Published online May 14, 2019. doi: 10.3748/wjg.v25.i18.2264
Peer-review started: March 6, 2019
First decision: April 5, 2019
Revised: April 17, 2019
Accepted: April 19, 2019
Article in press: April 20, 2019
Published online: May 14, 2019
Polycystic liver disease (PCLD) with a large cystic volume deteriorates the quality of life of patients through substantial effects on the adjacent organs, recurrent cyst infections, cyst rupture, and hemorrhage. Surgical or radiological intervention is usually needed to alleviate these symptoms. We report a rare case of the cystic metastasis of renal cell carcinoma (RCC), which was misdiagnosed as PCLD, as a result of the clinical and radiological similarity between these disorders.
A 74-year-old female who had undergone nephrectomy for papillary-type RCC (PRCC) was suffering from abdominal pain and the recurrent intracystic hemorrhage of multiple cysts in the liver. Imaging studies and aspiration cytology of the cysts showed no evidence of malignancy. With a diagnosis of autosomal dominant polycystic liver disease, the patient received hepatectomy for the purpose of mass reduction and infectious cyst removal. Surgery was performed without complications, and the patient was discharged on postoperative day 14. Postoperatively, the pathology revealed a diagnosis of recurrent PRCC with cystic formation.
This case demonstrates the importance of excluding the cystic metastasis of a cancer when liver cysts are observed.
Core tip: Polycystic liver disease (PCLD) usually exhibits typical presentations in imaging studies, but the diagnosis is sometimes challenging because of the late onset of this genetic disorder and the atypical presentations of other diseases. In a case of cystic metastasis of renal cell carcinoma, the disease could be misdiagnosed as PCLD due to the clinical and radiological similarity between these disorders. This case demonstrates that when multiple cystic lesions are observed in the liver, it is important to first exclude the cystic metastasis of a cancer. Additionally, some specific types of cancer can have different presentations at metastasis and recurrence.
- Citation: Liang C, Takahashi K, Kurata M, Sakashita S, Oda T, Ohkohchi N. Recurrent renal cell carcinoma leading to a misdiagnosis of polycystic liver disease: A case report. World J Gastroenterol 2019; 25(18): 2264-2270
- URL: https://www.wjgnet.com/1007-9327/full/v25/i18/2264.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i18.2264
Polycystic liver disease (PCLD) is defined as the presence of more than 20 cysts in the liver or the presence of more than 4 cysts in the liver with a family history of the disease. PCLD is a relatively rare disease, which is estimated to be present in 0.05%-0.53% of the total population[1]. PCLD manifests as clinical symptoms such as tiredness, fullness, shortness of breath, dissatisfaction with the abdomen size, limited mobility and early satiety, which significantly deteriorate the patient’s quality of life[2]. PCLD is classified as one of two inherited disorders, i.e., autosomal dominant polycystic kidney disease (ADPKD) and autosomal dominant polycystic liver disease (ADPLD). ADPLD is distinguished from ADPKD by the absence of polycystic kidneys. The mutations present in polycystic kidney disease (PKD1 and PKD2) are causative genes for ADPKD, while 20% of ADPLD is caused by mutations in the protein kinase C substrate 80K-H (PRKCSH) or SEC63, leaving the other 80% with unknown etiologies. PCLD, together with congenital hepatic fibrosis, is a type of disease that is characterized by the dysfunction of the primary cilium[3]. Current radiological and surgical interventions for “symptomatic” PCLD patients include aspiration-sclerotherapy, fenestration, hepatectomy and liver transplantation. Hepatectomy is usually indicated for Gigot type II PCLD, in which one liver segment is retained with unaffected liver parenchyma[4].
The diagnosis of PCLD is sometimes challenging. Typical differential diagnoses include ciliated hepatic foregut cysts, hepatobiliary cystadenomas, and parasitic cysts. However, in very rare cases, the cystic metastasis of a cancer becomes an important differential diagnosis that significantly changes the treatment strategy. The origins of cystic metastasis include colon, pancreas, ovary, kidney, neuroendocrine, and prostate cancer[1]. Here, we describe a rare case of the cystic metastasis of renal cell carcinoma (RCC), for which hepatectomy was performed due to the misdiagnosis of ADPLD.
A 74-year-old female complained of right upper quadrant abdominal pain when she presented at our hospital. Computed tomography (CT) with intravenous contrast demonstrated the local recurrence of RCC in the ipsilateral lymph nodes and multiple liver cysts.
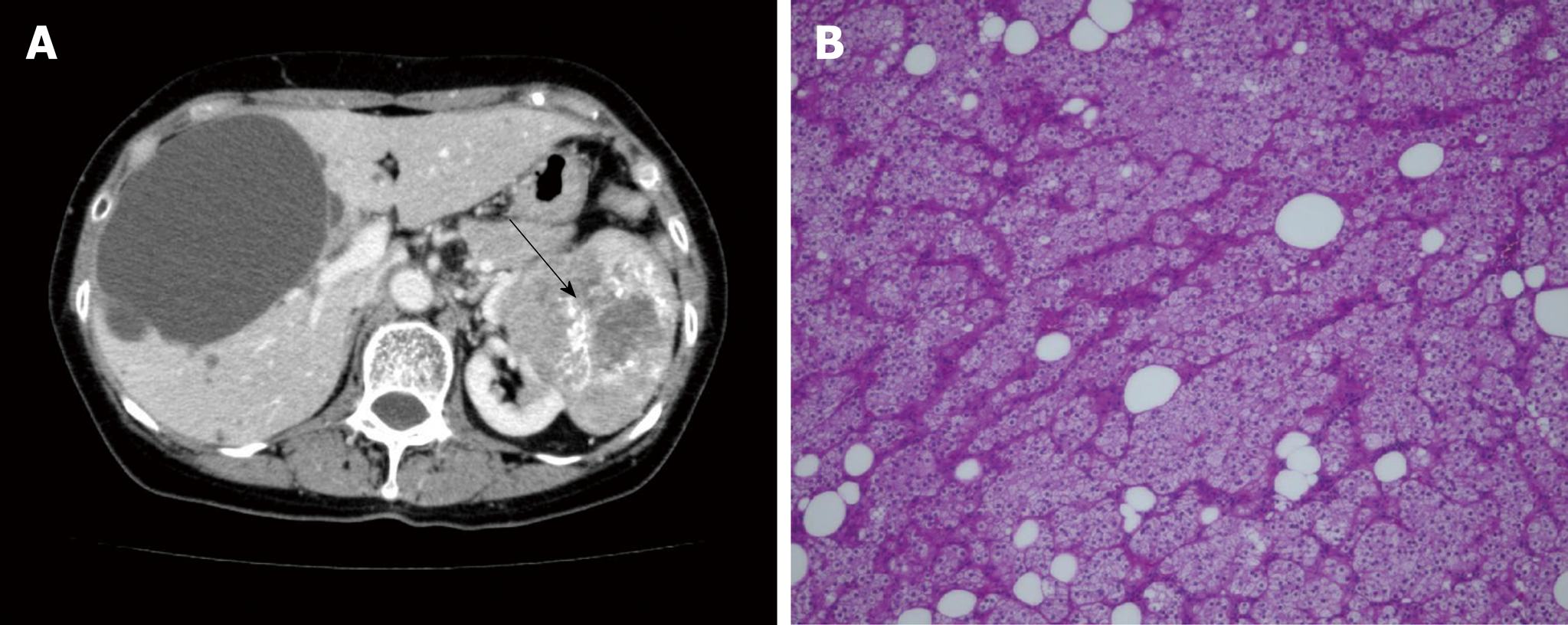
The patient was diagnosed with left renal cancer and liver cysts in the bilateral lobes 4 years prior (Figure 1A). There was one large cyst and several small cysts, as demonstrated by a CT scan. The liver cysts appeared as well-demarcated and water-dense sacs without mural nodules. The patient had not received health screening for 20 years. Left nephrectomy with ipsilateral adrenalectomy was performed. Pathology revealed PRCC, G2, INF-α, pT2a, N0, M0, and v (-) (Figure 1B). No cysts were found in the excision. Eight months prior to the surgery, the patient complained of right upper quadrant pain secondary to recurrent intracystic hemorrhage and received cyst aspiration and sclerotherapy. Aspiration cytology showed no evidence of malignancy, and the pain recurred soon after the treatment.
The patient had a medical history of hypertension and hyperlipemia.
The patient did not have a family history of PCLD.
The patient’s temperature was 36.2 °C, heart rate was 82 bpm, respiratory rate was 14 breaths per minute, blood pressure was 140/99 mmHg and oxygen saturation in room air was 98%. A surgical scar was located in the left upper abdomen. In the abdominal examination, an abdominal mass was observed in the umbilical region, shifting dullness was present and liver cysts were palpable.
Blood analysis revealed anemia with a hemoglobin level of 7.8 g/dL, white blood cells at 2.3 × 109/L, with a normal hematocrit and platelet count. The prothrombin, partial thromboplastin times and d-dimers were normal. The serum albumin was low, at 3.2 mg/dL. In the blood biochemistry analysis, the lactate dehydrogenase was high, at 262 U/L, with a high alkaline phosphatase level, at 626 U/L, and a γ-glutamyl transpeptidase rate of 101 U/L. The alanine aminotransferase and aspartate aminotransferase were normal. The urine analysis was normal. The electrocardiogram, chest X-ray and arterial blood gas were also normal.
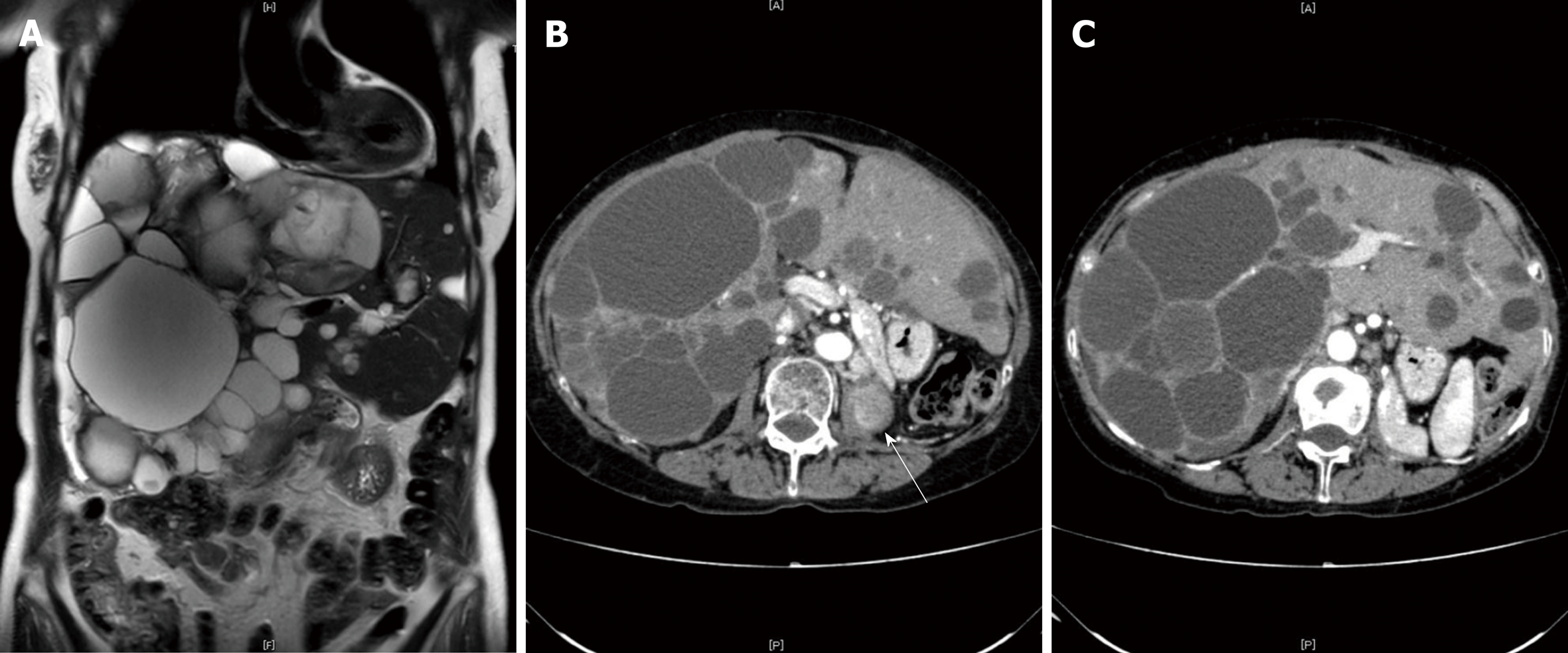
CT with intravenous contrast demonstrated the local recurrence of RCC in the ipsilateral lymph nodes and multiple liver cysts, which had increased in size and number (Figure 2A and B). The cysts were various in sizes, but the borders were clear, and there was no sign of enhancement in the cystic walls.
Considering the fact that multiple cysts existed, mainly in the right lobe, while normal liver areas were retained in the lateral section, the patient was classified as having Type II PCLD, based on Gigot's classification (Figure 2B and C).
Tumor resection with lymphadenectomy was planned to ensure the complete resection of the locally recurrent RCC. Further, with a diagnosis of PCLD, right lobectomy combined with cyst fenestration was planned to provide an optimized method for alleviating the symptoms.
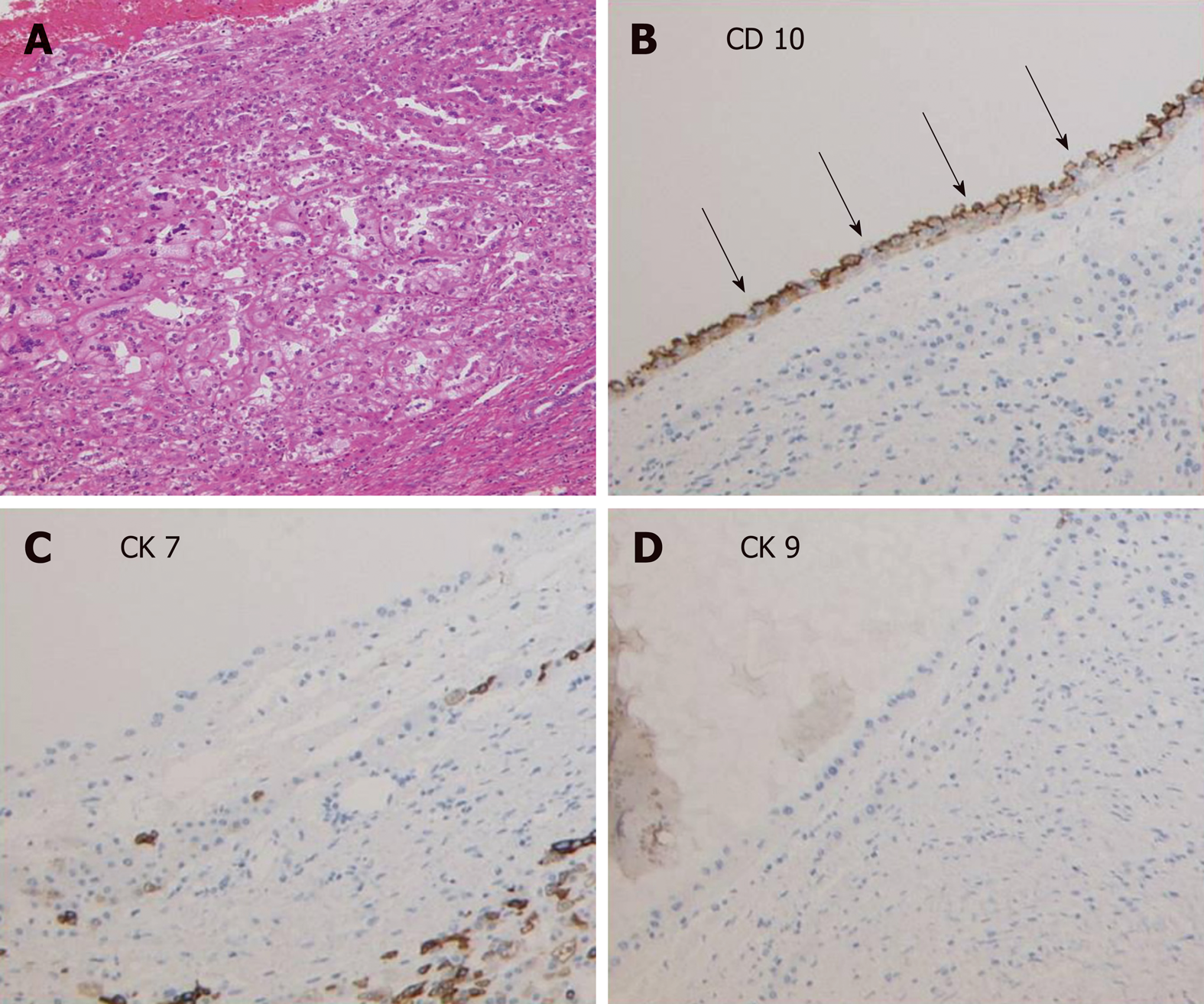
The surgery was completed without major intraoperative complications. In the pathological report of the liver specimens, cells located focally around the cysts formed tubule-papillary structures that were mostly lined with single-cuboidal or low-columnar epithelial cells with scant cytoplasm and uniform nuclei, which were morphologically similar to PRCC by hematoxylin-eosin staining (Figure 3A)[5]. Immunohistochemical staining showed the presence of CD10+ cells in the edges of the cysts (Figure 3B), while CK7+ and CK19+ cells were absent (Figure 3B-D). Combined with the results of H&E staining, the diagnosis of PRCC liver metastasis was verified. The patient was discharged on postoperative day 14 and started sunitinib treatment 1 mo after the hepatectomy. It has been 2 years since the surgery was performed. The pain was relived after the surgery, and the patient is still alive. However, lymph node metastases and lung metastases appeared after a few months, and the cysts occupying the remnant liver are growing, causing recurrent abdominal pain. The patient is now managed with symptomatic treatment.
PRCC is the second most frequent RCC subtype, followed by clear cell-type RCC (CRCC). PRCC is known to metastasize less frequently than CRCC, with a reported incidence of 5.7%-11%[6]. The lung and bone are common sites of metastases, whereas the metastasis of the liver is less frequent (16.1%)[6,7]. PRCC can be classified as having solid or cystic masses. Cystic-type PRCC may be a result of its inherent architecture or of cystic degeneration[6]. Cystic metastasis of RCC is very rare[7]. Some cases with cystic lymph node metastases or dissemination have been previously reported in the literature[8-11].
Liver cysts can be classified as developmental, neoplastic, inflammatory, or miscellaneous[12]. The differential diagnosis of liver cysts is sometimes challenging, and excluding metastasis is one of the highest priorities. In typical cases of cystic metastatic cancers, the borders of the cystic lesions are typically heterogeneous and ill-defined[13]. The cystic walls are irregular, and the vessels are amputated; however, these characteristics are not observed in cases of PCLD. Cystic metastases usually have a peripheral enhancing rim on the arterial phase of a CT scan and magnetic resonance imaging, while PCLD usually does not have this feature[14]. Peribiliary cysts, which are also seen with high incidence, are usually located at the hilum and adjacent to the hepatic ducts, and the cyst sizes are smaller than 10 mm[13,14]. The misdiagnosis of cystic liver disease can be critical since the treatment strategy is completely different.
We described a rare case of the cystic metastasis of PRCC, which was misdiagnosed as PCLD. This case is important in that it demonstrates that PRCC can manifest with different presentations at metastasis and recurrence, i.e., multiple cyst formation, imitating PCLD[7,13]. In our case, the preoperative images of the liver cysts indicated that there were clear borders with no signs of enhancement in the cystic walls, which were characteristically similar to the cysts of PCLD, and the aspiration cytology was negative for cancer cells. Pathologically, the cystic walls were irregular, and the cells around the cysts were morphologically compatible with RCC. The diagnosis of RCC was confirmed by the presence of CD10+ cells around the cysts, which is a characteristic surface marker of RCC. It was quite unlikely that cystic liver metastasis occurred to a liver affected by PCLD, since all the cysts that were pathologically investigated showed the malignant characteristics described above. Since CA 50 was reported as a potential tumor marker for cystic RCC[15], we could have tested the CA 50 in the blood and the cystic fluid from repeated fine-needle aspiration. Further, 18F-fluorodeoxyglucose (FDG) positron emission tomography/CT (PET/CT) is highly sensitive and accurate, with a sensitivity of 97% and a specificity of 75% for hepatic metastasis[10]. It has been reported to show intense uptake in cystic liver metastasis compared with that in benign cysts[16]. We could have checked PET/CT scan to confirm the malignancy of the cysts if we had taken metastasis into consideration.
The treatment for metastatic renal cancer is debated, and the therapeutic options are limited. The National Health Service of England Guidelines stated that sunitinib and pazopanib are first-line systemic therapies for metastatic RCC[17]. Complete resection is the only choice for patients with metastatic RCC to have a satisfactory prognosis, with 5-year overall survival rates of 15 to 60%[18]. However, patients with bilateral multiple metastatic RCC, such as our patient, are not candidates for hepatectomy because radical resection cannot be performed, and the surgery will not prolong the overall survival time of the patients[19,20]. In our case, since the patient suffered from acute abdominal pain and recurrent intracystic hemorrhage, we performed hepatectomy following the diagnosis of PCLD. However, as the cysts were located diffusely in the bilateral lobe, the patient would have had no indication for hepatectomy if she had been correctly diagnosed with PRCC preoperatively. We should have started with chemotherapy and palliative care, using opioids and additional adjuvants to alleviate the abdominal symptoms[21]. This case reminds us of the following: first, when multiple cystic lesions are observed throughout the liver parenchyma in patients with cancer, it is important to first exclude metastasis; and second, some specific types of cancer can show different presentations at metastasis and recurrence, which could cause preoperative misdiagnosis.
In a patient with cystic neoplasms of the liver, the diagnosis remains challenging in everyday practice. Cystic lesions may present as solitary or multiple cysts and may range from benign to malignant. To the best of our knowledge, this is the first report of cystic liver metastasis from PRCC. The most important implication of this case is that PCLD must be distinguished from the cystic metastasis of a cancer, which can contribute to a profoundly better prognosis as a result of the use of an optimal treatment approach.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen LW, Li S S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL
| 1. | Chen KW, Chen HW, Ou TM, Tsai WC, Hsieh TY. Hepatic cystic metastatic tumors from a locally controlled nasopharyngeal carcinoma. Advances in Digestive Medicine. 2016;3:69-72. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Neijenhuis MK, Kievit W, Verheesen SM, D'Agnolo HM, Gevers TJ, Drenth JP. Impact of liver volume on polycystic liver disease-related symptoms and quality of life. United European Gastroenterol J. 2018;6:81-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Wills ES, Roepman R, Drenth JP. Polycystic liver disease: ductal plate malformation and the primary cilium. Trends Mol Med. 2014;20:261-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Gevers TJ, Drenth JP. Diagnosis and management of polycystic liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:101-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Peckova K, Martinek P, Pivovarcikova K, Vanecek T, Alaghehbandan R, Prochazkova K, Montiel DP, Hora M, Skenderi F, Ulamec M, Rotterova P, Daum O, Ferda J, Davidson W, Ondic O, Dubova M, Michal M, Hes O. Cystic and necrotic papillary renal cell carcinoma: prognosis, morphology, immunohistochemical, and molecular-genetic profile of 10 cases. Ann Diagn Pathol. 2017;26:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Vikram R, Ng CS, Tamboli P, Tannir NM, Jonasch E, Matin SF, Wood CG, Sandler CM. Papillary renal cell carcinoma: radiologic-pathologic correlation and spectrum of disease. Radiographics. 2009;29:741-754; discussion 755-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Psutka SP, Master VA. Role of metastasis-directed treatment in kidney cancer. Cancer. 2018;124:3641-3655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Dwivedi AND, Mourya C. Disseminated cystic nodal metastasis in renal cell carcinoma mimicking systemic hydatidosis on imaging. J Cancer Res Ther. 2018;14:441-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 9. | Ishii N, Yonese J, Tsukamoto T, Maezawa T, Ishikawa Y, Fukui I. Retroperitoneal cystic metastasis from a small clear cell renal carcinoma. Int J Urol. 2001;8:637-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Rastogi R. Retroperitoneal cystic metastases from renal cell carcinoma. Saudi J Kidney Dis Transpl. 2008;19:244-246. [PubMed] [Cited in This Article: ] |
| 11. | Yamashita T, Morozumi M, Higashi M, Momose S, Tamaru JI. Retroperitoneal Cystic Nodal Metastasis of Renal Cell Carcinoma. Case Rep Urol. 2018;2018:1605102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Mortelé KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics. 2001;21:895-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 347] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Del Poggio P, Buonocore M. Cystic tumors of the liver: a practical approach. World J Gastroenterol. 2008;14:3616-3620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 92] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Borhani AA, Wiant A, Heller MT. Cystic hepatic lesions: a review and an algorithmic approach. AJR Am J Roentgenol. 2014;203:1192-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Ljungberg B, Holmberg G, Sjödin JG, Hietala SO, Stenling R. Renal cell carcinoma in a renal cyst: a case report and review of the literature. J Urol. 1990;143:797-799. [PubMed] [Cited in This Article: ] |
| 16. | Radhakrishnan V, Thulkar S, Karunanithi S, Tanveer N, Bakhshi S. Nasopharyngeal carcinoma with splenic and cystic liver metastases in a pediatric patient: 18F-FDG PET-CT findings. Pediatr Radiol. 2010;40 Suppl 1:S79-S82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | National Health Service of England. Guidelines for the management of renal cancer. Available from: https://www.england.nhs.uk/mids-east/wp-content/uploads/sites/7/2018/05/guidelines-for-the-management-of-renal-cancer.pdf. [Cited in This Article: ] |
| 18. | Krabbe LM, Bagrodia A, Margulis V, Wood CG. Surgical management of renal cell carcinoma. Semin Intervent Radiol. 2014;31:27-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Grimes NG, Devlin JM, Dunne DF, Jones RP, Poston GJ, Fenwick SW, Malik HZ. A systematic review of the role of hepatectomy in the management of metastatic renal cell carcinoma. Eur J Surg Oncol. 2014;40:1622-1628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Bakoyiannis A, Delis S, Triantopoulou C, Dervenis C. Rare cystic liver lesions: a diagnostic and managing challenge. World J Gastroenterol. 2013;19:7603-7619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 33] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Are M, McIntyre A, Reddy S. Global disparities in cancer pain management and palliative care. J Surg Oncol. 2017;115:637-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |