Published online Apr 21, 2019. doi: 10.3748/wjg.v25.i15.1828
Peer-review started: January 21, 2019
First decision: February13, 2019
Revised: February 21, 2019
Accepted: March 1, 2019
Article in press: March 2, 2019
Published online: April 21, 2019
Gastric cancer (GC) is one of the main causes of cancer mortality worldwide. Recent studies on tumor microenvironments have shown that tumor metabolism exerts a vital role in cancer progression.
To investigate whether lysyl oxidase (LOX) and hypoxia-inducible factor 1α (HIF1α) are prognostic and predictive biomarkers in GC.
A total of 80 tissue and blood samples were collected from 140 patients admitted to our hospital between August 2008 and March 2012. Immunohistochemical staining was performed to measure the expression of LOX and HIF1α in tumor and adjacent tissues collected from patients with GC. Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis was used to detect the mRNA expression levels of LOX and HIF1α in patients with GC. In addition, single-factor analysis was applied to analyze the relationship between LOX, HIF1α and prognosis of GC.
Immunohistochemical staining suggested that the expression levels of LOX and HIF1α increased in tumor tissues from patients with GC. QRT-PCR analysis indicated that mRNA expression of LOX and HIF1α was also upregulated in tumor tissues, which was in accordance with the above results. We also detected expression of these two genes in blood samples. The expression level of LOX and HIF1α was higher in patients with GC than in healthy controls. Additional analysis showed that the expression level of LOX and HIF1α was related to the clinicopathological characteristics of GC. Expression of LOX and HIF1α increased with the number of lymph node metastases, deeper infiltration depth and later tumor–node–metastasis stages. Single-factor analysis showed that high expression of LOX and HIF1α led to poor prognosis of patients with GC.
LOX and HIF1α can be used as prognostic and predictive biomarkers for GC.
Core tip: Lysyl oxidase (LOX) is a secreted extracellular matrix protein that plays an important role in remodeling the extracellular matrix and promoting tumor progression. The LOX family comprises the prototypic LOX, as well as the LOX-like proteins that are involved in carcinogenesis. Hypoxia-inducible factor 1α (HIF1α) is a type of transcription factor complex that has the capacity to regulate oxygen tension. In addition, HIF1α is able to activate or bind to multiple target genes and participates in inflammatory and other diseases. HIF1α accelerates the growth and metastasis of hepatocellular carcinoma.
- Citation: Han YL, Chen L, Qin R, Wang GQ, Lin XH, Dai GH. Lysyl oxidase and hypoxia-inducible factor 1α: biomarkers of gastric cancer. World J Gastroenterol 2019; 25(15): 1828-1839
- URL: https://www.wjgnet.com/1007-9327/full/v25/i15/1828.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i15.1828
Gastric cancer (GC) is one of the main causes of cancer mortality worldwide[1]. Current epidemiological data indicate that the incidence rate and mortality of GC rank among the top three of all malignant tumors[2]. The morbidity of GC rises gradually with age, and people aged 50-70 years account for the majority of cases. There is no gender differences in patients with GC, and the occurrence of GC is related to geographic variation in many countries. The clinical manifestations mainly include stomach ache, abdominal distension, loss of appetite and weight loss[3]. GC at early stages is free of symptoms, and advanced GC or metastasis sites could be pathological diagnosed by endoscopy or endoscopic ultrasound[4-7]. Patients who suffer from GC have a poor quality of life, and the survival rates are reported to be low[8]. As estimated in the statistics of GLOBOCAN 2012, about 410,000 patients are newly found to suffer from the pain caused by GC in China[9]. Among them, 330,000 patients die of tumor-related causes.
While several therapies have emerged in recent years, the effects of chemotherapy and surgery for GC remain limited[10]. These interventions do not improve prognosis, increase survival rate or prolong survival time[11]. In addition, there are disadvantages to current treatment methods, including inconvenience, increased prevalence of complications, and side-effects[12]. It has been reported that many factors are involved in the progression of GC. More seriously, approximately 60% of patients with GC have metastases when they are diagnosed[13]. Furthermore, the outcome of patients with late stage GC is poor, and most die within 1 year. Therefore, determination of predictive biomarkers for early diagnosis of GC is important.
Recent studies on tumor microenvironments have shown that tumor metabolism plays a vital role in cancer progression. The role of the microenvironment in tumor metabolism is currently attracting significant attention. Lysyl oxidase (LOX) and hypoxia-inducible factor 1α (HIF1α) affect the tumor microenvironment, and play a crucial role in cancer occurrence and development[14-18]. LOX is a secreted extracellular matrix protein that plays an important role in remodeling the extracellular matrix and promoting tumor progression. The LOX family comprises the prototypic LOX, as well as LOX-like proteins that are involved in carcinogenesis. LOX is a copper-dependent monoamine oxidase, and its overexpression is related to both poor survival and the development of multiple types of cancers[19-22]. HIF1α is a transcription factor complex that can regulate oxygen tension[23]. In addition, HIF1α can activate or bind to multiple target genes, and can contribute to inflammatory and other diseases[22,20]. HIF1α accelerates the growth and metastasis of hepatocellular carcinoma[17].
The present study investigated the expression of LOX and HIF1α in patients with GC and determined whether LOX and HIF1α act as prognostic and predictive biomarkers of GC. Research on the interaction between cancer and factors in the metabolic microenvironment is necessary to investigate the progression of GC and determine predictive biomarkers. Our study showed that LOX and HIF1α can act as biomarkers for the diagnosis and prediction of GC.
A total of 80 tissue and blood samples were collected from 140 patients admitted to our hospital between August 2008 and March 2012. The tumor and adjacent tissues were obtained from patients with GC. The blood samples were obtained from 80 patients with GC and 80 healthy controls. The use of human tissues in this research was agreed to by the volunteers and their relatives. All participants gave written informed consent. This study was conducted with permission from the Institutional Research Ethics Committee of our hospital. GC patients were aged > 18 years, and were diagnosed pathologically and clinically with GC. Clinicians measured and analyzed the conditions according to the Response Evaluation Criteria in Solid Tumors.
Total RNA from tissues and blood samples was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA, United States). The samples were added to TRIzol for 30 min in a 4 °C refrigerator. Total RNA was extracted from samples using chloroform, isopropanol, 75% absolute ethanol (all from Beijing Shiji Tuoxin Fine Chemical Industry Co. Ltd., Beijing, China) and diethyl-pyrocarbonate-treated water (Biosharp, Heifei City, China). RNA was stored in a - 80 °C freezer. Concentration and purity were measured using a Nanodrop machine (Thermo Scientific, Carlsbad, CA, United States). As described previously, to synthesize cDNAs, PrimeScript RT reagent kits (TaKaRa, Dalian City, China) were purchased and used for reverse transcription (RT)[21]. cDNA synthesis was conducted using a RT apparatus (Applied Biosystems, Foster City, CA, United States). The cDNA samples were stored at - 20 °C, and used for additional analysis.
To determine the expression of LOX and HIF1α in tissues and blood samples from patients with GC, qRT-PCR analysis was performed using SYBR reagents (Vazyme, Nanjing City, China) on an Applied Biosystems Real-Time PCR machine (Thermo Scientific). The cDNAs, SYBR reagents, double-distilled water and corresponding primers were used to measure expression of LOX and HIF1α. In addition, β-actin was used as a housekeeping gene. The sequences of primers used in this study are listed in Table 1.
| Target gene | Primer sequences | |
| HIF1α | Forward | CATAAAGTCTGCAACATGGAAGGT |
| Reverse | ATTTGATGGGTGAGGAATGGGTT | |
| LOX | Forward | CAGGCACCGACCTGGATATGG |
| Reverse | CGTACGTGGATGCCTGGATGTAGT | |
| β-actin | Forward | TGGCACCCAGCACAATGAA |
| Reverse | CTAAGTCATAGTCCGCCTAGAAGCA |
Immunohistochemistry
Immunohistochemical staining was performed as described previously[22,23]. Tissue specimens were fixed in 4% paraformaldehyde, and embedded in paraffin for subsequent analysis. Antibodies against LOX and HIF1α were purchased from Abcam (Cambridge, United Kingdom). The concentrations of LOX and HIF1α antibodies were 1:100 and 1:400 and the antibodies were diluted in Primary antibody dilution buffer. The paraffin specimens were incubated with these two antibodies that were used to detect the expression of LOX and HIF1α in the GC tissues. Tissue sections were incubated with antibodies against LOX or HIF1α, and then incubated with peroxidase-conjugated goat anti-rabbit secondary antibody (Cell Signaling Technology, Boston, MA, United States). All specimens were fixed in 4% paraformaldehyde, embedded in paraffin, and stored in the Department of Pathology at our hospital.
All experiments were repeated at least three times. Statistical analyses were performed with SPSS software (IBM, Armonk, NY, United States). Pearson’s χ2 tests for categorical variables. Survival rate was calculated by Kaplan-Meier method, Log-rank tests was used to confirm the relationship between LOX, HIF1α and the development of GC. Analysis of LOX and HIF1α expression was conducted using GraphPad Prism software. P < 0.05 was considered statistically significant.
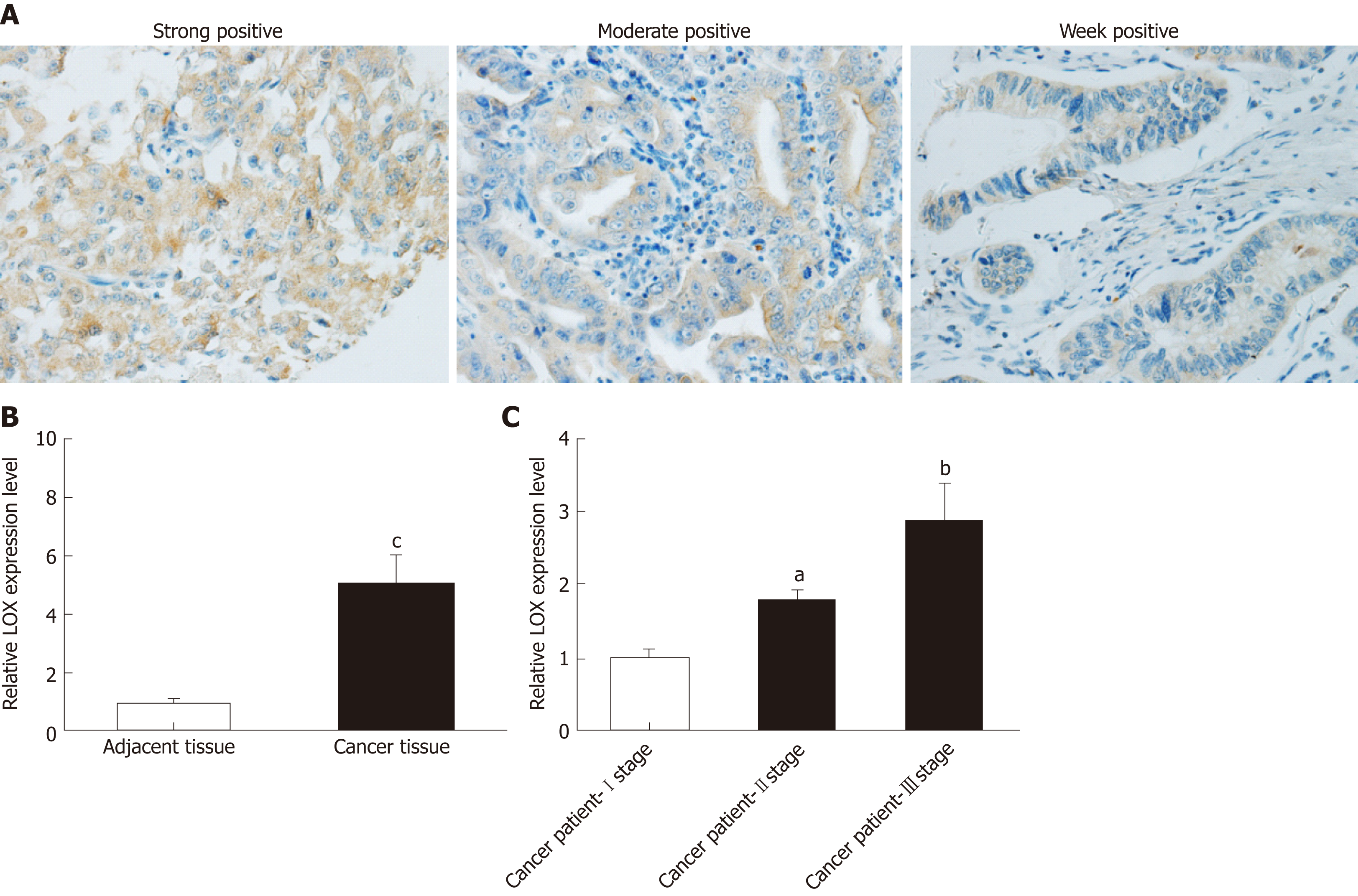
Immunohistochemical staining and qRT-PCR analysis were performed to determine whether expression of LOX was dysregulated in tissues and blood samples from patients with GC versus healthy controls. Immunohistochemical staining indicated that LOX was mainly located in the cytoplasm of tumor cells. Most of the cytoplasm of tumor cells exhibited brown positively stained particles, which displayed a scattered particle distribution. The same results were seen in the extracellular matrix (Figure 1A). The high expression rate of LOX in GC tissues was 35.7% (50/140), which was significantly higher than that in adjacent tissues (18.6%, 26/140) . QRT-PCR showed that mRNA expression of LOX was markedly increased in GC tissues; about four times higher than in adjacent tissues (Figure 1B). We further detected the expression level of LOX in blood samples from GC patients and healthy controls. Expression of LOX was higher in patients with GC than in the control group,Additional analysis showed that the later the clinicopathological stage, the higher the expression level of LOX in the blood samples (Figure 1C). Taken together, these results suggested that expression of LOX was significantly higher in patients with GC.
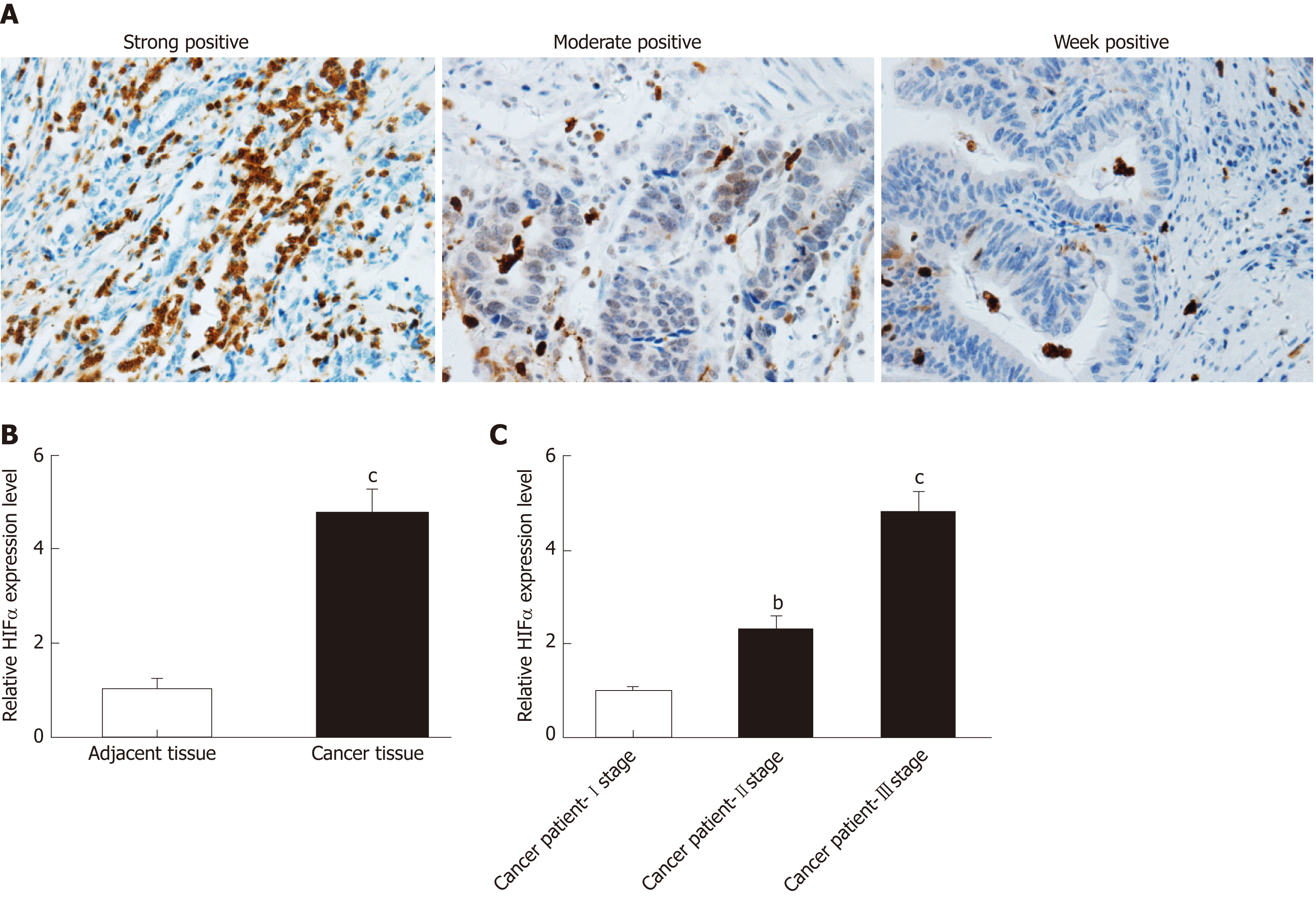
Further analysis was performed to detect expression of HIF1α in patients with GC. Immunohistochemistry revealed that HIF1α was mainly expressed in the nuclei of tumor cells (Figure 2A). The nuclei of HIF1α-positive cells presented with a brown color, and HIF1α exhibited occasional limited expression in the cytoplasm (Figure 2A). Scattered light brown granules were revealed by immunohistochemical staining (Figure 2A). The high expression rate of HIF1α in GC tissues was 33.6% (47/140), which was significantly higher than in adjacent tissues (12.1%, 17/140). The high expression of HIF1α in tumor compared with adjacent tissues was measured by qRT-PCR (Figure 2B). Expression of HIF1α in GC tissues was approximately four times higher than in adjacent tissues (Figure 2B). Additional analysis showed that the later the clinicopathological stage, the higher the expression level of HIF1α in the blood samples (Figure 2C). Thus, we concluded that expression of HIF1α was upregulated in tumor tissues and blood samples from patients with GC.
Expression of LOX and clinicopathological characteristics are shown in Table 2. In various groups with different clinical characteristics, expression of LOX was different. Expression of the LOX gene correlated with lymph node metastasis of GC, the tumor
| Characteristics | Sample size, n | LOX | P | |
| Low expression (%) | High expression (%) | |||
| Gender | 90 (64.3) | 50 (35.7) | ||
| Male | 112 | 73 (65.2) | 39 (34.8) | 0.659 |
| Female | 28 | 17 (60.7) | 11 (39.3) | |
| Age | ||||
| < 60 yr | 67 | 44 (65.7) | 23 (34.3) | 0.743 |
| ≥ 60 yr | 73 | 46 (63.0) | 27 (37.0) | |
| Tumor location | ||||
| Upper part | 57 | 37 (64.9) | 20 (35.1) | 0.978 |
| Middle part | 34 | 21 (61.8) | 13 (38.2) | |
| Lower part | 44 | 29 (65.9) | 15 (34.1) | |
| Total stomach | 5 | 3 (60.0) | 2 (40.0) | |
| Tumor size | ||||
| < 5 cm | 57 | 39 (68.4) | 18 (31.6) | 0.397 |
| ≥ 5 cm | 83 | 51 (61.4) | 32 (38.6) | |
| Depth of invasion | ||||
| T1 | 5 | 5 (100.0) | 0 (0.0) | 0.005 |
| T2 | 13 | 12 (92.3) | 1 (7.7) | |
| T3 | 85 | 56 (65.9) | 29 (34.1) | |
| T4 | 37 | 17 (45.9) | 20 (54.1) | |
| Lymphatic metastasis | ||||
| 0 | 34 | 33 (97.1) | 1 (2.9) | 0.000 |
| 1-2 | 32 | 24 (75.0) | 8 (25.0) | |
| 3-6 | 31 | 21 (67.7) | 10 (32.3) | |
| 7-15 | 37 | 12 (32.4) | 25 (67.6) | |
| ≥ 16 | 6 | 0 (0.0) | 6 (100.0) | |
| Borrmann classification | ||||
| Type I | 16 | 12 (75.0) | 4 (25.0) | 0.340 |
| Type II or III | 110 | 67 (60.9) | 43 (39.1) | |
| Type IV | 11 | 8 (72.7) | 3 (27.3) | |
| Type V | 3 | 3 (100.0) | 0 (0.0) | |
| WHO histological classification | ||||
| Adenocarcinoma | 77 | 48 (62.3) | 29 (37.7) | 0.862 |
| Signet-ring cell carcinoma | 17 | 13 (76.5) | 4 (23.5) | |
| Mucinous adenocarcinoma | 8 | 5 (62.5) | 3 (37.5) | |
| Mixed carcinoma | 32 | 20 (62.5) | 12 (37.5) | |
| Neuroendocrine carcinoma | 6 | 4 (66.7) | 2 (33.3) | |
| Lauren parting | ||||
| Intestinal type | 65 | 42 (64.6) | 23 (35.4) | 0.909 |
| Diffuse type | 68 | 43 (63.2) | 25 (36.8) | |
| Mixed type | 7 | 5 (71.4) | 2 (28.6) | |
| Differentiation grade | ||||
| Low and middle | 107 | 68 (63.6) | 39 (36.4) | 0.744 |
| High | 33 | 22 (66.7) | 11 (33.3) | |
| Cancer embolus | ||||
| Yes | 51 | 29 (56.9) | 22 (43.1) | 0.165 |
| No | 89 | 61 (68.5) | 28 (31.5) | |
| Affect neural | ||||
| Yes | 50 | 29 (58.0) | 21 (42.0) | 0.247 |
| No | 90 | 61 (67.8) | 29 (32.2) | |
| TNM staging | ||||
| I | 10 | 10 (100.0) | 0 (0.0) | 0.000 |
| II | 49 | 44 (89.8) | 5 (10.2) | |
| III | 81 | 36 (44.4) | 45 (55.6) | |
infiltration depth and the tumor-node-metastasis (TNM) stage. The P values for the correlation between LOX expression and lymph node metastasis of GC, between LOX expression and the infiltration depth of GC, and between LOX expression and clinical stage were 0.000, 0.005 and 0.000, respectively. When the number of lymph node metastasis was >16 in patients with GC, the rate of high expression of LOX was 100.0%. When the tumor infiltration depth reached T4, the high expression rate of LOX was 54.1%. When the tumor was clinicopathological stage III, the high expression rate of LOX was 55.6%. However, expression of LOX did not show any relationship with other clinical features, such as age, gender, tumor size, and tumor location. The results showed that the expression of LOX increased with the number of lymph node metastases, deeper infiltration depth and the late TNM stage.
Expression of HIF1α and clinicopathological characteristics are shown in Table 3. Expression of HIF1α differed among various groups. Expression of HIF1α was related to lymph node metastasis (P = 0.000). When the number of lymph node metastasis was > 16, the rate of high expression of HIF1α was 100.0%. When the tumor infiltration depth reached T4, high expression of HIF1α was 56.8% (P = 0.001). When they had stage III disease, the rate of high expression of HIF1α was 51.9% (P = 0.000). These results suggested that expression of HIF1α correlated with the number of metastatic lymph nodes,the tumor infiltration depth and the TNM stage. However, expression of HIF1α was not associated with other clinical characteristics, such as age, gender, tumor size, and tumor location.
| Characteristics | Sample size, n | HIF1α | P | |
| Low expression (%) | High expression (%) | |||
| Gender | 93 (66.4) | 47 (33.6) | ||
| Male | 112 | 76 (67.9) | 36 (32.1) | 0.474 |
| Female | 28 | 17 (60.7) | 11 (39.3) | |
| Age | ||||
| < 60 yr | 67 | 47 (70.1) | 20 (29.9) | 0.372 |
| ≥ 60 yr | 73 | 46 (63.0) | 27 (37.0) | |
| Tumor location | ||||
| Upper part | 57 | 41 (71.9) | 16 (28.1) | 0.702 |
| Middle part | 34 | 19 (55.9) | 15 (44.1) | |
| Lower part | 44 | 30 (68.2) | 14 (31.8) | |
| Total stomach | 5 | 3 (60.0) | 2 (40.0) | |
| Tumor size | ||||
| < 5 cm | 57 | 41 (71.9) | 16 (28.1) | 0.253 |
| ≥ 5 cm | 83 | 52 (62.7) | 31 (37.3) | |
| Depth of invasion | ||||
| T1 | 5 | 5 (100.0) | 0 (0.0) | 0.001 |
| T2 | 13 | 12 (92.3) | 1 (7.7) | |
| T3 | 85 | 60 (70.6) | 25 (29.4) | |
| T4 | 37 | 16 (43.2) | 21 (56.8) | |
| Lymphatic metastasis | ||||
| 0 | 34 | 29 (85.3) | 5 (14.7) | 0.000 |
| 1-2 | 32 | 24 (75.0) | 8 (25.0) | |
| 3-6 | 31 | 23 (74.2) | 8 (25.8) | |
| 7-15 | 37 | 17 (45.9) | 20 (54.1) | |
| ≥ 16 | 6 | 0 (0.0) | 6 (100.0) | |
| Borrmann classification | ||||
| Type I | 16 | 11 (68.8) | 5 (31.2) | 0.183 |
| Type II or III | 110 | 76 (69.1) | 34 (30.9) | |
| Type IV | 11 | 4 (36.4) | 7 (63.6) | |
| Type V | 3 | 2 (66.7) | 1 (33.3) | |
| WHO histological classification | ||||
| Adenocarcinoma | 77 | 55 (71.4) | 22 (28.6) | 0.725 |
| Signet-ring cell carcinoma | 17 | 12 (70.6) | 5 (29.4) | |
| Mucinous adenocarcinoma | 8 | 6 (75.0) | 2 (25.0) | |
| Mixed carcinoma | 32 | 20 (62.5) | 12 (37.5) | |
| Neuroendocrine carcinoma | 6 | 4 (66.7) | 2 (33.3) | |
| Lauren parting | ||||
| Intestinal type | 65 | 42 (64.6) | 23 (35.4) | 0.155 |
| Diffuse type | 68 | 44 (64.7) | 24 (35.3) | |
| Mixed type | 7 | 7 (100.0) | 0 (0.0) | |
| Differentiation grade | ||||
| Low and middle | 107 | 72 (67.3) | 35 (32.7) | 0.698 |
| High | 33 | 21 (63.6) | 12 (36.4) | |
| Cancer embolus | ||||
| Yes | 51 | 32 (62.7) | 19 (37.3) | 0.485 |
| No | 89 | 61 (68.5) | 28 (31.5) | |
| Affect neural | ||||
| Yes | 50 | 30 (60.0) | 20 (40.0) | 0.230 |
| No | 90 | 63 (70.0) | 27 (30.0) | |
| TNM staging | ||||
| I | 10 | 10 (100.0) | 0 (0.0) | 0.000 |
| II | 49 | 44 (89.8) | 5 (10.2) | |
| III | 81 | 39 (48.1) | 42 (51.9) | |
Single-factor analysis showed that disease-free survival (DFS) in the LOX-low expression group was 26.7 mo, which was significantly longer than in the LOX-high expression group at 15.6 mo (P = 0.037) (Figure 3A and Table 4). Overall survival (OS) of patients in the LOX-low expression group was significantly longer than that in the LOX-high expression group (P = 0.033) (Figure 3B and Table 4). These results demonstrated that high expression of LOX was associated with poor prognosis.
| Gene | Expression | Cases, n | Disease free survival | Overall survival | ||||
| mo | P | mo | P | |||||
| LOX | High | 50 | 15.6 | 0.037 | 4.352 | 22.9 | 0.033 | 4.524 |
| Low | 90 | 26.7 | 40.2 | |||||
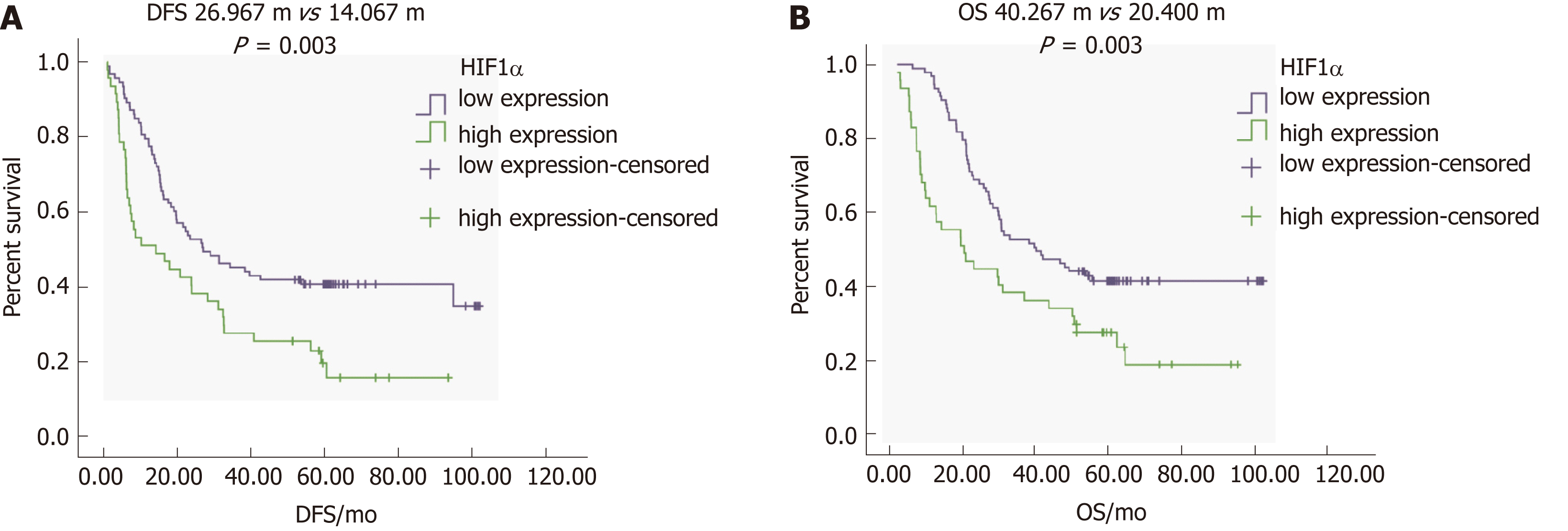
| HIF1α | High | 47 | 14.0 | 0.003 | 8.712 | 20.4 | 0.003 | 8.992 |
| Low | 93 | 26.9 | 40.2 | |||||
To determine the correlation between HIF1α expression and the prognosis of GC, we focused on DFS and OS in patients with different levels of HIFα expression. Single-factor survival analysis showed that DFS and OS in the HIF1α-low expression group were 26.9 mo and 40.2 mo, respectively, which were significantly longer than in the HIF1α-high expression group at 14.0 mo and 20.4 mo (both P = 0.003) (Figure 4A, 4B and Table 4). High expression of HIF1α was associated with poor prognosis.
The occurrence of GC has been increasing rapidly worldwide[24]. GC is related to the presence of tumor-suppressor or tumor-associated genes[25]. There is no accurate and efficient clinical biomarker of GC. Other gastrointestinal biomarkers or early detection methods were also not applied in GC[26-28]. Therefore, the aim of our study was to investigate whether LOX and HIF1α could be used as biomarkers of GC.
LOX participates in the osteoclastogenesis of breast cancer, which suggests a therapeutic tool for osteolytic bone destruction[21]. MiRNA-31-5p inhibits expression of HIF1α and strengthens the Warburg effect by inhibiting its target HIF-1α inhibitor. In addition, hepatitis transactivator protein X accelerates extracellular matrix modification by activating the HIF/LOX pathway and promoting the metastasis of hepatocellular carcinoma[13]. Therefore, we predicted that LOX and HIF1α could act as biomarkers of GC.
In the present study, immunohistochemical staining suggested that expression of LOX and HIF1α increased in tumor tissues from patients with GC. QRT-PCR analysis indicated that mRNA expression of LOX and HIF1α was upregulated in tumor tissues. We also detected expression of these two genes in blood samples. The results revealed that the expression levels of LOX and HIF1α were higher in GC patients than in healthy controls. Additional analysis showed that the expression levels of LOX and HIF1α were related to the clinicopathological characteristics of GC. The expression of LOX and HIF1α increased with the number of lymph node metastases, deeper infiltration depth and the later TNM stage. Single-factor analysis showed that high expression of LOX and HIF1α led to poor prognosis of GC patients.
In conclusion, our results demonstrated that expression of LOX and HIF1α was higher in patients with GC than in healthy controls. Expression of LOX and HIF1α was associated with clinicopathological characteristics and prognosis of GC. Thus, we concluded that LOX and HIF1α could be used as prognostic and predictive biomarkers for GC. Our study provided a link between LOX, HIF1α and GC, which contributes to the development and progression of GC.
To study whether lysyl oxidase (LOX) and hypoxia-inducible factor 1α (HIF1α) can be used as prognostic and predictive biomarkers in gastric cancer (GC).
To provide the prognostic and predictive biomarkers for treating GC.
To explore the interaction between cancer and factors in the metabolic microenvironment and determine predictive biomarkers of GC.
Patients and samples: This work is difficult and requires patient approval.
RNA extraction and cDNA synthesis: This work requires a lot of time, and so the manipulator should be patient. Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR): The manipulator should add the samples accurately during qRT-PCR analysis. Immunohistochemistry: The experiments take a long time, and the manipulator should perform the experiments step by step. Statistical analysis: All of the experiments were repeated at least three times, and the statistical analysis was then performed.
The expression levels of LOX and HIF1α increased in the tumor tissues and blood samples of patients with GC. The expression levels of LOX and HIF1α were related to the clinicopathological characteristics and prognosis of GC.
LOX and HIF1α can be used as prognostic and predictive biomarkers for the treatment of GC. Our study indicated that the expression of LOX and HIF1α was upregulated in patients with GC compared with the control group. In addition, the expression of LOX and HIF1α was related to the clinicopathological characteristics and prognosis of GC.
The present study suggested that LOX and HIF1α might be used as both prognostic and predictive biomarkers for GC, and provided a link between LOX, HIF1α and GC.
We appreciate the technical support and help from the Tumor Center Laboratory of PLA General Hospital.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Czubkowski P, Morling JR, Schievenbusch S S-Editor: Yan JP L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Liu F, Li C, Zhu J, Ren L, Qi X. ABO blood type and risk of hepatocellular carcinoma: A meta-analysis. Expert Rev Gastroenterol Hepatol. 2018;12:927-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Unal E, Karaosmanoglu AD, Ozmen MN, Akata D, Karcaaltincaba M. Computed Tomography-Based Diagnosis of Gastric Vein Invasion in Patients with Gastric Cancer. Eurasian J Med. 2018;50:91-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Ma C, Olevian DC, Lowenthal BM, Jayachandran P, Kozak MM, Chang DT, Pai RK. Loss of SATB2 Expression in Colorectal Carcinoma Is Associated With DNA Mismatch Repair Protein Deficiency and BRAF Mutation. Am J Surg Pathol. 2018;42:1409-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Okasha HH, Naguib M, El Nady M, Ezzat R, Al-Gemeie E, Al-Nabawy W, Aref W, Abdel-Moaty A, Essam K, Hamdy A. Role of endoscopic ultrasound and endoscopic-ultrasound-guided fine-needle aspiration in endoscopic biopsy negative gastrointestinal lesions. Endosc Ultrasound. 2017;6:156-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Sharma V, Rana SS, Ahmed SU, Guleria S, Sharma R, Gupta R. Endoscopic ultrasound-guided fine-needle aspiration from ascites and peritoneal nodules: A scoping review. Endosc Ultrasound. 2017;6:382-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Hocke M, Ignee A, Dietrich C. Role of contrast-enhanced endoscopic ultrasound in lymph nodes. Endosc Ultrasound. 2017;6:4-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Mizuno S, Nakai Y, Isayama H, Suzuki T, Saito K, Uchino R, Takahara N, Kogure H, Tada M, Koike K. EUS-FNA of gastric cancer metastatic to the head of pancreas using a forward oblique viewing echoendoscope in a case with Roux-en-Y anatomy. Endosc Ultrasound. 2018;7:420-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Xu W, Wei Q, Han M, Zhou B, Wang H, Zhang J, Wang Q, Sun J, Feng L, Wang S, Ye Y, Wang X, Zhou J, Jin H. CCL2-SQSTM1 positive feedback loop suppresses autophagy to promote chemoresistance in gastric cancer. Int J Biol Sci. 2018;14:1054-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Goetze OT, Al-Batran SE, Chevallay M, Mönig SP. Multimodal treatment in locally advanced gastric cancer. Updates Surg. 2018;70:173-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | El Hajj II, Lawrence KA, Tirkes T, Shahda S, Sherman S. Metachronous gastric metastasis from lung primary, with synchronous pancreatic neuroendocrine carcinoma. Clin Case Rep. 2018;6:1368-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Kim HG, Kim DY, Jeong O. Transition from Conventional to Reduced-Port Laparoscopic Gastrectomy to Treat Gastric Carcinoma: A Single Surgeon's Experience from a Small-Volume Center. J Gastric Cancer. 2018;18:172-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Zhou Z, Liu Y, Meng K, Guan W, He J, Liu S, Zhou Z. Application of spectral CT imaging in evaluating lymph node metastasis in patients with gastric cancers: Initial findings. Acta Radiol. 2018;284185118786076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Lee YI, Jeon HK, Im JW, Oh SY, Kim KB, Kim B. Primary Gastric Small Cell Carcinoma: A Case Identified as a Large Subepithelial Tumor from Invisible State in 6 Months. Clin Endosc. 2019;52:76-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Liang H, Ge F, Xu Y, Xiao J, Zhou Z, Liu R, Chen C. miR-153 inhibits the migration and the tube formation of endothelial cells by blocking the paracrine of angiopoietin 1 in breast cancer cells. Angiogenesis. 2018;21:849-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Meléndez-Rodríguez F, Roche O, Sanchez-Prieto R, Aragones J. Hypoxia-Inducible Factor 2-Dependent Pathways Driving Von Hippel-Lindau-Deficient Renal Cancer. Front Oncol. 2018;8:214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Li Q, Li Y, Liang L, Li J, Luo D, Liu Q, Cai S, Li X. Klotho negatively regulated aerobic glycolysis in colorectal cancer via ERK/HIF1α axis. Cell Commun Signal. 2018;16:26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Tse AP, Sze KM, Shea QT, Chiu EY, Tsang FH, Chiu DK, Zhang MS, Lee D, Xu IM, Chan CY, Koh HY, Wong CM, Zheng YP, Ng IO, Wong CC. Hepatitis transactivator protein X promotes extracellular matrix modification through HIF/LOX pathway in liver cancer. Oncogenesis. 2018;7:44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Liu Y, Wang G, Liang Z, Mei Z, Wu T, Cui A, Liu C, Cui L. Lysyl oxidase: A colorectal cancer biomarker of lung and hepatic metastasis. Thorac Cancer. 2018;9:785-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Li T, Wu C, Gao L, Qin F, Wei Q, Yuan J. Lysyl oxidase family members in urological tumorigenesis and fibrosis. Oncotarget. 2018;9:20156-20164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Leo C, Cotic C, Pomp V, Fink D, Varga Z. Overexpression of Lox in triple-negative breast cancer. Ann Diagn Pathol. 2018;34:98-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Kim J, Shin Y, Lee S, Kim M, Punj V, Lu JF, Shin H, Kim K, Ulmer TS, Koh J, Jeong D, An W. Regulation of Breast Cancer-Induced Osteoclastogenesis by MacroH2A1.2 Involving EZH2-Mediated H3K27me3. Cell Rep. 2018;24:224-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Tanaka N, Yamada S, Sonohara F, Suenaga M, Hayashi M, Takami H, Niwa Y, Hattori N, Iwata N, Kanda M, Tanaka C, Kobayashi D, Nakayama G, Koike M, Fujiwara M, Fujii T, Kodera Y. Clinical Implications of Lysyl Oxidase-Like Protein 2 Expression in Pancreatic Cancer. Sci Rep. 2018;8:9846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Zhang Z, Li P, Wang Y, Yan H. Hypoxia‑induced expression of CXCR4 favors trophoblast cell migration and invasion via the activation of HIF‑1α. Int J Mol Med. 2018;42:1508-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Zhu B, Cao X, Zhang W, Pan G, Yi Q, Zhong W, Yan D. MicroRNA-31-5p enhances the Warburg effect via targeting FIH. FASEB J. 2019;33:545-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Zhang J, Xu J, Dong Y, Huang B. Down-regulation of HIF-1α inhibits the proliferation, migration, and invasion of gastric cancer by inhibiting PI3K/AKT pathway and VEGF expression. Biosci Rep. 2018;38:pii: BSR20180741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Zhou Y, Hu Z. The Functions of Circulating Tumor Cells in Early Diagnosis and Surveillance During Cancer Advancement. J Transl Int Med. 2017;5:135-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Schizas D, Kapsampelis P, Mylonas KS MD. Adenosquamous Carcinoma of the Esophagus: A Literature Review. J Transl Int Med. 2018;6:70-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Montagnani F, Di Leonardo G, Pino M, Perboni S, Ribecco A, Fioretto L. Protracted Inhibition of Vascular Endothelial Growth Factor Signaling Improves Survival in Metastatic Colorectal Cancer: A Systematic Review. J Transl Int Med. 2017;5:18-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |