Published online Aug 28, 2018. doi: 10.3748/wjg.v24.i32.3583
Peer-review started: May 7, 2018
First decision: May 17, 2018
Revised: June 5, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: August 28, 2018
Despite a decrease in gastric cancer incidence, the development of novel biologic agents and combined therapeutic strategies, the prognosis of gastric cancer remains poor. Recently, the introduction of modern immunotherapy, especially using immune checkpoint inhibitors, led to an improved prognosis in many cancers. The use of immunotherapy was also associated with manageable adverse event profiles and promising results in the treatment of patients with gastric cancer, especially in heavily pretreated patients. These data have led to an accelerated approval of some checkpoint inhibitors in this setting. Understanding the complex relationship between the host immune microenvironment and tumor and the immune escape phenomenon leading to cancer occurrence and progression will subsequently lead to the identification of prognostic immune markers. Furthermore, this understanding will result in the discovery of both new mechanisms for blocking tumor immunosuppressive signals and pathways to stimulate the local immune response by targeting and modulating different subsets of immune cells. Due to the molecular heterogeneity of gastric cancers associated with different clinico-biologic parameters, immune markers expression and prognosis, novel immunotherapy algorithms should be personalized and addressed to selected subsets of gastric tumors, which have been proven to elicit the best clinical responses. Future perspectives in the treatment of gastric cancer include tailored dual immunotherapies or a combination of immunotherapy with other targeted agents with synergistic antitumor effects.
Core tip: The use of modern immunotherapy, including adoptive cell therapy, vaccines, and especially immune therapy using checkpoint inhibitors, has led to encouraging results in clinical trials including gastric cancer patients. This review analyzes the relationship between immune microenvironment profile of the host and tumor development, identification of the immune prognostic markers and future perspectives of immunotherapeutic strategies. The treatment algorithm should be adapted to the specific molecular profile of the gastric cancer subtype in order to obtain maximum clinical benefits.
- Citation: Lazăr DC, Avram MF, Romoșan I, Cornianu M, Tăban S, Goldiș A. Prognostic significance of tumor immune microenvironment and immunotherapy: Novel insights and future perspectives in gastric cancer. World J Gastroenterol 2018; 24(32): 3583-3616
- URL: https://www.wjgnet.com/1007-9327/full/v24/i32/3583.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i32.3583
Early estimates situated gastric cancer in the first place as being the most frequent neoplasia (1975). Despite a decrease in gastric cancer incidents during the last decades, it remains a major health problem globally, with almost one million new cases diagnosed in 2012 (6.8% of the total), after tumors of the lung, breast, colorectum and prostate. There is a high geographical variation in gastric cancer incidence. Approximately 70% of cases occur in developing countries; half of the total number occurs in Eastern Asia, especially China; the incidence rates are approximately twice in men vs women. Overall, this type of tumor represents the third leading cause of cancer death in both sexes, accounting for 723,000 deaths in 2012 (8.8% of the total number of cases). The highest mortality rates are seen in Eastern Asia, whereas the lowest rates occur in Northern America; also, high mortality rates are encountered in Central and Eastern Europe and in Central and South America, respectively[1].
Most gastric cancers are diagnosed at an advanced stage, whereas another 25%-50% of cases will develop metastases during the outcome of the disease. Although surgical resection remains the main treatment with curative-intent in gastric cancer patients, there is a poor associated 5-year survival rate of approximately 20%-25%. Therefore, additional treatments (neoadjuvant/adjuvant), such as chemotherapy and radiotherapy where associated with tumor resection, unfortunately lead to only modest survival benefits. In advanced stages, approximately 50% of cases present local/systemic recurrence after adjuvant treatment, and only 10%-15% of cases achieve a 5-year overall survival[2]. In the metastatic stage, the backbone of treatment is represented by palliative chemotherapy, associated with a poor median overall survival, of approximately 8-10 mo[3]. Despite recent advances using novel biologic therapeutic agents, with the exception of trastuzumab [anti-human growth factor receptor 2 (HER2) monoclonal antibody] and ramucirumab [fully humanized monoclonal antibody receptor antagonist to bind vascular endothelial growth factor receptor 2 (VEGFR-2)], showing beneficial results by improving overall survival (OS), and therefore approved in first-line (in association with standard chemotherapeutic regimens) and second-line settings, respectively (as monotherapy, or in association with chemotherapy), in advanced and metastatic gastric cancers, clinical trials assessing other targeted agents showed disappointing results in gastric cancer[4-6].
Recently, the therapeutic algorithm and prognosis of many tumors changed radically by introducing immunotherapy, especially using immune checkpoint inhibitors, and the first drug of this class approved by the United States Food and Drug Administration (FDA) was ipilimumab, an anticytotoxic T lymphocyte antigen-4 (CTLA-4) antibody, used in the treatment of advanced melanoma (2011)[7,8]. Afterwards, immune checkpoint inhibitors, which are antagonists of the programmed death (PD)-1/PD-ligand 1 (PD-L1) pathway, were approved by the FDA for the treatment of different tumors, such as melanoma, non-small cell lung cancer (NSCLC), urothelial/renal cell carcinoma, squamous cell carcinoma of the head and neck, Merkel cell carcinoma and Hodgkin’s lymphoma[9].
The following main histological classifications of gastric cancer have routinely been used: the World Health Organization (WHO) classification[10] that categorizes four histological subtypes, namely, papillary, tubular, mucinous and poorly cohesive, and Lauren’s classification, dividing gastric cancers into intestinal, diffuse and mixed type[11].
Because these two classifications are not able to direct specific therapeutic strategies and, additionally, because the group of gastric cancers includes heterogeneity of tumors, there was a need to elaborate new classifications capable of stratifying patients regarding tumor behavior, prognosis and response to specific treatments. For the first time, the molecular assessment of gastric cancer patients was proven to add benefits in the context of the TOGA trial in which a combined treatment with classical chemotherapy and trastuzumab showed an improvement of survival in the subgroup of patients overexpressing HER2[4]. Moreover, the behavior of the tumor and the outcome proved to be different in cases of Asian patients vs Caucasians included in several clinical trials[12].
In 2013, Singapore researchers identified three different molecular subtypes of gastric cancer: proliferative (high genomic instability, TP53 mutation), metabolic (high response to 5-FU chemotherapy), and mesenchymal (stem cell-like cancers that are sensitive to PIK3CA-mTOR inhibitors)[13].
The aim of “The Cancer Genome Atlas (TCGA)” project (2014) was to develop a new molecular classification of gastric cancer with clinical impact and to identify the main dysregulated pathways of each subtype of gastric tumors. The TGCA research group divided gastric cancer into four genomic subtypes:
Chromosomal instability (CIN): Includes approximately 50% of cases, most of the tumors are located at the gastro-esophageal junction or cardia[14]; leads to a loss or gain of some oncogenes and tumor suppressor genes[15]; has a high frequency of TP53 gene and receptor tyrosine kinase mutations and amplifications of cell cycle genes[16]; and has amplifications in oncogene pathways (HER2, BRAF, EGFR, MET, and RAS)[17].
Microsatellite instability: Tumor testing methodologies include immunohistochemistry for abnormal absence of MMR protein expression or polymerase chain reaction (PCR) for Microsatellite instability analysis (MSI), to evaluate for MSI-H on a tumor specimen. Immunohistochemistry can predict which gene is most likely to be mutated, the gene for the affected protein. Interpretation of immunohistochemical reports can sometimes be confusing, as “positive” should mean the abnormal absence of MMR protein expression, in contrast to normal presence of expression. MSI testing panels may consist of mononucleotide and dincleotid markers. For classifying MSI, a panel of five markers for the analysis of MSI was recommended by the National Cancer Institute, including two mononucleotide and two dinucleotide repeats. In the case that ≥ 2 of the five markers show instability, the genotypes are grouped into highfrequency (MSIH); when only one marker shows instability, into lowfrequency (MSIL), and when no marker shows instability, into microsatellite stable (MSS). MSIH consists of 30%40% instability markers, while MSIL of < 30%40%. Bethesda Guidelines have stated that MSIH can be defined when instability is present at mononucleotide loci and MSIL when instability is limited at only dinucleotide loci; mononucleotide repeats were demonstrated to be more sensitive vs dinucleotide loci in detecting MSI. Some studies have shown that both immunohistochemistry and MSI are cost-effective and useful for selecting high-risk patients. A review showed that the sensitivities of MSI and immunohistochemical testing are 77% to 89%, and 83%, respectively; specificities are 90% and 89%, respectively. Some patients may have MSI or abnormal immunohistochemistry due to sporadic development of cancer, rather than an underlying genetic (germline) mutation. MSI accounts for 15%-30% of gastric cancers; most often includes tumors of intestinal type, antral location, females and older patients[18,19]; shows mutations in DNA mismatch repair genes, such as MLH1 or MLH2, that lead to the dysfunction of DNA mismatch repair enzymes[20]; is reported in a meta-analysis that demonstrated a 37% reduced mortality risk and prolonged OS in gastric cancer patients with MSI-high (MSI-H) vs MSI-low (MSI-L)[21]; is associated in non-colorectal cancer with an increased frequency of somatic mutation and amplification of tumor antigens; therefore, an increased sensitivity to treatment with PD-1 immune checkpoint inhibitors[22,23]; has increased intratumor infiltrate[24]; is reported in studies that show an increased activity of pembrolizumab in gastric cancer[25] and assess the efficacy of nivolumab ± ipilimumab in MSI-H gastrointestinal cancers[26]; and is possibly being considered in the development of a preventive vaccine using neopeptides affecting MSI carcinogenesis[27].
Genomic stability: Accounts for approximately 20% of gastric cancer cases; has an increased frequency of the diffuse type; indicates main somatic genomic alterations: CDH1, ARIDIA, RHOA; and presents a recurrent interchromosomal translocation involved in cellular motility[28].
Epstein Barr virus-associated: Epstein Barr virus (EBV) is detected by in situ hybridization or PCR in approximately 10% of gastric cancer patients[29]; it seems to increase ten times the gastric cancer risk, especially in Far East Asian patients[30], and is more frequent in younger patients[29]; it has a better response to immunotherapy and better survival[31]; it shows that the PD-L1 gene is frequently amplified; approximately 15% of EBV+ tumors present amplification of chromosomal region 9p24.1 (locus of PD-L1, PD-L2)[32]; EBV+ is found in approximately 50% of tumor cells and 94% of immune cells; therefore, PD-1 testing could predict a response to immunotherapy in this subset of patients[12]; and it shows PIK3CA mutations that could be targeted using PI(3)-kinase inhibition, DNA hypermethylation and JAK2 mutations[28].
Taking into account specific characteristics of the four subtypes of gastric cancer, it was highlighted that EBV-associated and MSI categories are associated with the best responses to immune therapeutic strategies.
The Asian Cancer Research Group (ACRG) proposed a molecular classification of four molecular subtypes for gastric cancer (2015): one subtype was related to the epithelial-to-mesenchymal transition (MSS/EMT) phenotype, which was associated with highest recurrence frequency, and another subtype was related to the phenotype of MSI, cytokine signaling, cell proliferation and methylation signals, including hypermutated tumors, which was associated with the best overall prognosis. The remaining of the non-EMT and non-MSI patients were further divided into MSS/p53- and MSS/p53+ molecular subtypes and were associated with an intermediate overall prognosis and recurrence[33,34].
Comparing TCGA vs ACRG subtypes of gastric cancers, one may notice a resemblance between MSI tumors, GS and MSS/EMT subtypes, EBV and MSS/TP53+ subtypes, and CIN and MSS/TP53- tumors, respectively[13].
The development of genotyping different subtypes of gastric cancer will provide a guide to molecular targeted drugs that should be investigated in large clinical trials on specific subsets of gastric tumor patients in the future.
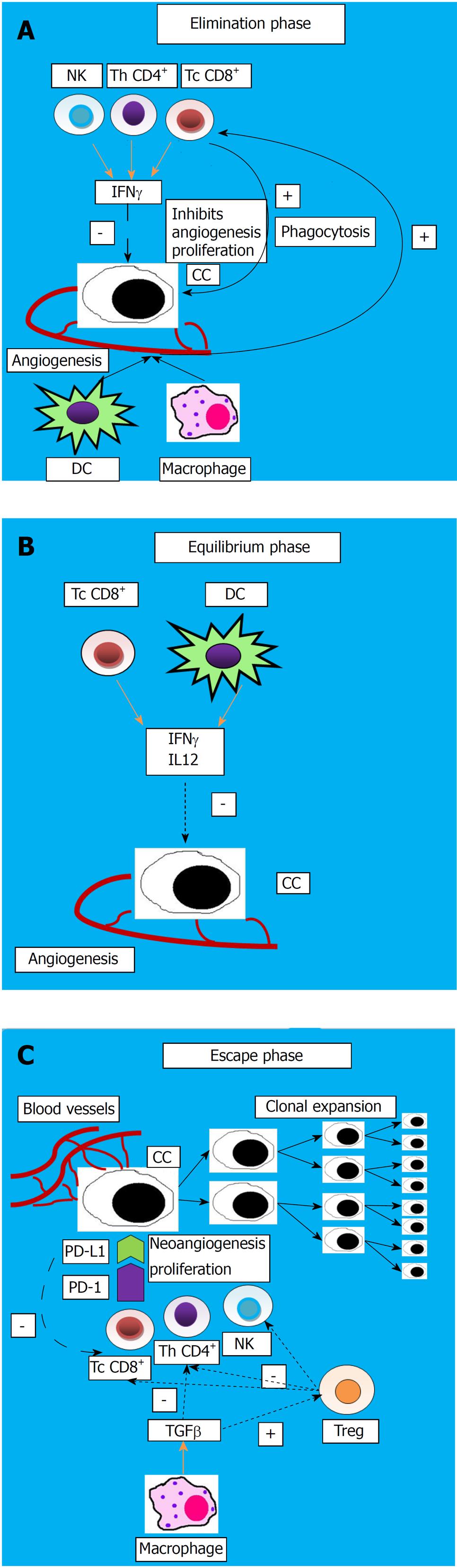
The term “immunosurveillance” refers to the capacity of the host immune system to identify the tumor cells as “non self”, and subsequently to kill them[35]. The immune response includes both innate immunity (represented by macrophages, dendritic cells, and natural killer cells), and specific adaptive immunity (T and B lymphocytes).
The host protective response and the capacity of the tumor to surpass the host immune response are defined as “cancer immunoediting”, which is a process comprising three progressive steps:
Elimination phase: In this stage, natural killer (NK) cells and T lymphocytes (helper and cytotoxic) secrete interferon IFNγ, leading to a reduction of angiogenesis and proliferation of cancerous cells; moreover, macrophages and dendritic cells (DC) secrete cytokines that activate immune cells to phagocytize dead tumor cells.
Equilibrium phase: Residual cancerous cells remain in a dormancy state because DC and cytotoxic T cells secrete IFNγ and inhibitory cytokines (IL12), suppressing them.
Escape phase: Tumor cells change their features which will be transmitted to the daughter cells, therefore escaping immunosuppression and proliferating, along with the apoptosis of the effector immunocytes[36]. This process is illustrated in Figure 1.
The tumor mechanisms to evade suppression by the immune system may include a reduced expression of the tumor antigens and major histocompatibility class I (MHC) antigen, Fas-L modulation, increased synthesis of inhibitory cytokines such as TGFβ1, IL10, IL6, VEGF, prostaglandin, and an increased number of T regulatory lymphocytes (Treg) and myeloid-derived suppressor cells (MDSC)[37].
The most important components of the tumor immunosuppressive microenvironment are represented by the regulatory T cells (Tregs) and mesenchymal- or bone marrow-derived stem cells (BM-MSCs). Tregs represent CD4+CD25+FOXP3+ T lymphocytes that determine reduced activity of cytotoxic and helper T cells, and of NK cells and are involved in the immunological tolerance to self-antigen and the persistence of Helicobacter pylori (H. pylori)-related inflammation[38]. BM-MSCs migrate to cancerous tissues and, in some animal models, were shown to create an immunosuppressive environment in chronic H. pylori infection and to represent a “seeding point” for gastric carcinogenesis[39-41]. In this regard, immunotherapeutic strategies directed against Tregs and BM-MSCs and against immunosuppressive cytokines seem to be promising.
Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed death-1 (PD-1) play essential roles in the immune checkpoint modulation. Normally, these molecules modulate the response of T lymphocytes to antigens. CTLA-4 represents an inhibitory receptor exhibited by T cells, whereas PD-1 represents a co-inhibitory receptor located on the cell surface, suppressing T cell activity in peripheral tissues in the context of inflammation. PD-1 is widely expressed on T and B lymphocytes, monocytes, and natural killer cells; conversely to CTLA-4, PD-1 is involved in subsequent phases of immune responses[42]. It has been shown that various tumor cells upregulate PD-L1. In gastrointestinal cancers, PD-L1 upregulation has been identified in pancreatic, gastric, and colorectal cancers, correlating in several studies with a poor prognosis[43,44].
Tumor-infiltrating lymphocytes (TILs) comprise the presence of T cells, B cells and NK cells[45]. T cells include cytotoxic lymphocytes (CD8+), helper T cells (CD4+), memory T cells (CD45RO+) and T regulatory cells (FOXP3+). Specific cell membrane antigens of TILs bind to specific cellular types: CD3, CD4, CD8 and FOXP3 bind to T cells, CD20 to B cells and CD57 to NK cells[46].
In the complex relationship between the host immune microenvironment and cancer occurrence and progression, TILs seem to gain bidirectional regulation abilities. In one way, tumor neoantigens captured by the DC are presented on MHC molecules to the T cells, leading to the activation of effector T cells, with subsequent infiltration of the tumor and destruction of the cancerous cells. In addition, these activated cells secrete inhibitory cytokines, with the augmentation of antitumor effects[47,48]. On the other hand, TILs may help cancer to proliferate, either by creating an appropriate environment for tumor growth or by protecting tumor cells to survive[49].
The stromal TILs represent the mononuclear inflammatory cells infiltrating tumor stroma, whereas intratumor TILs define the intraepithelial lymphocytes/mononuclear cells within the tumor. Stromal TILs were shown to predict disease-free survival (DFS) of patients[50].
The assessment of TILs as a prognostic biomarker in gastric cancer patients has led to controversial conclusions. The presence of various subsets of cells seems to differently influence the patient’s prognosis. Studies have shown that high density of intratumor TILs are associated with better prognosis (HR = 0.55)[51-53]. Additionally, some data in the literature revealed that an increased number of CD8+ T cells, both intra- or extra-tumor located, is associated with an improved DFS and OS[54-57]; in contrast, the results of a recent study showed that an increased number of CD8+ cells correlate with poor overall survival and increased expression of programmed death ligand 1 (PD-L1)[58]. Other studies showed that a high density of intratumor FOXP3+ Treg is correlated with a poor OS, whereas an extratumor high density of this cell type leads to an increased OS[59-64]. The increased intratumor Treg/CD8+ lymphocytes ratio is correlated with a decreased OS[65] and the presence of T helper 17 and T helper 22 with tumor progression[66]. Moreover, an increased T helper 1/T helper 2 of CD4+ T lymphocytes represents a favorable prognostic factor[67]. A better OS was associated with an increased intratumor presence of various immunocytes, such as CD3+ T cells[57,68], CD57 NK [51,57,69,70], CD45RO+ (memory T cells)[71], and T-bet+ (marker for T helper 1 lymphocytes)[72]; in addition, an increased DFS was observed in the case of high intratumor density of CD20 (surface marker of B cells)[73]. The data show a decrease in CD3+ TILs density along with tumor progression[74]. On the other hand, the subgroup of CD45RO+ T lymphocytes seems to prevent peritoneal spreading of gastric neoplasias[75]. All the data from the literature demonstrate that high densities of CD8+, CD3+, and CD57+ TILs and low densities of FOXP3+ TILs represent favorable prognostic factors in gastric neoplasia.
CD4+ T cells secrete various cytokines, such as IL-17, the role for which the data from the literature reveal controversial results. Some of the studies showed that this cytokine could stimulate tumor angiogenesis, growth, and spreading[76], while other studies show that IL-17 exhibits anticancer effects, either by stimulating the cytotoxic activity of TIL[77] or by stimulating the maturation of DC[78].
The study of Yuan et al[79] revealed that gastric cancers present an increased percentage of CD4+ T lymphocytes and lower CD8+ T cells (with an increased CD4+/CD8+ ratio) compared to blood and, further, to paraneoplastic tissue. In addition, the number of TILs of effector and memory T cell type is significantly higher than in the case of circulating T cells. As we have already mentioned, the involvement of Tregs in antitumor immunity is controversial, and their role may differ according to the type of cancer[80,81]. These authors considered CD4+CD25 highCD127low as being the most specific marker to define the Treg population, with the percentage of these cells being increased among TILs, demonstrating the accumulation of immunosuppressive Tregs at the site of a gastric tumor[79]. Recent studies show that the coexpression of PD-1+ and Tim-3+ define the most hypo-functional T lymphocytes[32,82]. The percentage of these cells among TILs was significantly increased in gastric tumors, especially in patients with advanced stages, suggesting that they may be implicated in a tumor immune escape phenomenon and that TILs in gastric cancer show T cell dysfunction. The combined blockade of these molecules seemed to have a synergistic effect on IFNγ production and, therefore, may provide new promising immune modulating strategies[79].
Finally, the strategies of immunotherapy in gastric cancer are directed on TILs and are based on augmenting anticancer immunity by blocking the interaction of CD8+ T lymphocyte-related receptors such as CTLA-4 and PD-1 and their ligands situated on tumor cells (PD-L2 and PD-L1). Furthermore, it may include the stimulation of the local immune response by targeting and modulating different subsets of CD8+ TILs.
Immunotherapy needs the activation of the cellular immune responses following the presentation of the tumor antigen peptides by DCs to T cells[83].
It has been demonstrated that the density of tumor infiltrating DCs correlates with the staging and prognosis in gastric neoplasias, and patients with a high amount of DCs present an improved OS compared with patients with a lower density of DCs[69]. The study of Tsujitani et al[84] showed that the use of postoperative adjuvant immunotherapy exhibits beneficial results on the survival of gastric cancer patients with reduced tumor DC infiltration.
Based on the existence of tumor-infiltrating lymphocytes (TILs) and PD-L1 expression, it has been proposed to categorize the tumors into four types[85] as follows: type I (TILs+ PD-L1+), presenting adaptive immune resistance; type II (TILs- PD-L1-), revealing immune ignorance; type III (TILs- PD-L1+), showing intrinsic induction; and type IV (TILs+ PD-L1-) in which other suppressors have a role in initiating immune tolerance. This stratification may have a certain prognostic role and may guide efforts towards a specific immunotherapeutic strategy.
Because tumor response to immunotherapy using PD-1 blockade requires the presence of CD8+ TILs that are downregulated by PD-1/PD-L1 activity[86], this type of therapeutic strategy in type I cancer might improve prognosis. As tumors included in type II and III lack TILs, the combination of immune checkpoint inhibitors with a vaccine that is capable to induce T cell activation, migration and infiltration at the tumor site might improve the clinical outcome in these patients[87].
Among the four molecular subtypes of gastric cancer, EBV+ and MSI tumors often show the activation of immune mechanisms, being associated with a high density of TILs, which has been correlated with an improved cancer-specific survival[88]. An increased number of CD8+ and FOXP3+ TILs were associated with improved OS in MSI-H gastric cancers[55] and EBV-associated cancers[87]. In addition, another study showed that a high number of CD3+ and CD8+ TILs in EBV-associated and MSI gastric cancer subtypes are associated with better survival[56].
Advanced TNM stages of EBV+ tumors were correlated with a reduced density of CD4+, CD8+ and Foxp3+ TILs and PD-1 expression. Additionally, PD-L1 expression was shown to predict a reduced survival in EBV-associated cancers. Approximately two-thirds of EBV+ gastric cancers were proved to present a type I or IV microenvironment associated with a better prognosis by inducing adaptive immune responses (type IV showed the best 5-year OS), whereas more than 70% of negative EBV tumors belong to the type II and III microenvironment, showing an absence of an immune response and a poor prognosis[87].
All these results are indicating the possibility of different subsets of TILs to be used as prognostic markers in these specific categories of patients. Moreover, EBV-associated and MSI gastric cancer categories might become potential targets of immunotherapy.
Myeloid-derived suppressor cells (MDSCs) represent immune suppressive cells with the ability to inhibit T cell (CD4+ and CD8+) activation and increase T cell apoptosis[89]. The data showed that high intratumor density of MDSCs is related to a poor prognosis in gastric cancer[80,90]. Peripheral blood granulocyte MDSCs are significantly increased in cancerous patients[91]. Shoji et al[92] noted that an increased proportion of granulocyte MDSCs prior to chemotherapy represents a negative prognostic factor for PFS in advanced gastric cancer patients receiving cisplatin-based chemotherapy and tends to be associated with poor OS.
Several papers showed that IL-8 is involved in gastrointestinal carcinogenesis by its ability to recruit MDSCs[93]. In addition, an increased number of circulating MDSCs was associated with advanced tumor stages, increased serum IL-8 levels, and dysfunction of T cells[94]. In this light, IL-8 seems to determine an adverse immune status[92].
Currently, numerous papers target defining the host inflammatory response to cancer. Due to a release of cytokines, the systemic inflammatory response seems to be responsible for the promotion of angiogenesis, DNA alteration, and tumor proliferation[95].
Studies have demonstrated that the neutrophil to lymphocyte ratio (NLR) has a prognostic value in patients with solid cancers[96-98]. In oncologic patients, lymphopenia is a marker of deficient cell-mediated immunity, while neutrophilia is a sign of response to systemic inflammation. Furthermore, malnutrition/ hypoalbuminemia show correlation both with immune suppression and systemic inflammation, which are phenomena overexpressed in advanced tumor stages[99,100]. Gonda et al[101] consider that NLR might be a useful biomarker for assessing tumor response to chemotherapy, immune suppression, malnutrition and unfavorable prognosis in gastric cancer patients. Moreover, it has been suggested that combining anti-inflammatory agents with chemotherapy provides an enhanced efficiency of the treatment.
The concept of immune modulating treatment was used for the first time by Edward Jenner (1798), who demonstrated that inoculating humans with cowpox could prevent smallpox occurrence. Over time, this strategy was implemented in developing serum and vaccinations. The efficacy of immunotherapy in cancer treatment was demonstrated by WB Coley (1891) who, by injecting streptococcal germs into a patient with unresectable cancer, determined the decrease of the tumor size[102]. Furthermore, Ehrlich (1909) has suggested that the host immune system could suppress the tumor growth by recognizing cancerous cells as non-self. Half a century later, Burnett has proposed the theory of tumor immune surveillance[35], recently completed by Schreiber et al[49] with the concept of cancer immunoediting.
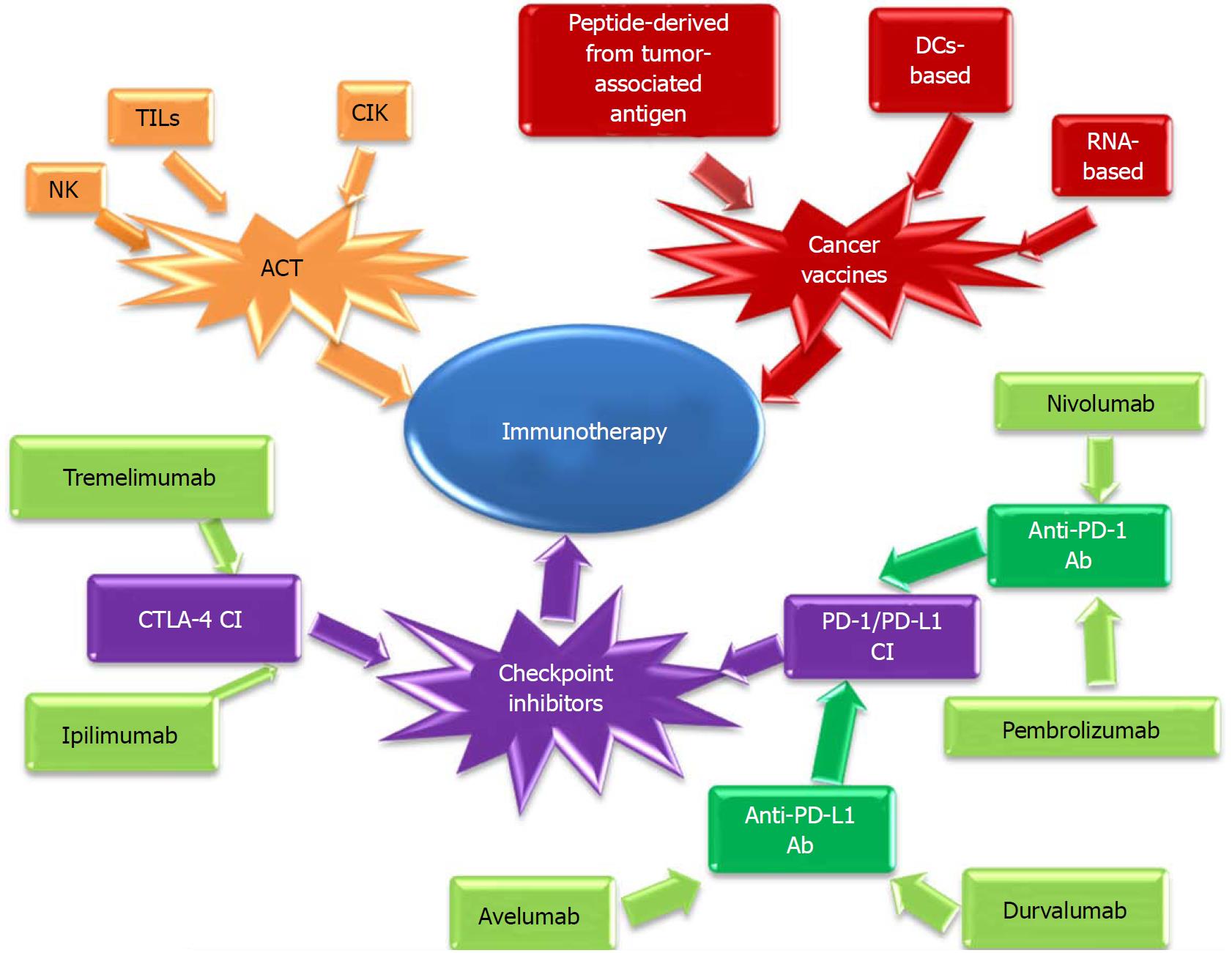
In recent years, many immunotherapeutic strategies have been developed, including treatments using monoclonal antibodies, cytokines, cytotoxic cells, T cells infusions and gene transferred vaccines[36], having the aim of either increasing the host antitumoral response capacity or increasing the immunogenicity and susceptibility to treatment of the tumor cells[103].
The development of an effective immunotherapeutic management for digestive cancers has evolved relatively slowly, with the majority of these immune-modulating approaches still under assessment in early phase clinical trials, mostly because of their well-known lack of antitumor effector T lymphocyte responses and their decreased immunogenicity[104]. Despite these specific aspects, some immunotherapeutic approaches, such as those using anti-PD/PD-L1 immune checkpoint inhibitors, proved to also be effective in cancers defined by a poor immunogenic nature[105].
Immunotherapeutic strategies may be classified into[42]:
Active immunization strategies, including: (1) Adoption of cytokines (e.g., IFNγ, IL-10, IL-2) to date, leading to inconclusive results[103]. (2) Vaccination strategies that include: vaccines using peptides/proteins recognized by CD8+ and CD4+ lymphocytes, such as various tumor-rejection antigens (including melanoma-associated antigen (MAGE-3) or HER-2/neu)[106]; new vaccines using a compound formed by an immunogenic protein fused with peptide (Z12) that determines a persistent antitumor T cell response[107]; DC-based vaccines; RNA-based vaccines, etc. (3) Immune checkpoint inhibitors (anti-CTLA4, anti-PD/PD-L1). (4) Combination of different immunotherapeutic strategies. (5) Combination of immunotherapy with standard treatment.
Passive immunization strategies: Adoptive cell therapy (ACT) using TILs- refers to the passive transfer of antitumor T lymphocytes into a tumor-bearing host followed subsequently by the direct destruction of this cancer[108]. Current immunotherapeutic strategies are presented in Figure 2.
Currently, immunotherapy in gastric cancer patients includes cell-based strategies aimed either to activate cytotoxic T lymphocytes directed against cancer cells or to bind molecules expressed by tumor cells.
ACT: This technique refers to injection of different tumor-specific T lymphocytes into a cancer patient, such as cytokine- and anti-CD3 monoclonal antibody- induced killer cells, as well as TILs.
(1) Cytotoxic T cell therapy
In a preclinical study, cytotoxic activity of peripheral blood lymphocytes extracted from gastric tumor patients or from healthy individuals was induced using different HLA-A matched allogeneic gastric cancer cells, exhibiting antitumor efficacy against HLA-A2 and HLA-A24 gastric cancer cell lines[109]. Furthermore, Kawamoto et al[110] demonstrated the efficiency of peptide-based immunotherapeutic strategies by proving that cytotoxic T lymphocytes were able to kill HLA-A-0201/2402 colon and gastric cancer cells, which were positive for mitotic centromere-associated kinesin (MCAK) (a new cancer antigen) in an HLA-I restricted way. In addition, MHC-1 restricted T cells were obtained from primary tumors, metastatic lymph nodes, and ascites of autologous gastric cancer and were proved to detain different recognition characteristics towards gastric cancer antigens[111]. Moreover, splenic MAGE-specific cytotoxic T lymphocytes against HLA-A2 cancer cell antigen, existing in testis and several neoplasias (including gastric cancer), were successfully obtained and tested[112].
Another preclinical study has revealed that cytokine-induced killer (CIK) cells, mostly by stimulating IFN-γ and tumor necrosis factor-alpha, exhibit antiproliferative effects against the MGC-803 gastric cancer cell line[113]. In addition, Kim et al[114] demonstrated the benefits of using ACT with CIK cells in gastric cancer patients. These CIK cells, isolated from the human peripheral blood mononuclear cells and activated by IL-2 and anti-CD3 antibody, were able to suppress the MKN74 human gastric cancer cell line in vitro and inhibit tumor proliferation in a nude mouse model.
As gastric cancers usually show paucity of stroma infiltration, in vivo studies have suggested administration of immunotherapy using ACT combined with chemotherapy[115]. Furthermore, the chemotherapeutic drug oxaliplatin, by stimulating high-mobility group box 1 protein to induce anticancerous T lymphocytes, is capable of producing an immunogenic cancer cell death[116]. In several in vitro and in vivo studies on drug-resistant gastric cancer, a high amount of cytokines was induced by combining this drug with CIK cells[117]. It was suggested that chemotherapy associated with T lymphocyte reduction would be capable of enhancing the results of ACT therapy by stimulating the persistence of endogenous T cells in circulation, while depleting autoimmune reactions on healthy tissues. However, these results unfortunately occurred at the expense of severe infectious adverse events in these patients[118].
Moreover, the results obtained by combining ACT treatment with an antibody directed against both anti-epidermal growth factor receptor (EGFR) and anti-epithelial cell adhesion molecule (EpCAM), that specifically targets the simian virus 40 (SV40) T antigen-specific T cells (previously transduced with a truncated human EGFR), showed a better tumor reduction and OS than with ACT alone [119].
Du et al[120] used a mouse model of gastric cancer and concluded that ACT by peritoneal injection of CIK might be both a beneficial and minimally invasive strategy for treating this type of cancer.
The first clinical trial proving the beneficial results of ACT in humans used lymphokine-activated killer cells plus IL-2 in patients with metastatic melanoma, leading to the approval of the treatment for this group of patients[121-123]. Furthermore, this strategy determined a significant tumor reduction in patients with different tumors[124].
Zhang et al[125] showed that administration of expanded activated autologous lymphocytes, which were stimulated by anti-CD3 monoclonal antibody (mAb) and IL-2, to gastric neoplasia patients led to a prolonged OS compared to the group that received only standard chemotherapy.
The efficiency assessment of combined treatment using CIK cells and chemotherapy as adjuvant therapy in stage II/III of gastric neoplasia after curative gastric resection revealed a significantly prolonged OS vs the group using chemotherapy alone[126]. In a similar context, a clinical trial analyzed the possible toxicities of adjuvant ACT associated with chemotherapy (including 5-fluorouracil/capecitabine), showing an improvement in both DFS and OS, without the development of severe adverse reactions[127]. Moreover, a clinical trial assessing this type of combined adjuvant treatment in stage III/IV (M0) gastric cancer patients after R0/D2 resection showed a significantly longer 5-year OS and DFS vs adjuvant chemotherapy[128].
In cases of advanced gastric cancer, several clinical studies have also proven an increased response rate, better quality of life and even an increased OS in patients treated with chemotherapy (FOLFOX4) plus CIK cells vs chemotherapy alone[129,130].
By assessing the administration of a chemotherapeutic regimen followed by autologous CIK cells, the results highlighted an improved remission rate in gastric cancer patients, associated with tolerable and reversible side effects. Standard chemotherapy using XELOX plus CIK cells administered intraperitoneally in gastric cancer patients showed a marked decrease in ascites volume and OS prolongation[131].
In a pilot study, patients with advanced gastric cancer, who received gamma delta T cells with zoledronate intraperitoneally as a local treatment for carcinomatous ascites, showed a significant reduction both in the number of peritoneal tumor cells and ascetic volume with no serious side effects[132].
Several clinical trials are currently investigating the tumor responses after adjuvant administration of ACT plus chemotherapy after surgical resection in advanced gastric cancer patients[133]. In a phase II clinical study involving gastric cancer patients in stages I-III, the adjuvant combination of autologous tumor lysate-pulsed dendritic and CIK cells (Ag-D-CIK) plus chemotherapy is currently being evaluated following curative resection[134].
A clinical trial is currently investigating the safety and efficiency of infusing chimeric antigen receptor (CAR) T cells specific for EpCAM into relapsed or refractory gastric cancer patients[135].
A phase I/II clinical trial is currently investigating the benefits of infusing CAR T cells targeting mucin 1 (MUC1) in several solid cancers (including gastric tumors), as its overexpression leads to chemotherapeutic-refractory tumors[136].
A phase Ib clinical trial on advanced gastric cancer expressing CEA assesses the efficacy of injecting anti-CEA CAR T cells into the hepatic artery targeting liver metastasis[137].
Additionally, a phase I/II clinical study on HER2+ gastric cancer patients (defined as HER2 in immunohistochemical tumor tissue greater than or equal to 2 levels) with liver metastasis is analyzing the cytotoxic potency of engineered pluripotent stem cells and T cells, which specifically bind to HER2[138]. In addition, another phase I clinical trial assesses the safety profile of administrating autologous T cells equipped with a bi-specific antibody (HER2 Bi-Armed T cells) in gastric and esophageal neoplasias[139].
Patients with metastatic gastric cancer are also investigated regarding a combination of S-1 plus dendritic cell activated CIK (DC-CIK) (phase I/II clinical trial)[140].
(2) Adoptive immunotherapy using TILs
The use of ACT with TILs is not associated with an immediate effect because this therapeutic protocol requires approximately six wks, as T cells must undergo the following preparation steps before infusion: first, they are isolated from tumor tissue; next, they are in vitro expanded; and finally, tumor-specific T cells are selected[141].
The immunotherapy using TILs has led to encouraging results in preclinical models[142], but not in all clinical studies (except for melanoma)[143,144].
In most gastric cancer patients, TILs exhibit a specific type-1 T cell response to cancer antigens. It is important to note that in order to obtain “in vivo” destruction of the tumor cells, the efficacy of tumor-specific T cells usually needs to be enhanced by combining vaccination using specific cancer antigens/peptides or by injecting in vitro expanded autologous cancer-specific T cells [103].
Moreover, TILs can sometimes stimulate proliferation of tumor cells. Studies show that HP0175-specific TILs in gastric cancer patients infected with H. pylori determine gastric Th17 response, exhibiting a pro-inflammatory low cytotoxic TIL response; Th17 cells promote tumor progression through the promotion of inflammation by secretion of IL-17 and other interleukins, which could induce proliferation and migration of cancer cells; therefore, TILs reveal a correlation between H. pylori infection and gastric cancer development[145].
Because studies have demonstrated that a high Tregs/CD8+ ratio in the tumor areas represents an independent factor for poor OS, a combination of the deletion of Tregs plus the stimulation of effector T cells may represent an effective immunotherapy in gastric cancer patients[65].
ACT using TILs isolated from the patient’s tumor was also assessed in cases with gastric neoplasia[146]; the results of a clinical trial showed a longer OS using a combined treatment of ACT using TILs and standard chemotherapy vs chemotherapy alone[143].
(3) Adoptive immunotherapy using NK cells
The data show that NK cells exhibit cytotoxic activity against allogeneic and autologous cancer cell lines, including gastric cancer cells lines[147], and could prevent tumor metastatic spreading[148,149]; additionally, intra-tumor infiltration of NK cells is associated with a longer survival in neoplastic patients[150]. Patients with advanced gastric adenocarcinoma having a high density of NK cells demonstrated a prolonged survival rate vs those with the low density NK[151]. The number of apoptotic NK cells (Fas+ NK cells) is significantly higher in gastric neoplasia patients vs normal controls and is correlated to the tumor progression[152].
Clinical data revealed a favorable prognostic role of NK cells in gastric cancer patients, with a high level of CD57 antibody expression in gastric tumors correlated with a reduced size of tumors, N0 tumors, more surgical resections and prolonged 5-year OS[51].
By culturing autologous peripheral blood mononuclear cells with K562 cells, researchers were able to obtain cytotoxic NK cells from cancer patients[147], suggesting a possible role of immunotherapy using autologous expanded NK cells in clinical practice. In this regard, a clinical study using a combination of cell-based immunotherapy with autologous NK cells, γδ T cells, and CIK cells plus chemotherapy showed a statistically significant improvement of the 2-year progression-free survival and quality of life, but without demonstrating a significantly prolonged OS in gastric cancer patients[153].
The safety and efficacy of therapy with trastuzumab and NK cells in the treatment of gastric cancer is currently assessed in a clinical trial[154].
It has been demonstrated that lupeol, which exhibits a curative effect on various diseases, has the ability not only to stimulate the proliferation and the cytolytic activity of NK cells against gastric tumor cells, but also to inhibit the proliferation of some gastric cancer cell lines. Therefore, this agent might be included (either alone or in combination with ACT) using NK cells in the therapeutic strategies of gastric tumors[155].
Cancer vaccines have the aim of activating and increasing the number of effector T lymphocytes, leading to the augmentation of existing immunity, development of novel immunological response, and therefore an improved anticancer immunity[36].
Peptides derived from tumor-associated antigens [HER2/neu-derived peptide[156] and MAGE[157] are captured by antigen-presenting cells (such as DC)] and presented by means of MHC type I for presentation to cytotoxic T cells, and by means of MHC type II for presentation to T helper cells, determine their activation, followed by the destruction of tumor cells.
There are several types of vaccinations that have shown promising results in gastric cancer patients:
(1) HLA-A*2402-restricted URLC10-A24-177 and vascular epidermal growth factor receptor (VEGFR1-A12-9 1084) epitope peptide cancer vaccines in advanced chemotherapy-resistant gastric cancer patients - these are safe and induce enhanced specific cytotoxic T cell immune responses[158].
(2) Vaccination using survivin epitope peptide - a recent study suggested an excellent efficiency in gastric cancer patients upon inducing survivin-derived peptide-specific cytotoxic T lymphocytes from mononuclear cells isolated from blood of healthy individuals[159].
(3) Autologous gp96 vaccine in addition to chemotherapy as an adjuvant treatment in patients with resected gastric cancer (phase II study) - glycoprotein (gp) 96, belonging to the group of autologous tumor-derived heat shock proteins (HSPs), binds tumor-associated antigens, constituting the HSPs-peptide complexes that promote the activation of APCs, as well as the release of various cytokines, enhancing T cell antitumor immune response; the vaccination seemed be well tolerated and is associated with a potential for prevention of gastric cancer recurrence[160].
(4) Trials using DC-based anticancer vaccines - ex vivo expanded DCs were able to generate antigen-specific T lymphocytes responses both in animal models[161] and in clinical trials[162]: (1) vaccination using dendritic cells pulsed with HER-2/neu peptide generated tumor shrinkage (phase I study)[143]; (2) vaccination using DC pulsed with nanoparticles MAGE-3 peptide-loaded stopped tumors from proliferating in a mouse model of gastric cancer (phase I study)[163]; and (3) vaccination with cancer-loaded autologous DCs (stimulated by autologous apoptotic gastric tumor cells) determined the activation of memory T cells[164].
RNA-based DC vaccines: By using stabilized mRNA, DCs transfected with mRNA coding for tumor-associated antigen/whole tumor RNA were able to generate potent immune responses both in mouse models[165,166] and clinical studies involving melanomas and renal cell carcinomas[167,168].
These RNA-based vaccines seem to present some potential benefits over the classical vaccination techniques: they are pharmaceutically safer because of the presence of transient and cytosolic active mRNA (lack of genomic integration); they have the ability to target multiple tumor-associated antigens; they are not associated with severe adverse events; and they are not MHC-restricted[36].
Because the clinical efficiency of DC vaccines is limited because of the short survival of DCs, mostly due to the cytolytic properties of DC-activated CD8+ T cells[169], their efficiency has been increased by using small interfering RNA (siRNA)-targeting phosphatase and tensin homolog (PTEN), which is involved in negative feedback in the signal transduction of the PI3K/AKT pathway[170]. This technique has increased the number of cancer-specific cytotoxic T cells and the antitumor immune response[171].
The results were improved by targeting the immunosuppressive IL-10 receptor in association with the siRNA DC vaccine[172], by using GM-CSF gene-modified DC[173], and by removing Tregs along with DC vaccination[174].
Strategies of combining vaccination with chemotherapy: The combination of an adjuvant Bacille Calmette-Guérin (BCG) vaccine with chemotherapy vs chemotherapy alone resulted in a better OS in patients with radically resected locally advanced gastric cancer[175].
Vaccination using gastrin-17 diphtheria toxoid (G17DT)-targeting gastrin peptide combined with chemotherapy (cisplatin plus fluorouracil) increased the OS of patients with advanced gastric cancer (phase II clinical trial)[176].
Vaccination using peptides derived from human VEGF receptors 1 and 2 combined with standard chemotherapy (S1+ cisplatin) improved the OS in patients with advanced gastric cancer[177].
Literature data show that vaccination is a safe and tolerable strategy that is associated with better prognosis in gastric cancer patients, especially when it is performed in addition to classical chemotherapy.
Immune checkpoints represent inhibitory pathways that are critical for maintaining self-tolerance and physiological homeostasis by controlling the intensity of physiological immune responses to prevent tissue injury, particularly when the immune system is fighting an infection. Additionally, they may also allow immune escape of cancer cells[36].
Immune checkpoint molecules, such as cytotoxic T lymphocyte protein-4 (CTLA-4) and programmed cell death protein-1 (PD-1), are involved in the inhibition of T cell activation via different pathways.
CTLA-4 is a co-inhibitory molecule exhibited on activated T lymphocytes and T regulatory cells, whose receptor on T cells interacts with its B7-1/B7-2 ligands located on antigen-presenting cells (APCs), subsequently suppressing the T cell stimulatory signal mediated by CD28[178]. CTLA-4 expression is stimulated only in the context of T cell activation; afterwards, it competes with CD28 to bind to B7 molecules and decrease the immune response. By inhibiting this interaction using an anti-CTLA-4 antibody, T cell activation and proliferation is promoted, along with a decrease in immunosuppressive Treg cells among TILs[179].
PD-1 represents a co-inhibitory receptor that is found on the surface of several types of cells, such as activated T cells, Treg cells, and monocytes. It has two ligands, PD-L1 and PD-L2. PD-L1 is expressed on both immune and tumor cells, while PD-L2 is mostly expressed on APCs. In tumors, PD-L1 that is expressed on tumor cells binds to PD-1 on activated T cells that reach the tumor and generates a suppression signal for the activation of T cells, which become unable to destroy tumor cells, leading to a decrease in both cellular and humoral immune responses[180,181]. Unlike CTLA-4, which is considered to be necessary for T cell activation, the PD-1/PD-L1/2 pathway seems to protect tumor cells from attack by T lymphocytes. It has been demonstrated that by inducing antibody-mediated blockage of the PD-1/PD-L pathway followed by the inhibition of this checkpoint, treatment is able to enhance the anticancer immune response of the host[22,182].
Several genetic studies have shown a possible correlation between PD-1 or CTLA-4 polymorphisms and the development of gastric cancer[183-185]. Additionally, the role of CTLA-4 gene promoter hypermethylation has been demonstrated as a risk factor for gastric tumor development, with CTLA-4 expression being significantly higher in gastric tumor samples vs normal tissue[186].
Prognostic significance of PD-1/PD-L1 and CTLA-4 expression in gastric cancer: Saito et al[187] showed that PD-1 expression in CD8+ and CD4+ T cells in gastric tumors was significantly higher vs in normal gastric mucosa. Wu et al[188] showed that PD-L1 expression was encountered in 42% of gastric cancer tissues but not in normal gastric mucosa.
A meta-analysis by Gu et al[189] that was based on 15 studies (most of them conducted in Asia) including 3291 patients, showed a high variability of PD-L1 immunohistochemical expression among studies, ranging between 14.3% and 69.4%, due to the differences in cut-off values (between > 1% and > 50%).
Unlike in other tumors, such as lung cancer or melanoma, there is scattered PD-L1 expression in gastric cancer cells, which mostly occurs in infiltrating myeloid cells at the tumor invasive front[25,58].
There are several papers suggesting that PD-L1 expression in tumor cells may be upregulated by genomic alterations and oncogenic signaling, either through the phosphatidylinositol-3-kinase-protein kinase B (PI3K-AKT) or signal transducers and activators of the transcription (STAT) 3 pathway. Moreover, PD-L1 is upregulated by the microRNA-200/zinc-finger E-box-binding homeobox 1 (ZEB-1) axis, which is closely related to epithelial-mesenchymal transition (EMT) conversion and to IFN-γ produced by TILs[190-192]. Mimura et al[193] showed that membranous PD-L1 immunohistochemical expression in gastric tumor cells was significantly correlated with the number of CD8+ TILs and IFN-γ positive cells in the tumor. Additionally, an enhanced cytotoxic effect of anti-PD-L1 monoclonal antibody treatment was observed after prior IFN-γ exposure.
Literature data regarding the expression of PD-1/PD-L1 as a prognostic in gastric cancer showed controversial results. Dai et al[53] reported an association between PD-L1 expression and an increased density of TILs; this increased density of TILs and higher levels of PD-L1 mRNA in gastric cancer was significantly correlated with a better prognosis. Some studies showed a significantly improved prognosis in patients with PD-L1 positive tumors[194,195]; conversely, others reported an association between PD-1/PD-L1 expression in cancer cells and TILs and advanced tumors, increased tumor size, the presence of deep invasion, lymph node metastasis, and perineural invasion and a significantly worse prognosis in these patients[196-198]; and the third group of papers found no influence of PD-L1 on the prognosis of gastric cancer[199].
A few recent meta-analyses have shown a correlation between PD-L1 and gastric cancer prognosis, demonstrating that PD-L1 overexpression is a worse prognostic factor in these patients[200-202]. Additionally, Zhang et al[203] performed a recent meta-analysis that included ten studies with 1901 gastric cancer patients and showed that PD-L1 expression was associated with a shorter OS and a poor clinicopathological status.
Fang et al[204] highlighted that PD-L1 was expressed both in tumor cells and in TILs. PD-L1 positivity in tumor cells was associated with differentiation, while its expression in TILs was correlated with a late stage of the disease, no surgery and the OS. Patients with PD-L1+ TILs had a significantly poorer 5-year OS than those without PD-L1 expression (14.2 vs 18.3; P = 0.001). A study by Gao et al[205] showed that PD-L1 and PD-L2 positivity in primary tumors and metastatic lymph nodes decreased the number of CD8+ T cells, and the amount of PD-1 positive expression on CD8+ T cells in primary tumors were prognostic factors that were correlated with a poor prognosis in stage II/III gastric cancer patients.
Again, the results of the meta-analysis by Gu and collaborators[189], showed that gastric cancer patients with deeper tumor infiltration, lymph node metastasis, venous invasion, and EBV+ and MSI subtypes were more likely to be PD-L1 positive. Moreover, for the subgroups of Asian patients and patients with stage II/III gastric cancer, cut-off values greater than 50% and cytoplasm/nuclear PD-L1 expression within tumor cells were positively associated with the OS. The results of this meta-analysis demonstrated that PD-L1 overexpression represented a significantly adverse prognostic factor in gastric cancer, fitting the theory of the cancer immunity cycle[47].
Many other studies[32,206] have also demonstrated that EBV+ and MSI gastric tumors tend to be positive for PD-L1 expression. These subtypes have a rich infiltration of lymphocytes, especially CD8+ T cells, in the tumor stroma; therefore, they may be categorized as medullary carcinomas. Moreover, in this context, PD-L1+ expression is associated with a significant increase in the number of CD8+ T cells at the tumor invasive front and with the ability of immune cells to infiltrate the center of the tumor[32]. Both EBV+ gastric cancers and MSI+ gastric cancers have IFN-γ response genes, therefore, PD-1 pathway signaling seems to be a crucial mechanism for controlling a previous cytotoxic anticancer immune response[189]. The EBV+ subtype is associated with amplification of the 9p24.1 locus, which harbors the PD-L1/PD-L2 genes; PD-L1 positivity is found in 50% of the cancer cells and 94% of immune cells in this subgroup[194,207]. Additionally, approximately 33% and 45% PD-L1+ expression levels are encountered on tumors cells and immune cells, respectively, in MSI gastric tumors[208]. These data suggest that EBV+ and MSI gastric cancers may be preferred candidates for PD-1 blockade immunotherapy. These types of neoplasias that are associated with rich inflammatory infiltrates are considered “hot” or inflamed tumors, while poorly immunogenic cancers are termed “cold.” “Hot” tumors are characterized by the expression of immune-inhibitory signals, such as PD-L1, indoleamine-2,3-dioxygenase (IDO), and Treg cells[209,210], which counterbalance the effects of cytotoxic T lymphocytes. Therefore, using combined treatments to convert cold into hot tumors may increase the proportion of patients who benefit from immunotherapy[211].
While some data from non-small cell lung cancer (NSCLC) and other cancers have revealed that PD-L1 immunohistochemical positivity in cancer and/or in immune cells (bioptic specimens) is correlated with beneficial results after checkpoint inhibitor immunotherapy using monoclonal antibodies[212], other studies have shown tumor responses to PD-L1 therapies in tumors with PD-L1- cancer cells[213].
The location of PD-L1 expression in TILs situated at the invasive front or even at the tumor center may negatively influence its use as a biomarker. Furthermore, stromal rather than membranous expression of PD-L1 may be the cause for the slightly poorer responses to single-agent PD-1 checkpoint inhibitors in gastric cancer vs other tumor types[208].
Because there are variations in the techniques, the antibody clones used, and the cutoff values for PD-L1 positivity that limit cross-trial comparisons of different tumor types, including gastric cancer, some researchers have proposed harmonization of PD-L1 testing to standardize the results. Recently, Jiang et al[214] elaborated an immunohistochemical-based immunoscore on a cohort of 879 Chinese gastric cancer patients and demonstrated that a high immunoscore corresponded to lower recurrence rates and improved OS after adjuvant therapy.
Data suggest that there is racial and geographical variability in the tumor-immune microenvironment that is related to different responses to immunotherapy; for example, non-Asian gastric cancer patients have tumors that are rich in TILs and are associated with high CTLA-4 signaling[215].
The study by Schlößer et al[198] found positive CTLA-4 expression in the tumor microenvironment of 86% of gastric cancer patients that was correlated with poor OS.
Clinical trials using checkpoint inhibitors: Clinical trials using CTLA-4 and PD/PD-L1 checkpoint inhibitors are listed in Table 1.
| Checkpoint inhibitors | Study number | Phase | Status | Study design | Study population | Primary outcome measures | Secondary outcome measures | No. of patients (estimated enrollment) | Final results |
| Nivolumab, Ipilimumab | NCT03342417 | II | Recruiting | NIVO + IPI | -Neoadjuvant breast cancer -Platinum-resistant advanced ovarian/GC | - % AE | - DOR, OS, QOL, recurrence rate | 60 | Pending |
| Nivolumab, Ipilimumab | NCT02872116 (CHECKMATE-649) | III | Recruiting | - NIVO + IPI/ -NIVO + chemo vs chemotherapy (XELOX/FOLFOX) | -Naive advanced/metastatic GC/GEJ | -OS NIVO + IPI vs chemo (PD-L1 + tumors) -OS NIVO + chemo vs chemo -ORR, PFS nivo + chemo vs chemo | -OS NIVO + IPI vs chemo -PFS NIVO + IPI/ NIVO + chemo vs chemo (PD-L1+) -ORR NIVO + chemo vs chemo | 1349 | Pending |
| Nivolumab, Ipilimumab | NCT02935634 (FRACTION-GC) | II | Recruiting | - NIVO + IPI -NIVO + RELATLIMAB -NIVO + BMS-986205 | -Advanced GC | -ORR, DOR, PFS | -% AE, SAE, discontinuation/death due to treatment | 300 | Pending |
| Nivolumab, Ipilimumab | NCT03044613 | Ib | Recruiting | - NIVO/NIVO + IPI prior to chemoradiation + NIVO | -Neoadjuvant treatment, resectable stage II/III EC/GEJ | -% AE | -Feasibility of induction treatment -Path CR -Quantity of NIVO bound to PD1 receptor -Changes in expression of immune markers -OS, RFS | 32 | Pending |
| Nivolumab, Ipilimumab | NCT03443856 (VESTIGE) | II | Not yet recruiting | - NIVO + IPI | -Adjuvant treatment GC/GEJ adenocarcinoma stage Ib-IVa, ↑risk of recurrence (ypN1-3 + /R1) after neoadjuvant treatment + resection | -DFS | -OS -Relapse rate -Loco-regional/distant failure rates -% AE, QOL, global health status | 240 | N/A |
| Nivolumab, Ipilimumab | NCT03409848 (INTEGA) | II | Recruiting | -IPI/FOLFOX + NIVO and TRAS | -Advanced/metastatic GC adenocarcinoma, previously untreated | -OS | -% AE -PFS, RR, QOL -Translational research -Central imaging review | 97 | Pending |
| Nivolumab, Ipilimumab | NCT02834013 | II | Recruiting | -NIVO + IPI | -Rare tumors, including gastric NET, SqCC, GIST | -ORR-RECIST | -Toxicities -OS, PFS -Clinical benefit rate -ir-ORR/PFS | 707 | Pending |
| Nivolumab, Ipilimumab | NCT03126110 | I/II | Recruiting | -NIVO + INCAGN01876 -IPI + INCAGN01876 -NIVO + IPI + INCAGN01876 | -Advanced/metastatic malignancies, including GC | -Toxicities (%AE) -ORR-RECIST | -DR, DDC, PFS, OS- RECIST | 450 | Pending |
| Nivolumab, Ipilimumab | NCT03241173 | I/II | recruiting | -NIVO + INCAGN0949 -IPI + INCAGN0949 -NIVO + IPI + INCAGN0949 | -Advanced/ metastatic malignancies, including GC | -Toxicities (%AE) -ORR-RECIST | -DR, DDC, PFS, OS- RECIST | 651 | Pending |
| Ipilimumab | NCT01585987 | II | Completed | -IPI vs BSC (5-FU) | -Unresectable/ metastatic GC/GEJ adenocarcinoma (following Ist line treatment) | -ir-PFS (ir RECIST) -% BOR | -PFS (mWHO) -OS | 143 | -ir-PFS ↓ (2.92 vs 4.89 mo); -mWHO-PFS↓ (2.72 vs 4.89) (P = 0.03); -OS at study completion 12.68 vs 12.06 mo |
| Tremelimumab, Durvalumab | NCT02658214 | I | Recruiting | -TREME + DURVA + chemo (platinum-based SOC) | -Ist line locally advanced/ metastatic solid tumor, including GC/GEJ | -Laboratory findings -%AE, safety, tolerability -Tumor assessment (RECIST) | - | 42 | Pending |
| Nivolumab | NCT03453164 (CIRCUIT) | I/II | Recruiting | NIVO + radiotherapy | -Unresectable recurrent GC (3 rd line) | -DCR: -PD: on CT/ MRI/ PET-CT -CR/PR/SD | -Mean survival -% AE -Local control rate -% PL-L1+, MHCI- tumor cells -Cytokines serum concentration -% regT cells -% Ag-specific CTL | 40 | Pending |
| Nivolumab | NCT02267343 | III | Active, not recruiting | NIVO vs placebo | -Refractory, unresectable, advanced/ recurrent GC/GEJ | -OS, PFS | -ORR, DOR, %AE, %SAE, safety | 480 | Pending |
| Nivolumab | NCT02746796 | II/III | Recruiting | NIVO + chemo | -Ist line therapy, unresectable advanced/ recurrent GC/GEJ | -PFS -OS | -ORR, DOR, DCR -TTR, BOR -% AE, SAE, laboratory abnormalities | 680 | Pending |
| Nivolumab | NCT03006705 | III | Recruiting | NIVO + chemo vs placebo + chemo | -Adjuvant treatment p stage III GC/GEJ (after D2 resection) | -RFS | -OS -Safety- % AE, SAE, laboratory abnormalities | 700 | Pending |
| Nivolumab | NCT02999295 | I/II | Recruiting | -NIVO + RAMUCIRUMAB | -Advanced/ recurrent unresectable GC/GEJ | -No. of pts with DLT -6 mo PFS | -%AE -ORR -DCR -OS, PFS | 44 | Pending |
| Nivolumab | NCT02946671 | I | Recruiting | -NIVO + MOGAMULIMUMAB (KW-0761 = anti-CCR4) | -Preoperator treatment against solid cancers, including GC | -% AE - FOXp3 + tumors by immunohistochemistry | -ORR-RECIST -% ↓Treg | 18 | Pending |
| Nivolumab | NCT02951091 (Biomarker – integrated Umbrella) | Observational | Recruiting | -NIVO/ AFATINIB/ GSK2636771 + PACLITAXEL | -Advanced GC -Different molecular cohorts: PD-L1+, MSI-H, EBV+ → NIVO | -PFS | - | 400 | Pending |
| Nivolumab | NCT02465060 (The MATCH screening trial) | II | Recruiting | -NIVO/ other agents (according to genetic testing) | -Advanced/ metastatic solid tumors (including GC), lymphomas, multiple myelomas → mismatch repair deficiency (loss of MLH1/ MLH2) | -ORR | -OS, PFS -TTP | 6452 | Pending |
| Nivolumab | NCT02862535 | Ib | Active, non-recruiting | -ANDECALIXIMAB (GS-5745) ± NIVO/chemo | -Previously treated, advanced GC/GEJ adenocarcinoma (Japan) | -Safety (% AE) | - Serum concentration of Andecaliximab -% Ab-anti Andecaliximab | 36 | N/A |
| Pembrolizumab | NCT03382600 (MK-3475-659/ KEYNOTE 659) | II | Recruiting | -PEMBRO + OXALIPLATIN + TS-1 vs -PEMBRO + CISPLATIN + TS-1 | -Advanced CG/GEJ adenocarcinoma, HER2(-), PD-L1+ | -ORR-RECIST | -ORR (i-RECIST) -DCR, DOR, TTR, PFS (RECIST, iRECIST) -OS, % AE | 90 | Pending |
| Pembrolizumab | NCT02901301 | I/II | Recruiting | -PEMBRO + TRASTUZUMAB + chemo | -Ist line advanced, GC HER2+ | -Recommended dose -ORR-RECIST | -DOR -TTR | 49 | Pending |
| Pembrolizumab | NCT03342937 | II | Recruiting | PEMBRO + XELOX | -Ist line metastatic GC adenocarcinoma | -PFS | -OS - % AE | 50 | Pending |
| Pembrolizumab | NCT02918161 | II | Recruiting | PEMBRO + chemo (SOC) | -Perioperative setting GC/GEJ | -2 years DFS | - Pathol CR -OS, ORR (RECIST) -DFS | 40 | Pending |
| Pembrolizumab | NCT02689284 | I/II | Recruiting | PEMBRO + MARGETUXIMAB | -HER2 + advanced, metastatic GC/GEJ | - Expansion phase dose of Margetuximab -Antitumor activity: RD, ORR (RECIST, ir-RECIST) | -OS -PFS | 72 | Pending |
| Pembrolizumab | NCT03257163 | II | Recruiting | -PEMBRO + CAPECITABINE + radiotherapy (perioperative) | -Mismatch repair deficient, EBV+, operable GC | -RFS | - | 40 | Pending |
| Pembrolizumab | NCT03064490 (PROCEED) | II | Recruiting | -PEMBRO + chemoradiotherapy | -Neoadjuvant treatment, locally advanced EG cancers | -Pathol CR | -Toxicity (% AE) | 38 | Pending |
| Pembrolizumab | NCT02563548 | Ib | Recruiting | -PEMBRO + PEGPH2O (Pegylated Recombinant Human Hyaluronidase) | -Hyaluronan-high (HA-H) patients with relapsed/ refractory cancers (adenocarcinoma) | -ORR | -DCR, DOR, PFS (RECIST, ir-RECIST) | 81 | Pending |
| Pembrolizumab | NCT02954536 | II | Recruiting | -PEMBRO+TRASTUZUMAB+chemo | -Advanced, metastatic HER2+, EG (Ist line) | -PFS (RECIST) | - | 37 | Pending |
| Pembrolizumab | NCT03221426 (MK-3475-585) (KEYNOTE-585) | III | Recruiting | -PEMBRO + cemo vs placebo + chemo | -Neoadjuvant/adjuvant previously untreated GC/GEJ adenocarcinoma | -OS - EFS event-free survival) -Pathol CR -% AE + discontinuation of treatment | -DFS | 860 | Pending |
| Pembrolizumab | NCT03196232 | II | Recruiting | -PEMBRO + EPACADOSTAT | -Metastatic/ unresectable GEJ | -6-mo PFS | -ORR (RECIST) -OS -RR | 30 | Pending |
| Pembrolizumab | NCT03019588 (MK-3475-063/ KEYNOTE-063) | III | Recruiting | -PEMBRO vs chemo (PACLITAXEL) | -Progression after Ist line platinum-fluoropyridine chemo, advanced GC/GEJ adenocarcinoma, PD-L1+ (Asia) | -OS, PFS (RECIST) | -ORR-RECIST -% AE, % discontinuation due to AE | 360 | Pending |
| Pembrolizumab | NCT03488667 | II | Not yet recruiting | PEMBRO + mFOLFOX | -Neoadjuvant treatment GEJ adenocarcinoma -adjuvant treatment GC | -yp RR (pathologic response) -toxicity (% AE) | -ORR, DFS, OS -PET scan response rate - % PD-L1 + in tumor cells | 40 | N/A |
| Pembrolizumab | NCT03413397 | II | Recruiting | PEMBRO + LENVATINIB MESYLATE | -Metastatic/ recurrent GC/GEJ | -ORR-RECIST | -PFS, OS -Characteristic immunologic changes | 29 | Pending |
| Pembrolizumab | NCT02730546 | I/II | Recruiting | PEMBRO + chemoradiotherapy | -Locally advanced, operable, GEJ/gastric cardia adenocarcinoma (neoadjuvant setting) | -Path CR -PFS | -R0 resection, DSF -Dose-limiting AE, % surgical complications, OS, PFS, time to relapse | 68 | Pending |
| Pembrolizumab | NCT02318901 | Ib/II | Active, not recruiting | PEMBRO-TRASTUZUMAB/ADO-TRASTUZUMAB-ETAMSINE/CETUXIMAB | -Unresectable HER2+, advanced GC/GEJ | -Dose of mAb combined with PEMBRO | -% grade 3-4 AE -RR (RECIST, ir-RECIST) -OS, PFS -Circulating tumor DNA -Imaging changes | 90 | N/A |
| Pembrolizumab | NCT03095781 | I | Recruiting | PEMBRO + XL888 (= Hsp90 inhibitor) | -Advanced gastrointestinal cancer (including GC) | -Recommended dose for combined treatment | -ORR, PFS, RS (RECIST) -OS | 50 | Pending |
| Pembrolizumab | NCT02346955 | I | Terminated | -CM-24[MK-6018 = mAb against CEACAM1] ± PEMBRO | -Advanced/recurrent malignancies (including GC) | -% AE, discontinuation due to AE, DLT | -Maximum drug concentration, half-life elimination, ORR, DOR | 27 | Pending |
| Pembrolizumab | NCT02178722 (KEYNOTE-037/ECHO-202) | I/II | Recruiting | -PEMBRO + EPACADOSTAT | -Selected carcinomas (including GC) | -% DTL -ORR | -PFS -% AE -OS | 508 | Pending |
| Pembrolizumab | NCT02903914 | I/II | Recruiting | -PEMBRO + ARGINASE INHIBITOR INCB001158 | - Advanced/metastatic solid tumors (including GC) | -% AE | - Rrecommended dose of arginase Inhibitor ± PEMBRO -Pharmacokinetic profile -Antitumor activity of drugs (RECIST, ir RECIST) | 346 | Pending |
| Pembrolizumab | NCT03122548 | II | Active, not recruiting | PEMBRO + CRS-207 | -Recurrent/metastatic GC/EG (1-2 prior lines of systemic treatment) | -% AE | -Tumor response (RECIST) -OS -Characterization of immune response -Analysis of biomarker expression | 79 | N/A |
| Pembrolizumab | NCT02393248 | I/II | Recruiting | -INCB054828 + PEMBRO/ chemo/ TRASTUZUMAB | -Advanced malignancies (including GC), progression after prior treatment | -Maximum tolerated dose, pharmacodynamic of INCB054828 | -ORR -Maximum/minimum plasma Concentration of NCB054828 | 280 | Pending |
| Pembrolizumab | NCT02494583 | III | Active, not recruiting | -PEMBRO vs -PEMBRO + chemo vs -Placebo + chemo | -Ist line treatment, advanced GC/GEJ | -PFS (RECIST) -OS | -ORR, DOR (RECIST) -QOL | 764 | N/A |
| Pembrolizumab | NCT02370498 (KEYNOTE-061) | III | Active, not recruiting | -PEMBRO vs chemo (PACLITAXEL) | -Advanced GC/GEJ adenocarcinoma, Progressed after Ist line (platinum + fluoropyrimidine), PD-L1+ | -PFS, OS- in PD-L1+ | -PFS, OS -TTP, ORR | 592 | Pending |
| Pembrolizumab | NCT02335411 (KEYNOTE-059) | II | Active, not recruiting | -PEMBRO or PEMBRO + chemo (CISPLATIN + 5-FU/CAPECITABINE) | -Recurrent/metastatic GC/GEJ adenocarcinoma | -% AE, discontinuation of treatment due to AE -ORR | - | 316 | Pending |
| Pembrolizumab | NCT03277352 | I/II | Recruiting | -INCAGN01876 + PEMBRO + EPACADOSTAT | -Advanced/ metastatic malignancies | -% AE -ORR, CRR (RECIST) | -ORR, DCR, DOR, PFS, OS (rECIST, mRECIST) | 166 | Pending |
| Pembrolizumab | NCT02443324 | I | Active, not recruiting | -PEMBRO + RAMUCIRUMAB | -GC/GEJ (NSCLC, transitional urothelial cancer, biliary tract cancer) | -DTL | -% BOR of CR/PR, ORR -% SD -DOR, time to response -PFS, OS -Pharmacokinetics | 155 | Pending |
| Avelumab | NCT02625623 (JAVELIN GASTRIC 300) | III | Active, not recruiting | -AVE + BSC vs -chemo+BSC/BSC | -Unresectable, recurrent, locally advanced/ metastatic GC/GEJ adenocarcinoma (3rd line) | -OS | -PFS, BOR -QOL | 37 | Pending |
| Avelumab | NCT03399071 (ICONIC) | II | Recruiting | -AVE + chemo (FLOT) | -Perioperative setting, operable EC/GC | -Pathol CR | -% grade 3-4 AE -Radiologic response (RECIST) -median PFS, OS | 40 | Pending |
| Avelumab | NCT02625610 (JAVELIN GASTRIC 100) | III | Active, not recruiting | -AVE maintenance vs Ist line continuation of chemo (OXALIPLATIN + FLUOROPYRIMIDINE) | -Unresectable, locally advanced/ metastatic GC/GEJ adenocarcinoma | -OS, PFS | -BOR (RECIST) -QOL -% AE | 499 | Pending |
| Avelumab | NCT01943461 (JAVELIN SOLID TUMOR JPN) | I | Active, not recruiting | -AVE | -Locally advanced/ metastatic solid tumors (Japan) → expansion part GC patients (Asia) | -DLT | -Concentration assessment, elimination half-life -% PD-L1 -BOR+ irBOR, PFS +irPFS -OS % Ab-anti AVE -% AE | 57 | Pending |
| Avelumab | NCT03475953 (REGOMUNE) | I/II | Not yet recruiting | -AVE + REGORAFENIB | -Advanced/metastatic digestive solid tumors (including GC) | -Recommended doses -Assessment of Regorafenib antitumor activity -Pharmacokinetics | -Maximum tolerated dose -DLT -% AE -BOR, ORR, PFS, OS -Blood/tumor growth biomarkers | 212 | Pending |
| Avelumab | NCT02554812 (JAVELINE Medley) | Ib/II | Recruiting | -AVE + other immunotherapies → AVE + PD 0360324 (M-CSF mAb) (gastric cancer) | -Locally advanced/ metastatic solid tumors (including GC) | -% DLT -ORR | -Serum concentration of drugs -Ab-anti drugs -TTR, DOR, PFS, OS -Tumor biomarkers (PD-L1, CD8+T cells) | 560 | Pending |
| Durvalumab | NCT02734004 (MEDIOLA) | I/II | Active, not recruiting | -DURVA + OLAPARIB (PARP inhibitor) | -Advanced solid tumors (including GC) | -DCR (at CT/MRI) -% AE -Safety- vital signs, blood samples | -% PD-L1 -DCR (mRECIST) -Time to treatment discontinuation) -OS -% change in tumor size (CT/MRI) -Serum concentration of Ab-anti drug -Pharmacokinetics -ORR, DOR, PFS (mRECIST) | 148 | Pending |
| Durvalumab | NCT02572687 | I | Active, not recruiting | DURVA + RAMUCIRUMAB | -Locally advanced unresectable/ metastatic gastrointestinal (including GC/GEJ adenocarcinoma) and thoracic malignancies | -% DLT | -ORR, DCR -DOR, TTR -PFS -OS -Pharmacokinetics -% Ab-anti drug | 114 | Pending |
| Durvalumab | NCT02678182 (PLATFORM) | II | Recruiting | -DURVA vs. CAPECITABINE vs. TRASTUZUMAB vs. RUCAPARIB vs. surveillance | -Maintenance treatment, locally advanced/ metastatic HER2+/-EG, adenocarcinoma (after Ist line chemo) | -PFS (RECIST) | -PFR -OS, ORR (RECIST) -% AE -PFS, PFR, OS, ORR according to PD-L1 immunohistochemical status | 770 | Pending |
| Durvalumab | NCT02264678 | I/II | Recruiting | -AZD6738 ± Chemo/OLAPARIB/DURVA | -Advanced malignancies (including GC) | -Safety, tolerability (AE, SAE) | -Pharmacodynamics, biomarker changes -Serum concentration of drug, half-life, etc. -BOR, ORR, % change in tumor size | 250 | Pending |
(1) CTLA-4 checkpoint inhibitors
Tremelimumab
Tremelimumab (formerly ticilimumab, CP-675,206) is a fully human monoclonal antibody against CTLA-4 that received FDA approval for the treatment of mesothelioma. It was the first immune checkpoint inhibitor that was investigated in patients with gastroesophageal tumors.
A phase II study[216] investigated tremelimumab (at a dose of 15 mg/kg every 90 d, which is presently considered sub-therapeutic) in 18 patients with advanced esophageal, gastroesophageal junction or gastric adenocarcinoma. Although the median time to progression and OS were relatively short, at 2.83 and 4.83 mo, respectively, approximately one-third of patients were alive at one year. Currently, there is an ongoing clinical trial assessing whether tremelimumab is associated with the anti-PD-L1 antibody durvalumab; the dose of tremelimumab used as a monotherapy is 10 mg/kg every 4 wk[9].
Ipilimumab
Ipilimumab (trade name Yervoy, previously known as MDX-010 and MDX-101) was approved by FDA for the treatment of patients with unresectable/metastatic melanoma, after at least one line of systemic treatment has been performed (2011)[217]; the indications for this drug were recently extended to include pediatric patients (12 years and older) (2017) and adjuvant treatment of patients with cutaneous melanoma with pathologic involvement of regional lymph nodes greater than 1 mm in size who have undergone complete resection (including total lymphadenectomy); in addition, it was recently approved as the first-line treatment, in combination with nivolumab, for patients with intermediate or poor risk with advanced renal cell carcinoma (2018).
A randomized phase II trial enrolled patients with unresectable, locally advanced/metastatic gastric or gastro-esophageal junction cancer with partial response/stable disease after first-line chemotherapy with a combined fluoropyrimidine plus platinum regimen to receive either the best supportive care (consisting of continuation of fluoropyrimidine) or ipilimumab. The primary end-point of the study was immune-related PFS. Unfortunately, the study was ended earlier due to the lack of clinical efficiency of ipilimumab. The PFS was shorter, and the toxicities were higher in the ipilimumab group, with similar OS for both groups[218].
(2) PD-1 and PD-L1 checkpoint inhibitors
Anti-PD-1 antibodies - Nivolumab
Nivolumab is a humanized immunoglobulin G4 monoclonal antibody (mAb) that targets PD-1 and has shown efficacy in various cancer types. The FDA approved nivolumab for the treatment of recurrent/metastatic melanoma, metastatic non-small cell lung carcinoma/recurrent or metastatic squamous cell carcinoma of the head and neck (after progression on platinum-based chemotherapy), advanced renal cell carcinoma after antiangiogenic treatment, and Hodgkin lymphoma that has relapsed/progressed after autologous hematopoietic stem cell transplantation.
The phase I/II CheckMate-032 clinical trial analyzed the safety and efficacy of nivolumab as monotherapy or combined with ipilimumab in heavily pretreated patients with advanced/metastatic solid cancers, including gastric tumors (NCT01928394)[219]. The partial results assessing 160 patients included in three different arms [nivolumab 3 mg/kg (N3), nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (N1+I3), and nivolumab 3 mg/kg + ipilimumab 1 mg/kg (N3+I1)] showed more frequent toxicities in the N1+I3 group. The most commonly encountered adverse events in all the subsets were fatigue, pruritus, nausea, diarrhea and loss of appetite; thyroid damage was more frequent in the N1+I3 group. The overall objective response rate (ORR) was 16%, which was the highest in the N1+I3 group (26%); the disease control rate (DCR) was 38%. The median OS was longest in the N1+I3 arm (6.9 mo), followed by the N3 (5.0 mo) and the N3+I1 arms (4.8 mo). The response rates for patients with PD-L1+ tumors (> 1% of immunohistochemically-positive cells), both in patients treated with nivolumab monotherapy and combined treatment N1+I3 and N3+I1, were superior compared to patients with negative tumors (19% vs 12%, 40% vs 22% and 23% vs 0%, respectively).
Because of the promising results obtained using N1+I3 combination, the CheckMate-649 phase III clinical trial (NCT02872116) was initiated. In this study, the clinicians plan to investigate 1349 patients with naive advanced or metastatic gastric/gastroesophageal junction (GEJ) cancer, with both positive/negative PD-L1 expression to receive first line nivolumab plus ipilimumab or nivolumab plus standard chemotherapy (XELOX or FOLFOX) vs standard chemotherapy (XELOX or FOLFOX), having as a primary endpoint the OS in patients with PD-L1+ tumors[220]. The data from the double-blinded, randomized, multicentric phase III trial ONO-12 (ATTRACTION 2) were presented at the ASCO Gastrointestinal Cancers Symposium 2017, demonstrating the benefits of nivolumab as a salvage treatment (third or later line) in patients with advanced unresectable or recurrent gastric or gastroesophageal junction cancer, either PD-1/L1+ or - vs placebo (NCT022673430). It was the first time that a prolonged OS was obtained for patients with heavily pretreated tumors using PD-1 inhibition[221]. There were 493 patients randomly assigned (2:1) to receive nivolumab (n = 330) or placebo (n = 163). The median OS was significantly increased with nivolumab vs placebo (5.26 mo vs 4.14 mo with placebo). The risk of death was lower in the nivolumab group vs placebo group (HR = 0.63; P < 0.0001); 68.5% of the patients in the nivolumab group died vs 86.5% in the placebo group. Additionally, nivolumab treatment was associated with a significantly better OS rates at 6 and 12 mo compared to placebo (46.1% vs 34.7% and 26.2% vs 10.9%), Nivolumab treatment led to a longer median PFS (1.61 mo vs 1.45 mo) and higher ORR than placebo (11.2% with nivolumab vs 0%). Among the approximately 40% (n = 192) of patients with tumor samples, 12.3% in the nivolumab group and 16.1% in the placebo group had PD-L1 positive tumors. The analysis of PD-L1 expression status showed that median OS in patients with PD-L1+ tumors was 5.22 mo in the nivolumab group and 3.83 mo in the placebo group (HR = 0.51). In patients with PD-L1- tumors, median OS was 6.05 mo in the nivolumab group, and 4.19 in the placebo group (HR = 0.72). Nivolumab resulted in an absolute survival benefit of 1.1 mo in median OS vs placebo. Nivolumab led to durable OS benefit that was sustained beyond one year vs placebo in heavily pretreated gastric cancer patients, regardless of PD-L1 expression status[222]. Because of the survival benefit demonstrated by the ATTRACTION-2 trial in this subset of difficult-to-treat gastric cancer patients, the approval of nivolumab in Japan was granted as a new treatment option that is beneficial for heavily pretreated advanced gastric cancer.
Anti-PD-1 antibodies - Pembrolizumab
Pembrolizumab (formerly MK-3475, trade name Keytruda) is a humanized IgG4 isotype antibody that targets the PD-1 receptors on lymphocytes. It has FDA approval for the treatment of unresectable or metastatic melanoma, metastatic non-small cell lung cancer (NSCLC) (certain situations), metastatic non-squamous NSCLC (PDL1+/-), as a second-line treatment for head and neck squamous cell carcinoma (HNSCC), after platinum-based chemotherapy, and for the treatment of adult/pediatric patients with refractory classic Hodgkin’s lymphoma.
The large multi-cohort, multicenter, nonrandomized, open-label phase Ib KEYNOTE-012 first demonstrated the efficacy of pembrolizumab as monotherapy (administered in a dose of 10 mg/kg every 2 wk or a 200 mg fixed dose every 3 wk) in PD-L1+ recurrent or metastatic gastric or gastroesophageal cancer (NCT01848834)[25]. Of all the patients assessed, 39 patients (40%) had PD-L1+ tumors, most of them being heavily pretreated patients. Treatment with single-agent pembrolizumab determined partial response (PR) in 22% and sustained disease (SD) in 13% of patients. The median PFS was 1.9 mo, with a 6 mo PFS of 26% and a median OS of 11.4 mo. The most common adverse events included loss of appetite, fatigue, pruritus, arthralgia and hypothyroidism. Pembrolizumab was well-tolerated, with 13% of patients developing grade 3-4 toxicity. It is important to stress that approximately 60% of patients enrolled in this trial have previously received more than three lines of chemotherapy, representing a group of patients without any therapeutic response demonstrated by other studies. Almost two-thirds of the tumors revealed genomic profiling of a microsatellite-instability high (MSI-H) status. This genomic status correlates with high tumor mutational burden, and patients with MSI-H cancers, colorectal and non-colorectal, have developed encouraging responses to anti-PD1 therapy in various solid tumors[23]. This aspect remains to be established if present in gastroesophageal cancers. Additionally, in this study, tumor response correlated with an increased interferon-γ gene expression[223].
With the goal of improving immune treatment efficacy and having the knowledge that chemotherapy is capable of promoting immunogenic cell death[224], PD-1 inhibitors were associated with standard chemotherapy, which is a combined regimen proven to increase response rates in lung cancer[225]. The KEYNOTE-059 phase II clinical trial included 259 patients with advanced gastric or gastroesophageal cancer. Cohort 1 assessed the efficacy and safety of pembrolizumab monotherapy in patients with previously treated advanced gastric cancer. Approximately half the patients who enrolled in cohort 1 had received two or more prior treatments for metastatic cancer, whereas others received three or more prior therapies. Among 259 patients, 148 (57.1%) were PD-L1+ (by immunohistochemistry). Pembrolizumab elicited sustained ORR in 30 of 259 patients (11.6%) and complete response in 2.3%. These responses were observed irrespective of PD-L1 expression. The ORR was higher in patients with PD-L1+vs PD-L1- tumors, 23 of 148 (15.5%) vs 7 of 109 (6.4%), respectively. A T cell-inflamed gene expression profiling score was developed in this study, demonstrated to be significantly associated with pembrolizumab response; also, a significant nonlinear association was found between this score and PD-L1 expression. Of 174 patients (67.2%) assessed for MSI, seven patients (4.0%) had samples that were MSI-high. It was observed a higher ORR in patients with MSI-high tumors than in patients with non-MSI-high tumors (57.1% vs 9.0%). However, prevalence of MSI high tumors was very low in this population (4%), and most responses were observed in non-MSI-high patients. Thus, in cohort 1, pembrolizumab monotherapy showed good responses and manageable toxicities after ≥ 2 prior lines of treatment[226,227]; these results led to an accelerated FDA approval of pembrolizumab for PD-L1+ advanced gastric cancer patients as third-line treatment. In cohort 2, the enrollees were HER2- naïve patients with advanced gastric/gastroesophageal junction adenocarcinoma, who receive pembrolizumab associated with combined chemotherapy (5-fluorouracil/capecitabine plus cisplatin) for six cycles, and subsequent maintenance treatment with pembrolizumab plus 5-FU/capecitabine for a maximum period of two years/until disease progression[228]. The preliminary data showed that 94% of patients have experienced adverse events (e.g., anorexia, nausea, neutropenia); of these patients, two thirds developed grade 3-4 toxicities. The results of the study proved manageable adverse event profiles for patients receiving combined therapeutic schemes, with none of the patients discontinuing the treatment. The data showed an ORR was 60% and 68.8% in PD-L1+ patients[229].
The KEYNOTE-028 phase Ib study evaluated the role of pembrolizumab, administered in up to two years or until progression, in PD-L1+ advanced solid tumors, including esophageal and gastroesophageal junction cancers (adenocarcinoma and squamous cell cancer), with most of them having at least two prior lines of chemotherapy[230]. The interim data on 23 patients showed an ORR of 30%, with 6- and 12-mo PFS rates of 30.4% and 21.7%, respectively. The response rates were better for adenocarcinomas.
An ongoing phase II study is assessing the efficiency of pembrolizumab monotherapy, along with the analysis of immune-related gene profiles and PD-L1 expression as biomarkers for treatment response in patients with advanced cancer of the esophagus or gastroesophageal junction (adenocarcinoma or squamous cell carcinoma) (KEYNOTE-180, NCT02559687)[231], progressing on standard chemotherapy.
KEYNOTE-061 is an ongoing phase III open-label clinical trial comparing pembrolizumab versus paclitaxel in the second-line setting for patients with advanced gastric or gastroesophageal cancer (progression after first-line therapy with a platinum plus fluoropyrimidine combination) (NCT02370498)[232]. The treatment administration continues until the disease progression or the occurrence of severe toxicities; the primary endpoints are PFS and OS in the PD-L1+ population. The study randomized 592 patients to receive pembrolizumab or standard-dose paclitaxel. PD-L1 positivity was encountered in 395/592 patients enrolled. Median OS was 9.1 mo with pembrolizumab vs 8.3 mo with paclitaxel (HR = 0.82, P = 0.042); 12-mo OS rates in pembrolizumab group compared with paclitaxel group were 39.8% vs 27.1%, and 18-mo rates were 25.7% vs 14.8%. There was no difference in PFS or ORR, but pembrolizumab responses proved more durable, and the treatment effect was more prominent in patients with ECOG PS 0 (HR = 0.69), gastroesophageal junction tumors (HR 0.61) and with increasing PD-L1 expression. The safety profile observed in KEYNOTE-061 was consistent with that observed in previously reported studies of pembrolizumab, this drug demonstrating a better safety profile than paclitaxel. The agent reduced the risk of death by 18% vs paclitaxel in patients with previously treated gastric cancer and PD-L1+, although this difference did not achieve statistical significance[233].
Furthermore, the ongoing phase III KEYNOTE-062 study is randomizing PD-L1+/HER2- advanced, metastatic gastric/GEJ adenocarcinoma patients to receive as a first-line regimen either pembrolizumab or pembrolizumab, associated with fluorouracil and cisplatin (NCT02494583)[234]. The primary endpoints are represented by OS and PFS.
A phase III study (KEYNOTE-181) aims to include approximately 600 patients with previously treated advanced adenocarcinoma or squamous cell carcinoma of the esophagus or gastroesophageal junction to receive either pembrolizumab or monochemotherapy (paclitaxel, docetaxel, or irinotecan) (NCT02564263)[235].
Anti-PD-L1 antibodies - Avelumab
Avelumab (MSB0010718C, trade name Bavencio) represents a fully human anti-PD-L1 IgG1 antibody which is approved by the FDA for the treatment of metastatic Merkel cell carcinoma (patients older than 12 years) and locally advanced/metastatic urothelial carcinoma under progression, after platinum-containing treatment (2017).
Avelumab is currently assessed in JAVELIN program (NCT01772004)[236]. Patients received 10 mg/kg of avelumab every two wks as second-line treatment. For Japanese gastric cancer patients, the obtained ORR was 15%, with 43.3% of patients presenting PFS at 12 wk[237]. In this context, phase Ib trials are currently investigating avelumab in patients with advanced gastric/gastroesophageal junction cancers. The results, presented at the American Society of Clinical Oncology (ASCO) 2016 meeting, showed that the most encountered adverse events were infusion-related reactions and fatigue. In the first context (patients who had at least one prior therapy), the ORR, DCR, and median PFS were 9.7%, 29.0%, and 6.0 wk, respectively, whereas in the second setting (patients who received avelumab as first-line switch maintenance after chemotherapy), they were 9%, 57.3%, and 12.0 wk, respectively. ORR in the PD-L1+ tumors was higher vs PD-L1- tumors (18.2% vs 9.1% for the third-line treatment group and 10% vs 3.1% for the maintenance group). Starting from these data, there are two phase III ongoing studies assessing avelumab in the treatment of gastric cancer patients. One study is comparing avelumab as a third-line of treatment to best supportive care (phase III JAVELIN Gastric 300 trial) (NCT02625623), while another is assessing patients receiving avelumab as a switch maintenance after chemotherapy with 5-FU or capecitabine plus oxaliplatin (phase III JAVELIN Gastric 100 study) (NCT02625610)[238]. The Javelin Gastric 300 trial enrolled 371 patients from 147 sites in Asia, Australia, Europe, North America and South America. Recently, the companies stated that the pivotal phase III Javelin trial investigating avelumab as third-line treatment for patients with unresectable, recurrent or metastatic gastric cancer did not meet its pre-specified primary endpoint of superior OS vs chemotherapy. It represents the first trial of a checkpoint inhibitor vs an active chemotherapy comparator rather than placebo in this hard-to-treat patient population. The safety profile was consistent with that observed in previously reported studies of avelumab. The JAVELIN Gastric 300 data will be further evaluated in an effort to better understand the results.
Anti-PD-L1 antibodies - Durvalumab (MEDI4736)
Durvalumab represents an engineered human anti-PD-L1 IgG1 antibody that prevents PD-L1 binding to PD-1 and CD80. It has received FDA approval for previously treated advanced bladder carcinoma and unresectable stage III NSCLC.
A phase I study assessed the efficiency of administering durvalumab in doses up to 10 mg/kg intravenously every two weeks for up to one year in patients with solid tumors, including gastric cancer (NCT01693562)[239]. Fatigue, nausea, vomiting, rash and pyrexia were the most frequent adverse events. An ORR of 25% was obtained in patients with gastric tumors. Additionally, two patients with heavily pretreated tumors surpassed the current median PFS that was obtained with standard treatments.
An ongoing phase IB/II study is investigating patients with recurrent/metastatic gastric/gastroesophageal junction adenocarcinomas for monotherapy with durvalumab, tremelimumab, or a combination of durvalumab and tremelimumab (anti-CTLA-4) in second- or third-line setting[240].
An algorithm of current treatment in unresectable locally advanced, recurrent or metastatic gastric cancer is presented in Figure 3.
Checkpoint inhibitors in the adjuvant /neoadjuvant setting (stage II or III tumors): Because multiple lines of standard chemotherapy may harm the immune system, and immune biomarkers may be stimulated by previous chemoradiation, it was suggested that addition of checkpoint inhibition in the adjuvant or even neoadjuvant setting in earlier stages of the disease may induce a significantly higher tumor response rate[241]. It has been demonstrated that after chemoradiation neoadjuvant therapy, there is a higher number of TILs that are agglomerated in perivascular areas and arranged in lymphoid-like structures; these are features that favor the tumor response to immunotherapy[58].
A randomized phase III study (CheckMate-577) NCT02743494 is assessing the role of adjuvant nivolumab for patients with resected esophageal/gastroesophageal cancer. Investigators aim to enroll approximately 760 patients to receive either nivolumab or placebo. The primary endpoints are OS and DFS[242]. Moreover, a phase I study is currently investigating nivolumab combined with an anti-CCR4 (mogamulizumab) in the preoperative setting (NCT02946671)[243].
The combination of nivolumab and ipilimumab is going to be assessed in the adjuvant treatment of gastric/gastroesophageal junction adenocarcinomas with a high risk of recurrence - a phase II study (NCT03443856) (VESTIGE)[244].
Several ongoing phase I/II clinical trials are assessing the safety and efficacy of neoadjuvant treatment using different PD-1/PD-L1 checkpoint inhibitors, administered either concomitantly or sequentially with neoadjuvant chemoradiation. For example, a phase I trial is assessing neoadjuvant administration of nivolumab and ipilimumab in stage II/III patients (NCT03044613)[245]; another Ib phase clinical trial is assessing pembrolizumab and chemoradiation in the neoadjuvant setting of locally advanced esogastric tumors (NCT03064490/PROCEED)[246]. Several II and III phase ongoing studies are investigating the efficacy of pembrolizumab in association with chemotherapy as neoadjuvant/adjuvant treatment in gastric cancer patients[247-249]. Additionally, a phase II study is evaluating the results of administering avelumab plus chemotherapy (FLOT) in the perioperative setting[250].
The synergistic action of the PD-1/PD-L1 checkpoint inhibitors and the vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor (VEGFR) blockade was demonstrated by preclinical data[251]; the combined treatment induced an improved tumor response and better OS. This combination treatment assessed in phase I/II studies was associated with an acceptable safety profile [252,253].
A phase Ia/Ib study (NCT02443324)[254] is investigating the safety and efficacy of combining anti-PD-L1 (durvalumab) and anti-VEGFR2 antibodies (ramucirumab) in patients with refractory gastric/gastroesophageal junction tumors. The interim data of 40 patients showed a 45% DCR and a median PFS of 2.60 mo. The most frequently encountered toxicities included fatigue, infusion-related reaction, loss of appetite, pruritus, rash, and hypertension; 25% of patients had severe toxicities.
Additionally, the efficacy of associating nivolumab with ramucirumab in patients with advanced, unresectable gastric/gastroesophageal junction cancers is currently under investigation in a phase I/II study (NCT02999295)[255]. The clinical trial of nivolumab with paclitaxel and ramucirumab in patients with advanced, unresectable gastric cancer is ongoing under investigation in a phase I/II study (UMIN000025947)[256].
A phase II study is currently recruiting patients with metastatic/recurrent gastric/gastroesophageal cancer to receive pembrolizumab plus lenvatinib mesylate (anti-VEGFR2 tyrosine kinase inhibitor) (NCT03413397)[257], whereas another phase I/II study is planning to combine avelumab with regorafenib (another anti-VEGFR2 tyrosine kinase inhibitor) in advanced gastric tumors (NCT03475953/REGOMUNE)[258].
Because HER2+ tumors develop resistance to trastuzumab, studies have been evaluating a combination of PD-1 blockade plus anti-HER2 agents. Preclinical studies indicate that the HER2 blockade stimulates T cell activation and enhances interferon-γ secretion by NK cells and antibody-dependent cellular toxicity; therefore, it boosts the PD-1/L1 inhibitor efficacy[259].
The combination of pembrolizumab plus the anti-HER2 monoclonal antibody margetuximab in patients with advanced HER2+ gastric cancers that are resistant to classical trastuzumab-based chemotherapy is currently under evaluation in a phase Ib/II dose-escalation trial (NCT02689284)[260].
Other promising options include dual immunotherapies, such as the administration of PD-1/PD-L1 checkpoint inhibitors combined with agents suppressing other immune checkpoints (e.g., TIM3, LAG3) or T cell costimulatory antibodies (e.g., GITR, OX40, and 4-1BB). Additionally, other directions investigate the combination of the PD-1/PD-L1 blockade with radiation and other cytotoxic and targeted treatments (PARP inhibitors, ATR serine/threonine protein kinase inhibitors, pegylated recombinant human hyaluronidase, anti-CEACAM1, arginase inhibitor INCB001158, FGFR inhibitors, immunotherapy using Listeria bacteria to activate an immune response against specific tumor-associated antigens, claudiximab, etc.), and enzymatic inhibitors such as IDO-1[211].
Therapeutic intervention upon IDO1-mediated immune suppression involves inhibition of the catalytic activity of IDO1. Preliminary data from the ongoing studies investigating dual inhibition of both PD-1 and IDO1 in solid tumors are promising, showing an increased efficiency of the checkpoint blockade[261,262]. Currently, there are two studies of phase I/II (NCT02178722/KEYNOTE-037/ECHO-202)[263] and II (NCT03196232)[264], respectively investigating the IDO1 inhibitor epacadostat in combination with pembrolizumab in gastric tumors.
Additionally, CDC20 encoding cell division cycle protein 20 homologue[265], as well as PLK1 and TTK, which represent checkpoints in mitosis, may be used as therapeutic targets[266]. PLK1 is upregulated in tumors, and data reported from both preclinical and clinical studies suggest encouraging benefits from PLK-1 inhibition[267]. Maternal embryonic leucine zipper kinase (MELK) decreases the apoptosis of tumor cells; therefore, additional MELK inhibition may also be offered in PD-L1+ cancers[268].
The negative checkpoint regulator VISTA represents a V-domain Ig suppressor of T cell activation (VISTA), known as PD1 homolog, which belongs to the B7 family. VISTA is mostly exhibited on hematopoietic cells[269]. In murine tumor models, anti-VISTA monoclonal antibodies activated intratumoral T cells, enhancing antitumor immunity. Moreover, the combined VISTA/PD-1 blockade was proven efficient[270,271]. A phase I study, combining VISTA and PD-L1/PD-L2 inhibition in solid tumors (CA-170 molecule; NCT02812875), has been initiated[272]. Böger et al[273], investigated VISTA expression in 464 gastric cancer patients, with an increased expression associated with the intestinal type (VISTA is a regulator of differentiation), proximal gastric tumors, and KRAS- and PIK3CA mutant cancers. Additionally, a significantly higher VISTA expression in immune cells was noticed in association with PD-L1 expression and in EBV+ tumors. These observations might stress that a subset of tumors may use multiple checkpoint pathways to escape immunity and that the combined VISTA/PD-1 inhibition may be a promising novel cancer approach.
Another study is investigating nivolumab plus a matrix metalloproteinase 9 inhibitor (GS-5745 = andecaliximab) in patients with unresectable/recurrent gastric or gastroesophageal junction adenocarcinoma (NCT02864381)[274].
Due to the permanent development of novel immunotherapeutic agents, classical trials do not have the ability to appropriately assess all possible drug combinations. In this regard, FRACTION (Fast Real-time Assessment of Combination Therapies in Immuno-Oncology) is a new clinical trial program with a dynamic design that provides the possibility both for the inclusion of new immunotherapeutic combinations, as well as exclusion of ineffective ones[275].
Cancer radiotherapy may seldom exhibit the clinical phenomenon of the abscopal response, meaning that no irradiated metastases diminish after radiation to the primary tumor. Data from preclinical trials highlighted that radiotherapy plus PD-1/PD-L1 blockade exhibited synergistic anticancer effects. Currently, trials on gastric cancer patients are investigating the efficiency of pembrolizumab plus palliative radiotherapy (metastatic tumors), and additional neoadjuvant chemoradiotherapy (resectable tumors) (NCT02730546)[276], respectively. A phase I/II trial is also investigating nivolumab+ radiotherapy in unresectable, recurrent gastric cancer (as third-line treatment) (NCT03453164) (CIRCUIT)[277].
The combination of immune checkpoint inhibitors with other types of immunotherapies, targeted agents, radio- or chemotherapies, seems to generate promising results against gastrointestinal cancers but at the expense of the occurrence of immune-related side effects, some of them potentially fatal[278,279]. Therefore, identification of prognostic biomarkers and genetic profiles to define subgroups of gastric cancer patients who are most likely to respond to specific immunotherapeutic regimens is an urgent need[280].
In this regard, an ongoing observational study NCT02951091 (Biomarker - integrated Umbrella)[281] is investigating different molecular cohorts in oncologic patients, the cases of PD-L1+, MSI-H, and EBV+ advanced gastric cancers being assigned to receive either nivolumab or other agents, such as AFATINIB (EGFR tyrosine kinase inhibitor or GSK2636771 (PI3K beta inhibitor) plus PACLITAXEL). A phase II study compares nivolumab with other novel agents, according to genetic testing, in gastric cancer patients with mismatch repair deficiency (loss of MLH1/ MLH2) (NCT02465060 - The MATCH screening trial)[282].
Because of the well-known heterogeneity of tumors, it is essential to assess the particular molecular biology of different subtypes of gastric cancers that are associated with different clinico-biologic parameters and prognosis to identify innovative treatment approaches that will improve current results in gastric cancer. Modern treatments, such as the promising immunotherapy, should be applied in this way to selected patients who have been proven to have the best response to a specific therapy (individualized treatment). In this context, it is also mandatory to discover prognostic tumor markers and predictive biomarkers for treatment response.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lee WJ, Saglam S, Sunakawa Y S- Editor: Gong ZM L- Editor: Donna Fox E- Editor: Yin SY
| 1. | Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray . Estimated incidence, mortality and prevalence worldwide in 2012. 2013. [Internet]. International Agency for Research on Cancer. Accessed May 4, 2018 Available from: http://globocan.iarc.fr/Default.aspx. [Cited in This Article: ] |
| 2. | Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi57-vi63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 3. | Okines AF, Norman AR, McCloud P, Kang YK, Cunningham D. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol. 2009;20:1529-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4615] [Cited by in F6Publishing: 4839] [Article Influence: 345.6] [Reference Citation Analysis (1)] |
| 5. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1541] [Cited by in F6Publishing: 1463] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 6. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1613] [Cited by in F6Publishing: 1583] [Article Influence: 158.3] [Reference Citation Analysis (0)] |
| 7. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10799] [Cited by in F6Publishing: 11106] [Article Influence: 793.3] [Reference Citation Analysis (0)] |
| 8. | Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3331] [Cited by in F6Publishing: 3269] [Article Influence: 251.5] [Reference Citation Analysis (0)] |
| 9. | Lyons TG, Ku GY. Systemic therapy for esophagogastric cancer: immune checkpoint inhibition. Chin Clin Oncol. 2017;6:53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Lauwers GY, Carneiro F, Graham DY. Gastric carcinoma. Classification of Tumours of the Digestive System. Lyon: IARC 2010; . [Cited in This Article: ] |
| 11. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4011] [Cited by in F6Publishing: 4108] [Article Influence: 146.7] [Reference Citation Analysis (0)] |
| 12. | Garattini SK, Basile D, Cattaneo M, Fanotto V, Ongaro E, Bonotto M, Negri FV, Berenato R, Ermacora P, Cardellino GG. Molecular classifications of gastric cancers: Novel insights and possible future applications. World J Gastrointest Oncol. 2017;9:194-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 43] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Lei Z, Tan IB, Das K, Deng N, Zouridis H, Pattison S, Chua C, Feng Z, Guan YK, Ooi CH. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology. 2013;145:554-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 313] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 14. | Lim B, Kim JH, Kim M, Kim SY. Genomic and epigenomic heterogeneity in molecular subtypes of gastric cancer. World J Gastroenterol. 2016;22:1190-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 47] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Aprile G, Giampieri R, Bonotto M, Bittoni A, Ongaro E, Cardellino GG, Graziano F, Giuliani F, Fasola G, Cascinu S. The challenge of targeted therapies for gastric cancer patients: the beginning of a long journey. Expert Opin Investig Drugs. 2014;23:925-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Chen T, Xu XY, Zhou PH. Emerging molecular classifications and therapeutic implications for gastric cancer. Chin J Cancer. 2016;35:49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Tan P, Yeoh KG. Genetics and Molecular Pathogenesis of Gastric Adenocarcinoma. Gastroenterology. 2015;149:1153-1162.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 311] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 18. | Pedrazzani C, Corso G, Velho S, Leite M, Pascale V, Bettarini F, Marrelli D, Seruca R, Roviello F. Evidence of tumor microsatellite instability in gastric cancer with familial aggregation. Fam Cancer. 2009;8:215-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Velho S, Fernandes MS, Leite M, Figueiredo C, Seruca R. Causes and consequences of microsatellite instability in gastric carcinogenesis. World J Gastroenterol. 2014;20:16433-16442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 54] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Chung DC, Rustgi AK. DNA mismatch repair and cancer. Gastroenterology. 1995;109:1685-1699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 125] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 21. | Zhu L, Li Z, Wang Y, Zhang C, Liu Y, Qu X. Microsatellite instability and survival in gastric cancer: A systematic review and meta-analysis. Mol Clin Oncol. 2015;3:699-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8900] [Cited by in F6Publishing: 9307] [Article Influence: 775.6] [Reference Citation Analysis (0)] |
| 23. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6096] [Cited by in F6Publishing: 6542] [Article Influence: 726.9] [Reference Citation Analysis (0)] |
| 24. | Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A, von Knebel Doeberitz M. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 25. | Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 838] [Article Influence: 104.8] [Reference Citation Analysis (1)] |
| 26. | Bristol-Myers Squibb. A Study of Nivolumab and Nivolumab Plus Ipilimumab in Recurrent and Metastatic Colon Cancer (CheckMate 142). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/ct2/show/NCT02060188 ClinicalTrials.gov Identifier: NCT02060188. [Cited in This Article: ] |
| 27. | Buckowitz A, Knaebel HP, Benner A, Bläker H, Gebert J, Kienle P, von Knebel Doeberitz M, Kloor M. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer. 2005;92:1746-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 28. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4230] [Cited by in F6Publishing: 4299] [Article Influence: 429.9] [Reference Citation Analysis (2)] |
| 29. | Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 348] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 30. | Camargo MC, Murphy G, Koriyama C, Pfeiffer RM, Kim WH, Herrera-Goepfert R, Corvalan AH, Carrascal E, Abdirad A, Anwar M. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis. Br J Cancer. 2011;105:38-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 31. | Kim SY, Park C, Kim HJ, Park J, Hwang J, Kim JI, Choi MG, Kim S, Kim KM, Kang MS. Deregulation of immune response genes in patients with Epstein-Barr virus-associated gastric cancer and outcomes. Gastroenterology. 2015;148:137-147.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, Sessa F, Fleitas T, Freeman GJ, Rodig SJ. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7:32925-32932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 33. | Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1071] [Cited by in F6Publishing: 1332] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 34. | Yingyan Y. A new molecular classification of gastric cancer proposed by Asian Cancer Research Group (ACRG). Transl Gastrointest Cancer. 2016;5:55-57. [DOI] [Cited in This Article: ] |
| 35. | Burnet M. Cancer; a biological approach. I. The processes of control. Br Med J. 1957;1:779-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 347] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol. 2014;20:1657-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 64] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6747] [Cited by in F6Publishing: 6673] [Article Influence: 317.8] [Reference Citation Analysis (0)] |
| 38. | Kandulski A, Malfertheiner P, Wex T. Role of regulatory T-cells in H. pylori-induced gastritis and gastric cancer. Anticancer Res. 2010;30:1093-1103. [PubMed] [Cited in This Article: ] |
| 39. | Haraguchi N, Inoue H, Tanaka F, Mimori K, Utsunomiya T, Sasaki A, Mori M. Cancer stem cells in human gastrointestinal cancers. Hum Cell. 2006;19:24-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567-1578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 41. | Lin R, Ma H, Ding Z, Shi W, Qian W, Song J, Hou X. Bone marrow-derived mesenchymal stem cells favor the immunosuppressive T cells skewing in a Helicobacter pylori model of gastric cancer. Stem Cells Dev. 2013;22:2836-2848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Procaccio L, Schirripa M, Fassan M, Vecchione L, Bergamo F, Prete AA, Intini R, Manai C, Dadduzio V, Boscolo A. Immunotherapy in Gastrointestinal Cancers. Biomed Res Int. 2017;2017:4346576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 933] [Cited by in F6Publishing: 1006] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 44. | Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 415] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 45. | Balch CM, Riley LB, Bae YJ, Salmeron MA, Platsoucas CD, von Eschenbach A, Itoh K. Patterns of human tumor-infiltrating lymphocytes in 120 human cancers. Arch Surg. 1990;125:200-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 193] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Zheng X, Song X, Shao Y, Xu B, Chen L, Zhou Q, Hu W, Zhang D, Wu C, Tao M, Zhu Y, Jiang J. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget. 2017;8:57386-57398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 47. | Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3297] [Cited by in F6Publishing: 4078] [Article Influence: 370.7] [Reference Citation Analysis (1)] |
| 48. | Kang BW, Kim JG, Lee IH, Bae HI, Seo AN. Clinical significance of tumor-infiltrating lymphocytes for gastric cancer in the era of immunology. World J Gastrointest Oncol. 2017;9:293-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 41] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3828] [Cited by in F6Publishing: 4143] [Article Influence: 318.7] [Reference Citation Analysis (0)] |
| 50. | Kang BW, Seo AN, Yoon S, Bae HI, Jeon SW, Kwon OK, Chung HY, Yu W, Kang H, Kim JG. Prognostic value of tumor-infiltrating lymphocytes in Epstein-Barr virus-associated gastric cancer. Ann Oncol. 2016;27:494-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 51. | Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 52. | Dirican A, Ekinci N, Avci A, Akyol M, Alacacioglu A, Kucukzeybek Y, Somali I, Erten C, Demir L, Can A. The effects of hematological parameters and tumor-infiltrating lymphocytes on prognosis in patients with gastric cancer. Cancer Biomark. 2013;13:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Dai C, Geng R, Wang C, Wong A, Qing M, Hu J, Sun Y, Lo AW, Li J. Concordance of immune checkpoints within tumor immune contexture and their prognostic significance in gastric cancer. Mol Oncol. 2016;10:1551-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 54. | Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, Distel LV. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol. 2009;9:65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 55. | Kim KJ, Lee KS, Cho HJ, Kim YH, Yang HK, Kim WH, Kang GH. Prognostic implications of tumor-infiltrating FoxP3+ regulatory T cells and CD8+ cytotoxic T cells in microsatellite-unstable gastric cancers. Hum Pathol. 2014;45:285-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 56. | Chiaravalli AM, Feltri M, Bertolini V, Bagnoli E, Furlan D, Cerutti R, Novario R, Capella C. Intratumour T cells, their activation status and survival in gastric carcinomas characterised for microsatellite instability and Epstein-Barr virus infection. Virchows Arch. 2006;448:344-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Liu K, Yang K, Wu B, Chen H, Chen X, Chen X, Jiang L, Ye F, He D, Lu Z. Tumor-Infiltrating Immune Cells Are Associated With Prognosis of Gastric Cancer. Medicine (Baltimore). 2015;94:e1631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 58. | Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66:794-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 328] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 59. | Zhou S, Shen Z, Wang Y, Ma H, Xu S, Qin J, Chen L, Tao H, Zhen Z, Chen G. CCR7 expression and intratumoral FOXP3+ regulatory T cells are correlated with overall survival and lymph node metastasis in gastric cancer. PLoS One. 2013;8:e74430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Wang B, Xu D, Yu X, Ding T, Rao H, Zhan Y, Zheng L, Li L. Association of intra-tumoral infiltrating macrophages and regulatory T cells is an independent prognostic factor in gastric cancer after radical resection. Ann Surg Oncol. 2011;18:2585-2593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 61. | Perrone G, Ruffini PA, Catalano V, Spino C, Santini D, Muretto P, Spoto C, Zingaretti C, Sisti V, Alessandroni P. Intratumoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur J Cancer. 2008;44:1875-1882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 62. | Kim KJ, Wen XY, Yang HK, Kim WH, Kang GH. Prognostic Implication of M2 Macrophages Are Determined by the Proportional Balance of Tumor Associated Macrophages and Tumor Infiltrating Lymphocytes in Microsatellite-Unstable Gastric Carcinoma. PLoS One. 2015;10:e0144192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 63. | Hu M, Li K, Maskey N, Xu Z, Peng C, Wang B, Li Y, Yang G. Decreased intratumoral Foxp3 Tregs and increased dendritic cell density by neoadjuvant chemotherapy associated with favorable prognosis in advanced gastric cancer. Int J Clin Exp Pathol. 2014;7:4685-4694. [PubMed] [Cited in This Article: ] |
| 64. | Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. Localisation pattern of Foxp3+ regulatory T cells is associated with clinical behaviour in gastric cancer. Br J Cancer. 2008;98:148-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 65. | Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136:1585-1595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 66. | Liu T, Peng L, Yu P, Zhao Y, Shi Y, Mao X, Chen W, Cheng P, Wang T, Chen N. Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J Clin Immunol. 2012;32:1332-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 67. | Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010;102:742-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 68. | Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704-1711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 69. | Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Xiangming C, Iwashige H, Aridome K, Hokita S, Aikou T. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett. 2000;159:103-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Mimura K, Kamiya T, Shiraishi K, Kua LF, Shabbir A, So J, Yong WP, Suzuki Y, Yoshimoto Y, Nakano T. Therapeutic potential of highly cytotoxic natural killer cells for gastric cancer. Int J Cancer. 2014;135:1390-1398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Wakatsuki K, Sho M, Yamato I, Takayama T, Matsumoto S, Tanaka T, Migita K, Ito M, Hotta K, Nakajima Y. Clinical impact of tumor-infiltrating CD45RO+ memory T cells on human gastric cancer. Oncol Rep. 2013;29:1756-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Chen LJ, Zheng X, Shen YP, Zhu YB, Li Q, Chen J, Xia R, Zhou SM, Wu CP, Zhang XG. Higher numbers of T-bet(+) intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer Immunol Immunother. 2013;62:553-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Hennequin A, Derangère V, Boidot R, Apetoh L, Vincent J, Orry D, Fraisse J, Causeret S, Martin F, Arnould L. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients. Oncoimmunology. 2015;5:e1054598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 74. | Arigami T, Uenosono Y, Ishigami S, Matsushita D, Hirahara T, Yanagita S, Okumura H, Uchikado Y, Nakajo A, Kijima Y. Decreased density of CD3+ tumor-infiltrating lymphocytes during gastric cancer progression. J Gastroenterol Hepatol. 2014;29:1435-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in F6Publishing: 493] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 76. | Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593-2603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1119] [Cited by in F6Publishing: 1104] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 77. | Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautès-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114-2121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 263] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 78. | Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, Thomson AW. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577-584. [PubMed] [Cited in This Article: ] |
| 79. | Yuan L, Xu B, Yuan P, Zhou J, Qin P, Han L, Chen G, Wang Z, Run Z, Zhao P. Tumor-infiltrating CD4+ T cells in patients with gastric cancer. Cancer Cell Int. 2017;17:114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 80. | Choi HS, Ha SY, Kim HM, Ahn SM, Kang MS, Kim KM, Choi MG, Lee JH, Sohn TS, Bae JM. The prognostic effects of tumor infiltrating regulatory T cells and myeloid derived suppressor cells assessed by multicolor flow cytometry in gastric cancer patients. Oncotarget. 2016;7:7940-7951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 81. | Raziorrouh B, Ulsenheimer A, Schraut W, Heeg M, Kurktschiev P, Zachoval R, Jung MC, Thimme R, Neumann-Haefelin C, Horster S. Inhibitory molecules that regulate expansion and restoration of HCV-specific CD4+ T cells in patients with chronic infection. Gastroenterology. 2011;141:1422-1431, 1431.e1-1431.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 82. | Xu B, Yuan L, Gao Q, Yuan P, Zhao P, Yuan H, Fan H, Li T, Qin P, Han L. Circulating and tumor-infiltrating Tim-3 in patients with colorectal cancer. Oncotarget. 2015;6:20592-20603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 83. | Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 739] [Cited by in F6Publishing: 728] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 84. | Tsujitani S, Kakeji Y, Orita H, Watanabe A, Kohnoe S, Baba H, Anai H, Maehara Y, Sugimachi K. Postoperative adjuvant immunochemotherapy and infiltration of dendritic cells for patients with advanced gastric cancer. Anticancer Res. 1992;12:645-648. [PubMed] [Cited in This Article: ] |
| 85. | Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139-2145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 879] [Cited by in F6Publishing: 1039] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 86. | Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4180] [Cited by in F6Publishing: 4769] [Article Influence: 529.9] [Reference Citation Analysis (0)] |
| 87. | Ma J, Li J, Hao Y, Nie Y, Li Z, Qian M, Liang Q, Yu J, Zeng M, Wu K. Differentiated tumor immune microenvironment of Epstein-Barr virus-associated and negative gastric cancer: implication in prognosis and immunotherapy. Oncotarget. 2017;8:67094-67103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 88. | Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol. 2003;16:641-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 89. | Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 681] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 90. | Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol. 2013;190:794-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 91. | Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 994] [Cited by in F6Publishing: 978] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 92. | Shoji H, Tada K, Kitano S, Nishimura T, Shimada Y, Nagashima K, Aoki K, Hiraoka N, Honma Y, Iwasa S. The peripheral immune status of granulocytic myeloid-derived suppressor cells correlates the survival in advanced gastric cancer patients receiving cisplatin-based chemotherapy. Oncotarget. 2017;8:95083-95094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Asfaha S, Dubeykovskiy AN, Tomita H, Yang X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA, Muthupalani S. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144:155-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 94. | Chi N, Tan Z, Ma K, Bao L, Yun Z. Increased circulating myeloid-derived suppressor cells correlate with cancer stages, interleukin-8 and -6 in prostate cancer. Int J Clin Exp Med. 2014;7:3181-3192. [PubMed] [Cited in This Article: ] |
| 95. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10123] [Cited by in F6Publishing: 10572] [Article Influence: 480.5] [Reference Citation Analysis (0)] |
| 96. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6713] [Cited by in F6Publishing: 7489] [Article Influence: 534.9] [Reference Citation Analysis (0)] |
| 97. | McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 631] [Cited by in F6Publishing: 692] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 98. | Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5-14. [PubMed] [Cited in This Article: ] |
| 99. | Shibata M, Nezu T, Kanou H, Abe H, Takekawa M, Fukuzawa M. Decreased production of interleukin-12 and type 2 immune responses are marked in cachectic patients with colorectal and gastric cancer. J Clin Gastroenterol. 2002;34:416-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 100. | Ohki S, Shibata M, Gonda K, Machida T, Shimura T, Nakamura I, Ohtake T, Koyama Y, Suzuki S, Ohto H. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep. 2012;28:453-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 101. | Gonda K, Shibata M, Sato Y, Washio M, Takeshita H, Shigeta H, Ogura M, Oka S, Sakuramoto S. Elevated neutrophil-to-lymphocyte ratio is associated with nutritional impairment, immune suppression, resistance to S-1 plus cisplatin, and poor prognosis in patients with stage IV gastric cancer. Mol Clin Oncol. 2017;7:1073-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154-158. [PubMed] [Cited in This Article: ] |
| 103. | Niccolai E, Taddei A, Prisco D, Amedei A. Gastric cancer and the epoch of immunotherapy approaches. World J Gastroenterol. 2015;21:5778-5793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 64] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 104. | Bonotto M, Garattini SK, Basile D, Ongaro E, Fanotto V, Cattaneo M, Cortiula F, Iacono D, Cardellino GG, Pella N. Immunotherapy for gastric cancers: emerging role and future perspectives. Expert Rev Clin Pharmacol. 2017;10:609-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1770] [Cited by in F6Publishing: 1999] [Article Influence: 181.7] [Reference Citation Analysis (0)] |
| 106. | Hanna MG Jr, Hoover HC Jr, Vermorken JB, Harris JE, Pinedo HM. Adjuvant active specific immunotherapy of stage II and stage III colon cancer with an autologous tumor cell vaccine: first randomized phase III trials show promise. Vaccine. 2001;19:2576-2582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 107. | Derouazi M, Di Berardino-Besson W, Belnoue E, Hoepner S, Walther R, Benkhoucha M, Teta P, Dufour Y, Yacoub Maroun C, Salazar AM. Novel Cell-Penetrating Peptide-Based Vaccine Induces Robust CD4+ and CD8+ T Cell-Mediated Antitumor Immunity. Cancer Res. 2015;75:3020-3031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 108. | Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550-4557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1428] [Cited by in F6Publishing: 1518] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 109. | Nie Y, Wu K, Yang J, Tian F, Li L, Chen B, Fan D. Induction of T lymphocytes specific to human gastric cancer using HLA-A matched allogeneic gastric tumor cells. J Immunother. 2003;26:403-411. [PubMed] [Cited in This Article: ] |
| 110. | Kawamoto M, Tanaka F, Mimori K, Inoue H, Kamohara Y, Mori M. Identification of HLA-A*0201/-A*2402-restricted CTL epitope-peptides derived from a novel cancer/testis antigen, MCAK, and induction of a specific antitumor immune response. Oncol Rep. 2011;25:469-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 111. | Kono K, Ichihara F, Iizuka H, Sekikawa T, Matsumoto Y. Differences in the recognition of tumor-specific CD8+ T cells derived from solid tumor, metastatic lymph nodes and ascites in patients with gastric cancer. Int J Cancer. 1997;71:978-981. [PubMed] [Cited in This Article: ] |
| 112. | Fujie T, Tanaka F, Tahara K, Li J, Tanaka S, Mori M, Ueo H, Takesako K, Akiyoshi T. Generation of specific antitumor reactivity by the stimulation of spleen cells from gastric cancer patients with MAGE-3 synthetic peptide. Cancer Immunol Immunother. 1999;48:189-194. [PubMed] [Cited in This Article: ] |
| 113. | Sun S, Li XM, Li XD, Yang WS. Studies on inducing apoptosis effects and mechanism of CIK cells for MGC-803 gastric cancer cell lines. Cancer Biother Radiopharm. 2005;20:173-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Kim YJ, Lim J, Kang JS, Kim HM, Lee HK, Ryu HS, Kim JY, Hong JT, Kim Y, Han SB. Adoptive immunotherapy of human gastric cancer with ex vivo expanded T cells. Arch Pharm Res. 2010;33:1789-1795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 115. | Bourquin C, von der Borch P, Zoglmeier C, Anz D, Sandholzer N, Suhartha N, Wurzenberger C, Denzel A, Kammerer R, Zimmermann W. Efficient eradication of subcutaneous but not of autochthonous gastric tumors by adoptive T cell transfer in an SV40 T antigen mouse model. J Immunol. 2010;185:2580-2588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 116. | Gebremeskel S, Johnston B. Concepts and mechanisms underlying chemotherapy induced immunogenic cell death: impact on clinical studies and considerations for combined therapies. Oncotarget. 2015;6:41600-41619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 117. | Zhao Q, Zhang H, Li Y, Liu J, Hu X, Fan L. Anti-tumor effects of CIK combined with oxaliplatin in human oxaliplatin-resistant gastric cancer cells in vivo and in vitro. J Exp Clin Cancer Res. 2010;29:118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 118. | Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2149] [Cited by in F6Publishing: 2055] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 119. | Kobold S, Steffen J, Chaloupka M, Grassmann S, Henkel J, Castoldi R, Zeng Y, Chmielewski M, Schmollinger JC, Schnurr M. Selective bispecific T cell recruiting antibody and antitumor activity of adoptive T cell transfer. J Natl Cancer Inst. 2014;107:364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 120. | Du X, Jin R, Ning N, Li L, Wang Q, Liang W, Liu J, Xu Y. In vivo distribution and antitumor effect of infused immune cells in a gastric cancer model. Oncol Rep. 2012;28:1743-1749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 121. | Kevin T, Mark H, Craig L, inventors; Alan J, assignee; Cytotoxic T Lymphocyte-stimulation peptides for prevention, treatment, and diagnosis of melanoma. International patent WO2001032193. 2001;May 10. [Cited in This Article: ] |
| 122. | Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park). 2009;23:488-496. [PubMed] [Cited in This Article: ] |
| 123. | Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168-16173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 913] [Cited by in F6Publishing: 883] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 124. | Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889-897. [PubMed] [Cited in This Article: ] |
| 125. | Zhang GQ, Zhao H, Wu JY, Li JY, Yan X, Wang G, Wu LL, Zhang XG, Shao Y, Wang Y. Prolonged overall survival in gastric cancer patients after adoptive immunotherapy. World J Gastroenterol. 2015;21:2777-2785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 126. | Zhao H, Fan Y, Li H, Yu J, Liu L, Cao S, Ren B, Yan F, Ren X. Immunotherapy with cytokine-induced killer cells as an adjuvant treatment for advanced gastric carcinoma: a retrospective study of 165 patients. Cancer Biother Radiopharm. 2013;28:303-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 127. | Chen Y, Guo ZQ, Shi CM, Zhou ZF, Ye YB, Chen Q. Efficacy of adjuvant chemotherapy combined with immunotherapy with cytokine-induced killer cells for gastric cancer after d2 gastrectomy. Int J Clin Exp Med. 2015;8:7728-7736. [PubMed] [Cited in This Article: ] |
| 128. | Shi L, Zhou Q, Wu J, Ji M, Li G, Jiang J, Wu C. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61:2251-2259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 129. | Jiang J, Xu N, Wu C, Deng H, Lu M, Li M, Xu B, Wu J, Wang R, Xu J. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res. 2006;26:2237-2242. [PubMed] [Cited in This Article: ] |
| 130. | Jiang JT, Shen YP, Wu CP, Zhu YB, Wei WX, Chen LJ, Zheng X, Sun J, Lu BF, Zhang XG. Increasing the frequency of CIK cells adoptive immunotherapy may decrease risk of death in gastric cancer patients. World J Gastroenterol. 2010;16:6155-6162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 52] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 131. | Jäkel CE, Vogt A, Gonzalez-Carmona MA, Schmidt-Wolf IG. Clinical studies applying cytokine-induced killer cells for the treatment of gastrointestinal tumors. J Immunol Res. 2014;2014:897214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 132. | Wada I, Matsushita H, Noji S, Mori K, Yamashita H, Nomura S, Shimizu N, Seto Y, Kakimi K. Intraperitoneal injection of in vitro expanded Vγ9Vδ2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med. 2014;3:362-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 133. | Cellular Biomedicine Group Ltd. The Study of Surgery, Chemotherapy and Autologous T Cells-Based Immunotherapy for Advanced Gastric Cancer. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT02704299 ClinicalTrials.gov Identifier: NCT02704299. [Cited in This Article: ] |
| 134. | Shenzhen Hornetcorn Bio-technology Company, LTD. “Study of Autologous Tumor Lysate-pulsed D-CIK Combined With Chemotherapy for Gastric Cancer”. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT02215837 ClinicalTrials.gov Identifier: NCT02215837. [Cited in This Article: ] |
| 135. | First Affiliated Hospital of Chengdu Medical College. A Clinical Research of CAR T Cells Targeting EpCAMPositive Cancer (CARTEPC). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03013712 ClinicalTrials.gov Identifier: NCT03013712. [Cited in This Article: ] |
| 136. | PersonGen BioTherapeutics (Suzhou) Co., Ltd. CAR-T Cell Immunotherapy in MUC1 Positive Solid Tumor. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT02617134 ClinicalTrials.gov Identifier: NCT02617134. [Cited in This Article: ] |
| 137. | Roger Williams Medical Center. CAR-T Hepatic Artery Infusions for CEA-Expressing Liver Metastases (HITM-SURE). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT02850536 ClinicalTrials.gov Identifier: NCT02850536. [Cited in This Article: ] |
| 138. | University SMM, Immunotherapy Using Pluripotent Killer-Human Epidermal Growth Factor Receptor-2 (PIK-HER2) Cells for the Treatment of Advanced Gastric Cancer With Liver Metastasis. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT02632201 ClinicalTrials.gov Identifier: NCT02632201. [Cited in This Article: ] |
| 139. | Miao Y. T Cell Mediated Adaptive therapy for Her2-positive Neoplasms of Digestive System, in ClinicalTrials. gov [Internet], Bethesda (MD): National Library of Medicine (US) 2000; Available from: http://clinicaltrials.gov/show/NCT02662348. [Cited in This Article: ] |
| 140. | Capital Medical University. Study of S-1 Plus DC-CIK for Patients With Advanced Gastric Cancer. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT01783951 ClinicalTrials.gov Identifier: NCT01783951. [Cited in This Article: ] |
| 141. | Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 541] [Cited by in F6Publishing: 511] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 142. | Alexander RB, Rosenberg SA. Long-term survival of adoptively transferred tumor-infiltrating lymphocytes in mice. J Immunol. 1990;145:1615-1620. [PubMed] [Cited in This Article: ] |
| 143. | Kono K, Takahashi A, Ichihara F, Amemiya H, Iizuka H, Fujii H, Sekikawa T, Matsumoto Y. Prognostic significance of adoptive immunotherapy with tumor-associated lymphocytes in patients with advanced gastric cancer: a randomized trial. Clin Cancer Res. 2002;8:1767-1771. [PubMed] [Cited in This Article: ] |
| 144. | Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 705] [Cited by in F6Publishing: 652] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 145. | Amedei A, Munari F, Bella CD, Niccolai E, Benagiano M, Bencini L, Cianchi F, Farsi M, Emmi G, Zanotti G. Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern Emerg Med. 2014;9:303-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 146. | Yamaue H, Tanimura H, Tsunoda T, Iwahashi M, Tani M, Inoue M, Tamai M. [Clinical application of adoptive immunotherapy by cytotoxic T lymphocytes induced from tumor-infiltrating lymphocytes]. Nihon Gan Chiryo Gakkai Shi. 1990;25:978-989. [PubMed] [Cited in This Article: ] |
| 147. | Voskens CJ, Watanabe R, Rollins S, Campana D, Hasumi K, Mann DL. Ex-vivo expanded human NK cells express activating receptors that mediate cytotoxicity of allogeneic and autologous cancer cell lines by direct recognition and antibody directed cellular cytotoxicity. J Exp Clin Cancer Res. 2010;29:134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 148. | Langers I, Renoux VM, Thiry M, Delvenne P, Jacobs N. Natural killer cells: role in local tumor growth and metastasis. Biologics. 2012;6:73-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 149. | Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci USA. 2000;97:2731-2736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 339] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 150. | Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320-2328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 151. | Rosso D, Rigueiro MP, Kassab P, Ilias EJ, Castro OA, Novo NF, Lourenço LG. [Correlation of natural killer cells with the prognosis of gastric adenocarcinoma]. Arq Bras Cir Dig. 2012;25:114-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 152. | Saito H, Takaya S, Osaki T, Ikeguchi M. Increased apoptosis and elevated Fas expression in circulating natural killer cells in gastric cancer patients. Gastric Cancer. 2013;16:473-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 153. | Cui J, Li L, Wang C, Jin H, Yao C, Wang Y, Li D, Tian H, Niu C, Wang G. Combined cellular immunotherapy and chemotherapy improves clinical outcome in patients with gastric carcinoma. Cytotherapy. 2015;17:979-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 154. | National University Hospital, Singapore. NK cell infusions with trastuzumab for patients with HER2+ breast and gastric cancer” . [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT02030561 ClinicalTrials.gov Identifier: NCT02030561. [Cited in This Article: ] |
| 155. | Wu XT, Liu JQ, Lu XT, Chen FX, Zhou ZH, Wang T, Zhu SP, Fei SJ. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int Immunopharmacol. 2013;16:332-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 156. | Kono K, Rongcun Y, Charo J, Ichihara F, Celis E, Sette A, Appella E, Sekikawa T, Matsumoto Y, Kiessling R. Identification of HER2/neu-derived peptide epitopes recognized by gastric cancer-specific cytotoxic T lymphocytes. Int J Cancer. 1998;78:202-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 157. | Tanaka F, Fujie T, Tahara K, Mori M, Takesako K, Sette A, Celis E, Akiyoshi T. Induction of antitumor cytotoxic T lymphocytes with a MAGE-3-encoded synthetic peptide presented by human leukocytes antigen-A24. Cancer Res. 1997;57:4465-4468. [PubMed] [Cited in This Article: ] |
| 158. | Higashihara Y, Kato J, Nagahara A, Izumi K, Konishi M, Kodani T, Serizawa N, Osada T, Watanabe S. Phase I clinical trial of peptide vaccination with URLC10 and VEGFR1 epitope peptides in patients with advanced gastric cancer. Int J Oncol. 2014;44:662-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 159. | Gang Y, Zhang X, He Y, Zheng J, Wu K, Ding J, Fan D. Efficient induction of specific cytotoxic T lymphocytes against gastric adenocarcinoma by a survivin peptide. Biochem Cell Biol. 2012;90:701-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 160. | Zhang K, Peng Z, Huang X, Qiao Z, Wang X, Wang N, Xi H, Cui J, Gao Y, Huang X. Phase II Trial of Adjuvant Immunotherapy with Autologous Tumor-derived Gp96 Vaccination in Patients with Gastric Cancer. J Cancer. 2017;8:1826-1832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 161. | Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD Jr. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996;183:283-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 569] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 162. | Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 437] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 163. | Yang J, Li ZH, Zhou JJ, Chen RF, Cheng LZ, Zhou QB, Yang LQ. Preparation and antitumor effects of nanovaccines with MAGE-3 peptides in transplanted gastric cancer in mice. Chin J Cancer. 2010;29:359-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 164. | Galetto A, Contarini M, Sapino A, Cassoni P, Consalvo E, Forno S, Pezzi C, Barnaba V, Mussa A, Matera L. Ex vivo host response to gastrointestinal cancer cells presented by autologous dendritic cells. J Surg Res. 2001;100:32-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 165. | Kreiter S, Selmi A, Diken M, Koslowski M, Britten CM, Huber C, Türeci O, Sahin U. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 2010;70:9031-9040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 166. | Fotin-Mleczek M, Duchardt KM, Lorenz C, Pfeiffer R, Ojkić-Zrna S, Probst J, Kallen KJ. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J Immunother. 2011;34:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 167. | Weide B, Carralot JP, Reese A, Scheel B, Eigentler TK, Hoerr I, Rammensee HG, Garbe C, Pascolo S. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother. 2008;31:180-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 168. | Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, Pawelec G, Hoerr I, Rammensee HG, Garbe C. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32:498-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 169. | Kim TW, Hung CF, Ling M, Juang J, He L, Hardwick JM, Kumar S, Wu TC. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003;112:109-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 170. | Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene. 2008;27:5464-5476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 171. | Kim JH, Kang TH, Noh KH, Kim SH, Lee YH, Kim KW, Bae HC, Ahn YH, Choi EY, Kim JS. Enhancement of DC vaccine potency by activating the PI3K/AKT pathway with a small interfering RNA targeting PTEN. Immunol Lett. 2010;134:47-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 172. | Kim JH, Kang TH, Noh KH, Bae HC, Ahn YH, Lee YH, Choi EY, Chun KH, Lee SJ, Kim TW. Blocking the immunosuppressive axis with small interfering RNA targeting interleukin (IL)-10 receptor enhances dendritic cell-based vaccine potency. Clin Exp Immunol. 2011;165:180-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 173. | He SB, Sun K, Wang L, Li DC, Zhang YY. [GM-CSF gene-modified dendritic cell vaccine enhances antitumor immunity in vitro]. Zhonghua Zhong Liu Za Zhi. 2010;32:410-414. [PubMed] [Cited in This Article: ] |
| 174. | Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623-3633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 776] [Cited by in F6Publishing: 799] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 175. | Popiela T, Kulig J, Czupryna A, Szczepanik AM, Zembala M. Efficiency of adjuvant immunochemotherapy following curative resection in patients with locally advanced gastric cancer. Gastric Cancer. 2004;7:240-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 176. | Ajani JA, Hecht JR, Ho L, Baker J, Oortgiesen M, Eduljee A, Michaeli D. An open-label, multinational, multicenter study of G17DT vaccination combined with cisplatin and 5-fluorouracil in patients with untreated, advanced gastric or gastroesophageal cancer: the GC4 study. Cancer. 2006;106:1908-1916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 177. | Masuzawa T, Fujiwara Y, Okada K, Nakamura A, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Osawa R. Phase I/II study of S-1 plus cisplatin combined with peptide vaccines for human vascular endothelial growth factor receptor 1 and 2 in patients with advanced gastric cancer. Int J Oncol. 2012;41:1297-1304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 178. | Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704-2715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 667] [Cited by in F6Publishing: 618] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 179. | Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695-1710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 980] [Cited by in F6Publishing: 1059] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 180. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3572] [Cited by in F6Publishing: 3735] [Article Influence: 155.6] [Reference Citation Analysis (0)] |
| 181. | Wang J, Yuan R, Song W, Sun J, Liu D, Li Z. PD-1, PD-L1 (B7-H1) and Tumor-Site Immune Modulation Therapy: The Historical Perspective. J Hematol Oncol. 2017;10:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 182. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5599] [Cited by in F6Publishing: 5891] [Article Influence: 490.9] [Reference Citation Analysis (0)] |
| 183. | Savabkar S, Azimzadeh P, Chaleshi V, Nazemalhosseini Mojarad E, Aghdaei HA. Programmed death-1 gene polymorphism (PD-1.5 C/T) is associated with gastric cancer. Gastroenterol Hepatol Bed Bench. 2013;6:178-182. [PubMed] [Cited in This Article: ] |
| 184. | Tang W, Chen Y, Chen S, Sun B, Gu H, Kang M. Programmed death-1 (PD-1) polymorphism is associated with gastric cardia adenocarcinoma. Int J Clin Exp Med. 2015;8:8086-8093. [PubMed] [Cited in This Article: ] |
| 185. | Yan Q, Chen P, Lu A, Zhao P, Gu A. Association between CTLA-4 60G/A and -1661A/G polymorphisms and the risk of cancers: a meta-analysis. PLoS One. 2013;8:e83710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 186. | Kordi-Tamandani DM, Davani SK, Baranzehi T, Hemati S. Analysis of promoter methylation, polymorphism and expression profile of cytotoxic T-lymphocyte-associated antigen-4 in patients with gastric cancer. J Gastrointestin Liver Dis. 2014;23:249-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 187. | Saito H, Kuroda H, Matsunaga T, Osaki T, Ikeguchi M. Increased PD-1 expression on CD4+ and CD8+ T cells is involved in immune evasion in gastric cancer. J Surg Oncol. 2013;107:517-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 188. | Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 382] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 189. | Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, Zhong X, Li X, Qian H, Wang X. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0182692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 176] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 190. | Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 970] [Cited by in F6Publishing: 1051] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 191. | Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A. 2008;105:20852-20857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 493] [Cited by in F6Publishing: 571] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 192. | Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 708] [Cited by in F6Publishing: 703] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 193. | Mimura K, Teh JL, Okayama H, Shiraishi K, Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109:43-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 194. | Böger C, Behrens HM, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269-24283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 214] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 195. | Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH, Kim SH, Chang H, Lee JO, Kim YJ, Lee HS. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19:42-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 196. | Chang H, Jung WY, Kang Y, Lee H, Kim A, Kim HK, Shin BK, Kim BH. Programmed death-ligand 1 expression in gastric adenocarcinoma is a poor prognostic factor in a high CD8+ tumor infiltrating lymphocytes group. Oncotarget. 2016;7:80426-80434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 197. | Sun J, Xu K, Wu C, Wang Y, Hu Y, Zhu Y, Chen Y, Shi Q, Yu G, Zhang X. PD-L1 expression analysis in gastric carcinoma tissue and blocking of tumor-associated PD-L1 signaling by two functional monoclonal antibodies. Tissue Antigens. 2007;69:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 198. | Schlößer HA, Drebber U, Kloth M, Thelen M, Rothschild SI, Haase S, Garcia-Marquez M, Wennhold K, Berlth F, Urbanski A. Immune checkpoints programmed death 1 ligand 1 and cytotoxic T lymphocyte associated molecule 4 in gastric adenocarcinoma. Oncoimmunology. 2015;5:e1100789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 199. | Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A, Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20:407-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 200. | Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS One. 2015;10:e0131403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 201. | Xu F, Feng G, Zhao H, Liu F, Xu L, Wang Q, An G. Clinicopathologic Significance and Prognostic Value of B7 Homolog 1 in Gastric Cancer: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2015;94:e1911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 202. | Liu YX, Wang XS, Wang YF, Hu XC, Yan JQ, Zhang YL, Wang W, Yang RJ, Feng YY, Gao SG. Prognostic significance of PD-L1 expression in patients with gastric cancer in East Asia: a meta-analysis. Onco Targets Ther. 2016;9:2649-2654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 203. | Zhang M, Dong Y, Liu H, Wang Y, Zhao S, Xuan Q, Wang Y, Zhang Q. The clinicopathological and prognostic significance of PD-L1 expression in gastric cancer: a meta-analysis of 10 studies with 1,901 patients. Sci Rep. 2016;6:37933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 204. | Fang W, Chen Y, Sheng J, Zhou T, Zhang Y, Zhan J, Liu L, Huang J, Peng P, Zhang L. Association between PD-L1 Expression on Tumour-Infiltrating Lymphocytes and Overall Survival in Patients with Gastric Cancer. J Cancer. 2017;8:1579-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 205. | Gao Y, Li S, Xu D, Chen S, Cai Y, Jiang W, Zhang X, Sun J, Wang K, Chang B. Prognostic value of programmed death-1, programmed death-ligand 1, programmed death-ligand 2 expression, and CD8(+) T cell density in primary tumors and metastatic lymph nodes from patients with stage T1-4N+M0 gastric adenocarcinoma. Chin J Cancer. 2017;36:61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 206. | Ma C, Patel K, Singhi AD, Ren B, Zhu B, Shaikh F, Sun W. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated With Epstein-Barr Virus or Microsatellite Instability. Am J Surg Pathol. 2016;40:1496-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 207. | Budczies J, Denkert C, Győrffy B, Schirmacher P, Stenzinger A. Chromosome 9p copy number gains involving PD-L1 are associated with a specific proliferation and immune-modulating gene expression program active across major cancer types. BMC Med Genomics. 2017;10:74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 208. | Ronan JK. Immunotherapy for Esophageal and Gastric Cancer. ASCO EDUCATIONAL BOOK | asco.org/edbook. 2017;292-300. [Cited in This Article: ] |
| 209. | Spranger S, Gajewski TF. Tumor-intrinsic oncogene pathways mediating immune avoidance. Oncoimmunology. 2015;5:e1086862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 210. | Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin Oncol. 2015;42:663-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 211. | Tran PN, Sarkissian S, Chao J, Klempner SJ. PD-1 and PD-L1 as emerging therapeutic targets in gastric cancer: current evidence. Gastrointest Cancer. 2017;7:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 212. | Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-1833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5948] [Cited by in F6Publishing: 6679] [Article Influence: 834.9] [Reference Citation Analysis (0)] |
| 213. | Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064-5074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1633] [Cited by in F6Publishing: 1860] [Article Influence: 186.0] [Reference Citation Analysis (0)] |
| 214. | Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, Ye G, Deng H, Mou T, Cai S. ImmunoScore Signature: A Prognostic and Predictive Tool in Gastric Cancer. Ann Surg. 2018;267:504-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 215. | Lin SJ, Gagnon-Bartsch JA, Tan IB, Earle S, Ruff L, Pettinger K, Ylstra B, van Grieken N, Rha SY, Chung HC. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut. 2015;64:1721-1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 216. | Ralph C, Elkord E, Burt DJ, O’Dwyer JF, Austin EB, Stern PL, Hawkins RE, Thistlethwaite FC. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res. 2010;16:1662-1672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 217. | Culver ME, Gatesman ML, Mancl EE, Lowe DK. Ipilimumab: a novel treatment for metastatic melanoma. Ann Pharmacother. 2011;45:510-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 218. | Moehler MH, Cho JY, Kim YH, Kim JW, Di Bartolomeo M, Ajani JA, Yamaguchi K, Balogh A, Kong-Sanchez MT, Bang YJ. A randomized, open-label, two-arm phase II trial comparing the efficacy of sequential ipilimumab (ipi) versus best supportive care (BSC) following first-line (1L) chemotherapy in patients with unresectable, locally advanced/metastatic (A/M) gastric or gastro-esophageal junction (G/GEJ) cancer. Alexandria:. J Clin Oncol. 2016;34:abstr 4011. [Cited in This Article: ] |
| 219. | Janjigian YY, Ott PA, Calvo E, Kim JW, Ascierto PA, Sharma P, Peltola KJ, Jaeger D, Evans TRJ, De Braud FG. Nivolumab ± ipilimumab in pts with advanced (adv)/metastatic chemotherapy refractory (CTx-R) gastric (G), esophageal (E), or gastroesophageal junction (GEJ) cancer: CheckMate 032 study. J Clin Oncol. 2017;35:abstract 4014. [Cited in This Article: ] |
| 220. | Janjigian YY, Adenis A, Aucoin JS, Barone C, Boku N, Chau I, Cleary JM, Feeney K, Franke FA, Moehler M. Checkmate 649: a randomized, multicenter, open-label, phase 3 study of nivolumab (Nivo) plus ipilimumab (Ipi) versus oxaliplatin plus fluoropyrimidine in patients (Pts) with previously untreated advanced or metastatic gastric (G) or gastroesophageal junction (GEJ) cancer. J Clin Oncol. 2017;35:TPS4132. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 221. | Kang YK, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yoshikawa T. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): a double-blinded, randomized, phase III trial. J Clin Oncol. 2017;35:abstr 2. [Cited in This Article: ] |
| 222. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1283] [Cited by in F6Publishing: 1496] [Article Influence: 213.7] [Reference Citation Analysis (0)] |
| 223. | Charalampakis N, Economopoulou P, Kotsantis I, Tolia M, Schizas D, Liakakos T, Elimova E, Ajani JA, Psyrri A. Medical management of gastric cancer: a 2017 update. Cancer Med. 2018;7:123-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 224. | Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3:436-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 545] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 225. | Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497-1508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 877] [Cited by in F6Publishing: 1076] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 226. | Fuchs CS, Doi T, Woo-Jun Jang R, Muro K, Satoh T, Machado M. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol. 2017;35:4003. [Cited in This Article: ] |
| 227. | Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 883] [Cited by in F6Publishing: 1235] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 228. | Fuchs CS, Ohtsu A, Tabernero J, Van Cutsem E, Wang JD, Lam B, Dalal R, Koshiji M, Banget YJ. Preliminary safety data from KEYNOTE-059: pembrolizumab plus 5-fluorouracil (5-FU) and cisplatin for first-line treatment of advanced gastric cancer. J Clin Oncol. 2016;34:abstr 4037. [Cited in This Article: ] |
| 229. | Bang YJ, Muro K, Fuchs CS, Golan T, Geva R, Hara H, Jalal SA, Borg C, Doi T, Wainberg ZA. KEYNOTE-059 cohort 2: safety and efficacy of pembrolizumab (pembro) plus 5-fluorouracil (5-FU) and cisplatin for first-line (1L) treatment of advanced gastric cancer. J Clin Oncol. 2017;35:4012. [Cited in This Article: ] |
| 230. | Doi TP, Piha-Paul SA, Jalal SI, Mai-Dang H, Saraf S, Koshiji M, Csiki I, Bennouna J. Updated results for the advanced esophageal carcinoma cohort of the phase Ib KEYNOTE-028 study of pembrolizumab (MK-3475). J Clin Oncol. 2016;34:abstr 7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 231. | Merck Sharp & Dohme Corp. Study of Pembrolizumab (MK-3475) in Previously-Treated Participants With Advanced Carcinoma of the Esophagus or Esophagogastric Junction (MK-3475-180/KEYNOTE-180). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: clinicaltrials.gov/ct2/show/NCT02559687?term=NCT02559687rank=1 ClinicalTrials.gov Identifier: NCT02559687. [Cited in This Article: ] |
| 232. | Ohtsu A, Tabernero J, Bang YJ, Fuchs CS, Sun L, Wang Z, Csiki I, Koshiji M, Van Cutsem E. Pembrolizumab (MK-3475) versus paclitaxel as second-line therapy for advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: phase 3 KEYNOTE-061 study. J Clin Oncol. 2016;34:abstr TPS183. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 233. | Fuchs CS, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC. Pembrolizumab (pembro) vs paclitaxel (PTX) for previously treated advanced gastric or gastroesophageal junction (G/GEJ) cancer: Phase 3 KEYNOTE-061 trial. J Clin Oncol. 2018;36:abstr 4062. [Cited in This Article: ] |
| 234. | Merck Sharp Dohme Corp. Study of Pembrolizumab (MK-3475) as First-Line Monotherapy and Combination Therapy for Treatment of Advanced Gastric or Gastroesophageal Junction Adenocarcinoma (MK-3475-062/KEYNOTE-062). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02494583?term=NCT02494583rank=1 ClinicalTrials.gov Identifier: NCT02494583. [Cited in This Article: ] |
| 235. | Merck Sharp Dohme Corp. Study of Pembrolizumab (MK-3475) Versus Investigator’s Choice Standard Therapy for Participants With Advanced Esophageal/Esophagogastric Junction Carcinoma That Progressed After First-Line Therapy (MK-3475-181/KEYNOTE-181). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02564263?term=NCT02564263rank=1 ClinicalTrials.gov Identifier: NCT02564263. [Cited in This Article: ] |
| 236. | Chung HC, Arkenau H-T, Wyrwicz L, Oh DY, Lee KW, Infante JR, Lee SS, Lee J, Keilholz U, Mita AC. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced gastric or gastroesophageal junction cancer from JAVELIN solid tumor phase Ib trial: analysis of safety and clinical activity. J Clin Oncol. 2016;34:abstr 4009. [Cited in This Article: ] |
| 237. | Nishina T, Shitara K, Iwasa S, Hironaka S, Muro K, Esaki T, Satoh T, Yamaguchi T, Machida N, von Heydebreck A. Safety, PD-L1 expression, and clinical activity of avelumab (MSB0010718C), an anti-PD-L1 antibody, in Japanese patients with advanced gastric or gastroesophageal junction cancer. J Clin Oncol. 2016;34:abstr 168. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 238. | Moehler MH, Taïeb J, Gurtler JS, Xiong H, Zhang J, Cuillerot JM, Bokuet N. Maintenance therapy with avelumab (MSB0010718C; anti-PD-L1) vs continuation of first-line chemotherapy in patients with unresectable, locally advanced or metastatic gastric cancer: the phase 3 JAVELIN Gastric 100 trial. J Clin Oncol. 2016;34:TPS4134). [Cited in This Article: ] |
| 239. | Segal NH, Antonia SJ, Brahmer JR, Maio M, Blake-Haskins A, Li X. Khleif S. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J Clin Oncol. 2014;32:5s. [Cited in This Article: ] |
| 240. | Kelly RJ, Chung , Gu Y, Steele KE, Rebelatto MC, Robbins PB, Almhanna K. (2015). Phase Ib/II study to evaluate the safety and antitumor activity of durvalumab (MEDI4736) and tremelimumab as monotherapy or in combination, in patients with recurrent or metastatic gastric/gastroesophageal junction adenocarcinoma. J Immunotherapy Cancer. 2015;32:P157. [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 241. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8693] [Cited by in F6Publishing: 9313] [Article Influence: 776.1] [Reference Citation Analysis (0)] |
| 242. | Bristol-Myers Squibb. An Investigational Immuno-therapy Study of Nivolumab or Placebo in Patients With Resected Esophageal or Gastroesophageal Junction Cancer (Checkmate 577). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02743494?term=CheckMate-577rank=1 ClinicalTrials.gov Identifier: NCT02743494. [Cited in This Article: ] |
| 243. | Wada H. Department of Clinical Research in Tumor Immunology, Graduate School of Medicine, Osaka University. Study of Pre-operative Combination Therapy With Mogamulizumab and Nivolumab Against Solid Cancer Patients. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02946671?term=NCT02946671rank=1 ClinicalTrials.gov Identifier: NCT0294667 ClinicalTrials.gov Identifier: NCT02946671. [Cited in This Article: ] |
| 244. | European Organisation for Research and Treatment of Cancer - EORTC. Postoperative Immunotherapy vs Standard Chemotherapy for Gastric Cancer With High Risk for Recurrence (VESTIGE). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03443856?term=NCT03443856rank=1 ClinicalTrials.gov Identifier: NCT03443856. [Cited in This Article: ] |
| 245. | Bristol-Myers Squibb. Ronan K, Johns Hopkins University. Nivolumab or Nivolumab/Ipilimumab Prior to Chemoradiation Plus Nivolumab With II/III Gastro/Esophageal Cancer. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03044613?term=NCT03044613rank=1 ClinicalTrials.gov Identifier: NCT03044613. [Cited in This Article: ] |
| 246. | Palta M, Duke University. Pembrolizumab, Radiotherapy, and Chemotherapy in Neoadjuvant Treatment of Malignant Esophago-gastric Diseases (PROCEED). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03064490?term=NCT03064490cond=gastric+cancerrank=1 ClinicalTrials.gov Identifier: NCT03064490. [Cited in This Article: ] |
| 247. | Merck Sharp Dohme Corp. Perioperative Chemo and Pembrolizumab in Gastric Cancer. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02918162?term=pembrolizumabcond=gastric+cancerrank=4 ClinicalTrials.gov Identifier: NCT02918162. [Cited in This Article: ] |
| 248. | Merck Sharp Dohme Corp. Study of Pembrolizumab (MK-3475) Plus Chemotherapy Versus Placebo Plus Chemotherapy in Participants With Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (MK-3475-585/KEYNOTE-585). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03221426?term=KEYNOTE-585cond=gastric+cancerrank=1 ClinicalTrials.gov Identifier: NCT03221426. [Cited in This Article: ] |
| 249. | Merck Sharp Dohme Corp. University of Kansas Medical Center. Perioperative mFOLFOX Plus Pembrolizumab in Gastroesophageal Junction (GEJ) and Stomach Adenocarcinoma. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03488667?term=NCT03488667cond=gastric+cancerrank=1 ClinicalTrials.gov Identifier: NCT03488667. [Cited in This Article: ] |
| 250. | Royal Marsden NHS Foundation Trust, Merck KgaA. Study Title: Peri-operative Immuno-Chemotherapy in Operable Oesophageal and Gastric cancer (ICONIC). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03399071?term=NCT03399071cond=gastric+cancerrank=1 ClinicalTrials.gov Identifier: NCT03399071. [Cited in This Article: ] |
| 251. | Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, Terme M. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 240] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 252. | Dudek AZ, Sica A, Sidani A, Jha GG, Xie H, Alva AS, Stein MN, Singer EA. Phase Ib study of pembrolizumab in combination with bevacizumab for the treatment of metastatic renal cell carcinoma: Big Ten Cancer Research Consortium BTCRC-GU14003. J Clin Oncol. 2016;34:suppl.559. [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 253. | Solmaz S, Johnstone PA, Forsyth PA. Safety and antitumor activity of hypofractionated stereotactic irradiation (HFSRT) with pembrolizumab (Pembro) and bevacizumab (Bev) in patients (pts) with recurrent high grade gliomas: preliminary results from phase I study. J Clin Oncol. 2016;34:abstr 2041. [Cited in This Article: ] |
| 254. | Chau I, Calvo E, Santana-Davila R, Ahnert JR, Penel N. Interim safety and clinical activity in patients (pts) with advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma from a multicohort phase 1 study of ramucirumab (R) plus pembrolizumab (P). J Clin Oncol. 2017;35:abstract 102. [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 255. | Kato K, Department of Gastrointestinal Medical Oncology, National cancer center hospital Japan. A Phase 1/2 Study of Ramucirumab Plus Nivolumab in Participants With Gastric or GEJ Cancer. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02999295?term=NCT02999295cond=gastric+cancerrank=1 ClinicalTrials.gov Identifier: NCT02999295. [Cited in This Article: ] |
| 256. |
St. Marianna University School of Medicine Hospital, Department of Clinical Oncology.
|
| 257. | New York University School of Medicine, National Cancer Institute (NCI). Lenvatinib Mesylate and Pembrolizumab in Treating Patients With Metastatic or Recurrent Gastric or Gastroesophageal Cancer. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03413397?term=nct03413397cond=gastric+cancerrank=1 ClinicalTrials.gov Identifier: NCT03413397. [Cited in This Article: ] |
| 258. | Institut Bergonié, Merck KgaA. A Phase I/II Study of Regorafenib Plus Avelumab in Digestive Tumors (REGOMUNE). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03475953?term=nct03475953cond=gastric+cancerrank=1 ClinicalTrials.gov Identifier: NCT03475953. [Cited in This Article: ] |
| 259. | Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1085] [Cited by in F6Publishing: 1032] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 260. | Catenacci DV, Kim SS, Gold PJ. A phase 1b/2, open label, dose-escalation study of margetuximab (M) in combination with pembrolizumab (P) in patients with relapsed/refractory advanced HER2+ gastroesophageal (GEJ) junction or gastric (G) cancer. J Clin Oncol. 2017;35:TPS219. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 261. | Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ, Wainwright DA. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clin Cancer Res. 2015;21:5427-5433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 262. | Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J Immunother Cancer. 2017;5:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 263. | Incyte Corporation. Merck Sharp Dohme Corp. A Phase 1/2 Study Exploring the Safety, Tolerability, and Efficacy of Pembrolizumab (MK-3475) in Combination With Epacadostat (INCB024360) in Subjects With Selected Cancers (INCB 24360-202 / MK-3475-037 / KEYNOTE-037/ ECHO-202). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine.Available from: https://clinicaltrials.gov/ct2/show/NCT02178722?term=NCT02178722rank=1 ClinicalTrials.gov Identifier: NCT02178722. [Cited in This Article: ] |
| 264. | Kunz P, Stanford University. Epacadostat and Pembrolizumab in Treating Patients With Metastatic or Unresectable Gastroesophageal Junction or Gastric Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT03196232?term=nct03196232rank=1. [Cited in This Article: ] |
| 265. | Kidokoro T, Tanikawa C, Furukawa Y, Katagiri T, Nakamura Y, Matsuda K. CDC20, a potential cancer therapeutic target, is negatively regulated by p53. Oncogene. 2008;27:1562-1571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 266. | Maire V, Baldeyron C, Richardson M, Tesson B, Vincent-Salomon A, Gravier E, Marty-Prouvost B, De Koning L, Rigaill G, Dumont A. TTK/hMPS1 is an attractive therapeutic target for triple-negative breast cancer. PLoS One. 2013;8:e63712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 267. | Yim H. Current clinical trials with polo-like kinase 1 inhibitors in solid tumors. Anticancer Drugs. 2013;24:999-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 268. | Gray D, Jubb AM, Hogue D, Dowd P, Kljavin N, Yi S, Bai W, Frantz G, Zhang Z, Koeppen H. Maternal embryonic leucine zipper kinase/murine protein serine-threonine kinase 38 is a promising therapeutic target for multiple cancers. Cancer Res. 2005;65:9751-9761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 269. | Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu LF, Gondek D, Wang Y, Fava RA. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 270. | Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, Noelle RJ, Wang L. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014;74:1933-1944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 333] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 271. | Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, Miller HE, Guleria I, Barth RJ, Huang YH. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci USA. 2015;112:6682-6687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 239] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 272. | Curis , Inc . A Study of CA-170 (Oral PD-L1, PD-L2 and VISTA Checkpoint Antagonist) in Patients With Advanced Tumors and Lymphomas. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02812875?term=NCT02812875rank=1 ClinicalTrials.gov Identifier: NCT02812875. [Cited in This Article: ] |
| 273. | Böger C, Behrens HM, Krüger S, Röcken C. The novel negative checkpoint regulator VISTA is expressed in gastric carcinoma and associated with PD-L1/PD-1: A future perspective for a combined gastric cancer therapy? Oncoimmunology. 2017;6:e1293215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 274. | Gilead Sciences. Andecaliximab Combined With Nivolumab Versus Nivolumab Alone in Adults With Unresectable or Recurrent Gastric or Gastroesophageal Junction Adenocarcinoma. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02864381?term=NCT02864381rank=1 ClinicalTrials.gov Identifier: NCT02864381. [Cited in This Article: ] |
| 275. | Aanur P, Gutierrez M, Kelly RJ, Ajani JA, Ku GY, Denlinger CS. FRACTION (fast real- time assessment of combination therapies in immuno- oncology)- gastric cancer (GC): a randomized, open-label, adaptive, phase 2 study of nivolumab in combination with other immuno- oncology (IO) agents in patients with advanced GC. J Clin Oncol. 2017;35:TPS4137. [Cited in This Article: ] |
| 276. | Mayo Clinic, National Cancer Institute (NCI). Pembrolizumab, Combination Chemotherapy, and Radiation Therapy Before Surgery in Treating Adult Patients With Locally Advanced Gastroesophageal Junction or Gastric Cardia Cancer That Can Be Removed by Surgery. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02730546?term=NCT02730546rank=1 ClinicalTrials.gov Identifier: NCT02730546. [Cited in This Article: ] |
| 277. | Kono K, Fukushima Medical University Hospital. Checkpoint Inhibitor and Radiotherapy for Recurrent Gastric Cancer (CIRCUIT). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03453164?term=NCT03453164rank=1 ClinicalTrials.gov Identifier: NCT03453164. [Cited in This Article: ] |
| 278. | Läubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015;3:11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 279. | Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375:1749-1755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1305] [Cited by in F6Publishing: 1468] [Article Influence: 183.5] [Reference Citation Analysis (0)] |
| 280. | Myint ZW, Goel G. Role of modern immunotherapy in gastrointestinal malignancies: a review of current clinical progress. J Hematol Oncol. 2017;10:86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 281. | Yonsei University, Korea. Biomarker-Integrated Umbrella, Advanced Gastric Cancer. [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02951091?term=NCT02951091rank=1 ClinicalTrials.gov Identifier: NCT0295109. [Cited in This Article: ] |
| 282. | National Cancer Institute (NCI). [accessed 2018 May 4]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02465060?term=NCT02465060rank=1 ClinicalTrials.gov Identifier: NCT02465060. [Cited in This Article: ] |