Published online Jul 28, 2018. doi: 10.3748/wjg.v24.i28.3171
Peer-review started: May 16, 2018
First decision: June 6, 2018
Revised: July 8, 2018
Accepted: July 16, 2018
Article in press: July 16, 2018
Published online: July 28, 2018
To study the published evidence on the impact of colectomy in preventing recurrent primary sclerosing cholangitis (rPSC).
An unrestricted systematic literature search in PubMed, EMBASE, Medline OvidSP, ISI Web of Science, Lista (EBSCO) and the Cochrane library was performed on clinical studies investigating colectomy in liver transplantation (LT) recipients with and without rPSC in the liver allograft. Study quality was evaluated according to a modification of the methodological index for non-randomized studies (MINORS) criteria. Primary endpoints were the impact of presence, timing and type of colectomy on rPSC. Overall presence of inflammatory bowel disease (IBD), time of IBD diagnosis, posttransplant IBD and immunosuppressive regimen were investigated as secondary outcome.
The literature search yielded a total of 180 publications. No randomized controlled trial was identified. Six retrospective studies met the inclusion criteria of which 5 studies were graded as high quality articles. Reporting of IBD was heterogenous but in four publications, either inflammatory bowel disease, ulcerative colitis or in particular active colitis post-LT significantly increased the risk of rPSC. The presence of an intact (i.e., retained) colon at LT was identified as risk factor for rPSC in two of the high quality studies while four studies found no effect. Type of colectomy was not associated with rPSC but this endpoint was underreported (only in 33% of included studies). Neither tacrolimus nor cyclosporine A yielded a significant benefit in disease recurrence of primary sclerosing cholangitis (PSC).
The data favours a protective role of pre-/peri-LT colectomy in rPSC but the current evidence is not strong enough to recommend routine colectomy for rPSC prevention.
Core tip: There are no known treatments that can alter the development and/or progression of recurrent primary sclerosing cholangitis (rPSC) after liver transplantation (LT). Shared leukocyte recruitment pathways of the gut-liver axis, bacterial translocation into the portal circulation from an inflamed gut and intestinal dysbiosis might contribute to the pathogenesis of rPSC. Indeed, inflammatory bowel disease, ulcerative colitis and in particular active colitis post-LT significantly increase the risk of rPSC and the available data favours a protective role of pre-/peri-LT colectomy in rPSC. Prospective studies and randomized trials are needed to further elucidate a possible mechanistic link between retained colon and rPSC.
- Citation: Buchholz BM, Lykoudis PM, Ravikumar R, Pollok JM, Fusai GK. Role of colectomy in preventing recurrent primary sclerosing cholangitis in liver transplant recipients. World J Gastroenterol 2018; 24(28): 3171-3180
- URL: https://www.wjgnet.com/1007-9327/full/v24/i28/3171.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i28.3171
Primary sclerosing cholangitis (PSC), a chronic inflammatory disease of the liver resulting in multi-focal strictures of the biliary tree[1], is estimated to recur in 20%-25% of liver allograft recipients during the first decade after liver transplantation (LT) with a mean interval to recurrent PSC (rPSC) of 6 mo to 5 years[2,3]. The diagnosis of rPSC is usually based on the Mayo criteria consisting of histological or cholangiographic features of PSC more than 90 d after LT in the absence of hepatic artery thrombosis/ stenosis, chronic ductopenic rejection, ABO incompatibility, anastomotic biliary strictures only, or non-anastomotic biliary strictures within 90 d after LT[4].
LT remains the only therapeutic option in patients with end stage liver disease from PSC and there are no known treatments that can alter the development and/or progression of rPSC after LT which oftentimes requires re-transplantation[1,5]. Different risk factors for rPSC have been identified by individual groups: Male recipient gender, cholangiocarcinoma identified on explant histology, extended criteria donor allograft, first-degree-related donor and acute cellular rejection[6]. Although the association of ulcerative colitis (UC) and PSC is widely acknowledged[7], there is a lack of understanding with regards to the effect of UC on the risk of developing rPSC in LT recipients.
Some authors have suggested that the presence of the colon at LT is associated with an increased risk of rPSC[8]. Three recent discoveries support a mechanistic link between the colon and PSC, and the same pathways that drive PSC in the non-transplant population might lead to the development of rPSC after LT. First, intestinal inflammation may fuel hepatic inflammation via shared leukocyte recruitment pathways of the gut-liver axis[9]. Homing molecules such as mucosal vascular addressin cell adhesion molecule 1 (MADCAM1) and the gut-associated chemokine (C-C motif) ligand 25, which are normally restricted to the gut, are abnormally upregulated in the liver of PSC patients and facilitate the recruitment of enteric-primed destructive α4β7-positive lymphocytes into the liver[10,11]. Additionally, as a consequence of intestinal inflammation, failure of the gut mucosal barrier with translocation of enteric pathogens to the portal circulation can drive hepatic inflammation via epithelial pattern recognition receptor activation[9]. Third, independent from inflammatory bowel disease (IBD), intestinal dysbiosis was found to be associated with PSC, indicating that the intestinal microbiome might play a role in PSC pathogenesis[12]. This article reviews the published evidence on the role of colectomy in preventing rPSC in LT recipients.
A comprehensive literature search was conducted using the databases of Pubmed, EMBASE, Medline OvidSP, ISI Web of Science, Lista (EBSCO) and the Cochrane library. Title, abstract, keywords and full-text of articles published in the time period from 1945 until 29th of April 2018 were screened for the search terms which were stratified in blocks with rPSC (block 1), colectomy and ulcerative colitis (block 2), and liver transplant, immunology and inflammatory bowel disease (block 3). All combinations were explored with one term each from block 1 and 2, subsequently combined with search terms from block 3 (Supplementary Figure 1). All languages and all publications on human subjects were considered. This unrestricted and unfiltered literature search was independently conducted by 2 authors (Buchholz BM and Lykoudis PM). Any disagreement was resolved by a third author (Fusai GK). The references of the identified publications were assessed for further reports pertinent to the topic.
Articles were selected for final review if they compared LT recipients with and without rPSC, and reported at least one of the defined outcome endpoints. If the same patient cohort was included in multiple publications, only the most recent update with the largest number of patients was retained.
The quality of publications identified by the above literature search was evaluated using a modification of the methodological index for non-randomized studies (MINORS) criteria, consisting of 9 items categorized into study design (clearly stated aim, inclusion of consecutive patients, prospective collection of data, endpoints appropriate to the aim of the study), data recording and data quality (appropriate follow-up period, loss to follow-up reporting, baseline equivalence of groups) and study assessment (outcome evaluation bias, adequate statistical analysis)[13]. The items were scored as 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate) with the ideal score being 18. The study evaluation for the MINORS criteria was independently performed by 2 authors (Buchholz BM and Lykoudis PM), and discrepancies were resolved by consensus with a neutral referee (Fusai GK). Given the above outlined natural history of rPSC, a follow-up time of 5 years after liver transplantation was set as appropriate.
The primary outcome was the impact of presence (yes or no), timing (pre-/peri-LT or post-LT) and type of colectomy (pan-proctocolectomy, segmental/subtotal or other) on rPSC in the liver allograft. The following endpoints were assessed as secondary outcomes: (1) Presence of IBD, time of IBD diagnosis (pre-LT, de novo), and post-transplant presence of IBD; and (2) primary and secondary immunosuppression. Further parameters were collected on recipient characteristics (recipient age at LT, recipient gender, MELD at LT, time to diagnosis of rPSC, and follow-up period) and donor demographics [donor age, donor gender and type of donation (DBD, DCD)]. The data of interest was extracted and tabulated from the relevant studies texts, tables and figures, by 2 independent authors (Buchholz BM and Lykoudis PM). Data are reported as n (%) and median (interquartile range) unless otherwise indicated.
The statistical methods of this study were reviewed by one of the authors (Lykoudis PM) Apart of the descriptive median MINORS score, no meaningful statistical analysis was feasible in this systematic review given the heterogeneity of the statistical methods applied for the described endpoints in the included original studies.
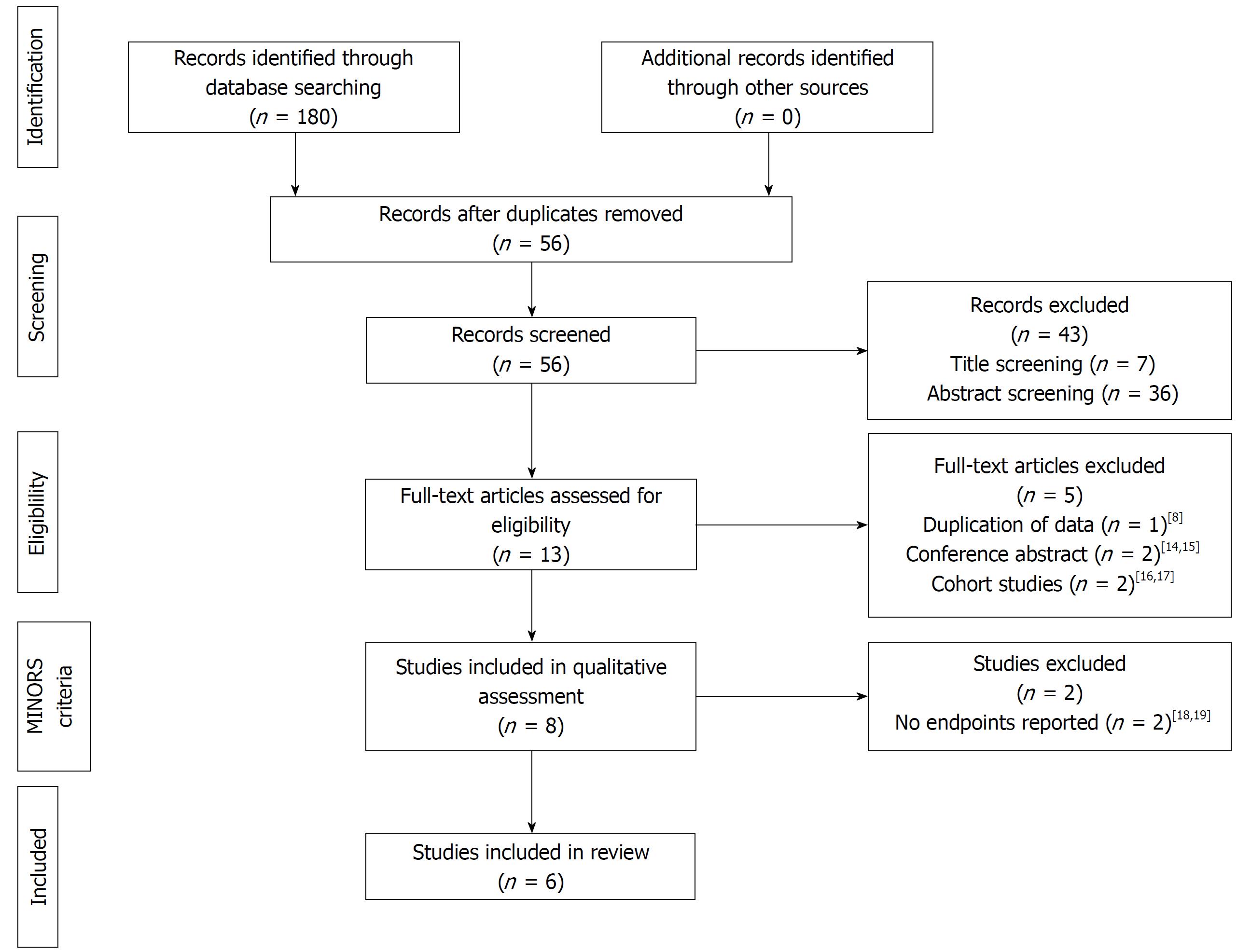
The systematic literature search of the databases yielded a total of 180 publications (Figure 1). No article was identified in the Cochrane Library or Lista (EBSCO) and no additional records were retrieved from the manual search of the reference lists. Following removal of duplicates, 56 studies were screened of which 43 were excluded on title screening (n = 7) and abstract screening (n = 36) as not pertinent to the topic. The full-text of the remaining 14 publications was assessed for eligibility. Publications reporting on a smaller proportion of the same patient population as larger studies[8], conference abstracts[14,15] and cohort studies[16,17] were further excluded leaving a selection of 8 studies eligible for quality assessment.
All retrieved publications had an observational and retrospective design; no randomized controlled trial was identified. Two studies did not report any relevant endpoints and were therefore excluded in retrospect[18,19]. Hence, a total of 6 reports comparing LT recipients with and without rPSC in contemporary groups were assessed by MINORS criteria[20-25]. The median MINORS score was 11 (IQR 8.75-12.25), with incomplete outcome reporting, lack of equivalent groups and failure to adequately state loss to follow up accounting primarily for lower scores (Table 1). There was evidence in outcome evaluation bias in all but two studies[20,25]. The study design was overall good with reporting of consecutive patients in all publications and a clearly formulated study aim in most reports, but only one study reported all endpoints[25]. Follow-up period and statistical methods were appropriate apart from one study published by Gelley et al[22]. The latter study therefore scored overall low quality (5 points) while 5 publications were deemed of good quality with a MINORS score ranging from 10 to 13. These included single-centre experiences[20,21,24] as well as two large multi-centre cohorts[23,25]. The patient cohort of the multi-centre report by Ravikumar et al[25] overlaps in part with two single-centre studies[20,21] but does not completely capture the patient data; therefore, all three publications were retained.
| Study design | Data recording and data quality | Study assessment | MINORS score | ||||||||
| Ref. | Year | Study aim | Consecutive patients | Data collection | Reported endpoints | Equivalent groups | Follow-up period | Loss tofollow-up | Outcome evaluation bias | Statistical methods | |
| Cholongitas et al[21] | 2008 | 2 | 2 | 1 | 1 | 0 | 2 | 0 | 1 | 2 | 11 |
| Alabraba et al[20] | 2009 | 2 | 2 | 1 | 1 | 0 | 2 | 0 | 2 | 2 | 12 |
| Moncrief et al[24] | 2010 | 1 | 2 | 1 | 1 | 0 | 2 | 0 | 1 | 2 | 10 |
| Gelley et al[22] | 2014 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 5 |
| Ravikumar et al[25] | 2015 | 2 | 2 | 1 | 2 | 0 | 2 | 0 | 2 | 2 | 13 |
| Hildebrand et al[23] | 2016 | 2 | 2 | 1 | 1 | 0 | 2 | 0 | 1 | 2 | 11 |
The retrieved studies on rPSC were published within the last decade and captured patient data from 1986 to 2011. The indication for LT was PSC in all patients and LT recipients were followed up for a median time of 5.7 to 9.1 years (Table 2). Follow-up was restricted by patient death but extended up to 8 to 22 years in the various studies. Diagnosis of rPSC was guided by Mayo criteria[4] in all studies and median time to diagnosis of rPSC ranged from 3.4 to 5 years as reported by 4 studies.
| Ref. | Year | Study period | Number n(rPSC vs no rPSC) | Time to diagnosis of rPSC (yr) | Follow-up (yr) | Recipient age at LT (yr) | Recipient gender (male) | MELD at LT | Donor age (yr) | Donor gender (male) | Donor type (DBD) |
| Cholongitas et al[21] | 2008 | 1989-2004 | 7 (13) vs 46 (87) | 5 (0.3-10)1 | 9.1 (1-15.4)1 | 35 ± 15 vs 42 ± 132 | 5 (71) vs 25 (54) | NR | 33 ± 18 vs 44 ± 142 | 6 (86) vs 27 (59) | NR |
| Alabraba et al[20] | 2009 | 1986-2006 | 61 (23) vs 202 (77) | 4.6 (0.5-12.9)1 | 6.9 (0-19.9)1 | 48 (16-72)1 | 50 (82) vs 149 (74) | NR | NR | NR | 61 (100) vs 202 (100) |
| Moncrief et al[24] | 2010 | 1989-2006 | 15 (25) vs 44 (75) | 3.4 (1.5-5.5) | 5.7 (2.8-8.8) | 45 (36-53) vs 47 (37-52) | 13 (87) vs 33 (75) | 18 (13-21) vs 14 (10-21) | NR | NR | NR |
| Gelley et al[22] | 2014 | 1995-2011 | 6 (24) vs 19 (76) | NR | NR | 27 ± 7 vs 37 ± 122 | 3 (50) vs 13 (68) | 16 ± 5 vs 11 ± 42 | 39 ± 14 vs 35 ± 112 | NR | NR |
| Ravikumar et al[25] | 2015 | 1990-2010 | 81 (14) vs 484 (86) | NR | 9 (5-14) | 43 (34-55) vs 49 (41-57) | 61 (75) vs 344 (71) | NR | 39 (28-53) vs 43 (32-54) | 46 (57) vs 268 (55) | 80 (99) vs 467 (96) |
| Hildebrand et al[23] | 2016 | 1990-2006 | 62 (20) vs 243 (80) | 4.6 (0.5-14.3)3 | 8.2 (0-22)3 | 39 ± 11.5 vs 39 ± 10.82 | 48 (77) vs 160 (66) | 16 ± 6 vs 14 ± 72 | 43.6 ± 16 vs 40.1 ± 16.92 | NR | NR |
Demographics were analyzed and described by different statistical methods in the various studies and can therefore only be compared in a descriptive manner. rPSC was more prevalent in patients undergoing a first liver transplant at a younger age. Similarly, there was a predominance of male gender in the rPSC group, with a higher MELD at LT. Liver grafts from younger donors were also associated with rPSC. Donor gender and donor type were rarely reported; however, the available data suggested no relevant differences between groups. It is noticeable that almost exclusively livers from donors after brain death were utilized, a fact that is likely explained by era of transplantation prior to increased transplantation of organs from donors after circulatory death.
Recipient factors such as younger age and advanced severity of liver disease were described as independent risk factors for rPSC (Table 3) but are given at the time of transplantation; it is logical that these cannot be addressed with the aim to improve outcome. The studies further suggest that a better graft selection with avoidance of donors with extended criteria, higher age and higher body mass index (BMI) could aid in preventing rPSC.
| Ref. | Year | Colectomy | IBD-specific risk factor for rPSC | non-IBD risk factor for rPSC |
| 1Cholongitas et al[21] | 2008 | No effect | Presence of UC post-LT | Need for maintenance steroids post-LT |
| Alabraba et al[20] | 2009 | Protective (pre- and peri-LT) | Presence of intact (i.e., retained) colon (independent of IBD or UC) | EDC grafts |
| Moncrief et al[24] | 2010 | No effect | None | At least one episode of ACR; CMV mismatch |
| Gelley et al[22] | 2014 | No effect | Severe active IBD | Higher donor BMI Younger recipient age |
| 1Ravikumar et al[25] | 2015 | Protective (univariate analysis) | Presence of UC post-LT | Younger recipient age |
| Hildebrand et al[23] | 2016 | No effect | IBD, UC, and in particular active colitis post-LT | Higher donor age; Higher INR at LT |
PSC develops in 2.4% to 7.5% of patients with inflammatory bowel disease (IBD) while 70% to 85% of patients with PSC will manifest symptoms of IBD during their lifetime[26]. Of the two distinct subtypes of IBD, ulcerative colitis has the strongest association with PSC accounting for 90% of the cases[7]. In line with this, data from Hildebrand et al[23] indicate that rates of IBD exceed the presence of UC in both rPSC and non-rPSC groups by approximately 10%. The majority of the studies (3 out of 4) stated a higher rate of IBD in LT recipients with rPSC ranging between 86% to 100% compared to the non-rPSC group (71%-79%) with the exception of Alabraba et al[20] who observed an equivalent rate of IBD presence between groups reaching 72% and 70%, respectively (Table 4). Two studies exclusively reported on ulcerative colitis (UC) with a higher prevalence of UC in rPSC (78% -100%) vs non-rPSC (52%-56%)[21,25].
| Ref. | Year | Presence of IBD(ever) | Time of IBD diagnosis (pre-LT/de novo) | Presence of IBD (post-LT) | Time of colectomypre- and peri-LT/post-LT | Type of colectomy | Primary immunosuppression | Secondary immunosuppression |
| 1Cholongitas et al[21] | 2008 | 7 (100) vs 26 (56) | 5 (71)/2 (29) vs 25 (54)/1 (2) | 7 (100) vs 26 (56) | 0 (0)/1 (14) vs 6 (13)/6 (13) | NR | TAC 2 (29) vs 25 (54) CyA 5 (71) vs 21 (46) | AZA 3 (43) vs 22 (48) OKT3 or ATG 2 (29) vs 11 (24) |
| Alabraba et al[20] | 2009 | 39 (72) vs 123 (70) | NR | NR | 1 (2)/14 (23) vs 28 (16)/18 (10) | Panproctocolectomy 7 (13) vs 15 (8) Segmental + subtotal 5 (9) vs 21 (12) Ileoanal pouch 3 (6) vs 10 (6) | TAC 16 (26) vs 104 (51) CyA 44 (72) vs 95 (47) | None 1 (2) vs 3 (2) OKT3 0 (0) vs 2 (2) |
| Moncrief et al[24] | 2010 | 15 (100) vs 33 (75) | 11 (73)/4 (27) vs 31 (70)/2 (5) | 13 (87) vs 24 (55) | 1 (7)/NR vs 9 (20)/NR | NR | TAC 7 (47) vs 28 (64) CyA 8 (53) vs 14 (32) | NR |
| Gelley et al[22] | 2014 | 6 (100) vs 15 (79) | 5 (83)/1 (17) vs 14 (74)/1 (5) | 6 (100) vs 15 (79) | 0 (0)/2 (34) vs 4 (21)/2 (11) | NR | TAC 4 (67) vs 14 (74) CyA 2 (33) vs 5 (26) | NR |
| 1Ravikumar et al[25] | 2015 | 51 (78) vs 220 (52) | 42 (65)/9 (13) vs 193 (46)/27 (6) | 43 (66) vs 181 (42) | 5 (6)/14 (17) vs 40 (8)/46 (10) | Panproctocolectomy: 2 (2) vs 22 (5) Segmental + subtotal: 16 (20) vs 41 (8) Other: 1 (1) vs 17 (4) | TAC 36 (44) vs 330 (68) CyA 20 (25) vs 55 (11) | AZA 26 (32) vs 212 (44) MMF 10 (12) vs 67 (14) Steroids 38 (47) vs 285 (59) |
| Hildebrand et al[23] | 2016 | 53 (86) vs 167 (71) | NR | 48 (77) vs 138 (59) | NR | NR | TAC 41 (67) vs 150 (66) CyA 32 (53) vs 124 (55) | Steroids 41 (70) vs 133 (60) |
The available results of 4 studies on timing of IBD/UC diagnosis demonstrates that IBD/UC occurs more frequently in LT recipients with rPSC both prior to transplantation (65%-83% vs 46%-74%) and de novo after transplantation (13%-29% vs 2%-6%). There is also consensus amongst reports that the overall presence of IBD/UC after LT is considerably higher in cases of rPSC (65%-100% vs 42%-79%). IBD, either by presence of UC post-LT[21,23,25] or severe active IBD[22,23], was reported as an independent risk factor for disease recurrence of PSC in the liver allograft in four studies which included the two multi-centre studies (Table 3).
None of the identified publications focused on the role of colectomy in prevention of rPSC, but some of them examined it within a broader assessment of risk factors for rPSC. Colectomy at any time was carried out at lower (7%-14% vs 20%-26%)[21,24] or equal rates (23%-34% vs 18%-32%)[20,22,25] in LT recipients with rPSC compared to the PSC cohort without recurrent disease in the liver allograft. The timing of colectomy, reported in 5 out of 6 studies, differed consistently between the two groups: colectomy in patients with rPSC was performed mainly post-LT (14%-34% vs 10%-13%) and less often pre- and peri-LT (0-7% vs 8%-21%) (Table 4). Two of the high quality studies conclude that (pre-/peri-LT) colectomy has a protective effect on rPSC in the liver allograft as the presence of a non-resected colon at transplantation was identified as a risk factor for rPSC on univariable analysis (Table 3)[20,25]. The remaining four publications reported no significant effect of colectomy on rPSC[21-24]. Still, as a trend towards a role of colectomy in preventing rPSC was found in some of the aforementioned studies, the lack of significance may be related to a type 2 statistical error as frequently seen in underpowered studies.
Data on the type of colectomy in the identified publications is scarce and contradictory. Segmental/subtotal colectomy was more often described in the multi-centre study by Ravikumar et al[25] while pan-proctocolectomy (specifically post-LT) was the preferred colorectal approach in LT recipients with rPSC in the work of Alabraba et al[20]. Pan-proctocolectomy was compared to other forms of colectomy in which remnant colorectal tissue remained in situ (segmental/subtotal colectomies and ileoanal pouch). Disregarding timing of colectomy, the overall assessment of type of colectomy revealed no significant difference in the risk for rPSC in LT recipients in both of the aforementioned studies. However, in the subgroup of post-LT colectomies, the risk of rPSC was significantly lower in patients who had undergone either pan-proctocolectomy or subtotal colectomy compared with LT recipients with ileoanal pouch[20].
Tacrolimus and cyclosporine A were used as mainstay immunosuppression in all identified studies. In the group with rPSC, the main choice of calcineurin inhibitor (CNI) was cyclosporine A (71%-72%) in two reports[20,21] and tacrolimus (44%-67%) in three publications[22,23,25], while the two drugs were utilized in a similar fashion in the study of Moncrief et al[24] (Table 4). Compared to LT recipients without rPSC, cyclosporine A was more often administered in the group with rPSC in 4 studies (25%-72% vs 11%-47%)[20,21,24,25] and to an equal extent in the two remaining publications (33%-53% vs 26%-55%)[22,23]. The choice of CNI had no effect on the risk of rPSC in 5 out of 6 studies while Ravikumar et al[25] found that maintenance immunosuppression with cyclosporine A carried a 2-fold increased risk for rPSC on univariate analysis; however, the association between cyclosporine A use and rPSC was lost when adjusted for transplantation era and in the multivariable model.
Azathioprine and steroids were less frequently used long-term in LT recipients with rPSC in the multi-centre study by Ravikumar et al[25], yet none of secondary immunosuppressant agents used were significantly associated with rPSC. Three further publications mention choice and frequency of secondary immunosuppression and describe no difference in the use of OKT3, anti-thymocyte globulin and steroids between the two study groups[20,21,23].
PSC is a complex liver disease characterized by chronic inflammation of the biliary epithelium[1]. The pathogenesis is not fully understood but immune dysregulation in genetically susceptible individuals is thought to play a major role[27]. Noticeably, PSC recurs in only one fifth of the transplant population[28] which implies that the natural course of PSC is altered after LT. As universally required in solid organ transplantation, management of the transplanted patient includes immunosuppression which might partially downregulate the immune pathways involved in the development of PSC. The CNI tacrolimus and cyclosporine A remain the cornerstone of modern treatment regimens to reduce allograft rejection[29]. Both drugs inhibit different stages of the T-lymphocyte and B-lymphocyte activation cycles by interfering with the interleukin-2 pathway but bind to diverse intracellular target molecules[30] and differ in potency and spectrum of immune modulation[31].
However, none of the immunosuppressive regimen utilized in the selected studies conveyed a significant benefit in disease recurrence of PSC. Assuming that the analyzed studies reflect real world practise and that no true differences exist between the two CNI affecting the development of rPSC, this would imply that both drugs either do not target the hypothesized immune mechanisms of rPSC or do so in a similar fashion. The latter seems unlikely given the fact that the use of tacrolimus is associated with increased IBD activity and the development of de novo IBD post-LT[32]. In line with this, a large Nordic cohort study identified tacrolimus as independent risk factor for rPSC[17]. On the other hand, bearing in mind that tacrolimus is more potent in preventing liver allograft rejection[31] and that acute cellular rejection can drive rPSC (Table 3)[24,33], one would choose tacrolimus as the preferred immunosuppressive regimen with regard to prevention of rPSC. It remains possible that the significance of cylosporine A as a risk factor for rPSC was lost in the work of Ravikumar et al[25] due to high statistical dependence with another variable, something that could be further investigated by Spearman’s rank correlations in future studies[34].
Suggestions have been made that the selection of better quality grafts could aid in preventing PSC[22,23,25]; however, the translation of such an approach might prove difficult in the era of organ shortage as more marginal grafts are utilized to expand the donor pool[35]. It is especially recognized that the donor age has significantly increased over the past decades and continues to rise[36]. The impact of new technologies such as normothermic machine perfusion of marginal liver grafts on rPSC will have to be awaited[37]. Yet, it is encouraging that newer studies have reported that liver grafts from donors after circulatory death can be safely utilized in LT candidates with PSC and, although the incidence of ischemic-type biliary lesions was increased in the donor group after circulatory death compared to donors after brain death, the incidence of anastomotic strictures or rPSC was not different between donor groups[38].
Considering the overall limitations in donor selection, the main targets in preventing rPSC appear to be factors in the management of the LT recipient such as frequently co-existing inflammatory bowel disease. Interestingly, it has been recently described that the coexistence of both ulcerative colitis and PSC in the same patient is associated with an increased risk of native liver disease progression and either need for liver transplantation or death[39]. Given the overall increased incidence of IBD in rPSC, it may therefore be hypothesized that the same pathways that drive PSC in the non-transplant population are engaged in the development of rPSC after LT such as shared leukocyte recruitment pathways of the gut-liver axis[10,11] and bacterial translocation into the portal circulation from an inflamed gut[9]. While it was reported that active colitis and the need for maintenance steroids (> 3 mo), mainly reflecting UC activity and not graft dysfunction, significantly predispose to rPSC[21,23], the distinct impact of disease severity of IBD on rPSC has not been well assessed in the present studies. Cholongitas et al[21] are the only study that reported in more detail that UC disease extension (distal or total), UC activity per se, and the post-LT course of UC in terms of severity, number of admissions for UC and utilization of immunosuppression for UC exacerbation was not associated with rPSC. Controversy however exists about the clinical course of pre-existing IBD post-LT in general as conflicting data either point towards ameliorated bowel disease[40,41] or reports poorly-controlled IBD exacerbation[42,43]. De novo IBD after LT occurs at a variable incidence of 1.3%-31.3%[42]. The 10-year risk to develop de novo IBD in the transplanted PSC population is estimated to be 14%-30% with a median time to onset of 4 years[44]. Even though de novo IBD usually presents later after transplantation and responds better to medical therapy compared to IBD recurrence[43], it has paradoxically been shown in the multi-centre study by Ravikumar et al[25] that the diagnosis of de novo UC was associated with a higher risk of rPSC compared to UC diagnosis prior LT. Liver cirrhosis attenuates T-cell function and the aforementioned finding raises the interesting question whether rPSC is driven by restored T-cell function as it has been implemented for increased IBD disease activity of previously quiescent colitis[45].
The leading indications for colectomy in patients with concomitant UC and PSC are the presence of colorectal neoplasia and severe colonic inflammation in both the pre- and post-transplant setting[32,46]. The overall necessity for colectomy due to disease activity of UC was found to be reduced in patients with advanced PSC needing liver transplantation[47]. Yet, colectomy prior to but not after PSC diagnosis is beneficial leading to a decreased risk of liver transplantation or death[48]. It was therefore especially relevant to analyze the identified studies regarding the impact of timing and type of colectomy on rPSC as it can be hypothesized that the prognosis of the liver allograft is altered depending on exposure to the UC environment or complete eradication of intestinal inflammatory factors and the intestinal microbiome prior to graft implantation. Within the limitations of retrospective publications, the data overall supported a protective role of colectomy in rPSC if carried out prior to or at the time of LT. The fact that intestinal dysbiosis has been implemented in the pathogenesis of PSC[12] and therefore possibly also rPSC, could explain why the presence of an intact (i.e., retained) colon post-LT significantly increased the risk of rPSC in the Birmingham series independently of colonic inflammation[20]. The included studies have not investigated whether the specific subpopulation of LT recipients with rPSC undergoing re-transplantation for graft failure represent a highly selected subgroup of patients that would in particular benefit from colectomy prior to or at the time of regrafting. This is something that could be investigated in future prospective studies or within the setting of a controlled study.
Peri-transplant morbidity and mortality associated to the previous colectomy procedure and complications arising from colectomy after LT were not investigated by any of the included studies. It is however known from the literature that the postoperative morbidity is high in colectomy in cirrhotic PSC patients. In particular, haemorrhagic complications and worsening liver function as well as sever liver failure requiring rescue LT are frequently encountered and are therefore of major concern[46,49,50]. Although historic reports on proctocolectomy in patients with PSC/UC have described no effect on patient survival in the pre-transplant setting[51], it is now recognized that mortality due to septic shock and hepatic failure occurs in pre-LT colectomy indicating that a simultaneous approach with colectomy at the time of transplantation might be better considered in patients with advanced PSC[46,49]. The type of colectomy appears without effect on the risk of rPSC, although one study found a higher risk of rPSC in LT recipients who underwent ileoanal pouch formation post-LT[20]. Although not reported in the selected studies and unrelated to rPSC, it should be taken into consideration that poor functional outcome as well as high rates of pouchitis (up to 90%) and pouch failure occur after ileoanal pouch formation in PSC patients[42,52].
It is a limitation of this review that only retrospective and observational reports were available to investigate the role of colectomy in rPSC. Furthermore, compared to other topics, a rather small number of studies were identified and included in our analysis. However, the selected studies were mainly of high quality and therefore reliably present the best evidence available.
In conclusion, this systematic review revealed no prospective or matched studies with comparative data on rPSC and non-rPSC. The identified retrospective, observational reports were mainly of high quality and examined the impact of colectomy on liver disease recurrence within a broader assessment of risk factors for rPSC. Within the limitations of scarce retrospective publications, the available data supports a protective role of colectomy in rPSC if carried out prior to or at the time of LT. This was indirectly underpinned by the strong association of rPSC with IBD, UC and in particular active colitis post-LT. The heterogeneity in the presentation of UC and its progression as well as the general difficulty in grading severity of IBD explain the lack of publications correlating bowel disease severity and colectomy in the development of rPSC. Choice of immunosuppression did not affect rPSC and pan-proctocolectomy was not superior compared to other forms of colectomy (ileoanal pouch and segmental-/-subtotal colectomies) in which residual colorectal tissue or intestinal microbiota could fuel immunopathogenic pathways. Taken together, the overall evidence is not strong enough to advocate routine colectomy. Prospective, matched studies and randomized trials on timing and type of colectomy in LT candidates with PSC, which should include analysis of peri-colectomy morbidity, are warranted for an adequate risk-benefit decision. However, as graft selection is limited in the era of marginal organ utilization, pre- or peri-LT timing of colectomy in LT candidates that are likely to require colectomy in due time based on IBD activity is a route that could be further explored with a view to improve outcomes after LT for PSC.
Recurrence of PSC (rPSC) following liver transplantation occurs in up to a quarter of transplant recipients. Prophylactic colectomy has been proposed as a strategy to reduce the incidence of rPSC.
Current literature on the benefit of prophylactic colectomy for prevention of rPSC post liver transplantation does not include any randomized controlled trials. Findings of reported studies need thus to be examined in a critical way, to assess strength of current evidence and to highlight areas for future improvement.
This study aims to critically review the existing evidence regarding prophylactic colectomy for prevention of post liver transplant rPSC, to evaluate reported studies and to identify shortcomings that should be addressed in future studies.
A systematic review was carried out, using structured search terms and a reproducible study selection procedure. Data were extracted and tabulated. The quality of the included studies was evaluated according to modified methodological index for non-randomized studies criteria.
From a total of 180 publications, 6 were included in the final analysis and all of them were retrospective cohort studies. There was significant heterogeneity in the studied samples, regarding other prognostic factors as well as timing and type of colectomy, but the overall evidence favoured a protective role of pre-/peri- liver transplantation (LT) colectomy in rPSC.
This study reviews and reports the results of the existing literature in a systematic and objective way. In the absence of randomized prospective studies, such an approach is indicated for drawing conclusions based on findings of retrospective cohort studies. It confirms the overall impression that colectomy might convey protection against rPSC after LT, but the current literature cannot provide definite answers. Finally, our work identifies a lack of comparable groups and failure to report loss to follow-up as the main limitations of reported studies.
According to the findings of the present study, prophylactic colectomy seems to play a protective role in rPSC post LT, but the existing evidence is not strong. The question would be better answered through prospective randomized trials. It is understood though that such attempt might face several difficulties, particularly in terms of sample size. Alternatively, if retrospective studies were to be carried out, they should include comparison between two groups, those who undergo prophylactic colectomy and those who don’t, and patients’ characteristics, follow-up and outcomes should be reported in a more detailed way.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gencdal G, Rodríguez-Perálvarez M S- Editor: Gong ZM L- Editor: A E- Editor: Yin SY
| 1. | Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 2. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 888] [Cited by in F6Publishing: 792] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 3. | Duclos-Vallee JC, Sebagh M. Recurrence of autoimmune disease, primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis after liver transplantation. Liver Transpl. 2009;15 Suppl 2:S25-S34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Graziadei IW, Wiesner RH, Batts KP, Marotta PJ, LaRusso NF, Porayko MK, Hay JE, Gores GJ, Charlton MR, Ludwig J. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology. 1999;29:1050-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 217] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 449] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 6. | Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol. 2014;60:210-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Loftus EV Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 515] [Cited by in F6Publishing: 477] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 8. | Vera A, Moledina S, Gunson B, Hubscher S, Mirza D, Olliff S, Neuberger J. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet. 2002;360:1943-1944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Trivedi PJ, Adams DH. Gut-liver immunity. J Hepatol. 2016;64:1187-1189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Grant AJ, Lalor PF, Hübscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). Hepatology. 2001;33:1065-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 196] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hübscher SG, Briskin M, Salmon M, Adams DH. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 229] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Sabino J, Vieira-Silva S, Machiels K, Joossens M, Falony G, Ballet V, Ferrante M, Van Assche G, Van der Merwe S, Vermeire S. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016;65:1681-1689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 270] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 13. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3743] [Cited by in F6Publishing: 4543] [Article Influence: 216.3] [Reference Citation Analysis (0)] |
| 14. | Srivasta B, Rushbrook S, Gelson W, Alexander G. Outcomes of liver transplantation in primary sclerosing cholangitis (PSC) - a single UK centre experience. J Hepatol. 2011;54:S363-S534. [DOI] [Cited in This Article: ] |
| 15. | Dekkers N, Meeteren MWV, Inderson A, Laleman W, Desschans B, Hoek BV, Jong MD, Sabino J, Sarasqueta AF, Vermeire S. Does mucosal inflammation drive recurrence of PSC in liver transplant recipients with ulcerative colitis? J. Crohn’s Colitis. 2018;12:P216. [DOI] [Cited in This Article: ] |
| 16. | Graziadei IW, Wiesner RH, Marotta PJ, Porayko MK, Hay JE, Charlton MR, Poterucha JJ, Rosen CB, Gores GJ, LaRusso NF. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology. 1999;30:1121-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Lindström L, Jørgensen KK, Boberg KM, Castedal M, Rasmussen A, Rostved AA, Isoniemi H, Bottai M, Bergquist A. Risk factors and prognosis for recurrent primary sclerosing cholangitis after liver transplantation: a Nordic Multicentre Study. Scand J Gastroenterol. 2018;53:297-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Gordon FD, Goldberg DS, Goodrich NP, Lok AS, Verna EC, Selzner N, Stravitz RT, Merion RM. Recurrent primary sclerosing cholangitis in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study: Comparison of risk factors between living and deceased donor recipients. Liver Transpl. 2016;22:1214-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Joshi D, Bjarnason I, Belgaumkar A, O’Grady J, Suddle A, Heneghan MA, Aluvihare V, Rela M, Heaton N, Agarwal K. The impact of inflammatory bowel disease post-liver transplantation for primary sclerosing cholangitis. Liver Int. 2013;33:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, Neuberger J. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Cholongitas E, Shusang V, Papatheodoridis GV, Marelli L, Manousou P, Rolando N, Patch D, Rolles K, Davidson B, Burroughs AK. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008;14:138-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Gelley F, Zádori G, Görög D, Kóbori L, Fehérvári I, Gámán G, Gerlei Z, Nagy P, Sárváry E, Nemes B. Recurrence of primary sclerosing cholangitis after liver transplantation - The Hungarian experience. Interv Med Appl Sci. 2014;6:16-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Hildebrand T, Pannicke N, Dechene A, Gotthardt DN, Kirchner G, Reiter FP, Sterneck M, Herzer K, Lenzen H, Rupp C. Biliary strictures and recurrence after liver transplantation for primary sclerosing cholangitis: A retrospective multicenter analysis. Liver Transpl. 2016;22:42-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Moncrief KJ, Savu A, Ma MM, Bain VG, Wong WW, Tandon P. The natural history of inflammatory bowel disease and primary sclerosing cholangitis after liver transplantation--a single-centre experience. Can J Gastroenterol. 2010;24:40-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Ravikumar R, Tsochatzis E, Jose S, Allison M, Athale A, Creamer F, Gunson B, Iyer V, Madanur M, Manas D. Risk factors for recurrent primary sclerosing cholangitis after liver transplantation. J Hepatol. 2015;63:1139-1146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Broomé U, Glaumann H, Lindstöm E, Lööf L, Almer S, Prytz H, Sandberg-Gertzén H, Lindgren S, Fork FT, Järnerot G. Natural history and outcome in 32 Swedish patients with small duct primary sclerosing cholangitis (PSC). J Hepatol. 2002;36:586-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Gidwaney NG, Pawa S, Das KM. Pathogenesis and clinical spectrum of primary sclerosing cholangitis. World J Gastroenterol. 2017;23:2459-2469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Carbone M, Neuberger J. Liver transplantation in PBC and PSC: indications and disease recurrence. Clin Res Hepatol Gastroenterol. 2011;35:446-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Rush D. The impact of calcineurin inhibitors on graft survival. Transplant Rev (Orlando). 2013;27:93-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Stepkowski SM. Molecular targets for existing and novel immunosuppressive drugs. Expert Rev Mol Med. 2000;2:1-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | McAlister VC, Haddad E, Renouf E, Malthaner RA, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant. 2006;6:1578-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Jørgensen KK, Lindström L, Cvancarova M, Karlsen TH, Castedal M, Friman S, Schrumpf E, Foss A, Isoniemi H, Nordin A. Immunosuppression after liver transplantation for primary sclerosing cholangitis influences activity of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:517-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Kugelmas M, Spiegelman P, Osgood MJ, Young DA, Trotter JF, Steinberg T, Wachs ME, Bak T, Kam I, Everson GT. Different immunosuppressive regimens and recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2003;9:727-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Lehman A, O’Rourke N, Hatcher L, Stepanski E. JMP for basic univariate and multivariate statistics: a step-by-step guide. Cary, NC: SAS, 2005.. . [Cited in This Article: ] |
| 35. | Attia M, Silva MA, Mirza DF. The marginal liver donor--an update. Transpl Int. 2008;21:713-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Jochmans I, van Rosmalen M, Pirenne J, Samuel U. Adult Liver Allocation in Eurotransplant. Transplantation. 2017;101:1542-1550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 37. | Ravikumar R, Leuvenink H, Friend PJ. Normothermic liver preservation: a new paradigm? Transpl Int. 2015;28:690-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 38. | Trivedi PJ, Scalera I, Slaney E, Laing RW, Gunson B, Hirschfield GM, Schlegel A, Ferguson J, Muiesan P. Clinical outcomes of donation after circulatory death liver transplantation in primary sclerosing cholangitis. J Hepatol. 2017;67:957-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Weismüller TJ, Trivedi PJ, Bergquist A, Imam M, Lenzen H, Ponsioen CY, Holm K, Gotthardt D, Färkkilä MA, Marschall HU. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology. 2017;152:1975-1984.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 306] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 40. | Navaneethan U, Choudhary M, Venkatesh PG, Lashner BA, Remzi FH, Shen B, Kiran RP. The effects of liver transplantation on the clinical course of colitis in ulcerative colitis patients with primary sclerosing cholangitis. Aliment Pharmacol Ther. 2012;35:1054-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Fattahi MR, Malek-Hosseini SA, Sivandzadeh GR, Safarpour AR, Bagheri Lankarani K, Taghavi AR, Ejtehadi F. Clinical Course of Ulcerative Colitis After Liver Transplantation in Patients with Concomitant Primary Sclerosing Cholangitis and Ulcerative Colitis. Inflamm Bowel Dis. 2017;23:1160-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015;21:1956-1971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 120] [Cited by in F6Publishing: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Verdonk RC, Dijkstra G, Haagsma EB, Shostrom VK, Van den Berg AP, Kleibeuker JH, Langnas AN, Sudan DL. Inflammatory bowel disease after liver transplantation: risk factors for recurrence and de novo disease. Am J Transplant. 2006;6:1422-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Indriolo A, Ravelli P. Clinical management of inflammatory bowel disease in the organ recipient. World J Gastroenterol. 2014;20:3525-3533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Rust C, Brand S. PSC: Protect and serve with colitis: does it help the liver to have severe ulcerative colitis? Gut. 2011;60:1165-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Treeprasertsuk S, Björnsson E, Sinakos E, Weeding E, Lindor KD. Outcome of patients with primary sclerosing cholangitis and ulcerative colitis undergoing colectomy. World J Gastrointest Pharmacol Ther. 2013;4:61-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Navaneethan U, Venkatesh PG, Mukewar S, Lashner BA, Remzi FH, McCullough AJ, Kiran RP, Shen B, Fung JJ. Progressive primary sclerosing cholangitis requiring liver transplantation is associated with reduced need for colectomy in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:540-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Nordenvall C, Olén O, Nilsson PJ, von Seth E, Ekbom A, Bottai M, Myrelid P, Bergquist A. Colectomy prior to diagnosis of primary sclerosing cholangitis is associated with improved prognosis in a nationwide cohort study of 2594 PSC-IBD patients. Aliment Pharmacol Ther. 2018;47:238-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Lian L, Menon KV, Shen B, Remzi F, Kiran RP. Inflammatory bowel disease complicated by primary sclerosing cholangitis and cirrhosis: is restorative proctocolectomy safe? Dis Colon Rectum. 2012;55:79-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Poritz LS, Koltun WA. Surgical management of ulcerative colitis in the presence of primary sclerosing cholangitis. Dis Colon Rectum. 2003;46:173-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Cangemi JR, Wiesner RH, Beaver SJ, Ludwig J, MacCarty RL, Dozois RR, Zinsmeister AR, LaRusso NF. Effect of proctocolectomy for chronic ulcerative colitis on the natural history of primary sclerosing cholangitis. Gastroenterology. 1989;96:790-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Block M, Jørgensen KK, Oresland T, Lindholm E, Grzyb K, Cvancarova M, Vatn MH, Boberg KM, Börjesson L. Colectomy for patients with ulcerative colitis and primary sclerosing cholangitis - what next? J Crohns Colitis. 2014;8:421-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |