Published online Feb 14, 2017. doi: 10.3748/wjg.v23.i6.1106
Peer-review started: October 28, 2016
First decision: December 01, 2016
Revised: December 17, 2016
Accepted: January 4, 2017
Article in press: January 4, 2017
Published online: February 14, 2017
High-grade colonic neuroendocrine carcinomas (NECs) are uncommon but extremely aggressive. Their co-existence with tubular adenoma (TA) has rarely been reported. We present a 68-year-old man who was found on routine colonoscopy to have multiple colorectal TAs and an ulcerated lesion in the ascending colon. Microscopically, a poorly-differentiated invasive carcinoma juxtaposed with a TA was identified. Differential diagnosis included a poorly-differentiated adenocarcinoma, medullary carcinoma, high-grade NEC and lymphoma. The immunohistochemical profile showed positive staining for keratins, synaptophysin and chromogranin but negative for LCA, CDX2, CK7, CK20, TTF-1 and PSA, supporting the NEC diagnosis. Upon subsequent laparoscopic right hemicolectomy, the tumor was identified as a 3.0 cm umbilicated and ulcerated mass with an adjacent TA. Both TA and NEC showed positive staining for β-catenin indicating a shared colonic origin. The mitotic counts (77/10 high power fields) and a high proliferation rate (75% by Ki-67) corroborated a high-grade stratification. Mutational analysis indicated a wild-type BRAF and KRAS with mismatch repair proficiency. The AJCC (7th edition) pathologic stage is pT3, pN0, pMx. The patient received adjuvant chemotherapy with cisplatin/etoposides for three cycles and will be followed up for a year to detect recurrence. In conclusion, the co-existence of TA with high grade-NEC in our case allowed early identification and intervention of the otherwise asymptomatic but aggressive tumor. In addition, the finding of a high-grade NEC within a large TA in this case suggests a link between the two lesions and could represent a shared stem cell origin.
Core tip: This is a case report of a patient with a high-grade large cell neuroendocrine carcinoma in the ascending colon with an overlying tubular adenoma discovered during routine colonoscopic screening in absence of clinical symptoms. This is a unique case where the contiguity of the neuroendocrine carcinoma to the tubular adenoma allowed for the diagnosis of the otherwise asymptomatic high-grade carcinoma. Being aware of this association bears practical implication where it can be conducive to the early and correct diagnosis of invasive cancer. In addition, we review the literature citing pertinent cases.
- Citation: Soliman ML, Tiwari A, Zhao Q. Coexisting tubular adenoma with a neuroendocrine carcinoma of colon allowing early surgical intervention and implicating a shared stem cell origin. World J Gastroenterol 2017; 23(6): 1106-1112
- URL: https://www.wjgnet.com/1007-9327/full/v23/i6/1106.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i6.1106
Neuroendocrine tumors (NET) is a distinct group of neoplasms that arises from enterochromaffin/neuroendocrine cells throughout the body and display unique histomorphology. The NETs are composed of neuroendocrine secretory granules with neurosecretory capacity that can be detected immunohistochemically on tissue sections and serologically in peripheral blood, i.e., chromogranin A, which can be used as tumor surrogate marker. The 2010 World Health Organization (WHO) classification, divides NET into well- and poorly-differentiated categories. The well-differentiated NET include low-grade (G1) and intermediate-grade (G2), whereas the poorly-differentiated tumors are high-grade (G3) and are called neuroendocrine carcinoma (NEC), including large cell and small cell types[1]. Both mitotic count and Ki-67 labeling index for measuring proliferation rate are required for tumor classification.
NETs in gastrointestinal tract account for 2% of all GI malignancies, and majority of them are well- to moderately-differentiated. High-grade NECs are extremely rare in GI tract, but they are extremely aggressive with poor prognosis when compared to colorectal adenocarcinoma of similar pathological stages due to their advanced and widely metastatic disease at the time of diagnosis[2,3]. The coexistence of tubular adenoma (TA) with high-grade NECs has been reported in only a few articles in the GI tracts such as in the ampulla of Vater[4], stomach[5] and rectum[6,7]. Most of the reported cases showed liver or lymph nodes metastases at the time of diagnosis. We present a unique case of a large cell high-grade NEC of ascending colon that was identified during a routine colonoscopic surveillance, due to its coexistence with a TA.
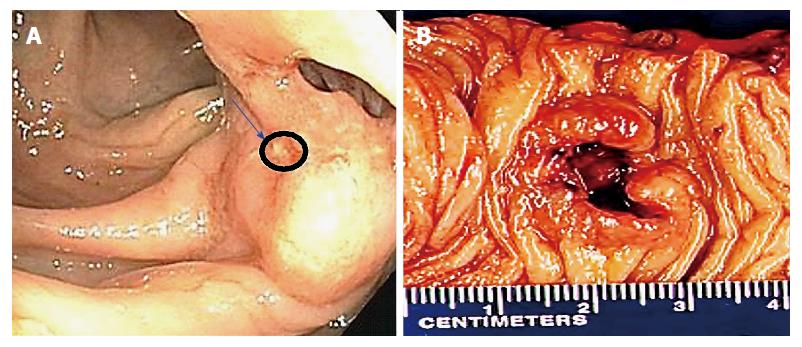
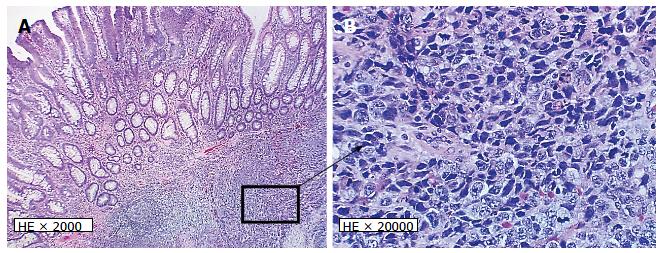
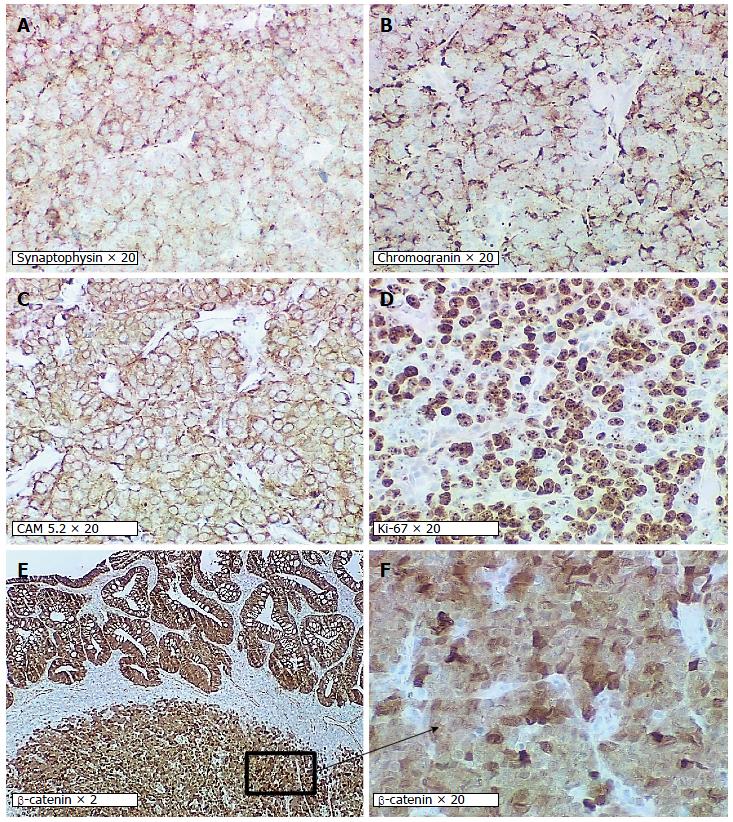
A 68-year-old male undergoing a routine colonoscopy was found to have multiple TAs measuring 5-8 mm, including one in ascending colon with surrounding ulcerated, irregular and slightly raised mucosa (Figure 1A) which was also biopsied. Under microscopic examination, an infiltrating poorly-differentiated malignant tumor was identified in the ulcerated lesion in close vicinity with an overlying TA. The tumor demonstrated pathological findings suggestive of a high-grade malignancy such as increased mitotic figures and apoptosis. The differential diagnosis included a poorly-differentiated adenocarcinoma, medullary carcinoma, NEC, high-grade lymphoma and sarcoma. Immunohistochemical studies performed on formalin-fixed paraffin-embedded tissue sections revealed positive staining for keratin Cam5.2, neuroendocrine markers synaptophysin and chromogranin but negative for lymphoma (LCA/CD45), colorectal adenocarcinoma (CDX2, CK20, CK7), lung adenocarcinoma (TTF-1) and prostatic adenocarcinoma (PSA) markers. A diagnosis of high-grade NEC large cell type with associated TA was rendered. Further evaluation by computed tomography (CT) and magnetic resonance imaging (MRI) for tumor staging revealed no metastatic disease or lymphadenopathy. Three months after the biopsy, the patient underwent laparoscopic right hemicolectomy. A 3-cm umbilicated tumor mass was identified in the proximal ascending colon with slightly raised border and central ulceration (Figure 1B). The tumor was grossly and microscopically seen invading into and through the muscularis propria into subserosal soft tissue, but not the serosa. The microscopic examination revealed solid sheets and nests of high-grade tumor cells with medium to large-sized vesicular nuclei and prominent nucleoli as well as prominent lymphocytic infiltration; a pattern morphologically consistent with the large-cell variant of high-grade NEC (Figure 2A and B). Moreover, the residual overlying TA is again identified in the raised mucosal surface with no high-grade dysplasia (Figure 2A). In addition to the classic morphology of a high-grade large-cell neuroendocrine carcinoma, the diagnosis was confirmed using immunohistochemical studies showing positive staining of the tumor cells for synaptophysin and chromogranin (Figure 3A and B). The number of mitotic figures [up to 77/10 high power fields (hpf)], the presence of focal tumor necrosis and the Ki-67 proliferation index of 75% (Figure 3D) confirmed the stratification as a high-grade NEC. Mutational analysis studies indicated that the NEC tumor cells expressed wild type BRAF and KRAS in addition to intact expression of mismatch repair proteins (MLH1, MSH2, PMS2 and MSH6) and therefore negative screening for Lynch syndrome. Immunohistochemical studies showed strong and diffuse positive staining for CAM5.2 and nuclear staining for β-catenin in the NEC tumor cells and TA (Figure 3C, E and F), indicating both components are related. No regional lymph node metastases were noted in 24 examined lymph nodes. According to the American Joint Committee on Cancer Staging Manual, 7th edition, the tumor is staged as pT3, pN0, pMx. The patient received adjuvant chemotherapy with cisplatin/etoposides for three cycles and has not shown any recurrence. The patient will be followed up for a year to detect recurrence.
We described a clinical scenario of a 68-year-old male patient who was found to have a mixed high-grade large cell NEC with an overlying TA discovered during routine colonoscopic screening in absence of clinical symptoms. The invasive large cell NEC was located underneath the TA, deeply involving the muscularis propria, without liver or distant metastatic disease or lymph nodes involvement at the time of primary tumor resection. The patient received postsurgical chemotherapy due to the high-grade nature of the carcinoma. As of the time of the article preparation, no recurrence was identified. Long-term follow up will be necessary to report any potential recurrence. We suspect that the clinical outcome will be shaped by the behavior of the clinically predominant tumor, which is neuroendocrine tumor in the present case.
The sequence of adenoma to adenocarcinoma in colorectal cancer is well established and both are believed to originate from the same precursor cells. A mixed or collision tumor is composed of both adenocarcinoma and NEC. The notion that the two malignancies as well as the TA originate from common multipotent stem cells simultaneously differentiating into glandular and neuroendocrine lineages is likely and plausible. However, when a TA is mixed with a high-grade NEC, their association becomes less clear. Following the examination of genome-wide loss of heterozygosity, it was proposed that the common genetics of the glandular and neuroendocrine components indicate origin from a single precursor[8]. This suggests that the two components of mixed tumors arise from multi-potential stem cells and show bi-phenotypic differentiation after carcinogenesis is initiated[9,10]. An alternative hypothesis is that mixed tumors are a neuroendocrine phenotype of dedifferentiated tubular adenocarcinoma[11]. Certain cases are reported of cecum and rectosigmoid collision adenocarcinoma and neuroendocrine tumors which were found to metastasize to lymph nodes as juxtaposed glandular and neuroendocrine components[12,13]. Likewise, a case of collision tumor located in transverse colon (adenocarcinoma and large-cell NEC) presented three years after resection with a retroperitoneal metastasis with 50% NEC and 50% adenocarcinoma[14]. These latter examples support the counter argument against the double primaries theory.
Poorly differentiated NEC may lose their intestinal differentiation and show negative immunostaining for CK20 and CDX2 as in our case. However, we found CK20 and CDX2 to be positive in the TA component as expected. The dedifferentiation and/or loss of intestinal differentiation may be a late event.
The current case presented as an ulcerative mass in the right colon, with TA (30% of the mass) juxtaposed with NEC (70% of the mass). In a review of 67 previously reported cases and 23 new cases, mixed neoplasms were found to present as a polypoid growth (57%), mass (30%) and ulcerating lesion (9%). The glandular component varied from adenoma (17%) to adenoma with well- or moderately-differentiated adenocarcinoma (35%), or adenoma with poorly-differentiated adenocarcinoma (48%). Furthermore, 56% of mixed colo-rectal tumors arise in the right colon with the tumors being 58% collision tumors (two distinct neoplastic components), 42% composite (i.e., intermixed neoplastic components) and less than 1% amphicrine (where individual cells demonstrate both exocrine and endocrine features) and with mean age of presentation of 61.9[15]. Nevertheless, mixed neoplasms have also been reported in various locations like the gallbladder[16], pancreatic ampulla[17], stomach[10] and cecum[18]. Based on the WHO 2010 classification, the neuroendocrine component in the present case is G3 based on the mitotic count of 77/10 hpf and the Ki-67 index of 75%, which far exceed the 20/10 hpf and 20% proliferation index, respectively used to grade NECs.
The patient under study has not presented with symptoms suggestive of carcinoid syndrome. Whereas many neuroendocrine tumors present with secretory syndromes characterized by flushing, diarrhea, wheezes, sweating, palpitations and right-sided heart valve lesions, gastrointestinal neuroendocrine tumors are less likely to present with carcinoid syndrome[19]. Serum chromogranin A elevation can be detected in patient with neuroendocrine tumor in the absence of carcinoid syndromes.
The large cell NEC is a poorly-differentiated neoplasm that is quite and belongs to the poorest prognostic subgroup among primary colorectal cancers. In a study of 6495 patients with colorectal cancer, only 0.65% had NEC and only 0.2% had large cell NEC. Despite its rarity, it is important to differentiate colorectal NEC from other tumor types because patients may benefit from alternative cytotoxic chemotherapy[20]. In this regard, unlike the colorectal adenocarcinoma, large cell NEC is managed primarily with platinum based chemotherapy such as cisplatin/etoposide and cisplatin/irinotecan. Based on various studies, the median survival rate for patients with colorectal NEC is between 5 and 11 mo with one-year survival rate between 10% and 15%. Patients with colorectal large cell neuroendocrine carcinoma were found an overall median survival of 9.4 mo[20].
Well-differentiated neuroendocrine tumors (also known as carcinoid) or microcarcinoid tumor nests had been reported in association with colonic tubular or tubulovillous adenoma. Those neuroendocrine components are well-differentiated either forming tumor nodules or scattered in the lamina propria within the adenoma lesion[21,22]. High-grade NECs arising in association with tubulovillous or tubular adenoma have only been seen in a handful of cases[23-26]. Whereas more than 50% of cases were found to have liver, bone or nodal metastasis at the time of diagnosis even when the tumor was microscopic, some primary tumors showed invasion limited to the muscularis mucosa. Therefore, colorectal NECs tend to behave aggressively and the prognosis is extremely poor with survival lasting a few months. Our case represents a unique situation where the presence of a TA warranted a polypectomy and endoscopic evaluation led to the adjacent ulcerated mucosa to be noticed and biopsied. In other words, the contiguity of the NEC to the TA allowed for early diagnosis of the otherwise asymptomatic NEC. Being aware of this association bears practical implication where it can be conducive to the early and correct diagnosis of high-grade tumor such as the NEC in the current case, and avoiding overlooking other fragments distinct from the adenoma.
Another implication to be born in mind is that superficial biopsy specimens may not provide the adequate representation of underlying neuroendocrine tumor found in the mixed tumor. Even when present in the biopsy sample, pathologists must be vigilant of the possibility of a mixed tumor and resist the tendency to diagnose an adenoma before careful inspection of the tissue for additional lesions. The overlying large TA or tubulovillous adenomas may mask a deep invasive tumor, making it imperative to collect adequate and deep tissue samples. Furthermore, surgical pathologist must also be aware of the differential diagnosis of poorly differentiated neoplasms that may involve an adenoma. This includes poorly-differentiated adenocarcinoma, medullary carcinoma, high-grade neuroendocrine carcinoma and lymphoma. Because the prognosis and management varies significantly amongst these entities, careful attention must be paid to the morphology of the tumor cells and general architecture. In addition, a panel of immunohistochemical stains that includes neuroendocrine, lymphocytic and adenocarcinoma markers and DNA mismatch repair enzyme screening should be considered to use for rendering the correct diagnosis.
An asymptomatic 68-year-old male patient undergoing routine colonoscopy.
Multiple ascending colon tubular adenomas, one of which is surrounded by an ulcerated and irregular mucosa of a suspicious lesion.
The tumor demonstrated morphological features of high-grade and poorly differentiated malignancy. Broad differential diagnoses included a poorly-differentiated adenocarcinoma, medullary carcinoma, neuroendocrine carcinoma, high-grade lymphoma and sarcoma.
Laboratory results were unremarkable.
Computed tomography and magnetic resonance imaging evaluation for tumor staging revealed no metastatic disease or lymphadenopathy.
Immunohistochemical studies performed on formalin-fixed paraffin-embedded tissue sections revealed positive staining of the tumor cells for the neuroendocrine markers (synaptophysin and chromogranin) but negative staining for lymphoma (LCA/CD45), colorectal adenocarcinoma (CDX2, CK20, CK7) and prostatic adenocarcinoma markers, rendering a diagnosis of high-grade neuroendocrine large-cell type with associated tubular adenoma.
Laparoscopic right hemicolectomy followed by three cycles of adjuvant chemotherapy with cisplatin/etoposides.
Neuroendocrine carcinomas are malignant neoplasms of the enterochromaffin tissue.
For patients undergoing colonoscopy screening, all biopsy fragments should be examined at all levels with high suspicion in order not to miss indolent high grade malignancies.
Colocalization of tubular adenoma and high-grade neuroendocrine carcinoma is rarely encountered; early diagnosis of a high-grade albeit asymptomatic neuroendocrine carcinoma carries a better long-term survival benefit.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liu SH S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Klimstra DS. Pathologic Classification of Neuroendocrine Neoplasms. Hematol Oncol Clin North Am. 2016;30:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3022] [Cited by in F6Publishing: 3036] [Article Influence: 189.8] [Reference Citation Analysis (0)] |
| 3. | Ilett EE, Langer SW, Olsen IH, Federspiel B, Kjær A, Knigge U. Neuroendocrine Carcinomas of the Gastroenteropancreatic System: A Comprehensive Review. Diagnostics (Basel). 2015;5:119-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Sun JH, Chao M, Zhang SZ, Zhang GQ, Li B, Wu JJ. Coexistence of small cell neuroendocrine carcinoma and villous adenoma in the ampulla of Vater. World J Gastroenterol. 2008;14:4709-4712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | De Marco L, Carlinfante G, Botticelli L, Di Maira PV, Putrino I, Cavazza A. Mixed neoplasia of the stomach: description of a case of tubular adenoma combined with carcinoid. Pathologica. 2003;95:214-216. [PubMed] [Cited in This Article: ] |
| 6. | Yoshikane H, Arakawa D, Kawashima H, Sakakibara A, Hidano H, Takahashi H, Yokoi T. Small neuroendocrine carcinoma of the rectum entirely covered by an adenomatous component. Endoscopy. 2001;33:298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Khamidullina GA, Kapuller LL, Sereda EN, Izbagambetov NA, Zharkov NV. Small-cell rectal carcinoma coexisted with tubular-villous adenoma. Arkh Patol. 2006;68:37-39. [PubMed] [Cited in This Article: ] |
| 8. | Kim KM, Kim MJ, Cho BK, Choi SW, Rhyu MG. Genetic evidence for the multi-step progression of mixed glandular-neuroendocrine gastric carcinomas. Virchows Arch. 2002;440:85-93. [PubMed] [Cited in This Article: ] |
| 9. | Kitajima T, Kaida S, Lee S, Haruta S, Shinohara H, Ueno M, Suyama K, Oota Y, Fujii T, Udagawa H. Mixed adeno(neuro)endocrine carcinoma arising from the ectopic gastric mucosa of the upper thoracic esophagus. World J Surg Oncol. 2013;11:218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Kim JJ, Kim JY, Hur H, Cho YK, Han SU. Clinicopathologic significance of gastric adenocarcinoma with neuroendocrine features. J Gastric Cancer. 2011;11:195-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Gurzu S, Kadar Z, Bara T, Bara T, Tamasi A, Azamfirei L, Jung I. Mixed adenoneuroendocrine carcinoma of gastrointestinal tract: report of two cases. World J Gastroenterol. 2015;21:1329-1333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 51] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Pecorella I, Memeo L, Ciardi A, Rotterdam H. An unusual case of colonic mixed adenoendocrine carcinoma: collision versus composite tumor. A case report and review of the literature. Ann Diagn Pathol. 2007;11:285-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Meşină C, Vasile I, Ciobanu D, Calotă F, Gruia CL, Streba L, Mogoantă SŞ, Pârvănescu H, Georgescu CV, Tarniţă DN. Collision tumor of recto-sigmoidian junction - case presentation. Rom J Morphol Embryol. 2014;55:643-647. [PubMed] [Cited in This Article: ] |
| 14. | Minaya-Bravo AM, Garcia Mahillo JC, Mendoza Moreno F, Noguelares Fraguas F, Granell J. Large cell neuroendocrine - Adenocarcinona mixed tumour of colon: Collision tumour with peculiar behaviour. What do we know about these tumours? Ann Med Surg (Lond). 2015;4:399-403. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Li Y, Yau A, Schaeffer D, Magliocco A, Gui X, Urbanski S, Waghray R, Owen D, Gao ZH. Colorectal glandular-neuroendocrine mixed tumor: pathologic spectrum and clinical implications. Am J Surg Pathol. 2011;35:413-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Paniz Mondolfi AE, Slova D, Fan W, Attiyeh FF, Afthinos J, Reidy J, Pang Y, Theise ND. Mixed adenoneuroendocrine carcinoma (MANEC) of the gallbladder: a possible stem cell tumor? Pathol Int. 2011;61:608-614. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Huang Z, Xiao WD, Li Y, Huang S, Cai J, Ao J. Mixed adenoneuroendocrine carcinoma of the ampulla: two case reports. World J Gastroenterol. 2015;21:2254-2259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 18. | Jain A, Singla S, Jagdeesh KS, Vishnumurthy HY. Mixed adenoneuroendocrine carcinoma of cecum: a rare entity. J Clin Imaging Sci. 2013;3:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Wang YH, Lin Y, Xue L, Wang JH, Chen MH, Chen J. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: A single-institution analysis (1995-2012) in South China. BMC Endocr Disord. 2012;12:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 20. | Bernick PE, Klimstra DS, Shia J, Minsky B, Saltz L, Shi W, Thaler H, Guillem J, Paty P, Cohen AM. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum. 2004;47:163-169. [PubMed] [Cited in This Article: ] |
| 21. | Pulitzer M, Xu R, Suriawinata AA, Waye JD, Harpaz N. Microcarcinoids in large intestinal adenomas. Am J Surg Pathol. 2006;30:1531-1536. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Ito H, Ito M, Tahara E. Minute carcinoid arising in gastric tubular adenoma. Histopathology. 1989;15:96-99. [PubMed] [Cited in This Article: ] |
| 23. | Aponte Aponte IE, Caceres W. Anal large cell neuroendocrine carcinoma with tubulovillous adenoma coexistence: A case report. Bol Asoc Med P R. 2014;106:27-28. [PubMed] [Cited in This Article: ] |
| 24. | Kuratate S, Inoue S, Chikakiyo M, Kaneda Y, Harino Y, Hirose T, Yagi T, Saitoh S, Sumitomo M, Fujino R. Coexistent poorly-differentiated neuroendocrine cell carcinoma and non-invasive well-differentiated adenocarcinoma in tubulovillous adenoma of the rectum: report of a case. J Med Invest. 2010;57:338-344. [PubMed] [Cited in This Article: ] |
| 25. | Ispas C, Yu J, Tarantino DR, Lara JF. Pathologic quiz case: a 44-year-old woman with a tubulovillous adenoma of the colon and liver and bone lesions. Small cell (neuroendocrine) carcinoma of the colon with metastasis and an associated, overlying villous adenoma. Arch Pathol Lab Med. 2005;129:412-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 26. | Ubiali A, Benetti A, Papotti M, Villanacci V, Rindi G. Genetic alterations in poorly differentiated endocrine colon carcinomas developing in tubulo-villous adenomas: a report of two cases. Virchows Arch. 2001;439:776-781. [PubMed] [Cited in This Article: ] |