelial barrier do not disseminate to liver and spleen, blocked by a second barrier[52]. These experiments definitively prove the existence of a gut-vascular barrier.
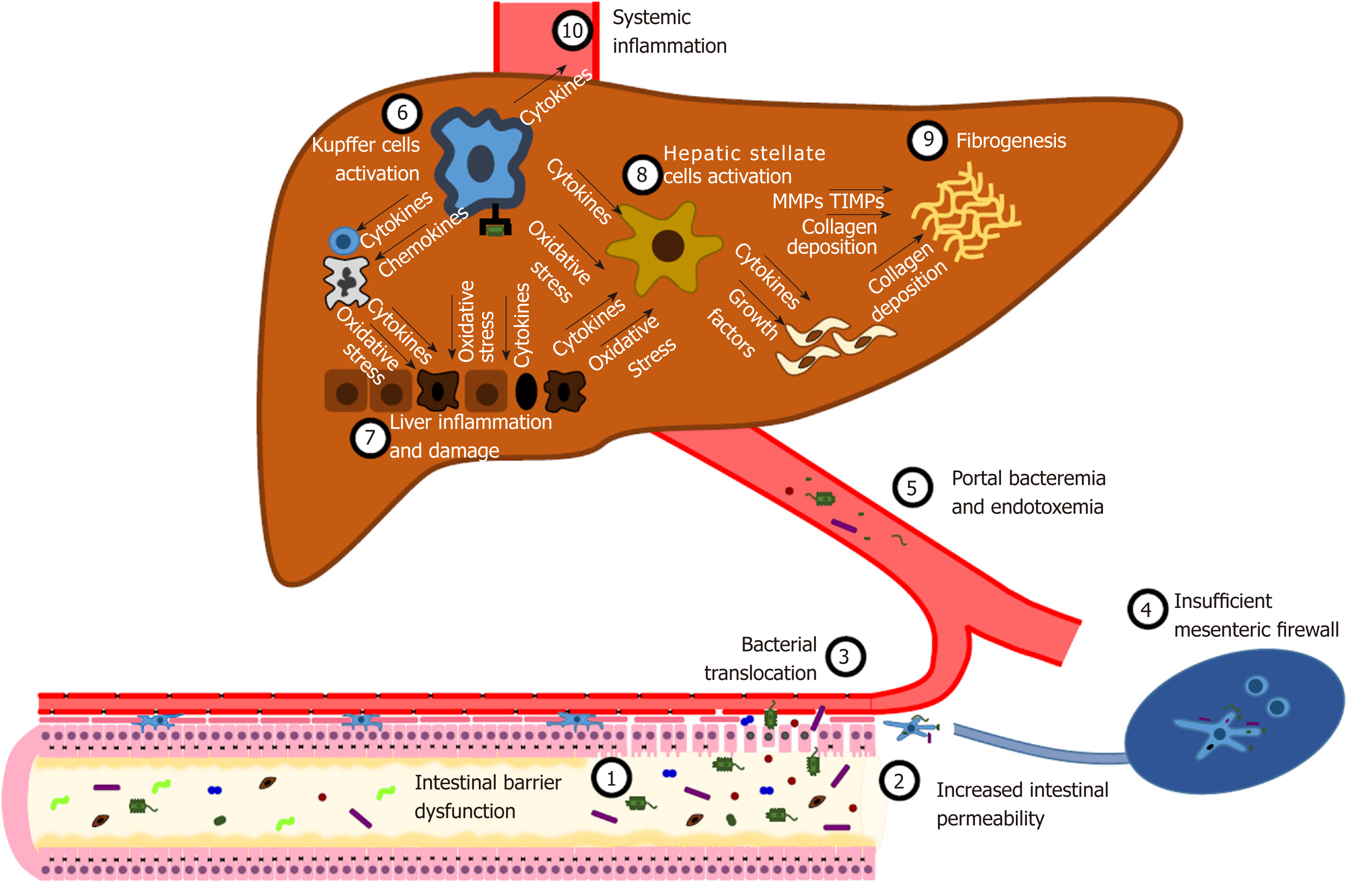
In liver diseases, increased IP is the consequence of multiple disorders that affect the homeostasis of the barrier. Several studies in animal models and in human pathology correlated liver damage and dysfunction to alterations of the gut microbiota composition[57], mucus quality and quantity[24], gastrointestinal motility[33], intestinal epithelial barrier and TJ[45], and the immune system[58].
Nevertheless, bacterial translocation (BT) is a physiological process that consists in the passage of small amounts of microorganisms and their constituents from the intestinal lumen to the MLNs[5]. At this site, microbial killing occurs without systemic inflammatory response[59,60]. This process is crucial for the modulation of the immune system and the development of immune tolerance[2,3]. Despite the fact that the liver is usually devoid of bacteria[61], in healthy individuals it is physiologically exposed to trace amounts of bacterial mRNAs and lipopolysaccharide (LPS)[4,62,63], mainly acting as a firewall detoxifying bacterial components[61,64]. In healthy mice, it has been demonstrated that the liver can act as a second firewall for microorganisms penetrated after mucosal damage and escaped from MLNs surveillance activity[4,61,64]. This function is supposed to be mainly exerted by the hepatic sinusoids, where Kupffer cells - representing over the 80% of all tissue macrophages - are able to phagocytize and kill microbes derived from the bloodstream[4,61,65-67]. Several experiments have demonstrated the importance of liver resident macrophages in the clearance of microorganisms and microbial- and pathogen- associated molecular patterns (MAMPs and PAMPs). In fact, 3H- and 14C-labelled endotoxin purified from E. coli is actively processed by Kupffer cells[68]. Similarly, lipopolysaccharide binding protein (LBP), an acute-phase protein synthesized in the liver and secreted after interleukin-1 (IL-1), interleukin-6 (IL-6), and glucocorticoids stimulation, after binding with LPS mediates the activation of liver mononuclear cells in a way that is dependent on the presence of functional Toll-like receptor 4 (TLR4)[69,70]. CD14, either expressed on myeloid cells (mCD14) or the isoform secreted into the bloodstream by monocytes and hepatocytes (sCD14), acts as a co-receptor of TLR4 binding the LPS-LBP complex and allowing its uptake by liver resident myeloid cells[71-73]. Moreover, an elegant imaging-based study by Lee et al[65] documented the ability of Kupffer cells to perform filtration of blood, phagocytosis and killing of green fluorescent protein expressing B. burgdorferii and antigen presentation to natural killer (NK) cells. Finally, in Kupffer cells depleted mice, the clearance of E. coli K-12 during bacteremia is delayed[61].
Yet, the “liver buffer” is exhaustible too. The disruption of the intestinal barrier at any level leads to an increase inIP (Figure 2). Thus, harmful substances, such as MAMPs and PAMPs (LPS, microbial DNA, peptidoglycans and lipopeptides), metabolic products, and whole bacteria massively reach local MLNs, that are unable to provide an adequate clearance[74-77]. Hence, a variable amount of detrimental products is delivered to the liver through the mesenteric and portal circulation[4]. The maintenance of a damaging insult triggers a systemic inflammatory response, developing from the liver[78-81]. Kupffer cells play a pivotal role in orchestrating this mechanism[67,80,82-84]. Indeed, the interaction between pathogen-associated molecular patterns and TLRs activate intracellular molecular pathways, either MyD88-dependent or MyD88-independent, resulting in the activation of NF-κB and the expression of inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-12, IL-18), chemokines (CXCL1, CXCL2, CCL2, CCL5, CCL3, CCL4), vasoactive factors [nitric oxide (NO)] and reactive oxygen species (ROS)[85]. This local inflammatory storm leads to the recruitment of systemic leukocytes, such as neutrophils, CD4+ T cells and monocytes, that perpetuate liver inflammation[80,82]. Net result of this process is the induction of hepatocyte apoptosis and necrosis[86]. Both inflammatory cytokines and cell death cause the activation and proliferation of hepatic stellate cells (HSC) and the development of fibrosis under the stimulation of transforming growth factor-β (TGFβ)[84,87].
As a consequence of inflammatory cytokines, HSCs and several other liver cells upregulate the expression of matrix metalloproteinases (MMPs). The overexpression and hyperactivation of MMPs result in the destruction of the hepatic tissue[88,89].
Tissue inhibitors of matrix metalloproteinases (TIMPs) are the main modulators of the activity of MMPs. While a decrease in the levels of TIMPs have been associated with liver damage in acute liver injury, an increase in their expression in chronic liver diseases favor the accumulation of collagen and liver fibrogenesis, by inhibiting degradation of collagen[88-91]. Furthermore, as proof of the relevance of these enzymes in the pathogenesis of liver damage, TIMP-1 has been identified as a predictive marker for the presence of non-alcoholic steatohepatitis (NASH)[92]
Oxidative stress plays a critical role in the development of liver damage[93]. The production of reactive oxygen species is a physiological consequence of aerobic life. Hence, organisms have developed antioxidant mechanisms in order to face the harmful effects of these agents. The detrimental effect of these species depends on the balance with antioxidant elements[94].
When this equilibrium is deranged, ROS can negatively affect both sides of the gut-liver axis. On the one hand, oxidative stress is responsible for intestinal barrier damage. Indeed, diet[95], alcohol[96], infectious[97] and primary inflammatory diseases[98], and drugs[99] are able to cause an imbalance in the redox state in the gut, resulting in increased IP. Furthermore, in advanced liver diseases PHT causes hypoperfusion of the intestinal mucosa. Subsequent hypoxia enhances the activity of xanthine oxidase, resulting in increased ROS release and oxidative damage[100]. On the other hand, the liver is an important scavenger of free radicals, since it plays a crucial role in the restoration of endogenous antioxidants and metabolism of exogenous ones[101,102]. A significant increase in the level of oxidative stress has been observed in all chronic liver diseases, irrespective of the etiology of the liver disorder. Moreover, all the liver cells are sensitive to oxidative stress-related molecules[93,103,104]. The activation of TLR causes the generation of ROS by Kupffer cells[105]. ROS signaling causes the activation and proliferation of HSC[106]. Conversely, as a consequence to the exposure to ROS, Kupffer cells produce cytokines and chemokines, which further stimulate HSCs[104].
Nevertheless, there are some protective mechanisms. IL-10 mediates remarkable protective effects towards the intestinal mucosa and liver. At the intestinal level, the release of IL-10 by macrophages modulates innate immune activation, preventing an excessive response and consequent tissue damage[107]. Hence, adequate IL-10 levels improve the integrity of the gut barrier, resulting in a decrease in endotoxin absorption[108]. In the liver, IL-10 reduces liver inflammations and fibrosis, inhibiting several Kupffer cells functions[109,110].
Similarly, NK cells regulate fibrogenetic mechanisms in the liver. Indeed, NK cells perform immunosurveillance activity by killing early activated and senescent HSCs, thus limiting fibrogenesis[111,112]. Interestingly, TIMP-1-expressing HSCs are resistant to NK cells activity[113].
Coeliac disease is the hallmark of the pathogenic mechanism linking increased IP and liver inflammation[45]. Liver damage is a common disorder associated with coeliac disease[114-119]. In a recent meta-analysis, the prevalence of cryptogenic hypertransaminasaemia in newly diagnosed coeliac disease is 27%[120]. In coeliac patients, increased permeability has been proved as well[121]. Although the pathogenesis is poorly understood, the theory that liver involvement could be secondary to increased IP and BT is widely accepted[114,115,118]. Bardella et al[115] reported a normalization of transaminases levels in about 90% of patients with increased levels at the time of coeliac disease diagnosis after six months of gluten free diet (GFD). In the remaining 10% other possible causes of liver damage were proven by liver biopsy. Another study demonstrated a significant correlation between serum transaminases levels and IP, assessed with lactulose/mannitol test. The authors found similar response to GFD (64/72 patients, 88.9%) and reported that IP index significantly decreased in conjunction with the normalization of serum transaminases levels within one year of diet. Conversely, in patients who were not compliant with GFD, liver injury persisted and permeability tests remained altered[122]. Furthermore, histological alterations in the liver of patients with newly diagnosed coeliac disease and transaminases elevation suggest that increased IP could be responsible for liver damage in this setting. As reported by Jacobsen et al[119], among 37 liver biopsies performed in coeliac patients, 25 showed non-specific patterns, 7 were diagnostic for other diseases, 5 were classified as normal. Liver histological features of the 25 non-specific specimens documented an increased number of Kupffer cells (52.0%), expanded portal tracts (48.0%) and parenchymal or portal mononuclear infiltration (36% and 20% respectively). Interestingly, some of these alterations are comparable to those observed in other experiments reproducing liver damage in context of increased IP[123]. Thus, these results are consistent with the hypothesis that IP per se could trigger the development of liver damage.
Also in the setting of primary liver disease, increasing evidence is linking IP to liver damage. Occludin deficient (Ocln−/−) mice do not show intestinal TJ alteration[124], but ethanol feeding induces a decrease in E-cadherin and β-catenin distribution, which are other proteins involved in the maintenance of TJ integrity, causing gut barrier dysfunction[125]. Although both ethanol fed Ocln−/− and wild type mice had increased plasma transaminase levels, liver damage was worse in occludin deficient mice, and histopathological examination of the liver confirmed the presence of inflammatory lesions only in Ocln−/− mice[125]. As for human studies, Cariello et al[126] demonstrated that plasma levels of inflammatory cytokines (TNF-α and IL-6) are higher in patients with both liver disease and increased IP compared to those with normal IP. A positive correlation between altered IP and liver inflammation and fibrosis was observed in a population of children with non-alcoholic fatty liver disease (NAFLD)[127]. Finally, a recent meta-analysis showed that patients with NAFLD, particularly those with increased liver injury markers, more frequently exhibit altered IP[128]. Altogether, these data suggest a pathogenic mechanism that determines liver damage through the alteration of the gut barrier.
The pathogenesis of liver damage in patients with NAFLD is still incompletely understood. However, a growing body of experimental and clinical data suggests a primary role of the gut-liver axis dysfunction. Traditionally, a “double-hit” pathogenetic model has been hypothesized for NAFLD development. Lipid accumulation into the liver (steatosis) represents the first step. Then, a second insult is needed to cause liver injury and inflammation[129,130]. The discovery of a linkage between small intestinal bacterial overgrowth (SIBO) and NAFLD[131-133] and the observation that endotoxin triggers liver inflammation in mice with steatosis[134] brought to the formulation of this hypothesis[130]. Several experiments in animal and human models confirmed the influence of increased IP both in the development of liver steatosis and in the pathogenesis of liver inflammation and fibrosis.
Brun et al[62] reported gut barrier dysfunction, tested as higher epithelial permeability to horseradish peroxidase in obese mice, both genetically deficient in leptin (C57BL/6Job/ob) and functionally deficient for the long-form leptin receptor (C57BL/6Jdb/db). Immunochemistry and Western blot confirmed important alterations of TJ proteins (ZO-1 and Occludin) distribution in obese mice. Hence, endotoxin in portal circulation and levels of circulating proinflammatory cytokines (IL-1, IL-6, INF-γ, and TNF-α) were significantly higher both in ob/ob and in db/db mice compared to controls. Interestingly, HSC isolated from obese mice showed enhanced sensitivity to LPS and produced higher levels of cytokines.
Junctional adhesion molecule A (JAM-A) is a constituent of the TJ encoded by the murine gene F11r. It modulates the epithelial barrier function, regulating IP and inflammation[135-138]. F11r−/− mice, fed a diet high in saturated fat, fructose and cholesterol (HFCD) for 8 weeks, developed a severe steatohepatitis, assessed by the presence of histological features of liver inflammation (hepatocyte ballooning and inflammatory cells infiltration) and fibrogenesis and increase in serum transaminases compared to controls[123].
In a recent study, male C57BL/6 mice were fed with dextran sulfate sodium (DSS), a chemical compound able to determine gut inflammation, and a high-fat diet (HFD) for 12 wk. Fat vacuoles and leukocyte infiltration in the liver were higher in DSS and HFD-fed mice compared to HFD-fed mice. Concordantly, levels of hepatic mRNA coding for inflammatory cytokines (IL-1, IL-6, TNF-α, MCP-1) were increased as well. Moreover, DSS + HFD showed higher expression of collagen I and profibrogenic factors mRNA (TGF-β, Actin α2, tissue inhibitor of metalloproteinase-1 and plasminogen activator inhibitor-1). Although there were no significant differences in the levels of serum endotoxin, an upregulation of TLR4 and TLR 9 was observed in DSS HFD mice. Finally, the downregulation of ZO-1 and Claudin-1 and the increased expression of PV1 confirmed both the intestinal and gut-vascular barrier dysfunction after DSS treatment[139].
As for human models, the first strong evidence of increased IP in NAFLD patients emerged from a study testing the intestinal absorption and urine excretion of orally administered 51Cr-EDTA[45]. Indeed, 51Cr-EDTA is normally not metabolized and poorly absorbed (1%-3%) from the gastrointestinal tract and it crosses the intestinal barrier through the paracellular pathway in the presence of TJ disruption[27,140,141]. 51Cr-EDTA excretion levels were significantly higher than values of healthy volunteers in a fashion that resulted proportional to the degree of liver steatosis. Furthermore, duodenal histology showed reduced ZO-1 expression in patients with NAFLD. In this population of patients, the prevalence of SIBO was about three times compared to controls, an observation that confirmed findings of previous studies[142]. However, increased IP was not associated with the severity of liver inflammation, fibrosis and the presence of NASH[45]. Similarly, in children with NALFD liver damage has been linked to alterations of the gut barrier. The ratio between urinary excretion of lactulose and mannitol (L/M ratio) after oral administration was used to measure the degree of IP[27,127,143]. L/M ratio was significantly higher in NAFLD children and further increased in NASH patients. In order to ascertain the presence of BT, serum LPS was quantified and resulted significantly higher in children with confirmed liver damage. Interestingly, the extent of hepatic inflammation and fibrosis was proportional to the degree of IP[127]. The association between SIBO and NAFLD and the finding of increased endotoxemia across the studies underlines the role of the gut microbiota in the initiation and development of metabolic liver disease[45,127,142,144]. Once increased IP is established, dysbiosis affects liver homeostasis through different mechanisms. Gut microorganisms directly cause liver damage either by means of MAMPs and PAMPs (e.g., LPS) or by products of their metabolism (e.g., ethanol, short-chain fatty acids (SCFAs) and trimethylamine)[145].
Proteobacteria, particularly Enterobacteriaceae, can ferment carbohydrates to ethanol[146]. In the presence of adequate conditions, the amount produced can be remarkable[147]; indeed, a significant correlation between ethanol-producing bacteria abundance, blood ethanol concentration and liver inflammation has been demonstrated[146]. Besides causing direct toxic effects to the liver, this overproduction determine the activation of hepatic ethanol metabolic pathways and increases liver oxidative stress[148]. These evidences have confirmed the relevance of endogenous ethanol production in the pathogenesis of NASH.
Acetic, propionic and butyric acid are the main SCFAs produced by the gut microbiota in physiological conditions as a result of carbohydrates fermentation[149]. Following the intestinal absorption, SCFAs reach the liver through the portal circulation, where they serve as energy source and exert a relevant role in lipogenesis and gluconeogenesis[145,150,151]. Interacting with G‑protein coupled receptors GPR41 and GPR43 of intestinal enteroendocrine L cells, SCFAs stimulate the release of the peptide YY (PYY), a hormone able to slow gastric emptying and intestinal transit and favor energy absorption[152]. Another important consequence is the release of glucagon-like peptide-1, which enhances glucose-dependent insulin release[153]. Altogether, these effects may favor the development of NAFLD and NASH[145].
Furthermore, the intestinal microbiota inhibits the production and secretion of fasting-induced adipocyte factor (FIAF) by the intestinal L cells and the enterocytes. FIAF is an inhibitor of lipoprotein lipase (LPL), which determines, when suppressed, the activation of LPL and the increase in triglyceride accumulation in the liver and the adipocytes[154]. Hence, increased hepatic lipid storage activates the carbohydrate-responsive element-binding protein and the sterol regulatory element-binding protein 1, perpetuating fat accumulation[155].
Finally, choline is implicated in the synthesis of very-low density lipoprotein (VLDL). Hence, choline deficiency cause a decrease in the production and release of VLDL and triglyceride accumulation in the liver[156]. Bacteria of the taxa Erysipelotrichia are able to metabolize choline to methylamines, toxic compounds that have been correlated to liver damage[157,158]. In NAFLD patients, augmented intestinal metabolism of choline, choline deficiency and abundance of Erysipelotrichia taxa have been observed[157].
Recent studies reported qualitative alterations of the gut microbiota composition in patients with NAFLD. Particularly, Bacteroides genus is correlated with NASH and a parallel decrease in Prevotella abundance was found[159,160]. In fact, diet enriched in fat, proteins of animal origin and simple sugars, like Western one, promotes Bacteroides abundance, whilst an increase in Prevotella abundance is favored by a diet rich in fibers and vegetal carbohydrates[159,161]. Ruminococcus genus has been positively associated with significant liver fibrosis (≥ F2) in humans[159], and a correlation between the abundance of this genus and the development of metabolic impairment has been observed in animal models[162]. Alcohol production, due to the ability of Ruminococcus to ferment complex carbohydrates, may be responsible for further liver damage[163]. An increase in Proteobacteria/Enterobacteriaceae/Escherichia abundance has been described in NASH and correlates with serum levels of alcohol[146].
Furthermore, NAFLD-related liver cirrhosis patients showed a low gut microbiota diversity compared to healthy controls. At the genus level, an abundance in Lactobacillus, Bacteroides, Ruminococcus, Klebsiella, Prevotella, Enterococcus, Haemophilus, Pseudomonas, Parabacteroides, Phascolarctobacterium, Veillonella, Streptococcus, Atopobium, Dialister, Christensenella, and decrease in Methanobrevibacter and Akkermansia was observed[164].
It is well known that diet also is a key regulator of IP[165]. In animal models of NAFLD, adaptation of a high-fat diet or high-fructose intake has been associated with increased gut permeability[166,167]. Elevated concentrations of saturated fat or fructose favors pro-inflammatory microbiota; on one hand, suppressing production of SCFAs that are essential for intestinal barrier function, on the other hand recruiting macrophages and leading to the release of TNF-α and other cytokines causing mucosal inflammation[168,169]. The consequence is a decreased expression of TJ proteins and a higher permeability of the gut barrier[170]. Diet-induced increases in blood LPS levels are known as metabolic endotoxaemia and play an important role in the activation of TLR-mediated low-grade liver inflammation, which are associated with NAFLD and NASH[171]. Current evidence from animal studies suggests that a high-fat diet or a high-fructose diet can induce metabolic endotoxaemia by altering the intestinal TJ proteins, mainly ZO-1 and occluding[62,172-174]. In NAFLD adolescents, postprandial endotoxin levels were increased compared to healthy subjects in response to fructose, but not glucose, beverages (consumed with meals) in a 24-h feeding challenge[175].
There are currently no data concerning diet modulation of IP in patients with NAFLD, and it is plausible that a healthy diet can reduce IP in patients with NAFLD by restoring the integrity of tight junctions. The Mediterranean diet contains a high intake of mono- and polyunsaturated fatty acids, fibres, polyphenols, antioxidants and phytochemicals; many of these components promote short-chain fatty acid-producing gut bacteria and have significant prebiotic effects[176]. As such, Mediterranean diet was an attractive tool for reducing impaired IP in patients with NAFLD. In a cross-over pilot study[177], twenty patients with NAFLD underwent 16 weeks of a Mediterranean diet and 16 weeks of a low-fat diet; although the majority of patients presented at baseline, as expected, high IP evaluated according to 51Cr-EDTA, none of the two diets were sufficient to modulate it. Diet-modulation of IP in humans is much more difficult to obtain than in animal models and further research is needed.
Increased IP and BT are hallmarks of liver cirrhosis[5,27]. As previously described, the contribution of BT to liver damage could be crucial for the progression to liver cirrhosis. On the other hand, the once liver cirrhosis is establishment it further enhances IP. The magnitude of BT is proportional to the stage of the disease[5] and correlates with prognosis[178].
PHT can reasonably be considered the primary determinant of the onset of altered IP in the setting of advanced liver disease. Indeed, increased splanchnic vasodilation induces a decrease in the blood flow and venous congestion at the intestinal mucosa level, leading to ischemia and edema, up to the disruption of the TJ and epithelial barrier dysfunction[179,180]. Consequently, BT is enhanced and in most cases it becomes clinically relevant, due to the large extent of the mucosa involved in the pathogenic mechanism[181-184]. To confirm of the importance of PHT in the pathogenesis of increased IP, the reduction of hepatic venous pressure gradient by non-selective beta-blocker therapy decreases IP[180].
Endotoxemia further worsens the hemodynamics of cirrhotic patients. In fact, the systemic inflammatory response activated by bacteria and their products/fragments leads to the release of cytokines and the consequent synthesis of (NO) by inducible nitric oxide synthase (iNOS)[185-187]. The result is a decrease in systemic vascular resistance and the secondary development of hyperdynamic circulation[74,75,188] that further worsen IP and BT[189]. In fact, there is evidence that intestinal decontamination improves the hyperdynamic state in liver cirrhosis[190,191].
Furthermore, increased IP and consequent BT are fundamental pathogenic steps in the development of complications of chronic liver disease[74]. In cirrhotic patients, impaired hemodynamics in advanced phases may negatively affect renal function, causing the hepatorenal syndrome (HRS). LPS per se leads to renal vasoconstriction, but it can worsen renal function via the increase of plasma levels of endothelin[192-194]. Furthermore, TLR4 may play a role in the pathogenesis of HRS via the consequent activation of NF-κB and TNF-α pathways, since it is overexpressed in the kidney during endotoxemia[195]. The importance of this pathogenic mechanism in the development of HRS is highlighted by the fact that in both animal and human studies, intestinal decontamination, achieved either by norfloxacin, paromomycin or rifaximin, showed beneficial effects on renal function[195-197]. Similarly, among the ancillary effects of albumin infusion, the scavenging of LPS is involved in the amelioration of renal hemodynamics[198].
In the first clinical reports of spontaneous bacterial peritonitis (SBP) in the 1960s, a pathogenetic mechanism involving BT from the gastrointestinal tract has already been hypothesized[199-202]. However, clear scientific evidence was only produced in the 1990s. These experiments showed in murine models of liver cirrhosis a high correspondence between the isolation of bacteria from cultures of MLNs and ascites. Positive cultures were obtained from both mice with or without SBP, demonstrating that BT is a frequent event in advanced liver disease[203-205]. Another evidence that elucidates the causal association between intestinal dysbiosis, impaired IP, BT and SBP is the decrease in the incidence of SBP (-72%) in patients with ascites treated with rifaximin[206]. Similar results in SBP primary and secondary prophylaxis have been obtained with norfloxacin[207,208].
In liver cirrhosis, the liver capacity to detoxify ammonia, neurotoxic substances and false neurotransmitters, produced by the gut microbiota from the catabolism of dietary proteins, is insufficient[209,210]. On the other hand, the formation of portosystemic shunts further decrease the part of blood depurated[211]. Thus, entering the bloodstream, these substances are delivered to the brain, where they have detrimental effects, causing edema and altering neurotransmission, causing hepatic encephalopathy (HE)[209,210].
A perturbation in the gut microbiota composition has been linked to the development of HE. In particular, Alcaligeneceae, Porphyromonadaceae, Enterobacteriaceae abundance has been correlated with cognitive impairment and neuroinflammation in cirrhotic patients[212]. Moreover, the systemic inflammatory state resulting from the perpetuation of BT independently affects brain functions and worsens cognitive performance[213-217], and finally, inflammation secondarily extends to the brain, where a self-maintaining process is then established[214,218-220]. Hence, the modulation of the gut microbiota and its metabolism represents the basis for the treatment and prevention of overt HE[221-223].
The pathogenesis of portal vein thrombosis (PVT) is incompletely understood. However, besides reduced portal vein flow velocity and prothrombotic state, BT into portal vein could favor the activation of the coagulative cascade[224,225]. Indeed, it is known that endotoxin is able to increase thrombin generation via the increased production of tissue factor (TF)[226]. Similarly, LPS stimulates the release of factor VIII and von Willebrand factor release, in a way that could be mediated by TLR4 activation[227]. Since the liver acts as a firewall towards BT[61], there is a gradient between the concentration of LPS in the portal vein and in the systemic circulation[228]. Hence, this could be a significant pathogenic mechanism for the development of PVT in cirrhotic patients[224,225]. Interestingly, endotoxin-induced prothrombotic state in the portal system can cause microembolism to hepatic sinusoids, contributing to liver damage and inflammation[229].
Increasing evidence supports the involvement of the gut-liver axis in hepatocarcinogenesis. As aforementioned, intestinal hyperpermeability and consequent BT activate TLRs through the binding with LPS[85]. The subsequent activation of NF-κB signaling initiates the inflammatory cascade that favors carcinogenesis[230,231]. Indeed, in animal models, it has been demonstrated that the infusion of LPS stimulates the development as well as the growth of liver tumors[232,233]. Conversely, the lack of IKK-b, a kinase that frees NF-κB from inhibitory proteins, decreases hepatocarcinogenesis[234]. An inflammatory environment is crucial for the development of hepatocellular carcinoma (HCC). Cytokines modify the micro-enviroment by recruiting innate immune cells and altering the extracellular matrix[231,235]. Moreover, the production of ROS cause direct DNA damage[236] and inflammation stimulate cell turnover and proliferation, favoring the accumulation of DNA mutations[231,235].
Other MAMPs and PAMPs and microbial metabolites have also been proposed as potential carcinogens[237,238]. Hence, recent studies have analyzed the gut microbiota of patients with HCC in order to find a microbial fingerprint of the disease. Ponziani et al[164] described the gut microbiota of NAFLD cirrhotic patients with HCC. At the genus level, a significant increased abundance of the Phascolarctobacterium, Enterococcus, Streptococcus, Gemella, Bilophila genera was observed. In another recent study, the abundance of the Haemophilus, Eggerthella, Bifidobacterium, Butyricimonas, Christensella, Odoribacter genera, an unknown genus from Tenericutes phylum and an unknown genus from Firmicutes phylum was significantly increased by 2-3 fold in the HCC group. Interestingly, the authors found a correlation between changes in the gut microbiota and liver inflammation[239].
Finally, as regards the gut microbiome in liver cirrhosis, a decreased bacterial diversity has been observed compared to healthy controls. At the phylum level, the abundance of Bacteroidetes is reduced, whilst Proteobacteria and Fusobacteria are increased. The increase in the abundance of potentially pathogenic bacteria, such as Streptococcus, Veilonella, and Enterobacteriaceae, may explain the frequent involvement of these bacteria in the pathogenesis of infectious complications in these patients[240,241]. A relocation in the distribution of microorganisms along the gastrointestinal tract has been correlated with the onset of the complications of liver cirrhosis, as well[240]. In particular, a higher abundance of Streptococcus salivarius has been correlated with the minimal HE[242]. In parallel, a decrease in the abundance of potentially beneficial Lachnospiraceae and Clostridium cluster XIVa has been reported[240,241].
PHT, which is responsible for increased IP in the setting of liver cirrhosis, is reverted by liver transplantation (LT)[243,244]. Accordingly, IP should decrease after LT. In a study analyzing IP 2 to 3 years after LT in patients on immunosuppressant drugs (tacrolimus and cyclosporine), Parrilli et al[245] reported an increase in lactulose /rhamnose ratio (Lacl/L-Rh ratio) that was only due to a decrease in L-Rh excretion. The authors concluded that IP was restored, in spite of the effects of antirejection drugs on intestinal barrier function. Moreover, serum endotoxin levels were similar between LT patients and controls. Another study soon after LT in patients receiving tacrolimus therapy showed that IP, assessed with L/R ratio, was elevated compared to healthy controls. Furthermore, about 50% of the patients had increased serum levels of endotoxin[246]. Therefore, IP could still be impaired soon after LT and improve later. However, further studies are needed to analyze the modification of IP in patients with cirrhosis after LT.
Few studies analyzed the alterations of the gut microbiota after LT. In particular, a decrease in Eubacteria, Bifidobacterium spp, Fecalibacterium prausnitzii and Lactobacillus spp abundance and a decrease in Enterobacteriaceae and Enterococcus spp has been observed[247]. Interestingly, in a recent study microbial diversity did not show significant modification during the first week after LT. Instead, during postoperative days 8 to 14 the influence of surgical operation, antibiotics and antirejection therapy reduced microbial diversity[248]. Afterwards diversity was progressively restored[247,248]. No association was been found between intestinal dysbiosis and acute cellular rejection, post-transplant bloodstream infections and/or the recurrence of liver disease[248,249].
Increased IP, BT and alterations of the gut microbiota composition are important pathogenetic elements responsible for the development of liver damage, the initiation of fibrosis changes up to the development of liver cirrhosis and its complications. At present, there are very few evidences of the efficacy of the role of the gut microbiota modulation in the modification of the natural course of liver disease. Further studies are needed to investigate the efficacy of these strategies.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hilmi I, Hori T, Kohla MAS, Pop TL S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
| 1. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2121] [Cited by in F6Publishing: 2385] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 2. | Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1099] [Cited by in F6Publishing: 1067] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 3. | Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 952] [Cited by in F6Publishing: 1015] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 4. | Brandl K, Kumar V, Eckmann L. Gut-liver axis at the frontier of host-microbial interactions. Am J Physiol Gastrointest Liver Physiol. 2017;312:G413-G419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 5. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 506] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 6. | Okumura R, Takeda K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm Regen. 2018;38:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 7. | Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369-2379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1826] [Cited by in F6Publishing: 1887] [Article Influence: 235.9] [Reference Citation Analysis (0)] |
| 8. | Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787-8803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 1421] [Cited by in F6Publishing: 1407] [Article Influence: 156.3] [Reference Citation Analysis (43)] |
| 9. | Jakobsson HE, Rodríguez-Piñeiro AM, Schütte A, Ermund A, Boysen P, Bemark M, Sommer F, Bäckhed F, Hansson GC, Johansson ME. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16:164-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 441] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 10. | Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3003] [Cited by in F6Publishing: 2739] [Article Influence: 228.3] [Reference Citation Analysis (0)] |
| 11. | Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1039] [Cited by in F6Publishing: 1151] [Article Influence: 143.9] [Reference Citation Analysis (0)] |
| 12. | Britanova L, Diefenbach A. Interplay of innate lymphoid cells and the microbiota. Immunol Rev. 2017;279:36-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2488] [Cited by in F6Publishing: 2827] [Article Influence: 282.7] [Reference Citation Analysis (0)] |
| 14. | Rescigno M. Mucosal immunology and bacterial handling in the intestine. Best Pract Res Clin Gastroenterol. 2013;27:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Rescigno M. Intestinal microbiota and its effects on the immune system. Cell Microbiol. 2014;16:1004-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 821] [Cited by in F6Publishing: 881] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 17. | Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GM, Schütte A, van der Post S, Svensson F, Rodríguez-Piñeiro AM, Nyström EE, Wising C, Johansson ME, Hansson GC. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 646] [Cited by in F6Publishing: 742] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 18. | Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064-15069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1326] [Cited by in F6Publishing: 1388] [Article Influence: 86.8] [Reference Citation Analysis (1)] |
| 19. | Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1288] [Cited by in F6Publishing: 1294] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 20. | Vereecke L, Beyaert R, van Loo G. Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol Med. 2011;17:584-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, Hebert H, Sjövall H, Hansson GC. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209:1263-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 22. | Schultsz C, Van Den Berg FM, Ten Kate FW, Tytgat GN, Dankert J. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology. 1999;117:1089-1097. [PubMed] [Cited in This Article: ] |
| 23. | Tawiah A, Cornick S, Moreau F, Gorman H, Kumar M, Tiwari S, Chadee K. High MUC2 Mucin Expression and Misfolding Induce Cellular Stress, Reactive Oxygen Production, and Apoptosis in Goblet Cells. Am J Pathol. 2018;188:1354-1373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, Stärkel P, Belzer C, Hellerbrand C, Tsukamoto H, Ho SB, Schnabl B. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 25. | Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, Adams R, Kato M, Nelms KA, Hong NA, Florin TH, Goodnow CC, McGuckin MA. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 521] [Cited by in F6Publishing: 541] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 26. | Strocchi A, Corazza G, Furne J, Fine C, Di Sario A, Gasbarrini G, Levitt MD. Measurements of the jejunal unstirred layer in normal subjects and patients with celiac disease. Am J Physiol. 1996;270:G487-G491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Ponziani FR, Zocco MA, Cerrito L, Gasbarrini A, Pompili M. Bacterial translocation in patients with liver cirrhosis: physiology, clinical consequences, and practical implications. Expert Rev Gastroenterol Hepatol. 2018;12:641-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 28. | Ponziani FR, Gerardi V, Gasbarrini A. Diagnosis and treatment of small intestinal bacterial overgrowth. Expert Rev Gastroenterol Hepatol. 2016;10:215-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1372] [Cited by in F6Publishing: 1352] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 30. | Duparc T, Plovier H, Marrachelli VG, Van Hul M, Essaghir A, Ståhlman M, Matamoros S, Geurts L, Pardo-Tendero MM, Druart C, Delzenne NM, Demoulin JB, van der Merwe SW, van Pelt J, Bäckhed F, Monleon D, Everard A, Cani PD. Hepatocyte MyD88 affects bile acids, gut microbiota and metabolome contributing to regulate glucose and lipid metabolism. Gut. 2017;66:620-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 31. | Lorenzo-Zúñiga V, Bartolí R, Planas R, Hofmann AF, Viñado B, Hagey LR, Hernández JM, Mañé J, Alvarez MA, Ausina V, Gassull MA. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 228] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 32. | Bertók L. Bile acids in physico-chemical host defence. Pathophysiology. 2004;11:139-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Theocharidou E, Dhar A, Patch D. Gastrointestinal Motility Disorders and Their Clinical Implications in Cirrhosis. Gastroenterol Res Pract. 2017;2017:8270310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Yamazaki Y, Okawa K, Yano T, Tsukita S, Tsukita S. Optimized proteomic analysis on gels of cell-cell adhering junctional membrane proteins. Biochemistry. 2008;47:5378-5386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213-C1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1008] [Cited by in F6Publishing: 1013] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 36. | Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014;36:157-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 344] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 37. | Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 652] [Cited by in F6Publishing: 700] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 38. | Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 39. | Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1346-C1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 285] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 40. | Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969-4976. [PubMed] [Cited in This Article: ] |
| 41. | Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology. 1998;114:657-668. [PubMed] [Cited in This Article: ] |
| 42. | Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 568] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 43. | Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378-C1385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 373] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 44. | Hartmann P, Haimerl M, Mazagova M, Brenner DA, Schnabl B. Toll-like receptor 2-mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology. 2012;143:1330-1340.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 45. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 938] [Cited by in F6Publishing: 982] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 46. | Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, Spiliopoulou I, Kaltezioti V, Charonis A, Nikolopoulou VN, Scopa CD, Thomopoulos KC. Altered intestinal tight junctions' expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest. 2012;42:439-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 123] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 47. | Bennett KM, Walker SL, Lo DD. Epithelial microvilli establish an electrostatic barrier to microbial adhesion. Infect Immun. 2014;82:2860-2871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson LG, Midtvedt T, Pütsep K, Andersson M. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57:764-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 49. | Gautreaux MD, Deitch EA, Berg RD. T lymphocytes in host defense against bacterial translocation from the gastrointestinal tract. Infect Immun. 1994;62:2874-2884. [PubMed] [Cited in This Article: ] |
| 50. | Gautreaux MD, Gelder FB, Deitch EA, Berg RD. Adoptive transfer of T lymphocytes to T-cell-depleted mice inhibits Escherichia coli translocation from the gastrointestinal tract. Infect Immun. 1995;63:3827-3834. [PubMed] [Cited in This Article: ] |
| 51. | Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, Curtiss R, McCoy KD, Macpherson AJ. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 533] [Cited by in F6Publishing: 556] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 52. | Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, Penna G, Dejana E, Rescigno M. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 381] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 53. | Spadoni I, Fornasa G, Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol. 2017;17:761-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 54. | Spadoni I, Pietrelli A, Pesole G, Rescigno M. Gene expression profile of endothelial cells during perturbation of the gut vascular barrier. Gut Microbes. 2016;7:540-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189-201. [PubMed] [Cited in This Article: ] |
| 56. | Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci USA. 2001;98:13306-13311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 243] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 57. | Betrapally NS, Gillevet PM, Bajaj JS. Gut microbiome and liver disease. Transl Res. 2017;179:49-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 58. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 691] [Cited by in F6Publishing: 726] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 59. | Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662-1665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1095] [Cited by in F6Publishing: 1056] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 60. | Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222-2226. [PubMed] [Cited in This Article: ] |
| 61. | Balmer ML, Slack E, de Gottardi A, Lawson MA, Hapfelmeier S, Miele L, Grieco A, Van Vlierberghe H, Fahrner R, Patuto N, Bernsmeier C, Ronchi F, Wyss M, Stroka D, Dickgreber N, Heim MH, McCoy KD, Macpherson AJ. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med. 2014;6:237ra66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 307] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 62. | Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518-G525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 592] [Cited by in F6Publishing: 600] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 63. | Etienne-Mesmin L, Vijay-Kumar M, Gewirtz AT, Chassaing B. Hepatocyte Toll-Like Receptor 5 Promotes Bacterial Clearance and Protects Mice Against High-Fat Diet-Induced Liver Disease. Cell Mol Gastroenterol Hepatol. 2016;2:584-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 64. | Wood NJ. Liver: the liver as a firewall--clearance of commensal bacteria that have escaped from the gut. Nat Rev Gastroenterol Hepatol. 2014;11:391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, Chaconas G, Kubes P. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol. 2010;11:295-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 255] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 66. | Knook DL, Barkway C, Sleyster EC. Lysosomal enzyme content of Kupffer and endothelial liver cells isolated from germfree and clean conventional rats. Infect Immun. 1981;33:620-622. [PubMed] [Cited in This Article: ] |
| 67. | Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886-1900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 332] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 68. | Fox ES, Thomas P, Broitman SA. Clearance of gut-derived endotoxins by the liver. Release and modification of 3H, 14C-lipopolysaccharide by isolated rat Kupffer cells. Gastroenterology. 1989;96:456-461. [PubMed] [Cited in This Article: ] |
| 69. | Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, Remick DG, Wang SC. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology. 2000;31:932-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 70. | Schumann RR, Kirschning CJ, Unbehaun A, Aberle HP, Knope HP, Lamping N, Ulevitch RJ, Herrmann F. The lipopolysaccharide-binding protein is a secretory class 1 acute-phase protein whose gene is transcriptionally activated by APRF/STAT/3 and other cytokine-inducible nuclear proteins. Mol Cell Biol. 1996;16:3490-3503. [PubMed] [Cited in This Article: ] |
| 71. | Frey EA, Miller DS, Jahr TG, Sundan A, Bazil V, Espevik T, Finlay BB, Wright SD. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665-1671. [PubMed] [Cited in This Article: ] |
| 72. | Pugin J, Schürer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744-2748. [PubMed] [Cited in This Article: ] |
| 73. | Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762-1769. [PubMed] [Cited in This Article: ] |
| 74. | Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165-172. [PubMed] [Cited in This Article: ] |
| 75. | Bellot P, García-Pagán JC, Francés R, Abraldes JG, Navasa M, Pérez-Mateo M, Such J, Bosch J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology. 2010;52:2044-2052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 76. | Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, Fuster J, García-Valdecasas JC, Lacy A, Suárez MJ, Rimola A, Rodés J. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32-37. [PubMed] [Cited in This Article: ] |
| 77. | Garcia-Tsao G, Lee FY, Barden GE, Cartun R, West AB. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology. 1995;108:1835-1841. [PubMed] [Cited in This Article: ] |
| 78. | Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol. 2009;51:168-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 79. | Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 650] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 80. | Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 668] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 81. | Ramadori G, Moriconi F, Malik I, Dudas J. Physiology and pathophysiology of liver inflammation, damage and repair. J Physiol Pharmacol. 2008;59 Suppl 1:107-117. [PubMed] [Cited in This Article: ] |
| 82. | Wenfeng Z, Yakun W, Di M, Jianping G, Chuanxin W, Chun H. Kupffer cells: increasingly significant role in nonalcoholic fatty liver disease. Ann Hepatol. 2014;13:489-495. [PubMed] [Cited in This Article: ] |
| 83. | Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 702] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 84. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1361] [Cited by in F6Publishing: 1440] [Article Influence: 84.7] [Reference Citation Analysis (1)] |
| 85. | Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 86. | Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 430] [Article Influence: 39.1] [Reference Citation Analysis (1)] |
| 87. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1221] [Cited by in F6Publishing: 1570] [Article Influence: 224.3] [Reference Citation Analysis (0)] |
| 88. | Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 358] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 89. | Roderfeld M. Matrix metalloproteinase functions in hepatic injury and fibrosis. Matrix Biol. 2018;68-69:452-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 90. | Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48-60. [PubMed] [Cited in This Article: ] |
| 91. | Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 371] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 92. | Miele L, Forgione A, La Torre G, Vero V, Cefalo C, Racco S, Vellone VG, Vecchio FM, Gasbarrini G, Rapaccini GL, Neuman MG, Grieco A. Serum levels of hyaluronic acid and tissue metalloproteinase inhibitor-1 combined with age predict the presence of nonalcoholic steatohepatitis in a pilot cohort of subjects with nonalcoholic fatty liver disease. Transl Res. 2009;154:194-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 93. | Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082-8091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 652] [Cited by in F6Publishing: 654] [Article Influence: 65.4] [Reference Citation Analysis (3)] |
| 94. | Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8502] [Cited by in F6Publishing: 8379] [Article Influence: 465.5] [Reference Citation Analysis (0)] |
| 95. | Gil-Cardoso K, Ginés I, Pinent M, Ardévol A, Terra X, Blay M. A cafeteria diet triggers intestinal inflammation and oxidative stress in obese rats. Br J Nutr. 2017;117:218-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 96. | Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 97. | van Ampting MT, Schonewille AJ, Vink C, Brummer RJ, van der Meer R, Bovee-Oudenhoven IM. Intestinal barrier function in response to abundant or depleted mucosal glutathione in Salmonella-infected rats. BMC Physiol. 2009;9:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Novak EA, Mollen KP. Mitochondrial dysfunction in inflammatory bowel disease. Front Cell Dev Biol. 2015;3:62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 99. | Utzeri E, Usai P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:3954-3963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 53] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 100. | Ramachandran A, Prabhu R, Thomas S, Reddy JB, Pulimood A, Balasubramanian KA. Intestinal mucosal alterations in experimental cirrhosis in the rat: role of oxygen free radicals. Hepatology. 2002;35:622-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 101. | Casas-Grajales S, Muriel P. Antioxidants in liver health. World J Gastrointest Pharmacol Ther. 2015;6:59-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 83] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 102. | Maellaro E, Casini AF, Del Bello B, Comporti M. Lipid peroxidation and antioxidant systems in the liver injury produced by glutathione depleting agents. Biochem Pharmacol. 1990;39:1513-1521. [PubMed] [Cited in This Article: ] |
| 103. | Luangmonkong T, Suriguga S, Mutsaers HAM, Groothuis GMM, Olinga P, Boersema M. Targeting Oxidative Stress for the Treatment of Liver Fibrosis. Rev Physiol Biochem Pharmacol. 2018;175:71-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 104. | Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci. 2015;16:26087-26124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1066] [Cited by in F6Publishing: 909] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 105. | Liang S, Kisseleva T, Brenner DA. The Role of NADPH Oxidases (NOXs) in Liver Fibrosis and the Activation of Myofibroblasts. Front Physiol. 2016;7:17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 106. | Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of [corrected] Kupffer cells on stellate cells. Hepatology. 2006;44:1487-1501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 107. | Krause P, Morris V, Greenbaum JA, Park Y, Bjoerheden U, Mikulski Z, Muffley T, Shui JW, Kim G, Cheroutre H, Liu YC, Peters B, Kronenberg M, Murai M. IL-10-producing intestinal macrophages prevent excessive antibacterial innate immunity by limiting IL-23 synthesis. Nat Commun. 2015;6:7055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 108. | Gómez-Hurtado I, Moratalla A, Moya-Pérez Á, Peiró G, Zapater P, González-Navajas JM, Giménez P, Such J, Sanz Y, Francés R. Role of interleukin 10 in norfloxacin prevention of luminal free endotoxin translocation in mice with cirrhosis. J Hepatol. 2014;61:799-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597-1606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 189] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 110. | de Souza-Cruz S, Victória MB, Tarragô AM, da Costa AG, Pimentel JP, Pires EF, Araújo Lde P, Coelho-dos-Reis JG, Gomes Mde S, Amaral LR, Teixeira-Carvalho A, Martins-Filho OA, Victória Fda S, Malheiro A. Liver and blood cytokine microenvironment in HCV patients is associated to liver fibrosis score: a proinflammatory cytokine ensemble orchestrated by TNF and tuned by IL-10. BMC Microbiol. 2016;16:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 111. | Melhem A, Muhanna N, Bishara A, Alvarez CE, Ilan Y, Bishara T, Horani A, Nassar M, Friedman SL, Safadi R. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 112. | Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1263] [Cited by in F6Publishing: 1362] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 113. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Fukui H. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 2002;36:850-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 114. | Volta U. Pathogenesis and clinical significance of liver injury in celiac disease. Clin Rev Allergy Immunol. 2009;36:62-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Bardella MT, Fraquelli M, Quatrini M, Molteni N, Bianchi P, Conte D. Prevalence of hypertransaminasemia in adult celiac patients and effect of gluten-free diet. Hepatology. 1995;22:833-836. [PubMed] [Cited in This Article: ] |
| 116. | Grieco A, Miele L, Pignatoro G, Pompili M, Rapaccini GL, Gasbarrini G. Is coeliac disease a confounding factor in the diagnosis of NASH? Gut. 2001;49:596. [PubMed] [Cited in This Article: ] |
| 117. | Duggan JM, Duggan AE. Systematic review: the liver in coeliac disease. Aliment Pharmacol Ther. 2005;21:515-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Casella G, Antonelli E, Di Bella C, Villanacci V, Fanini L, Baldini V, Bassotti G. Prevalence and causes of abnormal liver function in patients with coeliac disease. Liver Int. 2013;33:1128-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Jacobsen MB, Fausa O, Elgjo K, Schrumpf E. Hepatic lesions in adult coeliac disease. Scand J Gastroenterol. 1990;25:656-662. [PubMed] [Cited in This Article: ] |
| 120. | Sainsbury A, Sanders DS, Ford AC. Meta-analysis: Coeliac disease and hypertransaminasaemia. Aliment Pharmacol Ther. 2011;34:33-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 121. | van Elburg RM, Uil JJ, Mulder CJ, Heymans HS. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut. 1993;34:354-357. [PubMed] [Cited in This Article: ] |
| 122. | Novacek G, Miehsler W, Wrba F, Ferenci P, Penner E, Vogelsang H. Prevalence and clinical importance of hypertransaminasaemia in coeliac disease. Eur J Gastroenterol Hepatol. 1999;11:283-288. [PubMed] [Cited in This Article: ] |
| 123. | Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, Smith T, Neish AS, Li H, Tan S, Wu P, Liu X, Yu Y, Farris AB, Nusrat A, Parkos CA, Anania FA. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology. 2016;151:733-746.e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 124. | Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131-4142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 875] [Cited by in F6Publishing: 852] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 125. | Mir H, Meena AS, Chaudhry KK, Shukla PK, Gangwar R, Manda B, Padala MK, Shen L, Turner JR, Dietrich P, Dragatsis I, Rao R. Occludin deficiency promotes ethanol-induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice. Biochim Biophys Acta. 2016;1860:765-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 126. | Cariello R, Federico A, Sapone A, Tuccillo C, Scialdone VR, Tiso A, Miranda A, Portincasa P, Carbonara V, Palasciano G, Martorelli L, Esposito P, Cartenì M, Del Vecchio Blanco C, Loguercio C. Intestinal permeability in patients with chronic liver diseases: Its relationship with the aetiology and the entity of liver damage. Dig Liver Dis. 2010;42:200-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 127. | Giorgio V, Miele L, Principessa L, Ferretti F, Villa MP, Negro V, Grieco A, Alisi A, Nobili V. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis. 2014;46:556-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 128. | Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, Motola DL, Luther S, Bohr S, Jeoung SW, Deshpande V, Singh G, Turner JR, Yarmush ML, Chung RT, Patel SJ. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol Gastroenterol Hepatol. 2015;1:222-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 129. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [PubMed] [Cited in This Article: ] |
| 130. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1756] [Cited by in F6Publishing: 1769] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 131. | Nazim M, Stamp G, Hodgson HJ. Non-alcoholic steatohepatitis associated with small intestinal diverticulosis and bacterial overgrowth. Hepatogastroenterology. 1989;36:349-351. [PubMed] [Cited in This Article: ] |
| 132. | Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414-423. [PubMed] [Cited in This Article: ] |
| 133. | Lichtman SN, Keku J, Schwab JH, Sartor RB. Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology. 1991;100:513-519. [PubMed] [Cited in This Article: ] |
| 134. | Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 135. | Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, Dermody TS, Nusrat A, Parkos CA. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067-3076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 374] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 136. | Monteiro AC, Sumagin R, Rankin CR, Leoni G, Mina MJ, Reiter DM, Stehle T, Dermody TS, Schaefer SA, Hall RA, Nusrat A, Parkos CA. JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol Biol Cell. 2013;24:2849-2860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 137. | Ménard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010;3:247-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 138. | Vetrano S, Rescigno M, Cera MR, Correale C, Rumio C, Doni A, Fantini M, Sturm A, Borroni E, Repici A, Locati M, Malesci A, Dejana E, Danese S. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135:173-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 139. | Cheng C, Tan J, Qian W, Zhang L, Hou X. Gut inflammation exacerbates hepatic injury in the high-fat diet induced NAFLD mouse: Attention to the gut-vascular barrier dysfunction. Life Sci. 2018;209:157-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 140. | DeMeo MT, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385-396. [PubMed] [Cited in This Article: ] |
| 141. | Arslan G, Atasever T, Cindoruk M, Yildirim IS. (51)CrEDTA colonic permeability and therapy response in patients with ulcerative colitis. Nucl Med Commun. 2001;22:997-1001. [PubMed] [Cited in This Article: ] |
| 142. | Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206-211. [PubMed] [Cited in This Article: ] |
| 143. | Dastych M, Dastych M, Novotná H, Cíhalová J. Lactulose/mannitol test and specificity, sensitivity, and area under curve of intestinal permeability parameters in patients with liver cirrhosis and Crohn's disease. Dig Dis Sci. 2008;53:2789-2792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 144. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4095] [Cited by in F6Publishing: 4129] [Article Influence: 242.9] [Reference Citation Analysis (0)] |
| 145. | Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 520] [Cited by in F6Publishing: 603] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 146. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1015] [Cited by in F6Publishing: 1108] [Article Influence: 100.7] [Reference Citation Analysis (1)] |
| 147. | Dawes EA, Foster SM. The formation of ethanol in Escherichia coli. Biochim Biophys Acta. 1956;22:253-265. [PubMed] [Cited in This Article: ] |
| 148. | Baker SS, Baker RD, Liu W, Nowak NJ, Zhu L. Role of alcohol metabolism in non-alcoholic steatohepatitis. PLoS One. 2010;5:e9570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 149. | Duncan SH, Louis P, Thomson JM, Flint HJ. The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol. 2009;11:2112-2122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 457] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 150. | Brüssow H, Parkinson SJ. You are what you eat. Nat Biotechnol. 2014;32:243-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 151. | Subramanian S, Goodspeed L, Wang S, Kim J, Zeng L, Ioannou GN, Haigh WG, Yeh MM, Kowdley KV, O'Brien KD, Pennathur S, Chait A. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J Lipid Res. 2011;52:1626-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 152. | Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010;33:2277-2284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 422] [Cited by in F6Publishing: 422] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 153. | Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, Pacetti D, Vivarelli M, Nicolini D, Garelli P, Casini A, Manco M, Mingrone G, Risaliti A, Frega GN, Benedetti A, Gastaldelli A. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 307] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 154. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718-15723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3967] [Cited by in F6Publishing: 4117] [Article Influence: 205.9] [Reference Citation Analysis (4)] |
| 155. | Alex S, Lange K, Amolo T, Grinstead JS, Haakonsson AK, Szalowska E, Koppen A, Mudde K, Haenen D, Al-Lahham S, Roelofsen H, Houtman R, van der Burg B, Mandrup S, Bonvin AM, Kalkhoven E, Müller M, Hooiveld GJ, Kersten S. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor γ. Mol Cell Biol. 2013;33:1303-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 156. | Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3327] [Cited by in F6Publishing: 3627] [Article Influence: 279.0] [Reference Citation Analysis (0)] |
| 157. | Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 514] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 158. | Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, Mitchell SC, Holmes E, McCarthy MI, Scott J, Gauguier D, Nicholson JK. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511-12516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 810] [Cited by in F6Publishing: 770] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 159. | Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 763] [Cited by in F6Publishing: 865] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 160. | de Wit NJ, Afman LA, Mensink M, Müller M. Phenotyping the effect of diet on non-alcoholic fatty liver disease. J Hepatol. 2012;57:1370-1373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 161. | Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4098] [Cited by in F6Publishing: 4113] [Article Influence: 316.4] [Reference Citation Analysis (0)] |
| 162. | O'Sullivan A, He X, McNiven EM, Haggarty NW, Lönnerdal B, Slupsky CM. Early diet impacts infant rhesus gut microbiome, immunity, and metabolism. J Proteome Res. 2013;12:2833-2845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 163. | Christopherson MR, Dawson JA, Stevenson DM, Cunningham AC, Bramhacharya S, Weimer PJ, Kendziorski C, Suen G. Unique aspects of fiber degradation by the ruminal ethanologen Ruminococcus albus 7 revealed by physiological and transcriptomic analysis. BMC Genomics. 2014;15:1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 164. | Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:107-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 374] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 165. | Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 893] [Cited by in F6Publishing: 1021] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 166. | Serino M, Luche E, Gres S, Baylac A, Bergé M, Cenac C, Waget A, Klopp P, Iacovoni J, Klopp C, Mariette J, Bouchez O, Lluch J, Ouarné F, Monsan P, Valet P, Roques C, Amar J, Bouloumié A, Théodorou V, Burcelin R. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61:543-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 440] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 167. | Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 428] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 168. | Lambertz J, Weiskirchen S, Landert S, Weiskirchen R. Fructose: A Dietary Sugar in Crosstalk with Microbiota Contributing to the Development and Progression of Non-Alcoholic Liver Disease. Front Immunol. 2017;8:1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 169. | Ray K. NAFLD. Leaky guts: intestinal permeability and NASH. Nat Rev Gastroenterol Hepatol. 2015;12:123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 170. | Miele L, Marrone G, Lauritano C, Cefalo C, Gasbarrini A, Day C, Grieco A. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. 2013;19:5314-5324. [PubMed] [Cited in This Article: ] |
| 171. | Kirpich IA, Marsano LS, McClain CJ. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin Biochem. 2015;48:923-930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 172. | Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470-1481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3224] [Cited by in F6Publishing: 3232] [Article Influence: 202.0] [Reference Citation Analysis (0)] |
| 173. | Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 496] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 174. | Kavanagh K, Wylie AT, Tucker KL, Hamp TJ, Gharaibeh RZ, Fodor AA, Cullen JM. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am J Clin Nutr. 2013;98:349-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 175. | Jin R, Willment A, Patel SS, Sun X, Song M, Mannery YO, Kosters A, McClain CJ, Vos MB. Fructose induced endotoxemia in pediatric nonalcoholic Fatty liver disease. Int J Hepatol. 2014;2014:560620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 176. | Bifulco M. Mediterranean diet: the missing link between gut microbiota and inflammatory diseases. Eur J Clin Nutr. 2015;69:1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 177. | Biolato M, Manca F, Marrone G, Cefalo C, Racco S, Miggiano GA, Valenza V, Gasbarrini A, Miele L, Grieco A. Intestinal permeability after Mediterranean diet and low-fat diet in non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25:509-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 44] [Cited by in F6Publishing: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 178. | Zapater P, Francés R, González-Navajas JM, de la Hoz MA, Moreu R, Pascual S, Monfort D, Montoliu S, Vila C, Escudero A, Torras X, Cirera I, Llanos L, Guarner-Argente C, Palazón JM, Carnicer F, Bellot P, Guarner C, Planas R, Solá R, Serra MA, Muñoz C, Pérez-Mateo M, Such J. Serum and ascitic fluid bacterial DNA: a new independent prognostic factor in noninfected patients with cirrhosis. Hepatology. 2008;48:1924-1931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 179. | Iwao T, Toyonaga A, Ikegami M, Oho K, Sumino M, Harada H, Sakaki M, Shigemori H, Aoki T, Tanikawa K. Reduced gastric mucosal blood flow in patients with portal-hypertensive gastropathy. Hepatology. 1993;18:36-40. [PubMed] [Cited in This Article: ] |
| 180. | Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, Lammert F, Trauner M, Peck-Radosavljevic M, Vogelsang H; Vienna Hepatic Hemodynamic Lab. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 181. | Garcia-Tsao G, Albillos A, Barden GE, West AB. Bacterial translocation in acute and chronic portal hypertension. Hepatology. 1993;17:1081-1085. [PubMed] [Cited in This Article: ] |
| 182. | De Palma GD, Rega M, Masone S, Persico F, Siciliano S, Patrone F, Matantuono L, Persico G. Mucosal abnormalities of the small bowel in patients with cirrhosis and portal hypertension: a capsule endoscopy study. Gastrointest Endosc. 2005;62:529-534. [PubMed] [Cited in This Article: ] |
| 183. | Higaki N, Matsui H, Imaoka H, Ikeda Y, Murakami H, Hiasa Y, Matsuura B, Onji M. Characteristic endoscopic features of portal hypertensive enteropathy. J Gastroenterol. 2008;43:327-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 184. | Baffy G. Potential mechanisms linking gut microbiota and portal hypertension. Liver Int. 2019;39:598-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 185. | Francés R, Muñoz C, Zapater P, Uceda F, Gascón I, Pascual S, Pérez-Mateo M, Such J. Bacterial DNA activates cell mediated immune response and nitric oxide overproduction in peritoneal macrophages from patients with cirrhosis and ascites. Gut. 2004;53:860-864. [PubMed] [Cited in This Article: ] |
| 186. | Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 187. | Mishima S, Xu D, Lu Q, Deitch EA. Bacterial translocation is inhibited in inducible nitric oxide synthase knockout mice after endotoxin challenge but not in a model of bacterial overgrowth. Arch Surg. 1997;132:1190-1195. [PubMed] [Cited in This Article: ] |
| 188. | McAvoy NC, Semple S, Richards JM, Robson AJ, Patel D, Jardine AG, Leyland K, Cooper AS, Newby DE, Hayes PC. Differential visceral blood flow in the hyperdynamic circulation of patients with liver cirrhosis. Aliment Pharmacol Ther. 2016;43:947-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 189. | Du Plessis J, Vanheel H, Janssen CE, Roos L, Slavik T, Stivaktas PI, Nieuwoudt M, van Wyk SG, Vieira W, Pretorius E, Beukes M, Farré R, Tack J, Laleman W, Fevery J, Nevens F, Roskams T, Van der Merwe SW. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol. 2013;58:1125-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 190. | Zhu Q, Zou L, Jagavelu K, Simonetto DA, Huebert RC, Jiang ZD, DuPont HL, Shah VH. Intestinal decontamination inhibits TLR4 dependent fibronectin-mediated cross-talk between stellate cells and endothelial cells in liver fibrosis in mice. J Hepatol. 2012;56:893-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 191. | Guarner C, Runyon BA, Heck M, Young S, Sheikh MY. Effect of long-term trimethoprim-sulfamethoxazole prophylaxis on ascites formation, bacterial translocation, spontaneous bacterial peritonitis, and survival in cirrhotic rats. Dig Dis Sci. 1999;44:1957-1962. [PubMed] [Cited in This Article: ] |
| 192. | O'Hair DP, Adams MB, Tunberg TC, Osborn JL. Relationships among endotoxemia, arterial pressure, and renal function in dogs. Circ Shock. 1989;27:199-210. [PubMed] [Cited in This Article: ] |
| 193. | Huang LT, Hung JF, Chen CC, Hsieh CS, Yu HR, Hsu CN, Tain YL. Endotoxemia exacerbates kidney injury and increases asymmetric dimethylarginine in young bile duct-ligated rats. Shock. 2012;37:441-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 194. | Uchihara M, Izumi N, Sato C, Marumo F. Clinical significance of elevated plasma endothelin concentration in patients with cirrhosis. Hepatology. 1992;16:95-99. [PubMed] [Cited in This Article: ] |
| 195. | Shah N, Dhar D, El Zahraa Mohammed F, Habtesion A, Davies NA, Jover-Cobos M, Macnaughtan J, Sharma V, Olde Damink SW, Mookerjee RP, Jalan R. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol. 2012;56:1047-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 196. | Tarao K, Moroi T, Hirabayashi Y, Ikeuchi T, Endo O, Takamura Y. Effect of paromomycin sulfate on endotoxemia in patients with cirrhosis. J Clin Gastroenterol. 1982;4:263-267. [PubMed] [Cited in This Article: ] |
| 197. | Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, Tsianos EV. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol. 2012;10:815-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 198. | Garcia-Martinez R, Noiret L, Sen S, Mookerjee R, Jalan R. Albumin infusion improves renal blood flow autoregulation in patients with acute decompensation of cirrhosis and acute kidney injury. Liver Int. 2015;35:335-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 199. | Matz R, Jurmann J. Spontaneous peritonitis in cirrhosis of the liver. Lancet. 1966;1:1242-1243. [PubMed] [Cited in This Article: ] |
| 200. | Kerr DN, Pearson DT, Read AE. Infection of ascitic fluid in patients with hepatic cirrhosis. Gut. 1963;4:394-398. [PubMed] [Cited in This Article: ] |
| 201. | Conn HO. Spontaneous peritonitis and bacteremia in laennec's cirrhosis caused by enteric organisms. A relatively common but rarely recognized syndrome. Ann Intern Med. 1964;60:568-580. [PubMed] [Cited in This Article: ] |
| 202. | Whipple RL, Harris JF. B. coli septicemia in Laennec's cirrhosis of the liver. Ann Intern Med. 1950;33:462-466. [PubMed] [Cited in This Article: ] |
| 203. | Runyon BA, Squier S, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol. 1994;21:792-796. [PubMed] [Cited in This Article: ] |
| 204. | Llovet JM, Bartolí R, Planas R, Cabré E, Jimenez M, Urban A, Ojanguren I, Arnal J, Gassull MA. Bacterial translocation in cirrhotic rats. Its role in the development of spontaneous bacterial peritonitis. Gut. 1994;35:1648-1652. [PubMed] [Cited in This Article: ] |
| 205. | Llovet JM, Bartolí R, March F, Planas R, Viñado B, Cabré E, Arnal J, Coll P, Ausina V, Gassull MA. Translocated intestinal bacteria cause spontaneous bacterial peritonitis in cirrhotic rats: molecular epidemiologic evidence. J Hepatol. 1998;28:307-313. [PubMed] [Cited in This Article: ] |
| 206. | Hanouneh MA, Hanouneh IA, Hashash JG, Law R, Esfeh JM, Lopez R, Hazratjee N, Smith T, Zein NN. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J Clin Gastroenterol. 2012;46:709-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 207. | Soriano G, Guarner C, Tomás A, Villanueva C, Torras X, González D, Sainz S, Anguera A, Cussó X, Balanzó J. Norfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage. Gastroenterology. 1992;103:1267-1272. [PubMed] [Cited in This Article: ] |
| 208. | Ginés P, Rimola A, Planas R, Vargas V, Marco F, Almela M, Forné M, Miranda ML, Llach J, Salmerón JM. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716-724. [PubMed] [Cited in This Article: ] |
| 209. | Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375:1660-1670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 237] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 210. | Butterworth RF, Giguère JF, Michaud J, Lavoie J, Layrargues GP. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol. 1987;6:1-12. [PubMed] [Cited in This Article: ] |
| 211. | Olde Damink SW, Deutz NE, Dejong CH, Soeters PB, Jalan R. Interorgan ammonia metabolism in liver failure. Neurochem Int. 2002;41:177-188. [PubMed] [Cited in This Article: ] |
| 212. | Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168-G175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 213. | Shawcross DL, Sharifi Y, Canavan JB, Yeoman AD, Abeles RD, Taylor NJ, Auzinger G, Bernal W, Wendon JA. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol. 2011;54:640-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 214. | Murta V, Farías MI, Pitossi FJ, Ferrari CC. Chronic systemic IL-1β exacerbates central neuroinflammation independently of the blood-brain barrier integrity. J Neuroimmunol. 2015;278:30-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 215. | Jain L, Sharma BC, Sharma P, Srivastava S, Agrawal A, Sarin SK. Serum endotoxin and inflammatory mediators in patients with cirrhosis and hepatic encephalopathy. Dig Liver Dis. 2012;44:1027-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 216. | Luo M, Li L, Yang EN, Dai CY, Liang SR, Cao WK. Correlation between interleukin-6 and ammonia in patients with overt hepatic encephalopathy due to cirrhosis. Clin Res Hepatol Gastroenterol. 2013;37:384-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 217. | Shawcross DL, Davies NA, Williams R, Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol. 2004;40:247-254. [PubMed] [Cited in This Article: ] |
| 218. | Kang DJ, Betrapally NS, Ghosh SA, Sartor RB, Hylemon PB, Gillevet PM, Sanyal AJ, Heuman DM, Carl D, Zhou H, Liu R, Wang X, Yang J, Jiao C, Herzog J, Lippman HR, Sikaroodi M, Brown RR, Bajaj JS. Gut microbiota drive the development of neuroinflammatory response in cirrhosis in mice. Hepatology. 2016;64:1232-1248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 219. | Jayakumar AR, Rama Rao KV, Norenberg MD. Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol. 2015;5:S21-S28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 220. | Rodrigo R, Cauli O, Gomez-Pinedo U, Agusti A, Hernandez-Rabaza V, Garcia-Verdugo JM, Felipo V. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010;139:675-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 221. | American Association for the Study of Liver Diseases. European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61:642-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 281] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 222. | Ponziani FR, Gerardi V, Pecere S, D'Aversa F, Lopetuso L, Zocco MA, Pompili M, Gasbarrini A. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J Gastroenterol. 2015;21:12322-12333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 53] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 223. | Garcovich M, Zocco MA, Roccarina D, Ponziani FR, Gasbarrini A. Prevention and treatment of hepatic encephalopathy: focusing on gut microbiota. World J Gastroenterol. 2012;18:6693-6700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 39] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 224. | Violi F, Ferro D, Basili S, Lionetti R, Rossi E, Merli M, Riggio O, Bezzi M, Capocaccia L. Ongoing prothrombotic state in the portal circulation of cirrhotic patients. Thromb Haemost. 1997;77:44-47. [PubMed] [Cited in This Article: ] |
| 225. | Violi F, Lip GY, Cangemi R. Endotoxemia as a trigger of thrombosis in cirrhosis. Haematologica. 2016;101:e162-e163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 226. | Moore KL, Andreoli SP, Esmon NL, Esmon CT, Bang NU. Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J Clin Invest. 1987;79:124-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 342] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 227. | Carnevale R, Raparelli V, Nocella C, Bartimoccia S, Novo M, Severino A, De Falco E, Cammisotto V, Pasquale C, Crescioli C, Scavalli AS, Riggio O, Basili S, Violi F. Gut-derived endotoxin stimulates factor VIII secretion from endothelial cells. Implications for hypercoagulability in cirrhosis. J Hepatol. 2017;67:950-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 228. | Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. 1988;8:232-236. [PubMed] [Cited in This Article: ] |
| 229. | Shibayama Y. Sinusoidal circulatory disturbance by microthrombosis as a cause of endotoxin-induced hepatic injury. J Pathol. 1987;151:315-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 230. | Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1879] [Cited by in F6Publishing: 1950] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 231. | Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 232. | Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 854] [Cited by in F6Publishing: 901] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 233. | Liu WT, Jing YY, Gao L, Li R, Yang X, Pan XR, Yang Y, Meng Y, Hou XJ, Zhao QD, Han ZP, Wei LX. Lipopolysaccharide induces the differentiation of hepatic progenitor cells into myofibroblasts constitutes the hepatocarcinogenesis-associated microenvironment. Cell Death Differ. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 234. | Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 910] [Cited by in F6Publishing: 906] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 235. | Nakagawa H, Maeda S. Inflammation- and stress-related signaling pathways in hepatocarcinogenesis. World J Gastroenterol. 2012;18:4071-4081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 102] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 236. | Ma-On C, Sanpavat A, Whongsiri P, Suwannasin S, Hirankarn N, Tangkijvanich P, Boonla C. Oxidative stress indicated by elevated expression of Nrf2 and 8-OHdG promotes hepatocellular carcinoma progression. Med Oncol. 2017;34:57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 237. | Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1318] [Cited by in F6Publishing: 1455] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 238. | Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, Ozawa T, Nakamura M, Kumagai M, Watashi K, Taketo MM, Aoki T, Narumiya S, Oshima M, Arita M, Hara E, Ohtani N. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017;7:522-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 239. | Piñero F, Vazquez M, Baré P, Rohr C, Mendizabal M, Sciara M, Alonso C, Fay F, Silva M. A different gut microbiome linked to inflammation found in cirrhotic patients with and without hepatocellular carcinoma. Ann Hepatol. 2019;18:480-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 240. | Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1230] [Cited by in F6Publishing: 1330] [Article Influence: 133.0] [Reference Citation Analysis (2)] |
| 241. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 701] [Article Influence: 53.9] [Reference Citation Analysis (1)] |
| 242. | Zhang Z, Zhai H, Geng J, Yu R, Ren H, Fan H, Shi P. Large-scale survey of gut microbiota associated with MHE via 16S rRNA-based pyrosequencing. Am J Gastroenterol. 2013;108:1601-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 243. | Korda D, Deák Kiss G, Gerlei Z, Kóbori L, Görög D, Fehérvári I, Piros L, Máthé Z, Doros A. Management of Portal Hypertension After Liver Transplantation. Transplant Proc. 2017;49:1530-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 244. | Unger LW, Berlakovich GA, Trauner M, Reiberger T. Management of portal hypertension before and after liver transplantation. Liver Transpl. 2018;24:112-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 245. | Parrilli G, Abazia C, Sarnelli G, Corsaro MM, Coccoli P, Viglione L, Cuomo R, Budillon G. Effect of chronic administration of tacrolimus and cyclosporine on human gastrointestinal permeability. Liver Transpl. 2003;9:484-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 246. | Gabe SM, Bjarnason I, Tolou-Ghamari Z, Tredger JM, Johnson PG, Barclay GR, Williams R, Silk DB. The effect of tacrolimus (FK506) on intestinal barrier function and cellular energy production in humans. Gastroenterology. 1998;115:67-74. [PubMed] [Cited in This Article: ] |
| 247. | Wu ZW, Ling ZX, Lu HF, Zuo J, Sheng JF, Zheng SS, Li LJ. Changes of gut bacteria and immune parameters in liver transplant recipients. Hepatobiliary Pancreat Dis Int. 2012;11:40-50. [PubMed] [Cited in This Article: ] |
| 248. | Kato K, Nagao M, Miyamoto K, Oka K, Takahashi M, Yamamoto M, Matsumura Y, Kaido T, Uemoto S, Ichiyama S. Longitudinal Analysis of the Intestinal Microbiota in Liver Transplantation. Transplant Direct. 2017;3:e144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 249. | Doycheva I, Leise MD, Watt KD. The Intestinal Microbiome and the Liver Transplant Recipient: What We Know and What We Need to Know. Transplantation. 2016;100:61-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |