Published online Apr 7, 2018. doi: 10.3748/wjg.v24.i13.1373
Peer-review started: January 28, 2018
First decision: February 10, 2018
Revised: February 16, 2018
Accepted: February 26, 2018
Article in press: February 26, 2018
Published online: April 7, 2018
Drug-induced liver injury (DILI) has become a major topic in the field of Hepatology and Gastroenterology. DILI can be clinically divided into three phenotypes: hepatocytic, cholestatic and mixed. Although the clinical manifestations of DILI are variable and the pathogenesis complicated, recent insights using improved preclinical models, have allowed a better understanding of the mechanisms that trigger liver damage. In this review, we will discuss the pathophysiological mechanisms underlying DILI. The toxicity of the drug eventually induces hepatocellular damage through multiple molecular pathways, including direct hepatic toxicity and innate and adaptive immune responses. Drugs or their metabolites, such as the common analgesic, acetaminophen, can cause direct hepatic toxicity through accumulation of reactive oxygen species and mitochondrial dysfunction. The innate and adaptive immune responses play also a very important role in the occurrence of idiosyncratic DILI. Furthermore, we examine common forms of hepatocyte death and their association with the activation of specific signaling pathways.
Core tip: Drug-induced liver injury (DILI) represents a broad spectrum of clinical manifestations, and is generally divided into two subtypes: intrinsic and idiosyncratic hepatotoxicity. Drugs and their reactive metabolites covalently bind to mitochondria and cause direct hepatic toxicity through accumulation of oxidative stress (ROS and RNS), endoplasmic reticulum stress and mitochondrial dysfunction, ultimately leading to cell death. The innate and adaptive immune responses also play an important role in the occurrence of idiosyncratic immunological reactions towards the drugs. In this review, we discuss the pathophysiological mechanisms underlying DILI, specific signaling pathways and the common forms of hepatocyte death.
- Citation: Ye H, Nelson LJ, Gómez del Moral M, Martínez-Naves E, Cubero FJ. Dissecting the molecular pathophysiology of drug-induced liver injury. World J Gastroenterol 2018; 24(13): 1373-1385
- URL: https://www.wjgnet.com/1007-9327/full/v24/i13/1373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i13.1373
Drug-induced liver injury (DILI) is the most common cause of acute liver failure (ALF) in the United States and Europe[1], and is a leading reason for drug withdrawal and the high attrition rates in drug development (Table 1). In addition, the incidence of DILI has continued to rise and is therefore recognized as a major public health concern[2]. DILI is one of the most common and serious adverse drug reactions (ADRs)[3], and is defined as a chemical insult resulting in injury to the liver[4]. It can be triggered by the parent drug and/or its metabolites, or as a reaction of hypersensitivity to the compound. A wide variety of drugs can cause DILI, including anti-tumor chemotherapy drugs, anti-tuberculosis drugs, antipyretic analgesics, immunosuppressive agents, hypoglycaemic therapies, or anti-bacterial, anti-fungal and antiviral drugs. DILI leads to multiple presentations in the clinic, including elevated liver enzymes, hepatitis, hepatocellular necrosis, cholestasis, fatty liver and liver cirrhosis. Occasionally, the clinical symptoms are not specific and they are indistinguishable from other hepatic disorders. In some patients, liver injury is easily detected by blood tests. The wide range of clinical manifestation, the complication of aetiology and the lack of effective tests make its diagnosis and treatment particularly challenging.
| Country | France | Iceland | South Korea | Spain | United Kingdom | United States | Sweden |
| DILI incidence (‰) | 0.139 | 0.191 | 0.12 | 0.03 | 0.007-0.013 | 0.10-1.50 | 0.023 |
DILI is generally divided into two subtypes according to the hepatotoxicity of the drug: “intrinsic” hepatotoxicity and “idiosyncratic” hepatotoxicity. The former refers to dose-dependent hepatotoxicity that is predictable in humans or animal models, while idiosyncratic DILI (iDILI) is an unpredictable injury that cannot be explained by the known pharmacological properties. Recently, the screening of new drugs has become more stringent and the monitoring of ADRs improved. Problems associated with DILI have become a major driver in the development of new medications, and for the withdrawal, restriction or project termination of existing drugs and drug compound candidates. In developed countries, iDILI is less common, occurring only very rarely among treated patients, while intrinsic hepatotoxicity is still a main cause of DILI[5,6]. The pathogenesis of DILI is a complex process that has recently attracted much attention. Some researchers recently proposed a new hypothesis, providing a clear framework and direction for the further study of DILI[7]. According to this hypothesis, drug-induced liver injury can be divided into three steps: an initial insulting stimulation causes the mitochondrial dysfunction, and ultimately leads to cell death. However, until now, the exact mechanism remains unclear. For the purpose of preventing DILI and improving clinical management, the study of the pathogenesis of DILI is particularly important. In this article, we will review and discuss recent progress towards understanding the underlying mechanisms triggering DILI.
The liver plays an important role in the metabolism of drugs or exogenous toxicants, and the majority of drugs are biologically transformed in the liver. The pathological state of the liver can affect drug metabolism, thus changing the efficacy and the ADRs, whilst the metabolic products of drugs can cause liver damage.
The cytochrome P450s (CYP) are a superfamily of iron porphyrin proteins, which are key factors involved in drug oxidative and reduction reactions. Through the P450s, drugs are metabolized and can form ions, oxygen free radicals and other active substances. The balance between toxic formation and detoxification is essential for DILI. Toxins are inactivated by the detoxification phase I-III pathways of the liver. However, once the amount of toxins exceeds the capacity of the hepatic detoxification function, drugs and their reactive metabolites impact cell function - leading to liver cell damage - eventually causing apoptotic or necrotic cell death. At present, the most frequently studied drug, which causes intrinsic DILI, is acetaminophen (APAP), which is also known as paracetamol[8].
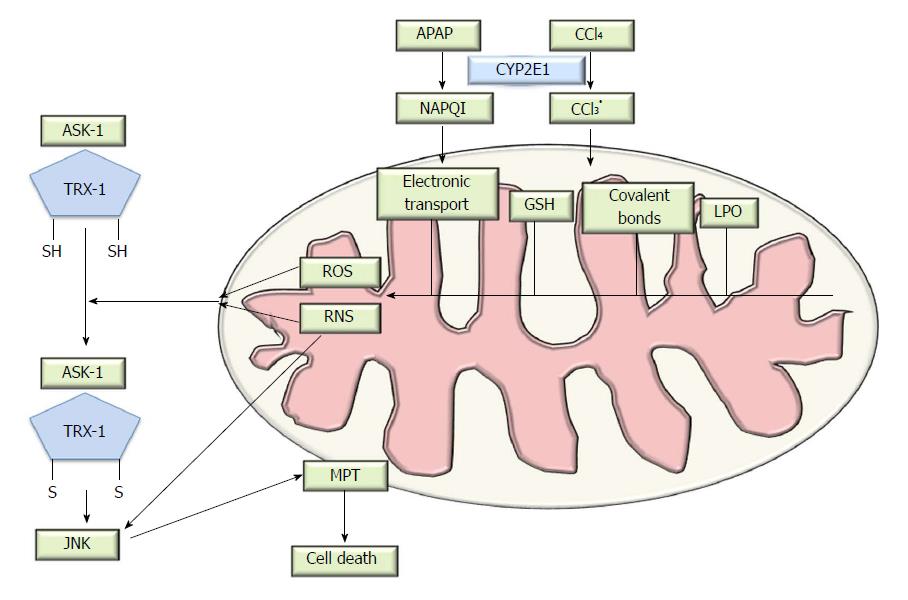
Upon rapid absorption, APAP is mainly metabolized via Phase-I reactions (sulfation or glucuronidation) and then excreted into the urine. APAP toxicity is caused mainly by the excess formation of the reactive intermediate, N-acetyl-p-benzoquinone imine (NAPQI)[9], as a result of CYP (predominately CYP2E1 and CYP1A2) metabolism. Under normal circumstances, NAPQI is detoxified by rapid conjugation with the hepatic glutathione (GSH) and excreted into the bile, thus, APAP usage is nontoxic. Following overdose, APAP saturates both the sulfation and the glucuronidation pathways[10], enhanced NAPQI production depletes mitochondrial GSH, and the excess NAPQI then reacts with sulfhydryl groups of proteins to form protein adducts[11]. The interaction of NAPQI with target DNA and proteins in the mitochondria and the formation of protein adducts is thought to be critical for the development of hepatic toxicity[12,13], leading to oxidative stress, mitochondrial dysfunction[14,15] and mitogen-activated protein kinase (MAPK) activation (Figure 1). Specific targets in the mitochondria, including glutathione peroxidase (GPx) and the alpha subunit of adenosine triphosphate (ATP) synthase, participate in adduct formation, which was identified using proteomic approaches[16]. Furthermore, some drugs lead to the obstruction of the bile duct and mediate inhibition of hepatobiliary transporter systems[17]. Bile salt export pump (BSEP) is an efflux transporter of bile acids (BAs) transport and responsible for the clearance of drugs from liver and the secretion of bile salts into bile. The inhibition of BSEP expression has profound effects on bile acid homeostasis[18]. The cytotoxic bile acids accumulating in the liver results in liver cell damage, and potentially cirrhosis[17].
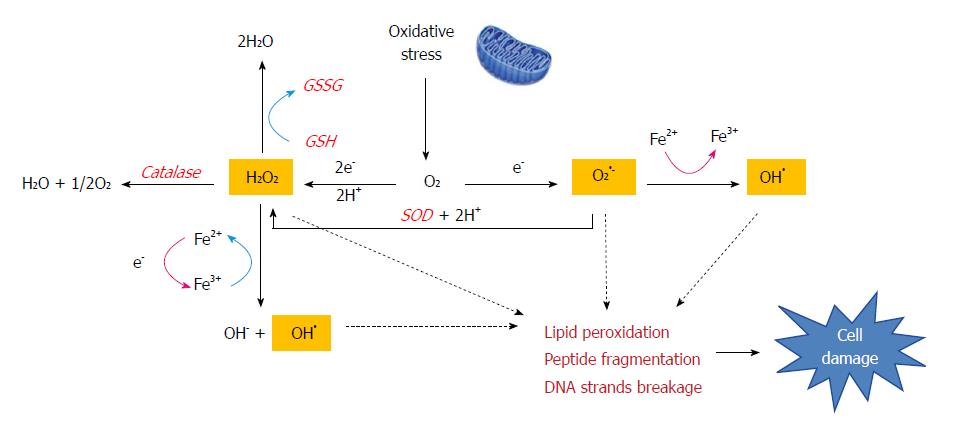
Oxidative stress is the result of the generation of ROS, which are a by-product of normal metabolism and have roles in cell signaling and homeostasis. Some DILI-causing drugs increase ROS accumulation through a variety of mechanisms[19]. Iron overload also amplifies oxidative stress as a catalyst for ROS formation via the Fenton reaction, in which H2O2 splits into hydroxyl radicals (OH•) and hydroxide (OH-) (Figure 2). Free radical metabolites participate in the redox process and are capable of inducing cell damage by covalently binding to macromolecules[20]. Moreover, radical species can oxidize essential cell components and result in mutations in genomic and mitochondrial DNA (p21, p53) and tumor generation.
The role of lipid peroxidation (LPO) remains controversial in APAP hepatoxicity, and is often considered to be involved in cell death[21]. However, APAP overdose causes severe liver damage but a minor increase in the levels of LPO in normal animals[22]. Thus it seems that lipid peroxidation is not a critical event in APAP-induced hepatotoxicity. The cell injury induced by LPO requires not only oxidant formation but also impairment of the antioxidant defense systems. Additionally, LPO can be a consequence of tissue injury rather than the cause[23].
Given a toxic dose of APAP, histological necrosis is evident in the liver at 4 h, and tyrosine nitration occurs, indicating peroxynitrite formation[24]. Enhanced production of superoxide radicals (O2●-) reacts with nitric oxide (NO), produced by inducible nitric oxide synthase (iNOS), forming peroxynitrite (ONOO-)[25]. Since the O2●- anion scarcely passes through the hepatocyte cell membrane, this process occurs exclusively within the mitochondria. The highly reactive and potent oxidant ONOO- also causes nitration of protein tyrosine residues[26] which induces damage to mitochondrial DNA and the opening of the mitochondrial membrane pore.
Mitochondrial oxidative stress alone is not sufficient to ultimately trigger mitochondrial membrane permeability transition (MPT) and induce cell death. A group of protein kinases known as the mitogen-activated protein kinases (MAPKs), one of the most actively studied kinases or signaling pathways, participates in this process. Conventional studies have shown that MAPK pathways include many proteins such as the extracellular signal-related kinases (ERK), c-Jun N-terminal kinases (JNKs) and p38[27]. The JNK genes, JNK1 and JNK2, are expressed in the liver. A dysregulation of JNK1 and JNK2 protein expression is characteristic in both human and murine models of DILI, and is a potential therapeutic target[28]. JNK activation occurs early after APAP overdose and is sustained during the process, inducing hepatocyte death. JNK activation has been found in both hepatocytes and infiltrating cells, and is mediated by MAP kinase kinases (MAP2K)[29], which in turn are phosphorylated and activated by MAP kinase kinase kinases (MAP3K). The apoptosis signal-regulating kinase-1 (ASK1) is involved in APAP-induced JNK elevation[30] and activated by the dissociation with thioredoxin-1(Trx-1). The mixed-lineage kinase-3 (MLK3), a member of serine/threonine protein kinases family, mediates the initial phase of JNK activation[31]. ASK1 and MLK belong to the MAP3K group and different MAP3K group members function cooperatively in the response to oxidative stress. The role of MAP3K in JNK regulation still requires to be further investigated; whilst the activation of JNK can also be influenced by the dose of APAP[32]. The fact that RIP3-deficiency prevented oxidant stress suggests that RIPK3 acted upstream of JNK activation[33]. After JNK activation and phosphorylation in the cytosol, JNK binds to the Sab protein on the outer mitochondrial membrane[34,35], leading to the inactivation of p-Src on the inner mitochondrial membrane, which inhibits electron transport and increases ROS generation and further mitochondrial injury[36]. Ultimately, the pJNK translocates to the mitochondria and results in downstream signaling events[34].
Mitochondrial dysfunction is the main cause of hepatocellular necrosis. The amplification of mitochondrial oxidative stress can reduce the synthesis of mitochondrial proteins and increase mitochondrial permeability transition. The induction of MPT increases mitochondrial membrane permeability allowing the exit of molecules less than 1500 Daltons[37], which causes mitochondria to become further depolarized, thus reducing the proton gradient leading to the collapse of the mitochondrial membrane potential (MMP). The mitochondria then swell, rupture and release proteins from the inter-membrane space[38], a sequence implicated in cell death pathways such as apoptosis[39,40]. ROS are also produced because of the opening of the MPT pore, in turn, exaggerating oxidative stress and inducing DNA damage. Furthermore, the β-oxidation respiratory chain is compromised, and the process of ATP production is disrupted, resulting in reduced energy [41].
Various cellular stresses such as ROS or alteration in the cellular calcium (Ca2+) concentration can impair protein folding and initiate the endoplasmic reticulum (ER) stress, which plays a critical role in APAP-induced hepatotoxicity[42]. Efficient protein folding in the ER requires tight coupling between the subunits of new proteins in the ER lumen and the ER folding capacity[43]. If the demand for protein folding increases, unfolded or misfolded proteins in the lumen also increase. ER stress is induced late after APAP intoxication (500 mg/kg) in murine models, and becomes significant 12 h following APAP administration[44]. The mechanisms by which APAP induces ER stress are poorly understood. One hypothesis is the alteration in the microsomes secondary to NAPQI generation. It has been reported that APAP induces an oxidative shift of the ER oxidoreductases, Erp72 and protein disulfide isomerase (PDI) in liver microsomes[45]. Furthermore, NAPQI can covalently bind to several microsomal proteins such as PDI and calreticulin, which have a significant role in protein folding in the ER, thus inducing ER stress. Another hypothesis suggests that ER stress might be due to ROS overproduction and mitochondrial dysfunction[46], including loss of the MMP and increase in intracellular Ca2+ concentration. Inhibition of BSEP results in not only cholestasis in some cases, but importantly, via bile acid retention, causes mitochondrial and ER stress, which may amplify injury or sensitize hepatocytes to other injury mechanisms[47].
iDILI is a rare ADR[48], and occurs with a variable latency to onset, usually after several weeks or months of continuous treatment with the offending drug but, more importantly, it is unpredictable[49]. The incidence of iDILI ranges from 1/1000 to 1/200000[50], depending on the agent. The diagnosis of iDILI relies on the exclusion of other causes of liver injury and detailed medical history. The mechanisms of iDILI have not yet been elucidated. Although it is thought that iDILI is not dose-related, recent studies have supported the prediction of dose-response to some extent[51]. In general, it is associated with host condition, behavioural factors and drug exposure. Amongst behavioural factors, excessive alcohol consumption and smoking are very common triggers of iDILI. The host factors include genetic and non-genetic-derived iDILI. For example, genetically, it is considered that iDILI is caused by the deficiency or low activity of drug-metabolizing enzymes and an abnormal immune response. Non-genetic types include existing disease states, pregnancy, age and host gender. In some iDILI reactions, the same mechanisms of intrinsic DILI are involved: ROS, mitochondrial dysfunction and altered bile acid homeostasis. The typical drugs are tacrine and stavudine. Additionally, in some iDILI, after exposure to certain drugs, neoantigens are produced in the liver and can mobilize the immune cells and result in idiosyncratic immunological reactions towards the drugs.
As a result of hepatocyte damage, iDILI triggers the inflammatory reaction, which involves the innate immune system. The innate immune system in the human liver is mainly composed of Kupffer cells (KCs), neutrophils, monocytes and natural killer cells/natural killer T cells (NK/NKT cells)[52,53]. In recent years, increasingly studies have confirmed that the innate immune system participates in the pathogenesis of iDILI, but the specific mechanism is still on the controversy. The main hypothesis is that neoantigen stimulates the cells of the innate immune system and creates inflammation by binding to Toll-like receptors (TLRs), scavenger receptors (SCRs) and mannitol receptors (MRs) of macrophages. In patients with iDILI, a large number of macrophages are mobilized in the blood and assemble around the damaged hepatocytes via adhesion factors. The proliferation of macrophages is also seen in the bone marrow[54]. The depletion of KC reduces the expression of IL-10, IL-6 and other mediators, and increases APAP-induced liver injury. Overall, the activation of KC is beneficial because the anti-inflammatory effects outweigh potential toxic effects[55]. Antigens derived from damage associated molecular patterns (DAMPs)[56] act as signals to activate innate immune cells. High mobility group box 1 protein (HMGB1) is one of the previously identified DAMPs. HMGB1 induces the infiltration of neutrophils, associates with TLRs and promotes the release of cytokines such as TNFα, IFNγ and IL-1, thereby activating the KC[57] and aggravating iDILI. In addition, the controversy surrounds the role of NK/NKT cells. Some researchers believe that NK/NKT cells ameliorate the liver injury caused by drugs through secreting IFNγ, IL-4 and other cytokines. However, some authors have reported no significant differences in the expression level of protective cytokines from the liver of NKT-cell-deficient mice[58]. In addition, the released cytokines and chemokines can enhance the adaptive immune response through a variety of mechanisms.
The finding that the liver injury recurs promptly after the iDILI patient is re-exposed to the offending drug, reflects the involvement of an adaptive immune response. This is in fact predictable, given that the antigen-specific lymphocytes still remain in the body. During the process of drug metabolism, drug metabolite covalently binds to hepatic protein or modified proteins expressed on the surface of hepatocytes and form protein haptens (essentially an incomplete antigen). The hapten is released after hepatocyte death or damage and presented by antigen-presenting cells (APCs) in complex with major histocompatibility complex (MHC) class II molecules to cluster of differentiation 4 CD4+ T cells. When recognized as ‘foreign’ by T cells and following binding to T-cell receptors of CD4+T cells, it then activates cluster of differentiation 8 CD8+ T cytotoxic cells via secretion of TNFα and IFNγ. CD8+T cytotoxic cells mediate cytotoxic reactions though FasL or perforin and induce hepatocellular apoptosis. The anaesthetic drug Halothane exactly triggers this mechanism. Under normal conditions, hapten alone is not sufficient to activate the immune response, therefore activation of the adaptive immune system requires other cell/ tissue threatening events. This is termed the ‘Hypothesis of danger signalling’. Indeed, it has been shown that the presence of an inflammatory background is associated with increased susceptibility to iDILI[59,60]. Infection and inflammation act as the danger signal and further augment the immune response by cell death or cytokine release[61]. However, the specific mechanisms still need further study.
The “hapten” hypothesis is a dominant mechanism proposed for the creation of neoantigens after drug exposure[62]. More recently, the ‘pharmacological interaction’ or ‘p-i’ model suggests a new hypothesis for activating T-cell-mediated immune responses[63]. The drug directly binds to either the T-cell receptor (TCR) or human leucocyte antigen (HLA) without intracellular antigen processing[64-66], and activates T cells in a peptide-independent manner[67]. This hypothesis might also explain the rapid reaction of T-cells after drug exposure in vitro, which is inconsistent with the time-course of antigen processing in vivo. Classic drugs that are considered to respond in this way are sulfamethoxazole, lamotrigine and carbamazepine.
A genome-wide association study (GWAS) proved that iDILI is associated with the HLA region on chromosome 6[68,69]. The HLA polymorphism results in the human body to be more prone to produce adaptive immune responses to certain drugs[70]. HLA genotyping of 75 amoxicillin-clavulanate hepatotoxicity cases in Spain has also demonstrated phenotype-specific HLA association[71]. Abacavir, a human immunodeficiency virus reverse transcriptase inhibitor, induces multi-organ toxicity exclusively in patients carrying the HLA-B*57:01 allele[72]. A GWAS on flucloxacillin hepatotoxicity (FLUX-DILI) has revealed a strong association with the HLA-B*57:01 allele[73]. Flucloxacillin is an effective antimicrobial drug against staphylococcal infections and widely used in Europe and Australia. The incidence of cholestatic hepatitis, which is induced by the use of flucloxacillin, is estimated to be 8.5 per 100000 in the first 1 to 45 d after start of treatment[74]. However, the incidence in the HLA-B*57:01 allele carriers raises more than 3-fold, indicating the HLA-B*57:01 have an added effect on FLUX-DILI[75].
Hepatocyte stress can be detected in the majority of individuals exposed to the insulting drug, especially at high concentrations. However, injury occurs in only a very small number of individuals. Although the liver is considered itself to be an immune-tolerant organ, the variation of susceptibility to the ensuing stress response(s) still exists; only in individuals with low tolerance, will DILI occur. The tolerance phenomenon due to liver immunity can be explained by: apoptosis of activated T cells, immune deviation and immune active suppression[53]. Antigen-specific CD8+ T-cell populations accumulate transiently within the liver before apoptosis[76]. It is possible that the liver can induce apoptosis of activated T cells through toxic molecules or the deprivation of survival signals[77]. Hepatocytes can attract apoptotic T cells because specific markers are expressed in the membrane and are recognized by KCs or other cells in the liver. During the hepatic immune responses, there is immune deviation occurring. Klugewitz et al[78], reported that the liver sinusoidal endothelial cells (LSECs) can selectively inhibit T helper-1 (Th1) cells and reduce the production of INF-γ, but LSECs can also activate T helper-2 (Th2) cells leading to an increase in IL-4 secretion. The third mechanism is the result of the unique composition of tolerogenic APCs in the liver. The tolerogenic APCs within the liver include LSECs, KCs, and hepatic dendritic cells (DCs). Although recognized as APCs, these cells are incapable of stimulating antigen-specific T-cell responses[79]. On the contrary, they trap and interact with the inactive T cells in the liver sinusoid, thereby promoting tolerance. LSECs can act as APCs to some extent, but CD4+ or CD8+ T cells activated by them cannot further differentiate into Th1 cells or cytotoxic cells. Moreover, hepatic KCs can also suppress T-cell activation through the production of prostaglandins[80].
The traditionally recognized forms of cell death are apoptosis and necrosis: apoptosis is a highly regulated and controlled cell death process that does not cause inflammation, while necrosis is a traumatic mode of cell death that induces inflammation and can promote tissue fibrosis[81]. Recently, increasing evidence has shown that there is a specific subtype of necrosis, termed necroptosis[82]. Autophagy was first observed by Keith R Porter and his student in 1962[83] and has become a controversial topic in the occurrence of DILI, which is not only a protective pathway but also associated with cell death (discussed below).
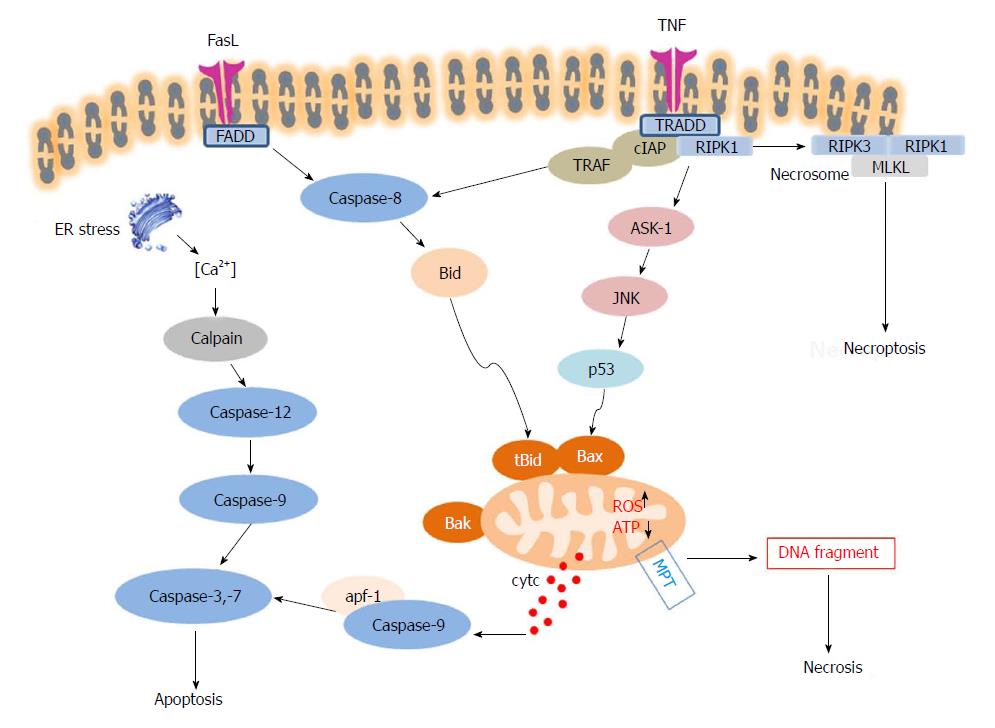
Apoptosis is a process of programmed cell death, which maintains physiological homeostasis in the normal human liver[84]. Characteristic apoptotic morphology includes cell shrinkage, nuclear fragmentation, chromatin condensation, chromosomal DNA fragmentation and global mRNA decay. Apoptosis can be initiated through two pathways: the intrinsic pathway (also called the mitochondrial pathway), and the extrinsic pathway. The intrinsic pathway is activated by intracellular signals generated when cells are stressed and depends on the release of proteins from the mitochondrial intermembrane space. The extrinsic pathway is activated by extracellular ligands binding to cell-surface death receptors (DRs). Both pathways induce cell death by activating executioner caspases (caspase 3 and 7) or enzymes that degrade protein (e.g., non-caspases, cathepsins, calpains, granzymes, and the proteasome complex, also have roles in mediating and promoting cell death).
Death receptors belong to the TNF family, comprising TNF receptor (TNFR), FAS and TNF-related apoptosis-inducing ligand receptor (TRAIL-R)[85]. The most widely expressed on the hepatocellular membrane are CD95 (APO-1/FAS) and TNFR1 (CD120a). When DRs are engaged by their ligands, the death domains of the receptors are oligomerized and form a membrane-bound supramolecular structure termed death-inducing signaling complex (DISC), including TNFR-associated death domain (TRADD), receptor interacting protein kinase-1 (RIPK1), cellular inhibitor of apoptosis 1 and 2 (cIAP1 and 2) and TNFR-associated factor 2 (TRAF2) or TRAF5[86], thereby recruiting caspase-8[87], and transducing a downstream signal cascade resulting in apoptosis[88]. The intrinsic pathway is commonly triggered via Bid, a protein of the B-cell lymphoma 2 (Bcl-2) family. Caspase-8 mediates the cleavage of Bid and cleaved Bid (tBid) would translocate to mitochondria, lead to mitochondrial outer membrane permeabilization (MOMP) via Bax and Bak and induce cytochrome C release to the cytoplasm, which binds to Apaf-1, forming a complex with caspase-9. The activation of procaspase-9 initiates the caspase cascade, promoting cell death. Several intracellular factors can activate this pathway, including ER stress and P53 activation[89]. ER stress induces an intrinsic cell death pathway termed lipoapoptosis mediated by JNK activation, whereas p53 induces apoptosis though regulation of specific target genes such as Bax. In the liver, the extrinsic and intrinsic pathways are linked, because hepatocytes require mitochondrial amplification activating caspase-3 for cell death execution[90].
Conventionally necrosis is thought to be ‘unprogrammed’ cell death caused by factors external to the cell or tissue, such as infection, virus, toxins, drugs or trauma. This results in the loss of cell membrane integrity with an uncontrolled release of cellular constituents into the extracellular space, thus eliciting an inflammatory response in the surrounding tissue[91]. The typical features of necrosis include depletion of ATP, ion imbalance and mitochondrial dysfunction. Similar to the intrinsic pathway of apoptosis, mitochondrial injury is the key factor of early-stage necrosis. The change of cell size and the formation of membrane “blebs” are reversible, but once MPT is changed and cellular constituents are released, the cascade is irreversible, and leads to cell rupture. Hepatocellular necrosis also requires the participation of proteases, one of which is calpain-mediating necrosis. Furthermore, recent work has demonstrated that necrosis can be regulated by MPT inhibitor or caspase inhibitors[92]. RIPK3-mediated mitochondrial fission seems to be also a feature of APAP-induced hepatocyte necrosis. Drp1 translocates to the mitochondria mediated by RIPK3, polymerizes and constricts mitochondria to facilitate organelle division[33].
Necroptosis is a “programmed” form of necrosis, which incorporates features of necrosis and apoptosis (Figure 3)[93]. Necroptosis shares the upstream pathway with apoptosis, and leads to cellular leakage, as seen in necrosis. Necroptosis can lead to cell death without the facilitation of caspase, in the presence of caspase inhibitors[93]. The typical signaling pathway of necroptosis is mediated by TNF super family member receptor. TNFα can stimulate its receptor TNFR1, and the TNFR-associated death protein TRADD signals to RIPK1 - which recruits RIPK3, to form the necrosome through the interaction of RIP-homology interaction motif (RHIM). RIPK3 then activates mixed lineage kinase domain like pseudokinase (MLKL) by phosphorylation, and p-MLKL subsequently drives oligomerization of MLKL, allowing MLKL to insert into and permeabilize plasma membranes and organelles[94]. The pro-inflammatory factors are then released and elicit immune responses ensue. The role of necroptosis in APAP-derived DILI is still controversial. Although TNF receptor signalling pathway is the best studied initiating event for necroptosis, there are multiple mechanisms to trigger this mode of cell death and further studies are needed to identify potential activators[95]. Many studies showed that Nec-1, an inhibitor of RIPK1, protects against APAP hepatotoxicity in vivo and in vitro[33,96]. However, RIPK3 and MLKL seem to be dispensable in APAP-derived DILI, whilst RIPK1 is essential for APAP toxicity via JNK activation[97].
Autophagy functions in a wide variety of physiological and pathophysiological roles as a complex, destructive mechanism of the cell that disassembles unnecessary or dysfunctional components[98]. Lysosomes are responsible for intracellular autophagy and the degradation of the cell. Autophagy is observable with the formation of autophagosomes, which are double-membrane vesicles originating from rough ER and contain part of the cytoplasm, the organelles and the proteins need to be degraded. Then autophagosomes fuse with lysosomes and initiate the orderly degradation and recycling of cellular components[99]. In disease, autophagy is considered to be an adaptive response to stress, which promotes survival and plays a vital role in cellular reconstruction. Ni et al[100] found that autophagy is important for the regulation of APAP protein adducts levels in hepatocytes, and this selective autophagic removal is mediated by ubiquitin and p62[100,101]. It is thought that autophagy activated by 5’-adenosine monophosphate-activated protein kinase (AMPK), can lead to adiponectin accumulation, which, in turn, removes damaged mitochondria, thereby ameliorating oxidative stress and hepatotoxicity[102]. And Parkin-induced mitophagy is also a mechanism of protection against APAP-induced liver injury and necrosis by negatively regulating JNK activity[36,103] and Mcl-1 degradation and increasing hepatocyte proliferation[104]. However, chronic deletion and acute knockdown of Parkin have different regulation towards mitophagy and liver injury, the mechanism of which is still unidentified and need further study[104]. Autophagy has emerged as an exciting new field in DILI and warrants further investigation.
The clinical manifestations of DILI are usually non-specific. Many patients may have no significant symptoms and only present with elevations in the level of hepatic biochemical indexes. For about the last half century, the traditional serum biomarkers for detecting DILI in clinics are alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total bilirubin (TBIL). However, elevations in these biomarkers take place when hepatocyte injury has already occurred and cannot be used to identify a potential for DILI. In recent years, with the further understanding of the mechanisms of DILI, several new biomarkers have been reported, including apoptosis-related caspase cleaved keratin 18 (cCK18)[105], necrosis-related HMGB1[106,107] and microRNA (especially microRNA-122)[108,109], specific mitochondrial injury biomarker glutamate dehydrogenase (GLDH)[110], biomarkers reflecting cholestasis (e.g. BAs) as well as genetic biomarkers reflecting the susceptibility to DILI, such as the genetic polymorphisms of HLA, drug metabolizing enzymes and drug transport proteins[2]. MicroRNA-122 and GLDH have been proposed as more sensitive and specific biomarkers of liver injury than ALT[111]. APAP-protein adducts and NAPQI are specific biomarkers of APAP-mediated DILI[112]. And apolipoprotein-A1 and haptoglobin have significant predictive values for the prediction of recovery in DILI patients[113]. Some of these biomarkers are already being used in early clinical trials. Though current biomarker are not specific to DILI and their value for clinical use still needs to be widely verified, their addition to conventional measurements could soon transform DILI prediction and detection, thereby promoting earlier treatment.
The liver works as a central detoxifying organ towards xenobiotics and chemicals. However, during the process of biotransformation to less toxic substances, molecules that can induce liver injury through various pathways are produced. The pathogenesis of DILI is very complex, and the occurrence of DILI is the consequence of multiple factors. Generally, important mechanisms involved in drug-induced hepatic injury can be divided into: (1) reactive metabolite formation via metabolism; (2) covalent binding between cellular components with drug; (3) reactive oxygen species generation in the cells; (4) activation of signal transduction pathways that modulate cell death or survival; and (5) cellular mitochondrial damage[114]. The common forms of hepatocyte death include apoptosis, necrosis, necroptosis and autophagy. The clinical characteristics of DILI are variable, and no specific laboratory tests are predictable for DILI, thereby presenting a major challenge for clinical diagnosis and treatment. Research on the molecular mechanisms underlying DILI will contribute greatly to early-stage screening of new drugs, predicting hepatotoxicity, and the monitoring of drug side-effects, eventually reducing the incidence of DILI, but clinical translation of the numerous mechanisms remains a challenge, requiring a considerable investment.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Higuera-de la Tijera F, Xiao J, Tanaka N S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17:575-586, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 2. | Yu YC, Mao YM, Chen CW, Chen JJ, Chen J, Cong WM, Ding Y, Duan ZP, Fu QC, Guo XY, Hu P, Hu XQ, Jia JD, Lai RT, Li DL, Liu YX, Lu LG, Ma SW, Ma X, Nan YM, Ren H, Shen T, Wang H, Wang JY, Wang TL, Wang XJ, Wei L, Xie Q, Xie W, Yang CQ, Yang DL, Yu YY, Zeng MD, Zhang L, Zhao XY, Zhuang H; Drug-induced Liver Injury (DILI) Study Group; Chinese Society of Hepatology (CSH); Chinese Medical Association (CMA). CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int. 2017;11:221-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 169] [Article Influence: 24.1] [Reference Citation Analysis (1)] |
| 3. | Miguel A, Azevedo LF, Araújo M, Pereira AC. Frequency of adverse drug reactions in hospitalized patients: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2012;21:1139-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran‘s Gastrointestinal and Liver Disease. In: Chitturi S TN, Farrell GC, editor Hepatic drug metabolism and liver disease caused by drugs. Philadelphia 2016; . [Cited in This Article: ] |
| 5. | Bell LN, Chalasani N. Epidemiology of idiosyncratic drug-induced liver injury. Semin Liver Dis. 2009;29:337-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ; Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950-66; quiz 967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 479] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 7. | Vinken M, Maes M, Vanhaecke T, Rogiers V. Drug-induced liver injury: mechanisms, types and biomarkers. Curr Med Chem. 2013;20:3011-3021. [PubMed] [Cited in This Article: ] |
| 8. | Park K, Williams DP, Naisbitt DJ, Kitteringham NR, Pirmohamed M. Investigation of toxic metabolites during drug development. Toxicol Appl Pharmacol. 2005;207:425-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279-288. [PubMed] [Cited in This Article: ] |
| 10. | Xie Y, McGill MR, Cook SF, Sharpe MR, Winefield RD, Wilkins DG, Rollins DE, Jaeschke H. Time course of acetaminophen-protein adducts and acetaminophen metabolites in circulation of overdose patients and in HepaRG cells. Xenobiotica. 2015;45:921-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269:240-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 12. | McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol. 2012;264:387-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 13. | Hu J, Ramshesh VK, McGill MR, Jaeschke H, Lemasters JJ. Low Dose Acetaminophen Induces Reversible Mitochondrial Dysfunction Associated with Transient c-Jun N-Terminal Kinase Activation in Mouse Liver. Toxicol Sci. 2016;150:204-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32:8-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 321] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 15. | Jaeschke H, Xie Y, McGill MR. Acetaminophen-induced Liver Injury: from Animal Models to Humans. J Clin Transl Hepatol. 2014;2:153-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273:17940-17953. [PubMed] [Cited in This Article: ] |
| 17. | Pauli-Magnus C, Meier PJ. Hepatobiliary transporters and drug-induced cholestasis. Hepatology. 2006;44:778-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Qiu X, Zhang Y, Liu T, Shen H, Xiao Y, Bourner MJ, Pratt JR, Thompson DC, Marathe P, Humphreys WG. Disruption of BSEP Function in HepaRG Cells Alters Bile Acid Disposition and Is a Susceptive Factor to Drug-Induced Cholestatic Injury. Mol Pharm. 2016;13:1206-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Gómez-Lechón MJ, Tolosa L, Donato MT. Metabolic activation and drug-induced liver injury: in vitro approaches for the safety risk assessment of new drugs. J Appl Toxicol. 2016;36:752-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Srivastava A, Maggs JL, Antoine DJ, Williams DP, Smith DA, Park BK. Role of reactive metabolites in drug-induced hepatotoxicity. Handb Exp Pharmacol. 2010;196:165-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T. Pathological aspects of lipid peroxidation. Free Radic Res. 2010;44:1125-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 470] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 22. | Knight TR, Fariss MW, Farhood A, Jaeschke H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci. 2003;76:229-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 563] [Cited by in F6Publishing: 640] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 24. | Hinson JA, Pike SL, Pumford NR, Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol. 1998;11:604-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 154] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30:463-488. [PubMed] [Cited in This Article: ] |
| 27. | Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1732] [Cited by in F6Publishing: 2043] [Article Influence: 157.2] [Reference Citation Analysis (0)] |
| 28. | Cubero FJ, Zoubek ME, Hu W, Peng J, Zhao G, Nevzorova YA, Al Masaoudi M, Bechmann LP, Boekschoten MV, Muller M. Combined Activities of JNK1 and JNK2 in Hepatocytes Protect Against Toxic Liver Injury. Gastroenterology. 2016;150:968-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 292] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 30. | Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 31. | Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, Jaeschke H. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2015;286:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099-2108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 34. | Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565-13577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 414] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 35. | Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071-35078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 36. | Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 37. | Karch J, Kwong JQ, Burr AR, Sargent MA, Elrod JW, Peixoto PM, Martinez-Caballero S, Osinska H, Cheng EH, Robbins J. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife. 2013;2:e00772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 38. | Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 268] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 39. | Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42:110-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45:156-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Will Y, Dykens J. Mitochondrial toxicity assessment in industry--a decade of technology development and insight. Expert Opin Drug Metab Toxicol. 2014;10:1061-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 42. | Kalinec GM, Thein P, Parsa A, Yorgason J, Luxford W, Urrutia R, Kalinec F. Acetaminophen and NAPQI are toxic to auditory cells via oxidative and endoplasmic reticulum stress-dependent pathways. Hear Res. 2014;313:26-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Halperin L, Jung J, Michalak M. The many functions of the endoplasmic reticulum chaperones and folding enzymes. IUBMB Life. 2014;66:318-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 44. | Uzi D, Barda L, Scaiewicz V, Mills M, Mueller T, Gonzalez-Rodriguez A, Valverde AM, Iwawaki T, Nahmias Y, Xavier R. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J Hepatol. 2013;59:495-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 45. | Letelier ME, López-Valladares M, Peredo-Silva L, Rojas-Sepúlveda D, Aracena P. Microsomal oxidative damage promoted by acetaminophen metabolism. Toxicol In Vitro. 2011;25:1310-1313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Vineetha VP, Soumya RS, Raghu KG. Phloretin ameliorates arsenic trioxide induced mitochondrial dysfunction in H9c2 cardiomyoblasts mediated via alterations in membrane permeability and ETC complexes. Eur J Pharmacol. 2015;754:162-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Morgan RE, Trauner M, van Staden CJ, Lee PH, Ramachandran B, Eschenberg M, Afshari CA, Qualls CW Jr, Lightfoot-Dunn R, Hamadeh HK. Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010;118:485-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 269] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 48. | Cosgrove BD, Alexopoulos LG, Hang TC, Hendriks BS, Sorger PK, Griffith LG, Lauffenburger DA. Cytokine-associated drug toxicity in human hepatocytes is associated with signaling network dysregulation. Mol Biosyst. 2010;6:1195-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Fontana RJ, Hayashi PH, Gu J, Reddy KR, Barnhart H, Watkins PB, Serrano J, Lee WM, Chalasani N, Stolz A. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology. 2014;147:96-108.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 50. | Goldman L, Schafer AI. Goldman-Cecil medicine. In: WM L, editor 152-Toxin- and drug-induced liver disease. Philadelphia 2012; 979-984. [Cited in This Article: ] |
| 51. | Uetrecht J, Naisbitt DJ. Idiosyncratic adverse drug reactions: current concepts. Pharmacol Rev. 2013;65:779-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 52. | Tujios S, Fontana RJ. Mechanisms of drug-induced liver injury: from bedside to bench. Nat Rev Gastroenterol Hepatol. 2011;8:202-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 53. | Ju C, Reilly T. Role of immune reactions in drug-induced liver injury (DILI). Drug Metab Rev. 2012;44:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Fisher JE, McKenzie TJ, Lillegard JB, Yu Y, Juskewitch JE, Nedredal GI, Brunn GJ, Yi ES, Malhi H, Smyrk TC. Role of Kupffer cells and toll-like receptor 4 in acetaminophen-induced acute liver failure. J Surg Res. 2013;180:147-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Jaeschke H. Innate immunity and acetaminophen-induced liver injury: why so many controversies? Hepatology. 2008;48:699-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett. 2010;192:387-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 57. | Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 483] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 58. | Martin-Murphy BV, Kominsky DJ, Orlicky DJ, Donohue TM Jr, Ju C. Increased susceptibility of natural killer T-cell-deficient mice to acetaminophen-induced liver injury. Hepatology. 2013;57:1575-1584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Laverty HG, Antoine DJ, Benson C, Chaponda M, Williams D, Kevin Park B. The potential of cytokines as safety biomarkers for drug-induced liver injury. Eur J Clin Pharmacol. 2010;66:961-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Maiuri AR, Breier AB, Gora LF, Parkins RV, Ganey PE, Roth RA. Cytotoxic Synergy Between Cytokines and NSAIDs Associated With Idiosyncratic Hepatotoxicity Is Driven by Mitogen-Activated Protein Kinases. Toxicol Sci. 2015;146:265-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Jiang J, Mathijs K, Timmermans L, Claessen SM, Hecka A, Weusten J, Peters R, van Delft JH, Kleinjans JCS, Jennen DGJ. Omics-based identification of the combined effects of idiosyncratic drugs and inflammatory cytokines on the development of drug-induced liver injury. Toxicol Appl Pharmacol. 2017;332:100-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Tailor A, Faulkner L, Naisbitt DJ, Park BK. The chemical, genetic and immunological basis of idiosyncratic drug-induced liver injury. Hum Exp Toxicol. 2015;34:1310-1317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | White KD, Chung WH, Hung SI, Mallal S, Phillips EJ. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: The role of host, pathogens, and drug response. J Allergy Clin Immunol. 2015;136:219-34; quiz 235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 64. | Zanni MP, von Greyerz S, Schnyder B, Brander KA, Frutig K, Hari Y, Valitutti S, Pichler WJ. HLA-restricted, processing- and metabolism-independent pathway of drug recognition by human alpha beta T lymphocytes. J Clin Invest. 1998;102:1591-1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Zanni MP, von Greyerz S, Schnyder B, Wendland T, Pichler WJ. Allele-unrestricted presentation of lidocaine by HLA-DR molecules to specific alphabeta+ T cell clones. Int Immunol. 1998;10:507-515. [PubMed] [Cited in This Article: ] |
| 66. | Schnyder B, Mauri-Hellweg D, Zanni M, Bettens F, Pichler WJ. Direct, MHC-dependent presentation of the drug sulfamethoxazole to human alphabeta T cell clones. J Clin Invest. 1997;100:136-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 219] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 67. | Schnyder B, Burkhart C, Schnyder-Frutig K, von Greyerz S, Naisbitt DJ, Pirmohamed M, Park BK, Pichler WJ. Recognition of sulfamethoxazole and its reactive metabolites by drug-specific CD4+ T cells from allergic individuals. J Immunol. 2000;164:6647-6654. [PubMed] [Cited in This Article: ] |
| 68. | Spraggs CF, Budde LR, Briley LP, Bing N, Cox CJ, King KS, Whittaker JC, Mooser VE, Preston AJ, Stein SH. HLA-DQA1*02:01 is a major risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. J Clin Oncol. 2011;29:667-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 69. | Lucena MI, Molokhia M, Shen Y, Urban TJ, Aithal GP, Andrade RJ, Day CP, Ruiz-Cabello F, Donaldson PT, Stephens C. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 317] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 70. | Alfirevic A, Gonzalez-Galarza F, Bell C, Martinsson K, Platt V, Bretland G, Evely J, Lichtenfels M, Cederbrant K, French N. In silico analysis of HLA associations with drug-induced liver injury: use of a HLA-genotyped DNA archive from healthy volunteers. Genome Med. 2012;4:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | Stephens C, López-Nevot MÁ, Ruiz-Cabello F, Ulzurrun E, Soriano G, Romero-Gómez M, Moreno-Casares A, Lucena MI, Andrade RJ. HLA alleles influence the clinical signature of amoxicillin-clavulanate hepatotoxicity. PLoS One. 2013;8:e68111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 72. | Song B, Aoki S, Liu C, Susukida T, Ito K. An animal model of abacavir-induced HLA-mediated liver injury. Toxicol Sci. 2018;162:713-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, Daly MJ, Goldstein DB, John S, Nelson MR. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 799] [Cited by in F6Publishing: 693] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 74. | Russmann S, Kaye JA, Jick SS, Jick H. Risk of cholestatic liver disease associated with flucloxacillin and flucloxacillin prescribing habits in the UK: cohort study using data from the UK General Practice Research Database. Br J Clin Pharmacol. 2005;60:76-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 75. | Andrews E, Armstrong M, Tugwood J, Swan D, Glaves P, Pirmohamed M, Aithal GP, Wright MC, Day CP, Daly AK. A role for the pregnane X receptor in flucloxacillin-induced liver injury. Hepatology. 2010;51:1656-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Holt MP, Ju C. Mechanisms of drug-induced liver injury. AAPS J. 2006;8:E48-E54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 77. | Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 78. | Klugewitz K, Blumenthal-Barby F, Schrage A, Knolle PA, Hamann A, Crispe IN. Immunomodulatory effects of the liver: deletion of activated CD4+ effector cells and suppression of IFN-gamma-producing cells after intravenous protein immunization. J Immunol. 2002;169:2407-2413. [PubMed] [Cited in This Article: ] |
| 79. | Ju C, McCoy JP, Chung CJ, Graf ML, Pohl LR. Tolerogenic role of Kupffer cells in allergic reactions. Chem Res Toxicol. 2003;16:1514-1519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 80. | You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 81. | Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205-219. [PubMed] [Cited in This Article: ] |
| 82. | Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 821] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 83. | Ashford TP, Porter KR. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962;12:198-202. [PubMed] [Cited in This Article: ] |
| 84. | Wang J, Yuan L, Xiao H, Xiao C, Wang Y, Liu X. Momordin Ic induces HepG2 cell apoptosis through MAPK and PI3K/Akt-mediated mitochondrial pathways. Apoptosis. 2013;18:751-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 85. | Iorga A, Dara L, Kaplowitz N. Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis. Int J Mol Sci. 2017;18:pii: E1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 86. | Ashkenazi A, Salvesen G. Regulated cell death: signaling and mechanisms. Annu Rev Cell Dev Biol. 2014;30:337-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 87. | Reinehr R, Häussinger D. CD95 death receptor and epidermal growth factor receptor (EGFR) in liver cell apoptosis and regeneration. Arch Biochem Biophys. 2012;518:2-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181-190. [PubMed] [Cited in This Article: ] |
| 89. | Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol. 2011;23:143-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 90. | Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2314] [Cited by in F6Publishing: 2203] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 91. | Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371-1387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 461] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 92. | Schwab BL, Guerini D, Didszun C, Bano D, Ferrando-May E, Fava E, Tam J, Xu D, Xanthoudakis S, Nicholson DW. Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis. Cell Death Differ. 2002;9:818-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 93. | Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1071] [Cited by in F6Publishing: 1266] [Article Influence: 126.6] [Reference Citation Analysis (0)] |
| 94. | Su L, Quade B, Wang H, Sun L, Wang X, Rizo J. A plug release mechanism for membrane permeation by MLKL. Structure. 2014;22:1489-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 95. | Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 96. | Takemoto K, Hatano E, Iwaisako K, Takeiri M, Noma N, Ohmae S, Toriguchi K, Tanabe K, Tanaka H, Seo S. Necrostatin-1 protects against reactive oxygen species (ROS)-induced hepatotoxicity in acetaminophen-induced acute liver failure. FEBS Open Bio. 2014;4:777-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 97. | Dara L, Johnson H, Suda J, Win S, Gaarde W, Han D, Kaplowitz N. Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology. 2015;62:1847-1857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 98. | Klionsky DJ. Autophagy revisited: a conversation with Christian de Duve. Autophagy. 2008;4:740-743. [PubMed] [Cited in This Article: ] |
| 99. | Kobayashi S. Choose Delicately and Reuse Adequately: The Newly Revealed Process of Autophagy. Biol Pharm Bull. 2015;38:1098-1103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 100. | Ni HM, McGill MR, Chao X, Du K, Williams JA, Xie Y, Jaeschke H, Ding WX. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J Hepatol. 2016;65:354-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 101. | Katsuragi Y, Ichimura Y, Komatsu M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J. 2015;282:4672-4678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 486] [Cited by in F6Publishing: 564] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 102. | Lin Z, Wu F, Lin S, Pan X, Jin L, Lu T, Shi L, Wang Y, Xu A, Li X. Adiponectin protects against acetaminophen-induced mitochondrial dysfunction and acute liver injury by promoting autophagy in mice. J Hepatol. 2014;61:825-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 103. | Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 104. | Williams JA, Ni HM, Haynes A, Manley S, Li Y, Jaeschke H, Ding WX. Chronic Deletion and Acute Knockdown of Parkin Have Differential Responses to Acetaminophen-induced Mitophagy and Liver Injury in Mice. J Biol Chem. 2015;290:10934-10946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 105. | Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, Kitteringham NR, Park BK. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112:521-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 106. | Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 305] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 107. | Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 108. | Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci USA. 2009;106:4402-4407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 891] [Cited by in F6Publishing: 910] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 109. | Dear JW, Antoine DJ, Starkey-Lewis P, Goldring CE, Park BK. Early detection of paracetamol toxicity using circulating liver microRNA and markers of cell necrosis. Br J Clin Pharmacol. 2014;77:904-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 110. | McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 480] [Cited by in F6Publishing: 522] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 111. | Church RJ, Watkins PB. The transformation in biomarker detection and management of drug-induced liver injury. Liver Int. 2017;37:1582-1590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 112. | Fannin RD, Russo M, O’Connell TM, Gerrish K, Winnike JH, Macdonald J, Newton J, Malik S, Sieber SO, Parker J. Acetaminophen dosing of humans results in blood transcriptome and metabolome changes consistent with impaired oxidative phosphorylation. Hepatology. 2010;51:227-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 113. | Peta V, Tse C, Perazzo H, Munteanu M, Ngo Y, Ngo A, Ramanujam N, Verglas L, Mallet M, Ratziu V. Serum apolipoprotein A1 and haptoglobin, in patients with suspected drug-induced liver injury (DILI) as biomarkers of recovery. PLoS One. 2017;12:e0189436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Han D, Shinohara M, Ybanez MD, Saberi B, Kaplowitz N. Signal transduction pathways involved in drug-induced liver injury. Handb Exp Pharmacol. 2010;267-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |