INTRODUCTION
With approximately 1.0 million new cases diagnosed in 2018, gastric cancer (GC) is currently the 5th most common cancer worldwide[1]. However, it is the third leading cause of cancer-related death as the majority of patients are diagnosed at an advanced stage. The detection of GC at an early stage is crucial, as the 5-year survival rate of GC patients is significantly better when it is managed in the early stages[2]. Furthermore, therapeutic endoscopy techniques, such as endoscopic mucosal resection and endoscopic submucosal dissection, have been successfully applied to cure patients suffering from early GC, thereby allowing the patients to avoid the risks associated with surgery.
Diagnosing the disease at an early stage is challenging, as many GC patients are asymptomatic in the early stages and some patients with advanced-stage disease may have no alarming features[3-5]. As the development of GC is usually preceded by the decades-long progression of a precancerous lesion[6] and the progression of GC from the early to advanced stages takes an average of 44 mo[7], it is important to identify high-risk individuals and to offer them a proper surveillance program.
Current evidence shows that individuals with gastric dysplasia, high-stage gastritis [according to the Operative Link on Gastritis Assessment (OLGA) or Operative Link for Gastric Intestinal Metaplasia (OLGIM)], severe endoscopic gastric atrophy (EGA), extensive gastric intestinal metaplasia (GIM) and the incomplete subtype of GIM are at increased risk for GC development[8-12]. In addition, it is reported that Helicobacter pylori (H. pylori) eradication does not reduce the GC risk of these individuals[13]. Thus, individuals with these conditions are at high risk for developing GC and should be identified and offered proper surveillance.
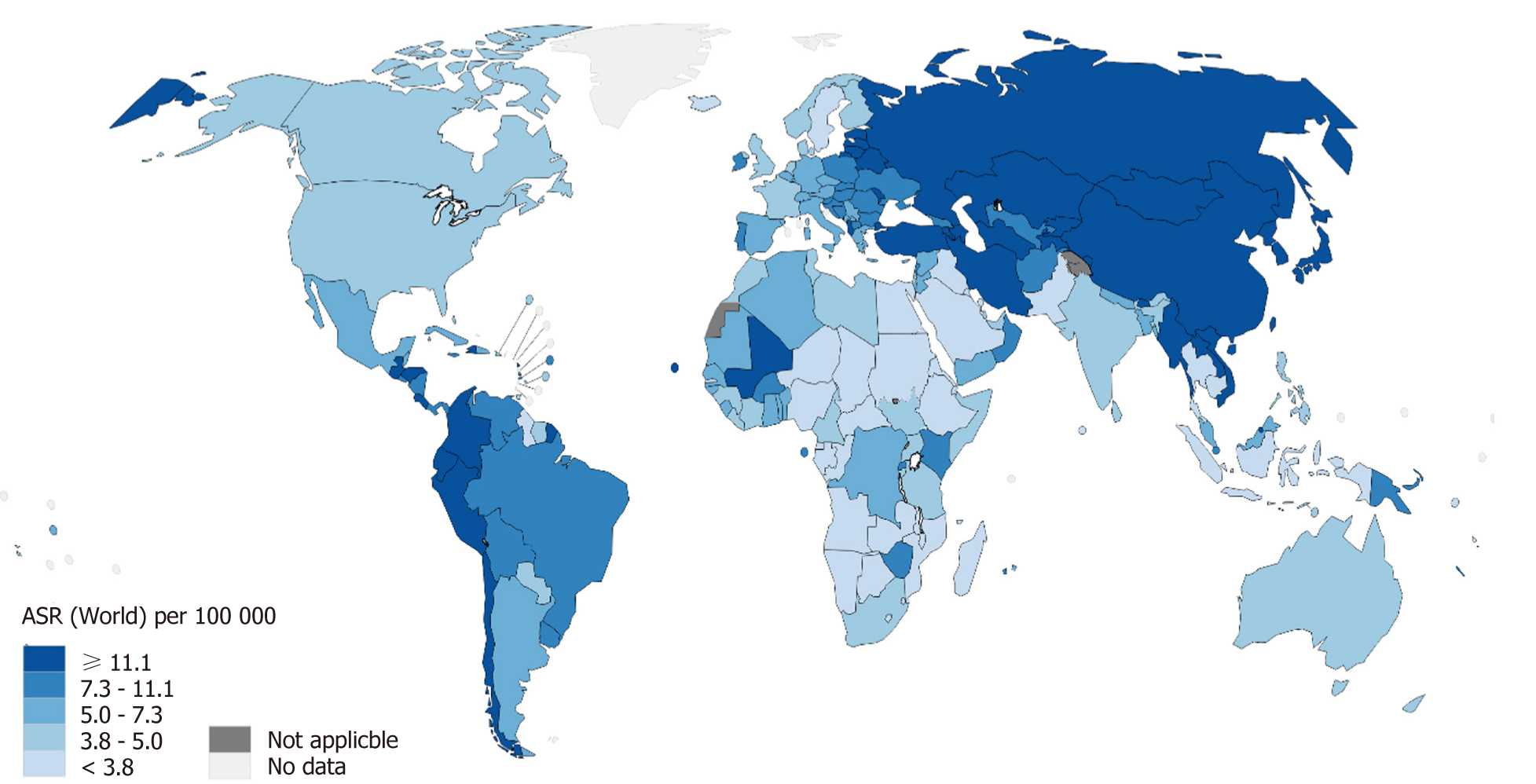
There are several approaches to identifying high-risk individuals, including noninvasive methods, esophagogastroduodenoscopy (EGD) and histology. The main approach in western countries is histology-based while that in Eastern countries with high prevalence of GC is endoscopy-based. One important issue that affects these approaches is cost-effectiveness. Another important and challenging issue, which has not received much attention in current literature, is local resources, as there are some regions within which the risk of GC is high but resources are limited (Figure 1). In this review, we discuss the current understanding and approaches from western and eastern perspectives and the possibility of implementing an integrated, resource-sensitive approach.
Figure 1 The estimated age-standardized incidence rate for gastric cancer in 2018 (both sexes, all ages)[1].
IDENTIFYING HIGH-RISK INDIVIDUALS FOR GASTRIC CANCER DEVELOPMENT
There are several approaches to identify subjects at high risk for GC development, including noninvasive methods, EGD and histology. A histological examination is traditionally required for the diagnosis of precancerous gastric lesions. However, endoscopy, especially with modern endoscopic technologies, and biomarkers have been reported to have acceptable accuracy in the diagnosis of precancerous gastric lesions. Currently, the main approach in Western countries is histology-based while that in Eastern countries with a high prevalence of GC is endoscopy-based.
Histological approach
Gastric atrophy and the risk of GC development: One of the first systematic reviews to describe the risk of GC in patients with histologically diagnosed gastric atrophy was recently published[14]. This study, which consisted of 5 studies from Europe and three studies from Asia, found that the annual incidence of GC among patients with gastric atrophy ranged from 0.1% to 0.5%. In addition, it found that patients with gastric atrophy in Asia had a higher risk of GC in comparison to those in Europe.
Atrophy of the gastric mucosa was traditionally defined as the loss of glands[15]. However, agreement among pathologists on the recognition and grading of gastric atrophy has remained elusive when using this definition[16]. Subsequently, it was re-defined as the loss of appropriate glands, which led to a high level of agreement among gastrointestinal pathologists trained in different cultural contexts[17]. In addition, although the updated Sydney system of gastritis classification has been accepted worldwide, it lacks prognostic information. Consequently, the OLGA gastritis staging system, which is based on the understanding that the risk levels of GC are directly related to the extent and severity of gastric atrophy, was proposed[18]. This atrophy-based staging system combines the antral and oxyntic mucosal atrophy scores using the updated Sydney system visual analog scales with the aim of offering clinicians information about the risk of GC[19]. A multi-center study has been conducted to test the correlation between the stages of gastritis, classified according to this staging system, with the risk of GC in different populations[20]. This study showed that the OLGA stage of gastritis mirrored the incidence of GC in populations with different levels of GC risk. Observational studies from populations with different levels of GC risk also consistently showed that neoplastic gastric lesions clustered in high stages (i.e., stage III and IV) OLGA gastritis, supporting the potentially useful application of the system in clinical practice[21,22]. In a prospective cross-sectional study of 439 consecutive dyspeptic outpatients in Italy who underwent endoscopy with standardized biopsy sampling, benign conditions were consistently clustered in OLGA stages 0-II, whereas all neoplastic gastric lesions were clustered in OLGA stages III-IV[21]. In another cross-sectional study to assess the distribution of the OLGA gastritis stages of 280 non-ulcer dyspeptic patients in Vietnam, neoplastic lesions were found to cluster in patients with OLGA stages III-IV as opposed to OLGA stages 0-II[22]. A meta-analysis of 6 case-control studies and 2 cohort studies from Europe and Asia also showed that there was a significant association between OLGA stages III-IV and GC[12]. Recently, two large and long-term follow-up Italian studies confirmed that this staging system reliably predicted the risk of GC development[23,24]. The Kyoto global consensus on H. pylori gastritis strongly recommends the use of the OLGA and OLGIM grading systems for GC risk stratification[10].
In summary, the application of the new definition of gastric atrophy as the loss of inappropriate glands has led to a higher agreement in the assessment of gastric atrophy. The risk of GC is significantly associated with the extent and severity of gastric atrophy, and the OLGA staging system has been shown to be correlated with risk of GC in long-term cohort studies.
GIM and the risk of GC development: A nationwide cohort study in the Netherlands reported that the overall annual incidence of GC development in patients with GIM was 0.25% at 5 years[25]. A population-based cohort study in Sweden reported that approximately 1 in 39 patients with GIM who underwent EGD with gastric biopsy for non-malignant indications developed GC within 20 years[26]. In a recent systematic review consisting of 9 cohorts (4 from the United States, 4 from Western European countries and 1 from South Korea), the incidence of GC among patients with GIM ranged from 0.38 to 17.08 per 1000 person-years[14]. However, the majority of the included cohorts reported incidence rates between 1.26 and 4.10 per 1000 person-years.
GIM subtypes and the risk of GC development: GIM can be classified into complete and incomplete subtypes. The complete subtype (type I) is characterized by goblet cells scattered among columnar absorptive cells. The incomplete subtype is characterized by goblet cells interspersed among mucin-secreting columnar cells, which can be further divided into type II (sialomucin-secreting cells; presence of Paneth cells) and type III (sulphomucin-secreting columnar cells; absence of Paneth cells) by high-iron diamine staining[27].
A cancer registry-based study in Slovenia reported that the cumulative incidence of GC in patients previously diagnosed with GIM was 1.3% in complete GIM type I, 2.8% in incomplete GIM type II and 9.8% in incomplete GIM type III[27]. A Spanish study reported that GC developed in 18.2% of patients with incomplete GIM and in only 0.9% of patients with complete GIM after a mean follow-up period of 12.8 years[28]. This study showed—based on a multivariate analysis—that incomplete GIM was associated with the highest risk of developing GC (Hazard ratio 11.3, 95%CI: 3.8-33.9). In South Korea, GIM subtyping was not found to play a major role in the prediction of GC development[29]. However, these observations were derived from cross-sectional studies and results from follow-up studies are awaited.
In a literature review on the association between incomplete GIM and GC in studies published between 1980 and 2010, 13 of the 14 cross-sectional studies and 6 of the ten follow-up studies found a statistically significant association between incomplete GIM and the risk of GC[30]. Among the studies that reported the magnitude of the risk, the relative risk (RR) of GC in patients with incomplete GIM was 4- to 11-fold higher than that in patients with complete GIM or without incomplete GIM.
In a recent retrospective cohort study in Thailand, 91 patients with GIM were recruited for surveillance EGD every 6-12 mo until a diagnosis of GC was made or the planned 5‐-year follow-up period was completed[31]. By the end of the study, incomplete GIM and male sex were found to be significantly associated with the development of gastric neoplasia. None of the 81 patients with complete GIM at the time of recruitment developed GC. In contrast, 5 of the 10 patients exhibiting incomplete GIM progressed to high-grade dysplasia (HGD) and GC.
In summary, most of the scientific evidence supports that incomplete GIM is a risk factor for GC.
The extent of GIM and the risk of GC development: The extent of GIM is a very important risk factor for the development of GC. There are four patterns of GIM distribution[32]. The “focal” GIM pattern consists of scattered foci, mostly in the lesser curvature and incisura. The “antrum-predominant” GIM pattern involves most of the antrum and incisura angularis. The “magenstraße” GIM pattern spreads throughout the lesser curvature from the cardia to the pylorus, also involving the greater curvature of the pre-pyloric antrum. Finally, the “diffuse” GIM pattern involves the entire gastric mucosa, with the exception of the fundic areas. In comparison to the focal or antral-predominant GIM patterns, the magenstraße GIM and diffuse GIM patterns are associated with a 5.7-fold and 12.2-fold increase in the risk of GC development, respectively. An Italian study also reported that the extension of GIM was associated with the risk of GC, and that ≥ 20% baseline GIM extension was a sensitive first screening parameter for identifying subjects with a higher risk of GC[33]. Recently, a Japanese cohort study followed 573 patients for 6.2 years and found that GC developed in 21 patients[34]. The cumulative 5-year incidence of GC was 1.5% in patients without GIM, 5.3% in those with GIM limited to the antrum and 9.8% in those with GIM in the corpus.
The OLGIM staging system: The proposed OLGIM staging system is based on the OLGA staging system, which provides clinically relevant information about GIM[35]. The main parameter of this system is the severity and extent of GIM, rather than gastric atrophy. The rationale for this system is that previous studies reported that the degree of interobserver agreement in the assessment of gastric atrophy was lower in comparison that for the assessment of GIM[36,37].
A prospective multicenter study conducted in the Netherlands found that replacement of gastric atrophy by GIM in the staging of gastritis considerably increased interobserver agreement while the correlation with the severity of gastritis remained at least as strong[35]. A systematic review and meta-analysis on the association between the OLGIM gastritis stage and the GC risk has been recently published[12]. The meta-analysis, which was based on three case-control studies from Eastern countries, showed that the GC risk was significantly higher among patients with OLGIM stage III-IV [Odds ratio (OR) = 3.99; 95%CI: 3.05-5.21: P < 0.001]. The only prospective cohort study, which was conducted in the Netherlands, found that patients with OLGIM stage III-IV were more likely to develop HGD (RR = 16.67; 95%CI: 0.8-327.53).
Gastric dysplasia and the risk of GC development: Gastric dysplasia is usually classified as low or high grade[38]. A nationwide cohort study in the Netherlands reported that the annual incidence of GC in patients with LGD and HGD within 5 years after the diagnosis was 0.6% and 6%, respectively[39]. A recent population based cohort study in Sweden reported that 1 in 19 patients with dysplasia progressed to GC within 20 years, although no differentiation was made between those with low-grade dysplasia (LGD) or HGD[26]. Notably, there is a remarkably histological discrepancy between biopsy specimens and material obtained from endoscopic resection. A recent study from Japan found that a substantial proportion of biopsy-proven gastric LGD specimens were diagnosed as GC after endoscopic resection[40]. The strategy for managing patients with gastric dysplasia is, therefore, more straightforward in comparison to that for gastric atrophy and GIM. The resection of endoscopically visible dysplastic lesions is now recommended worldwide, regardless of the grade of dysplasia[9,11,41]. However, a considerable number of patients have endoscopically invisible gastric dysplasia. These patients are still at high risk and need to be strictly followed up[22,42]. A recent literature review reported that LGD persisted in 19% to 50% of patients and that the risk of GC development in these patients ranged from 0% to 23% over 10-48 mo[11]. Regarding endoscopically invisible HGD, immediate endoscopic reassessment with extensive biopsy sampling and surveillance at 6- to 12-mo intervals is mandatory. Furthermore, the disappearance or assumed disappearance of dysplastic lesions, as assessed by follow-up endoscopic biopsy, does not rule out possible progression to invasive GC[9,41].
Endoscopic approaches
White light endoscopy: Gastric atrophy: The endoscopic diagnosis of gastric atrophy based on good visualization of the submucosal vessels, even in the hands of experienced endoscopists, is not reliable[43]. The sensitivity and specificity were only 61.5% and 57.7%, respectively in the antrum; and 46.8% and 76.4%, respectively in the corpus. However, the assessment of EGA according to the Kimura-Takemoto classification has been consistently confirmed to have a good correlation with histological gastric atrophy[22,44]. In addition, several long-term cohort studies confirmed its value in predicting the risk of GC development in subjects with and without H. pylori infection, as well as after the successful eradication of H. pylori[8,34,45].
The key point in assessing EGA according to the Kimura-Takemoto classification is to identify the location of the so-called endoscopic atrophic border of the stomach[46]. Based on the location of the endoscopic atrophic border, an endoscopic classification of gastric atrophy pattern was proposed which consists of two main types: Closed type (C-type) and open type (O-type). These two types are further subdivided in into three C-types (C-1, C-2 and C-3) and three O-types (O-1, O-2 and O-3). The severity of EGA is often classified into three grades: Mild (C-1, C-2), moderate (C-3, O-1) and severe (O-2, O-3)[8,22,34,45]. Our previous study in Vietnam showed that the severity of EGA was significantly correlated with the OLGA gastritis stage[22]. As EGA assessment has not been widely applied in Western countries and it was unclear whether the EGA findings were correlated with histological atrophy in Western patients, another study was conducted in the United Kingdom[44]. In this study, EGA was compared with histological atrophy using the updated Sydney classification system. The strength of agreement on the extent of atrophy between the endoscopic and histological findings was good, with a weighted kappa value of 0.76. In addition, the strength of agreement between endoscopic and histological atrophy, as assessed by cancer risk-oriented grading (i.e., none, limited atrophy in antrum and angulus or pan-atrophy) was good, with a kappa value of 0.81.
Several studies have consistently confirmed that the severity of EGA at baseline is associated the risk of GC development. A prospective cohort study that followed 1,603 consecutive Japanese patients with benign gastroduodenal diseases for an average of 8 years found that GC only developed in patients with H. pylori infection, and the RR of GC in patients with severe EGA at baseline was 4.9 times higher than that of those with no or mild EGA at baseline[8]. A recent cohort study that included 573 Japanese patients who underwent follow-up endoscopy after successful H. pylori eradication therapy found that the cumulative 5-year incidence of GC was 0.7%, 1.9%, and 10% in patients with none/mild, moderate, and severe EGA, respectively[34]. Based on the current evidence, the Kyoto global consensus on gastritis suggested that EGA assessment can be used initially in regions with proven expertise in EGA assessment; however, histological confirmation is still recommended[10].
GIM: The endoscopic diagnosis of GIM by standard endoscopy is also unreliable even in the hands of experienced endoscopists. A study in Korea reported that the sensitivity and specificity of endoscopy for the diagnosis of GIM were 24.0% and 91.9%, respectively for the antrum; 24.2% and 88.0%, respectively for the body[47]. A another study in Greece reported that the sensitivity and specificity of endoscopy for the diagnosis of GIM and LGD were 74.6% and 94%, respectively[48].
Gastric dysplasia: The endoscopic diagnosis of gastric dysplasia by endoscopy is even more unreliable, especially for LGD. In a Finnish study that included a series of 101 patients with histologically diagnosed gastric dysplasia graded into three categories (mild, moderate, or severe), all severe dysplastic lesions were detected in visible lesions but 3 (22%) moderate dysplastic lesions and 57 (68%) mild dysplastic lesions were endoscopically invisible and were only detected in random biopsy specimens[42]. Our previous study of 280 dyspeptic patients in Vietnam identified LGD in 7 (2.5%) patients and all of these dysplastic lesions were endoscopically invisible[22].
IEE and magnifying endoscopy: Several studies have shown the significantly higher accuracy of IEE and magnification endoscopy in the diagnosis of gastric atrophy, GIM, dysplasia and GC in comparison to white light endoscopy (WLE). A multicenter prospective randomized study in the Asia-Pacific region was conducted to compare narrow-band imaging (NBI) and high-definition WLE (HD-WLE) in the detection of GIM[49]. This study found that a significantly higher proportion of patients with GIM was detected by NBI compared with HD-WLE (17.7% vs 7.7%, P < 0.001). Similarly, a recent prospective blinded trial in the United States reported higher proportions of patients with GIM were detected by NBI (65%) and mapping (76%) vs HD-WLE (29%) (P < 0.005 for both comparisons). In addition, there were also higher proportions of sites with GIM detected with NBI (53%) and mapping biopsies (67%) than HD-WLE (28%) (P < 0.005 for both comparisons). A recent consensus developed by expert endoscopists in Asia strongly recommends to use IEE in addition to WLE to improve the detection rate of precancerous gastric lesions[50].
The development of NBI magnifying endoscopy (NBI-ME) has helped endoscopists to better observe the gastric mucosa endoscopically. The Light Blue Crest (LBC) sign, defined as a fine, blue-white line on the crests of the epithelial surface/gyri, was found to correlate with histological evidence of GIM[51]. A recent meta-analysis on the diagnostic yield of LBC in GIM reported that the sensitivity and specificity values of this finding were 0.90 (95%CI: 0.86-0.92) and 0.90 (95%CI: 0.86-0.93), respectively[52]. The practicality of NBI-ME for gastritis staging has been reported in Japan[53]. In this study, the NBI-ME score classification was established from images obtained beforehand, and then biopsy specimens taken from the observed areas were scored according to histological findings. The NBI-ME and histological stages were assessed using a combination of scores for the antrum and corpus, and were divided into low-risk and high-risk groups. This study found that the agreement between NBI-ME and histological scores was 69.1% for the antrum and 72.7% for the corpus, and that between the high- and low-risk groups was 89.1%. NBI-ME procedures, however, are generally time-consuming and require appropriate training and gastroscope. Therefore, it could not be widely used in daily practice.
A simplified classification system using NBI without magnification has been proposed and validated in Western countries, and its accuracy and reliability in the diagnosis of GIM and dysplasia have been demonstrated[54]. According to the simplified classification, pattern A (regular vessels with circular mucosa) was associated with normal histology (accuracy 83%; 95%CI: 75%-90%), pattern B (tubulo-villous mucosa) was associated with GIM (accuracy 84%; 95%CI: 77%-91%); and pattern C (irregular vessels and mucosa) was associated with dysplasia (accuracy 95%; 95%CI: 90%-99%). The reproducibility of these patterns was high (k = 0.62). Non-experienced endoscopists showed lower agreement (k = 0.6 vs k = 0.75) and accuracy (74 % vs 86%) than experienced endoscopists, suggesting that appropriate training is required. The real-time validity of HD-WLE with and without NBI in the diagnosis of precancerous gastric lesions and the possibility of deriving a classification for the endoscopic grading of GIM (EGGIM), a score (0-10) resulting from the sum of endoscopic assessments of GIM, have been reported[55,56]. In a later multicenter prospective study, NBI based on the simplified classification was found to significantly increase sensitivity in the diagnosis of GIM and gastric dysplasia (87% vs 53% and 92% vs 74%, respectively). The area under the curve (AUC) of the receiver operating characteristic curve for EGGIM in the diagnosis of extensive GIM was 0.98[55]. Another study was conducted by the same group to externally validate the EGGIM classification[56]. Consecutive patients underwent HD-WLE followed by NBI to estimate the EGGIM classification. The score was 0, 1, or 2 for no GIM, ≤ 30%, or > 30% of the mucosa, respectively, in five areas (lesser and greater curvature of both the antrum and corpus, and incisura). If GIM was endoscopically suspected, targeted biopsies were performed. If GIM was not noticeable, random biopsies were performed according to the Sydney system to estimate the OLGIM stage. For the diagnosis of high-stage OLGIM gastritis, the AUC was 0.96 (95%CI: 0.93-0.98) and the sensitivity and specificity using a cutoff of > 4 were 89% and 95%, respectively. This study shows the promise of the endoscopic approach in determining the risk of GC development without the need for biopsies. Further studies in other populations should be performed to validate the results.
Non-invasive approaches
Serum pepsinogen (PG) is the most intensively investigated biomarker for precancerous gastric lesions and GC. Serum PG consists of two distinct types, namely, pepsinogen I (PGI) and pepsinogen II (PGII). PG I is exclusively produced by chief and mucous neck cells in the fundic glands, while PG II is secreted by these cells as well as by cells in the pyloric glands and Brunner’s glands. Both of serum PGI and PGII levels initially increase on the progression of gastritis. However, as the fundic gland mucosa is reduced, PGI levels gradually decrease while PGII levels remain fairly constant. Consequently, the serum PG I/II ratio (sPGr) decreases in a stepwise manner which is closely correlated with the progression of atrophic gastritis[57]. The low serum PG I level and sPGr, therefore, reflect the severity of gastric atrophy. The measurement of serum PG I and sPGr alone or in combination with H. pylori serum antibody (HpAb) test, and/or Gastrin-17 has been investigated to identify high-risk individuals[58].
Non-invasive approaches in western countries: A study was conducted in 284 dyspeptic patients from 14 European countries to evaluate the role of sPGr as a screening test for moderate-to-severe and multifocal atrophic gastritis[59]. The best cut-off point of sPGr was 5.6, which showed 65.0% sensitivity and 77.9% specificity. Another study, which was one of a few population-based studies in Western countries, was conducted to investigate the serum levels of PGI and sPGr in asymptomatic individuals in northern Portugal, a region with high incidence of GC[60]. The participants, whose ages ranged from 40 to 79 years, were classified into a positive test group (PG I ≤ 70 ng/mL and sPGr ≤ 3) and a negative test group (all others). All participants with a positive test result and a consecutive random sample of participants with negative test results underwent EGD and were followed up in 5 years. In the detection of GC development during the follow-up period, this test showed 67% sensitivity, 47% specificity, a positive predictive value of 2% and a negative predictive value of 99%. Recently, a study investigating the cost-effectiveness of population screening strategies based on biomarkers and endoscopy was conducted in the United States[61]. This study found that although one-time serum PG testing at 50 years of age could prevent one in four cases of GC among men, it was not of high value in improving the outcomes of GC. However, targeting the high-risk group (i.e., male, smokers of > 50 years of age) could be a cost-effective approach for reducing GC-related mortality.
Non-invasive approaches in eastern countries: A meta-analysis was conducted to evaluate the prediction of GC development by sPGr, HpAb tests, and a risk-prediction model based on these two tests[62]. This model categorized patients into four groups: low risk (A: HpAb -, sPGr-), moderate risk (B: HpAb+ and sPGr-), and high risk (C: HpAb+ and sPGr+; D: HpAb-and sPGr+). This study included 9 prospective cohorts from Eastern Asian countries with a total of 33741 asymptomatic participants in GC screening programs. The mean ages of the participants at enrollment ranged from 45 to 57 years, while the mean follow-up ranged from 3.9 to 14 years. This study found that adults with a positive sPGr test had an approximately four-fold higher risk of developing GC than those with a negative test. In addition, the four-risk-group prediction model had the potential to stratify middle-aged presumptively healthy adults according to the risk of GC development.
Another meta-analysis, which consisted of over 30000 individuals across 13 different western and eastern countries, was conducted to assess the accuracy of serum PG testing in the diagnosis of GC and chronic atrophic gastritis (CAG)[63]. This study showed that serum PG testing had 69% sensitivity and 73% specificity in the diagnosis of GC, and 69% sensitivity and 88% specificity in the diagnosis of CAG. However, there were significant variations in serum PG measurement methods and cut-off values of PGI and sPGr among the included studies.
One other meta-analysis was conducted to assess the combination of sPGr, gastrin-17 and HpAb tests in the diagnosis of CAG. As Gastrin-17 is only secreted by the G cells of the antral mucosa, a low serum Gastrin-17 level in combination with a positive HpAb test would indicate the presence of antral CAG; and a combination of sPGr, Gastrin-17 and HpAb tests would help to detect the presence and site of CAG[64]. This meta-analysis included 20 eligible studies with a total of 4241 subjects. The median prevalence of CAG across the included studies was 27%. The test sensitivity, specificity, negative predictive value and positive predictive value for the diagnosis of CAG were 74.7%, 95.6%, 91% and 86%, respectively. However, that only six of the 20 included studies considered moderate-to-severe gastric atrophy as a gold standard might have adversely affected the practical usefulness of the study results.
There may be some exceptions regarding the use of the serum PG test in predicting the development of GC in different populations. Notably, a study from Singapore was conducted to examine whether racial differences in the prevalence of H. pylori infection and serum PG level could account for racial differences in the incidence of GC[65]. This study found that Indian subjects had a lower incidence of GC but a significantly higher prevalence of low PG in comparison to Chinese and Malay subjects. The study highlighted the limited usefulness of serum PG testing in the Indian population. In addition, the rPGr level has been recently reported to return to the normal range in Japanese patients after successful H. pylori eradication[66]. Thus, test results may be misleading in populations in which a large percentage of participants have undergone H. pylori eradication (intended and unintended).
LESSONS FROM WESTERN AND EASTERN PERSPECTIVES AND THE POSSIBILITY OF DEVELOPING AN INTEGRATED RESOURCE-SENSITIVE APPROACH TO IDENTIFY HIGH-RISK INDIVIDUALS
Pre-endoscopic risk assessment
The detection of high-risk precancerous gastric lesions generally requires endoscopy with biopsy. However, there are demographic and clinical features that are helpful for predicting the presence of these lesions, including ethnicity, gender, age, family history of GC, H. pylori status and serum PG level[4,50,67,68].
A recent review found that individuals in the United States who were immigrants from high-risk regions (East Asia, Russia, or South America) had a higher risk of GC development in comparison to other Americans[68]. In multi-ethnic Southeast Asia countries, it was reported that some ethnic groups, including Chinese, Batak and Minahasanese, had higher risk in comparison to the other ethnic groups[4]. H. pylori infection and having first-degree relatives diagnosed with GC have been reported as important risk factors for GC development worldwide[8,67,68]. In addition, male sex, smoking and advanced age are also associated with a higher risk of developing GC[61,69,70]. The age threshold may differ depending on the GC risk level in each region: it is approximately 40 years in high-risk regions and approximately 50 years in low-risk regions[67-69]. In subjects with family history of GC, the age threshold is approximately 10 years younger than the age of the first-degree relative at the diagnosis or 50 years of age (whichever is earlier)[68]. The application of the PG test in subjects with clinical high-risk characteristics has been shown to be promising but, as mentioned above, should be locally validated.
In summary, pre-endoscopic risk assessment is possible and it is helpful for selecting subjects with a high pre-test probability for a possibly cost-effective approach, especially in intermediate- and low-risk regions.
Approaches for asymptomatic individuals
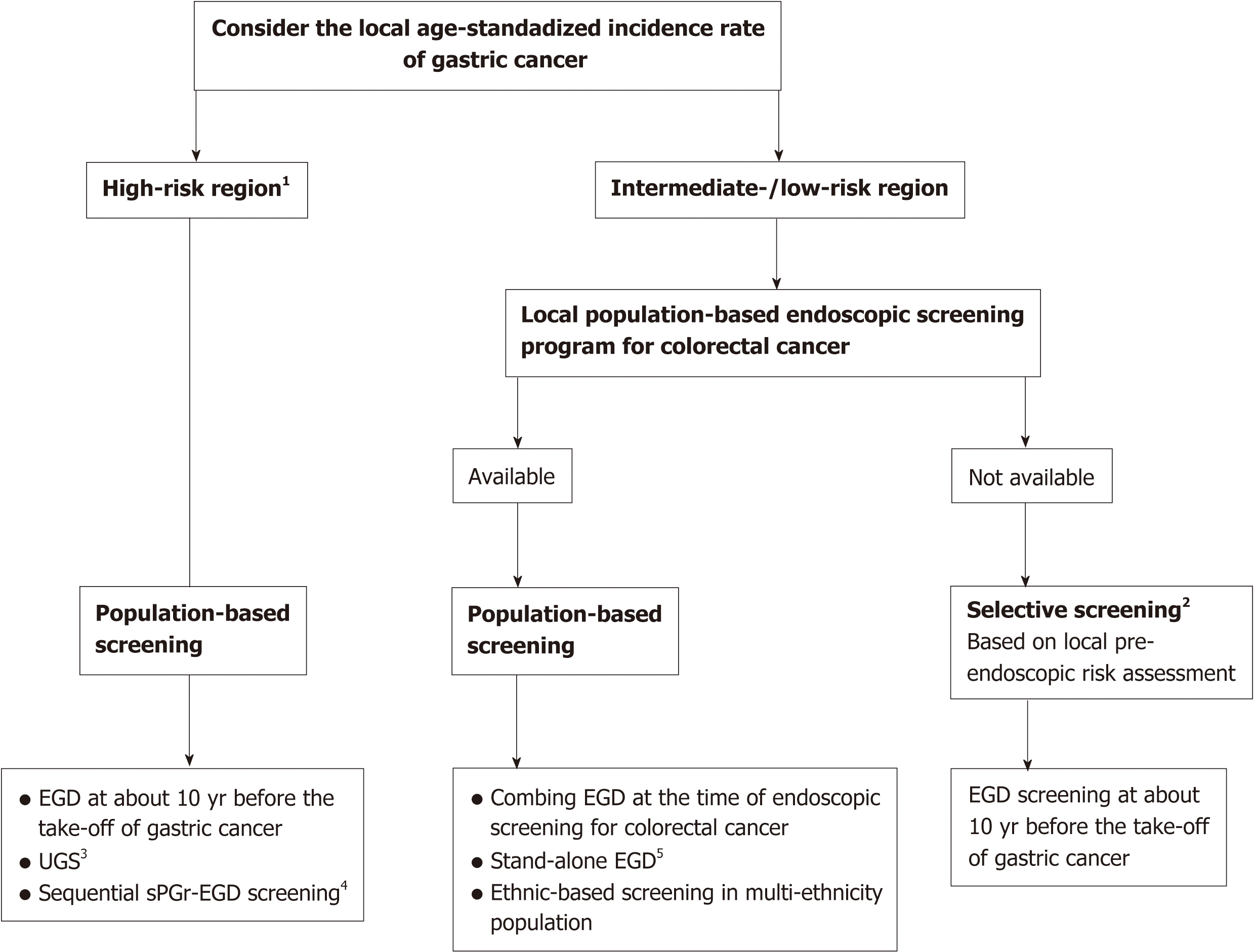
The two key issues in selecting applicable approaches for asymptomatic individuals are the ability to reduce GC mortality, especially in high-risk regions, and the cost-effectiveness of the approach, especially in low- and intermediate-risk regions. The possibly applicable approaches based on current evidence worldwide are summarized in Figure 2.
Figure 2 Possibly cost-effective approaches for identifying asymptomatic individuals with a high risk of gastric cancer development.
1The age-standardized incidence rate is greater than 20 per 100000. 2Applying selective screening for subjects with high-risk pre-endoscopic features. 3Not yet shown to reduce gastric cancer mortality. 4The performance of serum PG I/II ratio should be locally validated. 5Cost-effectiveness should be considered based on the local age-standardized incidence and the cost of esophagogastroduodenoscopy. In multi-ethnic populations, ethnicity-based screening for high-risk ethnic groups should be considered. EGD: Esophagogastroduodenoscopy; UGS: Upper gastrointestinal series; sPGr: Serum PG I/II ratio.
Approaches for asymptomatic individuals in eastern countries: At present, population-based screening programs have only been applied in some Asian countries with a high incidence of GC. In South Korea, the national cancer screening program for GC by EGD or upper gastrointestinal series (UGS) was launched in 1999 as a Medicaid program, but it has since expanded to all subjects of ≥ 40 years of age since 2005[71]. A recent nested case-control study was conducted to assess the effectiveness of this program in reducing GC mortality. The study used data from 16584283 subjects who participated in the screening program since 2002, and found that the subjects who received EGD were less likely to die from GC in comparison to those who received UGS[72]. In Japan, a recent cohort study reported that the survival rate and GC mortality among Japanese patients with screening-detected GC were not significantly different from those with interval GC in the annual endoscopic screening program, which also suggested the benefit of endoscopic screening in reducing GC mortality[73]. The current Japanese guidelines for GC screening recommend the use of EGD or UGS for population-based and opportunistic screenings, but emphasize that the former method is more sensitive than the later[74]. Serum PG and HpAb tests are currently not recommended for population-based screening in Japan due to insufficient evidence on the reduction of GC mortality. In China, there have been no national screening programs for GC but some screening programs have been applied in high-risk regions of the country. Among these programs, the so-called two-step examination (i.e., the sequential sPGr-EGD screening method) has been reported to have reasonable cost-effectiveness and good participant compliance[5]. A recent meta-analysis and systematic review, which included 6 cohort studies and 4 nested case-control studies from high-risk Asian countries (2 from South Korea, 2 from China, and 6 from Japan) with 342013 individuals, has been conducted to investigate how endoscopic screening affected the incidence of GC or GC mortality. This study found that endoscopic screening may reduce GC mortality, regardless of the incidence of GC in the included populations. The subgroup analysis showed significant reductions in GC mortality after endoscopic screening in comparison to no screening[75].
In other Asian countries, there have been no national screening programs. We recently conducted a survey about the management of H. pylori and GC across 9 South-East Asian countries[4], and found that most lesions were diagnosed in an advanced stage and that the prognosis of GC patients was very poor. Resource limitations are among the most challenging issues for countries with limited resources but a high prevalence of GC. In Singapore’s multi-ethnic population, the risk in Chinese is higher in comparison to Malaysians and Indians. A cost utility analysis was conducted to determine whether endoscopic screening for GC would be cost-effective and to better define the high-risk group[69]. This study found that screening of the high-risk group of Chinese men (age-standardized rate, 25.9/100000) from 50-70 years of age was highly cost-effective.
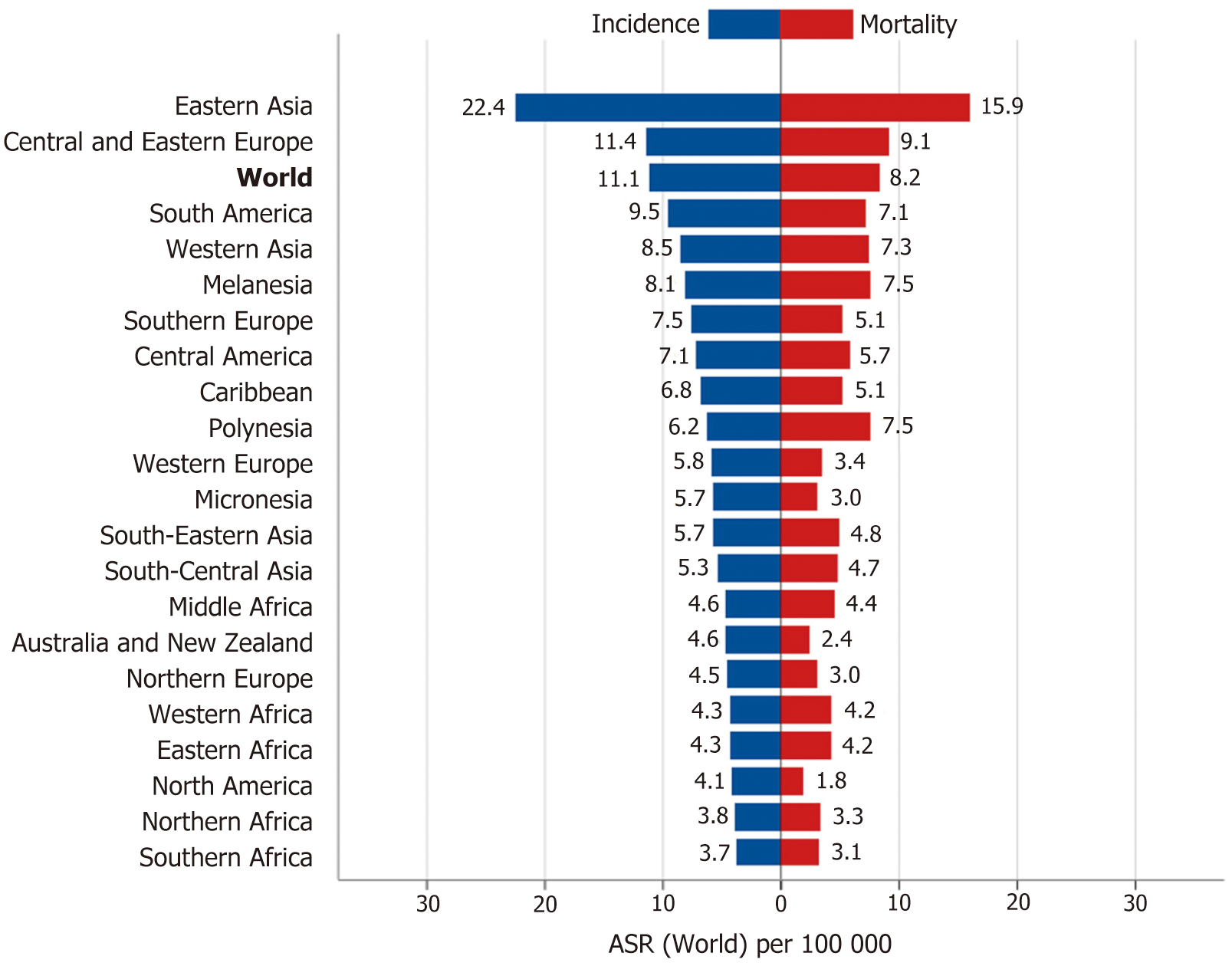
Approaches for asymptomatic individuals in western countries: Most western countries have low or intermediate GC risk (Figure 3). Thus, cost-effectiveness is the main issue concerning the selection of a suitable approach.
Figure 3 The age-standardized incidence and mortality rates of gastric cancer[1].
ASR: Age-standardized rate[1].
The longest follow-up study using serum PG screening tests for GC detection in the West was conducted in the northern part of Portugal, the area with the highest incidence of GC in Western Europe[76]. This cohort included 5913 individuals, of 40-74 years of age, who were subjected to the PG test (PGI = 70 ng/mL and sPGr ≥ 3). This study found that the PG test was suboptimal as a screening test for GC as its sensitivity was only 35% at the initial stage and 58% after 3 years of follow-up.
National screening programs for colon cancer have been well-developed in many western countries. Thus, in this region, there have been intensive investigations to determine a reasonable approach for combining gastric and colorectal cancer screening.
An analysis using a Markov model to determine the cost-utility of screening strategies for GC in Portugal was recently reported[77]. The three following screening strategies were compared vs no screening: Stand-alone EGD, EGD combined with a colorectal cancer screening colonoscopy after a positive fecal occult blood test or a positive serum PG test. This study found that endoscopic GC screening in Europe could be cost-effective if combined with screening colonoscopy in countries with an age-standardized rate (ASR) of ≥ 10 per 100000. Based on the cost of EGD alone (< €75), the provision of only three EGDs per patient or an ASR > 25/100000 would make stand-alone endoscopic screening cost-effective. Interestingly, this analysis of cost efficacy also supports the national endoscopic screening programs that are currently running in Japan and South Korea (ASR 27.5 and 39.6 per 100.000, respectively)[1].
The marked differences in the prevalence of GC among different ethnic groups in multi-ethnic countries may also affect the cost-effectiveness of the approach. In the United States, a recent study investigated whether selected non-cardia GC screening for members of high-risk ethnic groups was cost-effective[70]. A decision analytic Markov model was developed with the base case of a 50-year-old person of non-Hispanic white, non-Hispanic black, Hispanic, or Asian ethnicity. The cost effectiveness of a no-screening strategy (current standard) for non-cardia GC was compared with that of two endoscopic screening modalities initiated at the time of screening colonoscopy for colorectal cancer: EGD with biopsy examinations and continued surveillance, only if GIM or more severe histological findings were identified, or EGD with biopsy examinations continued every 2 years even in the absence of these histological findings. Compared with biennial and no screening, EGD screening with continued surveillance only when indicated was cost effective for non-Hispanic blacks, Hispanics, and Asians, but not for non-Hispanic whites. The cost-effectiveness was highest for Asians. A selective screening approach based on ethnicity, family history of GC and the age threshold has also been recently proposed in the United States[68].
Approaches for patients who have indications for EGD according to the current guidelines
Definitely, symptomatic patients who have indications for EGD according to the current guidelines for upper gastrointestinal symptoms are the subjects who benefit the most from opportunistic screening, regardless of the GC risk levels in their countries. However, we believe that the importance of opportunistic screening should be further emphasized in guidelines for dyspepsia and gastroesophageal reflux disease management and should always be considered whenever EGD is performed.
The combination of endoscopic and histological approaches should always be considered as it has several advantages. First, endoscopy provides a real-time assessment of the patient’s risk of GC development. As endoscopists tend to focus on endoscopic findings that explain patient symptoms, it is important to be cautious that precancerous gastric lesions and even early GCs may already exist. These subtle lesions are often not the causes of the symptom(s) and are very easy to miss[5]. Second, the results of histological examination greatly depend on the location from which the specimens are taken. New endoscopic technologies have helped to improve the endoscopist’s ability to identify subtle changes in the gastric mucosa and facilitate targeted biopsies instead of mapping biopsies, which results in a better correlation between endoscopic and histological findings[50,52,55,56].
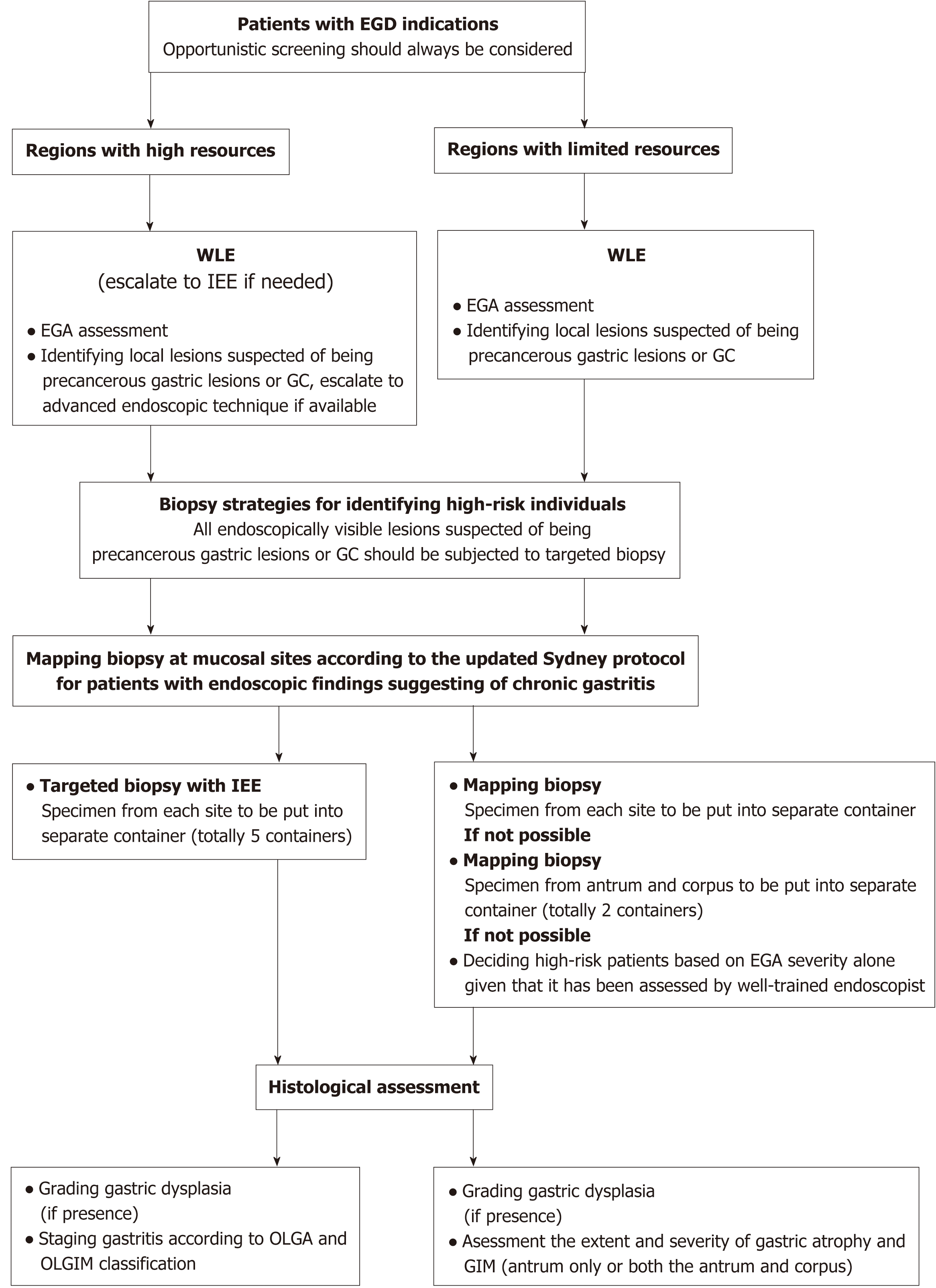
How to make this combined approach widely applicable in daily practice is a crucial issue. Obviously, an ideal approach should be accurate as well as feasible; it should not be time-consuming or require special expertise or equipment. Currently, the best evidence supports the use of IEE for endoscopy and OLGIM/OLGA grading systems for histological examination whenever applicable. However, resource limitation is an important barrier in many regions worldwide. Notably, there are several countries with a high risk of GC but limited resources, including Mongolia and Vietnam[1]. Resources may also be quite different within the same country, as reported in our recent survey of 9 Southeast Asian countries[78]. Thus, a suitable approach should not only be evidence-based but also resource-sensitive (Figure 4).
Figure 4 Resource-sensitive approaches to identifying high-risk patients who undergo esophagogastroduodenoscopy for any reason.
WLE: White-light endoscopy; EGA: Endoscopic gastric atrophy; GC: Gastric cancer; IEE: Image-enhanced endoscopy.
Endoscopic strategy: Regions with high resources: Start with WLE and escalate to IEE if necessary. Recent studies from the east and west strongly support that IEE, in addition to WLE, improves the rate at which gastric premalignant mucosal changes such as gastric atrophy and GIM are endoscopically detected[50,55,56].
Regions with limited resources (IEE is unavailable): Start with WLE and evaluate the severity of EGA. Recently, several cohort studies in Asia have shown that EAG assessment can help to effectively identify high-risk individuals with a higher risk of GC development among patients with moderate-to-severe EGA[8,34,45]. A recent global consensus on gastritis recommends that EGA be used if expert endoscopists are available[10]. We previously reported good to excellent intraobserver agreement and a moderate interobserver agreement among experienced endoscopists in the assessment of the severity of EGA[79]. A recent study reported that the interobserver agreement for the diagnosis and grading of EGA significantly improved after proper training and that it remained stable after intervention, irrespective of the endoscopist’s experience level[80]. Thus, this endoscopic assessment, which requires no additional equipment, is potentially useful in regions with limited resources. One limitation of EGA assessment that should be kept in mind is that the improvement of the severity of EGA may not be parallel with histological gastric atrophy after successful H. pylori eradication[81]. In such situations, it is necessary to obtain biopsy specimens for histological examination, especially if the baseline histological results are not available.
Biopsy strategy: Obviously, endoscopically visible gastric lesions that are suspected to represent precancerous gastric lesions and GC should be biopsied. However, the strategy of taking mapping biopsies may differ depending on the local resources (Figure 4).
In regions with high resources, the performance of a mapping biopsy at mucosal sites is recommended, according to the Sydney protocol, for patients with endoscopic findings suggestive of chronic gastritis[10]. A biopsy specimen from the angularis angular is essential in order not to downgrade the OLGA and OLGIM gastritis stage and miss high-risk individuals[82]. Specimens from each site should be put into separate container. Whenever available, IEE should be used for detection and obtaining targeted biopsy specimens[50]. Regarding histological assessment, the OLGIM and OLGA staging systems should be applied[9,10]. However, a recent South Korean study found that only about one quarter of GC patients in this high-risk population had high-stage OLGA and OLGIM gastritis. Thus, the sensitivity of these staging systems as indicators for GC in Asians, may be lower in comparison to the sensitivity reported in western populations, and local validation is required[83]. A GIM subtype analysis may be considered but is not a necessity, as the presence of incomplete GIM is significantly associated with extensive GIM, which is a documented marker that is easier to evaluate[32,33,84].
In regions with limited resources, mapping biopsy is also recommended and specimens from each site should be put into separate containers as mentioned above. In some developing countries, the cost of histological examinations is not currently reimbursed and the cost increment of additional containers may not be affordable for many self-paid patients[4]. As patients with extensive gastric atrophy and/or GIM have a higher risk of GC development[32,34,35], a reasonable option is to take 5 biopsies at mucosal sites according to the Sydney protocol and put them into 2 separate containers for the antrum and the corpus. Another option is to define high-risk patients based on EGA severity alone, if well-trained endoscopists are available.
Approach for patients with upper gastrointestinal symptoms who do not yet have indications for EGD according to the current guidelines
The indications for EGD according to the current guidelines on dyspepsia or gastroesophageal reflux disease are mainly to detect organic diseases that cause the patient’s symptoms, especially to rule out upper gastrointestinal malignancies. For a long time, the role of EGD as an opportunistic screening tool for these malignancies, including GC, has not been sufficiently emphasized. In our opinion, the benefits from opportunistic screening by EGD should also be considered whenever we decide whether patients with upper gastrointestinal symptoms should undergo EGD. There is currently no evidence on this topic and it represents an important and interesting direction for future research.