Published online Jun 21, 2019. doi: 10.3748/wjg.v25.i23.2961
Peer-review started: March 22, 2019
First decision: April 4, 2019
Revised: April 27, 2019
Accepted: May 8, 2019
Article in press: May 8, 2019
Published online: June 21, 2019
Recently, the American Association for the Study of Liver Disease suggested no preference between tenofovir (TDF) and entecavir (ETV) regarding potential long-term risks of renal complications. Over the years, renal safety has become a critical concern in nucleos(t)ide analog-treated patients due to the long-term use of these drugs. However, existing studies do not show significant differences in renal dysfunction between these two drugs. Further, there is a paucity of studies comparing the long-term renal effects of TDF and ETV.
To investigate the effects of TDF and ETV on renal function, we performed systematic review and meta-analysis.
Two investigators independently searched the Cochrane Library, MEDLINE, and Embase databases for randomized controlled trials and nonrandomized studies (NRSs) using the keywords “CHB”, “Tenofovir”, and “Entecavir”, and additional references were obtained from the bibliographies of relevant articles published through December 2017. The quality of each study was assessed using the Newcastle-Ottawa scale and the Grading of Recommendations Assessment, Development and Evaluation criteria. The primary outcome was the change in serum creatinine level in the TDF and ETV groups at baseline, 6 mo, 12 mo and 24 mo.
Nine NRSs comprising 2263 participants met the inclusion criteria. Changes in creatinine levels were higher in the TDF group than in the ETV group at 6 mo [mean difference (MD) = 0.03 mg/dL; 95%CI: 0.02-0.04; I2 = 0%], 12 mo (MD = 0.05 mg/dL; 95%CI: 0.02-0.08; I2 = 78%), and 24 mo (MD = 0.07 mg/dL; 95%CI: 0.01-0.13; I2 = 93%). The change in estimated glomerular filtration rate (eGFR) was significantly higher in the TDF group than in the ETV group at 6 mo [standardized mean difference (SMD), -0.22; 95%Cl: -0.36--0.08; I2 = 0%], 12 mo (SMD = -0.24; 95%Cl: -0.43--0.05; I2 = 50%), and 24 mo (-0.35; 95%Cl: -0.61- -0.09; I2 = 67%).
TDF statistically significantly increased serum creatinine levels and decreased the eGFR in 6-24 mo compared to ETV, with moderate to low quality of evidence. However, the differences are negligible.
Core tip: Recently, the American Association for the Study of Liver Disease suggested no preference between tenofovir (TDF) and entecavir (ETV) regarding potential long-term risks of renal complications. Over the years, renal safety has become a critical concern in nucleos(t)ide analog-treated patients due to the long-term use of these drugs. However, the existing studies do not show significant differences between the two drugs in renal dysfunction. We believe that our study could resolve the existing debate. This is the first meta-analysis comparing the influence of the two drugs on renal function using continuous variables. TDF statistically significantly decreases renal function compared to ETV, but the difference is inappreciable.
- Citation: Lee HY, Oh H, Park CH, Yeo YH, Nguyen MH, Jun DW. Comparison of renal safety of tenofovir and entecavir in patients with chronic hepatitis B: Systematic review with meta-analysis. World J Gastroenterol 2019; 25(23): 2961-2972
- URL: https://www.wjgnet.com/1007-9327/full/v25/i23/2961.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i23.2961
An estimated 257 million people worldwide are infected with the hepatitis B virus (HBV)[1]. Recently, research addressing chronic kidney disease among chronic hepatitis B patients has emerged[2]. Interest in drugs that can affect renal function has also increased. Tenofovir (TDF) and entecavir (ETV) are two drugs preferred as first-line treatments for chronic hepatitis B (CHB). In clinical trials, both have been proven to be safe and well-tolerated with short term follow-up; however, as the treatment period is indefinite, adverse events associated with these drugs over the long term remain worrisome[3,4]. Over the years, published studies have provided increasing evidence that TDF can negatively influence renal function and bone health[5-7]. Further confusing the issue, two recently announced guidelines make conflicting suggestions. The European Association for the Study of the Liver (EASL) recommended con-sidering switching from TDF to ETV in CHB patients with underlying renal disease, especially when exposed to LAM. EASL also suggested selecting ETV (or tenofovir alafenamide fumarate) over TDF for CHB patients with estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2, patients with albuminuria, and patients on hemodialysis. However, the American Association for the Study of Liver Disease (AASLD) suggested no preference between TDF and ETV use with regard to renal safety issues[3,4].
Most physicians acknowledge renal concerns associated with TDF use, although precise data on the degree of renal impairment are still limited. Results of previous studies have varied, mainly limited by small sample sizes and inadequate follow-up periods. Previous meta-analyses on antiviral therapy for CHB primarily focused on therapeutic efficacy rather than renal safety issues[8-10]. Hence, data regarding creatinine level and eGFR were not reported comprehensively. Moreover, the formulas for eGFR calculation were inconsistent, as some studies used the Mo-dification of Diet in Renal Disease (MDRD) formula, while others used the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation[8,9]. In addition, previous studies demonstrated the incidence of acute kidney injury (AKI) as di-chotomous data but not the precise changing values of renal function during the treatment period.
In this study, we aimed to conduct a systematic review and meta-analysis to provide a clear comparison of the renal safety of TDF and ETV in patients with CHB using continuous variables. To enhance the clinical importance, we performed subgroup analyses and sensitivity analysis to determine changes in renal function by study and patient characteristics.
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) statement[11] and Meta‐analysis of Observational Studies in Epidemiology (MOOSE) statements[12].
Two authors (Lee HY and Oh H) independently searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception to December 31, 2017, without language restriction. Additionally, we examined con-ference abstracts from the AASLD, the EASL, the Asian Pacific Association for the Study of the Liver (APASL), Digestive Disease Week (DDW), and The Liver Weeks (Korean Association for the Study of the Liver) published between 2013 and 2017 (Supplemental Table 1).
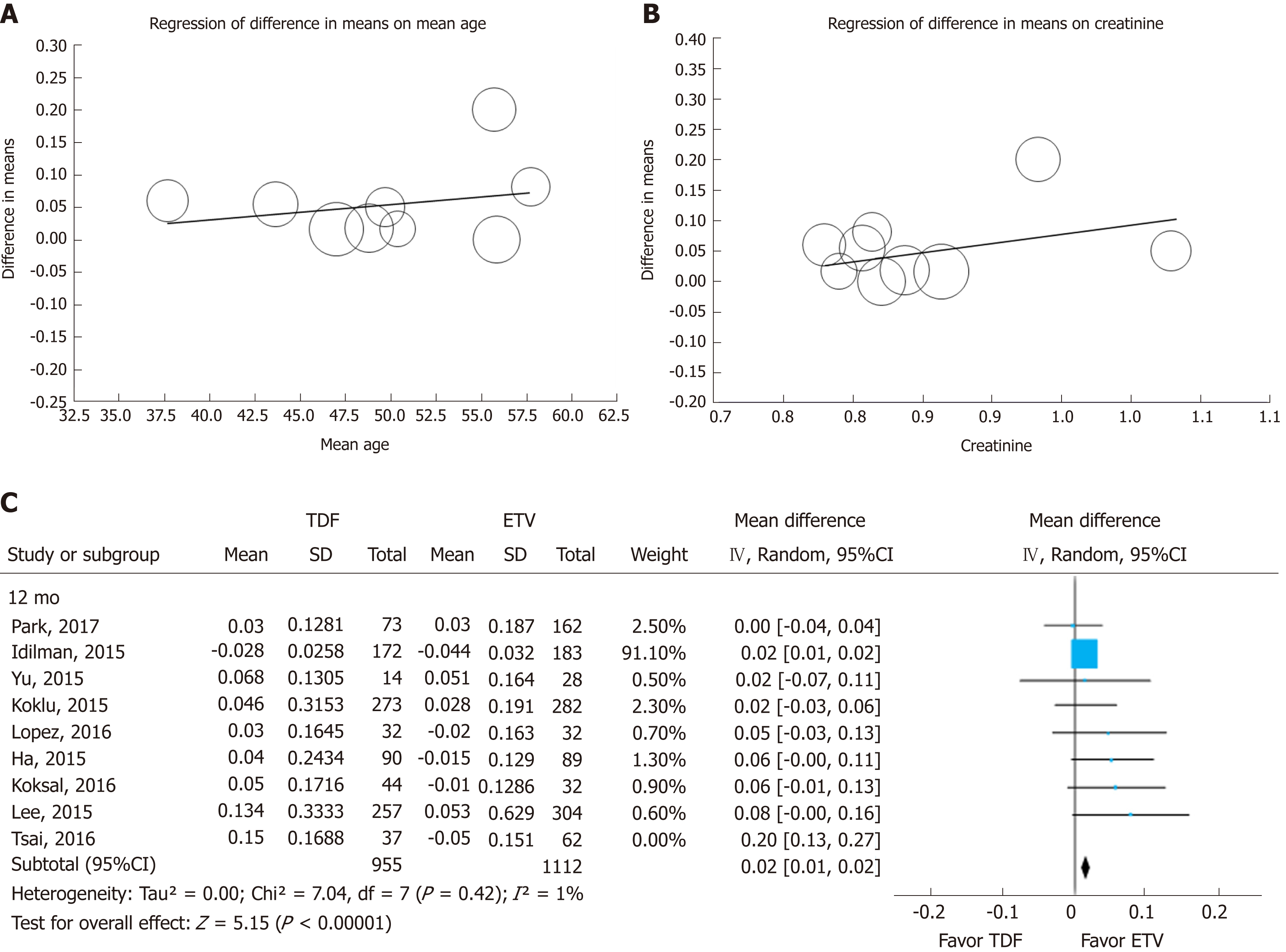
| Author, year country | Design | Duration (mo) | Cirrhosis (%) | Patients (n) | Age (mean) | Sex (M/F) | HBV_DNA | End point |
| TDF | TDF | TDF | ||||||
| ETV | ETV | ETV | (log10 IU/Ml) | |||||
| Ha et al[14] 2015 United States | Matched case-cohort | 24(18-66) | 9.20% | 103 | 43.5 | 65/38 | 5.3 ± 1.5 | RMSRC Decrease in eGFR 20% |
| 103 | 43.8 | 65/38 | 6.15 ± 1.9 | |||||
| Lee et al[15] 2015 South Korea | Prospective | ≥ 6 | NA | 258 | 66.4 | 173/85 | 6.40 ± 1.31 | NA |
| 308 | 50.5 | 190/118 | 6.72 ± 1.22 | |||||
| Yu et al[16] 2015 South Korea | Retrospective | 8.45/18.7 | 50% | 49 | 48.8 | 22/27 | 6.98 ± 1.55 | Creatinine ≥ 0.3 mg/dL or 1.5 times above baseline |
| 58 | 51.7 | 33/25 | 7.05 ± 1.33 | |||||
| Park et al[20] 2017 South Korea | Retrospective | 24 | 100% | 73 | 56.4 | 45/28 | 5.4 ± 1.3 | Serum creatinine increase > 0.2 mg/dLeGFR < 60 mL/min (CKD-EPI)Decrease in eGFR > 20% (CKD-EPI) |
| 162 | 55.6 | 110/52 | 5.6 ± 1.6 | |||||
| Koklu et al [24] 2015 Turkey | Prospective | 24 | 34% | 273 | 47.7 | 183/90 | 6.54 ± 1.74 | Shift from ≥ 90 to 60-89 mL/min per 1.73 m2> 25% increased creatinine |
| 282 | 49.9 | 197/85 | 6.69 ± 1.79 | |||||
| Idilman et al[25] 2015 Turkey | Retro-prospective | 36 (6-78) | 39% | 172 | 47 (15-79) | NA | 5.97 ± 1.72 | Serum creatinine > 0.5 mg/dL from baseline, eGFR < 50 mL/min |
| 183 | NA | |||||||
| Koksal 2016 et al[23] Turkey | Prospective | ≥ 24 | NA | 44 | 36 (29-43.7) | 19/25 | 6.8 ± 1.0 | |
| 32 | 40 (27.2-46.5) | 17/15 | 7.0 ± 1.2 | |||||
| Lopez et al [22] 2016 Spain | Retrospective | 12 | 33.90% | 32 | 50.2 | 25/7 | 1127.4 (19-2463,121) | eGFR < 60 mL/min per 1.73 m2 eGFR reduction > 25%Increase in creatinine > 1.4 mg/dl |
| 32 | 49.2 | 23/9 | 29311.4 (376.2-4660,135) | |||||
| Tsai et al[21] 2016 Taiwan | Retro-prospective | ≥ 24 | 52% | 37 | 56.6 | 32/5 | 6.3 ± 1.3 | Change of eGFR by 25% |
| 62 | 55.2 | 46/16 | 6.4 ± 1.2 |
The following keywords, MeSH, and free text were searched through MEDLINE: Tenofovir, Entecavir, chronic hepatitis B, and multiple synonyms (Supplemental Table 2). After the initial electronic search, further relevant articles and bibliographies were manually identified using reference lists from included studies. The identified articles were assessed individually for inclusion (Supplemental Table 3).
Two authors (Lee HY and Oh H) independently identified articles eligible for inclusion based on a two-level screening using the Population Intervention Com-parison Outcome (PICO) framework[13]. At the first level, titles and abstracts were screened, while at the second level, search queries of the full text of articles were made. The observed agreement between reviewers for eligibility of articles on initial screening was 98.2%, corresponding to substantial agreement (k = 0.79), and that in the second screening was 94.6%, corresponding to almost perfect agreement (k = 0.89). Disagreements between reviewers were resolved by consensus with a third author (Jun DW).
The inclusion criteria were (1) Human subject study design, including randomized control trials (RCTs) and non-RCTs with more than two arms, (2) CHB infection, (3) Intervention therapies of either ETV or TDF monotherapy, (4) Over 6 months of treatment duration, (5) Over 18 years of age, and (6) Documented data of repeated measures of serum creatinine level or eGFR every six months.
Exclusion criteria were (1) Coinfection with other hepatitis viruses (A, C, D, or E), human immunodeficiency virus, cytomegalovirus, or Epstein-Barr virus; (2) Other liver diseases such as alcoholic liver disease, autoimmune hepatitis, drug-induced liver injury, or Wilson’s disease; (3) Acute hepatitis and acute exacerbation; (4) Combination therapy or sequential therapy; (5) Unreported renal parameter data (serum creatinine or eGFR); (6) Exclusion of either TDF or ETV; (7) Pregnancy and/or breastfeeding; (8) Complication of decompensated cirrhosis (variceal bleeding, refractory ascites, hepatorenal syndrome, spontaneous bacterial peritonitis, hepatoencephalopathy); and (9) Organ transplantation.
Two authors (Lee HY and Oh H) independently extracted data from each study with a predefined data extraction form using the Cochrane Methods to minimize random and bias errors. Any disagreement or unresolved concern was independently reviewed by a third author (Jun DW). The following variables were extracted from the selected studies: (1) To evaluate the renal side effect, we compared changes in serum creatinine level and eGFR at baseline, 6 mo, 12 mo and 24 mo. The patient’s eGFR was calculated using the MDRD formula and CKD-EPI equation (Supplemental Table 4). All outcomes were assessed for changes due to intervention between treated and control groups. The results are expressed as the means and standard deviations. (2) When the study presented data on renal function using a graph rather than measured numerical data, we extracted comparable data from the graph. (3) When the data of interest were not available in the published reports, we contacted investigators of original studies via e-mail to request unpublished data, and 3 investigators responded to our request[14-16]. And (4) If necessary, we modified the data (combining two data or converting standard error to standard deviation) to enable comparison according to the equation presented in the Cochrane Handbook (Supplemental Table 5)[17].
Two authors (Lee HY and Oh H) independently evaluated the quality of the included studies using the Newcastle-Ottawa scale (NOS) for nonrandomized studies (Supplemental Table 6)[18] and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria to appraise quality of evidence (GRADEpro, Version 20. McMaster University, 2014) (Supplemental Table 7)[19]. Any disagreements between reviewers were resolved through discussion or review by the third author (Jun DW).
The primary outcome was the change in serum creatinine levels in the TDF and ETV groups at baseline, 6 mo, 12 mo and 24 mo. We derived the mean differences in creatinine levels between the aforementioned time points and the baseline. Subsequently, we estimated and pooled the differences in the mean between the TDF and ETV groups using a random effects model. A significant difference was defined as having a P-value of Z-score smaller than 0.05. To assess for heterogeneity, we estimated the proportion of inconsistencies due to true differences between studies (rather than differences due to random error or chance) using the I2 statistic, with values of 25%, 50%, and 75% considered low, moderate, and high, respectively. Publication bias was assessed by funnel plots and Egger’s test.
The secondary outcome was the change in serum eGFR. As eGFR was estimated by different formulas (e.g., MDRD or CKD-EPI), we standardized the mean difference of eGFR in each included article before pooling them using a random effects model.
Subgroup meta-analyses stratified by proportion of cirrhosis, history of treatment, region, mean age, and other factors were performed subsequently. Meta-regression was performed to identify the source of heterogeneity and to investigate the nature of the studies required to estimate the therapeutic effect. Meta-analyses were performed using the Review Manager (RevMan, version 5.3; The Cochrane Collaboration, Oxford, United Kingdom). Publication bias and meta-regression were analyzed using Comprehensive Meta-Analysis (CMA) statistical software (Version 3, BioStat Solutions, Inc.).
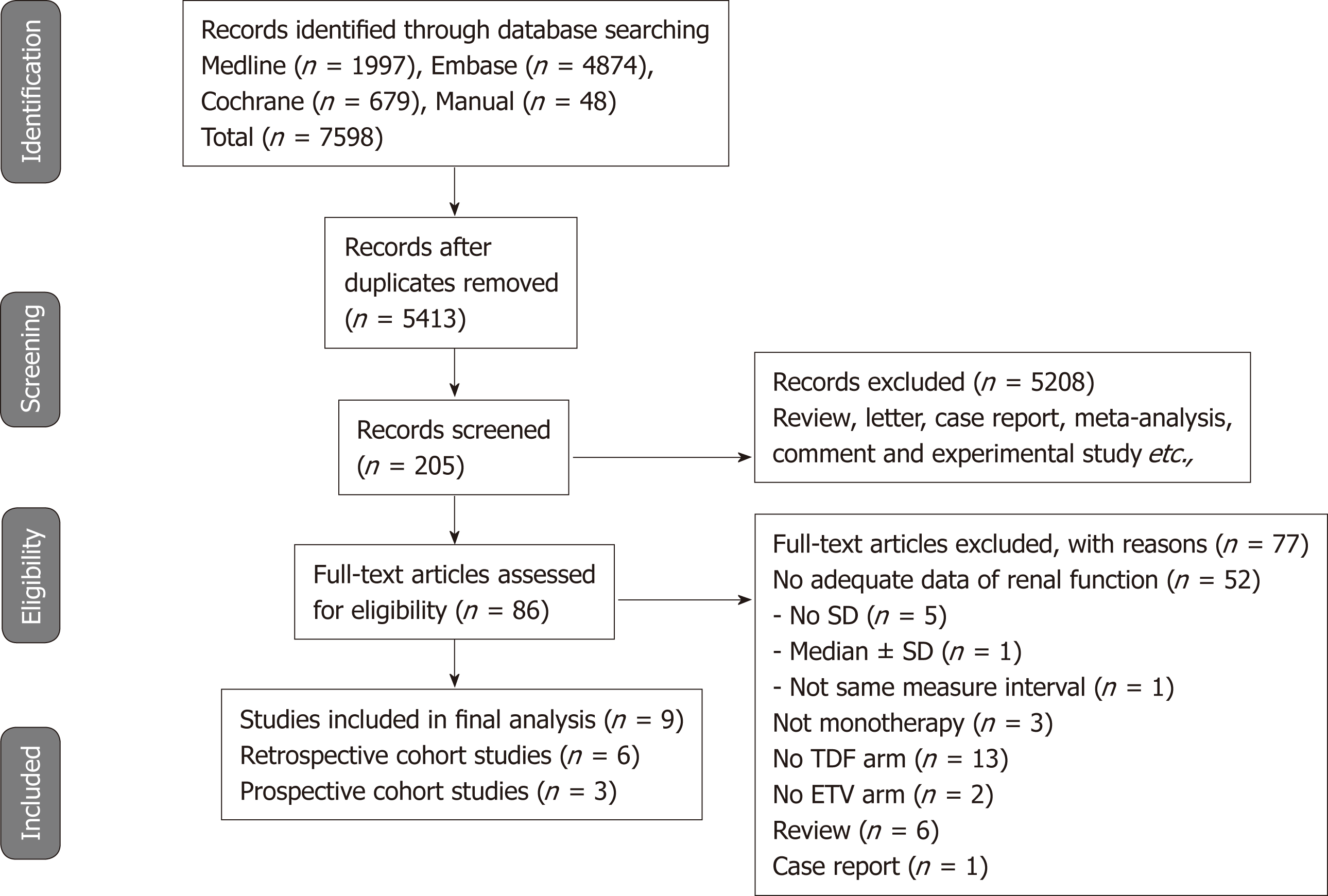
Figure 1 shows the details of the literature search and study selection. The initial search strategy identified 5413 articles (Supplemental Table 3). Of these, 5327 publications were excluded, as they did not fulfill the selection criteria by title and abstract screening. We performed full manuscript reviews of the remaining 86 articles. Nine reports (three prospective cohort studies and six retrospective cohort studies) were deemed eligible and included in the meta-analysis[14-16,20-25].
Table 1 describes the characteristics of the 9 included studies. A total of 2263 participants who received either TDF (n = 1041) or ETV (n = 1222) were included. Both drugs were administered for 6 mo to 2 years. Three studies were conducted in South Korea; one each in United States, Taiwan, and Spain; and three in Turkey. Serum creatinine data were provided in 9 studies and eGFR data in 6 studies. Except for one study[22], all of the studies recruited treatment-naive patients.
The quality of evidence was assessed using the NOS and GRADE guidelines. The level of evidence and grade of recommendation for each outcome are summarized in Supplemental Table 6 and 7.
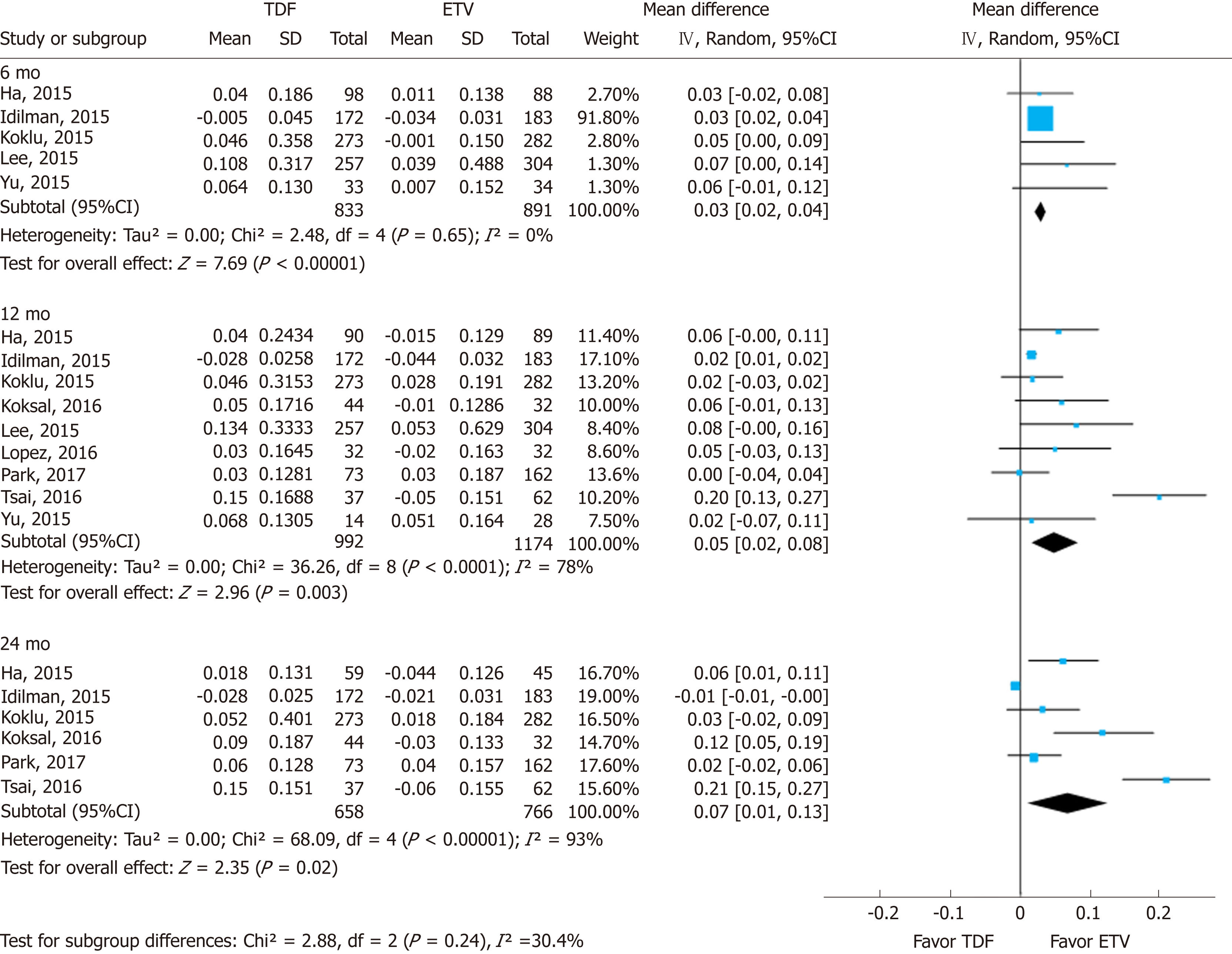
Renal function was assessed using serum creatinine levels at 6, 12, and 24 mo of treatment and its decrease from baseline. Using a random effects approach, changes in serum creatinine level compared to baseline increased more in the TDF group than in the ETV group at 6 mo (MD = 0.03 mg/dL; 95%CI: 0.02-0.04; I2 = 0%), 12 mo (MD = 0.05 mg/dL; 95%CI: 0.02- 0.08; I2 = 78%), and 24 mo (MD = 0.07 mg/dL; 95%CI: 0.01-0.13; I2 = 93%) (Figure 2). The subgroup difference among different time points was not significant.
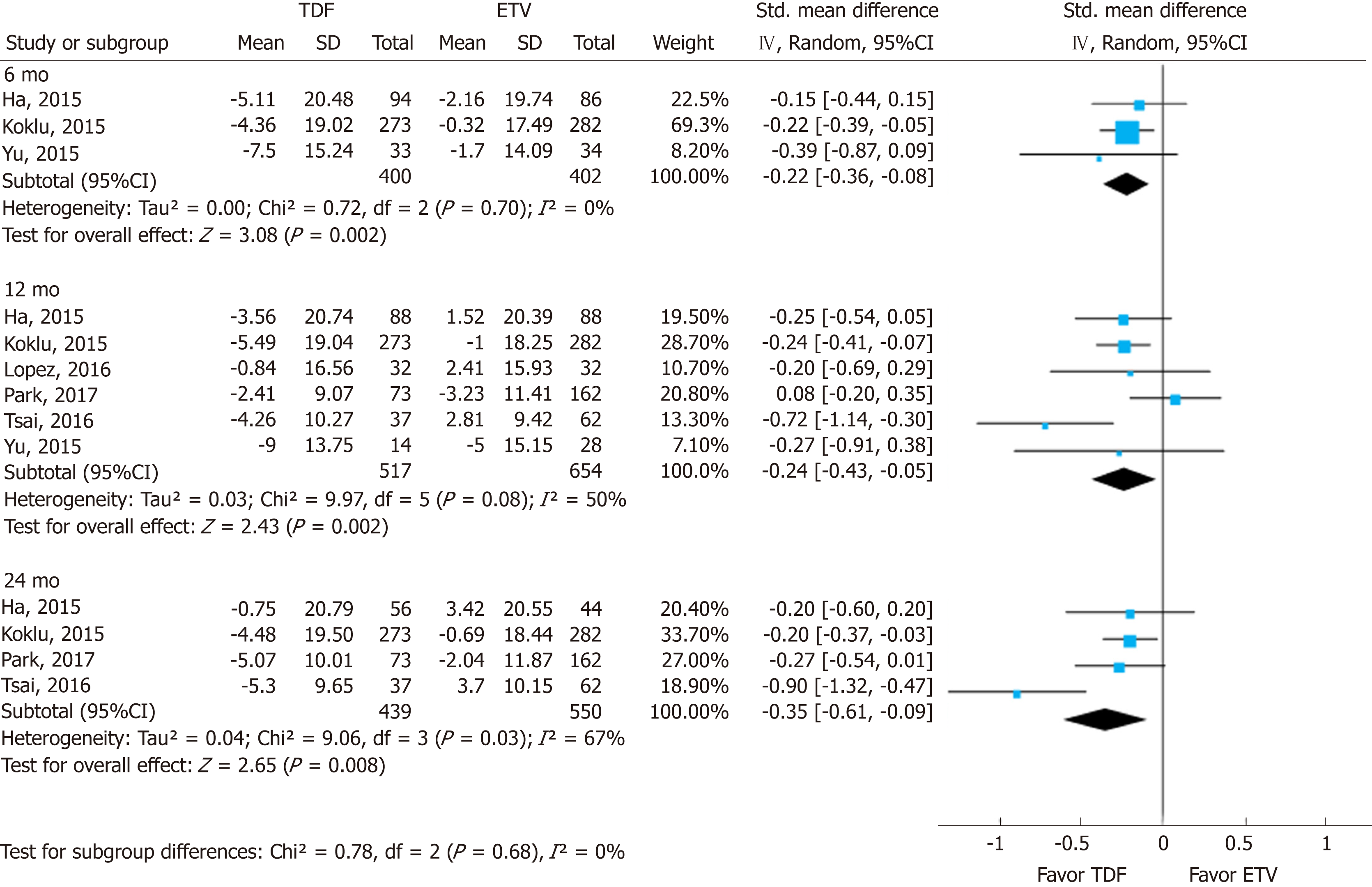
Changes in eGFR were significantly higher in the TDF group than in the ETV group. Most studies used various eGFR formulas (Supplement Table 8). The standardized mean differences (SMDs) of serum eGFR between the TDF and ETV groups at 6, 12, and 24 mo were -0.22 (95%CI: -0.36--0.08; I2 = 0%), -0.24 (95%CI: -0.43--0.05; I2 = 50%), and -0.35 (95%CI: -0.61--0.09; I2 = 67%), respectively (Figure 3). There was no significant difference in SMD among different time points.
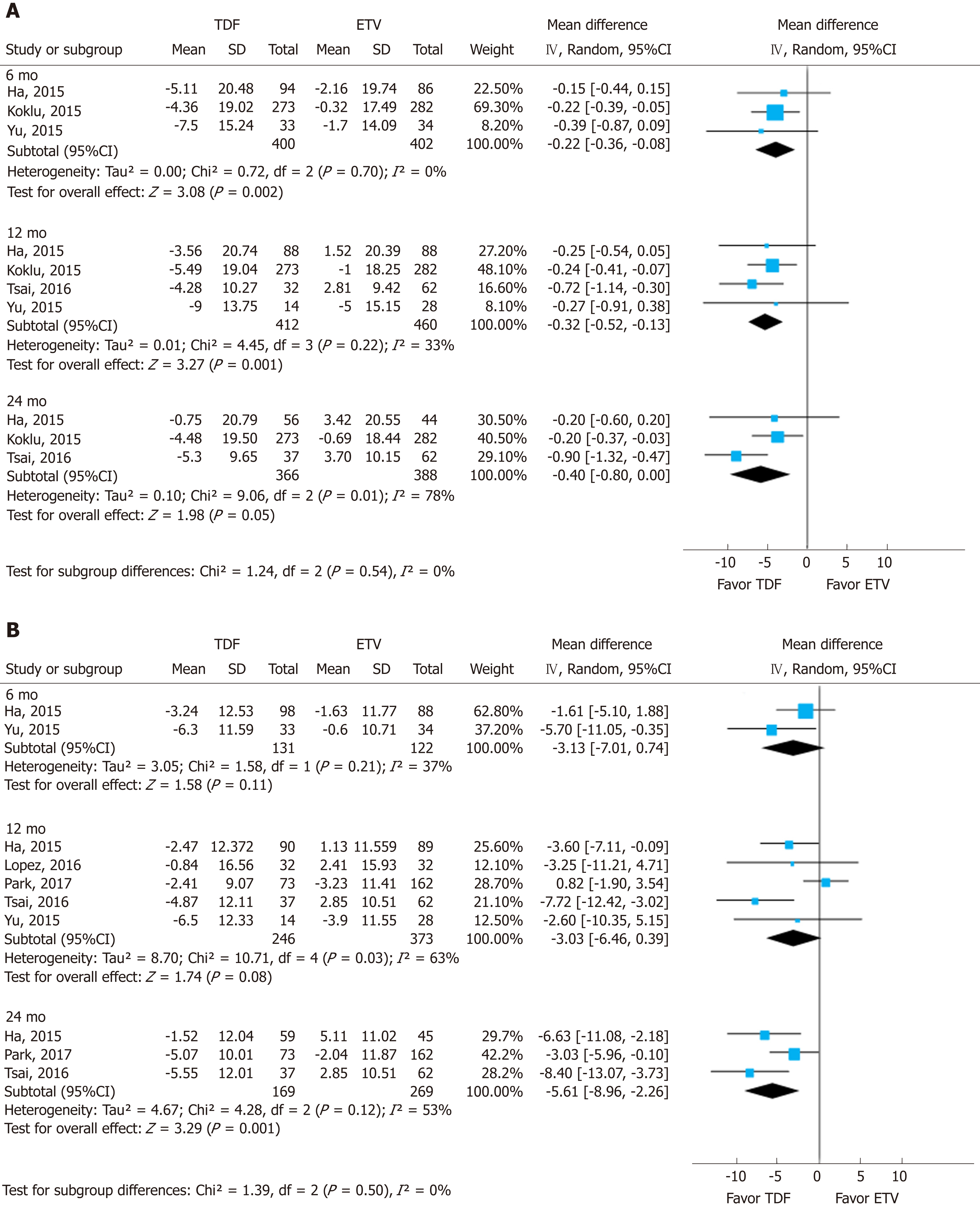
To assess the effect size, we conducted subgroup analysis of calculated MDRD and CKD-EPI after securing raw data on creatinine (Figure 4). The MDRD and CKD-EPI equations both showed the same directivity without subgroup difference in each follow-up period (I2 = 0%). However, the studies used for the analysis of MDRD and CKD-EPI were different, and the number of studies was insufficient.
We tested the heterogeneity of studies with 12-mo and 24-mo enrollment categories, as shown in Figure 2. To determine the cause of heterogeneity, we compared the inclusion criteria of the enrolled studies (Supplemental Table 8) and conducted meta-regression (Figure 5A and B). We considered the possibility that (mean age, creatinine) may have been a major source of substantial heterogeneity observed across the studies by research-level factors. In the meta-regression analysis, we did not find any statistically significant tendency dependent on available study characteristics. However, individual patient-level data could have been weak due to aggregation bias. But through meta-regression we identified two studies as potential sources of heterogeneity. Park et al[20] analyzed only cirrhotic patients in their study, and included 22% of decompensated cirrhotic patients, diabetics and patients who take diuretics. Tsai et al[21] showed greater heterogeneity than other studies. Tsai included patients with all impaired renal function (eGFR 30-90 mL/min per 1.73). When we excluded the study by Tsai et al[21], heterogeneity decreased from 78% to 1% in the 12-mo group and from 93% to 82% in the 24-mo group. After excluding the Tsai et al[21] study, the effect size remained statistically significant (Figure 5C).
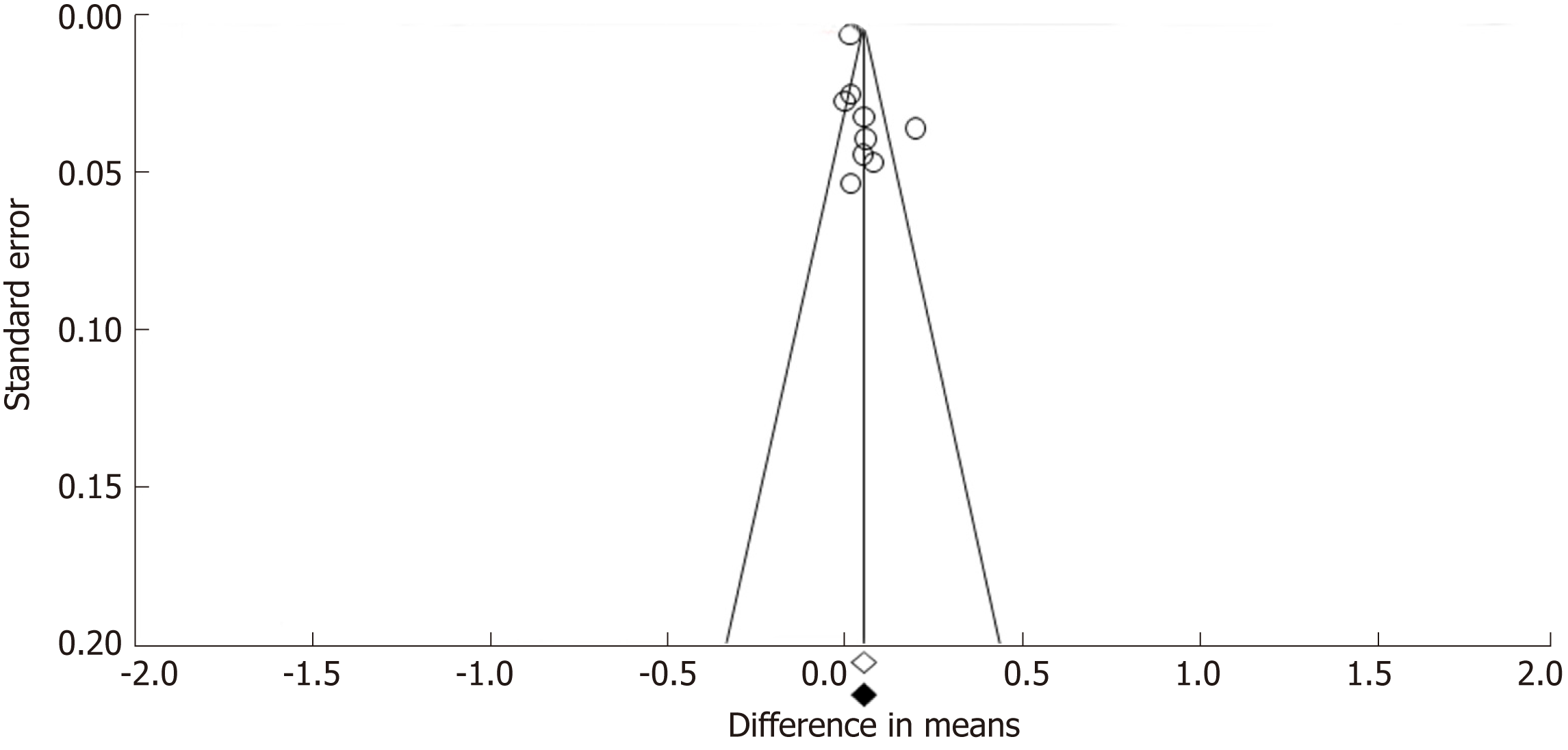
Funnel plots constructed with observed studies showed symmetry (Figure 6). In Egger’s test, publication bias revealed no significant evidence [bias = 1.967 (95%CI: -2.067-5.980), P = 0.285]. None of the studies trimmed in the Trim & Fill method with the random effect model. There was no significant publication bias detected by funnel plots.
In this systematic review and meta-analysis of prior studies, (1) The mean differences in serum creatinine levels between TDF and ETV were 0.03 mg/dL (95%CI: 0.02-0.04; I2 = 0%), 0.05 mg/dL (95%CI: 0.02 to 0.08; I2 = 78%), and 0.07 mg/dL (95%CI: 0.01-0.13; I2 = 93%) at 6, 12, and 24 mo, respectively. Although the mean difference was statistically significant, a gap of 0.03-0.07 mg/dL does not reach clinical significance. Although the mean difference seemed to increase gradually over time, there was no significant difference among the estimates. (2) Similarly, there was a significant standardized mean difference in serum eGFR levels between the two drugs over the same time periods. TDF showed a greater decrease than ETV after 6, 12, and 24 mo use. And (3) No significant difference was found through subgroup analysis and sensitivity analysis by research-level factors and patient-level factors.
Our research is different from existing studies for the following reasons: (1) We used continuous variables when comparing the influence of drugs on renal function. (2) We enhanced clinical relevance by using qualified research quality assessment methods and sensitivity analysis with meta-regression by study and patient characteristics. Although there have been many studies and efforts to clarify the influence of anti-HBV agents on renal function, these have resulted in conflicting data[8-10]. To our knowledge, there were three systematic reviews for efficacy and safety comparing TDF and ETV, but two of them primarily focused on efficacy rather than renal safety issues[8,9]. Some critical manuscripts and abstracts were not included in the previous studies. Moreover, most studies used various eGFR formula and different AKI definitions. Lok et al[9] only listed dichotomous data that consisted of an arbitrary definition of deterioration of renal function by NAs and change in eGFR values without statistical analysis. Han et al[8] suggested more complex conclusions. In their work, there was a statistically significant difference between TDF and ETV in eGFR at the endpoint (RR = 1.601, 95%CI: 1.035-2.478, I2 = 0.0%), but no significant difference in the change of eGFR from baseline (RR = 0.929, 95%CI: 0.616-1.4601, I2 = 0.0%). However, the authors did not clearly show how many studies were included in the analysis. Because renal safety was defined as a secondary outcome, there were several missing papers, and selection bias might have occurred. Moreover, in case of the study by Gish et al, which was one of the studies included by Han et al[8], nephrotoxicity was defined by an increase in serum creatinine of ≥ 0.2 mg/dL. There was no difference between the TDF- and ETV-treated groups, but more patients in the TDF group experienced eGFR decreases than in the ETV group. In this case, changes in renal function could be underestimated. An article by Chan et al[10] showed effect size with a continuous variable. They compared the effect of various NAs on renal function using a network analysis. However, the authors did not include articles regarding the effects of NAs on renal function comprehensively, with only three articles making direct comparisons between TDF and ETV. Moreover, the definition of AKI was inconsistent in the three included articles.
In this study, we determined that while TDF has been linked to declines in renal function, the difference is not clinically definitive. Although there was a statistically significant decrease in the short- and medium-term, our study does not guarantee long-term stability. Moreover, some studies found no significant change in renal function in TDF users in the longer term[26-28]. In sensitivity analysis, we conjectured that the use of TDF is associated with mild renal impairment. Although there are very few studies on CKD patients, many studies have performed a subgroup analysis of CKD patients in CHB. Recently, Trinh et al[29] showed that in the absence of underlying disease, the use of TDF did not significantly impair renal function, but the use of TDF in patients with CKD and over 60 years of age exacerbated a decline in renal function. In the study by Wong et al[30], the TDF-treated group had a significantly increased risk of CKD progression (HR = 1.21) and showed a more rapid progression of CKD.
Our study has several limitations: (1) Using GRADE criteria, we found that overall confidence in estimates was low. Due to the nature of the nonrandomized designs used in these studies, there was serious inconsistency and the level of quality of most of the studies was low (Supplemental Table 7). (2) The heterogeneity of several subgroups were high. Through the sensitivity analysis, we found the weight and effect size of one specific paper was large and have tried to explain the reason for this specificity. (3) We used SMD to perform the meta-analysis, as the formulas for eGFR calculation varied across studies. Therefore, the results need to be interpreted with caution. (4) There was a fundamental clinical heterogeneity. Although we performed sensitivity analysis, Child-Turcotte-Pugh score, hepatocellular carcinoma, hyper-tension, and diabetes mellitus were not controlled perfectly. (5) Our findings should be interpreted cautiously, since the studies included in our meta-analysis adopted different definitions and inclusion criteria for renal impairment. And (6) Finally, the total number of studies and patients is small. Larger scale cohort studies with long-term follow-up are warranted to provide more precise data on long-term renal adverse events.
In summary, our meta-analysis of observational studies reveals that TDF stati-stically significantly increased serum creatinine levels and decreased the eGFR over 6-24 mo in comparison with ETV. However, the quality of the evidence was moderate to low quality and the difference was inappreciable.
Tenofovir (TDF) and entecavir (ETV) are preferred first-line treatments for chronic hepatitis B (CHB). The long-term safety issue of nucleos(t)ide analog is very important because CHB patients should take it indefinitely. In addition, a number of researchers have recently reported many CHB patients suffer from CKD through large-scale studies.
Over the years, several studies have been conducted to compare renal safety of the two drugs, but the results varied and sometimes conflicted with each other. Confusedly, the recom-mendations of the recent two guidelines are contradictory.
We aimed to conduct a systematic review and meta-analysis to assess renal safety of TDF and ETV in patients with CHB using continuous variables.
Calculating the change of creatinine and estimated glomerular filtration rate (eGFR), we secured the distinction from the prior meta-analysis that using dichotomous data. To enhance the clinical importance, we performed sensitivity analysis with meta-regression.
With nine NRSs, we conducted meta-analysis. Changes in creatinine levels were higher in the TDF group than in the ETV group at 6 mo [mean difference (MD) = 0.03 mg/dL; 95%CI: 0.02-0.04; I2 = 0%], 12 mo (MD = 0.05 mg/dL; 95%CI: 0.02 to 0.08; I2 = 78%), and 24 mo (MD = 0.07 mg/dL; 95%CI: 0.01-0.13; I2 = 93%). The change in eGFR was significantly higher in the TDF group than in the ETV group at 6 mo [standardized mean difference (SMD) = -0.22; 95%Cl: -0.36--0.08; I2 = 0%], 12 mo (SMD = -0.24; 95%Cl: -0.43 to -0.05; I2 = 50%), and 24 mo (-0.35; 95%Cl: -0.61--0.09; I2 = 67%).
Until now, in studies comparing the effect on renal function between the two drugs, the di-fferences varied greatly. However, our study found that the difference was negligible.
The value of creatinine and eGFR in our meta-analysis was a secondary outcome in most of the included studies. And most of the studies used various eGFR formula and different AKI definitions. We need further research comparing renal function as a primary outcome and using universal definition of AKI, if possible, through large-scale RCT.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Razek AA, Hann HW, Yoshioka K S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
| 1. | World Health Organization. Global Hepatitis Report, 2017. Geneva, Switzerland: World Health Organization. Available from: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. [Cited in This Article: ] |
| 2. | Nguyen MH, Lim JK, Burak Ozbay A, Fraysse J, Liou I, Meyer N, Dusheiko G, Gordon SC. Advancing Age and Comorbidity in a US Insured Population-Based Cohort of Patients With Chronic Hepatitis B. Hepatology. 2019;69:959-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2290] [Cited by in F6Publishing: 2239] [Article Influence: 373.2] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver;. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2771] [Cited by in F6Publishing: 3100] [Article Influence: 442.9] [Reference Citation Analysis (0)] |
| 5. | Wang HM, Hung CH, Lee CM, Lu SN, Wang JH, Yen YH, Kee KM, Chang KC, Tseng PL, Hu TH, Chen CH. Three-year efficacy and safety of tenofovir in nucleos(t)ide analog-naïve and nucleos(t)ide analog-experienced chronic hepatitis B patients. J Gastroenterol Hepatol. 2016;31:1307-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Petersen J, Heyne R, Mauss S, Schlaak J, Schiffelholz W, Eisenbach C, Hartmann H, Wiese M, Boeker K, Loehr HF, John C, Leuschner M, Trautwein C, Felten G, Trein A, Krause W, Ruppert S, Warger T, Hueppe D. Effectiveness and Safety of Tenofovir Disoproxil Fumarate in Chronic Hepatitis B: A 3-Year Prospective Field Practice Study in Germany. Dig Dis Sci. 2016;61:3061-3071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Marcellin P, Zoulim F, Hézode C, Causse X, Roche B, Truchi R, Pauwels A, Ouzan D, Dumortier J, Pageaux GP, Bourlière M, Riachi G, Zarski JP, Cadranel JF, Tilliet V, Stern C, Pétour P, Libert O, Consoli SM, Larrey D. Effectiveness and Safety of Tenofovir Disoproxil Fumarate in Chronic Hepatitis B: A 3-Year, Prospective, Real-World Study in France. Dig Dis Sci. 2016;61:3072-3083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and Meta-analysis. Int Immunopharmacol. 2017;42:168-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Lok AS, McMahon BJ, Brown RS, Wong JB, Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA, Singh S, Mohamed EA, Abu Dabrh AM, Prokop LJ, Wang Z, Murad MH, Mohammed K. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 10. | Chan HL, Shaikh J, Gupta S, Hamed K. Renal Function in Nucleos(t)ide Analog-Treated Patients With Chronic Hepatitis B: A Systematic Literature Review and Network Meta-Analysis. Adv Ther. 2016;33:862-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16777] [Cited by in F6Publishing: 16214] [Article Influence: 1080.9] [Reference Citation Analysis (1)] |
| 12. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14425] [Cited by in F6Publishing: 15634] [Article Influence: 651.4] [Reference Citation Analysis (0)] |
| 13. | Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc. 2006;359-363. [PubMed] [Cited in This Article: ] |
| 14. | Ha NB, Ku K, Ha NB, Chaung KT, Trinh HN, Nguyen MH. Renal Function in Chronic Hepatitis B Patients Treated With Tenofovir Disoproxil Fumarate or Entecavir Monotherapy: A Matched Case-Cohort Study. J Clin Gastroenterol. 2015;49:873-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Lee SW, Kwon JH, Lee J, Han NI, Kim YW, Nam SW, Chung KW, Jang JW. Comparison of Efficacy between Tenofovir and Entecavir in Treatment Naive Chronic hepatitis B Patients. Korean Association for the Study of the Liver. 2015;2015:108. [Cited in This Article: ] |
| 16. | Yu HM, Kwon SY, Kim J, Chung HA, Kwon SW, Jeong TG, An SH, Jeong GW, Yun SU, Min JK, Kim JH, Choe WH. Virologic response and safety of tenofovir versus entecavir in treatment-naïve chronic Hepatitis B patients. Saudi J Gastroenterol. 2015;21:146-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Julian PTH, Sally G. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, 2008. . [Cited in This Article: ] |
| 18. | Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 770] [Cited by in F6Publishing: 1222] [Article Influence: 122.2] [Reference Citation Analysis (0)] |
| 19. | Schünemann HBJ, Brozek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group, 2013. Available from: https://gdt.gradepro.org/app/handbook/handbook.html. [Cited in This Article: ] |
| 20. | Park J, Jung KS, Lee HW, Kim BK, Kim SU, Kim DY, Ahn SH, Han KH, Park JY. Effects of Entecavir and Tenofovir on Renal Function in Patients with Hepatitis B Virus-Related Compensated and Decompensated Cirrhosis. Gut Liver. 2017;11:828-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Tsai MC, Chen CH, Tseng PL, Hung CH, Chiu KW, Chang KC, Yen YH, Lin MT, Hu TH. Does Nucleos(t)ide Analogues Treatment Affect Renal Function in Chronic Hepatitis B Patients Who Have Already Decreased eGFR? A Longitudinal Study. PLoS One. 2016;11:e0149761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | López Centeno B, Collado Borrell R, Pérez Encinas M, Gutiérrez García ML, Sanmartin Fenollera P. Comparison of the effectiveness and renal safety of tenofovir versus entecavir in patients with chronic hepatitis B. Farm Hosp. 2016;40:279-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 23. | Koksal AR, Alkim H, Boga S, Iyisoy MS, Sen I, Tekin Neijmann S, Alkim C. Value of Cystatin C-Based e-GFR Measurements to Predict Long-Term Tenofovir Nephrotoxicity in Patients With Hepatitis B. Am J Ther. 2019;26:e25-e31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Koklu S, Gulsen MT, Tuna Y, Koklu H, Yuksel O, Demir M, Guner R, Dogan Z, Kucukazman M, Poyrazoglu OK, Biyik M, Ozturk NA, Aydogan T, Coban S, Kocaman O, Sapmaz F, Gokturk SH, Karaca C, Demirezer A, Tanoglu A, Yildirim B, Altinbas A, Atak BM, Cosar AM, Alkan E; Other collaborators. Differences in nephrotoxicity risk and renal effects among anti-viral therapies against hepatitis B. Aliment Pharmacol Ther. 2015;41:310-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Idilman R, Gunsar F, Koruk M, Keskin O, Meral CE, Gulsen M, Elhan AH, Akarca US, Yurdaydin C. Long-term entecavir or tenofovir disoproxil fumarate therapy in treatment-naïve chronic hepatitis B patients in the real-world setting. J Viral Hepat. 2015;22:504-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Buti M, Tsai N, Petersen J, Flisiak R, Gurel S, Krastev Z, Aguilar Schall R, Flaherty JF, Martins EB, Charuworn P, Kitrinos KM, Subramanian GM, Gane E, Marcellin P. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60:1457-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 27. | Sriprayoon T, Mahidol C, Ungtrakul T, Chun-On P, Soonklang K, Pongpun W, Laohapand C, Dechma J, Pothijaroen C, Auewarakul C, Tanwandee T. Efficacy and safety of entecavir versus tenofovir treatment in chronic hepatitis B patients: A randomized controlled trial. Hepatol Res. 2017;47:E161-E168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1228] [Cited by in F6Publishing: 1226] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 29. | Trinh S, Le AK, Chang ET, Hoang J, Jeong D, Chung M, Lee MH, Wang U, Henry L, Cheung R, Nguyen MH. Changes in Renal Function in Patients With Chronic HBV Infection Treated With Tenofovir Disoproxil Fumarate vs Entecavir. Clin Gastroenterol Hepatol. 2019;17:948-956.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Wong GL, Chan HL, Tse YK, Yip TC, Lam KL, Lui GC, Szeto CC, Wong VW. Chronic kidney disease progression in patients with chronic hepatitis B on tenofovir, entecavir, or no treatment. Aliment Pharmacol Ther. 2018;48:984-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |