Published online Mar 28, 2019. doi: 10.3748/wjg.v25.i12.1502
Peer-review started: December 25, 2018
First decision: January 30, 2019
Revised: February 21, 2019
Accepted: February 22, 2019
Article in press: February 23, 2019
Published online: March 28, 2019
Risk factors for local recurrence after polypectomy, endoscopic mucosal resection (EMR), and endoscopic submucosal dissection (ESD) have not been identified. Additionally, the appropriate interval for endoscopic surveillance of colorectal tumors at high-risk of local recurrence has not been established.
To clarify the clinicopathological characteristics of recurrent lesions after endoscopic colorectal tumor resection and determine the appropriate interval.
Three hundred and sixty patients (1412 colorectal tumors) who underwent polypectomy, EMR, or ESD and received endoscopic surveillance subsequently for more than one year to detect local recurrence were enrolled in this study. The clinicopathological factors associated with local recurrence were determined via univariate and multivariate analyses.
Local recurrence was observed in 31 of 360 (8.6%) patients [31 of 1412 (2.2%) lesions] after colorectal tumor resection. Piecemeal resection, tumor size of more than 2 cm, and the presence of villous components were associated with colorectal tumor recurrence after endoscopic resection. Of these three factors, the piecemeal resection procedure was identified as an independent risk factor for recurrence. Colorectal tumors resected into more than five pieces were associated with a high risk of recurrence since the average period from resection to recurrence in these cases was approximately 3 mo. The period to recurrence in cases resected into more than 5 pieces was much shorter than that in those resected into less than 4 pieces (3.8 ± 1.9 mo vs 7.9 ± 5.0 mo, P < 0.05).
Local recurrence of endoscopically treated colorectal tumors depends upon the outcome of first endoscopic procedure. Piecemeal resection was the only significant risk factor associated with local recurrence after endoscopic resection.
Core tip: Local recurrence of endoscopically treated colorectal tumors depends on the outcome of the first endoscopic procedure. Local recurrence was observed in 31 of 360 (8.6%) patients [31 of 1412 (2.2%) lesions] after colorectal tumor resection. Piecemeal resection was the only significant risk factor associated with local recurrence. Average time between the initial resection and recurrence in all cases was 6 mo. Time to recurrence in cases resected into > 5 pieces was much shorter than that in those resected into < 4 pieces. The interval between endoscopic resection and surveillance colonoscopy should be determined based on the number of pieces.
- Citation: Komeda Y, Watanabe T, Sakurai T, Kono M, Okamoto K, Nagai T, Takenaka M, Hagiwara S, Matsui S, Nishida N, Tsuji N, Kashida H, Kudo M. Risk factors for local recurrence and appropriate surveillance interval after endoscopic resection. World J Gastroenterol 2019; 25(12): 1502-1512
- URL: https://www.wjgnet.com/1007-9327/full/v25/i12/1502.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i12.1502
Colorectal cancer (CRC) is one of the leading causes of cancer-related deaths in both Western and Asian countries[1-3]. It is now generally accepted that most, if not all, CRCs arise from adenomas[4]. Based on this notion called the “adenoma-carcinoma sequence”, colorectal adenomas are considered the precursor lesions of CRCs. Thus, their endoscopic removal is strongly recommended for the prevention of CRCs[5]. Several reports provide evidence that adenoma removal reduces the incidence of CRC and thereby improves patient survival[4,6]. Currently, three types of endoscopic tech-niques are performed for the resection of colorectal tumors; these are polypectomy, endoscopic mucosal resection (EMR), and endoscopic submucosal dissection (ESD)[7-12]. The introduction of ESD, which allows en bloc resection of flat or depressed colorectal tumors of more than 20 mm, has enabled endoscopists to remove adenomas as well as to remove early CRCs[13]. Thus, a wide variety of colorectal tumors, including adenomas and early CRCs, can be endoscopically resected using a selection of polypectomy, EMR, and ESD depending on the size and macroscopy of the tumors.
As the indication for endoscopic colorectal tumor resection has expanded in terms of tumor size and depth of tumor invasion, it has become apparent that local recurrence occurs in a significant proportion of patients treated via polypectomy, EMR, or ESD[14]. According to a previous meta-analysis, a high incidence of local recurrence of up to 50% after EMR has been reported[15]. Although tumor size, intra-procedural bleeding, piecemeal resection, and high-grade dysplasia have been shown to be associated with local recurrence of colorectal adenoma after EMR[16], the related risk factors after polypectomy, EMR, and ESD have not been identified. Additionally, the appropriate interval of endoscopic surveillance for colorectal tumors with a high risk of local recurrence has not been established, although the American Cancer Society recommends an interval of 3 to 6 mo for follow-up endoscopic examinations after piecemeal resection for large or sessile polyps[17]. In this retrospective study, we attempted to clarify the clinicopathological characteristics of recurrent lesions after endoscopic colorectal tumor resection and propose the appropriate follow-up interval of colonoscopic surveillance.
A total of 1020 patients underwent polypectomy, EMR, or ESD for 4236 lesions at Kindai University Hospital from January 2010 to December 2015. Among these patients, 360 patients (1412 lesions) received endoscopic surveillance for local recurrence for more than one year. Various factors associated with local recurrence after endoscopic resection was retrospectively analyzed in these patients. The factors used for the analysis included age, sex, past history of CRC, diabetes, macroscopic tumor type, tumor size and location, resection methods, number of adenomas, and histology. Although most previous reports[14-17] successfully identified lesional factors associated with the local recurrence of colonic tumors after endoscopic treatments, such as tumor sizes, endoscopic findings, and tumor locations, few reports have tried to identify patient factors, such as age, sex, history of colonic tumors, and diabetes. In this study, we performed univariate and multivariate analysis to identify patient factors associated with local recurrence. To this end, the largest tumor in size was selected in each patient for the analysis when more than 2 polyps are detected. Moreover, the most advanced type of histology was selected in each patient when more than 2 polyps were removed. Pathological diagnosis of the colorectal tumors was performed by experienced pathologists as described previously[18]. Cases diagnosed as intra-mucosal carcinoma by Japanese pathologists were categorized into high-grade dysplasia as described previously[19]. Invasive cancer was defined as invasion beyond the muscularis propria. Polypectomy, EMR, and ESD were performed for 157, 1130, and 125 colorectal tumors, respectively. Patients with inflammatory bowel disease or polyposis were excluded from the study. Ethical permission for this study was granted by the review board of Kindai University Faculty of Medicine (approval number: 30-157).
Polypectomy and EMR were performed as previously described by Komeda et al[20] and ESD was performed as previously described by Okamoto et al[18]. In our institute, the basic strategy for endoscopic resection is en bloc resection; piecemeal resection is avoided as much as possible. We performed ESD for colorectal tumors of the flat or depressed type when EMR may result in piecemeal resection. The patients’ endoscopic and medical records were retrospectively analyzed. The procedures followed were in accordance with the guidelines of the World Medical Association’s Declaration of Helsinki.
Imputed data were statistically assessed using SPSS version 12.0 for Windows (SPSS Inc, Chicago, IL, United States). Odds ratios and 95% confidence intervals (95%CI) were evaluated for each category of variable indicators, including age, sex, past history of CRC, diabetes, macroscopic tumor type, tumor size, tumor location, resection method, number of adenomas, and histology. The statistical significance of the differences between the two groups was determined via Student’s t-test. A probability value of 0.05 or less was considered significant.
Local recurrence was observed in 31/360 (8.6%) patients who received endoscopic surveillance for more than one year after colorectal tumor resection. The median observation period was 795 d. Of 31 patients included in the study, 24 and 7 were males and females, respectively. The locations of the recurrent lesions were distributed throughout the colorectum: 6, 9, 4, 2, 5, and 5 in the cecum, ascending colon, transverse colon, descending colon, sigmoid, and rectum, respectively.
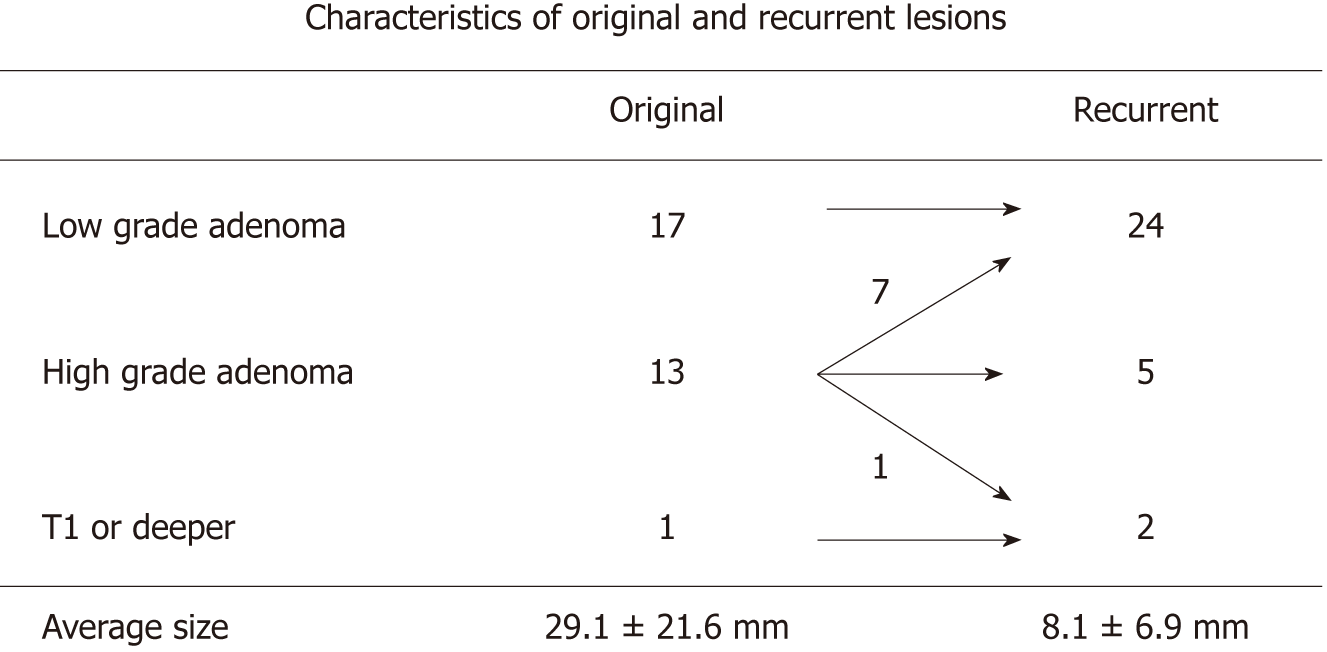
The characteristics of primary and recurrent lesions are shown in Figure 1. The sizes of the lesions were 29.1 ± 21.6 mm and 8.1 ± 6.9 mm (mean ± standard error), respectively. Primary colorectal tumors comprised 17 low-grade adenomas, 13 high-grade adenomas, and one submucosal invasion cancer (T1). Pathological examinations of the initial recurrent lesions showed that 24, 5, and 2 cases were diagnosed as low-grade adenoma, high-grade adenoma, and submucosal invasion cancer (T1), respectively. Intra-mucosal cancer was regarded as high-grade adenoma in accordance with previous reports[19]. Interestingly, 17 cases (70.8%) and 7 cases (29.2%) of recurrent low-grade adenoma arose from primary low-grade and high-grade adenomas, respectively. Five cases (100%) of recurrent high-grade adenomas originated from primary high-grade adenomas. Two cases of recurrent submucosal invasion cancer originated from one submucosal invasion cancer and one high-grade adenoma (Figures 2 and 3). These pathological findings strongly suggested that primary low-grade adenomas and submucosal invasion cancer manifest their recurrent lesions as primary pathological features. In contrast, primary high-grade adenomas may develop into a wide pathological variety of colorectal tumors, ranging from low-grade adenomas to submucosal invasion cancers.
Having obtained the pathological relationship between primary and recurrent colorectal tumors, we attempted to identify the clinicopathological risk factors associated with recurrence using univariate analysis. As shown in Table 1, no significant correlation was observed between recurrence and age, sex, history of colorectal tumors, or diabetes mellitus.
| Factors | Recurrence | Odds ratio | 95%CI | P value |
| Age | ||||
| < 60 yr | 2/60 | 1 | ||
| ≥ 60 yr | 29/300 | 3.07 | 0.73-12.8 | 0.12 |
| Sex | ||||
| Male | 24/228 | 1 | ||
| Female | 7/132 | 0.48 | 0.21-1.11 | 0.09 |
| History of CRC | ||||
| - | 31/341 | 1 | ||
| + | 0/19 | 0.046 | 0-50.0 | 0.38 |
| Diabetes | ||||
| - | 29/338 | 1 | ||
| + | 2/22 | 1.07 | 0.25-4.49 | 0.92 |
| Growth type | ||||
| LST-G | 8/58 | 1 | ||
| LST-NG | 9/47 | 1.395 | 0.53-3.61 | 0.49 |
| II a, II c | 1/45 | 0.693 | 0.34-1.38 | 0.30 |
| Ip, Is | 13/210 | 0.655 | 0.42-1.01 | 0.06 |
| Size | ||||
| < 2 cm | 11/239 | 1 | ||
| ≥ 2 cm | 20/121 | 3.77 | 1.80-7.88 | < 0.001 |
| Location | ||||
| Rectum | 4/67 | 1 | ||
| Colon | 27/293 | 0.626 | 0.21-1,79 | 0.38 |
| Resection methods | ||||
| En bloc | 6/297 | 1 | ||
| Piecemeal | 25/63 | 23.7 | 9.72-57.8 | < 0.001 |
| No of adenoma | ||||
| < 3 | 19/227 | 1 | ||
| ≥ 3 | 12/133 | 1.09 | 0.53-2.26 | 0.79 |
| Histology | ||||
| Low grade adenoma | 18/159 | 1 | ||
| High grade adenoma | 12/163 | 0.65 | 0.31-1.36 | 0.26 |
| T1 carcinoma or deeper | 1/38 | 0.46 | 0.17-1.27 | 0.13 |
| Histology villous type | ||||
| - | 25/333 | 1 | ||
| + | 6/27 | 2.09 | 1.10-3.97 | 0.023 |
Next, we examined whether the macroscopic appearance of tumors or the endoscopic procedures were associated with the recurrence of colorectal tumors. Analysis of the correlation between the endoscopic procedures and recurrence showed that all the patients who experienced recurrence (31 patients) were those in which a previous treatment resulted in piecemeal resection (25 patients, 80.6%, Table 1) or those who had a positive margin (6 patients, 19.4%). The 25 patients included 15 and 10 patients who underwent EMR and ESD, respectively. As shown in Table 2, the recurrence rate of colorectal tumors that were treated via piecemeal resection was much higher (25/63, 39.7%) than that of those treated via en bloc resection (6/297, 2.0%). Of the 31 recurrent lesions, 30 lesions were successfully treated using endoscopy without complications and one lesion was surgically treated due to cancerous submucosal invasion. Regarding the recurrent colorectal tumors, owing to the much higher rate of piecemeal resection than of en bloc resection, piecemeal resection was identified as a risk factor for recurrence.
| Factors | Odds ratio | 95%CI | P value |
| Size ≥ 2 cm | 0.93 | 0.41-2.11 | 0.87 |
| Histology villous type | 1.03 | 0.51-2.07 | 1.03 |
| Piecemeal resection | 24.3 | 9.07-65.4 | < 0.001 |
Subsequently, we focused on the relationship between the macroscopic appearance of colorectal tumors and recurrence. As shown in Table 2, the recurrence rate of colorectal tumors of more than 2 cm was much higher (20/121, 16.5%) than that of tumors of less than 2 cm (6/297, 4.6%).
In contrast, no significant correlation was identified between recurrence and the macroscopic appearance of colorectal tumors in terms of growth type: Laterally spreading tumor granular type (LST-G), LST-non granular type (LST-NG) IIa, IIc, Ip, and Is. Consistent with the high rate of previous piecemeal resection and the recurrent colorectal tumor size of more than 2 cm, univariate analysis identified piecemeal resection and tumor size of more than 2 cm as risk factors for recurrence.
We attempted to determine the relationship between the pathological features and recurrence of colorectal tumors. As shown in Table 1, neither the number of adenomas nor the pathological diagnosis of the tumors (low-grade adenoma, high-grade adenoma, and submucosal invasion cancer) was associated with tumor recurrence. Interestingly, the recurrence rate of colorectal tumors with villous components was much higher (6/27, 22.2%) than that of those without villous components (25/333, 7.5%). Univariate analysis identified the presence of villous components as a risk factor for tumor recurrence.
Having identified piecemeal resection, tumor size of more than 2 cm, and the presence of villous components as possible risk factors for the recurrence of endoscopically-treated colorectal tumors, we performed multivariate analysis. As shown in Table 2, the piecemeal resection technique was the only independent risk factor for colorectal tumor recurrence.
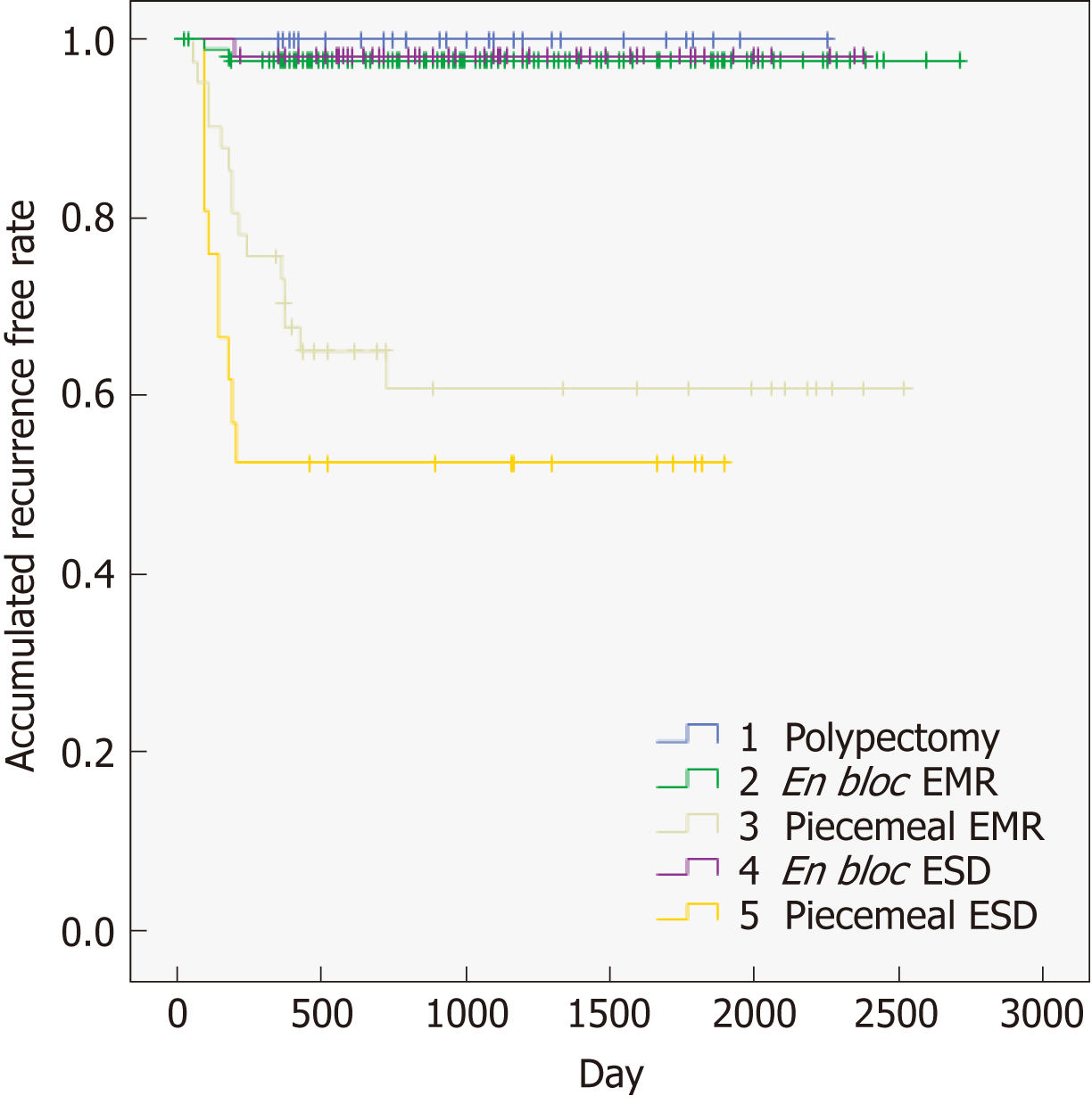
Table 3 shows the different types of techniques (polypectomy, en bloc EMR, piecemeal EMR, en bloc ESD, and piecemeal ESD), and provides evidence that endoscopic procedures are associated with local recurrence. Piecemeal resection (piecemeal EMR, piecemeal ESD) had a clear and significant relationship with local recurrence compared to en block resection. We also tried to confirm these results with another approach. As shown in Figure 4, we performed a sub-analysis to identify the types of techniques (polypectomy, en bloc EMR, piecemeal EMR, en bloc ESD, and piecemeal ESD) associated with local recurrence. This analysis also showed clearly that piecemeal resection (piecemeal EMR, piecemeal ESD) was highly related to local recurrence compared to en block resection.
| Therapy | Recurrence rate (recurrence/total) |
| Polypectomy | 0% (0/29) |
| En bloc EMR | 2.4% (5/209) |
| Piecemeal EMR | 36.6% (15/41) |
| En bloc ESD | 1.7% (1/60) |
| Piecemeal ESD | 52.4% (11/21) |
Finding that piecemeal resection was the strongest risk factor for colorectal tumor recurrence prompted us to examine the recurrence periods from the initial endoscopic procedure. The average period between the initial resection and recurrence for all cases was 6.1 ± 4.5 mo (range, 2 to 15 mo). Table 4 summarizes the relationship between the number of resected pieces and the time to recurrence. The period from the initial endoscopic procedure to the recurrence of colorectal tumors resected into 2, 3, and 4 pieces were 9.0 ± 3.8, 10.1 ± 6.3, and 5 mo, respectively. In contrast, the period to recurrence in the 13 cases resected into 5 or more pieces was 3.8 ± 1.9 mo. Thus, the period from the initial endoscopic procedure to the local recurrence for tumors resected into more than 5 pieces was very short. Therefore, such cases might require endoscopic surveillance soon after the initial endoscopic procedure.
| No of pieces | Time to recurrence (average months) |
| 1 piece but unclear margin (6 cases) | 6.0 ± 1.4 |
| 2 pieces (5 cases) | 9.0 ± 3.8 |
| 3 pieces (7 cases) | 10.1 ± 6.3 |
| 4 pieces (1 case) | 5 |
| ≥ 5 pieces (12 cases) | 3.8 ± 1.9 |
In this study, we attempted to determine the risk factors associated with the recurrence of endoscopically-treated colorectal tumors. For this purpose, we analyzed 360 patients (1412 lesions) in a retrospective manner; the local recurrence rate was 31/360 (8.6%). Among a variety of factors including clinical characteristics, endoscopic procedure, and pathological features, univariate analysis identified piecemeal resection, tumor size of more than 2 cm, and the presence of villous tumor components as possible risk factors for recurrence. Further multivariate analysis identified piecemeal resection as an independent risk factor for recurrence. In this study, the recurrence rate of colorectal tumors treated using piecemeal resection was 39.7% and that of lesions beyond 2 cm in size was 16.5%. These data are in line with a recent report that showed that piecemeal resection is the important risk factor for local recurrence in colorectal tumors treated via EMR or ESD[21]. Moreover, our results are consistent with a recent meta-analysis that showed that recurrence varies from 10% to 55% after endoscopic piecemeal mucosal resection in tumors measuring more than 2 cm [15]. However, we need to be cautious in the comparison of our data with those obtained in Western countries due to the difference in endoscopic resection methods. In our facility, the strategy for endoscopic removal of colorectal tumors is en bloc resection via ESD; in contrast, wide-filed EMR rather than ESD is widely used in Western countries[22]. In any case, it is likely that tumors of more than 2 cm and piecemeal resection both increase the risk of local recurrence.
En bloc resection via the ESD technique is recommended for the endoscopic resection of large colorectal tumors of more than 2 cm since accumulating evidence provides a reduced rate of local recurrence in patients treated using ESD[21]. However, colonic ESD does not always result in en bloc resection in difficult situations such as bowel peristalsis, fibrosis in the submucosa, bleeding, or inexperience skill of the endoscopist[23-25]. In this study, 10 and 21 cases treated with ESD and EMR, respectively, exhibited local recurrence. Among the 21 cases, 15 tumors were resected via the piecemeal technique and 6 tumors had a positive resection margin. The 10 cases treated via ESD were resected using piecemeal resection. Therefore, our study strongly suggests that piecemeal resection increases the risk of local colorectal tumor recurrence regardless of the endoscopic resection method employed.
Colon adenomas with villous components are considered high-risk tumors with the ability to differentiate into CRC[26]. In fact, in this study, the rate of local recurrence was higher for colorectal adenomas bearing villous components. However, the presence of villous components in colon adenomas was not defined as an independent risk factor for recurrence in the multivariate analysis. Future prospective studies are necessary to determine whether the presence of villous components in colon adenomas increases the risk of recurrence after endoscopic treatment.
The identification of piecemeal resection as an independent risk factor for the recurrence of colorectal tumors led us to investigate the periods from the endoscopic resection to recurrence. Interestingly, the period to recurrence in cases resected into more than 5 pieces was much shorter than that in those resected into less than 4 pieces (3.8 ± 1.9 mo vs 7.9 ± 5.0 mo, P < 0.05). Thus, colorectal tumors endoscopically resected into more than 5 pieces bear a very high risk of recurrence within 3 mo post-resection while those resected into less than 4 pieces bear a high risk for recurrence within 8 mo. Consistent with these data, previous reports[11,14] provide the evidence that the recurrence rate is higher and the interval to recurrence tends to be shorter for colorectal tumors resected into more than 5 pieces. Our analysis of the periods from the initial endoscopic treatment to tumor recurrence prompts us to propose appropriate intervals for endoscopic surveillance. Because the period to recurrence in cases resected into 1 piece with unclear margin (incomplete margin) was 6.1 ± 4.5 months in this study, surveillance endoscopy needed to be performed within 1 to 3 mo after the resection. In addition, surveillance endoscopy needed to be performed within 1 to 3 mo in cases resected into more than 5 pieces, and within 4 to 6 mo in cases resected into less than 4 pieces. Based on these, we strongly suggest that not only the piecemeal resection procedure but also the number of the resected pieces increase the risk of colorectal tumor recurrence (Table 4). In Table 5, we provide our recommendation for the interval preceding repeat colonoscopy.
| Interval before a repeat colonoscopy |
| 1-3 mo after piecemeal resection ≥ 5 pieces (high risk for recurrence) |
| 4-6 mo after piecemeal resection ≤ 4 pieces (moderate risk for recurrence) |
| 6 mo after en bloc resection for cancer (low risk for recurrence) |
| ≥ 12 mo after en bloc resection for adenoma (very low risk for recurrence) |
There are some limitations to this study. Firstly, it was retrospective and was conducted at a single university hospital. Secondly, each endoscopist determined the schedule of the endoscopic surveillance as no Japanese guideline was available. Some of the patients who underwent complete endoscopic resection did not receive careful endoscopic surveillance.
In conclusions, local recurrence of endoscopically treated colorectal tumors depends on the outcome of the first endoscopic procedure. Piecemeal resection was the only significant risk factor associated with local recurrence after endoscopic resection. The interval between endoscopic resection for colorectal tumors and surveillance colonoscopy need to be determined based on the number of pieces.
As the indication for endoscopic colorectal tumor resection has expanded in terms of tumor size and depth of tumor invasion, it has become apparent that local recurrence occurs in a significant proportion of patients treated via polypectomy, endoscopic mucosal resection (EMR), or endoscopic submucosal dissection (ESD). According to a previous meta-analysis, a high incidence of local recurrence of up to 50% after EMR has been reported. Although tumor size, intraprocedural bleeding, piecemeal resection, and high-grade dysplasia have been shown to be associated with local recurrence of colorectal adenoma after EMR, the related risk factors after polypectomy, EMR, and ESD have not been identified. Additionally, the appropriate interval of endoscopic surveillance for colorectal tumors with a high risk of local recurrence has not been established, although the American Cancer Society recommends an interval of 3 to 6 mo for follow-up endoscopic examinations after piecemeal resection for large or sessile polyps.
We need to be cautious in the comparison of our data with those obtained in Western countries due to the difference in endoscopic resection methods.
We attempted to clarify the clinicopathological characteristics of recurrent lesions after endoscopic colorectal tumor resection and determine the appropriate interval.
Three hundred and sixty patients (1412 colorectal tumors) who underwent polypectomy, EMR, or ESD and received endoscopic surveillance subsequently for more than one year to detect local recurrence were enrolled in this study. Although most previous reports successfully identified lesional factors associated with the local recurrence of colonic tumors after endoscopic treatments, such as tumor sizes, endoscopic findings, and tumor locations, few reports have tried to identify patient factors, such as age, sex, history of colonic tumors, and diabetes. In this study, we performed univariate and multivariate analysis to identify patient factors associated with local recurrence.
Local recurrence was observed in 31 of 360 (8.6%) patients [31 of 1412 (2.2%) lesions] after colorectal tumor resection. Piecemeal resection, tumor size of more than 2 cm, and the presence of villous components were associated with colorectal tumor recurrence after endoscopic resection. Of these three factors, the piecemeal resection procedure was identified as an independent risk factor for recurrence. Colorectal tumors resected into more than five pieces were associated with a high risk of recurrence since the average period from resection to recurrence in these cases was approximately 3 mo. The period to recurrence in cases resected into more than 5 pieces was much shorter than that in those resected into less than 4 pieces (3.8 ± 1.9 mo vs 7.9 ± 5.0 mo, P < 0.05).
Local recurrence of endoscopically treated colorectal tumors depends upon the outcome of first endoscopic procedure. Piecemeal resection was the only significant risk factor associated with local recurrence after endoscopic resection. The interval between endoscopic resection for colorectal tumors and surveillance colonoscopy need to be determined based on the number of pieces.
The interval between endoscopic resection for colorectal tumors and surveillance colonoscopy need to be determined based on the number of pieces in prospective study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lorenzo-Zúñiga V S-Editor: Yan JP L-Editor: A E-Editor: Song H
| 1. | Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1761] [Cited by in F6Publishing: 1687] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6112] [Cited by in F6Publishing: 5922] [Article Influence: 348.4] [Reference Citation Analysis (0)] |
| 3. | Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H; Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2009: A study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 438] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 4. | Bonnington SN, Rutter MD. Surveillance of colonic polyps: Are we getting it right? World J Gastroenterol. 2016;22:1925-1934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 45] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 5. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1952] [Cited by in F6Publishing: 2085] [Article Influence: 173.8] [Reference Citation Analysis (1)] |
| 6. | Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG 2nd, Park WG, Rizk MK, Sawhney MS, Shaheen NJ, Wani S, Weinberg DS. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 649] [Cited by in F6Publishing: 734] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 7. | Hurlstone DP, Sanders DS, Cross SS, Adam I, Shorthouse AJ, Brown S, Drew K, Lobo AJ. Colonoscopic resection of lateral spreading tumours: A prospective analysis of endoscopic mucosal resection. Gut. 2004;53:1334-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Tanaka S, Oka S, Chayama K. Colorectal endoscopic submucosal dissection: Present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol. 2008;43:641-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T, Fujii T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 10. | Woodward TA, Heckman MG, Cleveland P, De Melo S, Raimondo M, Wallace M. Predictors of complete endoscopic mucosal resection of flat and depressed gastrointestinal neoplasia of the colon. Am J Gastroenterol. 2012;107:650-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Terasaki M, Tanaka S, Oka S, Nakadoi K, Takata S, Kanao H, Yoshida S, Chayama K. Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J Gastroenterol Hepatol. 2012;27:734-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Chen RY, Byth K. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909-1918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 418] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 13. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisasbe T, Matsuda T, Ishikawa H, Sugihara K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 14. | Hotta K, Saito Y, Matsuda T, Shinohara T, Oyama T. Local recurrence and surveillance after endoscopic resection of large colorectal tumors. Dig Endosc. 2010;22 Suppl 1:S63-S68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: Systematic review and meta-analysis. Endoscopy. 2014;46:388-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 16. | Tate DJ, Desomer L, Klein A, Brown G, Hourigan LF, Lee EY, Moss A, Ormonde D, Raftopoulos S, Singh R, Williams SJ, Zanati S, Byth K, Bourke MJ. Adenoma recurrence after piecemeal colonic EMR is predictable: The Sydney EMR recurrence tool. Gastrointest Endosc. 2017;85:647-656.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O'Brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR, Bond JH, Brooks D, Byers T, Hyman N, Kirk L, Thorson A, Simmang C, Johnson D, Rex DK; US Multi-Society Task Force on Colorectal Cancer; American Cancer Society. Guidelines for colonoscopy surveillance after polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872-1885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 546] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 18. | Okamoto K, Watanabe T, Komeda Y, Kono T, Takashima K, Okamoto A, Kono M, Yamada M, Arizumi T, Kamata K, Minaga K, Yamao K, Nagai T, Asakuma Y, Takenaka M, Sakurai T, Matsui S, Nishida N, Chikugo T, Kashida H, Kudo M. Risk Factors for Postoperative Bleeding in Endoscopic Submucosal Dissection of Colorectal Tumors. Oncology. 2017;93 Suppl 1:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau H, Nakamura SI, Quirke P, Riboli E, Sobin LH. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro FT, Hruban RH, Theise ND. WHO classification of tumors of digestive system. 4th ed. Lyon: IARC; 2010; 134-142. [Cited in This Article: ] |
| 20. | Komeda Y, Suzuki N, Sarah M, Thomas-Gibson S, Vance M, Fraser C, Patel K, Saunders BP. Factors associated with failed polyp retrieval at screening colonoscopy. Gastrointest Endosc. 2013;77:395-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Oka S, Tanaka S, Saito Y, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisabe T, Tsuruta O, Sano Y, Yamano H, Shimizu S, Yahagi N, Watanabe T, Nakamura H, Fujii T, Ishikawa H, Sugihara K; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: A multicenter prospective study in Japan. Am J Gastroenterol. 2015;110:697-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 22. | Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Burgess NG, Sonson R, Byth K, Bourke MJ. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: Results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 23. | Taku K, Sano Y, Fu KI, Saito Y, Matsuda T, Uraoka T, Yoshino T, Yamaguchi Y, Fujita M, Hattori S, Ishikawa T, Saito D, Fujii T, Kaneko E, Yoshida S. Iatrogenic perforation associated with therapeutic colonoscopy: A multicenter study in Japan. J Gastroenterol Hepatol. 2007;22:1409-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Oka S, Tanaka S, Kanao H, Ishikawa H, Watanabe T, Igarashi M, Saito Y, Ikematsu H, Kobayashi K, Inoue Y, Yahagi N, Tsuda S, Simizu S, Iishi H, Yamano H, Kudo SE, Tsuruta O, Tamura S, Saito Y, Cho E, Fujii T, Sano Y, Nakamura H, Sugihara K, Muto T. Current status in the occurrence of postoperative bleeding, perforation and residual/local recurrence during colonoscopic treatment in Japan. Dig Endosc. 2010;22:376-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Brenner H, Chang-Claude J, Rickert A, Seiler CM, Hoffmeister M. Risk of colorectal cancer after detection and removal of adenomas at colonoscopy: Population-based case-control study. J Clin Oncol. 2012;30:2969-2976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Sakamoto T, Matsuda T, Otake Y, Nakajima T, Saito Y. Predictive factors of local recurrence after endoscopic piecemeal mucosal resection. J Gastroenterol. 2012;47:635-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |