Published online Aug 7, 2018. doi: 10.3748/wjg.v24.i29.3239
Peer-review started: May 4, 2018
First decision: May 17, 2018
Revised: June 28, 2018
Accepted: June 30, 2018
Article in press: June 30, 2018
Published online: August 7, 2018
Antibody-mediated rejection (AMR) in liver transplantation has long been underestimated. The concept of the liver as an organ susceptible to AMR has emerged in recent years, not only in the context of the major histocompatibility complex with the presence of HLA donor-specific antibodies, but also with antigens regarded as “minor”, whose role in AMR has been demonstrated. Among them, antibodies against glutathione S-transferase T1 have been found in 100% of patients with de novo autoimmune hepatitis (dnAIH) when studied. In its latest update, the Banff Working Group for liver allograft pathology proposed replacing the term dnAIH with plasma cell (PC)-rich rejection. Antibodies to glutathione S-transferase T1 (GSTT1) in null recipients of GSTT1 positive donors have been included as a contributory but nonessential feature of the diagnosis of PC-rich rejection. Also in this update, non-organ-specific anti-nuclear or smooth muscle autoantibodies are no longer included as diagnostic criteria. Although initially found in a proportion of patients with PC-rich rejection, the presence of autoantibodies is misleading since they are not disease-specific and appear in many different contexts as bystanders. The cellular types and proportions of the inflammatory infiltrates in diagnostic biopsies have been studied in detail very recently. PC-rich rejection biopsies present a characteristic cellular profile with a predominance of T lymphocytes and a high proportion of PCs, close to 30%, of which 16.48% are IgG4+. New data on the relevance of GSTT1-specific T lymphocytes to PC-rich rejection will be discussed in this review.
Core tip: The purpose of this review is to update the reader with recent knowledge about a disease of the liver allograft, whose definition has evolved from “de novo autoimmune hepatitis” to “plasma cell-rich rejection”. During the last 20 years, several groups have contributed new data that has prompted the liver transplant community to reconsider several aspects of the disease. It is not the intention of this review to go over details of the histological features or the role of autoantibodies in this disease, which have been well described in other reviews. Instead, more recent aspects, such as the composition of infiltrates in biopsies and T cell involvement will be discussed.
- Citation: Aguilera I, Aguado-Dominguez E, Sousa JM, Nuñez-Roldan A. Rethinking de novo immune hepatitis, an old concept for liver allograft rejection: Relevance of glutathione S-transferase T1 mismatch. World J Gastroenterol 2018; 24(29): 3239-3249
- URL: https://www.wjgnet.com/1007-9327/full/v24/i29/3239.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i29.3239
Antibody-mediated rejection (AMR) in liver transplantation is becoming increasingly relevant after being considered an immune privileged organ for many years. Indeed, a good HLA match between donor and recipient - very important in other settings such as kidney transplants - was never considered as essential in liver donations. A few years ago, a number of publications describing a pathogenic role for HLA donor-specific antibodies (DSA) came out indicating that the liver was prone to experience AMR like any other organ[1]. Earlier, in 1998, a new liver transplant-associated disease termed de novo autoimmune hepatitis (dnAIH) was described[2] and many groups reported cases of patients with similar characteristics but with different prevalence[3-25]. The diagnostic criteria are well described, particularly with regard to histological features that are essential for differential diagnosis in adult[26-32] and pediatric[9,33] cases. To complete the characterization of this special type of immune response, now generally accepted as rejection but with disconcerting similarities with autoimmunity[34], there are a few aspects that still need to be investigated.
For example, one important issue that has yet to be addressed is the role of allelic disparity of glutathione S-transferase T1 (GSTT1) or other minor histocompatibility antigen mismatches in the development of dnAIH in pediatric liver transplant. There are very few studies about the long-term consequences of dnAIH in the liver allograft of children. Ekong et al[14] reported their observations from a retrospective multicenter study that included 29 children from 5 centers. The authors showed that half of the patients did not experience rejection prior to diagnosis and the response to steroid therapy was good in general but not in all the cases. Interestingly, 38% of the children had abnormal liver enzymes over 2-fold the upper limit of normal, especially gamma-glutamyltransferase (GGT) at the time of last follow-up, indicating bile duct injury. This result contradicts one of the main arguments against considering dnAIH a type of rejection, namely the absence of bile duct involvement.
Well-established immunological criteria for diagnosing AMR in kidney transplantation include detection of complement component 4d (C4d) deposits in peritubular capillaries concomitantly with antidonor serology[35]. Presently, C4d deposition in portal capillaries is accepted as a distinctive feature of dnAIH/PC hepatitis[36,37] although it is not currently considered to be a diagnostic criterion.
IgG4 has been traditionally considered a benign antibody although this concept has changed due to the growing number of IgG4-related diseases described in the literature during the last few years. International experts in the field held a symposium in Boston in 2012 and generated consensus guidelines for the diagnosis of IgG4-related diseases[38]. Since an important presence of IgG4+ plasma cells (PCs) has been detected in subgroups of patients with dnAIH/PC-rich rejection, this aspect will be discussed later in this review.
When pathologists first identified the transplant-associated pathology dnAIH, the histological features described were very similar to those found in autoimmune hepatitis[2]. Following this first study in 1998[2], dnAIH became the term of choice[6-8,11,12,17,22,25,39,40]. However, from the beginning, clinicians found it difficult to admit progression to autoimmunity in a patient’s graft without a previous history of autoimmune phenomena and under the effects of immunosuppressive therapy. Moreover, the graft targeted by the immune reaction was not self but coming from a donor with a completely different genetic background, given the fact that selection was very uncommon, even for the HLA antigens. Soon after the first description, some authors started using other terms such as “de novo hepatitis”[3], “graft dysfunction mimicking autoimmune hepatitis”[16], “posttransplant immune hepatitis”[4] or de novo immune hepatitis[18]. In 2008, a study performed by Fiel et al[23] introduced the term “plasma cell hepatitis” with the suggestion that dnAIH could be a variant of rejection; this report was highlighted by an editorial comment[34]. Even though all these terms have been used historically, for simplicity, in this review we will refer to dnAIH or PC-rich rejection.
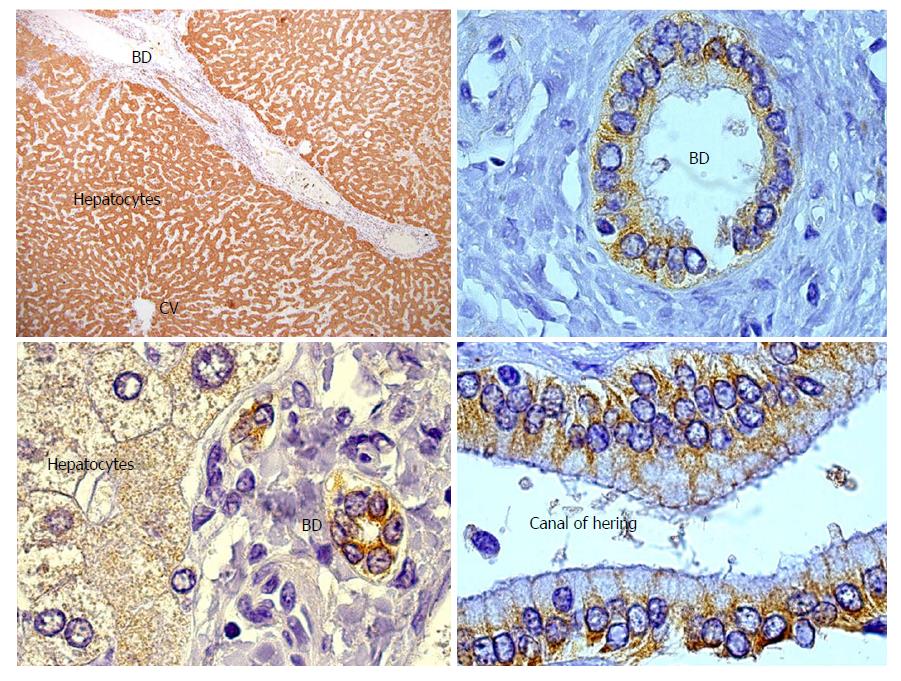
GSTT1 is a phase II drug metabolizing enzyme involved in protection against toxic compounds. Vital for this function is its abundant expression in liver and kidney (Figure 1), although GSTT1 is also expressed in other tissues to a lesser degree. Importantly, the GSTT1 antigen is present in red blood cells. It has been demonstrated that any healthy person with the GSTT1*0/0 genotype might produce anti-GSTT1 antibodies if they experience one of two sensitization events: blood transfusions from a GSTT1-positive donor or pregnancy of a GSTT1-positive fetus[41]. Mismatch of the GSTT1 alleles was first reported by Aguilera et al[42] and subsequently confirmed in patients with PC-rich rejection[18,21,24,43]. Czaja included GSTT1 antibodies as a feature in dnAIH patients and proposed a hypothesis that will be discussed in the PATHOGENESIS OF PC-RICH REJECTION section of this manuscript[44]. Controversially, one report did not find GSTT1 mismatch in a patient with dnAIH; however, the genotyping results were not very convincing since they lacked an internal control band and GSTT1 antibodies were not tested[22]. To the best of our knowledge, anti-GSTT1 antibodies have never been tested in serum samples from liver transplanted children.
We studied the cases of two female patients with preformed anti-GSTT1 antibodies who needed a liver transplant and received a GSTT1 expressing graft. Their original diseases were primary and secondary biliary cirrhosis, respectively. The first patient presented high titers of GSTT1 antibodies and was diagnosed with dnAIH 12 mo after the transplant with a good response to prednisone plus mycophenolate mofetil. She died of pneumonia 4 years after the transplant. The second patient had low antibody titers in different samples until exitus, 7 years after the transplant. This patient was never diagnosed correctly and the only biopsy, performed one month before exitus, revealed dnAIH as the cause of exitus. Since these pre-sensitized patients are not frequent, it is difficult to conclude whether the presence of preformed antibodies accelerates the onset or the severity of the disease.
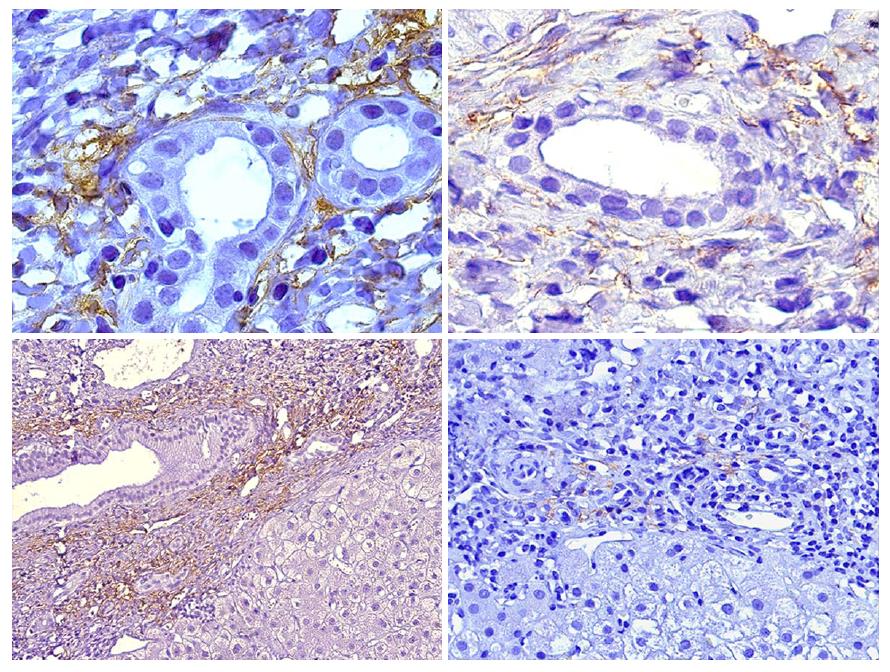
At the beginning of this decade a report by Aguilera et al[36], that was worth an editorial comment[45] was the first to link C4d immunopositivity in liver biopsies to donor-specific alloreactivity not related to HLA DSA or ABO incompatibility[46]. Patients with anti-GSTT1 DSA showed a very characteristic C4d immunostaining pattern restricted to portal capillaries (Figure 2), very distinct to the sinusoidal pattern described by Kozlowski et al[47] in association with HLA DSA, which was similar to our own findings in chronic rejection biopsies[36].
Dumortier et al[48] described a case of refractory dnAIH that did not respond to standard therapy in a patient with anti-LKM (liver-kidney microsomal) antibodies at the time of diagnosis. Recovery after treatment with plasmapheresis supported an involvement of humoral factors in the pathogenesis of the disease.
In the last publication by the Banff Working Group for liver allograft pathology, the proposed criteria included HLA DSA and GSTT1 in null recipients of GSTT1- positive donors as contributory but nonessential features for the diagnosis of PC-rich rejection[1].
HLA DQ DSAs have been described as a predictive variable for late allograft dysfunction in children[49]. Unfortunately, the number of patients in this study with dnAIH (n = 3) was too low to reach a conclusion and, even with the inclusion of 10 more patients with overlapping features of dnAIH and late ACR, the results should be considered carefully since it is not possible to analyze both events separately. We studied HLA DSA in a group of adult patients with dnAIH and found that 4 out of 14 (28.6%) produced de novo HLA DQ DSA, but always coexisting with GSTT1 DSAs (unpublished results), making it impossible to differentiate the real impact of HLA DSA on the development of PC-rich rejection. It would be very interesting to validate these results in larger cohorts in order to determine the pathogenic role of HLA DSA in the absence of anti-GSTT1 antibodies.
Although finally accepted as a form of allograft rejection, the liver transplant-associated disease formerly known as dnAIH is a special kind of rejection and one that is difficult to understand. It has many histological similarities with classical AIH, features that were misleading at the time this pathology was discovered. Moreover, the first-line therapy of choice is corticosteroids for both pathological processes. All of this supports the concept that an alloantigenic immune response may be difficult to distinguish from an autoimmune response in which the mechanisms of liver damage are probably similar. Interestingly, we noted a female predominance among patients who present with one autoimmune feature, since of the cases of dnAIH diagnosed at our center, 10 of 14 are female (71.4%), a feature that is not easy to explain if we contemplate that 75% of the liver transplanted patients in our hospital are males. Moreover, if we consider the 122 patients within the risk group with GSTT1 mismatch (positive donor/null recipient), 85 were males (69.7%) and 37 were females (30.3%). In accordance with female predominance are the reports by Miyagawa-Hayashino[7,19], in which 12 of 14 cases were females, and Pongpaibul et al[13] with 32 females vs 19 males diagnosed with dnAIH, although in the majority of publications this predominance does not appear. Ward et al[50] established that women may be more susceptible to PC hepatitis after liver transplant than men. A reasonable explanation could be sensitization through pregnancies. In fact, we have two female patients with GSTT1 antibodies in pre-transplant serum samples, although we do not have a sufficient number of cases to support this hypothesis.
It is generally accepted that dnAIH develops in patients transplanted for non-autoimmune diseases. This is not exactly true since there are a number of patients whose original disease was either primary biliary cirrhosis (PBC) or primary sclerosing cholangitis (PSC) (Table 1). Neuberger’s group found that PBC was an independent predictor of late acute rejection in a large cohort of adult liver transplant patients[51].
| Ref. | de novo AIH/total patients | Original disease (immune-mediated) | Initial calcineurine inhibitor | Pediatric/adult |
| Kerkar et al[2] (1998) | 7/180 | 4 BA | 4 CyA, 3 Tac | P |
| Gupta et al[3] (2001) | 6/115 | 5 BA | 6 CyA | P |
| Andries et al[4] (2001) | 11/471 | 7 BA | 10 Cya, 1 Tac | P |
| Hernández et al[5] (2001) | 5/155 | 4 BA, 1 PSC | 5 CyA | P |
| Petz et al[6] (2002) | 18/155 | 16 BA | 9 Cya, 9 Tac | P |
| Miyagawa-Hayashino et al[7] (2003) | 1 | BA | Tac | P |
| Gibelli et al[8] (2006) | 2/206 | 1 BA | CyA | P |
| Evans et al[9] (2006) | 4/158 | 4 PSC | 4 CyA | P |
| Riva et al[10] (2006) | 9/247 | 9 BA | 5 CyA, 4 Tac | P |
| Oya et al[11] (2009) | 1 | - | Tac | P |
| Cho et al[12] (2011) | 4/148 | 1 BA | - | P |
| Pongpaibul et al[13] (2012) | 51/685 | 29 BA | 22 CyA, 29 Tac | P |
| Ekong et al[14] (2017) | 29/1833 | 17 BA | 13 CyA,16 Tac | P |
| Jones et al[15] (1999) | 2 | 2 PBC | 2 CyA | A |
| Heneghan et al[16] (2001) | 7/1000 | 1 PBC, 2 PSC | 7 CyA | A |
| Salcedo et al[17] (2002) | 12/350 | 1 BA 1 PSC | 12 CyA | A |
| Aguilera et al[18] (2004) | 6/110 | None | 5 CyA, 1 Tac | A |
| 1Update 2017 | 8 | 1 PBC, 1 PSC | 8 CyA | A |
| Miyagawa-Hayashino et al[19] (2004) | 13/633 | 11 BA | 13 Tac | A |
| Tsuji et al[20] (2005) | 1 | PBC | CyA | A |
| Rodriguez-Diaz et al[21] (2006) | 1 | PBC | CyA | A |
| Yoshizawa et al[22] (2008) | 1 | PBC | Tac | A |
| Fiel et al[23] (2008) | 38/? | none (HCV) | 11 CyA, 27 Tac | A |
| Zhang et al[24] (2010) | 1 | PBC | Tac | A |
| Montano-Loza et al[25] (2012) | 17/576 | 5 PBC, 1 BA | 5 CyA, 7 Tac | A |
In children, the major indication for liver transplant is biliary atresia (BA), a process of unknown etiology with several potential causative factors. Among them, there is evidence in favor of autoimmune-mediated injury of bile duct epithelial cells in a subset of patients[52-54]. Very recently, a study described that BA is initiated before birth and the presence of maternal microchimerism in the BA liver supports graft versus host disease (GvHD)-like immune response[55].
A possible role of GSTT1-specific T lymphocytes in PC-rich rejection has been recently investigated. The authors provided the first evidence of memory specific T cells in samples of peripheral blood mononuclear cells (PBMC) of patients after recall with the GSTT1 antigen. Activation of CD8+ and CD4+ T cells with production of IL-4 and/or IFNγ was observed[56]. Most intriguing was the finding of highly activated CD4+ cells that retained CD8low expression with a mean value of 25% activated Th0 type cells (3.44%-78.95%). These results are particularly significant considering the immunosuppressed status of the patients. In silico analysis of the ability of patients’ HLA class I and class II alleles to present these peptides with optimal percentile ranks supported the experimental results[56].
Similar to autoimmune hepatitis, we observe damage to hepatocytes that is believed to be coordinated by CD4+ T lymphocytes recognizing a self-antigen presented by HLA-class II molecules to Th0 type cells[57], although with the substantial difference that, in this case, the target is an alloantigen.
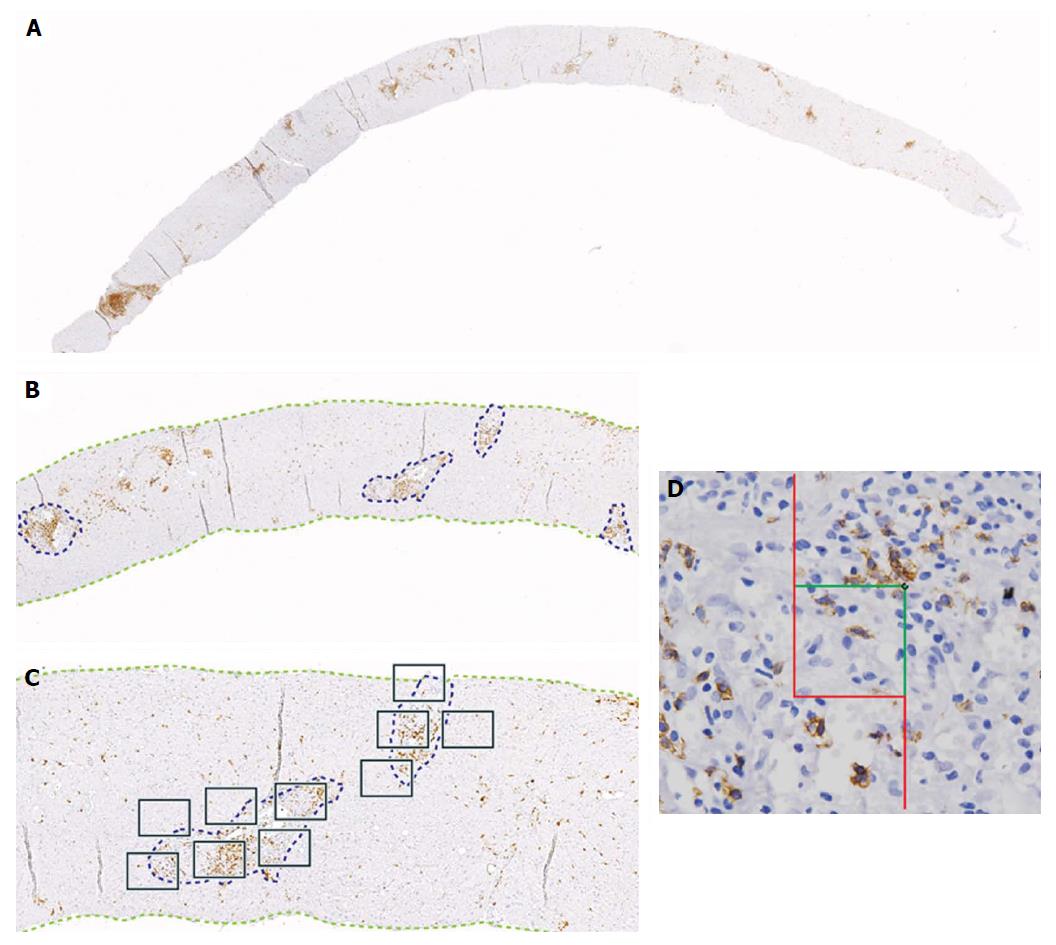
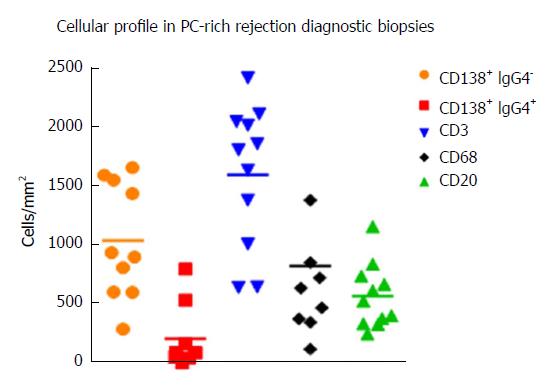
Diagnosis by liver biopsy is challenging because PC-rich rejection shares some histological and clinical features with late onset acute rejection or with other post-transplant pathologies such as recurrent hepatitis C. Moreover, very little is known about the cellular composition of the inflammatory infiltrates in portal tracts other than an important presence of plasma cells. Direct visualization of the cell types with different markers and quantification of cells/mm2 of tissue in diagnostic biopsies using computer-assisted system technology (newCAST) has proved to be an unbiased way to evaluate the situation directly in the organ and not only in peripheral blood (Figure 3). Very recent data allowed us to define a cellular profile characteristic of PC-rich rejection at the time of diagnosis[58]. We observed a predominance of T lymphocytes (mean 36.6%); followed by PCs (mean 28.8%), with 17% of them IgG4+; B cells (mean 14.9%); and macrophages (mean 19.7%) (Figure 4). This profile was very different to the one defined in a chronic rejection group included in the same study[58]. It remains to be confirmed whether the cellular composition in acute rejection biopsies would be useful to support diagnosis since HCV recurrence no longer has the same impact in the transplant community that as in the past.
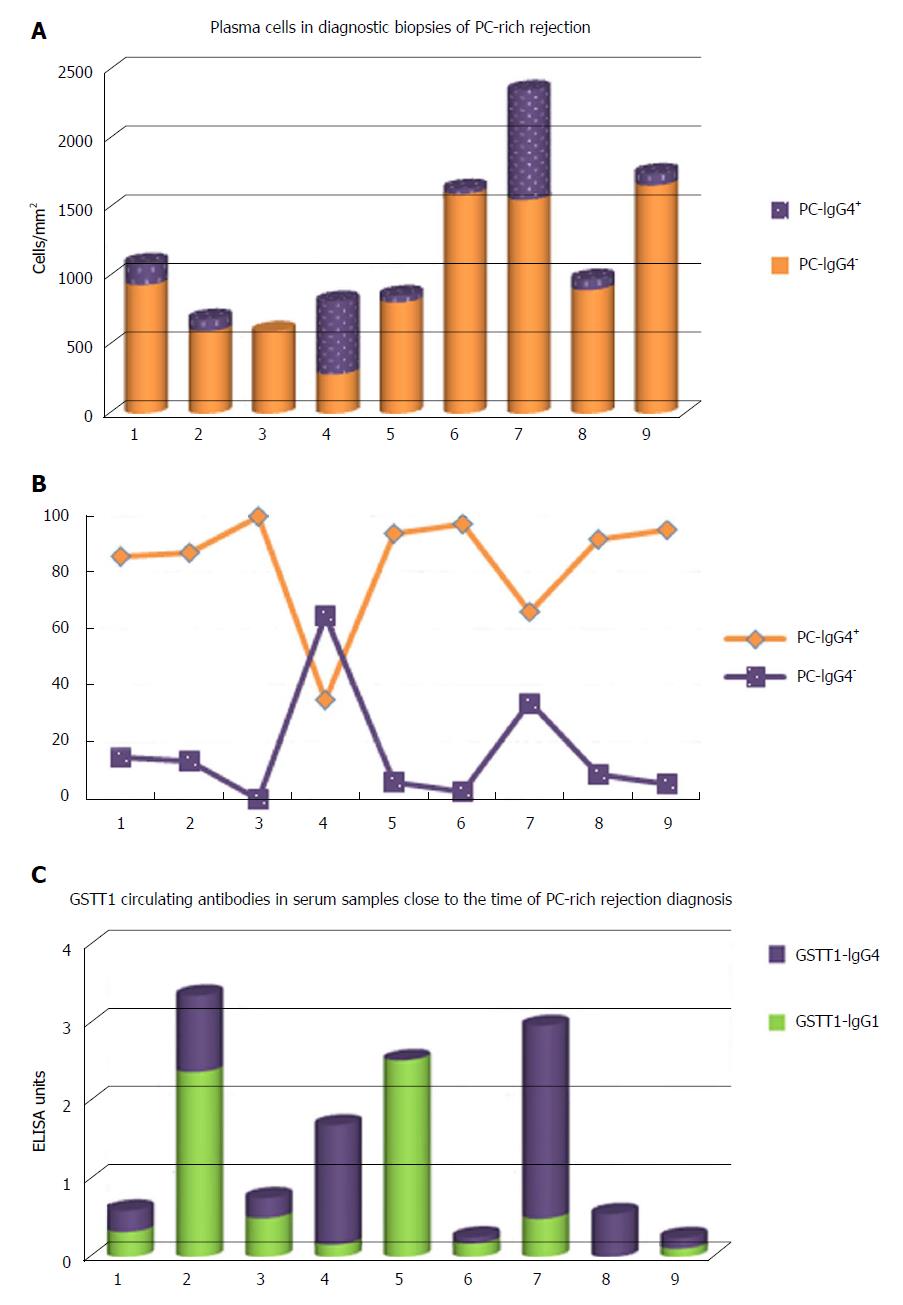
The IgG4 subclass has traditionally been considered a modulator of the immune response, linked to long-term exposure and high antigen concentration. Some authors analyzed the presence of IgG4 antibodies in dnAIH, mostly in children, but the results were contradictory. While Eguchi et al[59] described the absence of IgG4+ plasma cells in the biopsies of 4 patients and the values in serum were within normal limits, Castillo-Rama et al[60] detected IgG4+ PC over-representation in a subgroup of patients with PC hepatitis that showed a more severe disease course. The origin of these IgG4+ PC infiltrates in the allografts is unknown but it could be due to persistent alloimmune responses. We also found an important number of IgG4+ PC, representing 16.48% (2.45%-65%) of the total PCs in diagnostic biopsies of patients with PC-rich rejection (Figure 5A and B); however, higher numbers of IgG4+cells was not associated with worse clinical outcome. We also found an over-representation of serum levels of GSTT1-specific IgG4 antibodies, almost equaling the levels of GSTT1-IgG1, while GSTT1-IgG2 and -IgG3 were completely absent (Figure 5C)[61].
There is very little information about the representation of T cells in PC-rich rejection biopsies. A study performed by Ekong et al[62] found that around 3000 CD3+ cells/mm2 were present in the dnAIH pediatric group during disease activity. Our data in adults showed that CD3+ cells were less numerous, with a mean value of 1600 (641-2422) cells/mm2 at the time of diagnosis, before steroid treatment. Indirect evidence from biopsies suggests a potential role for Th17 cells in the prolongation of inflammation in dnAIH[63] although this needs further study.
PC-rich rejection has also been identified after hematopoietic cell transplantation (HCT) between HLA-identical siblings. In this context, the GSTT1 mismatch, defined as null donor/positive recipient, not only had a deleterious effect in HCT and constituted a risk factor for acute and chronic hepatic GvHD[64] but is also the basis of a true PC-rich rejection[65]. Together with the skin and intestine, the liver is a preferred target of the donor cells which are able to recognize disparate antigens expressed by the recipient. Differential diagnosis of hepatic GvHD versus PC-rich rejection must be done in biopsy because histological features can only be analyzed in tissue to permit the differentiation of both processes. As it happens, the cellular composition of the inflammatory infiltrates in portal areas of HCT patient with PC-rich rejection was very similar to that of diagnostic biopsies of liver transplanted patients[58].
From an immunological point of view, this finding has important implications because it demonstrates an identical immune response against the GSTT1 antigen as a result of donor/recipient mismatch in two completely different conditions: Solid organ and HCT.
It is clear that during surgery, intracellular antigens such as the GSTT1 protein are released and recognized by B cells, starting a process of affinity maturation and differentiation into memory cells, which requires collaboration with specific T helper cells in the lymph nodes. If this occurs, a subset of these cells will progress to plasma cells and anti-GSTT1 antibodies of the IgG class will be detected concurrently with mature GSTT1-specific CD4+ cells. Our experimental results sustain indirect presentation of the donor GSTT1 protein by recipient APCs[56].
In general terms, we could use the model proposed by Czaja[44] with some suggestions. Briefly, inflammatory stimuli induce expression of MHC class II in hepatocytes and cholangiocytes and, since both cellular types contain abundant GSTT1 enzyme, these liver cells could directly act as APCs and serve as targets of effector CD4+ T cells, whose existence has been also demonstrated in the PBMC of patients with the disease. The presence of an antigen mimicking GSTT1 on the surface of the cell cannot be ruled out but it does not seem necessary and so far has not been identified.
GSTT1-specific B cells have a critical role as antigen presenting cells (APCs) in the maintenance of a long-lasting immunological response, providing the signals required for specific T cell activation when professional APCs become exhausted. GSTT1 antibodies are, in principle and until a direct pathogenic role can be demonstrated, a signal of the existence of memory B cells. The mechanisms controlling GSTT1-specific T cell response in patients that, in spite of the production of GSTT1 antibodies, do not develop the disease are still not known. Alternatively, there are patients with GSTT1-specific T cells that lack the B cell response; they will never develop PC-rich rejection. In our experience, if PC-rich rejection is not initiated during the first 3 years, it will never occur even though anti-GSTT1 antibodies persist (longest follow-up > 20 years) (unpublished results).
Impairment of Treg cells has been described in patients with classical AIH[57]. In the transplant setting, a similar role for Tregs in the development of dnAIH has been suggested. Kerkar and Yanni proposed that Treg function could be impaired in dnAIH since calcineurin inhibitors reduce the production of IL-2, which is required for the survival and proliferation of Tregs[66]. In the same sense, a study by Arterbery et al[67] sustains that the Tregs of patients with dnAIH are functionally impaired and produce increased levels of proinflammatory cytokines.
On the other hand, important questions about dnAIH have yet to be answered. One of the most intriguing aspects is why some patients do not develop a GSTT1-specific B cell response, as this is going to be critical to the prevention of PC-rich rejection. In our hands, the choice of tacrolimus instead of cyclosporine can be decisive in avoiding the humoral response[68]. However, there is no consensus among the scientific community on this matter. While some studies support the observation of tacrolimus as a protective factor[3-5,8,9,15-17,20,21], others have shown that the use of tacrolimus seems to be a risk factor for dnAIH[13,14,19,22-25]; therefore, this point remains controversial (Table 1).
We are now closer than ever to clarification of the mechanisms leading to plasma cell-rich rejection. The demonstration of memory T cells specific for the GSTT1 antigen as well as B lymphocytes and GSTT1 antibody-producing plasma cells after GSTT1-mismatched liver transplants support the hypothesis in which both cell types are required to develop the immune response leading to PC-rich rejection. This chronic disease has a significant impact on survival of those patients that are not correctly diagnosed.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hann HW, Kamal SA, Mendez-Sanchez N S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Demetris AJ, Bellamy C, Hübscher SG, O’Leary J, Randhawa PS, Feng S, Neil D, Colvin RB, McCaughan G, Fung JJ. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am J Transplant. 2016;16:2816-2835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 361] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 2. | Kerkar N, Hadzić N, Davies ET, Portmann B, Donaldson PT, Rela M, Heaton ND, Vergani D, Mieli-Vergani G. De-novo autoimmune hepatitis after liver transplantation. Lancet. 1998;351:409-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 328] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Gupta P, Hart J, Millis JM, Cronin D, Brady L. De novo hepatitis with autoimmune antibodies and atypical histology: a rare cause of late graft dysfunction after pediatric liver transplantation. Transplantation. 2001;71:664-668. [PubMed] [Cited in This Article: ] |
| 4. | Andries S, Casamayou L, Sempoux C, Burlet M, Reding R, Bernard Otte J, Buts JP, Sokal E. Posttransplant immune hepatitis in pediatric liver transplant recipients: incidence and maintenance therapy with azathioprine. Transplantation. 2001;72:267-272. [PubMed] [Cited in This Article: ] |
| 5. | Hernandez HM, Kovarik P, Whitington PF, Alonso EM. Autoimmune hepatitis as a late complication of liver transplantation. J Pediatr Gastroenterol Nutr. 2001;32:131-136. [PubMed] [Cited in This Article: ] |
| 6. | Petz W, Sonzogni A, Bertani A, Spada M, Lucianetti A, Colledan M, Gridelli B. A cause of late graft dysfunction after pediatric liver transplantation: de novo autoimmune hepatitis. Transplant Proc. 2002;34:1958-1959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Miyagawa-Hayashino A, Haga H, Sakurai T, Shirase T, Manabe T, Egawa H. De novo autoimmune hepatitis affecting allograft but not the native liver in auxiliary partial orthotopic liver transplantation. Transplantation. 2003;76:271-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Gibelli NE, Tannuri U, Mello ES, Cançado ER, Santos MM, Ayoub AA, Maksoud-Filho JG, Velhote MC, Silva MM, Pinho-Apezzato ML. Successful treatment of de novo autoimmune hepatitis and cirrhosis after pediatric liver transplantation. Pediatr Transplant. 2006;10:371-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Evans HM, Kelly DA, McKiernan PJ, Hübscher S. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology. 2006;43:1109-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Riva S, Sonzogni A, Bravi M, Bertani A, Alessio MG, Candusso M, Stroppa P, Melzi ML, Spada M, Gridelli B. Late graft dysfunction and autoantibodies after liver transplantation in children: preliminary results of an Italian experience. Liver Transpl. 2006;12:573-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Oya H, Sato Y, Yamamoto S, Kobayashi T, Watanabe T, Kokai H, Hatakeyama K. De novo autoimmune hepatitis after living donor liver transplantation in a 25-day-old newborn baby: a case report. Transplant Proc. 2009;41:433-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Cho JM, Kim KM, Oh SH, Lee YJ, Rhee KW, Yu E. De novo autoimmune hepatitis in Korean children after liver transplantation: a single institution’s experience. Transplant Proc. 2011;43:2394-2396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Pongpaibul A, Venick RS, McDiarmid SV, Lassman CR. Histopathology of de novo autoimmune hepatitis. Liver Transpl. 2012;18:811-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Ekong UD, McKiernan P, Martinez M, Lobritto S, Kelly D, Ng VL, Alonso EM, Avitzur Y. Long-term outcomes of de novo autoimmune hepatitis in pediatric liver transplant recipients. Pediatr Transplant. 2017;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Jones DE, James OF, Portmann B, Burt AD, Williams R, Hudson M. Development of autoimmune hepatitis following liver transplantation for primary biliary cirrhosis. Hepatology. 1999;30:53-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Heneghan MA, Portmann BC, Norris SM, Williams R, Muiesan P, Rela M, Heaton ND, O’Grady JG. Graft dysfunction mimicking autoimmune hepatitis following liver transplantation in adults. Hepatology. 2001;34:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Salcedo M, Vaquero J, Bañares R, Rodríguez-Mahou M, Alvarez E, Vicario JL, Hernández-Albújar A, Tíscar JL, Rincón D, Alonso S. Response to steroids in de novo autoimmune hepatitis after liver transplantation. Hepatology. 2002;35:349-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Aguilera I, Sousa JM, Gavilán F, Bernardos A, Wichmann I, Nuñez-Roldán A. Glutathione S-transferase T1 mismatch constitutes a risk factor for de novo immune hepatitis after liver transplantation. Liver Transpl. 2004;10:1166-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Miyagawa-Hayashino A, Haga H, Egawa H, Hayashino Y, Sakurai T, Minamiguchi S, Tanaka K, Manabe T. Outcome and risk factors of de novo autoimmune hepatitis in living-donor liver transplantation. Transplantation. 2004;78:128-135. [PubMed] [Cited in This Article: ] |
| 20. | Tsuji H, Hiramatsu K, Minato H, Kaneko S, Nakanuma Y. Auxiliary partial orthotopic liver transplantation with de novo autoimmune hepatitis in the allograft and leftover primary biliary cirrhosis in the native liver. Semin Liver Dis. 2005;25:371-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Rodríguez-Diaz Y, Reyes-Rodriguez R, Dorta-Francisco MC, Aguilera I, Perera-Molinero A, Moneva-Arce E, Aviles-Ruiz JF. De novo autoimmune hepatitis following liver transplantation for primary biliary cirrhosis. Transplant Proc. 2006;38:1467-1470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Yoshizawa K, Shirakawa H, Ichijo T, Umemura T, Tanaka E, Kiyosawa K, Imagawa E, Matsuda K, Hidaka E, Sano K. De novo autoimmune hepatitis following living-donor liver transplantation for primary biliary cirrhosis. Clin Transplant. 2008;22:385-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Fiel MI, Agarwal K, Stanca C, Elhajj N, Kontorinis N, Thung SN, Schiano TD. Posttransplant plasma cell hepatitis (de novo autoimmune hepatitis) is a variant of rejection and may lead to a negative outcome in patients with hepatitis C virus. Liver Transpl. 2008;14:861-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Wang B, Wang T. De novo autoimmune hepatitis with centrilobular necrosis following liver transplantation for primary biliary cirrhosis: a case report. Transplant Proc. 2010;42:3854-3857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Montano-Loza AJ, Vargas-Vorackova F, Ma M, Bain VG, Burak K, Kumar T, Mason AL. Incidence and risk factors associated with de novo autoimmune hepatitis after liver transplantation. Liver Int. 2012;32:1426-1433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Banff Working Group. Demetris AJ, Adeyi O, Bellamy CO, Clouston A, Charlotte F, Czaja A, Daskal I, El-Monayeri MS, Fontes P, Fung J, Gridelli B, Guido M, Haga H, Hart J, Honsova E, Hubscher S, Itoh T, Jhala N, Jungmann P, Khettry U, Lassman C, Ligato S, Lunz JG 3rd, Marcos A, Minervini MI, Mölne J, Nalesnik M, Nasser I, Neil D, Ochoa E, Pappo O, Randhawa P, Reinholt FP, Ruiz P, Sebagh M, Spada M, Sonzogni A, Tsamandas AC, Wernerson A, Wu T, Yilmaz F. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44:489-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Shaikh OS, Demetris AJ. Idiopathic posttransplantation hepatitis? Liver Transpl. 2007;13:943-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Schreuder TC, Hübscher SG, Neuberger J. Autoimmune liver diseases and recurrence after orthotopic liver transplantation: what have we learned so far? Transpl Int. 2009;22:144-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 29. | Hübscher SG. Antibody-mediated rejection in the liver allograft. Curr Opin Organ Transplant. 2012;17:280-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Hübscher SG. What is the long-term outcome of the liver allograft? J Hepatol. 2011;55:702-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Sebagh M, Castillo-Rama M, Azoulay D, Coilly A, Delvart V, Allard MA, Dos Santos A, Johanet C, Roque-Afonso AM, Saliba F. Histologic findings predictive of a diagnosis of de novo autoimmune hepatitis after liver transplantation in adults. Transplantation. 2013;96:670-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Vukotic R, Vitale G, D’Errico-Grigioni A, Muratori L, Andreone P. De novo autoimmune hepatitis in liver transplant: State-of-the-art review. World J Gastroenterol. 2016;22:2906-2914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Nagai S, Ito M, Kamei H, Nakamura T, Ando H, Kiuchi T. Indirect immunohistochemical evaluation of graft fibrosis and interface hepatitis after pediatric liver transplantation. Pediatr Transplant. 2010;14:342-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Demetris AJ, Sebagh M. Plasma cell hepatitis in liver allografts: Variant of rejection or autoimmune hepatitis? Liver Transpl. 2008;14:750-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1514] [Cited by in F6Publishing: 1473] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 36. | Aguilera I, Sousa JM, Gavilan F, Gomez L, Alvarez-Márquez A, Núñez-Roldán A. Complement component 4d immunostaining in liver allografts of patients with de novo immune hepatitis. Liver Transpl. 2011;17:779-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Trivedi A, Schiano TD, Ward SC, Thung SN, Fiel MI, Levitsky J. C4d is present in portal venules in plasma cell hepatitis and may also be used as a predictor for its development. Hepatology. 2014;60:457A. [Cited in This Article: ] |
| 38. | Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Klöppel G, Heathcote JG, Khosroshahi A, Ferry JA. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1714] [Cited by in F6Publishing: 1637] [Article Influence: 136.4] [Reference Citation Analysis (0)] |
| 39. | Tamaro G, Sonzogni A, Torre G. Monitoring “de novo” autoimmune hepatitis (LKM positive) by serum type-IV collagen after liver transplant: a paediatric case. Clin Chim Acta. 2001;310:25-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Tripathi D, Neuberger J. Autoimmune hepatitis and liver transplantation: indications, results, and management of recurrent disease. Semin Liver Dis. 2009;29:286-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Wichmann I, Aguilera I, Sousa JM, Bernardos A, García Núñez EJ, Vigil E, Magariño R, Magariño I, Torres A, Núñez-Roldán A. Antibodies against glutathione S-transferase T1 in non-solid organ transplanted patients. Transfusion. 2006;46:1505-1509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Aguilera I, Wichmann I, Sousa JM, Bernardos A, Franco E, García-Lozano JR, Núñez-Roldán A. Antibodies against glutathione S-transferase T1 (GSTT1) in patients with de novo immune hepatitis following liver transplantation. Clin Exp Immunol. 2001;126:535-539. [PubMed] [Cited in This Article: ] |
| 43. | Rodriguez-Mahou M, Salcedo M, Fernandez-Cruz E, Tiscar JL, Bañares R, Clemente G, Vicario JL, Alvarez E, Rodriguez-Sainz C. Antibodies against glutathione S-transferase T1 (GSTT1) in patients with GSTT1 null genotype as prognostic marker: long-term follow-up after liver transplantation. Transplantation. 2007;83:1126-1129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Czaja AJ. Diagnosis, pathogenesis, and treatment of autoimmune hepatitis after liver transplantation. Dig Dis Sci. 2012;57:2248-2266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Bellamy CO. Complement C4d immunohistochemistry in the assessment of liver allograft biopsy samples: applications and pitfalls. Liver Transpl. 2011;17:747-750. [PubMed] [Cited in This Article: ] |
| 46. | Haga H, Egawa H, Fujimoto Y, Ueda M, Miyagawa-Hayashino A, Sakurai T, Okuno T, Koyanagi I, Takada Y, Manabe T. Acute humoral rejection and C4d immunostaining in ABO blood type-incompatible liver transplantation. Liver Transpl. 2006;12:457-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Kozlowski T, Rubinas T, Nickeleit V, Woosley J, Schmitz J, Collins D, Hayashi P, Passannante A, Andreoni K. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver Transpl. 2011;17:357-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 48. | Dumortier J, Scoazec JY, Guillaud O, Hequet O, Hervieu V, Boillot O. Treatment of severe refractory de novo auto-immune hepatitis after liver transplantation with plasmapheresis. Clin Res Hepatol Gastroenterol. 2015;39:e83-e85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Wozniak LJ, Hickey MJ, Venick RS, Vargas JH, Farmer DG, Busuttil RW, McDiarmid SV, Reed EF. Donor-specific HLA Antibodies Are Associated With Late Allograft Dysfunction After Pediatric Liver Transplantation. Transplantation. 2015;99:1416-1422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 50. | Ward SC, Schiano TD, Thung SN, Fiel MI. Plasma cell hepatitis in hepatitis C virus patients post-liver transplantation: case-control study showing poor outcome and predictive features in the liver explant. Liver Transpl. 2009;15:1826-1833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Neuberger J. An update on liver transplantation: A critical review. J Autoimmun. 2016;66:51-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Mack CL. What Causes Biliary Atresia? Unique Aspects of the Neonatal Immune System Provide Clues to Disease Pathogenesis. Cell Mol Gastroenterol Hepatol. 2015;1:267-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Alvarez F. Is biliary atresia an immune mediated disease? J Hepatol. 2013;59:648-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Pang SY, Dai YM, Zhang RZ, Chen YH, Peng XF, Fu J, Chen ZR, Liu YF, Yang LY, Wen Z. Autoimmune liver disease-related autoantibodies in patients with biliary atresia. World J Gastroenterol. 2018;24:387-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Muraji T, Ohtani H, Ieiri S. Unique manifestations of biliary atresia provide new immunological insight into its etiopathogenesis. Pediatr Surg Int. 2017;33:1249-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Martínez-Bravo MJ, Sánchez B, Sousa JM, Acevedo MJ, Gómez-Bravo MA, Núñez-Roldán A, Aguilera I. T-cell allorecognition of donor glutathione S-transferase T1 in plasma cell-rich rejection. World J Hepatol. 2017;9:1115-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Vergani D, Mieli-Vergani G. The impact of autoimmunity on hepatocytes. Semin Liver Dis. 2007;27:140-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Aguado-Domínguez E, Gómez L, Sousa JM, Gómez-Bravo MÁ, Núñez-Roldán A, Aguilera I. Identification of the cellular components involved in de novo immune hepatitis: a quantitative immunohistochemical analysis. J Transl Med. 2018;16:62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Eguchi S, Takatsuki M, Hidaka M, Tajima Y, Zen Y, Nakanuma Y, Kanematsu T. De novo autoimmune hepatitis after living donor liver transplantation is unlikely to be related to immunoglobulin subtype 4-related immune disease. J Gastroenterol Hepatol. 2008;23:e165-e169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Castillo-Rama M, Sebagh M, Sasatomi E, Randhawa P, Isse K, Salgarkar AD, Ruppert K, Humar A, Demetris AJ. “Plasma cell hepatitis” in liver allografts: identification and characterization of an IgG4-rich cohort. Am J Transplant. 2013;13:2966-2977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Aguilera I, Martinez-Bravo MJ, Sousa JM, Pozo-Borrego AJ, Núñez-Roldán A. IgG subclass profile among anti-Glutathione S-transferase T1 antibodies in post-transplant de novo immune hepatitis. Clin Transplant. 2016;30:210-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Ekong UD, Mathew J, Melin-Aldana H, Wang D, Alonso EM. Successful resolution of inflammation and increased regulatory T cells in sirolimus-treated post-transplant allograft hepatitis. Pediatr Transplant. 2012;16:165-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Edmunds C, Ekong UD. Autoimmune Liver Disease Post-Liver Transplantation: A Summary and Proposed Areas for Future Research. Transplantation. 2016;100:515-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Martínez-Bravo MJ, Calderón-Cabrera C, Márquez-Malaver FJ, Rodríguez N, Guijarro M, Espigado I, Núñez-Roldán A, Pérez-Simón JA, Aguilera I. Mismatch on glutathione S-transferase T1 increases the risk of graft-versus-host disease and mortality after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1356-1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Aguado-Dominguez E, Sousa JM, Perez-Simon JA, Aguilera I. Clinical association of anti-glutathione S-transferase T1 antibodies and de novo immune hepatitis after hematopoietic cell transplantation. Dig Liver Dis. 2018;50:418-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Kerkar N, Yanni G. ‘De novo’ and ‘recurrent’ autoimmune hepatitis after liver transplantation: A comprehensive review. J Autoimmun. 2016;66:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Arterbery AS, Osafo-Addo A, Avitzur Y, Ciarleglio M, Deng Y, Lobritto SJ, Martinez M, Hafler DA, Kleinewietfeld M, Ekong UD. Production of Proinflammatory Cytokines by Monocytes in Liver-Transplanted Recipients with De Novo Autoimmune Hepatitis Is Enhanced and Induces TH1-like Regulatory T Cells. J Immunol. 2016;196:4040-4051. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Aguilera I, Sousa JM, Praena JM, Gómez-Bravo MA, Núñez-Roldan A. Choice of calcineurin inhibitor may influence the development of de novo immune hepatitis associated with anti-GSTT1 antibodies after liver transplantation. Clin Transplant. 2011;25:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |