Published online Sep 7, 2018. doi: 10.3748/wjg.v24.i33.3749
Peer-review started: March 1, 2018
First decision: April 18, 2018
Revised: June 14, 2018
Accepted: June 16, 2018
Article in press: June 16, 2018
Published online: September 7, 2018
To establish cell line and patient-derived xenograft (PDX) models for neuroendocrine carcinomas (NEC) which is highly desirable for gaining insight into tumor development as well as preclinical research including biomarker testing and drug response prediction.
Cell line establishment was conducted from direct in vitro culturing of colonic NEC tissue (HROC57). A PDX could also successfully be established from vitally frozen tumor samples. Morphological features, invasive and migratory behavior of the HROC57 cells as well as expression of neuroendocrine markers were vastly analyzed. Phenotypic analysis was done by microscopy and multicolor flow cytometry. The extensive molecular-pathological profiling included mutation analysis, assessment of chromosomal and microsatellite instability; and in addition, fingerprinting (i.e., STR analysis) was performed from the cell line in direct comparison to primary patient-derived tissues and the PDX model established. Drug responsiveness was examined for a panel of chemotherapeutics in clinical use for the treatment of solid cancers.
The established cell line HROC57 showed distinct morphological and molecular features of a poorly differentiated large-cell NEC with KI-67 > 50%. Molecular-pathological analysis revealed a CpG island promoter methylation positive cell line with microsatellite instability being absent. The following mutation profile was observed: KRAS (wt), BRAF (mut). A high sensitivity to etoposide, cisplatin and 5-FU could be demonstrated while it was more resistant towards rapamycin.
We successfully established and characterized a novel patient-derived NEC cell line in parallel to a PDX model as a useful tool for further analysis of the biological characteristics and for development of novel diagnostic and therapeutic options for NEC.
Core tip: Since incidence of G3 poorly differentiated neuroendocrine carcinomas (NEC) is very low, data is substantially scarcer than on G1 or G2 neuroendocrine tumors. Herein we describe an ultra-low passage NEC cell line and corresponding patient-derived xenograft model established directly from patient derived colonic tumor samples. We characterized our model according to phenotype, molecular, morphological and growth characteristics, as well as drug response and radiation response profiles. We present a useful tool for further analysis of the biological characteristics and for development of novel diagnostic and therapeutic options for NEC. The model is available on request.
- Citation: Gock M, Mullins CS, Harnack C, Prall F, Ramer R, Göder A, Krämer OH, Klar E, Linnebacher M. Establishment, functional and genetic characterization of a colon derived large cell neuroendocrine carcinoma cell line. World J Gastroenterol 2018; 24(33): 3749-3759
- URL: https://www.wjgnet.com/1007-9327/full/v24/i33/3749.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i33.3749
Neuroendocrine tumors (NET) are a heterogeneous group of tumors arising from cells which are derived from the embryonic neural crest, neuroectoderm, and endoderm[1]. The age-adjusted incidence of all NETs increased from 1.9 to 5.25 cases per 100000 people[2] which is probably due to improvements in surveillance and diagnostic endoscopy[3]. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NEN) are a subgroup of NET that derive from neuroectodermal cells, such as the enterochromaffin-like cells of the intestine[4]. These tumors can produce a multitude of different markers and peptide hormones; i.e., neuron-specific enolase, synaptophysin and chromogranin A[5,6]. Based on the actual revised 2010 WHO classification, NEN are classified according to their differentiation and proliferation. In addition, the European Neuroendocrine Tumor Society has proposed a tumor-node-metastasis staging and grading system for various types of GEP-NET[7]. Well-differentiated NEN are classified together as NET G1 or G2 (G1: < 2 mitoses/10 high power fields; Ki-67 index ≤ 2%, G2: 2-20 mitoses/10 high power fields; Ki-67 index 3%-20%)[8,9]. While NET G1 can be equated with carcinoid, all poorly differentiated G3 NEN are termed neuroendocrine carcinoma (NEC). This NEC group is further subdivided into a small-cell and a large-cell variant. Regarding their proliferation activity, all NEC are poorly differentiated actively proliferating G3 tumors (NEC; G3: > 20 mitoses/10 high power fields; Ki-67 index > 20%)[7,8]. Recently, a proportion of NET could be identified presenting a high proliferation with either mitoses or Ki-67 index cutoff above 20% and most important a well-differentiated morphology. This novel category was called well-differentiated grade 3 NET (NET G-3)[10].
In local or locoregional disease all patients should be considered for curative resection of the primary tumor and locoregional lymph nodes according to actual guidelines[11]. In advanced or metastatic disease, cytoreductive surgery should be considered when metastatic disease is localized or if > 70% of tumor load is thought to be resectable[11]. In metastatic disease involving high-grade G3 NEC combination chemotherapy consisting of cisplatinum and etoposide is suggested[12]. Nonetheless response rates to chemotherapy are low and there is no established second line therapy[11].
For further research on the biological characteristics of these tumors, for development and preclinical testing of new treatment modalities and potential molecular therapeutical targets, establishment of new in vitro and in vivo models is mandatory. In the last decades, only a very few GEP-NEN cell lines have been established and characterized[13] but pathological terminology is very heterogeneous especially regarding the actual revised 2010 WHO classification[13]. For colorectal adenocarcinoma, many patient-individual tumor models, which have been generated in the last decade by us and others[14-16], have proven extremely helpful in deciphering colorectal cancers’ molecular heterogeneity[16] and in the identification of novel druggable targets and combinatorial treatment strategies[17,18].
In this study, we describe the establishment and functional characterization of a novel NEC colon derived cell line with corresponding patient-derived xenograft (PDX) model. A broad analysis of tumor biology, genetic alterations and assessment of chemosensitivity towards an extensive range of chemotherapeutic drugs and of radiosensitivity has been performed. Considering these aspects, such characterized matched in vitro and in vivo tumor models represent excellent tools for further development of individual therapy regimens and are a valuable tool to gain additional insight in the tumor biology of NEC.
Primary NEC resection specimens of HROC57 were received fresh from surgery, with informed written patient consent. All procedures were approved by the Ethics Committee of the University of Rostock (reference number II HV 43/2004) in accordance with generally accepted guidelines for the use of human material. Tumor samples were cut into small pieces. Parts of the tumor were immediately frozen in freezing medium [foetal calf serum (FCS) containing 10% DMSO] at -80 °C for subsequent xenografting. Other pieces were frozen in liquid nitrogen for molecular analysis. Cell line establishment protocol was adapted according to Maletzki et al[19], 2012.
For in vivo engraftment, six-week-old female NMRI nu/nu mice were used as recipients. Mice were bred in the university’s animal facility and maintained in specified pathogen-free conditions. All surgical interventions were performed under Ketamin/Xylazin anaesthesia (dose: 90/25 mg/kg body weight), and all efforts were made to minimize suffering. Subcutaneous tumour implantation was performed as previously described[19]. Established xenografts (> 1.500 mm3) were removed and underwent in vitro culture protocols as described above. All in vivo experimental procedures were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Rostock (Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg-Vorpommern; Thierfelder Str. 18, Rostock 18059, Germany; permit number: LALLF M-V/TSD/7221.3-1.1-071-10).
Histopathological examination of HE-stained primary tumors and corresponding PDX was done according to standard protocols for clinicopathological tumor staging[20], and additional staging information was compiled from patients’ clinical charts. Supplementary, immunostainings from paraffin-embedded primary tumors were done for cytokeratin 20, cytokeratin MNF116, CEA, synaptophysin and chromogranin.
Molecular classification was done according to Ostwald et al[21]. Mutations in tumor-associated APC, P53, KRAS, and BRAFV600E genes were analyzed as described. DNA-methylation in CIMP-sensitive promoters was traced by the MethyLight technology with a modified marker panel originally published by Ogino et al[22]. The degree of chromosomal instability was assessed using the SNP Array 6.0 from Affymetrix (Cleveland, OH) according to manufacturer’s instructions.
Genomic DNA was isolated from cell lines at different passages, matched tumor and normal tissue, as well as corresponding B cells using Wizard® Genomic DNA Purification Kit provided by Promega (Mannheim, Germany). Highly polymorphic short tandem repeat (STR) DNA marker (CSF1PO, TPOX, THO1, vWA, D16S539, D13S317, D5S818 and D7S820) and the gender marker amelogenin were amplified in standard PCR reactions and analyzed on an ABI Prism 3100 system. PCR primers were based on the original publication[23].
Peripheral blood lymphocytes were purified by Ficoll density-gradient centrifugation. B-lymphoid cell lines (B-LCLs) were generated by Epstein-Barr virus (EBV)-transformation as described before[24]. Outgrowing B-LCL cultures were harvested, expanded, characterized, and frozen.
Doubling time of HROC57 cells was determined from serial passages. Therefore, 5 × 105 viable cells were seeded into 25 cm2 flasks and viable cells (defined by trypan blue exclusion) were daily counted for seven days. Cultures were fed every 3 or 4 d. Ploidy and cell cycle analysis was performed by flow cytometry (FACSCalibur, BD Biosciences, Heidelberg, Germany) using fixed cells (70% ethanol), after RNase A digestion (100 μg/mL; Sigma Aldrich, Munich, Germany) and propidium iodide (10 μg/mL) addition. 10000 events were measured for each sample and cell cycle analysis was done by applying Modfit software (Verity Software House, Topsham, United States). Matched B-LCLs were used as diploid controls.
Cell surface marker expression on NEC line was traced by flow cytometry with and without IFN-γ pre-treatment using a panel of FITC-, PE- or APC-conjugated Abs: CD44, CD56, CD71, CD90, CD326, chromogranin A, synaptophysin, neuron specific enolase, Ki-67, MHC I, MHC II, and HLA-A2 (cell culture supernatant clone BB7.2). For HLA-A2, a secondary FITC-conjugated anti-mouse Ab was applied. Samples were analyzed using CellQuest software (BD Biosciences).
Mycoplasma contamination was tested by the 16S-rRNA-gene-based polymerase chain reaction (PCR) amplification method from whole cell lysates. For determining potential polyomavirus infection (JC/BK and SV40) gDNA was isolated using Wizard genomic DNA purification kit (Promega). All procedures were done as described in[19].
Analysis of tumour cell invasion was performed using a classical Boyden chamber test (8 μm pore size in a 24-well plate format) with Matrigel-coating (BD Biosciences) according to the manufacturer’s instructions. Cells were suspended to yield a cell number of 2 × 105 cells per upper Boyden chamber in 500 μL serum-free medium. Medium in the lower Boyden chamber was supplemented with 10% heat-inactivated FCS serving as chemo-attractant. After a 72 h incubation period, the non-invading cells on the upper surface of the inserts were removed with a cotton swab, and viability of cells on the lower surface was measured by the colorimetric 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,6-benzene disulfonate (WST-1) test (Roche Diagnostics, Mannheim, Germany)[25]. Quantification of migration was performed in parallel using the same protocol but with uncoated upper Boyden chambers.
For chemosensitivity, cells were seeded into 96-well microtiter plates at 5 × 103 or 1 × 104 cells/well. When cells reached 30%-40% confluency, cultures were exposed to increasing concentrations of etoposide, cisplatin, 5-FU, oxaliplatin, irinotecan and rapamycin (pharmacy of the University Hospital Rostock). After three days of exposure, media were removed and replaced by fresh medium supplemented with therapeutics. Following another three days, medium was removed; plates were carefully washed and stained with crystal violet (0.2%, 10 min). Finally, drug effects from triplicate wells were determined at the level of 50% inhibition (IC50) in comparison to control, measured at 570 nm (reference wavelength: 620 nm). For radiosensitivity analysis, cells radiated with 50 Gy using a 137Cs-source were seeded into 96-well microtiter plates in triplicates (1 × 105 cells per well and six serial two-fold dilutions). Control cells were not radiated. After 4 and 7 d, duplicate plates were analyzed for total cell growth using crystal violet as described above.
Western blot was done as previously described[26]. Antibodies specific for the following targets were from Santa Cruz (Heidelberg, Germany): Histone deacetylase (HDAC) 2 (sc-7899), p53 (sc-126) and p21 (sc-6246). Anti-HDAC1 (05-100) was obtained from Millipore (Burlington, MA, United Atates). Anti-HSP90 (ADI-SPA-830) was purchased from Enzo Life Sciences (Farmingdale, NY, United States). Anti-survivin (NB500-201) was obtained from Novus Biologicals (Cambridge, United Kingdom). Anti-acetyl-K382-p53 (ab75754) was from Abcam (Cambridge, United Kingdom), while phospho-S15-p53 (9284) was purchased from Cell Signaling Technology (Cambridge, United Kingdom). Western Blots were detected with the Odyssey Infrared Imaging System (Licor) using IRDye® 680RD- or IRDye® 800CW-coupled secondary antibodies.
Values are reported as the mean ± SD. After proving the assumption of normality, differences were determined by using the unpaired Student’s t-test. If normality failed, the nonparametric Mann-Whitney U-Test was applied. The tests were performed by using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, United States). The criterion for significance was set to P < 0.05.
We report on a 43-year-old patient with a history of weight loss of 12 kg and upper abdominal pain. CT scan showed a tumor of the ascending colon with diffuse liver metastasis and colonoscopy revealed a tumor of 5 cm length with tumor stenosis underneath the right flexure. First tumor biopsies showed an undifferentiated carcinoma. A right hemicolectomy was performed and intraoperatively several liver metastases in both lobes could be confirmed. Macroscopically, the tumor size was 9 cm × 5 cm × 5 cm. Microscopically, a poorly differentiated large cell neuroendocrine carcinoma with deep infiltration of pericolic fatty tissue, lymphatic tract invasion and major lymph node involvement was revealed [UICC G3 pT3 pN2 (16/28) L1 V1 cM1 (HEP)]. The patient was dismissed 8 d after the operation and received several courses of chemotherapy with cisplatin etoposide at last but died one year after first diagnosis due to tumor progression with lung and liver metastases.
The tumor showed a poorly differentiated large cell neuroendocrine cytology and distinct neuroendocrine and epithelial markers stained positive (Table 1).
| Immunohistochemistry | |
| Cytokeratin 20 | Positive |
| Pan cytokeratin MNF116 | Positive |
| Synaptophysin | Positive |
| Chromogranin | Negative |
| CEA | Negative |
In vitro and in vivo approaches were combined as described previously for establishment of a permanent cell line growing 2D[19] and a subcutaneous PDX[27]. Outgrowth of cells in culture occurred immediately and doubling time of the outgrowing cell line was 26.13 h.
Tumor formation in immunodeficient mice could be observed 37 d after the tumor engraftment. Similar to previous findings with adenocarcinomas, histological analysis revealed preserved tumor architecture in the PDX compared to the original patient tumor architecture (data not shown).
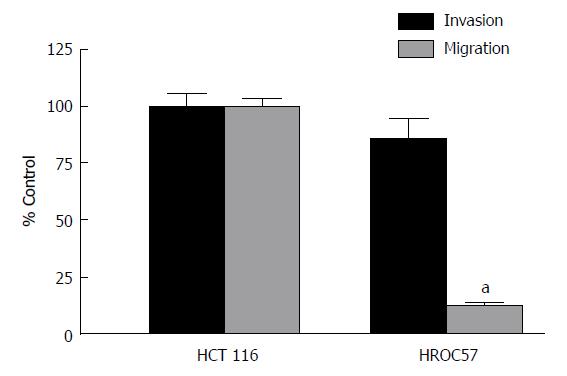
Analysis of invasion and migration revealed a slight non-significant difference in the infiltrative activity of the patient derived HROC57 compared to HCT116, which served as positive control. In contrast, patient-derived HROC57 revealed a significantly lower migratory activity (t-test, P < 0.0001) compared to HCT116 (Figure 1).
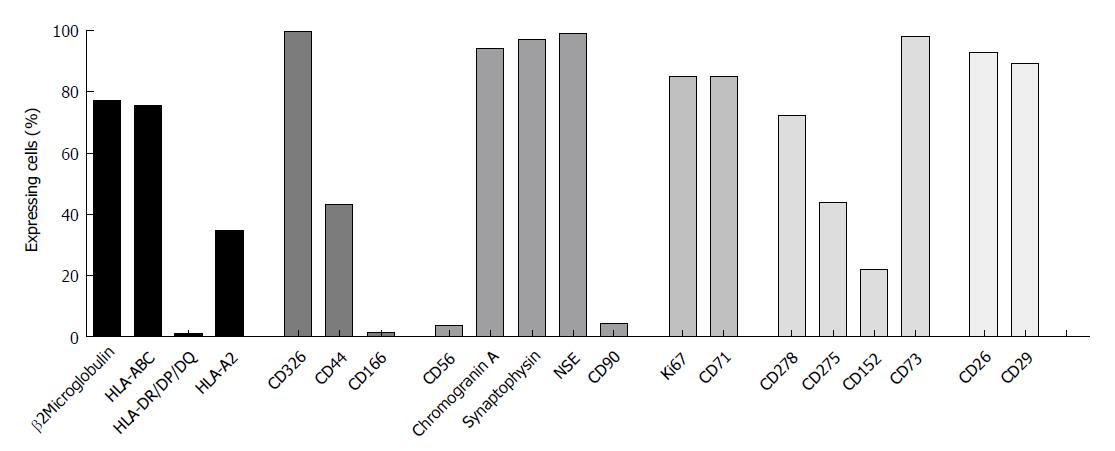
To investigate whether or not HROC57 cells recapitulate a neuroendocrine phenotype, expression of neuroendocrine markers was analyzed by flow cytometry. Chromogranin A, synaptophysin and neuron specific enolase were highly expressed by HROC57, whereas CD56 (neural cell adhesion molecule, NCAM) was only expressed to a very low extend (Figure 2). In addition to neuroendocrine markers, HROC57 expressed several epithelial and so-called stem-cell markers. We found a very high expression of CD326 (EpCAM) reflecting an epithelial origin of the tumor, moderate expression of the cellular migration and adhesion marker CD44 but almost no expression of CD166. CD26 and CD29, which have been described as stem cell and metastasis-promoting surface receptors[28,29], were highly expressed; CD90 only marginally.
Further characterization showed a high expression of the proliferation markers KI-67 and CD71 (Figure 2) reflecting the high proliferative activity of the tumor.
Regarding HLA molecules which play an important role in specific immune recognition and tumor cell defense, expression of HLA class I (ß2M and pan-HLA-ABC) could be observed, but no expression of HLA class II (Figure 2). The latter could also not be reversed by interferon pretreatment (data not shown). HROC57 cells additionally express high levels of CD73, which has been described as immune-evasion molecule[30]. Lower levels were observed for the other immune evasion molecules CD278 (ICOS), CD275 (B7-H2) and CD152 (CTLA-4) (Figure 2).
For further characterization, a comprehensive molecular analysis of the parental tumor and the HROC57 cell line was performed.
The tumor and the cell line showed a distinct degree of aneuploidy, moderate CpG island methylation and absent microsatellite instability. Mutational analysis revealed a wild-type KRAS status and, of note, a mutant BRAF status.
For assessment of larger genomic aberrations, single nucleotide polymorphisms and general gene expression, microarray analyses were performed. While the correspondent HROC57 B cell line showed no distinctive features, the HROC57 tumor cell line showed several aberrations. The tip of chromosome 8p showed losses, whereas gains in 7q and 19p were observed. Thus, HROC57 can be classified as only mildly chromosomal instable.
For further discrimination of HROC57, we performed an RNA expression analysis and compared HROC57 with 23 cell lines from colorectal adenocarcinomas also established in our lab. Within the 20 most over expressed genes, two neuroendocrine markers could readily be identified (CHGB and CPLX2). In addition, three genes involved in tumor immune modulation could be recognized (TRBC1, CTLA4 and LAT). Contrary to that, HROC57 did not express NOX1 and OLFM4, genes known for promoting colorectal adenocarcinoma.
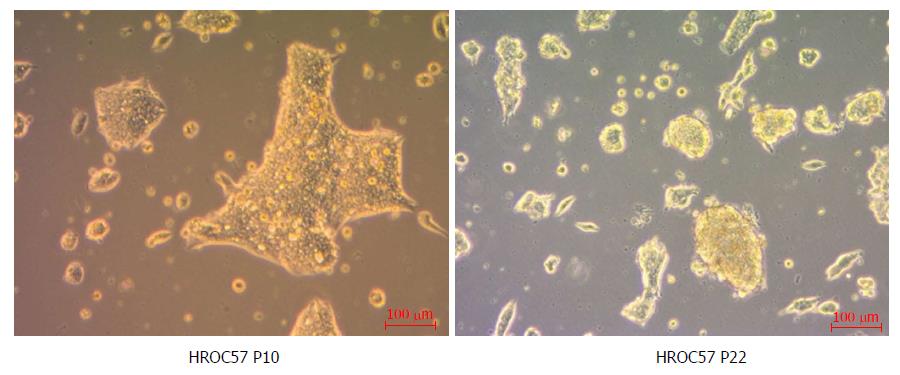
As determined by phase contrast microscopy, cells adhered tightly to the cell culture flask. The cell line was growing as monolayers on conventional tissue culture plastic and showed a stable outgrowth as defined by passaging > 50 times. HROC57 cells proliferated as polygonal cell colonies forming small grape-like cell clusters (Figure 3). Morphology did not change during long term passage (up to 50 passages). As determined by semi-quantitative PCR, HROC57 cell line was free of mycoplasma and several other potential contaminants (JC/BK and SV40) which have been described for colorectal cell lines (data not shown)
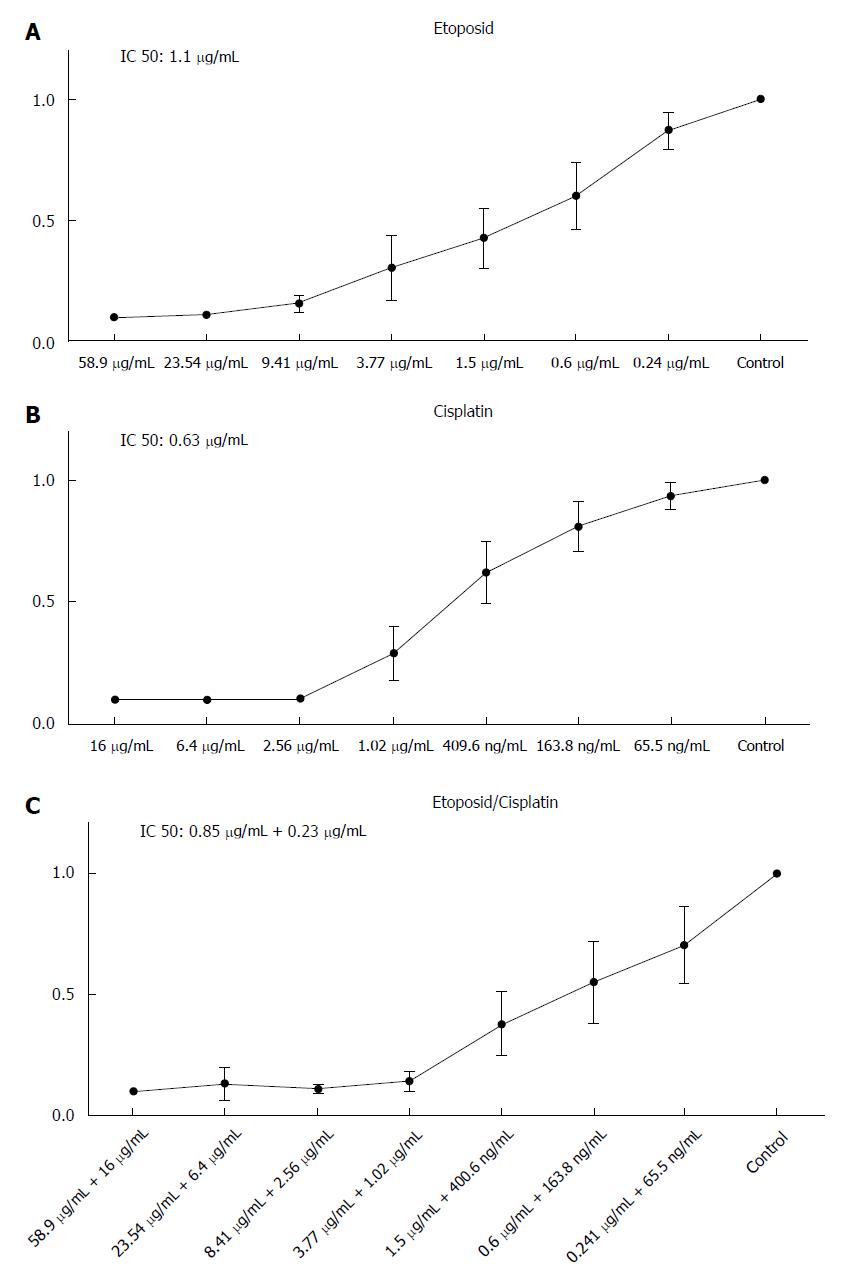
To assess the sensitivity of HROC57 cells to a variety of current chemotherapeutic drugs, standard proliferation and cytotoxicity assays were performed. In particular, sensitivity to etoposide and cisplatin was analyzed as actual guidelines suggest the combination of etoposide and cisplatin as first line chemotherapy for advanced NEC[11].
HROC57 showed a high sensitivity towards etoposide and cisplatin with a distinct increase of sensitivity after combination of cisplatin and etoposide (Figure 4) especially if patient plasma drug levels are set for reference (Table 2). Examination of the sensitivity of HROC57 cells to further chemotherapeutic drugs showed their high sensitivity to 5-FU, oxaliplatin, irinotecan, the histone deacetylase inhibitor (HDACi) suberoylanilide hydroxamic acid (SAHA), the mTOR inhibitor rapamycin and the drug combinations Folfox and Folfiri (Table 2). Again, when patient plasma levels are set for reference, measured IC50 concentrations were below those plasma concentrations (Table 2).
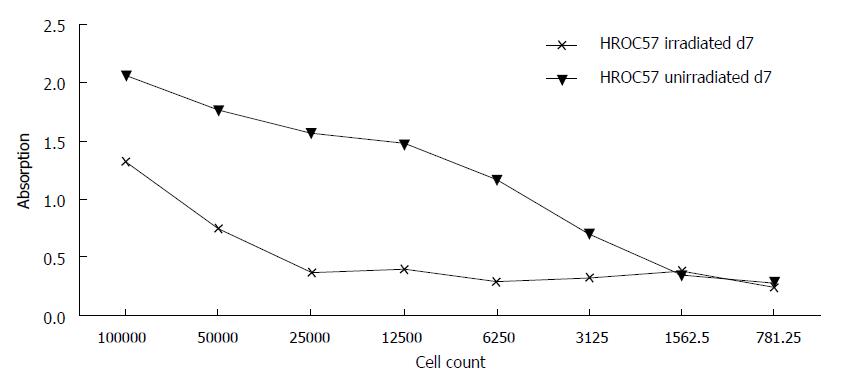
HROC57 cells were not completely resistant towards γ-radiation but even a radiation with 50Gy did not completely abolish further growth of the cell line (Figure 5). In comparison to several other patient-derived HROC cell lines established in our lab, they can be ranked as intermediate radiation sensitive (data not shown).
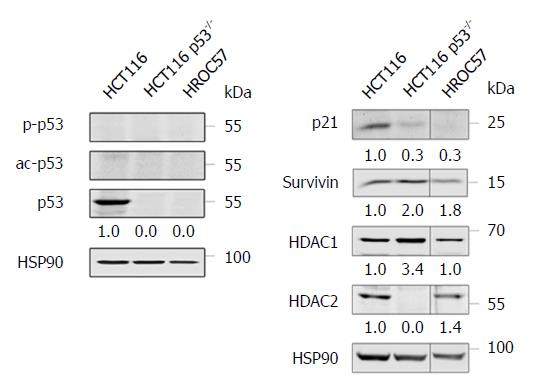
Remarkably, the sensitivity of HROC57 cells to chemotherapeutics is independent from the presence of wild-type p53 (Figure 6), since HROC57 cells (and p53-negative HCT116 cells used as controls) lack p53 protein expression as analyzed by Western-blot (Figure 4A). P53-negative tumor cells typically express less of the p53-activated factor p21 and more of the p53-repressed factor survivin[31] - as can easily be depicted in direct comparison to p53-positive HCT116 cells (Figure 4B). Moreover, HDAC1 and HDAC2 are both expressed by HROC57 cells; and this correlates with their sensitivity to SAHA (Table 2).
Currently, only a small number of GEP-NEN cell lines have been established and most of them are insufficiently characterized, especially with regard to the latest WHO classification and its proliferation based grading system[13].
Here, we report on the first characterization of a poorly differentiated large cell NEC that could be established directly from a primary tumor in the ascending colon. HROC57 cells showed a typical neuroendocrine cytology and a profile of neuro-endocrine markers that are commonly used for diagnosis of neuroendocrine tumors. Notably, flow cytometry revealed high expression of chromogranin A, synaptophysin and neuron specific enolase, which are common markers of neuroendocrine tumors. Only CD56 was expressed to a very low extend. Reflecting the high proliferative activity of the tumor cells, the proliferation markers Ki-67 and CD71 were highly expressed. Gene expression analysis unveiled the upregulation of CPLX2, which was recently identified as a new prognostic biomarker in human lung high grade neuroendocrine tumors[32] and thus might be worth being tested as a prognostic biomarker in GEP-NET as well.
As therapeutical interference with tumor immune escape mechanisms plays a more and more important role in clinical oncologic, we analyzed several key molecules of immune escape. HROC57 cells express high levels of CD73, which has been described as immune-evasion molecule and overexpression was observed in various tumor tissues[30]. CD73 functions as a rate-limiting enzyme in the generation of extracellular adenosine[30] and recent studies could show that increased adenosine levels result in immune tolerance by accumulating in the tumor environment and contribute to a cancer growth promoting environment. Adenosine not only functions in the tumor microenvironment, but is also involved in the regulation of proliferation, differentiation and apoptosis of cancer cells[33]. Additionally, we could demonstrate an upregulation of CTLA-4 on HROC57 cells. CTLA-4 is an immunoglobulin superfamily receptor and was the first immune-checkpoint receptor that was clinically targeted using blocking antibodies[34]. PD-1 is another member of this superfamily. This finding might open new therapeutical opportunities for NET/NEC. Tumor models like HROC57 could also help to develop immune checkpoint inhibiting strategies and subsequently optimize therapeutical applications.
In addition to the neuroendocrine markers, HROC57 cells highly express EpCAM (CD326), thus reflecting a possible epithelial, rather than neural crest provenance as was thought of these tumors[35]. Khan et al[36] analyzed EpCAM expression in several neuroendocrine tumors and found high expression of EpCAM in all analyzed midgut NET (ileal, pancreatic and gastric) but no colonic NET were included in this study[36]. Hence, this is the first report of high EpCAM expression in a colon-derived NEC. EpCAM plays an important role in promoting cell cycling and proliferation by upregulating c-myc and cyclins[37]. Therefore, EpCAM directed therapy might be an additional option in this tumor entity[38]. Moreover, expression of EpCAM in NET/NEC opens up opportunities to be further developed as biomarker and relapse prediction by screening for circulating tumor cells[39].
Molecular analysis of HROC57 revealed mild chromosomal instability with several chromosomal gains and losses. In particular, gains in 7q und 19p and a prominent loss in the tip of 8q were found. Little is known of the prevalence of these events in colonic NEC. Krieg et al[13] also described gains in 7q but not in 19p in their lymph node metastasis-derived NEC cell line NEC-DUE2, while gains in 19p are reported up to 50% in small intestine NEN[40]. Interestingly, we could not observe the reported deletions of 18q that are characteristic of midgut NEN[41]. Mutational analysis revealed wild-type KRAS but a mutant BRAF status. Karkouche and coworkers analyzed these mutations in a consecutive series of 12 colonic NECs[42]. They demonstrated a KRAS/BRAF mutation in six out of twelve tumors. They concluded that these KRAS/BRAF mutations might indicate that colorectal NEC may correspond to a high-grade transformation of colorectal carcinoma[42].
Generally, gastrointestinal NEC shows an aggressive and chemoresistant phenotype that often results in a rapid progressive course of disease[11]. A basic requirement for determining initial drug sensitivity and for predicting response is the maintenance of the original tumors’ signature[14]. With this principal condition fulfilled by the freshly established HROC57 cell line, the sensitivity towards a large panel of chemotherapeutic drugs was tested. Etoposide and cisplatin were included as actual guidelines suggest this combination as first line chemotherapy for advanced NEC[11]. Of note, HROC57 cells showed high sensitivity towards etoposide and cisplatin alone and even a distinct increase in sensitivity when both drugs were combined. While this sensitivity does not require the expression and activity of wild-type p53, the poor radiation sensitivity may be attributable to the lack of wild-type p53[43]. We should though mention that the ex vivo sensitivity of HROC57 cells does not recapitulate the clinical course of the patient who died within one year after diagnosis and operation due to rapid progressive disease. This discrepancy can best be explained by the fact that this potentially beneficial chemotherapeutic regimen was only applied in a situation with vast tumor recurrence, diffuse peritoneal and lung metastases and could not prevent the death of the patient one month later.
Examination of further chemotherapeutic drugs showed interestingly a likewise high sensitivity to 5-FU, SAHA, oxaliplatin, irinotecan, rapamycin and the drug combinations Folfox and Folfiri. This finding is in contrast to Krieg et al[13] who reported a high chemoresistance of their colonic NEC-cell line. A detailed analysis of these differences in chemosensitivity is recommended in the near future in order to identify similarities and disparities between primary and metastatic NEC cells.
At last, analysis of radiosensitivity revealed that HROC57 cells were relatively resistant towards γ-radiation but even a radiation with 50 Gy did not completely abolish further growth of the cell line. This observation is well in line with actual treatment algorithms of NEC which no longer recommend radiotherapy[11].
Our data emphasize the use of the HROC57 models for further basic and preclinical research to gain insight into the tumor biology of large cell neuroendocrine carcinoma, to develop and to optimize therapy regimens or to identify novel biomarkers. Albeit not emphasized in this manuscript, a non-tumorous B cell line of HROC57 is available beside the tumor models. Thus, comparative analyses of NET cells and matching normal cells are easily possible.
Only a few gastrointestinal neuroendocrine tumor lines have been published in the last decades. A major problem is the very heterogeneous pathological terminology when trying to classify these cell lines according to the lately revised WHO classification with regard to their original tumors. Particularly, reports of poorly differentiated neuroendocrine carcinoma (NEC) are very scarce mainly due to their low incidence.
Gaining insights in the biology of large cell NEC is essential for the identification of potentially therapeutic molecular targets of this highly malignant neoplasia. Individual tumor models deliver exceptional tools for further research of these objectives. However, well characterized and low passage NEC models are still rare.
Main objective of the study was the establishing and profound characterization of an patient derived ultra-low passage NEC cell line and corresponding patient-derived xenograft (PDX) model that allows drug response testing and prediction.
Cell line establishment could be realized from direct in vitro culturing of colonic NEC tissue. In addition, a PDX model could be established from frozen tumor samples. Profound analysis of morphological features, invasive and migratory behavior as well as expression of neuroendocrine markers was done. Detailed phenotypic analysis was performed by microscopy and multicolor flow cytometry. Chromosomal aberrations were mapped by array comparative genomic hybridization and DNA profiling was analyzed by DNA fingerprinting. At last drug responsiveness was evaluated and the sensitivity against chemotherapeutic agents assessed.
The cell line displayed characteristic morphological and molecular features of large cell NEC with KI-67 > 50%. In vitro and in vivo experiments demonstrated that the cell line retained their malignant properties. Molecular-pathological analysis revealed a CpG island promoter methylation positive cell line with microsatellite instability being absent. The KRAS gene was not mutated whereas a BRAF V600E mutation was detected. A high sensitivity to such drugs as etoposide, cisplatin and 5-FU could be observed with a more resistant phenotype to rapamycin.
Taken together, this study describes the development and basic characterization of powerful matched in vitro and in vivo patient-derived models not only to perform basic research to better understand the biology of NECs, but also to establish novel therapeutic options.
This descriptive study exemplifies the methodology and characterization of a large cell NEC cell line directly from original patients’ tumor material. This will help to improve the ability for personalizing tumor therapy in the near future.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Adachi Y S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Van Buren G 2nd, Rashid A, Yang AD, Abdalla EK, Gray MJ, Liu W, Somcio R, Fan F, Camp ER, Yao JC, Ellis LM. The development and characterization of a human midgut carcinoid cell line. Clin Cancer Res. 2007;13:4704-4712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3022] [Cited by in F6Publishing: 3036] [Article Influence: 189.8] [Reference Citation Analysis (0)] |
| 3. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1131] [Cited by in F6Publishing: 1117] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 4. | Schnirer II, Yao JC, Ajani JA. Carcinoid--a comprehensive review. Acta Oncol. 2003;42:672-692. [PubMed] [Cited in This Article: ] |
| 5. | Wiedenmann B, John M, Ahnert-Hilger G, Riecken EO. Molecular and cell biological aspects of neuroendocrine tumors of the gastroenteropancreatic system. J Mol Med (Berl). 1998;76:637-647. [PubMed] [Cited in This Article: ] |
| 6. | Bishop AE, Polak JM, Facer P, Ferri GL, Marangos PJ, Pearse AG. Neuron specific enolase: a common marker for the endocrine cells and innervation of the gut and pancreas. Gastroenterology. 1982;83:902-915. [PubMed] [Cited in This Article: ] |
| 7. | Schott M, Klöppel G, Raffel A, Saleh A, Knoefel WT, Scherbaum WA. Neuroendocrine neoplasms of the gastrointestinal tract. Dtsch Arztebl Int. 2011;108:305-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Bosman FT, Caneiro F. WHO classification of tumours of the digestive system. Lyon: IARC Press 2010; . [Cited in This Article: ] |
| 9. | Klöppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, Scarpa A, Scoazec JY, Wiedenmann B, Papotti M. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90:162-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 10. | Öberg K, Knigge U, Kwekkeboom D, Perren A; ESMO Guidelines Working Group. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii124-vii130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 351] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 11. | Coriat R, Walter T, Terris B, Couvelard A, Ruszniewski P. Gastroenteropancreatic Well-Differentiated Grade 3 Neuroendocrine Tumors: Review and Position Statement. Oncologist. 2016;21:1191-1199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Fazio N, Spada F, Giovannini M. Chemotherapy in gastroenteropancreatic (GEP) neuroendocrine carcinomas (NEC): a critical view. Cancer Treat Rev. 2013;39:270-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Krieg A, Mersch S, Boeck I, Dizdar L, Weihe E, Hilal Z, Krausch M, Möhlendick B, Topp SA, Piekorz RP. New model for gastroenteropancreatic large-cell neuroendocrine carcinoma: establishment of two clinically relevant cell lines. PLoS One. 2014;9:e88713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Maletzki C, Gock M, Randow M, Klar E, Huehns M, Prall F, Linnebacher M. Establishment and characterization of cell lines from chromosomal instable colorectal cancer. World J Gastroenterol. 2015;21:164-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 22] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 15. | Kuehn F, Mullins CS, Krohn M, Harnack C, Ramer R, Krämer OH, Klar E, Huehns M, Linnebacher M. Establishment and characterization of HROC69 - a Crohn´s related colonic carcinoma cell line and its matched patient-derived xenograft. Sci Rep. 2016;6:24671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1313] [Cited by in F6Publishing: 1487] [Article Influence: 185.9] [Reference Citation Analysis (0)] |
| 17. | Wiegering A, Matthes N, Mühling B, Koospal M, Quenzer A, Peter S, Germer CT, Linnebacher M, Otto C. Reactivating p53 and Inducing Tumor Apoptosis (RITA) Enhances the Response of RITA-Sensitive Colorectal Cancer Cells to Chemotherapeutic Agents 5-Fluorouracil and Oxaliplatin. Neoplasia. 2017;19:301-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Medico E, Russo M, Picco G, Cancelliere C, Valtorta E, Corti G, Buscarino M, Isella C, Lamba S, Martinoglio B. The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets. Nat Commun. 2015;6:7002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 19. | Maletzki C, Stier S, Gruenert U, Gock M, Ostwald C, Prall F, Linnebacher M. Establishment, characterization and chemosensitivity of three mismatch repair deficient cell lines from sporadic and inherited colorectal carcinomas. PLoS One. 2012;7:e52485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Fielding LP, Arsenault PA, Chapuis PH, Dent O, Gathright B, Hardcastle JD, Hermanek P, Jass JR, Newland RC. Clinicopathological staging for colorectal cancer: an International Documentation System (IDS) and an International Comprehensive Anatomical Terminology (ICAT). J Gastroenterol Hepatol. 1991;6:325-344. [PubMed] [Cited in This Article: ] |
| 21. | Ostwald C, Linnebacher M, Weirich V, Prall F. Chromosomally and microsatellite stable colorectal carcinomas without the CpG island methylator phenotype in a molecular classification. Int J Oncol. 2009;35:321-327. [PubMed] [Cited in This Article: ] |
| 22. | Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, Giovannucci EL, Fuchs CS. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 591] [Cited by in F6Publishing: 611] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 23. | Goodenough DJ. The use of ROC curves in testing the proficiency of individuals in classifying pneumoconiosis. Radiology. 1975;114:472-473. [PubMed] [Cited in This Article: ] |
| 24. | Klier U, Maletzki C, Klar E, Linnebacher M. Generation of highly pure fusions of colorectal carcinoma and antigen-presenting cells. Langenbecks Arch Surg. 2010;395:365-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Ramer R, Hinz B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J Natl Cancer Inst. 2008;100:59-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Beyer M, Kiweler N, Mahboobi S, Krämer OH. How to Distinguish Between the Activity of HDAC1-3 and HDAC6 with Western Blot. Methods Mol Biol. 2017;1510:355-364. [PubMed] [Cited in This Article: ] |
| 27. | Gock M, Kühn F, Mullins CS, Krohn M, Prall F, Klar E, Linnebacher M. Tumor Take Rate Optimization for Colorectal Carcinoma Patient-Derived Xenograft Models. Biomed Res Int. 2016;2016:1715053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Davies S, Beckenkamp A, Buffon A. CD26 a cancer stem cell marker and therapeutic target. Biomed Pharmacother. 2015;71:135-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Vassilopoulos A, Chisholm C, Lahusen T, Zheng H, Deng CX. A critical role of CD29 and CD49f in mediating metastasis for cancer-initiating cells isolated from a Brca1-associated mouse model of breast cancer. Oncogene. 2014;33:5477-5482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245-2255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 330] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 31. | Brandl A, Wagner T, Uhlig KM, Knauer SK, Stauber RH, Melchior F, Schneider G, Heinzel T, Krämer OH. Dynamically regulated sumoylation of HDAC2 controls p53 deacetylation and restricts apoptosis following genotoxic stress. J Mol Cell Biol. 2012;4:284-293. [PubMed] [Cited in This Article: ] |
| 32. | Komatsu H, Kakehashi A, Nishiyama N, Izumi N, Mizuguchi S, Yamano S, Inoue H, Hanada S, Chung K, Wei M. Complexin-2 (CPLX2) as a potential prognostic biomarker in human lung high grade neuroendocrine tumors. Cancer Biomark. 2013;13:171-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Antonioli L, Blandizzi C, Pacher P, Haskó G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13:842-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 517] [Cited by in F6Publishing: 538] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 34. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8693] [Cited by in F6Publishing: 9313] [Article Influence: 776.1] [Reference Citation Analysis (0)] |
| 35. | Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann NY Acad Sci. 2004;1014:1-12. [PubMed] [Cited in This Article: ] |
| 36. | Khan MS, Tsigani T, Rashid M, Rabouhans JS, Yu D, Luong TV, Caplin M, Meyer T. Circulating tumor cells and EpCAM expression in neuroendocrine tumors. Clin Cancer Res. 2011;17:337-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11:162-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 522] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 38. | Raffel A, Eisenberger CF, Cupisti K, Schott M, Baldus SE, Hoffmann I, Aydin F, Knoefel WT, Stoecklein NH. Increased EpCAM expression in malignant insulinoma: potential clinical implications. Eur J Endocrinol. 2010;162:391-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, Riethdorf S, Wege H. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer. 2013;133:2165-2171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 40. | Tönnies H, Toliat MR, Ramel C, Pape UF, Neitzel H, Berger W, Wiedenmann B. Analysis of sporadic neuroendocrine tumours of the enteropancreatic system by comparative genomic hybridisation. Gut. 2001;48:536-541. [PubMed] [Cited in This Article: ] |
| 41. | Löllgen RM, Hessman O, Szabo E, Westin G, Akerström G. Chromosome 18 deletions are common events in classical midgut carcinoid tumors. Int J Cancer. 2001;92:812-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Karkouche R, Bachet JB, Sandrini J, Mitry E, Penna C, Côté JF, Blons H, Penault-Llorca F, Rougier P, Saint André JP. Colorectal neuroendocrine carcinomas and adenocarcinomas share oncogenic pathways. A clinico-pathologic study of 12 cases. Eur J Gastroenterol Hepatol. 2012;24:1430-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Tomicic MT, Christmann M, Kaina B. Topotecan triggers apoptosis in p53-deficient cells by forcing degradation of XIAP and survivin thereby activating caspase-3-mediated Bid cleavage. J Pharmacol Exp Ther. 2010;332:316-325. [PubMed] [Cited in This Article: ] |
| 44. | Bocci G, Danesi R, Di Paolo AD, Innocenti F, Allegrini G, Falcone A, Melosi A, Battistoni M, Barsanti G, Conte PF. Comparative pharmacokinetic analysis of 5-fluorouracil and its major metabolite 5-fluoro-5,6-dihydrouracil after conventional and reduced test dose in cancer patients. Clin Cancer Res. 2000;6:3032-3037. [PubMed] [Cited in This Article: ] |
| 45. | Culy CR, Clemett D, Wiseman LR. Oxaliplatin. A review of its pharmacological properties and clinical efficacy in metastatic colorectal cancer and its potential in other malignancies. Drugs. 2000;60:895-924. [PubMed] [Cited in This Article: ] |
| 46. | Chabot GG. Clinical pharmacokinetics of irinotecan. Clin Pharmacokinet. 1997;33:245-259. [PubMed] [Cited in This Article: ] |
| 47. | Widmer N, Bardin C, Chatelut E, Paci A, Beijnen J, Levêque D, Veal G, Astier A. Review of therapeutic drug monitoring of anticancer drugs part two--targeted therapies. Eur J Cancer. 2014;50:2020-2036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |