Published online Apr 7, 2018. doi: 10.3748/wjg.v24.i13.1478
Peer-review started: February 5, 2018
First decision: February 26, 2018
Revised: February 26, 2018
Accepted: March 7, 2018
Article in press: March 7, 2018
Published online: April 7, 2018
To investigate the real-world efficacy and safety of sofosbuvir/ribavirin (SOF/RBV) therapy for Japanese patients with genotype 2 hepatitis C virus (GT2-HCV).
A total of 182 patients with GT2-HCV infection who received SOF/RBV therapy for 12 wk at our hospital were enrolled. The patients comprised 122 men and 60 women (age range: 17-84 years; mean age ± SD: 60.1 ± 12.1 years). Relationships between virological response and clinical data were examined by logistic regression analyses.
The proportions of patients with liver cirrhosis and history of hepatocellular carcinoma (HCC) were 29.0% and 17.3%, respectively. The proportion of patients with prior interferon (IFN)-based therapy was 25.6%. SOF/RBV therapy rapidly decreased HCV RNA levels. Several patients required RBV dose reduction because of anemia or fatigue. Four patients discontinued the therapy. The rates of sustained virological response at 12 wk after the end of treatment were 87.9% (intention to treat: 160/182) and 94.1% (per protocol: 159/169). Multivariate analyses showed that history of HCC or IFN-based therapy independently reduced the efficacy of SOF/RBV therapy.
SOF/RBV therapy for GT2-HCV is safe, highly tolerated, and effective. History of HCC or IFN-based therapy independently reduces the efficacy of this treatment.
Core tip: The real-world efficacy of sofosbuvir/ribavirin therapy for genotype 2 hepatitis C virus infection in Japan is high. Sofosbuvir/ribavirin therapy is safe and highly tolerated. History of hepatocellular carcinoma or interferon-based therapy independently reduces the efficacy of sofosbuvir/ribavirin therapy. Progressive liver fibrosis may attenuate the antiviral effect of sofosbuvir/ribavirin therapy.
- Citation: Yada M, Miyazaki M, Tanaka K, Masumoto A, Motomura K. Hepatocellular carcinoma or interferon-based therapy history attenuates sofosbuvir/ribavirin for Japanese genotype 2 hepatitis C virus. World J Gastroenterol 2018; 24(13): 1478-1485
- URL: https://www.wjgnet.com/1007-9327/full/v24/i13/1478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i13.1478
Hepatitis C virus (HCV) infection is a leading cause of chronic hepatitis, liver cirrhosis (LC), and hepatocellular carcinoma (HCC). In Global hepatitis report 2017[1], World Health Organization (WHO) described that 71 million persons were living with chronic HCV infection in 2015 (the global prevalence of HCV infection was 1.0%). Approximately 399000 people died each year from HCV, mostly from LC and HCC. In 2011, Japanese estimated number of persons with chronic HCV infection was 1.25 million containing 64% with genotype 1B and 35% with genotype 2, respectively[2]. WHO targets 80% of persons with HCV will be treated by 2030[1]. Combination therapy with peg-interferon (PEG-IFN) and ribavirin (RBV) was the first-line therapy for genotype 2 HCV (GT2-HCV) infection, but only 80% of patients achieved elimination of HCV with this treatment[3]. The introduction of direct-acting antiviral agents has drastically improved the efficacy of treatments for chronic HCV infection. In Japan, telaprevir (TVR), a first-generation NS3/4A protease inhibitor for GT2-HCV, was approved for clinical use in 2013. Patients received TVR (750 mg, every 8 h) for 12 wk and PEG-IFN/RBV for 24 wk. The sustained virological response (SVR) rate in patients receiving triple therapy (TVR/PEG-IFN/RBV) was reported to be 85%[4].
Subsequently, combination therapy with NS5B RNA-dependent RNA polymerase inhibitor sofosbuvir (SOF) and RBV for patients with GT2-HCV infection was approved for clinical use in June 2015. This therapy showed improved efficacy and was well tolerated in a phase 3 trial[5]. We conducted a prospective study to investigate the efficacy and safety of SOF/RBV therapy for Japanese patients with GT2-HCV infection in a real-world clinical setting.
A total of 182 patients with chronic GT2-HCV infection who received SOF/RBV therapy at Iizuka Hospital from September 2015 to January 2017 were enrolled. The patients comprised 122 men and 60 women with an age range of 17-86 years (mean ± SD: 60.1 ± 14.1 years). HCV genotype was determined by sequencing the NS5B region of the HCV genome. The results revealed 109 patients with genotype 2A and 70 patients with genotype 2B. Genotype was not determined in 3 patients. Presence of resistance-associated substitution (RAS) was analyzed by sequence determination around S282 in the NS5B region of the HCV genome. Two single nucleotide polymorphisms (SNPs), rs8099917 in the interleukin-28B (IL28B) locus associated with interferon (IFN) therapy[6] and rs1127354 in the inosine triphosphate pyrophosphatase (ITPA) locus associated with RBV-induced hemolytic anemia[7-9], were analyzed. fibrosis (FIB)-4 index was calculated as a noninvasive marker of liver fibrosis: FIB-4 index = age (years) × aspartate aminotransferase (IU/L)/[platelet count × 109/L × (alanine aminotransferase IU/L)1/2]. FIB-4 index of > 3.25 was defined as progressive fibrosis, based on a previous report[10].
SOF (Sovaldi®, Gilead Sciences Inc., Durham, NC, United States) was administered orally at a dose of 400 mg once daily, and RBV (Rebetol®, MSD, Tokyo, Japan or Copegus®, Chugai, Shizuoka, Japan) was administered orally at a dose of 200-1000 mg for 12 wk. The starting RBV dose was determined by body weight (600 mg for < 60 kg; 800 mg for 60-80 kg; 1000 mg for > 80 kg) (Figure 1).
HCV RNA levels were measured by a COBAS TaqMan test (Roche Diagnostics, Tokyo, Japan) with a lower limit of quantitation of 1.2 logIU/mL. HCV RNA was measured at screening, at day 1 and weeks 4, 8, and 12 of treatment, and at 12 wk after the end of treatment. The primary endpoint was the rate of SVR at 12 wk after the end of treatment (SVR12), defined as undetectable serum HCV RNA at this time point. Relapse and breakthrough were defined based on the guidelines of the American Association for the Study of Liver Diseases[11].
Safety evaluations included reported adverse events and serious adverse events, clinical laboratory tests, and physical examinations.
Statistical analyses were performed using JMP software version 8.0.2 (SAS Institute Inc., Cary, NC, United States). Hemoglobin levels and estimated glomerular filtration rate were compared by the Tukey honestly significant difference test. Categorical data were compared by Chi-squared test. To identify independent factors predicting non-SVR, sex, age, liver cirrhosis, and variables with values of P < 0.05 in univariate analyses were entered into a multiple logistic regression analysis. Values of P < 0.05 were considered statistically significant.
The pretreatment characteristics of all patients enrolled in the study are presented in Table 1. There were 48 patients (26.4%) with LC, 43 (23.7%) with history of IFN-based therapy, and 28 (15.4%) with history of HCC. Presence of the SNPs in IL28B (rs8099917) and ITPA (rs1127354) was examined in 174 of 182 patients. HCV RAS of S282 in the NS5B domain was not detected in the 138 patients examined by the RAS test.
| Pretreatment characteristics | Values |
| Age1, yr | 60.1 ± 14.1 |
| Age ≥ 70 yr | 27.40% |
| Sex, M : F | 122:60 |
| Body height1, cm | 163.0 ± 8.9 |
| Body weight1, kg | 62.3 ± 12.1 |
| Liver cirrhosis | 26.60% |
| History of HCC | 15.90% |
| FIB-4 index2 | 2.63 (0.45-19.03) |
| FIB-4 index > 3.25 | 40.70% |
| Wisteria floribunda agglutinin+-Mac-2 binding protein2 | 1.94 (0.20-18.51) |
| Hyaluronic acid2, ng/mL | 97.4 (10.0-2750.0) |
| History of IFN-based therapy | 23.60% |
| IL28B-SNP rs8099917 TT/non TT | 136/38/8 |
| ITPA-SNP rs1127354 CC | 129/45/8 |
| HCV genotype 2A/2B/ND | 109/70/3 |
| HCV RNA2, logIU/mL | 6.1 (1.2-7.6) |
| HCV RNA > 6 logIU/mL | 58.80% |
| White blood cell count2, /mm3 | 4575 (1700-12010) |
| Hemoglobin2, g/dL | 13.7 (10.1-17.6) |
| Platelet count2, /mm3 | 165 (38-389) × 103 |
| Aspartate aminotransferase2, IU/L | 41 (14-336) |
| Alanine aminotransferase2, IU/L | 40 (5-391) |
| Albumin2, g/dL | 4.0 (2.6-5.0) |
| Total bilirubin2, mg/dL | 0.8 (0.3-3.0) |
| Blood urea nitrogen2, mg/dL | 13 (5-28) |
| Creatinine2, mg/dL | 0.71 (0.32-1.23) |
| Estimated glomerular filtration rate2, mL/min/1.73 m2 | 78.9 (42.6-164.2) |
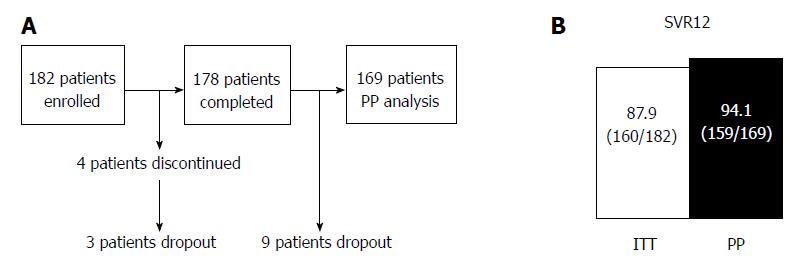
Of the 182 patients, 178 (97.8%) completed the treatment. The causes of treatment discontinuation were fatigue (n = 1; 0.5%) and self-withdrawal (n = 3; 1.6%). The rates of SVR12 were 87.9% [intention to treat (ITT): 160/182] and 94.1% [per protocol (PP): 159/169]. SVR12 was not evaluated in 12 patients (6.7%) because of dropout from the study (Figure 2).
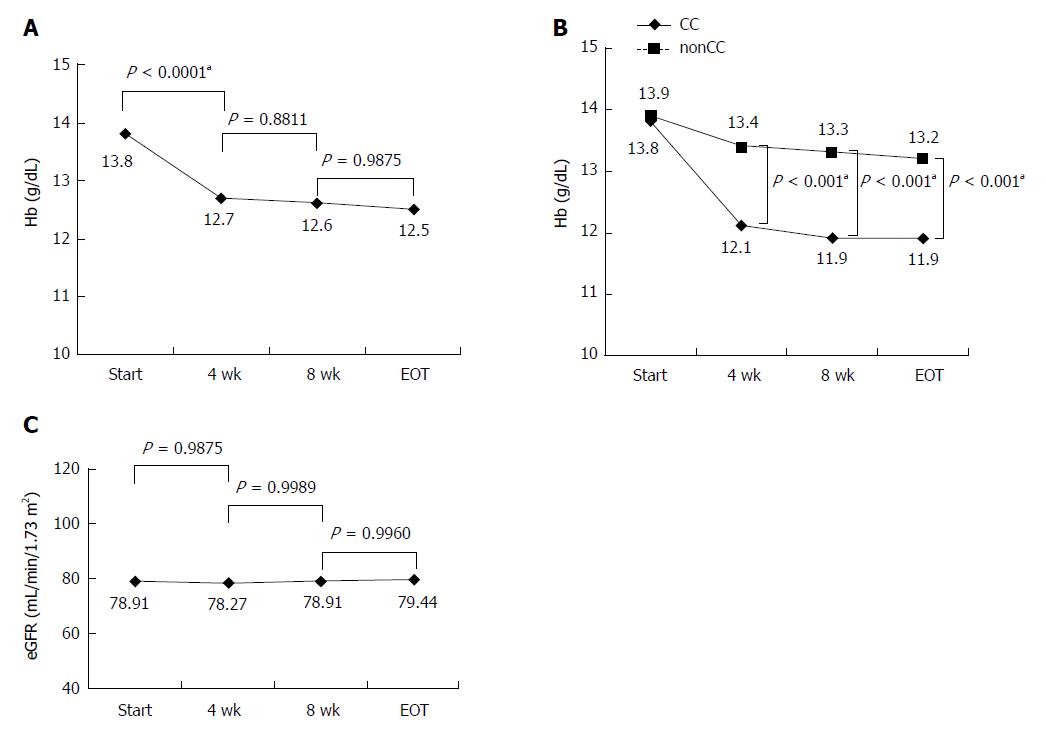
Hemoglobin levels during the treatment period were significantly reduced until 4 wk after the start of administration (Figure 3A). CC of the ITPA allele strongly contributed to anemia (Figure 3B). Twenty-nine (15.9%) of 182 patients needed RBV dose reduction because of anemia, but no patients discontinued the treatment for this reason. Although SOF and RBV cannot be used in patients with decreased renal function and we were concerned about SOF/RBV therapy decreasing the renal function of our patients, the estimated glomerular filtration rate did not change significantly during the treatment period (Figure 3C).
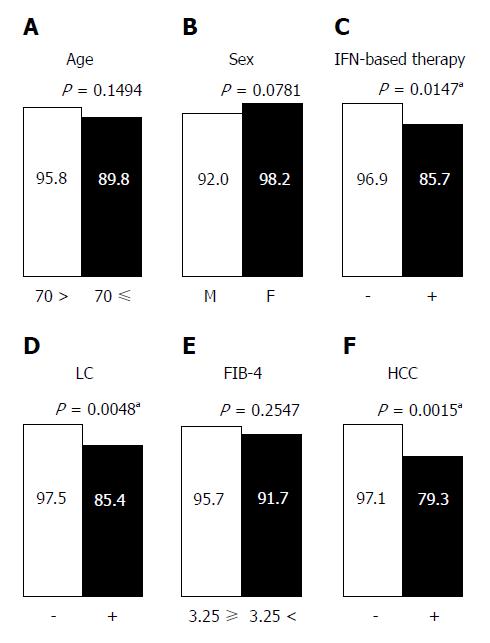
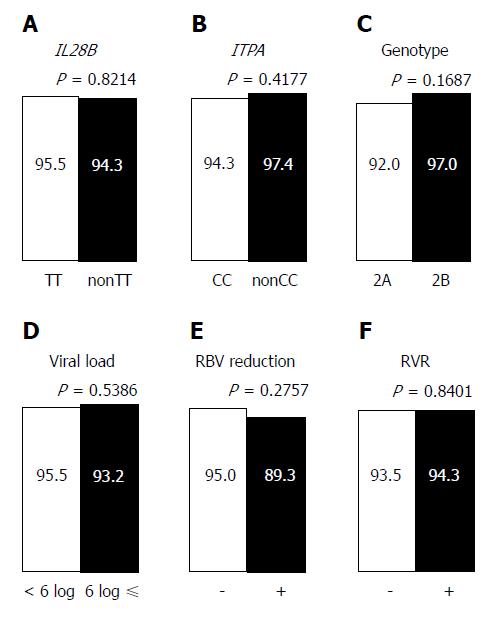
In the PP analysis, 13 of 182 patients who discontinued treatment and/or were not evaluated for SVR12 were excluded (Figure 2A). One hundred twenty three of 169 patients (72.8%) achieved rapid virological response (RVR), defined as undetectable serum HCV RNA after 4 wk of treatment. In univariate analyses, history of IFN-based therapy, LC, and history of HCC reduced the virological response, while age (≥ 70 years), sex, FIB-4 index, IL28B SNP, ITPA SNP, HCV genotype, pretreatment viral load, RBV dose reduction, and RVR had no effect on the treatment efficacy (Figures 4 and 5). The SNPs of IL28B and ITPA were determined for 161 patients in the PP analysis. The HCV viral genotype was determined in 166 patients. Multivariate analysis showed that history of HCC or IFN-based therapy was independently related to non-SVR (Table 2).
| Univariate | Multivariate | |||||
| Odds ratio | 95%CI | P value | Odds ratio | 95%CI | P value | |
| Age, ≥ 70 yr | 2.61 | 0.70, 9.82 | 0.1494 | |||
| Sex, male | 4.76 | 0.85, 88.89 | 0.0781 | |||
| History of IFN-based therapy | 5.12 | 1.39, 20.98 | 0.0147a | 7.05 | 1.65, 36.08 | 0.0084a |
| Liver cirrhosis | 6.72 | 1.78, 32.29 | 0.0048a | 2.13 | 0.31, 14.34 | 0.4315 |
| FIB-4 index, > 3.25 | 2.11 | 0.58, 8.54 | 0.2546 | |||
| History of HCC | 8.87 | 2.36, 37.04 | 0.0015a | 7.67 | 1.30, 60.30 | 0.0233a |
| IL28B-SNP, non TT | 1.21 | 0.17, 5.55 | 0.8214 | |||
| ITPA-SNP, non CC | 0.45 | 0.02, 2.63 | 0.4177 | |||
| HCV genotype, 2A | 2.78 | 0.67, 18.84 | 0.1687 | |||
| Viral load, ≥ 6 logIU/mL | 1.53 | 0.41, 7.31 | 0.5478 | |||
| RBV dose reduction | 2.29 | 0.47, 8.89 | 0.2757 | |||
| Rapid virological response | 0.86 | 0.22, 4.15 | 0.8401 | |||
We assessed 29 patients with history of HCC. We ascertained that their HCC was not detected by dynamic computed tomography or dynamic magnetic resonance imaging before initiation of SOF/RBV therapy. We compared alpha fetoprotein (AFP), latest therapy for HCC, and HCC recurrence within 1 year after the end of SOF/RBV therapy according to SVR or non-SVR (Table 3). The AFP level did not differ significantly between the two groups. Patients achieving SVR tended to be treated by surgical resection or radiofrequency ablation, and also tended to have no HCC recurrence.
| SVR (n = 23) | Non SVR (n = 6) | P value | |
| Alpha fetoprotein | 9.6 (1.7-348.9) | 5.3 (2.6-65.7) | 0.2815 |
| Latest treatment for HCC | 0.2558 | ||
| Surgical resection or Radiofrequency ablation | 73.9% (17) | 66.6% (6) | |
| Transcatheter arterial chemoembolization | 26.1% (6) | 16.7% (1) | |
| Radiation for bone metastasis | 0% (0) | 16.7% (1) | |
| Recurrent HCC within 1 yr from the end of SOF/RBV | 65. 2% (15) | 83.3% (5) | 0.6328 |
Combination therapy with SOF/RBV was the first IFN-free therapy for GT2-HCV infection approved for clinical use in Japan. In a phase 3 trial, 97% of patients achieved SVR12 and no patients discontinued the treatment[5]. Thus, we expected high efficacy and tolerability, and conducted a prospective study in a real-world clinical practice setting to investigate the efficacy and safety of this combination therapy for Japanese patients with GT2-HCV infection.
Hemoglobin levels rapidly reduced until 4 wk after the start of treatment. ITPA CC allele significantly contributed to anemia in this therapy, similar to the case for combination therapy of PEG-IFN and RBV[7-9]. Therefore, patients with the ITPA CC allele should be monitored frequently for their hemoglobin levels until 4 wk after the start of treatment. Despite our concern that SOF and RBV may decrease renal function of the patients, renal function degeneration was not observed in any of the patients. Many elderly patients (27.4% were aged ≥ 70 years) and cirrhotic patients (26.6% had liver cirrhosis) were enrolled in this study. Thus, the present findings demonstrate that SOF/RBV therapy is safe and highly tolerated regardless of age and LC.
The ITT rate in this study was low compared with the rates in phase 3 clinical trials of SOF/RBV for Japanese[5] and European[12] patients with GT2-HCV infection. The reason for the difference was that SVR12 could not be evaluated in 12 patients (6.7%) because of dropout from the study. It is important to monitor carcinogenesis after virus elimination, and it is therefore necessary to provide patients with instructions that promote periodic examinations. Meanwhile, the PP rate was almost equal to the rates in the phase 3 clinical trial of SOF/RBV for Japanese patients with GT2-HCV infection[5] and in real-world cohorts in North America and Europe[13]. This finding shows that SOF/RBV therapy for Japanese patients with GT2-HCV infection is highly effective in real-world clinical practice.
In a meta-analysis, Rangnekar et al[14] reported that IL28B SNP was predictor of SVR in Caucasian patients with GT2-HCV infection receiving PEG-IFN/RBV for 24 wk. In contrast, IL28B SNP was not associated with SVR in Asian patients with GT2-HCV infection. This study also showed Japanese IL28B SNP had no relation to efficacy of SOF/RBV for GT2-HCV infection. Morisco et al[15] reported RVR was the only independent predictive factor of SVR in triple therapy (TVR/PEG-IFN/RBV) regardless of LC. In the present study, RVR did not affect efficacy of SOF/RBV for GT2-HCV infection. The multivariate regression analysis showed that history of HCC or IFN-based therapy was independently related to non-SVR. LC also reduced the efficacy in univariate analyses. It was previously reported that the efficacy of IFN-based therapy was inferior in LC patients[16,17]. Furthermore, it was reported that patients with progressive liver fibrosis have a high probability of hepatocellular carcinogenesis[18-21]. Prenner et al[22] reported that presence of active HCC at the start of direct acting antivirals, including SOF/RBV therapy, decreased the SVR rate. In our study, the proportion of non-SVR patients treated by surgical resection or radiofrequency ablation tended to be lower than the proportion of non-SVR patients treated by transcatheter arterial chemoembolization or radiation for bone metastasis. Meanwhile, the rate of HCC recurrence within 1 year tended to be higher in non-SVR patients than that in SVR patients. These findings show a high probability that active HCC was not detected by dynamic computed tomography or dynamic magnetic resonance imaging at the start of SOF/RBV therapy. Prenner et al[22] discussed that a putative biological explanation could be inadequate drug delivery by decreased blood flow or local fibrosis arising from HCC treatment. The present results suggested that liver fibrosis attenuated the antiviral effect of SOF/RBV in patients with history of IFN-based therapy or HCC. Although the FIB-4 index, a fibrosis marker[10], had no effect on efficacy in the present study, the reason was unclear. History of IFN-based therapy and LC had no significant effect on SOF/RBV therapy in the phase 3 clinical trial for European patients with GT2-HCV infection[12]. However, it was reported that LC and lower serum albumin decreased the SVR rate in the real-world cohorts in North America and Europe[13]. Treatment-experienced patients tended to have ineffective outcomes in that study. These findings were similar to those in the present study. The real-world studies included larger populations of LC (26.6% in the present study and 26.8% in the North American and European study) than the phase 3 trials (11% in the Japanese trial[5] and 15% in the European trial[12]. Our study also enrolled patients with history of HCC (15.9%) and elderly patients aged ≥ 70 years (27.4%). It is suggested that the real-world studies enrolled more patients with severe fibrosis than the phase 3 clinical trials. As viral breakthrough did not occur in any of the non-SVR patients and all of these patients had relapses, it is suggested that SOF/RBV therapy was not ineffective for these patients. Extension of the treatment period for patients with history of IFN-based therapy, HCC, or LC should be considered to increase the efficacy of the therapy. As active HCC not detected by imaging was probably related to non-SVR, monitoring is required for carcinogenesis and recurrence of HCC after the end of SOF/RBV therapy, especially in non-SVR patients.
In conclusion, SOF/RBV therapy for Japanese patients with GT2-HCV infection is safe, highly tolerated, and effective. History of HCC or IFN-based therapy independently reduces the efficacy of the treatment.
Combination therapy with peg-interferon (PEG-IFN) and ribavirin (RBV) was the first-line therapy for genotype 2 hepatitis C virus (HCV) infection, but only 80% of patients achieved elimination of HCV with this treatment. The introduction of direct-acting antiviral agents has drastically improved the efficacy of treatments for chronic HCV infection. Combination therapy with NS5B RNA-dependent RNA polymerase inhibitor sofosbuvir (SOF) and RBV for patients with genotype 2 hepatitis C virus (GT2-HCV) infection was approved for clinical use in June 2015.
This therapy showed improved efficacy and was well tolerated in a phase 3 trial. However, predictive factor of sustained virological response (SVR) is not unclear.
We conducted a prospective study to investigate the efficacy and safety of sofosbuvir/ribavirin (SOF/RBV) therapy for Japanese patients with GT2-HCV infection in a real-world clinical setting.
A total of 182 patients with GT2-HCV infection who received SOF/RBV therapy for 12 wk at our hospital were enrolled. The patients comprised 122 men and 60 women (age range: 17-84 years; mean age ± SD: 60.1 ± 12.1 years). One hundred sixty nine of 182 patients completed 12 wk treatment and were examined their virological response. To investigate predictive factors of SVR, we examined the relationships between virological response and clinical data by logistic regression analyses.
The rates of SVR at 12 wk after the end of treatment were 87.9% (intention to treat: 160/182) and 94.1% (per protocol: 159/169). Multivariate analyses showed that history of hepatocellular carcinoma (HCC) or IFN-based therapy independently reduced the efficacy of SOF/RBV therapy.
This study showed Japanese IL28B single nucleotide polymorphisms had no relation to efficacy of SOF/RBV for GT2-HCV infection. Morisco et al[15] reported rapid virological response (RVR) was the only independent predictive factor of SVR in triple therapy (Telaprevir/PEG-IFN/RBV) regardless of cirrhosis. In the present study, RVR did not affect efficacy of SOF/RBV for GT2-HCV infection. The multivariate regression analysis showed that history of HCC or IFN-based therapy was independently related to non-SVR. In our study, the proportion of non-SVR patients treated by surgical resection or radiofrequency ablation tended to be lower than the proportion of non-SVR patients treated by transcatheter arterial chemoembolization or radiation for bone metastasis. Meanwhile, the rate of HCC recurrence within 1 year tended to be higher in non-SVR patients than that in SVR patients.
Prenner et al reported that presence of active HCC at the start of direct acting antivirals, including SOF/RBV therapy, decreased the SVR rate. As active HCC not detected by imaging was probably related to non-SVR, monitoring is required for carcinogenesis and recurrence of HCC after the end of SOF/RBV therapy, especially in non-SVR patients.
We are grateful to Ishibashi Y for assistance with manuscript preparation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abenavoli L, Dogan UB, El-Shabrawi MHF, Esmat S, Waheed Y S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | World Health Organization. Global hepatitis report, 2017. 2017; Available from: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. [Cited in This Article: ] |
| 2. | Liakina V, Hamid S, Tanaka J, Olafsson S, Sharara AI, Alavian SM, Gheorghe L, El Hassan ES, Abaalkhail F, Abbas Z. Historical epidemiology of hepatitis C virus (HCV) in select countries - volume 3. J Viral Hepat. 2015;22 Suppl 4:4-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Sato K, Hashizume H, Yamazaki Y, Horiguchi N, Kakizaki S, Takagi H, Mori M; Gunma Liver Study Group. Response-guided peginterferon-alpha-2b plus ribavirin therapy for chronic hepatitis C patients with genotype 2 and high viral loads. Hepatol Res. 2012;42:854-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Kumada H, Sato K, Takehara T, Nakamuta M, Ishigami M, Chayama K, Toyota J, Suzuki F, Nakayasu Y, Ochi M. Efficacy of telaprevir-based therapy for difficult-to-treat patients with genotype 2 chronic hepatitis C in Japan. Hepatol Res. 2015;45:745-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, Toyoda H, Yokosuka O, Nirei K, Genda T. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21:762-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1747] [Cited by in F6Publishing: 1746] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 7. | Fellay J, Thompson AJ, Ge D, Gumbs CE, Urban TJ, Shianna KV, Little LD, Qiu P, Bertelsen AH, Watson M. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature. 2010;464:405-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 359] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 8. | Ochi H, Maekawa T, Abe H, Hayashida Y, Nakano R, Kubo M, Tsunoda T, Hayes CN, Kumada H, Nakamura Y. ITPA polymorphism affects ribavirin-induced anemia and outcomes of therapy--a genome-wide study of Japanese HCV virus patients. Gastroenterology. 2010;139:1190-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Thompson AJ, Fellay J, Patel K, Tillmann HL, Naggie S, Ge D, Urban TJ, Shianna KV, Muir AJ, Fried MW. Variants in the ITPA gene protect against ribavirin-induced hemolytic anemia and decrease the need for ribavirin dose reduction. Gastroenterology. 2010;139:1181-1189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 2998] [Article Influence: 166.6] [Reference Citation Analysis (0)] |
| 11. | Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2252] [Cited by in F6Publishing: 2192] [Article Influence: 146.1] [Reference Citation Analysis (1)] |
| 12. | Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993-2001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 638] [Cited by in F6Publishing: 629] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 13. | Welzel TM, Nelson DR, Morelli G, Di Bisceglie A, Reddy RK, Kuo A, Lim JK, Darling J, Pockros P, Galati JS. Effectiveness and safety of sofosbuvir plus ribavirin for the treatment of HCV genotype 2 infection: results of the real-world, clinical practice HCV-TARGET study. Gut. 2017;66:1844-1852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Rangnekar AS, Fontana RJ. IL-28B polymorphisms and the response to antiviral therapy in HCV genotype 2 and 3 varies by ethnicity: a meta-analysis. J Viral Hepat. 2013;20:377-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Morisco F, Masarone M, Rosato V, Camera S, Granata R, Tartaglione MT, Coppola C, Coppola N, Salomone-Megna A, Gentile I. Impact of Telaprevir in HCV Patients with Cirrhosis and RVR: Real-Life Data from Boceprevir or Telaprevir based “Triple Therapy” Experience in Southern Italy. Rev Recent Clin Trials. 2016;11:306-316. [PubMed] [Cited in This Article: ] |
| 16. | Kaserer K, Fiedler R, Steindl P, Müller CH, Wrba F, Ferenci P. Liver biopsy is a useful predictor of response to interferon therapy in chronic hepatitis C. Histopathology. 1998;32:454-461. [PubMed] [Cited in This Article: ] |
| 17. | Akuta N, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Arase Y. Predictors of viral kinetics to peginterferon plus ribavirin combination therapy in Japanese patients infected with hepatitis C virus genotype 1b. J Med Virol. 2007;79:1686-1695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Zaman SN, Melia WM, Johnson RD, Portmann BC, Johnson PJ, Williams R. Risk factors in development of hepatocellular carcinoma in cirrhosis: prospective study of 613 patients. Lancet. 1985;1:1357-1360. [PubMed] [Cited in This Article: ] |
| 19. | Shiffman ML. Natural history and risk factors for progression of hepatitis C virus disease and development of hepatocellular cancer before liver transplantation. Liver Transpl. 2003;9:S14-S20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Ikeda M, Fujiyama S, Tanaka M, Sata M, Ide T, Yatsuhashi H, Watanabe H. Risk factors for development of hepatocellular carcinoma in patients with chronic hepatitis C after sustained response to interferon. J Gastroenterol. 2005;40:148-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Tokita H, Fukui H, Tanaka A, Kamitsukasa H, Yagura M, Harada H, Okamoto H. Risk factors for the development of hepatocellular carcinoma among patients with chronic hepatitis C who achieved a sustained virological response to interferon therapy. J Gastroenterol Hepatol. 2005;20:752-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Prenner SB, VanWagner LB, Flamm SL, Salem R, Lewandowski RJ, Kulik L. Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. J Hepatol. 2017;66:1173-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |