Published online Apr 7, 2019. doi: 10.3748/wjg.v25.i13.1618
Peer-review started: January 28, 2019
First decision: February 13, 2019
Revised: February 20, 2019
Accepted: February 22, 2019
Article in press: February 23, 2019
Published online: April 7, 2019
Chronic radiation proctitis (CRP) is a complication which occurs in 1%-5% of patients who undergo radiotherapy for pelvic malignancies. Although a wide range of therapeutic modalities are available, there is no literature to date showing any particularly appropriate therapeutic modality for each disease stage. Argon plasma coagulation (APC) is currently recommended as the first-choice treatment for hemorrhagic CRP, however, its indication based on long-term follow-up is still unclear. On the hypothesis that the long-term efficacy and safety of APC are not fully understood, we reviewed APC treatment for patients with hemorrhagic CRP from a single center.
To assess the long-term efficacy and safety of APC for hemorrhagic CRP.
This is a retrospective study of consecutive patients treated with APC for hemorrhagic CRP from January 2013 to October 2017. Demographics, clinical variables, and typical endoscopic features were recorded independently. Success was defined as either cessation of bleeding or only occasional traces of bloody stools with no further treatments for at least 12 mo after the last APC treatment. We performed univariate and multivariate analyses to identify factors associated with success and risk factors for fistulas.
Forty-five patients with a median follow-up period of 24 mo (range: 12-67 mo) were enrolled. Fifteen (33.3%) patients required blood transfusion before APC. Successful treatment with APC was achieved in 31 (68.9%) patients. The mean number of APC sessions was 1.3 (1-3). Multivariate analysis showed that APC failure was independently associated with telangiectasias present on more than 50% of the surface area [odds ratio (OR) = 6.53, 95% confidence interval (CI): 1.09-39.19, P = 0.04] and ulcerated area greater than 1 cm2 (OR = 8.15, 95%CI: 1.63-40.88, P = 0.01). Six (13.3%) patients had severe complications involving rectal fistulation. The only factor significantly associated with severe complications was ulcerated area greater than 1 cm2 (P = 0.035).
The long-term efficacy of APC for hemorrhagic CRP is uncertain in patients with telangiectasias present on > 50% of the surface area and ulceration > 1 cm2.
Core tip: Argon plasma coagulation (APC) is currently recommended as the first-choice treatment for hemorrhagic chronic radiation proctitis, however, its indication based on long-term follow-up is still unclear. The purpose of this study was to review APC’s long-term efficacy and safety. Forty-five patients with a median follow-up period of 24 mo were enrolled. Successful treatment was achieved in 31 (68.9%) patients. APC failure was independently associated with telangiectasias present on > 50% of the surface area and ulceration > 1 cm2. Six (13.3%) patients experienced severe complications involving rectal fistulation. The only factor significantly associated with severe complications was ulceration > 1 cm2.
- Citation: Zhong QH, Liu ZZ, Yuan ZX, Ma TH, Huang XY, Wang HM, Chen DC, Wang JP, Wang L. Efficacy and complications of argon plasma coagulation for hemorrhagic chronic radiation proctitis. World J Gastroenterol 2019; 25(13): 1618-1627
- URL: https://www.wjgnet.com/1007-9327/full/v25/i13/1618.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i13.1618
Chronic radiation proctitis (CRP) is a complication that occurs in 1%-5% of patients who undergo radiotherapy for pelvic malignancies[1]. Hemorrhagic CRP is a syndrome characterized by rectal bleeding, tenesmus, mucus discharge, and fecal incontinence[1,2]. It persists beyond three months after the completion of radiotherapy or begins three months after the initiation of radiotherapy[3]. The underlying pathological mechanisms of CRP are endarteritis obliterans and submucosal fibrosis, which lead to ischemia[4]. Telangiectasias, typical endoscopic findings of hemorrhagic CRP, are considered to be a compensatory mechanism for ischemia[5]. However, these superficial vascular lesions may bleed occasionally or even cause severe rectal bleeding requiring transfusion[6].
Current treatment modalities for hemorrhagic CRP include three main categories: medical, interventional, and surgical. Medical treatments mainly include formalin application[7,8] and sucralfate retention enemas[9,10]. Interventional treatments mainly include endoscopic argon plasma coagulation (APC)[11,12] and hyperbaric oxygen therapy[13,14]. Nonsurgical therapy is preferable to surgical treatment, as the latter may cause high morbidity or mortality[15]. Surgery should be reserved for refractory bleeding or cases complicated by fistulas, abscesses, or strictures. Although a wide range of therapeutic modalities are available, there is no literature to date showing any particularly appropriate therapeutic modality for each disease stage. APC is currently recommended as the first-choice treatment for hemorrhagic CRP, due to its coagulation depth control, easy accessibility, relatively high effectiveness, and low cost[16,17]. However, the indication of APC for hemorrhagic CRP is still unclear. The purpose of our study was to review the long-term efficacy and safety of APC for hemorrhagic CRP, and to evaluate the prognostic and risk factors.
We retrospectively reviewed consecutive patients with hemorrhagic CRP treated with APC between January 2013 and October 2017 at the Sixth Affiliated Hospital of Sun Yat-sen University. The indications for APC were persistent rectal bleeding despite several treatment attempts with various topical agents including sucralfate, almagate, corticosteroids, and 5-aminosalicylic acid enemas. The exclusion criteria for the present study included the following: (1) patients had received treatments other than medical therapy prior to APC, such as APC, formalin irrigation, fecal diversion, and proctectomy; (2) primary tumor residue/relapse or large bowel cancer occurring during follow-up; and (3) patients had causes of rectal bleeding other than CRP. Patients agreed to undergo treatment by written consent. Informed consent for this study was waived due to its retrospective nature, and the study was approved by the Institutional Review Board of the Sixth Affiliated Hospital of Sun Yat-sen University.
Patients maintained a clear fluid diet for 24 h before the APC procedure and underwent standard bowel preparation with 2-L polyethylene glycol. Preparation with enemas was performed in a few patients: 8.9% (4/45) for the first APC procedure and 29.4% (5/17) for the latter procedures. Patients received sedation with individual doses of midazolam, pethidine, or propofol if required. A total large bowel evaluation was not essential for patients who had received a complete evaluation previously or who could not tolerate a complete colonoscopy. A front-firing APC probe with a diameter of 2.3 mm was inserted through the working channel of a therapeutic colonoscope (PCF-Q260J, Olympus). If blood or other contaminating material was present, a volume of water was used to rinse the mucosal surface of the affected colorectum to prepare the surface for APC application. An argon flow of 1.0-3.0 L/min at a power of 40-60 W was applied to the lesions in 1-2 s pulses by the endoscopist, while an argon flow of 1.8 L/min at a power of 50 W was routinely adopted. The endoscopist aimed to ablate all the visible telangiectasia that might require multiple endoscopic procedures. During the APC procedure, adequate endoscopic aspiration was required to avoid overdistension with argon gas. Patients received a low or no residue diet for at least one week after the APC procedure. Repeat procedures, if necessary, were often performed at intervals of 3-4 wk.
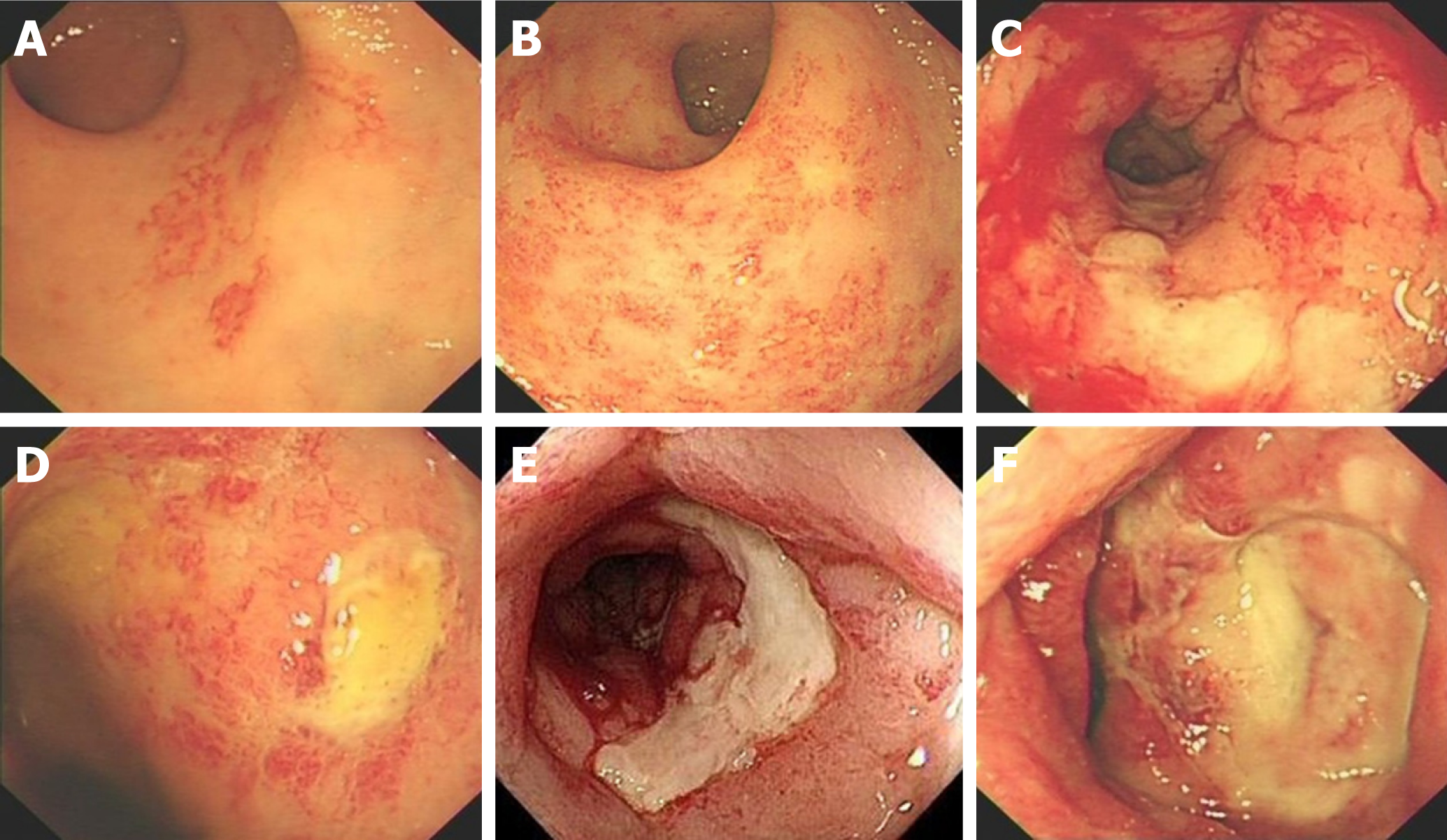
Follow-up was scheduled through outpatient clinic or by telephone at 6 and 12 mo after the procedure and thereafter at 12-mo intervals. Patients were advised to contact our departments in the event of recurrence of hemorrhage or anemia. Medical records were reviewed retrospectively. Endoscopic severity of CRP was derived from the highly-detailed endoscopic images in combination with the description on the endoscopic reports. CRP was endoscopically characterized according to the system advocated by Zinicola et al[18]. In addition, ulceration was an important feature according to the Vienna grading system[19]. Four factors were recorded independently: telangiectasia distribution, the surface area involved, the presence of fresh blood and ulceration (Figure 1).
Success was defined either as cessation of bleeding or only occasional traces of bloody stools with no further treatment for at least 12 mo after the last APC treatment[20].
Categorical variables were compared using the χ2 test or Fisher's exact test. A two-sided P-value < 0.05 was considered significant. We performed univariate and multivariate analyses to identify factors associated with success and risk factors for fistulas. Multivariate models were developed using the enter stepwise method with a removal cutoff of P = 0.10. All statistical analyses were performed with SPSS version 20 (SPSS, Inc., Chicago, IL, United States).
Between January 2013 and October 2017, 52 consecutive patients had not received treatments other than medical therapy before undergoing APC treatment for hemorrhagic CRP. After exclusion, a cohort of 45 patients were enrolled in this study. Reasons for exclusion were: (1) the patient was lost to follow-up (n = 6), and (2) the patient had a tumor relapse (n = 1). Approximately 88.9% of patients were treated with radiotherapy for gynecological malignancies, including cervical (n = 39) and vaginal (n = 1) cancers. The remaining five patients had prostate cancer. The median age at the time of the first APC treatment was 60 years (range: 43-88 years). The median duration between cessation of radiotherapy and onset of radiation proctitis was 8 mo (range: 0-78 mo). The median follow-up time from the most recent APC procedure was 24 mo (range: 12-67 mo). The median hemoglobin level at the time of first APC treatment was 10.1 g/dL (2.8-13.1 g/dL), and 15 (33.3%) patients required blood transfusion.
At the time of first APC treatment, 14 (31.1%) patients had extensive telangiectasia distributed more than 10 cm from the anal verge, 26 (57.8%) had more than 50% of the surface area covered by telangiectasias, 29 (64.4%) had fresh blood in the lumen, and 12 (26.7%) had ulceration greater than 1 cm2 (Table 1).
| Variable | No. of failure/total patients | P-value |
| Distribution of telangiectasias | ||
| Distal rectum (within 10 cm from anal verge) | 7/31 | 0.1361 |
| Entire rectum +/− sigmoid (more than 10 cm from anal verge) | 7/14 | |
| Surface area covered by telangiectasias | ||
| Less than 50% | 2/19 | 0.0112 |
| More than 50% | 12/26 | |
| Presence of fresh blood | ||
| No | 3/16 | 0.3201 |
| Yes | 11/29 | |
| Ulceration | ||
| < 1 cm2 | 6/33 | 0.0061 |
| > 1 cm2 | 8/12 | |
| Gender | ||
| Female | 14/40 | 0.3053 |
| Male | 0/5 | |
| Hypertensive | ||
| No | 11/39 | 0.3563 |
| Yes | 3/6 | |
| Diabetic | ||
| No | 11/40 | 0.1663 |
| Yes | 3/5 | |
| Abdominal surgery | ||
| No | 11/33 | 0.8651 |
| Yes | 3/12 | |
| Acute radiation injury | ||
| No | 14/39 | 0.1563 |
| Yes | 0/6 | |
| Requiring blood transfusions | ||
| No | 7/30 | 0.2101 |
| Yes | 7/15 | |
| Number of APC | ||
| 1 | 11/33 | 0.8651 |
| ≥ 2 | 3/12 | |
| Age, yr | ||
| < 60 | 8/22 | 0.4572 |
| ≥ 60 | 6/23 | |
| BMI at the first treatment of APC, kg/m2 | ||
| < 21 | 7/21 | 0.7632 |
| ≥ 21 | 7/24 | |
| Time from the end of radiotherapy to bleeding, mo | ||
| < 8 | 6/17 | 0.6372 |
| ≥ 8 | 8/28 | |
| Time from the end of radiotherapy to the first time of APC, mo | ||
| < 14 | 8/23 | 0.5862 |
| ≥ 14 | 6/22 |
Successful treatment with APC was achieved in 31 (68.9%) patients. The mean number of APC sessions was 1.3 (1-3). Bleeding was not successfully controlled by APC treatment in the remaining 14 (31.1%) patients. The univariate analysis of clinical and endoscopic variables showed statistically significant associations between APC failure and telangiectasias present on more than 50% of the surface area (P = 0.011) and ulceration greater than 1 cm2 (P = 0.006). Further multivariate analysis showed that APC failure was independently associated with telangiectasias present on more than 50% of the surface area [odds ratio (OR) = 6.53, 95% confidence interval (CI): 1.09-39.19, P = 0.04] and ulceration greater than 1 cm2 (OR = 8.15, 95%CI: 1.63-40.88, P = 0.01) .
For all 14 patients for whom APC was unsuccessful, bleeding was successfully controlled after fecal diversion.
Six (13.3%) patients had severe complications involving rectal fistula formation (including rectovaginal fistula and rectourethral fistula) at 1, 1, 2, 4, 9, and 9 mo after the first treatment session. They were treated with surgical interventions, including fecal diversion (n = 5) and restorative resection with pull-through coloanal anastomosis (n = 1). In the univariate analysis, the only factor significantly associated with severe complications was ulceration greater than 1 cm2 (P = 0.035) (Table 2).
| Variable | No. of complication/total patients | P-value |
| Distribution of telangiectasias | ||
| Distal rectum (within 10 cm from anal verge) | 4/31 | 1.0002 |
| Entire rectum +/− sigmoid (more than 10 cm from anal verge) | 2/14 | |
| Surface area covered by telangiectasias | ||
| Less than 50% | 2/19 | 1.0002 |
| More than 50% | 4/26 | |
| Presence of fresh blood | ||
| No | 1/16 | 0.3992 |
| Yes | 5/29 | |
| Ulceration | ||
| < 1 cm2 | 2/33 | 0.0352 |
| > 1 cm2 | 4/12 | |
| Gender | ||
| Female | 5/40 | 0.5292 |
| Male | 1/5 | |
| Hypertensive | ||
| No | 4/39 | 0.3671 |
| Yes | 2/6 | |
| Diabetic | ||
| No | 4/40 | 0.1252 |
| Yes | 2/5 | |
| Abdominal surgery | ||
| No | 6/33 | 0.1712 |
| Yes | 0/12 | |
| Acute radiation injury | ||
| No | 6/39 | 0.6991 |
| Yes | 0/6 | |
| Requiring blood transfusions | ||
| No | 3/30 | 0.3842 |
| Yes | 3/15 | |
| Number of APC | ||
| 1 | 5/33 | 1.0002 |
| ≥ 2 | 1/12 | |
| Age, yr | ||
| < 60 | 1/22 | 0.1872 |
| ≥ 60 | 5/23 | |
| BMI at the first treatment of APC, kg/m2 | ||
| < 21 | 3/21 | 1.0002 |
| ≥ 21 | 3/24 | |
| Time from the end of radiotherapy to bleeding, mo | ||
| < 8 | 2/17 | 1.0002 |
| ≥ 8 | 4/28 | |
| Time from the end of radiotherapy to the first time of APC, mo | ||
| < 14 | 4/23 | 0.6652 |
| ≥ 14 | 2/22 |
Radiation therapy is widely used for pelvic cancer. Although radiation techniques have made substantial advances in delivering more targeted radiation to tumors, as many as 5% of patients treated with radiotherapy for pelvic cancer will suffer from hemorrhagic CRP. Several treatment modalities, including APC, are strongly recommended by the American Society of Colon and Rectal Surgeons (ASCRS); however, none of them are based on high-quality evidence[17]. APC has been widely reported as an effective and safe modality for the treatment of hemorrhagic CRP; however, reported effectivity and complication rates vary across studies, with effectivity rates from 50% to 100% and complication rates from 0% to 63.6%[21]. Few studies have investigated the prognostic factors and risk factors of APC for the treatment of hemorrhagic CRP. Based on the strict definition of success, which states that the follow-up time should be at least 12 mo from the last treatment of APC, our study confirmed that APC was an effective modality with the complete control of bleeding in 68.9% of all patients. The independent prognostic factors were both endoscopic features prior to APC, including telangiectasias present on more than 50% of surface area and ulceration greater than 1 cm2. Our study also showed that APC was not a risk-free procedure, with 13.3% of patients developing rectal fistulas. The only risk factor identified was ulceration greater than 1 cm2.
The efficacy of APC in our study seemed to be less than that observed in some prior studies[22-24]. Two main reasons may contribute to this. First, we defined treatment success on the basis of the long-term effect (at least 12 mo of follow-up from the last treatment of APC). A study in Japan analyzed 64 patients who developed hemorrhagic CRP, with a median follow-up period of 35 mo (range: 12-69 mo) to assess treatment efficacy[25]. In this study, 12 patients received APC therapy, and 5 of them (42%) successfully had their bleeding stopped. Second, 24 (53.3%) patients in our study were categorized as having severe radiation proctitis prior to APC therapy according to the endoscopic severity of hemorrhagic CRP developed by Zinicola et al[18]. Half of the patients were treated successfully by APC. The results of this study were consistent with Zinicola et al[18], in which only one of the three patients with severe radiation proctitis was treated successfully by APC. The authors concluded that success was not certain for patients with severe hemorrhagic CRP.
Whether endoscopic severity has predictive value in the treatment of hemorrhagic CRP with APC remains controversial. In a series of 50 patients, Swan et al[26] found that the endoscopic grade did not predict the likelihood of treatment success. A study by Siow et al[22] also suggested that endoscopic grading was not a predictor of the number of APC sessions needed to achieve hemostasis. However, Zinicola et al[18] suggested that endoscopic severity could predict the success of APC. The endoscopic score they developed was based on the telangiectasia distribution, surface area covered by telangiectasias, and presence of fresh blood. APC failed in 2 of 14 patients, both of whom had more than 50% of the surface area covered by telangiectasias and fresh blood in the rectum. The authors considered these two factors to be significant in predicting the treatment success of APC. Karamanolis et al[20] also confirmed a statistically significant correlation between endoscopic severity and the treatment success of APC. They used a modified 2-grade scale instead of the 3 grades proposed by Zinicola et al[18] to assess the endoscopic severity. The modified scale included telangiectasia distribution and surface area covered by telangiectasias. Our study also confirmed that telangiectasias present on more than 50% of surface area was an independent prognostic factor for treatment failure of APC. We did not find a significant correlation between the presence of fresh blood and the treatment failure of APC. Some authors argued that luminal blood might result in an unclear view and prevent adequate telangiectasia ablation, which could reduce the efficacy of APC. During the APC procedure, we used a water pump (OFP-2; Olympus) to rinse away blood or other contaminating material, which might have minimized the adverse impact of the blood. According to the Vienna grading system, ulceration represents a severity feature of radiation proctitis. Goldner et al[27] thought that patients who had received high doses at a certain volume could develop histopathological changes such as ulcers in addition to congested mucosa or telangiectasia. Our study found that ulceration greater than 1 cm2 was another independent prognostic factor. The explanation for this is still unclear. One possible explanation is that ulceration greater than 1 cm2 indicates more severe disease; another is that the endososcopist might restrict the application of APC in terms of argon flow, power, and time in the case of a large ulceration.
Some gastroenterologists consider APC to be a safe and "risk-free" treatment modality. The major complications reported are mucus discharge, rectal pain, and rectal ulcerations, which are most likely self-limiting and rarely require intervention[24]. Severe complications, including fistulation and stricture formation, were reported to affect approximately 3% of patients in several studies[22]. However, Andreyev et al suggested that APC should be used with caution in patients with CRP. They warned that the severe complication rate of APC could reach as high as 26% when used in patients with severe ischemia in the rectum[28]. Weiner et al[29] reported the long-term results of 35 patients who received APC treatment for hemorrhagic CRP, with a median follow-up time of 56 mo (range: 3-112 mo). In their study, two (5.7%) patients developed fistulation, with one mortality. In our study, six (13.3%) patients developed severe complications involving rectal fistulation; however, all of them survived. We further identified ulceration greater than 1 cm2 as the only risk factor for severe complications. APC is a noncontact thermal coagulation technique, in which the thermal energy is delivered to the superficial blood vessels by ionized gas. The depth of coagulation is limited to approximately 0.5-3 mm; thus, the risk of perforation is generally considered to be low. Rectums with ulcers developing after pelvic radiation could be considered as having fragile, ischemic, and poor healing tissue. This may partly explain why patients with large ulceration have a higher risk of developing fistulation.
There is no consensus on the optimal APC settings for hemorrhagic CRP. A recent systematic review summarized different APC settings in 32 trials, with the electric power ranging from 25 W to 80 W (median 50 W), and the argon flow ranging from 0.6 L/min to 3.0 L/min (median 1.5 L/min)[21]. In one study, the optimal APC settings were determined by using a swine rectum at an argon flow of 1.2 L/min and a power of 40 W with application to the lesions in 2-s pulse. Sato et al[30] concluded that this setting was sufficient to ablate telangiectasia but did not damage the muscle layer. However, the review showed that there was no difference in the corresponding complications rates (0%-63.6% vs 0%-58.1%) between two electric power settings (50-80 W vs 30-50 W, respectively). In addition, four studies using a current of 60 W and an argon flow of > 1.5 L/min reported complications rates of 0%, 0%, 13.3%, and 35.7%. Peng et al[21] noted that APC settings seemed to have no correlation with complication rates. In our study, an argon flow of 1.0-3.0 L/min at a power of 40-60 W with application to the lesions in 1-2 -s pulses was determined by the endoscopist, while an argon flow of 1.8 L/min at a power of 50 W was routinely adopted. This is consistent with the guidelines advocated by ASCRS.
The present study was limited by its retrospective design and a relatively small number of cases. Further large-cohort prospective studies are therefore required to confirm our findings.
In conclusion, the long-term efficacy of APC for hemorrhagic CRP is uncertain in patients with telangiectasias present on more than 50% of the surface area and ulcerated area greater than 1 cm2. Ulcerated area greater than 1 cm2 is also a risk factor for severe complications.
Radiotherapy is widely used in the treatment of pelvic malignancies. Hemorrhagic chronic radiation proctitis (CRP) is one of the most concerning complication that occurs in 1%-5% of patients who received pelvic radiotherapy for cancer. Current treatment modalities for hemorrhagic CRP include three main categories: medical, interventional, and surgical. Although a wide range of therapeutic modalities are available, there is no literature to date showing any particularly appropriate therapeutic modality for each disease stage.
Argon plasma coagulation (APC) is currently recommended as the first-choice treatment for hemorrhagic CRP, due to its coagulation depth control, easy accessibility, relatively high effectiveness, and low cost. However, its indication based on long-term follow-up is still unclear.
This study aimed to review the long-term efficacy and safety of APC for hemorrhagic CRP, and to evaluate the prognostic and risk factors.
We retrospectively analyzed demographics, clinical and endoscopic characteristics, and long-term outcomes of consecutive patients who had received APC treatment for hemorrhagic CRP from January 2013 to October 2017. Success was defined as either cessation of bleeding or only occasional traces of bloody stools with no further treatments for at least 12 mo after the last APC treatment.
This study enrolled 45 patients with a median 24-mo follow-up period (range: 12-67 mo), 33.3% of whom required blood transfusion before APC. The success rate was 68.9%, with the mean number of APC sessions being 1.3 (1-3). This study showed that telangiectasias present on more than 50% of the surface area [odds ratio (OR) = 6.53, 95% confidence interval (CI): 1.09-39.19, P = 0.04] and ulcerated area greater than 1 cm2 (OR = 8.15, 95%CI: 1.63-40.88, P = 0.01) were poor prognostic indicators for APC treatment of hemorrhagic CRP. Six (13.3%) patients had severe complications involving rectal fistulation. The only risk factor for severe complications was ulcerated area greater than 1 cm2 (P = 0.035). Further large-cohort prospective studies are required to confirm our findings.
Endoscopic severity could predict the success of APC. The long-term efficacy of APC for hemorrhagic CRP is uncertain in patients with telangiectasias present on more than 50% of the surface area and ulcerated area greater than 1 cm2. APC is not a "risk-free" treatment modality. Ulcerated area greater than 1 cm2 is also a risk factor for severe complications.
Although APC is currently recommended as the first-choice treatment for hemorrhagic CRP, its long-term efficacy and safety are still not well understood. Our study showed that endoscopic characteristics could predict the success and severe complications of APC. Prospective, multicenter, large-scale studies involving different APC settings ought to be conducted in the follow-up research.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barret M, Souza JL S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Nelamangala Ramakrishnaiah VP, Krishnamachari S. Chronic haemorrhagic radiation proctitis: A review. World J Gastrointest Surg. 2016;8:483-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Dearnaley DP, Khoo VS, Norman AR, Meyer L, Nahum A, Tait D, Yarnold J, Horwich A. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet. 1999;353:267-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 499] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Weiner JP, Wong AT, Schwartz D, Martinez M, Aytaman A, Schreiber D. Endoscopic and non-endoscopic approaches for the management of radiation-induced rectal bleeding. World J Gastroenterol. 2016;22:6972-6986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 4. | Hasleton PS, Carr N, Schofield PF. Vascular changes in radiation bowel disease. Histopathology. 1985;9:517-534. [PubMed] [Cited in This Article: ] |
| 5. | Andreyev HJ. Argon plasma coagulation in chronic radiation proctitis: Postgate et al. Endoscopy. 2007;39:751-2; author reply 752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Dent OF, Galt E, Chapuis PH, Yuile P, Sinclair G, Bokey EL. Quality of life in patients undergoing treatment for chronic radiation-induced rectal bleeding. Br J Surg. 1998;85:1251-1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Haas EM, Bailey HR, Faragher I. Application of 10 percent formalin for the treatment of radiation-induced hemorrhagic proctitis. Dis Colon Rectum. 2007;50:213-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Kujawski R, Mik M, Berut M, Dziki A, TrzciÅski R. Formalin therapy for hemorrhagic radiation proctitis. Pharmacol Rep. 2015;67:896-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | McElvanna K, Wilson A, Irwin T. Sucralfate paste enema: a new method of topical treatment for haemorrhagic radiation proctitis. Colorectal Dis. 2014;16:281-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Gul YA, Prasannan S, Jabar FM, Shaker AR, Moissinac K. Pharmacotherapy for chronic hemorrhagic radiation proctitis. World J Surg. 2002;26:1499-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Sultania S, Sarkar R, Das K, Dhali GK. Argon plasma coagulation is an effective treatment for chronic radiation proctitis in gynaecological malignancy: an observational study. Colorectal Dis. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Postgate A, Saunders B, Tjandra J, Vargo J. Argon plasma coagulation in chronic radiation proctitis. Endoscopy. 2007;39:361-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Oscarsson N, Arnell P, Lodding P, Ricksten SE, Seeman-Lodding H. Hyperbaric oxygen treatment in radiation-induced cystitis and proctitis: a prospective cohort study on patient-perceived quality of recovery. Int J Radiat Oncol Biol Phys. 2013;87:670-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Clarke RE, Tenorio LM, Hussey JR, Toklu AS, Cone DL, Hinojosa JG, Desai SP, Dominguez Parra L, Rodrigues SD, Long RJ, Walker MB. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;72:134-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | McCrone LF, Neary PM, Larkin J, McCormick P, Mehigan B. The surgical management of radiation proctopathy. Int J Colorectal Dis. 2017;32:1099-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Sebastian S, O'Connor H, O'Morain C, Buckley M. Argon plasma coagulation as first-line treatment for chronic radiation proctopathy. J Gastroenterol Hepatol. 2004;19:1169-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Paquette IM, Vogel JD, Abbas MA, Feingold DL, Steele SR; Clinical Practice Guidelines Committee of The American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Chronic Radiation Proctitis. Dis Colon Rectum. 2018;61:1135-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Zinicola R, Rutter MD, Falasco G, Brooker JC, Cennamo V, Contini S, Saunders BP. Haemorrhagic radiation proctitis: endoscopic severity may be useful to guide therapy. Int J Colorectal Dis. 2003;18:439-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Wachter S, Gerstner N, Goldner G, Pötzi R, Wambersie A, Pötter R. Endoscopic scoring of late rectal mucosal damage after conformal radiotherapy for prostatic carcinoma. Radiother Oncol. 2000;54:11-19. [PubMed] [Cited in This Article: ] |
| 20. | Karamanolis G, Psatha P, Triantafyllou K. Endoscopic treatments for chronic radiation proctitis. World J Gastrointest Endosc. 2013;5:308-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Peng Y, Wang H, Feng J, Fang S, Zhang M, Wang F, Chang Y, Shi X, Zhao Q, Liu J. Efficacy and Safety of Argon Plasma Coagulation for Hemorrhagic Chronic Radiation Proctopathy: A Systematic Review. Gastroenterol Res Pract. 2018;2018:3087603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Siow SL, Mahendran HA, Seo CJ. Complication and remission rates after endoscopic argon plasma coagulation in the treatment of haemorrhagic radiation proctitis. Int J Colorectal Dis. 2017;32:131-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Yeoh E, Tam W, Schoeman M, Moore J, Thomas M, Botten R, Di Matteo A. Argon plasma coagulation therapy versus topical formalin for intractable rectal bleeding and anorectal dysfunction after radiation therapy for prostate carcinoma. Int J Radiat Oncol Biol Phys. 2013;87:954-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Chruscielewska-Kiliszek MR, Rupinski M, Kraszewska E, Pachlewski J, Regula J. The protective role of antiplatelet treatment against ulcer formation due to argon plasma coagulation in patients treated for chronic radiation proctitis. Colorectal Dis. 2014;16:293-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Takemoto S, Shibamoto Y, Ayakawa S, Nagai A, Hayashi A, Ogino H, Baba F, Yanagi T, Sugie C, Kataoka H, Mimura M. Treatment and prognosis of patients with late rectal bleeding after intensity-modulated radiation therapy for prostate cancer. Radiat Oncol. 2012;7:87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Swan MP, Moore GT, Sievert W, Devonshire DA. Efficacy and safety of single-session argon plasma coagulation in the management of chronic radiation proctitis. Gastrointest Endosc. 2010;72:150-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Goldner G, Tomicek B, Becker G, Geinitz H, Wachter S, Zimmermann F, Wachter-Gerstner N, Reibenwein J, Glocker S, Bamberg M, Feldmann H, Pötzi R, Molls M, Pötter R. Proctitis after external-beam radiotherapy for prostate cancer classified by Vienna Rectoscopy Score and correlated with EORTC/RTOG score for late rectal toxicity: results of a prospective multicenter study of 166 patients. Int J Radiat Oncol Biol Phys. 2007;67:78-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Andreyev HJ, Davidson SE, Gillespie C, Allum WH, Swarbrick E; British Society of Gastroenterology; Association of Colo-Proctology of Great Britain and Ireland; Association of Upper Gastrointestinal Surgeons; Faculty of Clinical Oncology Section of the Royal College of Radiologists. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut. 2012;61:179-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 29. | Weiner J, Schwartz D, Martinez M, Safdieh J, Aytaman A, Schreiber D. Long-term results on the efficacy of argon plasma coagulation for patients with chronic radiation proctitis after conventionally fractionated, dose-escalated radiation therapy for prostate cancer. Pract Radiat Oncol. 2017;7:e35-e42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Sato Y, Takayama T, Sagawa T, Hirakawa M, Ohnuma H, Miyanishi K, Sato T, Takimoto R, Kobune M, Okamoto K, Takeuchi H, Kato J. Argon plasma coagulation treatment of hemorrhagic radiation proctopathy: the optimal settings for application and long-term outcome. Gastrointest Endosc. 2011;73:543-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |