Published online Jan 21, 2018. doi: 10.3748/wjg.v24.i3.397
Peer-review started: November 4, 2017
First decision: November 14, 2017
Revised: November 30, 2017
Accepted: December 4, 2017
Article in press: December 4, 2017
Published online: January 21, 2018
To evaluate the association of Helicobacter pylori (H. pylori), cagA genotype, and type of gastric pathology with ghrelin, leptin and nutritional status.
Fasted dyspeptic adults (18-70 years) referred for an upper digestive endoscopy were enrolled in this cross-sectional study. Height and weight were assessed for body mass index (BMI) calculation. A sociodemographic survey was administered and nutrient intake was evaluated with 24 h dietary recalls. Serum total ghrelin and leptin levels were analyzed by enzyme-linked immunosorbent assay. 13C-Urea Breath Test was performed and four gastric biopsies were obtained during endoscopy for histopathology and H. pylori DNA amplification and genotyping. Data analysis was performed using χ2, Mann-Whitney U, Kruskal-Wallis tests, Spearman’s correlation and linear regression.
One hundred and sixty-three patients (40.8 ± 14.0 years), 98/65 females/males, were included. Overall, persistent H. pylori prevalence was 53.4% (95%CI: 45.7%-65.8%). Neither nutrient intake nor BMI differed significantly between H. pylori positive and negative groups. Serum ghrelin was significantly lower in infected patients [median 311.0 pg/mL (IQR 230.0-385.5)] than in uninfected ones [median 355.0 pg/mL (IQR 253.8-547.8)] (P = 0.025), even after adjusting for BMI and gender (P = 0.03). Ghrelin levels tended to be lower in patients carrying cagA positive strains both in the antrum and the corpus; however, differences with those carrying cagA negative strains did not reach statistical significance (P = 0.50 and P = 0.49, respectively). In addition, the type and severity of gastric pathology in the corpus was associated with lower serum ghrelin (P = 0.04), independently of H. pylori status. Conversely, leptin levels did not differ significantly between infected and uninfected patients [median 1.84 ng/mL (0.80-4.85) vs 1.84 ng/mL (0.50-5.09), (P = 0.51)].
H. pylori infection and severity of gastric corpus pathology are associated with lower serum ghrelin. Further studies could confirm a lower ghrelin prevalence in cagA-positive patients.
Core tip: The relationship between Helicobacter pylori (H. pylori) infection and hormonal modulation of food intake is still controversial. We conducted this study to evaluate the association between H. pylori infection, the genotype of infecting strains and the type of gastric pathology, with serum ghrelin and leptin concentrations and anthropometric nutritional status of dyspeptic patients. Our study demonstrated that H. pylori infection and the severity of gastric pathology of the corpus are associated with lower ghrelin serum concentrations. We also observed lower, but not significantly different, ghrelin levels in patients carrying cagA positive strains, an observation that should be evaluated further in future studies.
- Citation: Mantero P, Matus GS, Corti RE, Cabanne AM, Zerbetto de Palma GG, Marchesi Olid L, Piskorz MM, Zubillaga MB, Janjetic MA, Goldman CG. Helicobacter pylori and corpus gastric pathology are associated with lower serum ghrelin. World J Gastroenterol 2018; 24(3): 397-407
- URL: https://www.wjgnet.com/1007-9327/full/v24/i3/397.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i3.397
Since its discovery by Drs. Marshall and Warren[1], the bacterium Helicobacter pylori (H. pylori) has been associated with the development of diverse gastroduodenal pathologies of the host, from chronic superficial gastritis to gastric cancer, being classified as a class I carcinogen for its definite role in this latter outcome[2,3]. It is already known that the outcome of H. pylori colonization is determined by the confluence of different factors, related not only with its presence within the stomach, but with colonizing strains, environmental factors, and susceptibility of the host[4,5]. Consequently, only a low proportion of H. pylori persistently infected individuals develop gastroduodenal pathology. Recent data demonstrate 85%-90% of H. pylori positive individuals are asymptomatic; 6%-20% are at risk of developing peptic-ulcer disease; and 0.1%-1% develop gastric cancer[6].
The stomach plays an important role in food intake regulation through the production of ghrelin and leptin, two neuroendocrine hormones which exert hypothalamic actions regulating appetite and satiety[7-9]. Ghrelin is an orexigenic hormone predominantly produced by the ghrelin producing cells in the gastric oxyntic mucosa, which stimulates food intake, decreases energy expenditure and promotes body weight gain[10]. Its main action at the central nervous system consists in the stimulation of growth hormone (GH) release, affecting several physiological processes. In the gastrointestinal tract, it stimulates gastrin release, gastric acid secretion and gastric emptying via vagal activation[11]. In contrast, leptin exerts anorexigenic effects by acting on the hypothalamus, suppressing food intake and increasing energy metabolism. Circulating leptin is mainly provided by adipocytes production; however, a low proportion is produced by chief and endocrine P cells in the gastric tissue[7]. Gastric leptin also regulates intestinal nutrient absorption, delays gastric emptying and signals short-term satiety[9].
Recently, controversial results on the influence of H. pylori colonization on the gastric regulation of food intake and body mass index (BMI) have been reported. Some authors described lower gastric and/or plasmatic ghrelin levels in H. pylori positive patients[12-14], whereas others reported similar ghrelin serum concentrations for H. pylori positive and negative individuals[15], or even higher ghrelin gastric production in infected patients[16]. On the other hand, it has been described that leptin plasmatic concentrations are not effected by H. pylori colonization[14]; however, lower serum leptin levels have been reported in infected patients without a variation in gastric biopsies[15]. After a systematic review of the literature with a meta-analysis, Nweneka and Prentice concluded that circulating ghrelin levels were lower in H. pylori infected than in H. pylori negative individuals; nevertheless, its variation after H. pylori eradication remained controversial[17]. Two important aspects that would be involved in appetite hormone levels are the severity and topology of gastric mucosal affection, although these are not often evaluated. Liew et al[12] and Chuang et al[14] reported an association between chronic gastric inflammation histology scores and lower plasma ghrelin levels, and more recently an association has been described between plasma ghrelin levels and the severity of atrophy related to H. pylori infection in hemodialysis patients[18]. Evaluation of H. pylori cagA genotype would be also central because CagA protein producing strains have a higher interaction with the host[5], exerting cellular effects including the induction of pro-inflammatory signals[19]; however, the association of H. pylori colonizing strains genotype with ghrelin levels has been scarcely studied[15,20].
This present study was conducted to evaluate the association of persistent H. pylori infection, the genotype of infecting H. pylori strain, the type of gastric pathology and serum ghrelin and leptin concentrations, in dyspeptic patients with a known nutritional status.
This Cross-Sectional study included 12-h fasted dyspeptic adults (18-70 years) referred for an upper digestive endoscopy to the Esophagus-Stomach Section of the Hospital de Gastroenterología “Dr. Carlos Bonorino Udaondo”, and the Gastroenterology Unit of the Hospital de Clínicas “José de San Martín”, both located in Buenos Aires City, Argentina. Inclusion criteria were the presence of upper gastrointestinal signs and symptoms (gastroesophageal reflux, oesophagitis, dispepsia and abdominal pain), while exclusion criteria were antecedents of gastric surgery, neoplastic disease, diabetes, celiac disease, thyroid, renal or hepatic pathologies, drug abuse, coagulopathies, pregnancy, previous H. pylori treatment and use of antimicrobials or acid suppressants during the month before enrollment. The protocol was approved by the ethics committees of the two hospitals in which patients were recruited and it was conducted according to the guidelines laid down in the Declaration of Helsinki and the Guidelines of Good Clinical Practice. Written informed consent was obtained from the patients for inclusion in the study, in which the objectives, procedures and outcomes were detailed. Patients were informed about the results of the diagnostic tests and received the appropriate treatment after an individual basis.
A sociodemographic survey was administered to the patients in order to obtain information about possible predictive variables for H. pylori positivity. The questionnaire was focused on ethnicity, socio-demographic factors and sanitary conditions.
Body weight and height were obtained at enrollment to calculate the Body Mass Index (BMI) of each patient as their weight in kilograms divided by the square of their height in meters. Height was recorded using a stadiometer (Stanley, Morangis, France) to the nearest 0.1 cm, and weight was measured with a portable mechanical scale (CAM, Buenos Aires, Argentina) to the nearest 100 g. Underweight, stunting, overweight and obesity were defined according to the classification of the World Health Organization[21]. Anthropometric techniques were previously standardized according to the CDC anthropometry procedures manual[22]. Waist circumference was measured with a stretch-resistant tape to determine abdominal adiposity as a predictor of cardiovascular disease risk[23].
Energy and macronutrient intake were assessed with 24 h dietary recalls administered to the patients. A book of picture charts was used to aid respondents in portion size estimation[24]. Data analysis was performed using the food composition database compiled in 2007 by the Argentine Ministry of Health[25].
The 13C-UBT was performed using a commercial kit (TAU-KIT, Isomed Pharma, Madrid, Spain). Briefly, fasted patients were instructed to drink a 100 mL beverage enriched in citric acid. After 10 min, two pre-dose basal exhaled air samples were obtained in hermetically sealed containers. Each patient was given a 50 mL water solution in which a soluble tablet of 100 mg 13C-urea had been dissolved. Two breath samples were collected after 30 min. Samples were measured in an isotope ratio mass spectrometer coupled to a gas chromatograph (Finnigan MAT GmbH, Thermo Fisher Scientific, Bremen, Germany) as previously described[26,27]. A change of 3.5‰ in the Delta Over Baseline values was considered positive[28]. Urea Hydrolysis Rate (UHR) was calculated from the 13C-UBT to normalize the results by the endogenous CO2 production per body size[29], as previously described[30].
Venous blood samples were collected just before endoscopy. Serum was obtained by centrifugation and kept at -80 °C until assay. Serum total ghrelin levels were analyzed in duplicate samples by Enzyme-linked Immunosorbent Assay (ELISA) using a commercial kit (EMD Millipore Corporation, MO, United States), and serum leptin concentrations with a commercial Enzyme Amplified Sensitivity Immunoassay (EASIA) kit (DIAsource ImmunoAssays SA, Belgium). Absorbance was measured in a plate reader (Multiskan EX, Thermo Scientific INC, United States) and the results were processed with the Cembal 2.2® program (Cembal Applications 2000-2001, Argentina) to calculate hormonal concentrations.
Subjects underwent a routine endoscopic evaluation of the upper gastrointestinal tract during which two gastric biopsies from the antrum and two from the corpus were obtained. One of the samples of each gastric site was used for histological assessment and the other one for molecular biology evaluation.
Gastric biopsies were processed with a spin tissue processor (MicromSTP120, ThermoScientific Corp., Walldorf, Germany) comprising the following steps: Formol immersion (2 h), dehydration in alcohol 96% (6 h), alcohol 100% (4 h) and xylene (3 h), and paraffin immersion at 56-58 °C (3 h) and at 62 °C (3 h). Samples were then embedded in paraffin at 62 °C, from which 4 μm consecutive sections were obtained for haematoxylin-eosin and Giemsa histologic staining. Microscopic assessment was classified according to the updated Sydney System Classification[31].
DNA was extracted from gastric biopsies from the antrum and corpus using the QIAamp Mini Kit (QIAGEN, INC., CA, United States) and evaluation of H. pylori vacA and cagA genotypes was performed by PCR amplification. Primer sequences (5’-3’) and product base pair sizes were as follows: va1F (ATGGAAATACAACAAACACAC) and va1XR (CCTGAGACCGTTCCTACAGC) for vacAS1 allele (176 bp product) and vacAS2 (203 bp product)[32]; cagA22 (GATCCTGCTAGTTTGTCAGCGA) and cagA23 (CTTATCATTCACGAGTTTGAGC) for the cagA gene (127 bp product)[33]. Amplification was carried out in a total volume of 50 μL containing 1XTaq polymerase buffer, 1.5 mmol/L MgCl2, 0.2 mmol/L (each) deoxynucleotide, 1.0 U of Platinum® Taq DNA Polymerase (Invitrogen Argentina, Buenos Aires, Argentina), 0.1 μg each oligonucleotide primer, and 5 μL of DNA template. PCR [94 °C for 3 min; 35 cycles of 94 °C for 30 s, 50 °C for 45 s (vacA) or 54 °C for 30 s (cagA), and 72 °C for 45 s (vacA) or for 30 s (cagA); 72 °C for 5 min] was performed with an automatic thermocycler (MyCycler, BioRad, CA, United States) and a 10 μL aliquot was analyzed by electrophoresis through a 1.5% (wt/vol) agarose gel stained with ethidium bromide. PCR products were visualized by excitation under UV light.
H. pylori infection was determined by the three methodologies described above: 13C-UBT, histology and vacA PCR amplification from gastric biopsies. Patients were considered H. pylori positive with positive results from at least two of the three diagnostic methods.
A sample size of 160 individuals was calculated to be included in the study using the StatCalc program (Epi Info Version 3.2, Georgia, United States), setting an α error of 0.05, a β error of 0.20, an estimated 50% H. pylori infection prevalence in adult patients and a 25% expected frequency of ghrelin hormonal variation between the H. pylori positive and negative groups. Statistical analyses were performed by the χ2, Mann-Whitney U and Kruskal-Wallis tests, Spearman correlation and linear regression. Significance levels were set at α < 0.05. Statistical analyses were performed using SPSS software version 17.0 (IBM SPSS). The statistical methods of this study were reviewed by Janjetic MA from the Universidad de Buenos Aires and CONICET.
The present study included 163 patients (40.8 ± 14.0 years of age), 98/65 females/males. Prevalence of H. pylori infection was 53.4% (95%CI: 45.7%-65.8%). H. pylori positive and negative patients did not differ significantly in terms of age (P = 0.48) or gender (P = 0.46). Sociodemographic variables which proved to be associated with the infection were ethnicity (P = 0.007), with a higher H. pylori prevalence in South American Indians, and poorer sanitary conditions denoted by the type of house (P = 0.01) and type of flooring (P = 0.03).
Dietary recalls were collected from all of the participating adults. Table 1 summarizes the macronutrient and energy intake of the patients according to H. pylori status. Energy, carbohydrate, protein and fat intake were not associated with H. pylori infection.
| Nutrient | Helicobacter pylori negative | Helicobacter pylori positive | P value | ||
| Median | IQR | Median | IQR | ||
| Energy (kcal/d) | 1627.6 | 1187.4-2063.0 | 1748.4 | 1089.4-2308.8 | 0.65 |
| Carbohydrate (g/d) | 196.4 | 151.9-251.1 | 202.5 | 135.9-311.7 | 0.42 |
| Protein (g/d) | 72.7 | 47.6-88.5 | 68.8 | 42.7-104.6 | 0.91 |
| Fat (g/d) | 59.8 | 37.8-78.5 | 60.0 | 34.0-81.1 | 0.94 |
Anthropometric measurements in H. pylori positive and negative patients are described in Table 2. BMI of the patients did not differ significantly according to H. pylori status (P = 0.09), and neither did the percentage of patients with abdominal adiposity, denoted by a high waist circumference (47.1% of the infected patients vs 43.4% of the uninfected patients; P = 0.63). Anthropometric indicators of nutritional status showed that 30/87 (34.5%; 95%CI: 25.3%-44.9%) of the H. pylori positive patients had under/normo-weight, 35/87 (40.2%; 95%CI: 30.6%-50.7%) overweight, and 22/87 (25.3%; 95%CI: 17.3%-35.3%) obesity, whereas 38/76 (50.0%; 95%CI: 39.0%-61.0%) of the H. pylori negative patients had under/normo-weight, 24/76 (31.6%; 95%CI: 22.2%-42.7%) overweight and 14/76 (18.4%; 95%CI: 11.3%-28.6%) obesity, showing no statistically significant differences between the infected and the uninfected group (P = 0.13).
| Variable | Helicobacter pylori negative | Helicobacter pylori positive | P value |
| Weight (kg)1 | 66.43 (14.15) | 69.52 (12.71) | 0.08 |
| Height (m)1 | 1.61 (0.09) | 1.61 (0.08) | 0.93 |
| BMI (kg/m2)1 | 25.78 (5.13) | 26.93 (4.26) | 0.09 |
| Waist Circunference (cm)1 | 83.78 (11.89) | 87.04 (11.09) | 0.08 |
| Ghrelin (pg/mL)2 | 355.0 (253.8-547.8) | 311.0 (230.0-385.5) | 0.025a |
| Leptin (ng/mL)2 | 1.84 (0.50-5.09) | 1.84 (0.80-4.85) | 0.87 |
Appetite hormones serum concentrations are summarized in Table 2. Both ghrelin and leptin serum levels were found to be higher in females than in males (P = 0.020 and P < 0.0001). Statistical analysis demonstrated that the infection was associated with lower serum ghrelin concentrations (P = 0.025), remaining associated after adjusting for BMI and gender in a linear regression analysis (P = 0.03). On the other hand, leptin levels did not differ significantly between the infected and the uninfected group (P = 0.51), even after adjusting for BMI and gender (P = 0.10).
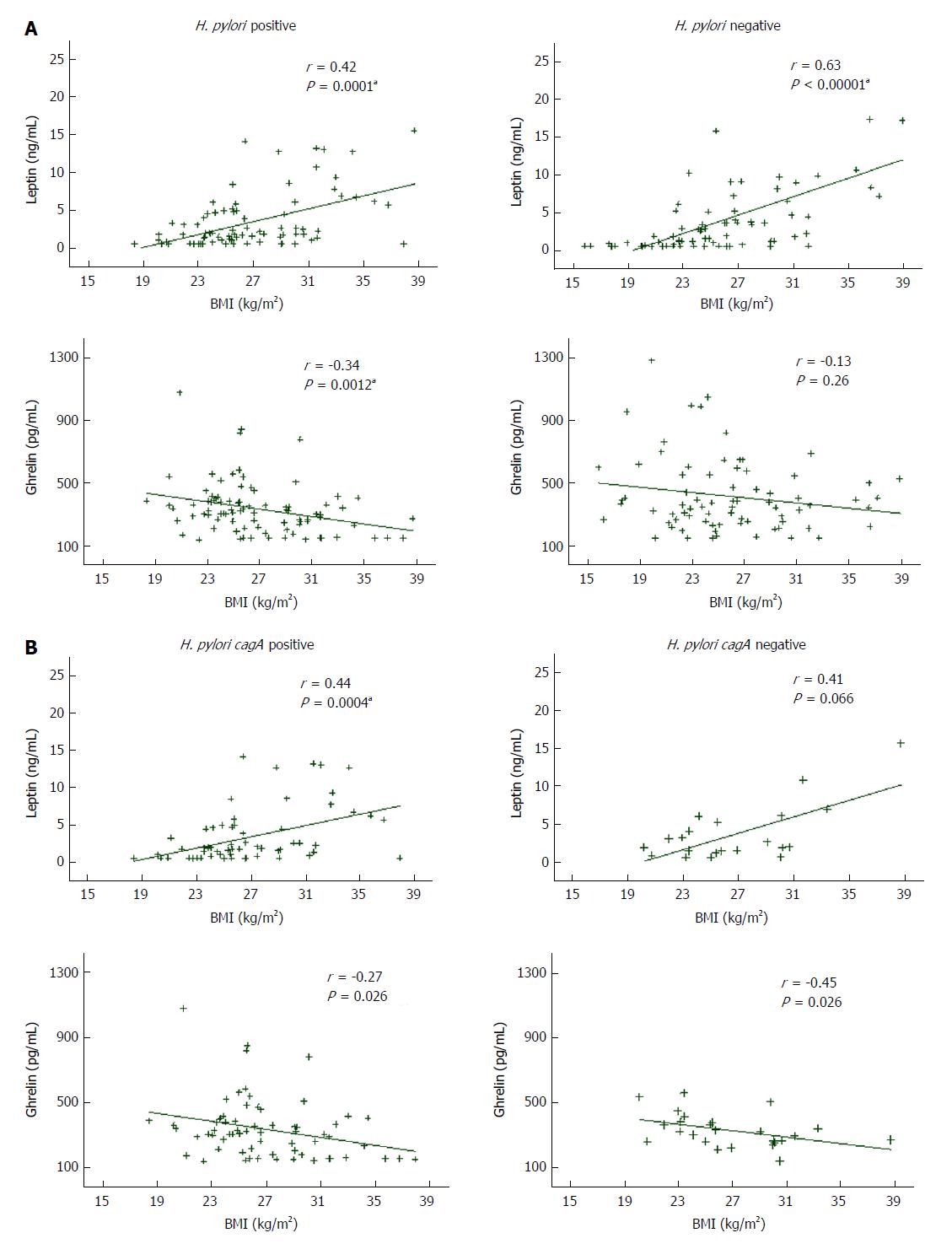
Figure 1 illustrates the correlation between appetite hormones serum concentrations and BMI in the whole population and according to H. pylori status. Serum leptin values positively correlated with BMI in the total population (r = 0.52; P < 0.00001), remaining correlated in the H. pylori negatives (r = 0.63; P < 0.00001) and the H. pylori positives (r = 0.42; P = 0.0001), in which the correlation was obtained only for infected patients carrying H. pylori cagA positive strains (r = 0.44; P = 0.0004). In contrast, a week inverse correlation was found between serum ghrelin values and BMI in the whole population (r = -0.23; P = 0.0036) and in the H. pylori positive group (r = -0.34; P = 0.0012), while a lack of correlation was obtained for ghrelin values and BMI in uninfected patients (r = -0.13; P = 0.26). When the results of the infected group were analyzed by cagA genotype, ghrelin levels remained inversely correlated with BMI both in the cagA positive (r = -0.27; P = 0.026) and the cagA negative group (r = -0.45; P = 0.026) (Figure 1).
Results of histopathology analysis of gastric biopsies are presented in Table 3. Histological analysis from gastric biopsies could not be performed in 11/163 (6.7%) antrum samples and 28/163 (17.2%) body samples. Presence of H. pylori was associated with the type of gastric pathology both in the antrum (P < 0.0001) and the corpus (P < 0.0001), with a higher prevalence of active chronic gastritis among H. pylori positive patients, as has been widely described[34]. Table 4 summarizes the results obtained from the analysis of serum ghrelin concentrations according to the type of gastric pathology of the antrum and the corpus in all the patients, independently of their H. pylori status. The type and severity of gastric pathology in the corpus were associated with lower serum ghrelin levels (P = 0.04). The Kruskal Wallis post-hoc analysis revealed that ghrelin levels differed significantly among all the types of gastric pathology in the corpus except in the chronic inactive and active gastritis groups. On the other hand, gastric pathology of the antrum was not associated with ghrelin levels (P = 0.08).
| Type of gastric pathology | Helicobacter pylori positive (%) | Helicobacter pylori negative (%) | P value |
| Antrum | n = 82 | n = 70 | |
| Normal | 0.0 | 78.6 | |
| Chronic inactive gastritis | 4.9 | 12.8 | < 0.0001a |
| Chronic active gastritis | 78.0 | 5.7 | |
| Atrophy or intestinal metaplasia | 17.1 | 2.9 | |
| Corpus | n = 77 | n = 58 | |
| Normal | 2.6 | 77.6 | |
| Chronic inactive gastritis | 9.1 | 8.6 | < 0.0001a |
| Chronic active gastritis | 84.4 | 6.9 | |
| Atrophy or intestinal metaplasia | 3.9 | 6.9 |
| Type of gastric pathology | Ghrelin levels (pg/mL)1 | P value | |||
| Normal | Chronic inactive gastritis | Chronic active gastritis | Atrophy/intestinal metaplasia | ||
| Antrum | 365.5 (256.0-594.0) | 334.5 (223.3-368.0) | 306.8 (255.3-389.6) | 294.0 (182.8-441.1) | 0.08 |
| Corpus | 334.5 (242.0-549.0) | 323.8 (238.5-371.0) | 305.0 (234.0-395.5) | 161.0 (150.0-289.0) | < 0.04a |
The distribution of H. pylori vacA and cagA genotypes from infected patients were as follows: The vacA S1 allele was detected in 77.0% of the H. pylori positive patients, while the vacA S2 allele was amplified in 23.0% of the positive group. Overall prevalence of H. pylori cagA positive genotype in the antrum and the corpus was 74.7% (95%CI: 64.4%-82.8%). From 62 H. pylori vacAS1 gastric biopsies, 53 (85.5%) were cagA positive and 9 (14.5%) were cagA negative both in the antrum and the corpus; while from 18 H. pylori vacAS2 antrum gastric biopsies and 19 corpus gastric biopsies, 5 (27.8%) and 8 (42.1%) were cagA positive, whereas 13 (72.2%) and 11 (57.9%) were cagA negative. Due to the association found in this study between H. pylori infection and lower ghrelin serum levels, we investigated whether ghrelin levels of infected patients differed according to their cagA genotype (Table 5). Although a tendency towards lower ghrelin levels could be observed from antrum and corpus cagA positive patients, differences with cagA negative patients did not reach statistical significance (P = 0.50 and P = 0.49, respectively). On the other hand, no statistically significant difference was obtained for the BMI of infected patients carrying a cagA negative genotype from those carrying cagA positive strains in the gastric antrum (P = 0.94) or corpus (P = 0.65).
The relationship between H. pylori infection and hormonal modulation of food intake, although lately investigated, is still controversial. We conducted this study to evaluate the presence of an association, in dyspeptic patients, between persistent H. pylori infection, the genotype of infecting strain, the type of gastric pathology, and the serum ghrelin and leptin levels in patients with a measured anthropometric nutritional status. Our study demonstrated that H. pylori infected patients had lower serum ghrelin concentrations than the uninfected ones, independently of their BMI or gender, supporting the findings from several groups[12-14,17,35]. It has been demonstrated that ghrelin exhibits gastroprotective antioxidant and anti-inflammatory effects[36-38], modulating gastric mucosal inflammation induced by H. pylori lipopolysacaccharide (LPS) through several mechanisms which were recently reviewed[39]. Ghrelin circulating levels were described to rise in response to severe gastric oxidative stress induced during acute gastritis or peptic ulcer disease; however, its concentration decreases concomitantly with injury of the gastric glands[36]. Our results of lower ghrelin levels in persistently infected patients are consistent with these findings, and with the ones from other studies[35,40]. In contrast, leptin levels were not associated to H. pylori infection in our studied population, as reported by other authors[14], but contrary to previous reports that found lower serum leptin levels in infected individuals[15]. Despite the lower ghrelin serum concentrations in H. pylori positive patients, their food intake and BMI did not differ when compared to uninfected patients. A lack of association between H. pylori infection and dietary intake was previously reported by our group in dyspeptic children[30], and by a Japanese group which described a tendency towards higher energy and carbohydrate intake in H. pylori seropositive adults but no statistically significant difference in relation to seronegative adults values[41], which is consistent with the findings of the present study. We used the 24-hour dietary recall for dietary assessment, which might limit our results particularly due to the patients’ unintentional misreport[42]. However, we consider that the complex and multifactorial nature of food intake regulation would be a more accurate explanation for these findings[9]. In this way, a study by Carrasco et al[43] showed that the greater decrease in ghrelin levels one year after resective bypass was not associated with differences in dietary intake or weight loss at the same time point. The authors suggest that the restriction of the stomach capacity along with other hormonal mechanisms would be more relevant than the decrease of ghrelin levels on food intake and weight loss[43].
Circulating ghrelin and leptin levels were described to be similar between gender[44]; however, we found higher hormonal levels in women than in men, as described in previous reports[16]. Our results also showed that circulating leptin concentrations positively correlated with BMI of the patients independently of their H. pylori status; however, when the results were analyzed according to H. pylori genotype, leptin serum levels remained positively correlated with BMI only in infected patients carrying cagA positive strains. Such results are coincident with those reported by Roper et al[15]. In addition, ghrelin serum levels inversely correlated with BMI of infected patients independently of their cagA genotype.
H. pylori positivity was associated with lower ghrelin concentrations; however, we were not able to demonstrate a statistically significant difference in hormonal levels according to the cagA genotype despite finding a tendency towards lower ghrelin levels in cagA positive patients. It should be pointed out this study was powered to detect differences in hormonal concentrations between H. pylori infected and uninfected patients. Consequently, the high prevalence of the cagA positive genotype in our population (74.7%) and the low number of patients carrying the vacA S2 cagA positive less frequent allele, along with an insufficient sample size of infected individuals, may have prevented the observation of these differences, if any.
Another important aspect evaluated in this study was the relationship between the type of gastric pathology and appetite hormones level. The results demonstrated that the type and severity of gastric pathology in the corpus were associated with lower ghrelin serum levels, independently of H. pylori status. Our findings are similar to the ones of Isomoto et al[40], and to those recently reported by Ichikawa et al[18], which described a decrease in plasma acyl- and desacyl-ghrelin levels according to the severity of atrophy in hemodialysis patients. The location of ghrelin producing cells in the gastric fundus-corpus[10] is consistent with these results. The small number of patients with gastric atrophy or intestinal metaplasia in the H. pylori positive and negative groups did not allow us to seek an association between ghrelin levels and gastric atrophy according to H. pylori status. In addition, the histological evaluation of one biopsy from each gastric compartment should be also taken into consideration as a biopsy-based sampling error that could limit our results. Future studies should be performed with a different study design or a higher sample size, where the number of patients with this pathological condition would allow the investigation of that relationship.
In conclusion, our study demonstrated that persistent H. pylori infection and the severity of gastric pathology of the corpus are associated with lower ghrelin serum concentrations in dyspeptic patients. Future studies are needed to determine if significantly lower ghrelin levels are observed in cagA positive patients.
The stomach participates in the production of ghrelin and leptin, two important neuroendocrine hormones in food intake modulation. Helicobacter pylori (H. pylori) infection has been associated with several pathologies affecting the gastroduodenal mucosa, for which reason it would be important to find out whether it could alter circulating levels of these hormones and ultimately, the body mass index (BMI).
Although the influence of H. pylori infection on the hormonal regulation of food intake has been addressed lately, the results are controversial.
The present study aimed to evaluate the relationship between H. pylori infection, cagA genotype, type of gastric pathology, serum ghrelin and leptin concentrations and nutritional status in patients with gastrointestinal symptoms.
This cross-sectional study included fasted dyspeptic adults (18-70 y) referred for an upper digestive endoscopy. We conducted a survey for sociodemographic variables evaluation and a 24 h dietary recall for food intake estimation. H. pylori status was determined by three methods: histological analysis, PCR amplification of the vacA constitutive H. pylori gene and 13C -Urea Breath Test. Total ghrelin and leptin serum concentrations were measured by enzyme-linked immunosorbent assay and enzyme amplified sensitivity immunoassay respectively. During an upper gastrointestinal endoscopy, four gastric biopsies were obtained. One sample of each gastric site was used for histological assessment and the others for PCR amplification of H. pylori vacA and cagA genes. Statistical analysis was performed using χ2, Mann-Whitney U, Kruskal-Wallis tests, Spearman’s correlation and linear regression.
Prevalence of persistent H. pylori infection was 53.4% (95%CI: 45.7%-65.8%) in our population of 163 adults. Mean age was 40.8 ± 14.0 years, and 98 (60.1%) were female. Nutrient intake did not differ significantly between H. pylori positive and negative patients, neither did BMI. We observed significantly lower serum ghrelin levels in infected patients [median 311.0 pg/mL (IQR 230.0-385.5)] than in uninfected ones [median 355.0 pg/mL (IQR 253.8-547.8)] (P = 0.025), even after adjusting for BMI and gender (P = 0.03). A tendency towards lower ghrelin levels could be detected from antrum and corpus cagA positive patients; however, differences with cagA negative patients did not reach statistical significance (P = 0.50 and P = 0.49, respectively). Lower serum ghrelin concentration was associated with the type and severity of gastric pathology in the corpus (P = 0.04), independently of H. pylori status. Serum leptin levels did not differ significantly between H. pylori positive and negative patients [median 1.84 ng/mL (0.80-4.85) vs 1.84 ng/mL (0.50 - 5.09), (P = 0.51)].
Our study demonstrated that H. pylori infection and the severity of gastric pathology of the corpus are associated with lower ghrelin serum concentrations. We also observed lower, but not significantly different ghrelin levels in patients carrying cagA positive strains, an observation that should be evaluated further in future studies.
Our conclusions highlight the importance of investigating the effect of H. pylori eradication on ghrelin circulating levels regarding the genotype of infecting strains.
We thank the medical team of the Esophagus-Stomach Section, Endoscopy and Pathology Units and the administrative personnel of the Hospital de Gastroenterología “Dr. Carlos Bonorino Udaondo” for their cooperation with our protocol. Our thanks are also due to Dr. Roger A Feldman, who has kindly revised this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Jonaitis LV, Pellicano R, Slomiany BL, Sugimoto M, Ulasoglu C, Xu CF S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [PubMed] [Cited in This Article: ] |
| 2. | Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] [Cited in This Article: ] |
| 3. | Czinn SJ. Helicobacter pylori infection: detection, investigation, and management. J Pediatr. 2005;146:S21-S26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 626] [Cited by in F6Publishing: 656] [Article Influence: 32.8] [Reference Citation Analysis (1)] |
| 5. | Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest. 2009;119:2475-2487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 378] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 6. | Frenck RW Jr, Clemens J. Helicobacter in the developing world. Microbes Infect. 2003;5:705-713. [PubMed] [Cited in This Article: ] |
| 7. | Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y. The stomach is a source of leptin. Nature. 1998;394:790-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 793] [Cited by in F6Publishing: 757] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 8. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5961] [Cited by in F6Publishing: 5702] [Article Influence: 228.1] [Reference Citation Analysis (0)] |
| 9. | Konturek PC, Konturek JW, Cześnikiewicz-Guzik M, Brzozowski T, Sito E, Konturek SJ. Neuro-hormonal control of food intake: basic mechanisms and clinical implications. J Physiol Pharmacol. 2005;56 Suppl 6:5-25. [PubMed] [Cited in This Article: ] |
| 10. | Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255-4261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 919] [Cited by in F6Publishing: 936] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 11. | Kojima M, Kangawa K. Ghrelin: more than endogenous growth hormone secretagogue. Ann N Y Acad Sci. 2010;1200:140-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Liew PL, Lee WJ, Lee YC, Chen WY. Gastric ghrelin expression associated with Helicobacter pylori infection and chronic gastritis in obese patients. Obes Surg. 2006;16:612-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Salles N, Ménard A, Georges A, Salzmann M, de Ledinghen V, de Mascarel A, Emeriau JP, Lamouliatte H, Mégraud F. Effects of Helicobacter pylori infection on gut appetite peptide (leptin, ghrelin) expression in elderly inpatients. J Gerontol A Biol Sci Med Sci. 2006;61:1144-1150. [PubMed] [Cited in This Article: ] |
| 14. | Chuang CH, Sheu BS, Yang HB, Lee SC, Kao AW, Cheng HC, Chang WL, Yao WJ. Gender difference of circulating ghrelin and leptin concentrations in chronic Helicobacter pylori infection. Helicobacter. 2009;14:54-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Roper J, Francois F, Shue PL, Mourad MS, Pei Z, Olivares de Perez AZ, Perez-Perez GI, Tseng CH, Blaser MJ. Leptin and ghrelin in relation to Helicobacter pylori status in adult males. J Clin Endocrinol Metab. 2008;93:2350-2357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Stec-Michalska K, Malicki S, Michalski B, Peczek L, Wisniewska-Jarosinska M, Nawrot B. Gastric ghrelin in relation to gender, stomach topography and Helicobacter pylori in dyspeptic patients. World J Gastroenterol. 2009;15:5409-5417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Nweneka CV, Prentice AM. Helicobacter pylori infection and circulating ghrelin levels - a systematic review. BMC Gastroenterol. 2011;11:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Ichikawa H, Sugimoto M, Sakao Y, Sahara S, Ohashi N, Kato A, Sugimoto K, Furuta T, Andoh A, Sakao T. Relationship between ghrelin, Helicobacter pylori and gastric mucosal atrophy in hemodialysis patients. World J Gastroenterol. 2016;22:10440-10449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 19. | Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. [PubMed] [Cited in This Article: ] |
| 20. | Isomoto H, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, Ohnita K, Mizuta Y, Ohtsuru A, Yamashita S. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am J Gastroenterol. 2005;100:1711-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | World Health Organization. Report of a WHO Expert Committee. Physical Status: The use and interpretation of anthropometry. Geneva: WHO Technical Report Series 854 1995; 4-33. [Cited in This Article: ] |
| 22. | CDC. National Health and Nutrition Examination Survey. Anthropometry Procedures Manual. Atlanta: CDC 2007; 1.1-3.26. [Cited in This Article: ] |
| 23. | World Health Organization. Report of a WHO Expert Consultation. Waist Circumference and Waist-Hip Ratio. Geneva: World Health Organization 2008; 1-34. [Cited in This Article: ] |
| 24. | Avila T, Chiappe C. Atlas Fotográfico de Preparaciones de Alimentos. 1st ed. Buenos Aires: Editorial Akadia 2010; 1-11. [Cited in This Article: ] |
| 25. | Ministerio de Salud. Presidencia de la Nación Argentina. SARA: Sistema de Análisis y Registro de Alimentos. Buenos Aires: Ministerio de Salud de la Nación Argentina 2006; Available from: http://datos.dinami.gov.ar/produccion/sara/. [Cited in This Article: ] |
| 26. | Goldman C, Barrado A, Janjetic M, Balcarce N, Cueto Rua E, Oshiro M, Calcagno ML, Sarrasague MM, Fuda J, Weill R. Factors associated with H. pylori epidemiology in symptomatic children in Buenos Aires, Argentina. World J Gastroenterol. 2006;12:5384-5388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Janjetic MA, Goldman CG, Barrado DA, Cueto Rua E, Balcarce N, Mantero P, Zubillaga MB, López LB, Boccio JR. Decreasing trend of Helicobacter pylori infection in children with gastrointestinal symptoms from Buenos Aires, Argentina. Helicobacter. 2011;16:316-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection -- a critical review. Aliment Pharmacol Ther. 2004;20:1001-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Slater C, Preston T, Weaver LT. Is there an advantage in normalising the results of the Helicobacter pylori [13C]urea breath test for CO2 production rate in children? Isotopes Environ Health Stud. 2004;40:89-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Janjetic MA, Mantero P, Cueto Rua E, Balcarce N, Zerbetto de Palma G, Catalano M, Zubillaga MB, Boccio JR, Goldman CG. Dietary and anthropometric indicators of nutritional status in relation to Helicobacter pylori infection in a paediatric population. Br J Nutr. 2015;113:1113-1119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] [Cited in This Article: ] |
| 32. | van Doorn LJ, Figueiredo C, Sanna R, Pena S, Midolo P, Ng EK, Atherton JC, Blaser MJ, Quint WG. Expanding allelic diversity of Helicobacter pylori vacA. J Clin Microbiol. 1998;36:2597-2603. [PubMed] [Cited in This Article: ] |
| 33. | Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, Reece CA, Bukanov NO, Drazek ES, Roe BA, Berg DE. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37-53. [PubMed] [Cited in This Article: ] |
| 34. | Cover TL. Helicobacter pylori Diversity and Gastric Cancer Risk. MBio. 2016;7:e01869-e01815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 35. | Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, Tameda M, Shiraki K, Ito M, Takei Y. Changes in plasma ghrelin and leptin levels in patients with peptic ulcer and gastritis following eradication of Helicobacter pylori infection. BMC Gastroenterol. 2016;16:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Suzuki H, Matsuzaki J, Hibi T. Ghrelin and oxidative stress in gastrointestinal tract. J Clin Biochem Nutr. 2011;48:122-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Slomiany BL, Slomiany A. Induction in gastric mucosal prostaglandin and nitric oxide by Helicobacter pylori is dependent on MAPK/ERK-mediated activation of IKK-β and cPLA2: modulatory effect of ghrelin. Inflammopharmacology. 2013;21:241-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Slomiany BL, Slomiany A. Role of amplification in phospholipase Cγ2 activation in modulation of gastric mucosal inflammatory responses to Helicobacter pylori: effect of ghrelin. Inflammopharmacology. 2015;23:37-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Slomiany BL, Slomiany A. Role of LPS-elicited signaling in triggering gastric mucosal inflammatory responses to H. pylori: modulatory effect of ghrelin. Inflammopharmacology. 2017;25:415-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Isomoto H, Ueno H, Nishi Y, Yasutake T, Tanaka K, Kawano N, Ohnita K, Mizuta Y, Inoue K, Nakazato M. Circulating ghrelin levels in patients with various upper gastrointestinal diseases. Dig Dis Sci. 2005;50:833-838. [PubMed] [Cited in This Article: ] |
| 41. | Toyonaga A, Okamatsu H, Sasaki K, Kimura H, Saito T, Shimizu S, Fukuizumi K, Tsuruta O, Tanikawa K, Sata M. Epidemiological study on food intake and Helicobacter pylori infection. Kurume Med J. 2000;47:25-30. [PubMed] [Cited in This Article: ] |
| 42. | Willett W. Issues in analysis and presentation of dietary data. Nutritional Epidemiology. New York, NY: Oxford University Press 1998; 321-346. [Cited in This Article: ] |
| 43. | Carrasco F, Rojas P, Csendes A, Codoceo J, Inostroza J, Basfi-fer K, Papapietro K, Watkins G, Rojas J, Ruz M. Changes in ghrelin concentrations one year after resective and non-resective gastric bypass: associations with weight loss and energy and macronutrient intakes. Nutrition. 2012;28:757-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Ulasoglu C, Isbilen B, Doganay L, Ozen F, Kiziltas S, Tuncer I. Effect of Helicobacter pylori eradication on serum ghrelin and obestatin levels. World J Gastroenterol. 2013;19:2388-2394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |