Published online Nov 7, 2018. doi: 10.3748/wjg.v24.i41.4617
Peer-review started: August 19, 2018
First decision: October 9, 2018
Revised: October 11, 2018
Accepted: October 21, 2018
Article in press: October 21, 2018
Published online: November 7, 2018
Hepatitis C virus (HCV) infection is associated with extrahepatic manifestations, among these there is an increased risk of atherosclerosis and cardiovascular disease as well as an increased cardiovascular mortality. Several direct and indirect HCV pro-atherogenic mechanisms have been proposed. HCV lives and replicates within carotid plaques, promoting a local environment of pro-atherogenic factors. In addition, it causes conditions such as insulin resistance, diabetes, hepatic steatosis, cryoglobulinemia and endotoxinemia that are associated with the development of atherosclerosis and cardiovascular disease. Therapeutic regimens based on direct-acting antiviral agents (DAA) are currently available with high efficacy in HCV clearance and improvement of liver disease, but does HCV eradication also improve atherosclerosis and the risk of cardiovascular disease? Recently, a multi-center study has shown that elimination of HCV improves carotid atherosclerosis. Two studies have shown that DAA treatments significantly reduce the risk of cardiovascular events. Several studies have assessed the impact of HCV clearance on pro-atherosclerosis metabolic conditions showing improvement in cardiovascular risk biomarkers, disappearance or improvement of insulin resistance, reduction of risk of developing diabetes and improvement of glycemic control. There are also evidences that HCV clearance promotes the recovery of cytokines and inflammatory markers associated with atherosclerosis and the disappearance of cryoglobulinemia. Available data show that clearance of HCV by DAAs is associated with an improvement in atherosclerosis and metabolic and immunological conditions that promote the development of cardiovascular disease. However, the data are not sufficient to allow definitive conclusions and further studies will be needed to definitively clarify the impact of HCV clearance on atherosclerosis and cardiovascular disease.
Core tip: Chronic hepatitis C (HCV) infection is associated with an increased risk of atherosclerosis, cardiovascular disease and cardiovascular mortality. HCV lives within carotid plaques, promotes pro-atherogenic conditions such as pro-inflammatory cytokines, oxidative stress, insulin resistance, diabetes, steatosis, cryoglobulinemia and endotoxinemia. Direct-acting antiviral agents (DAA) are highly effective and safe therapeutic regimens. There are a number of studies showing that HCV clearance by DAAs is associated with an improvement in atherosclerosis and a reduced risk of cardiovascular events. In addition, HCV clearance is associated with improvement of metabolic and immunological conditions that promote cardiovascular disease. Further studies will be needed to confirm these promising data.
- Citation: Adinolfi LE, Rinaldi L, Nevola R. Chronic hepatitis C, atherosclerosis and cardiovascular disease: What impact of direct-acting antiviral treatments? World J Gastroenterol 2018; 24(41): 4617-4621
- URL: https://www.wjgnet.com/1007-9327/full/v24/i41/4617.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i41.4617
The global prevalence of hepatitis C virus (HCV) infection was estimated at 1% in 2015 and therefore around 71 million people worldwide are still considered to be infected[1]. Chronic HCV infection beyond the liver is a systemic disease, so its clinical impact and prognosis depend not only on the risk of complications and deaths related to liver disease, but also on HCV-related extrahepatic manifestations. Among the latter, HCV infection was associated with an increased risk of sub-clinical atherosclerosis, myocardial injury, peripheral artery disease, cerebro- and cardio-vascular events and increased cardiovascular mortality[2,3]. Two meta-analysis, although conducted on heterogeneity data, confirm the overall negative impact of HCV infection on cardiovascular changes and mortality, but suggest that a better understanding of this potential association is still needed[4-6].
Atherosclerosis is a chronic inflammatory disease and the mechanisms by which HCV can induce or facilitate the development of atherosclerosis are not fully understood. Chronic HCV infection causes hepatic and systemic inflammation[7] and it has been hypothesized that direct and indirect mechanisms can be involved in the development of atherosclerosis via increased levels of pro-atherogenic chemokines and cytokines[7] and inducing pro-atherogenic metabolic factors[7]. HCV has been shown to live and replicate within carotid plaques[8], supporting the hypothesis that HCV plays a direct pro-atherogenic role by inducing arterial inflammation, likely via the pro-inflammatory cytokine interleukin 1β[3]. Furthermore, HCV structural and non-structural proteins play an important role in initiating and maintaining chronic inflammation and in generating oxidative stress, which trigger atherogenesis[3]. HCV also interferes with glucose and lipid metabolism, leading to insulin resistance (IR), diabetes and liver steatosis, which are known factors that induce atherosclerosis[9]. A high TNF-α/adiponectin ratio was found in HCV-infected patients that is related to the development of IR and atherosclerosis[10]. It has been reported that atherosclerosis in HCV patents was also associated with advanced liver fibrosis[11].
Cryoglobulinemia occurs with high frequency in patients with chronic HCV infection and the virus plays an etiological and pathogenic role in its development[12]. The presence of cryoglobulinemia is associated with vasculitis and cardiovascular events[13]. Recently, it has been shown that endotoxinemia induces a pro-atherogenic inflammatory condition and an increase in oxidative stress levels that contribute to the development of atherosclerosis in chronic HCV patients[12].
In this complex scenario, a question arises: Can the eradication of HCV improve atherosclerosis and reduce the risk of cardiovascular disease?
Most of the available data on the impact of HCV clearance on matter is derived from patient populations receiving interferon (IFN)-based therapies[14-16]. The data showed that HCV clearance by IFN reduced both cardiovascular events and mortality, but these studies do not allow to discriminate whether the effects observed after sustained virological response (SVR) are a direct consequence of the elimination of the virus, or an effect of IFN, or associated with the selection bias of patients to be treated with IFN.
The mystifying aspects of IFN treatment should be overcome by the recent introduction of regimens based on direct-acting antiviral agents (DAAs) for the treatment of HCV infection that have a short duration, are safe and effective in 92%-98% of cases. DAAs, which have no particular limitations to their use and are therefore feasible for almost all patients with HCV infection regardless of their stage of hepatic injury, have direct antiviral mechanisms and do not directly interfere with other conditions that potentially affect atherosclerosis and therefore a positive impact would underline the role of the virus on arteriosclerosis. These characteristics favor the possibility of assessing whether the clearance of HCV can reduce cardiovascular morbidity and mortality in patients with chronic HCV infection. It is obvious that the follow-up time after treatment with SVR by DAAs is relatively short to give definitive answers. Furthermore, few data have been published in the field, although preliminary results seem to indicate a positive impact of HCV clearance on sub-clinical atherosclerosis, cardiovascular events and pro-atherogenic conditions.
The most surprising and perhaps unexpected data have been published recently and concern the direct impact of HCV clearance by DAAs on sub-clinical atherosclerosis[17]. A multicenter Italian study of 182 consecutive HCV patients with advanced fibrosis (F3) or compensated cirrhosis (66% of patients) evaluated the effect of HCV clearance on subclinical carotid arteriosclerosis vs an untreated control group. Carotid atherosclerosis was evaluated using a high-resolution B-mode ultrasound with a multifrequency linear probe. Carotid measurements were performed at baseline, before initial treatment and 9-12 mo after withdrawal of DAA treatment. Fifty-six percent of the patients were male, mean age 63 years, obesity was present in 14%, hypertension in 42% and type 2 diabetes in 20% of patients. The control group showed similar characteristics. At baseline, carotid atherosclerosis was found in 42.8% of patients and the mean carotid thickness (IMT) was 0.94 mm. At the end of follow-up, a significant decrease in carotid IMT was observed (from 0.94 mm to 0.81 mm, P < 0.001), while no effect was observed on carotid plaques. The BMI of these patients did not change during follow-up, while a significant increase in serum cholesterol levels was observed. There were no significant changes in the IMT in the control group. The results of the study have shown that the eradication of HCV by DAA has led to an improvement in carotid atherosclerosis and in particular a significant reduction of the IMT. It is also important to note that in patients with advanced atherosclerosis, as evidenced by the presence of carotid plaques, the clearance of HCV did not lead to significant changes in plaque value. Results were also confirmed when patient analysis was stratified by different cardiovascular risk factors. This is an important study that demonstrates how the clearance of HCV by DAA improves carotid atherosclerosis in patients with advanced liver fibrosis, but also emphasizes that patients with advanced atherosclerosis do not benefit from this effect and therefore the need for early treatment.
Because of the short period of time, there are few published articles that assessed the impact of DAA on cardiovascular outcome[18,19]. A large prospective study[18] evaluated the cardiovascular outcomes in a cohort of patients with HCV infection with compensated cirrhosis treated with IFN or DAAs. Data showed that patients with SVR had a significant reduction in cardiovascular events, although the data had not been analyzed for the type of regimen used (IFN or DAAs). A second retrospective study was published only in abstract form[19] and evaluated a large real-world cohort of HCV-infected patients. The authors assessed cardio and cerebro-vascular events in patients treated or not treated with DAAs. After adjustment for age, sex, cirrhosis of the liver, other comorbidities and use of cardiovascular drugs, data showed that DAA treatments significantly reduced the risk of cardio- and cerebro-vascular events (cumulative HR: 0.86, 95%CI: 0.74-0.99) compared to untreated patients.
These data, although preliminary, seem to confirm the results reported in patients treated with IFN and indicate a key role of HCV in the development of both subclinical atherosclerosis and cardiovascular disease and of a greater concern that the clearance of HCV is associated with an improvement of cardiovascular damage.
Several studies have assessed the impact of HCV clearance by DAAs on metabolic conditions considered pro-atherosclerosis[20-22]. A recent post-hoc analysis was conducted using a large populations of patients with chronic hepatitis C genotype 1 infection and treated with DAA (3D) ± ribavirin (RBV) in order to evaluate the impact of treatment on cardiovascular and metabolic markers during a follow-up period of 52 wk post-treatment[20]. Serum triglyceride and glucose fasting levels were evaluated as markers, which are known to be risk factors for coronary heart disease associated with vascular complications and all-cause long-term mortality[23-25]. The results of this analysis showed that HCV patients treated with 3D ± RBV who obtained SVR had a significant improvement, at least for 1 year after treatment, of the studied cardiovascular risk biomarkers[20]. The authors suggest that treatment with DAAs may lead to a possible beneficial effect on cardiovascular outcomes.
A recent prospective study evaluated IR changes three months after SVR from DAA treatments in non-diabetic patients with HCV genotype 1 infection and advanced liver disease[21]. Data showed that virus eradication was associated with the disappearance or significant reduction of IR in 76% of cases. Furthermore, these patients also showed a significant improvement in glucose metabolism[21]. The results also demonstrated that improvement in glucose metabolism was negligible in patients with advanced cirrhosis[21]. A large study[22] in the real world showed that SVR of DAAs is associated with a significant reduction in the risk of developing diabetes, in addition many studies have shown significant improvement in glycemic control in type 2 diabetic patients after HCV clearance by the DAAs[22].
Overall, the above data showing that clearance of HCV by DAAs has a positive impact on pro-atherogenic metabolic factors and appears to indicate that SVR may have an effect in reducing the development of atherosclerosis and cardiovascular disease.
In addition to the above data demonstrating that the elimination of HCV from treatment with DAAs has a direct impact on atherosclerosis and on the metabolic conditions associated with atherosclerosis, there are preliminary evidences that the elimination of HCV by DAA induces a restoration of cytokines and inflammatory markers implicated in the development of atherosclerosis[26,27], although the data seem to indicate that this improvement was less sustained in HCV patients with advanced liver disease. Furthermore, several studies have shown that clearance of HCV by DAAs improves or eliminates cryoglobulinemia and related vasculitis syndromes[28,29] suggesting a possible reduction of cardiovascular consequences associated with the presence of cryoglobulinemia.
At present there are no data on the effect of clearance of HCV by DAA on endotoxinemia. However, it is possible to hypothesize that the improvement of liver disease and changes in the microbiota can improve endotoxinemia and reduce the risk of atherosclerosis. Likewise, improvement in liver disease should improve the atherogenic environment associated with HCV-related liver disease and reduce the progression of atherosclerosis.
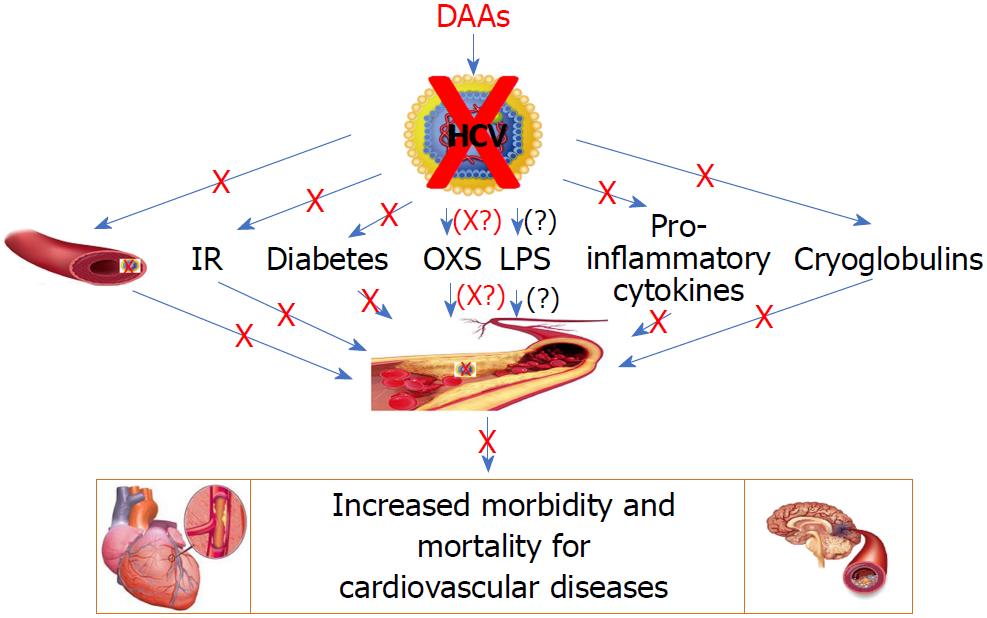
By making an overall assessment of the current data available in the field, we can underline that, on the one hand, it is evident that data on the subject are relatively poor and do not allow definitive conclusions on the impact of HCV clearance on atherosclerosis and cardiovascular disease and that further studies will be needed to definitively clarify this aspect; on the other hand, it is undeniable that all the data currently available show that the clearance of HCV by DAAs, at least in the short term, is associated with an improvement of atherosclerosis and of metabolic and immunological conditions that favor the development of arteriosclerosis. Figure 1 shows the various direct and indirect factors considered responsible for the atherosclerosis and cardiovascular disease associated with chronic HCV infection, as well as the effect of the clearance of HCV by DAAs.
The data seem to indicate that the elimination of HCV by DAAs improves atherosclerosis and pro-atherogenic metabolic, inflammatory and immunological conditions and therefore should improve and prevent cardiovascular damage. However, the data also show that the treatment of advanced forms of hepatic and atherosclerotic damage is poorly effective and therefore, the results underscore the importance of an early treatment. Considering the limited number of studies on the subject, further studies are needed to confirm that the elimination of HCV not only leads to an improvement in liver disease, but also has an important positive effect on an extrahepatic manifestation of HCV which is atherosclerosis and consequent cardiovascular disease. In addition, future studies should also evaluate whether short-term results will be maintained over the long term, as some cardiovascular risk factors such as BMI and serum cholesterol tend to increase after HCV clearance[17,30]. In view of the occurrence of these predisposing factors to atherosclerosis, it might be useful to advise patients, who recover from HCV infection, to control body weight by avoiding its increase and by eating a low-fat diet.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: García-Elorriaga G, Kharlamov AN, Zampino R S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
| 1. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1330] [Cited by in F6Publishing: 1384] [Article Influence: 197.7] [Reference Citation Analysis (0)] |
| 2. | Babiker A, Jeudy J, Kligerman S, Khambaty M, Shah A, Bagchi S. Risk of Cardiovascular Disease Due to Chronic Hepatitis C Infection: A Review. J Clin Transl Hepatol. 2017;5:343-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Adinolfi LE, Zampino R, Restivo L, Lonardo A, Guerrera B, Marrone A, Nascimbeni F, Florio A, Loria P. Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol. 2014;20:3410-3417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 121] [Cited by in F6Publishing: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Petta S, Maida M, Macaluso FS, Barbara M, Licata A, Craxì A, Cammà C. Hepatitis C Virus Infection Is Associated With Increased Cardiovascular Mortality: A Meta-Analysis of Observational Studies. Gastroenterology. 2016;150:145-155.e4; quiz e15-e16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | He Huang, Kang R, Zhao Z. Hepatitis C virus infection and risk of stroke: a systematic review and meta-analysis. PLoS One. 2013;8:e81305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Huang H, Kang R, Zhao Z. Is hepatitis C associated with atherosclerotic burden? A systematic review and meta-analysis. PLoS One. 2014;9:e106376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Zampino R, Marrone A, Restivo L, Guerrera B, Sellitto A, Rinaldi L, Romano C, Adinolfi LE. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013;5:528-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 157] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 8. | Boddi M, Abbate R, Chellini B, Giusti B, Giannini C, Pratesi G, Rossi L, Pratesi C, Gensini GF, Paperetti L. Hepatitis C virus RNA localization in human carotid plaques. J Clin Virol. 2010;47:72-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, Riello F, Loria P, Florio A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, Cioffi M, Ruggiero G, Adinolfi LE. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Petta S, Torres D, Fazio G, Cammà C, Cabibi D, Di Marco V, Licata A, Marchesini G, Mazzola A, Parrinello G. Carotid atherosclerosis and chronic hepatitis C: a prospective study of risk associations. Hepatology. 2012;55:1317-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Zampino R, Marrone A, Rinaldi L, Guerrera B, Nevola R, Boemio A, Iuliano N, Giordano M, Passariello N, Sasso FC. Endotoxinemia contributes to steatosis, insulin resistance and atherosclerosis in chronic hepatitis C: the role of pro-inflammatory cytokines and oxidative stress. Infection. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Ragab G, Hussein MA. Vasculitic syndromes in hepatitis C virus: A review. J Adv Res. 2017;8:99-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Hsu YC, Lin JT, Ho HJ, Kao YH, Huang YT, Hsiao NW, Wu MS, Liu YY, Wu CY. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293-1302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 15. | Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, Marcellin P, Guyader D, Fontaine H, Larrey D, De Lédinghen V. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology. 2017;152:142-156.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 345] [Cited by in F6Publishing: 365] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 16. | Maruyama S, Koda M, Oyake N, Sato H, Fujii Y, Horie Y, Murawaki Y. Myocardial injury in patients with chronic hepatitis C infection. J Hepatol. 2013;58:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Petta S, Adinolfi LE, Fracanzani AL, Rini F, Caldarella R, Calvaruso V, Cammà C, Ciaccio M, Di Marco V, Grimaudo S. Hepatitis C virus eradication by direct-acting antiviral agents improves carotid atherosclerosis in patients with severe liver fibrosis. J Hepatol. 2018;69:18-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 18. | Cacoub P, Nahon P, Layese R, Blaise L, Desbois AC, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S. Prognostic value of viral eradication for major adverse cardiovascular events in hepatitis C cirrhotic patients. Am Heart J. 2018;198:4-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Singer AW, Osinusi AO, Brainard DM, Chokkalingam AP. Risk of cardiovascular and cerebrovascular events in hepatitis C patients following completion of direct-acting antiviral therapy: a retrospective cohort study. J Hepatol. 2017;66:S95-S332. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Mehta DA, Cohen E, Charafeddine M, Cohen DE, Bao Y, Sanchez Gonzalez Y, Tran TT. Effect of Hepatitis C Treatment with Ombitasvir/Paritaprevir/R+ Dasabuvir on Renal, Cardiovascular and Metabolic Extrahepatic Manifestations: A Post-Hoc Analysis of Phase 3 Clinical Trials. Infect Dis Ther. 2017;6:515-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Adinolfi LE, Nevola R, Guerrera B, D’Alterio G, Marrone A, Giordano M, Rinaldi L. Hepatitis C virus clearance by direct-acting antiviral treatments and impact on insulin resistance in chronic hepatitis C patients. J Gastroenterol Hepatol. 2018;33:1379-1382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Adinolfi LE, Rinaldi L, Marrone A, Giordano M. The effect of sustained virological response by direct-acting antivirals on insulin resistance and diabetes mellitus in patients with chronic hepatitis C. Expert Rev Anti Infect Ther. 2018;16:595-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993-2000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1866] [Cited by in F6Publishing: 1971] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 24. | Klempfner R, Erez A, Sagit BZ, Goldenberg I, Fisman E, Kopel E, Shlomo N, Israel A, Tenenbaum A. Elevated Triglyceride Level Is Independently Associated With Increased All-Cause Mortality in Patients With Established Coronary Heart Disease: Twenty-Two-Year Follow-Up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl:S5-S20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1635] [Cited by in F6Publishing: 2041] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 26. | Saraiva GN, do Rosário NF, Medeiros T, Leite PEC, Lacerda GS, de Andrade TG, de Azeredo EL, Ancuta P, Almeida JR, Xavier AR. Restoring Inflammatory Mediator Balance after Sofosbuvir-Induced Viral Clearance in Patients with Chronic Hepatitis C. Mediators Inflamm. 2018;2018:8578051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Mascia C, Vita S, Zuccalà P, Marocco R, Tieghi T, Savinelli S, Rossi R, Iannetta M, Pozzetto I, Furlan C. Changes in inflammatory biomarkers in HCV-infected patients undergoing direct acting antiviral-containing regimens with or without interferon. PLoS One. 2017;12:e0179400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Sise ME, Bloom AK, Wisocky J, Lin MV, Gustafson JL, Lundquist AL, Steele D, Thiim M, Williams WW, Hashemi N. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology. 2016;63:408-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 29. | Gragnani L, Visentini M, Fognani E, Urraro T, De Santis A, Petraccia L, Perez M, Ceccotti G, Colantuono S, Mitrevski M. Prospective study of guideline-tailored therapy with direct-acting antivirals for hepatitis C virus-associated mixed cryoglobulinemia. Hepatology. 2016;64:1473-1482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 30. | Meissner EG, Lee YJ, Osinusi A, Sims Z, Qin J, Sturdevant D, McHutchison J, Subramanian M, Sampson M, Naggie S. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients. Hepatology. 2015;61:790-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |