Published online Sep 7, 2018. doi: 10.3748/wjg.v24.i33.3806
Peer-review started: May 18, 2018
First decision: June 6, 2018
Revised: June 11, 2018
Accepted: July 21, 2018
Article in press: July 21, 2018
Published online: September 7, 2018
Neurofibromatosis type 1 (NF-1) is commonly associated with benign or malignant tumors in both the central and peripheral nervous systems. However, rare cases of NF-1-associated multiple rectal neuroendocrine tumors have been reported. This report describes a case of a 39 year old female with NF-1 and intermittent hematochezia as a primary symptom. Physical examination showed multiple subcutaneous nodules and café au lait spots with obvious scoliosis of the back. Imaging examinations and colonoscopy found malformation of the left external iliac vein and multiple gray-yellow nodules with varying sizes and shapes in the rectal submucosal layer. Histological and immunohistochemical results suggested multiple rectal neuroendocrine tumors, a rare disease with few appreciable symptoms and a particularly poor prognosis. The patient with NF-1 presented here had not only multiple rectal neuroendocrine neoplasms but also vascular malformations, scoliosis and other multiple system lesions. This case therefore contributes to improving clinical understanding, diagnosis and treatment of related complications for patients with NF-1 who present with associated medical conditions.
Core tip: Neurofibromatosis type 1 (NF-1) is commonly complicated with either benign or malignant tumors in both the central and peripheral nervous systems. However, there are rare reported cases of NF-1 associated with multiple rectal neuroendocrine tumors. This study reports a case of a 39 year old female NF-1 patient with not only multiple rectal neuroendocrine neoplasms but also vascular malformations and scoliosis.
- Citation: Xie R, Fu KI, Chen SM, Tuo BG, Wu HC. Neurofibromatosis type 1-associated multiple rectal neuroendocrine tumors: A case report and review of the literature. World J Gastroenterol 2018; 24(33): 3806-3812
- URL: https://www.wjgnet.com/1007-9327/full/v24/i33/3806.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i33.3806
Neurofibromatosis (NF) is an autosomal dominant genetic disorder that includes three subtypes: type 1 NF (NF-1), type 2 NF, and schwannoma[1,2]. Approximately 50% of patients have a family history. The incidence rate of NF-1 is approximately 1/3000-1/4000, and it is associated with a pathogenic mutation on chromosome 17q11.2[3]. Physiologically, the disease is characterized by abnormal skin pigmentation (i.e., milk coffee spots), Lisch nodules, and multiple skin nodules. NF could affect many tissues and organs, including the peripheral and central nervous systems, bones, and internal organs[4,5]. Previous studies have reported that NF-1 can lead to Ras pathway abnormalities, which may also result in peripheral neurilemmomas, central nervous system tumors, stromal tumors, neuroendocrine tumors, and other benign and malignant tumors[6-8]. However, the incidence of NF-1 in combination with gastrointestinal neuroendocrine tumor is less than two percent. Neuroendocrine tumors are commonly found in the duodenum and pancreas[9-12] and usually present as a single malignant lesion. Multiple rectal neuroendocrine tumors are particularly rare, with diverse and non-specific clinical symptoms. Common symptoms include changes in bowel habits, hematochezia, and abdominal pain, which are similar to those of more common rectal diseases such as hemorrhoids, rectal polyps, and colorectal cancers, thus complicating the accuracy of disease diagnosis[13]. Our department has received and treated a patient diagnosed with NF-1 that was combined with multiple rectal neuroendocrine tumors, vascular malformations, and scoliosis.
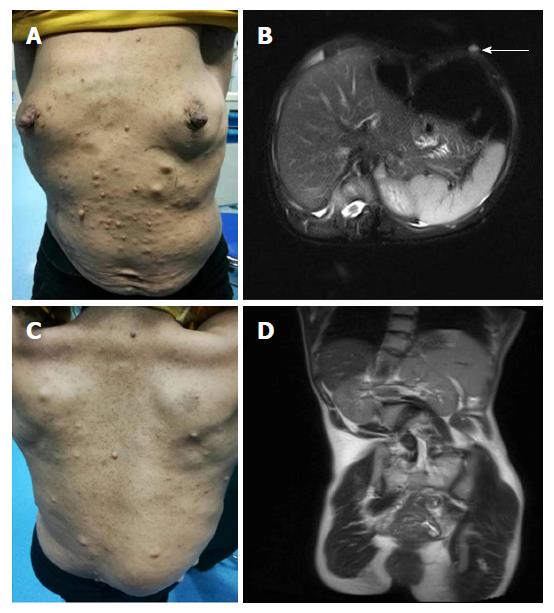
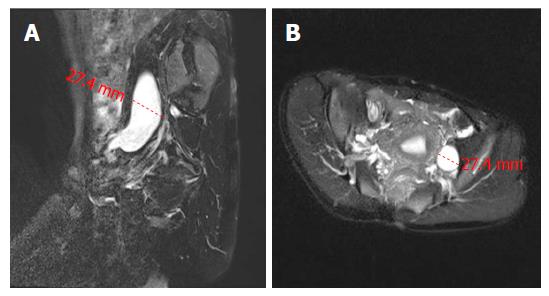
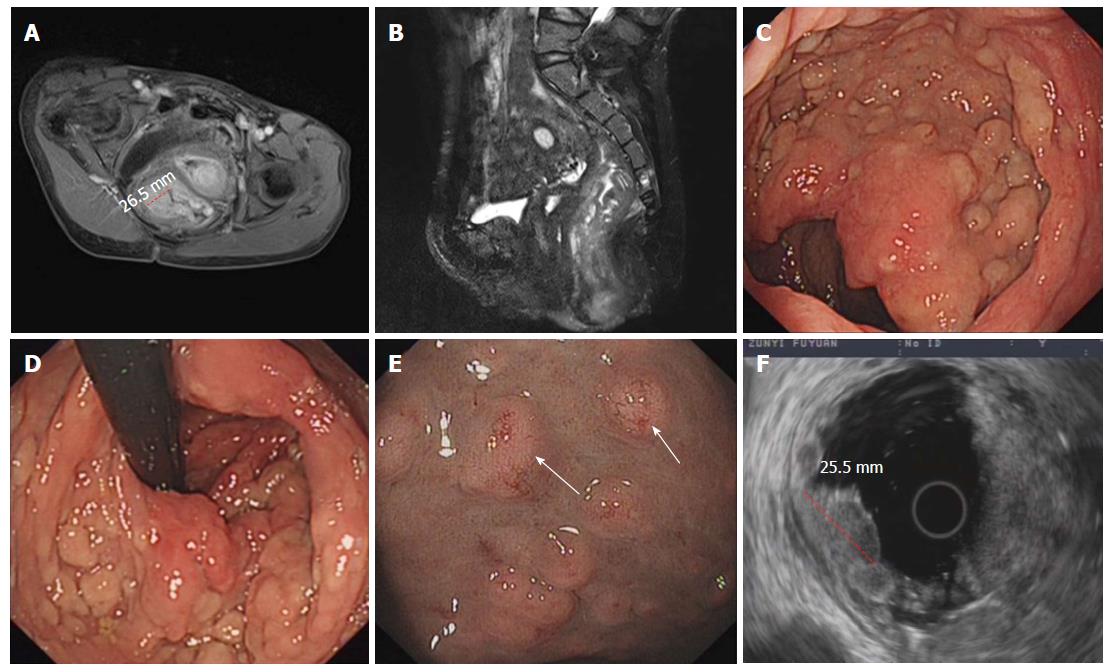
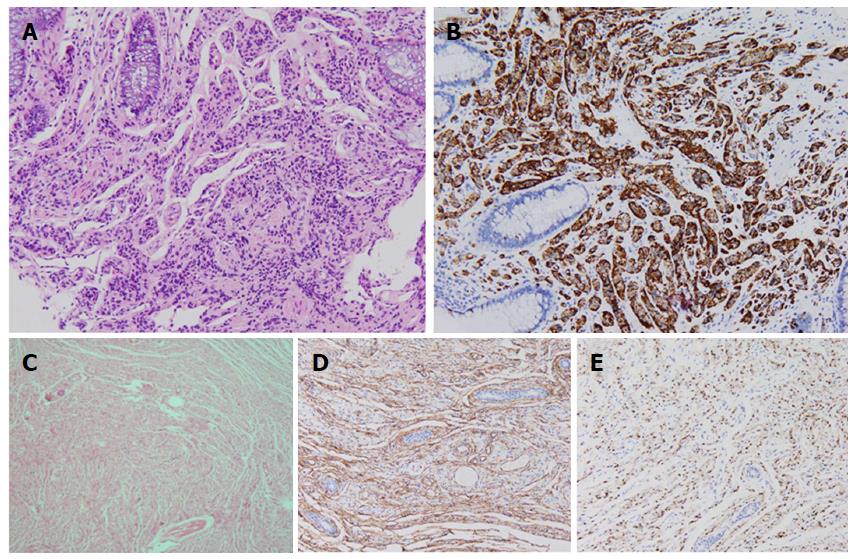
A 39 year old woman was admitted to our department because of intermittent bloody stools without vomiting, abdominal pain, diarrhea, skin flushes, etc. The patient had suffered from a slightly curved spinal column since childhood, with the abnormal curvature becoming noticeable 13 years prior. Systemic skin pimples then occurred gradually without pain or itching. Physical examination showed multiple hemispherical subcutaneous nodules with varying sizes and with soft and clear boundaries on the chest and abdomen. There were coffee pigment spots with varied sizes and colors between these nodules, with a maximal size of 3 cm × 2 cm (Figure 1A). The patient’s father also had definitive NF-1. Blood examination showed the hemoglobin level of the patient was 101 g/L, with no other abnormalities. Computed tomography and magnetic resonance imaging (MRI) of the chest revealed enlarged mediastinal lymph nodes, dermatologic nodules with long T1 and T2 values, uniform densities, clear boundaries, diameters of < 10 mm (Figure 1B), thoracolumbar scoliosis and thoracic deformities (Figure 1C-D). A pelvic MRI detected segmental thickening of the right external iliac vein, with a thickness of 27.4 mm and a sausage-like appearance (Figure 2). The middle and lower rectal mucosae were irregularly thickened, with 26.5 mm at the widest point and an irregular signal with long T1 and slightly longer T2 values. Obvious uneven enhancement was noted in the post-contrast arterial phase, while separation and necrosis were visible in parts. These presentations suggested a diagnosis of multiple rectal lesions (Figure 3A and B). No obvious abnormalities were noted in the computed tomography of the head. Colonoscopy revealed multiple yellow-white nodular uplifts under the rectal mucosa at approximately 1-10 cm from the anal verge. These uplifts varied between 0.3-2.5 cm in diameter and presented with a patchy distribution. The lesion involved the entire rectal lumen (Figure 3C and D). The uplifted surface was smooth but congestive, and blood vessels were apparent on the surface of the nodules under the narrow-band imaging (Figure 3E). Endoscopic ultrasound revealed multiple hypoechoic lesions in the mucosa and submucosa, with enlarged lymph nodes in the outer membrane (Figure 3F). Pathohistological and immunohistochemical examinations (n = 10) at many sites from rectal samples showed that tumor cells were present in the lesions and mutually linked to form cord, nest, or gland-like structures. The tumor cells were round, oval or columnar, of varying sizes, with round nuclei, and without obvious mitosis. Cells were CD117 (-), CD56 (+), CK (+), CgA (+), Syn (+), and TTF-1 (-), with a Ki-67 index of < 2%, thus supporting the diagnosis of a grade 1 rectal neuroendocrine tumor (Figure 4A-B). Specimens from many nodules were taken throughout the body and were examined by pathohistology and immunohistochemistry. The subdermal nerve fibers were in a disordered arrangement, and the cells were elongated, spindle-shaped and oddly distributed in the light-stained collagen matrix. Immune staining revealed CD34 (+) and S-100 (+) expression, deep and S-shaped nuclei, and scattered mast cells. These pathological features corresponded to a diagnosis of type I neurofibromatosis (Figure 4C-E). This patient had multiple rectal neuroendocrine tumors with a diameter > 20 mm. The probability of lymph node metastasis and distant metastasis was considered to be very high. Surgical intervention was advised, however the patient rejected surgery and favored surveillance by regular follow-ups every 3-6 mo.
NF-1 is an autosomal dominant genetic disorder caused by abnormal ectodermal development that results in peripheral and central nervous system impairment. This disease has a 68.6% and 31.4% likelihood of maternal and paternal heritability, respectively. The etiology of NF-1 is not fully understood. NF-1 is currently considered to be related to gene mutations, hormones, telomerase, angiogenic factors, tumor microenvironment, electrophysiological changes and other factors related to tumor promotion[7]. Neurofilament, encoded by the NF-1 gene, is a negative regulator of the Ras pathway, and the GAP-related domains encoded by exons 21-27 are homologous to the GTPase-activating protein family. This protein may convert the active form of Ras-GTP into the inactive form of Ras-GDP, thereby inhibiting the activation of Ras and its downstream signaling pathways including Raf-MEK-ERK and Paf-MAPK-PI3-K/Akt[6,7]. Therefore, patients with an NF-1 mutation could present with complications such as spinal malformations, vascular malformations, and benign and malignant tumors in both the central and peripheral nervous systems due to excessive Ras pathway activation. The clinical symptoms are diverse, complex and difficult to treat. Neuroendocrine tumors refer to a group of heterogeneous tumors that originate from neuroendocrine cells. They grow slowly with malignant potential and can occur in multiple systems throughout the body, although they are most commonly found in the gastrointestinal tract[14]. Clinical data confirmed that approximately two percent of patients diagnosed with NF-1 also have neuroendocrine tumors, which may be related to Ras-PI3K over-activation that leads to an imbalance of rapamycin (mTOR) expression[15]. The case presented in this report contradicts previous studies claiming that complicated neuroendocrine tumors are commonly located in the region around the ampulla of the duodenum and pancreas[9-12]. There are very few cases of NF-1 that are associated with multiple rectal neuroendocrine tumors. Rectal neuroendocrine neoplasms (NENs) are often derived from peptidergic neurons and neuroendocrine cells of the rectal mucosal epithelium, and are often divided into functional or non-functional types[16]. The clinical symptoms of functional NENs are most often related to peptides and hormones secreted from the primary site, while non-functional NENs have no specific clinical symptoms. Imaging, endoscopic ultrasound and biopsy are used as the main diagnostic methods for non-functional NENs.
Clinically, the rectal neuroendocrine tumors are mostly non-functional. In addition, rectal neuroendocrine tumors are usually single-onset, with only two to four percent being multiple-onset. Previous research suggests that the MEN1 (neuroendocrine tumor) gene, PI3-K/AKT, Raf/MEK/ERK, Notch, GSK-3β and other signaling pathways may be involved in the occurrence and metastasis of multiple rectal tumors[17]. We have summarized the relevant literature in the past 20 years and found that only one case, combined with NF-1 in 14 cases, reports of multiple rectal neuroendocrine tumors (Table 1)[18-26]. Moreover, compared with Ghassemi[26]’s case report in 2010, the number of rectal endocrine tumors in our patient significant increased (n > 30), and the lesions were more widely spread over the entire lumen of the rectum. The tumor size was also unprecedented. According to the data in Table 1, most rectal endocrine tumor diameters were between 4-10 mm, while the largest tumor diameter in this case measured up to 25 mm, with the deepest lesion invading into the submucosa. Clinical evidence revealed that the diameters of rectal endocrine tumors are greater than 20 mm, the rate of lymph node metastasis may be as high as 60%-80%, and the rate of distant metastasis can reach up to 40%[27,28]. Surgical resection is considered to be the most appropriate treatment. Common surgical methods include endoscopic submucosal dissection, endoscopic mucosal resection, transanal endoscopic microsurgery, anterior resection and abdominoperineal resection. The choice of surgical methods depends on the size of the tumor, the depth of invasion, the regional lymph node, the distant metastasis and the malignancy grade of the tumor. The 2010 guide for the diagnosis and treatment of rectal neuroendocrine cancer suggested that endoscopic resection or endoscopic dissection was the first choice for rectal carcinoids that had lower levels of malignancy with sizes of no more than 2 cm in the mucosal or submucosal layers. However, cases with tumors > 2 cm in diameter that show intravascular myometrium infiltration and vascular metastasis require surgical treatment. In addition, in light of the malignant tendency and metastasis of most gastrointestinal NENs, in addition to surgery, rectal neuroendocrine tumors require a combination of multidisciplinary and multiple interventions. For example, somatostatin analogs and molecular targeting drugs like sunitinib and everolimus inhibit tumor growth, are anti-angiogenic, and have been successfully applied in clinical applications. Additionally, the chemotherapeutic drug streptozocin, as well as similar types of temozolomides, have certain effects on patients who have failed with standardized treatments of neuroendocrine carcinomas. In recent years, peptide receptor-mediated radio receptor therapy has proven to have a definite effect on alleviating symptoms and shrinking tumors, however its severe side effects restrict its use and promotion. Although the long-term effects of the aforementioned adjuvant therapies are still not fully confirmed, multidisciplinary and multi-system combination therapy is an inevitable trend in the treatment of neuroendocrine tumors. However, the patient rejected surgery and so the pathological data are therefore not available in this case. The risk of malignancy and metastasis in this patient is very high, and she should receive regular follow-ups every 3-6 mo.
| Case | Sex | Age | Number | Size (mm) | The depth of invasion | Lymph node metastasis | Histological stage | Treatment | Complicated with NF-1 |
| Kato et al[18] | M | 61 | 52 | 1-6 | SM | NA | NA | NA | No |
| Maruyama et al[19] | M | 52 | 5 | 4-10 | M3 | No | NA | AR | No |
| Okamoto et al[20] | M | 54 | 4 | < 6 | SM | NA | NA | ESMR-L | No |
| Haraguchi et al[21] | M | 69 | 30 | < 10 | SM | Yes | NA | APR | No |
| Sasou et al[22] | M | 51 | 7 | < 8 | SM | Yes | G1 | APR | No |
| M | 58 | 3 | < 7 | M3 | Yes | G2 | AR | No | |
| Zhou et al[23] | M | 47 | 3 | 5-8 | SM | No | G1 | TEM | No |
| Park et al[24] | M | 52 | 2 | 4 | SM | No | G1 | ESMR-L | No |
| M | 32 | 3 | 5-7 | SM | No | G1 | ESMR-L | No | |
| F | 65 | 3 | 5-7 | SM | No | NA | EMR | No | |
| M | 62 | 2 | 5 | SM | No | G1 | ESMR-L | No | |
| F | 48 | 2 | NA | SM | No | G1 | ESMR-L | No | |
| Hua et al[25] | F | 61 | 12 | 3-10 | SM | No | G1 | TEM | No |
| Ghassemi et al[26] | F | 53 | 6 | 2-3 | SM | No | G1 | NA | Yes |
In addition to rectal neuroendocrine tumors, the patient also presented with malformations of the external iliac veins and the spinal column. A pelvic MRI revealed segmental thickening of the right external iliac vein, which was nearly double the normal diameter and showed a sausage-like appearance. However, there was no obvious stenosis below the venous expansion, suggesting a congenital deformity instead of a compensatory increase caused by stenosis. Neurofibromatosis is associated with a one to three percent risk of vascular lesions. The lesions can affect varying sizes of blood vessels and present as stenosis, occlusions, hemorrhages, aneurysms, arteriovenous malformations and arteriovenous fistulas. The renal artery is the most vulnerable blood vessel, followed by the superior mesenteric artery, intracranial artery, cardiovascular, etc[29]. NF-1 patients often present with hypertension or arteriovenous malformations and bleeding prior to definitive diagnosis. To our knowledge, this is the first case of NF-1 that is complicated with abdominal iliac vein malformation. Previous studies have suggested that vascular dysplasia may be associated with mutations in the NF-1 gene, which may lead to dysregulated vascular development in the mesoderm. Concentric growth, rupture of elastic fibers, and nodule hyperplasia occur in the intima of blood vessels. The reduction of smooth muscle, decrease in elastic components in the media, and increase in brittleness of the vessel wall are all observed, ultimately leading to thinning of the blood vessel wall, poor elasticity, and formation of a large number of sinus cavities in the diseased tissue, which can cause bleeding[29,30]. Currently, the preferred treatment for vascular malformations includes symptomatic treatment, surgical resection or other surgical interventions. However, no clinical symptoms can be observed in this patient, and follow-up observations can be continued. Conversely, both the patient and her daughter were diagnosed with scoliosis during childhood, which supported the possibility of heredity. Scoliosis is a common clinical manifestation of NF-1, with 10%-33% of children simultaneously diagnosed with NF-1 and scoliosis [31]. The incidence of scoliosis in adult patients with NF-1 has been reported to be between 10%-77%[32]. The pathogenesis of NF-1-associated spinal deformity is not yet clear and may encompass several factors, including the direct erosion of neurofibroma, dural dilatation of the spinal canal, osteoporosis, precocious puberty, and mesoderm dysplasia[31,32]. Patients with scoliosis may also develop lung damage as time progresses. Surgical correction of NF-1-related spinal deformities may improve the clinical curative effect.
In summary, this case suggests that an NF-1 diagnosis may be complicated by multiple system diseases. The clinical symptoms are complex, non-specific, and not easily identified. We thus need to develop individualized treatment based on the different symptoms of NF-1 patients. Although surgical and symptomatic treatments are currently preferred for multiple rectal neuroendocrine tumors, patients often require multi-system and multi-disciplinary comprehensive treatment. It is necessary to formulate the most appropriate intervention based on individual complications, with the comprehensive application of various technologies and inspection methods, in order to reduce the psychological burden on patients and improve overall quality of life.
A 39 year old woman was admitted to our department because of intermittent bloody stools. The diagnosis was confirmed to be neurofibromatosis type I(NF-1) with multiple rectal neuroendocrine neoplasms, vascular malformations and scoliosis.
A female woman had a primary symptom of intermittent hematochezia without vomiting, abdominal pain, diarrhea, skin flushes, etc.
There were three different diagnoses considered: hemorrhoids, rectal polyps and colorectal cancer.
Blood examination showed the hemoglobin levels of the patient was 101 g/L, without other abnormalities such as liver and renal function, tumor markers, etc.
Computed tomography and magnetic resonance imaging of the chest revealed enlarged mediastinal lymph nodes, dermatologic nodules with long T1 and T2 values, uniform density, clear boundaries and diameters of < 10 mm. A pelvic MRI detected segmental thickening of the right external iliac vein. The middle and lower rectal mucosae were irregularly thickened, with 26.5 mm at the widest point and an irregular signal with long T1 and slightly longer T2 values.
Pathohistological and immunohistochemical examinations showed that neuroendocrine tumor cells were present in the lesions and mutually linked to form cord, nest, or gland-like structures. The tumor cells were round, oval or columnar, of varying sizes, with round nuclei, and without obvious mitosis. Cells were CD117 (-), CD56 (+), CK (+), CgA (+), Syn (+), and TTF-1 (-), with a Ki-67 index of < 2%. The subdermal nerve fibers were in a disordered arrangement, and the cells were elongated, spindle-shaped and oddly distributed in the light-stained collagen matrix. Immune staining revealed CD34 (+) and S-100 (+) expression, deep and S-shaped nuclei, and scattered mast cells.
Surgical intervention was advised, however the patient rejected surgery and favored surveillance by regular follow-ups every 3-6 mo.
Neuroendocrine tumors are commonly found in the duodenum and pancreas, and rare cases of NF-1-associated multiple rectal neuroendocrine tumors have been reported. We have summarized the relevant literature in the past 20 years and found that only one case, combined with NF-1 in 14 cases, reports of rectal multiple neuroendocrine tumors. In addition, this is the first case where NF-1 is complicated by abdominal iliac vein malformation.
Rectal neuroendocrine neoplasms (NENs) are often derived from peptidergic neurons and neuroendocrine cells of the rectal mucosal epithelium, and are often divided into functional and non-functional types. Non-functional NENs have no specific clinical symptoms. Imaging, endoscopic ultrasound and biopsy are used as the main diagnostic methods for non-functional NENs.
NF-1 diagnosis may be complicated by multiple system diseases. The clinical symptoms are complex, non-specific, and not easily identified. We need to develop individualized treatment based on the different symptoms of NF-1 patients. Although surgical and symptomatic treatments are currently preferred for multiple rectal neuroendocrine tumors, patients often require multi-system and multi-disciplinary comprehensive treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology andhepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Serban ED S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
| 1. | Huffman JL, Gahtan V, Bowers VD, Mills JL. Neurofibromatosis and arterial aneurysms. Am Surg. 1996;62:311-314. [PubMed] [Cited in This Article: ] |
| 2. | Ilgit ET, Vural M, Oguz A, Ozdogan ME. Peripheral arterial involvement in neurofibromatosis type 1--a case report. Angiology. 1999;50:955-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | O’Connell P, Leach RJ, Ledbetter DH, Cawthon RM, Culver M, Eldridge JR, Frej AK, Holm TR, Wolff E, Thayer MJ. Fine structure DNA mapping studies of the chromosomal region harboring the genetic defect in neurofibromatosis type I. Am J Hum Genet. 1989;44:51-57. [PubMed] [Cited in This Article: ] |
| 4. | Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123:124-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 392] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 5. | Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1-14; quiz 15-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 274] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23:3061-3071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 250] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Gottfried ON, Viskochil DH, Fults DW, Couldwell WT. Molecular, genetic, and cellular pathogenesis of neurofibromas and surgical implications. Neurosurgery. 2006;58:1-16; discussion 1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Pasmant E, Vidaud M, Vidaud D, Wolkenstein P. Neurofibromatosis type 1: from genotype to phenotype. J Med Genet. 2012;49:483-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Mao C, Shah A, Hanson DJ, Howard JM. Von Recklinghausen’s disease associated with duodenal somatostatinoma: contrast of duodenal versus pancreatic somatostatinomas. J Surg Oncol. 1995;59:67-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 93] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Clements WM, Martin SP, Stemmerman G, Lowy AM. Ampullary carcinoid tumors: rationale for an aggressive surgical approach. J Gastrointest Surg. 2003;7:773-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Klein A, Clemens J, Cameron J. Periampullary neoplasms in von Recklinghausen’s disease. Surgery. 1989;106:815-819. [PubMed] [Cited in This Article: ] |
| 12. | Relles D, Baek J, Witkiewicz A, Yeo CJ. Periampullary and duodenal neoplasms in neurofibromatosis type 1: two cases and an updated 20-year review of the literature yielding 76 cases. J Gastrointest Surg. 2010;14:1052-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1131] [Cited by in F6Publishing: 1117] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 14. | Mandair D, Caplin ME. Colonic and rectal NET’s. Best Pract Res Clin Gastroenterol. 2012;26:775-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Rosner M, Hanneder M, Siegel N, Valli A, Fuchs C, Hengstschläger M. The mTOR pathway and its role in human genetic diseases. Mutat Res. 2008;659:284-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Blonski WC, Reddy KR, Shaked A, Siegelman E, Metz DC. Liver transplantation for metastatic neuroendocrine tumor: a case report and review of the literature. World J Gastroenterol. 2005;11:7676-7683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Ning L, Chen H, Kunnimalaiyaan M. Focal adhesion kinase, a downstream mediator of Raf-1 signaling, suppresses cellular adhesion, migration, and neuroendocrine markers in BON carcinoid cells. Mol Cancer Res. 2010;8:775-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Kato M, Yonemura Y, Sugiyama K, Hashimoto T, Shima Y, Miyazaki I, Sugiura H, Kurumaya H, Hoso M, Yao T. [Multiple rectal carcinoids--with special reference to the histogenesis of these lesions]. Gan No Rinsho. 1986;32:1894-1900. [PubMed] [Cited in This Article: ] |
| 19. | Maruyama M, Fukayama M, Koike M. A case of multiple carcinoid tumors of the rectum with extraglandular endocrine cell proliferation. Cancer. 1988;61:131-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 20. | Okamoto Y, Fujii M, Tateiwa S, Sakai T, Ochi F, Sugano M, Oshiro K, Masai K, Okabayashi Y. Treatment of multiple rectal carcinoids by endoscopic mucosal resection using a device for esophageal variceal ligation. Endoscopy. 2004;36:469-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Haraguchi M, Kinoshita H, Koori M, Tsuneoka N, Kosaka T, Ito Y, Furui J, Kanematsu T. Multiple rectal carcinoids with diffuse ganglioneuromatosis. World J Surg Oncol. 2007;5:19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Sasou S, Suto T, Satoh T, Tamura G, Kudara N. Multiple carcinoid tumors of the rectum: report of two cases suggesting the origin of carcinoid tumors. Pathol Int. 2012;62:699-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Zhou JL, Lin GL, Zhao DC, Zhong GX, Qiu HZ. Resection of multiple rectal carcinoids with transanal endoscopic microsurgery: case report. World J Gastroenterol. 2015;21:2220-2224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Park CS, Lee SH, Kim SB, Kim KO, Jang BI. Multiple rectal neuroendocrine tumors: report of five cases. Korean J Gastroenterol. 2014;64:103-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Bai H, Meng F, Yang X. One case of multiple rectal carcinoids and literature review. Zhongguo Yiyao Daokan. 2016;18:1282-1283. [Cited in This Article: ] |
| 26. | Ghassemi KA, Ou H, Roth BE. Multiple rectal carcinoids in a patient with neurofibromatosis. Gastrointest Endosc. 2010;71:216-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, Plöckinger U, Papotti M, Salazar R, Pascher A; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 28. | Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H; Japanese Society for Cancer of the Colon and Rectum. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut. 2007;56:863-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Serranito-García R, Gelabert M, García-Allut A. [Aneurysm of anterior communicating artery associated with type 1 neurofibromatosis]. Neurologia. 2007;22:547-550. [PubMed] [Cited in This Article: ] |
| 30. | Deans WR, Bloch S, Leibrock L, Berman BM, Skultety FM. Arteriovenous fistula in patients with neurofibromatosis. Radiology. 1982;144:103-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 69] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Abdel-Wanis ME, Kawahara N. Aetiology of spinal deformities in neurofibromatosis 1: new hypotheses. Med Hypotheses. 2001;56:400-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Tsirikos AI, Saifuddin A, Noordeen MH. Spinal deformity in neurofibromatosis type-1: diagnosis and treatment. Eur Spine J. 2005;14:427-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |