Published online Sep 28, 2018. doi: 10.3748/wjg.v24.i36.4132
Peer-review started: March 25, 2018
First decision: April 18, 2018
Revised: April 24, 2018
Accepted: May 6, 2018
Article in press: May 6, 2018
Published online: September 28, 2018
Lysophosphatidic acid (LPA), a glycerophospholipid, consists of a glycerol backbone connected to a phosphate head group and an acyl chain linked to sn-1 or sn-2 position. In the circulation, LPA is in sub-millimolar range and mainly derived from hydrolysis of lysophosphatidylcholine, a process mediated by lysophospholipase D activity in proteins such as autotaxin (ATX). Intracellular and extracellular LPAs act as bioactive lipid mediators with diverse functions in almost every mammalian cell type. The binding of LPA to its receptors LPA1-6 activates multiple cellular processes such as migration, proliferation and survival. The production of LPA and activation of LPA receptor signaling pathways in the events of physiology and pathophysiology have attracted the interest of researchers. Results from studies using transgenic and gene knockout animals with alterations of ATX and LPA receptors genes, have revealed the roles of LPA signaling pathways in metabolic active tissues and organs. The present review was aimed to summarize recent progresses in the studies of extracellular and intracellular LPA production pathways. This includes the functional, structural and biochemical properties of ATX and LPA receptors. The potential roles of LPA production and LPA receptor signaling pathways in obesity, insulin resistance and liver fibrosis are also discussed.
Core tip: Lysophosphatidic acid (LPA) is mainly derived from hydrolysis of lysophosphatidylcholine, a process mediated by lysophospholipase D activity in proteins such as autotaxin (ATX). The binding of LPA to its receptors LPA1-6 activates multiple cellular signaling pathways and leads to changes. Studies using genetically modified animals have begun to reveal the roles of LPA pathways in metabolic active tissues and organs. The present review summarized recent progresses in the studies of extracellular and intracellular LPA production pathways; the functions, structural and biochemical properties of ATX and LPA receptors. Furthermore, the potential roles of LPA production and LPA receptor signaling pathways in obesity, insulin resistance and liver fibrosis are discussed.
- Citation: Yang F, Chen GX. Production of extracellular lysophosphatidic acid in the regulation of adipocyte functions and liver fibrosis. World J Gastroenterol 2018; 24(36): 4132-4151
- URL: https://www.wjgnet.com/1007-9327/full/v24/i36/4132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i36.4132
Lysophosphatidic acid (1- or 2-acyl-sn-glycerol 3-phosphate/radyl-glycerol-phosphate, LPA) is one type of water-soluble glycerophospholipid with molecular mass about 430-480 Da. All LPA molecules contain a glycerol backbone linked to a phosphate head group at sn-3 position and an acyl chain esterified to sn-1 or sn-2 position. Due to variation of the fatty acyl chain, LPA molecules are in different forms and derived from multiple sources, such as membrane lipids[1]. The LPA molecules produced extracellularly exert a variety of physiological responses after binding to their receptors as shown in Figure 1.
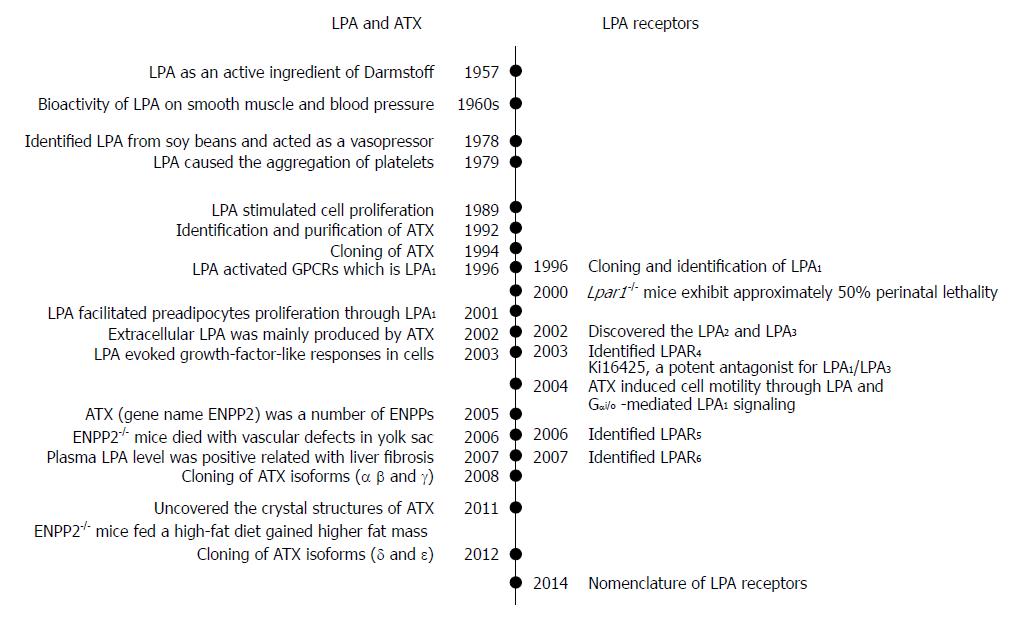
LPA was first identified as an active ingredient of Darmstoff by Vogt in 1957[2,3]. The term Darmstoff was used to describe a smooth-muscle-stimulating substance which was first observed with bath fluid of isolated intestine preparations[4]. The substance was acidic and soluble in many organic solvents, properties that distinguish Darmstoff from amines and polypeptides[5]. Results of acidic hydrolysis and paper chromatography showed that the smooth-muscle-stimulating activity of Darmstoff was due to a mixture of acidic phospholipids (PLs), one of which is an acetal phosphatidic acid[2,3]. In the 1960s, studies on smooth muscle and blood pressure suggested that LPA had biological activities[6,7]. Later on, various molecules of LPA species were isolated and identified from soy beans. It was shown that intravenous injection of LPA from crude soybean lecithin caused hypertension in rats and guinea pigs, but hypotension in cats and rabbits[8]. This raised intriguing questions regarding the activation mechanism of this lipid specie. Since then, the myriad biological effects of LPA have drawn attention of biomedical scientists.
Subsequently, LPA in incubated serum at 36 °C for 18-24 h was shown to cause aggregation of feline and human platelets[9]. Whether LPA acts through its detergent-like physical property or its interaction with a specific receptor remained a critical question. Later, LPA was shown to stimulate cell proliferation in a pertussis toxin-sensitive manner[10]. This finding suggested that LPA acts through G protein-coupled receptor (GPCR). This information led to the cloning and identification of a GPCR, which is now known as LPA receptor 1 (LPA1)[11]. It is known now that as a bioactive lipid mediator, LPA activates at least 6 specific GPCRs, named as LPA1-6. These GPCRs are coupled with several Gα proteins such as Gα12/13, Gαq/11, Gαi/o, and Gαs. The binding of LPA to these receptors stimulates the activations of small GTPases, Ras, Rho, and Rac, and induces downstream actions[12].
The existence of extracellular LPA indicates its production outside a cell. Since autotaxin (ATX) was first identified from human plasma and found to be a lysophosphatidic acid-producing enzyme in 2002[13], the ATX-LPA receptor signaling pathway has been implicated in a variety of disease processes including the vascular and neural development, hair follicle development, tumor progression, lymphocyte trafficking, bone development, pulmonary fibrosis, fat mass regulation, cholestatic pruritus, neuropathic pain, embryo implantation, obesity and glucose homeostasis, spermatogenesis, fetal hydrocephalus, chronic inflammation, cellular proliferation, and smooth muscle contraction during development[14-18]. Both ATX and LPA have attracted the interest of researchers in an effort to understand their roles in pathophysiology and to develop new agents to treat above-mentioned pathological conditions.
LPA and its common precursor lysophosphatidylcholine (LPC) can be found both extracellular and intracellular as signaling mediators and membrane components, respectively. Structurally, LPA is an acyl group esterified to the sn-1 or sn-2 position of the glycerol backbone. Due to the differences of acyl chain length, saturation and backbone position, various LPA chemical forms can be found in tissues and cells. Extracellular LPA is thought to mediate bioactive effects through LPA receptors[19]. Intracellular LPA is an important intermediate for the de novo biosynthesis of complex glycerolipids, including mono-, di-, and triglycerides, as well as PLs[20]. In addition, it has been thought that LPA can function as a ligand for transcription factor peroxisome proliferator-activated receptor γ (PPARγ)[21]. This indicates that LPA may play important roles in the regulation of gene expression.
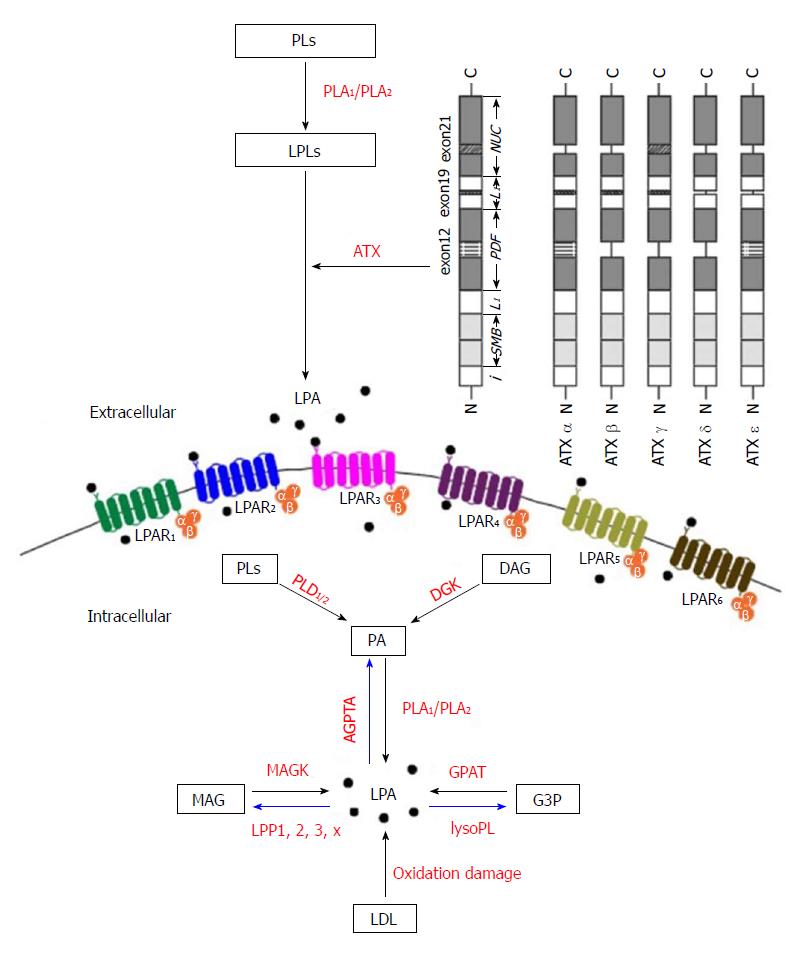
As shown in Figure 2, there are five major pathways for LPA production, (1) the lysophospholipids-ATX (LPLs-ATX) pathway, (2) the phosphatidic acid - phospholipase A1 or A2 (PA-PLA1/PLA2) pathway, (3) the de novo glycerophosphate acyltransferase (GPAT) synthesis pathway, (4) the monoacylglycerol kinase (MAGK) pathway, and (5) the oxidative modification of low-density lipoprotein (LDL) pathway. Despite recent advances in the identification of the enzymes responsible for LPA production, the regulation of these enzymes still remains obscure.
In the first pathway, LPLs generated from PLs by PLA1 or PLA2 are converted to LPA by a plasma enzyme ATX[22,13], which we will describe in later part of this article. A major source of extracellular LPA is LPC, other LPLs such as lysophosphatidylserine and lysophosphatidylethanolamine can also be enzymatically processed to produce LPA. This pathway accounts for the majority of circulating LPA.
LPA is also produced intracellularly as an intermediate for the synthesis of other glycerolipids[20]. LPA can be produced enzymatically from intracellular organelles such as mitochondria and endoplasmic reticulum. Phosphatidic acid (PA) is first generated from PLs or diacylglycerol by phospholipase D enzymes (PLD1 and PLD2) and diacylglycerol kinase (DGK) activities, respectively. Then, one acyl group is removed from the sn-1 position by PLA1 or at the sn-2 position by PLA2 enzymes to generate LPA. This pathway may be more important in specific tissues with expression of DGK such as the brain and skin[23].
GPATs catalyze the first step in glycerolipid synthesis, i.e., the conversion of glycerol-3-phosphate (G3P) to LPA by the transfer of fatty acids from acyl-CoA. Since GPAT exhibits the lowest specific activity of enzymes in the de novo triacylglycerol (TAG) and PLs synthesis pathways, it has been considered to be the rate limiting enzyme for them[24]. Many studies have been published on the regulation of TAG synthesis and its relevance to obesity and insulin resistance. GPAT activity in mitochondria was shown to be regulated by fatty acid-binding protein (FABP)[25,26]. It has been shown that mitochondrial GPAT activity was inhibited by LPA. FABP reversed the inhibition of LPA through the binding and extracting LPA from the mitochondrial outer membrane. The extracted LPA was converted to PA by microsomes, where acylglycerophosphate acyltransferases (AGPATs) are located[25,26]. These results suggested that FABP regulated the de novo synthesis of PA through the stimulation of mitochondrial GPAT and transport of LPA from mitochondria to microsomes.
Lipid phosphate phosphatases (LPPs) are also involved in the LPA turnover. LPPs can be found extracellularly or intracellularly in endoplasmic reticulum or Golgi, where they dephosphorylate LPA, which leads to the formation of monoacylglycerol (MAG)[27]. MAG may then be phosphorylated by MAGK and thus participate in another round of LPA signaling[20]. Thus, the production of LPA is regulated by the availability of precursors as well as the expression of catalytic enzymes.
LPA was found as an active molecule on oxidized and modified LDL, in where it may contribute to platelet activation, endothelial cell stress-fiber and gap formation[28,29]. LPAs on these lipoproteins activate platelets through G-protein coupled LPA receptors and a Rho/Rho kinase signaling pathway, which leads to platelet shape change and subsequent aggregation. The biologically active LPA-like products generated by this non-enzymatic oxidation co-migrated with an authentic LPA standard in thin layer chromatography. LPA was found to be accumulated in atherosclerotic plaques, which might act to activate platelets. The level of LPA is very high in the human carotid atherosclerotic lesion, suggesting the roles in thrombogenesis and rupture[28,29]. An alternative explanation to the generation of LPA from oxidized LDL is that ATX might be activated. The acyl/alkyl composition, the precursor of LPA and the mechanism responsible for LPA generation in the oxidized LDL remain to be addressed in the future.
There are three major pathways that degrade LPA as shown in Figure 2. The first is the removal of phosphate to form MAG by LPPs[30]. LPA has a half-life of 3 min when it is added to cells expressing LPP[31]. Four isoforms of LPP have been cloned and characterized in mammals, LPP1/PAP-2α/PAP-2α1[32], LPP1α/PAP-2α2[33], LPP2/PAP-2c/PAP-2α[34] and LPP3/PAP-2β/PAP-2β[32]. The second LPA degradation pathway involves the action of AGPAT enzymes, also known as lysophosphatidic acid acyltransferase. These microsomal enzymes catalyze the transfer of an acyl group from acyl-CoA to LPA to form PA. Proteins with AGPAT activity include a family of transmembrane enzymes[35], and membrane associated proteins involved in membrane fission such as endophilin[36] and C-terminal-binding protein/brefeldin A-ADP ribosylated substrate[37]. The third pathway for LPA degradation involves the hydrolysis of the acyl group from the G3P head group by the action of lysophospholipases. The majority of characterized lysophospholipases act on LPC[38].
The ecto-nucleotide pyrophosphatase/phosphodiesterase (ENPP) family contains seven members with structurally similar catalytic domains that hydrolyze phosphodiester bonds in various substrates, including nucleoside triphosphates, LPLs, and choline phosphate esters[39,40]. ATX, or ENPP2, is the best-characterized member of ENPP family. ENPPs are defined by their ability to hydrolyze phosphodiester bonds of various nucleotides in vitro[41-43]. ATX/ENPP2 was originally identified as a tumor cell-motility-stimulating factor from the conditional medium of A2058 human melanoma cells[44]. Since the addition of pertussis toxin reduced cellular motility, ATX’s effects were thought to involve Gi/o-mediated signaling[39,44]. ATX can be secreted as a 100 kDa glycoprotein. It is produced by multiple tissues including adipose tissue[45,46]. It is believed that the circulated ATX/ENPP2 is degraded by the liver[47]. Extracellular LPA was found to be present in sub-micromolar ranges. The responsible enzyme was identified to be ATX[13,22]. ATX-mediated autocrine signaling induces cell motility through LPA production and Gi/o-mediated LPA receptor signaling[48].
From both a structural and evolutionary point of view, ENPP family members have been categorized into two subgroups, ENPP1-3 and ENPP4-7[39]. ENPP1-3 all have two N-terminal somatomedin B-like (SMB) domains, a central phosphodiesterase (PDE) domain and a C-terminal nuclease (NUC)-like domain as shown in Figure 2. ENPP4-7 only have similarity in the PDE domain. The crystal structures of mouse[49] and rat[50] ATX show loops on both sides of the catalytic domain, which may help to determine the binding specificity. The SMB and PDE domains are connected by the first loop (L1 linker region), whereas the PDE and NUC domains are connected by the second loop (L2 linker region)[49].
The secreted ATX is a constitutively active glycoprotein with a N-terminal signal peptide sequence containing a furin cleavage site[46]. The other ENPPs are transmembrane or anchored proteins. In addition to its pyrophosphatase/phosphodiesterase activities, ATX has lysophospholipase D (lysoPLD) activity. The N-terminal signal peptide of the ATX precursor is removed first, and then, the remaining part is cleaved by proprotein convertases before the active ATX with lysoPLD activity is released into the extracellular environment, which converts LPC into LPA and choline[47]. The structure of PDE domain has a lipid binding pocket and a nearby tunnel allowing entry of substrates and release of products[51]. The NUC domain has been thought to maintain the rigidity of the PDE domain, and the two N-terminal SMB domains mediate binding of ATX to integrin[52]. This binding brings ATX to the cell membrane, which allows the production of LPA in a location close to its receptors[51,53,54].
The structures of ATX in complex with diverse LPAs show distinct conformations after different acyl chains occupy the binding pocket[49]. LPAs with saturated chains bind in the hydrophobic pocket in a more elongated fashion, whereas LPAs with unsaturated chains have a bent conformation due to the presence of carbon-carbon double bond(s). For LPA (22:6), the acyl chain shows a U-shaped conformation in the binding pocket[49]. ATX prefers LPC species with shorter and unsaturated acyl chain as substrates, and the rank order is 14:0 > 16:0 > 18:3 > 18:1 > 18:0. All these show that ATX is able to hydrolyze LPCs with different lengths and saturations of acyl chains to produce the corresponding LPAs.
The cDNA of ATX/ENPP2 was cloned in 1994[55]. After that, its homology with phosphodiesterases was revealed, and the cloning and tissue distribution of the three human and mouse isoforms (α, β and γ) were determined in 2008[50]. Two more isoforms (δ and ε) were identified in 2012[56]. The ATX gene is located on mouse chromosome 15 and on human chromosome 8. The human and mouse ATX gene structures are conserved[50]. The mouse ATX gene spans more than 80 kb and contains at least 27 exons. The three splicing sites in exons 12, 19 and 21 can theoretically result in eight isoforms, in which five were detected. These isoforms are catalytically active (ATX α-δ) and expressed in different tissues. They are ATXα (ATXm), ATXβ (ATXt), ATXγ (PD-Iα)[50], ATXδ and ATXε[56]. ATXβ and ATXδ, which are the most and second most abundant isoforms, respectively, share similar biochemical characteristics (Figure 2). Houben et al[53] characterized that a 52-residue polybasic insertion corresponding to exon 12 in ATXα isoform confers specific binding to heparan sulfate proteoglycans thereby targeting LPA production to the plasma membrane. This is another potential mechanism for localizing ATXα to cell membranes and for LPA production in close proximity to LPA receptors. Exon 12 encodes a 52-amino acid insertion of the mouse ATXα and ATXε isoforms (amino acids 324-375), whereas exon 21 encodes an additional 25-amino acid of the murine ATXγ isoform (amino acids 593-617). Novel isoforms ATXδ and ATXε have a 4-amino acid deletion on exon 19. This complex way of exon arrangement has been maintained through evolution. Human ATX exhibit 93% sequence identity with rodent ATX while all important residues are highly conserved[49].
ATX has a broad profile of tissue expression, with relatively high levels in the blood, brain, kidney, and lymphoid organs[57-59]. Secretion of ATX leads to high concentration in cerebrospinal fluid and in the endothelial venules of lymphoid tissues[60-62]. The cellular sources of plasma ATX are incompletely understood. Nevertheless, adipocytes may be a source[63,64]. ATX is also stored in platelets and released during their activation[65,66]. Circulating ATX is rapidly taken up by the scavenger receptors of liver sinusoidal endothelial cells, and then degraded by the liver[46]. Thus, like insulin, ATX is largely removed from the circulation through first passage by the liver. For the ATX isoforms, high expression levels of ATXβ and ATXγ mRNA were detected in peripheral tissues and the brain, whereas ATXα was shown the lowest expression level in both the central nervous system and peripheral tissues among the three isoforms in human. In mice, ATXβ is widely expressed in the brain and peripheral tissues, and ATXγ and ATXα showed little variation in their distribution[50]. Human brain and retina showed relatively higher expression level of ATXα than that of ATXβ and ATXγ, whereas the expression levels of ATXδ and ATXε in the small intestine and spleen are higher than that in other tissues[56].
LPA has been quantified in a variety of species, tissues, and fluids, including neural tissue, cerebrospinal fluid, fertilized hen white, seminal fluid, tears, plasma, serum, urine, saliva, and aqueous humor[67-69]. The formation of LPA species depends on the precursor PLs, which can vary by acyl chain length and degree of saturation. The term LPA most often refers to 18:1 oleoyl-LPA (1-acyl-2-hydroxy-sn-glycero-3-phosphate), as it is the most commonly one. Other chemical forms of LPA can be observed in various biological systems that have concentrations ranging from low nanomolar to micromolar levels[67,70]. LPA concentrations in human and rat blood can range from 0.1 μmol/L in plasma and up to 10 μmol/L in serum, which is well over the apparent nanomolar kDa of LPA1–6[71-74]. The LPA molecules containing 18:2, 20:4, 16:1, 16:0, and 18:1 acyl chains are particularly abundant in plasma[75-77]. Current methods to detect LPA include indirect enzymatic assays[73], TLC-GC, LC-MS, and LC-MS/MS[78-80].
LPA acts as a potent mitogen, which was previously known as “ventricular zone gene-1 (vzg-1)” due its high level in the embryonic neuroproliferative layer of the cerebral cortex[11,12]. The cloning and functional identification of LPA1 led to determination of other receptor genes based upon sequence homology[81-83]. This is particularly true for the “endothelial differentiation gene” (EDG) members[84] that include LPA and sphingosine 1-phosphate receptors. Then, two other LPA receptors, LPA2 and LPA3 (also known as EDG4, and EDG7), were subsequently discovered based on shared homology with LPA1(EDG2)[85]. Later on, LPA4 (P2RY9, GPR23)[86], LPA5 (GPR92)[87] and LPA6 (P2RY5, GPR87)[88] were identified. They share 35% amino acid homology to the purinergic (P2Y) family of GPCRs, as compared to less than 20% homology to LPA1, suggesting that LPA4-6 are more closely related to the P2Y receptors[52]. Here, LPA1-LPA6 are for proteins, and their gene symbols are LPAR1-LPAR6 for human and Lpar1-Lpar6 for non-human[89].
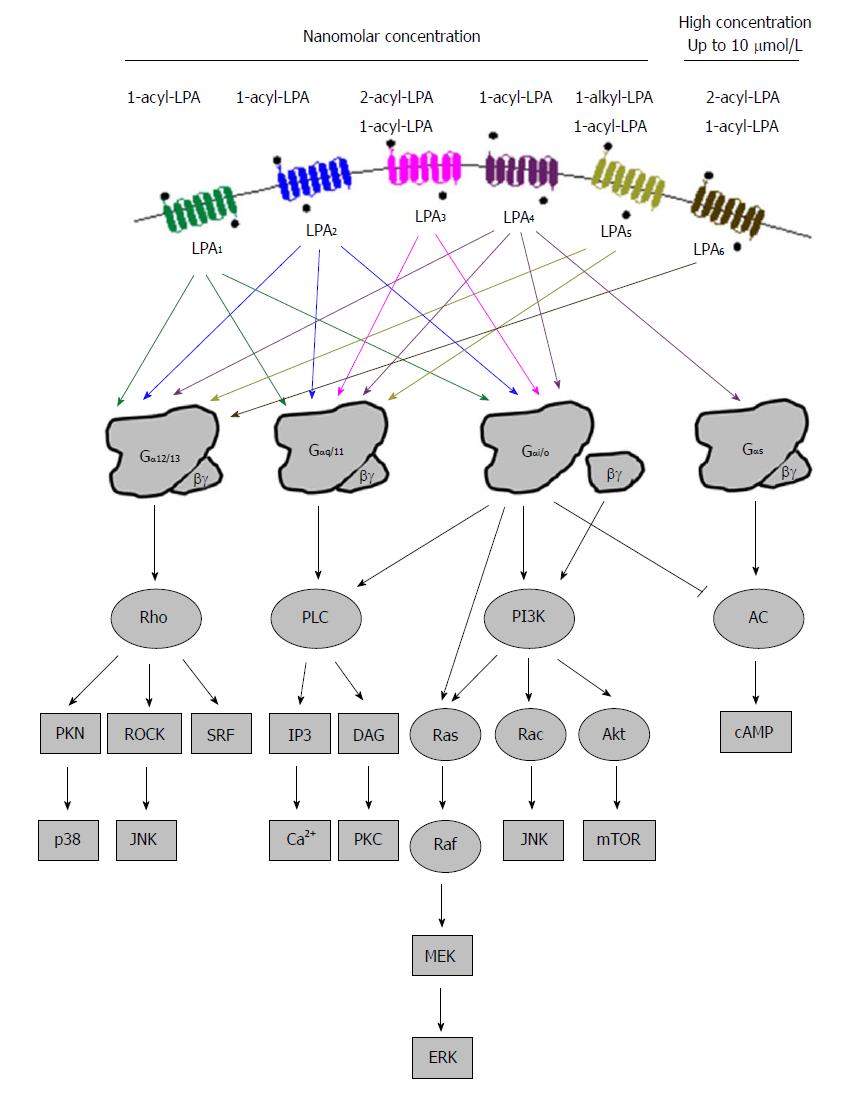
All LPA receptors signal through at least one of the four heterotrimeric Gα proteins (Gα12/13, Gαq/11, Gαi/o, and Gαs)[12,90], resulting in downstream signals that produce diverse physiological and pathophysiological effects (Figure 3). Gα12/13-mediated LPA signaling regulates cytoskeletal remodeling, cell migration and invasion through activation of Rho pathway proteins[91]. Rho signals to c-jun N-terminal kinase (JNK) and p38 through Rho-associated kinase (ROCK) and protein kinase N. The LPA-coupled Gαq/11 protein primarily regulates Ca2+ homeostasis through phospholipase C (PLC), which generates the second messengers IP3 and diacylglycerol (DAG)[92-94]. Gβγ and Gαi/o subunits mediate the activation of phosphatidylinositol 3-kinase (PI3K) which results in the stimulation of the Akt pathway and increase of protein translation after the activation of the mammalian target of rapamycin (mTOR) signaling pathway. Activation of PI3K by Gβγ subunits also stimulates the activity of Rac, leading to cell migration and JNK regulation of pro-inflammatory gene expression, and Ras activity, leading to the stimulation of Raf- mitogen-activated protein kinase (MEK)-extracellular signal-regulated kinase (ERK) pathway to promote the expression of genes involved in proliferation and invasion. Gαi/o, besides PI3K, also stimulates the Ras-Raf-MEK-ERK pathway promoting cell survival and other functions[95,96]. Gαs can activate adenylyl cyclase and increase cAMP concentration upon LPA stimulation[97]. However, the same enzyme is also inhibited by Gαi/o, showing the complexity of signaling pathways after the activation of LPA receptors[98].
All six LPA receptors can be stimulated by 1-acyl-LPAs, which show different potencies. LPA3 and LPA6 prefer unsaturated 2-acyl-LPA, while LPA5 likes ether-linked 1-alkyl-LPA species[99,100]. In addition, lysophosphatidylserine, lysophosphatidylinositol, and lysophosphatidylethanolamine, have been thought to activate these receptors as well[101]. Different LPA molecules may have preference to different subtypes of LPA receptors[102]. Table 1 summarizes PLA receptors expression profiles and their known physiological functions in humans and mice.
| Name | Information | Previous orphan names | Major expression tissue (high to low level) | Knockout effects in mouse | Biological functions | Ref. |
| LPA1 | Human chromosome locus 9q31.3; 41.1 kDa1; 364 aa2; Identity3 97.3% | vzg-1, edg-2, 39.4 kDa; 344 aa mrec1.3, lpA1 | Brain, placenta, urinary bladder, uterus, testis, lung, small intestine, heart, stomach, kidney, spleen, thymus, and skeletal muscle. | Perinatal lethality, retarded growth, defective olfaction, reduced body size, craniofacial dysmorphism with blunted snouts, and increased apoptosis in sciatic nerve Schwann cells. | Neurodevelopment regulation, cell proliferation, differentiation, apoptosis and survival, cell-cell contact through serum-response element activation, cell migration and cytoskeletal organization, Ca2+ homeostasis, cAMP-regulated cellular processes and adenylyl cyclase inhibition | Yung et al[68], 2014; Archbold et al[138], 2014; Choi et al[139], 2008; Anliker et al[103], 2013; Sakai et al[104], 2013; Wittpoth et al[98], 1999; An et al[82], 1998; Contos et al[107], 2000; Contos et al[106], 2000; Fukushima et al[84], 2001. |
| Mouse chromosome locus 4, 32.2 cM; 41.1 kDa; 364 aa | Brain, heart, lungs, stomach, intestine, placenta, kidneys, spleen, uterus, testes. | |||||
| LPA2 | Human chromosome 19p13.11; 39.1 kDa; 351 aa; Identity 83.5% | edg-4, lpA2 | Leukocytes, testis, prostate, spleen, thymus and pancreas. | Normal | Cell migration, viable and healthy, nervous system development and immune system regulation. | Yung et al[68], 2014; An et al[82], 1998; Contos et al[106], 2000b; Archbold et al[138], 2014; Ohuchi et al[111], 2008; Choi et al[110], 2010; Valentine et al[140], 2008; Xu et al[113], 2004; Lai et al[112], 2005; Contos et al[115], 2002; Choi et al[139], 2008. |
| Mouse chromosome 8, 33.91 cM; 38.7 kDa; 348 aa | Kidney, testis, uterus, lung, stomach, spleen, thymus, postnatal brain, and heart. | |||||
| LPA3 | Human chromosomal locus 1p22.3; 40.1 kDa; 353 aa; Identity 91.2% | edg-7, lpA3 | Heart, testis, prostate, pancreas, lung, ovary, and brain. | Delayed embryo implantation, embryo crowding, and reduced litter size for female null mutants. | Male and female reproductive physiology, inflammation, cell Ca2+ homeostasis and cAMP regulation, vertebrate left-right patterning during embryogenesis. | Yung et al[68], 2014; Bandoh et al[83], 1999; Im et al[116], 2000; Contos et al[107], 2000a; Zhao et al[117], 2015; Ye et al[118], 2010; Hama et al[119], 2010; Lai et al[120], 2012 |
| Mouse chromosome locus 3, 71.03 cM; 40.3 kDa; 354 aa | Lung, kidney, uterus, testis, small intestine, brain, heart, stomach, placenta, spleen, and thymus. | |||||
| LPA4 | Human chromosome Xq21.1; 41.9 kDa; 370 aa; Identity 98.4% | P2Y9/GPR23 | Ovaries, thymus, pancreas, brain, heart, small intestine, testis, prostate, colon, and spleen. | Inhibition of its differentiation into osteoblasts in human mesenchymal stem cell line; For mouse: increased trabecular bone volume, number, and thickness; pericardial effusions, severe edema and hemorrhage, abnormally dilated blood and lymphatic vessels and lymph sacs, and impaired pericyte recruitment. | ROCK-dependent cell aggregation and N-cadherin-dependent cell adhesion, cAMP accumulation, differentiation of immortalized hippocampal progenitor cells, negatively cell motility regulation and osteogenesis. | Yung et al[68], 2014; Ohuchi et al[111], 2008; Choi et al[110], 2010; Liu et al[123], 2010; Mansell et al[124], 2010; Liu et al[125], 2009; Sumida et al[126], 2010; Yanagida et al[71]; Lee et al[97], 2007; Rhee et al[121], 2006; Lee et al[122], 2008 |
| Mouse chromosome X region D; 41.9 kDa; 370 aa | Heart, ovary, skin, thymus, and bone Marrow. | |||||
| LPA5 | Human chromosome 12p13.31; 41.3 kDa; 372 aa; Identity 79.0% | GPR92 | Spleen, heart, small intestine, placenta, colon, and liver. | Reduced lung metastasis by melanoma cells. | Neurite retraction, stress fiber formation, receptor internalization, water absorption, Ca2+ mobilization and cAMP accumulation, LPA-induced release of chemokine ligand 4 in mast cells. | Yung et al[68], 2014; Lee et al[87], 2006; Lee et al[141], 2015; Amisten et al[142], 2008; Lundequist et al[129], 2011; Araki et al[130], 2014; Lin et al[128], 2010; Yanagida et al[143], 2013 |
| Mouse chromosome 6, 59.21 cM; 41.4 kDa; 372 aa | Small intestine, lung, heart, stomach, colon, spleen, thymus, skin, liver, platelets, mast cells, gastrointestinal lymphocytes, and dorsal root ganglia. | |||||
| LPA6 | Human chromosome 13q14.2; 39.4 kDa; 344 aa; Identity 93.0% | P2Y5 | Hair, skin. | Hypotrichosis | Hair development, increased intracellular Ca2+, reduced forskolin-stimulated cAMP accumulation, and ERK1/2 activation | Yanagida et al[144], 2011; Yanagida et al[143], 2013; Raza et al[135], 2014; Dong et al[145], 2014; Lee et al[141], 2015; Lee et al[133], 2009 |
| Mouse chromosome 14, region D3; 39.4 kDa; 344 aa | Hair, immune cells. |
LPA1 is the first LPA receptor identified based on studies of LPA in the brain[11]. LPA1 couples to three Gα proteins - Gα12/13, Gαq/11, and Gαi/o, which can result in the activation of downstream pathways including Akt, Rho, Ras, and PLC (Figure 3). These pathways mediate many cellular responses initiated by LPA1 such as neurodevelopment regulation, cell proliferation, differentiation, apoptosis and survival, cell-cell contact through a variety of mechanisms[68,84,103-106]. Lpar1-/- mice exhibit about 50% perinatal lethality, which was attributed to the defective development of olfaction. The survived ones had reduction of body size, craniofacial dysmorphism, and loss of Schwann cells[107]. Dysregulation at glutamatergic synapses was observed in Lpar1-/- mice[108]. When the original Lpar1-/- mouse line was expanded, a spontaneous variant named “Málaga LPA1” arose. They showed more severe brain defects than the original Lpar1-/- line mice did[109]. The loss of LPA1 in animals seems to modulate the development of several diseases including cancer, obesity, neuropathic pain, fibrosis and male infertility[110].
The amino acid sequence of LPA2 is about 50% identical to that of LPA1, and it associates with Gαi/o, Gαq/11, and Gα12/13, the same as LPA1[106] (Figure 3). These G proteins use Ras, PI3K/Rac, PLC/DGA and Rho to mediate their down-stream signals, which may regulate cell survival and migration[107]. LPA2 regulates cell survival and cell migration in the development of nervous system and functions of immune system[68,90,106,110,111]. The focal adhesion molecule thyroid receptor-interacting protein 6[112,113] and several PDZ-domain and zinc finger proteins[114] interact with LPA2. The PDZ-binding domain of LPA2 regulates Na+/H+ exchanger regulatory factor 2 activity, and activates PLC-3 and Akt/ERK signaling pathways. These pathways stimulate cell migration, enhance survival, and alter gene expression, accounting for the functions attributed to LPA2. Lpar2-/- mice are viable and healthy, while those null for both Lpar1 and Lpar2 show features essentially consistent with those of Lpar1-/-[115]. These data suggest functional redundancy of LPA2 with LPA1.
LPAR3/Lpar3 was cloned based upon homology to already identified LPA receptor genes using degenerated primers in a PCR-based cloning strategy[83,116]. LPA3 couples with Gαq/11 and Gαi/o to mediate adenylyl cyclase inhibition, PLC activation and Ca2+ mobilization, and Ras activation[105] (Figure 3). LPA3 prefers 2-acyl-LPAs containing unsaturated fatty acids[83]. It mediates the activation of a series of physiological processes such as male and female reproductive physiology, inflammation, cell Ca2+ homeostasis and cAMP regulation[107,117-119]. LPA3 appears to determine vertebrate left-right patterning during embryogenesis as downregulation of Lpar3 or inhibition of LPA3 activity disrupted patterning process in zebrafish[120]. Lpar3-/- mice are viable with no reported neural deficits, even though LPA3 is found in the frontal cortex, hippocampus, and amygdala[83,116]. On the other hand, female Lpar3-/- mice have a delayed embryo implantation, and reduced litter size[117].
The first so-called non-EDG LPA receptor was identified in 2003, and named as LPA4. It shares homology (approximately 20%) with LPA1-3, and it is more closer to the P2Y receptor family[86]. LPA4 was identified by screening orphan receptors using calcium mobilization as a readout for ligand-induced signals. LPA4 couples with Gα12/13, Gαq/11, Gαi/o and Gαs[97], and activates Rho/ROCK to induce neurite retraction and stress fiber formation[71,97] (Figure 3). It induces ROCK-dependent cell aggregation and N-cadherin-dependent cell adhesion[71]. LPA4 is only LPA receptor that activates Gαs to induce cAMP level[97]. The activation of LPA4 was thought to regulate the differentiation of immortalized hippocampal progenitor cells[121]. In addition, the activation of LPA4 could inhibit LPA-induced cell migration, but LPA exposure increased lamellipodia formation and transwell movement of LPA4 null cells, indicating an increased sensitivity[122]. It shows the ability of LPA4 to negatively regulate cell motility and indicates that differential effects may be achieved by simultaneously expressing multiple LPA receptors. LPAR4-deficient human mesenchymal stem cells lost ability to differentiate into osteoblasts[123]. While adult Lpar4-/- mice appear grossly normal[122], they exhibit increased trabecular bone volume, number, and thickness[124,125]. LPA4 pathway seems to inhibit osteogenesis. Lpar4-/- mice had reduction of prenatal survival rate during embryo development, which is accompanied by changes such as pericardial effusions, severe edema and hemorrhage[126].
LPA5, the fifth LPA receptor, was identified in 2006[87,127]. It shares about 35% homology with LPAR4, and 22% homology with LPAR1-3[87]. LPA5 couples with Gα12/13 and Gαq/11, which mediate neurite retraction, stress fiber formation, and receptor internalization in LPA5-expressing cell lines[87] (Figure 3). It also activates Gαq/11 to increase intracellular calcium mobilization, and cAMP accumulation via a non-Gαs mechanism, suggesting the involvement of other G-proteins[87,127]. LPA5 signaling may also affect intestinal water absorption[128]. This is achieved through the LPA-induced recruitment of Na+/H+ exchanger 3 to the microvilli mediated by the interaction between LPA5 and Na+/H+ exchanger regulatory factor 2. Additionally, LPA5 is the main LPA receptor responsible for LPA-induced release of chemokine ligand 4 in mast cells[129]. Interestingly, LPA5 in B16 melanoma cells, prefers alkyl-LPA (18:1) to acyl-LPA (18:1)[99]. Lpar5-/- null mice exhibit reduced lung metastasis by melanoma cells compared with wild type ones[130].
The most recently identified LPA receptor is LPA6. It was first isolated from a chicken T cell library and named receptor 6H1 in 1993[131], and then, renamed to P2Y5 because of sequence homology with P2Y receptors in 1996[132]. LPA6 couples with Gα-protein Gα12/13 (Figure 3). Its activation by LPA causes cAMP accumulation, changes in cell morphology, and guanosine 5′-3-O-(thio) triphosphate binding[71]. When LPA6 was expressed together with a Gα protein, LPA stimulation increased intracellular Ca2+ level, and decreased forskolin-induced cAMP level and ERK activation in intestinal cells[133]. LPA6 has been thought to be involved in familial hair loss[134,135]. Mutations of lipase member H and LPA6 in patients with hypotrichosis are respectively associated with a decrease in LPA production and abnormal LPA6 activation in cells[134,136,137]. These findings demonstrate the roles of LPA6 and LPA signaling may be therapeutic targets for the treatment or prevention of human hair loss[138-146].
Recently, obesity has become major public health concern, particularly in the United States. According to 2015 Center of Disease Control and Prevention estimates, more than one-third of adults (34.9% or 78.6 million) and 17% of youth in the United States were obese in 2011-2014[147]. Obesity is associated with the development of chronic metabolic diseases including diabetes, heart disease, stroke, and some types of cancer. The long-term effects of being overweight correlate with premature death, cardiovascular disease, metabolic morbidities, and asthma, among other problems[148]. Both environmental factors and genetic factors contribute to the obesity development. Many factors modulate the propensity to accumulate fat in cells, including an increased ratio of adipocyte precursor cells to differentiated adipocytes[149].
Obesity is associated with adipocyte hypertrophy and hyperplasia. Hypertrophy results in excessive TAG accumulation in adipocytes. Hyperplasia results in recruitment of new adipocytes via proliferation and differentiation. LPA was found to induce proliferation of 3T3F442A preadipocytes, indicating the role of LPA signaling in fat storage[150]. LPA stimulation increases the growth of 3T3F442A cells via LPA1, which activates the Ras-Raf-MEK-ERK pathway, and of the focal adhesion kinase[20,151].
It has been reported that Lpar1-/- mice exhibited greater adiposity than the control mice without alteration of feeding behavior, despite of lowered body weight[107]. Interestingly, Lpar1-/- mice were resistant to diet-induced obesity that may result at least in part from alterations in leptin production[64]. Mature adipocytes express more ATX than preadipocytes. When secreted from adipose tissue, ATX may promote preadipocyte proliferation. Its expression was up-regulated during adipocyte differentiation, and in db/db mice[44,45].
The serum levels of LPC, the precursors of LPA, increases gradually in rabbits fed a high-cholesterol diet for 12 wk. The levels of individual LPAs formed after the incubation of serum for 24 h elevated with the increase of the length of time that rabbits were fed a high cholesterol diet[152]. These studies indicate that feeding of a high-fat diet can cause an increase in the circulating level of LPA. Preadipocytes mainly express LPA1[153], and the mRNA level of Lpar1 expression in preadipocytes is higher than that in mature 3T3-L1 adipocytes[154]. However, in human adipose tissue, obesity does not influence on LPAR1 expression[155]. This discrepancy of LPA1 expression levels between human and mouse adipose tissues suggest that obesity promotes LPA synthesis rather than activation in adipose tissue.
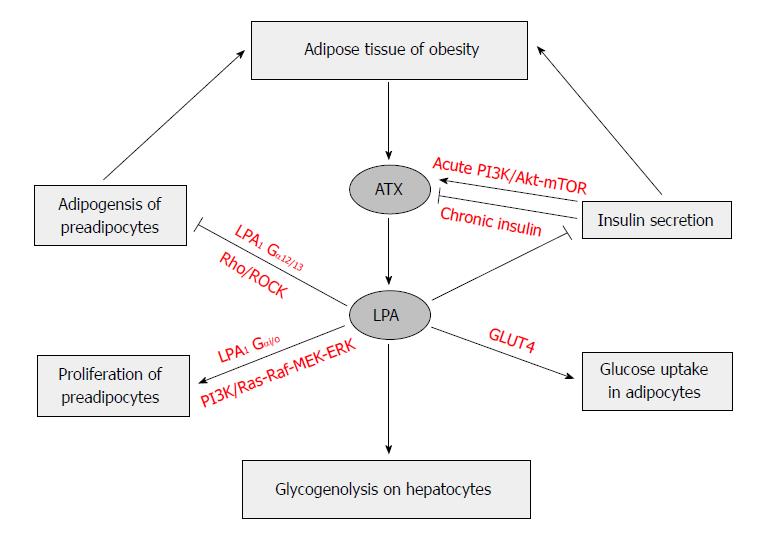
The LPA-induced proliferation of preadipocytes[20,153,154] has been thought to be mediated through LPA1 and the activation of the Ras-Raf-MEK-ERK pathway[154,156,157]. LPA inhibits differentiation of white and brown preadipocyte cell lines, which include porcine preadipocyte cell line; mouse preadipocyte cell line, 3T3-L1 and 3T3F442A; and human Simpson-Golabi-Behmel Syndrome preadipocyte cells[153,154,158,159]. This inhibition is mediated by LPA1via the Rho-ROCK pathway[160,161]. All these result in a down-regulation of PPARγ, and impaired responses of PPARγ-targeted genes to its ligands, which leads to reduced TAG accumulation, and expression levels of adipogenic genes[153,154].
The activation of Rho-ROCK pathway delayed the activation of the Wnt-signaling pathway, which has been partially attributed to the inhibited PPARγ expression and adipogenesis. When mice with the adipocyte-specific knockout of ATX gene (FATX-KO) were fed a high-fat diet, they had more fat mass and larger adipocyte size, but not adipocyte number, than the control mice did in the absence of any change of food intake. The deletion of ATX in mice appeared to lead sensitivity to diet-induced obesity, which might be due to elevated expression levels of PPARγ and its down-stream adipogenic genes in subcutaneous white adipose tissue. Interestingly, those knockout mice had improved glucose tolerance and less systemic insulin resistance than the control mice fed the same diet[63,161]. LPA stimulation seems to have anti-adipogenic effect in white adipocytes[153] and in brown preadipocytes[159]. Aforementioned experiments seem to indicate that ATX-LPA receptor signaling pathway may inhibit the development of adipose tissue (Figure 4).
On the other hand, others reported that ATX promotes preadipocytes proliferation and differentiation into adipocytes, thereby promoting adipocyte hyperplasia and obesity. It was showed that deletion of ATX results in smaller body weight gain, smaller fat pad weights and adipocyte numbers, less insulin resistance and glucose tolerance in heterozygous Enpp2+/- mice and adipocyte-specific FATX-KO mice fed a high-fat diet than their littermates controls[162]. Moreover, the FATX-KO improved brown adipose tissue function, increased energy expenditure, and improved systemic metabolism. Transgenic mice expressing the human ATX/ENPP2 gene under the control of α1 antitrypsin gene promoter became sensitive to diet-induced obesity due to reduced expression of brown adipose tissue-related genes in peripheral white adipose tissue and accumulated significantly more fat without any change of locomotor activities, thermogenic profiles, and systemic metabolism[159]. In mice, ATX is highly expressed in visceral white adipose tissue and brown adipose tissue and is downregulated in adipose tissue hypertrophy[162]. In human, ATX expression is higher in subcutaneous than in visceral fat, and the latter fat pad in obese subjects has higher ATX expression level than that in non-obese subjects, which is correlated with leptin expression[155]. The circulating ATX levels correlated negatively with body mass index, and mRNA levels of ATX were reduced in subcutaneous fat from obese subjects[162]. Moreover, ATX expression in adipose tissues may be negatively regulated by LPA through a feedback regulatory mechanism, which may involves inflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin-1β[163].
Earlier studies found that increases in adipocyte size correlated with insulin resistance, and increased risk of type 2 diabetes[164,165]. The expression of ATX is increased in the adipose tissue of obese and insulin-resistant subjects and mice[44,155,166]. It has been shown that LPA also regulates glucose metabolism[167,168]. LPA was found to enhance glucose uptake in a dose-dependent manner in both GLUT4myc L6 myotubes and 3T3-L1 adipocytes, a process that was attributed to the increase of GLUT4 translocation in a PI3K dependent manner. Moreover, the effect of LPA on glucose uptake was completely inhibited by pretreating cells with LPA1/3 receptor antagonist Ki16425 and Gi inhibitor pertussis toxin[169]. LPA significantly lowered blood glucose levels in normal mice and streptozotocin-induced diabetic mice, suggesting the promotion of glucose usage, but not stimulation of insulin secretion[169].
The elevation of ATX expression in adipocytes of db/db mice occurred simultaneously with the development of hyperglycemia, and only 3 wk after the emergence of hyperinsulinemia in them[166]. ATX expression was up-regulated by treatment with TNFα, and down-regulated by rosiglitazone in 3T3F442A adipocytes[166]. The upregulation of ATX expression in adipocytes of db/db mice seems to be associated with the emergence of hyperglycemia rather than fat accumulation or hyperinsulinemia[166].
The plasma levels of LPC, as the precursor of LPA, are reduced in obese and type 2 diabetic mice, suggesting that it may regulate blood glucose level. This reduction may contribute to the impairment of glucose homeostasis[170]. Interestingly, adipocyte specific ATX knockout mice fed with a high-fat diet showed greater adiposity and better tolerance to glucose challenge than control mice[63], suggesting a negative effect of LPA on glucose homeostasis. Similarly, LPA production appears to impair glucose disposal probably through a reduction of plasma insulin as pharmacological inhibition of LPAR1/3 activation improves glucose homeostasis in obese and prediabetic mice[171]. Another possibility is that the progression of diabetes affects ATX expression in adipose tissue[162]. It has been shown that treatments with high concentrations of glucose and insulin led to ATX secretion in adipocytes. Short-term insulin treatment increased ATX activity, whereas long-term insulin treatment reduced the levels of ATX mRNA and protein, and its activity[172].
In humans, ATX expression in adipose tissue significantly increased in diabetes patients in contrast with obese-only subjects[158,166]. Its expression in subcutaneous fat is higher than that in visceral fat. Nevertheless, ATX in visceral, but not subcutaneous, fat of obese subjects is higher than that in non-obese patients[155]. Interestingly, the circulating ATX levels in the blood were reduced in obese subjects[162]. The females have higher blood ATX level than males[173].
The variations of ATX expression were correlated with some clinical parameters. In obese patients, visceral fat ATX was positively correlated with diastolic arterial blood pressure, plasma leptin level, and expression levels of inducible nitric oxide synthase and apelin receptor[155]. In older and obese humans, plasma ATX correlated with fasting glucose, fasting insulin, and glucose level 2 h after an oral glucose tolerance test, and body mass index[173]. LPA produced by ATX in obesity has a tonic inhibitory effect on glucose homeostasis through inhibition of insulin secretion in isolated pancreas islets, increase of glucose transport in myocyte and adipocytes, and elevation of glycogenolysis in hepatocytes[164]. LPA was reported to activate glycogenolysis in hepatocytes in vitro[174], suggesting that LPA’s effects on glucose homeostasis may be mediated by the liver. All these indicate that LPA production via ATX and its receptors activation may impact glucose homeostasis (Figure 4).
The liver plays a critical role in the control of glucose and lipid homeostasis. The disturbance of this homeostasis may lead to development of metabolic diseases such as type 2 diabetes[175] and nonalcoholic fatty liver disease (NAFLD)[176]. Liver fibrosis is a process that leads to the alteration of the hepatic architecture marked by the accumulation of proteins such as collagen in extracellular matrix. This is generally associated with the development of liver diseases such as NAFLD and hepatitis. If left untreated, the further development of these diseases and liver fibrosis will lead to cirrhosis, and liver failure, which needs liver transplantation for the treatment. Factors causing damages of hepatocytes result in activation of hepatic stellate cells (HSCs) and production of pro-inflammatory and pro-fibrotic factors, which will stimulate formation of accumulation of proteins in extracellular matrix[177].
The injuries caused by nutritional and environmental factors alter liver structures and functions, which may lead to the liver fibrosis[178]. The hepatic matrix is remodeled by the inflammatory responses after liver injury. Upon the stimulation, the generation of the liver matrix such as collagen, elastin, hyaluronan, proteoglycans and fibronectin is elevated, which is followed by remodeling processes. All these are associated with the activation of HSCs, and the change of local architecture and the reduction of liver functions. Excessive production and accumulation of extracellular matrix in the liver results in fibrosis, which can lead to liver cirrhosis[178]. In addition to HSCs, other cells responsible for the fibrosis include fibrocytes from hematopoietic stem cells, portal fibroblasts, bone marrow derived mesenchymal cells, epithelial-mesenchymal transition and endothelial to mesenchymal transition[178].
The excessive accumulation of lipids and alterations of their metabolism have been used to explain the etiology of type 2 diabetes, which is associated with profound changes of hepatic gene expression[175,179]. This alteration of hepatic lipid metabolism may cause the development of fibrosis. For example, the elevation of lipid peroxidation in zone 3 hepatocytes has been suggested with the development of fibrosis[180]. On the other hand, changes of fatty acid compositions in plasma phospholipids have been observed in subjects with fibrosis[181,182]. All these show that the alterations of plasma phospholipids in patients with metabolic diseases may play a role in the development of fibrosis.
It has been shown that serum ATX activity and LPA level increase with the development of liver fibrosis in patients with chronic hepatitis C[74,183-185], and with cholestasis and pruritus[186,187]. The association of elevated plasma ATX level with chronic liver disease (CLD) in patients suggests a shorter overall survival in a 10-year follow-up study[185]. Moreover, the increased expression level of hepatic ATX mRNA was found in the majority of publically available CLD and hepatocellular carcinoma (HCC) microarray data sets, suggesting an association of ATX with liver pathophysiology[185]. ATX and LPA levels increased in the plasma of patients with hepatitis C virus (HCV) infection, and positively associate with liver fibrosis stages[183,184,188,189]. HCV infection may stabilize the activity of hypoxia inducible factor in a PI3K dependent manner, which may increase ATX expression, and in turn induce liver fibrosis[190].
It has been shown that serum ATX level correlated with fibrosis grade, and is useful as its marker in liver fibrosis[191]. The higher expression level of LPA2 mRNA has been associated with the poorer differentiation of HCC cells, and a higher LPA6 mRNA level is associated with microvascular invasion of HCC. The high expression levels of LPA2 and LPA6 mRNA in HCC predict a high potential for malignancy. The elevated levels of LPA6 and LPA6 mRNA in conjunction with plasma ATX predict higher rate of recurrence after surgical removal of the tumors[192]. In addition, the plasma level of ATX has been considered as a potential pathogenic factor and/or biomarker for nonalcoholic fatty liver disease in nondiabetic and obese women[193]. Moreover, the plasma ATX levels correlated with prognosis of cirrhosis (Child-Pugh score), showing the link of ATX and the severity of cirrhosis in patients with CLD[194].
In rats, plasma LPA level and serum ATX activity were increased in liver injury and were correlated with severity of the damage; the former in relation to the extent of fibrosis, and the latter in relation to the extent of hepatocyte damage[186,195]. In mice, different hepatotoxic stimuli linked with the development of different forms of CLD were shown to stimulate hepatocyte ATX expression, leading to increased LPA production, HSCs activation, and signals for fibrosis development[185].
LPA was first shown to stimulate rat HSCs proliferation through MAP kinase activation in 1998[196]. Then, LPA was shown to enhance HSCs contractility through modulation of cellular morphology and attachment to extracellular matrices via Rho-kinase[197,198]. LPA also inhibits the apoptosis of those cells through Rho/Rho kinase activation[199], suggesting its involvement in the pathogenesis of liver fibrosis. Moreover, LPA was shown to induce nuclear translocation of inducible nitric-oxide synthase in hepatocytes[200]. These findings demonstrate the possible involvement of LPA in the development of liver fibrosis.
LPA is a highly bioactive lipid mediator with a number of cellular sources and exerts its actions through a family of receptors coupling with GPCRs in various cell types. Here, we have discussed recent advances in pathways for extracellular and intracellular production of LPA, the functions as well as structural and biochemical properties of ATX and LPA receptors. For the past 20-30 years, the cloning and identification of proteins mediating LPA production and signal transduction pathways open a new field for us to understand relevance of these proteins in physiology and disease development. The association of LPA production and signal pathways with chronic metabolic diseases has been gradually realized. We have highlighted the roles of LPA signaling pathways in the obesity, insulin resistance and liver fibrosis.
The realization of the importance of LPA-mediated functions leads to more open questions begging for answers. (1) The regulations of enzymes involved in LPA synthesis and degradation pathways remain to be further investigated. Whether intracellularly produced LPA can cross the plasma membrane into the extracellular compartment is currently unclear. Additional enzymes or pathways for the production of LPA are still worth exploring. For example, phosphatidylglycerol was shown to be converted to LPA under the catalytic action of GPAT as reported[201]. (2) For ATX, an important player for the extracellular LPA production, how its activity is regulated, and how the newly produced LPA is released remain to be addressed. Future analysis will undoubtedly shed some light on these. (3) There are many factors contributing to the pathophysiology of obesity and metabolic diseases. Therefore, the precise role of LPA signaling pathways in these diseases remains to be investigated further. In addition, mechanisms by which the LPA and its receptor signaling pathways in the differentiation of both white and brown adipocytes remain to be clarified. This may help for the control of lipid metabolism. (4) LPA seems to have a negative effect on glucose homeostasis in obesity. This was observed only in obese patients, but not in non-obese subjects. So future human studies should focus on more heathy subjects and compare with those parameters of obese patients. (5) LPA appears to inhibit insulin secretion. Whether this inhibitory effect is due to a direct action of LPA on pancreas islets or a possible regulation of liver glycogen mobilization and/or muscle glucose oxidation remains to be clarified. And (6) It was shown that the plasma LPA level and serum ATX activity both were increased in association with liver fibrosis. The underlying mechanism remains to be determined.
Taken together, the LPA signaling pathways contain multiple points that potentially involve in the development of obesity, liver fibrosis and related pathologies. The development of novel pharmacological modulators targeting intervention points may open new research fields and provide potential medicinal therapies to reduce human suffering. The prospects are bright for expanding insights and contributions in LPA biology.
The authors wish to thank Dr. Elizabeth (Betsy) Anderson Steeves (Department of Nutrition at the University of Tennessee, Knoxville, TN, United States) for her critical reading and editing of this manuscript. In addition, we also would like to thank Dr. Jianan Qi (Southeast University, China) for figures editorial assistance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bourgoin SG, Tajiri K, Yun CC S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4496] [Cited by in F6Publishing: 4821] [Article Influence: 301.3] [Reference Citation Analysis (0)] |
| 2. | Vogt W. The chemical nature of Darmstoff. J Physiol. 1957;137:154-167. [PubMed] [Cited in This Article: ] |
| 3. | Vogt W. Pharmacologically active lipidsoluble acids of natural occurrence. Nature. 1957;179:300-304; passim. [PubMed] [Cited in This Article: ] |
| 4. | Vogt W. [Not Available]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1949;206:1-11. [PubMed] [Cited in This Article: ] |
| 5. | Vogt W. [Properties and preparation of Darmstoff]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1955;227:224-233. [PubMed] [Cited in This Article: ] |
| 6. | Vogt W. Pharamacologically active acidic phospholipids and glycolipids. Biochem Pharmacol. 1963;12:415-420. [PubMed] [Cited in This Article: ] |
| 7. | Sen S, Smeby RR, Bumpus FM. Antihypertensive effect of an isolated phospholipid. Am J Physiol. 1968;214:337-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Tokumura A, Fukuzawa K, Tsukatani H. Effects of synthetic and natural lysophosphatidic acids on the arterial blood pressure of different animal species. Lipids. 1978;13:572-574. [PubMed] [Cited in This Article: ] |
| 9. | Schumacher KA, Classen HG, Späth M. Platelet aggregation evoked in vitro and in vivo by phosphatidic acids and lysoderivatives: identity with substances in aged serum (DAS). Thromb Haemost. 1979;42:631-640. [PubMed] [Cited in This Article: ] |
| 10. | van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45-54. [PubMed] [Cited in This Article: ] |
| 11. | Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071-1083. [PubMed] [Cited in This Article: ] |
| 12. | Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2010;62:579-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 245] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436-39442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 578] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 14. | Aikawa S, Hashimoto T, Kano K, Aoki J. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J Biochem. 2015;157:81-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Rancoule C, Dusaulcy R, Tréguer K, Grès S, Attané C, Saulnier-Blache JS. Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie. 2014;96:140-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Barbayianni E, Kaffe E, Aidinis V, Kokotos G. Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer. Prog Lipid Res. 2015;58:76-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Sheng X, Yung YC, Chen A, Chun J. Lysophosphatidic acid signalling in development. Development. 2015;142:1390-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Velasco M, O’Sullivan C, Sheridan GK. Lysophosphatidic acid receptors (LPARs): Potential targets for the treatment of neuropathic pain. Neuropharmacology. 2017;113:608-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Okudaira S, Yukiura H, Aoki J. Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie. 2010;92:698-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Pagès C, Simon MF, Valet P, Saulnier-Blache JS. Lysophosphatidic acid synthesis and release. Prostaglandins Other Lipid Mediat. 2001;64:1-10. [PubMed] [Cited in This Article: ] |
| 21. | McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci USA. 2003;100:131-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 434] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 22. | Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 741] [Cited by in F6Publishing: 747] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 23. | Nakamura K, Kishimoto T, Ohkawa R, Okubo S, Tozuka M, Yokota H, Ikeda H, Ohshima N, Mizuno K, Yatomi Y. Suppression of lysophosphatidic acid and lysophosphatidylcholine formation in the plasma in vitro: proposal of a plasma sample preparation method for laboratory testing of these lipids. Anal Biochem. 2007;367:20-27. [PubMed] [Cited in This Article: ] |
| 24. | Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43:134-176. [PubMed] [Cited in This Article: ] |
| 25. | Vancura A, Carroll MA, Haldar D. A lysophosphatidic acid-binding cytosolic protein stimulates mitochondrial glycerophosphate acyltransferase. Biochem Biophys Res Commun. 1991;175:339-343. [PubMed] [Cited in This Article: ] |
| 26. | Vancura A, Haldar D. Regulation of mitochondrial and microsomal phospholipid synthesis by liver fatty acid-binding protein. J Biol Chem. 1992;267:14353-14359. [PubMed] [Cited in This Article: ] |
| 27. | Brindley DN, Pilquil C. Lipid phosphate phosphatases and signaling. J Lipid Res. 2009;50 Suppl:S225-S230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96:6931-6936. [PubMed] [Cited in This Article: ] |
| 29. | Siess W. Platelet interaction with bioactive lipids formed by mild oxidation of low-density lipoprotein. Pathophysiol Haemost Thromb. 2006;35:292-304. [PubMed] [Cited in This Article: ] |
| 30. | Pyne S, Kong KC, Darroch PI. Lysophosphatidic acid and sphingosine 1-phosphate biology: the role of lipid phosphate phosphatases. Semin Cell Dev Biol. 2004;15:491-501. [PubMed] [Cited in This Article: ] |
| 31. | Jasinska R, Zhang QX, Pilquil C, Singh I, Xu J, Dewald J, Dillon DA, Berthiaume LG, Carman GM, Waggoner DW. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem J. 1999;340:677-686. [PubMed] [Cited in This Article: ] |
| 32. | Kai M, Wada I, Imai S, Sakane F, Kanoh H. Identification and cDNA cloning of 35-kDa phosphatidic acid phosphatase (type 2) bound to plasma membranes. Polymerase chain reaction amplification of mouse H2O2-inducible hic53 clone yielded the cDNA encoding phosphatidic acid phosphatase. J Biol Chem. 1996;271:18931-18938. [PubMed] [Cited in This Article: ] |
| 33. | Leung DW, Tompkins CK, White T. Molecular cloning of two alternatively spliced forms of human phosphatidic acid phosphatase cDNAs that are differentially expressed in normal and tumor cells. DNA Cell Biol. 1998;17:377-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Roberts R, Sciorra VA, Morris AJ. Human type 2 phosphatidic acid phosphohydrolases. Substrate specificity of the type 2a, 2b, and 2c enzymes and cell surface activity of the 2a isoform. J Biol Chem. 1998;273:22059-22067. [PubMed] [Cited in This Article: ] |
| 35. | Aguado B, Campbell RD. Characterization of a human lysophosphatidic acid acyltransferase that is encoded by a gene located in the class III region of the human major histocompatibility complex. J Biol Chem. 1998;273:4096-4105. [PubMed] [Cited in This Article: ] |
| 36. | Modregger J, Schmidt AA, Ritter B, Huttner WB, Plomann M. Characterization of Endophilin B1b, a brain-specific membrane-associated lysophosphatidic acid acyl transferase with properties distinct from endophilin A1. J Biol Chem. 2003;278:4160-4167. [PubMed] [Cited in This Article: ] |
| 37. | Weigert R, Silletta MG, Spanò S, Turacchio G, Cericola C, Colanzi A, Senatore S, Mancini R, Polishchuk EV, Salmona M. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 246] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Wang A, Dennis EA. Mammalian lysophospholipases. Biochim Biophys Acta. 1999;1439:1-16. [PubMed] [Cited in This Article: ] |
| 39. | Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30:542-550. [PubMed] [Cited in This Article: ] |
| 40. | Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;437-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 794] [Cited by in F6Publishing: 753] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 41. | Boutin JA, Ferry G. Autotaxin. Cell Mol Life Sci. 2009;66:3009-3021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Nakanaga K, Hama K, Aoki J. Autotaxin--an LPA producing enzyme with diverse functions. J Biochem. 2010;148:13-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 43. | Yuelling LM, Fuss B. Autotaxin (ATX): a multi-functional and multi-modular protein possessing enzymatic lysoPLD activity and matricellular properties. Biochim Biophys Acta. 2008;1781:525-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, Liotta LA. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524-2529. [PubMed] [Cited in This Article: ] |
| 45. | Ferry G, Tellier E, Try A, Grés S, Naime I, Simon MF, Rodriguez M, Boucher J, Tack I, Gesta S. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem. 2003;278:18162-18169. [PubMed] [Cited in This Article: ] |
| 46. | Gesta S, Simon MF, Rey A, Sibrac D, Girard A, Lafontan M, Valet P, Saulnier-Blache JS. Secretion of a lysophospholipase D activity by adipocytes: involvement in lysophosphatidic acid synthesis. J Lipid Res. 2002;43:904-910. [PubMed] [Cited in This Article: ] |
| 47. | Jansen S, Andries M, Vekemans K, Vanbilloen H, Verbruggen A, Bollen M. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 2009;284:216-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Lee HY, Clair T, Mulvaney PT, Woodhouse EC, Aznavoorian S, Liotta LA, Stracke ML. Stimulation of tumor cell motility linked to phosphodiesterase catalytic site of autotaxin. J Biol Chem. 1996;271:24408-24412. [PubMed] [Cited in This Article: ] |
| 49. | Nishimasu H, Okudaira S, Hama K, Mihara E, Dohmae N, Inoue A, Ishitani R, Takagi J, Aoki J, Nureki O. Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat Struct Mol Biol. 2011;18:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 50. | Giganti A, Rodriguez M, Fould B, Moulharat N, Cogé F, Chomarat P, Galizzi JP, Valet P, Saulnier-Blache JS, Boutin JA. Murine and human autotaxin alpha, beta, and gamma isoforms: gene organization, tissue distribution, and biochemical characterization. J Biol Chem. 2008;283:7776-7789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Moolenaar WH, Perrakis A. Insights into autotaxin: how to produce and present a lipid mediator. Nat Rev Mol Cell Biol. 2011;12:674-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 52. | Hausmann J, Kamtekar S, Christodoulou E, Day JE, Wu T, Fulkerson Z, Albers HM, van Meeteren LA, Houben AJ, van Zeijl L. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat Struct Mol Biol. 2011;18:198-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 53. | Houben AJ, van Wijk XM, van Meeteren LA, van Zeijl L, van de Westerlo EM, Hausmann J, Fish A, Perrakis A, van Kuppevelt TH, Moolenaar WH. The polybasic insertion in autotaxin α confers specific binding to heparin and cell surface heparan sulfate proteoglycans. J Biol Chem. 2013;288:510-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Fulkerson Z, Wu T, Sunkara M, Kooi CV, Morris AJ, Smyth SS. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J Biol Chem. 2011;286:34654-34663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 55. | Murata J, Lee HY, Clair T, Krutzsch HC, Arestad AA, Sobel ME, Liotta LA, Stracke ML. cDNA cloning of the human tumor motility-stimulating protein, autotaxin, reveals a homology with phosphodiesterases. J Biol Chem. 1994;269:30479-30484. [PubMed] [Cited in This Article: ] |
| 56. | Hashimoto T, Okudaira S, Igarashi K, Hama K, Yatomi Y, Aoki J. Identification and biochemical characterization of a novel autotaxin isoform, ATXδ, with a four-amino acid deletion. J Biochem. 2012;151:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Bächner D, Ahrens M, Betat N, Schröder D, Gross G. Developmental expression analysis of murine autotaxin (ATX). Mech Dev. 1999;84:121-125. [PubMed] [Cited in This Article: ] |
| 58. | Nimitphong H, Holick MF. Vitamin D status and sun exposure in southeast Asia. Dermatoendocrinol. 2013;5:34-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 59. | Savaskan NE, Rocha L, Kotter MR, Baer A, Lubec G, van Meeteren LA, Kishi Y, Aoki J, Moolenaar WH, Nitsch R. Autotaxin (NPP-2) in the brain: cell type-specific expression and regulation during development and after neurotrauma. Cell Mol Life Sci. 2007;64:230-243. [PubMed] [Cited in This Article: ] |
| 60. | Brock K, Cant R, Clemson L, Mason RS, Fraser DR. Effects of diet and exercise on plasma vitamin D (25(OH)D) levels in Vietnamese immigrant elderly in Sydney, Australia. J Steroid Biochem Mol Biol. 2007;103:786-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Nakasaki T, Tanaka T, Okudaira S, Hirosawa M, Umemoto E, Otani K, Jin S, Bai Z, Hayasaka H, Fukui Y. Involvement of the lysophosphatidic acid-generating enzyme autotaxin in lymphocyte-endothelial cell interactions. Am J Pathol. 2008;173:1566-1576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Bai Z, Cai L, Umemoto E, Takeda A, Tohya K, Komai Y, Veeraveedu PT, Hata E, Sugiura Y, Kubo A. Constitutive lymphocyte transmigration across the basal lamina of high endothelial venules is regulated by the autotaxin/lysophosphatidic acid axis. J Immunol. 2013;190:2036-2048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 63. | Dusaulcy R, Rancoule C, Grès S, Wanecq E, Colom A, Guigné C, van Meeteren LA, Moolenaar WH, Valet P, Saulnier-Blache JS. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res. 2011;52:1247-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 64. | Dusaulcy R, Daviaud D, Pradère JP, Grès S, Valet P, Saulnier-Blache JS. Altered food consumption in mice lacking lysophosphatidic acid receptor-1. J Physiol Biochem. 2009;65:345-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Pamuklar Z, Federico L, Liu S, Umezu-Goto M, Dong A, Panchatcharam M, Fulkerson Z, Berdyshev E, Natarajan V, Fang X. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J Biol Chem. 2009;284:7385-7394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 66. | Leblanc R, Lee SC, David M, Bordet JC, Norman DD, Patil R, Miller D, Sahay D, Ribeiro J, Clézardin P. Interaction of platelet-derived autotaxin with tumor integrin αVβ3 controls metastasis of breast cancer cells to bone. Blood. 2014;124:3141-3150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 67. | Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004;15:477-489. [PubMed] [Cited in This Article: ] |
| 68. | Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res. 2014;55:1192-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 489] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 69. | Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokumura A. Lysophosphatidic acid, a growth factor-like lipid, in the saliva. J Lipid Res. 2002;43:2049-2055. [PubMed] [Cited in This Article: ] |
| 70. | Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokumura A, Hanahan DJ. Occurrence of lysophosphatidic acid and its alkyl ether-linked analog in rat brain and comparison of their biological activities toward cultured neural cells. Biochim Biophys Acta. 1999;1440:194-204. [PubMed] [Cited in This Article: ] |
| 71. | Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, Taguchi R, Shimizu T, Ishii S. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem. 2009;284:17731-17741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 72. | Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem. 2002;277:48737-48744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 344] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 73. | Hosogaya S, Yatomi Y, Nakamura K, Ohkawa R, Okubo S, Yokota H, Ohta M, Yamazaki H, Koike T, Ozaki Y. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: strong correlation with lysophospholipase D activity. Ann Clin Biochem. 2008;45:364-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 74. | Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Aoki J, Hama K, Okudaira S, Tanaka M, Tomiya T. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J Clin Gastroenterol. 2007;41:616-623. [PubMed] [Cited in This Article: ] |
| 75. | Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002;277:21197-21206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 76. | Yatomi Y, Igarashi K, Nakamura K, Ohkawa R, Masuda A, Suzuki A, Kishimoto T, Ikeda H, Aoki J. Clinical introduction of lysophosphatidic acid (LPA) and autotaxin assays. In Lysophospholipid Receptors Signaling and Biochemistry. Wiley: Hoboken Pub 2013; 709-735. [Cited in This Article: ] |
| 77. | Scherer M, Schmitz G, Liebisch G. High-throughput analysis of sphingosine 1-phosphate, sphinganine 1-phosphate, and lysophosphatidic acid in plasma samples by liquid chromatography-tandem mass spectrometry. Clin Chem. 2009;55:1218-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 78. | Smyth SS, Cheng HY, Miriyala S, Panchatcharam M, Morris AJ. Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim Biophys Acta. 2008;1781:563-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | Aaltonen N, Laitinen JT, Lehtonen M. Quantification of lysophosphatidic acids in rat brain tissue by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1145-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Lee JW, Nishiumi S, Yoshida M, Fukusaki E, Bamba T. Simultaneous profiling of polar lipids by supercritical fluid chromatography/tandem mass spectrometry with methylation. J Chromatogr A. 2013;1279:98-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 81. | An S, Bleu T, Huang W, Hallmark OG, Coughlin SR, Goetzl EJ. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279-282. [PubMed] [Cited in This Article: ] |
| 82. | An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem. 1998;273:7906-7910. [PubMed] [Cited in This Article: ] |
| 83. | Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, Tsujimoto M, Arai H, Inoue K. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274:27776-27785. [PubMed] [Cited in This Article: ] |
| 84. | Fukushima N, Ishii I, Contos JJ, Weiner JA, Chun J. Lysophospholipid receptors. Annu Rev Pharmacol Toxicol. 2001;41:507-534. [PubMed] [Cited in This Article: ] |
| 85. | Lynch KR. Lysophospholipid receptor nomenclature. Biochim Biophys Acta. 2002;1582:70-71. [PubMed] [Cited in This Article: ] |
| 86. | Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600-25606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 464] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 87. | Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589-23597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 376] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 88. | Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363:861-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 89. | Kihara Y, Maceyka M, Spiegel S, Chun J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br J Pharmacol. 2014;171:3575-3594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 242] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 90. | Fukushima N, Ishii S, Tsujiuchi T, Kagawa N, Katoh K. Comparative analyses of lysophosphatidic acid receptor-mediated signaling. Cell Mol Life Sci. 2015;72:2377-2394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Xiang SY, Dusaban SS, Brown JH. Lysophospholipid receptor activation of RhoA and lipid signaling pathways. Biochim Biophys Acta. 2013;1831:213-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 92. | Hildebrandt JP. Lysophosphatidic acid induces inositol phosphate and calcium signals in exocrine cells from the avian nasal salt gland. J Membr Biol. 1995;144:49-58. [PubMed] [Cited in This Article: ] |
| 93. | Gennero I, Xuereb JM, Simon MF, Girolami JP, Bascands JL, Chap H, Boneu B, Sié P. Effects of lysophosphatidic acid on proliferation and cytosolic Ca++ of human adult vascular smooth muscle cells in culture. Thromb Res. 1999;94:317-326. [PubMed] [Cited in This Article: ] |
| 94. | Litosch I. Phosphatidic acid potentiates G(alpha)q stimulation of phospholipase C-beta1 signaling. Biochem Biophys Res Commun. 2009;390:603-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79-94. [PubMed] [Cited in This Article: ] |
| 96. | McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. G-protein signaling: back to the future. Cell Mol Life Sci. 2005;62:551-577. [PubMed] [Cited in This Article: ] |
| 97. | Lee CW, Rivera R, Dubin AE, Chun J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J Biol Chem. 2007;282:4310-4317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 98. | Wittpoth C, Scholich K, Yigzaw Y, Stringfield TM, Patel TB. Regions on adenylyl cyclase that are necessary for inhibition of activity by beta gamma and G(ialpha) subunits of heterotrimeric G proteins. Proc Natl Acad Sci U S A. 1999;96:9551-9556. [PubMed] [Cited in This Article: ] |
| 99. | Jongsma M, Matas-Rico E, Rzadkowski A, Jalink K, Moolenaar WH. LPA is a chemorepellent for B16 melanoma cells: action through the cAMP-elevating LPA5 receptor. PLoS One. 2011;6:e29260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, Siess W, Parrill AL, Tigyi G, Fujiwara Y. Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J Biol Chem. 2009;284:17304-17319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 101. | Makide K, Kitamura H, Sato Y, Okutani M, Aoki J. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipid Mediat. 2009;89:135-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 102. | Kano K, Arima N, Ohgami M, Aoki J. LPA and its analogs-attractive tools for elucidation of LPA biology and drug development. Curr Med Chem. 2008;15:2122-2131. [PubMed] [Cited in This Article: ] |
| 103. | Anliker B, Choi JW, Lin ME, Gardell SE, Rivera RR, Kennedy G, Chun J. Lysophosphatidic acid (LPA) and its receptor, LPA1 , influence embryonic schwann cell migration, myelination, and cell-to-axon segregation. Glia. 2013;61:2009-2022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 104. | Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 2013;27:1830-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 105. | Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321-354. [PubMed] [Cited in This Article: ] |
| 106. | Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000;58:1188-1196. [PubMed] [Cited in This Article: ] |
| 107. | Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci USA. 2000;97:13384-13389. [PubMed] [Cited in This Article: ] |
| 108. | Musazzi L, Di Daniel E, Maycox P, Racagni G, Popoli M. Abnormalities in α/β-CaMKII and related mechanisms suggest synaptic dysfunction in hippocampus of LPA1 receptor knockout mice. Int J Neuropsychopharmacol. 2011;14:941-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Estivill-Torrús G, Llebrez-Zayas P, Matas-Rico E, Santín L, Pedraza C, De Diego I, Del Arco I, Fernández-Llebrez P, Chun J, De Fonseca FR. Absence of LPA1 signaling results in defective cortical development. Cereb Cortex. 2008;18:938-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 110. | Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 614] [Cited by in F6Publishing: 631] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 111. | Ohuchi H, Hamada A, Matsuda H, Takagi A, Tanaka M, Aoki J, Arai H, Noji S. Expression patterns of the lysophospholipid receptor genes during mouse early development. Dev Dyn. 2008;237:3280-3294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 112. | Lai YJ, Chen CS, Lin WC, Lin FT. c-Src-mediated phosphorylation of TRIP6 regulates its function in lysophosphatidic acid-induced cell migration. Mol Cell Biol. 2005;25:5859-5868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 113. | Xu J, Lai YJ, Lin WC, Lin FT. TRIP6 enhances lysophosphatidic acid-induced cell migration by interacting with the lysophosphatidic acid 2 receptor. J Biol Chem. 2004;279:10459-10468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | Lin FT, Lai YJ. Regulation of the LPA2 receptor signaling through the carboxyl-terminal tail-mediated protein-protein interactions. Biochim Biophys Acta. 2008;1781:558-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 115. | Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol Cell Biol. 2002;22:6921-6929. [PubMed] [Cited in This Article: ] |
| 116. | Im DS, Heise CE, Harding MA, George SR, O’Dowd BF, Theodorescu D, Lynch KR. Molecular cloning and characterization of a lysophosphatidic acid receptor, Edg-7, expressed in prostate. Mol Pharmacol. 2000;57:753-759. [PubMed] [Cited in This Article: ] |
| 117. | Zhao C, Sardella A, Davis L, Poubelle PE, Bourgoin SG, Fernandes MJ. A transgenic mouse model for the in vivo bioluminescence imaging of the expression of the lysophosphatidic acid receptor 3: relevance for inflammation and uterine physiology research. Transgenic Res. 2015;24:625-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 118. | Ye X, Chun J. Lysophosphatidic acid (LPA) signaling in vertebrate reproduction. Trends Endocrinol Metab. 2010;21:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 119. | Hama K, Aoki J. LPA(3), a unique G protein-coupled receptor for lysophosphatidic acid. Prog Lipid Res. 2010;49:335-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 120. | Lai SL, Yao WL, Tsao KC, Houben AJ, Albers HM, Ovaa H, Moolenaar WH, Lee SJ. Autotaxin/Lpar3 signaling regulates Kupffer’s vesicle formation and left-right asymmetry in zebrafish. Development. 2012;139:4439-4448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 121. | Rhee HJ, Nam JS, Sun Y, Kim MJ, Choi HK, Han DH, Kim NH, Huh SO. Lysophosphatidic acid stimulates cAMP accumulation and cAMP response element-binding protein phosphorylation in immortalized hippocampal progenitor cells. Neuroreport. 2006;17:523-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 122. | Lee Z, Cheng CT, Zhang H, Subler MA, Wu J, Mukherjee A, Windle JJ, Chen CK, Fang X. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol Biol Cell. 2008;19:5435-5445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 123. | Liu YB, Kharode Y, Bodine PV, Yaworsky PJ, Robinson JA, Billiard J. LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J Cell Biochem. 2010;109:794-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 124. | Mansell JP, Barbour M, Moore C, Nowghani M, Pabbruwe M, Sjostrom T, Blom AW. The synergistic effects of lysophosphatidic acid receptor agonists and calcitriol on MG63 osteoblast maturation at titanium and hydroxyapatite surfaces. Biomaterials. 2010;31:199-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 125. | Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, Yu S, Stephens LC, Cui X, Murrow G. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009;15:539-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 126. | Sumida H, Noguchi K, Kihara Y, Abe M, Yanagida K, Hamano F, Sato S, Tamaki K, Morishita Y, Kano MR. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood. 2010;116:5060-5070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 127. | Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 128. | Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M, Lamprecht G, de Jonge HR, Tse M, Donowitz M. Lysophosphatidic acid stimulates the intestinal brush border Na(+)/H(+) exchanger 3 and fluid absorption via LPA(5) and NHERF2. Gastroenterology. 2010;138:649-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 129. | Lundequist A, Boyce JA. LPA5 is abundantly expressed by human mast cells and important for lysophosphatidic acid induced MIP-1β release. PLoS One. 2011;6:e18192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 130. | Araki M, Kitayoshi M, Dong Y, Hirane M, Ozaki S, Mori S, Fukushima N, Honoki K, Tsujiuchi T. Inhibitory effects of lysophosphatidic acid receptor-5 on cellular functions of sarcoma cells. Growth Factors. 2014;32:117-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 131. | Kaplan MH, Smith DI, Sundick RS. Identification of a G protein coupled receptor induced in activated T cells. J Immunol. 1993;151:628-636. [PubMed] [Cited in This Article: ] |
| 132. | Webb TE, Kaplan MG, Barnard EA. Identification of 6H1 as a P2Y purinoceptor: P2Y5. Biochem Biophys Res Commun. 1996;219:105-110. [PubMed] [Cited in This Article: ] |
| 133. | Lee M, Choi S, Halldén G, Yo SJ, Schichnes D, Aponte GW. P2Y5 is a G(alpha)i, G(alpha)12/13 G protein-coupled receptor activated by lysophosphatidic acid that reduces intestinal cell adhesion. Am J Physiol Gastrointest Liver Physiol. 2009;297:G641-G654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 134. | Pasternack SM, von Kügelgen I, Al Aboud K, Lee YA, Rüschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40:329-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 293] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 135. | Raza SI, Muhammad D, Jan A, Ali RH, Hassan M, Ahmad W, Rashid S. In silico analysis of missense mutations in LPAR6 reveals abnormal phospholipid signaling pathway leading to hypotrichosis. PLoS One. 2014;9:e104756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 136. | Shimomura Y, Garzon MC, Kristal L, Shapiro L, Christiano AM. Autosomal recessive woolly hair with hypotrichosis caused by a novel homozygous mutation in the P2RY5 gene. Exp Dermatol. 2009;18:218-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 137. | Nahum S, Morice-Picard F, Taieb A, Sprecher E. A novel mutation in LPAR6 causes autosomal recessive hypotrichosis of the scalp. Clin Exp Dermatol. 2011;36:188-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 138. | Archbold JK, Martin JL, Sweet MJ. Towards selective lysophospholipid GPCR modulators. Trends Pharmacol Sci. 2014;35:219-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 139. | Choi JW, Lee CW, Chun J. Biological roles of lysophospholipid receptors revealed by genetic null mice: an update. Biochim Biophys Acta. 2008;1781:531-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 140. | Valentine WJ, Fells JI, Perygin DH, Mujahid S, Yokoyama K, Fujiwara Y, Tsukahara R, Van Brocklyn JR, Parrill AL, Tigyi G. Subtype-specific residues involved in ligand activation of the endothelial differentiation gene family lysophosphatidic acid receptors. J Biol Chem. 2008;283:12175-12187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 141. | Lee SC, Fujiwara Y, Liu J, Yue J, Shimizu Y, Norman DD, Wang Y, Tsukahara R, Szabo E, Patil R. Autotaxin and LPA1 and LPA5 receptors exert disparate functions in tumor cells versus the host tissue microenvironment in melanoma invasion and metastasis. Mol Cancer Res. 2015;13:174-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 142. | Amisten S, Braun OO, Bengtsson A, Erlinge D. Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb Res. 2008;122:47-57. [PubMed] [Cited in This Article: ] |
| 143. | Yanagida K, Kurikawa Y, Shimizu T, Ishii S. Current progress in non-Edg family LPA receptor research. Biochim Biophys Acta. 2013;1831:33-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 144. | Yanagida K, Ishii S. Non-Edg family LPA receptors: the cutting edge of LPA research. J Biochem. 2011;150:223-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 145. | Dong Y, Hirane M, Araki M, Fukushima N, Tsujiuchi T. Lysophosphatidic acid receptor-5 negatively regulates cellular responses in mouse fibroblast 3T3 cells. Biochem Biophys Res Commun. 2014;446:585-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 146. | UniProt Consortium. Update on activities at the Universal Protein Resource (UniProt) in 2013. Nucleic Acids Res. 2013;41:D43-D47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 512] [Cited by in F6Publishing: 579] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 147. | Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011-2014. NCHS Data Brief. 2015;1-8. [PubMed] [Cited in This Article: ] |
| 148. | Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011;35:891-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1353] [Cited by in F6Publishing: 1372] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 149. | Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58:1550-1557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 258] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 150. | Valet P, Pagès C, Jeanneton O, Daviaud D, Barbe P, Record M, Saulnier-Blache JS, Lafontan M. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. A paracrine signal for preadipocyte growth. J Clin Invest. 1998;101:1431-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 151. | Pagès G, Girard A, Jeanneton O, Barbe P, Wolf C, Lafontan M, Valet P, Saulnier-Blache JS. LPA as a paracrine mediator of adipocyte growth and function. Ann N Y Acad Sci. 2000;905:159-164. [PubMed] [Cited in This Article: ] |
| 152. | Tokumura A, Kanaya Y, Kitahara M, Miyake M, Yoshioka Y, Fukuzawa K. Increased formation of lysophosphatidic acids by lysophospholipase D in serum of hypercholesterolemic rabbits. J Lipid Res. 2002;43:307-315. [PubMed] [Cited in This Article: ] |
| 153. | Simon MF, Daviaud D, Pradère JP, Grès S, Guigné C, Wabitsch M, Chun J, Valet P, Saulnier-Blache JS. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2. J Biol Chem. 2005;280:14656-14662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 154. | Nobusue H, Kondo D, Yamamoto M, Kano K. Effects of lysophosphatidic acid on the in vitro proliferation and differentiation of a novel porcine preadipocyte cell line. Comp Biochem Physiol B Biochem Mol Biol. 2010;157:401-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 155. | Rancoule C, Dusaulcy R, Tréguer K, Grès S, Guigné C, Quilliot D, Valet P, Saulnier-Blache JS. Depot-specific regulation of autotaxin with obesity in human adipose tissue. J Physiol Biochem. 2012;68:635-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 156. | Holmström TE, Mattsson CL, Wang Y, Iakovleva I, Petrovic N, Nedergaard J. Non-transactivational, dual pathways for LPA-induced Erk1/2 activation in primary cultures of brown pre-adipocytes. Exp Cell Res. 2010;316:2664-2675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 157. | Mattsson CL, Andersson ER, Nedergaard J. Differential involvement of caveolin-1 in brown adipocyte signaling: impaired beta3-adrenergic, but unaffected LPA, PDGF and EGF receptor signaling. Biochim Biophys Acta. 2010;1803:983-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 158. | Saulnier-Blache JS. Secretion and role of autotaxin and lysophosphatidic acid in adipose tissue. J Soc Biol. 2006;200:77-81. [PubMed] [Cited in This Article: ] |
| 159. | Federico L, Ren H, Mueller PA, Wu T, Liu S, Popovic J, Blalock EM, Sunkara M, Ovaa H, Albers HM. Autotaxin and its product lysophosphatidic acid suppress brown adipose differentiation and promote diet-induced obesity in mice. Mol Endocrinol. 2012;26:786-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 160. | Noguchi M, Hosoda K, Fujikura J, Fujimoto M, Iwakura H, Tomita T, Ishii T, Arai N, Hirata M, Ebihara K. Genetic and pharmacological inhibition of Rho-associated kinase II enhances adipogenesis. J Biol Chem. 2007;282:29574-29583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 161. | Li L, Tam L, Liu L, Jin T, Ng DS. Wnt-signaling mediates the anti-adipogenic action of lysophosphatidic acid through cross talking with the Rho/Rho associated kinase (ROCK) pathway. Biochem Cell Biol. 2011;89:515-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 162. | Nishimura S, Nagasaki M, Okudaira S, Aoki J, Ohmori T, Ohkawa R, Nakamura K, Igarashi K, Yamashita H, Eto K. ENPP2 contributes to adipose tissue expansion and insulin resistance in diet-induced obesity. Diabetes. 2014;63:4154-4164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 163. | Benesch MG, Zhao YY, Curtis JM, McMullen TP, Brindley DN. Regulation of autotaxin expression and secretion by lysophosphatidate and sphingosine 1-phosphate. J Lipid Res. 2015;56:1134-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 164. | Lundgren M, Svensson M, Lindmark S, Renström F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50:625-633. [PubMed] [Cited in This Article: ] |
| 165. | Arner E, Westermark PO, Spalding KL, Britton T, Rydén M, Frisén J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 412] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 166. | Boucher J, Quilliot D, Pradères JP, Simon MF, Grès S, Guigné C, Prévot D, Ferry G, Boutin JA, Carpéné C. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia. 2005;48:569-577. [PubMed] [Cited in This Article: ] |
| 167. | Coy PE, Taneja N, Lee I, Hecquet C, Bryson JM, Robey RB. LPA is a novel lipid regulator of mesangial cell hexokinase activity and HKII isoform expression. Am J Physiol Renal Physiol. 2002;283:F271-F279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 168. | Keller JN, Steiner MR, Mattson MP, Steiner SM. Lysophosphatidic acid decreases glutamate and glucose uptake by astrocytes. J Neurochem. 1996;67:2300-2305. [PubMed] [Cited in This Article: ] |
| 169. | Yea K, Kim J, Lim S, Park HS, Park KS, Suh PG, Ryu SH. Lysophosphatidic acid regulates blood glucose by stimulating myotube and adipocyte glucose uptake. J Mol Med (Berl). 2008;86:211-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 170. | Barber MN, Risis S, Yang C, Meikle PJ, Staples M, Febbraio MA, Bruce CR. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS One. 2012;7:e41456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 171. | Rancoule C, Attané C, Grès S, Fournel A, Dusaulcy R, Bertrand C, Vinel C, Tréguer K, Prentki M, Valet P. Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high-fat diet obese mice. Diabetologia. 2013;56:1394-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 172. | D’Souza K, Kane DA, Touaibia M, Kershaw EE, Pulinilkunnil T, Kienesberger PC. Autotaxin Is Regulated by Glucose and Insulin in Adipocytes. Endocrinology. 2017;158:791-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 173. | Reeves VL, Trybula JS, Wills RC, Goodpaster BH, Dubé JJ, Kienesberger PC, Kershaw EE. Serum Autotaxin/ENPP2 correlates with insulin resistance in older humans with obesity. Obesity (Silver Spring). 2015;23:2371-2376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 174. | Im DS, Fujioka T, Katada T, Kondo Y, Ui M, Okajima F. Characterization of sphingosine 1-phosphate-induced actions and its signaling pathways in rat hepatocytes. Am J Physiol. 1997;272:G1091-G1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 175. | McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7-18. [PubMed] [Cited in This Article: ] |
| 176. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3655] [Cited by in F6Publishing: 3609] [Article Influence: 164.0] [Reference Citation Analysis (2)] |
| 177. | Chen G. The link between Hepatic Vitamin A Metabolism and Nonalcoholic Fatty Liver Disease. Curr Drug Targets. 2015;16:1281-1292. [PubMed] [Cited in This Article: ] |
| 178. | Elpek GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J Gastroenterol. 2014;20:7260-7276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 240] [Cited by in F6Publishing: 242] [Article Influence: 24.2] [Reference Citation Analysis (4)] |
| 179. | Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 694] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 180. | MacDonald GA, Bridle KR, Ward PJ, Walker NI, Houglum K, George DK, Smith JL, Powell LW, Crawford DH, Ramm GA. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J Gastroenterol Hepatol. 2001;16:599-606. [PubMed] [Cited in This Article: ] |
| 181. | Drzymała-Czyż S, Szczepanik M, Krzyżanowska P, Duś-Żuchowska M, Pogorzelski A, Sapiejka E, Juszczak P, Lisowska A, Koletzko B, Walkowiak J. Serum Phospholipid Fatty Acid Composition in Cystic Fibrosis Patients with and without Liver Cirrhosis. Ann Nutr Metab. 2017;71:91-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 182. | Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827-1838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 470] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 183. | Cooper AB, Wu J, Lu D, Maluccio MA. Is autotaxin (ENPP2) the link between hepatitis C and hepatocellular cancer? J Gastrointest Surg. 2007;11:1628-34; discussion 1634-5. [PubMed] [Cited in This Article: ] |
| 184. | Schlatzer DM, Sugalski JM, Chen Y, Barnholtz-Sloan J, Davitkov P, Hazlett FE, Funderburg N, Rodriguez B, Lederman MM, Sieg SF. Plasma proteome analysis reveals overlapping, yet distinct mechanisms of immune activation in chronic HCV and HIV infections. J Acquir Immune Defic Syndr. 2013;63:563-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 185. | Kaffe E, Katsifa A, Xylourgidis N, Ninou I, Zannikou M, Harokopos V, Foka P, Dimitriadis A, Evangelou K, Moulas AN. Hepatocyte autotaxin expression promotes liver fibrosis and cancer. Hepatology. 2017;65:1369-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 186. | Kremer AE, Martens JJ, Kulik W, Ruëff F, Kuiper EM, van Buuren HR, van Erpecum KJ, Kondrackiene J, Prieto J, Rust C. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008-1018, 1018.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 291] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 187. | Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, Reiners KS, Raap U, van Buuren HR, van Erpecum KJ. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56:1391-1400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 201] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 188. | Wu JM, Xu Y, Skill NJ, Sheng H, Zhao Z, Yu M, Saxena R, Maluccio MA. Autotaxin expression and its connection with the TNF-alpha-NF-kappaB axis in human hepatocellular carcinoma. Mol Cancer. 2010;9:71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 189. | Kondo M, Ishizawa T, Enooku K, Tokuhara Y, Ohkawa R, Uranbileg B, Nakagawa H, Tateishi R, Yoshida H, Kokudo N. Increased serum autotaxin levels in hepatocellular carcinoma patients were caused by background liver fibrosis but not by carcinoma. Clin Chim Acta. 2014;433:128-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 190. | Farquhar MJ, Humphreys IS, Rudge SA, Wilson GK, Bhattacharya B, Ciaccia M, Hu K, Zhang Q, Mailly L, Reynolds GM. Autotaxin-lysophosphatidic acid receptor signalling regulates hepatitis C virus replication. J Hepatol. 2017;66:919-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 191. | Nakagawa H, Ikeda H, Nakamura K, Ohkawa R, Masuzaki R, Tateishi R, Yoshida H, Watanabe N, Tejima K, Kume Y. Autotaxin as a novel serum marker of liver fibrosis. Clin Chim Acta. 2011;412:1201-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 192. | Enooku K, Uranbileg B, Ikeda H, Kurano M, Sato M, Kudo H, Maki H, Koike K, Hasegawa K, Kokudo N. Higher LPA2 and LPA6 mRNA Levels in Hepatocellular Carcinoma Are Associated with Poorer Differentiation, Microvascular Invasion and Earlier Recurrence with Higher Serum Autotaxin Levels. PLoS One. 2016;11:e0161825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 193. | Rachakonda VP, Reeves VL, Aljammal J, Wills RC, Trybula JS, DeLany JP, Kienesberger PC, Kershaw EE. Serum autotaxin is independently associated with hepatic steatosis in women with severe obesity. Obesity (Silver Spring). 2015;23:965-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 194. | Pleli T, Martin D, Kronenberger B, Brunner F, Köberle V, Grammatikos G, Farnik H, Martinez Y, Finkelmeier F, Labocha S. Serum autotaxin is a parameter for the severity of liver cirrhosis and overall survival in patients with liver cirrhosis--a prospective cohort study. PLoS One. 2014;9:e103532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 195. | Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Tomiya T, Tejima K, Nishikawa T, Arai M, Yanase M. Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life Sci. 2007;81:1009-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 196. | Ikeda H, Yatomi Y, Yanase M, Satoh H, Nishihara A, Kawabata M, Fujiwara K. Effects of lysophosphatidic acid on proliferation of stellate cells and hepatocytes in culture. Biochem Biophys Res Commun. 1998;248:436-440. [PubMed] [Cited in This Article: ] |
| 197. | Yanase M, Ikeda H, Matsui A, Maekawa H, Noiri E, Tomiya T, Arai M, Yano T, Shibata M, Ikebe M. Lysophosphatidic acid enhances collagen gel contraction by hepatic stellate cells: association with rho-kinase. Biochem Biophys Res Commun. 2000;277:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 198. | Yanase M, Ikeda H, Ogata I, Matsui A, Noiri E, Tomiya T, Arai M, Inoue Y, Tejima K, Nagashima K. Functional diversity between Rho-kinase- and MLCK-mediated cytoskeletal actions in a myofibroblast-like hepatic stellate cell line. Biochem Biophys Res Commun. 2003;305:223-228. [PubMed] [Cited in This Article: ] |
| 199. | Ikeda H, Nagashima K, Yanase M, Tomiya T, Arai M, Inoue Y, Tejima K, Nishikawa T, Omata M, Kimura S. Involvement of Rho/Rho kinase pathway in regulation of apoptosis in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G880-G886. [PubMed] [Cited in This Article: ] |
| 200. | Gobeil F Jr, Zhu T, Brault S, Geha A, Vazquez-Tello A, Fortier A, Barbaz D, Checchin D, Hou X, Nader M, Bkaily G, Gratton JP, Heveker N, Ribeiro-da-Silva A, Peri K, Bard H, Chorvatova A, D’Orléans-Juste P, Goetzl EJ, Chemtob S. Nitric oxide signaling via nuclearized endothelial nitric-oxide synthase modulates expression of the immediate early genes iNOS and mPGES-1. J Biol Chem. 2006;281:16058-16067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 201. | Tigyi G, Parrill AL. Molecular mechanisms of lysophosphatidic acid action. Prog Lipid Res. 2003;42:498-526. [PubMed] [Cited in This Article: ] |