Published online Jan 7, 2019. doi: 10.3748/wjg.v25.i1.69
Peer-review started: October 10, 2018
First decision: November 14, 2018
Revised: November 17, 2018
Accepted: November 30, 2018
Article in press: November 30, 2018
Published online: January 7, 2019
Acute lower gastrointestinal bleeding (LGIB) is a common indication for hospital admission. Patients with LGIB often experience persistent or recurrent bleeding and require blood transfusions and interventions, such as colonoscopic, radiological, and surgical treatments. Appropriate decision-making is needed to initially manage acute LGIB, including emergency hospitalization, timing of colonoscopy, and medication use. In this literature review, we summarize the evidence for initial management of acute LGIB. Assessing various clinical factors, including comorbidities, medication use, presenting symptoms, vital signs, and laboratory data is useful for risk stratification of severe LGIB, and for discriminating upper gastrointestinal bleeding. Early timing of colonoscopy had the possibility of improving identification of the bleeding source, and the rate of endoscopic intervention, compared with elective colonoscopy. Contrast-enhanced computed tomography before colonoscopy may help identify stigmata of recent hemorrhage on colonoscopy, particularly in patients who can be examined immediately after the last hematochezia. How to deal with nonsteroidal anti-inflammatory drugs (NSAIDs) and antithrombotic agents after hemostasis should be carefully considered because of the risk of rebleeding and thromboembolic events. In general, aspirin as primary prophylaxis for cardiovascular events and NSAIDs were suggested to be discontinued after LGIB. Managing acute LGIB based on this information would improve clinical outcomes. Further investigations are needed to distinguish patients with LGIB who require early colonoscopy and hemostatic intervention.
Core tip: Several concerns exist when managing acute lower gastrointestinal bleeding (LGIB). Fortunately in recent years, novel findings in the acute LGIB setting have accumulated with respect to predictive scores for severe bleeding, the clinical significance of contrast-enhanced computed tomography before colonoscopy, the utility of early colonoscopy, and the management of direct-acting oral anticoagulants. Here, we review evidence for the initial management of acute LGIB.
- Citation: Aoki T, Hirata Y, Yamada A, Koike K. Initial management for acute lower gastrointestinal bleeding. World J Gastroenterol 2019; 25(1): 69-84
- URL: https://www.wjgnet.com/1007-9327/full/v25/i1/69.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i1.69
Traditionally, gastrointestinal bleeding (GIB) was classified into upper gastrointestinal bleeding (UGIB) and lower gastrointestinal bleeding (LGIB). LGIB was defined as bleeding from the lesion distal to the ligament of Treitz, including the small and large bowels. In the last decade, the availability of advanced diagnostic innovations such as capsule endoscopy and balloon-assisted enteroscopy has led to better understanding of the etiological profile of small bowel bleeding. Thus, some recent reports adopted three categories of GIB: Upper-, mid-, and lower GIB[1].
Acute LGIB has increased due to aging of the population, and with the increasing use of antithrombotic agents[2-4]. Although many UGIB events can be prevented by proton pump inhibitors (PPIs) and eradicating Helicobacter pylori, there are few effective methods for preventing LGIB recurrence. The estimated hospitalization rate for LGIB is 33-87 per 100000 population[2,5,6], with mortality rates of 2.5%-3.9% during hospitalization[7-9] and rebleeding rates of 13%-19% after 1 year[10,11].
Several concerns exist when managing acute-onset hematochezia suspected as acute LGIB. First, the causes of bleeding varied from many types of colonic diseases to UGIB and small-bowel bleeding. Most cases experience spontaneous cessation with conservative therapy. In contrast, patients with vascular diseases, such as diverticular bleeding and angioectasia, often suffer from continuous and/or recurrent bleeding, requiring hemostatic intervention and blood transfusion[9,12]. A few cases die during hospitalization. Therefore, risk stratification tools for severe LGIB would be useful for deciding on emergency hospitalization or an early intervention. However, unlike UGIB, predictive clinical scores for severe acute LGIB are not well defined.
Second, colonoscopy, which is essential for diagnosis and therapy of LGIB[13], requires bowel preparation to identify the bleeding source, unlike upper endoscopy. The timing of time-consuming and laborious colonoscopy should be optimized, but the utility of early colonoscopy remains controversial.
Third, there are numerous antithrombotic agents, including dual antiplatelet therapy and direct-acting oral anticoagulants (DOACs), and the management of antithrombotic agent use requires considering the conflicting risks of ongoing/recurrent bleeding and thromboembolic events.
Thus, appropriate decision-making is needed when managing acute LGIB. Fortunately, novel findings in the acute LGIB setting have accumulated in recent years, such as predictive scores for severe bleeding, the clinical significance of contrast-enhanced computed tomography (CE-CT) before colonoscopy, the utility of early colonoscopy, and the management of DOACs.
The purpose of this literature review is to summarize findings regarding the initial management of acute LGIB, in line with the 2016 American College of Gastroenterology guideline for acute LGIB and the 2016 American Society for Gastrointestinal Endoscopy guideline for the management of antithrombotic agents on gastrointestinal endoscopy[13,14].
History-taking, physical examination, and laboratory testing are important at the time of presentation of patients with presumed acute LGIB. The need for intravenous fluid resuscitation and blood transfusion should be determined by evaluating hemodynamic status according to history of syncope, level of consciousness, and vital signs, including postural changes. Hematochezia with hemodynamic instability requires attention, as brisk UGIB can also result in that type of stool. A blood urea nitrogen/creatinine (BUN/Cr) ratio > 30 (likelihood ratio, 7.5) and nasogastric aspirate/lavage with blood or coffee grounds (likelihood ratio, 9.6) are the features of UGIB[15], being useful to distinguish UGIB from LGIB. In addition, in a report of patients with hematochezia, the systolic blood pressure was significantly lower in UGIB than in LGIB (mean pressure, 114 mmHg vs 133 mmHg)[16]. If the likelihood of UGIB is high based on these factors, upper endoscopy is recommended.
The presence of certain symptoms can suggest the source of LGIB[13]. Patients with colitis (ischemia, infection, or inflammatory bowel disease) often present with diarrhea and abdominal tenderness, whereas those with vascular diseases, such as diverticular bleeding, hemorrhoid, angioectasia, and rectal ulcers, usually do not present with lower gastrointestinal symptoms. Weight loss and altered bowel habits suggest malignancy.
Although most patients with acute LGIB experience spontaneous hemostasis, some suffer from severe, persistent hemorrhage and rebleeding. The frequency of adverse outcomes varies by cause of LGIB. The causes of acute LGIB in the Western countries are as follows[17]: Diverticular bleeding (30%-65%), ischemic colitis (5%-20%), hemorrhoids (5%-20%), colorectal polyps/neoplasms (2%-15%), angioectasia (5%-10%), post-polypectomy bleeding (2%-7%), inflammatory bowel disease (3%-5%), infectious colitis (2%-5%), rectal ulcer (0-5%), colorectal varices (0-3%), radiation proctitis (0-2%), drug-induced colitis (0-2%), and Dieulafoy’s lesion (rare). On the other hand, in the tropical countries, colorectal polyps/neoplasms (29%-53%) and colitis (23%-38%) are the common causes, and diverticular bleeding is less common (4%-19%)[16,18]. The rate of rebleeding is 22%-38% in patients with diverticular bleeding[12]. Ischemic colitis cases have significantly lower blood transfusion requirements (4%) compared with other forms of LGIB[19].
Physicians must understand the predictive factors for severe LGIB to improve triage of appropriate patients for emergency hospitalization or early intervention. Several studies have investigated risk factors for adverse outcomes (rebleeding, severe bleeding, need for emergent hospitalization, need for intervention, adverse events, or death) in patients with acute LGIB[7,8,20-28]. These include older age, presenting symptoms (no abdominal tenderness, no diarrhea, altered mental status, or blood on rectal examination), vital signs, comorbidities, medication use [nonsteroidal anti-inflammatory drugs (NSAIDs) and antithrombotic agents], and laboratory data [hemoglobin (Hb), hematocrit, albumin, BUN, Cr, and prothrombin time (PT)] (Table 1). We also previously reported a predictive model of severe LGIB (NOBLADS score), which included NSAID use, no diarrhea, no abdominal tenderness, systolic blood pressure ≤ 100 mmHg, albumin level < 3.0 g/dL non-aspirin antiplatelet drug use, Charlson comorbidity index score ≥ 2, and syncope[24]. Several predictive models have been validated in other settings (Table 2)[21,22,24,27,28]. Applying these models to manage LGIB could improve clinical outcomes and resource utilization. However, compared with established models of severe UGIB[29,30] such as the Blatchford score, predictive models of severe LGIB require further validation and improvements in accuracy.
| Severe/recurrent bleeding | In-hospital complications3 | Adverse outcomes4 | Mortality | |
| Patient characteristic | ||||
| Older age | 2.31 | - | 4.21 | 4.92 |
| Male sex | 6 | - | - | 1.5-1.6 |
| Lower body mass index | - | - | - | 2.0 |
| Smoking | - | - | 0.5 | - |
| Comorbidities | ||||
| Charlson index > 2 or ≥ 2 | 1.7-1.9 | - | - | 3.0 |
| Unstable comorbid diseases | - | 2.9 | - | - |
| Congestive heart failure | - | - | - | 1.5 |
| Cardiovascular disease | 6 | - | - | - |
| Dementia | 6 | - | - | 5.2 |
| Metastatic cancer | - | - | - | 5.0 |
| Chronic kidney disease | - | - | - | 1.8-2.2 |
| Liver disease | - | - | - | 1.9 |
| Chronic pulmonary disease | - | - | - | 1.6 |
| History of colonic diverticulosis and/or angiodysplasia | 6 | - | - | 6 |
| Presenting symptom | ||||
| Syncope / altered mental status | 2.5-3.3 | 2.0 | - | 6 |
| No diarrhea | 2.2 | - | - | - |
| No abdominal tenderness | 2.4-3.0 | - | - | - |
| Ongoing bleeding | - | 3.1 | - | - |
| Bleeding in the first 4 h | 2.3 | - | - | - |
| Medication | ||||
| NSAIDs (non-aspirin)1 | 2.5 | - | - | 1.5 |
| Aspirin | 1.9-2.1 | - | - | - |
| Antiplatelet drugs (non-aspirin) | 2.0 | - | - | - |
| Anticoagulants | - | - | - | 1.5 |
| Physical examination | ||||
| Blood pressure ≤ 100 or ≤ 115 mmHg | 2.3-3.5 | 3.0 | - | 6 |
| Heart rate ≥ 100/min | 3.7 | - | - | - |
| Abnormal vital signs after 1 h | 4.3 | - | - | - |
| Abnormal hemodynamic parameters | - | - | 2.1 | - |
| Gross blood on rectal examination | 3.5-3.9 | - | - | 6 |
| Laboratory data | ||||
| Hemoglobin < 10 g/dL | 3.6 | - | - | - |
| Albumin < 3.0 or < 3.8 g/dL | 2.0-2.9 | - | - | 2.9 |
| Creatinine > 150 or > 133 µmol/L | 6 | - | 10.3 | 6 |
| Hematocrit < 35% or < 30% | 4.7-6.3 | - | - | 6 |
| Prothrombin time > 1.2 times control | - | 2.0 | - | - |
| Clinical course | ||||
| Rebleeding | - | - | 1.9 | - |
| Intestinal ischemia | - | - | - | 3.5 |
| Coagulation defects | - | - | - | 2.3 |
| Hypovolemia | - | - | - | 2.2 |
| Blood transfusion | - | - | - | 1.6-2.8 |
| Need for intervention5 | - | - | - | 2.3-2.4 |
| In-hospital onset LGIB | - | - | - | 2.4 |
| Derivation study | Outcomes | Risk factors | ROC-AUC | Validation study |
| Strate et al[21] | Severe bleeding | Syncope | 0.76 | Prospective cohort (n = 275) |
| (n = 252) | (continuous and/or recurrent bleeding) | No abdominal tenderness | ROC-AUC: 0.75 | |
| Aspirin use | ||||
| Heart rate ≥ 100/min | ||||
| Systolic blood pressure ≤ 115 mmHg | ||||
| Bleeding per rectum in the first 4 h | ||||
| Charlson comorbidity index > 2 | ||||
| Das et al[22] | Rebleeding | (19 factors) | 0.92 | Prospective cohort (n = 142) |
| (n = 120) | Need for intervention | Age | 0.93 | |
| Artificial neural network based model | In-hospital mortality | Comorbidity (5 factors) | 0.95 | |
| History (4 factors) | ||||
| Features at presentation (2 factors) | ||||
| Features at initial assessment (2 factors) | ||||
| Initial laboratory data (5 factors) | ||||
| Aoki et al[24] | Severe bleeding | (NOBLADS) | 0.77 | Prospective cohort (n = 161) |
| (n = 439) | (Continuous and/or recurrent bleeding) | NSAIDs use | ROC-AUC: 0.76 | |
| No diarrhea | Retrospective cohort (n = 511) | |||
| No abdominal tenderness | ROC-AUC: 0.74 | |||
| Blood pressure (systolic) ≤ 100 mmHg | ||||
| Albumin level < 3.0 g/dL | ||||
| Antiplatelet drugs use (non-aspirin) | ||||
| Disease score ≥ 21 | ||||
| Syncope | ||||
| Oakland et al[27] | Safe discharge | Age | 0.84 | Prospective cohort (n = 288) |
| (n = 2336) | (Absence of death, rebleeding, intervention, blood transfusion, | Male sex | ROC-AUC: 0.79 | |
| or 28 d readmission) | Blood on rectal examination | |||
| Heart rate | ||||
| Systolic blood pressure | ||||
| Hemoglobin level | ||||
| Previous LGIB admission | ||||
| Sengupta et al[28] | 30 d mortality | Age | 0.81 | Retrospective cohort (n = 2060) |
| (n = 4044) | Dementia | ROC-AUC: 0.72 | ||
| Metastatic cancer | ||||
| Chronic kidney disease | ||||
| Chronic pulmonary disease | ||||
| Anticoagulant use | ||||
| Hematocrit level | ||||
| Albumin level |
Intravenous fluid resuscitation with crystalloids should be initiated, particularly in hemodynamically unstable patients[13,31]. In a review of fluid administration in bleeding patients, the best fluid resuscitation strategy was not determined on the basis of the timing, volume, and type of fluid[32]. Another review on critically ill patients indicated that colloids do not improve the mortality rate and are more expensive compared with crystalloids[33].
Although patients with LGIB often require a blood transfusion[9], transfusion strategies specific to LGIB have not been investigated. In a recent meta-analysis of five randomized controlled trials (RCTs) comparing restrictive and liberal transfusion strategies in the acute UGIB setting, restrictive transfusion of red blood cells (Hb threshold, 7-8 g/dL) was associated with a lower risk of all-cause mortality [relative risk (RR): 0.65, 95% confidence interval (CI) 0.44-0.97] and rebleeding (RR: 0.58, 95%CI: 0.40-0.84) than liberal transfusion (Hb threshold 9-10 g/dL)[34]. The LGIB guideline applied this result to recommendations for LGIB[13].
However, it should be noted that a previous RCT and a meta-analysis indicated that for patients with cardiovascular disease which limits myocardial oxygen delivery, rates of mortality and cardiovascular events were higher in a restrictive transfusion group than in a liberal transfusion group[35,36]. LGIB guideline recommended that liberal transfusion be considered in patients with massive bleeding or cardiovascular disease[13].
With respect to platelet transfusion, a systematic review concluded that there are no data to inform optimal therapeutic platelet count targets in the acute gastrointestinal bleeding (GIB) setting[37]. Based on expert opinion and the standard in the hematology literature, a platelet count of 50 × 109/L is proposed as the LGIB guideline threshold[13,38].
Colonoscopy is the initial procedure for nearly all patients presenting with acute LGIB, because it has both diagnostic and therapeutic utility[13]. Common causes of acute LGIB include diverticular bleeding, ischemic colitis, angioectasia, and post-polypectomy bleeding. Other, less common causes include rectal ulcers, infectious colitis, inflammatory bowel disease, colorectal polyps/neoplasms, radiation proctitis, and hemorrhoids[13]. One of the most important issues in diagnostic colonoscopy for acute LGIB is identifying stigmata of recent hemorrhage (SRH), including active bleeding, a non-bleeding visible vessel, and an adherent clot[39,40]. SRH is regarded as an indication for endoscopic hemostasis because a prospective study showed that the rebleeding rate within 30 d in patients with SRH was 66% when endoscopic therapy was not performed, whereas patients without SRH had no rebleeding[40].
The optimal timing of colonoscopy remains controversial. The definition of early colonoscopy used in most studies was within 24 h of presentation, and the definition in a few prospective trials was within 6-12 h[41-45]. Two RCTs and three meta-analyses examined the utility of early colonoscopy compared with elective colonoscopy in the acute LGIB setting (Table 3). These studies showed that early colonoscopy had the possibility of improving identification of the bleeding source, and the rate of endoscopic intervention, compared with elective colonoscopy. However, there is no clear evidence that early colonoscopy reduces important clinical outcomes, such as rebleeding or mortality.
| Study | Study design | Sample size | Bleeding source localization | Endoscopic intervention | Surgery required | Rebleeding | Length of stay | Adverse events | Mortality |
| Green et al[41] | RCT1 | 100 | 2.6 (1.1-6.2)4 | - | NS | NS | NS | NS | NS |
| Laine et al[42] | RCT2 | 72 | NS | - | - | NS | NS | - | - |
| Sengupta et al[44] | Meta-analysis3 | 901 | 2.97 (2.11-4.19)4 | 3.99 (2.59-6.13)4 | NS | NS | - | - | NS |
| Kouanda et al[43] | Meta-analysis3 | 24,396 | NS | 1.70 (1.08-2.67)4 | - | NS | - | NS | NS |
| Seth et al[45] | Meta-analysis3 | 23,419 | SRH detection 2.85 (1.90-4.28)4 | NS | NS | NS | NS | - | NS |
The limitations of past studies may have affected these results. Previous RCTs were single-center studies and were terminated during enrollment because of difficulties in achieving the originally planned sample size. To address these issues, we are now conducting a multicenter RCT to examine the superiority of early colonoscopy over elective colonoscopy in patients with acute LGIB[46]. The primary outcome measure is identification of SRH. Secondary outcomes include 30-d rebleeding, the need for transfusion, and 30 d mortality. This trial will provide high-quality evidence of the benefits of early colonoscopy.
The safety of early colonoscopy in the acute LGIB setting has been reported. The rate of complications associated with bowel preparation was not significantly different between early colonoscopy (1.8%) and elective colonoscopy (1.2%) in a study based on a propensity score-matching analysis[47]. A literature review showed that the rate of complications associated with colonoscopic procedures is low for both early colonoscopy (0.6%) and elective colonoscopy (0.3%)[48].
The LGIB guideline recommends that patients with high-risk clinical features and signs of ongoing bleeding should undergo early colonoscopy, i.e., within 24 h of presentation[13]. One of the signs of ongoing bleeding is extravasation on a CT scan, which should lead to early colonoscopy. However, clinical factors remain uncertain which can be easily obtained at the presentation and can suggest the indication for early colonoscopy. Although the NOBLADS score, one of the predictive score for severe LGIB, indicated the need for intervention in a derivation cohort (P = 0.001 for trend)[24], the score was not a significant predictor of the need for intervention in an externally validated cohort (P = 0.060 for trend; area under the curve, 0.54)[49]. In a recent LGIB study, all seven previous models for predicting severe GIB were not useful for distinguishing patients who required therapeutic intervention[27]. No existing model directly predicts the need for intervention in the LGIB setting; thus, appropriate models and novel strategies are required.
Based on evidence to date, the primary purpose of early colonoscopy is to identify the bleeding site and perform endoscopic hemostatic therapy. In addition to earlier colonoscopy, adequate colon preparation, an expert colonoscopist, use of a cap, and use of a water-jet scope have been suggested to improve the rate of SRH identification in patients with diverticular bleeding[39,50]. Because more than half of SRH has been reported to locate in the right colon (71%)[51], cecal intubation with adequate colon preparation is required, even for early colonoscopy. A nasogastric tube can be placed for bowel preparation when patients with LGIB are unable to tolerate rapid colon preparation[39,41].
A systematic review showed high sensitivity (85.2%) and high specificity (92.1%) of CT angiography for diagnosing acute GIB[52]. The American College of Gastroenterology guideline suggest that CT angiography should be considered to localize the bleeding site before angiography or surgery, when the hemodynamics do not permit endoscopic evaluation and/or when patients are unable to tolerate the bowel preparation[13].
The clinical significance of performing CE-CT before colonoscopy has been examined in recent years. Our retrospective study of acute LGIB reported that the detection rate for vascular lesions was higher for colonoscopy following CT than for colonoscopy alone (35.7% vs 20.6%, P = 0.01), leading to more endoscopic therapies (34.9% vs 13.4%, P < 0.01)[53].
Furthermore, several studies have focused on the association between extravasation on CT and definitive diverticular bleeding on colonoscopy (Table 4). The colonoscopic detection rate of bleeding diverticula is significantly higher in patients with extravasation on CT than in those without (60%-76% vs 18%-31%)[54-56], suggesting that extravasation on CT is a reasonable indication for urgent colonoscopy to detect SRH. However, CT is not recommended for all cases due to the low rate of positive extravasation (15%-25%) documented in prospective studies of diverticular bleeding[56,57]. The intermittent nature of diverticular bleeding can reduce the sensitivity of CT for diagnosing diverticular bleeding. A prospective multicenter study suggested that patients who can be examined within 4 h of the last hematochezia would be candidates for urgent CT, because sensitivity is higher in this group than in those examined after 4 h (64.7% vs 33.3%, P < 0.01)[56].
| Study | Study design | Sample size1 | Detection rate of extravasation on CT (%) | SRH detection rate on CS after extravasation on CT (%) | SRH detection rate on CS after no extravasation on CT (%) | Predictors for extravasation on CT |
| Obana et al[57] | Prospective | 52 | 15 | 50 | 36 | History of diverticular bleeding |
| Within 2 h of last hematochezia | ||||||
| Nakatsu et al[54] | Retrospective | 346 | 30 | 68 | 20 | - |
| Nagata et al[53] | Retrospective | 77 | 31 | 63 | 38 | History of diverticular bleeding |
| Sugiyama et al[55] | Retrospective | 55 | 36 | 60 | 31 | - |
| Wada et al[118] | Retrospective | 100 | 23 | 70 | - | - |
| Umezawa et al[56] | Prospective | 202 | 25 | 76 | 18 | Within 4 h of last hematochezia |
The major advantage of angiography and embolization is that it can control severe bleeding without bowel preparation. A systematic review reported that super-selective angiographic embolization achieves immediate hemostasis in 40%-100% of diverticular bleeding with occasional rebleeding (15%)[58]. The disadvantages of angiography and embolization include the requirement for active bleeding and the risk of bowel ischemia and contrast-induced nephropathic complications. The rate of bowel ischemia following embolization was 1%-4% in recent studies[59,60]. The LGIB guideline recommends that this intervention should be reserved for patients with very brisk, ongoing bleeding who do not respond adequately to hemodynamic resuscitation efforts and are unlikely to tolerate bowel preparation and early colonoscopy[13].
Angiography localizes the LGIB source in 24%-70% of cases[59,61]. Angiography requires blood loss rates > 0.5 mL/min to localize a bleeding site[62].Transfusion of > 5 units of red blood cells or 4 units of fresh frozen plasma within 24 h, hemodynamic instability at the time of angiography, and older age are predictors of a positive angiography[63,64]. In addition, CT angiography may be useful as a noninvasive diagnostic tool before angiography, because it is more sensitive than transcatheter angiography and identifies bleeding at rates of 0.3 mL/min[65].
In a retrospective study of colonic diverticular bleeding with SRH on colonoscopy, the rate of interventional radiology and/or surgery due to failure of repeated colonoscopic hemostasis was higher for bleeding from the ascending colon (19%) than from other parts of the colon (0)[51]. Therefore, patients with bleeding from the ascending colon have a higher risk of being transferred for interventional radiology after colonoscopy.
Studies on surgery for acute LGIB have recently decreased, probably because of advances in endoscopic hemostasis and interventional radiology. The complication and mortality rates of surgery for acute LGIB are as high as 60% and 16%, respectively[66]. Given these high rates, surgery should be reserved for patients with brisk, ongoing LGIB. Indications for emergency surgery for severe LGIB include (1) the bleeding source has been clearly identified but non-surgical interventions have failed, and ii) continued bleeding (6 units of red blood cells transfused) and the lack of a diagnosis despite a thorough work-up using endoscopic and radiographic modalities[48,67].
Localizing the bleeding lesion before surgical resection is important to prevent rebleeding after surgery from an unresected culprit lesion, and to prevent excess mortality after a blind total colectomy. In previous studies of surgical management for acute LGIB[68-71], the rebleeding rate was higher after a limited colonic resection (4%-18%) than after a total colonic resection (0-4%). In most of these studies[68-70], the mortality rate was lower after limited colonic resection (7%-22%) than after total colonic resection (20%-40%).
High-dose barium impaction therapy using concentrated (200%) barium sulphate for diverticular bleeding has been reported. Evidence of the effectiveness of initial hemostasis is poor, due to the studies being case reports or case series. Nevertheless, these reports suggest that this therapy may have advantages for hemostasis in patients with uncontrolled or recurrent presumptive diverticular bleeding[72-75]. Novel barium impaction therapy using an enteroscopic overtube with a balloon has been reported for diverticular bleeding in the right-sided colon, and can be used to apply sufficient barium pressure to the deep colon[76].
The effectiveness of barium impaction therapy with respect to long-term prevention of rebleeding was demonstrated in an RCT[77]. The hazard ratio (HR) of rebleeding in the barium group, comparing barium therapy to conservative therapy after spontaneous cessation of diverticular bleeding, was 0.34 (95%CI: 0.12-0.98).
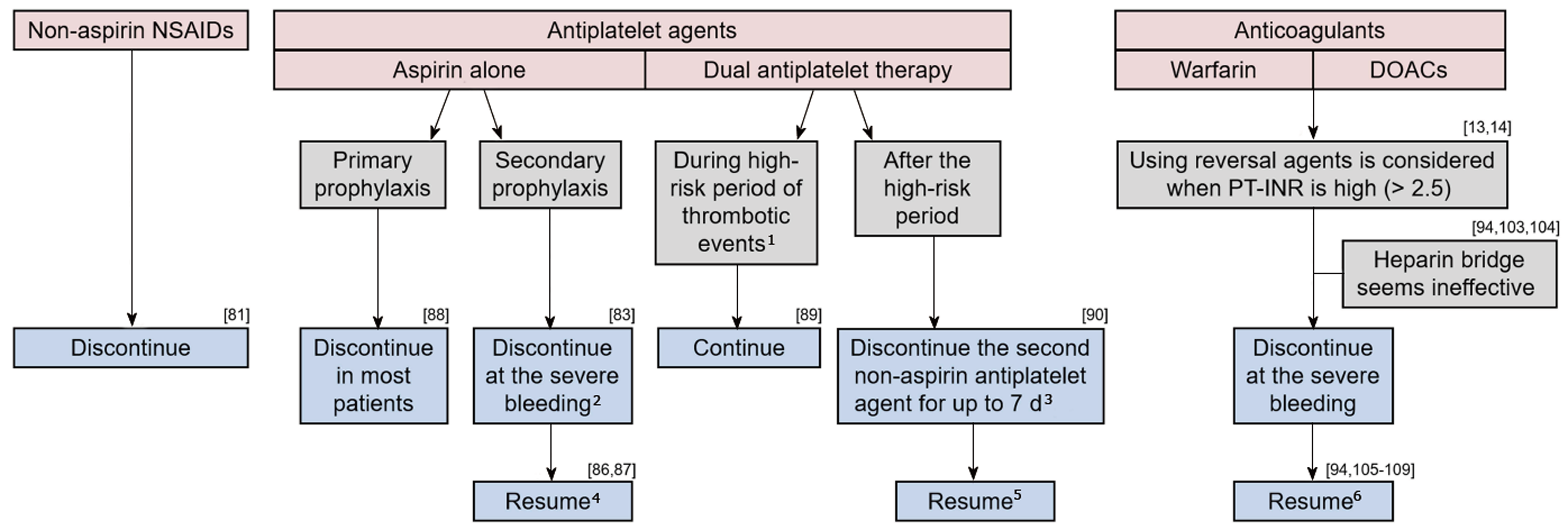
The management of medication use in the LGIB setting requires considering the risks of ongoing/recurrent bleeding and thromboembolic events (Figure 1). Cessation of these agents can be considered in patients on antithrombotic agents with life-threatening or serious bleeding. Although there are few data to guide the timing of the resumption of antithrombotic agents, current guidelines recommend resumption as soon as hemostasis is achieved[13,14]. A multidisciplinary approach involving cardiology, neurology, hematology, and gastroenterology is necessary, particularly for managing patients taking dual antiplatelet agents or anticoagulants.
Previous studies have indicated that NSAIDs increase the risk of both event and recurrence of LGIB[11,78-80]. In a retrospective cohort study of 342 patients with LGIB, the HR of NSAID use for recurrence was 2.0 (95%CI: 1.2-3.3)[11]. In a prospective study of 132 patients with diverticular bleeding, the recurrence rate at 12 mo was significantly higher in patients who continued NSAID use (77%) than in those who discontinued use (9%)[81]. Therefore, non-aspirin NSAIDs should be discontinued after acute LGIB, particularly in cases of diverticular bleeding. Unlike UGIB, changing from a non-selective NSAID to a cyclooxygenase-2 (COX-2) selective NSAID might be ineffective for preventing recurrence, because COX-2-selective and -non-selective agents increase the risk of LGIB[79,82].
Antiplatelet agents increase the risk of both event and recurrence of LGIB[11,79,80,83]. The risk of LGIB with antiplatelet agents use is approximately three times that for UGIB[84,85], probably because LGIB lacks prophylactic measures, such as H. pylori eradication and PPIs.
Available data on the influence of discontinuing aspirin in the GIB setting are as follows. A retrospective cohort study of patients with LGIB showed that the rate of cardiovascular events was significantly higher in those who discontinued aspirin (37%) than in those who continued the drug (23%), while the rate of recurrent LGIB was lower in the former cohort (7%) than in the latter cohort (19%) within 5 years[86]. In an RCT of peptic-ulcer bleeding, 60 d mortality was significantly higher in patients who discontinued aspirin after endoscopic therapy than in those who continued; the rate of rebleeding was not different between the groups[87]. Based on this evidence, aspirin for secondary prophylaxis in patients with established cardiovascular disease should not be interrupted to prevent thrombotic events in the LGIB setting. However, aspirin as the primary prophylaxis for patients who are not at high risk of cardiovascular events had little effect (0.07% absolute risk reduction)[88] and should be discontinued after LGIB.
The influence of short-term drug interruption in single antiplatelet users (aspirin or other antiplatelet agents) has not been determined. No difference in in-hospital rebleeding was observed in a retrospective study comparing patients who had their antiplatelet drug stopped for < 5 d with those who continued it throughout their admission[83]. In that study, cardiovascular events were too few to allow meaningful comparison.
There are some data that can guide the management of dual antiplatelet therapy. The risk of myocardial infarction and death after discontinuing dual antiplatelet therapy is high during the first 30 d following coronary stenting and during the first 90 d following acute coronary syndrome[89]. Such patients are advised to continue dual therapy. In contrast, discontinuing the second non-aspirin antiplatelet agent for up to 7 d is allowed for patients with more distant coronary stenting or coronary syndrome, because it seems to carry a relatively low risk as long as aspirin is continued[90].
Anticoagulants are classified into warfarin and DOACs. Two types of DOACs are currently available: thrombin inhibitors (dabigatran) and coagulation factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Current endoscopic and LGIB guidelines do not discuss the role of a heparin bridge sufficiently, nor management of DOACs in the acute GIB setting[13,14]. Evidence is mainly based on studies of UGIB or all types of GIB.
Prothrombin time-international normalized ratio and the reverse method: Guidelines[13,14] recommend INR < 2.5 as being reasonable for endoscopy in the acute GIB setting, based on reports that a moderate elevation in INR does not increase the risk of rebleeding following endoscopic therapy for nonvariceal UGIB[91-93]. Guidelines also recommend using reversal agents before endoscopy for patients with an INR > 2.5, but the evidence for this is not well-established. Indeed, some retrospective studies found that a higher INR does not increase the rebleeding rate in LGIB[94] or all types of GIB[95]. Thus, an elevated INR appears not to carry a risk of rebleeding. However, an elevated INR at onset has been reported to be a predictor of thromboembolism within 90 d of endoscopy for all GIB (INR > 2.5, OR: 7.9)[94], and of mortality for nonvariceal UGIB (INR > 1.5, OR: 5.6)[96]. This is presumably because INR is an indicator of underlying comorbid diseases. In a study of all types of GIB, other factors related to anticoagulant management, such as the difference in onset and pre-endoscopic INR, reversal agent use, and anticoagulant interruption, were associated with thromboembolism[94]. Therefore, it might be unnecessary to actively reduce the INR. Rather, early endoscopy without using a reversal agent or interrupting anticoagulant therapy may be warranted for acute GIB.
Reversal of the anticoagulant effect should be considered for ongoing severe bleeding via intravenous vitamin K, fresh frozen plasma, or prothrombin complex concentrate (PCC) for warfarin users[97,98], and via oral charcoal, hemodialysis, idarucizumab, or PCC for DOAC users[99-102]. Oral charcoal is considered if a DOAC was taken within 2 h. Hemodialysis or idarucizumab is considered for dabigatran users. The effect of PCC on bleeding of DOAC users has not been established.
Heparin Bridge: Previous reports suggest that a heparin bridge might be ineffective in the acute GIB setting. A heparin bridge did not significantly alter the risk of rebleeding or thromboembolism in a recent retrospective study of patients with GIB[94]. In an RCT of warfarin users undergoing invasive procedures, the heparin bridge group suffered from more major bleeding than the non-bridged group, without a difference in the thromboembolism rate during the periprocedural period[103]. Furthermore, a similar result was found in a prospective observational study of DOAC users undergoing interventional procedures[104].
Resumption of anticoagulants: A meta-analysis concluded that resuming anticoagulants reduces the rate of thrombotic events in patients with disrupted use of anticoagulants due to GIB (HR: 0.68, 95%CI: 0.52-0.88), and mortality (HR: 0.76, 95%CI: 0.66-0.88), without significantly increasing the rebleeding rate (HR: 1.20, 95%CI: 0.97-1.48)[105]. This result was consistent with other reports[106-108]. Studies that compared warfarin and DOAC users reported that the rate of thrombotic events was similar between the two groups, during the 90 d after GIB[94] and during the anticoagulant-interrupted period[109]. A retrospective cohort study on DOAC users reported that the rate of thromboembolism within 90 d of GIB did not differ between those who resumed DOAC and those who did not[110]. In that study, a history of venous thromboembolism was associated with thromboembolism events (HR: 3.30, 95%CI: 1.29-7.38).
The optimal duration before restarting anticoagulants after an episode of GIB remains uncertain. In a retrospective cohort study, the HRs of rebleeding, thromboembolism, and mortality in patients who resumed warfarin within 7 d were 3.27 (95%CI: 1.82-5.91), 0.76 (95%CI: 0.37-1.59), and 0.56 (95%CI: 0.33-0.93), respectively, compared with patients who resumed warfarin after 1 mo[107].
The bleeding risk of individual anticoagulants should be considered, when resuming anticoagulants in patients with high-risk GIB. Changing to apixaban, or reducing the dose of dabigatran to 110 mg b.i.d may reduce rebleeding in GIB patients taking warfarin, dabigatran (150 mg b.i.d) or rivaroxaban[111-115]. The HAS-BLED is a scoring system to evaluate bleeding risk among anticoagulants users[116]. However, the main outcome of the score is composite bleeding events, including intracerebral hemorrhage and GIB. One study focused specifically on the risk of acute GIB in anticoagulant users, and developed a new scoring model for acute GIB risk based on five factors (no PPI use, chronic kidney disease, chronic obstructive pulmonary disease, history of peptic ulcer disease, and liver cirrhosis). The c-statistic of the new score (0.65) was superior to that of the HAS-BLED score (0.57) for predicting acute GIB[117,118]. The utility of these scoring systems for predicting re-bleeding, and the strategy of changing anticoagulants would be important topics of study.
This literature review has summarized evidence for the initial management of acute LGIB. Assessing various clinical factors, including comorbidities, medication use, presenting symptoms, vital signs, and laboratory data is useful for risk stratification of severe LGIB. Early timing of colonoscopy could improve identification of the bleeding source and the rate of endoscopic intervention. CE-CT before colonoscopy may support identification, particularly for patients who can be examined immediately after the last hematochezia. How to deal with antithrombotic agents after hemostasis should be carefully considered. Further investigations are required to predict the need for early colonoscopy and hemostatic intervention in patients with LGIB.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Manguso F, Sharma V S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Gunjan D, Sharma V, Rana SS, Bhasin DK. Small bowel bleeding: a comprehensive review. Gastroenterol Rep (Oxf). 2014;2:262-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (2)] |
| 2. | Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Alonso-Abreu I, Perez-Aisa MA, Perez-Gisbert J, Bujanda L, Castro M, Muñoz M, Rodrigo L, Calvet X, Del-Pino D, Garcia S. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104:1633-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 379] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 3. | Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Quintero E, Perez-Aisa MA, Gisbert JP, Bujanda L, Castro M, Muñoz M, Del-Pino MD, Garcia S, Calvet X. The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011;33:585-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Nagata N, Niikura R, Aoki T, Shimbo T, Itoh T, Goda Y, Suda R, Yano H, Akiyama J, Yanase M, Mizokami M, Uemura N. Increase in colonic diverticulosis and diverticular hemorrhage in an aging society: lessons from a 9-year colonoscopic study of 28,192 patients in Japan. Int J Colorectal Dis. 2014;29:379-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. 2012;107:1190-1195; quiz 1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 207] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 6. | Hreinsson JP, Gumundsson S, Kalaitzakis E, Björnsson ES. Lower gastrointestinal bleeding: incidence, etiology, and outcomes in a population-based setting. Eur J Gastroenterol Hepatol. 2013;25:37-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 106] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 7. | Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6:1004-1010; quiz 955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Niikura R, Yasunaga H, Yamaji Y, Horiguchi H, Fushimi K, Yamada A, Hirata Y, Koike K. Factors affecting in-hospital mortality in patients with lower gastrointestinal tract bleeding: a retrospective study using a national database in Japan. J Gastroenterol. 2015;50:533-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Oakland K, Guy R, Uberoi R, Hogg R, Mortensen N, Murphy MF, Jairath V; UK Lower GI Bleeding Collaborative. Acute lower GI bleeding in the UK: patient characteristics, interventions and outcomes in the first nationwide audit. Gut. 2018;67:654-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Anthony T, Penta P, Todd RD, Sarosi GA, Nwariaku F, Rege RV. Rebleeding and survival after acute lower gastrointestinal bleeding. Am J Surg. 2004;188:485-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Aoki T, Nagata N, Niikura R, Shimbo T, Tanaka S, Sekine K, Kishida Y, Watanabe K, Sakurai T, Yokoi C, Akiyama J, Yanase M, Mizokami M, Uemura N. Recurrence and mortality among patients hospitalized for acute lower gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2015;13:488-494.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Mohammed Ilyas MI, Szilagy EJ. Management of Diverticular Bleeding: Evaluation, Stabilization, Intervention, and Recurrence of Bleeding and Indications for Resection after Control of Bleeding. Clin Colon Rectal Surg. 2018;31:243-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Strate LL, Gralnek IM. ACG Clinical Guideline: Management of Patients With Acute Lower Gastrointestinal Bleeding. Am J Gastroenterol. 2016;111:459-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 255] [Article Influence: 31.9] [Reference Citation Analysis (1)] |
| 14. | ASGE Standards of Practice Committee, Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fisher DA, Fonkalsrud L, Hwang JH, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Shergill AK, Wang A, Cash BD, DeWitt JM. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83:3-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 388] [Article Influence: 48.5] [Reference Citation Analysis (1)] |
| 15. | Srygley FD, Gerardo CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307:1072-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Sittichanbuncha Y, Senasu S, Thongkrau T, Keeratikasikorn C, Sawanyawisuth K. How to differentiate sites of gastrointestinal bleeding in patients with hematochezia by using clinical factors? Gastroenterol Res Pract. 2013;2013:265076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Gralnek IM, Neeman Z, Strate LL. Acute Lower Gastrointestinal Bleeding. N Engl J Med. 2017;376:1054-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Morkar DN, Hazare S. Spectrum of the causes of lower gastrointestinal bleeding in geriatric patients in tertiary care hospital. J Sci Soc. 2017;148-151 [doi:10.4103/jss.JSS_17_16]. [Cited in This Article: ] |
| 19. | Nagata N, Niikura R, Aoki T, Shimbo T, Kishida Y, Sekine K, Tanaka S, Okubo H, Watanabe K, Sakurai T, Yokoi C, Akiyama J, Yanase M, Mizokami M, Uemura N. Natural history of outpatient-onset ischemic colitis compared with other lower gastrointestinal bleeding: a long-term cohort study. Int J Colorectal Dis. 2015;30:243-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Kollef MH, O’Brien JD, Zuckerman GR, Shannon W. BLEED: a classification tool to predict outcomes in patients with acute upper and lower gastrointestinal hemorrhage. Crit Care Med. 1997;25:1125-1132. [PubMed] [Cited in This Article: ] |
| 21. | Strate LL, Orav EJ, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Arch Intern Med. 2003;163:838-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Das A, Ben-Menachem T, Cooper GS, Chak A, Sivak MV Jr, Gonet JA, Wong RC. Prediction of outcome in acute lower-gastrointestinal haemorrhage based on an artificial neural network: internal and external validation of a predictive model. Lancet. 2003;362:1261-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Velayos FS, Williamson A, Sousa KH, Lung E, Bostrom A, Weber EJ, Ostroff JW, Terdiman JP. Early predictors of severe lower gastrointestinal bleeding and adverse outcomes: a prospective study. Clin Gastroenterol Hepatol. 2004;2:485-490. [PubMed] [Cited in This Article: ] |
| 24. | Aoki T, Nagata N, Shimbo T, Niikura R, Sakurai T, Moriyasu S, Okubo H, Sekine K, Watanabe K, Yokoi C, Yanase M, Akiyama J, Mizokami M, Uemura N. Development and Validation of a Risk Scoring System for Severe Acute Lower Gastrointestinal Bleeding. Clin Gastroenterol Hepatol. 2016;14:1562-1570.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Newman J, Fitzgerald JE, Gupta S, von Roon AC, Sigurdsson HH, Allen-Mersh TG. Outcome predictors in acute surgical admissions for lower gastrointestinal bleeding. Colorectal Dis. 2012;14:1020-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Chong V, Hill AG, MacCormick AD. Accurate triage of lower gastrointestinal bleed (LGIB) - A cohort study. Int J Surg. 2016;25:19-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Oakland K, Jairath V, Uberoi R, Guy R, Ayaru L, Mortensen N, Murphy MF, Collins GS. Derivation and validation of a novel risk score for safe discharge after acute lower gastrointestinal bleeding: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:635-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 28. | Sengupta N, Tapper EB. Derivation and Internal Validation of a Clinical Prediction Tool for 30-Day Mortality in Lower Gastrointestinal Bleeding. Am J Med. 2017;130:601.e1-601.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321. [PubMed] [Cited in This Article: ] |
| 30. | Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 666] [Cited by in F6Publishing: 612] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 31. | Baradarian R, Ramdhaney S, Chapalamadugu R, Skoczylas L, Wang K, Rivilis S, Remus K, Mayer I, Iswara K, Tenner S. Early intensive resuscitation of patients with upper gastrointestinal bleeding decreases mortality. Am J Gastroenterol. 2004;99:619-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Kwan I, Bunn F, Chinnock P, Roberts I. Timing and volume of fluid administration for patients with bleeding. Cochrane Database Syst Rev. 2014;CD002245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013;CD000567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 34. | Odutayo A, Desborough MJ, Trivella M, Stanley AJ, Dorée C, Collins GS, Hopewell S, Brunskill SJ, Kahan BC, Logan RF, Barkun AN, Murphy MF, Jairath V. Restrictive versus liberal blood transfusion for gastrointestinal bleeding: a systematic review and meta-analysis of randomised controlled trials. Lancet Gastroenterol Hepatol. 2017;2:354-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 35. | Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, Srinivas V, Menegus MA, Marroquin OC, Rao SV, Noveck H, Passano E, Hardison RM, Smitherman T, Vagaonescu T, Wimmer NJ, Williams DO. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964-971.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 36. | Docherty AB, O’Donnell R, Brunskill S, Trivella M, Doree C, Holst L, Parker M, Gregersen M, Pinheiro de Almeida J, Walsh TS, Stanworth SJ. Effect of restrictive versus liberal transfusion strategies on outcomes in patients with cardiovascular disease in a non-cardiac surgery setting: systematic review and meta-analysis. BMJ. 2016;352:i1351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 37. | Razzaghi A, Barkun AN. Platelet transfusion threshold in patients with upper gastrointestinal bleeding: a systematic review. J Clin Gastroenterol. 2012;46:482-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Contreras M. Final statement from the consensus conference on platelet transfusion. Transfusion. 1998;38:796-797. [PubMed] [Cited in This Article: ] |
| 39. | Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 493] [Cited by in F6Publishing: 400] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 40. | Jensen DM, Ohning GV, Kovacs TO, Jutabha R, Ghassemi K, Dulai GS, Machicado GA. Natural history of definitive diverticular hemorrhage based on stigmata of recent hemorrhage and colonoscopic Doppler blood flow monitoring for risk stratification and definitive hemostasis. Gastrointest Endosc. 2016;83:416-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Green BT, Rockey DC, Portwood G, Tarnasky PR, Guarisco S, Branch MS, Leung J, Jowell P. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005;100:2395-2402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 42. | Laine L, Shah A. Randomized trial of urgent vs. elective colonoscopy in patients hospitalized with lower GI bleeding. Am J Gastroenterol. 2010;105:2636-2641; quiz 2642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 43. | Kouanda AM, Somsouk M, Sewell JL, Day LW. Urgent colonoscopy in patients with lower GI bleeding: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:107-117.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Sengupta N, Tapper EB, Feuerstein JD. Early Versus Delayed Colonoscopy in Hospitalized Patients With Lower Gastrointestinal Bleeding: A Meta-Analysis. J Clin Gastroenterol. 2017;51:352-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 45. | Seth A, Khan MA, Nollan R, Gupta D, Kamal S, Singh U, Kamal F, Howden CW. Does Urgent Colonoscopy Improve Outcomes in the Management of Lower Gastrointestinal Bleeding? Am J Med Sci. 2017;353:298-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Niikura R, Nagata N, Yamada A, Doyama H, Shiratori Y, Nishida T, Kiyotoki S, Yada T, Fujita T, Sumiyoshi T, Hasatani K, Mikami T, Honda T, Mabe K, Hara K, Yamamoto K, Takeda M, Takata M, Tanaka M, Shinozaki T, Fujishiro M, Koike K. A multicenter, randomized controlled trial comparing the identification rate of stigmata of recent hemorrhage and rebleeding rate between early and elective colonoscopy in outpatient-onset acute lower gastrointestinal bleeding: study protocol for a randomized controlled trial. Trials. 2018;19:214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Nagata N, Niikura R, Sakurai T, Shimbo T, Aoki T, Moriyasu S, Sekine K, Okubo H, Imbe K, Watanabe K, Yokoi C, Yanase M, Akiyama J, Uemura N. Safety and Effectiveness of Early Colonoscopy in Management of Acute Lower Gastrointestinal Bleeding on the Basis of Propensity Score Matching Analysis. Clin Gastroenterol Hepatol. 2016;14:558-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 48. | Strate LL, Naumann CR. The role of colonoscopy and radiological procedures in the management of acute lower intestinal bleeding. Clin Gastroenterol Hepatol. 2010;8:333-343; quiz e44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 49. | Aoki T, Yamada A, Nagata N, Niikura R, Hirata Y, Koike K. External validation of the NOBLADS score, a risk scoring system for severe acute lower gastrointestinal bleeding. PLoS One. 2018;13:e0196514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Niikura R, Nagata N, Aoki T, Shimbo T, Tanaka S, Sekine K, Kishida Y, Watanabe K, Sakurai T, Yokoi C, Yanase M, Akiyama J, Mizokami M, Uemura N. Predictors for identification of stigmata of recent hemorrhage on colonic diverticula in lower gastrointestinal bleeding. J Clin Gastroenterol. 2015;49:e24-e30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Ishii N, Hirata N, Omata F, Itoh T, Uemura M, Matsuda M, Suzuki S, Iizuka Y, Fukuda K, Fujita Y. Location in the ascending colon is a predictor of refractory colonic diverticular hemorrhage after endoscopic clipping. Gastrointest Endosc. 2012;76:1175-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | García-Blázquez V, Vicente-Bártulos A, Olavarria-Delgado A, Plana MN, van der Winden D, Zamora J; EBM-Connect Collaboration. Accuracy of CT angiography in the diagnosis of acute gastrointestinal bleeding: systematic review and meta-analysis. Eur Radiol. 2013;23:1181-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 53. | Nagata N, Niikura R, Aoki T, Moriyasu S, Sakurai T, Shimbo T, Shinozaki M, Sekine K, Okubo H, Watanabe K, Yokoi C, Yanase M, Akiyama J, Uemura N. Role of urgent contrast-enhanced multidetector computed tomography for acute lower gastrointestinal bleeding in patients undergoing early colonoscopy. J Gastroenterol. 2015;50:1162-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Nakatsu S, Yasuda H, Maehata T, Nomoto M, Ohinata N, Hosoya K, Ishigooka S, Ozawa S, Ikeda Y, Sato Y, Suzuki M, Kiyokawa H, Yamamoto H, Itoh F. Urgent computed tomography for determining the optimal timing of colonoscopy in patients with acute lower gastrointestinal bleeding. Intern Med. 2015;54:553-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Sugiyama T, Hirata Y, Kojima Y, Kanno T, Kimura M, Okuda Y, Haneda K, Ikeuchi H, Morikawa T, Mochizuki H, Takada H, Sobue S. Efficacy of Contrast-enhanced Computed Tomography for the Treatment Strategy of Colonic Diverticular Bleeding. Intern Med. 2015;54:2961-2967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Umezawa S, Nagata N, Arimoto J, Uchiyama S, Higurashi T, Nakano K, Ishii N, Sakurai T, Moriyasu S, Takeda Y, Nagase H, Komatsu H, Nakajima A, Mizuki A. Contrast-enhanced CT for Colonic Diverticular Bleeding before Colonoscopy: A Prospective Multicenter Study. Radiology. 2018;288:755-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Obana T, Fujita N, Sugita R, Hirasawa D, Sugawara T, Harada Y, Oohira T, Maeda Y, Koike Y, Suzuki K, Yamagata T, Kusaka J, Masu K. Prospective evaluation of contrast-enhanced computed tomography for the detection of colonic diverticular bleeding. Dig Dis Sci. 2013;58:1985-1990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Khanna A, Ognibene SJ, Koniaris LG. Embolization as first-line therapy for diverticulosis-related massive lower gastrointestinal bleeding: evidence from a meta-analysis. J Gastrointest Surg. 2005;9:343-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 59. | Ali M, Ul Haq T, Salam B, Beg M, Sayani R, Azeemuddin M. Treatment of nonvariceal gastrointestinal hemorrhage by transcatheter embolization. Radiol Res Pract. 2013;2013:604328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Yata S, Ihaya T, Kaminou T, Hashimoto M, Ohuchi Y, Umekita Y, Ogawa T. Transcatheter arterial embolization of acute arterial bleeding in the upper and lower gastrointestinal tract with N-butyl-2-cyanoacrylate. J Vasc Interv Radiol. 2013;24:422-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Yi WS, Garg G, Sava JA. Localization and definitive control of lower gastrointestinal bleeding with angiography and embolization. Am Surg. 2013;79:375-380. [PubMed] [Cited in This Article: ] |
| 62. | Krüger K, Heindel W, Dölken W, Landwehr P, Lackner K. Angiographic detection of gastrointestinal bleeding. An experimental comparison of conventional screen-film angiography and digital subtraction angiography. Invest Radiol. 1996;31:451-457. [PubMed] [Cited in This Article: ] |
| 63. | Lee L, Iqbal S, Najmeh S, Fata P, Razek T, Khwaja K. Mesenteric angiography for acute gastrointestinal bleed: predictors of active extravasation and outcomes. Can J Surg. 2012;55:382-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Rasuli P, Doumit J, Boulos M, Rizk C, Doumit G. Factors influencing the yield of mesenteric angiography in lower gastrointestinal bleed. World J Radiol. 2014;6:218-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Kuhle WG, Sheiman RG. Detection of active colonic hemorrhage with use of helical CT: findings in a swine model. Radiology. 2003;228:743-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 66. | Czymek R, Kempf A, Roblick UJ, Bader FG, Habermann J, Kujath P, Bruch HP, Fischer F. Surgical treatment concepts for acute lower gastrointestinal bleeding. J Gastrointest Surg. 2008;12:2212-2220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Farrell JJ, Friedman LS. Review article: the management of lower gastrointestinal bleeding. Aliment Pharmacol Ther. 2005;21:1281-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 68. | Britt LG, Warren L, Moore OF 3rd. Selective management of lower gastrointestinal bleeding. Am Surg. 1983;49:121-125. [PubMed] [Cited in This Article: ] |
| 69. | Leitman IM, Paull DE, Shires GT 3rd. Evaluation and management of massive lower gastrointestinal hemorrhage. Ann Surg. 1989;209:175-180. [PubMed] [Cited in This Article: ] |
| 70. | Parkes BM, Obeid FN, Sorensen VJ, Horst HM, Fath JJ. The management of massive lower gastrointestinal bleeding. Am Surg. 1993;59:676-678. [PubMed] [Cited in This Article: ] |
| 71. | Farner R, Lichliter W, Kuhn J, Fisher T. Total colectomy versus limited colonic resection for acute lower gastrointestinal bleeding. Am J Surg. 1999;178:587-591. [PubMed] [Cited in This Article: ] |
| 72. | Matsuhashi N, Akahane M, Nakajima A. Barium impaction therapy for refractory colonic diverticular bleeding. AJR Am J Roentgenol. 2003;180:490-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Iwamoto J, Mizokami Y, Shimokobe K, Matsuoka T, Matsuzaki Y. Therapeutic barium enema for bleeding colonic diverticula: four case series and review of the literature. World J Gastroenterol. 2008;14:6413-6417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Pausawasdi N, Al-Hawary M, Higgins PD. Therapeutic high-density barium enema in a case of presumed diverticular hemorrhage. Case Rep Gastroenterol. 2011;5:88-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Niikura R, Nagata N, Yamano K, Shimbo T, Uemura N. High-dose barium impaction therapy is useful for the initial hemostasis and for preventing the recurrence of colonic diverticular bleeding unresponsive to endoscopic clipping. Case Rep Gastrointest Med. 2013;2013:365954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Koga M, Kusano C, Gotoda T, Suzuki S, Sato T, Fukuzawa M, Itoi T, Moriyasu F. Barium impaction therapy with balloon occlusion for deep colonic diverticular bleeding: a three-case series. Endosc Int Open. 2016;4:E560-E563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 77. | Nagata N, Niikura R, Shimbo T, Ishizuka N, Yamano K, Mizuguchi K, Akiyama J, Yanase M, Mizokami M, Uemura N. High-dose barium impaction therapy for the recurrence of colonic diverticular bleeding: a randomized controlled trial. Ann Surg. 2015;261:269-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Yamada A, Sugimoto T, Kondo S, Ohta M, Watabe H, Maeda S, Togo G, Yamaji Y, Ogura K, Okamoto M, Yoshida H, Kawabe T, Kawase T, Omata M. Assessment of the risk factors for colonic diverticular hemorrhage. Dis Colon Rectum. 2008;51:116-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 79. | Nagata N, Niikura R, Aoki T, Shimbo T, Kishida Y, Sekine K, Tanaka S, Okubo H, Watanabe K, Sakurai T, Yokoi C, Akiyama J, Yanase M, Mizokami M, Uemura N. Lower GI bleeding risk of nonsteroidal anti-inflammatory drugs and antiplatelet drug use alone and the effect of combined therapy. Gastrointest Endosc. 2014;80:1124-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 80. | Lanas Á, Carrera-Lasfuentes P, Arguedas Y, García S, Bujanda L, Calvet X, Ponce J, Perez-Aísa Á, Castro M, Muñoz M, Sostres C, García-Rodríguez LA. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin Gastroenterol Hepatol. 2015;13:906-912.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 81. | Nagata N, Niikura R, Aoki T, Shimbo T, Sekine K, Okubo H, Watanabe K, Sakurai T, Yokoi C, Akiyama J, Yanase M, Mizokami M, Uemura N. Impact of discontinuing non-steroidal antiinflammatory drugs on long-term recurrence in colonic diverticular bleeding. World J Gastroenterol. 2015;21:1292-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Laine L, Curtis SP, Langman M, Jensen DM, Cryer B, Kaur A, Cannon CP. Lower gastrointestinal events in a double-blind trial of the cyclo-oxygenase-2 selective inhibitor etoricoxib and the traditional nonsteroidal anti-inflammatory drug diclofenac. Gastroenterology. 2008;135:1517-1525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 83. | Oakland K, Desborough MJ, Murphy MF, Schachter M, Jairath V. Re-bleeding and Mortality After Lower Gastrointestinal Bleeding in Patients Taking Anti-platelets or Anti-coagulants. Clin Gastroenterol Hepatol. 2017;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Casado Arroyo R, Polo-Tomas M, Roncalés MP, Scheiman J, Lanas A. Lower GI bleeding is more common than upper among patients on dual antiplatelet therapy: long-term follow-up of a cohort of patients commonly using PPI co-therapy. Heart. 2012;98:718-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Abraham NS, Hartman C, Richardson P, Castillo D, Street RL Jr, Naik AD. Risk of lower and upper gastrointestinal bleeding, transfusions, and hospitalizations with complex antithrombotic therapy in elderly patients. Circulation. 2013;128:1869-1877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 86. | Chan FK, Leung Ki EL, Wong GL, Ching JY, Tse YK, Au KW, Wu JC, Ng SC. Risks of Bleeding Recurrence and Cardiovascular Events With Continued Aspirin Use After Lower Gastrointestinal Hemorrhage. Gastroenterology. 2016;151:271-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 87. | Sung JJ, Lau JY, Ching JY, Wu JC, Lee YT, Chiu PW, Leung VK, Wong VW, Chan FK. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2010;152:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 88. | Antithrombotic Trialists' (ATT) Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2661] [Cited by in F6Publishing: 2438] [Article Influence: 162.5] [Reference Citation Analysis (0)] |
| 89. | Ho PM, Peterson ED, Wang L, Magid DJ, Fihn SD, Larsen GC, Jesse RA, Rumsfeld JS. Incidence of death and acute myocardial infarction associated with stopping clopidogrel after acute coronary syndrome. JAMA. 2008;299:532-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 90. | Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation. 2009;119:1634-1642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 91. | Choudari CP, Rajgopal C, Palmer KR. Acute gastrointestinal haemorrhage in anticoagulated patients: diagnoses and response to endoscopic treatment. Gut. 1994;35:464-466. [PubMed] [Cited in This Article: ] |
| 92. | Wolf AT, Wasan SK, Saltzman JR. Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol. 2007;102:290-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Shingina A, Barkun AN, Razzaghi A, Martel M, Bardou M, Gralnek I; RUGBE Investigators. Systematic review: the presenting international normalised ratio (INR) as a predictor of outcome in patients with upper nonvariceal gastrointestinal bleeding. Aliment Pharmacol Ther. 2011;33:1010-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Nagata N, Sakurai T, Moriyasu S, Shimbo T, Okubo H, Watanabe K, Yokoi C, Yanase M, Akiyama J, Uemura N. Impact of INR monitoring, reversal agent use, heparin bridging, and anticoagulant interruption on rebleeding and thromboembolism in acute gastrointestinal bleeding. PLoS One. 2017;12:e0183423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Rubin TA, Murdoch M, Nelson DB. Acute GI bleeding in the setting of supratherapeutic international normalized ratio in patients taking warfarin: endoscopic diagnosis, clinical management, and outcomes. Gastrointest Endosc. 2003;58:369-373. [PubMed] [Cited in This Article: ] |
| 96. | Jairath V, Kahan BC, Stanworth SJ, Logan RF, Hearnshaw SA, Travis SP, Palmer KR, Murphy MF. Prevalence, management, and outcomes of patients with coagulopathy after acute nonvariceal upper gastrointestinal bleeding in the United Kingdom. Transfusion. 2013;53:1069-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD; ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521-e643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 867] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 98. | Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e152S-e184S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 847] [Cited by in F6Publishing: 875] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 99. | van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A. Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in F6Publishing: 1091] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 100. | Khadzhynov D, Wagner F, Formella S, Wiegert E, Moschetti V, Slowinski T, Neumayer HH, Liesenfeld KH, Lehr T, Härtter S, Friedman J, Peters H, Clemens A. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost. 2013;109:596-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 101. | Pollack CV Jr, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, Kreuzer J, Levy JH, Sellke FW, Stangier J, Steiner T, Wang B, Kam CW, Weitz JI. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373:511-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1176] [Cited by in F6Publishing: 1053] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 102. | Weitz JI, Pollack CV Jr. Practical management of bleeding in patients receiving non-vitamin K antagonist oral anticoagulants. Thromb Haemost. 2015;114:1113-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 103. | Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK, Kong DF, Schulman S, Turpie AG, Hasselblad V, Ortel TL; BRIDGE Investigators. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med. 2015;373:823-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 695] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 104. | Beyer-Westendorf J, Gelbricht V, Förster K, Ebertz F, Köhler C, Werth S, Kuhlisch E, Stange T, Thieme C, Daschkow K, Weiss N. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J. 2014;35:1888-1896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 243] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 105. | Chai-Adisaksopha C, Hillis C, Monreal M, Witt DM, Crowther M. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114:819-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 106. | Witt DM, Delate T, Garcia DA, Clark NP, Hylek EM, Ageno W, Dentali F, Crowther MA. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172:1484-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 107. | Qureshi W, Mittal C, Patsias I, Garikapati K, Kuchipudi A, Cheema G, Elbatta M, Alirhayim Z, Khalid F. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. 2014;113:662-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 108. | Sengupta N, Feuerstein JD, Patwardhan VR, Tapper EB, Ketwaroo GA, Thaker AM, Leffler DA. The risks of thromboembolism vs. recurrent gastrointestinal bleeding after interruption of systemic anticoagulation in hospitalized inpatients with gastrointestinal bleeding: a prospective study. Am J Gastroenterol. 2015;110:328-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 109. | Sherwood MW, Douketis JD, Patel MR, Piccini JP, Hellkamp AS, Lokhnygina Y, Spyropoulos AC, Hankey GJ, Singer DE, Nessel CC, Mahaffey KW, Fox KA, Califf RM, Becker RC; ROCKET AF Investigators. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;129:1850-1859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 110. | Sengupta N, Marshall AL, Jones BA, Ham S, Tapper EB. Rebleeding vs Thromboembolism After Hospitalization for Gastrointestinal Bleeding in Patients on Direct Oral Anticoagulants. Clin Gastroenterol Hepatol. 2018;16:1893-1900.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 111. | Desai J, Kolb JM, Weitz JI, Aisenberg J. Gastrointestinal bleeding with the new oral anticoagulants--defining the issues and the management strategies. Thromb Haemost. 2013;110:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 112. | Lip GYH, Lane DA. Matching the NOAC to the Patient: Remember the Modifiable Bleeding Risk Factors. J Am Coll Cardiol. 2015;66:2282-2284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 113. | Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3181] [Cited by in F6Publishing: 3290] [Article Influence: 329.0] [Reference Citation Analysis (0)] |
| 114. | Abraham NS, Noseworthy PA, Yao X, Sangaralingham LR, Shah ND. Gastrointestinal Safety of Direct Oral Anticoagulants: A Large Population-Based Study. Gastroenterology. 2017;152:1014-1022.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 115. | Noseworthy PA, Yao X, Abraham NS, Sangaralingham LR, McBane RD, Shah ND. Direct Comparison of Dabigatran, Rivaroxaban, and Apixaban for Effectiveness and Safety in Nonvalvular Atrial Fibrillation. Chest. 2016;150:1302-1312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 116. | Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2971] [Cited by in F6Publishing: 3113] [Article Influence: 222.4] [Reference Citation Analysis (0)] |
| 117. | Shimomura A, Nagata N, Shimbo T, Sakurai T, Moriyasu S, Okubo H, Watanabe K, Yokoi C, Akiyama J, Uemura N. New predictive model for acute gastrointestinal bleeding in patients taking oral anticoagulants: A cohort study. J Gastroenterol Hepatol. 2018;33:164-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 118. | Wada M, Kato M, Hirai Y, Kubosawa Y, Sunata Y, Abe K, Hirata T, Takada Y, Banno S, Takatori Y, Kinoshita S, Mori H, Takabayashi K, Kikuchi M, Kikuchi M, Suzuki M, Kanai T, Uraoka T. Initial Management of Colonic Diverticular Bleeding: Observational Study. Digestion. 2018;98:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |