Published online Apr 14, 2019. doi: 10.3748/wjg.v25.i14.1764
Peer-review started: November 12, 2018
First decision: January 30, 2019
Revised: February 26, 2019
Accepted: March 1, 2019
Article in press: March 1, 2019
Published online: April 14, 2019
Identifying predictors of therapeutic response is the cornerstone of personalized medicine.
To identify predictors of long-term mucosal healing (MH) in patients with Crohn’s disease (CD) treated with tumor necrosis factor α (TNF-α) inhibitors.
Prospective single center study. Consecutive patients with clinically active CD requiring treatment with a TNF-α inhibitor were included. A baseline segmental CD Endoscopic Index of Severity (CDEIS) ≥ 10 in at least one segment or the presence of ulcerations were required for inclusion. Clinical, biological and endoscopic data were obtained at baseline, weeks 14 and 46. Endoscopic response (ER) was defined as a decrease ≥ 50% from baseline CDEIS and MH as partial CDEIS ≤ 5 in all segments.
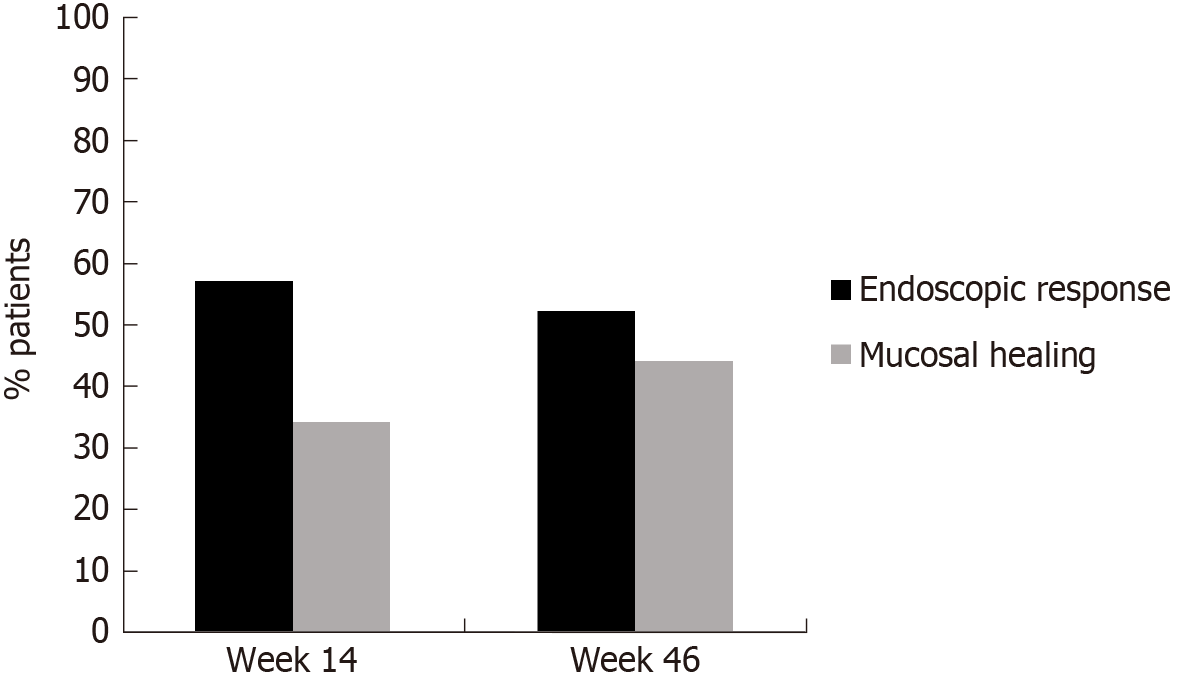
Of 62 patients were included. At baseline, median CD Activity Index and CDEIS were 201 and 6.7, respectively with a significant reduction after one year of treatment (53 and 3.0 respectively, P < 0.001). At week 14, 56% of patients achieved ER and 34% MH. At week 46, the corresponding percentages were 52% and 44%. Baseline disease characteristics or biomarkers did not predict MH. A decrease from baseline CDEIS at week 14 of at least 80% was the best predictor of MH at week 46 (59% sensitivity and 91% specificity; area under the curve = 0.778).
Clinical and biomarker data are not useful predictors of response to TNF-α inhibitors in CD, whereas ER to induction therapy, defined as 80% reduction in global CDEIS, is a robust predictor of long-term MH. Achievement of this endoscopic endpoint may be considered as a therapeutic target for anti-TNF-α therapy.
Core tip: In our study we report that endoscopic response after completion of induction treatment with a tumor necrosis factor inhibitor predicts mucosal healing at long term in patients with Crohn’s disease, and that endoscopic evaluation at this time point may be considered in clinical practice to predict long term outcomes and could contribute to perform treatment adjustments.
- Citation: Alfaro I, Masamunt MC, Planell N, López-García A, Castro J, Gallego M, Barastegui R, Giner A, Vara A, Salas A, Ricart E, Panés J, Ordás I. Endoscopic response to tumor necrosis factor inhibitors predicts long term benefits in Crohn’s disease. World J Gastroenterol 2019; 25(14): 1764-1774
- URL: https://www.wjgnet.com/1007-9327/full/v25/i14/1764.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i14.1764
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) characterized by a wide spectrum of disease phenotypes that typically alternates periods of active inflammation and remission. Persistent inflammation leads to the accumulation of intestinal damage and loss of function in the affected intestinal segments, resulting in disability in many patients[1]. The main treatment goal in CD is to achieve healing of inflammatory lesions to prevent damage progression by introducing highly effective therapies early during the course of the disease. Tumor necrosis factor α (TNF-α) inhibitors are one of the most effective therapies for induction and maintenance of remission in patients with CD[2].
Response to anti-TNF therapy is heterogeneous. In patients with CD several predictors of clinical response have been described, including age[3,4], disease duration[5], inflammatory phenotype[6], and smoking status[6-10], but the effect size of these factors is small and not sufficient to guide clinical practice. Furthermore, predictors of endoscopic healing remain to be definitively established. In the field of biomarkers, normalization of C reactive protein (CRP) and calprotectin has been associated with mucosal healing (MH)[6,11]. With regard to endoscopic lesions, achievement of MH after three months of treatment with a TNF-α inhibitor has been identified as a predictor of long-term endoscopic response[12,13]. However, to date, no observational prospective studies have been specifically designed to identify predictors of MH, which should be the therapeutic target[14].
The availability of new treatment agents with mechanisms of action different from TNF-α inhibition, has brought about the need for identifying predictors of efficacy, in particular predictors of MH, in order to individualize treatment strategies and facilitate a more personalized medicine. The aim of this study was to identify predictors of MH after one year of treatment with TNF-α inhibitors.
This is a prospective observational single center study. Recruitment of patients was performed from November 2012 until May 2016. The study was approved by the local ethics committee of Hospital Clinic de Barcelona on November 22 of 2012, and was performed according to the good clinical practice guidelines of the European Medicines Agency (CMPM/ICH/135/95, July 2002). All patients provided written informed consent before inclusion. Eligibility criteria were patients with clinically active luminal CD requiring treatment with an anti-TNF for induction of remission. At baseline, all patients underwent a colonoscopy for assessment of endoscopic disease activity. A baseline segmental CD Endoscopic Index of Severity (CDEIS) ≥ 10 in at least one segment, or the presences of ulcerations, was required for inclusion. Exclusion criteria were formal contraindication for anti-TNF therapy, intolerance or contraindication for undergoing an ileocolonoscopy, absence of endoscopic activity as described above or a patient’s refusal to participate.
The induction therapy for infliximab was 5 mg/kg at weeks 0, 2 and 6; for adalimumab 160 mg and 80 mg at weeks 0 and 2, respectively, followed by 40 mg every other week. For maintenance, 5 mg/kg infliximab was administered every 8 weeks and 40 mg of adalimumab every other week. Intensification of treatment was performed reactively when loss of clinical response was documented in association with objective evidence of active disease (increased fecal calprotectin or
CRP or presence of endoscopic lesions) and drug levels below recommended thresholds (infliximab < 3 μg/mL, adalimumab < 7 μg/mL). If remission was documented by endoscopy, no modification of treatment was performed.
Clinical evaluation was performed at baseline, and at weeks 6, 14, 30 and 46. At each visit the CD Activity Index (CDAI) was determined. Blood and fecal samples were also collected at each visit for assessment of biomarkers (CRP and calprotectin). Monitoring for adverse events and use of concomitant medications was registered at each visit, including unscheduled visits performed if the patient developed symptoms between scheduled visits. Drug levels and anti-drug antibodies were analyzed at baseline, week 14, week 46, and in case of loss of clinical response.
Ileocolonoscopy was performed at baseline, week 14 and week 46. All examinations were performed under anesthesia by an endoscopist experienced in the assessment of IBD (ER, IA, IO). Quantification of endoscopic disease activity was based on the CDEIS, with measurement of segmental and global scores. Investigators evaluating the endoscopic lesions were blinded to the patient’s symptoms. Endoscopic response was defined as a decrease of at least 50% from baseline in the global CDEIS and MH as segmental CDEIS ≤ 5 in all segments, which implies the disappearance of mucosal ulcerations.
A formal sample-size calculation was not performed. Precision of the predictors identified is provided by 95% confidence intervals. Quantitative variables are expressed as mean. Percentages are given for discrete variables. Analyses were performed according to the intention-to-treat principle including all recruited patients. Patients with missing data were classified as non-responders for clinical and endoscopic outcomes (Non-response imputation). Missing quantitative data at different time points was imputed using the last observation carried forward. For identification of clinical, biological and endoscopic predictors of endoscopic response, logistic regression analysis was performed. A receiver operating characteristic (ROC) curve was constructed to determine the best cut-off value for predicting MH after one year of treatment. Spearman rank-order correlation coefficient was performed to identify association between variables. Statistical significance was set at P < 0.05 for all tests. Statistical analysis was performed using the statistical package SPSS V.23. The Statistical methods of this study were review by one of the authors (Ingrid Ordas).
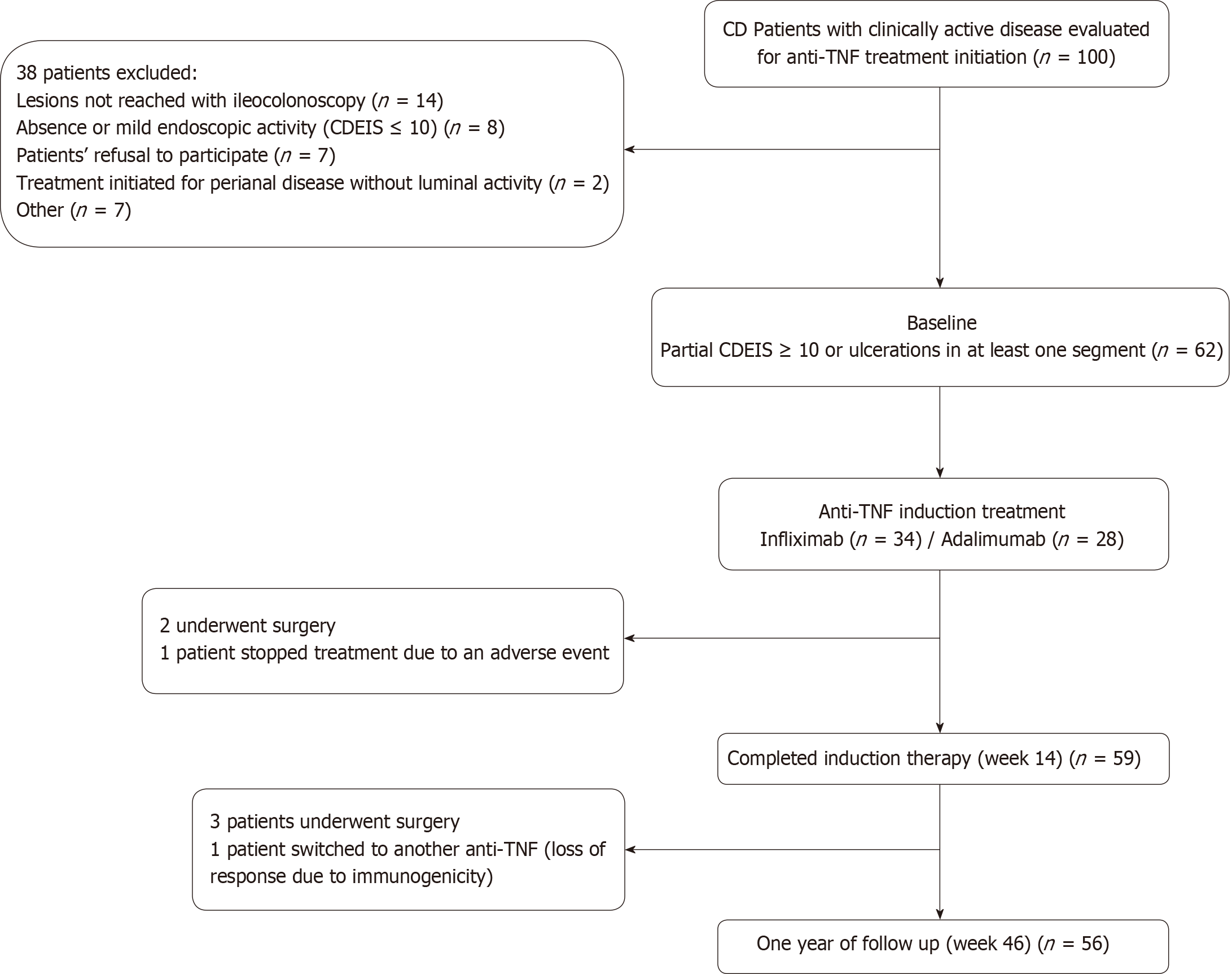
From 100 potentially eligible patients with clinically active disease, 62 were finally included. Thirty eight patients were excluded for the following reasons: colonoscopy could not reach the affected area (n = 14), absence or mild endoscopic activity with all segmental CDEIS < 10 (n = 8), patient´s refusal to participate (n = 7), spontaneous patient’s improvement without need of anti-TNF treatment initiation (n = 7) or because anti-TNF treatment was initiated for complex perianal disease without significant luminal activity (n = 2).
Seven patients dropped out from the study, three of them during induction and four during the maintenance period. In 5 cases because surgery was required, in one patient treatment was switched to another anti-TNF due to immunogenicity with secondary loss of response and in one case treatment was stopped due to an adverse event (infusion reaction). All seven cases were imputed as non-responders. Fifty-nine patients (95.2%) completed the 14 wk induction period. Of these, 53 underwent endoscopic evaluation. Fifty-six patients (90.3%) completed one year of follow up of whom forty-seven underwent endoscopic evaluation (Figure 1). Endoscopic assessment was not performed in some patients at weeks 14 or 46 due to patient’s refusal; all of them were considered as non-responders.
Demographic and baseline disease characteristics are summarized in Table 1. A majority of patients received combination therapy (86%). The proportion of patients achieving MH at week 46 under IFX and ADA were similar (46% vs 42%), the subsequent analysis was therefore performed in the pooled population.
| Patient’s demographic characteristics (n = 62) | |
| Female, n (%) | 31 (50) |
| Age at inclusion, mean (min-max) | 39 (18-72) |
| Disease duration (yr), mean (min-max) | 9 (0-33) |
| Location | |
| Terminal ileum | 32 (52) |
| Colonic | 15 (24) |
| Ileocolonic | 15 (24) |
| Associated upper involvement | 3 (5) |
| Phenotype | |
| Inflammatory | 39 (63) |
| Stricturing | 14 (22) |
| Penetrating | 9 (15) |
| Associated structuring + penetrating | 4 (7) |
| Perianal disease | 16 (26) |
| Current smokers | 19 (31) |
| Anti-TNFdrug used | |
| Infliximab | 34 (55) |
| Adalimumab | 28 (45) |
| Prior anti-TNF exposure | 16 (26) |
| Immunomodulators at baseline | 53 (86) |
| Steroids at baseline | 13 (21) |
| Previous CD surgery | 9 (15) |
Clinical, biological, pharmacokinetic, and endoscopic data at baseline and during follow up are presented in Table 2. At baseline, median CDAI was 201; treatment with anti-TNF resulted in a significant decrease in CDAI to 60 (P < 0.001) at week 14 and to 53 at week 46 (P < 0.001). Changes in biomarkers are summarized in Table 2. Calprotectin levels decreased progressively with significant differences relative to baseline at weeks 14 and 46. CRP value also decreased during follow-up reaching statistical significance at week 46 in the whole study population and also in the subgroup of patients with elevated CRP (> 1.0 mg/dL) at baseline (n = 26; 41%). Hemoglobin and albumin concentrations significantly increased at weeks 14 and 46 relative to baseline (P < 0.05).
| Baseline, n = 62 | Week 14, n = 59 | P value (week 14- baseline) | Week 46, n = 56 | P value (week 46- baseline) | |
| CDAI, median (IQR) | 201 (114-236) | 60 (28-94) | P < 0.001 | 53 (26-94) | P < 0.001 |
| CDEIS, median (IQR) | 6.7 (5-11.3) | 3.2 (0.8-5) | P < 0.001 | 3.0 (0.2-4.4) | P < 0.001 |
| CRP mg/dL, median (IQR) | 0.66 (0,16-1.72) | 0.2 (0.03-0.71) | P = 0.125 | 0.19 (0.03-0.76) | P < 0.05 |
| Hemoglobin g/L, median (IQR) | 127 (113-140) | 135 (121-141) | P < 0.05 | 134 (123-143) | P < 0.05 |
| Albumin g/L, median (IQR) | 41 (40-43) | 43 (40-45) | P < 0.05 | 43 (40-45) | P < 0.05 |
| Serum TNF-alpha pg/mL, median (IQR) | 5.5 (3.3-8) | 20 (11-36) | P < 0.001 | 17 (8.25-30) | P < 0.001 |
| Fecal Calprotectin μg/g, median (IQR) | 1044 (685-1800) | 610 (209-1646) | P < 0.05 | 940 (233-1747) | P < 0.05 |
| Infliximab μg/mL, median (IQR) | 0 (0-0) | 3.1 (1.2-5.6) | P < 0.001 | 1.8 (1-6.6) | P < 0.001 |
| ATIs, % patients (n) | 8 (5/62) | 3 (1/34) | P = 0.327 | 13 (4/32) | P = 0.230 |
| Adalimumab μg/mL, median (IQR) | 0 (0-0) | 8.9 (5.6-12) | P < 0.001 | 9.9 (7-12) | P < 0.001 |
| ATAs, % patients (n/total) | 10 (6/62) | 0 (0/25) | P = 0.117 | 4.1 (1/24) | P = 0.408 |
Median infliximab and adalimumab concentrations at week 14 were 3.1 μg/mL and 8.9 μg/mL, respectively. After one year of treatment median serum drug concentrations were 1.8 μg/mL and 9.9 μg/mL, respectively. At baseline 8% of patients presented antidrug antibodies to infliximab (ATIs) and 10% to adalimumab (ATAs); of them 60% and 50% respectively were naïve to anti-TNF therapy. In all cases, antibody levels were in the low range (3.9-98 μg/mL). At week 14, ATIs were detected in only one patient (3%) and ATAs in none of them (the test used, ELISA, cannot detect antibodies in the presence of circulating drug). After one year of treatment, ATIs were detected in 4 patients (13%) and ATAs in one patient (4%). All of them were under concomitant immunomodulatory therapy. Median CDEIS at baseline was 6.7 with significant decreases up to 3.2 at week 14 (P < 0.001) and up to 3.0 (P < 0.001) after one year of treatment. As illustrated in Figure 2, at week 14, 56% of patients achieved endoscopic response and 34% were on MH. At week 46, the corresponding percentages for endoscopic response and MH were 52% and 44%, respectively.
None of the demographic or baseline disease characteristics predicted endoscopic response or remission at week 14. Baseline disease characteristics with predictive value of long-term endoscopic response (wk46) in the univariate analysis included disease duration, CDAI and CDEIS. The week 14 variables that predicted endoscopic response at week 46 were: CRP, a decrease from baseline CDAI of 100 points, CDEIS, the percentage of CDEIS reduction from baseline and the achievement of endoscopic response or MH. Due to an elevated rate of missing values (35% at baseline and 42% at week 14), calprotectin was not included in the logistic regression analysis. In the multivariate analysis, only disease duration and the percentage of CDEIS reduction at week 14, independently predicted MH on the long term.
Predictors of MH after 46 wk of treatment were identified only at week 14 in the univariate analysis, including the CRP and the adalimumab concentration, the CDEIS score, the percentage of CDEIS reduction from baseline and the achievement of endoscopic response or MH at week 14. In the multivariate analysis all the endoscopic variables independently predicted MH at week 46.
The changes in clinical or biomarker variables from baseline to week 14 did not predict MH on the long term. Adalimumab or infliximab serum concentrations at week 14 were not associated with clinical or endoscopic outcomes at this time point of evaluation. Serum drug concentration at week 14 did not predict clinical outcomes or endoscopic response at week 46. Only adalimumab concentration at week 14 achieved statistical significance to predict MH on the long term (week 46). A concentration of at least 10 μg/mL at week (area under the curve: 0.76) predicts MH with moderate sensitivity (61%) and good specificity (84%). Drug concentration and anti-drug antibodies at week 46 were not correlated with clinical or endoscopic outcomes at that time of evaluation.
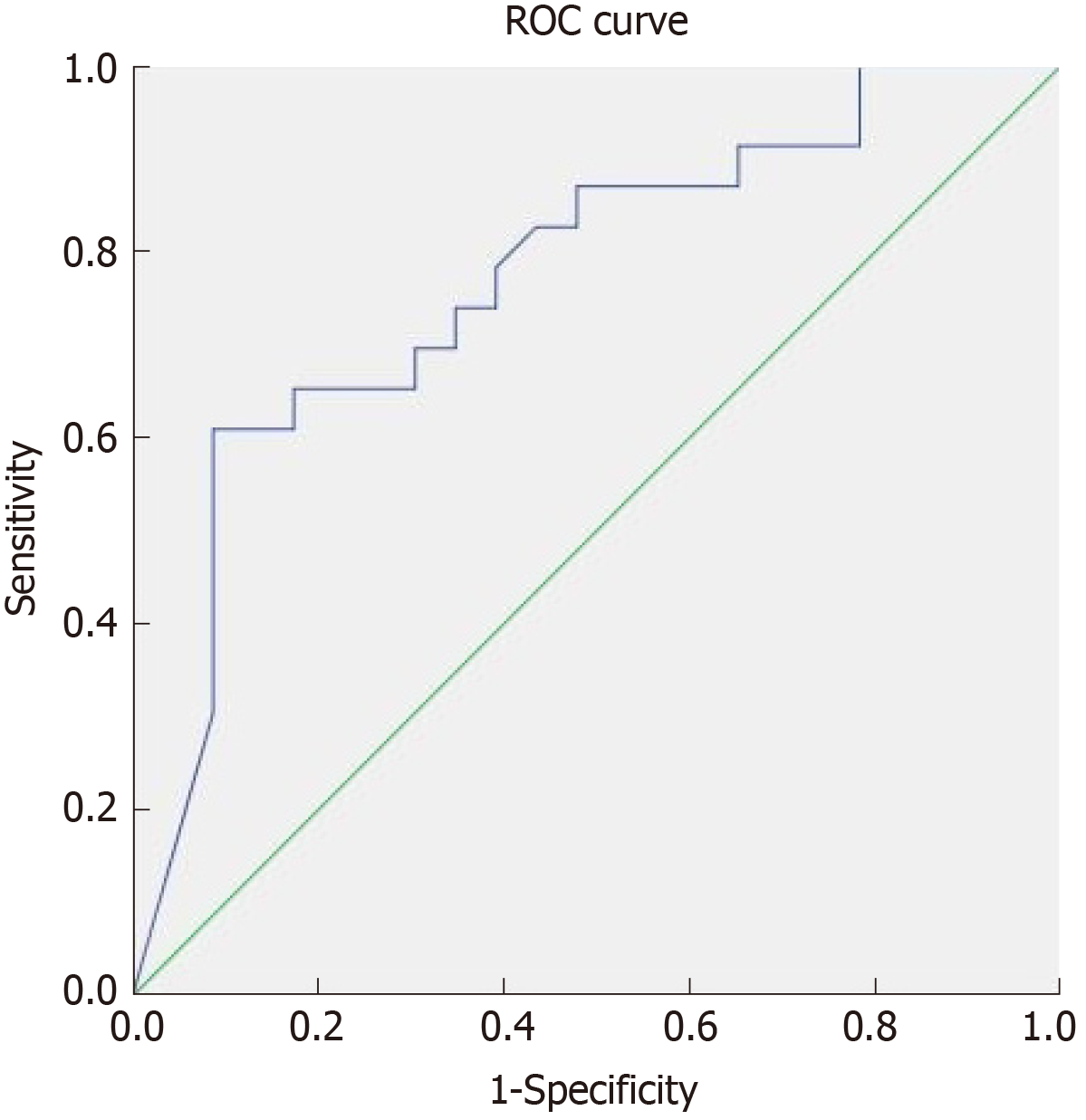
In our cohort, only 19% of patients required treatment intensification before achieving the endpoint of week 46. In the logistic regression analysis treatment intensification did not achieve statistical significance as a predictor of MH. To define the best cutoff value for predicting MH after one year of treatment, ROC curves were constructed for CDEIS scores at week 14 and for the percentage of CDEIS reduction from baseline to week 14. Sensitivity and specificity were also calculated for achievement of endoscopic response and MH at week 14 as predictors of long-term MH (Table 3). Relative changes in the endoscopic score from baseline to week 14 had higher accuracy in predicting MH at week 46 than absolute CDEIS values at week 14. Based on the clinical relevance of the evaluated cutoff values, a decrease of at least 80% from baseline CDEIS at week 14 was the best discriminative cutoff value for predicting MH at week 46 with a sensitivity of 59% and 91% specificity (Figure 3). As shown in Table 4, at week 14, a reduction of ≥ 80% from baseline CDEIS was documented in 19 patients (31%) with a positive predictive value (PPV) of 84% and a negative predicted value of 74%. Table 5 shows the comparison of diagnostic accuracy between 50% and 80% CDEIS reduction from baseline to week 14 as a predictor of MH at week 46.
| Variable | Sensitivity | Specificity |
| CDEIS < 3 | 69 | 67 |
| CDEIS < 4 | 62 | 78 |
| 80% reduction of CDEIS (baseline to week 14) | 59 | 91 |
| Endoscopic response at week 14 | 85 | 66 |
| Endoscopic remission at week 14 | 59 | 85 |
| Mucosal healing at week 46 | ||||
| No | Yes | |||
| ≥ 80% reduction in CDEIS at week 14 from baseline | No | n (%) | 32 (74.4) | 11(25.6) |
| Yes | n (%) | 3 (15.8) | 16 (84.2) | |
| CDEIS reduction | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | LR+ (95%CI) | LR- (95%CI) |
| 50% | 85 (66%-96%) | 66 (48%-81%) | 66 (54%-76%) | 85 (69%-93%) | 2.5 (1.5-4) | 0.23 (0.09-0.57) |
| 80% | 59 (39%-77%) | 91 (77%-98%) | 84 (63%-94%) | 74 (65%-82%) | 6.9 (2.24-21) | 0.45 (0.28-0.72) |
In this prospective observational study, we identified predictors of long-term MH in patients with CD treated with TNF-α inhibitors. After completing 14 weeks of treatment , we found that the absolute CDEIS value, the percentage of CDEIS reduction from baseline and the achievement of endoscopic response or MH significantly predict MH after one year of treatment. Of these four predictive factors, achievement of MH after induction treatment with an anti-TNF has already been described as a predictor of long-term MH in a series of patients retrospectively evaluated and prospectively validated in a subsequent study[12,13]. In this study, 42 patients with active luminal CD (defined as a SES-CD ≥ 3) treated with an anti-TNF were followed clinically and endoscopically after 3 months and one year of treatment. The authors concluded that MH (defined as a SES-CD ≤ 2) after 3 mo of treatment predicted long-term MH with 88% of sensitivity and 64% of specificity. Our study shows that not only achievement of MH is a predictor of long term MH, but also achievement of endoscopic response after completing induction predicts MH on the long term, expanding thus the number of patients in whom a recommendation of continued anti-TNF therapy is made after induction with high probability of achieving the therapeutic target of MH at week 46.
The percentage of CDEIS reduction as a predictor of clinical outcomes has been evaluated in a post-hoc analysis of the SONIC trial[15]. In this study, a decrease from the baseline CDEIS of at least 50% at week 26 predicted steroid-free clinical remission at week 50 with a sensitivity of 73% and a specificity of 46 %. We consider, though, that objective demonstration of MH by endoscopy is a more robust endpoint than steroid-free clinical remission. In addition, a specificity of 46% can lead to a high rate of false positives.
In our cohort, a decrease of at least 50% from baseline CDEIS at week 14 had a sensitivity of 85% and moderate specificity (66%). In our study, we chose a reduction of 80% from baseline CDEIS as a predictor of long term MH since it has better diagnostic accuracy, with moderate sensitivity (59%) but excellent specificity (91%). Moreover, compared with a 50% reduction, it demonstrated a better PPV and positive likelihood ratio. In cases in which sensitivity is more relevant, a 50% reduction can be chosen as the endpoint. In the current study, we observed that changes in CDEIS in response to induction therapy are better predictors of long-term efficacy of anti-TNF drugs than absolute values. Absolute values are highly influenced by disease location and extension, whereas a percentage reduction relative to baseline may be preferable because it is a dynamic parameter of response and integrates the magnitude of response to induction therapy.
In this cohort, 84% of patients that achieved an 80% reduction in CDEIS from baseline to week 14 were in MH after one year of treatment. In patients who do not achieve this endpoint, potential treatment optimization might be considered by the addition of immunosuppressive agents in case of anti-TNF monotherapy, intensification of anti-TNF treatment or by considering alternative treatment strategies other than TNF-α inhibitors. The effectiveness of these type of interventions remains to be assessed in prospective clinical trials. In our study neither demographic nor disease characteristic predicted achievement of endoscopic response at week 14, or MH after induction or at week 46. This was also true for clinical disease activity and CRP. Disease duration has been reported to be a predictor of clinical response[5] and in our study was identified as a predictor of endoscopic response in the long term (the longer the disease duration the less probabilities to achieve endoscopic response).
In our cohort, adalimumab serum concentration after completing treatment induction has predictive value for the long term MH diagnosis. Previous reports have described an association between adalimumab serum levels and achievement of MH. The cutoff point of this parameter has been situated in at least 4.9 μg/Ml[16] and some retrospective studies or studies with non-systematic measurement of adalimumab serum concentration suggest that the concentration needed to achieve this endoscopic outcome are even higher (> 7.1 μg/mL)[17-19]. In our prospective cohort, an adalimumab concentration of at least 10 μg/mL after induction was best correlated with long term MH with a moderate sensitivity and good specificity, suggesting that patients may need even higher concentrations than previously reported. Measurement of serum drug concentration after induction could be considered as a time point to perform this evaluation for predicting long term endoscopic outcomes. Infliximab serum concentration were not predictive of MH; lack of statistical power, and the fact that a considerable number of patients had low serum infliximab concentrations may have prevented the identification of the relationship between drug levels and therapeutic response.
When we analyzed the subgroup of patients that achieved MH at weeks 14 and 46, a recommended concentration of infliximab (equal or higher than 3.0 μg/mL) was observed in a 73% and 29% of patients respectively. The high proportion of patients in MH and low anti-TNF serum concentration during maintenance might indicate that lower drug levels may be sufficient after achieving healing. The current study has several strengths. It has been prospectively performed and all patients had documented endoscopic active disease at baseline. In addition, monitoring of disease activity was prospectively registered using clinical, biological, and endoscopic variables. Our cohort has a lower clinical activity at baseline compared to pivotal registration trials. Indeed, our population probably better reflects clinical practice, where anti-TNF therapies are used not only in patients with severe disease as in pivotal trials, but also in patients with milder forms.
This study has also some limitations that should be acknowledged. It is a single center study with a limited sample size. With regard to biomarkers as predictors of endoscopic response, calprotectin could not be incorporated in the analysis due to a considerable proportion of missing samples, despite proactively reminding the patients to collect them. This is an inherent limitation of this monitoring modality. Escalation of drug doses was triggered by clinical symptoms. This could be considered as a limitation, however, there is no evidence at this moment supporting that treatment intensification based on a target serum drug concentration[20,21] is superior to clinically based dosing. In this scenario, treatment intensification based on clinical decisions represent the current clinical practice in the majority of centers until new evidence is available.
In summary, in patients with CD treated with an anti-TNF, endoscopic evaluation following completion of induction treatment should be considered for determining endoscopic response and predicting long-term outcomes. After 14 weeks of treatment, achievement of a reduction of 80% from baseline CDEIS may be considered a target for optimization of anti-TNF therapy in clinical practice.
Tumor necrosis factor (TNF) inhibitors are one of the most effective therapies for induction and maintenance of remission in patients with Crohn’s disease (CD), however, to date, no observational prospective studies have been specifically designed to identify predictors of mucosal healing (MH), which should be the therapeutic target.
Improve knowledge about the predictive factors of response to TNF inhibitors and try to provide more tools to perform a personalized treatment.
The aim of this study was to identify predictors of MH after one year of treatment with TNF-α inhibitors.
Prospective observational single center study. Consecutive patients with clinically active CD requiring treatment with a TNF-α inhibitor were included. Clinical, biological and endoscopic data were obtained at baseline, weeks 14 and 46.
Endoscopic response to induction therapy, defined as 80% reduction in global CD Endoscopic Index of Severity (CDEIS), is a robust predictor of long-term MH. Endoscopic response is a predictor of MH. Achievement endoscopic response after induction may be considered as a therapeutic target for anti-TNF-α therapy. None, it was a observational study to identify predictors. After induction of remision with a TNF inhibitor perform a colonoscopy should be considered to predict long-term outocomes. Endoscopic response predict long-term outcomes. There were no hypotheses. After induction of remision with a TNF inhibitor perform a colonoscopy should be considered to predict long-term outocomes in patients with CD.
Clinical and biomarker data are not useful predictors of response to TNF-α inhibitors in CD, whereas endoscopic response to induction therapy, defined as 80% reduction in global CDEIS, is a robust predictor of long-term MH. Achievement of this endoscopic endpoint may be considered as a therapeutic target for anti-TNF-α therapy.
This work has been founded in part by Helmsley Charitable Trust 2015PG-IBD005.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sipahi AM, Tahan V S-Editor: Yan JP L-Editor: A E-Editor: Song H
| 1. | Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, Chowers Y, D'Haens G, Feagan BG, Hibi T, Hommes DW, Irvine EJ, Kamm MA, Loftus EV, Louis E, Michetti P, Munkholm P, Oresland T, Panés J, Peyrin-Biroulet L, Reinisch W, Sands BE, Schoelmerich J, Schreiber S, Tilg H, Travis S, van Assche G, Vecchi M, Mary JY, Colombel JF, Lémann M. Development of the Crohn's disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415-1422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 419] [Article Influence: 32.2] [Reference Citation Analysis (1)] |
| 2. | Hazlewood GS, Rezaie A, Borman M, Panaccione R, Ghosh S, Seow CH, Kuenzig E, Tomlinson G, Siegel CA, Melmed GY, Kaplan GG. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: A network meta-analysis. Gastroenterology. 2015;148:344-354.e5; quiz e14-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 3. | Grover Z, Biron R, Carman N, Lewindon P. Predictors of response to Infliximab in children with luminal Crohn's disease. J Crohns Colitis. 2014;8:739-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Sandborn WJ, Melmed GY, McGovern DP, Loftus EV, Choi JM, Cho JH, Abraham B, Gutierrez A, Lichtenstein G, Lee SD, Randall CW, Schwartz DA, Regueiro M, Siegel CA, Spearman M, Kosutic G, Pierre-Louis B, Coarse J, Schreiber S. Clinical and demographic characteristics predictive of treatment outcomes for certolizumab pegol in moderate to severe Crohn's disease: Analyses from the 7-year PRECiSE 3 study. Aliment Pharmacol Ther. 2015;42:330-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Ding NS, Hart A, De Cruz P. Systematic review: Predicting and optimising response to anti-TNF therapy in Crohn's disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 6. | Kiss LS, Szamosi T, Molnar T, Miheller P, Lakatos L, Vincze A, Palatka K, Barta Z, Gasztonyi B, Salamon A, Horvath G, Tóth GT, Farkas K, Banai J, Tulassay Z, Nagy F, Szenes M, Veres G, Lovasz BD, Vegh Z, Golovics PA, Szathmari M, Papp M, Lakatos PL; Hungarian IBD Study Group. Early clinical remission and normalisation of CRP are the strongest predictors of efficacy, mucosal healing and dose escalation during the first year of adalimumab therapy in Crohn's disease. Aliment Pharmacol Ther. 2011;34:911-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Cohen RD, Lewis JR, Turner H, Harrell LE, Hanauer SB, Rubin DT. Predictors of adalimumab dose escalation in patients with Crohn's disease at a tertiary referral center. Inflamm Bowel Dis. 2012;18:10-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Triantafillidis JK, Mantzaris G, Karagiannis J, Papavasilliou E, Papatheodoridis G, Fouskas J, Malgarinos G, Gikas A, Papamichael K, Mathou N, Symboulakis E, Karamanolis D. Similar response to adalimumab in patients with active Crohn's disease either naive to biologic agents or with prior loss of response or intolerance to infliximab. Rev Med Chir Soc Med Nat Iasi. 2010;114:85-90. [PubMed] [Cited in This Article: ] |
| 9. | Chaparro M, Panes J, García V, Mañosa M, Esteve M, Merino O, Andreu M, Gutierrez A, Gomollón F, Cabriada JL, Montoro MA, Mendoza JL, Nos P, Gisbert JP. Long-term durability of infliximab treatment in Crohn's disease and efficacy of dose "escalation" in patients losing response. J Clin Gastroenterol. 2011;45:113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Arnott ID, McNeill G, Satsangi J. An analysis of factors influencing short-term and sustained response to infliximab treatment for Crohn's disease. Aliment Pharmacol Ther. 2003;17:1451-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Røseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: A predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. 2004;39:1017-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | af Björkesten CG, Nieminen U, Sipponen T, Turunen U, Arkkila P, Färkkilä M. Mucosal healing at 3 months predicts long-term endoscopic remission in anti-TNF-treated luminal Crohn's disease. Scand J Gastroenterol. 2013;48:543-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | af Björkesten CG, Nieminen U, Turunen U, Arkkila PE, Sipponen T, Färkkilä MA. Endoscopic monitoring of infliximab therapy in Crohn's disease. Inflamm Bowel Dis. 2011;17:947-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D'Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV, Marteau P, Munkholm P, Murdoch TB, Ordás I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O'Donnell S, Pariente B, Winer S, Hanauer S, Colombel JF. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1160] [Cited by in F6Publishing: 1250] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 15. | Ferrante M, Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens GR, van der Woude CJ, Danese S, Diamond RH, Oortwijn AF, Tang KL, Miller M, Cornillie F, Rutgeerts PJ; International Organization for the Study of Inflammatory Bowel Diseases. Validation of endoscopic activity scores in patients with Crohn's disease based on a post hoc analysis of data from SONIC. Gastroenterology. 2013;145:978-986.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 128] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 16. | Roblin X, Marotte H, Rinaudo M, Del Tedesco E, Moreau A, Phelip JM, Genin C, Peyrin-Biroulet L, Paul S. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:80-84.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 17. | Yarur AJ, Jain A, Hauenstein SI, Quintero MA, Barkin JS, Deshpande AR, Sussman DA, Singh S, Abreu MT. Higher Adalimumab Levels Are Associated with Histologic and Endoscopic Remission in Patients with Crohn's Disease and Ulcerative Colitis. Inflamm Bowel Dis. 2016;22:409-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Zittan E, Kabakchiev B, Milgrom R, Nguyen GC, Croitoru K, Steinhart AH, Silverberg MS. Higher Adalimumab Drug Levels are Associated with Mucosal Healing in Patients with Crohn's Disease. J Crohns Colitis. 2016;10:510-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Ungar B, Levy I, Yavne Y, Yavzori M, Picard O, Fudim E, Loebstein R, Chowers Y, Eliakim R, Kopylov U, Ben-Horin S. Optimizing Anti-TNF-α Therapy: Serum Levels of Infliximab and Adalimumab Are Associated With Mucosal Healing in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2016;14:550-557.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 20. | Vande Casteele N, Ferrante M, Van Assche G, Ballet V, Compernolle G, Van Steen K, Simoens S, Rutgeerts P, Gils A, Vermeire S. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320-1329.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 642] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 21. | D’Haens G, Vermeire S, Lambrecht G, Baert F, Bossuyt P, Pariente B, Buisson A, Bouhnik Y, Filippi J, Vander Woude J, Van Hootegem P, Moreau J, Louis E, Franchimont D, De Vos M, Mana F, Peyrin-Biroulet L, Brixi H, Allez M, Caenepeel P, Aubourg A, Oldenburg B, Pierik M, Gils A, Chevret S, Laharie D; GETAID. Increasing Infliximab Dose Based on Symptoms, Biomarkers, and Serum Drug Concentrations Does Not Increase Clinical, Endoscopic, and Corticosteroid-Free Remission in Patients With Active Luminal Crohn's Disease. Gastroenterology. 2018;154:1343-1351.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |