Published online Jul 14, 2019. doi: 10.3748/wjg.v25.i26.3426
Peer-review started: April 8, 2019
First decision: April 30, 2019
Revised: May 7, 2019
Accepted: June 8, 2019
Article in press: June 8, 2019
Published online: July 14, 2019
In patients with cirrhosis, hepatic encephalopathy (HE) indicates a poor prognosis despite the use of artificial liver and liver transplantation, presenting as frequent hospitalizations and increased mortality rate.
To determine predictors of early readmission and mid-term mortality in cirrhotic patients discharged after the resolution of HE.
From January to February 2018, 213 patients were enrolled in this observational study assessing all the successive patients with cirrhosis discharged from Department of Gastroenterology and Department of Infectious and Liver Diseases, Second Affiliated Hospital of Chongqing Medical University after the resolution of HE. The patients were followed for 6 mo. For each subject, demographic, clinical, and laboratory variables were assessed at the time of diagnosis of HE, during hospital stay, at discharge, and during follow-up. The primary endpoints were incidence of early readmission and mid-term mortality.
During follow-up, 65 (31%) patients experienced an early readmission. International normalized ratio (INR) [odds ratio (OR) = 2.40; P = 0.003) at discharge independently predicted early readmission. The incidence of early readmission was significantly higher in patients with an INR > 1.62 at discharge than in those with an INR ≤ 1.62 (44% vs 19%; P < 0.001). Model for End-stage Liver Disease (MELD) score (OR = 1.11; P = 0.048) at discharge proved to be an independent predictor of early readmission caused by HE. Hemoglobin (OR = 0.97; P = 0.005) at discharge proved to be an independent predictor of non-early readmission. During 6 months of follow-up, 34 (16%) patients died. Artificial liver use (hazard ratio = 6.67; P = 0.021) during the first hospitalization independently predicted mid-term mortality.
INR could be applied to identify fragile cirrhotic patients, MELD score could be used to predict early relapse of HE, and anemia is a potential target for preventing early readmission.
Core tip: International normalized ratio at discharge predicts 30-d readmission in cirrhotic patients after the resolution of hepatic encephalopathy (HE) and Model for End-stage Liver Disease score at discharge predicts 30-d readmission caused by HE in these patients. Meantime, hemoglobin level at discharge predicts 30-d non-readmission in these patients.
- Citation: Hu XP, Gao J. International normalized ratio and Model for End-stage Liver Disease score predict short-term outcome in cirrhotic patients after the resolution of hepatic encephalopathy. World J Gastroenterol 2019; 25(26): 3426-3437
- URL: https://www.wjgnet.com/1007-9327/full/v25/i26/3426.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i26.3426
A high percentage of cirrhotic patients experience readmissions within 30 d after discharge[1]. Early readmission (30-d) indicates a lower survival rate for at least one year following initial hospitalization when taking account of other factors associated with mortality in cirrhotic patients[2]. The major causes of hospital readmissions are complications of this disease, such as upper gastrointestinal (GI) bleeding, spontaneous peritonitis, fluid and electrolyte imbalance, and hepatic encephalopathy (HE). In a multistate, population-based cohort study, HE was found to be most strongly associated with early readmission[1]. HE is an important feature of liver failure, and it is one of the common causes of emergency medical care. Once HE occurs in patients with chronic liver disease, the prognosis is very poor, with a 1-year survival rate of less than 50% and a 3-year survival rate of less than 25%. Liver transplantation should be considered for patients suffering from HE, particularly those who have suffered it twice in the past 6 mo, in case of no complications that hinder surgery[3]. However, “transplantation might not be feasible owing to contraindications or organ shortage”[4]; for one thing, the muscle volume usually decreases rapidly in patients who have HE with persistent or frequent relapse, and survival is not easy even for those who have experienced liver transplantation for another[5]. In addition, patients with HE may seek an artificial liver support system, which can reduce plasma ammonia, inflammatory cytokines, bilirubin, and other toxins[6], providing the liver with a chance of recovery and avoiding liver trans-plantation, or allowing patients to wait for transplantation. Nevertheless, three systematic reviews showed that artificial liver system has no significant effect on mortality of patients who have acute liver failure, and seven randomized controlled trials revealed that it does not increase survival rate. Apart from these, the system itself is both expensive and resource-consuming[7]. Taking all this into account, the final conclusion could be that close surveillance of cirrhotic patients with a high incidence of early readmission is compulsory, especially for those who suffer from HE during the first admission. Previous studies were not found to describe this specific cohort, thus leading us to conduct a prospective study to determine predictors of 30-d readmission in patients with cirrhosis after the resolution of HE.
The study was carried out by assessing all the successive patients with cirrhosis discharged from Department of Gastroenterology and Department of Infectious and Liver Diseases, Second Affiliated Hospital of Chongqing Medical University from January to February 2018 after hospitalization for HE. Patients were included if they were diagnosed with liver cirrhosis based on clinical, radiological, and endoscopic data, diagnosed with HE during the first hospitalization, and aged at least 18 years but not older than 80 years old. Patients were excluded if they met any of the following criteria: (1) Had active malignant tumors (except hepatocellular carcinoma [HCC] within the Milan criteria) or a previous malignancy with a negative follow-up period of less than five years; (2) Had acute liver failure without underlying liver cirrhosis; (3) Had chronic obstructive pulmonary disease of Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage ≥ 2; (4) Had chronic heart failure of New York Heart Association (NYHA) classification ≥ 2; (5) Had serious mental disorders; (6) Had hematological system diseases; (7) Had warfarin intake or received vitamin K therapy; (8) Died during the first hospital stay; and (9) Had HIV infection. This study was performed according to the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement and registered at http://www.chictr.org.cn (ChiCTR1800014275) (Supplementary Material, which contains study data of this study). The study protocol conformed to the provisions of the Declaration of Helsinki and was approved by the Ethics Committee of Second Affiliated Hospital of Chongqing Medical University, and informed consent was obtained from all patients.
Patients were admitted to hospital because of HE or other complications of liver cirrhosis. After confirming HE and obtaining informed consent, we screened patients during hospitalization. At this stage, demographic and clinical parameters (especially incentives and grading of HE) were collected. Occurrence of acute-on-chronic liver failure (ACLF), acute kidney injury (AKI), or hepatorenal syndrome (HRS) during hospitalization was also recorded. Treatments for HE were administered according to the international guideline (mainly including lactulose, rifaximin, and aspartate ornithine)[8]. The treatment of underlying conditions (including liver cirrhosis, liver failure, and HCC) was also based on the associated international guidelines[9,10]. Artificial liver (MARS system) was used according to the guideline from the European Association for the Study of the Liver[9]. The decision that a patient can be discharged was made by the attending physicians based on clinical and laboratory parameters. Patients who survived the first hospitalization were enrolled in the study, and their clinical parameters (especially the course of anti-HE treatment and discharge with medications to prevent HE) were collected; meantime, laboratory parameters were reassessed. After being discharged, patients were routinely followed for six months or until death or liver transplantation. During follow-up, data concerning other hospital admissions were collected in the study charts and data about daily medication were recorded in a form (see Supplementary Material, which contains a record of the daily medication of included patients after discharge). If patients did not participate in the visit, the patients, family members, or the attending physicians would receive a telephone call. In this study, “early readmission” was defined as unplanned, urgent readmission within 30 d from discharge. Hospitalizations due to programmed procedures (such as programmed endoscopy examination or programmed endoscopic surgery) were not considered readmissions. The data on the causes of readmissions were recorded. Furthermore, death and causes of death were recorded during the six-month follow-up. For each subject, demographic, clinical, and laboratory variables were assessed at the time of diagnosis of HE, during hospital stay, at discharge, and during follow-up. All authors had no access to information that could identify individual participants during or after data collection.
HE was diagnosed according to the American Association for the Study of Liver Diseases (AASLD) practice guideline[8].
Comorbidity was defined as diabetes, hypertension, or chronic kidney disease.
HRS and ACLF were determined based on the European Association for the Study of the Liver (EASL) practice guideline[9]. AKI was determined based on the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury[11].
The major endpoints were the incidence of early readmission (30 d) and mid-term mortality (6 mo). Patients lost to visit immediately after discharge were excluded from the analysis. We reported patient characteristics as the mean and standard deviation or frequency and percentage. We used the t-test for comparing continuous variables and the χ2 test for comparing categorical variables. A univariate analysis was carried out to assess variables associated with early readmission. The significant (P < 0.1) variables identified in the univariate analysis were then included in logistic regression analysis to determine the independent predictors of early readmission and generate odds ratios for each independent predictor.
The Kaplan–Meier method was used to plot the survival curves and the log rank test was used to compare them. A univariate analysis was carried out to assess variables associated with mid-term mortality, and the significant variables (P < 0.1) identified in the univariate analysis were included in Cox regression analysis to determine the independent predictors of mid-term mortality and generate hazard ratios for each independent predictor.
All tests were two-sided, and P < 0.05 was considered significant. All statistical analyses were performed with SPSS 19.0 (SPSS, Chicago, IL, United States). The statistical methods of this study were reviewed by Jian Gao, who is not only the corresponding author of this manuscript, but also a biomedical statistician.
A total of 260 patients with cirrhosis discharged after an episode of HE were enrolled. Forty-seven patients were excluded from the study because of loss to visit immediately after discharge, and finally we included 213 patients in the cohort. All patients showed good compliance with medication.
The baseline characteristics of the patients are summarized in Tables 1 and 2. The mean age was 57 ± 11 years and the majority were male (72%). HE without obvious incentive was the most common condition, followed by that caused by a high protein diet and infection (54, 22%, and 8%, respectively). These HE cases were graded as degree I in 139 (65%) patients, degree II in 49 (23%), and degree IV in just 4 (2.0%). In terms of treatments for HE, lactulose combined with aspartate ornithine was mostly used in 139 (65%) patients, and lactulose combined with rifaximin and aspartate ornithine as well as white vinegar enema combined with aspartate ornithine was least used, in just 1 (0.5%) patient. Among all the patients, 68 (32%) developed comorbidities, mainly including diabetes in 52 (24%) patients, hypertension in 24 (11%), and CKD in 6 (3%), and the mean Charlson comorbidity score was 3.6 ± 1.2 for all. During hospitalization, 0.5% of patients met the AKI criteria, 2% met the HRS criteria, and 4% met the ACLF criteria. Furthermore, 27 (13%) patients progressed to liver cancer by this hospitalization. At discharge, the average Model for End-stage Liver Disease (MELD) score was 12.9 ± 5.8, the average hospital stay was 16 ± 13 d, and the average course of anti-HE treatment was 11 ± 8 d. In addition, most patients (61%) were discharged with medications to prevent HE.
| Variable | Whole population | Readmitted | Non-readmitted | P-value |
| (n = 213) | (n = 65) | (n = 148) | ||
| Age (yr), mean (SD) | 57 (11) | 56 (11) | 57 (10) | 0.536 |
| Gender (male) | 153 (72) | 44 (68) | 109 (74) | 0.374 |
| Etiology, n (%) | 0.946 | |||
| HBV | 116 (54) | 37 (57) | 79 (53) | |
| HCV | 13 (6) | 4 (6) | 9 (6) | |
| Alcohol | 34 (16) | 9 (14) | 25 (17) | |
| Other | 50 (23) | 15 (23) | 35 (24) | |
| Comorbidity, n (%) | 68 (32) | 15 (23) | 53 (36) | 0.066 |
| Diabetes, n (%) | 52 (24) | 12 (18) | 40 (27) | 0.180 |
| Chronic kidney disease, n (%) | 6 (3) | 1 (2) | 5 (3) | 0.455 |
| Hypertension, n (%) | 24 (11) | 5 (8) | 19 (13) | 0.274 |
| Charlson comorbidity score, mean (SD) | 3.6 (1.2) | 3.6 (1.3) | 3.6 (1.2) | 0.936 |
| Albumin (g/L), mean (SD)1 | 30 (4) | 30 (3) | 30 (4) | 0.454 |
| Total bilirubin (μmol/L), mean (SD)1 | 48 (58) | 57 (75) | 44 (48) | 0.214 |
| WBC (×109/L), mean (SD)1 | 3.8 (2.0) | 3.9 (1.8) | 3.8 (2.1) | 0.895 |
| Serum sodium (mmol/L), mean (SD)1 | 139.0 (4.6) | 138.0 (3.5) | 139.0 (5.0) | 0.576 |
| Serum potassium (mmol/L), mean (SD)1 | 3.9 (0.5) | 3.9 (0.5) | 3.9 (0.5) | 0.786 |
| SCr (μmol/L), mean (SD)1 | 85 (95) | 95 (157) | 80 (45) | 0.441 |
| PTA (%), mean (SD)1 | 52 (17) | 47 (17) | 55 (16) | 0.001 |
| INR, mean (SD)1 | 1.7 (0.5) | 1.9 (0.6) | 1.6 (0.5) | 0.001 |
| Hb (g/L), mean (SD)1 | 92 (20) | 85 (15) | 95 (21) | 0.001 |
| PLT (×109/L), mean (SD)1 | 87 (68) | 94 (71) | 84 (67) | 0.350 |
| Ascites, n (%)1 | 21 (10) | 11 (17) | 10 (7) | 0.022 |
| HRS during hospital stay, n (%)1 | 4 (2) | 2 (3) | 2 (1) | 0.393 |
| AKI during hospital stay, n (%)1 | 1 (0.5) | 0 (0) | 1 (0.7) | 0.507 |
| ACLF during hospital stay, n (%)1 | 9 (4) | 2 (3) | 7 (5) | 0.581 |
| MELD score, mean (SD)1 | 12.9 (5.8) | 12.7 (5.2) | 13.0 (6.0) | 0.719 |
| Length of hospital stay (d), mean (SD)1 | 16 (13) | 15 (13) | 16 (13) | 0.646 |
| Artificial liver use, n (%)1 | 4 (2) | 2 (3) | 2 (1) | 0.393 |
| Anti-HE treatment course (d), mean (SD)1 | 11 (8) | 12 (9) | 10 (7) | 0.159 |
| Preventing HE with medications post discharge, n (%) | 130 (61) | 40 (62) | 90 (61) | 0.920 |
| Progression to HCC, n (%) | 27 (13) | 8 (12) | 19 (13) | 0.915 |
| Variable | Whole population | Readmitted | Non-readmitted | P-value |
| (n = 213) | (n = 65) | (n = 148) | ||
| Incentives of HE | 0.645 | |||
| No obvious incentive | 114 (54) | 35 (54) | 79 (53) | |
| High protein diet | 47 (22) | 12 (18) | 35 (24) | |
| Infection | 16 (8) | 6 (9) | 10 (7) | |
| GI bleeding | 15 (7) | 7 (11) | 8 (5) | |
| Electrolyte disturbances | 3 (1) | 1 (2) | 2 (1) | |
| Other | 18 (8) | 4 (6) | 14 (9) | |
| Degree of HE | 0.341 | |||
| I | 139 (65) | 39 (60) | 100 (68) | |
| II | 49 (23) | 15 (23) | 34 (23) | |
| III | 21 (10) | 10 (15) | 11 (7) | |
| IV | 4 (2.0) | 1 (2.0) | 3 (2.0) | |
| Treatment for HE | 0.523 | |||
| L + AO | 139 (65) | 48 (74) | 91 (62) | |
| L + R + AO | 1 (0.5) | 0 (0) | 1 (0.7) | |
| L | 2 (0.9) | 1 (1.5) | 1 (0.7) | |
| WVE + AO | 1 (0.5) | 0 (0) | 1 (0.7) | |
| AO | 61 (29) | 15 (23) | 46 (31) | |
| Nothing | 3 (1.4) | 0 (0) | 3 (2.0) | |
| L + AO + WVE | 6 (2.8) | 1 (1.5) | 5 (3.4) |
Sixty-five (31%) patients experienced a readmission within 30 days from discharge. Causes of readmissions included HE (n = 32), GI bleeding (n = 12), ascites (n = 6), infections (n = 6), abdominal distention (n = 4), edema of both lower extremities (n = 3), severe hepatitis (n = 1), and hepatic injury (n = 1).
Tables 1 and 2 show a comparison between groups readmitted and not readmitted. Patients readmitted early were more likely to have ascites (17% vs 7%; P = 0.022) at discharge than those not. Meantime, they had lower levels of prothrombin activity (PTA) (47% vs 55%; P = 0.001) and hemoglobin (Hb) (85 g/L vs 95 g/L; P = 0.001) and higher levels of international normalized ratio (INR) (1.9 vs 1.6; P = 0.001) at discharge than those not readmitted. There was no significant difference between the two groups in age; sex; cause of cirrhosis; comorbidity (diabetes, CKD, or hypertension); Charlson comorbidity score; albumin, total bilirubin; white blood cells (WBC), platelets; serum sodium, potassium, or creatinine; presence of HRS, AKI, ACLF, or liver cancer, MELD score; length of hospital stay; incentive, degree, or treatment of HE; anti-HE treatment course; artificial liver use or prevention of HE with me-dications after discharge.
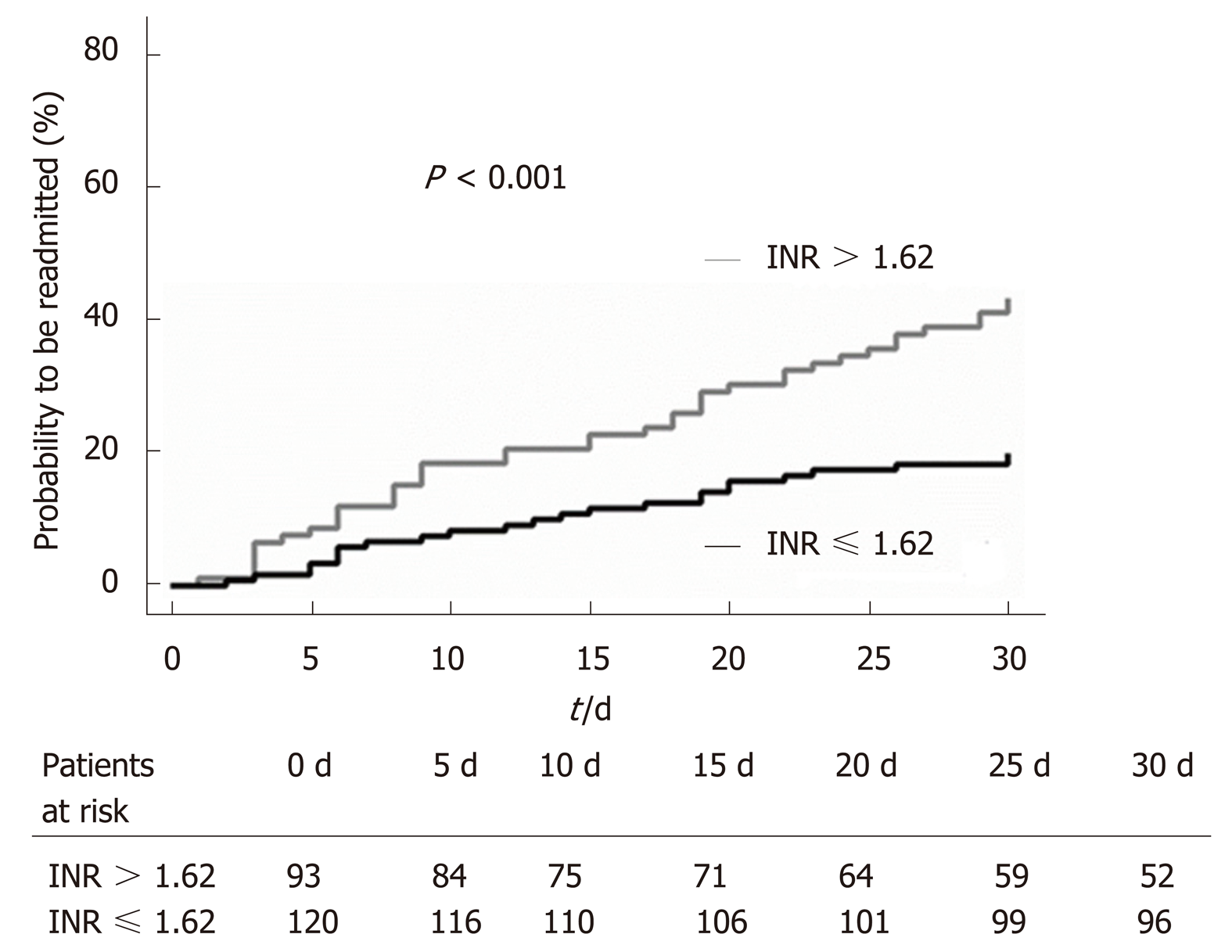
In the multivariate analysis, we found that INR (OR = 2.40; P = 0.003; Table 3) at discharge independently predicted early readmission. The incidence of early readmission was significantly higher in patients with an INR > 1.62 at discharge than in those with an INR ≤ 1.62 (44% vs 19%; P < 0.001; Figure 1). Meanwhile, hemoglobin (OR = 0.97; P = 0.005; Table 3) was found to be an independent predictor of non-readmission within 30 d.
| Variable | OR | 95%CI | P-value |
| Hb at discharge | 0.97 | 0.96-0.99 | 0.005 |
| INR at discharge | 2.40 | 1.36-4.26 | 0.003 |
| MELD score1 | 1.11 | 1.00-1.24 | 0.048 |
Among 65 patients, 33 (51%) were readmitted early because of HE. MELD score at discharge proved to be an independent predictor of early readmission when HE was considered the only cause (OR = 1.11; P = 0.048; Table 3).
During 6 months of follow-up, 34 (16%) patients died, and 179 (84%) survived. No one received a liver transplant or was lost to follow-up. Causes of death were GI bleeding (n = 12), terminal liver failure (n = 4), respiratory and circulatory failure (n = 4), HE (n = 3), multiple organ failure (n = 3), ACLF (n = 2), and one each for advanced HCC, terminal renal failure, hemorrhagic shock, sepsis, septic shock, and unknown.
Tables 4 and 5 show a comparison of characteristics between survivors and non-survivors within 6 months from discharge. Non-survivors had lower levels of serum sodium than survivors at 6 mo. AKI more frequently occurred in patients who died than in those who survived at 6 mo. Similar to the previous results, levels of INR were significantly higher in non-survivors than in survivors at 6 mo (2.0 vs 1.7; P = 0.012), whereas levels of hemoglobin were significantly lower in non-survivors than in survivors at 6 mo (84 g/L vs 94 g/L; P = 0.007). Finally, events that progressed to HCC and early readmission more frequently occurred among patients who died than in those who survived at 6 months.
| Variable | Survivors | Non-survivors | P-value |
| (n = 179) | (n = 34) | ||
| Age (yr), mean (SD) | 57 (11) | 57 (11) | 0.927 |
| Gender (male), n (%) | 130 (73) | 23 (68) | 0.554 |
| Etiology, n (%) | 0.743 | ||
| HBV | 95 (53) | 21 (62) | |
| HCV | 12 (7) | 1 (3) | |
| Alcohol | 29 (16) | 5 (15) | |
| Other | 43 (24) | 7 (21) | |
| Comorbidity, n (%) | 61 (34) | 7 (21) | 0.122 |
| Diabetes, n (%) | 46 (26) | 6 (18) | 0.316 |
| Chronic kidney disease, n (%) | 4 (21) | 2 (6) | 0.239 |
| Hypertension, n (%) | 21 (12) | 3 (9) | 0.623 |
| Charlson comorbidity score, mean (SD) | 3.6 (1.2) | 3.8 (1.3) | 0.286 |
| Albumin (g/L), mean (SD)2 | 30 (4) | 30 (4) | 0.204 |
| Total bilirubin (μmol/L), mean (SD)2 | 42 (44) | 76 (100) | 0.061 |
| WBC (×109/L), mean (SD)2 | 3.8 (1.9) | 4.0 (2.5) | 0.546 |
| Serum sodium (mmol/L), mean (SD)2 | 139 (5) | 137 (3) | 0.017 |
| Serum potassium (mmol/L), mean (SD)2 | 3.9 (0.5) | 3.9 (0.5) | 0.677 |
| SCr (μmol/L), mean (SD)2 | 82 (97) | 98 (79) | 0.390 |
| PTA (%), mean (SD)2 | 53 (16) | 47 (19) | 0.068 |
| INR, mean (SD)2 | 1.7 (0.4) | 2.0 (0.8) | 0.012 |
| Hb (g/L), mean (SD)2 | 94 (20) | 84 (18) | 0.007 |
| PLT (×109/L), mean (SD)2 | 87 (67) | 88 (73) | 0.937 |
| Ascites, n (%)2 | 16 (9) | 5 (15) | 0.301 |
| HRS during hospital stay, n (%)2 | 2 (1) | 2 (6) | 0.061 |
| AKI during hospital stay, n (%)2 | 0 (0) | 1 (3) | 0.021 |
| ACLF during hospital stay, n (%)2 | 7 (4) | 2 (6) | 0.600 |
| MELD score, mean (SD)2 | 13 (6) | 12 (6) | 0.006 |
| Length of hospital stay (d), mean (SD)2 | 15.8 (13.0) | 17.0 (12.5) | 0.621 |
| Artificial liver use, n (%)2 | 2 (1) | 2 (6) | 0.061 |
| Anti-HE treatment course (d), mean (SD)2 | 11 (8) | 10 (6) | 0.646 |
| Preventing HE with medications post discharge, n (%) | 112 (63) | 18 (53) | 0.291 |
| Progression to HCC, n (%) | 18 (10) | 9 (26) | 0.008 |
| Early readmission, n (%) | 47 (26) | 18 (53) | 0.002 |
| Variable | Survivors | Non-survivors | P-value |
| (n = 179) | (n = 34) | ||
| Incentive of HE | 0.689 | ||
| No obvious incentive | 92 (51) | 22 (65) | |
| High protein diet | 40 (22) | 7 (21) | |
| Infection | 15 (8) | 1 (3) | |
| GI bleeding | 13 (7) | 2 (6) | |
| Electrolyte disturbances | 3 (2) | 0 (0) | |
| Other | 16 (9) | 2 (6) | |
| Degree of HE | 0.935 | ||
| I | 116 (65) | 23 (68) | |
| II | 42 (23) | 7 (21) | |
| III | 18 (10) | 3 (9) | |
| IV | 3 (1.6) | 1 (2.9) | |
| Treatment for HE | 0.329 | ||
| L + AO | 116 (65) | 23 (68) | |
| L + R + AO | 1 (0.6) | 0 (0) | |
| L | 2 (1.1) | 0 (0) | |
| WVE + AO | 1 (0.6) | 0 (0) | |
| AO | 53 (30) | 8 (23) | |
| Nothing | 1 (0.6) | 2 (5.9) | |
| L + AO + WVE | 5 (2.8) | 1 (2.9) |
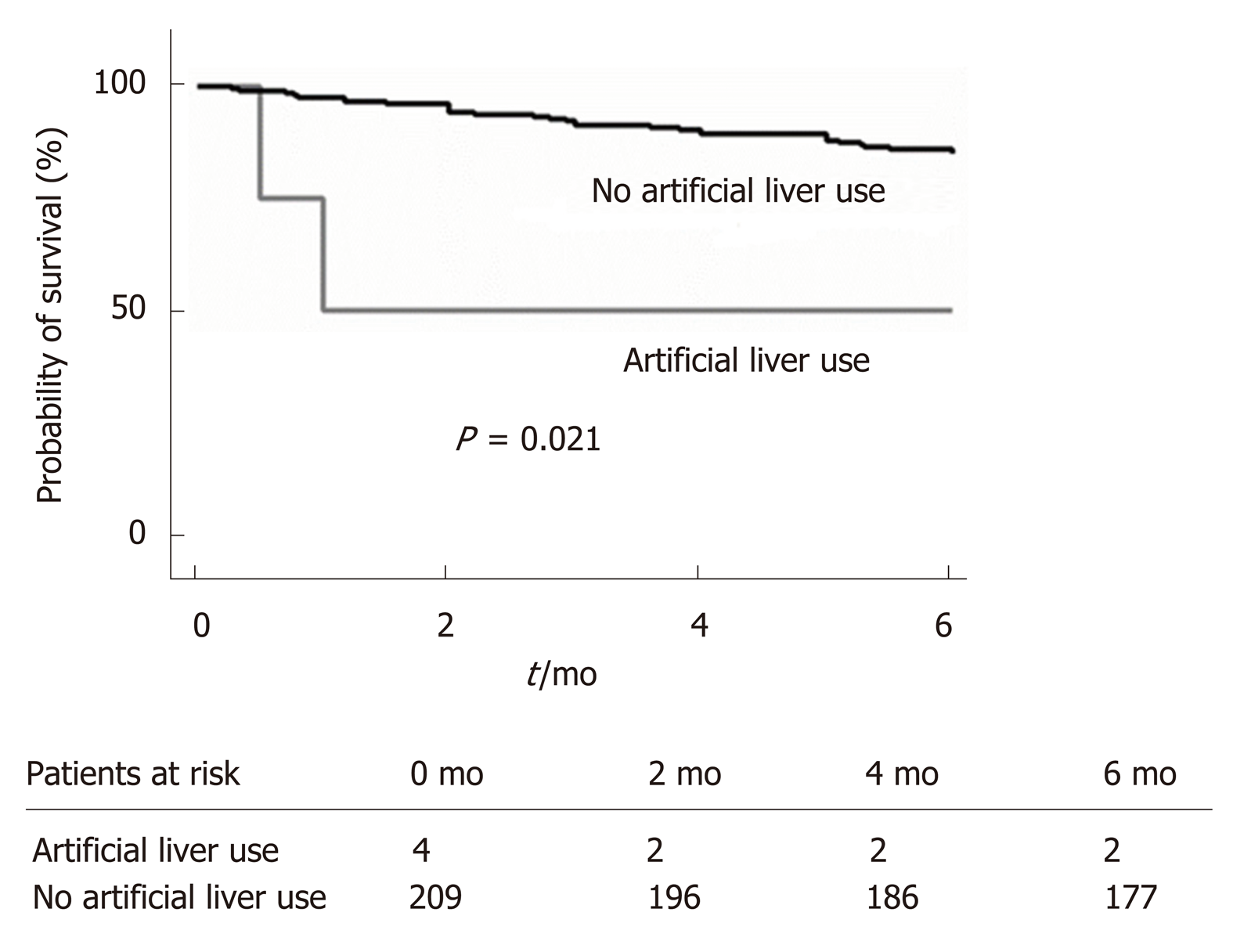
In the multivariate analysis, we also found that artificial liver use (HR = 6.67; P = 0.021; Table 6, Figure 2) during the first hospitalization independently predicted mortality by 6 months.
| Variable | HR | 95%CI | P-value |
| Artificial liver use | 6.67 | 1.33-33.49 | 0.021 |
In addition, there was no significant difference in survival rates between the two stratified groups when INR with a cut-off value of 1.62 was considered, probably because the sample size was insufficient (58.7% vs 41.3%; P = 0.117).
In this well-designed cohort, we found that INR independently predicted early readmission in patients with cirrhosis after an episode of HE. Increased INR is very common in patients with cirrhosis, especially in those with decompensated cirrhosis, and it was mentioned in a previous study that INR appeared to be a promising prognostic indicator of ACLF for assessment in the future studies[12]. Although not an ACLF cohort, the present finding verified conjecture of previous researchers to some extent in that patients who have decompensated liver cirrhosis often overlap with those who have ACLF. Remarkably, increased INR was stronger than all of the other parameters when all the readmissions were considered, and MELD score was the only predictor when HE was considered the only cause of readmission. These findings lead to potential explanations.
HE, GI bleeding, infections, ascites, hypoalbuminemia, abdominal distention, severe hepatitis, and hepatic injury were the causes of readmission in the cohort. Some complications reflect insufficiency of liver function reserve, whereas others reflect portal hypertension. From the perspective of pathophysiology, it is well known that elevated INR indirectly reflects the deficiency of liver function reserve in patients with decompensated cirrhosis except in those who take warfarin, and is also a predictor of variceal bleeding[13], which means that elevated INR is associated with significant portal hypertension. In addition, the levels of hemoglobin, WBC count, and platelet count were significantly lower in patients whose INR was more than 1.62 at discharge (P < 0.001, P < 0.001, P < 0.001, respectively), suggesting that patients whose liver function reserve remains less have more serious hypersplenism, which is caused by portal hypertension. Therefore, elevated INR may indicate portal hypertension. Liver dysfunction and portal hypertension are two major pathophysiological features of decompensated cirrhosis, and elevated INR may reflect the above two features. This interpretation makes it possible to apply the INR to identification of patients with a high incidence of early readmission to improve their prognosis. In the guideline, INR level greater than or equal to 1.5 is one of the diagnostic criteria for all types of liver failure[14] and the cut-off value of 1.5 is calculated from data with large sample size; while most of our patients (59.6%) had an INR level greater than or equal to 1.5, which should be considered the diagnosis of chronic liver failure or ACLF. Meantime, the cut-off value of 1.62 in this study, which is slightly above the classical cut-off value of 1.5 in liver failure, was figured out from data with limited sample size, thus leading to a narrowing of the range (>1.62 vs ≥1.5). Of note, patients with chronic liver failure or ACLF are more fragile than others among patients with cirrhosis, especially the latter, whose short-term outcome is rather poor (high 28-d mortality)[9]. Similarly to our finding, Shalimar et al[15] in 2015 mentioned that aggressive correction of coagulopathy must be undertaken to achieve an INR < 1.7 in controlling HE. Therefore, an INR value of 1.62 could be used to stratify cirrhotic patients who need close monitoring to prevent or reduce liver-related readmission. However, further evaluation of this finding is required in future clinical controlled trials, with primary endpoints such as 30-d and 3-mo hospital readmission rates and 30-d and 6-mo mortality rates.
In our cohort, MELD score at discharge was found to be the only predictor of readmission caused by HE. It is well known that MELD score can effectively predict short- and medium-term mortality in end-stage liver disease[16,17]. Although we did not find a significant correlation between readmission caused by HE and six-month mortality (r = -0.078, P = 0.535), the study by Scaglione et al[2] has already highlighted that an early readmission to hospital independently predicts mortality in patients with decompensated cirrhosis for at least one year following the first hospitalization. Therefore, MELD score at discharge could probably predict early readmission precipitated by HE. Further, the relationship between MELD score and early readmission because of HE should be verified in clinical controlled trials.
Hemoglobin, we found, was a protective factor of readmission, which means that low values of hemoglobin increase the rate of readmission. This finding is not difficult to understand. Similarly, anemia was found to predict ACLF independently in patients with cirrhosis[18], and ACLF is an independent predictor of early readmission in cirrhotic patients[19]. In terms of mechanism, “anemia may reduce peripheral oxygen delivery, directly and/or by further impairing the macrovascular function, thus favoring the development of liver failure”[16]. Accordingly, anemia is a potential target for preventing early readmission.
In addition, we found that artificial liver use during the first hospitalization independently predicted 6-month mortality. This finding is not completely new. In fact, previous studies have revealed that artificial liver does not increase survival rate[7]. Further, patients who use artificial liver are in a state of acute liver failure or ACLF, presenting as “acute exacerbation in liver function, multiple organ failure, and high short-term mortality”[20]. Thus, this indicator may be a predictor of 6-month mortality. However, we must recognize that the results of our study do not imply a cause-effect relationship between artificial liver use and increased 6-month mortality rate. In fact, artificial liver use may just be a marker of disease severity. In addition, the sample size of artificial liver use was insufficient (2 in the non-survival group vs 2 in the survival group), leading this finding to be further validated in larger sample trials.
In conclusion, INR could be applied to identify fragile patients with cirrhosis who develop HE to improve their outcomes, and MELD score could be used to predict early relapse of HE. The efficacy of these strategies in lowering early readmission rate when surveilling fragile patients should be verified in clinical trials. However, expect for patients with anemia, how those patients with an elevated INR and MELD score can be prevented from readmission remains a question that needs to be answered in future studies.
Among cirrhotic patients, hepatic encephalopathy (HE) indicates a poor prognosis despite the use of artificial liver and liver transplantation, presenting as frequent hospitalizations and increased mortality rate.
The aim of this study was to determine predictors of early readmission and mid-term mortality in patients with cirrhosis after an episode of HE, which may contribute to early recognition of fragile cirrhotic patients.
To determine predictors of early readmission and mid-term mortality in patients with cirrhosis after an episode of HE to provide theoretical support for the management of cirrhotic patients.
This is an observational study, and the total follow-up time was 6 mo. The primary endpoints were the incidence of early readmission (30 d) and mid-term mortality (6 mo). For each subject, demographic, clinical, and laboratory variables were assessed at the time of diagnosis of HE, during hospital stay, at discharge, and during follow-up.
International normalized ratio (INR) level at discharge predicted early readmission in cirrhotic patients after the resolution of HE and Model for End-stage Liver Disease score at discharge predicted early readmission caused by HE in these patients. Meanwhile, hemoglobin level at discharge predicted early non-readmission in these patients. Finally, artificial liver use during the first hospitalization independently predicted mid-term mortality.
INR could be applied to identify fragile cirrhotic patients, Model for End-stage Liver Disease score could be used to predict early relapse of HE, and anemia is a potential target for preventing early readmission.
Further controlled trials are needed to verify the efficacy of these strategies in lowering early readmission rate when surveilling fragile cirrhotic patients. Expect for patients with anemia, how those patients with an elevated INR and MELD score can be prevented from readmission remains a question that needs to be answered in future studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gencdal G, Mousa N S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Tapper EB, Halbert B, Mellinger J. Rates of and Reasons for Hospital Readmissions in Patients With Cirrhosis: A Multistate Population-based Cohort Study. Clin Gastroenterol Hepatol. 2016;14:1181-1188.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 2. | Scaglione SJ, Metcalfe L, Kliethermes S, Vasilyev I, Tsang R, Caines A, Mumtaz S, Goyal V, Khalid A, Shoham D, Markossian T, Luke A, Underwood H, Cotler SJ. Early Hospital Readmissions and Mortality in Patients With Decompensated Cirrhosis Enrolled in a Large National Health Insurance Administrative Database. J Clin Gastroenterol. 2017;51:839-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Ellul MA, Gholkar SA, Cross TJ. Hepatic encephalopathy due to liver cirrhosis. BMJ. 2015;351:h4187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Gonzalez HC, Jafri SM, Gordon SC. Management of Acute Hepatotoxicity Including Medical Agents and Liver Support Systems. Clin Liver Dis. 2017;21:163-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Prakash RK, Mullen KD, Schiff ER, Maddrey WC. Hepatic Encephalopathy. Schiff ER, Maddrey WC. Schiff's Diseases of the Liver. Beijing: Peking University Medical Press 2015; 401. [Cited in This Article: ] |
| 6. | Bañares R, Catalina MV, Vaquero J. Molecular adsorbent recirculating system and bioartificial devices for liver failure. Clin Liver Dis. 2014;18:945-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Flamm SL, Yang YX, Singh S, Falck-Ytter YT; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guidelines for the Diagnosis and Management of Acute Liver Failure. Gastroenterology. 2017;152:644-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1158] [Cited by in F6Publishing: 1225] [Article Influence: 122.5] [Reference Citation Analysis (1)] |
| 9. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1177] [Cited by in F6Publishing: 1439] [Article Influence: 239.8] [Reference Citation Analysis (1)] |
| 10. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3934] [Cited by in F6Publishing: 4903] [Article Influence: 817.2] [Reference Citation Analysis (0)] |
| 11. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1436] [Cited by in F6Publishing: 2488] [Article Influence: 207.3] [Reference Citation Analysis (0)] |
| 12. | Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RA. A systematic review on prognostic indicators of acute on chronic liver failure and their predictive value for mortality. Liver Int. 2013;33:40-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Matei D, Groza I, Furnea B, Puie L, Levi C, Chiru A, Cruciat C, Mester G, Vesa SC, Tantau M. Predictors of variceal or nonvariceal source of upper gastrointestinal bleeding. An etiology predictive score established and validated in a tertiary referral center. J Gastrointestin Liver Dis. 2013;22:379-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Liver Failure and Artificial Liver Group; Chinese Society of Infectious Diseases; Chinese Medical Association; Severe Liver Disease and Artificial Liver Group; Chinese Society of Hepatology; Chinese Medical Association. [Guideline for diagnosis and treatment of liver failure]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:18-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 39] [Reference Citation Analysis (0)] |
| 15. | Shalimar, Acharya SK. Management in acute liver failure. J Clin Exp Hepatol. 2015;5:S104-S115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 673] [Cited by in F6Publishing: 679] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 17. | Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1075] [Cited by in F6Publishing: 1118] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 18. | Piano S, Tonon M, Vettore E, Stanco M, Pilutti C, Romano A, Mareso S, Gambino C, Brocca A, Sticca A, Fasolato S, Angeli P. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J Hepatol. 2017;67:1177-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Piano S, Morando F, Carretta G, Tonon M, Vettore E, Rosi S, Stanco M, Pilutti C, Romano A, Brocca A, Sticca A, Donato D, Angeli P. Predictors of Early Readmission in Patients With Cirrhosis After the Resolution of Bacterial Infections. Am J Gastroenterol. 2017;112:1575-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Asrani SK, O'Leary JG. Acute-on-chronic liver failure. Clin Liver Dis. 2014;18:561-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |