Published online Aug 7, 2018. doi: 10.3748/wjg.v24.i29.3204
Peer-review started: April 9, 2018
First decision: April 26, 2018
Revised: May 19, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: August 7, 2018
Helicobacter pylori (H. pylori) infection is very common and affects approximately half of the world population. It causes gastric diseases, but some authors have reported an association of H. pylori infection with other systemic manifestations beginning in 1994. The list of potential effects of H. pylori outside the stomach includes a number of extragastric manifestations and we focused on neurological, dermatological, hematologic, ocular, cardiovascular, metabolic, allergic, and hepatobiliary diseases. This review discusses these important reported manifestations that are not related to the gastrointestinal tract.
Core tip:Helicobacter pylori (H. pylori) infection is a common infection that can cause gastric and extragastric diseases. A considerable amount of evidence links H. pylori infection with extragastric diseases, and in many of these diseases there is a clear beneficial effect of eradication therapy. This review summarizes the H. pylori-related extragastric manifestations of major interest that have been reported in the scientific literature, such as neurological, dermatological, hematologic, ocular, cardiovascular, metabolic, allergic and hepatobiliary disease manifestations.
- Citation: Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: A review. World J Gastroenterol 2018; 24(29): 3204-3221
- URL: https://www.wjgnet.com/1007-9327/full/v24/i29/3204.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i29.3204
Helicobacter pylori (H. pylori) is a gram-negative, microaerophilic, spiral-shaped and flagellated bacterium infecting about half the world’s population whose main reservoir is the human stomach. The prevalence of infection varies by geographic area, age, ethnicity and socioeconomic status; in fact, the prevalence is higher in developing countries and in those with poor socio-economic conditions[1-4]. H. pylori possesses microbiological characteristics that allow it to survive in extremely adverse conditions such as the gastric acidic environment. Transmission of the infection occurs mainly through the oral-fecal route[5], in particular through contaminated water and food. Oral-oral transmission is also possible, as shown by the isolation of the bacterium in saliva and dental plaque[6].
H. pylori infection is the main cause of chronic gastritis and peptic ulcer disease[7]. H. pylori has a determinant pathogenic role in the development of distal gastric adenocarcinoma and gastric mucosa associated lymphoid tissue (MALT) lymphoma; in fact, it can contribute to gastric carcinogenesis by stimulating gastric cell proliferation without counterbalancing with adequate apoptosis[8,9]. The spectrum of gastroduodenal diseases associated with the evolution of H. pylori infection is wide. A large proportion of infected subjects (approximately 80%-85%) develop mild antrum and body gastritis associated with an alteration of gastric homeostasis characterized by hypergastrinemia with normal levels of gastric acid secretion. These subjects are unlikely to develop severe clinical conditions. Approximately 10%-15% of infected individuals develop prevalent antrum gastritis, in which hypergastrinemia is associated with increased gastric secretion with the possible development of duodenal ulceration[10]. A lower percentage (approximately 1%-2%) develop a prevalent body gastritis in response to the infection that is associated with multifocal atrophic gastritis, increased gastrinemia, hypo-chlorhydria and the possible development of gastric adenocarcinoma. The reason why H. pylori infection is associated with different gastric phenotypes with different clinical outcomes is not fully known, but it is likely that the interaction among bacterial virulence factors (e.g., CagA, urease, VacA, and babA2), environmental factors (e.g., socioeconomic conditions, nutrition, and exposure to toxic substances) and genetic substrates of the host [e.g., IL1B gene cluster and tumor necrosis factor-α (TNFα) gene polymorphism] play an important role[11]. The first reports of the association between H. pylori infection and extra-gastric diseases were by Mendall et al[12] in 1994. There are several clinical extra-gastric manifestations associated with H. pylori infection that have been reported to date, making this a very interesting and debated topic. We summarize the data in the literature about extra-digestive diseases associated with H. pylori infection and focus our attention on the following conditions that are potentially linked to H. pylori infection: neurological, dermatological, hematologic, ocular, cardiovascular, metabolic and allergic diseases. For some of these diseases, only one association is described without a clear explanation of the pathogenic mechanism; however, for others, as we will see later, the association is so strong and the pathogenic mechanism is so clear that guidelines for the treatment of H. pylori infection suggest that in these conditions, H. pylori infection should be determined and, if present, should be treated with eradication therapy[13].
Several neurological disorders are associated with H. pylori infection. There are studies in the literature that report a positive predictive value between H. pylori infection and stroke; however, in 2013, in a prospective cohort analysis performed on 9885 subjects with stroke, Chen et al[14] not only reported no increased mortality but actually found that people infected with H. pylori had a lower mortality than the general population. In a recent metanalysis, Wang et al[15] demonstrated that chronic H. pylori infection and the presence of CagA-positive strains were statistically significant risk factors for ischemic stroke. The underlying pathogenic mechanism is not yet known, but it has been hypothesized that H. pylori increases the expression of a number of mediators of inflammation and activates platelets and factors involved in coagulation[16]. Difference in the study population and in the methods to assess H. pylori infection might partially explain the discrepancy between different studies.
Another neurologic disease that has been linked to H. pylori infection is Alzheimer’s disease (AD). There are several studies concerning H. pylori and dementia. Huang et al[17] showed a 1.6 times greater risk of developing AD in H. pylori-infected people than in non-infected people, supporting a possible role of H. pylori in the pathophysiology of AD. Roubaud Baudron et al[18] also reported a 1.5 times greater risk of developing dementia over a 20-year follow-up period in infected people than in non-infected people. Beydoun et al[19] also reported an association between H. pylori infection and reduced cognitive ability. There are also several studies that report that eradication of infection can positively influence the manifestations of AD[20-22]. In 2016, Kountouras et al[23] showed that in patients with AD and H. pylori infection there was an increased prevalence of apolipoprotein E (ApoE) 4 polymorphism compared with non-infected patients. The ApoE 4 polymorphism is the strongest genetic risk factor for AD[23,24]. In 2009, the same author reported significantly higher levels of anti-H. pylori-specific antibodies (anti-H. pylori IgG) in the cerebrospinal fluid (CSF) and serum from patients with AD than that in the CSF and serum from age-matched subjects with normal cognition. The same research group demonstrated a significant correlation between the severity of disease and levels of anti-H. pylori IgG in the CSF of these patients[25]. One hypothesis to explain the association between H. pylori and AD is that H. pylori might access the brain via an oral-nasal-olfactory pathway thus leading to neurodegeneration. In fact, olfactory performance is reduced in 90% of patients with AD, and significant olfactory bulb atrophy, as evaluated by imaging techniques, is present in these patients[26-29]. Another hypothesis is that H. pylori may access the brain via monocytes infected with H. pylori (due to H. pylori replication in autophagic vesicles) through a disrupted blood-brain barrier (BBB), which is also called the “Trojan horse theory”; this may lead to increased production of inflammatory mediators, such as TNF-α, which in turn may cause BBB disruption by up-regulation of metalloproteinases[30]. A third hypothesis is that H. pylori may access to brain through a fast retrograde neural pathway from the gastrointestinal tract (GIT), leading to neurodegeneration[30]. A study conducted in Japan[31] did not confirm the association between H. pylori infection and AD. However the high prevalence of H. pylori in controls might partially explain the difference with studies conducted in Western countries where the prevalence of the infection is lower. Furthermore, Chang et al[32] in partial support of a role for H. pylori in AD, demonstrated that eradication of the infection led to a decreased progression of the neurological disease.
Multiple sclerosis (MS) is a chronic demyelinating disease that affects the central nervous system. Mohebi et al[33] showed an inverse relationship between H. pylori infection and MS; however, other authors have shown positivity of markers for optic neuromyelitis in H. pylori-positive patients. The administration of H. pylori SS1 antigen in an experimental model of MS [experimental autoimmune encephalomyelitis (EAE)], indicates the presence of immunomodulating properties of H. pylori, suggesting a possible effect of H. pylori infection in the pathophysiology of MS[34]. Cook et al[35] showed a probable protective role of H. pylori against EAE by inhibiting both Th1 and Th17 responses. H. pylori infection seems to be a risk factor for the development of antiaquaporin 4 (AQP4) antibodies in MS, through molecular mimicry between AQP4 and bacterial AQP[14]. Therefore, based on these studies, whether H. pylori infection may cause MS is still controversial.
Another disease of great neurological interest for which an association with H. pylori infection has been reported is Parkinson’s disease (PD). PD is caused by the degeneration of the dopaminergic neurons of the substantia nigra pars compacta of the basal ganglia system. A meta-analysis by Shen et al[36] in 2017, evaluating eight eligible studies involving 33125 participants, demonstrated that H. pylori infection might be associated with the risk of PD. A recent study by Huang et al[37] in the general population in Taiwan demonstrated that H. pylori infection was significantly associated with an increased risk of PD among individuals who were ≥ 60 years old but not among those < 60 years old. H. pylori infection may affect L-3,4-dihydroxyphenylalanine (L-dopa) bioavailability, which is used in the treatment of PD, by disrupting the duodenal mucosa, which is the site of absorption of L-dopa[38]. The eradication of the infection appears to be associated with greater bioavailability of L-dopa and is associated with better clinical outcomes[39,40]. Several studies have demonstrated that pro-inflammatory cytokines associated with chronic gastrointestinal disease can induce brain inflammation through a disruption of the BBB and death of dopaminergic neurons and may eventually be responsible for parkinsonism[41-43]. The association between PD and H. pylori infection seems, therefore, to be strongly supported by literature reports.
Guillain-Barré syndrome (GBS) is an acute autoimmune neuropathy characterized by progressive paralysis of the limbs with a distal-proximal pattern (the legs being affected first and the arms later). It can cause life-threatening complications, particularly if there is respiratory muscle involvement or autonomic nervous system involvement. The disease is usually triggered by an infection. Campylobacter jejuni (C. jejuni), the most prevalent cause of bacterial gastroenteritis, has been associated with GBS and the possible pathogenic mechanism involves molecular mimicry between peripheral nerve gangliosides and C. jejuni lipopolysaccharides (LPSs). In fact sialic acid, a characteristic component of human gangliosides, is present among the surface antigens of C. jejuni. H. pylori has a high-molecular weight sequence in LPSs that is detected in one third of C. jejuni serotypes. Therefore a molecular mimicry between H. pylori antigens and peripheral nerve gangliosides might be responsible for the association between H. pylori infection and GBS[16,44,45]. Some authors demonstrated that the presence of serum anti- H. pylori IgG in patients with GBS was significantly higher than that in controls. They also demonstrated that the CSF was positive for anti-H. pylori IgG in 80% of patients and in only 20% of controls[16]. Chiba et al[46] demonstrated IgG antibodies to vacuolating cytotoxin A (VacA) of H. pylori in the CSF of patients with GBS. In this study, the authors found sequence homology between VacA and the human ATPase A subunit, suggesting that antibodies to VacA might bind to ion channels in Schwann cells, resulting in the demyelination of motor neurons in these patients. A limitation of all these studies is the small sample size and, for this reason, studies with a greater sample size are needed to demonstrate a real cause-effect relationship between H. pylori and GBS.
Rosacea is the most common dermatological disease associated with H. pylori infection. It is a chronic facial dermatitis that manifests as erythema and cutaneous lesions characterized by much dilated red superficial capillaries, called telangiectasia, and its etiology remains unknown. Although the etiopathogenesis is not fully known, an element commonly found in patients with rosacea is the presence of gastrointestinal disorders. Several studies have suggested a potential relationship to H. pylori infection; however, the correlation between H. pylori infection and rosacea is still debated. Our group has demonstrated H. pylori infection in 48.9% of patients with rosacea, while only 26.7% of patients in the control group were infected with H. pylori. We demonstrated an improvement with partial or total regression of rosacea skin lesions after successful H. pylori eradication in 96.9% of patients[47]. Argenziano et al[48] reported H. pylori infection in 81% of patients with rosacea, and almost all of the patients housed CagA-positive strains. El-Khalawany et al[49] demonstrated that papular rosacea responded better to eradication therapy than erythematous rosacea did. Based on the majority of studies one may therefore suggest to test for and eradicate H. pylori infection in patients with rosacea.
Psoriasis is an autoimmune chronic inflammatory disease of the skin that is not infectious or contagious and is usually of a chronic and recurring nature. The association between H. pylori infection and psoriasis is still controversial. In fact some authors demonstrated neither a significant relationship between psoriasis and H. pylori[50,51], nor a significant relationship between psoriasis severity and the serum levels of IgG anti-H pylori[51] . However, other authors demonstrated that H. pylori infection is more common in patients with psoriasis than in healthy controls[52,53] and that prevalence of H. pylori infection was higher in patients with severe psoriasis (79%) compared to those with moderate (69.5%) or mild (46.2%) disease[53,54]. Finally, in support of a causative role for H. pylori in psoriasis, an interventional study by Onsun et al[55] demonstrated that eradication of H. pylori infection caused more rapid improvement than treatment with acitretin alone.
Chronic urticaria (CU) is characterized by the appearance of a more or less itchy rash, and its unique lesion is the wheal. Some research groups have reported a higher prevalence of H. pylori infection in patients with CU[56,57]. Campanati et al[58] demonstrated no difference in the presence of H. pylori infection between patients with CU and apparently healthy people, but they reported a significant improvement in skin lesions after eradication therapy. Yoshimasu et al[59] demonstrated that patients with CU infected with H. pylori had a cure rate of approximately 56% and that none of the patients with H. pylori infection were cured if they were treated with antihistamine therapy alone.
Data regarding the association between alopecia aerata (AA) and H. pylori infection are discordant. In fact, some authors have reported a higher prevalence of infection in patients with AA, but other authors did not confirm this association[60,61]. Differences in methodology might account for the discrepancy of results related to the role of H. pylori in CU or AA and, therefore, larger population studies and controlled trials to obtain more accurate evidence about these associations are needed.
Autoimmune bullous diseases (AIBD) are a group of dermatological diseases including pemphigus, pemphigoid, epidermolysis bullosa acquisita, dermatitis herpetiformis, and linear immunoglobulin A disease[62,63]. Very few published studies have investigated the association between AIBD and H. pylori infection. Sagi et al[64] and Mortazavi et al[65] demonstrated that anti-H. pylori IgG was higher in patients with AIBD than in controls. In the Mortazavi et al[65] study, the prevalence of H. pylori infection was as high as 79.3% in the AIBD group.
Schöenlein-Henoch purpura (SHP) is an immune condition characterized by the deposition of immunoglobulin A in the skin and in other organs, such as the kidneys, joints, and gastrointestinal tract. The onset of this disease is characterized by the appearance of purple skin lesions. There are a few reports that support the association between H. pylori infection and SHP, demonstrating an improvement in skin lesions following the successful eradication of the infection[66-68]. Clinical studies with a great sample size are necessary prior to draw a firm conclusion regarding the role of H. pylori in SHP.
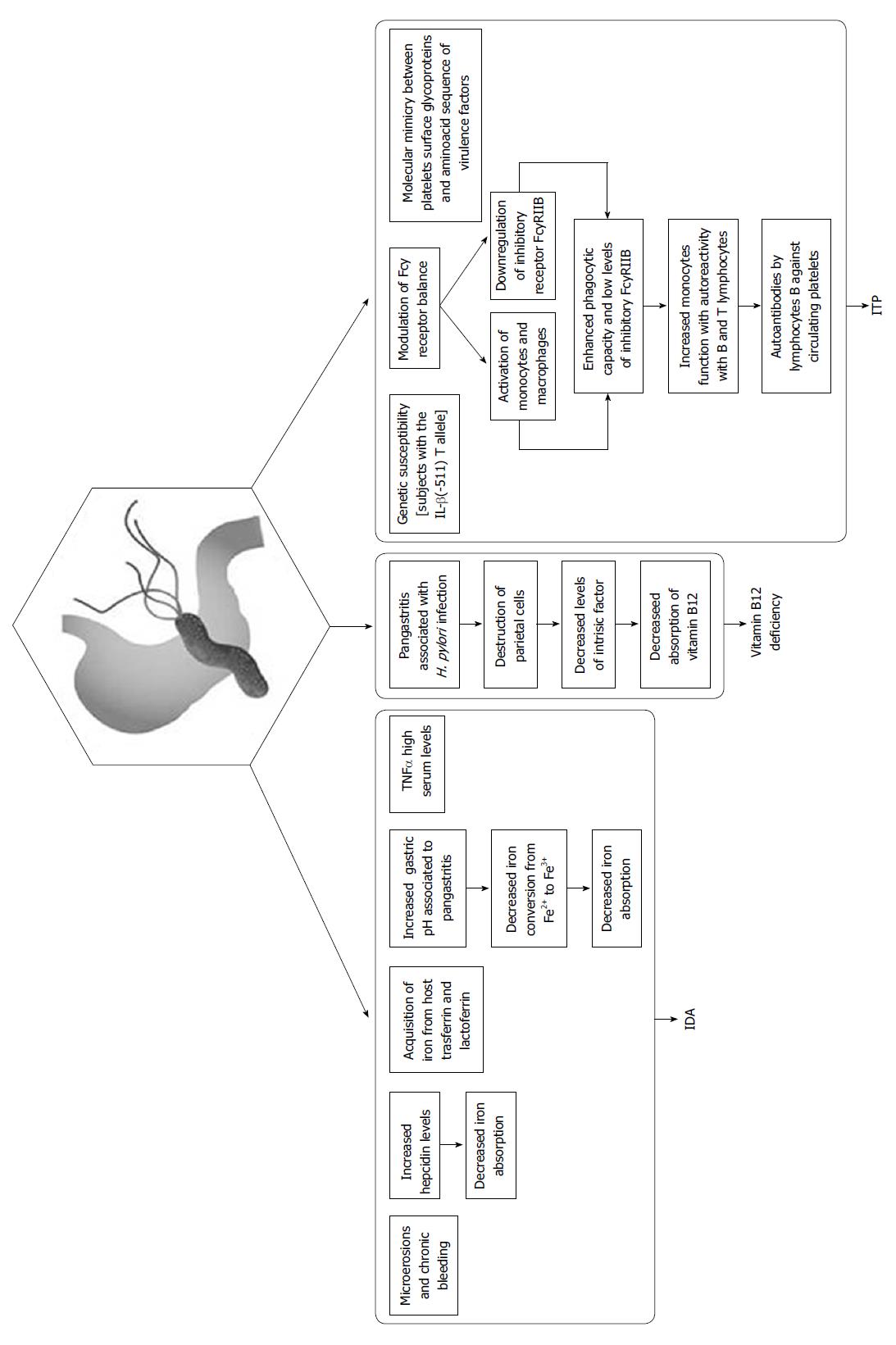
Iron deficiency anemia (IDA) is well known to be associated with H. pylori infection. In 1991, Blecker et al[69] described a case of hemorrhagic gastritis linked to H. pylori infection and showed a possible relationship between H. pylori infection and IDA. Subsequently, 5 meta-analyses concluded that there was a close association between infection and IDA[70-74]. Guidelines for the treatment of H. pylori infection indicate that the infection should be eradicated in cases of IDA[13]. The reason why IDA is not always associated with H. pylori infection is not known. Azab et al[75] studied the role of hepcidin, which is a small protein responsible for regulating iron recycling and the balance of iron in the body. Hepcidin, which is produced in the liver, regulates the absorption of iron from enterocytes and its release from macrophages[76]. H. pylori infection upregulates hepcidin serum levels, thus attenuating the response to iron therapy[77]. Senkovich et al[78] demonstrated that H. pylori is able to acquire iron from host transferrin and lactoferrin. Yokota et al[79] demonstrated that some polymorphisms within the H. pylori neutrophil-activating protein were more frequent in strains from IDA patients than in those from non-IDA patients. H. pylori that harbors these polymorphisms internalizes iron more rapidly than other strains[79]. Inorganic iron dissolves best in a highly acidic environment, and H. pylori infection may reduce the bioavailability of dietary iron. Patients infected with CagA-positive strains and those with refractory IDA have high serum levels of TNFα, which is a pro-inflammatory cytokine that can induce anemia[80,81]. Hershko et al[82] demonstrated that 64%-75% of patients with IDA and H. pylori infections had a complete disappearance of IDA after H. pylori eradication. Other possible causes of IDA in patients with H. pylori infections are chronic or acute bleeding due to erosive gastritis or concomitant therapy with non-steroidal anti-inflammatory drugs including aspirin[83-86].
Vitamin B12 is the coenzyme of many important enzymatic reactions in the human body that lead to DNA synthesis[87]. Lahner et al[88] showed an association between low serum levels of vitamin B12 and H. pylori infection in a systematic review evaluating 17 studies involving 2454 patients. Homocysteine is a component of the vitamin B12 metabolic pathway[89], and some authors have demonstrated a relationship between low serum levels of vitamin B12 and increases in serum homocysteine associated with H. pylori infection[90]. Increased serum levels of vitamin B12 and decreased serum levels of homocysteine have been reported after H. pylori eradication[87].The pathophysiological mechanism of the association between vitamin B12 deficiency and H. pylori infection is also unknown. The absorption of vitamin B12 may be compromised in corpus-predominant H. pylori-related gastritis. Chronic vitamin B12 deficiency can cause a pernicious anemia and peripheral neuropathy and lesions of the spinal cord[91].
Primary immune thrombocytopenia (ITP), which was previously called idiopathic thrombocytopenic purpura and autoimmune thrombocytopenic purpura, is an autoimmune disorder characterized by an isolated thrombocytopenia (a peripheral blood platelet count of 100 × 109/L) in the absence of other causes[87]. The first case of association between H. pylori infection and ITP was described in Spain in 1999[92]. In the world literature, there are many authors who have reported this association, and a significant increase in platelet count following the eradication of H. pylori infection varies from 32% to 100% in Italian studies[93,94] and from 26% to 100% in the overall literature[95,96]. There are only a few published studies that do not describe a positive association between H. pylori infection and ITP, but this may be explained by the low prevalence of the infection in these countries[97-100]. H. pylori-infected patients who have a particular polymorphism in IL-β, the IL-β (-511) T allele, are more likely to develop ITP than uninfected ITP patients[80,101]. The pathogenesis of ITP associated with H. pylori infection is most likely multifactorial. Various mechanisms involved in this autoimmune process in patients with H. pylori infection have been described, and one mechanism does not exclude the other. One of these mechanisms is the modulation of the Fcγ receptor balance, which is related to the activation of monocytes/macrophages and the relationship to the inhibitory receptor FcγRIIB. Monocytes from H. pylori-infected patients exhibited an enhanced phagocytic capacity and low levels of inhibitory FcγRIIB, leading to increased monocyte function with autoreactivity with B and T lymphocytes. This may cause the production of autoantibodies by lymphocyte B against circulating platelets. Eradication of H. pylori in ITP causes an upregulation of FcγRIIB expression in monocytes, increased platelet recovery and suppression of antigen presentation by macrophages with the subsequent inhibition of T- and B-cell responses to platelet antigens[87,102]. Another mechanism potentially involved in H. pylori-associated ITP is molecular mimicry between platelet surface glycoproteins, including glycoprotein IIIa, and amino acid sequences of virulence factors, such as VacA, CagA and urease B[87,103]. Anti-H. pylori IgG can induce opsonization of platelets by binding to H. pylori, von Willebrand’s factor, and GPIb[102,104].
The putative mechanisms responsible for the association between H. pylori infection, IDA, Vitamin B12 deficiency and ITP are summarized in Figure 1.
Other unrecognized hematologic disorders that are associated with H. pylori infection are autoimmune neutropenia (AN), anti-phospholipid syndrome (AS), and plasma cell dyscrasias (PCD). The association between AN and H. pylori infection was described for the first time by Cicconi et al[105] in 2001 and was subsequently described by other authors[105,106]. AN (defined as 400 neutrophils per μL) may dramatically improve after H. pylori eradication[87,107,108]. The association between H. pylori infection and PCD, including monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma, solitary plasmacytomas, plasma cell leukemia, Waldenstrom macroglobulinemia and other chronic myeloproliferative diseases of B lymphocytes, is supported by some authors but not by others[107,109-111]. This relationship maybe a result of chronic antigenic stimulation of B lymphocytes by H. pylori[84].
Ocular diseases described in association with H. pylori infection are open-angle glaucoma (OAG), central serous chorioretinitis (CSC) and blepharitis (B). There are studies that show a prevalence of H. pylori infection that is approximately two-fold higher in patients with OAG than in controls. However, other authors including Galloway et al[112] and Kurtz et al[113] did not find a higher prevalence of infection in patients with open-angle glaucoma. Zeng et al[114] published a meta-analysis about this association, evaluating ten studies, and suggested a statistically significant association between H. pylori infection and OAG. In further support of an association between H. pylori infection and OAG, successful eradication of the infection has been reported to lead to an improvement in ocular pressure parameters in treated patients compared with the patients who do not have the infection eradicated[80].
CSC is an ocular disease that causes a temporary reduction in central vision and usually affects only one eye. In the different phases of activity, it is characterized by the presence of liquid passing under the retina, which tends to accumulate under the macula. This results in blurred or distorted vision, with a reduction in visual acuity that can persist even after reabsorption of the fluid if it does not take place rapidly[115]. In a systematic review and meta-analysis about the risk factors for CSC published in 2016, Liu et al[116] concluded that H. pylori infection was a possible risk factor for the occurrence of CSC. Our research group also evaluated this association in a retrospective observational case series and demonstrated that the prevalence of H. pylori infection was 78.2% in CSC patients and 43.5% in control subjects[117]. H. pylori eradication may lead to an improvement in CSC[115,118,119]. In particular, Zavoloka et al[120] demonstrated that H. pylori eradication caused a decrease in the disease duration of 3 mo and in the recurrence frequency of 45.6 % and led to an improved long term prognosis. In fact, after two years, the visual acuity increased, the scotoma frequency decreased and the metamorphopsia frequency decreased.
Blepharitis is characterized by non-granulomatous inflammation of the eyelid margin. It remains difficult to ascertain whether its association with H. pylori infection is real because the data are highly discordant[121,122].
The association of H. pylori infection with cardiovascular diseases (CD) represents one of the fields of study that has attracted the most attention in the scientific community, despite the evident difficulties in identifying the specific role of H. pylori in the pathogenic processes of CD, including coronary atherosclerotic disease (CAD), stroke and myocardial infarction. Mendall et al[12] were the first to describe a significant association between H. pylori infection and the development of CAD in male subjects aged 45-65 years in a prospective study. CAD is due to dysfunction of the vessel endothelium associated with a simultaneous remodeling of the vessel wall, which is accompanied by an increase in blood pressure, a local inflammatory state and blood clotting; these are all phenomena that overall converge towards the formation of atherosclerotic plaques that are frequently unstable and susceptible to breakage. A possible rupture may compromise the blood circulation and lead to a myocardial infarction (MI). There are multiple causal factors that are involved in the pathogenesis during the initial stages of disease progression, including smoking, arterial hypertension, the presence of mixed dyslipidemia (alterations in total cholesterol levels, low density lipoprotein (LDL) and triglycerides) associated with a simultaneous reduction in high density lipoprotein (HDL) cholesterol, obesity, diabetes mellitus, and the presence of hyperhomocysteinemia and increased coagulation factors[123,124]. The hypothesis that infectious factors may play a role in the pathogenesis of CAD has developed only recently, probably stimulated by evidence that, despite the predisposing factor pattern, there is still a part of the population affected by CAD in which the origin and progression of the disease is inexplicable. Indeed, Lai et al[125] have shown that H. pylori infection increases the risk of acute coronary artery disease, even in the absence (or after the removal) of risk factors. The most likely pathogenic mechanism of the relationship between H. pylori and CAD is the initiation of a chronic inflammatory process associated with infection; however, it should be clarified if the role of H. pylori is to trigger the disease or only to accelerate its clinical course. The particular role of H. pylori in the pathogenesis of CAD is thought to be related to its ability to trigger a persistent chronic inflammatory state that is established within the gastric epithelium but that can also cause systemic inflammatory effects[126]. In the stomach, VacA and urease contribute to the destruction of tight junctions, and this may allow the bacterial agents that breach the lamina propria to come into contact with the cells of the immune system[127]. H. pylori antigens can either interact directly with the vascular endothelium or may form complexes with LDL/oxLDL cholesterol[128]. The subacute inflammation present in chronic diseases, such as CAD, can be determined by the activation of the cells of the immune system or epithelial cells, through pattern recognition receptors (PRRs) that specifically recognize pathogen-associated molecular pattern[129]. Both endothelial cells and macrophages present in atherosclerotic lesions show an overexpression of PRRs, such as TLR4, CD14 and TLR2, that are specialized for recognizing bacterial LPS[130,131]. Xu et al[131] suggested a possible pathophysiological link between lipids, H. pylori infection, inflammatory status and CAD based on the overexpression of TLR4 on the surface of macrophages induced by pro-oxidant oxidized LDL. The inflammatory hypothesis was confirmed by several studies conducted with the intent of identifying an association between the increase in inflammatory markers and CAD. Li et al[132] and Libby et al[133] have highlighted a relationship between CAD and some of the most important inflammation markers, such as C-reactive protein (CRP), interleukin-6 (IL-6) and TNF-α, which are all expressed at higher levels in patients with atherosclerosis than in controls. CRP is an important acute phase protein that is associated with infectious and inflammatory processes or tissue damage; therefore, it is a reliable marker of endothelial dysfunction in CAD[134,135]. A second hypothesis about the relationship between H. pylori infection and the pathogenesis of CAD is that bacterial antigens can induce T- and B-cell expansion and cause self-reactive antibody production by molecular mimicry. As an example of molecular mimicry, Matsuura et al[136] proposed that heat shock protein 60 derived from H. pylori (Hp-HSP60) may potentially be related to the pathogenesis of CAD through the stimulation of Th1 lymphocytes, which are induced to produce INF-γ and IL-12, or through the activation of macrophages important in forming atherosclerotic plaques. An analysis of the literature clearly shows that serum positivity for CagA is closely associated with heart disease, including atherosclerosis, unstable angina, cardiac syndrome X and coronary artery disease[137-139]. CagA is the most important virulence factor of H. pylori. CagA-positive strains are associated with increased IL-8 production, which is a marker of inflammation[140]. The atherosclerotic cascade is notoriously characterized by an increase in the expression levels of inflammation markers, such as CRP and some interleukins. Discordant data about the association between H. pylori infection and CAD are present in the literature. In fact, Manolakis et al[141] did not find a correlation between CRP levels and H. pylori infection. Similarly, Carter et al[142] did not find any correlation between H. pylori infection and fibrinogen or von Willebrand’s factor levels.
Discordant data about the relationship between H. pylori and myocardial infarction (MI) also exist. In 2013, Ikeda et al[143] demonstrated that only CagA-positive H. pylori strains were associated with an increased risk of MI. In a meta-analysis of 26000 patients, Liu et al[144] demonstrated the existence of a significant association between H. pylori infection and the risk of MI, whereas Hughes et al[145] showed a parallel decline of MI and duodenal ulcers in people born from 1930 to 1980. This study showed that the decline in MI was temporally related to a decline in duodenal ulcers and, by inference, H. pylori infection. The discordant data make it impossible to provide an unambiguous definition of H. pylori as a causal factor for cardiovascular diseases.
H. pylori infection has also been identified as related to alterations in glycolipid metabolism. The role of H. pylori infection in diabetes mellitus (DM), insulin resistance syndrome (IR), and metabolic syndrome (SMet) is still rather controversial, and in some cases, appears to bear marginal weight.
The association between H. pylori infection and DM has been suggested recently but still appears rather unclear[146]. In a study of a Chinese population, Hsieh et al[147]demonstrated how high levels of glycated hemoglobin (HbA1c) were significantly associated with H. pylori infection in patients aged > 65 years. Bégué et al[148] demonstrated that eradication of H. pylori infection in patients with type 1 DM might be associated with better control of glycemia by evaluating HbA1c, which is an important indicator of long-term glycemic control, within two years after the eradication of the infection. In contrast, in 141 patients with type 2 DM, Demir et al[149] demonstrated no significant differences in either blood glucose levels or HbA1c values in patients with H. pylori infections compared with controls. In a cross-sectional study including 1285 subjects aged 19-85 years, Yang et al[150] suggested the existence of a correlation between H. pylori infection and DM. According to Horikawa et al[151], the glycemic control of patients with DM was worse in the presence of H. pylori infections, in particular in infections with CagA-positive strains. A few studies demonstrated that eradication could lead to a benefit for glycemic control[152,153]. This has not been confirmed by other studies[154]. A meta-analysis by Wang et al[155] demonstrated the presence of higher levels of inflammatory markers in diabetic patients than in controls. Jeon et al[156] demonstrated that IL-6 levels and CRP levels, when used as inflammatory markers, appeared to be very similar in patients with DM, both in patients who were H. pylori positive and in those who were negative. As it is now known, chronic H. pylori infection is responsible for a low-level inflammatory state of the gastric mucosa, which is defined as the biological response of the mucosa to pathogens, that can involve different regions depending on the host phenotype. Chronic gastritis, such as that found in type 2 DM, atherosclerosis and SMet, is associated with an increase in circulating pro-inflammatory cytokine levels that are capable of interfering with both local and systemic metabolic processes[157]. Studies have also been conducted evaluating complications associated with DM. Wang et al[158] described a possible correlation between H. pylori infection and the risk of nephropathy or neuropathy in an Asian population; however, some other authors did not confirm this correlation[159]. Vafaeimanesh et al[160] have demonstrated the existence of an association between diabetic patients with microalbuminuria and H. pylori infection. Microalbuminuria, as well as neuropathy and heart disease, are very common complications in patients with DM, and patients with CagA-positive H. pylori infection are at a greater risk of developing these complications[152,161].
Vafaeimanesh et al[160] also focused on the possible correlation between H. pylori infection and the development of IR. IR and H. pylori infection share pathogenic mediators that are potentially involved in the pathophysiology of the SMet[161]. Zhou et al[162] showed that H. pylori may induce hepatic IR by interfering with the c-Jun/miR-203/SOCS3 pathway, both in humans and in animal models. An analysis of the literature shows that H. pylori eradication can improve IR, although this resolution seems to be associated with a progressive increase in BMI and cholesterol levels, as H. pylori suppresses ghrelin, which is the hormone responsible for increased appetite, which may explain the weight gain associated with the eradication of infection[154]. However, one can hypothesize that the resolution of the symptoms related to infection may lead to recovery of a normal weight. HOMA-IR, which is a model for measuring insulin homeostasis, is higher in patients with H. pylori infection than in control[163-165]. However, this finding has not been confirmed by other studies[166].
Considering the aforementioned role of H. pylori in IR, there have been several studies in the literature that demonstrate a higher prevalence of SMet in patients with H. pylori infection[167]. Some studies have revealed changes in the lipid profiles of patients associated with the low-level inflammatory status induced by H. pylori infection; this has been demonstrated by Niemelä et al[168] in a study involving a Finnish population, in which serum cholesterol and triglyceride levels were higher in infected male subjects. Several studies have demonstrated the presence of these serum lipid profile alterations that can promote atherogenic processes[169].
In the literature, a number of studies and meta-analyses have reported an inverse association between H. pylori infection and asthma, worldwide[170-172]. Blaser et al[173] showed the existence of an inverse relationship between H. pylori infection and the development of asthma or other allergic diseases, particularly in children and young people with an early onset of allergies. This relationship is controversial in adults, however, and has been confirmed mainly in cases in which the H. pylori strains are CagA-positive. Amberbir et al[174] showed that the relationship between H. pylori and rhinitis is not always inverse. H. pylori can almost completely protect against airway hyper-reactivity, broncho-alveolar eosinophilia, and lung inflammation, but this protection, which is strongly dependent on regulatory T (Treg) lymphocytes, is impaired by the complete eradication of the infection through antibiotic therapy[170]. The functionality of T reg cells depends on IL-18 production by dendritic cells (DCs) following exposure to H. pylori, in the absence of which, a neonatal tolerance to infection is not established. Treg cells are derived from cells with an IL-18 -/- or an IL-18R -/- phenotype that have no protective capabilities against asthma; moreover, IL-18 is fundamental for the conversion of CD4+ T-cells into CD25+ Foxp3+ Treg cells[175]. VacA toxin is the most important factor involved in protection against allergies. It appears to disrupt the T helper cell response to Treg cells. It stimulates Treg cells, but it also has the ability to interfere with antigen presentation and T-cell inhibition[176]. H. pylori strains expressing the active form of VacA are usually also CagA positive[177]. Most likely, CagA-positive strains elicit a greater response through modulation of inflammation by Treg cells, but it seems plausible that there is also a pronounced effect on the migration of Treg cells[178]. To validate this hypothesis, Engler et al[179] demonstrated that the administration of VacA to mouse models was able to provide allergy protection that was comparable to that of active infection. However, these effects seem to be more pronounced if this administration is carried out during the neonatal stage of life, probably because this represents a crucial period during which antigenic tolerance develops, both in mice and in humans. H. pylori influences the immune system by shifting the balance of cytokines to the Th1 type, which suppresses allergic diseases that are dependent on the Th2 cytotype[180,181], protecting infected individuals from developing atopic disease. This might also explain the low prevalence of eosinophilic esophagitis in H. pylori-infected subjects[182]. When analyzing the association between H. pylori infection and allergies one should take into account the hygiene hypothesis and the reduced prevalence of allergic diseases in pet owners. In fact, H. pylori infection is associated with poor hygiene, crowding and low socio-economic status. Poor hygiene conditions and low socioeconomic status together with pet ownership may expose to other bacteria or antigens which may reduce the risk of allergic diseases. Therefore, in the interpretation of epidemiologic studies between H. pylori infection and allergies, possible confounding factors should be considered before drawing conclusions on putative cause-effect relationship[180-182].
Recent studies have shown the involvement of H. pylori in the etiopathogenesis of a number of liver diseases[183,184]. Polyzos et al[185] showed a higher serum level of anti-H. pylori IgG in patients with nonalcoholic fatty liver disease (NAFLD) than in non-NAFLD patients. Many other studies have shown an association between NAFLD and H. pylori[186,187]. H. pylori could be related to a worsening of the inflammatory status of the liver, regardless of the etiology of the underlying liver disease[184]. Fukuda et al[188] have proposed that because of the increase in gastric and intestinal mucosal permeability, H. pylori antigens could have access to the blood stream and reach the liver through the portal vein, thus causing liver damage. Some authors, such as Sumida et al[189], suggest that the eradication of H. pylori may play an important role in the treatment of nonalcoholic steatohepatitis (NASH), through the decrease of TNFα, one of the pro-inflammatory cytokines that, together with IL-1β, IL-6 and IL-8, is also directly correlated with the etiopathogenesis of IR, and of NASH[190,191]. Adiponectin is also involved in the etiopathogenesis of NAFLD. In fact adiponectin deficiency is associated with a pro-inflammatory condition, as it is observed in obesity and other metabolic disorders[192]. According to Polyzos et al[185] a higher prevalence of low levels of circulating adiponectin, as well as of higher levels of anti-H. pylori IgG and elevated TNFα are observed in NAFLD patients compared to controls.
The correlation between H. pylori and hepatic fibrosis was analyzed mostly in animal models. Ki et al[193] demonstrated in a murine model that H. pylori infection may accelerate hepatic fibrosis through increased TGF-β1-induced pro-inflammatory signaling pathways in hepatic stellate cells and that H. pylori infection might increase the risk of TGF-β 1-mediated tumorigenesis by disturbing the balance between apoptosis and proliferation of hepatocytes. Senescence marker protein-30, a protein that prevents oxidative stress and hepatic cell apoptosis[194] is reduced in the liver of mice treated with CCL4 and H. pylori compared to those treated with CCL4 only[195].
H. pylori has been considered as putative a triggering factor for autoimmune extragastric conditions[196,197]. Goo et al[198] in 2008 showed that C57BL/6 mouse infected with H. pylori developed a form of primary biliary cirrhosis (PBC) very similar to that described in humans. Because of the high serum levels of IgG against H. pylori VacA, the authors suggested that anti-VacA antibodies might play a role in the development of PBC. Shapira et al[199] found higher prevalence of anti-H. pylori IgG in patients with PBC compared to controls. Nilsson et al[200] evaluated 24 liver biopsies obtained from patients with PBC and primary sclerosing cholangitis (PSC) and found that H. pylori was present in 20/24 patients. The authors a suggested that the pathogenic ling between H. pylori infection and autoimmune liver diseases might be a form of molecular mimicry between H. pylori and liver antigens. In fact, a similar amino acid sequence homology between the main mitochondrial autoepithopic region from the E2 subunit of the pyruvate dehydrogenase complex and the H. pylori urease was found. However Bogdanos et al[201] showed that the existence of this homology does not necessarily imply cross-reactivity. H. pylori DNA was detected in biliary epithelium in PSC patients compared to controls, corroborating the hypothesis that biliary reflux could lead to H. pylori contamination of the proximal biliary system, contributing to the development and / or progression of PSC in some patients[202]. Patients with PSC may also suffer from ulcerative colitis (UC). It has therefore been suggested that an increased intestinal permeability in UC patients may promote the translocation of H. pylori into the hepatobiliary system, thus triggering autoimmunity mechanisms[203].
The role of H. pylori in hepatic carcinogenesis is controversial. There are several known risk factors for HCC, such as alcoholism, PCB, PSC and chronic viral infections[204]. The finding of H. pylori in hepatic biopsies of patients with HCC led to the hypothesis that H. pylori might be playing a role also in the development of this infection[205]. In several studies, liver biopsies of patients with HCC or even cholangiocarcinoma were positive for H. pylori[206,207]. Dore et al[208] demonstrated H. pylori presence in patients with HCC, liver cirrhosis and chronic hepatitis, using both PCR on liver tissue and serology. Prevalence of H. pylori infection was as high as 73% in patients with HCC. Verhoef et al[209] and Pellicano et al[210] also found positivity for H. pylori in 45% of patients with HCC vs 10% of controls, and in 85% of patients with HCC vs 33% of controls, respectively. Finally, it has been suggested that infection by CagA positive H. pylori strains might play a major role in the development of H. pylori-associated liver diseases[211].
To date, there is no sufficient evidence to draw a firm conclusion on the relationship between H. pylori infection and liver diseases.
H. pylori is the cause of a number of gastroduodenal diseases including peptic ulcer disease and gastric adenocarcinoma which are the results of an interaction between bacterial virulence factors, host and environmental factors. Many extra-gastric manifestations have been reported to be linked to H. pylori infection (Table 1). However, most evidences derive from epidemiological studies where a number of confounding factors have not been analyzed in depth, thus making it impossible to establish a cause-effect correlation. As a result of this, to date, according to the last Consensus Report on the management of H. pylori infection (i.e., Maastricht V/Florence Guidelines[13]) H. pylori infection should be sought and, if present, eradicated only in patients with IDA, ITP and vitamin B12 deficiency.
| Neurologic diseases | Pro | Con |
| Stroke | Wang et al[15] | Chen et al[14] |
| Alvarez-Arellano et al[16] | ||
| Alzheimer’s disease | Huang et al[17] | Shiota et al[31] |
| Roubaud Baudron et al[18] | ||
| Beydoun et al[19] | ||
| Kountouras et al[21] | ||
| Kountouras et al[22] | ||
| Kountouras et al[23] | ||
| Santos et al[24] | ||
| Kountouras et al[25] | ||
| Zelaya et al[26] | ||
| Attems J et al[27] | ||
| Thomann et al[28] | ||
| Förster et al[29] | ||
| Chang et al[32] | ||
| Multiple sclerosis | Chen et al[14] | Mohebi et al[33] |
| Cook et al[35] | ||
| Parkinson’s disease | Shen X et al[36] | |
| Huang HK et al[37] | ||
| Fasano et al[38] | ||
| Tan et al[39] | ||
| Mridula et al[40] | ||
| Candelario-Jalil et al[41] | ||
| Lo et al[42] | ||
| Dobbs et al[43] | ||
| Guillain-Barré syndrome | Kountouras et al[44] | |
| Moran et al[45] | ||
| Chiba et al[46] | ||
| Dermatological diseases | Pro | Con |
| Rosacea | Gravina et al[47] | |
| Argenziano et al[48] | ||
| El-Khalawany et al[49] | ||
| Psoriasis | Qayoom et al[52] | Campanati et al[50] |
| Mesquita et al[53] | Azizzadeh et al[51] | |
| Ribaldone et al[54] | ||
| Onsun et al[55] | ||
| Chronic urticaria | Hizal et al[56] | Campanati et al[58] |
| Galadari et al[57] | ||
| Yoshimasu et al[59] | ||
| Alopecia areata | Behrangi et al[61] | Rigopoulos et al[60] |
| Autoimmune bullous diseases | Sagi et al[64] | |
| Mortazavi et al[65] | ||
| Schöenlein-Henoch purpura | Novák et al[66] | |
| Grivceva-Panovska et al[67] | ||
| Hoshino et al[68] | ||
| Hematologic diseases | Pro | Con |
| Iron deficiency anemia | Blecker et al[69] | |
| Muhsen et al[70] | ||
| Qu et al[71] | ||
| Huang et al[72] | ||
| Yuan et al[73] | ||
| Zhang et al[74] | ||
| Ozkasap et al[77] | ||
| Senkovich et al[78] | ||
| Yokota et al[79] | ||
| Testerman et al[80] | ||
| Afifi et al[81] | ||
| Hershko et al[82] | ||
| Hershko et al[83] | ||
| Kang et al[84] | ||
| Musumba et al[85] | ||
| De Leest et al[86] | ||
| Vitamin B12 deficiency | Campuzano-Maya et al[87] | |
| Lahner et al[88] | ||
| Marino et al[90] | ||
| Toh et al[91] | ||
| Primary immune thrombocytopenia | García Pérez et al[92] | Jarque et al[97] |
| Stasi et al[93] | Michel et al[98] | |
| Gasbarrini et al[94] | Michel et al[99] | |
| Suvajdzić et al[95] | Estrada-Gómez et al[100] | |
| Jackson et al[96] | ||
| Satoh et al[101] | ||
| Asahi et al[102] | ||
| Kodama et al[103] | ||
| Byrne et al[104] | ||
| Autoimmune neutropenia | Cicconi et al[105] | |
| Papadaki et al[107] | ||
| Papadaki et al[108] | ||
| Plasma cell dyscrasias | Tursi et al[109] | Papadaki et al[107] |
| Wolkersdörfer et al[110] | Rajkumar et al[111] | |
| Ocular diseases | Pro | Con |
| Open-angle glaucoma | Zeng J et al[114] | Galloway et al[112] |
| Testerman et al[80] | Kurtz et al[113] | |
| Central serous chorioretinitis | Casella et al[115] | |
| Liu et al[116] | ||
| Cotticelli et al[117] | ||
| Rahbani-Nobar et al[118] | ||
| Dang et al[119] | ||
| Zavoloka et al[120] | ||
| Blepharitis | Saccà et al[122] | Saccà et al[121] |
| Cardiovascular diseases | Pro | Con |
| Coronary atherosclerotic disease | Mendall et al[12] | Manolakis et al[141] |
| Lai et al[125] | Carter et al[142] | |
| Chmiela et al[126] | ||
| Chmiela et al[128] | ||
| Chalubinski et al[129] | ||
| Libby[130] | ||
| Xu et al[131] | ||
| Matsuura et al[136] | ||
| Khodaii et al[137] | ||
| Rasmi et al[138] | ||
| Zhang et al[139] | ||
| Myocardial infarction | Ikeda et al[143] | |
| Liu et al[144] | ||
| Hughes et al[145] | ||
| Metabolic disease | Pro | Con |
| Diabetes mellitus | Oldenburg et al[146] | Demir et al[149] |
| Hsieh et al[147] | Akanuma et al[154] | |
| Bégué et al[148] | Yanik et al[159] | |
| Yang et al[150] | ||
| Horikawa et al[151] | ||
| Ibrahim et al[152] | ||
| Chen et al[153] | ||
| Jeon et al[156] | ||
| Wang et al[158] | ||
| Vafaeimanesh et al[160] | ||
| Chung et al[161] | ||
| Insulin resistance syndrome | Vafaeimanesh et al[160] | Naja et al[166] |
| Chung et al[161] | ||
| Zhou et al[162] | ||
| Aydemir et al[163] | ||
| Gunji et al[164] | ||
| Eshraghian et al[165] | ||
| Metabolic syndrome | Chen et al[167] | |
| Niemelä et al[168] | ||
| Laurila et al[169] | ||
| Polyzos et al[185] | ||
| Allergic diseases | Pro | Con |
| Chen et al[170] | ||
| Wang et al[171] | ||
| Zhou et al[172] | ||
| Blaser et al[173] | ||
| Amberbir et al[174] | ||
| Oertli et al[175] | ||
| Oertli et al[176] | ||
| Cook et al[178] | ||
| Engler et al[179] | ||
| Grad et al[180] | ||
| Sheikh et al[181] | ||
| Molina-Infante et al[182] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Reyes VE, Testerman TL, Yuan Y S- Editor: Wang JL L- Editor: A E- Editor: Huang Y
| 1. | Mégraud F, Brassens-Rabbé MP, Denis F, Belbouri A, Hoa DQ. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol. 1989;27:1870-1873. [PubMed] [Cited in This Article: ] |
| 2. | Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16 Suppl 1:3-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 292] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Leclerc H. [Epidemiological aspects of Helicobacter pylori infection]. Bull Acad Natl Med. 2006;190:949-962. [PubMed] [Cited in This Article: ] |
| 4. | Mandeville KL, Krabshuis J, Ladep NG, Mulder CJ, Quigley EM, Khan SA. Gastroenterology in developing countries: issues and advances. World J Gastroenterol. 2009;15:2839-2854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 58] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Ahmed KS, Khan AA, Ahmed I, Tiwari SK, Habeeb MA, Ali SM, Ahi JD, Abid Z, Alvi A, Hussain MA. Prevalence study to elucidate the transmission pathways of Helicobacter pylori at oral and gastroduodenal sites of a South Indian population. Singapore Med J. 2006;47:291-296. [PubMed] [Cited in This Article: ] |
| 6. | Mladenova I, Durazzo M. Transmission of Helicobacter pylori. Minerva Gastroenterol Dietol. 2018;64:251-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 7. | Blaser MJ. Hypothesis: the changing relationships of Helicobacter pylori and humans: implications for health and disease. J Infect Dis. 1999;179:1523-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 238] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 8. | Lehours P, Mégraud F. Helicobacter pylori infection and gastric MALT lymphoma. Rocz Akad Med Bialymst. 2005;50:54-61. [PubMed] [Cited in This Article: ] |
| 9. | Romano M, Ricci V, Zarrilli R. Mechanisms of disease: Helicobacter pylori-related gastric carcinogenesis--implications for chemoprevention. Nat Clin Pract Gastroenterol Hepatol. 2006;3:622-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Censini S, Stein M, Covacci A. Cellular responses induced after contact with Helicobacter pylori. Curr Opin Microbiol. 2001;4:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1690] [Cited by in F6Publishing: 1614] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 12. | Mendall MA, Goggin PM, Molineaux N, Levy J, Toosy T, Strachan D, Camm AJ, Northfield TC. Relation of Helicobacter pylori infection and coronary heart disease. Br Heart J. 1994;71:437-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 452] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1710] [Cited by in F6Publishing: 1745] [Article Influence: 249.3] [Reference Citation Analysis (1)] |
| 14. | Chen Y, Segers S, Blaser MJ. Association between Helicobacter pylori and mortality in the NHANES III study. Gut. 2013;62:1262-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Wang ZW, Li Y, Huang LY, Guan QK, Xu DW, Zhou WK, Zhang XZ. Helicobacter pylori infection contributes to high risk of ischemic stroke: evidence from a meta-analysis. J Neurol. 2012;259:2527-2537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Alvarez-Arellano L, Maldonado-Bernal C. Helicobacter pylori and neurological diseases: Married by the laws of inflammation. World J Gastrointest Pathophysiol. 2014;5:400-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 65] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Huang WS, Yang TY, Shen WC, Lin CL, Lin MC, Kao CH. Association between Helicobacter pylori infection and dementia. J Clin Neurosci. 2014;21:1355-1358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Roubaud Baudron C, Letenneur L, Langlais A, Buissonnière A, Mégraud F, Dartigues JF, Salles N; Personnes Agées QUID Study. Does Helicobacter pylori infection increase incidence of dementia? The Personnes Agées QUID Study. J Am Geriatr Soc. 2013;61:74-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Beydoun MA, Beydoun HA, Shroff MR, Kitner-Triolo MH, Zonderman AB. Helicobacter pylori seropositivity and cognitive performance among US adults: evidence from a large national survey. Psychosom Med. 2013;75:486-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Goni E, Franceschi F. Helicobacter pylori and extragastric diseases. Helicobacter. 2016;21 Suppl 1:45-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Chatzigeorgiou S, Katsinelos P, Grigoriadis N, Giartza-Taxidou E, Venizelos I. Five-year survival after Helicobacter pylori eradication in Alzheimer disease patients. Cogn Behav Neurol. 2010;23:199-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Kountouras J, Boziki M, Gavalas E, Zavos C, Grigoriadis N, Deretzi G, Tzilves D, Katsinelos P, Tsolaki M, Chatzopoulos D. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. J Neurol. 2009;256:758-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Kountouras J, Tsolaki F, Tsolaki M, Gavalas E, Zavos C, Polyzos SA, Boziki M, Katsinelos P, Kountouras C, Vardaka E. Helicobacter pylori-related ApoE 4 polymorphism may be associated with dysphagic symptoms in older adults. Dis Esophagus. 2016;29:842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement (Amst). 2017;7:69-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 25. | Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Grigoriadis N, Tsolaki M, Chatzopoulos D, Katsinelos P, Tzilves D. Increased cerebrospinal fluid Helicobacter pylori antibody in Alzheimer’s disease. Int J Neurosci. 2009;119:765-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Zelaya MV, Pérez-Valderrama E, de Morentin XM, Tuñon T, Ferrer I, Luquin MR, Fernandez-Irigoyen J, Santamaría E. Olfactory bulb proteome dynamics during the progression of sporadic Alzheimer’s disease: identification of common and distinct olfactory targets across Alzheimer-related co-pathologies. Oncotarget. 2015;6:39437-39456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Attems J, Walker L, Jellinger KA. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 2014;127:459-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 28. | Thomann PA, Dos Santos V, Seidl U, Toro P, Essig M, Schröder J. MRI-derived atrophy of the olfactory bulb and tract in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2009;17:213-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Förster S, Vaitl A, Teipel SJ, Yakushev I, Mustafa M, la Fougère C, Rominger A, Cumming P, Bartenstein P, Hampel H. Functional representation of olfactory impairment in early Alzheimer’s disease. J Alzheimers Dis. 2010;22:581-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Doulberis M, Kotronis G, Thomann R, Polyzos SA, Boziki M, Gialamprinou D, Deretzi G, Katsinelos P, Kountouras J. Review: Impact of Helicobacter pylori on Alzheimer’s disease: What do we know so far? Helicobacter. 2018;23:e12454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Shiota S, Murakami K, Yoshiiwa A, Yamamoto K, Ohno S, Kuroda A, Mizukami K, Hanada K, Okimoto T, Kodama M. The relationship between Helicobacter pylori infection and Alzheimer’s disease in Japan. J Neurol. 2011;258:1460-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Chang YP, Chiu GF, Kuo FC, Lai CL, Yang YH, Hu HM, Chang PY, Chen CY, Wu DC, Yu FJ. Eradication of Helicobacter pylori Is Associated with the Progression of Dementia: A Population-Based Study. Gastroenterol Res Pract. 2013;2013:175729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Mohebi N, Mamarabadi M, Moghaddasi M. Relation of helicobacter pylori infection and multiple sclerosis in Iranian patients. Neurol Int. 2013;5:31-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Franceschi F, Gasbarrini A, Polyzos SA, Kountouras J. Extragastric Diseases and Helicobacter pylori. Helicobacter. 2015;20 Suppl 1:40-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Cook KW, Crooks J, Hussain K, O’Brien K, Braitch M, Kareem H, Constantinescu CS, Robinson K, Gran B. Helicobacter pylori infection reduces disease severity in an experimental model of multiple sclerosis. Front Microbiol. 2015;6:52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Shen X, Yang H, Wu Y, Zhang D, Jiang H. Meta-analysis: Association of Helicobacter pylori infection with Parkinson’s diseases. Helicobacter. 2017;22:e12398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Huang HK, Wang JH, Lei WY, Chen CL, Chang CY, Liou LS. Helicobacter pylori infection is associated with an increased risk of Parkinson’s disease: A population-based retrospective cohort study. Parkinsonism Relat Disord. 2018;47:26-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | Fasano A, Bove F, Gabrielli M, Petracca M, Zocco MA, Ragazzoni E, Barbaro F, Piano C, Fortuna S, Tortora A. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord. 2013;28:1241-1249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 39. | Tan AH, Mahadeva S, Marras C, Thalha AM, Kiew CK, Yeat CM, Ng SW, Ang SP, Chow SK, Loke MF. Helicobacter pylori infection is associated with worse severity of Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:221-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Mridula KR, Borgohain R, Chandrasekhar Reddy V, Bandaru VCh, Suryaprabha T. Association of Helicobacter pylori with Parkinson’s Disease. J Clin Neurol. 2017;13:181-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, Fiebich BL, Rosenberg GA. Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J Pharmacol Exp Ther. 2007;323:488-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 42. | Lo YC, Shih YT, Wu DC, Lee YC. In vitro effects of Helicobacter pylori-induced infection in gastric epithelial AGS cells on microglia-mediated toxicity in neuroblastoma SH-SY5Y cells. Inflamm Res. 2009;58:329-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Dobbs RJ, Charlett A, Purkiss AG, Dobbs SM, Weller C, Peterson DW. Association of circulating TNF-alpha and IL-6 with ageing and parkinsonism. Acta Neurol Scand. 1999;100:34-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 44. | Kountouras J, Deretzi G, Zavos C, Karatzoglou P, Touloumis L, Nicolaides T, Chatzopoulos D, Venizelos I. Association between Helicobacter pylori infection and acute inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2005;12:139-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Moran AP, Prendergast MM. Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: contribution of gastrointestinal infections to autoimmunity. J Autoimmun. 2001;16:241-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Chiba S, Sugiyama T, Yonekura K, Tanaka S, Matsumoto H, Fujii N, Ebisu S, Sekiguchi K. An antibody to VacA of Helicobacter pylori in cerebrospinal fluid from patients with Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 2002;73:76-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Gravina A, Federico A, Ruocco E, Lo Schiavo A, Masarone M, Tuccillo C, Peccerillo F, Miranda A, Romano L, de Sio C. Helicobacter pylori infection but not small intestinal bacterial overgrowth may play a pathogenic role in rosacea. United European Gastroenterol J. 2015;3:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 48. | Argenziano G, Donnarumma G, Iovene MR, Arnese P, Baldassarre MA, Baroni A. Incidence of anti-Helicobacter pylori and anti-CagA antibodies in rosacea patients. Int J Dermatol. 2003;42:601-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | El-Khalawany M, Mahmoud A, Mosbeh AS, A B D Alsalam F, Ghonaim N, Abou-Bakr A. Role of Helicobacter pylori in common rosacea subtypes: a genotypic comparative study of Egyptian patients. J Dermatol. 2012;39:989-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Campanati A, Ganzetti G, Martina E, Giannoni M, Gesuita R, Bendia E, Giuliodori K, Sandroni L, Offidani A. Helicobacter pylori infection in psoriasis: results of a clinical study and review of the literature. Int J Dermatol. 2015;54:e109-e114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Azizzadeh M, Nejad ZV, Ghorbani R, Pahlevan D. Relationship between Helicobacter pylori infection and psoriasis. Ann Saudi Med. 2014;34:241-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Qayoom S, Ahmad QM. Psoriasis and Helicobacter pylori. Indian J Dermatol Venereol Leprol. 2003;69:133-134. [PubMed] [Cited in This Article: ] |
| 53. | Mesquita PM, Diogo A Filho, Jorge MT, Berbert AL, Mantese SA, Rodrigues JJ. Relationship of Helicobacter pylori seroprevalence with the occurrence and severity of psoriasis. An Bras Dermatol. 2017;92:52-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Mesquita PMD, Diogo A Filho, Jorge MT, Berbert ALCV, Mantese SAO, Rodrigues JJ. Comment on Helicobacter pylori seroprevalence and the occurrence and severity of psoriasis - Reply. An Bras Dermatol. 2017;92:585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Onsun N, Arda Ulusal H, Su O, Beycan I, Biyik Ozkaya D, Senocak M. Impact of Helicobacter pylori infection on severity of psoriasis and response to treatment. Eur J Dermatol. 2012;22:117-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Hizal M, Tüzün B, Wolf R, Tüzün Y. The relationship between Helicobacter pylori IgG antibody and autologous serum test in chronic urticaria. Int J Dermatol. 2000;39:443-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Galadari IH, Sheriff MO. The role of Helicobacter pylori in urticaria and atopic dermatitis. Skinmed. 2006;5:172-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Campanati A, Gesuita R, Giannoni M, Piraccini F, Sandroni L, Martina E, Conocchiari L, Bendia E, Di Sario A, Offidani A. Role of small intestinal bacterial overgrowth and Helicobacter pylori infection in chronic spontaneous urticaria: a prospective analysis. Acta Derm Venereol. 2013;93:161-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Yoshimasu T, Furukawa F. Eradication therapy for urticaria with high titers of anti H. pylori IgG antibody. Allergol Int. 2014;63:37-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Rigopoulos D, Katsambas A, Karalexis A, Papatheodorou G, Rokkas T. No increased prevalence of Helicobacter pylori in patients with alopecia areata. J Am Acad Dermatol. 2002;46:141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Behrangi E, Mansouri P, Agah S, Ebrahimi Daryani N, Mokhtare M, Azizi Z, Ramezani Ghamsari M, Rohani Nasab M, Azizian Z. Association between Helicobacter Pylori Infection and Alopecia Areata: A Study in Iranian Population. Middle East J Dig Dis. 2017;9:107-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Ljubojevic S, Lipozenčić J. Autoimmune bullous diseases associations. Clin Dermatol. 2012;30:17-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Magen E, Delgado JS. Helicobacter pylori and skin autoimmune diseases. World J Gastroenterol. 2014;20:1510-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 37] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Sagi L, Baum S, Agmon-Levin N, Sherer Y, Katz BS, Barzilai O, Ram M, Bizzaro N, SanMarco M, Trau H. Autoimmune bullous diseases the spectrum of infectious agent antibodies and review of the literature. Autoimmun Rev. 2011;10:527-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Mortazavi H, Hejazi P, Khamesipour A, Mohebali M, Ehsani AH, Mohammadi Y, Farahani IV, Amirzargar AA. Frequency of seropositivity against infectious agents amongst pemphigus vulgaris patients: a case-control study on Strongyloides stercoralis, Helicobacter pylori, Toxoplasma gondii, Leishmania major, and Epstein-Barr virus. Int J Dermatol. 2015;54:e458-e465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Novák J, Szekanecz Z, Sebesi J, Takáts A, Demeter P, Bene L, Sipka S, Csiki Z. Elevated levels of anti-Helicobacter pylori antibodies in Henoch-Schönlein purpura. Autoimmunity. 2003;36:307-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Grivceva-Panovska V, Grivceva Stardelova K, Serafimoski V. Henoch-Schönlein purpura in an adult patient: extragastric, cutaneous manifestation of helicobacter pylori infection. Prilozi. 2008;29:291-301. [PubMed] [Cited in This Article: ] |
| 68. | Hoshino C. Adult onset Schönlein-Henoch purpura associated with Helicobacter pylori infection. Intern Med. 2009;48:847-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Blecker U, Renders F, Lanciers S, Vandenplas Y. Syncopes leading to the diagnosis of a Helicobacter pylori positive chronic active haemorrhagic gastritis. Eur J Pediatr. 1991;150:560-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Muhsen K, Cohen D. Helicobacter pylori infection and iron stores: a systematic review and meta-analysis. Helicobacter. 2008;13:323-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 71. | Qu XH, Huang XL, Xiong P, Zhu CY, Huang YL, Lu LG, Sun X, Rong L, Zhong L, Sun DY. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J Gastroenterol. 2010;16:886-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 62] [Reference Citation Analysis (0)] |
| 72. | Huang X, Qu X, Yan W, Huang Y, Cai M, Hu B, Wu L, Lin H, Chen Z, Zhu C. Iron deficiency anaemia can be improved after eradication of Helicobacter pylori. Postgrad Med J. 2010;86:272-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 73. | Yuan W, Li Yumin, Yang Kehu, Ma Bin, Guan Quanlin, Wang D, Yang L. Iron deficiency anemia in Helicobacter pylori infection: meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2010;45:665-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 74. | Zhang ZF, Yang N, Zhao G, Zhu L, Zhu Y, Wang LX. Effect of Helicobacter pylori eradication on iron deficiency. Chin Med J (Engl). 2010;123:1924-1930. [PubMed] [Cited in This Article: ] |
| 75. | Azab SF, Esh AM. Serum hepcidin levels in Helicobacter pylori-infected children with iron-deficiency anemia: a case-control study. Ann Hematol. 2013;92:1477-1483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Kroot JJ, Tjalsma H, Fleming RE, Swinkels DW. Hepcidin in human iron disorders: diagnostic implications. Clin Chem. 2011;57:1650-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 77. | Ozkasap S, Yarali N, Isik P, Bay A, Kara A, Tunc B. The role of prohepcidin in anemia due to Helicobacter pylori infection. Pediatr Hematol Oncol. 2013;30:425-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Senkovich O, Ceaser S, McGee DJ, Testerman TL. Unique host iron utilization mechanisms of Helicobacter pylori revealed with iron-deficient chemically defined media. Infect Immun. 2010;78:1841-1849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Yokota S, Toita N, Yamamoto S, Fujii N, Konno M. Positive relationship between a polymorphism in Helicobacter pylori neutrophil-activating protein a gene and iron-deficiency anemia. Helicobacter. 2013;18:112-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Testerman TL, Morris J. Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol. 2014;20:12781-12808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 188] [Cited by in F6Publishing: 188] [Article Influence: 18.8] [Reference Citation Analysis (2)] |
| 81. | Afifi MT, Abd El-Aziz HK, Hamed NA, Barghash NA, Abdo A, Gamal M. Role of Helicobacter pylori in refractory iron deficiency anaemia. Br J Biomed Sci. 2009;66:133-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood. 2014;123:326-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 83. | Hershko C, Ronson A. Iron deficiency, Helicobacter infection and gastritis. Acta Haematol. 2009;122:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Kang JM, Kim N, Lee BH, Park HK, Jo HJ, Shin CM, Lee SH, Park YS, Hwang JH, Kim JW. Risk factors for peptic ulcer bleeding in terms of Helicobacter pylori, NSAIDs, and antiplatelet agents. Scand J Gastroenterol. 2011;46:1295-1301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Musumba C, Jorgensen A, Sutton L, Van Eker D, Moorcroft J, Hopkins M, Pritchard DM, Pirmohamed M. The relative contribution of NSAIDs and Helicobacter pylori to the aetiology of endoscopically-diagnosed peptic ulcer disease: observations from a tertiary referral hospital in the UK between 2005 and 2010. Aliment Pharmacol Ther. 2012;36:48-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | De Leest HT, Steen KS, Bloemena E, Lems WF, Kuipers EJ, Van de Laar MA, Bijlsma JW, Janssen M, Houben HH, Kostense PJ. Helicobacter pylori eradication in patients on long-term treatment with NSAIDs reduces the severity of gastritis: a randomized controlled trial. J Clin Gastroenterol. 2009;43:140-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 87. | Campuzano-Maya G. Hematologic manifestations of Helicobacter pylori infection. World J Gastroenterol. 2014;20:12818-12838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 45] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 88. | Lahner E, Persechino S, Annibale B. Micronutrients (Other than iron) and Helicobacter pylori infection: a systematic review. Helicobacter. 2012;17:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Andrès E, Loukili NH, Noel E, Kaltenbach G, Abdelgheni MB, Perrin AE, Noblet-Dick M, Maloisel F, Schlienger JL, Blicklé JF. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ. 2004;171:251-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 296] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 90. | Marino MC, de Oliveira CA, Rocha AM, Rocha GA, Clementino NC, Antunes LF, Oliveira RA, Martins AS, Del Puerto HL, D’Almeida V. Long-term effect of Helicobacter pylori eradication on plasma homocysteine in elderly patients with cobalamin deficiency. Gut. 2007;56:469-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337:1441-1448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 427] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 92. | García Pérez A, Valverde de La Osa J, Giménez Samper M, Alonso García I. Resolution of an autoimmune thrombocytopenic purpura after eradicating treatment of Helicobacter pylori. Sangre (Barc). 1999;44:387-388. [PubMed] [Cited in This Article: ] |
| 93. | Stasi R, Rossi Z, Stipa E, Amadori S, Newland AC, Provan D. Helicobacter pylori eradication in the management of patients with idiopathic thrombocytopenic purpura. Am J Med. 2005;118:414-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 94. | Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352:878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 319] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 95. | Suvajdzić N, Stanković B, Artiko V, Cvejić T, Bulat V, Bakrac M, Colović M, Obradović V, Atkinson HD. Helicobacter pylori eradication can induce platelet recovery in chronic idiopathic thrombocytopenic purpura. Platelets. 2006;17:227-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Jackson SC, Beck P, Buret AG, O’Connor PM, Meddings J, Pineo G, Poon MC. Long term platelet responses to Helicobacter pylori eradication in Canadian patients with immune thrombocytopenic purpura. Int J Hematol. 2008;88:212-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Jarque I, Andreu R, Llopis I, De la Rubia J, Gomis F, Senent L, Jiménez C, Martín G, Martínez JA, Sanz GF. Absence of platelet response after eradication of Helicobacter pylori infection in patients with chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2001;115:1002-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Michel M, Khellaf M, Desforges L, Lee K, Schaeffer A, Godeau B, Bierling P. Autoimmune thrombocytopenic Purpura and Helicobacter pylori infection. Arch Intern Med. 2002;162:1033-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 99. | Michel M, Cooper N, Jean C, Frissora C, Bussel JB. Does Helicobater pylori initiate or perpetuate immune thrombocytopenic purpura? Blood. 2004;103:890-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 100. | Estrada-Gómez RA, Parra-Ortega I, Martínez-Barreda C, Ruiz-Argüelles GJ. Helicobacter pylori infection and thrombocytopenia: a single-institution experience in Mexico. Rev Invest Clin. 2007;59:112-115. [PubMed] [Cited in This Article: ] |
| 101. | Satoh T, Pandey JP, Okazaki Y, Asahi A, Kawakami Y, Ikeda Y, Kuwana M. Single nucleotide polymorphism of interleukin-1beta associated with Helicobacter pylori infection in immune thrombocytopenic purpura. Tissue Antigens. 2009;73:353-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 102. | Asahi A, Nishimoto T, Okazaki Y, Suzuki H, Masaoka T, Kawakami Y, Ikeda Y, Kuwana M. Helicobacter pylori eradication shifts monocyte Fcgamma receptor balance toward inhibitory FcgammaRIIB in immune thrombocytopenic purpura patients. J Clin Invest. 2008;118:2939-2949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Kodama M, Kitadai Y, Ito M, Kai H, Masuda H, Tanaka S, Yoshihara M, Fujimura K, Chayama K. Immune response to CagA protein is associated with improved platelet count after Helicobacter pylori eradication in patients with idiopathic thrombocytopenic purpura. Helicobacter. 2007;12:36-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Byrne MF, Kerrigan SW, Corcoran PA, Atherton JC, Murray FE, Fitzgerald DJ, Cox DM. Helicobacter pylori binds von Willebrand factor and interacts with GPIb to induce platelet aggregation. Gastroenterology. 2003;124:1846-1854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 105. | Cicconi V, Carloni E, Franceschi F, Nocente R, Silveri NG, Manna R, Servidei S, Bentivoglio AR, Gasbarrini A, Gasbarrini G. Disappearance of antiphospholipid antibodies syndrome after Helicobacter pylori eradication. Am J Med. 2001;111:163-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA. 2006;295:1050-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 107. | Papadaki HA, Pontikoglou C, Stavroulaki E, Minadakis G, Eliopoulos DA, Pyrovolaki K, Skordilis P, Eliopoulos GD. High prevalence of Helicobacter pylori infection and monoclonal gammopathy of undetermined significance in patients with chronic idiopathic neutropenia. Ann Hematol. 2005;84:317-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 108. | Papadaki HA, Pontikoglou C, Eliopoulos DG, Pyrovolaki K, Spyridaki R, Eliopoulos GD. Helicobacter pylori infection is probably the cause of chronic idiopathic neutropenia (CIN)-associated splenomegaly. Am J Hematol. 2006;81:142-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 109. | Tursi A, Modeo ME. Monoclonal gammopathy of undetermined significance predisposing to Helicobacter pylori-related gastric mucosa-associated lymphoid tissue lymphoma. J Clin Gastroenterol. 2002;34:147-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 110. | Wolkersdörfer GW, Haase M, Morgner A, Baretton G, Miehlke S. Monoclonal gammopathy of undetermined significance and Russell body formation in Helicobacter pylori gastritis. Helicobacter. 2006;11:506-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 111. | Rajkumar SV, Kyle RA, Plevak MF, Murray JA, Therneau TM. Helicobacter pylori infection and monoclonal gammopathy of undetermined significance. Br J Haematol. 2002;119:706-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 112. | Galloway PH, Warner SJ, Morshed MG, Mikelberg FS. Helicobacter pylori infection and the risk for open-angle glaucoma. Ophthalmology. 2003;110:922-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 113. | Kurtz S, Regenbogen M, Goldiner I, Horowitz N, Moshkowitz M. No association between Helicobacter pylori infection or CagA-bearing strains and glaucoma. J Glaucoma. 2008;17:223-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 114. | Zeng J, Liu H, Liu X, Ding C. The Relationship Between Helicobacter pylori Infection and Open-Angle Glaucoma: A Meta-Analysis. Invest Ophthalmol Vis Sci. 2015;56:5238-5245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 115. | Casella AM, Berbel RF, Bressanim GL, Malaguido MR, Cardillo JA. Helicobacter pylori as a potential target for the treatment of central serous chorioretinopathy. Clinics (Sao Paulo). 2012;67:1047-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Liu B, Deng T, Zhang J. RISK FACTORS FOR CENTRAL SEROUS CHORIORETINOPATHY: A Systematic Review and Meta-Analysis. Retina. 2016;36:9-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 117. | Cotticelli L, Borrelli M, D’Alessio AC, Menzione M, Villani A, Piccolo G, Montella F, Iovene MR, Romano M. Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol. 2006;16:274-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 118. | Rahbani-Nobar MB, Javadzadeh A, Ghojazadeh L, Rafeey M, Ghorbanihaghjo A. The effect of Helicobacter pylori treatment on remission of idiopathic central serous chorioretinopathy. Mol Vis. 2011;17:99-103. [PubMed] [Cited in This Article: ] |
| 119. | Dang Y, Mu Y, Zhao M, Li L, Guo Y, Zhu Y. The effect of eradicating Helicobacter pylori on idiopathic central serous chorioretinopathy patients. Ther Clin Risk Manag. 2013;9:355-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 120. | Zavoloka O, Bezditko P, Lahorzhevska I, Zubkova D, Ilyina Y. Clinical efficiency of Helicobacter pylori eradication in the treatment of patients with acute central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1737-1742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 121. | Saccà SC, Vagge A, Pulliero A, Izzotti A. Helicobacter pylori infection and eye diseases: a systematic review. Medicine (Baltimore). 2014;93:e216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 122. | Saccà SC, Pascotto A, Venturino GM, Prigione G, Mastromarino A, Baldi F, Bilardi C, Savarino V, Brusati C, Rebora A. Prevalence and treatment of Helicobacter pylori in patients with blepharitis. Invest Ophthalmol Vis Sci. 2006;47:501-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 123. | Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, Falush D, Stamer C, Prugnolle F, van der Merwe SW. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 682] [Cited by in F6Publishing: 607] [Article Influence: 35.7] [Reference Citation Analysis (3)] |
| 124. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3302] [Cited by in F6Publishing: 3116] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 125. | Lai CY, Yang TY, Lin CL, Kao CH. Helicobacter pylori infection and the risk of acute coronary syndrome: a nationwide retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2015;34:69-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 126. | Chmiela M, Gajewski A, Rudnicka K. Helicobacter pylori vs coronary heart disease - searching for connections. World J Cardiol. 2015;7:187-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 127. | Wroblewski LE, Peek RM Jr. Targeted disruption of the epithelial-barrier by Helicobacter pylori. Cell Commun Signal. 2011;9:29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 128. | Chmiela M, Miszczyk E, Rudnicka K. Structural modifications of Helicobacter pylori lipopolysaccharide: an idea for how to live in peace. World J Gastroenterol. 2014;20:9882-9897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 129. | Chalubinski M, Wojdan K, Dorantowicz R, Jackowska P, Gorzelak P, Broncel M. Comprehensive insight into immune regulatory mechanisms and vascular wall determinants of atherogenesis - emerging perspectives of immunomodulation. Arch Med Sci. 2013;9:159-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 130. | Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6017] [Cited by in F6Publishing: 5821] [Article Influence: 264.6] [Reference Citation Analysis (0)] |
| 131. | Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103-3108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 432] [Cited by in F6Publishing: 440] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 132. | Li J, Li JJ, Li Q, Li Z, Qian HY. A rational connection of inflammation with peripheral arterial disease. Med Hypotheses. 2007;69:1190-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 133. | Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129-2138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1352] [Cited by in F6Publishing: 1423] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 134. | Perkov S, Paro MMK, Vidjak V, Flegar-Mestric Z. The Evaluation of New Biomarkers of Inflammation and Angiogenesis in Peripheral Arterial Disease. Current trends in atherogenesis. London: IntechOpen 2013; 97-120. [DOI] [Cited in This Article: ] |
| 135. | Vahdat K, Jafari SM, Pazoki R, Nabipour I. Concurrent increased high sensitivity C-reactive protein and chronic infections are associated with coronary artery disease: a population-based study. Indian J Med Sci. 2007;61:135-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 136. | Matsuura E, Kobayashi K, Matsunami Y, Shen L, Quan N, Makarova M, Suchkov SV, Ayada K, Oguma K, Lopez LR. Autoimmunity, infectious immunity, and atherosclerosis. J Clin Immunol. 2009;29:714-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 137. | Khodaii Z, Vakili H, Ghaderian SM, Najar RA, Panah AS. Association of Helicobacter pylori infection with acute myocardial infarction. Coron Artery Dis. 2011;22:6-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 138. | Rasmi Y, Raeisi S, Seyyed Mohammadzad MH. Association of inflammation and cytotoxin-associated gene a positive strains of helicobacter pylori in cardiac syndrome x. Helicobacter. 2012;17:116-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 139. | Zhang S, Guo Y, Ma Y, Teng Y. Cytotoxin-associated gene-A-seropositive virulent strains of Helicobacter pylori and atherosclerotic diseases: a systematic review. Chin Med J (Engl). 2008;121:946-951. [PubMed] [Cited in This Article: ] |
| 140. | Fischer W, Prassl S, Haas R. Virulence mechanisms and persistence strategies of the human gastric pathogen Helicobacter pylori. Curr Top Microbiol Immunol. 2009;337:129-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 141. | Manolakis A, Kapsoritakis AN, Potamianos SP. A review of the postulated mechanisms concerning the association of Helicobacter pylori with ischemic heart disease. Helicobacter. 2007;12:287-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 142. | Carter AM, Moayyedi P, Catto A, Heppell RM, Axon AT, Grant PJ. The influence of Helicobacter pylori status on circulating levels of the coagulation factors fibrinogen, von Willebrand factor, factor VII, and factor VIII. Helicobacter. 1996;1:65-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 143. | Ikeda A, Iso H, Sasazuki S, Inoue M, Tsugane S; JPHC Study Group. The combination of Helicobacter pylori- and cytotoxin-associated gene-A seropositivity in relation to the risk of myocardial infarction in middle-aged Japanese: The Japan Public Health Center-based study. Atherosclerosis. 2013;230:67-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 144. | Liu J, Wang F, Shi S. Helicobacter pylori Infection Increase the Risk of Myocardial Infarction: A Meta-Analysis of 26 Studies Involving more than 20,000 Participants. Helicobacter. 2015;20:176-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 145. | Hughes WS. An hypothesis: the dramatic decline in heart attacks in the United States is temporally related to the decline in duodenal ulcer disease and Helicobacter pylori infection. Helicobacter. 2014;19:239-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 146. | Oldenburg B, Diepersloot RJ, Hoekstra JB. High seroprevalence of Helicobacter pylori in diabetes mellitus patients. Dig Dis Sci. 1996;41:458-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 147. | Hsieh MC, Wang SS, Hsieh YT, Kuo FC, Soon MS, Wu DC. Helicobacter pylori infection associated with high HbA1c and type 2 diabetes. Eur J Clin Invest. 2013;43:949-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 148. | Bégué RE, Gómez R, Compton T, Vargas A. Effect of Helicobacter pylori eradication in the glycemia of children with type 1 diabetes: a preliminary study. South Med J. 2002;95:842-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 149. | Demir M, Gokturk HS, Ozturk NA, Kulaksizoglu M, Serin E, Yilmaz U. Helicobacter pylori prevalence in diabetes mellitus patients with dyspeptic symptoms and its relationship to glycemic control and late complications. Dig Dis Sci. 2008;53:2646-2649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 150. | Yang GH, Wu JS, Yang YC, Huang YH, Lu FH, Chang CJ. Gastric Helicobacter pylori infection associated with risk of diabetes mellitus, but not prediabetes. J Gastroenterol Hepatol. 2014;29:1794-1799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 151. | Horikawa C, Kodama S, Fujihara K, Hirasawa R, Yachi Y, Suzuki A, Hanyu O, Shimano H, Sone H. High risk of failing eradication of Helicobacter pylori in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2014;106:81-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 152. | Ibrahim A, Zaher T, Ghonemy TA, El-Azim SA, El-Azim MA, Ramadan A. Impact of cytotoxin-associated gene A of Helicobacter pylori strains on microalbuminuria in type 2 diabetes. Saudi J Kidney Dis Transpl. 2010;21:694-700. [PubMed] [Cited in This Article: ] |
| 153. | Chen Y, Blaser MJ. Association between gastric Helicobacter pylori colonization and glycated hemoglobin levels. J Infect Dis. 2012;205:1195-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 154. | Akanuma M, Yanai A, Sakamoto K, Hirata Y, Yamaji Y, Kawazu S, Maeda S. Influence of Helicobacter pylori eradication on the management of type 2 diabetes. Hepatogastroenterology. 2012;59:641-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 155. | Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 532] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 156. | Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW, Aiello AE. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35:520-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 157. | Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38:183-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 158. | Wang F, Fu Y, Lv Z. Association of Helicobacter pylori infection with diabetic complications: a meta-analysis. Endocr Res. 2014;39:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 159. | Yanik S, Doğan Z, Sarikaya M, Ergul B, Filik L. Helicobacter pylori eradication reduces microalbuminuria in type-2 diabetic patients: a prospective study. Acta Gastroenterol Belg. 2014;77:235-239. [PubMed] [Cited in This Article: ] |
| 160. | Vafaeimanesh J, Parham M, Seyyedmajidi M, Bagherzadeh M. Helicobacter pylori infection and insulin resistance in diabetic and nondiabetic population. ScientificWorldJournal. 2014;2014:391250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 161. | Chung GE, Heo NJ, Park MJ, Chung SJ, Kang HY, Kang SJ. Helicobacter pylori seropositivity in diabetic patients is associated with microalbuminuria. World J Gastroenterol. 2013;19:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 162. | Zhou X, Liu W, Gu M, Zhou H, Zhang G. Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway. J Gastroenterol. 2015;50:1027-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 163. | Aydemir S, Bayraktaroglu T, Sert M, Sokmen C, Atmaca H, Mungan G, Gun BD, Borazan A, Ustundag Y. The effect of Helicobacter pylori on insulin resistance. Dig Dis Sci. 2005;50:2090-2093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 164. | Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter. 2009;14:144-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 165. | Eshraghian A, Hashemi SA, Hamidian Jahromi A, Eshraghian H, Masoompour SM, Davarpanah MA, Eshraghian K, Taghavi SA. Helicobacter pylori infection as a risk factor for insulin resistance. Dig Dis Sci. 2009;54:1966-1970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 166. | Naja F, Nasreddine L, Hwalla N, Moghames P, Shoaib H, Fatfat M, Sibai A, Gali-Muhtasib H. Association of H. pylori infection with insulin resistance and metabolic syndrome among Lebanese adults. Helicobacter. 2012;17:444-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 167. | Chen TP, Hung HF, Chen MK, Lai HH, Hsu WF, Huang KC, Yang KC. Helicobacter Pylori Infection is Positively Associated with Metabolic Syndrome in Taiwanese Adults: a Cross-Sectional Study. Helicobacter. 2015;20:184-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 168. | Niemelä S, Karttunen T, Korhonen T, Läärä E, Karttunen R, Ikäheimo M, Kesäniemi YA. Could Helicobacter pylori infection increase the risk of coronary heart disease by modifying serum lipid concentrations? Heart. 1996;75:573-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 169. | Laurila A, Bloigu A, Näyhä S, Hassi J, Leinonen M, Saikku P. Association of Helicobacter pylori infection with elevated serum lipids. Atherosclerosis. 1999;142:207-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 170. | Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 171. | Wang Q, Yu C, Sun Y. The association between asthma and Helicobacter pylori: a meta-analysis. Helicobacter. 2013;18:41-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 172. | Zhou X, Wu J, Zhang G. Association between Helicobacter pylori and asthma: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:460-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 173. | Blaser MJ, Chen Y, Reibman J. Does Helicobacter pylori protect against asthma and allergy? Gut. 2008;57:561-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 174. | Amberbir A, Medhin G, Erku W, Alem A, Simms R, Robinson K, Fogarty A, Britton J, Venn A, Davey G. Effects of Helicobacter pylori, geohelminth infection and selected commensal bacteria on the risk of allergic disease and sensitization in 3-year-old Ethiopian children. Clin Exp Allergy. 2011;41:1422-1430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 175. | Oertli M, Sundquist M, Hitzler I, Engler DB, Arnold IC, Reuter S, Maxeiner J, Hansson M, Taube C, Quiding-Järbrink M. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122:1082-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 176. | Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, Gerhard M, Taube C, Müller A. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci USA. 2013;110:3047-3052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 177. | Memon AA, Hussein NR, Miendje Deyi VY, Burette A, Atherton JC. Vacuolating cytotoxin genotypes are strong markers of gastric cancer and duodenal ulcer-associated Helicobacter pylori strains: a matched case-control study. J Clin Microbiol. 2014;52:2984-2989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 178. | Cook KW, Letley DP, Ingram RJ, Staples E, Skjoldmose H, Atherton JC, Robinson K. CCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosa. Gut. 2014;63:1550-1559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 179. | Engler DB, Reuter S, van Wijck Y, Urban S, Kyburz A, Maxeiner J, Martin H, Yogev N, Waisman A, Gerhard M. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc Natl Acad Sci USA. 2014;111:11810-11815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 180. | Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2012;175:54-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 181. | Sheikh A, Strachan DP. The hygiene theory: fact or fiction? Curr Opin Otolaryngol Head Neck Surg. 2004;12:232-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 182. | Molina-Infante J, Gutierrez-Junquera C, Savarino E, Penagini R, Modolell I, Bartolo O, Prieto-García A, Mauro A, Alcedo J, Perelló A, Guarner-Argente C, Alcaide N, Vegas AM, Barros-García P, Murzi-Pulgar M, Perona M, Gisbert JP, Lucendo AJ; Upper GI Tract Study Group from the Spanish Gastroenterological Association (AEG). Helicobacter pylori infection does not protect against eosinophilic esophagitis: results from a large multicenter case-control study. Am J Gastroenterol. 2018; [Epub ahead of print]. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 183. | Doğan Z, Filik L, Ergül B, Sarikaya M, Akbal E. Association between Helicobacter pylori and liver-to-spleen ratio: a randomized-controlled single-blind study. Eur J Gastroenterol Hepatol. 2013;25:107-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 184. | Waluga M, Kukla M, Żorniak M, Bacik A, Kotulski R. From the stomach to other organs: Helicobacter pylori and the liver. World J Hepatol. 2015;7:2136-2146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 185. | Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. 2011;16:79-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 186. | Chen CX, Mao YS, Foster P, Zhu ZW, Du J, Guo CY. Possible association between Helicobacter pylori infection and nonalcoholic fatty liver disease. Appl Physiol Nutr Metab. 2017;42:295-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 187. | Boutari C, Perakakis N, Mantzoros CS. Association of Adipokines with Development and Progression of Nonalcoholic Fatty Liver Disease. Endocrinol Metab (Seoul). 2018;33:33-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 188. | Fukuda Y, Bamba H, Okui M, Tamura K, Tanida N, Satomi M, Shimoyama T, Nishigami T. Helicobacter pylori infection increases mucosal permeability of the stomach and intestine. Digestion. 2001;63 Suppl 1:93-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 189. | Sumida Y, Kanemasa K, Imai S, Mori K, Tanaka S, Shimokobe H, Kitamura Y, Fukumoto K, Kakutani A, Ohno T. Helicobacter pylori infection might have a potential role in hepatocyte ballooning in nonalcoholic fatty liver disease. J Gastroenterol. 2015;50:996-1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 190. | Basso D, Plebani M, Kusters JG. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2010;15 Suppl 1:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 191. | Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665-668. [PubMed] [Cited in This Article: ] |
| 192. | Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153-6160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 454] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 193. | Ki MR, Goo MJ, Park JK, Hong IH, Ji AR, Han SY, You SY, Lee EM, Kim AY, Park SJ. Helicobacter pylori accelerates hepatic fibrosis by sensitizing transforming growth factor-β1-induced inflammatory signaling. Lab Invest. 2010;90:1507-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 194. | Lee A, O’Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386-1397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 745] [Cited by in F6Publishing: 738] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 195. | Goo MJ, Ki MR, Lee HR, Yang HJ, Yuan DW, Hong IH, Park JK, Hong KS, Han JY, Hwang OK. Helicobacter pylori promotes hepatic fibrosis in the animal model. Lab Invest. 2009;89:1291-1303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 196. | Smyk DS, Koutsoumpas AL, Mytilinaiou MG, Rigopoulou EI, Sakkas LI, Bogdanos DP. Helicobacter pylori and autoimmune disease: cause or bystander. World J Gastroenterol. 2014;20:613-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 108] [Cited by in F6Publishing: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 197. | Hasni S, Ippolito A, Illei GG. Helicobacter pylori and autoimmune diseases. Oral Dis. 2011;17:621-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 198. | Goo MJ, Ki MR, Lee HR, Hong IH, Park JK, Yang HJ, Yuan DW, Hwang OK, Do SH, Yoo SE. Primary biliary cirrhosis, similar to that in human beings, in a male C57BL/6 mouse infected with Helicobacter pylori. Eur J Gastroenterol Hepatol. 2008;20:1045-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 199. | Shapira Y, Agmon-Levin N, Renaudineau Y, Porat-Katz BS, Barzilai O, Ram M, Youinou P, Shoenfeld Y. Serum markers of infections in patients with primary biliary cirrhosis: evidence of infection burden. Exp Mol Pathol. 2012;93:386-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 200. | Nilsson HO, Taneera J, Castedal M, Glatz E, Olsson R, Wadström T. Identification of Helicobacter pylori and other Helicobacter species by PCR, hybridization, and partial DNA sequencing in human liver samples from patients with primary sclerosing cholangitis or primary biliary cirrhosis. J Clin Microbiol. 2000;38:1072-1076. [PubMed] [Cited in This Article: ] |
| 201. | Bogdanos DP, Baum H, Gunsar F, Arioli D, Polymeros D, Ma Y, Burroughs AK, Vergani D. Extensive homology between the major immunodominant mitochondrial antigen in primary biliary cirrhosis and Helicobacter pylori does not lead to immunological cross-reactivity. Scand J Gastroenterol. 2004;39:981-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 202. | Krasinskas AM, Yao Y, Randhawa P, Dore MP, Sepulveda AR. Helicobacter pylori may play a contributory role in the pathogenesis of primary sclerosing cholangitis. Dig Dis Sci. 2007;52:2265-2270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 203. | Casswall TH, Németh A, Nilsson I, Wadström T, Nilsson HO. Helicobacter species DNA in liver and gastric tissues in children and adolescents with chronic liver disease. Scand J Gastroenterol. 2010;45:160-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 204. | Rabelo-Gonçalves EM, Roesler BM, Zeitune JM. Extragastric manifestations of Helicobacter pylori infection: Possible role of bacterium in liver and pancreas diseases. World J Hepatol. 2015;7:2968-2979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 205. | Tu QV, Okoli AS, Kovach Z, Mendz GL. Hepatocellular carcinoma: prevalence and molecular pathogenesis of Helicobacter spp. Future Microbiol. 2009;4:1283-1301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 206. | Avenaud P, Marais A, Monteiro L, Le Bail B, Bioulac Sage P, Balabaud C, Mégraud F. Detection of Helicobacter species in the liver of patients with and without primary liver carcinoma. Cancer. 2000;89:1431-1439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 207. | Nilsson HO, Mulchandani R, Tranberg KG, Stenram U, Wadström T. Helicobacter species identified in liver from patients with cholangiocarcinoma and hepatocellular carcinoma. Gastroenterology. 2001;120:323-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 208. | Dore MP, Realdi G, Mura D, Graham DY, Sepulveda AR. Helicobacter infection in patients with HCV-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Dig Dis Sci. 2002;47:1638-1643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 209. | Verhoef C, Pot RG, de Man RA, Zondervan PE, Kuipers EJ, IJzermans JN, Kusters JG. Detection of identical Helicobacter DNA in the stomach and in the non-cirrhotic liver of patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2003;15:1171-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 210. | Pellicano R, Mazzaferro V, Grigioni WF, Cutufia MA, Fagoonee S, Silengo L, Rizzetto M, Ponzetto A. Helicobacter species sequences in liver samples from patients with and without hepatocellular carcinoma. World J Gastroenterol. 2004;10:598-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 74] [Cited by in F6Publishing: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 211. | Esmat G, El-Bendary M, Zakarya S, Ela MA, Zalata K. Role of Helicobacter pylori in patients with HCV-related chronic hepatitis and cirrhosis with or without hepatocellular carcinoma: possible association with disease progression. J Viral Hepat. 2012;19:473-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |