Published online Jul 14, 2018. doi: 10.3748/wjg.v24.i26.2867
Peer-review started: March 30, 2018
First decision: May 17, 2018
Revised: May 25, 2018
Accepted: June 9, 2018
Article in press: June 9, 2018
Published online: July 14, 2018
To explore the protective effects and underlying mechanisms of total polysaccharides of the Sijunzi decoction (TPSJ) on the epithelial barriers in vitro.
Caco-2 cell monolayers were treated with or without TPSJ in the presence or absence of TNF-α, and paracellular permeability and transepithelial electrical resistance (TEER) were measured to evaluate the epithelial barrier function. Immunofluorescence and western blotting were respectively used to evaluate the distribution and expression of the tight junction proteins claudin 1, claudin 2, zo3, and occludin in Caco-2 cells. Western blotting was also used to evaluate the cellular expression of myosin light chain (MLC), phosphorylated MLC (pMLC), MLC kinase (MLCK), and nuclear factor (NF)-κB p65.
TPSJ promoted the proliferation of Caco-2 cells and inhibited TNF-α-induced secretion of pro-inflammatory cytokines. Furthermore, TPSJ significantly ameliorated both the reduction of TEER and the increased paracellular permeability observed in tumor necrosis factor (TNF)-α-damaged Caco-2 monolayers. Furthermore, TPSJ remarkably attenuated TNF-α-induced morphological changes, downregulated the expression of claudin 1, claudin 2, zo3, and occludin, and markedly suppressed TNF-α-mediated upregulation of p-MLC and MLCK expression. Finally, TPSJ inhibited the activation and expression of NF-κB p65.
Our results demonstrate that TPSJ alleviates the TNF-α-induced impairment of the intestinal epithelial cell barrier function by suppressing NF-κB p65-mediated phosphorylation of MLCK and MLC.
Core tip: Total polysaccharides of the Sijunzi decoction (TPSJ) comprise the active ingredient of Sijunzi decoction, which has long been used to treat gastrointestinal tract disorders. However, the mechanisms by which TPSJ affects the intestinal epithelial barrier remain unclear. Our study results demonstrated that TPSJ attenuated tumor necrosis factor (TNF)-α-induced intestinal barrier dysfunction in a Caco-2 cell monolayer. Furthermore, TPSJ inhibited TNF-α-induced upregulation of myosin light chain (MLC) phosphorylation, which is mediated by MLC kinase and NF-κB, suggesting that this mechanism might underlie the protective effects of TPSJ against intestinal epithelial barrier dysfunction triggered by proinflammatory cytokines.
- Citation: Lu Y, Li L, Zhang JW, Zhong XQ, Wei JA, Han L. Total polysaccharides of the Sijunzi decoction attenuate tumor necrosis factor-α-induced damage to the barrier function of a Caco-2 cell monolayer via the nuclear factor-κB-myosin light chain kinase-myosin light chain pathway. World J Gastroenterol 2018; 24(26): 2867-2877
- URL: https://www.wjgnet.com/1007-9327/full/v24/i26/2867.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i26.2867
The intestinal epithelial barrier plays a crucial role in separating luminal microbes and antigenic molecules from the internal milieu. Accordingly, intestinal barrier dysfunction can destroy immune homeostasis and induce an inflammatory response[1,2]. Tight junctions (TJs), which are mediated by proteins such as claudins, occludin, and zonula occludens, are necessary for epithelial barrier maintenance[3-5]. Disruption of the intestinal epithelial barrier can increase intestinal permeability, a crucial pathogenic contributor to intestinal inflammation[6,7]. Intestinal epithelial barrier disruption is common to many inflammatory enteropathies, including Crohn’s disease (CD), ulcerative colitis (UC), and infectious diarrhea[8-11].
Many locally released pro-inflammatory cytokines and mediators, including tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-1β, Th17 type cytokines (IL-17, IL-23, IL-22, and IL-6), and nitric oxide (NO) have been recognized to contribute to intestinal barrier dysfunction during the course of inflammatory bowel disease in vitro and in vivo[12-17]. Additionally, myosin light chain kinase (MLCK), which mediates the phosphorylation of myosin light chain (MLC), is thought to play a critical role in proinflammatory cytokine-induced intestinal barrier disruption [18,19].
Sijunzi decoction (SJZD) is a traditional medicinal formula comprising four Chinese herbs: Ginseng Radix et Rhizoma or Codonopsispilosula, Atractylodes Macrocephalae Rhizoma, Poria, and Glycyrrhizae Radix et Rhizoma Praeparatecum Melle. This decoction, which is used to strengthen the spleen and tonify the qi, has been used to treat gastrointestinal tract diseases since ancient times[20-22]. In a previous study, SJZD was shown to regulate both digestive system and immune system function[23]. In mice, total polysaccharides of the Sijunzi decoction (TPSJ) were shown to antagonize cyclophosphamide-induced injury to the intestinal mucosal associated lymphoid tissues[24], suggesting that these polysaccharides could improve intestinal mucosal immune function. Another study found that polysaccharides of the SJZD can restore intestinal function and protect against indomethacin-induced damage to IEC-6 rat intestinal epithelial cells[25].
Our previous studies suggested that TPSJ could inhibit the proliferation of IEC-6 cells in vitro. However, no reports have discussed the ability of TPSJ to regulate the intestinal epithelial barrier. In the present study, we explored the effects of TPSJ on the intestinal barrier formed by Caco-2 human colon adenocarcinoma cells damaged by the proinflammatory cytokine TNF-α, as well as the underlying mechanism. Our results indicate that TPSJ could relieve the intestinal epithelial barrier dysfunction induced by TNF-α, and that this function was mediated by the downregulation of MLCK-dependent MLC phosphorylation in a manner dependent on nuclear factor (NF)-κB p65.
The Sijunzi Decoction (SJZD) used in this research comprised Ginseng Radix et Rhizoma or Codonopsispilosula, Atractylodes Macrocephalae Rhizoma, Poria and Glycyrrhizae Radix et Rhizoma Praeparatecum Melle. These four drugs were pharmacopoeia-grade. All herbs were obtained from Kangmei Pharmaceutical Company Ltd. (Guangzhou, Guangdong, China).
Dulbecco’s modified Eagle’s medium (DMEM), non-essential amino acids (NEAA), and fetal bovine serum (FBS) were obtained from GIBCO Laboratories (Grand Island, NY, United States). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and phenolsulfonphthalein were obtained from Sigma-Aldrich (St. Louis, MO, United States). Fluoroisothiocyanate (FITC)-conjugated Annexin V and propidium iodide (PI) were purchased from Lianke Biotechnology Co (Hangzhou, China). Antibodies specific for claudin 1, claudin 2, zo3, occludin, MLC (phospho S20), MLC (pan), and MLCK and the NF-κB p50/p65 transcription factor assay kit were all purchased from Abcam (Cambridge, MA, United States). Enzyme-linked immunosorbent (ELISA) kits for TNF-α, IL-6 and IL-8 were obtained from eBioscience (San Diego, CA, United States).
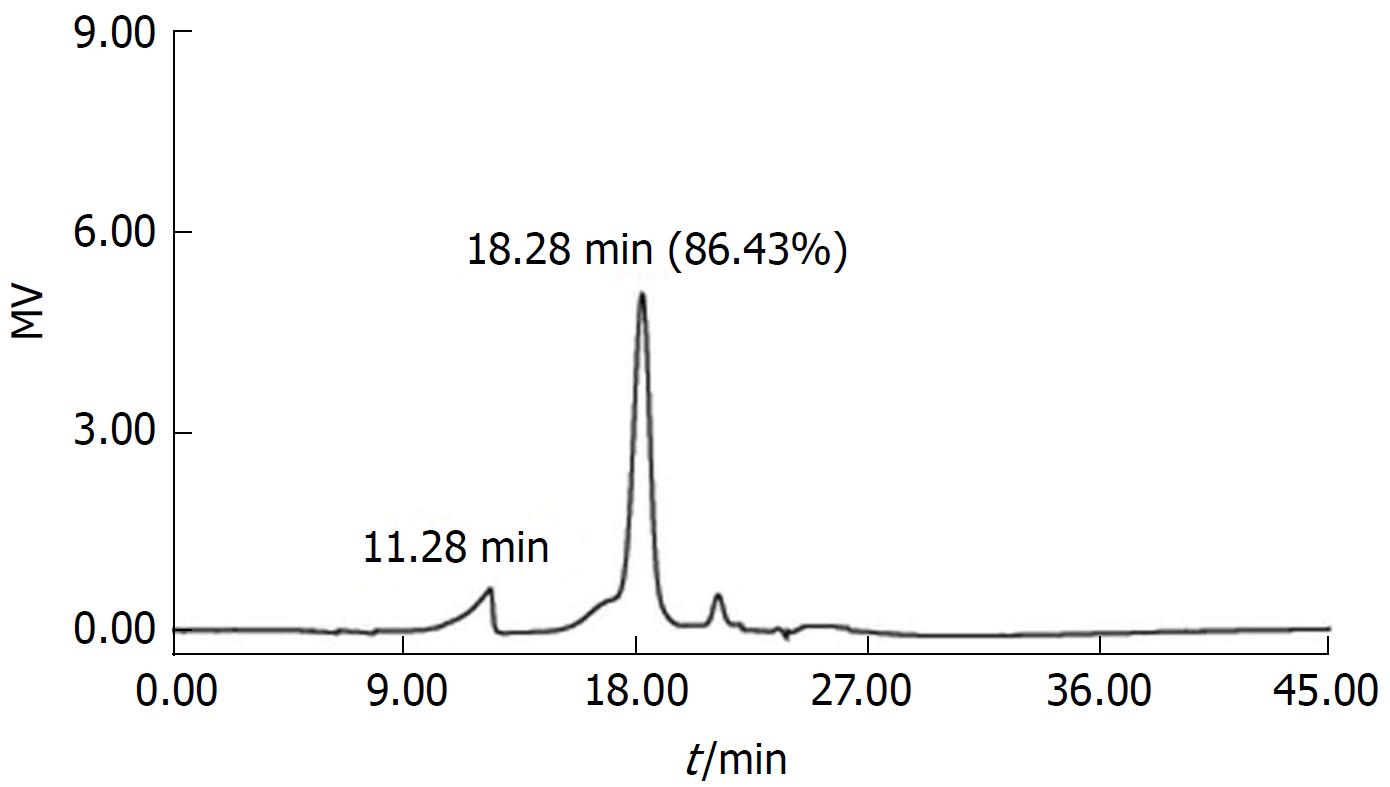
SJZD comprised Ginseng Radix et Rhizoma or Codonopsispilosula, Atractylodes Macrocephalae Rhizoma, Poria, and Glycyrrhizae Radix et Rhizoma Praeparatecum Melle at a ratio of 3:3:3:2 to yield a total weight of 1100 g. All of the herbs were placed in a container to which a volume of cold water approximately 12 times (7.2 L) the solid volume was added. After soaking the herbs for 2 h, we boiled the mixture for 30 min and filtered the herbs, reserving the filtrate. Subsequently, we added another volume of water approximately 5 times the volume of herbs to the container and boiled the mixture for 30 min, followed by filtration. We then mixed the two filtrates and concentrated the liquid to 1.4 L. Subsequently, we added ethanol to the filtrates to yield an alcohol concentration of 75% and stored them at 4 °C overnight. The next day, we filtered, precipitated, and dissolved the ethanol mixture in approximately 1.6 L of ultrapure water, followed by centrifugation at 8400 rpm for 15 min. The resulting supernatant was frozen and dried to yield the total polysaccharide. A phenol-sulfuric acid spectrophotometry method was used to measure the polysaccharide content (as glucose), which was 70.61% ± 1.70%, according to at least three independent experiments. Figure 1 depicts the gel permeation chromatography (GPC) analysis of TPSJ[26].
Caco-2 human colon adenocarcinoma cells were obtained from the Cell Culture Unit of Shanghai Science Academy (Shanghai, China). The cells were grown in DMEM supplemented with 10% FBS and 1% NEAA and incubated in a humidified atmosphere with 5% CO2 atmosphere at 37 °C.
Cell viability was determined using a MTT reduction assay. Cells were seeded into 96-well plates in DMEM + 10% FBS + 1% NEAA at a density of 5000 per well and treated with 100 ng/mL TNF-α. After a 24-h incubation, TPSJ or DMEM (control) was added to the wells, followed by another 24-h incubation. Subsequently, 10 μL of MTT solution was added to each well, and the plates were incubated for 4 h. Finally, we lysed the cells with 0.04 N HCl in isopropyl alcohol and read the absorbance of each well at 570 nm.
To assess apoptosis, we harvested Caco-2 cells. After two washes with phosphate-buffered saline (PBS), we resuspended the cells in 200 μL of Annexin-V binding buffer (10 mmol/L HEPES, 140 mmol/L NaCl, 2 mmol/L MgCl2, 5 mmol/L KCl, 2.5 mmol/L CaCl2, pH 7.4) and added 10 μL of FITC-conjugated Annexin V to each tube according to the manufacturer’s protocol. Following a 15 min incubation in the dark at room temperature, we added 10 μL of PI and 200 μL binding buffer to each tube. Finally, we analyzed the samples on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, United States).
We used an EVOM TEER meter (Millipore, Bedford, MA, United States) to monitor the transepithelial electrical resistance (TEER) of Caco-2 cells. Specifically, an increase in TEER to a steady state exceeding 200 Ω cm2 at day 7 indicated the complete formation of tight junctions and full epithelial barrier integrity. In our experiments, we treated cell monolayers with recombinant human TNF-α (100 ng/mL) for 24 h and subsequently added 150 μg/ml TPSJ or not to the wells. Monolayers treated with cytokine alone or DMEM alone were used as controls.
Caco-2 cells were grown on inserts. Firstly, we washed the cell monolayers with PBS. Next, we added phenolsulfonphthalein to the apical compartment to a final concentration of 20 mg/L in ultrapure water. We added only water to the basolateral compartment. After a 4-h incubation, we removed 150 μL aliquots from the basolateral compartment into tubes containing 1.5 mL NaOH (20 μmol/mL). We then analyzed the absorbance of each tube at 570 nm using a spectrophotometer.
We collected culture medium of from Caco-2 cells and used ELISA kits (eBioscience) to measure the amounts of TNF-α, IL-6, and IL-8 according to the manufacturer’s instruction.
We seeded Caco-2 cells on glass cover slips placed in the wells of a 6-well plate and treated the cells with TNF-α (100 ng/mL) for 24 h without or with 150 μg/mL TPSJ. The immunofluorescence assay was performed according to the protocol with antibodies specific for claudin 1, claudin 2, zo3, and occludin, followed by incubation with a FITC-conjugated anti-rabbit IgG (1:200 dilution). Images were captured using a fluorescence microscope (Olympus, BX51, Tokyo, Japan).
Measurement of NF-κB p65 activity
We used a NF-κB p50/p65 transcription factor assay kit to measure the NF-κB p65 activity in prepared cellular extracts according to the manufacturer’s instructions.
We plated Caco-2 cells in 6-well plates at a density of 1 × 106 per well. The cells were treated with recombinant human TNF-α (100 ng/mL) for 24 h without or with 150 μg/mL TPSJ for different time intervals.
At different time points, we lysed the cells in lysis buffer [50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 0.1% sodium dodecylsulfate (SDS), 1% sodium deoxycholate, 1 mmol/L phenylmethylsulphonyl fluoride, 1% Triton X-100 and protease inhibitors] and subsequently removed cell debris by centrifugation (15000 rpm, 15 min, 4 °C). After determining the protein concentrations of the samples, we separated equal amounts of protein by 12.5% SDS-PAGE and transferred the proteins to nitrocellulose membranes. After blocking the membranes, we incubated them overnight at 4 °C with different primary antibodies, followed by a 1 h incubation with secondary antibodies. Finally, protein expression was evaluated using a Bio-Rad Imaging System (Bio-Rad Biosciences, Hercules, CA, United States).
The results are expressed as means ± standard errors of the means (SEM). Student’s t-test was used for the statistical analysis, and a P value < 0.05 was considered significant. At least three independent experiments were performed.
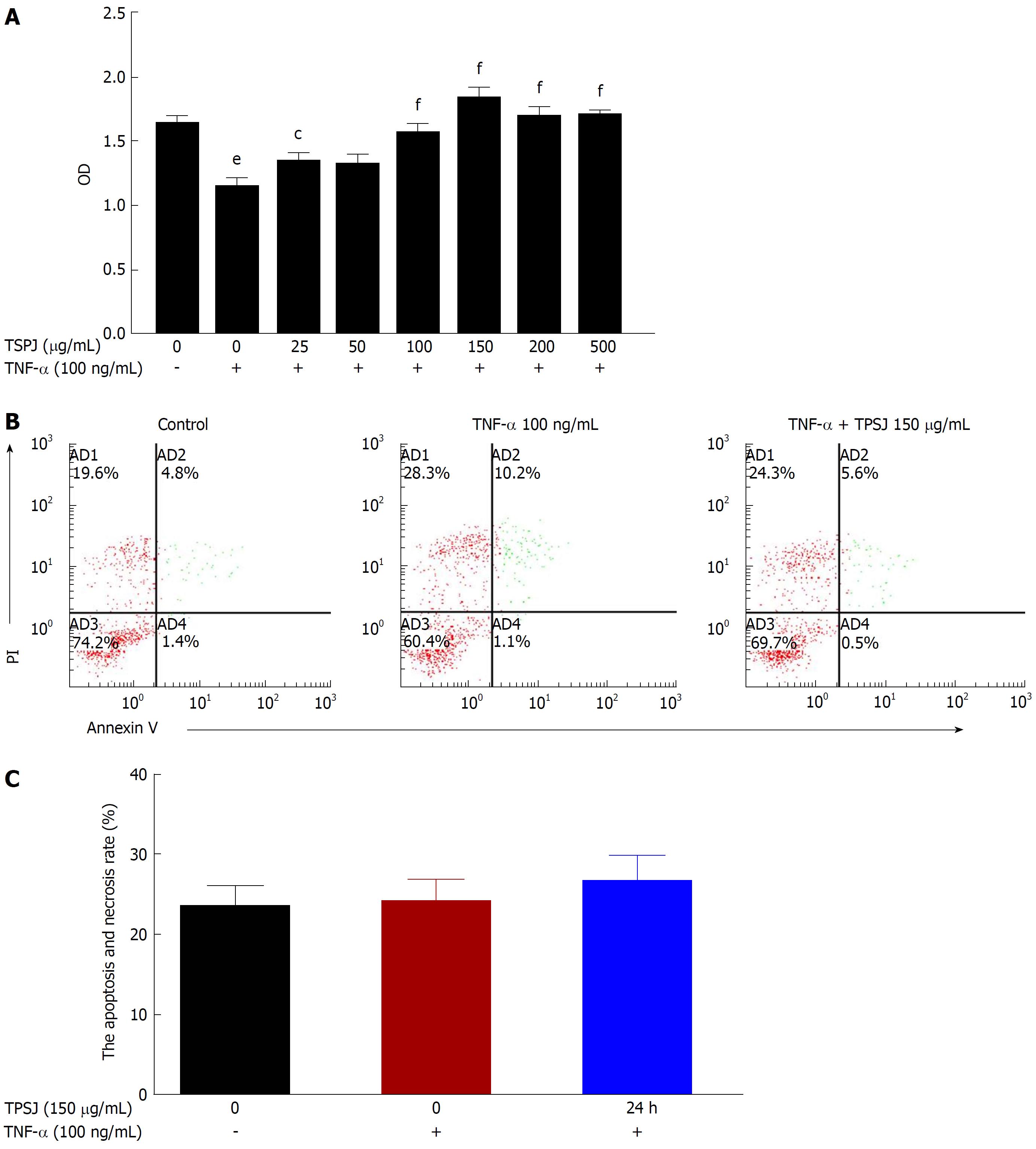
We first used a MTT assay to assess the effect of TPSJ on TNF-α-damaged Caco-2 cells. As shown in Figure 2A, TPSJ dramatically induced the growth of TNF-α-treated Caco-2 cells in a dose-dependent manner, particularly at a concentration of 150 μg/mL. However, TPSJ treatment had no significant effect on the frequency of cell apoptosis in comparison to the TNF-α control group (Figure 2B and C).
TPSJ ameliorates the intestinal epithelial barrier dysfunction induced by TNF-α
Many investigators have shown that TNF-α disrupts intestinal barrier function by decreasing the TEER and increasing paracellular permeability[19,27]. Therefore, to investigate the effects of TPSJ on intestinal barrier function, we treated a Caco-2 cell monolayer with TNF-α for 24 h and subsequently analyzed the TEER and permeability of the cells after TPSJ treatment.
As shown in Figure 3A, the TEER of the TNF-α-damaged Caco-2 cell monolayer decreased significantly compared with the control group, indicating that TNF-α upregulated the paracellular permeability of ionic solutes. By contrast, TPSJ treatment significantly increased the TEER.
As shown in Figure 3B, the phenolsulfonphthalein flux was significantly higher in the TNF-α-damaged Caco-2 cell monolayer than in the control monolayer, indicating this inflammatory cytokine increased the paracellular permeability of nonionic macromolecules. However, TPSJ markedly decreased the increased phenolsulfonphthalein flux induced by TNF-α. These results suggest that TPSJ can attenuate the intestinal epithelial barrier dysfunction induced by TNF-α.
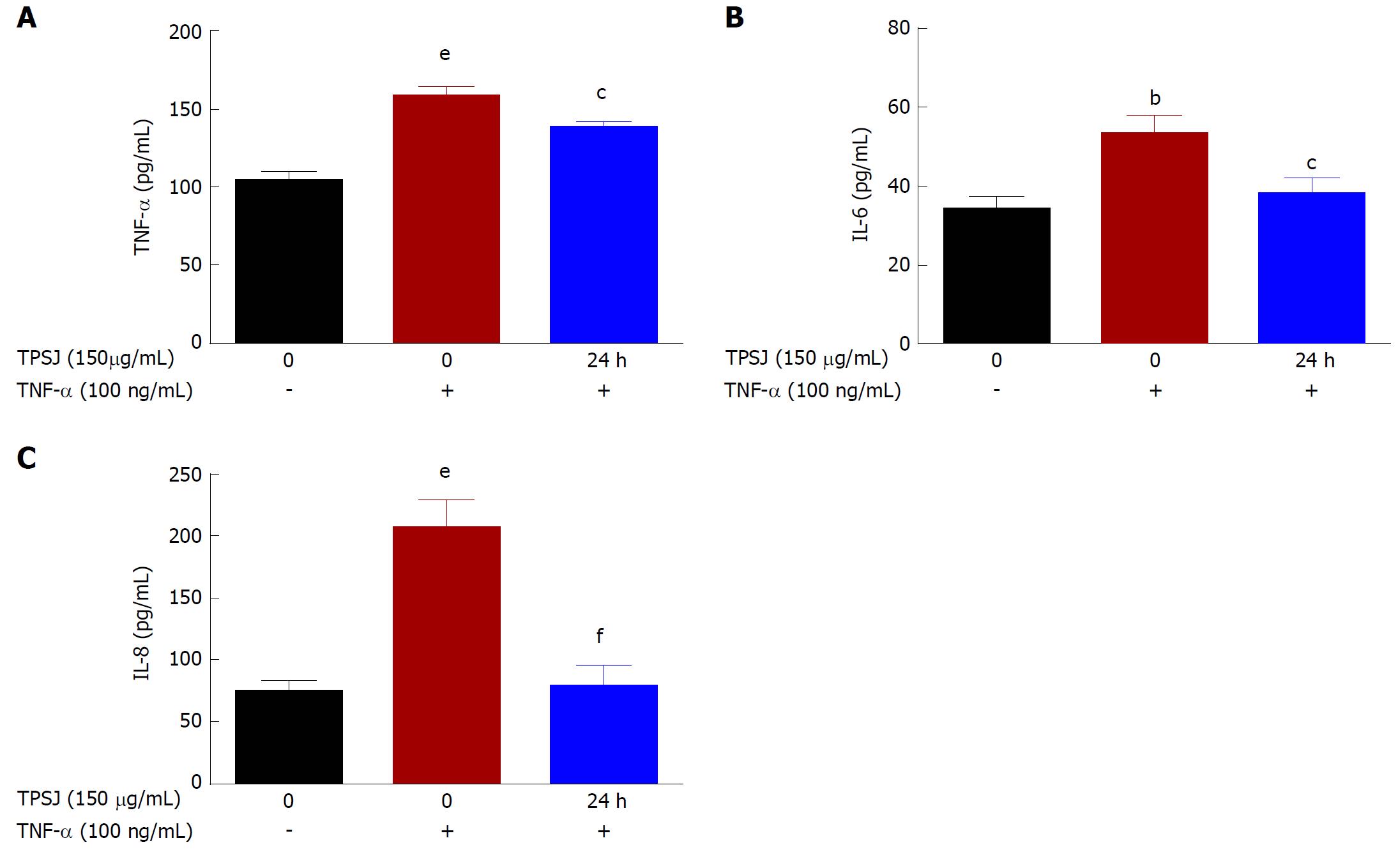
TPSJ decreased the secretion of pro-inflammatory cytokines by TNF-α-induced Caco-2 cells
The ELISA results shown in Figure 4 demonstrate significant increases in the levels of TNF-α, IL-6, and IL-8 secreted by Caco-2 cells into the culture medium after TNF-α treatment. However, TPSJ markedly decreased the secretion of these cytokines in response to TNF-α. These results indicate that TPSJ can regulate TNF-α-induced production of pro-inflammatory factors.
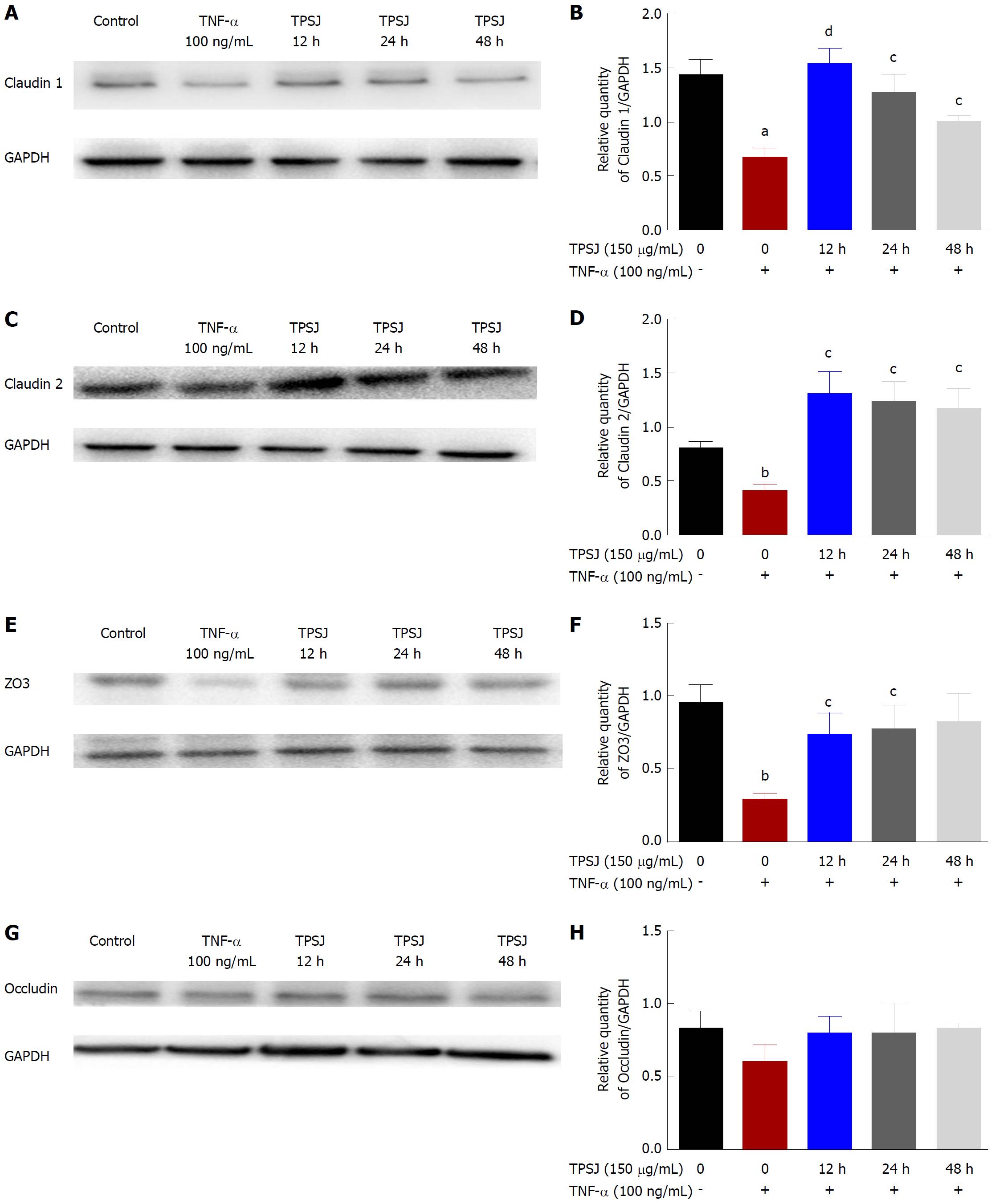
TPSJ protected a Caco-2 cell monolayer from TNF-α-induced barrier dysfunction by regulating tight junctions
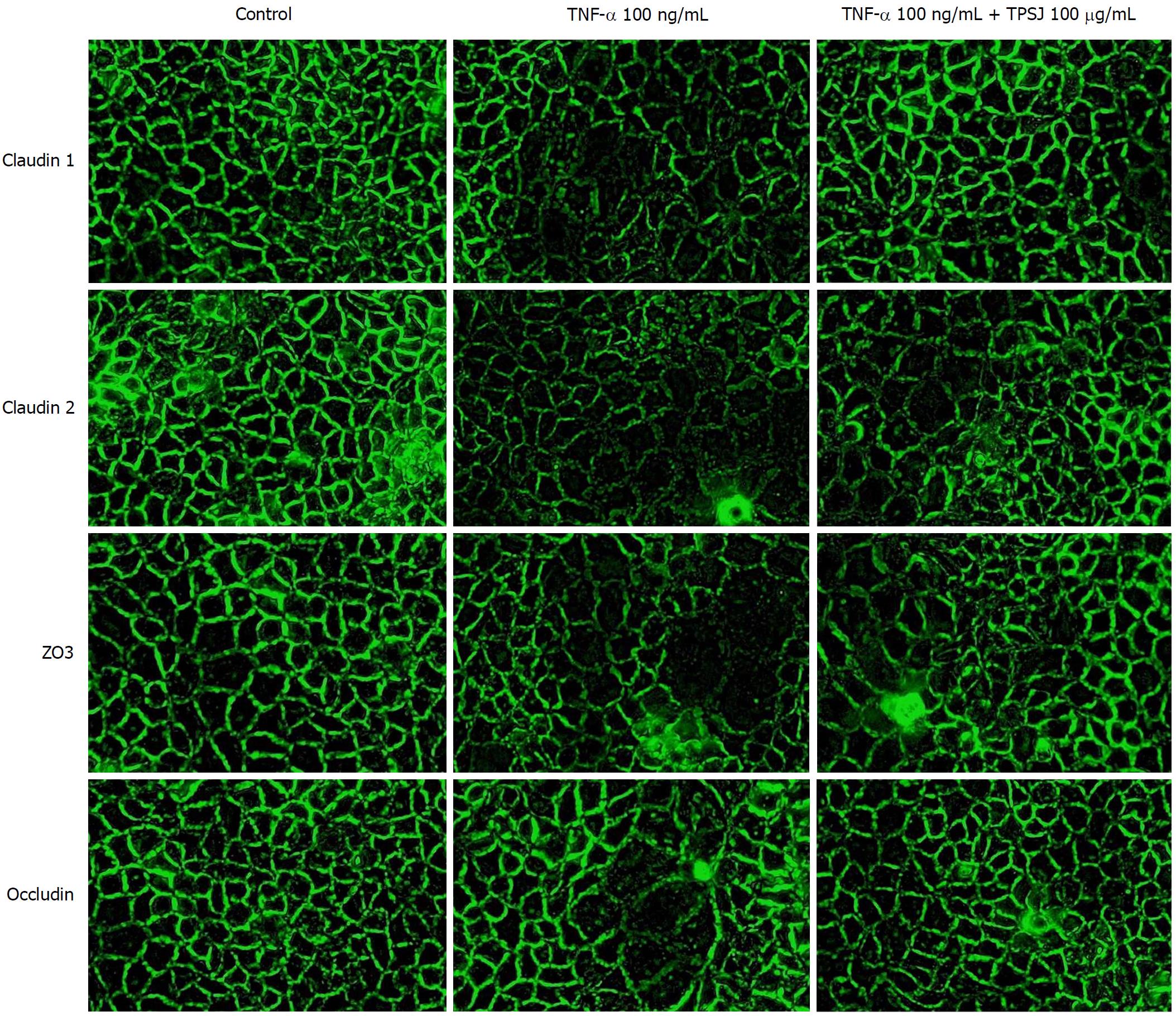
Increasing evidence suggests that altered tight junction protein expression contributes to the proinflammatory cytokine-induced disruption of barrier function[15,28]. Therefore, we examined the effects of TPSJ on the expression of the tight junction proteins claudin 1, claudin 2, zo3, and occludin in Caco-2 cell monolayers treated with or without TNF-α. As shown in Figure 5, the expression of claudin 1, claudin 2, and zo3 proteins were significantly downregulated by TNF-α. After TPSJ treatment, however, the expressions of all three proteins were upregulated markedly at different time points. By contrast, the expression of occludin was not significantly affected by treatment with or without TNF-α or in the absence or presence of TPSJ.
Reports have demonstrated an association of proinflammatory cytokine-induced intestinal barrier dysfunction with the morphological alterations and relocalization of the tight junction[29-31]. Thus, we next determined whether TPSJ affected the morphological localization of tight junctions in Caco-2 cell monolayers treated with or without TNF-α. As shown in Figure 6, claudin 1, claudin 2, zo3, and occludin were localized along the edges of cells in the control group. However, a 24-h treatment with TNF-α rendered the tight junction distribution irregular and discontinuous, and led to the partial internalization of occludin into cytoplasmic vesicles. After TPSJ treatment, however, the reorganization of claudin 1, claudin 2, zo3, and occludin was significantly attenuated. These results indicate that TPSJ can prevent the proinflammatory cytokine-induced reorganization of tight junctions in a Caco-2 cell monolayer.
TPSJ suppresses TNF-α-induced upregulation of MLC phosphorylation and MLCK expression
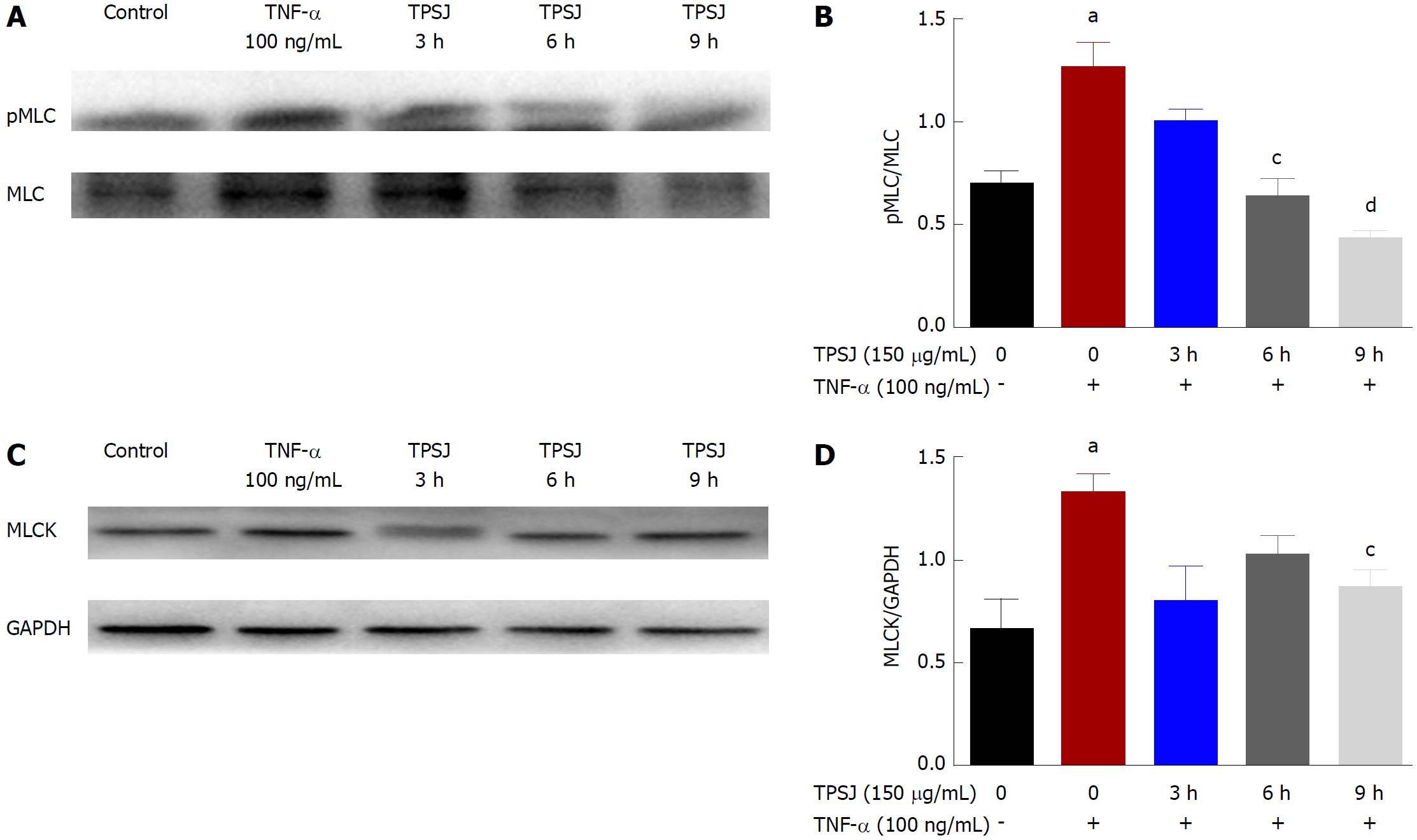
The MLCK-mediated phosphorylation of MLC has been reported to play a crucial role in the regulation of intestinal epithelial tight junctions and paracellular leakage pathways[3,4]. Given the protective effect of TPSJ on intestinal barrier function, we wished to explore whether TPSJ could alleviate TNF-α-induced barrier dysfunction and tight junction disruption by inhibiting MLC phosphorylation. As shown in Figure 7A and B, treatment of a Caco-2 monolayer with TNF-α induced a significant increase in the ratio of phosphorylated to total MLC. However, TPSJ treatment markedly downregulated this ratio at 6 h and 9 h (compared with the TNF-α control group).
MLCK is a well-known and predominant regulator of MLC phosphorylation, and many studies have indicated an association of MLCK upregulation with tight junction dysfunction and paracellular hyperpermeability[30,31]. Therefore, we next investigated the effect of TPSJ on MLCK expression in Caco-2 cell monolayers treated with or without TNF-α. As shown in Figure 7C and D, MLCK expression increased significantly in the TNF-α-treated monolayer. However, TPSJ treatment significantly reduced MLCK expression at 9 h, compared with the TNF-α control group. These results suggest that TPSJ attenuates TNF-α-induced intestinal barrier disruption by suppressing the MLCK-mediated phosphorylation of MLC.
TPSJ attenuated TNF-α-induced intestinal epithelial barrier dysfunction by inhibiting NF-κB p65
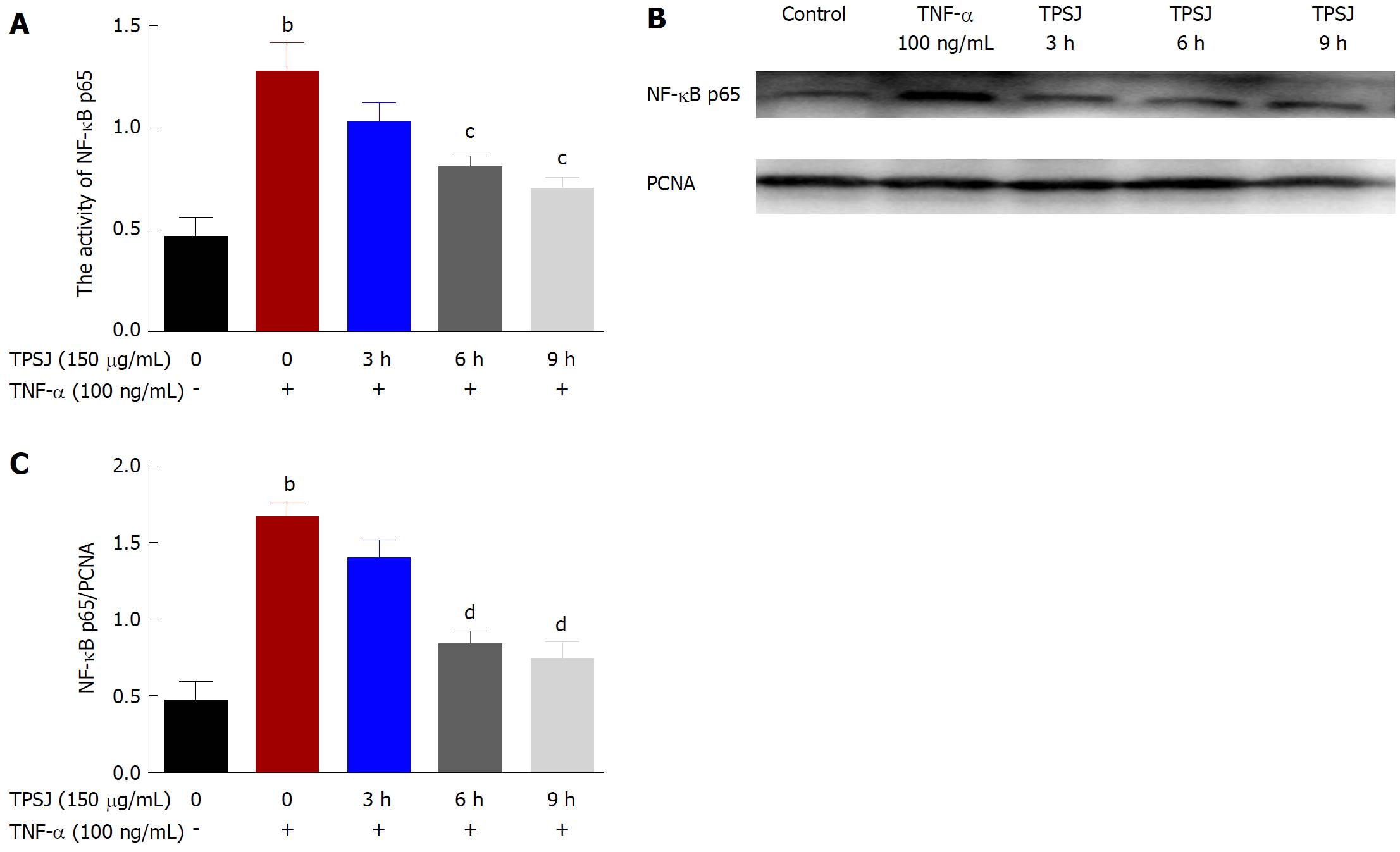
Studies have shown that NF-κB activation plays a role in intestinal barrier dysfunction as well as in the upregulation of MLCK in TNF-α treated intestinal epithelial cells[32,33]. Based on the above results, we aimed to investigate further the potential involvement of the NF-κB signaling pathway in the protective effect of TPSJ against TNF-α-induced epithelial barrier dysfunction. As demonstrated in Figure 8A, NF-κB p65 activity was upregulated in the TNF-α-treated Caco-2 cell monolayer. TPSJ treatment, however, significantly inhibited NF-κB p65 activity at 6 h and 9 h compared with the TNF-α-treated Caco-2 monolayer. Similarly, as shown in Figure 8B and C, the nuclear expression of NF-κB p65 increased following TNF-α treatment, whereas TPSJ markedly downregulated the nuclear expression at 6 h and 9 h.
Inflammatory bowel diseases, including UC and CD, are well-known chronic and recurring inflammatory diseases of the intestinal tract. Although the pathogenesis of inflammatory bowel diseases is not fully elucidated, all are characterized by the overproduction of proinflammatory cytokines within the mucosa and the disruption of epithelial barrier function. However, many research groups have demonstrated that proinflammatory cytokines may disrupt intestinal barrier function both in vivo and in vitro[28,29,34]. Therefore, restoration of the intestinal barrier function is a worthwhile strategy for the treatment of inflammatory bowel diseases.
As noted above, TPSJ was previously reported to antagonize cyclophosphamide-induced mucosal-associated lymphoid tissue injury in an animal model, suggesting a potential beneficial role in intestinal mucosal immune function[24]. In this study, we showed that TPSJ could attenuate intestinal epithelial barrier dysfunction caused by proinflammatory cytokines in a Caco-2 cell monolayer. Specifically, TPSJ alleviated the TNF-α-induced decrease in TEER and increase in paracellular permeability, enhanced the expression of claudin 1, claudin 2, and zo3, and preserved the morphological distributions of these three tight junction proteins and occludin.
The molecular mechanism by which TPSJ ameliorates the proinflammatory cytokine-induced intestinal barrier dysfunction is currently unknown. Many research groups have demonstrated that the upregulation of MLCK and subsequent increase in MLC phosphorylation are essential to the induction of intestinal barrier defects by proinflammatory cytokines[9,12]. In our study, we found that TPSJ inhibited increases in MLC phosphorylation and MLCK expression in Caco-2 cell monolayers exposed to TNF-α, suggesting that TPSJ may attenuate proinflammatory cytokine-induced intestinal barrier dysfunction by inhibiting MLCK activation and subsequent MLC phosphorylation. However, other potential molecular mechanisms remain to be investigated further.
According to previously published reports, activated NF-κB mediates the increased intestinal epithelial tight junction permeability induced by TNF-α[27] and contributes to MLCK upregulation in a Caco-2 cell monolayer exposed to proinflammatory cytokines[12,32]. In this study, we showed that TPSJ could suppress the activation and expression of NF-κB p65 in a Caco-2 cell monolayer treated with TNF-α. Our results suggest that the mechanism by which TPSJ attenuates TNF-α-induced barrier dysfunction in the Caco-2 cell monolayer is mediated by the NF-κB signaling pathway.
In conclusion, our results demonstrate that TPSJ attenuates the intestinal barrier dysfunction elicited by TNF-α treatment in a Caco-2 cell monolayer. We further demonstrate that TPSJ inhibits the TNF-α-induced upregulation of MLC phosphorylation, which is mediated by MLCK and NF-κB. These factors may comprise the mechanism by which TPSJ protects the intestinal epithelial barrier from destruction triggered by proinflammatory cytokines.
Sijunzi decoction (SJZD) is a traditional Chinese medicinal prescription that has been used to treat gastrointestinal tract diseases since ancient times. Our previous studies suggested that total polysaccharides of the Sijunzi decoction (TPSJ) could inhibit the proliferation of IEC-6 rat intestinal epithelial cells in vitro. However, no report has discussed the regulatory effects of TPSJ on the intestinal epithelial barrier.
Although TPSJ may inhibit intestinal epithelial cell proliferation, the mechanism by which it mediates barrier protection remains unclear.
To explore the protective effects of TPSJ on the epithelial barrier and the mechanism by which it mitigates tumor necrosis factor α (TNF-α)-induced damage in a Caco-2 cell monolayer.
We first used a MTT assay to assess the effect of TPSJ on TNF-α-damaged Caco-2 cells. Secondly, we treated a Caco-2 cell monolayer with TNF-α for 24 h and subsequently analyzed the TEER, permeability, and cytokines of the cells after TPSJ treatment. Third, we examined the effects of TPSJ on the expression of the tight junction proteins claudin 1, claudin 2, zo3, and occludin by immunofluorescence and western blotting. Finally, we investigated the NF-κB-MLCK-MLC pathway in TNF-α treated intestinal epithelial cells.
TPSJ promoted the growth of TNF-α-treated Caco-2 cells in a dose-dependent manner and decreased the secretion of pro-inflammatory cytokines in response to TNF-α. Secondly, TPSJ treatment significantly increased the TEER and decreased the increased phenolsulfonphthalein flux induced by TNF-α. Third, TPSJ markedly upregulated the expression of claudin 1, claudin 2, and zo3 proteins and attenuated the reorganization of claudin 1, claudin 2, zo3, and occludin. Finally, TPSJ suppressed the TNF-a-induced upregulation of myosin light chain (MLC) phosphorylation, MLC kinase (MLCK), and NF-κB p65.
TPSJ promoted proliferation of TNF-α-treated Caco2 cells. In Caco2 cell monolayers, TPSJ alleviated the TNF-α-induced decrease in TEER and increase in paracellular permeability, enhanced the expression of claudin 1, claudin 2, and zo3, and preserved the morphological distributions of these three tight junction proteins and occludin. Further, we found that the barrier protective effect of TPSJ was mediated through suppressing the NF-κB p65-mediated phosphorylation of MLCK and MLC.
Our findings provide evidence that TPSJ is a potential protective agent of intestinal barrier function. Further investigation into the mechanism of TPSJ on intestinal barrier as well as in vivo research is required.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Touil-Boukoffa C S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Yin SY
| 1. | Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000;35:1163-1169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437-1439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 462] [Cited by in F6Publishing: 432] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 588] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 4. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2121] [Cited by in F6Publishing: 2385] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 5. | Weber CR. Dynamic properties of the tight junction barrier. Ann N Y Acad Sci. 2012;1257:77-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2744] [Cited by in F6Publishing: 2693] [Article Influence: 141.7] [Reference Citation Analysis (2)] |
| 7. | Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2328] [Cited by in F6Publishing: 2214] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 8. | Martínez C, González-Castro A, Vicario M, Santos J. Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome. Gut Liver. 2012;6:305-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 432] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 10. | Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 11. | Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 777] [Cited by in F6Publishing: 827] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 12. | Rafa H, Benkhelifa S, AitYounes S, Saoula H, Belhadef S, Belkhelfa M, Boukercha A, Toumi R, Soufli I, Moralès O. All-Trans Retinoic Acid Modulates TLR4/NF-κB Signaling Pathway Targeting TNF-α and Nitric Oxide Synthase 2 Expression in Colonic Mucosa during Ulcerative Colitis and Colitis Associated Cancer. Mediators Inflamm. 2017;2017:7353252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Toumi R, Soufli I, Rafa H, Belkhelfa M, Biad A, Touil-Boukoffa C. Probiotic bacteria lactobacillus and bifidobacterium attenuate inflammation in dextran sulfate sodium-induced experimental colitis in mice. Int J Immunopathol Pharmacol. 2014;27:615-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Soufli I, Toumi R, Rafa H, Touil-Boukoffa C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016;7:353-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 210] [Cited by in F6Publishing: 219] [Article Influence: 27.4] [Reference Citation Analysis (5)] |
| 15. | Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263-31271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 377] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 16. | Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 464] [Cited by in F6Publishing: 506] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 17. | Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131:1153-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 243] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037-12046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383-2394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Cao J. 49 cases of Sijunzi decoction on functional dyspepsia of spleen deficiency and liver stagnation syndrome. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2008;16:126-128. [Cited in This Article: ] |
| 21. | Chen J. Effect of Sijunzi decoction and enteral nutrition in postoperative recovery of gastric cancer. Jilin Zhongyiyao. 2013;33:383-385. [Cited in This Article: ] |
| 22. | Li Y. Clinical observation of Sijunzi decoction in the treatment of spleen and stomach types of functional dyspepsia. Beijing Zhongyiyao. 2008;27:806-807. [Cited in This Article: ] |
| 23. | Shan T, Yu XY, Zhou ZL, Xie JL, Li L. Effect of Sijunzi decoction on restoration of gut barrier after relief of intestinal obstruction in rabbit intestine. Zhongguo Zhongxiyi Jiehe Waike Zazhi. 2010;16:319-323. [Cited in This Article: ] |
| 24. | Liu L, Zhou H, Wang PX, Hu YJ. Influence of total polysaccharide extracted from Sijunzi decoction on the intestinal mucosa-associated lymphoid tissues of mice. Zhongguo Mianyixue Zazhi. 2000;17:204-206. [Cited in This Article: ] |
| 25. | Liu L, Han L, Wong DY, Yue PY, Ha WY, Hu YH, Wang PX, Wong RN. Effects of Si-Jun-Zi decoction polysaccharides on cell migration and gene expression in wounded rat intestinal epithelial cells. Br J Nutr. 2005;93:21-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Deng J, Li RL, Cai JZ, Tu XH, Chen WW. Changes in IEC-6 cell migration ability are impacted by polysaccharides separated and purified from Sijunzi decoction. World Sci Tech/ Modern Trad Chin Med Mat Medi. 2016;18:600-606. [Cited in This Article: ] |
| 27. | Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367-G376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 613] [Cited by in F6Publishing: 675] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 28. | Amasheh M, Grotjohann I, Amasheh S, Fromm A, Söderholm JD, Zeitz M, Fromm M, Schulzke JD. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: a novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand J Gastroenterol. 2009;44:1226-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Interferon-gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol. 2008;126:67-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Liu H, Li M, Wang P, Wang F. Blockade of hypoxia-inducible factor-1α by YC-1 attenuates interferon-γ and tumor necrosis factor-α-induced intestinal epithelial barrier dysfunction. Cytokine. 2011;56:581-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Liu H, Wang P, Cao M, Li M, Wang F. Protective role of oligomycin against intestinal epithelial barrier dysfunction caused by IFN-γ and TNF-α. Cell Physiol Biochem. 2012;29:799-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Graham WV, Wang F, Clayburgh DR, Cheng JX, Yoon B, Wang Y, Lin A, Turner JR. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205-26215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Ye D, Ma TY. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-alpha-induced regulation of myosin light chain kinase gene activity. J Cell Mol Med. 2008;12:1331-1346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422-G430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 335] [Article Influence: 17.6] [Reference Citation Analysis (0)] |