Published online Mar 21, 2019. doi: 10.3748/wjg.v25.i11.1378
Peer-review started: December 3, 2018
First decision: January 30, 2019
Revised: February 13, 2019
Accepted: February 22, 2019
Article in press: February 22, 2019
Published online: March 21, 2019
Conventionally, the low luminous intensity, low image resolution, and difficulty in operation have been reported with the ultrathin endoscope. However, it has markedly advanced recently. The improvement of the diagnostic ability is expected.
To compare the early gastric cancer diagnostic ability of an ultrathin endoscope loaded with a laser light source and that of the conventional endoscope.
The target subjects were 375 consecutive patients who underwent endoscopy at our hospital for post-endoscopic submucosal dissection follow-up of gastric cancer from January to August 2018. During endoscopy, the ultrathin endoscope was used in 140 patients (37.3%), and the conventional endoscope was used in 235 patients (62.7%). Patient background was adjusted using the propensity score matching method, and gastric cancer detection ability was evaluated in the two groups.
The gastric cancer detection rate was 7.8% in the ultrathin endoscope group and 7.0% in the conventional endoscope group, and the mean intragastric observation time was 4.1 ± 1.7 min in the ultrathin endoscope group and 4.1 ± 1.9 min in the conventional endoscope group, showing no significant differences between the groups. Moreover, the biopsy implementation rate was 31.8% in the ultrathin endoscope group and 41.1% in the conventional endoscope group, and the biopsy prediction rate was 17.9% and 13.2%, respectively, showing no significant differences between the groups.
The gastric cancer diagnostic ability of the ultrathin endoscope loaded with a laser light source was comparable to that of the conventional endoscope. The observation time was also comparable. Thus, endoscopy using the ultrathin endoscope loaded with the laser light source would be the first option in screening examinations of gastric cancer due to its low invasion.
Core tip: The gastric cancer diagnostic ability of the ultrathin endoscope loaded with a laser light source is comparable to that of the conventional endoscope. From the view of low invasion, the ultrathin endoscope is superior to the conventional endoscope. Thus, endoscopy using the ultrathin endoscope loaded with the laser light source would be the first option in screening examinations of gastric cancer.
- Citation: Suzuki T, Kitagawa Y, Nankinzan R, Yamaguchi T. Early gastric cancer diagnostic ability of ultrathin endoscope loaded with laser light source. World J Gastroenterol 2019; 25(11): 1378-1386
- URL: https://www.wjgnet.com/1007-9327/full/v25/i11/1378.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i11.1378
Barium x-ray with photofluorography has been widely used in screening exami-nations including health checkups because it has the effect of decreasing gastric cancer fatality rate[1-3]. However, the sensitivity of this examination method was reportedly limited to 39% in the case of early gastric cancer[4]. In order to overcome this drawback, esophagogastroduodenoscopy (EGD) has adopted instead. Since the marked effect of EGD to suppress gastric cancer fatality rate was demonstrated recently, EGD has been widely used in gastric cancer diagnosis[5,6]. However, for the patients EGD is an unpleasant examination accompanied by afflictions such as nausea, gag reflex, and choking, and there are also cardiovascular loads[7-9]. Thus, diameter thinning has advanced in the evolution of endoscope, and even an examination using an ultrathin endoscope such as transnasal endoscopy has been promoted[10].
It was reported that less problems, higher patient acceptability, and lower loads would accompany an ultrathin endoscope to the heart and lung than those with the conventional endoscope[11-15]. In disseminating endoscopy, an ultrathin endoscope is highly needed. In contrast, the low luminous intensity, low image resolution, and difficulty in operation were reported with the ultrathin endoscope in comparison with the conventional endoscope, and it was also pointed out that the examination time would be longer with the ultrathin endoscope due to the dark field of vision and lower absorption capacity (small channel diameter)[16]. At present, an endoscope using a laser light source has been developed. In comparison with the conventional endoscope using the xenon light source, the field of vision is bright and broad, and high image quality has been achieved with a laser light source. Furthermore, operation has also been improved by expansion of the channel diameter (forceps diameter).
Some studies reported that the early gastric cancer diagnostic ability is comparable between the ultrathin and conventional endoscopes, but in other reports the inferiority of the ultrathin endoscope to the conventional endoscope was reported[17-21]. In such reports, the low diagnostic capacity of the ultrathin endoscope was pointed out, particularly in the case of small early gastric cancer or gastric cancer in the U region[22]. At this time, the aim was to compare the early gastric cancer diagnostic ability between the ultrathin endoscope and the conventional endoscope loaded with the laser light source.
This retrospective analysis of database on EGD was conducted at the Chiba Cancer Center, Japan. This was approved by the ethics committee of Chiba Cancer Center, and the contents were displayed on the notice board for in- and outpatients. The study was conducted in accordance with the World Medical Association’s Declaration of Helsinki. All patients provided informed consent to undergo EGD. All authors had access to the study data and approved the final manuscript.
The target patients were those who underwent endoscopic submucosal dissection (ESD) for gastric cancer at this hospital and were periodically undergoing endoscopy thereafter. At this hospital, post-ESD endoscopy is performed initially at 3 mo after ESD, at 1 yr after ESD, and thereafter once a year. Because gastric cancer develops metachronously, patients with a history of ESD have a high risk for gastric cancer. In this study, such patients were targeted. In addition, patients infected with Helicobacter pylori (H. pylori) must receive eradication therapy. There were 375 consecutive patients who underwent endoscopy at this hospital from January to August 2018 and were enrolled in this study. Patients with postoperative gastric remnant were excluded. At this hospital, the ultrathin endoscope was being used in the same manner as the conventional endoscope, and in the patients who prefer transnasal endoscopy, the ultrathin endoscope was used. In this study, the ultrathin endoscope was used in 140 patients (37.3%), and the conventional endoscope was used in 235 patients (62.7%). The endoscopists in charge were five specialists authorized by the Japan Gastroenterological Endoscopy Society and four non-specialists.
In order to investigate the diagnostic ability of the ultrathin endoscope, the backgrounds were adjusted with the group using the conventional endoscope by the propensity score (PS) matching method to compare gastric cancer detection rate as the primary evaluation item and intragastric observation time, biopsy implementation rate, biopsy prediction rate, and details of detected gastric cancers as the secondary evaluation items.
An endoscopic system using a laser light source (LASEREO, Fujifilm, Japan) was used. This system has two lasers with different wavelengths. One is a white light laser (wavelength 450 ± 10 nm) providing wide-spectrum white light illumination suitable for general observation. The other is a blue laser imaging mode laser (wavelength 410 ± 10 nm) with characteristics of short wavelength and narrowband. Therefore, in comparison with the endoscope system using the conventional xenon light source, it is feasible to visualize a brighter and higher-resolution image. The ultrathin endoscope (EG-L580NW) used in this system is 5.8 mm in scope outside diameter, 2.4 mm in forceps diameter, and 140° in viewing angle showing markedly improved performance as compared with the previous ultrathin endoscope. It can be used in not only the transoral but also transnasal route. As the conventional endoscope, EG-L590WR, EG-L590ZW, or EG-L600ZW was used.
We created a PS-matched cohort by attempting to match a patient who underwent the examination with an ultrathin endoscope with a patient who underwent the examination with a conventional endoscope (1:1 match) using a greedy nearest neighbor-matching technique. A caliper width of 0.05 of the standard deviation for the logit of the PS was used for the developed PS. After matching, crude comparisons of the matched cohorts were made. Upon matching, four covariates that could possibly influence the detection of gastric cancer were used, namely age, sex, degree of gastric mucosa atrophy, and endoscopist.
The clinical data were calculated as mean value with standard deviation, median value with range, and rate with 95% confidence interval (CI). In comparison between the two groups, Student’s t-test was used to analyze numerical values. Chi-square and Fisher’s exact tests were used to analyze rate values. A P-value < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS version 23.0 for Windows (IBM Japan, Ltd., Japan).
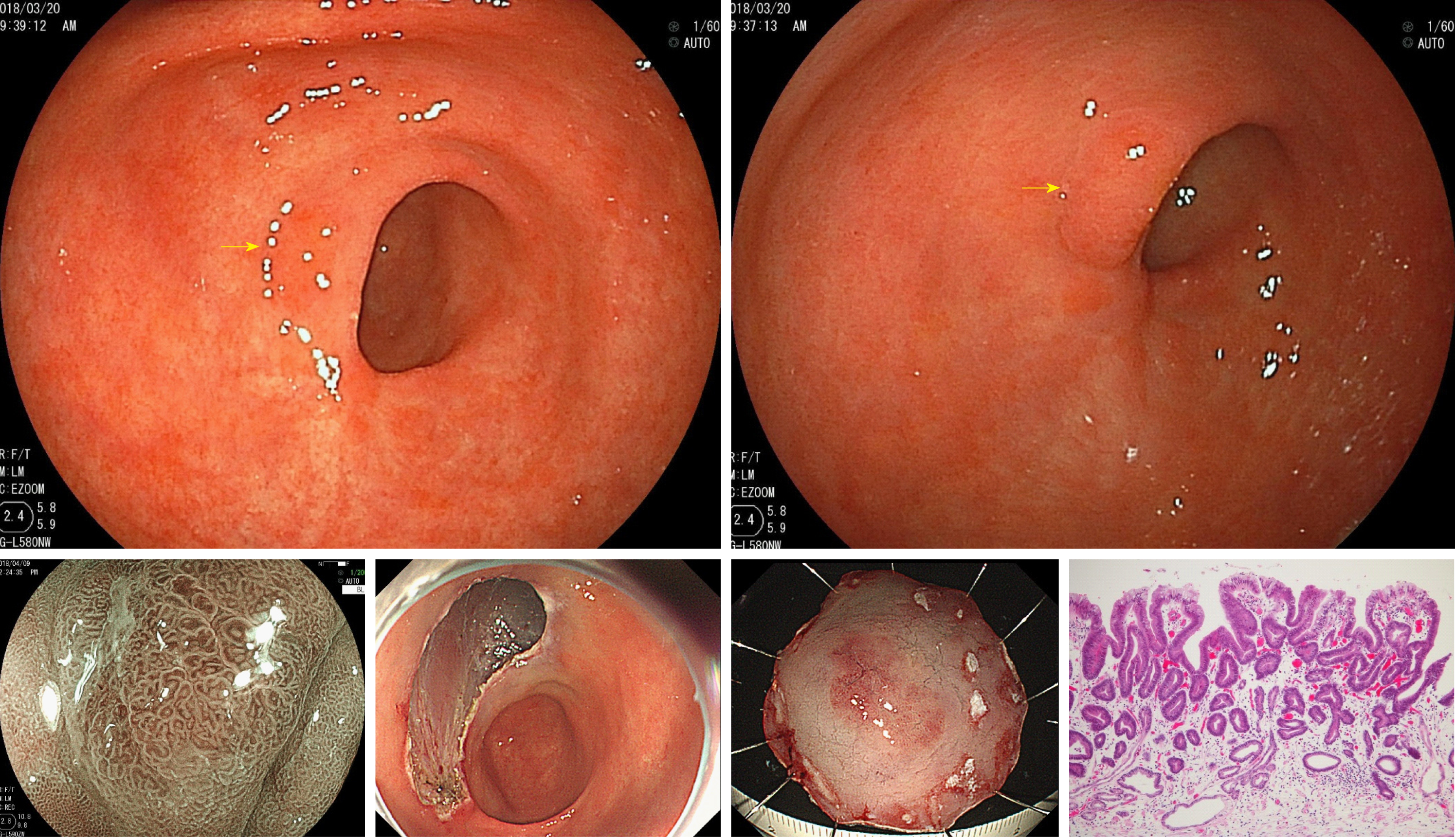
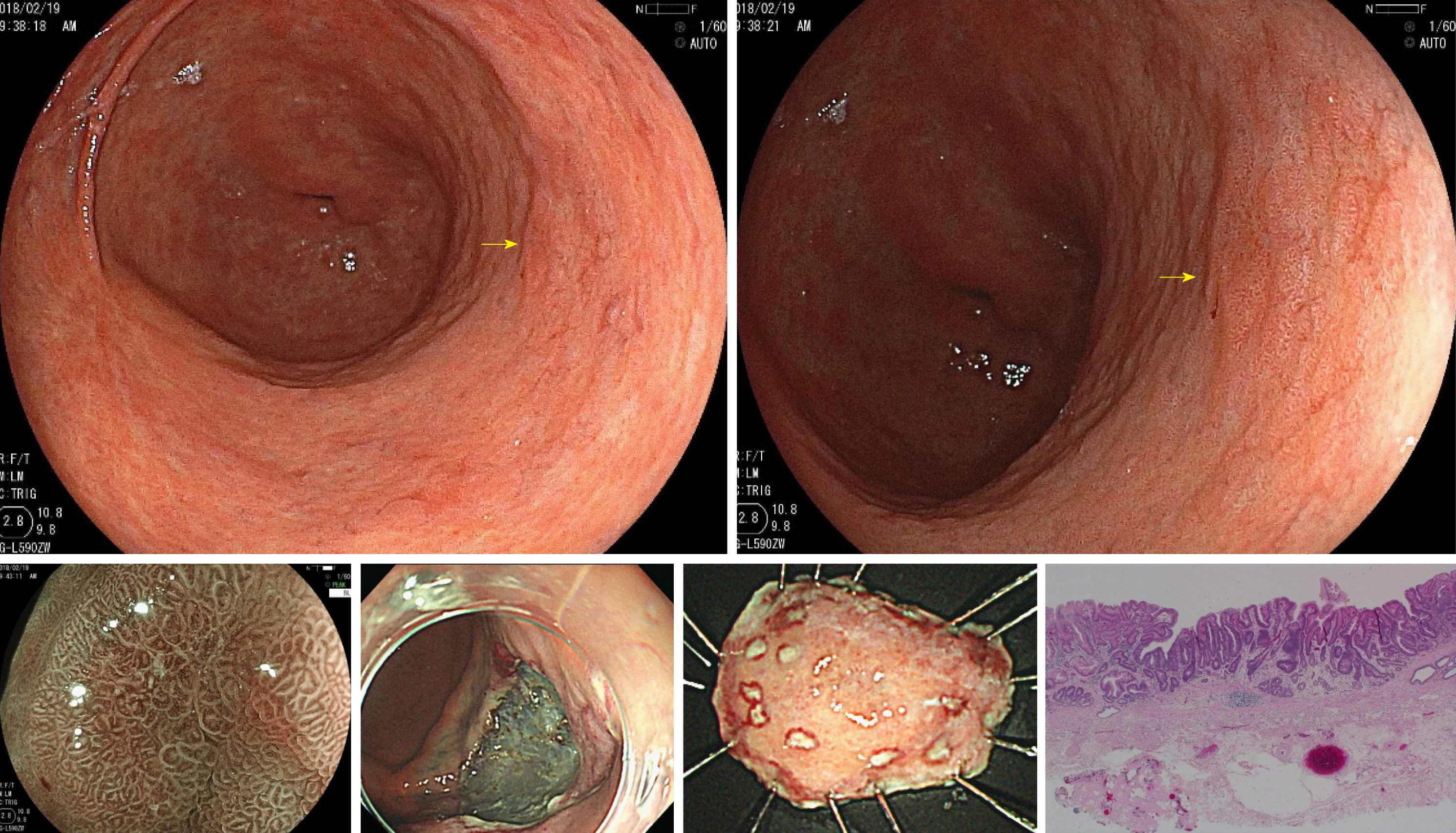
The ultrathin endoscope was used via the transnasal route in 41 patients (29.3%) and transoral route in 99 patients (70.7%). In the conventional endoscope group, a sedative agent was used in 74 patients (31.5%). Due to being post-ESD, the patients were supposed to have undergone H. pylori eradication therapy. H. pylori-positive patients accounted for only 3.7% of the whole. Table 1 shows the patient backgrounds and results of the two groups before matching. After matching 140 patients of the ultrathin endoscope group and 235 patients of the conventional endoscope group, 129 patients in each group were adopted for analysis. The C-statistic of PS score was 0.557. Table 2 shows the matching results and balances. Table 3 shows the patient backgrounds of the two groups after matching. Gastric cancer was detected in 10 patients (7.8%, 95%CI 3.1%-12.4%) of the ultrathin endoscope group and 9 patients (7.0%, 95%CI 2.6%-11.4%) of the conventional endoscope group, showing no significant difference between the two groups (P = 0.81). All detected gastric cancers were in the early stage, and the median tumor size (range) was 7.5 mm (3-30 mm) in the ultrathin endoscope group and 6.0 mm (3-15 mm) in the conventional endoscope group, showing no significant difference between the two groups (P = 0.42). The region (U/M+L) of detected gastric cancer was 4/6 in the ultrathin endoscope group and 2/7 in the conventional endoscope group, showing no significant difference (P = 0.41) (Table 4). In addition, the mean intragastric observation time was 4.1 ± 1.7 min in the ultrathin endoscope group and 4.1 ± 1.9 min in the conventional endoscope group, showing no significant difference (P = 0.96). The biopsy implementation rate was 31.8% (95%CI 23.8%-39.8%) in the ultrathin endoscope group and 41.1% (95%CI 32.6%-49.6%) in the conventional endoscope group, showing no significant difference (P = 0.12) but a tendency to be lower in the former. The biopsy prediction rate was 17.9% (95%CI 7.9%-27.9%) and 13.2% (95%CI 5.2%-21.2%) in the ultrathin and conventional endoscope groups, respectively, showing no significant difference (P = 0.48) but a tendency to be lower also in the latter (Table 5). Figures 1 and 2 show the patients in whom gastric cancer was actually detected.
| Ultrathin endoscope | Conventional endoscope | P value | |
| Number of screened subjects | 140 | 235 | |
| Transnasal | 41 (29.3%) | 0 (0%) | - |
| Sedation | 16 (11.4%) | 74 (31.5%) | < 0.001 |
| Age in yr, median (range) | 74 (43-89) | 73 (43-93) | 0.49 |
| Gender, male | 99 (70.7%) | 187 (79.6%) | 0.05 |
| Helicobacter pylori positive | 6 (4.3%) | 9 (3.8%) | 0.83 |
| Atrophy, open type | 108 (77.1%) | 197 (83.8%) | 0.11 |
| Operator (expert/nonexpert) | 99/41 | 178/57 | 0.28 |
| Number of gastric cancer | 12 | 20 | |
| Number of subjects with gastric cancer/detection rate | 12 (8.6%) | 18 (7.7%) | 0.75 |
| Location (U/M, L) | 4/4, 4 | 3/11, 6 | 0.22 |
| Size in mm, median (range) | 5 (3-30) | 6.5 (3-18) | 0.37 |
| Morphological type (I, IIa/IIb, IIc) | 0, 6/0, 6 | 2, 6/0, 12 | 0.09 |
| Depth of invasion (m/sm) | 10/2 | 20/0 | 0.06 |
| Full cohort | Propensity score-matched cohort | |||||
| Conventional | Ultrathin | ASD, % | Conventional | Ultrathin | ASD, % | |
| n | 235 | 140 | 129 | 129 | ||
| Age in yr | 73.1 ± 7.6 | 73.2 ± 7.6 | 1.9 | 73.2 ± 7.6 | 73.4 ± 7.1 | 2.7 |
| Male | 187, 79.6 | 99, 70.7 | 20.6 | 98, 76.0 | 97, 75.2 | 1.8 |
| Specialist | 178, 75.7 | 99, 70.7 | 11.4 | 95, 73.6 | 92, 71.3 | 5.2 |
| Atrophy | 198, 84.3 | 108, 77.1 | 18.1 | 103, 79.8 | 107, 82.9 | 8.0 |
| Ultrathin endoscope | Conventional endoscope | P value | |
| Number of screened subjects | 129 | 129 | |
| Age in yr, median (range) | 74 (52-89) | 74 (47-87) | 0.46 |
| Gender, males | 108 (77.1%) | 99 (76.7%) | 0.16 |
| Helicobacter pylori positive | 5 (3.9%) | 4 (3.1%) | 0.73 |
| Atrophy, open type | 107 (82.9%) | 103 (79.8%) | 0.52 |
| Operator, expert/nonexpert | 92/37 | 95/34 | 0.68 |
| Ultrathin endoscope | Conventional endoscope | P value | |
| Number of gastric cancer | 10 | 9 | |
| Number of subjects with gastric cancer/detection rate | 10 (7.8%) | 9 (7.0%) | 0.81 |
| Location (U/M + L) | 4/4 + 2 | 2/6 + 1 | 0.41 |
| Size in mm, median (range) | 7.5 (3-30) | 6.0 (3-15) | 0.42 |
| Morphological type (I, IIa/IIb, IIc) | 0, 6/0, 4 | 0, 2/0, 7 | 0.10 |
| Depth of invasion (m/sm) | 8/2 | 9/0 | 0.16 |
| Ultrathin endoscope | Conventional endoscope | P value | |
| Observation time of stomach in min | 4.1 ± 1.7 | 4.1 ± 1.9 | 0.96 |
| Biopsy implementation rate | 31.8% (41/129) (95%CI 23.8-39.8) | 41.1% (53/129) (95%CI 32.6-49.6) | 0.12 |
| Biopsy prediction rate | 17.9% (10/56) (95%CI 7.9-27.9) | 13.2% (9/68) (95%CI 5.2-21.2) | 0.48 |
In this study it was shown that the ultrathin endoscope loaded with a laser light source can detect gastric cancer at the same rate as the conventional endoscope. Until recently, the problems with the ultrathin endoscope were dark images, low image quality, and weak absorption power. The inferiority of the ultrathin endoscope to the conventional endoscope in gastric cancer detection rate has been reported[17-21]. However, the ultrathin endoscope loaded with the laser light source is markedly different from the conventional endoscope system using the xenon light source, and the image quality and brightness have been remarkably improved. In this study, patients with a history of ESD for gastric cancer were enrolled. Gastric cancer tends to multiply metachronously. In addition, according to the World Health Organization, H. pylori is the definite carcinogen of gastric cancer, and the majority of patients targeted in this study received H. pylori eradication therapy. The onset rate of gastric cancer is reduced by H. pylori eradication therapy, but a certain risk of gastric cancer remains even after eradication. Therefore, such patients have a higher risk of gastric cancer than the general population[23]. The ultrathin endoscope showed a diagnostic ability that was not inferior to that in the conventional endoscope even in such patients.
It was thus far unavoidable to extend the examination time due to the poor absorption capacity, dark image, and narrow viewing angle, but in the case of the ultrathin endoscope loaded with the laser light source, the examination time was comparable to the conventional endoscope, due to the improvement of absorption capacity brought about by expansion of the forceps diameter and the expansion of observation range brought about by the improvement of viewing angle and brightness. As one of the factors, it is estimated that the amount of highly viscous gastric mucosa was small after the aforementioned H. pylori eradication therapy and the time required for mucosa washing and accompanying absorption could be shortened. However, because the number of H. pylori-negative patients is expected to increase hereafter, the situation was considered to reflect the current status.
Detected gastric cancers were not different between the two groups in lesion site and size. The lower diagnostic ability of the ultrathin endoscope was pointed out in the previous studies in the diagnosis of small gastric cancers < 20 mm in the U region, but such tendency was not seen in this study[21,22]. Furthermore, the biopsy implementation and prediction rates were not significantly different between the two groups. There was rather a tendency of the biopsy implementation rate to be lower and the biopsy prediction rate to be higher in the ultrathin endoscope group. The reason for such tendency was not clear, but it was suggested that the ultrathin endoscope would be able to provide appropriate findings.
There are limitations in this study. First, this was a retrospective study, but the bias could be minimized in the investigation by matching the backgrounds using the PS matching method. Next, the ultrathin endoscope was used via not only the transnasal but also the transoral route in this study for pooled analysis, and a sedative agent was frequently used in the conventional endoscope group. Therefore, the influence of these factors on the analysis cannot be negated. Lastly, this study was performed in a limited number of patients at a single medical institution. It is therefore desired to perform a prospective multicenter study hereafter.
As EGD becomes widely known as a gastric cancer diagnostic procedure, the ultrathin endoscope may become the first-choice screening examination in gastric cancer diagnosis due to its low invasion.
An ultrathin endoscope has low luminous intensity, low image resolution, and difficulty in operation. However, the ultrathin endoscopy is a useful tool for screening endoscopy because of its low invasion.
Recently endoscopic technology has markedly advanced. The improvement of the diagnostic ability of ultrathin endoscope has also improved. Evaluating the diagnostic ability of the ultrathin endoscope is warranted.
In this study, early gastric cancer diagnostic ability of an ultrathin endoscope loaded with a laser light source was compared with that of the conventional endoscope.
The study subjects were 375 consecutive patients who underwent endoscopy at our hospital for post-endoscopic submucosal dissection follow-up of gastric cancer from January to August 2018. During endoscopy, the ultrathin endoscope was used in 140 patients (37.3%), and the conventional endoscope was used in 235 patients (62.7%). Patient background was adjusted using the propensity score matching method, and gastric cancer detection ability was evaluated in the two groups.
The gastric cancer detection rate was 7.8% in the ultrathin endoscope group and 7.0% in the conventional endoscope group, and the mean intragastric observation time was 4.1 ± 1.7 min in the ultrathin endoscope group and 4.1 ± 1.9 min in the conventional endoscope group, showing no significant differences between the groups. Moreover, the biopsy implementation rate was 31.8% in the ultrathin endoscope group and 41.1% in the conventional endoscope group, and the biopsy prediction rate was 17.9% and 13.2%, respectively, showing no significant differences between the groups.
The gastric cancer diagnostic ability of the ultrathin endoscope loaded with a laser light source was comparable to that of the conventional endoscope. The observation time was also comparable. Thus, endoscopy using the ultrathin endoscope loaded with the laser light source would be the first option in screening examinations of gastric cancer due to its low invasion.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Christodoulidis G, Sitkin S S- Editor: Yan JP L- Editor: Filipodia E- Editor: Huang Y
| 1. | Oshima A, Hirata N, Ubukata T, Umeda K, Fujimoto I. Evaluation of a mass screening program for stomach cancer with a case-control study design. Int J Cancer. 1986;38:829-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 104] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Fukao A, Tsubono Y, Tsuji I, HIsamichi S, Sugahara N, Takano A. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: A population-based case-control study. Int J Cancer. 1995;60:45-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Tsubono Y, Hisamichi S. Screening for gastric cancer in Japan. Gastric Cancer. 2000;3:9-18. [PubMed] [Cited in This Article: ] |
| 4. | Nisizawa M. Present status and prospect for cancer screening (in Japanese). J Gastroenterol Mass Surv. 1993;78:100-103. [Cited in This Article: ] |
| 5. | Hamashima C, Ogoshi K, Okamoto M, Shabana M, Kishimoto T, Fukao A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One. 2013;8:e79088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Matsumoto S, Yoshida Y. Efficacy of endoscopic screening in an isolated island: A case-control study. Indian J Gastroenterol. 2014;33:46-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Brandt LJ. Patients' attitudes and apprehensions about endoscopy: How to calm troubled waters. Am J Gastroenterol. 2001;96:280-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Hart R, Classen M. Complications of diagnostic gastrointestinal endoscopy. Endoscopy. 1990;22:229-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 139] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Bough EW, Meyers S. Cardiovascular responses to upper gastrointestinal endoscopy. Am J Gastroenterol. 1978;69:655-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 10. | Amin MR, Postma GN, Setzen M, Koufman JA. Transnasal esophagoscopy: A position statement from the American Bronchoesophagological Association (ABEA). Otolaryngol Head Neck Surg. 2008;138:411-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Yagi J, Adachi K, Arima N, Tanaka S, Ose T, Azumi T, Sasaki H, Sato M, Kinoshita Y. A prospective randomized comparative study on the safety and tolerability of transnasal esophagogastroduodenoscopy. Endoscopy. 2005;37:1226-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Preiss C, Charton JP, Schumacher B, Neuhaus H. A randomized trial of unsedated transnasal small-caliber esophagogastroduodenoscopy (EGD) versus peroral small-caliber EGD versus conventional EGD. Endoscopy. 2003;35:641-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Mori A, Ohashi N, Maruyama T, Tatebe H, Sakai K, Shibuya T, Inoue H, Okuno M, Fushimi N, Asano T. Cardiovascular tolerance in upper gastrointestinal endoscopy using an ultrathin scope: Prospective randomized comparison between transnasal and conventional oral procedures. Dig Endosc. 2008;20:79-83. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Nakata H, Oka M, Magari H, Inoue I, Iguchi M, Yanaoka K, Tamai H, Arii K, Ichinose M. Prospective study comparing transoral and transnasal upper gastrointestinal endoscopy on the cardiorespiratory parameter and tolerability (in Japanese). Gastroenterol Endosc. 2007;49:2684-2689. [DOI] [Cited in This Article: ] |
| 15. | Kawai T, Miyazaki I, Yagi K, Kataoka M, Kawakami K, Yamagishi T, Sofuni A, Itoi T, Moriyasu F, Osaka Y, Takagi Y, Aoki T. Comparison of the effects on cardiopulmonary function of ultrathin transnasal versus normal diameter transoral esophagogastroduodenoscopy in Japan. Hepatogastroenterology. 2007;54:770-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Tatsumi Y, Harada A, Matsumoto T, Tani T, Nishida H. Current status and evaluation of transnasal esophagogastroduodenoscopy. Dig Endosc. 2009;21:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Sorbi D, Gostout CJ, Henry J, Lindor KD. Unsedated small-caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: A comparative study. Gastroenterology. 1999;117:1301-1307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Catanzaro A, Faulx A, Isenberg GA, Wong RC, Cooper G, Sivak MV, Chak A. Prospective evaluation of 4-mm diameter endoscopes for esophagoscopy in sedated and unsedated patients. Gastrointest Endosc. 2003;57:300-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Murata A, Akahoshi K, Sumida Y, Yamamoto H, Nakamura K, Nawata H. Prospective randomized trial of transnasal versus peroral endoscopy using an ultrathin videoendoscope in unsedated patients. J Gastroenterol Hepatol. 2007;22:482-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Yoshida Y, Hayami Y, Matuoka M, Nakayama S. Comparison of endoscopic detection rate of early gastric cancer and gastric adenoma using transnasal EGD with that of transoral EGD. Dig Endosc. 2008;20:184-189. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Nakata H, Enomoto S, Maekita T, Inoue I, Ueda K, Deguchi H, Shingaki N, Moribata K, Maeda Y, Mori Y, Iguchi M, Tamai H, Yamamichi N, Fujishiro M, Kato J, Ichinose M. Transnasal and standard transoral endoscopies in the screening of gastric mucosal neoplasias. World J Gastrointest Endosc. 2011;3:162-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Hayashi Y, Yamamoto Y, Suganuma T, Okada K, Nego M, Imada S, Imai M, Yoshimoto K, Ueki N, Hirasawa T, Uragami N, Tsuchida T, Fujisaki J, Hoshino E, Takahashi H, Igarashi M. Comparison of the diagnostic utility of the ultrathin endoscope and the conventional endoscope in early gastric cancer screening. Dig Endosc. 2009;21:116-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M; Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet. 2008;372:392-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 850] [Article Influence: 53.1] [Reference Citation Analysis (0)] |