Published online Mar 21, 2018. doi: 10.3748/wjg.v24.i11.1216
Peer-review started: January 13, 2018
First decision: February 5, 2018
Revised: February 21, 2018
Accepted: March 3, 2018
Article in press: March 3, 2018
Published online: March 21, 2018
To describe the characteristics of people diagnosed with acute and chronic hepatitis B virus (HBV) infection in British Columbia (BC).
We used data from the BC Hepatitis Testers Cohort (BC-HTC), which includes all individuals tested for hepatitis C virus (HCV) or human immunodeficiency virus (HIV) or those diagnosed with HBV or active tuberculosis in BC since 1990. These data were integrated with prescription drug, medical visit, hospitalization and mortality data. HBV cases were classified as acute or chronic according to provincial guidelines. We compared characteristics of individuals by HBV infection group (acute, chronic and negative). Factors associated with acute or chronic HBV infection were assessed with multinomial logistic regression models in comparison to the HBV negative group.
46498 of the 1058056 eligible BC-HTC participants were diagnosed with HBV infection. 4.3% of HBV positive individuals were diagnosed with acute HBV infections while 95.7% had chronic infections. Problematic alcohol use, injection drug use, and HIV or HCV co-infection were more common among individuals diagnosed with acute HBV compared to those with chronic infections and HBV negative individuals. In multivariable multinomial logistic regression models, we observed significant associations between acute or chronic HBV diagnosis and being male, age at HBV diagnosis or birth cohort, South and East Asian ethnicity, HCV or HIV infection, and injection drug use. The odds of acute HBV decreased with increasing age among people who inject drugs, while the opposite was true for chronic HBV. Persons with acute HBV were predominantly White (78%) while those with chronic HBV were mostly East Asian (60%). Relative to Whites, East Asians had 12 times greater odds of being diagnosed with chronic HBV infection. These odds increased with increasing socioeconomic deprivation.
Differences in the profiles of people diagnosed with acute and chronic HBV infection necessitate differentiated screening, prevention, care and treatment programs.
Core tip: Substance use, major mental illness and hepatitis C virus or human immunodeficiency virus co-infection were more common among individuals with acute HBV compared with those diagnosed with chronic hepatitis B virus (HBV). Acute HBV was mainly diagnosed in the White population, while chronic HBV was mostly diagnosed among people with East Asian ethnicity. The risk of acute HBV was highest among the younger population who injected drugs, while the risk of chronic HBV infection was highest among East Asian people with lower socioeconomic status. Differences in the profiles of people diagnosed with acute and chronic HBV suggest the need for different interventions for both population groups.
- Citation: Binka M, Butt ZA, Wong S, Chong M, Buxton JA, Chapinal N, Yu A, Alvarez M, Darvishian M, Wong J, McGowan G, Torban M, Gilbert M, Tyndall M, Krajden M, Janjua NZ. Differing profiles of people diagnosed with acute and chronic hepatitis B virus infection in British Columbia, Canada. World J Gastroenterol 2018; 24(11): 1216-1227
- URL: https://www.wjgnet.com/1007-9327/full/v24/i11/1216.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i11.1216
Hepatitis B virus (HBV) affects 257 million people worldwide, including approximately 200000 Canadians[1-4]. Chronic HBV infection is associated with 66% of the 1.34 million viral hepatitis-related deaths reported worldwide and is responsible for a substantial disease burden from liver cancer and end-stage liver disease[2].
HBV vaccination is highly effective in preventing infection[1,3,5]. Mother to child transmission during childbirth is the most common mode of infection in HBV-endemic countries, while infection typically occurs through sexual transmission and injection drug use in Canada and other developed countries[1,3,5]. Over 90% of children and 50% of adults display no symptoms with acute infection[1,3,5]. The asymptomatic nature of this disease makes diagnosis difficult, leading to chronic illness in 5% of infected adults, 30% to 50% of children, and 90% of infected neonates[1,3,5]. Childhood vaccination programs have led to a dramatic reduction in the occurrence of acute infections in developed countries, including Canada and the United States[1,3,6]. The record low number of reported acute HBV infections in British Columbia (BC) in 2015 have been attributed to successful vaccination programs[7]. However, certain high risk groups, including men who have sex with men (MSM) and people who inject drugs (PWID), continue to acquire and transmit HBV infection[8,9]. As HBV infection is mostly asymptomatic, identifying the factors associated with acute and chronic infection could determine avenues for closing gaps in screening, vaccination and other prevention programs.
In many developed countries, a relatively larger number of people are diagnosed with chronic HBV, compared with acute HBV, each year[5,10-13]. Data from Canada, the United States and other developed countries indicate that most chronic HBV infections are diagnosed among immigrants from HBV-endemic Asia-Pacific countries[10,12-14]. Our previous work suggests that 49% of persons with chronic HBV and decompensated cirrhosis and 46% of those with HCC in BC were diagnosed late in the course of their infections[15]. The rate of late diagnosis has declined but is still substantially higher for HBV compared to hepatitis C virus (HCV)[15]. Therefore, establishing the characteristics of individuals who are more likely to be infected with HBV could enhance the planning of screening programs to further reduce late diagnoses within the province.
Individuals diagnosed with acute and chronic HBV infections may differ with regards to demographics and risk behavior[1,5]. These distinctions may have implications for interventions targeted at either population. Analyses based on these differences could also identify areas for the optimal integration of such HBV programs with currently available health services. We are unaware of any study comparing large population level data for both acute and chronic HBV. Previous studies have mainly focused on chronic HBV epidemiology, with limited data on acute HBV, or acute HBV only[10,11,16-18]. In this study, we describe the characteristics of individuals with acute and chronic HBV infections and identify the factors associated with HBV infection within the BC Hepatitis Testers Cohort (BC-HTC).
The BC-HTC includes over 1.7 million individuals tested for hepatitis C virus (HCV) or human immunodeficiency virus (HIV) at the BC Centre for Disease Control Public Health Laboratory, or reported to BC public health as a confirmed case of HCV, HBV, HIV/AIDS or active tuberculosis (TB) since 1990[19]. Cohort data are integrated with data on medical visits, hospitalizations, prescription drugs, cancers and deaths[19]. Details of cohort creation and epidemiological characteristics have been reported previously and are summarized in Supplementary Table 1[19]. This analysis is based on data collected between April 1, 1990 and December 31, 2015.
BC-HTC participants who were included in the provincial registry of reported HBV cases, those who tested positive for HBV DNA or HBeAg, and those who were recorded as having received treatment for HBV were considered as cases of HBV. HBV cases recorded in the BC provincial registry with an acute diagnosis were classified as acute HBV infections, while the remainder were designated as having chronic HBV (Supplementary Table 2). Individuals who were not diagnosed with HBV but were tested for HBsAg or anti HBV core total were denoted as being HBV negative.
We assessed potential risk factors at HBV diagnosis or at the last negative test, including age, birth cohort, sex, infection with HIV, HCV or TB, problematic alcohol use, illicit drug use, major mental illness, and material and social deprivation. HIV, HCV or TB diagnoses were based on laboratory confirmation or being reported as a confirmed case in the public health reportable disease database. Assessment of problematic alcohol and illicit drug use, and major mental illness was based on associated diagnostic codes in administrative health datasets evaluated across the entire dataset prior to the first positive or the last negative test (Supplementary Table 2).
Ethnicity classification was based on the validated name recognition software Onomap[20,21]. Onomap sensitivity and specificity relative to self-identified ethnicity, determined on a subset of the BC-HTC (n = 5962), was 93% and 98.6% for South Asians, and 67% and 99.5% for East Asians. Race/ethnicity was grouped as White, South Asian (Pakistani, Indians, Bangladeshis, Nepalese and Sri Lankans), East Asian (Chinese, Filipinos, Japanese, Korean and South-East Asians), and Others (Black, Central Asian, Latin American, Pacific Islander and West Asian individuals). Socioeconomic status was assessed using the Quebec Material and Social deprivation Index[22].
We compared characteristics of individuals by HBV infection group (acute, chronic and negative) with Pearson’s chi-square tests for categorical variables and Kruskal Wallis tests for median age. Factors associated with acute or chronic HBV infection were assessed with multivariable multinomial logistic regression models in comparison with the HBV negative group.
1058056 individuals were eligible for inclusion in this analysis. Of these, 46498 individuals were diagnosed with HBV infection while 1011558 were HBV negative. 4.3% of HBV positive individuals were diagnosed with acute HBV infections while 95.7% had chronic infections (Table 1).
| HBV positive | HBV negative | ||||
| Acute | Chronic | All positive | P value | ||
| n = 2015 | n = 44483 | n = 46498 | n = 1011558 | ||
| Sex | |||||
| Female | 590 (29.3) | 20094 (45.2) | 20684 (44.5) | 563440 (55.7) | 0.000 |
| Male | 1425 (70.7) | 24387 (54.8) | 25812 (55.5) | 448072 (44.3) | |
| Unknown | 0 (0.0) | 2 (0.0) | 2 (0.0) | 44 (0.0) | |
| Birth Cohort | |||||
| < 1945 | 204 (10.1) | 5385 (12.1) | 5589 (12.0) | 103498 (10.2) | 0.000 |
| 1945-1964 | 1001 (49.7) | 20316 (45.7) | 21317 (45.8) | 297768 (29.4) | |
| 1965-1974 | 577 (28.6) | 9852 (22.1) | 10429 (22.4) | 199121 (19.7) | |
| > 1974 | 233 (11.6) | 8930 (20.1) | 9163 (19.7) | 411171 (40.6) | |
| Age group at HBV diagnosis (yr) | |||||
| < 25 | 306 (15.2) | 5163 (11.6) | 5469 (11.8) | 137379 (13.6) | 0.000 |
| 25-34 | 653 (32.4) | 9963 (22.4) | 10616 (22.8) | 264809 (26.2) | |
| 35-44 | 552 (27.4) | 11835 (26.6) | 12387 (26.6) | 220745 (21.8) | |
| 45-54 | 317 (15.7) | 9202 (20.7) | 9519 (20.5) | 165884 (16.4) | |
| 55-64 | 115 (5.7) | 4954 (11.1) | 5069 (10.9) | 117550 (11.6) | |
| > 64 | 72 (3.6) | 3366 (7.6) | 3438 (7.4) | 105191 (10.4) | |
| Median [IQR] | 35 [28-45] | 41 [31-51] | 40 [31-50] | 39 [29-53] | 0.000 |
| Year of HBV diagnosis | |||||
| 1990-1999 | 1388 (68.9) | 14068 (31.6) | 15456 (33.2) | 47343 (4.7) | 0.000 |
| 2000-2004 | 362 (18.0) | 11246 (25.3) | 11608 (25.0) | 126715 (12.5) | |
| 2005-2009 | 196 (9.7) | 8968 (20.2) | 9164 (19.7) | 219195 (21.7) | |
| 2010-2015 | 69 (3.4) | 10201 (22.9) | 10270 (22.1) | 618305 (61.1) | |
| Ethnicity | |||||
| East Asian | 278 (13.8) | 26578 (59.7) | 26856 (57.8) | 139306 (13.8) | 0.000 |
| Other | 35 (1.7) | 1651 (3.7) | 1686 (3.6) | 41452 (4.1) | |
| South Asian | 125 (6.2) | 1420 (3.2) | 1545 (3.3) | 83200 (8.2) | |
| White | 1577 (78.3) | 14834 (33.3) | 16411 (35.3) | 747600 (73.9) | |
| HCV | |||||
| Negative | 1296 (64.3) | 39575 (89.0) | 40871 (87.9) | 964780 (95.4) | 0.000 |
| Positive | 719 (35.7) | 4908 (11.0) | 5627 (12.1) | 46778 (4.6) | |
| HIV | |||||
| Negative | 1888 (93.7) | 43370 (97.5) | 45258 (97.3) | 1006511 (99.5) | 0.000 |
| Positive | 127 (6.3) | 1113 (2.5) | 1240 (2.7) | 5047 (0.5) | |
| HCV and HIV | |||||
| Positive | 82 (4.1) | 712 (1.6) | 794 (1.7) | 1768 (0.2) | 0.000 |
| Active tuberculosis | |||||
| Negative | 2004 (99.5) | 44101 (99.1) | 46105 (99.2) | 1008555 (99.7) | 0.000 |
| Positive | 11 (0.5) | 382 (0.9) | 393 (0.8) | 3003 (0.3) | |
| Problematic alcohol use | |||||
| No | 1807 (89.7) | 42544 (95.6) | 44531 (95.8) | 959326 (94.8) | 0.000 |
| Yes | 208 (10.3) | 1939 (4.4) | 2147 (4.6) | 52232 (5.2) | |
| Illicit drug use | |||||
| No | 1617 (80.2) | 41558 (93.4) | 43175 (92.9) | 944910 (93.4) | 0.000 |
| Yes | 398 (19.8) | 2925 (6.6) | 3323 (7.1) | 66648 (6.6) | |
| Injection drug use | |||||
| No | 1718 (85.3) | 42040 (94.5) | 43758 (94.1) | 960849 (95.0) | 0.000 |
| Yes | 297 (14.7) | 2443 (5.5) | 2740 (5.9) | 50709 (5.0) | |
| Major mental illness | |||||
| No | 1788 (88.7) | 41410 (93.1) | 43198 (92.9) | 874387 (86.4) | 0.000 |
| Yes | 227 (11.3) | 3073 (6.9) | 3300 (7.1) | 137171 (13.6) | |
| Material deprivation quintile | |||||
| Q1 (most privileged) | 320 (15.9) | 7076 (15.9) | 7396 (15.9) | 228694 (22.6) | 0.000 |
| Q2 | 303 (15.0) | 6753 (15.2) | 7056 (15.2) | 189225 (18.7) | |
| Q3 | 320 (15.9) | 7797 (17.5) | 8117 (17.5) | 189823 (18.8) | |
| Q4 | 425 (21.1) | 9564 (21.5) | 9989 (21.5) | 198263 (19.6) | |
| Q5 (most deprived) | 585 (29.0) | 12226 (27.5) | 12811 (27.6) | 185193 (18.3) | |
| Unknown | 62 (3.1) | 1067 (2.4) | 1129 (2.4) | 20360 (2.0) | |
| Social deprivation quintile | |||||
| Q1 (most privileged) | 247 (12.3) | 9952 (22.4) | 10199 (21.9) | 184233 (18.2) | 0.000 |
| Q2 | 267 (13.3) | 9032 (20.3) | 9299 (20.0) | 180059 (17.8) | |
| Q3 | 314 (15.6) | 7574 (17.0) | 7888 (17.0) | 173461 (17.1) | |
| Q4 | 383 (19.0) | 7336 (16.5) | 7719 (16.6) | 199229 (19.7) | |
| Q5 (most deprived) | 742 (36.8) | 9522 (21.4) | 10264 (22.1) | 254216 (25.1) | |
| Unknown | 62 (3.1) | 1067 (2.4) | 1129 (2.4) | 20360 (2.0) | |
About two-thirds of acute infections (70.7%) and half of chronic HBV infections (54.8%) were diagnosed among males. In contrast, HBV negative individuals were predominantly female (55.7%). The 1945-1964 birth cohort formed the majority of persons with either acute or chronic HBV infections (acute: 49.7%; chronic: 45.7%) but represented a smaller proportion of the HBV negative group (29.4%). Age at HBV testing or diagnosis was significantly lower among persons with acute HBV compared to those with chronic infections and HBV negative individuals (median age: 35 vs 41 and 39 years; P < 0.001). Additionally, females were more likely to be tested or diagnosed at a younger age compared to males (median age: 37 vs 41 years, P < 0.001).
The majority of HBV negative individuals (73.9%) and those with acute HBV (78.3%) were White. Chronic HBV infections, however, were more frequently diagnosed among East Asians (59.7%). Material deprivation was more common among HBV positive individuals than among HBV negative persons [Q5 (most deprived): acute HBV: 29.0%; chronic HBV: 27.5%; HBV negative: 18.3%]. In contrast, social deprivation was predominant within each HBV group, though highest among individuals with acute HBV [Q5 (most deprived): acute HBV: 36.8%; chronic HBV: 21.4%; HBV negative: 25.1%] (Table 1).
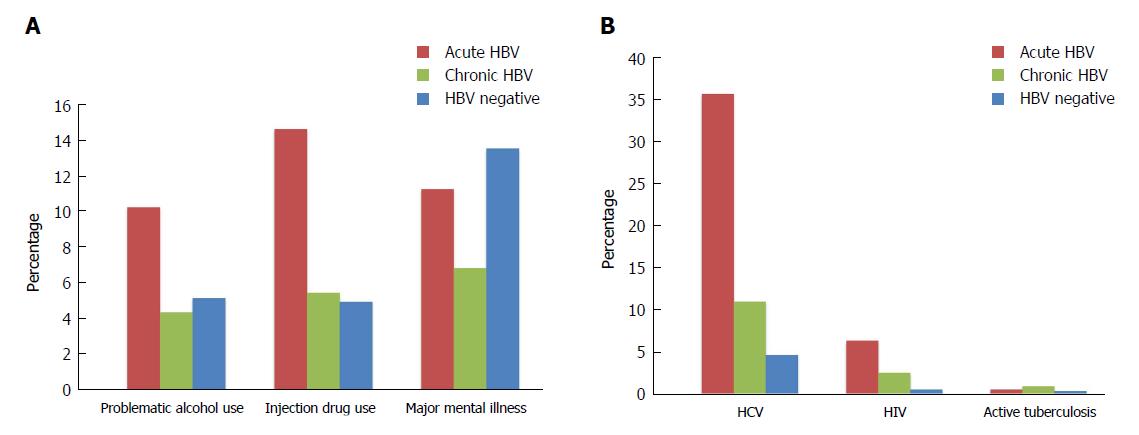
A larger proportion of persons with acute HBV experienced problematic alcohol use, illicit drug use, and injection drug use relative to those with chronic infections and HBV negative individuals (problematic alcohol use: 10.3%, 4.4%, 5.2%, P < 0.001; illicit drug use: 19.8%, 6.6%, 6.6%, P < 0.001; injection drug use: 14.7%, 5.5%, 5.0%, P < 0.001) (Figure 1A, Table 1). Conversely, major mental illness was most prevalent among HBV negative individuals (HBV negative: 13.6%; acute HBV: 11.3%, chronic HBV: 6.9%; P < 0.001).
HCV and HIV co-infections were more common among people diagnosed with acute HBV than among persons with chronic HBV and HBV negative individuals (HCV: 35.7%, 11.0%, 4.6%, P < 0.001; HIV: 6.3%, 2.5%, 0.5%, P < 0.001) (Figure 1B, Table 1). Active TB was less prevalent in the cohort, but more common among those with chronic HBV relative to HBV negative individuals and those with acute HBV (0.9%, 0.3%, 0.5%; P < 0.001). The cohort also consisted of 749 persons with HBV/HCV/HIV triple infection, who mostly had acute HBV infections (acute HBV: 4.1%, chronic HBV: 1.6%; HBV negative: 0.2%, P < 0.001) (Table 1).
In multivariable multinomial logistic regression models, we observed significant associations between acute or chronic HBV diagnosis and being male, age at HBV diagnosis or birth cohort, South and East Asian ethnicity, HCV or HIV infection, and injection drug use (Table 2 and Supplementary Table 3). However, the magnitude and direction of association for various variables differed for acute and chronic HBV.
| Adjusted OR (95%CI) | ||
| Acute HBV | Chronic HBV | |
| Sex | ||
| Male | 2.28 (2.06-2.52) | 1.43 (1.40-1.46) |
| Female | 1.00 | 1.00 |
| Age at HBV diagnosis (yr) | ||
| < 25 | 2.38 (1.97-2.87) | 0.89 (0.85-0.92) |
| 25-34 | 2.62 (2.21-3.09) | 1.09 (1.05-1.13) |
| 35-44 | 1.82 (1.53-2.16) | 1.19 (1.15-1.23) |
| 45-54 | 1.49 (1.23-1.79) | 1.22 (1.18-1.27) |
| 55 + | 1.00 | 1.00 |
| Ethnicity | ||
| East Asian | 1.76 (1.53-2.02) | 12.45 (12.15-12.77) |
| Other | 0.88 (0.63-1.24) | 3.06 (2.90-3.23) |
| South Asian | 1.66 (1.37-2.02) | 1.26 (1.19-1.33) |
| White | 1.00 | 1.00 |
| HCV | ||
| Positive | 5.23 (4.65-5.87) | 2.89 (2.77-3.01) |
| Negative | 1.00 | 1.00 |
| HIV | ||
| Positive | 5.29 (4.30-6.51) | 5.73 (5.29-6.20) |
| Negative | 1.00 | 1.00 |
| Active tuberculosis | ||
| Yes | 0.59 (0.32-1.09) | 0.97 (0.85-1.10) |
| No | 1.00 | 1.00 |
| Problematic alcohol use | ||
| Yes | 0.87 (0.74-1.04) | 1.02 (0.96-1.08) |
| No | 1.00 | 1.00 |
| Injection drug use | ||
| Yes | 1.84 (1.57-2.17) | 1.67 (1.58-1.77) |
| No | 1.00 | 1.00 |
| Major mental illness | ||
| Yes | 0.73 (0.62-0.85) | 0.74 (0.71-0.78) |
| No | 1.00 | 1.00 |
| Material deprivation quintile | ||
| Q1 (most privileged) | 1.00 | 1.00 |
| Q2-4 | 1.06 (0.94 -1.21) | 1.10 (1.06-1.13) |
| Q5 (most deprived) | 1.35 (1.17 -1.55) | 1.35 (1.30-1.39) |
| Social deprivation quintile | ||
| Q1 (most privileged) | 1.00 | 1.00 |
| Q2-4 | 1.20 (1.04-1.39) | 0.97 (0.95-1.00) |
| Q5 (most deprived) | 1.56 (1.34-1.82) | 1.00 (0.97-1.03) |
| Year of HBV diagnosis | ||
| 1990-1999 | 1.00 | 1.00 |
| 2000-2004 | 0.10 (0.09-0.12) | 0.27 (0.26-0.28) |
| 2005-2009 | 0.03 (0.03-0.04) | 0.14 (0.13-0.14) |
| 2010-2015 | 0.00 (0.00-0.01) | 0.05 (0.05-0.06) |
South and East Asians had higher odds of acute or chronic HBV compared to Whites, with East Asians having 12 times greater odds of chronic HBV (ORacute: East Asian: 1.76, 95%CI: 1.53-2.02; South Asian: 1.66, 95%CI: 1.37-2.02; ORchronic: East Asian: 12.45, 95%CI: 12.15-12.77; South Asian: 1.26, 95%CI: 1.19-1.33) (Table 2).
Individuals with HCV or HIV infection had 5 times the odds acute HBV infection compared to their HBV negative counterparts (OR: HIV: 5.29, 95%CI: 4.30-6.51; HCV: 5.23, 95%CI: 4.65-5.87). For chronic infections, however, the odds of infection were slightly elevated among people with HIV co-infection (OR: 5.73, 95%CI: 5.29-6.20), but much lower among those infected with HCV (OR: 2.89, 95%CI: 2.77-3.01) (Table 2).
Injection drug use was associated with increased odds of both acute and chronic HBV (ORacute: 1.84, 95%CI: 1.57-2.17; ORchronic: 1.67, 95%CI: 1.58-1.77). In contrast, individuals with major mental illness had lower odds of either infection (ORacute: 0.73, 95%CI: 0.62-0.85; ORchronic: 0.74, 95%CI: 0.71-0.78). Material deprivation was also associated with increased odds of acute and chronic HBV infection (Q5: ORacute: 1.35, 95%CI: 1.17-1.55; ORchronic: 1.35, 95%CI: 1.30-1.39) (Table 2). Although social deprivation was associated with higher odds of acute HBV infection (ORacute: 1.56, 95%CI: 1.34-1.82), it was not significantly associated with a chronic infection (ORchronic: 1.00, 95%CI: 0.97-1.03).
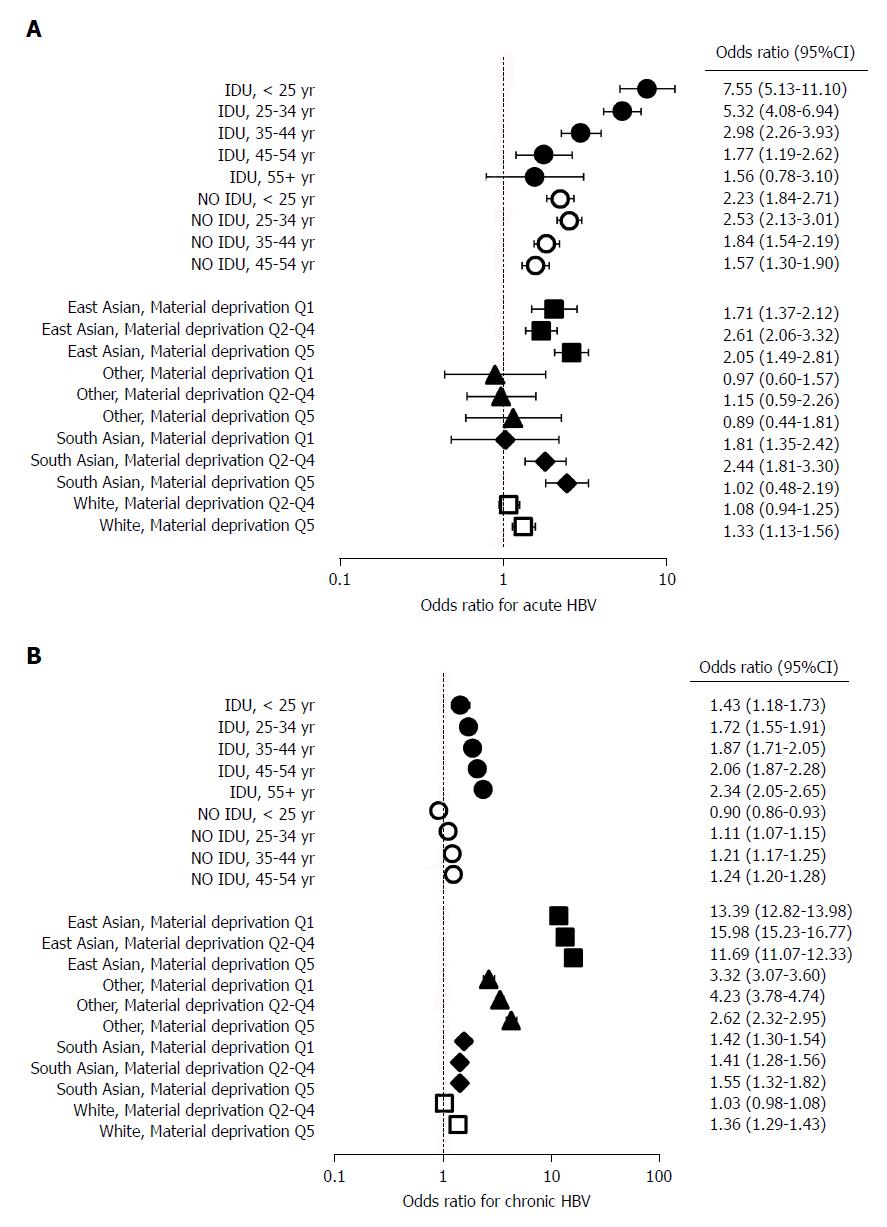
In an additional model, composite variables (age at HBV diagnosis and injection drug use, and race/ethnicity and material deprivation) were used to determine joint effects on HBV infection (Figure 2, Table 3). In this model, there was a graded decrease in the odds of acute HBV with increasing age at diagnosis among PWID (OR: < 25 years: 7.55, 95%CI: 5.13-11.10; 25-34 years: 5.32, 95%CI: 4.08-6.94; 35-44 years: 2.98, 95%CI: 2.26-3.9; 45-54 years: 1.77, 95%CI: 1.19-2.62; 55 + years: 1.56, 95%CI: 0.78-3.10) (Figure 2A, Table 3). The opposite pattern was observed for chronic HBV infections, as the odds of chronic HBV increased with age at diagnosis (OR: < 25 years: 1.43, 95%CI: 1.18-1.73; 25-34 years: 1.72, 95%CI: 1.55-1.91; 35-44 years: 1.87, 95%CI: 1.71-2.05; 45-54 years: 2.06, 95%CI: 1.87-2.28; 55 + years: 2.34, 95%CI: 2.05-2.65) (Figure 2B, Table 3). This model also demonstrated that the relatively high odds of chronic HBV among East Asians increased further with worsening material deprivation [OR: Material deprivation, Q1 (most privileged): 11.69, 95%CI: 11.07-12.33; Q2-Q4: 13.39, 95%CI: 12.82-13.98; Q5 (most deprived): 15.98, 95%CI: 15.23-16.77) (Figure 2B, Table 3).
| Adjusted OR (95% CI) | ||
| Acute HBV | Chronic HBV | |
| Sex | ||
| Male | 2.30 (2.08-2.54) | 1.43 (1.4-1.46) |
| Female | 1.00 | 1.00 |
| HCV | ||
| Positive | 5.22 (4.65-5.86) | 2.88 (2.77-3.00) |
| Negative | 1.00 | 1.00 |
| HIV | ||
| Positive | 5.25 (4.26-6.47) | 5.75 (5.31-6.22) |
| Negative | 1.00 | 1.00 |
| Active tuberculosis | ||
| Yes | 0.58 (0.31-1.08) | 0.97 (0.86-1.10) |
| No | 1.00 | 1.00 |
| Problematic alcohol use | ||
| Yes | 0.90 (0.75-1.07) | 1.01 (0.95-1.07) |
| No | 1.00 | 1.00 |
| Major mental illness | ||
| Yes | 0.72 (0.61-0.84) | 0.75 (0.71-0.78) |
| No | 1.00 | 1.00 |
| Social deprivation quintile | ||
| Q2-Q4 | 1.21 (1.05-1.40) | 0.97 (0.94-1.00) |
| Q5 (most deprived) | 1.58 (1.36-1.84) | 0.99 (0.96-1.03) |
| Q1 (most privileged) | 1.00 | 1.00 |
| IDU*Age at HBV diagnosis (yr) | ||
| IDU* < 25 | 7.55 (5.13-11.10) | 1.43 (1.18-1.73) |
| IDU*25-34 | 5.32 (4.08-6.94) | 1.72 (1.55-1.91) |
| IDU*35-44 | 2.98 (2.26-3.93) | 1.87 (1.71-2.05) |
| IDU*45-54 | 1.77 (1.19-2.62) | 2.06 (1.87-2.28) |
| IDU*55+ | 1.56 (0.78-3.10) | 2.34 (2.05-2.65) |
| No IDU* < 25 | 2.23 (1.84-2.71) | 0.90 (0.86-0.93) |
| No IDU*25-34 | 2.53 (2.13-3.01) | 1.11 (1.07-1.15) |
| No IDU*35-44 | 1.84 (1.54-2.19) | 1.21 (1.17-1.25) |
| No IDU*45-54 | 1.57 (1.30-1.90) | 1.24 (1.20-1.28) |
| No IDU*55+ | 1.00 | 1.00 |
| Ethnicity*Material Deprivation quintile | ||
| East Asian*Material deprivation Q2-Q4 | 1.71 (1.37-2.12) | 13.39 (12.82-13.98) |
| East Asian*Material deprivation Q5 | 2.61 (2.06-3.32) | 15.98 (15.23-16.77) |
| East Asian*Material deprivation Q1 | 2.05 (1.49-2.81) | 11.69 (11.07-12.33) |
| Other*Material deprivation Q2-Q4 | 0.97 (0.60-1.57) | 3.32 (3.07-3.60) |
| Other*Material deprivation Q5 | 1.15 (0.59-2.26) | 4.23 (3.78-4.74) |
| Other*Material deprivation Q1 | 0.89 (0.44-1.81) | 2.62 (2.32-2.95) |
| South Asian*Material deprivation Q2-Q4 | 1.81 (1.35-2.42) | 1.42 (1.30-1.54) |
| South Asian*Material deprivation Q5 | 2.44 (1.81-3.30) | 1.41 (1.28-1.56) |
| South Asian*Material deprivation Q1 | 1.02 (0.48-2.19) | 1.55 (1.32-1.82) |
| White*Material deprivation Q2-Q4 | 1.08 (0.94-1.25) | 1.03 (0.98-1.08) |
| White*Material deprivation Q5 | 1.33 (1.13-1.56) | 1.36 (1.29-1.43) |
| White*Material deprivation Q1 | 1.00 | 1.00 |
| Year of HBV diagnosis | ||
| 1990-1999 | 1.00 | 1.00 |
| 2000-2004 | 0.10 (0.09-0.12) | 0.27 (0.26-0.28) |
| 2005-2009 | 0.03 (0.03-0.04) | 0.14 (0.13-0.14) |
| 2010-2015 | 0.00 (0.00-0.01) | 0.05 (0.05-0.06) |
In this large population based cohort study, we assessed over 1 million individuals for risk factors associated with acute and chronic HBV infection in British Columbia. This study shows two distinct patterns of risk factors among people diagnosed with acute and chronic infections. Acute HBV infections, indicative of new transmission events, occurred predominantly among males, persons aged between 25 and 34 years, White individuals, and socioeconomically disadvantaged persons. Problematic alcohol use, injection drug use, HIV and HCV co-infection were also common within this group. Individuals diagnosed with chronic HBV infection had similar characteristics, but were predominantly older, that is, aged between 35 to 44 years, and East Asian. Additionally, substance use and HIV or HCV co-infection were relatively low within this group. These findings highlight distinct risk patterns for individuals with acute and chronic HBV infections within the province and underscore the need for different strategies to prevent, diagnose and treat HBV within these groups.
Persons with acute and chronic infections had distinct co-infections and concurrent social condition profiles. Problematic alcohol use, illicit drug use and major mental illness were more common among individuals diagnosed with acute HBV than among those with chronic HBV. Similarly, HBV/HCV, HBV/HIV and HBV/HCV/HIV co-infection occurred 3 times more frequently among individuals diagnosed with acute infections. These findings are consistent with those of studies in the United States and with observations made among seroconverters and chronically infected HCV positive individuals in the BC-HTC[17,18,23]. The HBV vaccination rate in BC is high and the incidence of acute HBV infections gradually declined to just 6 reported cases in 2015[7,24]. However, the current opioid epidemic may lead to localized HBV transmission among unvaccinated PWID, as was seen in suburban United States[18,25]. In our study, the odds of acute HBV were highest among younger persons and these age-dependent odds were greatly elevated by injection drug use among younger age groups. Younger PWID had 8-fold greater odds of being diagnosed with acute HBV than older non-PWID and up to 3 times the odds of their non-PWID of identical age. These findings demonstrate the interconnected nature of HBV, HCV and HIV infection, mental illness and alcohol/drug addiction and, therefore, highlight the need for a syndemic approach to significantly reducing new HBV infections. As the incidence of vaccine preventable HBV infection typically occurs among populations that are already affected by many social conditions and infections, such an approach should involve the integration of HBV prevention, screening and treatment programs with those of HIV, HCV, mental health and addiction programs.
Ethnicity was the most distinguishing factor between individuals diagnosed with acute and chronic HBV in BC. While over 75% of persons with acute HBV in the BC-HTC were White, 60% of chronically infected persons were East Asian. East Asian individuals had 12-fold greater odds of being diagnosed with chronic HBV than White persons, which worsened with declining socioeconomic status. Recent studies from the US on acute HBV infection also indicated that 57%-89% of acute infections were among the White population, while the majority of prevalent HBV infections were among people from the Asia-Pacific region[12,13,17,18]. Similarly, most individuals with chronic HBV in Australia in 2011 (38%) were born in the Asia-Pacific region[10]. These results mirror those observed in Canada (2007-2011), where rates of chronic HBV among foreign-born and non-White Canadians were estimated to be 4 and 5 times the national rates, respectively[4]. Low socioeconomic status within the East Asian community may act as a double-edged sword; increasing their risk of chronic HBV, while acting as a barrier to accessing health care. East Asian immigrants may also face additional barriers to health care, including language and cultural barriers, and insufficient information to make full use of the health care system[26-28]. Lack of awareness about HBV and its effects on health, a large proportion of unvaccinated individuals and low awareness of vaccination status may also affect HBV screening and treatment within this population[28,29]. In general, the asymptomatic nature of HBV infection leads to late diagnosis after complications have developed[1]. Studies have shown that immigrants in Canada are disproportionately affected by HCC and decompensated cirrhosis and have up to 5 times greater risk of death from these causes than their Canadian-born counterparts[14,30,31]. Therefore, screening for HBV within the East Asian population is vital for early diagnosis. As the East Asian population in BC faces the additional challenge of a high burden of TB, the integration of HBV screening with TB screening and treatment programs should create additional avenues for the identification of undiagnosed HBV carriers within the East Asian population in BC[32]. The ethnicity-related differences in acute and chronic HBV and related comorbidities and social conditions emphasize the need for differentiated programing for prevention, diagnosis and treatment by ethnicity.
The differences in HBV risk factor patterns among individuals diagnosed with acute and chronic HBV infection in BC are also mirrored by the distinct patterns of circulating HBV genotypes in both populations[33]. Between 2006 and 2012, genotype C viruses were predominantly isolated from individuals with chronic HBV, while genotype D viruses were prevalent among persons diagnosed with acute HBV in Canada[33]. As a high rate of chronic HBV diagnoses in Canada occur among immigrants form HBV endemic countries, these distinctions in circulating HBV genotypes may be related to the primary circulating strains in their home countries as well as to differing modes of acquisition[4,33]. In contrast to developed countries, major risk factors for HBV acquisition in HBV endemic developing countries include unsafe medical practices, for example, during circumcision and dental procedures[34,35]. These variations in circulating genotypes may have serious implications for disease progression and treatment outcomes among individuals diagnosed with either acute or chronic HBV in Canada[33,36]. Indeed, the elevated risk of HCC among African, Asian and Alaskan populations may be linked to circulating HBV genotypes[36]. This reinforces the need for ethnicity-based screening programs that go beyond the ongoing prenatal screening, and neonatal/preadolescent vaccination programs for immigrants originating from HBV endemic countries in certain parts of Canada[37].
The findings of this study should be interpreted in the light of following methodological considerations. As inclusion in the BC-HTC is dependent on either testing for HCV or HIV at the public health laboratory or being reported as confirmed cases of HIV, HCV, HBV or TB, individuals who test negative for HBV and do not meet any of the aforementioned criteria were excluded from our dataset. However, given the large number of HBV negative individuals in the study, we do not expect any major impact on our findings. Data on vaccination status, household contacts, sexual transmission and orientation were not available, which could have further enhanced our understanding of HBV epidemiology within the province. Additionally, ethnicity classifications in our analysis may be affected by the variable sensitivity of Onomap in assigning Asian ethnicities[20]. Onomap was validated on a subset of the BC-HTC and showed low sensitivity for Filipinos. This may have resulted in the misclassification of East Asians from the Philippines as Whites and in the underestimation of the HBV in East Asian population. Previous studies have shown that foreign-born and Indigenous Canadians have a higher prevalence of chronic HBV relative to Canadian-born and non-Indigenous Canadians, respectively[5,30,38]. Similar findings have been reported in Australia and the United States[10,39]. Therefore, future analyses incorporating Indigenous and immigration status would provide additional insights for more tailored prevention and screening programs.
In summary, our findings show distinct characteristics of people diagnosed with acute and chronic infection in BC. Persons diagnosed with acute infection had a high level of substance use, co-infection with HIV or HCV, and were predominantly young White males. As acute HBV infection co-occurs with other infections and social conditions, a syndemic approach, where HBV prevention, screening, and treatment programs are integrated with those of other sexually transmitted and blood-borne infections as well as with mental health and addiction care would be an optimal approach for further reducing the incidence of acute HBV in the province and providing care to those diagnosed with acute HBV.
In contrast, the majority of chronic HBV infections in BC were diagnosed among East and South Asian individuals, who had very low levels of illicit drug and problem alcohol use, major mental illness and co-infection with HIV or HCV. Since traditional risk behavior and viral co-infection are less common among persons with chronic HBV infections, risk-based screening and prevention programs may impact only a fraction of such individuals. Given the asymptomatic nature of the disease and the grave health-related consequences of untreated HBV infection, organized screening programs are urgently needed to facilitate early diagnosis within the immigrant population in BC. Therefore, HBV screening and treatment programs focusing specifically on foreign-born East and South Asian population within BC would be necessary to have a significant impact on HBV-related disease burden within the province.
Hepatitis B virus (HBV) affects approximately 200000 Canadians and 257 million people worldwide. In many developed countries, a relatively larger number of people are diagnosed with chronic HBV compared to acute HBV, each year. Chronic HBV infection is associated with 66% of the 1.34 million viral hepatitis-related deaths reported worldwide. It is responsible for a substantial disease burden from liver cancer and end-stage liver disease.
Data from Canada, the United States and other developed countries indicate that most chronic HBV infections are diagnosed among immigrants from HBV-endemic Asia-Pacific countries, while acute infections are predominant among White individuals. Persons diagnosed with acute and chronic HBV infections may differ with respect to demographics and risk behavior. These distinctions may have implications for interventions targeted at either population. Additionally, 49% of persons with chronic HBV and decompensated cirrhosis and 46% of those with HCC in British Columbia (BC) were diagnosed late in the course of their infections. Therefore, establishing the characteristics of individuals who are more likely to be infected with HBV could enhance the planning of prevention and screening programs to further reduce late diagnoses within the province.
In this study, we describe the characteristics of individuals diagnosed with acute and chronic HBV infections and identify the factors associated with HBV infection within the BC Hepatitis Testers Cohort (BC-HTC). We are unaware of any study comparing large population level data for both acute and chronic HBV. Study findings should inform prevention and screening programs within BC.
We used data from the BC Hepatitis Testers Cohort (BC-HTC), which includes all individuals tested for HCV or HIV and those diagnosed with HBV or TB in BC since 1990. These data were integrated with prescription drug, medical visit, hospitalization and mortality data. HBV cases were classified as acute or chronic in accordance with provincial guidelines. We compared characteristics of individuals by HBV infection group (acute, chronic and negative). Factors associated with acute or chronic HBV infection were assessed with multivariable multinomial logistic regression models in comparison with the HBV negative group.
46498 of the 1058056 eligible BC-HTC participants were diagnosed with HBV infection; 95.7% with chronic infections at HBV diagnosis. Acute HBV infections, indicative of new transmission events, were diagnosed predominantly among males, persons aged between 25 and 34 years, White individuals, and socioeconomically disadvantaged persons. Problematic alcohol use, injection drug use, HIV and HCV co-infection were also common within this group. Individuals diagnosed with chronic HBV infection were predominantly older and East Asian. Additionally, substance use and HIV or HCV co-infection were relatively low within this group. Relative to Whites, East Asians had 12 times greater odds of being diagnosed with chronic HBV infection. These odds increased with increasing socioeconomic deprivation.
These findings highlight distinct risk patterns for individuals with acute and chronic HBV infections and underscore the need for different strategies to prevent, diagnose and treat HBV within these groups. Optimal care for acute HBV would require the integration of HBV prevention, screening, and treatment programs with programs for mental health, addiction and other blood-borne infections. Managing chronic HBV, on the other hand, may require screening programs focusing on at-risk ethnic groups, including foreign-born East and South Asians with low prevalence of traditional risk factors, for early diagnosis and treatment initiation.
We found clear differences in the characteristics of individuals diagnosed with acute and chronic HBV in BC. Consequently, we propose two distinct interventions for the management of acute and chronic HBV in the province: the integration of HBV-related public health programs with those of blood borne infection programs and mental health services to provide optimal care for populations at risk for acquiring acute HBV, and the implementation of targeted screening programs for early diagnosis among ethnic groups at risk for chronic HBV.
We acknowledge the assistance of BCCDC, PHSA Performance measurement and reporting, Information Analysts, Ministry of Health Data Access, Research and Stewardship, and MSP, DAD and Medical Beneficiary and Pharmaceutical Services programme areas, BC Ministry of Health, and BC Cancer Agency and their staff involved in data access and procurement, and data management.
All inferences, opinions, and conclusions drawn in this publication are those of the authors, and do not necessarily reflect the opinions or policies of the (British Columbia) Ministry of Health.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gong ZJ, Roohvand F S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Centers for Disease Control and Prevention. Chapter 10: Hepatitis B. Jennifer Hamborsky, Kroger A, Wolfe C, editors. Epidemiology and prevention of vaccine preventable diseases. 13th ed. Washington, DC: Public Health Foundation; 2015; . [Cited in This Article: ] |
| 2. | World Health Organization. Global hepatitis report, 2017. 2017; Available from: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. [Cited in This Article: ] |
| 3. | Public Health Agency of Canada. Hepatitis B infection in Canada: Brief report; 2011. Available from: http://www.phac-aspc.gc.ca/id-mi/hepatitisBCan-hepatiteBCan-eng.php. [Cited in This Article: ] |
| 4. | Rotermann M, Langlois K, Andonov A, Trubnikov M. Seroprevalence of hepatitis B and C virus infections: Results from the 2007 to 2009 and 2009 to 2011 Canadian Health Measures Survey. Health Rep. 2013;24:3-13. [PubMed] [Cited in This Article: ] |
| 5. | Public Health Agency of Canada. Report on hepatitis B and C in Canada: 2013. 2016; Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/report-hepatitis-b-c-canada-2013.html. [Cited in This Article: ] |
| 6. | Meireles LC, Marinho RT, Van Damme P. Three decades of hepatitis B control with vaccination. World J Hepatol. 2015;7:2127-2132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (2)] |
| 7. | British Columbia Centre for Disease Control. British Columbia annual summary of reportable diseases, 2015. 2016; Available from: http://www.bccdc.ca/resourcegallery/Documents/Statistics%20and%20Research/Statistics%20and%20Reports/Epid/Annual%20Reports/2015CDAnnualReportFinal.pdf. [Cited in This Article: ] |
| 8. | McGuiness L. BCCDC Enhanced Hepatitis Strain Surveillance System (EHSSS) site report, 2000-2011. 2014; Available from: http://www.bccdc.ca/resourcegallery/Documents/Statistics%20and%20Research/Statistics%20and%20Reports/Hepatitis/EHSSS_BCCDC_Site_Report_200011Final_forWeb.ppt. [Cited in This Article: ] |
| 9. | Fang L, Yu A, Buxton JA. Identification of acute vaccine-preventable hepatitis in individuals with chronic hepatitis in British Columbia between 1991 and 2007. Can J Infect Dis Med Microbiol. 2011;22:10-14. [PubMed] [Cited in This Article: ] |
| 10. | MacLachlan JH, Allard N, Towell V, Cowie BC. The burden of chronic hepatitis B virus infection in Australia, 2011. Aust N Z J Public Health. 2013;37:416-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Wallace L, Yeung A, Trayner K, Cullen B, Templeton K, Aitken C. Surveillance report: Hepatitis B infection in Scotland: 2015. Health Prot Scotl. 2017;51:17-36. [Cited in This Article: ] |
| 12. | Tsang CA, Granseth G, Dudek J, Thomas J, Woodward P, Tran S. 2016 viral hepatitis epidemiologic profile for Arizona. Arizona Department of Health Services. 2016; Available from: http://www.azdhs.gov/documents/preparedness/epidemiology-disease-control/hepatitis/arizona-2016-viral-hepatitis-profile.pdf. [Cited in This Article: ] |
| 13. | Centers for Disease Control and Prevention. Viral hepatitis surveillance United States, 2015. 2017; Available from: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. [Cited in This Article: ] |
| 14. | Pottie K, Greenaway C, Feightner J, Welch V, Swinkels H, Rashid M, Narasiah L, Kirmayer LJ, Ueffing E, MacDonald NE. Evidence-based clinical guidelines for immigrants and refugees. CMAJ. 2011;183:E824-E925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 15. | Samji H, Yu A, Kuo M, Alavi M, Woods R, Alvarez M, Dore GJ, Tyndall M, Krajden M, Janjua NZ; BC Hepatitis Testers Cohort Team. Late hepatitis B and C diagnosis in relation to disease decompensation and hepatocellular carcinoma development. J Hepatol. 2017;67:909-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Ghany MG, Perrillo R, Li R, Belle SH, Janssen HL, Terrault NA, Shuhart MC, Lau DT, Kim WR, Fried MW. Characteristics of adults in the hepatitis B research network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol. 2015;13:183-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Iqbal K, Klevens RM, Kainer MA, Baumgartner J, Gerard K, Poissant T, Sweet K, Vonderwahl C, Knickerbocker T, Khudyakov Y. Epidemiology of Acute Hepatitis B in the United States From Population-Based Surveillance, 2006-2011. Clin Infect Dis. 2015;61:584-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Harris AM, Iqbal K, Schillie S, Britton J, Kainer MA, Tressler S, Vellozzi C. Increases in Acute Hepatitis B Virus Infections - Kentucky, Tennessee, and West Virginia, 2006-2013. MMWR Morb Mortal Wkly Rep. 2016;65:47-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Janjua NZ, Kuo M, Chong M, Yu A, Alvarez M, Cook D, Armour R, Aiken C, Li K, Mussavi Rizi SA. Assessing Hepatitis C Burden and Treatment Effectiveness through the British Columbia Hepatitis Testers Cohort (BC-HTC): Design and Characteristics of Linked and Unlinked Participants. PLoS One. 2016;11:e0150176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Lakha F, Gorman DR, Mateos P. Name analysis to classify populations by ethnicity in public health: validation of Onomap in Scotland. Public Health. 2011;125:688-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Ryan R, Vernon S, Lawrence G, Wilson S. Use of name recognition software, census data and multiple imputation to predict missing data on ethnicity: application to cancer registry records. BMC Med Inform Decis Mak. 2012;12:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Pampalon R, Hamel D, Gamache P, Simpson A, Philibert MD. Validation of a deprivation index for public health: a complex exercise illustrated by the Quebec index. Chronic Dis Inj Can. 2014;34:12-22. [PubMed] [Cited in This Article: ] |
| 23. | Janjua NZ, Yu A, Kuo M, Alvarez M, Cook D, Wong J, Tyndall MW, Krajden M. Twin epidemics of new and prevalent hepatitis C infections in Canada: BC Hepatitis Testers Cohort. BMC Infect Dis. 2016;16:334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Public Health Agency of Canada. Canadian immunization guide: Part 4-Active vaccines-Hepatitis B vaccine; 2017. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-7-hepatitis-b-vaccine.html. [Cited in This Article: ] |
| 25. | The Canadian Press. BC first in Canada to declare public health emergency after fentanyl overdoses. Maclean’s. 14 Apr. 2016; Available from: http://www.macleans.ca/news/canada/b-c-first-in-canada-to-declare-public-health-emergency-after-fentanyl-overdoses/. [Cited in This Article: ] |
| 26. | Kalich A, Heinemann L, Ghahari S. A Scoping Review of Immigrant Experience of Health Care Access Barriers in Canada. J Immigr Minor Health. 2016;18:697-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Newbold KB. Health care use and the Canadian immigrant population. Int J Health Serv. 2009;39:545-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Sherman M, Bilodeau M, Cooper C, Mackie D, Depew W, Villeneuve JP. Liver disease in Canada: A crisis in the making. Canadian Liver Foundation 2013. Available from: http://www.liver.ca/files/PDF/Liver_Disease_Report_2013/Liver_Disease_in_Canada_-_E.pdf. [Cited in This Article: ] |
| 29. | Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46:1034-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Ng E, Myers RP, Manuel D, Sanmartin C. Hospital stays for hepatitis B or C virus infection or primary liver cancer among immigrants: a census-linked population-based cohort study. CMAJ Open. 2016;4:E162-E168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | DesMeules M, Gold J, McDermott S, Cao Z, Payne J, Lafrance B, Vissandjée B, Kliewer E, Mao Y. Disparities in mortality patterns among Canadian immigrants and refugees, 1980-1998: results of a national cohort study. J Immigr Health. 2005;7:221-232. [PubMed] [Cited in This Article: ] |
| 32. | British Columbia Centre for Disease Control. TB in British Columbia: Annual report 2014; 2016. Available from: http://www.bccdc.ca/resource-gallery/Documents/Statistics%20and%20Research/Statistics%20and%20Reports/TB/TB_Annual_Report_2014.pdf. [Cited in This Article: ] |
| 33. | Osiowy C, Giles E, Trubnikov M, Choudhri Y, Andonov A. Characterization of Acute and Chronic Hepatitis B Virus Genotypes in Canada. PLoS One. 2015;10:e0136074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Olayinka AT, Oyemakinde A, Balogun MS, Ajudua A, Nguku P, Aderinola M, Egwuenu-Oladejo A, Ajisegiri SW, Sha’aibu S, Musa BO. Seroprevalence of Hepatitis B Infection in Nigeria: A National Survey. Am J Trop Med Hyg. 2016;95:902-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Mahtab MA, Rahman S, Karim MF, Khan M, Foster G, Solaiman S, Afroz S. Epidemiology of hepatitis B virus in Bangladeshi general population. Hepatobiliary Pancreat Dis Int. 2008;7:595-600. [PubMed] [Cited in This Article: ] |
| 36. | Sherman M, Bain V, Villeneuve JP, Myers RP, Cooper C, Martin S, Lowe C. The management of chronic viral hepatitis: a Canadian consensus conference 2004. Can J Gastroenterol. 2004;18:715-728. [PubMed] [Cited in This Article: ] |
| 37. | Villeneuve JP. The natural history of chronic hepatitis B virus infection. J Clin Virol. 2005;34 Suppl 1:S139-S142. [PubMed] [Cited in This Article: ] |
| 38. | Rossi C, Schwartzman K, Oxlade O, Klein MB, Greenaway C. Hepatitis B screening and vaccination strategies for newly arrived adult Canadian immigrants and refugees: a cost-effectiveness analysis. PLoS One. 2013;8:e78548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Liu SJ, Iqbal K, Shallow S, Speers S, Rizzo E, Gerard K, Poissant T, Klevens RM. Characterization of chronic hepatitis B cases among foreign-born persons in six population-based surveillance sites, United States 2001-2010. J Immigr Minor Health. 2015;17:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |