Published online Oct 21, 2018. doi: 10.3748/wjg.v24.i39.4499
Peer-review started: August 8, 2018
First decision: August 30, 2018
Revised: September 6, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: October 21, 2018
To analyse the postoperative survival of patients with portal hypertension and determine the factors that influence survival and construct nomograms.
We retrospectively followed 1045 patients who underwent splenectomy plus pericardial devascularisation (SPD) between January 2002 and December 2017. Two SPD types are used in our department: splenectomy plus simplified pericardial devascularisation (SSPD) and splenectomy plus traditional pericardial devascularisation (STPD). The Kaplan-Meier method and Cox regression analysis were used to evaluate the prognostic effects of multiple parameters on overall survival (OS), disease-specific survival (DSS) and bleeding-free survival (BFS). Significant prognostic factors were combined to build nomograms to predict the survival rate of individual patients.
Five hundred and fifty-seven (53.30%) patients were successfully followed with 192 in the SSPD group and 365 in the STPD group; 93 (16.70%) patients died, of whom 42 (7.54%) died due to bleeding. Postoperative bleeding was observed in 84 (15.10%) patients. The 5- and 10-year OS, DSS and BFS rates in the group of patients who underwent SSPD were not significantly different from those in patients who underwent STPD. Independent prognostic factors for OS were age, operative time, alanine transaminase level and albumin-bilirubin score. Independent prognostic factors for BFS were male sex, age, intraoperative blood loss and time to first flatus. Independent prognostic factors for DSS were the Comprehensive Complication Index and age. These characteristics were used to establish nomograms, which showed good accuracy in predicting 1-, 3- and 5-year OS and BFS.
SSPD achieves or surpasses the long-term survival effect of traditional pericardial devascularisation and is worthy of clinical promotion and application. Nomograms are effective at predicting prognosis.
Core tip: The mortality and re-bleeding rate are still extremely high among patients with portal hypertension after splenectomy plus pericardial devascularisation. This study aimed to analyse the postoperative survival, identify risk factors, construct nomograms, and explore the clinical effect of splenectomy plus simplified pericardial devascularisation (SSPD). Five hundred and fifty-seven (53.30%) patients were successfully followed, and the results suggested that the 5- and 10-year overall survival, disease-specific survival and bleeding-free survival rates were not significantly different between patients who underwent SSPD and patients who underwent splenectomy plus traditional pericardial devascularisation. Age, operative time, alanine transaminase level and albumin-bilirubin score were independent prognostic factors influencing overall survival. Male sex, age, intraoperative blood loss and time to first flatus were independent prognostic factors influencing bleeding-free survival. Comprehensive Complication Index and age were independent prognostic factors influencing disease-specific survival.
- Citation: Zhang YF, Ji H, Lu HW, Lu L, Wang L, Wang JL, Li YM. Postoperative survival analysis and prognostic nomogram model for patients with portal hypertension. World J Gastroenterol 2018; 24(39): 4499-4509
- URL: https://www.wjgnet.com/1007-9327/full/v24/i39/4499.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i39.4499
Portal hypertension (PH) is mainly caused by cirrhosis, of which most cases are posthepatitic cirrhosis in China and alcoholic cirrhosis in Western countries. Its clinical manifestations are splenomegaly and hypersplenism, and eventually oesophageal and gastric varices. The most important complication of PH is acute variceal bleeding, with a high mortality rate of more than 50%. And there is also a high risk of re-bleeding in surviving cases[1,2]. Due to a difference in aetiology, the therapy of PH is different. Shunts are the main method in the West. However, in oriental countries, such as China, where patients who develop PH after hepatitis cirrhosis have poor liver function, treatment mainly involves devascularisation. Splenectomy plus pericardial devascularisation (SPD) is the main operative method used to prevent and treat PH[3,4]. This operation does not reduce portal vein blood flow to the liver, does not damage liver function, can dislodge hypersplenism, and can effectively reduce the incidence of hepatic encephalopathy. However, splenectomy plus traditional pericardial devascularisation (STPD) is difficult and complicated, which will still cause greater tissue damage and increase the operative time, thereby increasing the damage of the liver and kidneys. By simplifying STPD, we put forward splenectomy plus simplified pericardial devascularisation (SSPD). The short-term curative effect of SSPD has been verified, but its effects on long-term survival are not clear[5,6].
Patients with PH still face high risks of re-bleeding and death after SPD. Therefore, the best postoperative treatment must be identified to improve the prognosis of these patients, and a determination of the parameters impacting survival by examining factors related to the disease course in patients with PH is useful. Moreover, the identification of prognostic factors in patients with PH is important for estimating outcomes and determining the appropriate treatments. Nomograms have been used to integrate a variety of prognostic factors, quantify the impact of different factors on survival and visualise the results to predict the survival rate of individual patients. Nomograms have been widely used to assess patient prognosis[7-9].
In the present study, 1045 patients with PH who underwent SPD were followed retrospectively to explore the long-term survival effect of SSPD and the prognostic factors for patients with PH, as well as construct a survival nomogram to predict the overall survival rate of patients with PH.
Patients with PH presenting with oesophagogastric varices and hypersplenism who were treated in our department from January 2002 to February 2017 were screened for this single-centre retrospective cohort study. Two SPD types are used in our department: SSPD and STPD. The surgical details are described in a previous publication[5,6]. The inclusion criteria were: (1) patients with PH who were diagnosed with oesophagogastric varices and hypersplenism based on clinical symptoms combined with laboratory, digestive endoscopy or image examinations; (2) patients with PH classified as grade A or B according to the Child-Pugh grading criteria or Child-Pugh grade C at admission and assigned a reduced classification to preoperative Child-Pugh grade A or B after liver preservation therapy to attain appropriate surgical indications; and (3) patients who were able to tolerate general anaesthesia and had no surgical contraindications. The exclusion criteria were: (1) patients with hepatocellular carcinoma, acute heart failure, shock, or other vital organ diseases; (2) patients in an acute haemorrhagic state with unstable vital signs; and (3) patients with poor heart, lung, liver or kidney function. This research was approved by the Ethical Committee of the Second Affiliated Hospital of Xi’an Jiaotong University. All procedures were conducted in accordance with the Declaration of Helsinki from the World Medical Association and with the ethical standards of the committees responsible for human experimentation (institutional and national). The requirement for written informed patient consent was waived due to the retrospective and anonymous nature of this study; all data were used only for statistical analysis.
Survival and postoperative bleeding conditions of the patients were monitored and recorded. The following primary data were collected: age, gender, aetiology, Charlson score, blood type, history of variceal ligation, history of abdominal surgery, smoking, drinking, history of variceal bleeding, body mass index (BMI), Child-Pugh score at admission, model for end-stage liver disease (MELD) score at admission, albumin-bilirubin (ALBI) score at admission, and Comprehensive Complication Index (CCI) score. The following laboratory data were collected at admission and during the perioperative period: White blood cell (WBC) count, haemoglobin (Hb) level, platelet count, prothrombin time (PT), international normalised ratio (INR), total bilirubin (TBIL) level, direct bilirubin (DBIL) level, alanine transaminase (ALT) level, aspartate transaminase (AST) level, albumin (ALB) level, globulin (GLB) level, serum creatinine (Scr) level, cystatin C (Cys C) level, duration of the preoperative hospital stay, duration of the postoperative hospital stay, total hospital stay, operative time, intraoperative blood loss, time to first flatus, and type of surgery. Information about the therapies used to correct a specific complication was also collected to calculate the CCI.
The ALBI, CP, and MELD scores were calculated using the relevant formulas[10-12]. The ALBI score was calculated as follows: ALBI score = 0.66 × log10 [total bilirubin (μmol/L)] - 0.085 × [albumin (g/L)][10]. The CP score included five parameters: presence or absence of encephalopathy and ascites, serum total bilirubin level, albumin level and prothrombin time[13]. The MELD score was calculated using the following formula: 11.2 × ln (international normalised ratio) + 9.57 × ln (creatinine, mg/dL) + 3.78 × ln (bilirubin, mg/dL) + 6.43 × (aetiology: 0 if cholestatic or alcoholic, 1 otherwise)[14]. Complications that occurred within 30 d after the operation were considered surgical complications and the severity of all complications was graded using the Centers for Disease Control (CDC) criteria[15]. The CCI was calculated as the severity-weighted sum of all postoperative complications (available at http://www.assessurgery.com). The CCI ranges from 0 (no complications) to 100 points (death)[16-18].
All included patients underwent routine follow-up examinations. Follow-up methods were mainly telephone calls or inpatient or outpatient re-examinations, and the last follow-up occurred on January 31, 2018. One of our primary endpoints of interest was overall survival (OS), which was defined as the time from surgery to death from any cause. In the analysis of OS, patients who were alive at the last follow-up date were counted as censored observations. The other primary endpoint of interest was disease-specific survival (DSS), which was defined as the time from surgery to death attributed to PH. In the DSS analysis, patients who died of other causes or were alive at the last follow-up date were counted as censored observations. Another primary endpoint was bleeding-free survival (BFS), which was defined as the time from surgery to the first appearance of initial oesophagogastric variceal bleeding. In the BFS analysis, patients who died of other causes or were alive at the last follow-up date were counted as censored observations. Univariate and multivariate Cox regression models were used to determine survival-related factors. Factors that were observed to have significant associations with survival in multivariate analyses (P < 0.05) were used to build the nomograms for OS and BFS.
Statistical analyses were performed using IBM SPSS statistics 22 software (SPSS Inc., Chicago, IL, United States) and R version 3.2.2 software (Institute for Statistics and Mathematics, Vienna, Austria; http://www.r-project.org/). Continuous variables are presented as means ± SD, and categorical variables are presented as frequencies and percentages. Survival curves were generated using the Kaplan-Meier method, and the significance of difference in survival among selected variables was verified using the log-rank test. A univariate Cox regression analysis with an Enter method was used to estimate the relative risk (RR). A multivariate Cox regression analysis with a Forward Condition method was used to estimate the RR and identify independent prognostic factors. The “rms” R library (cran.r-project.org/web/packages/ rms) was used to construct nomogram models. All P-values were two-sided, and P < 0.05 was considered statistically significant.
Five hundred and fifty-seven (53.30%) patients were successfully followed, with 192 (34.47%) in the SSPD group and 283 (50.81%) males. These included 48 patients who had been followed in 2011 and were alive at that time[5,6]. However, we were unable to reach these 48 patients now because of a change of contact or address, so we used this data as a censored data. The mean age of the patients was 48.79 ± 11.53 years. Ninety-three (16.70%) patients died, of whom 42 (7.54%), 21 (3.77%), 18 (3.23%) and 12 (2.15%) died of bleeding, liver failure, liver cancer, and cardiovascular and circulatory diseases, respectively. Postoperative bleeding was observed in 84 (15.10%) patients, and the bleeding mortality rate was 50.00%.
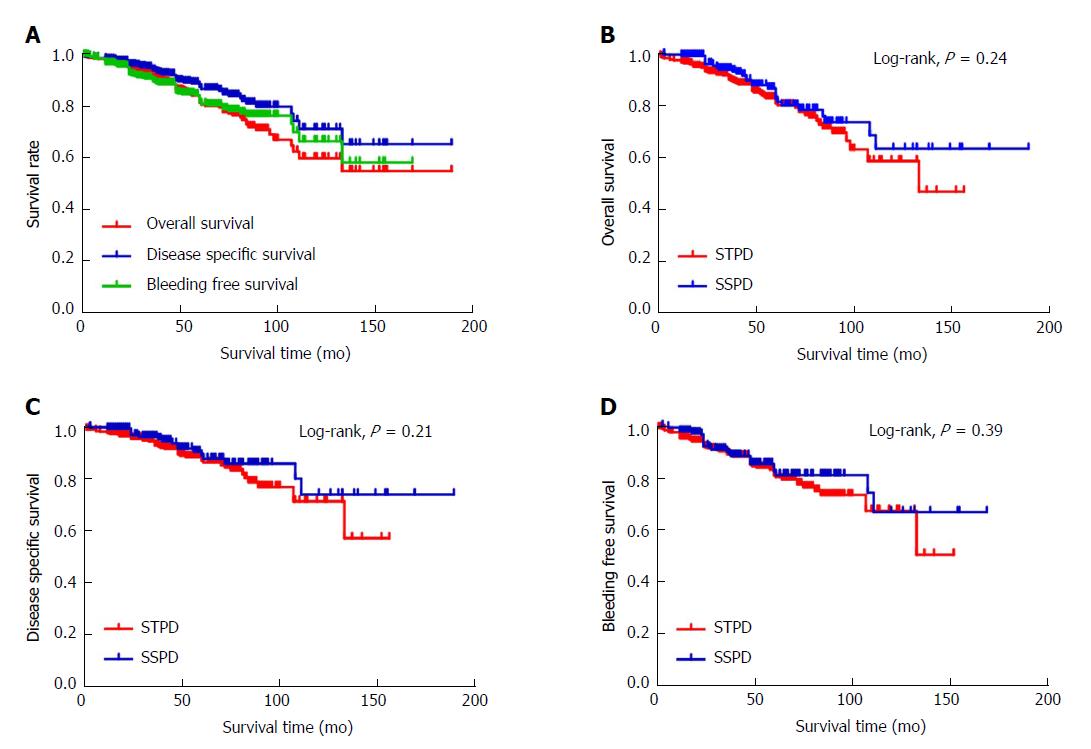
Figure 1 shows the Kaplan-Meier survival curves for the OS, DSS and BFS of patients with PH. The 5-year OS rate was 81.3% and the 10-year OS rate was 59.7%. The 5-year DSS rate was 87.3% and 10-year DSS rate was 71.0%. The 5-year BFS rate was 81.6% and 10-year BFS rate was 66.3%. Figure 1B shows the Kaplan-Meier survival curves for OS stratified by the type of operation. For the STPD group, the 5-year OS rate was 80.7% and 10-year OS rate was 58.4%. For the SSPD group, the 5-year OS rate was 82.5% and 10-year OS rate was 63.2%. The 5-year and 10-year OS rates in the SSPD group were higher than those in the STPD group, but the P-value of the log-rank analysis was 0.24 (P > 0.05), indicating that the difference was not statistically significant. Figure 1C shows the Kaplan-Meier survival curves for DSS stratified by the type of operation. For the STPD group, the 5-year DSS rate was 86.6% and 10-year DSS rate was 71.0%. For the SSPD group, the 5-year DSS rate was 88.7% and 10-year DSS rate was 73.6%. The 5-year and 10-year DSS rates in the SSPD group were higher than those in the STPD group, but the P-value of the log-rank analysis was 0.21 (P > 0.05), indicating that the difference was not statistically significant. Figure 1D shows the Kaplan-Meier survival curves for BFS stratified by the type of operation. For the STPD group, the 5-year BFS rate was 81.1% and 10-year BFS rate was 67.4%. The 5-year and 10-year BFS rates in the SSPD group were 82.6% and 66.9%, respectively. The P-value of the log-rank analysis was 0.39 (P > 0.05), indicating that the difference was not statistically significant.
In the follow-up analysis, we selected 319 patients with complete data to perform Cox regression analyses and to explore the prognostic factors for OS. Table 1 shows the demographics, laboratory information and perioperative characteristics of patients with PH.
| Parameter | STPD (n = 200) | SSPD (n = 119) | P value |
| Age (yr) | 49.79 ± 11.14 | 48.92 ± 10.09 | 0.49 |
| Gender (male) | 93 (46.50) | 54 (45.38) | 0.85 |
| Aetiology: Hepatitis B/hepatitis C/others | 131 (65.50)/23 (11.50)/46 (23.00) | 88 (73.95)/17 (14.29)/14 (11.76) | 0.04 |
| Charlson score: 0/1/2/3/≥ 3 | 105 (52.50)/54 (27.00)/21 (10.50)/ 20 (10.00) | 62 (52.10)/42 (35.29)/12 (10.08)/3 (2.52) | 0.06 |
| Blood type: A/B/O/AB | 57 (28.50)/62 (31.00)/59 (29.50)/22 (11.00) | 36 (30.25)/33 (27.73)/36 (30.25)/14 (11.76) | 0.94 |
| History of variceal ligation | 27 (13.50) | 9 (7.56) | 0.11 |
| History of abdominal surgery | 35(17.50) | 21(17.65) | 0.97 |
| Smoking | 57 (28.50) | 28 (23.53) | 0.33 |
| Drinking | 38 (19.00) | 25 (21.01) | 0.66 |
| History of variceal bleeding | 105 (52.50) | 43 (36.13) | 0.01 |
| BMI | 21.97 ± 3.04 | 21.62 ± 2.54 | 0.29 |
| Child-Pugh score | 6.56 ± 1.27 | 6.92 ± 1.29 | 0.02 |
| MELD score | 5.92 ± 0.40 | 5.97 ± 0.47 | 0.32 |
| ALBI score | -2.26 ± 0.50 | -2.10 ± 0.54 | 0.01 |
| CCI score | 18.64 ± 11.78 | 17.53 ± 9.53 | 0.38 |
| WBC count (109/L) | 2.79 ± 1.78 | 2.40 ± 1.38 | 0.04 |
| Hb (g/L) | 93.35 ± 24.77 | 94.74 ± 25.82 | 0.63 |
| Platelet count (109/L) | 49.32 ± 28.63 | 43.28 ± 21.20 | 0.05 |
| PT (s) | 13.85 ± 1.96 | 14.29 ± 1.82 | 0.04 |
| INR | 1.19 ± 0.18 | 1.32 ± 1.10 | 0.10 |
| TBIL (mmol/L) | 27.24 ± 16.22 | 29.37 ± 16.81 | 0.26 |
| DBIL (mmol/L) | 11.87 ± 7.67 | 12.33 ± 6.87 | 0.59 |
| ALT (IU/L) | 35.79 ± 55.76 | 38.20 ± 31.58 | 0.67 |
| AST (IU/L) | 43.72 ± 52.72 | 44.55 ± 33.76 | 0.88 |
| ALB (g/L) | 37.24 ± 5.50 | 35.61 ± 5.66 | 0.01 |
| GLB (g/L) | 28.36 ± 5.90 | 29.03 ± 5.91 | 0.32 |
| Scr (mmol/L) | 62.44 ± 18.02 | 58.71 ± 13.68 | 0.05 |
| Cys C (mg/L) | 1.12 ± 0.32 | 1.11 ± 0.25 | 0.71 |
| Duration of preoperative hospital stay (d) | 14.56 ± 9.43 | 16.08 ± 9.29 | 0.16 |
| Duration of postoperative hospital stay (d) | 15.75 ± 6.46 | 14.97 ± 5.30 | 0.27 |
| Total hospital stay (d) | 30.61 ± 12.20 | 31.77 ± 11.75 | 0.40 |
| Operative time, min | 139.76 ± 50.73 | 124.55 ± 42.49 | 0.00 |
| Intraoperative blood loss (mL) | 869.51 ± 692.77 | 591.34 ± 477.54 | 0.00 |
| Time to first flatus (d) | 4.69 ± 1.70 | 3.67 ± 1.18 | 0.00 |
As shown in Table 2, CCI; age; operative time; intraoperative blood loss; WBC, Hb, ALT, AST, and ALB levels; and Child-Pugh and ALBI scores at admission were remarkably correlated with OS in univariate Cox regression analyses (P < 0.05). CCI, age, intraoperative blood loss, platelet count, Scr level, Cys C level and MELD score were remarkably correlated with DSS in univariate Cox regression analyses (P < 0.05). Gender, age, history of variceal ligation, history of variceal bleeding, operative time, intraoperative blood loss, time to first flatus, Hb level, platelet count and GLB level at admission were remarkably correlated with BFS in univariate Cox regression analyses (P < 0.05).
| Parameter | OS | BFS | DSS | |||||||||
| B | P value | RR | 95%CI | B | P value | RR | 95%CI | B | P value | RR | 95%CI | |
| Type of operation | 0.16 | 0.62 | 1.18 | 0.62-2.23 | -0.39 | 0.33 | 0.68 | 0.31-1.48 | -0.45 | 0.17 | 0.64 | 0.34-1.21 |
| CCI | 0.04 | 0.00 | 1.04 | 1.01-1.07 | 0.02 | 0.36 | 1.02 | 0.98-1.05 | 0.06 | 0.00 | 1.06 | 1.02-1.09 |
| Gender | 0.01 | 0.99 | 1.01 | 0.54-1.89 | 0.94 | 0.02 | 2.56 | 1.20-5.43 | 0.06 | 0.91 | 1.06 | 0.43-2.60 |
| Age | 0.06 | 0.00 | 1.06 | 1.03-1.09 | 0.04 | 0.01 | 1.05 | 1.01-1.08 | 0.05 | 0.00 | 1.05 | 1.02-1.09 |
| Aetiology | 0.07 | 0.74 | 1.07 | 0.73-1.56 | 0.16 | 0.44 | 1.18 | 0.78-1.78 | -0.16 | 0.43 | 0.86 | 0.58-1.26 |
| History of variceal ligation | 0.75 | 0.10 | 2.12 | 0.87-5.19 | 1.07 | 0.01 | 2.92 | 1.24-6.88 | 0.54 | 0.21 | 1.72 | 0.73-4.02 |
| History of abdominal surgery | 0.33 | 0.42 | 1.39 | 0.63-3.03 | 0.16 | 0.73 | 1.17 | 0.48-2.87 | -1.28 | 0.20 | 0.28 | 0.04-1.96 |
| Smoking | 0.45 | 0.19 | 1.56 | 0.80-3.04 | 0.54 | 0.15 | 1.72 | 0.82-3.60 | 0.27 | 0.55 | 1.31 | 0.55-3.11 |
| Drinking | 0.17 | 0.65 | 1.19 | 0.56-2.51 | 0.71 | 0.07 | 2.04 | 0.96-4.33 | -0.12 | 0.81 | 0.89 | 0.34-2.35 |
| Charlson Score | 0.11 | 0.53 | 1.12 | 0.80-1.56 | 0.02 | 0.93 | 1.02 | 0.69-1.51 | 0.06 | 0.77 | 1.06 | 0.72-1.58 |
| History of variceal bleeding | 0.07 | 0.82 | 1.08 | 0.57-2.03 | 1.02 | 0.01 | 2.77 | 1.30-5.90 | -0.30 | 0.43 | 0.74 | 0.36-1.55 |
| Blood type | -0.20 | 0.25 | 0.82 | 0.58-1.15 | -0.15 | 0.41 | 0.86 | 0.60-1.23 | -0.16 | 0.29 | 0.85 | 0.63-1.15 |
| BMI | -0.06 | 0.34 | 0.94 | 0.83-1.07 | 0.00 | 0.97 | 1.00 | 0.88-1.14 | -0.03 | 0.57 | 0.97 | 0.86-1.09 |
| Total hospital stay | 0.01 | 0.36 | 1.01 | 0.99-1.04 | 0.00 | 0.79 | 1.00 | 0.98-1.03 | -0.03 | 0.26 | 0.97 | 0.93-1.02 |
| Duration of preoperative hospital stay | 0.01 | 0.43 | 1.01 | 0.98-1.05 | 0.01 | 0.59 | 1.01 | 0.97-1.05 | 0.04 | 0.20 | 1.04 | 0.98-1.09 |
| Duration of postoperative hospital stay | 0.03 | 0.20 | 1.03 | 0.98-1.09 | -0.01 | 0.71 | 0.99 | 0.92-1.06 | 0.00 | 0.91 | 1.00 | 0.94-1.08 |
| Operative time | 0.01 | 0.00 | 1.01 | 1.00-1.02 | 0.01 | 0.04 | 1.01 | 1.00-1.02 | 0.00 | 0.33 | 1.00 | 1.00-1.01 |
| Intraoperative blood loss | 0.00 | 0.01 | 1.00 | 1.00-1.01 | 0.00 | 0.00 | 1.00 | 1.00-1.01 | 0.00 | 0.09 | 1.00 | 1.00-1.00 |
| Time to first flatus | -0.01 | 0.94 | 0.99 | 0.81-1.21 | 0.23 | 0.03 | 1.26 | 1.03-1.53 | -0.06 | 0.57 | 0.94 | 0.78-1.15 |
| WBC count | 0.20 | 0.03 | 1.22 | 1.02-1.45 | 0.15 | 0.13 | 1.16 | 0.96-1.41 | -0.02 | 0.88 | 0.98 | 0.80-1.21 |
| Hb | -0.01 | 0.04 | 0.99 | 0.97-1.00 | -0.02 | 0.04 | 0.99 | 0.97-1 | -0.01 | 0.31 | 0.99 | 0.97-1.01 |
| Platelet count | 0.01 | 0.08 | 1.01 | 1.00-1.02 | 0.01 | 0.01 | 1.01 | 1.00-1.02 | 0.01 | 0.05 | 1.01 | 1.00-1.03 |
| PT | 0.07 | 0.46 | 1.07 | 0.90-1.27 | -0.08 | 0.44 | 0.92 | 0.74-1.14 | -0.26 | 0.75 | 0.77 | 0.16-3.66 |
| INR | -0.03 | 0.93 | 0.97 | 0.51-1.83 | -0.93 | 0.44 | 0.39 | 0.04-4.29 | -1.80 | 0.84 | 0.17 | 0.00-9821579.13 |
| TBIL | 0.01 | 0.22 | 1.01 | 0.99-1.03 | -0.01 | 0.45 | 0.99 | 0.97-1.02 | 0.00 | 0.97 | 1.00 | 0.96-1.04 |
| DBIL | 0.02 | 0.20 | 1.02 | 0.99-1.06 | -0.03 | 0.34 | 0.97 | 0.92-1.03 | 0.02 | 0.72 | 1.02 | 0.93-1.12 |
| ALT | 0.00 | 0.00 | 1.00 | 1.00-1.01 | -0.01 | 0.35 | 0.99 | 0.98-1.01 | 0.01 | 0.12 | 1.02 | 1.00-1.03 |
| AST | 0.00 | 0.01 | 1.00 | 1.00-1.01 | -0.02 | 0.10 | 0.98 | 0.96-1.00 | -0.01 | 0.27 | 0.99 | 0.97-1.01 |
| ALB | -0.11 | 0.00 | 0.90 | 0.84-0.96 | -0.03 | 0.45 | 0.98 | 0.91-1.04 | -0.39 | 0.25 | 0.68 | 0.35-1.31 |
| GLB | 0.02 | 0.53 | 1.02 | 0.96-1.07 | -0.07 | 0.04 | 0.93 | 0.87-1.00 | 0.03 | 0.28 | 1.03 | 0.97-1.10 |
| Scr | 0.01 | 0.32 | 1.01 | 0.99-1.03 | 0.02 | 0.07 | 1.02 | 1.00-1.03 | -0.08 | 0.01 | 0.93 | 0.87-0.98 |
| Cys C | 0.71 | 0.09 | 2.03 | 0.91-4.57 | 0.23 | 0.67 | 1.26 | 0.44-3.66 | 1.17 | 0.02 | 3.21 | 1.20-8.62 |
| Child-Pugh score | 0.29 | 0.01 | 1.33 | 1.08-1.65 | 0.13 | 0.36 | 1.13 | 0.87-1.48 | 0.14 | 0.54 | 1.15 | 0.74-1.81 |
| MELD score | 0.50 | 0.18 | 1.64 | 0.80-3.40 | 0.02 | 0.97 | 1.02 | 0.44-2.36 | 4.18 | 0.05 | 65.31 | 0.94-4536.32 |
| ALBI score | 1.07 | 0.00 | 2.92 | 1.49-5.76 | 0.19 | 0.59 | 1.21 | 0.60-2.46 | -3.86 | 0.33 | 0.02 | 0.00-48.67 |
A multivariate Cox regression analysis was used to further explore the influences of all variables that were significant in the univariate analysis. The multivariate analyses of OS showed that age, operative time, ALT levels and ALBI score were independent positive risk factors (P < 0.05); CCI and age were independent prognostic factors influencing DSS (P < 0.05), and male sex, age, intraoperative blood loss and time to first flatus were independent positive risk factors (P < 0.05) (Table 3).
| Parameter | B | SE | P value | RR | 95%CI |
| OS | |||||
| Age | 0.06 | 0.02 | 0.00 | 1.06 | 1.03-1.09 |
| Operative time | 0.01 | 0.00 | 0.01 | 1.01 | 1.00-1.02 |
| ALT | 0.01 | 0.00 | 0.00 | 1.01 | 1.00-1.01 |
| ALBI score | 1.03 | 0.37 | 0.01 | 2.79 | 1.36-5.72 |
| BFS | |||||
| Male | 1.17 | 0.40 | 0.00 | 3.22 | 1.48-7.01 |
| Age | 0.05 | 0.02 | 0.01 | 1.05 | 1.01-1.09 |
| Intraoperative blood loss | 0.00 | 0.00 | 0.05 | 1.00 | 1.00-1.01 |
| Time to first flatus | 0.23 | 0.10 | 0.02 | 1.26 | 1.04-1.53 |
| DSS | |||||
| CCI | 0.06 | 0.01 | 0.00 | 1.06 | 1.05-1.08 |
| Age | 0.04 | 0.01 | 0.00 | 1.04 | 1.02-1.06 |
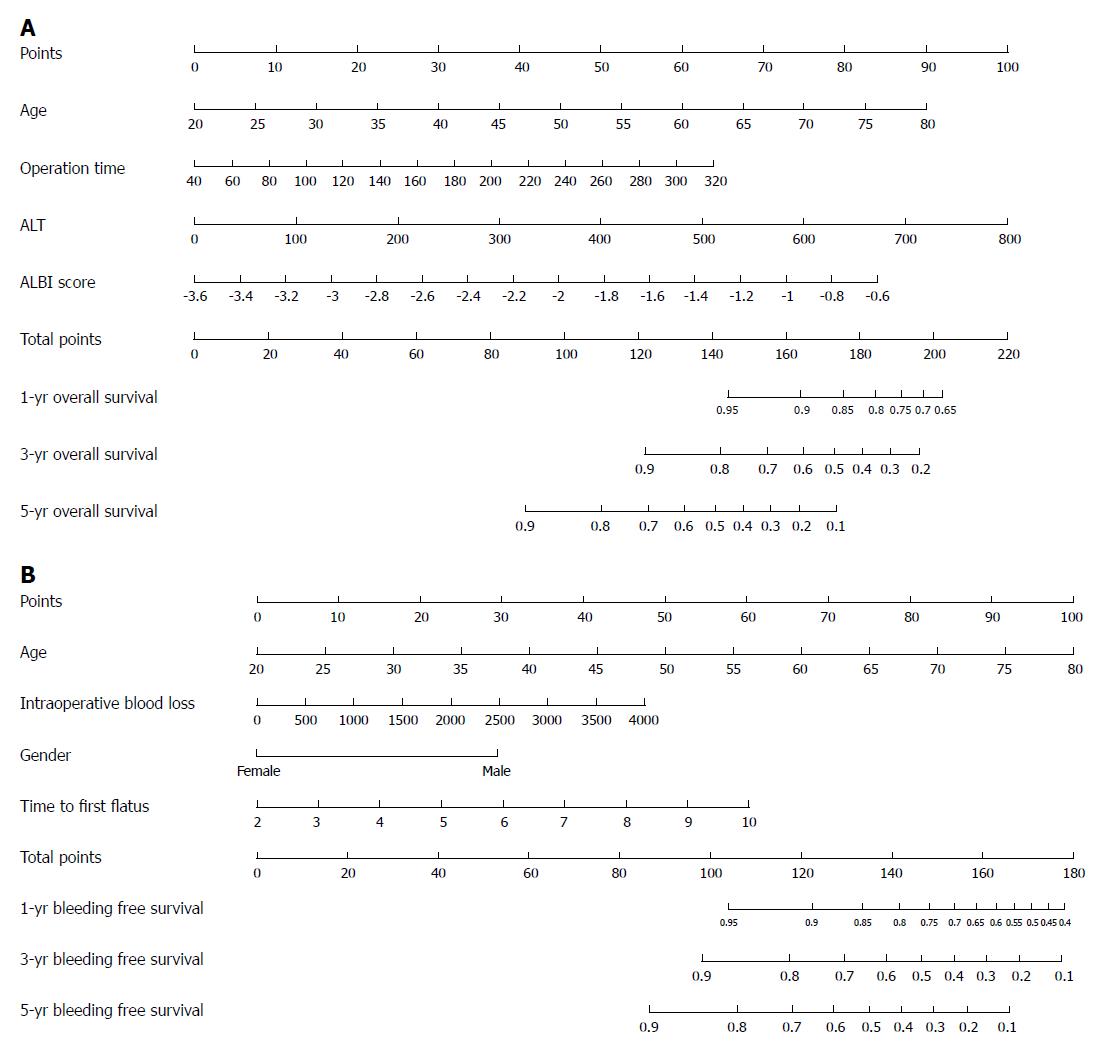
We recruited all independent prognostic factors identified in Cox regression analysis of OS and BFS to construct the nomograms. The prognostic nomograms for 1-, 3- and 5-year OS rates are shown in Figure 2A, and nomograms for 1-, 3- and 5-year BFS rates are shown in Figure 2B. Each variable is projected upward to the value of the small ruler (Points) to get the score of each parameter. The higher the score, the worse the prognosis of survival. The sum of all small rulers is the total score (Total Points). The 1-, 3- and 5-year OS and BFS rates can be obtained from the downward projection of the Total Points. This nomogram can predict the survival rate individually according to the different conditions of different patients, so as to improve the prediction efficiency and accuracy. The nomograms showed that the OS rates were higher for patients with younger age, patients with shorter operative time, patients with lower alanine transaminase levels and patients with lower albumin-bilirubin scores. The BFS rates were better for females, younger patients, patients with less intraoperative blood loss and patients with less time to the first flatus. Guided by nomograms, we can better predict the prognosis based on the different characteristics of each patient.
We simplified STPD and introduced SSPD in 2002, which includes cutting and ligating of the posterior gastric vessels, suturing left gastric vessels and suturing from the lesser curvature to the lower esophageal vessels. Holding the paraoesophageal vein and cutting off the perforating vein only can effectively block the reflux of esophageal vessels, lower the portal vein pressure and ensure thorough haemostasis. On the other hand, SSPD can reduce gastric mucosal congestion, so as to reduce the incidence of PHG and prevent postoperative re-bleeding. Changing to suture and refraining from incision of seromuscular layer minimises wound injuries and reduces intraoperative blood loss. In addition, the operative time is significantly shortened, which can reduce liver injury to some extent. Higher 5- and 10-year OS and DSS rates were reported for the SSPD group than the STPD group, but the difference was not statistically significant. Moreover, the 5- and 10-year BFS rates for the SSPD group were not statistically significant compared with the STPD group. Based on these results, SSPD achieved or surpassed the long-term survival effect of STPD. However, the procedure of SSPD is simple and prone to master, with less tissue injury and inflammatory reactions. Therefore, SSPD is a good method of treating PH patients, and it can and should be promoted and applied to hospitals at different levels.
Five hundred and fifty-seven (53.30%) patients were successfully followed; 93 (16.70%) patients died, of whom 42 (7.54%) died due to bleeding. Postoperative bleeding was observed in 84 (15.10%) patients, and the bleeding mortality rate was 50.00%. Acute variceal bleeding is the most life-threatening complication of PH, and despite the recent progress in management, this complication still occurs in approximately 20% of patients at 6 wk[19,20]. Therefore, the risk factors for OS, DSS and BFS must be determined to accurately predict the OS and BFS of patients with PH and to conduct individualised prevention and treatment as early as possible. The multivariate analyses of OS showed that age, operative time, ALT levels and ALBI score were independent positive risk factors. Not surprisingly, age and operative time were independent positive risk factors for OS, as older age and longer operative time are always accompanied by underlying disease and more severe conditions, respectively. ALT is a sensitive marker of acute hepatocyte damage[21], which may dramatically influence the OS. The ALBI score was recently established as an evidence-based model to assess the liver function of patients with hepatocellular carcinoma[10] and has already been validated and proven to be more objective and precise than CP and MELD scores in predicting postoperative efficacy and survival[22-28]. In clinical practice, we should pay more attention to older patients and patients with longer operative time and higher ALT levels and ALBI scores at admission to improve the preventative treatments for these patients during the perioperative period. According to the multivariate analyses of DSS, CCI and age were independent positive risk factors. The CCI is a novel method that mathematically integrates all complications graded by the conventional CDC criteria into one number, regardless of the number and severity of the complications, to capture the overall burden of an operation. Additionally, CCI is a continuous variable ranging from 0 (no complications) to 100 (death) points that easily quantifies complications and can be included in multifactor analyses. Thus, the CCI is the most attractive method for evaluating postoperative complications[29]. The CCI has been applied in abdominal surgery and in the context of randomised controlled trials for patients undergoing oesophagectomy and has achieved better results[30-32]. The multivariate analyses of BFS showed that male sex, age, intraoperative blood loss and time to first flatus were independent positive risk factors. Intraoperative blood loss and time to first flatus were common factors in the perioperative evaluation index. However, male sex was an independent positive risk factor that has not been reported in previous studies. This discrepancy may be more related to the smoking and drinking history of male patients. In clinical practice, we should pay more attention to older male patients and patients with larger amounts of intraoperative bleeding and longer postoperative exhaust time. We should strengthen the measures to protect these patients during the perioperative period, pay attention to the postoperative re-examination, and perform ligation in a timely manner to prevent bleeding. In addition, a precise survival and re-bleeding prognostic tool is urgently needed to guide therapy selection for high-risk patients.
Integrating independent prognostic parameters, a nomogram can provide individualised evaluation of the clinical event incidence, such as survival rate[7-9]. Compared with traditional methods, nomograms make predictions more quickly, conveniently and accurately. Its predictive value is better than other evaluation systems and is very important in clinical decision-making processes[33,34]. However, the application of nomogram in PH patients has rarely been reported. In the present report, the prognostic nomograms included all significant independent factors in the Cox regression analyses of OS and BFS. According to the nomograms, the OS rates were better for younger patients, patients with shorter operative time, patients with lower ALT levels and patients with lower ALBI scores. In addition, the BFS rates were better for females, younger patients, patients with less intraoperative blood loss and patients with less time to first flatus. Guided by nomograms, we can better predict the prognosis based on the different characteristics of each patient. To the best of our knowledge, our study is the first to construct nomograms to predict OS and BFS rates in patients with PH.
There are several limitations in the present study. First, potential bias may exist for the retrospective nature of our study[35]. However, a randomised clinical trial may be not realizable for the reason of ethics. Second, for the research included only Chinese patients, our results may not be directly applicable to other races. In some cases, it may need to be verified. Third, this study was just conducted in a single hospital. Our results may not be fully applicable to other hospitals for the difference in treatment modalities and medical conditions. In addition, the nomograms constructed in our study lacked external validation due to the limited number of cases, which may reduce the credibility of the nomograms. Despite the aforementioned limitations, the present study has identified the prognostic factors for patients with PH after SPD and is the first to construct a nomogram to forecast the postoperative survival and re-bleeding rates of PH patients.
In summary, SSPD achieves or surpasses the long-term survival effect of STPD and is worthy of clinical promotion and application, particularly in primary hospitals. In clinical practice, we should pay more attention to males, older patients, and patients with longer operative time, patients with higher CCI scores, ALT levels and ALBI scores at admission, and patients with larger amounts of intraoperative bleeding and longer postoperative exhaust time. Nomograms are effective in predicting prognosis according to individual patient characteristics. Further large-scale prospective studies are needed to confirm our findings.
Patients with portal hypertension (PH) still have higher re-bleeding rates and mortality after splenectomy plus pericardial devascularisation. We simplified splenectomy plus traditional pericardial devascularisation (STPD) and put forward splenectomy plus simplified pericardial devascularisation (SSPD), whose initial curative effects have been verified, but its long-term survival effects are not clear. Therefore, we need to identify the best postoperative treatment to improve the prognosis of these patients, and a determination of the underlying influencing factors is useful for estimating outcomes and determining the appropriate treatments.
SSPD achieves or surpasses the long-term survival outcome of STPD and is worthy of clinical promotion and application. In clinical practice, males and older patients, patients with longer operative time, patients with higher Comprehensive Complication Index (CCI), alanine transaminase (ALT) and albumin-bilirubin (ALBI) scores at admission, patients with larger amounts of intraoperative bleeding and patients with longer postoperative exhaust time should receive more attention.
The main aim of the retrospective research was to assess the postoperative survival rates of PH patients and identify the clinical efficacy of SSPD. Factors influencing survival and nomograms were also identified.
Five hundred fifty-seven (53.30%) patients were successfully followed. We performed a Kaplan-Meier analysis to construct survival curves. We also applied log-rank test to verify the significance of difference in survival rates. The risk factors were estimated using a univariate Cox regression analysis. A multivariate Cox regression analysis was used to estimate the relative risk and to identify independent prognostic factors. The “rms” R library was used to construct nomograms.
Five hundred and fifty-seven (53.30%) patients were successfully followed; 93 (16.70%) patients died, of whom 42 (7.54%) patients died due to bleeding. Postoperative bleeding was observed in 84 (15.10%) patients. There was no significant difference between SSPD and STPD in 5- and 10-year overall survival (OS), disease-specific survival (DSS) and bleeding-free survival (BFS) rates. Age, operative time, ALT level and the ALBI score were independent prognostic factors for OS. Male sex, age, intraoperative blood loss and time to the first flatus were independent prognostic factors for BFS. CCI and age were independent prognostic factors for DSS. Nomograms were established and were better at predicting 1-, 3-, and 5-year OS and BFS rates.
SSPD achieves or surpasses the long-term survival outcomes of STPD, which is worthy of clinical promotion and application. In clinical practice, males, older patients, patients with longer operative time, patients with higher CCI scores, ALT levels and ALBI scores at admission, and patients with larger amounts of intraoperative bleeding and longer postoperative exhaust time should receive more attention. Nomograms are better in predicting prognosis according to individual patient characteristics.
In the future, the long-term survival of patients with PH undergoing SSPD should be assessed in large-scale prospective studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Compean D, Li LQ S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC. Portal hypertension and gastrointestinal bleeding. Semin Liver Dis. 2008;28:3-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4902] [Cited by in F6Publishing: 4886] [Article Influence: 542.9] [Reference Citation Analysis (0)] |
| 3. | Yao HS, Wang WJ, Wang Q, Gao WC, Xiang HG, Hu ZQ, Gao JD, Chen XY, Wang WM. Randomized clinical trial of vessel sealing system (LigaSure) in esophagogastric devascularization and splenectomy in patients with portal hypertension. Am J Surg. 2011;202:82-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Zheng X, Liu Q, Yao Y. Laparoscopic splenectomy and esophagogastric devascularization is a safe, effective, minimally invasive alternative for the treatment of portal hypertension with refractory variceal bleeding. Surg Innov. 2013;20:32-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Zhang YF, Ji H, Lu HW, Lu L, Wang L, Wang JL, Li YM. Comparison of simplified and traditional pericardial devascularisation combined with splenectomy for the treatment of portal hypertension. World J Clin Cases. 2018;6:99-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Lu H, Liu S, Zhang Y, Shang H, Ji H, Li Y. Therapeutic effects and complications of simplified pericardial devascularization for patients with portal hypertension. Int J Clin Exp Med. 2015;8:14036-14041. [PubMed] [Cited in This Article: ] |
| 7. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1119] [Cited by in F6Publishing: 1875] [Article Influence: 208.3] [Reference Citation Analysis (0)] |
| 8. | Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, Wang Z, Zhu Z, Deng Q, Xiong X. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33:861-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 433] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 9. | Tang LQ, Li CF, Li J, Chen WH, Chen QY, Yuan LX, Lai XP, He Y, Xu YX, Hu DP. Establishment and Validation of Prognostic Nomograms for Endemic Nasopharyngeal Carcinoma. J Natl Cancer Inst. 2015;108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 254] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 10. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O’Beirne J, Fox R, Skowronska A, Palmer D. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1174] [Cited by in F6Publishing: 1636] [Article Influence: 163.6] [Reference Citation Analysis (0)] |
| 11. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] [Cited in This Article: ] |
| 12. | Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1075] [Cited by in F6Publishing: 1118] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 13. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] [Cited in This Article: ] |
| 14. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3462] [Cited by in F6Publishing: 3417] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 15. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] [Cited in This Article: ] |
| 16. | Slankamenac K, Nederlof N, Pessaux P, de Jonge J, Wijnhoven BP, Breitenstein S, Oberkofler CE, Graf R, Puhan MA, Clavien PA. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg. 2014;260:757-762; discussion 762-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 17. | Yamashita S, Sheth RA, Niekamp AS, Aloia TA, Chun YS, Lee JE, Vauthey JN, Conrad C. Comprehensive Complication Index Predicts Cancer-specific Survival After Resection of Colorectal Metastases Independent of RAS Mutational Status. Ann Surg. 2017;266:1045-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Tahiri M, Sikder T, Maimon G, Teasdale D, Hamadani F, Sourial N, Feldman LS, Guralnick J, Fraser SA, Demyttenaere S. The impact of postoperative complications on the recovery of elderly surgical patients. Surg Endosc. 2016;30:1762-1770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Sarin SK, Kumar A, Angus PW, Baijal SS, Baik SK, Bayraktar Y, Chawla YK, Choudhuri G, Chung JW, de Franchis R, de Silva HJ, Garg H, Garg PK, Helmy A, Hou MC, Jafri W, Jia JD, Lau GK, Li CZ, Lui HF, Maruyama H, Pandey CM, Puri AS, Rerknimitr R, Sahni P, Saraya A, Sharma BC, Sharma P, Shiha G, Sollano JD, Wu J, Xu RY, Yachha SK, Zhang C; Asian Pacific Association for the Study of the Liver (APASL) Working Party on Portal Hypertension. Diagnosis and management of acute variceal bleeding: Asian Pacific Association for Study of the Liver recommendations. Hepatol Int. 2011;5:607-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1229] [Cited by in F6Publishing: 1147] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 21. | Mangus RS, Fridell JA, Kubal CA, Davis JP, Tector AJ. Elevated alanine aminotransferase (ALT) in the deceased donor: impact on early post-transplant liver allograft function. Liver Int. 2015;35:524-531. [PubMed] [Cited in This Article: ] |
| 22. | Liu PH, Hsu CY, Hsia CY, Lee YH, Chiou YY, Huang YH, Lee FY, Lin HC, Hou MC, Huo TI. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J Gastroenterol Hepatol. 2017;32:879-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 23. | Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, Ueki H, Kaneto M, Aibiki T, Okudaira T, Kawakami T. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1031-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 184] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 24. | Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, Ma L, Qi LN, Ou BN, Li LQ. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103:725-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 25. | Guan CT, Zhao H, Li XQ, Qu CX, Cai JQ, Wei WW, Qiao YL. [Basic characteristics and survival analysis of patients with hepatocellular carcinoma]. Zhonghua Zhong Liu Za Zhi. 2017;39:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 26. | Toyoda H, Lai PB, O’Beirne J, Chong CC, Berhane S, Reeves H, Manas D, Fox RP, Yeo W, Mo F. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer. 2016;114:744-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 27. | Chan AW, Chan RC, Wong GL, Wong VW, Choi PC, Chan HL, To KF. New simple prognostic score for primary biliary cirrhosis: Albumin-bilirubin score. J Gastroenterol Hepatol. 2015;30:1391-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Li MX, Zhao H, Bi XY, Li ZY, Huang Z, Han Y, Zhou JG, Zhao JJ, Zhang YF, Cai JQ. Prognostic value of the albumin-bilirubin grade in patients with hepatocellular carcinoma: Validation in a Chinese cohort. Hepatol Res. 2017;47:731-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Kodali P, Wu P, Lahiji PA, Brown EJ, Maher JJ. ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am J Physiol Gastrointest Liver Physiol. 2006;291:G355-G363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Kim TH, Suh YS, Huh YJ, Son YG, Park JH, Yang JY, Kong SH, Ahn HS, Lee HJ, Slankamenac K. The comprehensive complication index (CCI) is a more sensitive complication index than the conventional Clavien-Dindo classification in radical gastric cancer surgery. Gastric Cancer. 2018;21:171-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 31. | Kalisvaart M, de Haan JE, Polak WG, Metselaar HJ, Wijnhoven BPL, IJzermans JNM, de Jonge J. Comparison of Postoperative Outcomes Between Donation After Circulatory Death and Donation After Brain Death Liver Transplantation Using the Comprehensive Complication Index. Ann Surg. 2017;266:772-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Nederlof N, Slaman AE, van Hagen P, van der Gaast A, Slankamenac K, Gisbertz SS, van Lanschot JJ, Wijnhoven BP, van Berge Henegouwen MI; CROSS-Study Group. Using the Comprehensive Complication Index to Assess the Impact of Neoadjuvant Chemoradiotherapy on Complication Severity After Esophagectomy for Cancer. Ann Surg Oncol. 2016;23:3964-3971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 597] [Cited by in F6Publishing: 777] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 34. | Wang ZX, Qiu MZ, Jiang YM, Zhou ZW, Li GX, Xu RH. Comparison of prognostic nomograms based on different nodal staging systems in patients with resected gastric cancer. J Cancer. 2017;8:950-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Kammerman LA, Grosser S. Statistical considerations in the design, analysis and interpretation of clinical studies that use patient-reported outcomes. Stat Methods Med Res. 2014;23:393-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |