Published online Mar 7, 2018. doi: 10.3748/wjg.v24.i9.1056
Peer-review started: October 28, 2017
First decision: November 22, 2017
Revised: December 4, 2017
Accepted: January 24, 2018
Article in press: January 24, 2018
Published online: March 7, 2018
Processing time: 52 Days and 17.4 Hours
Esophageal atresia (EA) is one of the most common congenital digestive malformations and requires surgical correction early in life. Dedicated centers have reported survival rates up to 95%. The most frequent comorbidities after EA repair are dysphagia (72%) and gastroesophageal reflux (GER) (67%). Chronic GER after EA repair might lead to mucosal damage, esophageal stricturing, Barrett’s esophagus and eventually esophageal adenocarcinoma. Several long-term follow-up studies found an increased risk of Barrett’s esophagus and esophageal carcinoma in EA patients, both at a relatively young age. Given these findings, the recent ESPGHAN-NASPGHAN guideline recommends routine endoscopy in adults born with EA. We report a series of four EA patients who developed a carcinoma of the gastrointestinal tract: three esophageal carcinoma and one colorectal carcinoma in a colonic interposition. These cases emphasize the importance of lifelong screening of the upper gastrointestinal tract in EA patients.
Core tip: Esophageal atresia (EA) is a common congenital malformation that requires surgical correction early in life. Improved perioperative care and surgical techniques have increased the survival rate. Gastroesophageal reflux and stasis are common after surgical repair and may be associated with an increased esophageal cancer risk. However, data on incidence and risk factors for esophageal carcinogenesis after EA repair are scarce. The recent ESPGHAN-NASPGHAN guideline recommends routine endoscopy in adults born with EA. Here we report four cancer cases at a relatively young age after EA repair: three esophageal carcinoma and one colorectal carcinoma in a colonic interposition.
- Citation: Vergouwe FW, Gottrand M, Wijnhoven BP, IJsselstijn H, Piessen G, Bruno MJ, Wijnen RM, Spaander MC. Four cancer cases after esophageal atresia repair: Time to start screening the upper gastrointestinal tract. World J Gastroenterol 2018; 24(9): 1056-1062
- URL: https://www.wjgnet.com/1007-9327/full/v24/i9/1056.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i9.1056
With a prevalence of 2.43 per 10000 births, esophageal atresia (EA) with or without a tracheoesophageal fistula (TEF) is one of the most common congenital digestive malformations[1]. Surgical correction needs to be performed shortly after birth. Due to advanced surgical techniques and improved perioperative care, survival rate has increased up to 95% in dedicated centers[2,3]. Follow-up studies have shown that most EA patients have a favourable long-term outcome despite persistant digestive and respiratory problems. Common gastrointestinal symptoms after EA repair are dysphagia and gastroesophageal reflux (GER) in up to 72% and 67% of the patients, respectively[4,5]. Chronic GER after EA repair might lead to mucosal damage, esophageal stricturing, Barrett’s esophagus and eventually esophageal adenocarcinoma (EAC)[5-8]. Data on incidence and risk factors for esophageal carcinogenesis after EA repair are scarce[8-10]. The recent ESPGHAN-NASPGHAN guideline recommends routine endoscopy in adults born with EA[11]. Until now, eight cases of esophageal cancer in young EA patients have been described: five esophageal squamous cell carcinoma (ESCC) and three EAC[10,12-15]. Here we report four EA patients who developed a carcinoma of the gastrointestinal tract: three esophageal carcinoma and one colorectal carcinoma in a colonic interposition. These cases emphasize the importance of lifelong screening and surveillance of the upper gastrointestinal tract in EA patients.
Patient A presented for the first time with esophageal carcinoma at age 45 years. He was born with EA Gross type C (with a distal TEF) which was surgically repaired with closing of the fistula and end-to-end anastomosis of the esophagus. In childhood he had undergone a number of esophageal dilations to treat an anastomotic stricture.
At the age of 37 years he developed progressive dysphagia. Upper endoscopy showed proximal esophagitis and a stenotic anastomosis, which then was dilated. No biopsies were taken. Eight years later, dysphagia for solid foods reoccurred with complaints of heartburn and weight loss of 6 kg in six months (BMI 21.6 kg/m2). He was a tobacco smoker (at least 27 pack years) and used 3-4 alcoholic beverages per day. Upper endoscopy showed a non-stenotic anastomosis at 30 cm from the incisors with a ¾ circular growing easily bleeding lesion from 33-42 cm from the incisors. Biopsies showed chronic inflammation. A chest CT scan revealed a stenotic esophagus extending from the aortic arch to the cardia with a malignant appearance and mediastinal lymph nodes (pre- and subcarinal). Due to the strong suspicion of esophageal cancer an esophageal resection with gastric tube reconstruction was performed. Pathology results confirmed the diagnosis of a squamous cell carcinoma (SCC) of the distal esophagus (pT2N0M0) which did not need further treatment.
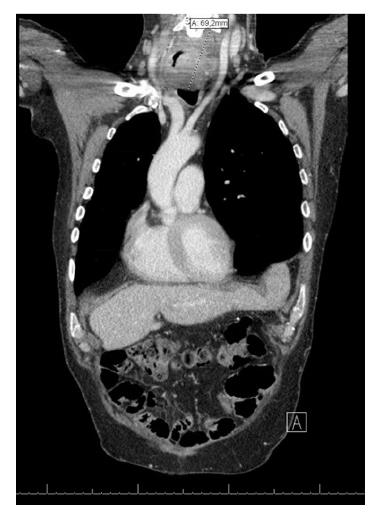
Fifteen years later, at the age of 60 years, he again developed dysphagia and odynophagia with 7 kg weight loss (BMI 23.2 kg/m2). Endoscopy revealed a circular tumor (17-21 cm from incisors) in the remaining cervical native esophagus eroding the constructed gastric tube and trachea. Biopsies showed a well-differentiated SCC. One suspicious supraclavicular and two mediastinal FDG-positive lymph nodes were seen on PET-CT scan images and tumor invasion in the left thyroid gland was suspected (Figure 1). Given the long interval between the two malignancies, this new tumor (T4bN2M0) was most likely a second primary tumor in the remaining cervical esophagus. In a multidisciplinary team discussion it was decided to treat with induction chemotherapy (carboplatin/paclitaxel). Initially the tumor responded well, but four months later he suffered from progressive disease with fistula formation to the trachea which was a contraindication for additional radiotherapy. An esophageal stent was placed to manage progressive dysphagia and palliative radiotherapy (13 × 3 Gy) was started to manage neuropathic pain caused by tumor invasion with imminent spinal cord compression. He died two days later.
Patient B was a 42-year old man born with VACTERL association (acronym: vertebral anomalies, anal atresia, cardiac anomalies, TEF, renal anomalies, and limb defects)[16] including EA Gross type A (long gap without TEF), anorectal malformation, coccyx agenesis and vertebral anomalies. Continuity of the esophagus was restored with a delayed end-to-end anastomosis.
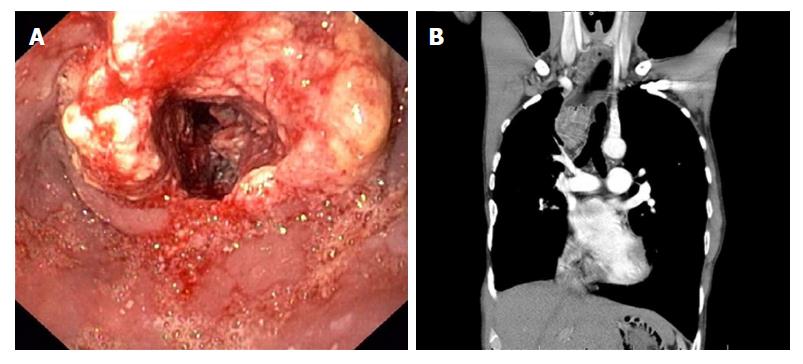
At 37 years of age he presented with dysphagia. Upper endoscopy revealed a stenotic anastomosis at 30 cm from the incisors, which could be easily dilated. In the next two years he underwent another three esophageal dilation procedures because of recurrent dysphagia. Biopsies revealed chronic and active inflammation with presence of hyphae. At the age of 42 years he presented with progressive dysphagia, without weight loss (BMI 17.6 kg/m2). He smoked tobacco and drank alcoholic beverages only in the weekend. This time upper endoscopy revealed a circular stenotic ulcerative ESCC in the proximal esophagus (22-29 cm, anastomosis not visible) (Figure 2A). Endoscopic ultrasound findings were suspicious for tumor invasion in the trachea and several potentially malignant regional lymph nodes (T4N2M0). The tumor was considered unresectable due to invasion of surrounding vital structures (cT4b) (Figure 2B), lymph node metastases, previous thoracotomies (both sides) and intra-mediastinal surgery. Induction chemotherapy (paclitaxel/carboplatin) was started to which the tumor evidently had responded after 2 mo. Concomitant chemoradiotherapy was given (28 × 1.8 Gy) with curative intent. Six years after treatment he shows no signs of recurrent or metastatic disease.
Case 3
Patient C presented at the age of 36 years. She was born with an EA Gross type A which was surgically repaired with an end-to-end anastomosis using Livaditis elongation procedure at one month of age. At one year of age she underwent a Nissen fundoplication for severe GER. At the age of 3 years, an anastomotic stricture developed which was treated with repeated esophageal dilations. At the age of 22 years she presented with chronic respiratory symptoms, severe pneumonia, persistent GER, and dysphagia complaints. Upper endoscopy with esophageal biopsies showed no abnormality. In view of the respiratory and gastrointestinal symptoms a duodenal diversion procedure (partial antrectomy with Roux-en-Y gastrojejunal anastomosis) was performed at the age of 23 years.
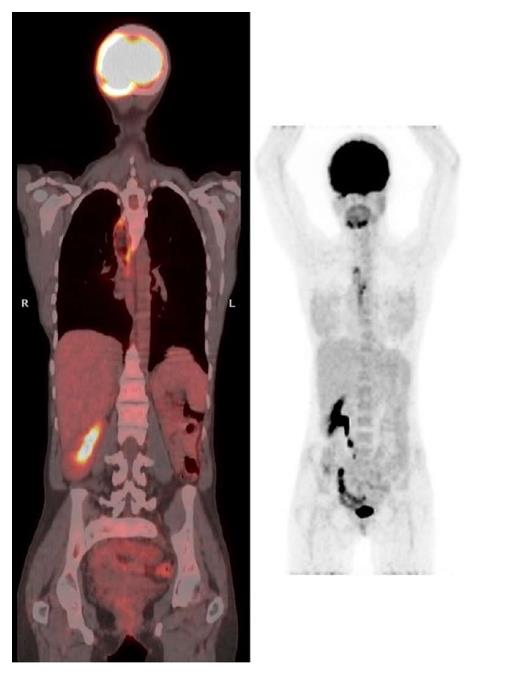
At 36 years of age she presented with food impaction and weight loss of 4 kg (BMI 14.9 kg/m2). She did not smoke tobacco and did not drink alcoholic beverages. Upper endoscopy revealed a stenotic ulcerative tumor in the distal esophagus with proximal dilation of the esophagus (25-32 cm from the incisors, gastroesophageal junction at 34 cm, anastomosis not visible). Biopsies revealed a well differentiated SCC. PET-CT scan (Figure 3) and bronchoscopy did not reveal any metastasis. She underwent a subtotal esophagectomy with total gastrectomy and a colonic interposition (pT1bN0M0). Within the following month she required reoperation for a cervical fistula and mediastinitis and underwent two endoscopic dilations of an anastomotic stricture without any evidence of tumor recurrence. Twelve months after surgery she was diagnosed with pleural and bone metastases for which she recently has started palliative chemotherapy.
Patient D presented at the age of 47 years. He was born with VACTERL association[16](EA Gross type C, anorectal malformation, congenital urethral valves with bilateral vesicoureteral reflux and hydronephrosis left kidney). At day 5 after birth a thoracotomy was performed with TEF closure, gastrostomy and cervical esophagostomy placement. In addition the anorectal malformation was corrected. Nine days later a recurrent TEF was ligated. At day 29 the distal esophagus was ligated directly above the stomach and after 7 mo a colonic interposition was constructed. The spleen was congested and therefore resected during this surgery. Revision was needed because of leakage of the proximal anastomosis 19 days later. At 2.5 year of age the gastrostomy was closed. Other medical history included asthmatic bronchitis, bilateral orchidopexy, transurethral resection of urethral valves and nephrectomy of an afunctional infected left kidney.
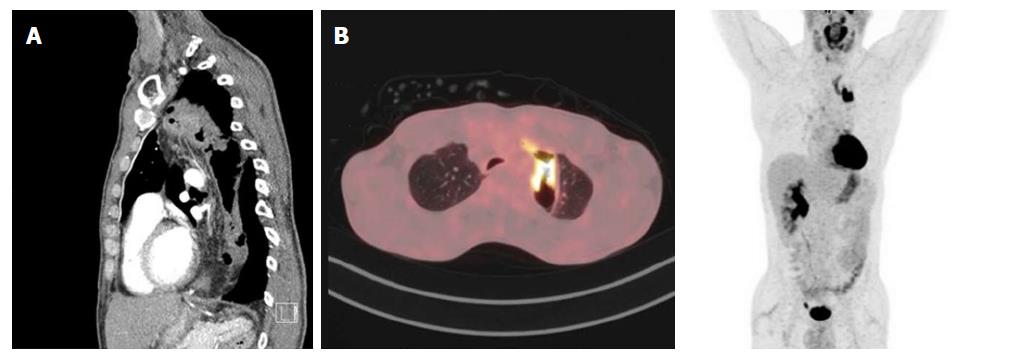
At presentation the patient suffered from pneumonia with a density in the lower lobe of the right lung. Subsequent PET-scan revealed a PET-positive thickening in the colonic interposition for which he had been referred to our center. He complained about progressive dysphagia without any weight loss (BMI 18.6 kg/m2). He was a cannabis smoker (2 joints/wk), had quit tobacco smoking just before presentation (a few cigarettes per day) and only sporadically drank alcoholic beverages. Upper endoscopy revealed the proximal and distal anastomosis of the colonic interposition at, respectively, 21 and 47 cm from incisors. From 26-30 cm from incisors a tumor was visible in the colon interposition which could be easily passed with the scope. Histology revealed a moderately differentiated adenocarcinoma. No abnormalities were found at colonoscopy. PET-CT scan showed circumferential thickening of the colonic interposition over a length of 10 cm, not clearly separated from the thyroid and left brachiocephalic vein, a small lesion in the lower right lobe of the lung (PET-negative) and a few locoregional lymph nodes (≤ 1 cm, PET-negative) (Figure 4 A and B).
Patient D was treated with induction chemotherapy (capecitabine/oxaliplatin) to enable maximum tumor regression. After six treatments, the colonic interposition was resected and an esophagostomy and jejunal fistula for feeding were created. Pathological examination confirmed the diagnosis of colonic adenocarcinoma with a maximum diameter of 4.1 cm, tumor free resection margins (≥ 1 cm) and one of 19 lymph nodes positive for metastasis (ypT2N1). Family history was negative for Lynch Syndrome. Both pentaplex microsatellite instability testing and mismatch repair gene expression analysis for MLH1, MSH2, MSH6 and PMS2 were normal.
After 4 mo continuity was restored by a subcutaneous gastric tube pull-up. At oncological follow-up one year after resection of the colonic interposition patient D did not experience any dysphagia, weight was stable (BMI 19.7 kg/m2) and ultrasound of the liver and CEA were normal (2.72 μg/L).
We presented four cases of gastrointestinal cancer that have developed more than 30 years after surgical treatment of EA: three esophageal carcinoma and one unusual presentation of colorectal carcinoma in a colonic interposition. These patients’ relatively young age, the fact that only few carcinogenic factors were identified and the high incidence of cancer development in a low prevalence disease suggest that EA carries an increased risk for esophageal cancer development and therefore screening and surveillance may be warranted, as recommended in the ESPGHAN-NASPGHAN guideline[11].
Esophageal cancer is the 8th most common cancer worldwide, with an incidence rate of 6.4 and 1.2 per 100000 males and females respectively in developed countries and 10.1 and 4.1 per 100000 males and females, respectively, in less developed countries[17]. ESCC and EAC have different etiologies. ESCC arrises from dysplastic squamous epithelium and is associated with a low socioeconomic status, use of tobacco or alcohol, several dietary factors, and human papilloma virus[18,19]. The main risk factors for EAC are GER, use of tobacco, obesity, and hiatal hernia[18]. Chronic GER might lead to gastric and intestinal metaplasia of the squamous epithelium in the esophagus, known as Barrett’s esophagus, which predisposes to dysplasia and EAC. GER is present in up to 67% of the adult EA patients and is likely to contribute to EAC development[5]. However, in literature - and also in our case series - ESCC is more common than EAC in EA patients[10,12-15]. The reason for this high risk of ESCC development has not yet been established. The pathogenesis might be the same as in achalasia, where ESCC is thought to result from stasis, causing bacterial overgrowth with nitrosamine production and subsequent esophageal inflammation, dysplasia and cancer[20,21]. Most of the ESCC in EA patients were found near or at the anastomosis (mid-distal esophagus). It has been suggested, therefore, that frequent dilation procedures with associated mucosal tears, scarring and inflammation may lead to development of ESCC in this patient group[10,12]. Mitomycin-C, an antifibrotic applicant used to prevent recurrence of strictures, may be an additional risk factor for ESCC, but this was not used in any of the patients in present case series[22]. Moreover, genetic predisposition may contribute to esophageal cancer in EA patients and is subject to future studies.
Endoscopic surveillance of EA patients is advocated to detect lesions at an early stage[11]. Those treated with a colonic interposition should not be excluded from surveillance, as carcinoma could arise in the cervical native esophagus or thoracic colon. More data on the actual incidence of esophageal cancer development in adulthood will hopefully become available soon when surveillance programs have been implemented. Together with the identification of risk factors this will help to optimize surveillance strategies in EA patients. Until then, pediatric surgeons and gastroenterologists who are involved in treatment of EA patients should be made aware of the cancer risk and be encouraged to reach consensus on optimal surveillance. When EA patients reach adulthood, they should be transferred to a gastroenterologist for endoscopic surveillance.
At presentation (age 36-47 years) all four patients born with esophageal atresia (EA) complained of progressive dysphagia, and two of them had lost weight.
Clinical diagnosis was made by upper endoscopy, revealing a for potentially malignant circular stenotic lesion in the esophagus in three cases and a tumor in the colonic interposition in one case.
The differential diagnosis included severe ulcerative esophagitis, benign stenotic anastomosis, and motility disorder.
Carcinoembryonic antigen (CEA) was measured (normal) in the patient with a colonic adenocarcinoma in the colonic interposition.
In addition to upper endoscopy a positron emission tomography-computed tomography scan (PET-CT scan) - in combination with endoscopic ultrasound in one case - was performed, which revealed a stenotic esophagus in three cases; a circumferential thickening of the colonic interposition in one case; potentially malignant lymph nodes in three cases; and suspected tumor invasion in two cases.
Histology and immunohistochemistry results confirmed the diagnosis of squamous cell carcinoma of the esophagus in three cases and adenocarcinoma of the colonic interposition in one case, in the latter case pentaplex microsatellite instability testing and mismatch repair gene expression analysis for MLH1, MSH2, MSH6 and PMS2 were normal.
The esophageal cancer patients underwent (sub)total esophagectomy with reconstruction (curative intent); received induction chemotherapy (paclitaxel/carboplatin) followed by chemoradiotherapy (curative intent); or received palliative radiotherapy or chemotherapy. The patient with colon cancer was treated with induction chemotherapy (capecitabine/oxaliplatin) followed by resection of the colonic interposition with construction of an esophagostoma and jejunal fistula for feeding.
Up to two-thirds of EA patients suffer from gastroesophageal reflux, which in the long-term might lead to mucosal damage including Barrett’s esophagus and esophageal adenocarcinoma. As dysphagia is common (up to 72%) after EA repair, this symptom may be neglected as an early warning symptom of esophageal cancer in these patients. Up till now, eight esophageal cancer cases have been described in young EA patients.
EA with or without tracheoesophageal fistula (TEF) is a common congenital malformation and requires surgical correction early in life. The Gross classification divides five types of EA: type A (isolated EA), type B (EA with proximal TEF), type C (EA with distal TEF), type D (EA with dual TEF’s) or type E (isolated TEF).
VACTERL is an acronym that describes a nonrandom association of birth defects: Vertebral anomalies, Anal atresia, Cardiac anomalies, TEF, Renal anomalies, and Limb defects.
These patients’ relatively young age, the fact that only few carcinogenic factors were identified and the high incidence of cancer development in a low prevalence disease suggest that EA carries an increased risk for esophageal cancer development. This emphasizes the importance of lifelong screening and surveillance of the upper gastrointestinal tract in EA patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Netherlands
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: De Lusong MAA, Thota PN, Wani HU S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Pedersen RN, Calzolari E, Husby S, Garne E; EUROCAT Working group. Oesophageal atresia: prevalence, prenatal diagnosis and associated anomalies in 23 European regions. Arch Dis Child. 2012;97:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 2. | Wang B, Tashiro J, Allan BJ, Sola JE, Parikh PP, Hogan AR, Neville HL, Perez EA. A nationwide analysis of clinical outcomes among newborns with esophageal atresia and tracheoesophageal fistulas in the United States. J Surg Res. 2014;190:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Sulkowski JP, Cooper JN, Lopez JJ, Jadcherla Y, Cuenot A, Mattei P, Deans KJ, Minneci PC. Morbidity and mortality in patients with esophageal atresia. Surgery. 2014;156:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Rintala RJ, Pakarinen MP. Long-term outcome of esophageal anastomosis. Eur J Pediatr Surg. 2013;23:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Vergouwe FW, IJsselstijn H, Wijnen RM, Bruno MJ, Spaander MC. Screening and Surveillance in Esophageal Atresia Patients: Current Knowledge and Future Perspectives. Eur J Pediatr Surg. 2015;25:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Huynh Trudeau V, Maynard S, Terzic T, Soucy G, Bouin M. Dysphagia among adult patients who underwent surgery for esophageal atresia at birth. Can J Gastroenterol Hepatol. 2015;29:91-94. [PubMed] |
| 7. | Taylor AC, Breen KJ, Auldist A, Catto-Smith A, Clarnette T, Crameri J, Taylor R, Nagarajah S, Brady J, Stokes K. Gastroesophageal reflux and related pathology in adults who were born with esophageal atresia: a long-term follow-up study. Clin Gastroenterol Hepatol. 2007;5:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Sistonen SJ, Koivusalo A, Nieminen U, Lindahl H, Lohi J, Kero M, Kärkkäinen PA, Färkkilä MA, Sarna S, Rintala RJ. Esophageal morbidity and function in adults with repaired esophageal atresia with tracheoesophageal fistula: a population-based long-term follow-up. Ann Surg. 2010;251:1167-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 9. | Sistonen SJ, Koivusalo A, Lindahl H, Pukkala E, Rintala RJ, Pakarinen MP. Cancer after repair of esophageal atresia: population-based long-term follow-up. J Pediatr Surg. 2008;43:602-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Jayasekera CS, Desmond PV, Holmes JA, Kitson M, Taylor AC. Cluster of 4 cases of esophageal squamous cell cancer developing in adults with surgically corrected esophageal atresia--time for screening to start. J Pediatr Surg. 2012;47:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Krishnan U, Mousa H, Dall’Oglio L, Homaira N, Rosen R, Faure C, Gottrand F. ESPGHAN-NASPGHAN Guidelines for the Evaluation and Treatment of Gastrointestinal and Nutritional Complications in Children With Esophageal Atresia-Tracheoesophageal Fistula. J Pediatr Gastroenterol Nutr. 2016;63:550-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 12. | Deurloo JA, van Lanschot JJ, Drillenburg P, Aronson DC. Esophageal squamous cell carcinoma 38 years after primary repair of esophageal atresia. J Pediatr Surg. 2001;36:629-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Adzick NS, Fisher JH, Winter HS, Sandler RH, Hendren WH. Esophageal adenocarcinoma 20 years after esophageal atresia repair. J Pediatr Surg. 1989;24:741-744. [PubMed] |
| 14. | Alfaro L, Bermas H, Fenoglio M, Parker R, Janik JS. Are patients who have had a tracheoesophageal fistula repair during infancy at risk for esophageal adenocarcinoma during adulthood? J Pediatr Surg. 2005;40:719-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Pultrum BB, Bijleveld CM, de Langen ZJ, Plukker JT. Development of an adenocarcinoma of the esophagus 22 years after primary repair of a congenital atresia. J Pediatr Surg. 2005;40:e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Solomon BD, Baker LA, Bear KA, Cunningham BK, Giampietro PF, Hadigan C, Hadley DW, Harrison S, Levitt MA, Niforatos N. An approach to the identification of anomalies and etiologies in neonates with identified or suspected VACTERL (vertebral defects, anal atresia, tracheo-esophageal fistula with esophageal atresia, cardiac anomalies, renal anomalies, and limb anomalies) association. J Pediatr. 2014;164:451-457.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21368] [Article Influence: 2136.8] [Reference Citation Analysis (3)] |
| 18. | Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27-57, vii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 19. | Zhang SK, Guo LW, Chen Q, Zhang M, Liu SZ, Quan PL, Lu JB, Sun XB. The association between human papillomavirus 16 and esophageal cancer in Chinese population: a meta-analysis. BMC Cancer. 2015;15:1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Sandler RS, Nyrén O, Ekbom A, Eisen GM, Yuen J, Josefsson S. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA. 1995;274:1359-1362. [PubMed] |
| 21. | Pajecki D, Zilberstein B, Cecconello I, Dos Santos MA, Yagi OK, Gama-Rodrigues JJ. Larger amounts of nitrite and nitrate-reducing bacteria in megaesophagus of Chagas’ disease than in controls. J Gastrointest Surg. 2007;11:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Chapuy L, Pomerleau M, Faure C. Topical mitomycin-C application in recurrent esophageal strictures after surgical repair of esophageal atresia. J Pediatr Gastroenterol Nutr. 2014;59:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |