Published online Feb 7, 2018. doi: 10.3748/wjg.v24.i5.613
Peer-review started: November 29, 2017
First decision: December 20, 2017
Revised: December 26, 2017
Accepted: January 15, 2018
Article in press: January 15, 2018
Published online: February 7, 2018
To assess the incidence of hepatocellular carcinoma (HCC) in chronic liver disease due to hepatitis B virus (HBV) or hepatitis C virus (HCV) coinfected with human immunodeficiency virus (HIV).
A retrospective cohort study was performed, including patients with chronic liver disease due to HBV or HCV, with and without HIV coinfection. Patients were selected in the largest tertiary public hospital complex in southern Brazil between January 2007 and June 2014. We assessed demographic and clinical data, including lifestyle habits such as illicit drug use or alcohol abuse, in addition to frequency and reasons for hospital admissions via medical records review.
Of 804 patients were included (399 with HIV coinfection and 405 monoinfected with HBV or HCV). Coinfected patients were younger (36.7 ± 10 vs 46.3 ± 12.5, P < 0.001). Liver cirrhosis was observed in 31.3% of HIV-negative patients and in 16.5% of coinfected (P < 0.001). HCC was diagnosed in 36 patients (10 HIV coinfected and 26 monoinfected). The incidence density of HCC in coinfected and monoinfected patients was 0.25 and 0.72 cases per 100 patient-years (95%CI: 0.12-0.46 vs 0.47-1.05) (long-rank P = 0.002), respectively. The ratio for the HCC incidence rate was 2.98 for HIV-negative. However, when adjusting for age or when only cirrhotic are analyzed, the absence of HIV lost statistical significance for the development of HCC.
In this study, the presence of HIV coinfection in chronic liver disease due to HBV or HCV showed no relation to the increase of HCC incidence.
Core tip: We conducted a retrospective cohort study with 804 patients with chronic viral hepatitis B or C, with and without human immunodeficiency virus (HIV) coinfection (399 HIV-coinfected and 405 monoinfected with HBV or HCV). The main objective was to assess the incidence of hepatocellular carcinoma in HIV-coinfected patients. Hepatocellular carcinoma (HCC) was observed in 36 patients (10 HIV-positive and 26 HIV-negative). When adjusted for age, the role of HIV was no longer statistical significant for the development of HCC. Moreover, when analyzing cirrhotic patients only, the HCC incidence had no difference between the groups. Only age and alcohol use were associated with risk of developing HCC.
- Citation: Marcon PDS, Tovo CV, Kliemann DA, Fisch P, Mattos AA. Incidence of hepatocellular carcinoma in patients with chronic liver disease due to hepatitis B or C and coinfected with the human immunodeficiency virus: A retrospective cohort study. World J Gastroenterol 2018; 24(5): 613-622
- URL: https://www.wjgnet.com/1007-9327/full/v24/i5/613.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i5.613
Liver disease performs an important public health issue with high costs for healthcare systems and causing a decrease in quality of life, representing the eighth cause of death in Brazil[1]. Among liver diseases, primary liver cancer receives particular attention for being the sixth most common malignant neoplasm worldwide and the second most common cause of death by cancer[2]. Hepatocellular carcinoma (HCC) is the most frequent neoplasm, and is responsible for 70%-90% of cases[2-4]. The American Cancer Society estimated 39200 new diagnoses for 2016, with 27170 deaths, affecting mostly men[5].
In general, 80%-90% of HCC cases are related to cirrhosis, regardless of etiology[2,3,6-10]. However, there is an important geographic variation with regard to HCC etiology. In endemic areas for the hepatitis B virus (HBV), such as in parts of Africa and Asia, HBV is the most common cause[8,10,11]. In the United States and in many countries of Europe, the main etiological factor is the hepatitis C virus (HCV)[12-17].
In Brazil, some authors found chronic HCV infection followed by alcohol liver disease as the most frequent causes of HCC[18,19]. Similar findings can be seen in a study conducted in Latin America[20].
The increase in HCC incidence in the past decade, along with the tendency that this increase will continue, is closely related to time of exposure to HBV and HCV[11]. Thus, the role of coinfection with the human immunodeficiency virus (HIV) has gained prominence, since the introduction of highly active retroviral therapies (HAART) against HIV has presented significant improvements in patient survival[21-35]. In this scenario, liver disease has become one of the main causes of hospitalization and death in HIV-positive patients, with HCC as a prominent factor. This phenomena is closely related to an increase in alcohol consumption and with the high prevalence of HBV and HCV in this population, which share similar transmission routes with HIV[34,36-40].
Current data suggests an unfavorable evolution both in the natural history of liver disease and in the outcome after hepatitis treatment in coinfected patients[41,42], suggesting that the incidence of cirrhosis and HCC is significantly higher in this population[34]. On the other hand, some authors found conflicting data. Recently, a study with 148 cirrhotic patients due to HCV, coinfected or not with HIV and monitored for an average period of 43 mo showed no significant difference in HCC incidence between HIV-positive and HIV-negative patients[43].
Thus, we believe it is extremely important to have data clarifying the profile of this association in our environment. Therefore, the main objective of this study is to assess the incidence of HCC in patients with chronic liver disease due to HBV or HCV and coinfected with HIV.
A retrospective cohort study was conducted with individuals with chronic viral hepatitis B or C, with and without HIV coinfection. Patients were selected from the viral hepatitis notification bank at the epidemiology department of Hospital Nossa Senhora da Conceição, a tertiary public care center in Porto Alegre - Brazil, between January 2007 and June 2014.
Initially, eligible patients were those with documented chronic infection by HBV or HCV. Chronic infection by HBV was considered in the presence of positive HBsAg and/or polymerase chain reaction (PCR) for HBV DNA for longer than six months. HCV chronic infection was defined by detection of the anti-HCV antibody and PCR for HCV RNA in the plasma for longer than six months. These individuals were split into two groups, according to their HIV infection status. Patients considered as infected by HIV were those with detection of anti-HIV antibodies in the plasma, repeated and confirmed via molecular method or Western blot.
Individuals under 18 years of age, patients with insufficient data on their medical records, those who did not attend monitoring exams, pregnant women, those with other types of hepatitis or who did not have at least one annual appointment during the monitoring period were excluded from the study.
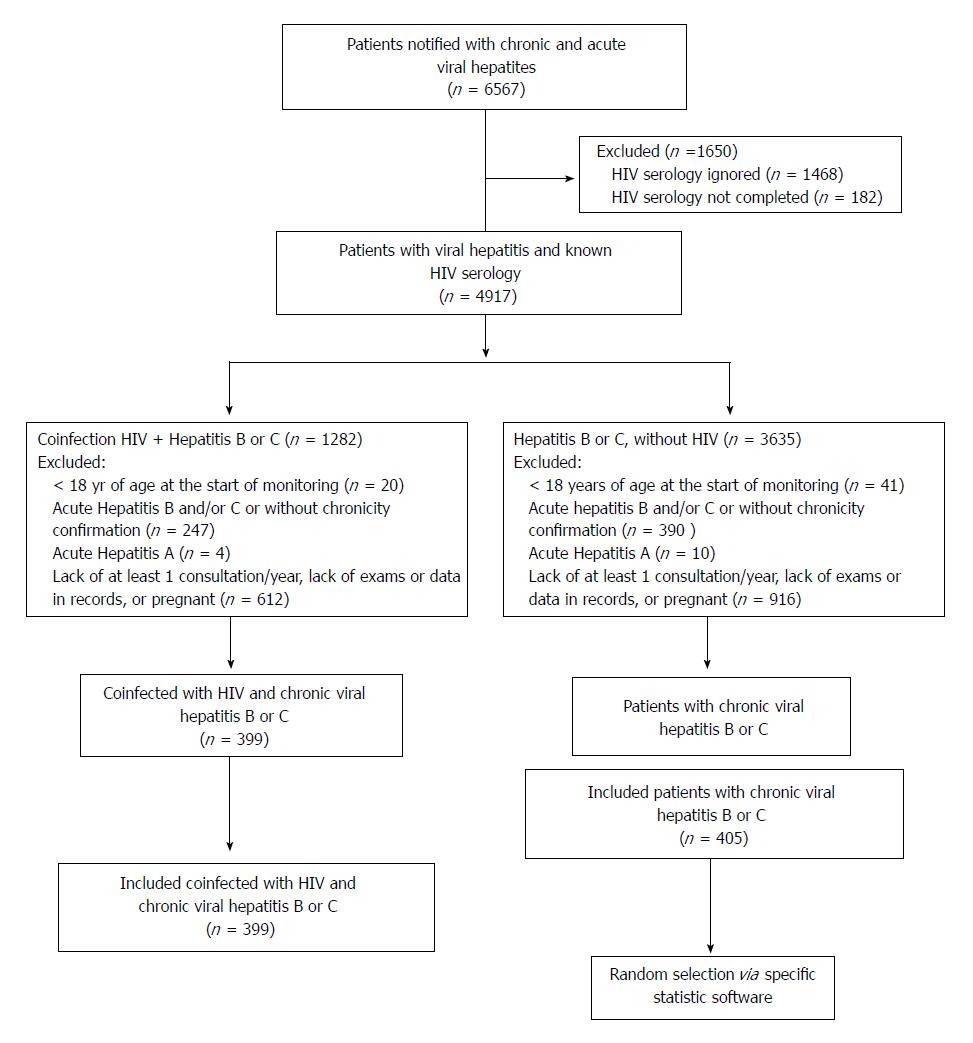
All patients who were coinfected with HIV and met the inclusion criteria were included in the study; for patients who were only infected with HBV or HCV, a simple random draw was conducted using IBM® SPSS software version 22.0, for later data collection of selected patients (Figure 1).
We assessed demographic and clinical data, including lifestyle habits such as illicit drug use or alcohol abuse, in addition to frequency and reasons for hospital admissions via medical records review. The diagnosis of diabetes mellitus (DM) or glucose intolerance was done according the criteria set by the American Diabetes Association[44].
Specific treatments for viral hepatitis were investigated, with the following criteria being considered as adequate responses to HBV and sustained virological response (SVR) to HCV: HBV treatment with any oral antiviral or conventional interferon, with negative viral load after 12 mo from the start of monitoring; HCV treatment with conventional or pegylated interferon, associated or not to ribavirin and to boceprevir or telaprevir, with negative viral load after at least 12 wk from the end of treatment[34,45].
In patients coinfected with HIV, the use of HAART was assessed, with the negativation of the HIV viral load being considered as adequate response to the treatment. The occurrence of opportunistic infections was also investigated.
All patients were investigated for presence of liver cirrhosis and its complications. The cirrhosis diagnosis was established in accordance with the association of clinical and laboratory findings, abdominal and/or endoscopic images and histopathological in cases of doubt.
The HCC diagnosis was based on the typical radiologic aspect in at least one image exam (CT or MRI), following the criteria established by the European Association for the Study of the Liver (EASL)[8]. The inconclusive cases were referred for biopsy of the suspected hepatic lesion, followed by anatomopathological examination for diagnostic confirmation. In the confirmed cases of HCC, the extent of the disease was assessed according to the Milan criteria[46]. Additionally, we determined the alpha-fetoprotein value and the Child-Pugh and Model for End-Stage Liver Disease scores (MELD) at the time of HCC diagnosis.
This project was submitted to and approved by the Ethical Committees of Universidade Federal de Ciências da Saúde de Porto Alegre and of Hospital Nossa Senhora da Conceição in Porto Alegre, Brazil, following the ethical precepts of the Helsinki Declaration, revised in 2013[47]. This is a retrospective cohort study, which did not present direct intervention in the patients and with the data analyzed only in numbers; thus the informed consent statement was waived.
Continuous variables were described as mean ± SD. When Gaussian assumptions were violated, we used median and interquartile range (25 percentile to 75 percentile). Categorical variables were expressed by frequencies and percentages. The comparison of means was performed by Student's t-test, and in the case of asymmetric data, by its non-parametric substitute (Mann-Whitney U test). The comparison of categorical variables was performed by chi-square test or by Fisher’s exact test when appropriate. The time for the event was calculated from the first event of medical care at the institution due to HIV and/or viral hepatitis until the final event, considered as HCC or death. The incidence of events (HCC or death) was estimated by the Kaplan-Meier method according to the presence or absence of HIV coinfection. The comparison between groups was performed by the log-rank test. Additionally, we elaborated a Cox proportional hazards model, with which we obtained the gross and adjusted rate ratios (hazard ratio) for potential confounders, followed by their respective confidence intervals. For all tests, the level of bicaudal significance of α/2 = 0.05 was considered. Data was stored in Microsoft® Office Excel 2010 and statistically analyzed by IBM® SPSS 22.0 software. The statistical methods of this study were reviewed by Mario B. Wagner from Universidade Federal do Rio Grande do Sul - Brazil.
A total of 6567 medical records of patients referred to tertiary care center with viral hepatitis were analyzed; of these, 804 patients were included in the study (399 coinfected with HIV/HBV or HCV and 405 monoinfected with HBV or HCV) (Figure 1).
The general characteristics of patients are shown in Table 1, where it can be seen that those coinfected with HIV were younger, more often male and less frequently Caucasian, besides showing a lower frequency of DM. In this group of patients, the use of illicit drugs was more frequent, although there was no difference regarding alcohol abuse. There was also a higher frequency of HBV in the coinfected patients, with higher rates of treatment, but with lower response rates (54.8% vs 92.3%, P = 0.012). In turn, HIV-negative patients had a higher frequency of HCV and a higher rate of treatments performed, with SVR seen in 56.2% of these cases (56.2% vs 44.3%, P = 0.083).
| Characteristics | HIV+ (n = 399) | HIV- (n = 405) | P value |
| Age, yr | 36.7 ± 10 | 46.3 ± 12.5 | < 0.001 |
| Male gender | 249 (62.4) | 197 (48.6) | < 0.001 |
| Caucasian | 285 (71.4) | 352 (86.9) | < 0.001 |
| n = 390 | n = 343 | ||
| Diabetes or glucose intolerance | 57 (14.6) | 100 (29.2) | < 0.001 |
| n = 247 | n = 184 | ||
| Illicit drugs | 161 (65.2) | 41 (22.3) | < 0.001 |
| n = 283 | n = 269 | ||
| Alcohol | 119 (42.0) | 96 (35.7) | 0.138 |
| n = 399 | n = 404 | ||
| Hepatitis B | 82 (20.6) | 49 (12.1) | 0.002 |
| n = 81 | n = 35 | ||
| Treatment | 77 (95.1) | 13 (37.1) | < 0.001 |
| n = 395 | n = 399 | ||
| Hepatitis C | 333 (84.3) | 361 (90.5) | 0.010 |
| n = 292 | n = 296 | ||
| Genotype 1 | 192 (65.8) | 157 (53.0) | 0.005 |
| Genotype 2 | 19 (6.5) | 17 (5.7) | |
| Genotype 3 | 79 (27.1) | 119 (40.2) | |
| Other1 | 2 (0.7) | 3 (1.0) | |
| n = 325 | n = 338 | ||
| Treatment | 102 (31.4) | 151 (44.7) | < 0.001 |
| n = 399 | n = 405 | ||
| Hospital admissions | 359 (90.0) | 249 (61.5) | < 0.001 |
| n = 399 | n = 399 | ||
| Cirrhosis | 66 (16.5) | 125 (31.3) | < 0.001 |
Most of the coinfected patients received HAART (94.2%), with only 59% of them presenting adequate response to treatment. It was observed that approximately 64% of the coinfected patients presented some type of opportunistic infection during the monitoring period and had a larger number of hospitalizations than the HIV-negative patients [3 (1-5) vs 1 (0-3), P < 0.001]. On the other hand, the presence of hepatic cirrhosis was higher in HIV-negative patients.
When only patients with cirrhosis were analyzed, coinfected patients were also younger (40.1 ± 10.4 years vs 50.9 ± 11.8 years, P < 0.001), not Caucasian (77.3% vs 92%, P = 0.006), with less occurrences of diabetes (18.2% vs 39.5%, P = 0.004) and more occurrences of alcohol abuse (64.6% vs 42.6%, P = 0.021). There was no statistical difference regarding the presence of HBV and HCV among the groups. Regarding the treatment of HBV, it was not possible to make considerations due to the small number of cases evaluated. Regarding HCV treatment, it was observed that in both groups, less than 50% of the patients received treatment, with a higher rate of SVR in the monoinfected group (48.8% vs 17.6%, P = 0.039). Regarding cirrhosis complications, no differences were observed in the incidence of ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, hepatorenal syndrome and variceal bleeding when comparing patients with and without HIV. The development of ascites (in about 60% of coinfected cirrhotic patients and 50% of HIV negative) was the most frequent complication in both groups. In cirrhotic patients, the frequency of hospital admissions was higher in the coinfected patients [3 (2-5) vs 2 (0-4), P = 0.047], but there was no statistical difference between the groups when analyzing the percentage of admissions due to hepatic causes (61.3% vs 76.1%, P = 0.072).
In general, patients were monitored for a median time of 10.54 years (95%CI: 9.58-11.50, P = 0.005). Coinfected patients were monitored for a median time 12.03 years (95% CI: 10.92 - 13.15), while monoinfected patients were monitored for a median time of 8.57 years (95%CI: 7.19-9.94), P = 0.005. The total follow-up was 7.498.8 patient-years (3.901.9 patient-years in HIV-positive patients and 3.596.9 patient-years in HIV-negative patients).
All patients who developed HCC had liver cirrhosis at the time of diagnosis. These patients’ characteristics are presented in Table 2, where it can be observed that the co-infected population was younger and had higher alpha-fetoprotein levels. There was no difference in other aspects evaluated.
| Characteristics | HIV+ (n = 10) | HIV- (n= 26) | P value |
| Age, yr | 42.1 ± 8.3 | 53.7 ± 10.6 | 0.004 |
| Male gender | 8 (80.0) | 18 (69.2) | 0.689 |
| Caucasian | 9 (90.0) | 24 (92.3) | > 0.999 |
| Diabetes or glucose intolerance | 2 (20.0) | 10 (41.7) | 0.432 |
| n = 9 | n = 22 | ||
| Alcohol | 7 (77.8) | 10 (45.5) | 0.132 |
| n = 10 | n = 26 | ||
| Hepatitis B | 1 (10.0) | 2 (7.7) | > 0.99 |
| Hepatitis C | 9 (90.0) | 24 (92.3) | > 0.99 |
| Child- Pugh | n = 8 | n = 25 | |
| A | 2 (25.0) | 11 (44.0) | 0.735 |
| B | 5 (62.5) | 11 (44.0) | |
| C | 1 (12.5) | 3 (12.0) | |
| n = 7 | n = 22 | ||
| MELD | 13.7 ± 5.1 | 10.9 ± 4.2 | 0.157 |
| n = 10 | n = 26 | ||
| Milan criteria2 | 3 (30.0) | 14 (53.8) | 0.157 |
| n = 10 | n = 24 | ||
| Alpha-fetoprotein, ng/mL | 276 (23-18750) | 20 (4-113) | 0.038 |
The development of HCC was observed in 36 patients - 10 cases in HIV-positive patients and 26 in HIV-negative patients, resulting in a cumulative incidence 2.5% e 6.4%, respectively. The incidence density of HCC in coinfected and monoinfected patients was 0.25 cases per 100 patient-years (95%CI: 0.12-0.46) and 0.72 cases per 100 patient-years (95%CI: 0.47-1.05) (long-rank P = 0.002), respectively (Table 3). The ratio of incidence rates of HCC of HIV negative when compared to HIV positive was 2.98. When adjusted for age, the role of HIV is no longer statistical significant for the development of HCC (Table 3).
| HIV | n | HCC | % | Patient-years | Rate × 100 patient-years | RR | 95%CI | P value |
| + | 399 | 10 | 2.5 | 3963.80 | 0.25 | - | - | - |
| - | 405 | 26 | 6.4 | 3624.50 | 0.72 | 2.98 | 1.43-6.18 | 0.003 |
| Model 2: adjusted for age | 1.29 | 0.58-2.87 | 0.529 | |||||
| Model 3: adjusted for age and DM | 1.27 | 0.56-2.88 | 0.571 | |||||
| Model 4: adjusted for age, DM and alcohol | 1.23 | 0.52-2.95 | 0.638 | |||||
When analyzing cirrhotic patients only, we observed an HCC incidence density of 1.54 cases per 100 patient-years (95%CI: 0.74-2.83) in coinfected patients and 2.31 cases per 100 patient-years (95%CI: 1.51-3.38) in monoinfected patients (long-rank p = 0.202) (Table 4). The HCC incidence rate in this case becomes 1.60, with no statistical significance (95%CI: 0.77-3.32, P = 0.207) (Table 4).
| HIV | n | HCC | % | Patient-years | Rate of 100 patient-years | RR | 95%CI | P value |
| + | 66 | 10 | 15.2 | 649.75 | 1.54 | - | - | - |
| - | 125 | 26 | 20.8 | 1125.88 | 2.31 | 1.60 | 0.77-3.32 | 0.207 |
Among the factors analyzed in cirrhotic patients, the only ones that presented statistical significance for the risk of developing HCC were age and alcohol use; in this sample, DM was unable to demonstrate such association (Table 5).
| Characteristic | RRc | 95%CI | P value | RRa | 95%CI | P value |
| HIV | 1.60 | 0.77-3.32 | 0.207 | 0.60 | 0.23-1.62 | 0.313 |
| Age (by 10 yr) | 1.80 | 1.75-1.86 | < 0.001 | 2.20 | 2.12-2.30 | 0.001 |
| DM | 0.96 | 0.48-1.95 | 0.913 | 0.97 | 0.44-2.14 | 0.930 |
| Alcohol | 1.29 | 0.64-2.62 | 0.480 | 2.31 | 1.02-5.23 | 0.046 |
The arrival of HAART against HIV in the 90s directly impacted the natural history of HIV infection. The improvement in the immunity and, consequently, the survival of these patients led to a decrease in diseases related to the human immunodeficiency syndrome (which would invariably lead to death within only a few months). This has led to an increase in the incidence of diseases not directly related to HIV, especially neoplasms and liver disease[24,34,35,38,42,43,48,49]. In this scenario, HBV, HCV and alcohol abuse - factors often related to HIV infection - deserve attention[9,49-51].
In recent years, evidence suggests that HIV infection might be related to negative consequences on the progression of liver diseases, particularly increasing the risk of HCC. Puoti et al[51] observed an unfavorable evolution of liver disease in patients with HIV, with an increase in HCC cases, mainly associating this diagnosis with HCV infection. In 2008, a case-control study associated the development of HCC in coinfected patients with low CD4 levels, demonstrating the influence of HIV-related immunodeficiency on the development of this neoplasm[52]. Beretta et al[42] (2011) reported a younger profile of patients coinfected with HIV and HCC patients, with a clear deterioration in the survival rates of these patients, inferring that the presence of HIV would accelerate the process of carcinogenesis. Several other authors have also suggested that the presence of HIV may increase the risk of developing HCC, even after treatment for viral hepatitis, raising questions about the role of the HIV virus or the drugs involved in its treatment in hepatocarcinogenesis, something that has not been properly clarified[34,48,53,54].
The present study found no significant association between the presence of HIV and the development of HCC. When evaluating the cohort as a whole, we found lower cumulative incidence rates in coinfected patients (2.5% vs 6.4%). However, the group of monoinfected patients were 10 years older than coinfected patients, which suggests a longer time of infection/exposure to viral hepatitis. This is in line with the findings of Beretta et al[42] (2011). On the other hand, when we corrected for age, no difference was observed in the incidence between HIV-positive and HIV-negative patients. Similarly, when we analyzed the subgroup with liver cirrhosis only, no difference in the incidence of HCC between monoinfected and coinfected patients was seen.
Other authors have also been unable to demonstrate a higher incidence of HCC in patients coinfected with HIV and HBV/HCV. Smukler et al[55] observed that despite an increase in the incidence of certain neoplasms in HIV-positive patients after the introduction of HAARTs, the incidence of HCC had no significant changes. Later, Kramer et al[56], in a retrospective cohort study, found no association between coinfection and HCC, regardless of the use of HAART. Likewise, García-García et al[57], in a study with over 1000 patients, comparing monoinfected patients with HCV and coinfected patients with HIV/HCV, observed a higher incidence of HCC in the group of patients without HIV. More recently, Benedetto et al[43] found similar results when, in a prospective cohort study with cirrhotic patients, they investigated 69 patients monoinfected with HCV and 79 patients coinfected with HIV/HCV. Patients in this study were monitored for an average of 43 mo, with an incidence density of 1.54 cases per 100 patient-years in the coinfected group and 3.03 cases per 100 patient-years in the monoinfected group, which are very similar numbers to those found in the present study, with the difference that our average monitoring was 126 mo. The Argentine study also found younger patients coinfected with HIV, which leads us to speculate that the time of exposure to hepatic injury factors, such as HBV and HCV, is much more relevant to the development of HCC than the presence of HIV per se.
Alcohol abuse is traditionally associated with the development and worsening of liver disease, both in HIV-positive and HIV-negative patients[34,51,58]. Data from the United States shows a prevalence of up to 35% of alcohol abuse in HIV-infected patients[34]. In the present study, the importance of this association was confirmed, as alcohol abuse was a risk factor for the development of HCC in cirrhotic patients.
In the present study, although around 94% of HIV-positive patients received some type of HAART, only 59% obtained an adequate response to treatment, probably due to the abandonment of the proposed therapy. This also explains the high incidence of opportunistic infections (64% presented some type of opportunistic infection during monitoring) as well as the large number of hospital admissions for these individuals.
Some limitations of the present study should be mentioned, such as those inherent to retrospective studies and regarding data collection from potentially incomplete medical records. In addition, because of the low HBV and HCC number , some analyzes were impaired, such as the subanalysis of the incidence of HCC categorized by the presence of HBV or HCV separately or by the SVR after treatment of hepatitis.
In conclusion, the data suggests that HIV coinfection in patients with chronic hepatitis related to HBV or HCV does not play a relevant role in the development of HCC.
Following the introduction of highly effective antiviral therapy (HAART) against human immunodeficiency virus (HIV), the survival of affected patients improved considerably. In this scenario, liver diseases have gained prominence, especially viral hepatitis and hepatocellular carcinoma (HCC). Current data suggest an unfavorable evolution both in the natural history of liver disease and in the outcome after hepatitis treatment in coinfected patients.
The data about the outcomes in HIV/viral hepatitis association are conflicting, especially with regard to the incidence of HCC, mainly in South America. Thus, we believe it is extremely important to have data clarifying the profile of this association in our environment.
The main objective of this study is to assess the incidence of HCC in patients with chronic liver disease due to HBV or HCV and coinfected with HIV. These data are extremely important in order to implement more appropriate policies of HCC screening and prevention in this population.
A retrospective cohort study was conducted with individuals with chronic viral hepatitis B or C, with and without HIV coinfection. Patients were selected from the viral hepatitis notification bank at the epidemiology department of Hospital Nossa Senhora da Conceição, a tertiary public care center in Porto Alegre - Brazil, between January 2007 and June 2014. These individuals were split into two groups, according to their HIV infection status. Individuals under 18 years of age, patients with insufficient data on their medical records, those who did not attend monitoring exams, pregnant women, those with other types of hepatitis or who did not have at least one annual appointment during the monitoring period were excluded from the study. We assessed demographic and clinical data, including lifestyle habits, specific treatments for viral hepatitis, HAART use in patients coinfected with HIV, presence of liver cirrhosis and its complications and the HCC diagnosis.
A total of 6567 medical records of patients referred to this tertiary care center with viral hepatitis were analyzed; of these, 804 patients were included in the study (399 coinfected with HIV/HBV or HCV and 405 monoinfected with HBV or HCV). In general, patients were monitored for a median time of 10.54 years (95%CI: 9.58-11.50, P = 0.005). The total follow-up was 7498.8 patient-years (3901.9 patient-years in HIV-positive patients and 3596.9 patient-years in HIV-negative patients). The development of HCC was observed in 36 patients - 10 cases in HIV-positive patients and 26 in HIV-negative patients. All patients who developed HCC had liver cirrhosis at the time of diagnosis. The incidence density of HCC in coinfected and monoinfected patients was 0.25 cases per 100 patient-years (95%CI: 0.12-0.46) and 0.72 cases per 100 patient-years (95%CI: 0.47-1.05) (long-rank P = 0.002), respectively. The ratio of incidence rates of HCC of HIV negative when compared to HIV positive was 2.98. When adjusted for age, the role of HIV is no longer statistical significant for the development of HCC.
The present study found no significant association between the presence of HIV and the development of HCC. The data from this study suggests that the time of exposure to hepatic injury factors, such as HBV and HCV, is much more relevant to the development of HCC than the presence of HIV per se.
The present study demonstrates the need to study more deeply the consequences of the association of HIV with chronic liver diseases, especially in the era of HAART. The data presented here indicate the need for further prospective studies to better evaluate the consequences of HIV/viral hepatitis coinfection.
We would like to express our gratitude to the statistician who contributed to this study, Dr. Mario Bernardes Wagner, Postdoc in Data Analysis Statistics in Clinical Research from King’s College School of Medicine and Dentistry, University of London, full Professor at Universidade Federal do Rio Grande do Sul, Brazil.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sharafi H, Tanaka Y S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Nader LA, de Mattos AA, Bastos GA. Burden of liver disease in Brazil. Liver Int. 2014;34:844-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 491] [Cited by in F6Publishing: 574] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 3. | Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP Jr. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 755] [Cited by in F6Publishing: 691] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 4. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18694] [Cited by in F6Publishing: 20838] [Article Influence: 2315.3] [Reference Citation Analysis (2)] |
| 5. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12135] [Cited by in F6Publishing: 12702] [Article Influence: 1587.8] [Reference Citation Analysis (2)] |
| 6. | John JA, de Mattos AA, da Silva Miozzo SA, Comerlato PH, Porto M, Contiero P, da Silva RR. Survival and risk factors related to death in outpatients with cirrhosis treated in a clinic in Southern Brazil. Eur J Gastroenterol Hepatol. 2015;27:1372-1377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Méndez-Sánchez N, Ridruejo E, Alves de Mattos A, Chávez-Tapia NC, Zapata R, Paraná R, Mastai R, Strauss E, Guevara-Casallas LG, Daruich J. Latin American Association for the Study of the Liver (LAASL) clinical practice guidelines: management of hepatocellular carcinoma. Ann Hepatol. 2014;13 Suppl 1:S4-S40. [PubMed] [Cited in This Article: ] |
| 8. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4059] [Cited by in F6Publishing: 4345] [Article Influence: 362.1] [Reference Citation Analysis (2)] |
| 9. | Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938-945, 945.e1-945.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 192] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 10. | Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2014;10:153-161. [PubMed] [Cited in This Article: ] |
| 11. | Hemming AW, Berumen J, Mekeel K. Hepatitis B and Hepatocellular Carcinoma. Clin Liver Dis. 2016;20:703-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Ren JS, Shi JF, Li N, Wang YT, Qu C, Zhang Y, Dai M. International trends in primary liver cancer incidence from 1973 to 2007. BMC Cancer. 2015;15:94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978-2007. Int J Cancer. 2016;139:1534-1545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 237] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 14. | Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011 [Online, 15 December 2016] 2012 Bethesda National Cancer Institute. . [Cited in This Article: ] |
| 15. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 770] [Cited by in F6Publishing: 832] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 16. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1196] [Cited by in F6Publishing: 1285] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 17. | Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, Richardson P, El-Serag HB. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182-1188.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 18. | Carrilho FJ, Kikuchi L, Branco F, Goncalves CS, Mattos AA; Brazilian HCC Study Group. Clinical and epidemiological aspects of hepatocellular carcinoma in Brazil. Clinics (Sao Paulo). 2010;65:1285-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Appel-da-Silva MC, Miozzo SA, Dossin IA, Tovo CV, Branco F, de Mattos AA. Incidence of hepatocellular carcinoma in outpatients with cirrhosis in Brazil: A 10-year retrospective cohort study. World J Gastroenterol. 2016;22:10219-10225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Fassio E, Díaz S, Santa C, Reig ME, Martínez Artola Y, Alves de Mattos A, Míguez C, Galizzi J, Zapata R, Ridruejo E, de Souza FC, Hernández N, Pinchuk L; Multicenter Group for Study of Hepatocarcinoma in Latin America; Asociación Latinoamericana para el Estudio del Hígado (ALEH). Etiology of hepatocellular carcinoma in Latin America: a prospective, multicenter, international study. Ann Hepatol. 2010;9:63-69. [PubMed] [Cited in This Article: ] |
| 21. | World Health Organization; UNAIDS. Report on the global AIDS epidemic 2013 [Online, December 20, 2016] 2013 Geneva, WHO. . [Cited in This Article: ] |
| 22. | Zaidi J, Grapsa E, Tanser F, Newell ML, Bärnighausen T. Dramatic increase in HIV prevalence after scale-up of antiretroviral treatment. AIDS. 2013;27:2301-2305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Aldaz P, Moreno-Iribas C, Egüés N, Irisarri F, Floristan Y, Sola-Boneta J, Martínez-Artola V, Sagredo M, Castilla J. Mortality by causes in HIV-infected adults: comparison with the general population. BMC Public Health. 2011;11:300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Hernando V, Perez-Cachafeiro S, Lewden C, Gonzalez J, Segura F, Oteo JA, Rubio R, Dalmau D, Moreno S, Amo JD; CoRIS. All-cause and liver-related mortality in HIV positive subjects compared to the general population: differences by HCV co-infection. J Hepatol. 2012;57:743-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996-2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 464] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 26. | Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384:258-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 484] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 27. | 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1-19. [PubMed] [Cited in This Article: ] |
| 28. | Spano JP, Costagliola D, Katlama C, Mounier N, Oksenhendler E, Khayat D. AIDS-related malignancies: state of the art and therapeutic challenges. J Clin Oncol. 2008;26:4834-4842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Cobucci RN, Lima PH, de Souza PC, Costa VV, Cornetta Mda C, Fernandes JV, Gonçalves AK. Assessing the impact of HAART on the incidence of defining and non-defining AIDS cancers among patients with HIV/AIDS: a systematic review. J Infect Public Health. 2015;8:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 30. | Calabresi A, Ferraresi A, Festa A, Scarcella C, Donato F, Vassallo F, Limina R, Castelli F, Quiros-Roldan E; Brescia HIV Cancer Study Group. Incidence of AIDS-defining cancers and virus-related and non-virus-related non-AIDS-defining cancers among HIV-infected patients compared with the general population in a large health district of Northern Italy, 1999-2009. HIV Med. 2013;14:481-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Castilho JL, Luz PM, Shepherd BE, Turner M, Ribeiro SR, Bebawy SS, Netto JS, McGowan CC, Veloso VG, Engels EA. HIV and cancer: a comparative retrospective study of Brazilian and U.S. clinical cohorts. Infect Agent Cancer. 2015;10:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, Maspoli M, Bouchardy C, Dehler S, Jundt G, Ess S. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer. 2010;103:416-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 33. | Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, Biggar RJ; HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645-1654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 551] [Cited by in F6Publishing: 518] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 34. | Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57:249-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 35. | Brugnaro P, Morelli E, Cattelan F, Petrucci A, Panese S, Eseme F, Cavinato F, Barelli A, Raise E. Non-AIDS definings malignancies among human immunodeficiency virus-positive subjects: Epidemiology and outcome after two decades of HAART era. World J Virol. 2015;4:209-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Soriano V, Vispo E, Fernandez-Montero JV, Labarga P, Barreiro P. Update on HIV/HCV coinfection. Curr HIV/AIDS Rep. 2013;10:226-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377:1198-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 38. | Dimitroulis D, Valsami S, Spartalis E, Pikoulis E, Kouraklis G. Hepatocellular carcinoma in patients co-infected with hepatitis C virus and human immunodeficiency virus. World J Hepatol. 2013;5:323-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 859] [Cited by in F6Publishing: 900] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 40. | Pineda JA, Aguilar-Guisado M, Rivero A, Girón-González JA, Ruiz-Morales J, Merino D, Ríos-Villegas MJ, Macías J, López-Cortés LF, Camacho A, Merchante N, Del Valle J; Grupo para el Estudio de las Hepatitis Víricas (HEPAVIR) de la Sociedad Andaluza de Enfermedades Infecciosas. Natural history of compensated hepatitis C virus-related cirrhosis in HIV-infected patients. Clin Infect Dis. 2009;49:1274-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | van der Helm J, Geskus R, Sabin C, Meyer L, Del Amo J, Chêne G, Dorrucci M, Muga R, Porter K, Prins M; CASCADE Collaboration in EuroCoord. Effect of HCV infection on cause-specific mortality after HIV seroconversion, before and after 1997. Gastroenterology. 2013;144:751-760.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Berretta M, Garlassi E, Cacopardo B, Cappellani A, Guaraldi G, Cocchi S, De Paoli P, Lleshi A, Izzi I, Torresin A. Hepatocellular carcinoma in HIV-infected patients: check early, treat hard. Oncologist. 2011;16:1258-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Di Benedetto N, Peralta M, Alvarez E, Schroder MT, Estepo C, Paz S, Fainboim H. Incidence of hepatocellular carcinoma in hepatitis C cirrhotic patients with and without HIV infection: a cohort study, 1999-2011. Ann Hepatol. 2013;13:38-44. [PubMed] [Cited in This Article: ] |
| 44. | American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14-S80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2830] [Cited by in F6Publishing: 2933] [Article Influence: 293.3] [Reference Citation Analysis (0)] |
| 45. | Phung BC, Sogni P, Launay O. Hepatitis B and human immunodeficiency virus co-infection. World J Gastroenterol. 2014;20:17360-17367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5110] [Cited by in F6Publishing: 4991] [Article Influence: 178.3] [Reference Citation Analysis (0)] |
| 47. | World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-2194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12095] [Cited by in F6Publishing: 14472] [Article Influence: 1315.6] [Reference Citation Analysis (0)] |
| 48. | Klein MB, Rockstroh JK, Wittkop L. Effect of coinfection with hepatitis C virus on survival of individuals with HIV-1 infection. Curr Opin HIV AIDS. 2016;11:521-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Antonello VS, Antonello IC, Zaltron RF, Tovo CV. HIV and hepatitis C virus coinfection. Who is this patient today? Arq Gastroenterol. 2016;53:180-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Soriano V, Puoti M, Sulkowski M, Cargnel A, Benhamou Y, Peters M, Mauss S, Bräu N, Hatzakis A, Pol S. Care of patients coinfected with HIV and hepatitis C virus: 2007 updated recommendations from the HCV-HIV International Panel. AIDS. 2007;21:1073-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 265] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 51. | Puoti M, Bruno R, Soriano V, Donato F, Gaeta GB, Quinzan GP, Precone D, Gelatti U, Asensi V, Vaccher E; HIV HCC Cooperative Italian-Spanish Group. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS. 2004;18:2285-2293. [PubMed] [Cited in This Article: ] |
| 52. | Clifford GM, Rickenbach M, Polesel J, Dal Maso L, Steffen I, Ledergerber B, Rauch A, Probst-Hensch NM, Bouchardy C, Levi F. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135-2141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 53. | Merchante N, Merino E, Rodríguez-Arrondo F, Tural C, Muñoz J, Delgado-Fernández M, Jover F, Galindo MJ, Rivero A, López-Aldeguer J. HIV/hepatitis C virus-coinfected patients who achieved sustained virological response are still at risk of developing hepatocellular carcinoma. AIDS. 2014;28:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Maor Y, Schapiro JM, Bashari D, Martinowitz U. Survival of hepatitis C-infected haemophilia patients is predicted by presence of cirrhosis but not by anti-viral treatment. Ann Hepatol. 2014;13:753-761. [PubMed] [Cited in This Article: ] |
| 55. | Smukler AJ, Ratner L. Hepatitis viruses and hepatocellular carcinoma in HIV-infected patients. Curr Opin Oncol. 2002;14:538-542. [PubMed] [Cited in This Article: ] |
| 56. | Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100:56-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | García-García JA, Romero-Gómez M, Girón-González JA, Rivera-Irigoin R, Torre-Cisneros J, Montero JL, González-Serrano M, Andrade RJ, Aguilar-Guisado M, Grilo I, Martín-Vivaldi J, Salmerón J, Caballero-Granado FJ, Macías J, Vergara-López S, Pineda JA; Grupo Andaluz para el Estudio de las Enfermedades Infecciosas (GAEI); Grupo Andaluz para el Estudio del Hígado (GAEH). Incidence of and factors associated with hepatocellular carcinoma among hepatitis C virus and human immunodeficiency virus coinfected patients with decompensated cirrhosis. AIDS Res Hum Retroviruses. 2006;22:1236-1241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, Corrao G, Boffetta P, La Vecchia C. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2014;25:1526-1535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |