Published online Apr 7, 2019. doi: 10.3748/wjg.v25.i13.1628
Peer-review started: February 6, 2019
First decision: February 21, 2019
Revised: March 7, 2019
Accepted: March 11, 2019
Article in press: March 12, 2019
Published online: April 7, 2019
Alcohol-related liver disease (ALD) is a leading cause of liver failure and indication for liver transplantation that arises in the setting of alcohol use disorder (AUD). Previous reviews of transplantation for ALD are limited in scope of outcomes and type of ALD studied. A comprehensive systematic review could improve use of transplantation in ALD and improve future research. We hypothesize that while transplanting ALD may improve mortality and relapse, findings will be limited by pre-specified causes of heterogeneity - assessment and treatment of AUD, definition of ALD, spectrum of ALD studied, assessment and rates of relapse, and study quality and bias.
To optimize liver transplantation for ALD, understanding existing research to guide future research, we conducted a systematic review with meta-analysis.
We conducted a systematic review, comparing liver transplant to no-transplant in patients with ALD, with a primary outcome of both short- and long-term mortality and relapse. We performed a comprehensive search of MEDLINE, EMBASE, Web of Science, and The Cochrane Library databases for peer-reviewed journal articles comparing use of liver transplant in ALD to no-transplant. Two reviewers independently conducted screening, full text review, and data extraction according to the PRISMA guidelines. We report the quality of the evidence according to the GRADE criteria.
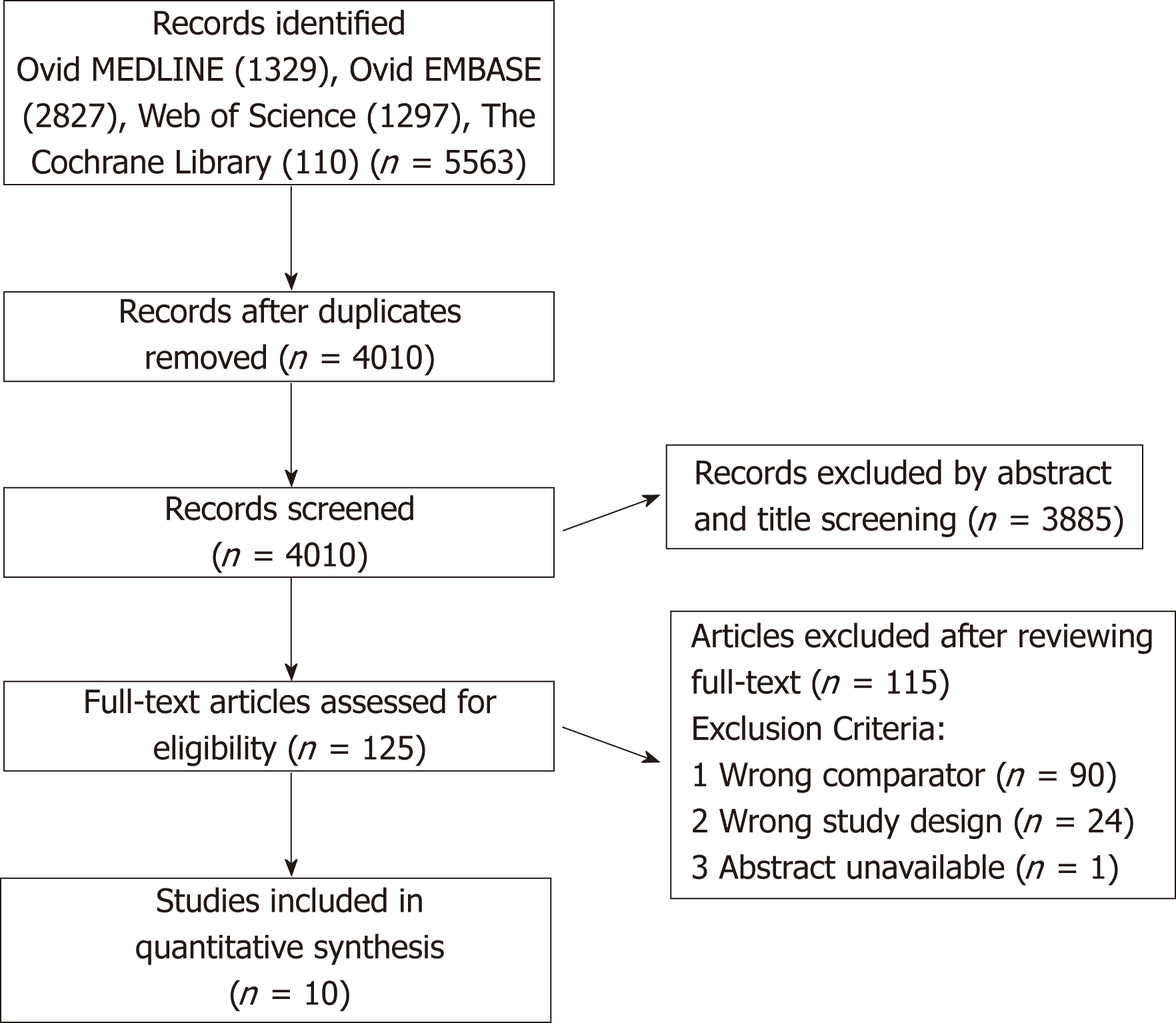
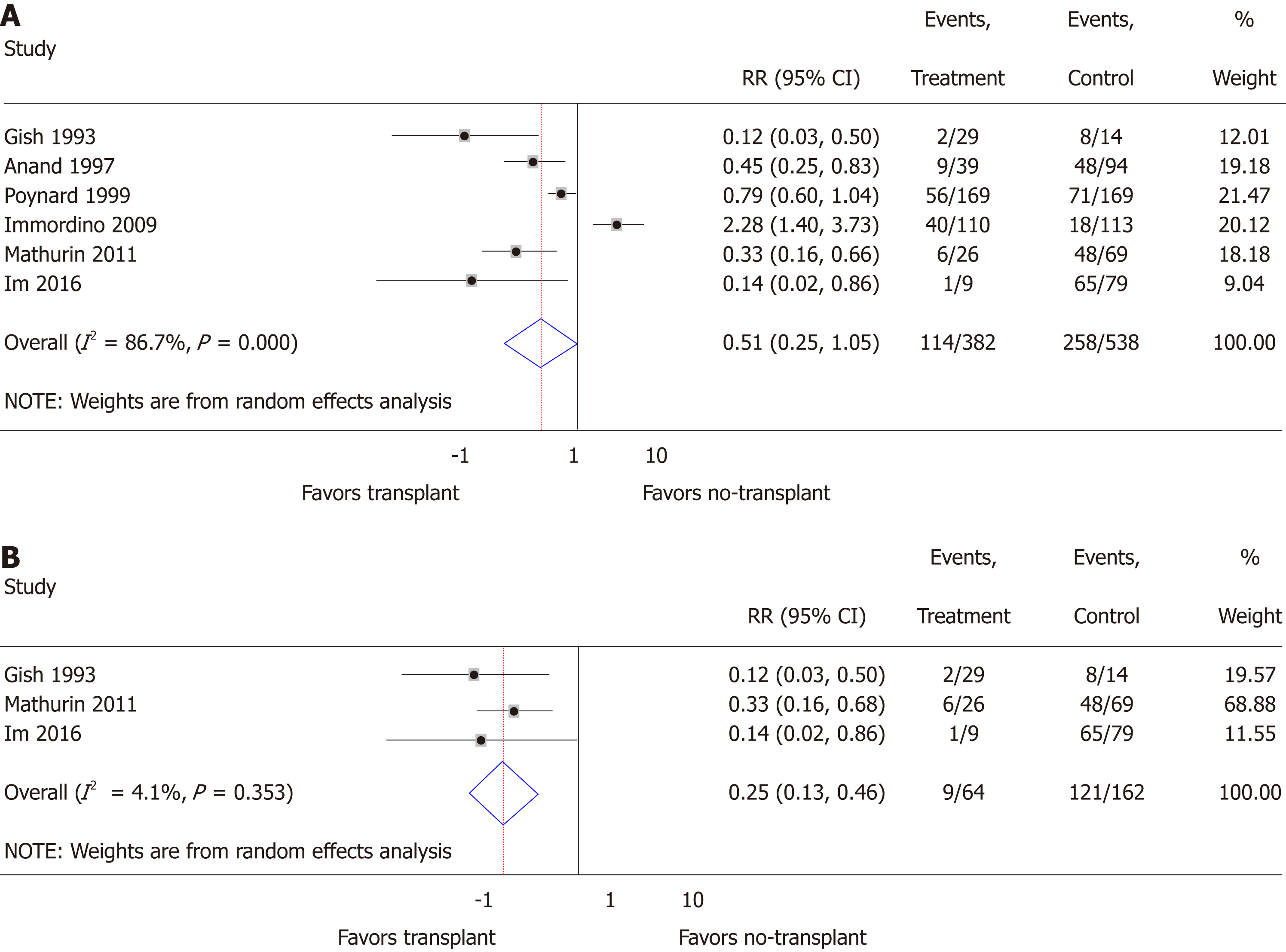
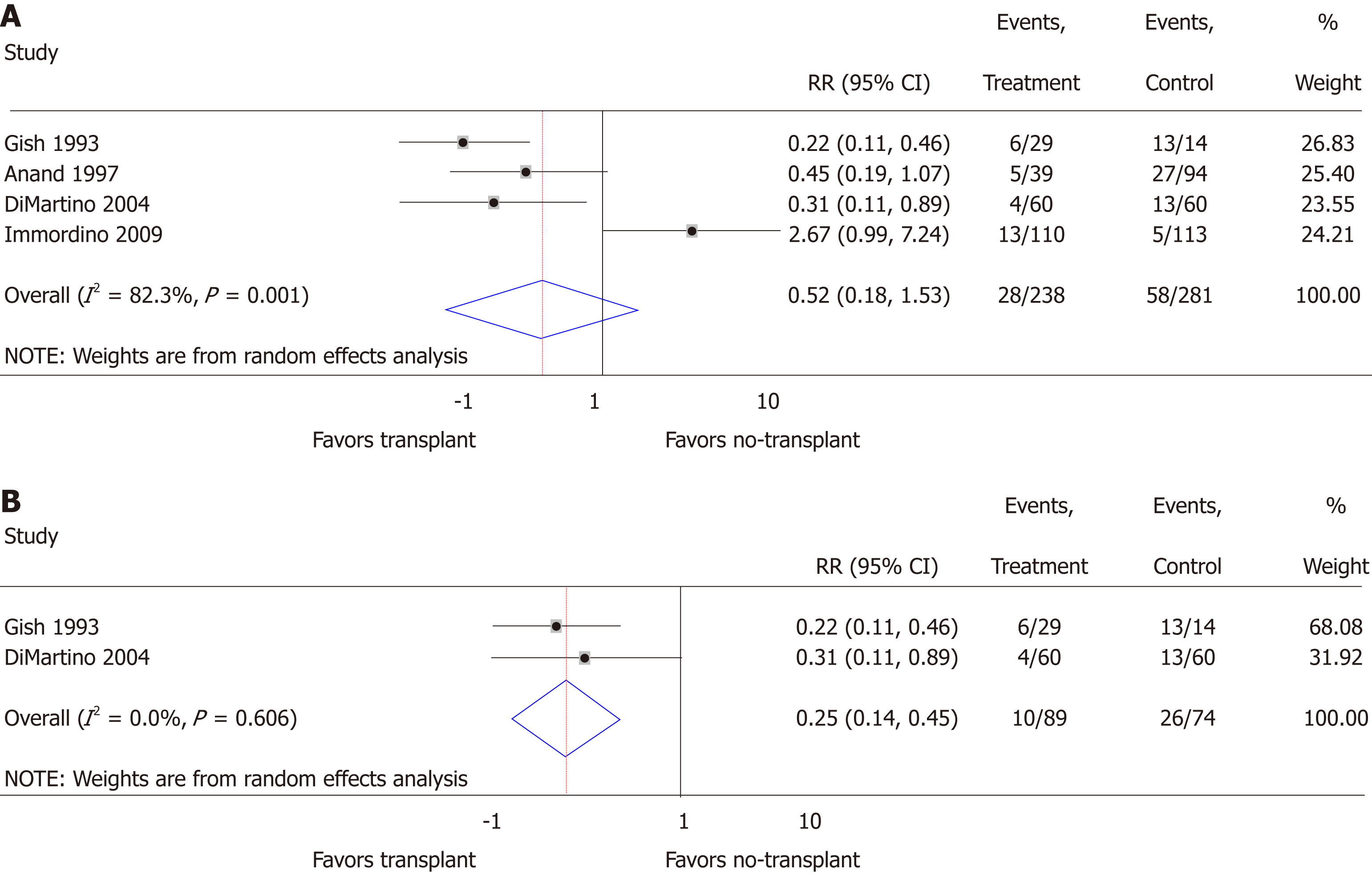
We analyzed data from 10 studies. Of 1332 participants, 34.2% (456/1332) had undergone liver transplantation, while 65.8% (876/1332) had not. While random effects meta-analysis suggested transplant in comparison to no-transplant had an association of reduced mortality that did not reach statistical significance, relative risk (RR) = 0.51 (0.25-1.05), but not relapse risk, RR = 0.52 (0.18-1.53), significant heterogeneity limited these findings. When restricted to prospective data, transplant compared to no-transplant significantly reduced mortality, RR = 0.25 (0.13-0.46, P < 0.01), and relapse, RR = 0.25 (0.14-0.45, P < 0.01), with insignificant heterogeneity but persistent small-study effects. The overall quality of the evidence was Very Low. Heterogeneity analysis suggested that AUD assessment and treatment was often not reported while ALD, relapse assessment and rate, and data collection were institutionally rather than standardly defined.
Systematic review of liver transplantation for ALD suggests reduced mortality and relapse in heterogeneous, institution-specific populations with inherent bias. To understand efficacy of transplanting ALD, our research approach must change.
Core tip: Our findings suggest the dearth of well-published literature on transplantation in alcohol-related liver disease (ALD) and the urgent need for rigorous standardization in studying ALD. Such standardization would enable global scale assessment on the efficacy of transplanting ALD. Standardization should include addressing the presence and treatment of alcohol use disorder, the clinical definition of ALD, reporting the spectrum of the population studied (acute, chronic, acute on chronic, hepatocellular carcinoma in the setting of ALD), data collection, and definition and detection of relapse.
- Citation: Shen NT, Londono C, Gold S, Wu A, Mages KC, Brown RSJ. Systematic review with meta-analysis on transplantation for alcohol-related liver disease: Very low evidence of improved outcomes. World J Gastroenterol 2019; 25(13): 1628-1639
- URL: https://www.wjgnet.com/1007-9327/full/v25/i13/1628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i13.1628
Alcohol-related liver disease (ALD) is a leading cause of liver failure in the United States that arises in the setting of alcohol use disorder (AUD)[1-3]. Recent studies report a rising prevalence on the transplant waiting list and among privately insured persons[1,2]. Additionally, population studies suggest rising ALD death rates, and most recently, ALD was found to have the greatest risk for death among gastrointestinal diseases with a rate of 6.8 per 100000[4-6]. Treatment options for ALD are limited and at the minimum involve treating the underlying AUD, possibly in combination with liver transplantation. Given the increasing burden of disease and mortality in the setting of a profound shortage of donor organs, it is imperative to understand our current use of transplantation in the ALD population in order to optimize care and future research.
ALD occurs on a pathological spectrum and assessment of transplant use within ALD is limited. ALD ranges from asymptomatic steatosis to symptomatic cirrhosis and its complications[7], and this process can be categorized into acute alcohol-related hepatitis (AH), severe alcohol-related hepatitis (SAH), chronic ALD, acute-on-chronic ALD, and hepatocellular carcinoma due to ALD[7]. While a prior published systematic review with meta-analysis investigated alcohol relapse as primary outcome and 6-mo mortality as a secondary outcome, this review has significant limitations that restrict clinical applicability[8]. The limitations include the narrow inclusion criteria of observational studies focused in AH, the inclusion of studies with a lack of comparator (potentially biasing the results in favor transplantation), the failure to extract or comment on AUD, the focus on short-term 6-mo mortality outcome, ambiguity if the PRISMA guidelines were followed, failure to report the overall quality of the evidence using GRADE (which is different than bias assessment), and limiting assessment of heterogeneity to removal of studies without “stringent criteria for selecting candidates for liver transplantation”[8]. Moreover, the review did not include data extraction of factors associated with abstinence, which were reported in a prior systematic review published by McCallum et al[9] - social stability, no nuclear family history of alcohol disease, older age, no prior rehabilitation treatment failure, no co-existing psychiatric problem[8,9]. Exploration of heterogeneity causes and use of pre-specified control for heterogeneity is necessary to accurately study clinical efficacy of liver transplantation.
Overall, a more comprehensive systematic review of the literature, thoroughly assessing both long-term outcomes and pre-specified causes of heterogeneity to identify best practices, was needed to improve care, establish future research priorities, in particular related to the use of transplant, in the context of underlying AUD and in the broader ALD population, and inform clinical practice guidance documents. We hypothesized that the literature would be limited by a lack of standardization of terminology, and that the use of transplantation in the ALD population would also be highly variable. To assess this, we systematically reviewed the use of transplantation in all forms of ALD, including all studies comparing transplant to no-transplant and investigating short- and long-term outcomes. All ALD populations were included without restriction. Placement of the cohort on the ALD disease spectrum, assessment of underlying AUD and treatment, definition of ALD and relapse, assessment of relapse, reporting of data associated with abstinence, and study quality and bias were collected. By systematically reviewing the published literature, in particular the definitions used to assess the ALD population and outcomes, we aimed to fully characterize any heterogeneity, with the goal of assessing and combating bias to optimize and standardize care when considering patients with ALD for transplant, allowing best use of a limited resource.
This study was constructed using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines[10]. Accordingly, a protocol was registered in PROSPERO, an international prospective register of systematic reviews (Registration #: CRD42017016195; URL: https://http://www.crd.york.ac.uk/prospero/display_record.php?recordid=16195), and the PRISMA checklist was submitted with our manuscript.
Electronic searches: A comprehensive literature search identifying studies investigating transplant compared to no-transplant for ALD was conducted. The initial search was performed on February 3, 2017 via Ovid MEDLINE® and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily and Versions®. Follow-up searches via Ovid EMBASE (1974 to present); Web of Science (Core Collection); and The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Methodology Register, Technology Assessments (HTA)) were run on February 22, 2017. Search terms included all subject headings and/or keywords associated with “Alcoholic Liver Disease”, “Liver Transplantation”, “Survival Rate”, “Mortality”, “Treatment Outcome”, “Prognosis”, “Recurrence”, “Recidivism”, “Alcohol Drinking”, “Patient Compliance”, “Temperance”, “Alcohol Abstinence”, and “Alcohol Cessation”. There were no language, publication date, or article-type restrictions implemented. These searches were re-run on February 28, 2018 to capture potentially relevant articles published after our initial search. The full OVID Medline Search strategy is available in the supporting information.
Types of studies: Randomized controlled trials, observational, and case-control studies investigating adults with ALD comparing those with and without transplant.
Types of participants: Adults aged ≥ 18 years with ALD.
Types of interventions: Use of liver transplant (intervention) in comparison to no-transplant (control).
Types of outcome measures: Primary outcomes assessed short-term (≤ 6 mo) and long-term (> 6 mo) mortality and rate of alcohol relapse. Secondary outcomes included adverse events such as graft dysfunction and/or failure, bacterial infection (ascites, pulmonary, urinary, bacteremia, other), hepatorenal syndrome, gastrointestinal bleeding, and mechanical ventilation.
Selection of studies: After excluding duplicates, two researchers (Londono C and Gold S) independently screened titles and abstracts. An independent, third investigator (NTS) resolved any conflicts. All articles were reviewed against pre-defined inclusion criteria. Employing the same process, articles underwent full-text review, and those meeting inclusion criteria moved on to data extraction.
Data extraction and management: Two investigators (Londono C and Gold S) independently extracted data using a standardized form separating the cohorts into transplant and no-transplant. Patients on the waitlist at time of data reporting were excluded from the analysis in order to not bias the results. Extracted data included trial design and methodology (assessment and treatment of AUD, definition of the diagnosis of ALD, required period of abstinence prior to transplant, definition of relapse, recognition of the presence of AH or SAH), patient demographics (age, sex, ethnicity, Child-Turcotte-Pugh (CTP) score, model for end-stage liver disease (MELD) score, history of prior alcohol-related decompensating events, medical management (pentoxifylline or steroids), Maddrey’s discriminant function, labs on presentation (bilirubin, prothrombin time), Lille score at 7 d, length of abstinence in months (pre-transplant where applicable), time to transplant listing, loss to follow-up, and length of follow-up), and primary and secondary outcomes. Where data was missing or unclear, the manuscript corresponding authors were contacted for further information.
Risk of bias and quality assessment: Two investigators (Londono C and Gold S) independently assessed trial risk for bias using the Newcastle-Ottawa Scale for cohort and case-control studies and the Cochrane Risk of Bias tool for randomized controlled trials[11,12]. The Newcastle Ottawa Scale assesses for bias using a star system across 3 categories - selection, comparability, and exposure[11]. The Cochrane Risk of Bias tool assesses for bias, categorizing the risk as high, low, or unclear across the following components: selection (randomization, allocation concealment), performance (blinding of participants and personnel), detection (blinding of outcome assessment), attrition (incomplete outcome data), reporting (selective reporting), and other (funding, etc)[12]. Studies lacking the maximum stars available across categories using the Newcastle Ottawa Scale or with low or unclear risk of bias according to the Cochrane Risk of Bias tool were considered to be at risk for bias. A third investigator (NTS) resolved disagreements. The overall quality of the evidence was evaluated using the GRADE system[13], downgrading based on study design, study limitations, inconsistency of results, indirectness of evidence, imprecision, and reporting bias.
Statistical analysis: Studies meeting the inclusion criteria were tabulated. Using random effects meta-analysis method of DerSimonian and Laird, statistically and clinically appropriate studies were combined to calculate a summary relative risk (RR) and 95% confidence interval (CI). Pre-specified subgroup analyses were undertaken to evaluate whether the estimated effect was modified by study design. Using the statistical software Stata, version 14 (StataCorp LP, College Station, TX, United States), data analysis was conducted.
Assessment of heterogeneity: To assess heterogeneity, the I2 statistic and the chi-squared test were computed[14]. Pre-specified explanations for heterogeneity included: study design, definition of ALD, definition and assessment of relapse, and inclusion criteria.
Assessment of publication bias: Funnel plots and Egger’s regression were used to evaluate for publication bias[15-17].
Included studies: Of the 4010 articles screened, 125 underwent full text review and 10 studies (6 prospective, 4 retrospective) met inclusion criteria (Figure 1). These 10 studies included a total of 1332 participants, of which 34.2% (456/1332) underwent liver transplantation and 65.8% (876/1332) did not. Table 1 shows details of the included studies and their transplant and no-transplant groups baseline characteristics, with weighted averages where applicable reported in Table 2. Included studies were performed between 1993 to 2017, with high representation of a French population, 40% (4/10), with later studies more likely to report findings restricted to SAH populations[18,19]. The majority of both transplant and no-transplant populations were men, 80.1% and 64.1%, respectively and had mean weighted ages of 46.7 and 49.6. Data reporting CTP and MELD broken down into transplant and no-transplant cohorts was limited (Tables 1 and 2); the study populations appeared to be CTP class B/C with lower MELD scores in studies not restricted to SAH populations[20,21] in comparison to those restricted to patients with SAH[18,19]. Most studies did not report ethnicity, history of prior alcohol-related decompensating events, use of medications, Maddrey’s discriminant function, labs on presentation, or Lille score (data not shown).
| Study | Place | Study design | Transplant | No-transplant | ||||||||
| n (%) | Age1 (SD or range) | Male sex n (%) | CTP1 (SD or range) | MELD (SD or range) | n (%) | Age1 (SD or range) | Male sex n (%) | CTP1 (SD or range) | MELD (SD or range) | |||
| Gish et al[24] | United States | P, C | 29 (62) | 472 | 30 (64)2 | 12 (9-15)2 | 14 (30) | 472 | 30 (64)2 | 13 (9-15)2 | ||
| Anand et al [22] | United Kingdom | R, C | 39 (28) | 48 (37-69) | 34 (87) | 113 | 94 (69) | 51 (30-71)3 | 67 (74) | 113 | ||
| Poynard et al [25] | France | R, CC | 169 (50) | 47 (39-55) | NS | 9 (5-15)3 | 169 (50) | 47 (39-54) | NS | 9 (5-15)3 | ||
| Veldt et al[26] | France | P, C | 2 (3) | 59 (37-82)2 | 47 (64)2 | 11 (10-15)2 | 72 (97) | 59 (37-82)2 | 47 (64)2 | 11 (10-15)2 | ||
| DiMartino et al[23] | France | P, RCT | 60 (50) | 502 | 92 (77)2 | 8.22 | 60 (50) | 502 | 92 (77)2 | 8.22 | ||
| Immordino et al[20] | Germany | R, C | 110 (45) | 53 (30-68) | 95 (86) | 10 (6-13) | 14 (4-35) | 113 (46) | 50 (33-64) | 64 (57) | 6.5 (5-13) | 12 (6-40) |
| Alvarez et al [21] | Spain | P, C | 5 (3) | < 65 | NS | > 8 | 14 (13-15)2 | 156 (95) | 56 (54-58)2 | 135 (82)2 | 9 (8-9)2 | 14 (13-15)2 |
| Mathurin et al[18] | France | P, CC | 26 (27) | 47 (35-61) | 15 (58) | NS | 30 (22-47) | 69 (73) | 52 (47-54) | 41 (59) | NS | NS |
| Im et al[19] | United States | P, CC | 9 (10) | 41 (30-60) | 5 (55) | NS | 39 (27-42) | 79 (84) | 48 (26-68)2 | 54 (57)2 | NS | 31 (16-52)2 |
| Onishi et al[27] | Japan | R, | 7 (7) | 44 (28-51) | 4 (57) | 10.1(2)2 | NS4 | 50 (75) | 52 (31-69) | 37 (74) | 10.1 (2)2 | NS4 |
| n (%) | Male n (%) | Age | CTP | MELD | |
| Transplant | 456 (34.2) | 153 (80.1) | 46.7 | 9.8 | 18.4 |
| No-transplant | 876 (65.8) | 183 (64.1) | 49.6 | 8.7 | 15.21 |
Gaps in reporting: Supplementary Table 1 shows individual trial details, highlighting gaps in reporting regarding assessment and treatment of underlying AUD, diagnosis of ALD, requirement of a period of abstinence prior to transplant, details of relapse (definition and assessment), and accounting for or restricting to the presence of AH and or SAH. Two of the studies did not report how ALD was diagnosed[22,23], and the remaining 8 studies used a range of diagnostic criteria - some studies included mixed liver etiology (alcohol and other causes of liver disease)[24]; others defined ALD by alcohol consumption ranging from 50 g/d for both men and women[25], greater than 3 units per day for men or 2 units per day for women[26], or greater than 80 g/d for men or 60 g/d for women[21]; and the most recent studies did not specifically quantify the amount[18,19,27]. The majority of studies did not require pathological diagnosis, specify a required period of abstinence, or differentiate where patients were classified on the spectrum of ALD. Relapse was rarely defined, mostly assessed for by interviews at non-standardized intervals, and only three studies accounted for quantity of alcohol consumed among those with relapse[18,19,26].
Risk of bias in the included studies: Supplementary Table 2 shows the study quality and risk of bias assessment. The randomized control trial[23], the only abstract included in the analysis, had an unclear risk of bias across the different categories. Four of the later studies appeared at substantial risk of bias - the two case-control studies had a higher risk of selection and outcome bias[18,19] while the other two were at risk of comparability bias[21,27]. Two earlier studies were additionally at risk of outcome bias[24,25]. Of the three studies that appeared to have low risk of bias[20,22,26], only one was prospectively conducted[26].
Mortality: Random effects meta-analysis suggested a trend toward transplantation reducing mortality risk in comparison to no-transplant (RR = 0.51; 95%CI: 0.25-1.05; P = 0.07), but heterogeneity (I2 = 86.7%; P < 0.01) was significant (Figure 2A). This heterogeneity was no longer significant (I2 = 4.1%; P = 0.35) and a statistically significant reduction in mortality (RR = 0.25; 95%CI: 0.13-0.46; P < 0.01) was observed when restricting the study population to prospectively collected data (Figure 2B)[18,19,24]. When restricting to studies reporting early mortality, the remaining two studies included prospectively collected data in steroid non-responsive SAH populations[18,19], suggesting significantly reduced 6-mo mortality (RR = 0.30; 95%CI: 0.15-0.58; P < 0.01) with insignificant heterogeneity (I2 = 0.0%; P = 0.35). All six studies with mortality data reported presence of AH patients, with three specifying presence of SAH[18,19,25], of which two studied only steroid non-responsive SAH[18,19]. The lack of details of the AH patients within the other studies prevented further meta-analyses. Graphical evidence of publication bias was observed when including all studies (Supplementary Figure 1A) and when restricted to prospectively collected studies (Supplementary Figure 1B), with suggestion of the presence of small-study effects, P = 0.14 and P = 0.07, respectively.
Relapse: Relapse risk in both the transplant and no-transplant cohorts was reported in 4 of the studies[20,22-24], and random effects meta-analysis was not significantly different (RR = 0.52; 95%CI: 0.18-1.53; P = 0.24) with significant heterogeneity (I2 = 82.3; P < 0.01) (Figure 3A). None of these studies differentiated between short or long-term relapse. When restricting the analysis to prospectively collected data[23,24], relapse risk significantly decreased for transplant patients (RR = 0.25; 95%CI: 0.14-0.45; P < 0.01) with insignificant heterogeneity (I2 = 0.0%; P = 0.61) (Figure 3B). Funnel plot suggested publication bias when including all studies (Supplementary Figure 2A) and when restricted to prospectively collected data (Supplementary Figure 2B). Of the 4 studies, 3 included a mixed population of AH that did not specify SAH and lacked details to allow additional meta-analyses (Supplementary Table 1)[20,22,24].
Adverse events: Four studies specified the occurrence of graft dysfunction and re-transplantation[18,19,25,27]. Of these four, the study with the longest follow-up observed graft dysfunction in two of their 31 transplant patients with relapse (6%), of which one (50%) underwent re-transplant, the other which died (Supplementary Table 3)[25]. Other adverse events including bacterial infection (ascites, pulmonary, urinary, bacteremia, other), hepatorenal syndrome, gastrointestinal bleeding, and mechanical ventilation were only reported in two studies (data not shown).
| Study | Transplant | No-transplant | ||||||||
| Abstinence length (SD or range)1 | Relapse n (%) | Mortality n (%) | Loss to follow-up n (%) | Follow-up length (SD or range)1 | Abstinence length (SD or range)1 | Relapse n (%) | Mortality n (%) | Loss to follow-up n (%) | Follow-up length (SD or range)1 | |
| Gish et al[24] | 21 | 6 (21) | 2 (7) | 0 (0) | 24 (12-41)2 | 5 | 13 (93) | 8 (57) | 5 (36) | 24 (12-41)2 |
| Anand et al[22] | 18 (6-130) | 5 (13) | 9 (23) | 0 (0) | 25 (7-63) | 11 (2-86)3 | 27 (37) | 48 (51) | 11 (12) | 24 (6-90) |
| Poynard et al[25] | ≥ 64 | 31 (18) | 56 (33) | 0 (0) | 28 (21-37) | NS | NS | 71 (42) | NS | NS |
| Veldt et al[26] | ≥ 6 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| DiMartino et al[23] | NS | 4 (6) | NS | NS | 422 | NS | 13 (21) | NS | NS | 422 |
| Immordino et al[20] | NS | 13 (12)5 | 40 (36) | NS | 120 | NS | 5 (6) | 18 (18) | 14 (12) | 120 |
| Alvarez et al[21] | NS | NS | 116 (70)2 | 9 (5)2 | 54 (19-96)2 | NS | NS | 116 (70)2 | 9 (5)2 | 54 (19-96)2 |
| Mathurin et al[18] | NS | 3 (12) | 6 (23) | NS | 24 | NS | NS | 48 (70) | NS | 24 |
| Im et al[19] | NS | 2 (22) | 1 (11) | NS | 25 (6-39) | NS | NS | 65 (76)6 | NS | NS |
| Onishi et al[27] | 21.2 (17.4)7 | 1 (14) | NS | NS | 61.2 | 8.8 (13.6) | NS | NS | NS | NS |
Quality of the evidence: Using the GRADE system, the quality of the evidence across studies was classified as Very Low for use of transplantation in ALD and relapse risk, with downgrading for study design, risk of bias, inconsistency, imprecision, and publication bias.
ALD is a leading indication for liver transplantation[28,29], with increasing prevalence[1] and incidence[30] on the liver transplant waiting list, but our understanding of the utility and application of transplant in this population compared to no-transplant is limited with no prior comprehensive systematic review. Random effects meta-analysis of the currently reported literature supports that transplantation for ALD reduced mortality, but not relapse risk, and significant heterogeneity limited these findings. The overall quality of evidence for both outcomes by GRADE criteria was very low. When analyzing prospectively collected data, transplant in comparison to no-transplant significantly reduced mortality and relapse with insignificant heterogeneity, but the suggestion of small-study effects driving these results persisted. Similar to our findings, significant heterogeneity observed in a prior systematic review focused on transplanted AH patients[8] corrected with restricting analysis to studies with strict candidate selection criteria and SAH. The prior systematic review failed to explore the causes for heterogeneity beyond this corrective maneuver, but in order to improve our study of ALD so that future studies report clear findings that allow larger scale compilation of data, a detailed understanding of prior causes of heterogeneity to allow successful future standardization is necessary.
None of the studies explicitly comment on the severity or treatment of AUD present in their study cohorts, which would impact outcomes of interest, in particular relapse. AUD when diagnosed according to the Diagnostic and Statistical Manual (DSM) 5th edition put forth by the American Psychiatric Association is defined by meeting two of eleven possible criteria over a 12-mo period and further categorized into mild (presence of 2-3 symptoms), moderate (presence of 4-5 symptoms) or severe (presence of 6 or more symptoms) AUD[31]. Characterization of underlying AUD is imperative as prior research suggests that patients with less severe AUD are less likely to relapse[32]. Additionally, effective treatments for AUD include medications and behavioral therapies, and a recent systematic review found that interventions increase abstinence[33]. Overall, this failure of studies to provide detailed information pertaining to underlying AUD severity and treatment likely contributed to heterogeneity, reducing the reproducibility of prior publications.
Furthermore, all of the studies included defined ALD inconsistently. The majority of published research studies rely on clinical history rather than pathology, even when transplant makes explant liver pathology easily available. Additionally, studies use varying alcohol consumption cut-offs, vague terminology such as “alcoholism” without elaboration, and include mixed disease processes (alcohol in combination with another cause of liver disease, e.g., hepatitis C). Similar to findings reported by McCallum et al[9], we report that this persistently vague definition of ALD inevitably leads to greater heterogeneity and prevents comparability among studies. Studies also fail to consistently characterize the clinical spectrum of ALD studied lie - acute, chronic, acute on chronic, hepatocellular carcinoma due to ALD - though this spectrum is commonly used in clinical practice[7]. This reduces the clinical applicability and external validity of the reported literature. The current literature investigating the use of transplantation in ALD defines ALD in a manner that not only introduces selection bias into the patients studied to date, but also may affect the study outcomes and raises the question of the presence of country and or transplant center bias in access to transplantation for patients with ALD.
In addition to heterogeneous disease definitions, the current reporting of pre-transplant abstinence and relapse rates in ALD allows significant under-reporting and variability[7,9]. Pre-transplant abstinence in the transplant population was not reported in the majority of studies (Table 3), and though detailed breakdown of relapse appears more clinically useful[34], only 4 of the studies defined relapse, varying from “any consumption” to “slips.” Additionally, though patient self-report, interviewing, and biochemical tests of blood, urine, or hair all present with limitations in terms of sensitivity, specificity, cost, and feasibility for monitoring for relapse[35], the reported studies likely suffered from underreporting; the majority of studies used “short” or “random” intervals and relied only on interviews to detect relapse. A standardized protocol for defining and detecting relapse pre- and post-transplant in general practice and in clinical trials is needed.
There appears to be a critical need for standardized data collection tools to capture underlying AUD severity and treatment, spectrum of ALD studied, patient demographics (e.g., race, ethnicity, socioeconomics) and adverse events that may influence mortality and relapse outcomes. While the National Institute on Alcohol Abuse and Alcoholism (NIAAA) made recommendations to help with standardization of the study of AH[36], the focus on AH and lack of incorporation of underlying AUD definitions and diagnoses limit the recommendations. This suggests the need for newer, more comprehensive recommendations, which were recently put forth by Shen et al[37], proposing a standardized flow chart approach to patients with ALD and a comprehensive data collection tool.
Not only does the heterogeneity due to lack of standardization limit our ability to fully assess transplantation in ALD, but this systematic review also suggests that the published data is of poor and limited quality - small studies with suggestion of small study effects in analysis, mostly observational or case-control cohort study design with only a single randomized controlled trial in abstract form, under-representation of many countries, and lack of long-term follow-up. Once definitions and data collection are standardized, within the spectrum of AUD and ALD, future multi-center prospective consortia and preferably controlled randomized clinical trials with long-term follow-up should be organized to capture and optimize the use of transplantation in the ALD population.
Our systematic review explores the extensiveness of study heterogeneity, even affecting the definition of ALD and lack of accounting for AUD. Additionally the majority of studies failed to report our pre-specified outcome of adverse events and lacked long-term follow-up to capture graft dysfunction or failure. Similarly, the definition of relapse, monitoring of it, and presence of social support or underlying demographics that might influence it were inconsistently reported. Despite the limitations, our analyses do suggest a short-term mortality benefit for transplantation in at least a subgroup of the ALD population, patients with steroid non-responsive SAH. Overall, the review highlights the need for more detailed studies in the ALD population, particularly the non-SAH.
Overall prior literature to date has focused on requirements of pre-transplant abstinence prior to listing and transplanting AH patients, but our systematic review suggests that this focus may be premature. Our findings suggest the urgent need for rigorous standardization in studying ALD, including the presence and treatment of AUD, the clinical definition of ALD, reporting the spectrum of the ALD population studied, data collection, and definition and detection of relapse. Only with such standardization can the needed international, large-scale, randomized controlled trials with long-term follow-up be conducted in a clinically useful manner.
Alcohol-related liver disease (ALD) is a leading cause of liver failure and indication for liver transplantation, thus optimizing use of liver transplantation in this patient population is imperative. Systematically reviewing the literature, comparing transplanting ALD to not transplanting ALD is necessary to understand how to optimize use of liver transplantation in ALD and to direct future research.
Systematically reviewing the existing literature on the use of liver transplant compared to no-transplant in patients with ALD could help guide clinical care and future directions of research.
To help inform optimal use of liver transplantation in ALD and understand limitations of existing research to guide future research, we conducted a comprehensive systematic review.
We systematically reviewed the existing literature for studies comparing liver transplant to no-transplant with a primary outcome of both short- and long-term mortality and relapse. Pre-specified causes of heterogeneity included assessment and treatment of alcohol use disorder (AUD), definition of ALD, spectrum of ALD studied, assessment and rates of relapse, and study quality and bias.
We analyzed data from 10 studies including 1332 participants. While meta-analysis comparing liver transplant to no-transplant suggested improved mortality, relapse was found to be insignificant and both meta-analyses were limited by significant heterogeneity. Outcomes and heterogeneity improved with restriction to prospectively collected data; liver transplant in comparison to no-transplant had significantly reduced mortality and relapse with insignificant heterogeneity, though results remained limited by small-study effects. Overall, the quality of the evidence was very low.
Current systematic review with meta-analysis comparing liver transplant to no-transplant suggests a mortality and relapse benefit in heterogeneous, institution-specific populations with inherent bias.
To understand efficacy of liver transplantation for ALD on a global scale, formal recognition of the dearth of well-published literature on transplantation in this population is necessary, and there is an urgent need to standardize our approach to studying ALD. Such standardization should include assessment of the presence and treatment of AUD, the clinical definition of ALD, reporting the spectrum of the ALD population studied, data collection, and definition and detection of relapse.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carter WG, Morales-González JA, Senturk H S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
| 1. | Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, Charlton M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152:1090-1099.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 430] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 2. | Mellinger JL, Shedden K, Winder GS, Tapper E, Adams M, Fontana RJ, Volk ML, Blow FC, Lok ASF. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology. 2018;68:872-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 3. | Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1532] [Cited by in F6Publishing: 1689] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 4. | Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: Final Data for 2014. Natl Vital Stat Rep. 2016;65:1-122. [PubMed] [Cited in This Article: ] |
| 5. | Rehm J, Shield KD. Global alcohol-attributable deaths from cancer, liver cirrhosis, and injury in 2010. Alcohol Res. 2013;35:174-183. [PubMed] [Cited in This Article: ] |
| 6. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 776] [Cited by in F6Publishing: 889] [Article Influence: 177.8] [Reference Citation Analysis (0)] |
| 7. | Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113:175-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 431] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 8. | Marot A, Dubois M, Trépo E, Moreno C, Deltenre P. Liver transplantation for alcoholic hepatitis: A systematic review with meta-analysis. PLoS One. 2018;13:e0190823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | McCallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol Alcohol. 2006;41:358-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12295] [Cited by in F6Publishing: 12046] [Article Influence: 803.1] [Reference Citation Analysis (0)] |
| 11. | Wells G SB, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa: Ottawa Hospital Research Institute 2012; . [Cited in This Article: ] |
| 12. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18487] [Cited by in F6Publishing: 21250] [Article Influence: 1634.6] [Reference Citation Analysis (2)] |
| 13. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11058] [Cited by in F6Publishing: 12497] [Article Influence: 781.1] [Reference Citation Analysis (0)] |
| 14. | Higgins JP. Cochrane Handbook for Systematic Reviews of Interventions In: Higgins JPT, Green S, editor. Section 95 Heterogenity. The Cochrane Collaboration® Version 5.1.0 Last updated March 2011. Available from: URL: http://handbook.cochrane.org. [Cited in This Article: ] |
| 15. | Langan D, Higgins JP, Gregory W, Sutton AJ. Graphical augmentations to the funnel plot assess the impact of additional evidence on a meta-analysis. J Clin Epidemiol. 2012;65:511-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] [Cited in This Article: ] |
| 17. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] [Cited in This Article: ] |
| 18. | Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, Duhamel A, Pageaux GP, Leroy V, Dharancy S, Louvet A, Boleslawski E, Lucidi V, Gustot T, Francoz C, Letoublon C, Castaing D, Belghiti J, Donckier V, Pruvot FR, Duclos-Vallée JC. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790-1800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 597] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 19. | Im GY, Kim-Schluger L, Shenoy A, Schubert E, Goel A, Friedman SL, Florman S, Schiano TD. Early Liver Transplantation for Severe Alcoholic Hepatitis in the United States--A Single-Center Experience. Am J Transplant. 2016;16:841-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 20. | Immordino G, Gelli M, Ferrante R, Ferrari C, Piaggio F, Ghinolfi D, Sturdevant M, Andorno E, Morelli N, Bottino G, Casaccia M, Valente U. Alcohol abstinence and orthotopic liver transplantation in alcoholic liver cirrhosis. Transplant Proc. 2009;41:1253-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Alvarez MA, Cirera I, Solà R, Bargalló A, Morillas RM, Planas R. Long-term clinical course of decompensated alcoholic cirrhosis: a prospective study of 165 patients. J Clin Gastroenterol. 2011;45:906-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Anand AC, Ferraz-Neto BH, Nightingale P, Mirza DF, White AC, McMaster P, Neuberger JM. Liver transplantation for alcoholic liver disease: evaluation of a selection protocol. Hepatology. 1997;25:1478-1484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | DiMartino V, Miguet M, Vanlemmens C, Monnet E, Gache P, Messner M, Hrusovsky S, Minello A, Hillon P, Bresson-Hadni S, Miguet JP. Impact of Liver Transplantation (LT) on Alcohol Consumption (AC) in Patients with Pugh B Cirrhosis: A Randomized Study. J Hepatol. 2004;40:44. [DOI] [Cited in This Article: ] |
| 24. | Gish RG, Lee AH, Keeffe EB, Rome H, Concepcion W, Esquivel CO. Liver transplantation for patients with alcoholism and end-stage liver disease. Am J Gastroenterol. 1993;88:1337-1342. [PubMed] [Cited in This Article: ] |
| 25. | Poynard T, Naveau S, Doffoel M, Boudjema K, Vanlemmens C, Mantion G, Messner M, Launois B, Samuel D, Cherqui D, Pageaux G, Bernard PH, Calmus Y, Zarski JP, Miguet JP, Chaput JC. Evaluation of efficacy of liver transplantation in alcoholic cirrhosis using matched and simulated controls: 5-year survival. Multi-centre group. J Hepatol. 1999;30:1130-1137. [PubMed] [Cited in This Article: ] |
| 26. | Veldt BJ, Lainé F, Guillygomarc'h A, Lauvin L, Boudjema K, Messner M, Brissot P, Deugnier Y, Moirand R. Indication of liver transplantation in severe alcoholic liver cirrhosis: quantitative evaluation and optimal timing. J Hepatol. 2002;36:93-98. [PubMed] [Cited in This Article: ] |
| 27. | Onishi Y, Kimura H, Hori T, Kishi S, Kamei H, Kurata N, Tsuboi C, Yamaguchi N, Takahashi M, Sunada S, Hirano M, Fujishiro H, Okada T, Ishigami M, Goto H, Ozaki N, Ogura Y. Risk of alcohol use relapse after liver transplantation for alcoholic liver disease. World J Gastroenterol. 2017;23:869-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 879] [Cited by in F6Publishing: 868] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 29. | Singal AK, Guturu P, Hmoud B, Kuo YF, Salameh H, Wiesner RH. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation. 2013;95:755-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 30. | Kling CE, Perkins JD, Carithers RL, Donovan DM, Sibulesky L. Recent trends in liver transplantation for alcoholic liver disease in the United States. World J Hepatol. 2017;9:1315-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association 2013; . [Cited in This Article: ] |
| 32. | Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 321] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 33. | O'Connor EA PL, Senger CA, Rushkin M, Patnode CD, Bean SI, Jonas DE. Screening and Behavioral Counseling Interventions to Reduce Unhealthy Alcohol Use in Adolescents and Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality. 2018;171 (Evidence Synthesis). [Cited in This Article: ] |
| 34. | Dom G, Wojnar M, Crunelle CL, Thon N, Bobes J, Preuss UW, Addolorato G, Seitz HK, Wurst FM. Assessing and treating alcohol relapse risk in liver transplantation candidates. Alcohol Alcohol. 2015;50:164-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Addolorato G, Mirijello A, Barrio P, Gual A. Treatment of alcohol use disorders in patients with alcoholic liver disease. J Hepatol. 2016;65:618-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 36. | Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, McClain C, McCullough A, Mitchell MC, Morgan TR, Nagy L, Radaeva S, Sanyal A, Shah V, Szabo G; NIAAA Alcoholic Hepatitis Consortia. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150:785-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 332] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 37. | Shen NT, Salajegheh A, Brown RS. A call to standardize definitions, data collection & outcome assessment to improve care in alcohol-related liver disease. Hepatology. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |