Published online Sep 14, 2018. doi: 10.3748/wjg.v24.i34.3834
Peer-review started: April 27, 2018
First decision: May 30, 2018
Revised: June 25, 2018
Accepted: July 16, 2018
Article in press: July 16, 2018
Published online: September 14, 2018
Colorectal cancer (CRC) is often diagnosed at an advanced stage when tumor cell dissemination has taken place. Chemo- and targeted therapies provide only a limited increase of overall survival for these patients. The major reason for clinical outcome finds its origin in therapy resistance. Escape mechanisms to both chemo- and targeted therapy remain the main culprits. Here, we evaluate major resistant mechanisms and elaborate on potential new therapies. Amongst promising therapies is α-amanitin antibody-drug conjugate targeting hemizygous p53 loss. It becomes clear that a dynamic interaction with the tumor microenvironment exists and that this dictates therapeutic outcome. In addition, CRC displays a limited response to checkpoint inhibitors, as only a minority of patients with microsatellite instable high tumors is susceptible. In this review, we highlight new developments with clinical potentials to augment responses to checkpoint inhibitors.
Core tip: Therapy resistance has been a culprit for colorectal cancer (CRC) treatment. Here, we review a novel therapeutic approach using α-amanitin antibody-drug conjugates inhibiting RNA polymerase II against CRC with hemizygous loss of p53. Since its mechanism of cell killing is independent of the generally used tubulin inhibitors and chemotherapy drugs, this approach shows the promise to overcome common drug resistance. In addition, we summarize the sensitivity of CRC to newly developed immune checkpoint inhibitors. While patients with microsatellite instability-high CRC remain the sole subgroup responsive to current checkpoint inhibitors so far, we highlight potentially new developments that may lead to promising results in treating patients with microsatellite-stable CRC, which constitutes the majority of this disease.
- Citation: Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol 2018; 24(34): 3834-3848
- URL: https://www.wjgnet.com/1007-9327/full/v24/i34/3834.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i34.3834
Colorectal cancer (CRC) is ranked third amongst the most common cancers affecting both men and women worldwide[1]. Over one million new cases are reported and around 600000 patients die from the disease every year[2]. The five-year survival prognosis is highly dependent on the stage of the disease. While displaying over 90 percent survival for patients with stage I CRC, it barely reaches 10 percent for patients with stage IV CRC. Thus, early detection of the disease has been a priority. For patients failing to be screened early enough, late-stage CRC remains an arduous disease to treat. The basis of CRC treatment consists of surgery, targeted therapy, neoadjuvant radiotherapy and adjuvant chemotherapy. Unfortunately, drug-resistance remains one of the deadlocks for the low survival rates of CRC patients. A better understanding in the intrinsic and acquired therapy resistance will be a great asset for drug development. Recently, the impact of the tumor microenvironment (TME) has gained attention in CRC, prompting the extensive analysis of clinical trials to assess immune-cell infiltration as prognostic and predictive markers. In addition, a promising avenue of clinical research for the treatment of CRC is the use of immunotherapy. Currently, encouraging results have been obtained with the use of immune checkpoint inhibitors in CRC in subgroups of patients. Discovery to improve the responsiveness to checkpoint inhibitors is one of the major points of focus for CRC treatment and will be a point of focus during this review.
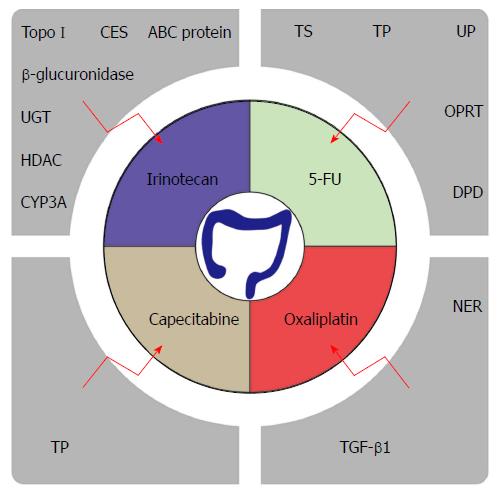
Since the 1950s, 5-fluorouracil (5-FU)-based chemotherapy remains the mainstay of therapy for patients with CRC[3,4]. In recent years, chemotherapy drugs such as oxaliplatin, irinotecan and capecitabine have been developed. Conventional treatment for advanced CRC encompasses the combination of 5-FU and leucovorin with oxaliplatin or irinotecan[5]. The medical treatment in CRC has made great strides with the advent of monoclonal antibodies such as Bevacizumab and Cetuximab. Despite the improvement in response rates with various modulation strategies such as monoclonal antibodies combined with chemotherapy, the five-year survival rate for metastatic CRC (mCRC) is only slightly over 12 percent[1]. One of the major obstacles for this observation is due to the appearance of drug resistance. Nearly half of mCRC patients are resistant to 5-FU-based chemotherapies[6]. With continuous research, multiple drug resistance mechanisms are being unraveled, such as enhanced DNA repair and increased drug metabolism.
In addition to the above-mentioned general resistance mechanisms, 5-FU also has its unique drug resistance. 5-FU is a synthetic fluorinated pyrimidine analog that inhibits DNA replication. This leads to the replacement of thymidine by fluorinated nucleotides into the DNA, hereby causing cell death. Therefore, it is not surprising that 5-FU resistance is closely related to the expression of thymidylate synthase (TS). Since TS is the primary target of 5-FU, patients with low TS expression display a better overall survival (OS) than patients with higher TS expression in tumor tissue[7,8]. As TS is encoded by the TYMS gene, the level of TYMS gene expression offers significant prognostic value[9]. Thymidine phosphorylase (TP), uridine phosphorylase (UP), orotate phosphoribosyl transferase (OPRT) and dihydropyrimidine dehydrogenase (DPD) are all involved in the metabolism and degradation of 5-FU. The relationship between their activity and the sensitivity of CRC to 5-FU has been demonstrated in several studies. Higher expression of TP, UP and OPRT levels displayed enhanced sensitivity to 5-FU therapy[10-12]. Similarly, as DPD contributes to the degradation of 5-FU, its expression level was inversely correlated with chemosensitivity[11]. Collectively, inhibition of the activity of these enzymes could allow enhanced sensitivity to 5-FU.
In 1996, irinotecan (CPT-11) was approved by the FDA for CRC treatment. Irinotecan is a semi-synthetic camptothecin derivative that selectively inhibits topoisomerase I (Topo I). In the cell, CPT-11 undergoes intracellular modifications such as the removal of the C10 group through carboxylesterase catalysis, and then is metabolized to become 7-ethyl-10-hydroxycamptothecin (SN-38). SN-38 possesses 100 to 1000 times stronger anticancer activity than CPT-11[13]. CPT-11 or its active metabolite SN-38 forms a topoisomerase-inhibitor-DNA complex affecting the DNA function. Therefore, the higher the concentration of Topo I, the more sensitive the cells becomes to irinotecan[14,15]. Carboxylesterases (CES), uridine diphosphate glucuronosyltransferase (UGT), hepatic cytochrome P-450 enzymes CYP3A, β-glucuronidase and ATP-binding cassette (ABC) transporter protein are involved in the uptake and metabolism of irinotecan. Consequently, they stand out as major players that determine drug resistance[16,17]. Additionally, targeting the MAPK signal transduction pathway via the inhibition of FGF2, FGF9, MECOM, PLA2G4C and PRKACB could also potentially improve responsiveness to irinotecan[18]. Epigenetic changes take part in development of irinotecan resistance. A change in histone acetylation, such as H4K16 acetylation, is associated with the resistance to irinotecan. Combinatory therapy with histone deacetylase (HDAC) inhibitors holds promise in overcoming irinotecan resistance[19].
Oxaliplatin, a platinum-based chemotherapeutic drug, is approved for the treatment of CRC. Itis most commonly combined with 5-FU and leucovorin, a folinic acid. The combination of these drugs as a treatment regimen is referred to as FOLFOX and has been the first-line chemotherapy strategy for mCRC. The chemical structure difference between oxaliplatin and other platinum-based chemotherapeutic drugs is that oxaliplatin possesses a 1,2-diaminocyclohexane ligand (DACH). DACH together with its platinum compound causes DNA to be more difficult to repair, hereby improving its tumor cell killing potential[20]. Oxaliplatin resistance is related to the nucleotide excision repair (NER) pathway. Gene expression levels of ERCC1, XRCC1 and XDP are correlated with resistance to oxaliplatin, and can be used together as a drug sensitivity predictor index[21]. In addition to NER, the WBSCR22 protein represents a novel oxaliplatin resistance biomarker as well as a possible drug target for therapeutic development[22]. Transforming growth factor-β1 (TGF-β1) is secreted abundantly by a variety of cells within the TME. TGF-β1 is thought to help the induction of resistance to oxaliplatin through epithelial to mesenchymal transition (EMT)[23]. Thus, interfering with TGF-β1 to abrogate EMT could potentially sensitize tumor cells towards oxaliplatin cell-mediated killing.
Capecitabine is the first oral chemotherapy drug for CRC. It is metabolized in the body and converted to 5’-deoxy-5-fluorocytidine (5’-OFCR) and 5’-deoxy-5-fluorouridine (5’-DFUR). Hereafter, 5’-DFUR is eventually hydrolyzed by TP to 5-FU, which will exert its cytotoxic effect. Many of the resistance mechanisms involved in 5-FU resistance are shared. In particular, TP, which is an essential enzyme for the conversion of capecitabine to 5-FU, plays a central role in its resistance. Patients with higher expression levels of TP will have better responses to capecitabine, while loss of function confers the resistance[24,25].The multinational phase III trial provided evidence for capecitabine and irinotecan combination therapy (XELIRI) with or without Bevacizumab as a second-line treatment option of mCRC[26,27].
In addition to the above described mechanisms, there is tremendous heterogeneity within CRC cells. The discovery of cancer stem cells and their therapy resistance as well as their self-renewal capacity has driven the attention towards this peculiar cell population. This specific subset of tumor cells has been shown to be prognostic for patients[28,29]. So far, CRC stem cells have been reported to be enriched for specific surface markers such as CD133, EphB2high, EpCAMhigh, CD44+, CD166+, ALDH+, LGR5+ and CD44v6+[30]. Aside from surface markers, cancer stem cells can be characterized through molecular features such as hyperactivated β-catenin pathway and functional traits such as self-renewal[31,32]. Another functional phenotype is their expression of efflux pumps such as the ATP binding cassette (ABC) family members, including ABCG2[28]. The presence of efflux pumps promotes the transport of drugs, such as chemotherapeutic compounds, outside the cell. Therefore, cancer stem cells are in part more resistant to chemotherapy. Cancer stem cells have shown an ability to respond to therapy challenges such as chemotherapy, radiotherapy and more recently immunotherapy[33-35].
Taken together, many chemotherapeutic regimens are currently being adopted for the treatment of CRC. However, this disease displays specific mechanisms rendering a lower therapeutic benefit (Figure 1). In-depth study of drug resistance and targeting the cancer stem cell population will eventually improve the clinical outcome.
Targeted therapies including monoclonal antibodies and small molecule inhibitors are effective treatments following chemotherapy. With the apparition of monoclonal antibodies against vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR), the OS for CRC increased up to three years[36-38]. Targeted therapies display significantly lower side effects as compared to chemotherapy. Bevacizumab is the first anti-angiogenic drug that can precisely target VEGF, leading to reduced tumor growth[39]. Kabbinavar and colleagues showed improved response rates and OS from data obtained from three clinical trials comparing patients treated with fluorouracil/leucovorin alone or in combination with Bevacizumab[40]. However, the survival benefit of anti-VEGF therapy in mCRC patients is limited to a few months due to acquired resistance. During Bevacizumab exposure, VEGF-A is decreased, but increased levels of VEGFR1 result in drug resistance. Decreased hepatocyte growth factor (HGF) levels are observed during acquired resistance, suggesting the potential implementation of strategies to counter HGF-ligand inhibition[41]. Findings by Carbone et al[42] propose a role for the transcription factor HOXB9 as one of the key mechanisms of anti-VEGF resistance. Silencing HOXB9 is thought to be a promising approach to modulate this resistance. Despite these few findings, the major mechanism of drug resistance to anti-VEGF therapy is not fully elucidated. Further research on drug resistance mechanisms, as well as on predictive biomarkers, is therefore essential.
EGFR is a key component involved in the regulation of cell proliferation. Anti-EGFR antibodies, such as Cetuximab and Panitumumab, inhibit downstream signaling pathways. This leads to an inhibition of proliferation and induction of apoptosis. KRAS or NRAS mutations are well-known predictors of resistance to anti-EGFR therapy[43]. The efficacy of Cetuximab was demonstrated in the CRYSTAL trial for patients with wild type KRAS in combination with FOLFIRI (leucovorin + 5-FU + irinotecan) or FOLFOX regimen. In this trial, the median OS was extended for more than three months. However, patients with CRC carrying KRAS mutations did not benefit from the combination therapy[36,44]. In addition, several clinical trials proved that either Cetuximab or Panitumumab significantly improved the OS in wild-type KRAS patients. The effect was more pronounced in wild-type RAS mCRC, delineating that RAS mutation could be a negative predictive marker for Panitumumab[45-47]. However, the G13D KRAS mutation in exon 2 codon 13 deserves particular attention. In several studies, patients harboring the G13D KRAS mutation as well as wild-type KRAS, significantly benefited from the addition of Cetuximab based on OS[48-50]. Some studies pointed out that BRAF, PTEN and PIK3CA (like KRAS) have predictive value for anti-EGFR treatment efficacy[36,51-54]. All of the clinical trials support KRAS and BRAF mutation assessment for mCRC patients before initiation of treatment with anti-EGFR therapy. In addition to the above-described targeted therapies, Regorafenib, a multikinase inhibitor, is also approved for the treatment of mCRC. It can inhibit the function of fms-related tyrosine kinase 1 (FLT1), kinase insert domain receptor (KDR), TEK receptor tyrosine kinase, KIT proto-oncogene receptor tyrosine kinase, Raf-1 proto-oncogene and serine/threonine kinase[55]. Two phase III clinic trails showed that Regorafenib treatment could improve the OS by 1.4 mo and 2.5 mo[56,57]. Even though these drugs are approved, they display limited progression free survival (PFS) and OS due to resistance[44,58]. Their major advantage is that they have limited toxicity side effects as compared to chemotherapeutic drugs.
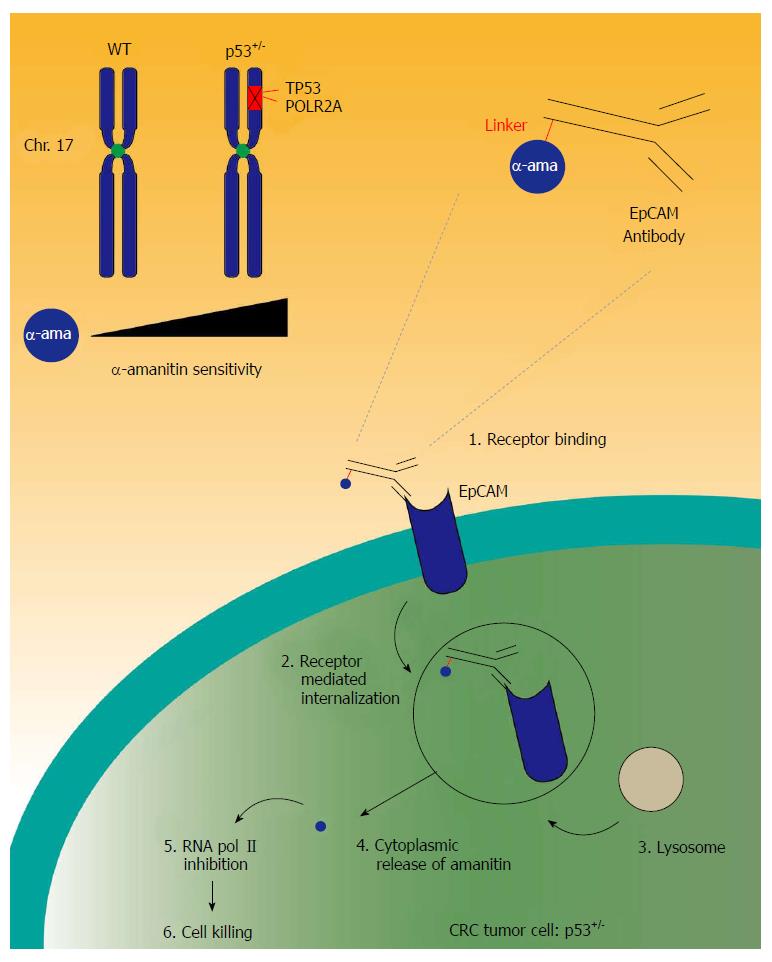
CRC development and progression is associated with acquired genomic events. The amount of mutations and genomic alterations is extensive. One of the main drivers for cancer development is gene copy number variation, such as the amplification of oncogenes or the deletion of tumor suppressor genes. Over the years, significant work has been done to tackle the tumor suppressor p53 activity in cancer therapies. Usually, promotion of cancer development can occur when a tumor suppressor gene, such as p53, undergoes a two-hit modification, being the mutation of the gene and the hemizygous loss of its other counterpart on the other arm of the chromosome. Unfortunately, no effective drug has reached the clinic due to its complex signaling pathway[59]. We demonstrate that genomic deletion of p53 frequently encompasses neighboring essential genes, rendering cancer cells with hemizygous p53 deletion vulnerable to further suppression of such genes. POLR2A is identified as such a gene that is always co-deleted with p53 in human cancers. Hemizygous loss of p53/POLR2A occurs in 53% of CRC. POLR2A encodes the largest and catalytic subunit of RNA polymerase II complex. It is specifically inhibited by α-amanitin, a cyclic 8-aa peptide toxin found in the death cap mushroom (Amanita phalloides)[60,61]. Suppression of POLR2A selectively inhibits proliferation, survival and tumorigenic potential of CRC cells with hemizygous p53 loss. Previous clinical applications of α-amanitin have been limited due to its liver toxicity. Free α-amanitin causes apoptosis and necrosis of hepatocytes by interacting with the hepatocyte-specific transporting protein OATP1B3[62]. However, α-amanitin is no longer a substrate for OATP1B3 when coupled to antibodies[63]. Therefore, α-amanitin-based antibody drug conjugates (ADCs) are highly effective therapeutic agents with significantly reduced toxicity. Our study has shown that low doses of α-amanitin-conjugated anti-epithelial cell adhesion molecule (EpCAM) antibody leads to complete tumor regression in murine models of human CRC with hemizygous deletion of POLR2A (Figure 2)[64]. The preclinical studies provide the foundation for future clinical trials. The major advantage for the use of such targeted therapy is that the function of POLR2A is essential for cell survival. Thus, no alternative “escape” pathway can be recruited, leading to drug resistance. In addition, this mode of action is not related to the proliferation of cancer cells. Cancer stem cells would be also targeted via this approach, leading to a potentially more pronounced therapeutic benefit. However, hypothetically, resistance could occur if the remaining POLR2A allele would undergo mutations, amplification or transcriptional activation as well as post-transcriptional or post-translation enhancement. In addition, tumor cells could downregulate the EpCAM receptor on their surface, hereby avoiding the binding of the ADC.
CRC is often diagnosed at a later stage when tumor cell dissemination has taken place. Over the last decades, metastatic disease, occurring in almost half of the patients, has been a challenge for clinicians to treat and remains an incurable disease. Unfortunately, most of the promising preclinical approaches have proven scarce in clinical translation. The metastatic process has been extensively investigated but has yet to be linked with specific genetic alterations of epithelial CRC cells[65]. Nevertheless, it has become clear that one of the key issues relies on the TME[66]. Therefore, blockage of cancer immunity and tumor-promoting inflammation have become hallmarks of cancer[67]. The TME plays a critical role at the different stages of the disease from a physiological colonic epithelium to an adenomatous polyp and eventually to a mCRC. The TME confers to CRC cell survival, immune evasion and a favorable environment to grow and metastasize.
The TME consists of a variety of cells that are continuously interacting with each other in a dynamic manner: Immune cells, extra-cellular matrix, cancer associated fibroblasts, endothelial cells, endothelial progenitor cells, platelets and mesenchymal stem cells, to name a few. The initial attraction of these cell types is mediated through inflammation, leading to the secretion by both the tumor cells and stromal cells of a variety of cytokines and chemokines. Stromal inflammation was shown to promote the evolution of adenomas to adenocarcinomas in nude mice[68]. Ultimately, the tumor breaches the equilibrium and the tumor becomes uncontrollable. At this stage tumor cells will abuse stromal cells to promote tumor survival, proliferation and metastasis.
Dendritic cells (DCs) are considered the most professional antigen presenting cells (APCs) and are essential to generate a proper adaptive immune response[69]. Ideally, DCs within the TME will engulf tumor associated antigens (TAAs) and migrate towards the draining lymph nodes, where they will elicit T-cell mediated responses. In CRC, no correlation was found between the frequency of DCs in the tumor and patient survival[70]. O'Toole et al[71], however, could link the capacity of tumor conditioned media to inhibit the lipopolysaccharide (LPS)-induced maturation of DCs with patient survival. The suppression of DCs was independent of the stage of the disease. Thus, the outcome could be rather linked with the potential functionality of DCs than their presence in the TME. DCs are very versatile based on their environment. In a suppressive environment that hampers their maturation, they become tolerogenic or regulatory DCs, which promote tumor cell survival. On the other hand, well-activated DCs will induce immunity and Th1 immune cell responses.
The role of tumor associated macrophages (TAMs) is of particular interest in CRC. Usually in most types of solid cancers, the infiltration of TAMs is linked with a poor survival and enhanced metastasis. However in CRC, the infiltration of TAMs is linked with better prognosis[72,73]. Recently, Zhang et al[74] conducted a meta-analysis of 55 studies with a total of 8692 patients in which they correlated the survival with the infiltration of TAMs using the pan-macrophage marker CD68. Strikingly, only in CRC were favorable clinical outcomes correlated with their infiltration. Moreover, TAM-rich tumors are accompanied with a lower amount of both lymph node and distant metastases[74].
A variety of chemoattractants are involved in the recruitment of monocytes in the TME. Chemokines such as C-C motif chemokine ligand-2 (CCL-2), CCL-5 and C-X-C motif chemokine ligand 12 (CXCL-12), cytokines such as colony stimulating factor1 (CSF-1) and VEGF family members, and complement components such as C5a contribute to the recruitment of macrophages in the TME[75-77]. These factors also lead to a differential activation status of macrophages[78]. TAMs can be subdivided into two categories based on their activation status: M1 (classically activated) or M2 (alternatively activated). Classically activated M1 TAMs are driven by interferon-γ (IFN-γ), whereas alternative M2 TAMs are driven by interleukin-4 (IL-4) and IL-13[75]. This nomenclature is based on the Th1 and Th2 concept, and thus reflects their role in adaptive immunity. Therefore, in most cases M2 TAMs are considered pro-tumorigenic through their contribution to tumor vascularization and dampening of adaptive immune responses, while M1 macrophages possess antitumor activities. To address the potential influence of TAMs in improved patient survival, Malesci et al[79] studied the impact of high TAM infiltration in stage III CRC patients treated with the adjuvant 5-FU chemotherapeutic drug. Their results showed a clear benefit of TAM infiltration when associated with 5-FU, while no benefit was observed in the untreated group. In addition, the effect of high-density TAM in metastatic lymph nodes is more pronounced in patient survival compared to the high TAM infiltration in the primary tumors. In vitro experiments pointed out that treatment with 5-FU resulted in M1 polarization of the macrophages[79]. However, further research into which subtype of macrophages is critical for patient outcome in CRC remains to be defined.
The correlation of T-cell infiltration on the OS at different stages of CRC has been well documented but remains controversial[80-86]. Numerous studies were made to assess the correlation of distinct T-cell populations (Including: CD3+, CD4+, CD8+, CD45RO+ and FoxP3+) with the clinical outcome. Most studies point out that T-cell infiltration is unlikely a predictive factor in CRC patients. The majority of studies encompassed only a low number of patients, which leads to debatable interpretation of the tumor-infiltrating lymphocytes (TILs) and specific subpopulations thereof on the clinical outcome. This has prompted the meta-analysis of TILs and their correlation with survival. Nosho et al[87] analyzed 768 CRC cases ranging from stage I to IV. They concluded that the density of memory T cells (CD45RO+) in the tumors was associated with improved survival. In addition, assessment was done on molecular alterations such as microsatellite instability (MSI), CpG island methylator phenotype (CIMP), BRAF, KRAS, PIK3CA and LINE-1 hypomethylation. The high-frequency of MSI (MSI-H) and high LINE-1 methylation were correlated with higher CD45RO+ cell density. These data are in accordance with previously published data showing the relationship between MSI and TILs. MSI is thought to induce truncated peptides that cause immunogenicity of tumor cells[85], which in turn contributes to the stimulation of adaptive immune responses. Taken together, the predictive value of TILs in CRC remains unclear.
The gut microbiome regulates the homeostasis of the digestive tract in a very dynamic way. Disruption in the latter can thus disturb this balance and cause major environmental changes leading to diseases such as inflammatory bowel disease (IBD) and cancer[88]. The gut microbiota consists of trillions of micro-organisms such as bacteria, viruses and fungi[89,90]. Recent studies have investigated the presence and functional roles of certain bacteria in CRC[91-96].
In the last three years, the gut microbiome has emerged as a potential key player in cancer immunotherapy. Initial findings by Vetizou et al[97] showed that the checkpoint inhibitor (CPI) ipilimumab (anti-cytotoxic T-lymphocyte antigen-4: Anti-CTLA4) could treat specific pathogen free (SPF) mice, but not germ-free mice. In addition, the anti-tumor effects of ipilimumab could be deteriorated by antibiotics. Analysis of murine feces revealed significant changes in the microbiome, leading to a decrease in the bacterial species Bacteroidales and Burkholderiales. Supplementation of these missing microbes could restore the anti-tumor effects of ipilimumab. In the same vein, Sivan et al[98] showed that mice obtained from two different providers responded distinctly to anti-programmed death-1 receptor ligand (anti-PD-L1) treatment. These mice were shown to harbor a different microbiome, and fecal transplants could reverse the treatment discrepancies. In their case, Bifidobacterium showed a positive correlation with anti-tumor T-cell responses.
Future research could potentially link different species of bacteria with an alternate immune cell infiltration. For instance, certain bacteria could potentially shape macrophage polarization towards a distinct phenotype and thus be used as a potential predictive marker for CRC patients. It could also be that the presence of bacteria species would be distinct based on the stage of the disease. Interestingly, the right or proximal colon more frequently exhibits MSI tumors, whereas the left or distal colon displays more chromosomal instability (CIN)[99]. It might be that the proximal or distal colon display different type of bacteria leading to these genetically different tumors. We expect that in the future, intestinal microbiota might serve as a standard of care biomarker for immunotherapies such as CPIs. Moreover, fecal transplant or supplementation of certain species of bacteria could potentially be co-administered with CPI, leading to improved responses towards CPI. Taken together, better understanding of the microbiota dysbiosis could serve as prognostic and predictive marker in CRC.
Several types of cancers have undergone a complete revolution thanks to immunotherapy. This has led the editors of Science calling cancer immunotherapy the “breakthrough of the year” in 2013[100]. Nonetheless, CRC has so far been a poor candidate for immunotherapy. Initial studies lacked objective clinical responses with nivolumab in unselected patients[101,102]. Previous observations noticed that immunotherapy works better in tumors containing a high mutational load as illustrated in melanoma and lung cancer. To further emphasize the importance of the mutational load in lung cancer, smoking patients displayed a better response rate to CPI compared to non-smokers[103]. Increased amount of mutations is associated with the production of neoantigens, which in turn enhance the tumor immunogenicity[104]. The better predictive value of smoking lung cancer patients to CPI was linked with an increased amount of neoantigens.
Consequently, this has prompted the application of CPI in patients with MSI-H CRC. In MSI, frameshift mutations in protein-coding sequences possess the capacity to generate different peptides with potential neoepitopes recognized as foreign by the immune system[85]. To further illustrate this, Saeterdal et al[105] found an immunogenic peptide derived from a frameshift mutation in transforming growth factor β receptor type II (TGFβRII) referred to as p538. This peptide is expressed in over 90 percent of tumors with DNA mismatch repair (dMMR), suggesting it is highly applicable in the field. Many of such genes in MSI-H tumors are shared by a majority of patients as they are thought to be part of the carcinogenesis process[105]. Therefore, both prophylactic for patients with a genetic predisposition and therapeutic cancer vaccinations could be done using such peptides.
Interestingly, the number of TILs is increased in MSI tumors compared to microsatellite stable (MSS) tumors[84,106,107]. Furthermore, TILs display an enhanced CD8+CD103+ phenotype[108]. CD103+ TILs were found in 27-fold higher amounts within the same patient tumor compared to normal epithelium. Increased objective response (OR), stable disease (SD) and PFS were observed in a phase II clinical trial using pembrolizumab (anti-PD-1) to treat MSI-H patients. Similarly, when nivolumab (anti-PD-L1) was administered to MSI-H patients, a clear benefit was observed which led to the approval for these selected patients. As for melanoma and other cancers, the combination of ipilimumab and nivolumab is currently being tested for MSI-H metastatic CRC patients[109].
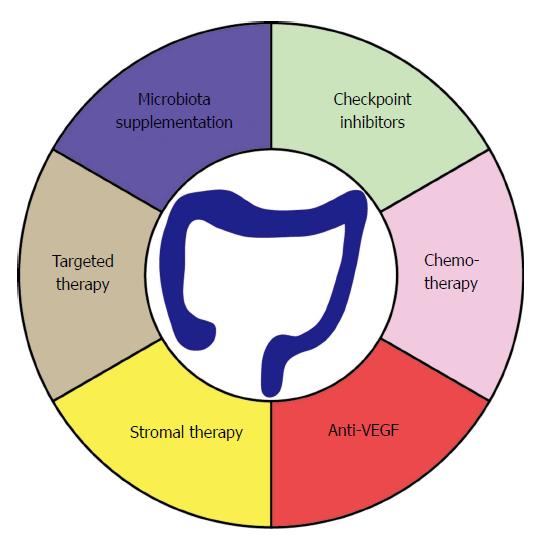
Of note, colon cancer cell lines derived from MSI tumors display a loss in human leukocyte antigen (HLA) class I expression[110]. This is due to genetic mutations in the β2m, which is an essential part of the HLA class I complex. The presentation of TAAs is considered a prerequisite for successful T-cell responses[111]. Therefore, it is thought that these surviving tumors were exposed to high selection pressure to escape T-cell surveillance. In many cancers, treatment with CPI fails to reach satisfying results[112,113]. Consequently, there is a growing interest in combining CPI together with chemotherapies or targeted therapies (Figure 3). The rationale is that certain chemotherapeutic drugs or targeted therapies could enhance the immunogenicity of the tumors. This process is dependent on induction of immunogenic cell death (ICD) of tumor cells[114]. When killed in an immunogenic way, tumor cells will express surface makers such as calreticulin and will secrete factors such a high-mobility group box 1 (HMGB1) in the extracellular milieu, hereby allowing the spontaneous generation of an adaptive immune response that might benefit from CPI. Preclinical evidence supported the oxaliplatin-induced immunogenic cell death in the murine BALB/c colon carcinoma model CT26[115].
Furthermore, a few ongoing clinical trials hold the promise to improve the outcome of PD1/PD-L1 blockade. VEGF, leading to angiogenesis, is frequently upregulated in CRC and is linked with poor OS. The latter can also influence the maturation of DCs. As described above, the DC maturation capacity was correlated with patient survival[70]. Therefore, blockage of VEGF through Bevacizumab could potentiate immune responses. In a phase Ib clinical trial, the combination of Bevacizumab and Atezolizumab displayed a clear benefit of the combination therapy for MSI-H patients[116]. Similarly, in the NCT02997228 clinical trial, 439 patients with MSI-H CRC will be treated with either: Atezolizumab as monotherapy; Atezolizumab combined with FOLFOX (a chemotherapy regimen consisting of folinic acid + 5-FU + oxaliplatin) and Bevacizumab; or FOLFOX and Bevacizumab. FOLFOX will also be tested with and without Atezolizumab in over 700 MSI-H CRC stage III patients (NCT02912559).
In addition to Bevacizumab, several targeted therapies have been approved for the treatment of CRC. Amongst them, Cetuximab was shown to display antibody-dependent cellular cytoxicity (ADCC). Interestingly, Cetuximab could induce antigen-spreading in head and neck cancer patients[117]. Antigen-spreading is a therapy induced phenomenon where secondary to therapy more antigens are released and can trigger the generation of antigen-specific immune responses against a broader number of antigens[118]. As tumor cells are known to evolve and “protect” themselves against any form of therapy, they will ultimately try to downregulate immune responses against a single antigen. Therefore, the generation of immune responses against several epitopes could lead to robust and long-lasting immune responses, which encouraged the clinical evaluation for the combination of Cetuximab with Pembrolizumab (NCT02713373).
Unfortunately, the presence of MSI solely accounts for 15% of CRC cases, while the frequency of MSI-H is even lower at 5.9% of the patients[119-122]. The amount of MSI-H varies only slightly based on the stage of the disease (0-I: 5.9%; II: 8.9%; III: 4.0% and IV: 3.7%). The overwhelming majority of CRC patients would thus remain out of scope for CPI. Ebert et al[123] studied the potential combination of mitogen-activated protein kinase kinase (MEK) inhibition using the MEK inhibitor G-38963, which is considered similar to the clinically used Cobimetinib, together with anti-PD-L1 in CT26 tumor-bearing mice[123-125]. Treatments with only MEK inhibitors lead to an initial delay in tumor growth. However, upon analysis of these tumors, an increased amount of antigen-specific T cells were present with a distinct T-bethigh phenotype. Therapeutic combination of MEK inhibition and PD-L1 blockage led to an impressive long-term survival, whereas single agents only displayed an initial delay of tumor growth. This MEK inhibitor was furthermore shown to act on the post-naïve stage of T-cell differentiation. Antigen-specific CD8+ T cells expressed Nur77, which is associated with exhaustive T-cell death. MEK inhibition was shown to counter the expression of Nur77 and thus could rescue T-cell exhaustion. This effect seemed to be in parallel with the PD-1 axis; therefore, a therapeutic rescue might only be possible in the case of blocking the PD1/PD-L1 axis. A potential risk to this strategy might be the prolonged exposure to MEK inhibition. The latter could lead to a depletion of the T-cell population. To address this issue, a period of two days without MEK treatment was introduced, which could bring back the amount of T-bethigh antigen-specific T cells to normal. Based on the findings that MEK inhibition leads to enhanced T-cell infiltration, synergy with blockade of the PD-1/PD-L1 axis and an upregulation of MHC class I on tumor cells clinical efficacy was assessed[123,126-129]. Phase Ib clinical results (NCT01988896) indicated a potential benefit for the combination of PD-L1 inhibition (Atezolizumab) and Cobimetinib on proficient MMR (pMMR), which is the equivalent of MSS tumors that were previously unresponsive to CPI. These data have prompted the more in depth analysis of this combination and is currently evaluated in a phase III clinical trial (NCT02788279). Such combination therapy trying to render MSS tumors sensible for CPI might open the avenue for CRC sensitivity towards CPI, and thus, long-term benefits.
Assessing a change in the TME is one of the major targets for current immunotherapeutic approaches. Depletion of myeloid-derived suppressor cells (MDSCs) using anti-CSF1R and anti-CTLA4 improved the survival of CT26 tumor-bearing mice[130]. Similarly, a decrease in the granulocyte fraction of the MDSCs was found to be a favorable factor for patients treated with FOLFOX and Bevacizumab[131]. Another option would be the local delivery of drugs directly in the TME to reduce the toxicity of the delivered drugs. The effectiveness of the local delivery also possesses multiple advantages[132-134]. Local delivery can induce systemic immune responses, leading to the eradication of tumors on multiple locations[135]. This phenomenon is also known as the abscopal effect. Aside from targeting cells within the tumors, it is also possible to modulate soluble factors such as cytokines and chemokines[133]. TGF-β, for example, is a very well-studied factor in CRC, which is linked with a poor prognosis[136,137]. Its function is somewhat controversial in CRC. In early stages, it possesses tumor suppressive properties, whereas in a later stage of the disease, it will promote tumor progression. TGF-β will initially lead to a cytostatic effect on epithelial cells. Calon et al[136] evidenced that pharmacological inhibition of stromal TGF-β signaling blocks metastasis. Local neutralization of soluble factors such as TGF-β have been previously reported[138]. Currently, intratumoral approaches for CRC are being tested, such as intratumoral injection of autologous activated DCs. The latter is being tested in a phase I clinical trial by Northwest Biotherapeutics (NCT01882946) using their DCVax-Direct. Another currently tested approach is the intratumoral injection of the adenoviral vector coding for the human IL-12 (cDNA) by Mount Sinai School of Medicine National Cancer Institute (NCI) for liver metastases secondary to colorectal cancer (NCT00072098).
The major drawback for the local injection remains the involvement of a surgical procedure, thus impairing large scalability of similar immunotherapeutic approaches. An elegant solution to avoid local injection is the use of tumor targeting antibodies linked with the component of interest. In the past, preclinical results were obtained using tumor targeting moieties such as anti-CD20, anti-Neu or anti-EGFR to name a few[139,140]. In CRC, an interesting clinical trial is ongoing using a T-cell bispecific (TCB) antibody targeting carcinoembryonic antigen (CEA) on CRC tumor cells and CD3 on T cells (NCT02324257 and NCT02650713). This molecule simultaneously binds to T-cells and tumor cells. This bispecific antibody is currently being tested both as a monotherapy and in combination with Atezolizumab in a majority of MSS patients. Preliminary results show promising disease control and stable disease with only a minority of progressive disease[141]. A better understanding in cancer immunotherapy drugs leading to an improved survival in the MSS subgroup of CRC will help further development of this currently unfavorable subset of patients constituting the majority of CRC patients.
The CRC field has been evolving over the last decades. Nevertheless, therapy resistance and unresponsiveness to immunotherapy remain major obstacles. Recent findings might give hope for targeted therapy resistance thanks to ADCs using RNA polymerase II inhibition as a different killing mechanism as compared to tubulin inhibitors. Less resistant mechanisms are expected; however, the future will tell us whether this holds true.
The potential use of CPI to treat CRC has been successful in a minority of patients displaying MSI-H tumors. However, recent findings point towards new avenues leading to potential enlargement of the CPI sensitive pool. Currently, a tremendous effort is being made in understanding the effects of the TME and microbiome on the outcome of CPI therapy. However, it has become clear that combination therapy will lead to tremendous benefits for patients in the future.
The authors would like to thank Michael Frieden for reviewing the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Grizzi F, Kim T, Paoluzi OA, Vynios D S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Bian YN
| 1. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 2025] [Article Influence: 202.5] [Reference Citation Analysis (0)] |
| 2. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1965] [Cited by in F6Publishing: 2133] [Article Influence: 213.3] [Reference Citation Analysis (1)] |
| 3. | Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman L, Diasio RB. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322-1327. [PubMed] [Cited in This Article: ] |
| 4. | Showalter SL, Showalter TN, Witkiewicz A, Havens R, Kennedy EP, Hucl T, Kern SE, Yeo CJ, Brody JR. Evaluating the drug-target relationship between thymidylate synthase expression and tumor response to 5-fluorouracil. Is it time to move forward? Cancer Biol Ther. 2008;7:986-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Yaffee P, Osipov A, Tan C, Tuli R, Hendifar A. Review of systemic therapies for locally advanced and metastatic rectal cancer. J Gastrointest Oncol. 2015;6:185-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 35] [Reference Citation Analysis (0)] |
| 6. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2407] [Cited by in F6Publishing: 2336] [Article Influence: 97.3] [Reference Citation Analysis (1)] |
| 7. | Qiu LX, Tang QY, Bai JL, Qian XP, Li RT, Liu BR, Zheng MH. Predictive value of thymidylate synthase expression in advanced colorectal cancer patients receiving fluoropyrimidine-based chemotherapy: evidence from 24 studies. Int J Cancer. 2008;123:2384-2389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Abdallah EA, Fanelli MF, Buim ME, Machado Netto MC, Gasparini Junior JL, Souza E Silva V, Dettino AL, Mingues NB, Romero JV, Ocea LM. Thymidylate synthase expression in circulating tumor cells: a new tool to predict 5-fluorouracil resistance in metastatic colorectal cancer patients. Int J Cancer. 2015;137:1397-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Zhou JY, Shi R, Yu HL, Zeng Y, Zheng WL, Ma WL. The association between two polymorphisms in the TS gene and risk of cancer: a systematic review and pooled analysis. Int J Cancer. 2012;131:2103-2116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Che J, Pan L, Yang X, Liu Z, Huang L, Wen C, Lin A, Liu H. Thymidine phosphorylase expression and prognosis in colorectal cancer treated with 5-fluorouracil-based chemotherapy: A meta-analysis. Mol Clin Oncol. 2017;7:943-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Sakowicz-Burkiewicz M, Przybyla T, Wesserling M, Bielarczyk H, Maciejewska I, Pawelczyk T. Suppression of TWIST1 enhances the sensitivity of colon cancer cells to 5-fluorouracil. Int J Biochem Cell Biol. 2016;78:268-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Yanagisawa Y, Maruta F, Iinuma N, Ishizone S, Koide N, Nakayama J, Miyagawa S. Modified Irinotecan/5FU/Leucovorin therapy in advanced colorectal cancer and predicting therapeutic efficacy by expression of tumor-related enzymes. Scand J Gastroenterol. 2007;42:477-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Hicks LD, Hyatt JL, Stoddard S, Tsurkan L, Edwards CC, Wadkins RM, Potter PM. Improved, selective, human intestinal carboxylesterase inhibitors designed to modulate 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin (Irinotecan; CPT-11) toxicity. J Med Chem. 2009;52:3742-3752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Palshof JA, Høgdall EV, Poulsen TS, Linnemann D, Jensen BV, Pfeiffer P, Tarpgaard LS, Brünner N, Stenvang J, Yilmaz M. Topoisomerase I copy number alterations as biomarker for irinotecan efficacy in metastatic colorectal cancer. BMC Cancer. 2017;17:48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Meisenberg C, Gilbert DC, Chalmers A, Haley V, Gollins S, Ward SE, El-Khamisy SF. Clinical and cellular roles for TDP1 and TOP1 in modulating colorectal cancer response to irinotecan. Mol Cancer Ther. 2015;14:575-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Nielsen DL, Palshof JA, Brünner N, Stenvang J, Viuff BM. Implications of ABCG2 Expression on Irinotecan Treatment of Colorectal Cancer Patients: A Review. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin Pharmacokinet. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 18. | Makondi PT, Chu CM, Wei PL, Chang YJ. Prediction of novel target genes and pathways involved in irinotecan-resistant colorectal cancer. PLoS One. 2017;12:e0180616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Meisenberg C, Ashour ME, El-Shafie L, Liao C, Hodgson A, Pilborough A, Khurram SA, Downs JA, Ward SE, El-Khamisy SF. Epigenetic changes in histone acetylation underpin resistance to the topoisomerase I inhibitor irinotecan. Nucleic Acids Res. 2017;45:1159-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Chaney SG, Campbell SL, Bassett E, Wu Y. Recognition and processing of cisplatin- and oxaliplatin-DNA adducts. Crit Rev Oncol Hematol. 2005;53:3-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 21. | Gnoni A, Russo A, Silvestris N, Maiello E, Vacca A, Marech I, Numico G, Paradiso A, Lorusso V, Azzariti A. Pharmacokinetic and metabolism determinants of fluoropyrimidines and oxaliplatin activity in treatment of colorectal patients. Curr Drug Metab. 2011;12:918-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Yan D, Tu L, Yuan H, Fang J, Cheng L, Zheng X, Wang X. WBSCR22 confers oxaliplatin resistance in human colorectal cancer. Sci Rep. 2017;7:15443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Mao L, Li Y, Zhao J, Li Q, Yang B, Wang Y, Zhu Z, Sun H, Zhai Z. Transforming growth factor-β1 contributes to oxaliplatin resistance in colorectal cancer via epithelial to mesenchymal transition. Oncol Lett. 2017;14:647-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Stark M, Bram EE, Akerman M, Mandel-Gutfreund Y, Assaraf YG. Heterogeneous nuclear ribonucleoprotein H1/H2-dependent unsplicing of thymidine phosphorylase results in anticancer drug resistance. J Biol Chem. 2011;286:3741-3754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Lin S, Lai H, Qin Y, Chen J, Lin Y. Thymidine phosphorylase and hypoxia-inducible factor 1-α expression in clinical stage II/III rectal cancer: association with response to neoadjuvant chemoradiation therapy and prognosis. Int J Clin Exp Pathol. 2015;8:10680-10688. [PubMed] [Cited in This Article: ] |
| 26. | Kotaka M, Xu R, Muro K, Park YS, Morita S, Iwasa S, Uetake H, Nishina T, Nozawa H, Matsumoto H. Study protocol of the Asian XELIRI ProjecT (AXEPT): a multinational, randomized, non-inferiority, phase III trial of second-line chemotherapy for metastatic colorectal cancer, comparing the efficacy and safety of XELIRI with or without bevacizumab versus FOLFIRI with or without bevacizumab. Chin J Cancer. 2016;35:102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Garcia-Alfonso P, Chaves M, Muñoz A, Salud A, García-Gonzalez M, Grávalos C, Massuti B, González-Flores E, Queralt B, López-Ladrón A, Losa F, Gómez MJ, Oltra A, Aranda E; Spanish Cooperative Group for the Treatment of Digestive Tumors (TTD). Capecitabine and irinotecan with bevacizumab 2-weekly for metastatic colorectal cancer: the phase II AVAXIRI study. BMC Cancer. 2015;15:327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Hu J, Li J, Yue X, Wang J, Liu J, Sun L, Kong D. Expression of the cancer stem cell markers ABCG2 and OCT-4 in right-sided colon cancer predicts recurrence and poor outcomes. Oncotarget. 2017;8:28463-28470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | de Sousa E Melo F, Colak S, Buikhuisen J, Koster J, Cameron K, de Jong JH, Tuynman JB, Prasetyanti PR, Fessler E, van den Bergh SP. Methylation of cancer-stem-cell-associated Wnt target genes predicts poor prognosis in colorectal cancer patients. Cell Stem Cell. 2011;9:476-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 30. | Zeuner A, Todaro M, Stassi G, De Maria R. Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell. 2014;15:692-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 275] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 31. | Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1301] [Cited by in F6Publishing: 1412] [Article Influence: 100.9] [Reference Citation Analysis (1)] |
| 32. | Paquet-Fifield S, Koh SL, Cheng L, Beyit LM, Shembrey C, Mølck C, Behrenbruch C, Papin M, Gironella M, Guelfi S. Tight Junction Protein Claudin-2 Promotes Self-Renewal of Human Colorectal Cancer Stem-like Cells. Cancer Res. 2018;78:2925-2938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Bialkowski L, Van der Jeught K, Bevers S, Tjok Joe P, Renmans D, Heirman C, Aerts JL, Thielemans K. Immune checkpoint blockade combined with IL-6 and TGF-β inhibition improves the therapeutic outcome of mRNA-based immunotherapy. Int J Cancer. 2018;143:686-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Hu X, Ghisolfi L, Keates AC, Zhang J, Xiang S, Lee DK, Li CJ. Induction of cancer cell stemness by chemotherapy. Cell Cycle. 2012;11:2691-2698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 463] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 36. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1314] [Cited by in F6Publishing: 1452] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 37. | Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1117] [Cited by in F6Publishing: 1227] [Article Influence: 122.7] [Reference Citation Analysis (0)] |
| 38. | Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS, Go WY. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240-2247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 474] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 39. | Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2741] [Cited by in F6Publishing: 2639] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 40. | Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706-3712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 528] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 41. | Pineda E, Salud A, Vila-Navarro E, Safont MJ, Llorente B, Aparicio J, Vera R, Escudero P, Casado E, Bosch C. Dynamic soluble changes in sVEGFR1, HGF, and VEGF promote chemotherapy and bevacizumab resistance: A prospective translational study in the BECOX (GEMCAD 09-01) trial. Tumour Biol. 2017;39:1010428317705509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Carbone C, Piro G, Simionato F, Ligorio F, Cremolini C, Loupakis F, Alì G, Rossini D, Merz V, Santoro R. Homeobox B9 Mediates Resistance to Anti-VEGF Therapy in Colorectal Cancer Patients. Clin Cancer Res. 2017;23:4312-4322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230-3237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 897] [Cited by in F6Publishing: 876] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 44. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2901] [Cited by in F6Publishing: 3021] [Article Influence: 201.4] [Reference Citation Analysis (1)] |
| 45. | Kim TW, Elme A, Kusic Z, Park JO, Udrea AA, Kim SY, Ahn JB, Valencia RV, Krishnan S, Bilic A. A phase 3 trial evaluating panitumumab plus best supportive care vs best supportive care in chemorefractory wild-type KRAS or RAS metastatic colorectal cancer. Br J Cancer. 2016;115:1206-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Shitara K, Yonesaka K, Denda T, Yamazaki K, Moriwaki T, Tsuda M, Takano T, Okuda H, Nishina T, Sakai K. Randomized study of FOLFIRI plus either panitumumab or bevacizumab for wild-type KRAS colorectal cancer-WJOG 6210G. Cancer Sci. 2016;107:1843-1850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Peeters M, Oliner KS, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F. Analysis of KRAS/NRAS Mutations in a Phase III Study of Panitumumab with FOLFIRI Compared with FOLFIRI Alone as Second-line Treatment for Metastatic Colorectal Cancer. Clin Cancer Res. 2015;21:5469-5479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 48. | De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M, Piessevaux H. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812-1820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 561] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 49. | Osumi H, Shinozaki E, Osako M, Kawazoe Y, Oba M, Misaka T, Goto T, Kamo H, Suenaga M, Kumekawa Y. Cetuximab treatment for metastatic colorectal cancer with KRAS p.G13D mutations improves progression-free survival. Mol Clin Oncol. 2015;3:1053-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Tejpar S, Celik I, Schlichting M, Sartorius U, Bokemeyer C, Van Cutsem E. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol. 2012;30:3570-3577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 272] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 51. | Pietrantonio F, Petrelli F, Coinu A, Di Bartolomeo M, Borgonovo K, Maggi C, Cabiddu M, Iacovelli R, Bossi I, Lonati V. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51:587-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 52. | De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1491] [Cited by in F6Publishing: 1585] [Article Influence: 113.2] [Reference Citation Analysis (1)] |
| 53. | Kaczirek K, Ciuleanu TE, Vrbanec D, Marton E, Messinger D, Liegl-Atzwanger B, Wrba F, Knittelfelder R, Lindner E, Zielinski CC. FOLFOX4 Plus Cetuximab for Patients With Previously Untreated Metastatic Colorectal Cancer According to Tumor RAS and BRAF Mutation Status: Updated Analysis of the CECOG/CORE 1.2.002 Study. Clin Colorectal Cancer. 2015;14:91-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Karapetis CS, Jonker D, Daneshmand M, Hanson JE, O’Callaghan CJ, Marginean C, Zalcberg JR, Simes J, Moore MJ, Tebbutt NC. PIK3CA, BRAF, and PTEN status and benefit from cetuximab in the treatment of advanced colorectal cancer--results from NCIC CTG/AGITG CO.17. Clin Cancer Res. 2014;20:744-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 55. | Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 838] [Cited by in F6Publishing: 922] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 56. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1808] [Cited by in F6Publishing: 1888] [Article Influence: 171.6] [Reference Citation Analysis (0)] |
| 57. | Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 490] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 58. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1296] [Cited by in F6Publishing: 1336] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 59. | Liu Y, Wang L, Lu X. A new way to target p53-defective colorectal cancer. Future Oncol. 2015;11:3101-3104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Bensaude O. Inhibiting eukaryotic transcription: Which compound to choose? How to evaluate its activity? Transcription. 2011;2:103-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 379] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 61. | Lindell TJ, Weinberg F, Morris PW, Roeder RG, Rutter WJ. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970;170:447-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 664] [Cited by in F6Publishing: 680] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 62. | Letschert K, Faulstich H, Keller D, Keppler D. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci. 2006;91:140-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 63. | Moldenhauer G, Salnikov AV, Lüttgau S, Herr I, Anderl J, Faulstich H. Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma. J Natl Cancer Inst. 2012;104:622-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 64. | Liu Y, Zhang X, Han C, Wan G, Huang X, Ivan C, Jiang D, Rodriguez-Aguayo C, Lopez-Berestein G, Rao PH. TP53 loss creates therapeutic vulnerability in colorectal cancer. Nature. 2015;520:697-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 65. | Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, Antal T, Traulsen A, Nowak MA, Siegel C, Velculescu VE. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci USA. 2008;105:4283-4288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 609] [Cited by in F6Publishing: 598] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 66. | Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2921] [Cited by in F6Publishing: 3034] [Article Influence: 252.8] [Reference Citation Analysis (0)] |
| 67. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39812] [Cited by in F6Publishing: 42955] [Article Influence: 3304.2] [Reference Citation Analysis (4)] |
| 68. | Okada F, Kawaguchi T, Habelhah H, Kobayashi T, Tazawa H, Takeichi N, Kitagawa T, Hosokawa M. Conversion of human colonic adenoma cells to adenocarcinoma cells through inflammation in nude mice. Lab Invest. 2000;80:1617-1628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1427] [Cited by in F6Publishing: 1421] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 70. | Michielsen AJ, Noonan S, Martin P, Tosetto M, Marry J, Biniecka M, Maguire AA, Hyland JM, Sheahan KD, O’Donoghue DP. Inhibition of dendritic cell maturation by the tumor microenvironment correlates with the survival of colorectal cancer patients following bevacizumab treatment. Mol Cancer Ther. 2012;11:1829-1837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | O’Toole A, Michielsen AJ, Nolan B, Tosetto M, Sheahan K, Mulcahy HE, Winter DC, Hyland JM, O’Connell PR, Fennelly D. Tumour microenvironment of both early- and late-stage colorectal cancer is equally immunosuppressive. Br J Cancer. 2014;111:927-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Cavnar MJ, Turcotte S, Katz SC, Kuk D, Gönen M, Shia J, Allen PJ, Balachandran VP, D’Angelica MI, Kingham TP. Tumor-Associated Macrophage Infiltration in Colorectal Cancer Liver Metastases is Associated With Better Outcome. Ann Surg Oncol. 2017;24:1835-1842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 73. | Koelzer VH, Canonica K, Dawson H, Sokol L, Karamitopoulou-Diamantis E, Lugli A, Zlobec I. Phenotyping of tumor-associated macrophages in colorectal cancer: Impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology. 2015;5:e1106677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 74. | Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 677] [Cited by in F6Publishing: 729] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 75. | Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1778] [Cited by in F6Publishing: 2340] [Article Influence: 334.3] [Reference Citation Analysis (0)] |
| 76. | Bonavita E, Galdiero MR, Jaillon S, Mantovani A. Phagocytes as Corrupted Policemen in Cancer-Related Inflammation. Adv Cancer Res. 2015;128:141-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 77. | Bonavita E, Gentile S, Rubino M, Maina V, Papait R, Kunderfranco P, Greco C, Feruglio F, Molgora M, Laface I. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell. 2015;160:700-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 78. | Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212:1043-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 451] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 79. | Malesci A, Bianchi P, Celesti G, Basso G, Marchesi F, Grizzi F, Di Caro G, Cavalleri T, Rimassa L, Palmqvist R. Tumor-associated macrophages and response to 5-fluorouracil adjuvant therapy in stage III colorectal cancer. Oncoimmunology. 2017;6:e1342918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 80. | Scurr M, Gallimore A, Godkin A. T cell subsets and colorectal cancer: discerning the good from the bad. Cell Immunol. 2012;279:21-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Baxevanis CN, Papamichail M, Perez SA. Immune classification of colorectal cancer patients: impressive but how complete? Expert Opin Biol Ther. 2013;13:517-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Senovilla L, Vacchelli E, Galon J, Adjemian S, Eggermont A, Fridman WH, Sautès-Fridman C, Ma Y, Tartour E, Zitvogel L. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology. 2012;1:1323-1343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 177] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 83. | Zlobec I, Terracciano LM, Lugli A. Local recurrence in mismatch repair-proficient colon cancer predicted by an infiltrative tumor border and lack of CD8+ tumor-infiltrating lymphocytes. Clin Cancer Res. 2008;14:3792-3797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4318] [Cited by in F6Publishing: 4590] [Article Influence: 255.0] [Reference Citation Analysis (0)] |
| 85. | Deschoolmeester V, Baay M, Lardon F, Pauwels P, Peeters M. Immune Cells in Colorectal Cancer: Prognostic Relevance and Role of MSI. Cancer Microenviron. 2011;4:377-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, Vermorken JB. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010;11:19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 87. | Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 88. | Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 540] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 89. | Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1659] [Cited by in F6Publishing: 1427] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 90. | Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, Collins MD, Doré J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799-4807. [PubMed] [Cited in This Article: ] |
| 91. | Sears CL, Geis AL, Housseau F. Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J Clin Invest. 2014;124:4166-4172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 92. | Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1659] [Cited by in F6Publishing: 1581] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 93. | Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1164] [Cited by in F6Publishing: 1324] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 94. | Gao Z, Guo B, Gao R, Zhu Q, Qin H. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol. 2015;6:20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 338] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 95. | Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1199] [Cited by in F6Publishing: 1323] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 96. | Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1511] [Cited by in F6Publishing: 1353] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 97. | Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1834] [Cited by in F6Publishing: 2167] [Article Influence: 240.8] [Reference Citation Analysis (0)] |
| 98. | Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1979] [Cited by in F6Publishing: 2382] [Article Influence: 264.7] [Reference Citation Analysis (0)] |
| 99. | Söreide K, Janssen EA, Söiland H, Körner H, Baak JP. Microsatellite instability in colorectal cancer. Br J Surg. 2006;93:395-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 100. | Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432-1433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1306] [Cited by in F6Publishing: 1399] [Article Influence: 139.9] [Reference Citation Analysis (0)] |
| 101. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5599] [Cited by in F6Publishing: 5881] [Article Influence: 490.1] [Reference Citation Analysis (0)] |
| 102. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8900] [Cited by in F6Publishing: 9301] [Article Influence: 775.1] [Reference Citation Analysis (0)] |
| 103. | Hiniker SM, Maecker HT, Knox SJ. Predictors of clinical response to immunotherapy with or without radiotherapy. J Radiat Oncol. 2015;4:339-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2935] [Cited by in F6Publishing: 3228] [Article Influence: 358.7] [Reference Citation Analysis (0)] |
| 105. | Saeterdal I, Bjørheim J, Lislerud K, Gjertsen MK, Bukholm IK, Olsen OC, Nesland JM, Eriksen JA, Møller M, Lindblom A. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci USA. 2001;98:13255-13260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 106. | Turksma AW, Coupé VM, Shamier MC, Lam KL, de Weger VA, Belien JA, van den Eertwegh AJ, Meijer GA, Meijer CJ, Hooijberg E. Extent and Location of Tumor-Infiltrating Lymphocytes in Microsatellite-Stable Colon Cancer Predict Outcome to Adjuvant Active Specific Immunotherapy. Clin Cancer Res. 2016;22:346-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 107. | Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. 2004;91:469-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 108. | Quinn E, Hawkins N, Yip YL, Suter C, Ward R. CD103+ intraepithelial lymphocytes--a unique population in microsatellite unstable sporadic colorectal cancer. Eur J Cancer. 2003;39:469-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Overman MJ, Kopetz S, McDermott RS, Leach J, Lonardi S, Lenz H-J, Morse MA, Desai J, Hill A, Axelson MD. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results. J Clin Oncol. 2016;34:3501-3501. [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 110. | Branch P, Bicknell DC, Rowan A, Bodmer WF, Karran P. Immune surveillance in colorectal carcinoma. Nat Genet. 1995;9:231-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 111. | Wallich R, Bulbuc N, Hämmerling GJ, Katzav S, Segal S, Feldman M. Abrogation of metastatic properties of tumour cells by de novo expression of H-2K antigens following H-2 gene transfection. Nature. 1985;315:301-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 339] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 112. | Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 394] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 113. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8693] [Cited by in F6Publishing: 9303] [Article Influence: 775.3] [Reference Citation Analysis (0)] |
| 114. | Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1356] [Cited by in F6Publishing: 1706] [Article Influence: 213.3] [Reference Citation Analysis (0)] |
| 115. | Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 692] [Cited by in F6Publishing: 821] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 116. | Hochster HS, Bendell JC, Cleary JM, Foster P, Zhang W, He X, Hernandez G, Iizuka K, Eckhardt SG. Efficacy and safety of atezolizumab (atezo) and bevacizumab (bev) in a phase Ib study of microsatellite instability (MSI)-high metastatic colorectal cancer (mCRC). J Clin Oncol. 2017;35:673-673. [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 117. | Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, López-Albaitero A, Gibson SP, Gooding WE, Ferrone S. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858-1872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 118. | Gulley JL, Madan RA, Pachynski R, Mulders P, Sheikh NA, Trager J, Drake CG. Role of Antigen Spread and Distinctive Characteristics of Immunotherapy in Cancer Treatment. J Natl Cancer Inst. 2017;109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 119. | Fujiyoshi K, Yamamoto G, Takenoya T, Takahashi A, Arai Y, Yamada M, Kakuta M, Yamaguchi K, Akagi Y, Nishimura Y. Metastatic Pattern of Stage IV Colorectal Cancer with High-Frequency Microsatellite Instability as a Prognostic Factor. Anticancer Res. 2017;37:239-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 120. | Merok MA, Ahlquist T, Røyrvik EC, Tufteland KF, Hektoen M, Sjo OH, Mala T, Svindland A, Lothe RA, Nesbakken A. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol. 2013;24:1274-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 121. | de la Chapelle A, Hampel H. Clinical relevance of microsatellite instability in colorectal cancer. J Clin Oncol. 2010;28:3380-3387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 122. | Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1267] [Cited by in F6Publishing: 1270] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 123. | Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, Gould SE, Maecker H, Irving BA, Kim JM. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity. 2016;44:609-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 499] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 124. | Tan N, Wong M, Nannini MA, Hong R, Lee LB, Price S, Williams K, Savy PP, Yue P, Sampath D. Bcl-2/Bcl-xL inhibition increases the efficacy of MEK inhibition alone and in combination with PI3 kinase inhibition in lung and pancreatic tumor models. Mol Cancer Ther. 2013;12:853-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 125. | Hoeflich KP, Merchant M, Orr C, Chan J, Den Otter D, Berry L, Kasman I, Koeppen H, Rice K, Yang NY. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012;72:210-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 126. | Brea EJ, Oh CY, Manchado E, Budhu S, Gejman RS, Mo G, Mondello P, Han JE, Jarvis CA, Ulmert D. Kinase Regulation of Human MHC Class I Molecule Expression on Cancer Cells. Cancer Immunol Res. 2016;4:936-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 127. | Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T, Yang J, Seestaller-Wehr L, Zhang SY, Hopson C. The BRAF and MEK Inhibitors Dabrafenib and Trametinib: Effects on Immune Function and in Combination with Immunomodulatory Antibodies Targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res. 2015;21:1639-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 329] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 128. | Pollack BP, Sapkota B, Cartee TV. Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin Cancer Res. 2011;17:4400-4413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 129. | Mimura K, Shiraishi K, Mueller A, Izawa S, Kua LF, So J, Yong WP, Fujii H, Seliger B, Kiessling R. The MAPK pathway is a predominant regulator of HLA-A expression in esophageal and gastric cancer. J Immunol. 2013;191:6261-6272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 130. | Holmgaard RB, Brachfeld A, Gasmi B, Jones DR, Mattar M, Doman T, Murphy M, Schaer D, Wolchok JD, Merghoub T. Timing of CSF-1/CSF-1R signaling blockade is critical to improving responses to CTLA-4 based immunotherapy. Oncoimmunology. 2016;5:e1151595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 131. | Limagne E, Euvrard R, Thibaudin M, Rébé C, Derangère V, Chevriaux A, Boidot R, Végran F, Bonnefoy N, Vincent J. Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer Res. 2016;76:5241-5252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 132. | Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res. 2014;20:1747-1756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 133. | Van der Jeught K, Bialkowski L, Daszkiewicz L, Broos K, Goyvaerts C, Renmans D, Van Lint S, Heirman C, Thielemans K, Breckpot K. Targeting the tumor microenvironment to enhance antitumor immune responses. Oncotarget. 2015;6:1359-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 134. | Van der Jeught K, Van Lint S, Thielemans K, Breckpot K. Intratumoral delivery of mRNA: Overcoming obstacles for effective immunotherapy. Oncoimmunology. 2015;4:e1005504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 135. | Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, Levy R. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 136. | Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, Sevillano M, Palomo-Ponce S, Tauriello DV, Byrom D. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015;47:320-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 745] [Cited by in F6Publishing: 756] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 137. | Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung P, Zhang XH. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 745] [Cited by in F6Publishing: 829] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 138. | Van der Jeught K, Joe PT, Bialkowski L, Heirman C, Daszkiewicz L, Liechtenstein T, Escors D, Thielemans K, Breckpot K. Intratumoral administration of mRNA encoding a fusokine consisting of IFN-β and the ectodomain of the TGF-β receptor II potentiates antitumor immunity. Oncotarget. 2014;5:10100-10113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 139. | Xuan C, Steward KK, Timmerman JM, Morrison SL. Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood. 2010;115:2864-2871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 140. | Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, Fu YX. Targeting the tumor microenvironment with interferon-β bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 141. | Tabernero J, Melero I, Ros W, Argiles G, Marabelle A, Rodriguez-Ruiz ME, Albanell J, Calvo E, Moreno V, Cleary JM. Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: Preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC). J Clin Oncol. 2017;35:3002-3002. [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |