Published online Dec 21, 2018. doi: 10.3748/wjg.v24.i47.5379
Peer-review started: July 13, 2018
First decision: August 27, 2018
Revised: September 1, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: December 21, 2018
To develop a screening test for celiac disease based on the coating of gold nanoparticles with a peptide sequence derived from gliadin, the protein that triggers celiac disease.
20 nm gold nanoparticles were first coated with NeutrAvidin. A long chain Polyethylene glycol (PEG) linker containing Maleimide at the Ω-end and Biotin group at the α-end was used to ensure peptide coating to the gold nanoparticles. The maleimide group with the thiol (-SH) side chain reacted with the cysteine amino acid in the peptide sequence and the biotinylated and PEGylated peptide was added to the NeutrAvidin coated gold nanoparticles. The peptide coated gold nanoparticles were then converted into a serological assay. We used the peptide functionalised gold nanoparticle-based assay on thirty patient serum samples in a blinded assessment and compared our results with the previously run serological and pathological tests on these patients.
A stable colloidal suspension of peptide coated gold nanoparticles was obtained without any aggregation. An absorbance peak shift as well as color change was caused by the aggregation of gold nanoparticles following the addition of anti-gliadin antibody to peptide coated nanoparticles at levels associated with celiac disease. The developed assay has been shown to detect anti-gliadin antibody not only in quantitatively spiked samples but also in a small-scale study on real non-hemolytic celiac disease patient’s samples.
The study demonstrates the potential of gold nanoparticle-peptide based approach to be adapted for developing a screening assay for celiac disease diagnosis. The assay could be a part of an exclusion based diagnostic strategy and prove particularly useful for testing high celiac disease risk populations.
Core tip: In the present study, we demonstrated that a peptide sequence derived from gliadin, a hydrophobic whole protein that induces celiac disease, in conjunction with gold nanoparticles can be used to detect a biomarker for celiac disease from serum. We confirmed our gold nanoparticle based serological assay can detect anti-gliadin antibody not only in quantitatively spiked samples but also in a small-scale study on real non-hemolytic celiac disease patient’s samples.
- Citation: Kaur A, Shimoni O, Wallach M. Novel screening test for celiac disease using peptide functionalised gold nanoparticles. World J Gastroenterol 2018; 24(47): 5379-5390
- URL: https://www.wjgnet.com/1007-9327/full/v24/i47/5379.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i47.5379
Sensing platforms based on the optical properties of gold nanoparticles (AuNPs) for the molecular detection and recognition of disease biomarkers is an important research challenge. Colorimetric sensors based on AuNPs have been applied for detecting targets, such as metal ions[1-4], DNA[5,6], protein conformations[7] and enzyme activity[8], where they have demonstrated high sensitivity and effectiveness.
In recent years, newer designs of nanoparticles with enhanced and controlled surface chemistry are being explored for sensing applications. Peptide-functionalized nanoparticles (PFNs) are one such emerging sensing element. PFNs have previously been used for effective drug delivery for treating brain tumors and for pancreatic cancer treatment as well as for kinase inhibitor screening[9,10,11-12]. Here we demonstrate the potential of PFNs as a colorimetric sensor for screening celiac disease.
Celiac disease (CD) is a small intestine enteropathy affecting genetically susceptible individuals, following the consumption of wheat prolamins (gliadin) and other prolamins of cereals[13]. Population based studies have predicted a high prevalence rate for the disease, with a large number of CD sufferers remaining undiagnosed[14]. The current diagnosis of CD is based on mucosal biopsy that remains the gold standard[15]. Serological testing for gliadin-induced antibodies using an enzyme-linked immunosorbent assay is being widely applied[16,17] and is usually the first line in clinical diagnosis for CD.
Gliadin antigenicity arises due to the higher content and repetitive arrangement of amino acids glutamine (~36 %) and proline (~17%-23%)[18]. This acts as the substrate for the enzyme tissue transglutaminase (tTG: EC 2.3.2.13), resulting in deamidation and formation of an irreversible isopeptidyl bond[19]. Human Leukocyte Antigen-DQ molecules (HLA-DQ2/8) present the deamidated gliadin peptides (DGP) to mucosal CD4+ T cells leading to an immunostimulatory effect[20]. Gluten reactive helper T cells support the activated CD4+ T cells in the intestinal mucosa leading to the release of autoantibodies that act as the serological biomarkers[21,22].
Non-treated CD patients have been shown to have increased concentration of anti-gliadin (AGA), tTG antibodies as well as Anti-DGP antibodies[23,24]. A deamidated peptide sequence derived from α-gliadin amino acids 57-73 has also been identified as an immunogenic peptide sequence that can act as a trigger for CD[25].
In this paper, we present a screening test for CD using gold nanoparticles (AuNPs) coated with a peptide sequence derived from the gliadin protein. We first established a stable suspension (without any significant level of aggregation) of peptide coated AuNPs, which enables us to translate it to a serological assay. We next assessed the sensitivity and specificity levels of the test using serum samples spiked with AGA. Furthermore, we tested our assay on thirty patient serum samples and found that the PFN-based assay could distinguish CD from non-CD patients.
This study highlights the potential of using immunodominant and biomarker specific peptide sequences that can be used for developing an efficient, easy to use screening test for pre-selecting CD cases, which can be then confirmed by mucosal biopsy for CD.
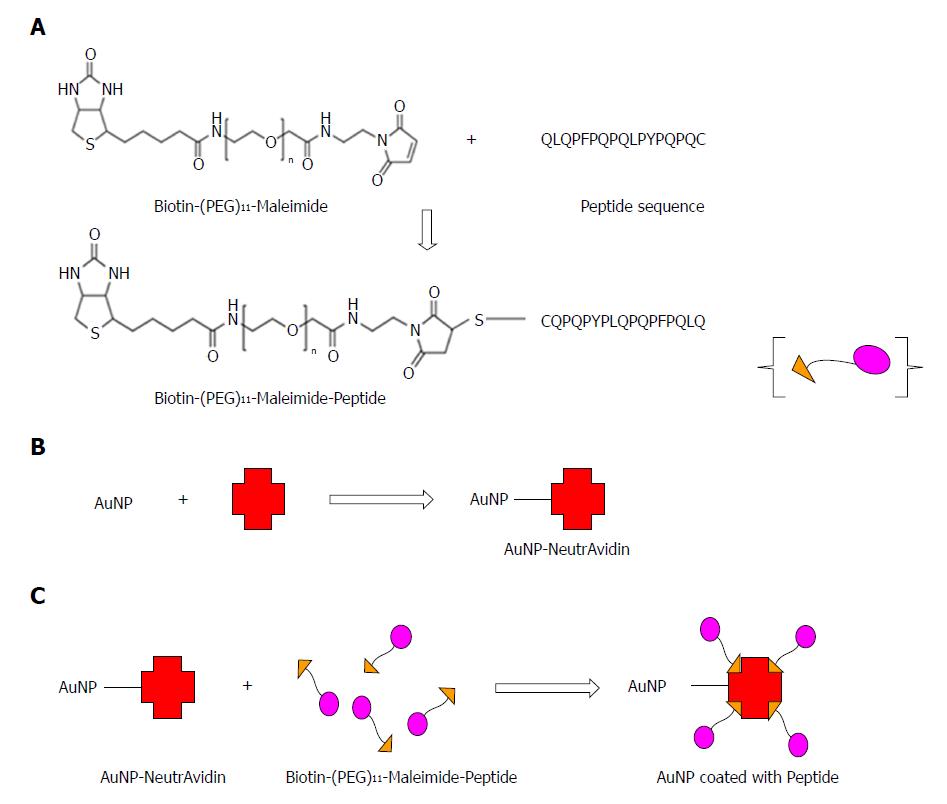
Reagents: 20 nm citrate stabilized gold nanoparticles (AuNPs), bovine serum albumin (BSA), AGA from rabbit, IgG antibody from whole normal rabbit serum, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), phosphate-buffered saline (PBS) were obtained from Sigma-Aldrich (Australia). Poly (ethylene glycol) [N-(2-maleimidoethyl) carbamoyl] methyl ether 2-(biotinylamino) ethane (i.e., Biotin-PEG11-Maleimide) and NeutrAvidin were obtained from Thermo Fisher Scientific (Australia).
Peptide: The Peptide (QLQPFPQPQLPYPQPQC) was synthesised from ChinaPeptides Co., Ltd. (China). The synthetic crude peptides were purified by reversed-phase liquid chromatography to give satisfactory peptide sequence (~90% homogeneity by analytical HPLC) with the correct amino acid sequences and mass spectra. The peptide contained residues 57-72 of α-gliadin and was designated as follows: Peptide: QLQPFPQPQLPYPQPQC.
The peptide (QLQPFPQPQLPYPQPQC) was coated on the surface of the AuNPs in two stages: First, coating with NeutrAvidin followed by a second step of binding of the peptide through a biotin-PEG11-Maleimide linker molecule.
Preparation of the AuNPs coated with NeutrAvidin: 300 μL of 20 nm AuNP were added dropwise to 200 μL of NeutrAvidin dissolved in 10mM HEPES (1 mg/mL) while vortexing. The tube was incubated for 60 min at room temperature with repeated vortexing. The solution was centrifuged for 30 min at 4500 × g, supernatant discarded, and the pellet was re-suspended in 200 μL MilliQ water. The process was repeated twice after which the pellet was re-suspended in 200 μL HEPES buffer followed by centrifugation at 4500 × g for 30 min using an Eppendorf® Microcentrifuge. The NeutrAvidin coated AuNPs were stored at 4 °C.
Binding of peptide to linker molecule: 300 μL of 0.5 mg/mL peptide (QLQPFPQPQLPYPQPQC) dissolved in MilliQ water was added dropwise to 700 μL of 0.5 mg/mL Poly (ethylene glycol) [N-(2-maleimidoethyl) carbamoyl] methyl ether 2-(biotinylamino) ethane (i.e., Biotin-PEG-Maleimide) dissolved in MilliQ water, (Mn 5400, MW 921 Da) having maleimide at the Ω-end and biotin at the α-end. This solution was left overnight at room temperature.
Preparation of the AuNPs coated with peptide using linker: The NeutrAvidin coated AuNPs were centrifuged at 4500 × g for 30 min using an Eppendorf® microcentrifuge, supernatant discarded, the pellet was re-suspended in 100 μL of the peptide-linker solution. The tube was incubated for 60 min at room temperature with repeated vortexing. The solution was centrifuged for 5 min at 4500 × g, supernatant was discarded, and the pellet was re-suspended in 100 μL of MilliQ water. The peptide coated AuNPs were stored at 4 °C for up to 4 wk.
Dynamic light scattering (DLS): The nanoparticle hydrodynamic radius was measured using Zetasizer Nano (Malvern Technologies, Inc.). Measurements were carried out at 25 °C in disposable cuvettes using a sample volume of 500 μL. Each sample was measured in duplicates and the mean value was calculated.
Transmission electron microscopy: High-resolution transmission electron microscopy (TEM) micrographs were obtained using a FEI Tecnai TEM 200V fitted with a Gatan (Pleasantville, CA, United States) CCD camera. Samples were prepared by placing 2 μL of AuNP coated with peptide onto a carbon-coated TEM grid (Agar Scientific, United Kingdom) and the film allowed to air dry for 15 min.
UV-vis measurements: UV-vis measurements were carried out using a Cary series UV-vis spectrophotometer (Agilent Technologies) using a standard 1 cm path-length quartz cuvette. Spectra were obtained from 200 nm to 800 nm. MilliQ water was used as the blank.
Anti-gliadin assay: The immunoassay used to assess the activity of AuNPs coated with peptide with the antibodies is outlined below. Assay steps were performed at room temperature. Briefly, 150 μL of AuNPs coated with peptide were added to 1.5 mL low protein binding Eppendorf® tubes. AGA (1 mg/mL) from rabbit was added to each of the tubes corresponding to concentrations ranging from 2 μg/mL to 20 μg/mL (i.e., 1 μL to 10 μL) to determine the specificity of the reaction between peptide coated AuNPs and AGA. IgG from normal rabbit serum (1 mg/mL) was used as a control antibody and added to 150 μL of AuNPs coated with peptide in concentrations ranging from 2 μg/mL to 20 μg/mL (i.e., 1 μL to 10 μL). MilliQ water was added to the tubes to bring the final volume in each Eppendorf tube up to 225 μL. The UV-vis absorption spectra of solutions containing peptide coated gold nanoparticles and AGA at increasing dilutions (2 μg/mL, 4 μg/mL, 6 μg/mL, 8 μg/mL, 10 μg/mL, 12 μg/mL, 14 μg/mL, 16 μg/mL, 18 μg/mL and 20 μg/mL) were studied using a Cary Series UV-vis spectrophotometer. Readings were taken in triplicate and the student’s t-test was used to determine the P value.
Anti-gliadin assay in spiked human serum: Normal human serum was diluted to 1:20 using 10 mmol/L HEPES buffer. 75 μL of serum from the dilution was spiked with AGA at various dilutions comparable to that seen in celiac patients.
To prevent non-specific binding, 1 μL of 20% BSA dissolved in MilliQ water and was added to 150 μL of 20 nm AuNPs coated with peptide. The tubes were incubated for 30 min room temperature. 75 μL of normal serum spiked with AGA at increasing dilutions of 2 μg/mL, 4 μg/mL, 6 μg/mL, 8 μg/mL and 10 μg/mL, 12 μg/mL, 14 μg/mL, 16 μg/mL, 18 μg/mL and 20 μg/mL was then added to AuNPs coated with peptide. The tubes were then incubated for 30 min room temperature.
Anti-gliadin assay in clinical human serum: Anonymised patient samples were provided by Dr Jason Tye-Din from the Walter and Eliza Hall Institute of Medical Research (WEHI Institute, Melbourne Parkville, Australia). They were collected with informed consent and approval of Melbourne Health and WEHI Human Research Ethic Committees (2003.009 and 03/04) respectively. Ethical approval was also obtained from the University of Technology Sydney Research Ethics committee (UTS HREC ETH16 - 0841) before testing the clinical samples. The clinical samples consisted of 30 human serum samples that were analyzed in a blinded assessment. No prior knowledge of the CD status or any other clinical condition for any of the patient samples was known while testing. Samples were collected from patients with active CD (pre-treatment), treated CD (on a gluten free diet) and controls without CD (Tables 1 and 2). All cases of CD were medically diagnosed and based on typical small intestinal histology usually in conjunction with positive CD serology. The histological interpretation and the serology levels for each of the clinical sample tested using biopsy and the existing commercially available serology tests is presented in Tables 1 and 2.
| Volunteer | Histology | tTG-IgA | DGP-IgG | AuNP-peptide-AGA test |
| n.1 | CD | 1 (< 4) | 3 (< 20) | CD positive |
| n.21 | Non-CD | 0.1 (0-6) | 0.2 (0-6) | CD positive |
| n.3 | CD | > 100 (< 4) | 33 (< 20) | CD positive |
| n.4 | CD | 121 (< 20) | CD positive | |
| n.5 | CD | > 100 (< 4) | > 100 (< 20) | CD positive |
| n.6 | CD | 217 (< 5) | > 150 (< 20) | CD positive |
| n.72 | CD | 13 (0-6) | 23 (0-6) | CD negative |
| n.8 | CD | > 100 (< 5) | > 100 (< 20) | CD positive |
| n.9 | CD | 11 (0-6) | 1.4 (0-6) | CD positive |
| n.10 | CD | 9 (< 4) | 97 (< 20) | CD positive |
| n.11 | CD | 18.2 (0 < 20) | 3 (0.20) | CD positive |
| n.12 | CD | 16 (0-20) | 7 (< 20) | CD positive |
| n.13 | Non-CD | 4 (0-20) | Non-CD | |
| n.14 | CD | 47 (< 5) | 86 (< 5) | CD positive |
| n.152 | CD | 74 (0-20) | CD negative | |
| n.16 | CD | 145 (0-20) | CD positive | |
| n.17 | CD | 57 (< 4) | 93 (< 20) | CD positive |
| n.18 | CD | 149 (<20) | 63 (< 20) | CD positive |
| n.19 | CD | > 100 (< 4) | > 100 (< 20) | CD positive |
| n.201 | Non-CD | 3.8 (< 6) | 37 (< 6) | CD positive |
| n.21 | Non-CD | < 5 (< 5) | < 20 (< 20) | Non-CD |
| n.22 | CD | 180 (0-6) | 21 (0-6) | CD positive |
| n.23 | CD | 20 (0-6) | 8.1 (0-6) | CD positive |
| Volunteer | Histology | tTG-IgA | DGP-IgG | Peptide-AuNP-AGA |
| n.24 | Mucosal lesions | 5 (< 20) | 17 (< 20) | CD positive |
| n.25 | Mucosal lesions | 28 (0-6) | 22 (0-6) | CD positive |
| n.26 | Mucosal lesions | 4.8 (0-6) | 22 (0-6) | CD positive |
| n.27 | Increased γδ+ IELs | < 5 (< 5) | 22 (< 20) | CD positive |
| n.28 | Increased γδ+ IELs | 12 (0-6) | 18 (0-6) | CD positive |
| n.29 | Increased γδ+ IELs | 11 (0-6) | 13 (0-6) | CD positive |
| n.30 | Increased γδ+ IELs | < 5 (< 5) | < 20 (< 20) | CD positive |
Prior to testing, each human serum sample was diluted to 1:10, 1:20 and 1:50 using 10 mmol/L HEPES buffer. 1 μL of 20% BSA dissolved in MilliQ water was added to AuNPs coated with peptide to prevent non-specific binding. 75 μL of serum from each of the dilutions and the tubes were incubated for 15 min at room temperature before the absorbance was measured using a UV-vis spectrophotometer.
Concentration of immunoglobulins in clinical human serum: 200 μL of each human serum sample was centrifuged at 3200 × g or 15 min. The supernatant was removed, and the serum samples were diluted with 200 μL of 10 mmol/L PBS. An equal volume of saturated ammonium sulphate solution was added slowly to achieve a 33% saturated (v/v) final concentration with continuous stirring of the tubes. Samples were kept at 4 °C for 30 min and then centrifuged again at 2500 × g for 15 min. The supernatant was removed, and the pellet was re-suspended by adding 200 μL of 10 mmol/L PBS. The concentrated serum solution was stored at 20 °C till further use.
Zeba™ Spin desalting columns (Thermo Scientific™) were used for the de salting of the immunoglobulins from the concentrated serum solution according to the manufacturer’s instructions. The final concentration of the total immunoglobulins was measured using the NanoDrop and stored at -20 °C until further use.
Colorimetric response curve: A colorimetric response curve was plotted to represent the sensitivity values obtained for the different dilution values for both the AGA and the control antibody (IgG from rabbit serum). The assay sensitivity was determined based on the colorimetric response values calculated as colorimetric response = Imax at 527 nm/ Iat 550 nm, i.e., spectral absorbance value obtained at 527 nm - the wavelength where AuNP coated with peptide show maximum absorbance by itself (no antibodies are added) - divided by the absorbance at 550 nm, where a shift in absorbance is observed following the interaction of the antibody to the AuNP coated with peptide.
The assay sensitivity in spiked serum was calculated as colorimetric response = Imax at 580nm/Iat 527 nm, i.e., absorbance value obtained at 580 nm. This is the wavelength where a shift in absorbance is observed following the interaction of the antibody to the AuNP coated with peptide in serum divided by the maximum absorbance value of AuNP coated with peptide in serum.
To ensure the peptide coating on AuNPs, we used a long chain PEG linker containing Maleimide at the Ω-end and Biotin group at the α-end. Following the reaction of maleimide group with the thiol (-SH) side chain of the cysteine amino acid in the peptide sequence, the biotinylated and PEGylated peptide was added to the NeutrAvidin coated AuNPs.
The coating of the peptide sequence to the colloidal AuNP using the Biotin-(PEG)11-Maleimide linker is represented in the schematic (Figure 1).
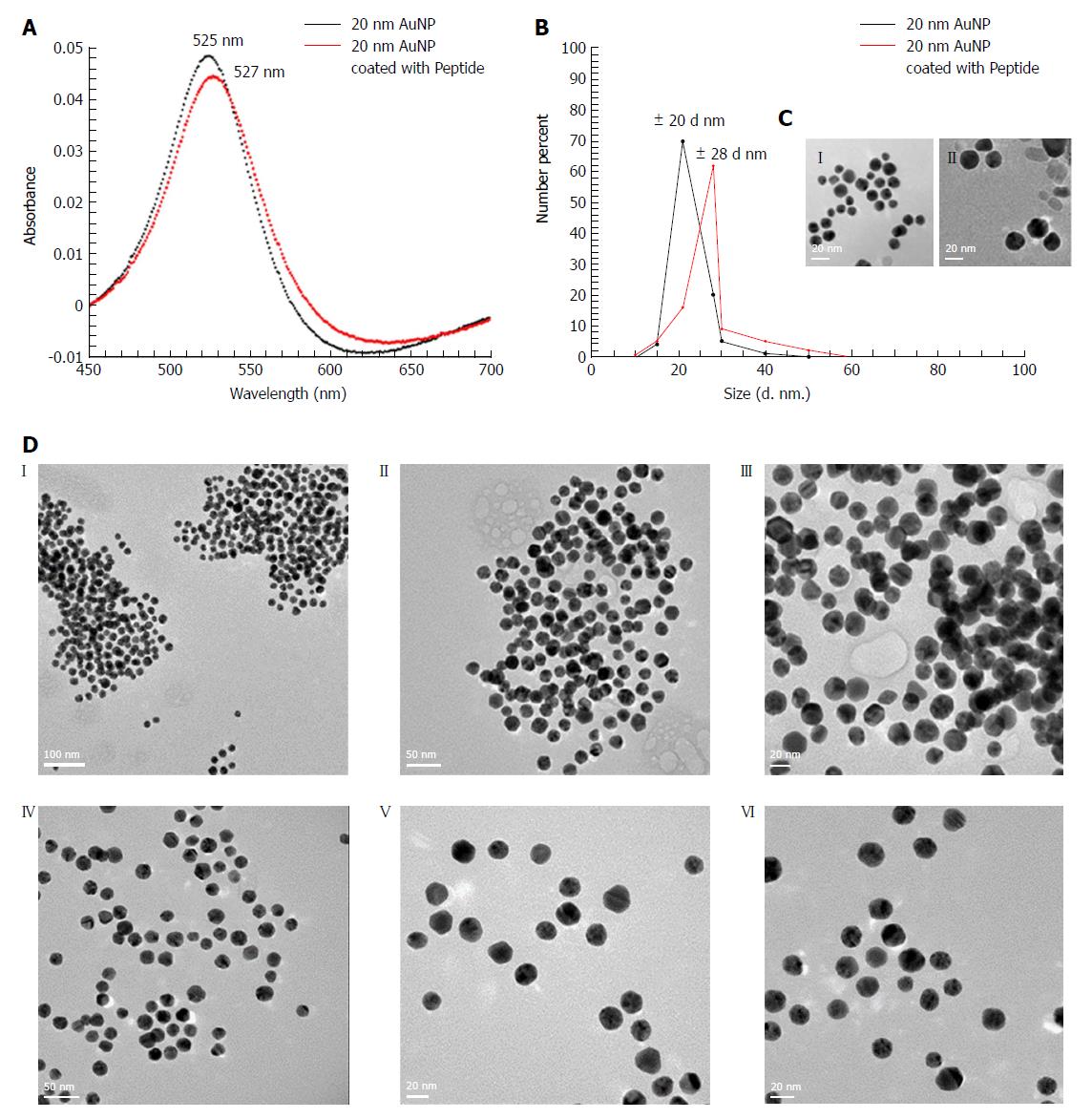
The coating of peptide onto the AuNPs was characterized using the UV-vis spectrophotometer. It was observed that upon coating of peptide onto the AuNPs, there was a shift in absorbance maximum from 525 nm to 527 nm (red-shift) (Figure 2A). The DLS showed an increase in hydrodynamic diameter from 20 nm to 28 nm following coating with peptide (Figure 2B). The hydrodynamic diameter of NeutrAvidin coated AuNPs is presented in Supplementary Figure 1.
To directly observe the coating of peptide onto the surface of the AuNPs, high-resolution TEM imaging was used. Our results showed the presence of a thin layer of material (< 1 nm) surrounding the nanoparticles [Figure 2C (II)], which was not observed on the surface of the uncoated nanoparticles [Figure 2C (I)] that indicated peptide coating. As the layer was very thin, peptide coated and uncoated AuNPs were incubated with AGA (12 μg/mL), wherein the peptide coated AuNPs aggregated [Figure 2D (I, II, III)] while uncoated AuNPs remained dispersed [Figure 2D (IV, V, VI)].
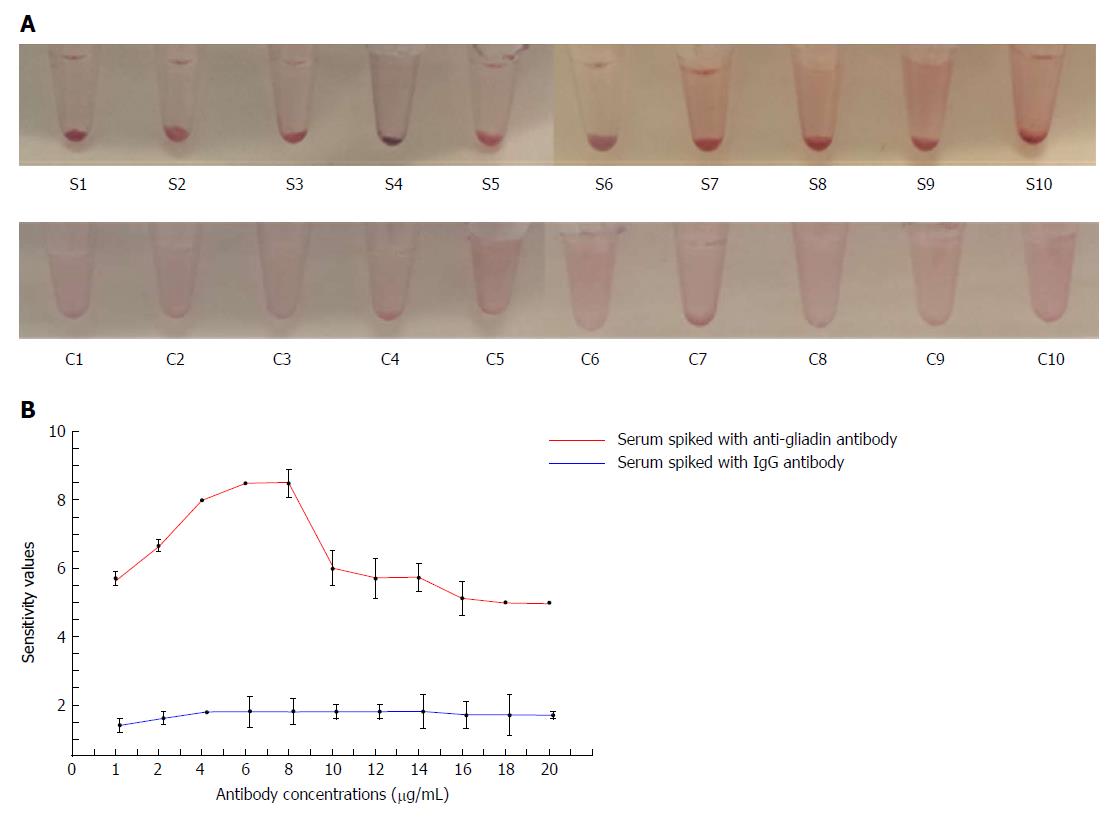
Peptide coated AuNPs were incubated with serial dilutions of rabbit anti-gliadin IgG polyclonal antibody, after 45 min incubation we observed a significant reduction in color as well as a shift in absorbance from 527 nm to 580 nm (Figure 3A and B, Supplementary Figure 2).
To confirm the specificity of the interactions, we tested normal IgG antibody with the peptide coated AuNPs as a control, where we observed no significant reduction in color or shift in the wavelength or aggregation of AuNPs. (Figure 3A and B, Supplementary Figure 3). At all the tested concentrations, the absorbance was significantly lower using AGA as compared to normal IgG (P < 0.005, Figure 3B, Supplementary Table 1).
To confirm the sensitivity of the AGA toward peptide coated AuNPs, we calculated a colorimetric response between our assay (Figure 3C). The colorimetric response reaches a maximum value at a concentration of 8 μg/mL of AGA meaning the highest sensitivity occurred at this concentration. This interaction of the peptide coated AuNPs with AGA resembles the precipitin reaction of antibody-antigen immune complexes. The near constant colorimetric response curve obtained for the control antibody as compared to the response curve obtained for AGA demonstrates the distinct sensitivity of the assay.
A variety of proteins, peptides as well as nucleic acids are constituents of human serum, making it a complex fluid. To reduce background binding, we used 1 μL of 20% BSA as a blocking agent, to lower the non-specific interaction with the peptide coated AuNPs. Spiked human serum containing 2-20 μg/mL AGA was incubated with the peptide coated AuNPs. The results showed an increase in aggregation and precipitation, which was easily detectable by eye, with a specificity up to a value of 2 μg/mL of AGA (Figure 4A). We also observed a reduction in the color of the solution from red to translucent. This change was supported by the increase in the colorimetric response reaching a maximum sensitivity at 8 μg/mL of AGA. The curve then begins to drop off at 10 μg/mL of AGA, however, it remains well above the control IgG level (Figure 4B). In comparison, when normal rabbit IgG was added to the human serum, no precipitate formation or change in color was observed. Normal serum itself did not show any precipitate formation or change in absorbance with a constant response curve at all IgG concentrations.
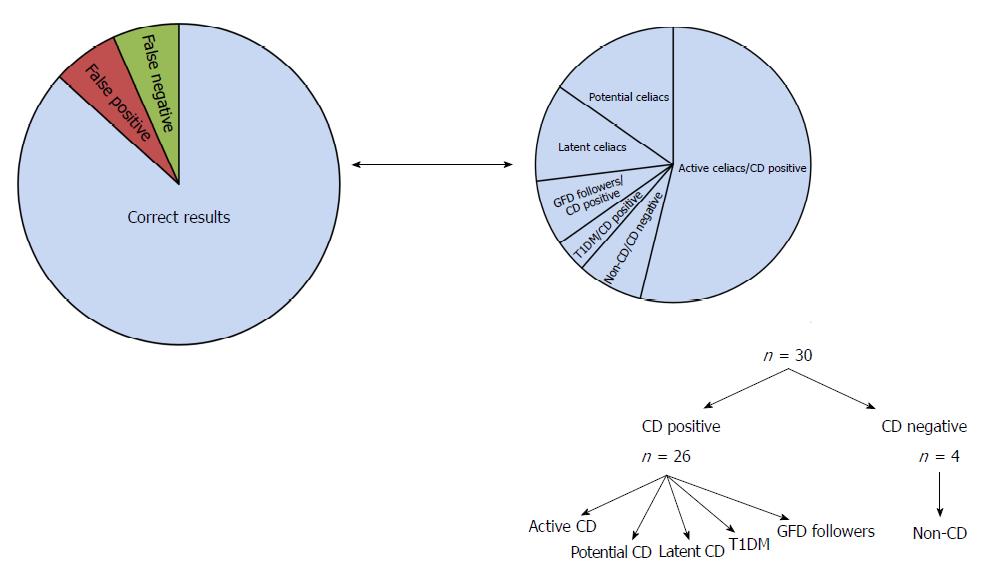
The results for the 30 non-haemolytic, clinical samples were recorded after the visual examination of precipitate formation and determination of shift or change in absorbance values using a UV-vis spectrophotometer. Based on the results observed by eye, the samples have been divided into three categories: clear precipitation, aggregation and colloidal suspension.
The assay sensitivity was determined based on the colorimetric response obtained for each serum sample and is calculated as colorimetric response = Imax at 580 nm/Iat 527 nm. Using this method, the calculated colorimetric response for normal serum (serum without AGA) is 1 and this acts as the cut-off value. Therefore, for the clinical samples, based on the spectral absorbance data, a value of ≤ 1 is indicated as negative for CD and a value above 1 is indicated as CD positive. Based on the observation and calculation of the colorimetric response curve, we summarized the outcomes in Tables 1 and 2 and Figure 5 along with the results reported using other serological methods, biopsy and histology.
The hexapeptide sequence QXQPFP (X being P, Q and L) within gliadin was previously identified as the dominant epitope for IgA and IgG antibodies against deamidated gliadin peptides (a-DGP)[26]. This sequence overlaps with residues 57-62 of native α-gliadin and has been shown to occur with high specificity in sera from CD individuals[27,28]. The 17-mer peptide sequence (QLQPFPQPQLPYPQPQC) used as the antigen in our study is a small, 2 kDa molecular weight hexapeptide containing sequence.
In order to use peptide to coat AuNPs and to develop an assay for AGA, we needed to overcome the potential problem of aggregation of the coated nanoparticles prior to adding the AGA in the test. This was achieved by using a long chain PEG linker containing Maleimide at the Ω-end and Biotin group at the α-end. Following the reaction of maleimide group with the thiol (-SH) side chain of the cysteine amino acid in the peptide sequence, the biotinylated and PEGylated peptide was added to the NeutrAvidin coated AuNPs leading to the formation of peptide coated AuNPs. The coating of peptide onto the AuNPs surface was examined by UV-vis measurements that was based on the observation of a change in the absorbance peak for the nanoparticle or a red-shift[29]. The UV-vis absorbance spectra showed that upon coating of peptide onto the AuNPs, there was a shift in absorbance maximum from 525 nm to 527 nm (red-shift) (Figure 2A). Also, we did not observe any measurable decrease of absorbance at peak wavelength or increase of absorbance at long wavelengths (600-700 nm). These results indicate that no strong AuNP-to-AuNP interactions took place after being coated with peptide and were stable in a dispersed colloidal state with minimal aggregation. In addition, a small peak at 210 nm was also observed following the binding of peptide to AuNPs, as observed in previous studies on peptide coating[30].
We further confirmed the absence of aggregation using DLS, that showed an increase in hydrodynamic diameter from 20 nm to 28 nm following coating with peptide (Figure 2B). The increase in hydrodynamic diameter is larger than that expected for a 2 kDa peptide sequence and can be attributed to the larger molecular weight (68 kDa) NeutrAvidin that coated the AuNPs (Supplementary information S1) through the biotin-maleimide PEG linker. Since the hydrodynamic radius in solution compares directly to the molar mass of the protein[31], in this case, being the peptide sequence as well as NeutrAvidin, an increase in hydrodynamic radius to 28 nm can be correlated. High resolution TEM images of AuNPs coated with peptide showed a “halo” layer surrounding the surface of the nanoparticles indicating that coating of the gold with the peptide had occurred and this helped to confirm the peptide coating to AuNPs. The peptide coats the AuNPs as a monolayer with a sharp border with constant thickness on the gold nanoparticle surface[32].
To assess the capability of peptide-coated AuNPs to detect AGA, serial dilutions of rabbit anti-gliadin IgG polyclonal antibody in a range that normally exists in human serum, were tested[33]. A significant reduction in color as well as a shift in absorbance from 527 nm to 580 nm with a decrease in the absorbance peak (Figure 3), indicated that in the presence of the AGA there was an increase in the intermolecular association of the peptide coated AuNPs. These inter-particle interactions caused aggregation, leading to precipitation and a drop-in absorbance. These results obtained for the peptide coated AuNPs-AGA interaction are consistent with previous work, on the addition of specific analytes, such as metal ions, to citrate stabilised gold nanoparticles[34,35]. Spiked human serum containing 2-20 μg/mL AGA was then incubated with the peptide coated AuNPs. An increased aggregation and precipitation, which was easily detectable by eye in the presence of the AGA was observed (Figure 4). No color change or precipitation was observed in the serum spiked with normal IgG rabbit.
Peptide-coated AuNPs were next tested in a selected set of human serum samples obtained from patients with CD or controls without CD. The main aim was to clearly distinguish the CD affected from the non-CD affected. To achieve that, serum was diluted with PBS, concentrated, purified and then allowed to react with AuNPs coated with peptide. This ensured that the range of the AGA in the sera fell in the peak sensitivity range (2-20 μg/mL).
Out of the thirty samples analysed, fourteen samples that were diagnosed with active CD with high antibody titres as shown by serology and intestinal damage as per biopsy were identified as CD positive using AuNP-Peptide-AGA assay as well. These samples showed the formation of a precipitate and had a clear shift as well as drop in UV-vis absorbance values as well as a high colorimetric response value (refer Table 1). The remaining samples were then classified into various sub-classes based on the analysis using the AuNP-Peptide-AGA assay as described below (refer Tables 1 and 2, Figure 5).
The AuNP-Peptide-AGA assay could correctly identify the patient suffering from another autoimmune disease, Type 1 diabetes (T1DM), as positive for CD and this result matched with the previously conducted biopsy and serology profile of the patient. In comparison, an existing commercially available point-of-care test, SimtomaX® blood drop (Augurix diagnostics) that uses a combination of three different peptides as the DGP antigens was found to be less specific in diagnosing CD in children suffering from T1DM[36].
The elevated risks of patients with T1DM to develop celiac disease arises because of multiple environmental and immunological factors and common genetic background[37]. The prevalence of celiac disease in T1DM is reported high with a mean prevalence rate of 8%[38] making regular monitoring of patients, particularly children with T1DM a necessity. AuNP-Peptide-AGA diagnostic assay shows improved specificity over the existing point-of-care test based on DGP. This test result shows immense potential of the assay in pre-selecting CD sufferers particularly those belonging to high-risk pediatric populations and, therefore, needs to be further explored in large clinical studies.
The cohort of tested samples included two cases where the patients had previously been diagnosed with CD and therefore followed a gluten free diet (GFD). While one person had been on a GFD for more than 8 wk (volunteer number n.11), the other person had been on a GFD for less than 2 wk (volunteer number n.12). While the conventional serology test identified these two patients as negative, the peptide-based assay obtained a positive for CD that is similar to the diagnosis based on mucosal biopsy.
A clear precipitate using the AuNP-Peptide-AGA assay was obtained for three samples (volunteer numbers n.24, n.25, n.26, Table 2) with patchy/irregular mucosal lesions, classified as a broad sub-type of potential or latent celiac. The UV-vis absorbance data supported the visual examination. These three samples were classified as positive for CD as these may be latent celiac sufferers that initially, display mild mucosal atrophy, that then develops to typical atrophy of small intestine mucosa along with a presentation of positive CD serology[39].
Aggregation of nanoparticles along with a drop in UV-vis absorbance values was demonstrated by four clinical samples (volunteer numbers n.27, n.28, n.29, n.30, Table 2). These samples had positive or low positive serology along with mucosal inflammation. The AuNP-Peptide-AGA assay showed results similar to those found using the existing serological assays. These four cases have been identified as “potential” CD positives, a subtype of CD that displays a normal villous architecture but also shows clinical symptoms, such as increased γδ+ intraepithelial lymphocytes along with the presence of gliadin specific antibodies, that are mostly at low titres (< 1:40)[40]. Potential CD cases are often difficult to diagnose by histology which has a lowered predictive value in recognizing such cases. Correct diagnosis for potential CD cases therefore necessitates an evaluation of both serological markers as well as pathological symptoms.
The cohort included four samples identified as negative for CD based on biopsy and existing serology tests (volunteer numbers n.2, n.13, n.20 and n.21, Table 1). Out of the four samples, two samples were correctly identified as CD negative by AuNP-Peptide-AGA assay while the other two samples showed the formation of aggregates and were identified as positive (volunteer numbers n.2 and n.20). As intestinal biopsy has been used as the gold standard for CD confirmation, these two samples have been referred to as a false positive.
Overall, upon comparing the results for the 30 clinical samples, while 26 samples were interpreted similar to the analysis using the existing serology and histology, 2 false positive results and 2 false negative results were obtained using the AuNP-Peptide-AGA assay giving the AuNP-Peptide-AGA assay an overall accuracy of 86.6%.
Celiac disease is induced by the protein gliadin, present in wheat and some other cereals causing damage to the small intestine. In the present study, we demonstrated that a peptide sequence derived from gliadin in conjunction with AuNPs can be used as an efficient tool to detect a biomarker for CD from serum. This was achieved by developing a methodology to coat the small antigenic peptide sequence on the surface of AuNPs without causing aggregation. A color change and absorbance peak shift caused by the aggregation of AuNPs was observed following the addition of AGA to peptide coated AuNPs at levels associated with CD. We confirmed that the developed assay can detect AGA not only in quantitatively spiked samples but also in a small-scale study on real non-hemolytic CD patient’s samples.
The present study shows that this novel peptide functionalised AuNP based assay is useful for pre-selecting CD diseases particularly in high-risk pediatric populations that can be then confirmed by mucosal biopsy. The developed assay has high accuracy levels and is relatively cheaper to develop, the assay format has potential to be adapted as point-of-care test that would be useful in an exclusion diagnostic strategy as positive result would strengthen the possibility of CD that can be confirmed using intestinal biopsy.
Celiac disease is a chronic immune mediated disorder of the small intestine caused by consuming the protein gluten present in wheat and some other cereals. Following the activation of the innate immune system, a number of cytokines as well as antibodies are released in celiac patients that can be used as specific biomarkers to develop diagnostic tests. Over the years, a number of diagnostic tests have been developed, however, in spite of the good initial results in terms of sensitivity and specificity, when these tests are used on a large scale they have lowered predictive values. In the present study, a novel assay using peptide functionalised gold nanoparticles was developed that can be useful in an exclusion based diagnostic strategy.
The number of celiac disease sufferers are rapidly increasing throughout the world, and there is an increased need for newer detection methods that are easy to use, accurate as well as cheaper to enable early identification of the disease. The present study addresses this issue through the development of a novel assay combining the unique properties of gold nanoparticles with the specificity of the antibodies. The developed assay shows great potential to be developed as a point-of-care test that would be beneficial for large scale screening of celiac disease.
In order to develop a celiac diagnostic assay based on the properties of the gold nanoparticles combined with the specificity of the antibodies from serum the following aims have been addressed in this work: (1) To develop method for the binding and adsorption of peptide derived from gliadin, the main antigenic protein causing celiac disease on the surface of gold nanoparticles; (2) to detect and measure the concentrations of antibodies to be used as biomarkers in serum; (3) to test and validate the developed test on real patient serum samples.
The peptide coated gold nanoparticles were characterized using UV-vis spectra, dynamic light scattering and transmission electron microscopy. The UV-vis absorbance readings of peptide coated AuNPs following interactions with AGA and IgG from rabbit serum (control antibody) were used to calculate the percentage absorbance values. The students t-test was used to compare the sets of quantitative data that were collected independently of one another to calculate the p value and determine statistical significance. The assay sensitivity was determined based on the colorimetric response values.
The clinical accuracy of the peptide coated AuNPs was determined using a selected, varied set of thirty human serum samples obtained from patients with celiac disease or controls without celiac disease. The results for the thirty clinical samples were recorded after the visual examination of precipitate formation and the determination of a shift or change in absorbance values using a UV-vis spectrophotometer. The assay sensitivity was determined based on the colorimetric response values obtained for each serum sample.
The peptide sequence was successfully coated to the AuNP and characterization methods indicated that a stable colloidal suspension of the peptide coated AuNPs was achieved that was sustained by the high affinity biotin-avidin interactions.
The addition of anti-gliadin antibody to peptide coated AuNPs as well as in spiked serum at a threshold level resulted in lowering as well as a shift of absorbance peaks that indicated the aggregation of AuNPs. On the other hand, in the presence of a non-specific, normal IgG antibody there was no interaction between the peptide coated AuNPs, with no reduction in color or shift in wavelength or aggregation of AuNPs.
The clinical accuracy of the peptide coated AuNPs was tested using 30 clinical samples, where 26 samples were shown to have the same analysis as that obtained with existing serology and histology, however, 2 false positive results and 2 false negative results were also obtained using the AuNP-peptide-AGA assay giving the AuNP-peptide-AGA assay an overall accuracy of 86.6%.
In this study, we demonstrate the potential of peptide functionalised gold nanoparticles as a colorimetric sensor for screening celiac disease. The AuNP-peptide assay seems promising for development as a point-of-care test, this is because it is based on the formation of a precipitate as well as a reduction in color for a positive sample in the presence of a celiac disease specific autoantibody, thereby, eliminating the need for secondary antibody. This greatly reduces the cost of development for the assay and would be a step in the direction of one-step detection for celiac disease based on single antibody detection.
The AuNP-Peptide based approach shows great potential and would be particularly useful in aiding large-scale screening of the general population, particularly in the pre-selection of young Celiac disease (CD) sufferers which can be then confirmed by mucosal biopsy. A positive result would strengthen the possibility of CD while a negative test would help avoid unnecessary intestinal biopsy thereby reducing the economic burden on healthcare resources resulting in cost savings.
The authors thank Dr. Jason Tye-Din, MBBS, PhD, FRACP, Immunology Division, The Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia, for providing clinical samples as well for his critical review and feedback on the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Ciaccio EJ, Prasad KK, Rostami K, Rostami-Nejad M S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
| 1. | Lee JS, Han MS, Mirkin CA. Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew Chem Int Ed Engl. 2007;46:4093-4096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1096] [Cited by in F6Publishing: 893] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 2. | Hung YL, Hsiung TM, Chen YY, Huang YF, Huang CC. 2010, Colorimetric Detection of Heavy Metal Ions Using Label-Free Gold Nanoparticles and Alkanethiols. J Phys Chem C. 2010;114:16329-16334. [DOI] [Cited in This Article: ] |
| 3. | Chen GH, Chen WY, Yen YC, Wang CW, Chang HT, Chen CF. Detection of mercury(II) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal Chem. 2014;86:6843-6849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 307] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 4. | Chen W, Cao F, Zheng W, Tian Y, Xianyu Y, Xu P, Zhang W, Wang Z, Deng K, Jiang X. Detection of the nanomolar level of total Cr[(iii) and (vi)] by functionalized gold nanoparticles and a smartphone with the assistance of theoretical calculation models. Nanoscale. 2015;7:2042-2049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science. 1997;277:1078-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3738] [Cited by in F6Publishing: 2759] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 6. | Wang Q, Liu R, Yang X, Wang K, Zhu J, He L, Li Q. Surface plasmon resonance biosensor for enzyme-free amplified microRNA detection based on gold nanoparticles and DNA supersandwich. Sens Actuators B Chem. 2016;223:613-620. [DOI] [Cited in This Article: ] |
| 7. | Tsai DH, DelRio FW, Keene AM, Tyner KM, MacCuspie RI, Cho TJ, Zachariah MR, Hackley VA. Adsorption and conformation of serum albumin protein on gold nanoparticles investigated using dimensional measurements and in situ spectroscopic methods. Langmuir. 2011;27:2464-2477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 8. | Wang J, Wu L, Ren J, Qu X. Visualizing human telomerase activity with primer-modified Au nanoparticles. Small. 2012;8:259-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Gao H, Xiong Y, Zhang S, Yang Z, Cao S, Jiang X. RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and neovasculature dual targeting delivery and elevated tumor penetration. Mol Pharm. 2014;11:1042-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Lee GY, Qian WP, Wang L, Wang YA, Staley CA, Satpathy M, Nie S, Mao H, Yang L. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano. 2013;7:2078-2089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 11. | Gupta S, Andresen H, Ghadiali JE, Stevens MM. Kinase-actuated immunoaggregation of Peptide-conjugated gold nanoparticles. Small. 2010;6:1509-1513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Gupta S, Andresen H, Stevens MM. Single-step kinase inhibitor screening using a peptide-modified gold nanoparticle platform. Chem Commun (Camb). 2011;47:2249-2251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, Hadjivassiliou M, Kaukinen K, Kelly CP, Leonard JN. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1002] [Cited by in F6Publishing: 1022] [Article Influence: 92.9] [Reference Citation Analysis (1)] |
| 14. | Marsh MN, Vincenzo V, Srivastava A. Histology of gluten related disorders. Gastroenterol Hepatol Bed Bench. 2015;8:171-177. [Cited in This Article: ] |
| 15. | Rostami K, Marsh MN, Johnson MW, Mohaghegh H, Heal C, Holmes G, Ensari A, Aldulaimi D, Bancel B, Bassotti G. ROC-king onwards: intraepithelial lymphocyte counts, distribution & role in coeliac disease mucosal interpretation. Gut. 2017;66:2080-2086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Dahlbom I, Nyberg BI, Berntson L, Hansson T. Simultaneous detection of IgA and IgG antibodies against tissue transglutaminase: The preferred pre-biopsy test in childhood celiac disease. Scand J Clin Lab Invest. 2016;76:208-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Benkebil F, Combescure C, Anghel SI, Besson Duvanel C, Schäppi MG. Diagnostic accuracy of a new point-of-care screening assay for celiac disease. World J Gastroenterol. 2013;19:5111-5117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 35] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Wieser H. Relation between gliadin structure and coeliac toxicity. Acta Paediatr Suppl. 1996;412:3-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Piper JL, Gray GM, Khosla C. High selectivity of human tissue transglutaminase for immunoactive gliadin peptides: implications for celiac sprue. Biochemistry. 2002;41:386-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Lundin KE, Scott H, Hansen T, Paulsen G, Halstensen TS, Fausa O, Thorsby E, Sollid LM. Gliadin-specific, HLA-DQ(alpha 1*0501,beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med. 1993;178:187-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 434] [Cited by in F6Publishing: 421] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Sollid LM, Jabri B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat Rev Immunol. 2013;13:294-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 22. | du Pré MF, Sollid LM. T-cell and B-cell immunity in celiac disease. Best Pract Res Clin Gastroenterol. 2015;29:413-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Dieterich W, Laag E, Schöpper H, Volta U, Ferguson A, Gillett H, Riecken EO, Schuppan D. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 410] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | Amarri S, Alvisi P, De Giorgio R, Gelli MC, Cicola R, Tovoli F, Sassatelli R, Caio G, Volta U. Antibodies to deamidated gliadin peptides: an accurate predictor of coeliac disease in infancy. J Clin Immunol. 2013;33:1027-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Anderson RP, Degano P, Godkin AJ, Jewell DP, Hill AV. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med. 2000;6:337-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 423] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 26. | Osman AA, Günnel T, Dietl A, Uhlig HH, Amin M, Fleckenstein B, Richter T, Mothes T. B cell epitopes of gliadin. Clin Exp Immunol. 2000;121:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Piaggio MV, Demonte AM, Sihufe G, Garcilazo S, Esper MC, Waggener M, Aleanzi M. Serological diagnosis of celiac disease: anti-gliadin peptide antibodies and anti-tissue transglutaminase. Medicina. 1999;59:693-697. [Cited in This Article: ] |
| 28. | Aleanzi M, Demonte AM, Esper C, Garcilazo S, Waggener M. Celiac disease: antibody recognition against native and selectively deamidated gliadin peptides. Clin Chem. 2001;47:2023-2028. [PubMed] [Cited in This Article: ] |
| 29. | Link S, El-Sayed MA. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J Phys Chem B. 1999;103:4212-4217. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2051] [Cited by in F6Publishing: 2058] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 30. | Slocik JM, Govorov AO, Naik RR. Plasmonic circular dichroism of Peptide-functionalized gold nanoparticles. Nano Lett. 2011;11:701-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 31. | Jans H, Liu X, Austin L, Maes G, Huo Q. Dynamic light scattering as a powerful tool for gold nanoparticle bioconjugation and biomolecular binding studies. Anal Chem. 2009;81:9425-9432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 32. | Jürgens L, Nichtl A, Werner U. Electron density imaging of protein films on gold-particle surfaces with transmission electron microscopy. Cytometry. 1999;37:87-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 33. | O'Farrelly C, Kelly J, Hekkens W, Bradley B, Thompson A, Feighery C, Weir DG. Alpha gliadin antibody levels: a serological test for coeliac disease. Br Med J (Clin Res Ed). 1983;286:2007-2010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 82] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Wang Z, Lee JH, Lu Y. Label-free colorimetric detection of lead ions with a nanomolar detection limit and tunable dynamic range by using gold nanoparticles and DNAzyme. Adv Mater. 2008;20:3263-3267. [DOI] [Cited in This Article: ] |
| 35. | Guo Y, Wang Z, Qu W, Shao H, Jiang X. Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens Bioelectron. 2011;26:4064-4069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 36. | Bienvenu F, Besson Duvanel C, Seignovert C, Rouzaire P, Lachaux A, Bienvenu J. Evaluation of a point-of-care test based on deamidated gliadin peptides for celiac disease screening in a large pediatric population. Eur J Gastroenterol Hepatol. 2012;24:1418-1423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Volta U, Tovoli F, Caio G. Clinical and immunological features of celiac disease in patients with Type 1 diabetes mellitus. Expert Rev Gastroenterol Hepatol. 2011;5:479-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Ventura A, Magazù G, Gerarduzzi T, Greco L. Coeliac disease and the risk of autoimmune disorders. Gut. 2002;51:897; author reply 897-897; author reply 898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Kaukinen K, Mäki M, Partanen J, Sievänen H, Collin P. Celiac disease without villous atrophy: revision of criteria called for. Dig Dis Sci. 2001;46:879-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Volta U, Villanacci V. Celiac disease: diagnostic criteria in progress. Cell Mol Immunol. 2011;8:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |