Published online Dec 21, 2017. doi: 10.3748/wjg.v23.i47.8405
Peer-review started: September 3, 2017
First decision: September 20, 2017
Revised: October 3, 2017
Accepted: October 26, 2017
Article in press: October 26, 2017
Published online: December 21, 2017
To evaluate the rate of adverse events (AEs) during consecutive gastric and duodenal polypectomies in several Spanish centers.
Polypectomies of protruded gastric or duodenal polyps ≥ 5 mm using hot snare were prospectively included. Prophylactic measures of hemorrhage were allowed in predefined cases. AEs were defined and graded according to the lexicon recommended by the American Society for Gastrointestinal Endoscopy. Patients were followed for 48 h, one week and 1 mo after the procedure.
308 patients were included and a single polypectomy was performed in 205. Only 36 (11.7%) were on prior anticoagulant therapy. Mean polyp size was 15 ± 8.9 mm (5-60) and in 294 cases (95.4%) were located in the stomach. Hemorrhage prophylaxis was performed in 219 (71.1%) patients. Nine patients presented AEs (2.9%), and 6 of them were bleeding (n = 6, 1.9%) (in 5 out of 6 AE, different types of endoscopic treatment were performed). Other 24 hemorrhagic episodes could be managed without any change in the outcome of the endoscopy and, consequently, were considered incidents. We did not find any independent risk factor of bleeding.
Gastroduodenal polypectomy using prophylactic measures has a rate of AEs small enough to consider this procedure a safe and effective method for polyp resection independently of the polyp size and location.
Core tip: The safety of polypectomy in the upper gastroduodenal tract is controversial because the reported rate in retrospective studies is higher than in colonic polypectomy but results come mainly from retrospective studies and they do not use the same standardized nomenclature and definitions for adverse events. To our knowledge, this is the first study using the ASGE lexicon for reporting adverse events of gastro-duodenal polypectomy and shows an acceptable low rate, confirming the safety of this procedure.
- Citation: Córdova H, Argüello L, Loras C, Naranjo Rodríguez A, Riu Pons F, Gornals JB, Nicolás-Pérez D, Andújar Murcia X, Hernández L, Santolaria S, Leal C, Pons C, Pérez-Cuadrado-Robles E, García-Bosch O, Papo Berger M, Ulla Rocha JL, Sánchez-Montes C, Fernández-Esparrach G. Rate of adverse events of gastroduodenal snare polypectomy for non-flat polyp is low: A prospective and multicenter study. World J Gastroenterol 2017; 23(47): 8405-8414
- URL: https://www.wjgnet.com/1007-9327/full/v23/i47/8405.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i47.8405
Gastric polyps are found in around 3%-6% of patients undergoing upper endoscopy[1,2]. According to these sources, the most common gastric polyps are the hyperplastic and fundic gland types (70%-90%) followed by adenomas, with a variable prevalence among countries depending on the use of proton pump inhibitor drugs (PPI) or the prevalence of Helicobacter pylori (H. pylori) infection. In western countries, where H. pylori infection is low and PPI use is very common, fundic gland polyps are seen more frequently[2,3]. However, two retrospective Spanish series showed that in Spain the most frequent were hyperplastic polyps (50.9%), followed by fundic gland polyps (7.4%), adenomas (3%), and adenocarcinomas (1.9%)[4,5].
Sporadic duodenal polyps are uncommon with a prevalence of 0.3% to 4.6%[6,7]. Whereas multiple, small polyps in the duodenal bulb are benign a substantial number of them located in the descending duodenum are adenomas[7]. Duodenal adenomas can occur sporadically or more commonly in patients with Familial Adenomatous Polyposis, occurring in 50% to 100%[8]. Most of gastric and duodenal polyps are asymptomatic and are incidentally found at endoscopy performed for unrelated reasons.
Gastric and duodenal polyps have a risk of malignant transformation that depends on histologic type. The risk of gastric polyps undergoing malignant transformation is near 2% for hyperplasic polyps, 5% for tubular adenoma, and up to 30% for tubulovillous adenoma[9-11]. However, polyp histology cannot be reliably distinguished by endoscopic appearance[12,13] and biopsy is not always representative of the entire polyp[14]. Therefore, polypectomy is warranted if feasible and clinically appropriate; this is supported by current guidelines[15,16].
Endoscopic polypectomy has become standard in the management of most polyps in the gastrointestinal tract. Gastric and duodenal polyps can be safely removed with snare depending on size, location and presence of a stalk. However, bleeding is the most common adverse event (AE) of snare polypectomy, with an incidence of 6% to 7.2% in gastric polyps and up to 13.9% in duodenal polyps[17,18]. Although these figures are higher than those reported in colonic polypectomies (0.3%-6%)[19,20], the available evidence is limited by the fact that it is often based on retrospective studies performed at a single center or with a small number of patients and the nomenclature and definitions used for AEs are different. The need for standardized nomenclature and agreement on definitions for AEs was addressed by the American Society for Gastrointestinal Endoscopy (ASGE) in a workshop celebrated in 2008 and whose recommendations were published in 2010[21]. Moreover, there are few studies that specifically evaluate risk factors and the efficacy of different hemostatic techniques in the prevention and control of post-polypectomy bleeding.
The aim of this study was to estimate the incidence and risk factors of several types of AEs associated with gastroduodenal polypectomy in several Spanish hospitals using a standardized lexicon specific for endoscopic procedures.
This is a prospective multicenter study performed at 15 Spanish hospitals. Patients with gastric and duodenal polyps that underwent endoscopic polypectomy were eligible for inclusion in the study. All patients included in the study had been previously diagnosed of gastric polyps and subsequently underwent a second endoscopy to perform the polypectomy. Therefore, when the physicians were aware that they had to perform the polypectomy, they previously asked the patient for consent to participate in the study. Inclusion criteria were: (1) protruded gastric or duodenal polyps ≥ 5 mm; and (2) polypectomy performed using an electrocautery snare. The exclusion criteria were: (1) age under 18 years; (2) prothrombin time < 50% or INR > 1.5 and platelet count < 50000 (blood test were only mandatory in patients with anticoagulation therapy or with conditions associated with coagulation disturbances); (3) aspirin intake during the previous 3 d; (4) clopidogrel intake during the previous 7 d; and (5) conditions associated with coagulation disturbances. The study protocol was approved by the Ethics Committee of each hospital and informed consent was obtained from all patients.
Three days before the procedure, oral anticoagulants were replaced by subcutaneous low-molecular weight heparin. The patients were guided to reintroduce them 24-h after the procedure (the dose depended on the value of the previous INR value). Aspirin and clopidogrel were also reintroduced at usual doses.
Snare polypectomy was performed according to the conventional method encircling the polyp with a polypectomy snare and applying electrocautery current[22]. Patients were placed in the left lateral decubitus position and sedation was administered according to the endoscopist or anaesthesiologist’s preference.
Variables were recorded in database templates. The database included demographic characteristics, medical and drug history, indication of upper endoscopy, endoscopists’ expertise (staff or fellow), morphological features and localization of polyps, technical information about the polypectomy procedure (bloc/peacemeal resection, cautery setting, hemorrhage prophylaxis technique), type of sedation, unexpected events and measures for correcting them, and patient outcome. Polyp size was determined endoscopically using an open biopsy forceps (7 mm in length, Boston Scientific Large Capacity with Needle Biopsy Forceps 2.8 mm). In cases with multiple polyps, the biggest one’s characteristics were recorded.
AEs were defined, following the lexicon of ASGE Workshop[21], as an event that prevents completion of the polypectomy (planned procedure) and/or results in admission to hospital, prolongation of existing hospital stay, another procedure (needing sedation/anesthesia), or subsequent medical consultation. Unplanned events that did not interfere with completion of the planned procedure or changed the plan of care were classified as incidents.
Severity of AEs was graded as mild, moderate, severe and fatal according to ASGE classification. AEs were defined as mild or moderate if patients required less than 4 nights or between 4 to 10 nights of hospitalization respectively. They were classified as severe if unplanned or prolonged hospitalization was required for more than 10 nights or requiring intensive care unit admission or surgery. Finally they were graded as fatal if death occurred in relationship of the procedure.
Based on timing, AEs were defined as “intra-procedure” if they occurred during the exploration or in the recovery area, “early” if they occurred within 14 d and "late" from day 15th onward after polypectomy.
AEs were assessed and recorded by a physician during and after the procedure while the patient was recovering from sedation or anesthesia and up to 24 h later in those admitted for observation. At 48 h, one week and day 30 after the procedure, a telephone call was made in order to ask the patient whether they had experienced any symptoms or required medical assistance. A standard questioner was used for the evaluation of late complications. Responses were recorded and entered into a database.
The completeness of data collection was monitored every 2 wk and missing data were proactively collected by contacting the patients and/or referring physicians, as far as this was possible.
Bleeding was recorded as a potential AE when it required any form of intervention, either immediately after polypectomy during the index endoscopy, or in a repeat endoscopy, regardless of obtaining hemostasis, hospital admission, blood transfusion, or surgery. Depending on its activity, bleeding was classified as spurting or oozing; depending on its timing, it was classified as immediate-onset bleeding (evident during the examination) or late-onset bleeding (evident after the examination).
Immediate postpolypectomy bleeding was graded from G1 to G4 in severity based on objective endoscopic findings based on the time and continuity of bleeding as previously described (G1: Spontaneous hemostasis within 60 s, G2: Continuous but decreased oozing over 60 seconds, G3: Continuous oozing over 60 s that needs endoscopic treatment and G4: Active spurting)[23].
After the procedure, bleeding was defined as a drop in Hb > 2 gr/dL or clinical evidence of bleeding (melena or hematemesis).
Prophylaxis of hemorrhage was allowed in the following situations:
Pedunculated polyps (Paris type 0-Ip): (1) Stalk ≥ 5 mm and/or head ≥ 20 mm: adrenaline injection or endoloop before or immediately after polypectomy; and (2) Visible vessel after polypectomy: adrenaline injection, endoloop or hemostatic clip.
Sessile polyps (Paris type 0-Is): oozing bleeding with spontaneous hemostasis in less than 30 s and polyp size > 20 mm: adrenaline injection, argon plasma coagulation (APC) or hemostatic clip[23]. The technique was selected based on physician’s preference.
Sample size calculation was performed assuming 10% of AE from the previous data published[24,25]. With these numbers, we calculated that a total of 300 patients were required to achieve statistical significance (α error = 0.05, β error = 0.1).
Continuous variables were expressed as mean ± SD. In cases with a multiple polypectomy, data provided correspond to the biggest one. Analysis was performed per patient and not per polyp. 95% confidence interval (CI) of AEs incidence was calculated by using standard formula. Comparisons were done using Fisher’s test for categorical variables and t test for continuous variables. The chi-squared test and the Mann-Whitney U test, or Student’s t-test were applied where appropriate for statistical analysis. In addition, a multivariate logistic regression analysis was carried out to assess the existence of predictive factors of AEs and the odds ratio (OR) was calculated to indicate the associated risk. P < 0.05 was considered statistically significant. All analyses were performed with SPSS for Windows, version 23.0 (SPSS Inc, Chicago, IL; United States).
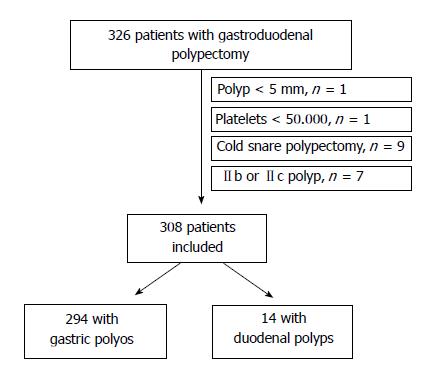
From September 2012 to March 2015, a total of 326 patients with gastroduodenal polyps agreed to participate in the study. 18 patients were excluded because they did not meet inclusion criteria (polyp < 5 mm, n = 1; platelets < 50.000, n = 1; cold snare polypectomy, n = 9; Paris classification IIb or IIc polyp, n = 7). Then, 308 patients were finally included (Figure 1). Most of them were ASA I-II (n = 231, 75%) and only 36 (11.7%) were on anticoagulants. The most frequent indication was iron-deficiency anemia (n = 103, 33.4%). Characteristics of the patients are described in Table 1.
| Characteristics | Value |
| Age (yr), mean ± SD (range) | 69.1 ± 11.8 (22-92) |
| Gender: M/F | 111/197 (36/64) |
| Smoker | 21 (6.8) |
| Alcohol | 35 (11.4) |
| Cirrhosis | 20 (6.5) |
| Anticoagulation | 36 (11.7) |
| Indication | |
| Iron-deficiency anemia | 103 (33.4) |
| Polyp follow-up | 68 (22.1) |
| Dyspepsia/ GERD | 51 (16.6) |
| Upper hemorrhage | 33 (10.7) |
| Pernicious anemia | 7 (2.3) |
| Dysphagia | 6 (1.9) |
| FAP | 4 (1.3) |
| Others | 36 (11.7) |
| ASA | |
| I | 34 (10.4) |
| II | 202 (62) |
| III | 88 (27) |
| IV | 2 (0.6) |
In 205 cases a single polypectomy was performed whereas in the other 103 it was multiple (mean 1.7 ± 1.3, range 1-7). Polyp mean size was 15 ± 8.9 mm (5-60) and 179 of them (58.1%) were > 10 mm. The majority of them were located in the stomach (n = 294, 95.4%). The most frequent histological type was hyperplastic (n = 224, 72.7%). Characteristics of the resected polyps are described in Table 2.
| Characteristics | Value |
| Paris classification of polyps | |
| 0- Is | 152 (49.4) |
| 0-Ip | 156 (50.6) |
| Size (mm), mean ± SD (range) | 15 ± 8.9 mm (5-60) |
| Size | |
| 5 mm | 17 (5.5) |
| 6-10 mm | 109 (35.4) |
| 11-20 mm | 132 (42.9) |
| > 20 mm | 47 (15.3) |
| Location | |
| Fundus | 50 (16.2) |
| Body | 112 (36.4) |
| Incisura | 5 (1.6) |
| Antrum | 119 (38.6) |
| Pylorus | 8 (2.6) |
| Duodenum | 14 (4.5) |
| Physician expertise | |
| Staff | 268 (87) |
| Fellow | 40 (13) |
| Polyp histology | |
| Hyperplastic | 224 (72.7) |
| Adenoma | 29 (9.4) |
| Fundic glands hyperplasia | 25 (8.1) |
| Adenocarcinoma | 8 (2.6) |
| Inflammatory fibroid | 7 (2.3) |
| Neuroendocrine tumor | 5 (1.6) |
| Others1 | 7 (2.3) |
| No retrieved | 2 (0.6) |
Table 3 shows the technical details of the endoscopy and polypectomy. Polypectomies were performed by a staff endoscopist in 268 cases (87%) and at university hospitals in 251 cases (81.5%). Hemorrhage prophylaxis was performed in 219 (71.1%) patients; the most common technique was injection of adrenaline alone or in combination with clips, endoloops and APC.
| Characteristics | n (%) |
| Sedation | 302 (98.1) |
| University Hospital | 251 (81.5) |
| Endoscopist Staff | 268 (87) |
| Number of polyps resected | |
| 1 | 205 (66.6) |
| 2 | 55 (17.9) |
| 3 | 20 (6.5) |
| 4 | 8 (2.6) |
| 5 | 7 (2.3) |
| > 5 | 13 (4.2) |
| Cautery settings | |
| Endocut | 236 (76.6) |
| Hemorrhage prophylaxis | 219 (71.1) |
| One technique | 149 (68) |
| Two or more | 70 (32) |
| Prophylactic technique | |
| Injection alone | 119 (54.3) |
| Clips | 16 (7.3) |
| Clips + injection | 60 (27.4) |
| Endoloop | 9 (4.1) |
| Endoloop + injection | 5 (2.3) |
| APC | 5 (2.3) |
| APC + injection | 2 (0.9) |
| APC+ clips + injection | 3 (1.4) |
All the patients were successfully contacted. A total of 41 patients (13.3%) presented 45 unexpected events: 30 bleeding, 10 abdominal pain, 2 respiratory desaturation, 1 spontaneous bacterial peritonitis, 1 esophageal laceration and 1 pneumothorax. However, following the ASGE lexicon, only 9 patients presented 9 (2.9%; 95%CI: 1-4.8) events that were considered AEs, and 6 of them were bleeding (5 in stomach and 1 in duodenum; 1.9%; 95%CI: 0.4-3.5). Severity and timing of these AEs are described in Table 4.
| Unexpected events | Time of presentation | Severity (intraprocedural hemorrhage) | Admission or prolongation of hospitalization | Repeat endoscopy | AEs | ||
| ASGE lexicon | |||||||
| Type | n = 45 | n = 9 | Severity | ||||
| Hemorrhage | 30 | Intraprocedural, n = 26 | Grade 1, n =11 | Yes, n = 2 | No | 2 | Mild |
| Grade 3, n =14 | |||||||
| Grade 4, n =1 | |||||||
| 3 d, n = 1 | No | Yes | 1 | Moderate | |||
| 7 d, n = 2 | Yes | Yes | 2 | Moderate (1) | |||
| Severe (1) | |||||||
| 30 d, n = 1 | Yes | Yes | 1 | Moderate | |||
| Abdominal pain | 10 | 24 h | No | No | |||
| Respiratory desaturation | 2 | Intraprocedural | No | No | |||
| Pneumothorax | 1 | Intraprocedural | Yes | No | 1 | Moderate | |
| SBP | 1 | 7 d | Yes | No | 1 | Severe | |
| Esophageal laceration | 1 | Intraprocedural | Yes | No | 1 | Mild | |
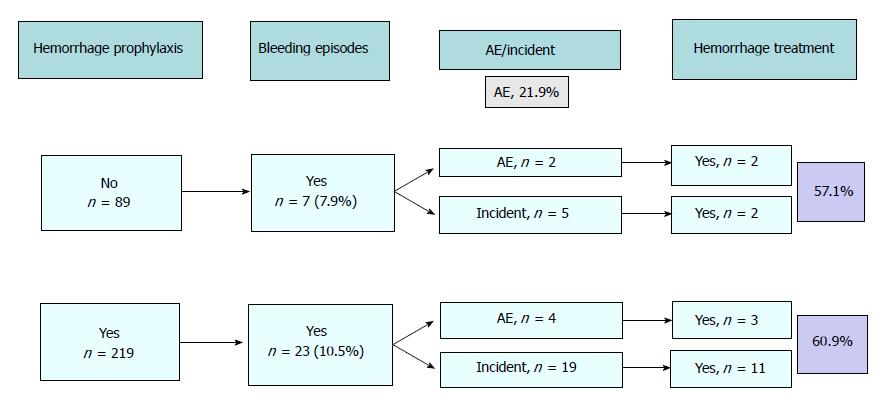
Bleeding was the most common unplanned event that occurred during the procedure (n = 30, 9.7%; 95%CI: 6.4-13.1). The majority of episodes could be managed without any change in the outcome of the endoscopy and, consequently, were considered incidents (24 out of 30, 80%). In 13 out of 24 incidents (54.1%) and in 5 out of 6 AE (83.3%), different types of endoscopic treatment were performed: injection alone in 3, clips alone in 3, injection plus clips in 10 and combination of injection, clips and APC in 2. In all the cases, bleeding was adequately controlled. Figure 2 shows the relationship between the use of prophylactic measures, the presence and severity of bleeding and the use of endoscopic treatment.
There were no statistically significant differences in terms of age, gender, polyp histology and location in stomach or duodenum, technical details of polypectomy, hospital characteristics and use of prophylactic measures between patients who developed hemorrhagic episodes and those who did not. Only polyp size and endoscopist expertise were statistically significant in the univariate but not in the multivariate analysis (Table 5).
| Bleeding, n = 30 | No bleeding, n = 278 | P value | |
| Age (yr), mean ± SD | 69.5 ± 10 | 69.1 ± 12 | 0.137 |
| Gender | 0.634 | ||
| Male | 12 (40) | 99 (35.6) | |
| Female | 18 (60) | 179 (64.4) | |
| Anticoagulation1 | 0.217 | ||
| Yes | 14 (46.7) | 98 (35.2) | |
| No | 16 (53.3) | 180 (64.7) | |
| ASA | 0.515 | ||
| I, II | 20 (66.7) | 201 (72.3) | |
| III, IV | 10 (33.3) | 77 (27.7) | |
| Paris classification of polyps1 | |||
| 0-Ip | 17 (56.7) | 135 (48.6) | 0.399 |
| 0-Is | 13 (43.3) | 143 (51.4) | |
| Polyp size1 | < 0.036 | ||
| ≤ 10 mm | 7 (23) | 120 (43.2) | |
| > 10 mm | 23 (77) | 158 (56.8) | |
| Location | 0.557 | ||
| Stomach | 28 (93.3) | 266 (95.7) | |
| Duodenum | 2 (6.7) | 12 (4.3) | |
| Polyp histology1 | 0.092 | ||
| Hyperplastic | 18 (60) | 206 (74.4) | |
| Others | 12 (40) | 72 (25.6) | |
| Polyp with dysplasia | 0.053 | ||
| Yes | 8 (26.7) | 36 (13.4) | |
| No | 22 (73.3) | 232 (86.6) | |
| Physician expertise1 | < 0.026 | ||
| Staff | 30 (100) | 238 (85.6) | |
| Fellow | 0 (0) | 40 (14.4) | |
| University hospital | 0.207 | ||
| Yes | 27 (90) | 224 (80.6) | |
| No | 3 (10) | 54 (19.4) | |
| Number of polyps resected | 0.989 | ||
| One | 20 (66.7) | 185 (66.5) | |
| More than one | 10 (33.3) | 93 (33.4) | |
| Use of endocut1 | 0.068 | ||
| Yes | 27 (90) | 209 (75.2) | |
| No | 3 (10) | 69 (24.8) | |
| Hemorrhage prophylaxis1 | 0.479 | ||
| Yes | 23 (76.7) | 196 (70.5) | |
| No | 7 (23.3) | 82 (29.5) |
Bleeding is the most common adverse event of snare polypectomy in the upper gastrointestinal tract. In our study we found a 2.6% AEs rate (1.9% considering only bleeding) after resection of gastric and duodenal polyps which is lower than data reported in other series. To the best of our knowledge, this study is the first multicenter and prospective evaluation of AEs after gastroduodenal snare polypectomy using the lexicon recommended by the ASGE. Our results confirm the safety of gastric polypectomy when applying preventive measures and emphasize the need of using standardized systems to report AEs.
For years, polypectomy in the upper gastrointestinal tract was considered less secure than that of colonic polyps. Two prospective studies with a lower number of patients evaluated the safety of gastric polypectomy. Muehldorfer et al[14] studied the use of biopsy for the histological diagnosis of gastric polyps and the assessment of AEs was a secondary aim and the reported an incidence of hemorrhagic events was 7.2%. However, the definition of bleeding was broad including all the cases in which a therapeutic intervention was required regardless of the need of hospitalization or transfusion. Following the ASGE lexicon definition of AEs, the rate of AEs in this study would have been of three (1.3%): two bleeding episodes that required a blood transfusion and one perforation. The other prospective study is a Taiwanese comparative study that assessed the efficacy of submucosal epinephrine injection before polypectomy of 151 sessile polyps (87 colonic and 64 upper GI) in the prevention of bleeding and perforation[18]. This study showed a total of nine (5.96%) episodes of post-polypectomy hemorrhage, eight of them were immediate, and two perforations, with a total of 7.3% complications. However, most of the hemorrhagic episodes occurred in foregut polyps (10.9% vs 2.3%), were immediate and were controlled with additional endoscopic treatment. Only two patients required blood transfusion, cutting down the number of hemorrhagic AEs in foregut polypectomies to 3.1%.
Bardan et al[26] performed a retrospective study (102 patients with gastric polyps) in which the primary outcome was the occurrence of immediate or delayed bleeding episodes. Although they reported seven episodes of bleeding (6.9%), six were detected immediately after polypectomy and were adequately treated by injection. Only one episode was considered severe because it required a blood transfusion 6 days after the polypectomy and fulfilled the definition of AEs by the ASGE lexicon, decreasing the rate of hemorrhagic AEs to 0.98%. The retrospective design of this study limits the conclusions and it could be argued that complication rate might be higher.
Kratzsch et al[27] in the largest retrospective analysis (1416 foregut polyps) also found a low complication rate (3.1%) that is close to our findings. However, there is a lack of relevant information concerning the definition of AEs and use of prophylactic measures, and the retrospective design of this study limits the conclusions since it may underestimate complications.
Information regarding the risks of duodenal resection is even scarcer. To date, the results of the two largest retrospective series treated with snare polypectomy showed a rate of hemorrhagic AEs of 7.8%-11%[28,29] which is much higher than ours. Although duodenal polypectomy is usually technically more challenging than gastric polypectomy, location in the duodenum is not significantly associated with more hemorrhagic episodes, as showed in our study. We did not include flat polyps because these should be removed with mucosectomy which is technically challenging, more difficult than standard polypectomy, and associated with more AE’s.
Polyp size has proved to be the main risk factor for significant unexpected events in colonic polypectomies. In fact, size is one factor that determines the complexity of polypectomy and as the complexity of polypectomy increases, a higher risk of complications is reported[30,31]. The overall perforation and bleeding rates in these series were very low (0.05% and 0.65%, respectively). However, when the analysis was limited to bleeding requiring transfusion, unplanned hospital admission, interventional radiology or endoscopy, or surgery, the rate dropped to 0.13%. Again, these results emphasize the importance of using standardized systems for reporting AEs. Because hemorrhage prophylaxis was allowed in polyps larger than 20 mm which have a high likelihood of bleeding, this fact could explain that size was not associated with a higher rate of hemorrhagic AEs in our series.
Although the rate of AEs in our study is low, the number of bleeding episodes is not negligible and many of them received prophylaxis (10.5%) or were treated endoscopically (60%) with injection, APC, hemostatic clips or a combination of methods which increases health care costs. Interestingly, the combination of two or more techniques did not improve the prophylactic effect of using one technique alone against bleeding. However, one could expect a higher number and more severe bleeding episodes if we had not systematically applied prophylactic measures, with an estimated high economical impact as well.
This study has several strengths. First, it is a multicenter study performed in many hospitals (tertiary and community) with a different volume of explorations that increase the generalizability of the results. Second, preventive measures for post polypectomy bleeding were applied systematically. Third, all patients were systematically evaluated and reached three times (at 48 h, 7 and 30 d after the procedure), avoiding drop-outs that could bias the results. Fourth, we only included protruded polyps in order to avoid the use of other endoscopic resection techniques such as mucosectomy or endoscopic submucosal dissection which are more technically demanding and have a higher risk of complications. And finally, we used a standardized lexicon for endoscopic AEs.
One limitation of the study is that multiple polyps in the same patient were not considered separately and it is not possible to attribute the bleeding episode to the one that received prophylaxis or not. However, prophylactic measures were applied to the polyps with more risk of bleeding and the number of polyps was considered a variable in the analysis. The second limitation is that the number of AEs found is lower than the estimated figure used for the sample size calculation, which underpowers the results of the study. Unfortunately, this low rate prevented us from studying risk factors for polypectomy-related AEs. Finally, we used definitions of hemorrhage and criteria for prophylaxis that apply to colonic polyps because we did not find any specific definition for gastric polyps. However, we assume that the mechanism of post-polypectomy hemorrhage must be similar regardless the localization of the polyp.
In conclusion, gastroduodenal polypectomy using prophylactic measures has a rate of AEs small enough to consider this procedure a safe and effective method for polyp resection independently of the polyp size and location.
Gastric and duodenal polypectomy is commonly performed. Although there is a theoretical increased risk of bleeding, there is scarce information regarding the potential adverse events (AEs) of polypectomy in this setting. The aim of this study was to evaluate the rate of AEs during consecutive gastric and duodenal polypectomies in several Spanish centers.
The safety of polypectomy in the upper GI tract is controversial because the reported rate in retrospective studies is higher than in colonic polypectomy but results come mainly from retrospective studies and they do not use the same standardized nomenclature and definitions for adverse events.
The aims of this study were to determine in a prospective study the rate of adverse events of gastroduodenal snare polypectomy for non-flat polyps; to evaluate the adverse events (early and late) that occur after a gastric and/or duodenal polypectomy as well as the predictive fractures for its development; to evaluate the different endoscopic techniques used in the prophylaxis of post-polypectomy hemorrhage.
The research methods: (1) Multicenter, longitudinal and prospective study of all patients undergoing polypectomy of gastric or duodenal polyps ≥ 5 mm using an electrocautery polypectomy snare; (2) Patients with PT < 50% and platelets < 50000 or clopidogrel in the 7 d prior to endoscopy were excluded; (3) Prophylactic measures of hemorrhage were allowed in certain predefined cases; (4) Intraprocedural hemorrhage was defined as bleeding that lasts more than 30 seconds and severity was graded from 1 to 4; (5) Late hemorrhage was defined as melena or hematochezia since discharge from endoscopy unit and up to 30 d. (6) Patients were followed during 30 d with serial phone calls; and (7) Predictive factors of complications were analyzed
308 patients were included and a single polypectomy was performed in 205. Hemorrhage prophylaxis was performed in 219 (71.1%) patients. Nine patients presented AEs (2.9%), and 6 of them were bleeding (n = 6, 1.9%) (In 5 out of 6 AEs, different types of endoscopic treatment were performed). Other 24 hemorrhagic episodes could be managed without any change in the outcome of the endoscopy and, consequently, were considered incidents. We did not find any independent risk factor of bleeding.
The rate of adverse events of gastroduodenal snare polypectomy for non-flat polyp is low. However, the number of bleeding episodes is not negligible and many of them receive prophylaxis or are treated endoscopically with injection, APC, hemostatic clips or a combination of methods which increases health care costs. Prophylactic measures do not reduce the risk of hemorrhage. To our knowledge, this is the first study using the ASGE lexicon for reporting adverse events of gastro-duodenal polypectomy and shows an acceptable low rate, confirming the safety of this procedure. Because AEs of gastroduodenal polypectomies are low, there is no need of using more than one prophylactic endoscopic technique (clips, sclerosis, APC…) with the consequent reduction of costs.
Gastroduodenal polypectomy using prophylactic measures has a rate of AEs small enough to consider this procedure a safe and effective method for polyp resection independently of the polyp size and location. The future research direction is to compare the use of prophylaxis or not before polypectomy in gastric polyps and the best method would be a prospective, comparative and randomized study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Emara MH, Qayed E, Rodrigo L, Slomiany BL S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Turner JR, Odze RD. Polyps of the stomach. In Odze RD, Goldblum JR, Crawford JM (eds). Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas , 1st edn Saunders, Elsevier: Philadelphia 2004; 267-294. [Cited in This Article: ] |
| 2. | Carmack SW, Genta RM, Schuler CM, Saboorian MH. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol. 2009;104:1524-1532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Jalving M, Koornstra JJ, Wesseling J, Boezen HM, DE Jong S, Kleibeuker JH. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther. 2006;24:1341-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Macenlle García R, Bassante Flores LA, Fernández Seara J. [Gastric epithelial polyps. A retrospective study 1995-2000]. Rev Clin Esp. 2003;203:368-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | García-Alonso FJ, Martín-Mateos RM, González Martín JA, Foruny JR, Vázquez-Sequeiros E, Boixeda de Miquel D. Gastric polyps: analysis of endoscopic and histological features in our center. Rev Esp Enferm Dig. 2011;103:416-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Culver EL, McIntyre AS. Sporadic duodenal polyps: classification, investigation, and management. Endoscopy. 2011;43:144-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Jepsen JM, Persson M, Jakobsen NO, Christiansen T, Skoubo-Kristensen E, Funch-Jensen P, Kruse A, Thommesen P. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 137] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Bleau BL, Gostout CJ. Endoscopic treatment of ampullary adenomas in familial adenomatous polyposis. J Clin Gastroenterol. 1996;22:237-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Schmitz JM, Stolte M. Gastric polyps as precancerous lesions. Gastrointest Endosc Clin N Am. 1997;7:29-46. [PubMed] [Cited in This Article: ] |
| 10. | Choudhry U, Boyce HW Jr, Coppola D. Proton pump inhibitor-associated gastric polyps: a retrospective analysis of their frequency, and endoscopic, histologic, and ultrastructural characteristics. Am J Clin Pathol. 1998;110:615-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 140] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Cristallini EG, Ascani S, Bolis GB. Association between histologic type of polyp and carcinoma in the stomach. Gastrointest Endosc. 1992;38:481-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Ginsberg GG, Al-Kawas FH, Fleischer DE, Reilly HF, Benjamin SB. Gastric polyps: relationship of size and histology to cancer risk. Am J Gastroenterol. 1996;91:714-717. [PubMed] [Cited in This Article: ] |
| 13. | Fujiwara Y, Arakawa T, Fukuda T, Kimura S, Uchida T, Obata A, Higuchi K, Wakasa K, Sakurai M, Kobayashi K. Diagnosis of borderline adenomas of the stomach by endoscopic mucosal resection. Endoscopy. 1996;28:425-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Muehldorfer SM, Stolte M, Martus P, Hahn EG, Ell C; Multicenter Study Group “Gastric Polyps”. Diagnostic accuracy of forceps biopsy versus polypectomy for gastric polyps: a prospective multicentre study. Gut. 2002;50:465-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 369] [Cited by in F6Publishing: 389] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 16. | Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol. 2009;6:331-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Lanza FL, Graham DY, Nelson RS, Godines R, McKechnie JC. Endoscopic upper gastrointestinal polypectomy. Report of 73 polypectomies in 63 patients. Am J Gastroenterol. 1981;75:345-348. [PubMed] [Cited in This Article: ] |
| 18. | Hsieh YH, Lin HJ, Tseng GY, Perng CL, Li AF, Chang FY, Lee SD. Is submucosal epinephrine injection necessary before polypectomy? A prospective, comparative study. Hepatogastroenterology. 2001;48:1379-1382. [PubMed] [Cited in This Article: ] |
| 19. | Van Gossum A, Cozzoli A, Adler M, Taton G, Cremer M. Colonoscopic snare polypectomy: analysis of 1485 resections comparing two types of current. Gastrointest Endosc. 1992;38:472-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 134] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Mühldorfer SM, Kekos G, Hahn EG, Ell C. Complications of therapeutic gastrointestinal endoscopy. Endoscopy. 1992;24:276-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1238] [Cited by in F6Publishing: 1532] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 22. | Fyock CJ, Draganov PV. Colonoscopic polypectomy and associated techniques. World J Gastroenterol. 2010;16:3630-3637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 50] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Kim HS, Kim TI, Kim WH, Kim YH, Kim HJ, Yang SK, Myung SJ, Byeon JS, Lee MS, Chung IK. Risk factors for immediate postpolypectomy bleeding of the colon: a multicenter study. Am J Gastroenterol. 2006;101:1333-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, Schulman J. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 427] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 25. | Wolfsen HC, Hemminger LL, Achem SR, Loeb DS, Stark ME, Bouras EP, DeVault KR. Complications of endoscopy of the upper gastrointestinal tract: a single-center experience. Mayo Clin Proc. 2004;79:1264-1267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Bardan E, Maor Y, Carter D, Lang A, Bar-Meir S, Avidan B. Endoscopic ultrasound (EUS) before gastric polyp resection: is it mandatory? J Clin Gastroenterol. 2007;41:371-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Kratzsch KH, Bosseckert H. [Results of endoscopic polypectomy in the upper gastrointestinal tract--a multicenter study]. Dtsch Z Verdau Stoffwechselkr. 1984;44:61-66. [PubMed] [Cited in This Article: ] |
| 28. | Apel D, Jakobs R, Spiethoff A, Riemann JF. Follow-up after endoscopic snare resection of duodenal adenomas. Endoscopy. 2005;37:444-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Eswaran SL, Sanders M, Bernadino KP, Ansari A, Lawrence C, Stefan A, Mattia A, Howell DA. Success and complications of endoscopic removal of giant duodenal and ampullary polyps: a comparative series. Gastrointest Endosc. 2006;64:925-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Chukmaitov A, Bradley CJ, Dahman B, Siangphoe U, Warren JL, Klabunde CN. Association of polypectomy techniques, endoscopist volume, and facility type with colonoscopy complications. Gastrointest Endosc. 2013;77:436-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Rutter MD, Nickerson C, Rees CJ, Patnick J, Blanks RG. Risk factors for adverse events related to polypectomy in the English Bowel Cancer Screening Programme. Endoscopy. 2014;46:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |