Published online Dec 21, 2017. doi: 10.3748/wjg.v23.i47.8263
Peer-review started: October 25, 2017
First decision: November 8, 2017
Revised: November 8, 2017
Accepted: December 4, 2017
Article in press: December 4, 2017
Published online: December 21, 2017
Nonalcoholic fatty liver disease (NAFLD) is defined as the presence of hepatic fat accumulation after the exclusion of other causes of hepatic steatosis, including other causes of liver disease, excessive alcohol consumption, and other conditions that may lead to hepatic steatosis. NAFLD encompasses a broad clinical spectrum ranging from nonalcoholic fatty liver to nonalcoholic steatohepatitis (NASH), advanced fibrosis, cirrhosis, and finally hepatocellular carcinoma (HCC). NAFLD is the most common liver disease in the world and NASH may soon become the most common indication for liver transplantation. Ongoing persistence of obesity with increasing rate of diabetes will increase the prevalence of NAFLD, and as this population ages, many will develop cirrhosis and end-stage liver disease. There has been a general increase in the prevalence of NAFLD, with Asia leading the rise, yet the United States is following closely behind with a rising prevalence from 15% in 2005 to 25% within 5 years. NAFLD is commonly associated with metabolic comorbidities, including obesity, type II diabetes, dyslipidemia, and metabolic syndrome. Our understanding of the pathophysiology of NAFLD is constantly evolving. Based on NAFLD subtypes, it has the potential to progress into advanced fibrosis, end-stage liver disease and HCC. The increasing prevalence of NAFLD with advanced fibrosis, is concerning because patients appear to experience higher liver-related and non-liver-related mortality than the general population. The increased morbidity and mortality, healthcare costs and declining health related quality of life associated with NAFLD makes it a formidable disease, and one that requires more in-depth analysis.
Core tip: Nonalcoholic fatty liver disease (NAFLD) is a term for a host of histological findings stemming from hepatic steatosis and remains the most common liver disease globally with increasing prevalence. The vast variation in disease presentation complicates diagnosis, leading to an underestimate of actual disease occurrence. NAFLD is associated with many metabolic comorbidities, including obesity, type II diabetes, dyslipidemia, and metabolic syndrome. Its potential to develop into more severe liver conditions, such as nonalcoholic steatohepatitis, advanced fibrosis, cirrhosis and hepatocellular carcinoma, can lead to a state in which liver transplantation is the only treatment option available. The population at risk of developing progressive liver disease creates a challenge to the healthcare system in terms of screening for this evolving epidemic of liver disease.
- Citation: Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol 2017; 23(47): 8263-8276
- URL: https://www.wjgnet.com/1007-9327/full/v23/i47/8263.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i47.8263
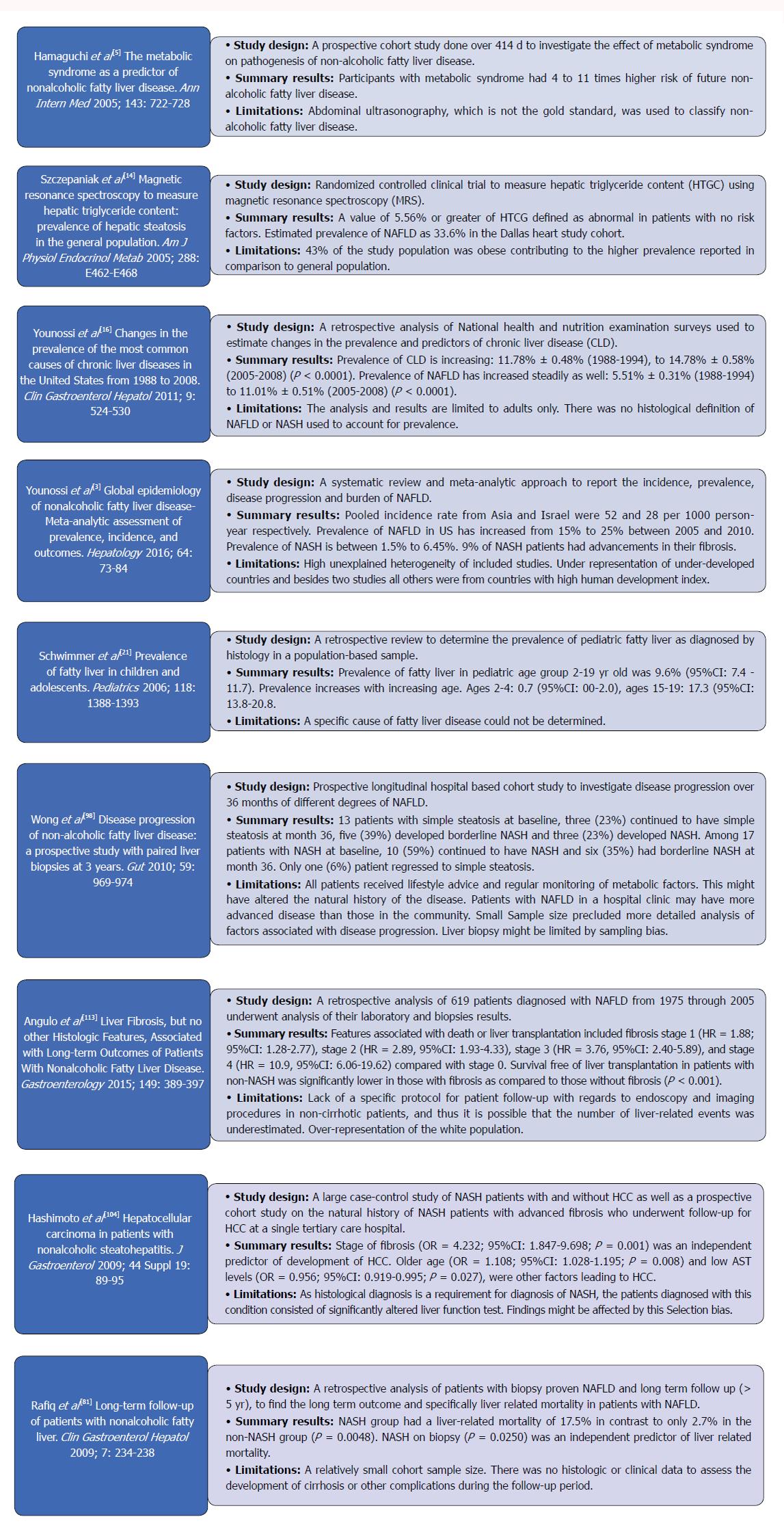
Nonalcoholic fatty liver disease (NAFLD) has become a common cause of chronic liver disease in the world[1] since its first description in 1980 as the “unnamed disease”[2]. It has been studied in-depth subsequently with continuous myriad of further investigations being carried into this soon to be common indication for liver transplantation (LT). Figure 1 summarizes some of the most landmark studies in the current literature on NAFLD.
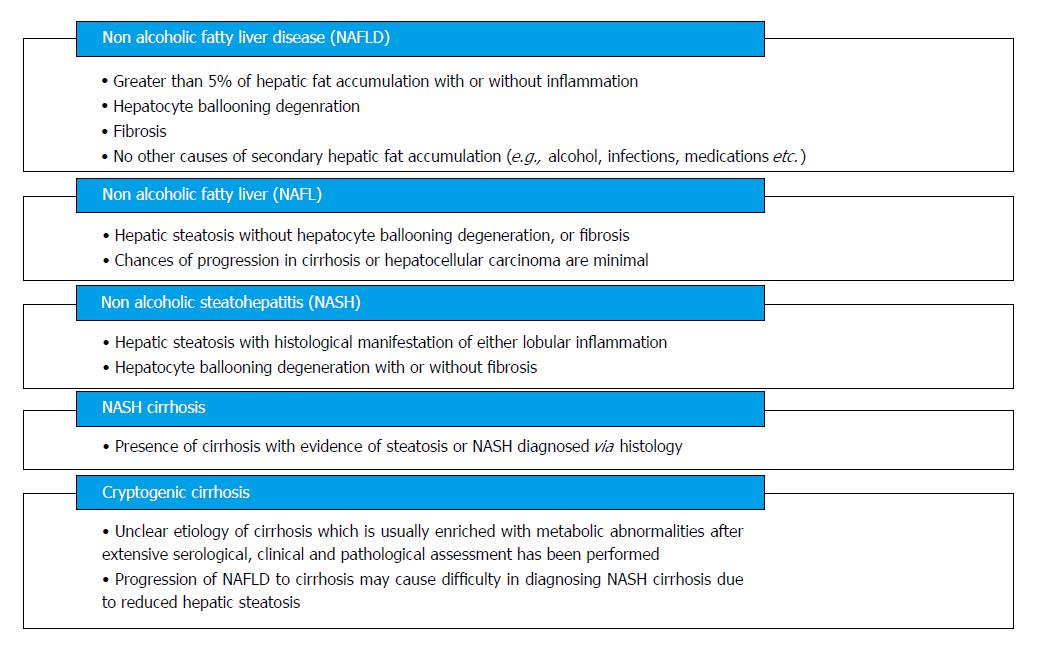
NAFLD encompasses a wide histological variety: Nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), fibrosis, NASH cirrhosis, and NASH-related hepatocellular carcinoma (HCC) (Figure 2). NAFLD is characterized by ≥ 5% of hepatic fat accumulation in the absence of any secondary causes and is a diagnosis of exclusion. Therefore, other etiologies leading to similar hepatic histology must be ruled out including excessive alcohol consumption; viral hepatitis; other chronic liver disease such as, Wilson’s disease, hemochromatosis, viral hepatitis, autoimmune hepatitis, cholestatic liver disease and other chronic liver diseases; starvation; lipodystrophy; celiac disease; Cushing’s disease; and medications (corticosteroids, methotrexate, diltiazem, oxaliplatin, amiodarone, isoniazid, highly active anti-retroviral therapy, etc.). Current guidelines recommend utilizing criteria requiring an alcohol exposure of less than 30 g/d for men and less than 20 g/d for women as a component of NAFLD diagnosis [1].
NAFLD has diverse manifestations described in all ethnicities all over the world and present in both sexes[3]. The variable presentations probably contribute to the underreported new and existing cases of NAFLD as well as the limited studies undertaken to elucidate the exact incidence and prevalence of NAFLD.
It is currently estimated that the global prevalence of NAFLD is as high as one billion[4]. In the United States, NAFLD is estimated to be the most common cause of chronic liver disease, affecting between 80 and 100 million individuals, among whom nearly 25% progress to NASH.
A study from Japan which followed 3147 patients over 414 d found a 10% annual incidence rate[5]. Another Japanese study evaluated elevated aminotransferase levels, weight gain and insulin resistance development over 5 years to classify patients with NAFLD and their incidence was reported as 31 per 1000 person-years[6]. A retrospective study done in England later demonstrated a much lower incidence of 29 per 100000 person-years[7]. A recent extensive meta-analysis described a pooled regional incidence of NAFLD in Asia and Israel to be 52 [95% confidence interval (CI): 28-97] per 1000 person-years and 28 (95%CI: 19-41) per 1000 person-years, respectively[3]. Current data on incidence for NAFLD are limited in some regions of the world due to the limited number of studies. Further studies seem warranted to determine the true incidence in general population.
In general, the prevalence of NAFLD has increased over the last 20 years. In addition to the gold standard diagnostic test of liver biopsy, there are some noninvasive modalities available to diagnose NAFLD. Hepatic ultrasonography, computed tomography (CT), and MRI are accepted modalities for detecting hepatic fatty infiltration. The difference in sensitivity of diagnostic modalities may account for the discrepancy in prevalence data for NAFLD. Using aminotransferase levels as a screening laboratory test for liver disease, prevalence of elevated aminotransferases was 7.9% in the United States general population (1988-1992) with unexplained liver disease in 69% of these subjects[8,9]. In a recent meta-analysis, hepatic ultrasonography allowed for the reliable and accurate detection of moderate-to-severe fatty liver and is now considered the screening modality of choice[10]. Prevalence of ultrasonographic diagnosis of NAFLD ranged between 17% in India to 46% in the United States[8,11,12]. MRS remains one of most sensitive and accurate noninvasive tests available with a NAFLD prevalence of 33% reported in the Dallas Heart Study[13,14]. The Middle East and South America have the highest NAFLD prevalence at 31% and 32% respectively with the lowest prevalence in Africa at 13.5%[3]. Recently, Asia has been facing the highest obesity epidemic and thus not surprisingly has been experiencing a rapid rate of increase in the prevalence of NAFLD. Chinese adolescents on a “westernized” diet have a greater than 25% prevalence of NAFLD. Studies from Korea, China, Japan and Taiwan have all reported a prevalence ranging from 11%-45%[15]. Along with the global drift, United States has not been immune to the uptrend in NAFLD. A recent United States-based study using the National Health and Nutrition Examination Surveys (NHANES) conducted between 1988 and 2008 found that the prevalence of NAFLD using elevated alanine aminotransferese (ALT) doubled in the United States during this time period (5.5% to 11.0%)[16]. Based on the NHANES-III data collected between 1988 and 1994, the prevalence of ultrasonography-diagnosed NAFLD was 34%[17]. Meta-regression of studies done globally also displayed an increased prevalence of NAFLD from 15% in 2005 to 25% in 2010[3]. The discrepancy in the prevalence of NAFLD among studies is most likely due to differences in sample selection, diagnostic modalities, dietary and lifestyle habits.
The current annual medical and societal costs of NAFLD are estimated at $292 billion in the United States[18]. The projected cost of caring for patients is expected to increase by 18% from 2000 to 2035 and health-related quality of life of NAFLD patients is described as declining[19,20].
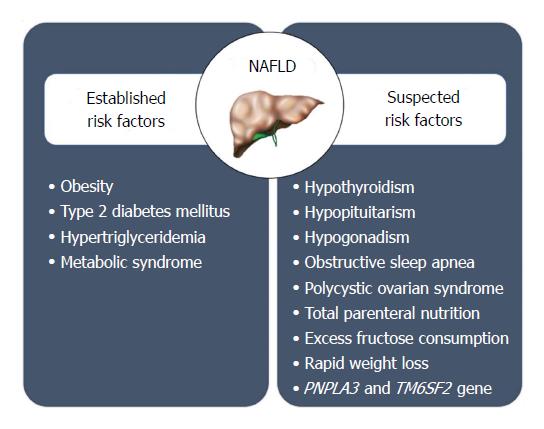
Based on our current knowledge, it appears that a combination of genetic, demographic, clinical and environmental factors may play a role in determining the likelihood of NAFLD in a given individual (Figure 3). Therefore, the pathogenesis of NAFLD is a multifactorial and multi-step process.
Although obesity, lifestyle variation, and insulin resistance are the most prevalent risk factors leading to the development of NAFLD in a person, NAFLD varies substantially among subjects with comparable lifestyle, environmental impact, and metabolic abnormalities, indicating that other factors contribute to pathogenesis. The heritability[21] and interethnic variations in susceptibility[13] suggest that genetic factors may play an important role in determining the phenotypic manifestation and overall risk for NAFLD. NAFLD clusters in families with certain genetic variants on or near TM6SF2, PNPLA3, NCAN, and PPP1R3B genes that increase the heritability of NAFLD by up to 27% within families[22,23]. One genetic variant that is associated with NAFLD is a missense mutation [Ile148 - > Met148 (I148M)] in the palatin-like phospholipase domain-containing 3 gene (PNPLA3)[24] A recent meta-analysis showed that PNPLA3 exerts a strong influence not only on hepatic fat accumulation (GG homozygous individuals showed a 73% higher hepatic fat content compared with CC homozygous individuals, P < 1 x 10-9) but also on the susceptibility to develop more severe histologic liver damage (GG homozygous individuals had a 3.24-fold greater risk of higher necro-inflammatory scores and a 3.2-fold greater risk of developing fibrosis compared with CC homozygous individuals, P < 1 x 10-9, respectively)[22]. These associations were maintained irrespective of the degree of obesity or the presence of diabetes[23,25,26]. A single variant in PNPLA3 gene (I148M ) has been observed in highest frequency in Hispanics, followed by non-Hispanic whites and least in African Americans[24]. A minor allele in transmembrane 5 superfamily member 2 (TM6SF2) was associated with MRS-measured hepatic triglyceride content from the Dallas Heart Study[27]. In addition, a minor allele of TM6SF2 was noted to increase the risk for hepatic fibrosis independent of age, obesity, diabetes, and PNPLA3 genotype[28].
Generally, gender differences exist in NAFLD. Prevalence of NAFLD and NASH was higher in men[12]. Women are at a reduced risk of NAFLD compared with men at their reproductive period, whereas after menopause women lose the protective effect and have a comparable prevalence of NAFLD as men[29]. These associations were consistent with children[30]. Superseding gender, age trends have been associated with NAFLD. Based on the NHANES data, suspected NAFLD prevalence defined as elevated ALT rose from 3.9% in 1988-1994 to 10.7% in 2007-2010, with increases among all race/ethnic subgroups, males and females ranging 12-19 years in age[30]. These trends were also consistent among adolescent and young adults aged 15-39 years[31]. Although the majority of studies are among people aged 30 to 70 years, the general trend of increased prevalence is observed with age with peak prevalence of NAFLD noted between age 50-60 in men[32]; with 16.1% in ages 30 to 40 years old, 22.3% in 41 to 50 years old, 29.3% in 51 to 60 years old, and 27.6% in over 60 years old based on NHANES III[33]. In women, prevalence of NAFLD increased with age especially after menopause; with 12.5% in ages 30 to 40 years old, 16.1% in 41 to 50 years old, 21.6% in 51 to 60 years old, and 25.4% in over 60 years old[33]. A study with octogenarians admitted in a geriatric hospital showed a higher than usual prevalence of 46%[34].
Race/ethnicity is another variable affecting the prevalence of NAFLD, with the highest prevalence among Hispanics followed by non-Hispanic whites, and lowest prevalence in African Americans[12,13,35]. The numbers cited are at times double for Hispanics (45%-58%) in comparison to African Americans (24%-35%), with Latinos of Mexican origin having the highest prevalence in a subgroup analysis of the Latino population[13,36]. These findings hold true even in studies in the pediatric population[30]. Underlying genetic and lifestyle variations amongst these ethnicities could further account for the skewed prevalence of NAFLD.
The prevalence of NAFLD among the obese population ranges from 30% to 37%[8]. Abdominal obesity with increased waist circumference is specifically more strongly correlated with NAFLD[37]. In a recent cohort study of 2017 subjects during a median 4.4 year follow-up, the visceral adiposity was associated with incident NAFLD in a dose-dependent manner, with an adjusted hazard ratio [HR, per 1-standard deviation (SD) increase] for incident NAFLD of 1.36 (1.16-1.59)[38]. In addition, this study found significant relationships with subcutaneous adiposity for regressed NAFLD of HR = 1.36 (95%CI: 1.08-1.72) independent of visceral adiposity[38]. Furthermore, a recent study reported that visceral adiposity increased the risk for NAFLD without significant fibrosis and NAFLD with significant fibrosis after adjusting for known risk factors[39]. Multivariate analysis showed that the visceral adipose tissue area was independently associated with increased risks of NASH and significant fibrosis[39]. These studies suggest that certain types of abdominal fat are risk factors for NAFLD and more advanced NAFLD-related fibrosis, whereas other types could reduce risk for NAFLD. In recent years, several cohort studies demonstrated an association between body weight change and incident NAFLD[40-43]. Even a modest gain in body weight of 2 kg within the normal range has been shown to increase the risk of developing NAFLD[41]. Obesity has also been noted to be an additive factor causing a two-fold increase in steatosis in the setting significant alcohol use[28]. While it is common to have NAFLD in obese population, it is even more common to have obesity in patients with NAFLD. The pooled prevalence of obesity in NAFLD globally is reported to be 51%[3].
Due to the evidence supporting that obesity is associated with NAFLD, some macro- and micro-nutrients contribute more to the epidemic of NAFLD. Fructose is a major player, either from sucrose or high fructose corn syrup found in beverages. Consumption of such beverages has increased five-fold in the United States since 1950, and drinking two average size sugar containing beverage servings for 6 mo ends up mirroring many features of NAFLD[44]. It is hypothesized that sugars promote de novo lipogenesis and trigger inflammatory response leading to hepatocyte apoptosis via the c-Jun-N-Terminal pathway[45].
Pre-existing metabolic disorders, specifically type 2 diabetes mellitus (T2DM), have a close association with NAFLD, with more than three-quarters of diabetic patients reportedly having NAFLD[46]. T2DM and insulin resistance promote lipolysis of the adipose tissue leading to release of free fatty acids and their deposition in the liver leading to steatosis[45]. T2DM is a significant risk factor to cause progressive NASH, fibrosis, cirrhosis and an independent risk factor of mortality in addition to liver-related mortality[47] .
Sleep disturbances and disorders are common medical problems in the current era. Epidemiological studies[48,49] have provided evidence that poor sleep quality and sleep deprivation is associated with obesity which plays a key role in the pathogenesis of NAFLD. Recently, population cohort studies [50-52] reported that sleep deprivation may be independently associated with NAFLD with odds ratio 1.28 (1.13-1.44) in men and 1.71 (1.38-2.13) in women. Further, poor quality sleep was found to be a positive predictor of NAFLD in men and women 1.10 (1.02-1.19) and 1.36 (1.17-1.59) respectively[52]. Biologic plausibility for this independent association has been explored by evaluating the role of inflammatory cytokines interleukin 6 and TNF-α[53,54]. These cytokines are increased by sleep disturbances and play a role in pathogenesis of NAFLD by increasing adipocyte lipolysis which in turn can cause hepatic overflow of free fatty acids[55]. Further, sleep deprivation can affect hypothalamus pituitary adrenal axis, which in turn affects cortisol metabolism leading to hepatic fat accumulation[56,57].
NAFLD is diagnosed based on clinical history, laboratory and radiographic studies which are further complemented by histologic information. Abdominal imaging revealing hepatic steatosis may be sufficient for diagnosis of NAFLD and liver biopsy may not be required if clinical and laboratory data have ruled out other causes of liver disease. However, role of liver biopsy is important in differentiating NASH from simple steatosis and this may have implications in management as NASH has a higher risk of disease progression as compared to simple steatosis[58]. NASH is confirmed when all four features viz. steatosis, inflammation, cellular ballooning and fibrosis are present on histology[58,59]. Apart from imaging and liver biopsy, certain non-invasive tests can help in clinical decision making regarding the presence of advanced fibrosis in NAFLD patients. NAFLD fibrosis score (NFS) is one of the most commonly employed non-invasive tests to assess severity of hepatic fibrosis by utilizing six variables: age, BMI, hyperglycemia, platelet count, albumin and aspartate aminotransferase (AST)/ALT ratio. It is calculated using the published formula available at (Hepatology 2007; 45: 846-854 DOI: 10.1002/hep.21496). A meta-analysis of 3064 patients reported that NFS has an area under the receiver operating curve (AUROC) of 0.85 for predicting bridging fibrosis with nodularity or cirrhosis. A score < -1.45 had 90% sensitivity to exclude advanced fibrosis, whereas a score > 0.67 had a 97% specificity to identify presence of advanced fibrosis[60]. FIB-4 index is another algorithmic score utilized in studies to predict advanced fibrosis. It is based on age, platelet count, AST and ALT and is calculated using published formula (Hepatology 2006; 43: 1317-1325 DOI: 10.1002/hep.21178). Using this formula, patients with score > 3.25 are likely to have advanced fibrosis whereas, those with score < 1.45 are unlikely to have advanced fibrosis. Imajo et al[61] compared various risk scores and elastography against liver histology and showed that NFS and FIB-4 were better than other non-invasive scoring indices like AST to platelet ration index and AST/ALT ratio. Further, NFS and FIB-4 were as good as MR elastography (MRE) in predicting advanced fibrosis in patients with biopsy-proven NAFLD.
A variety of imaging tools can be utilized for the diagnosis of NAFLD. Abdominal ultrasound is limited by low sensitivity in patients with less than 30% steatosis on histology[62]. However, it is noninvasive, widely available and does not require contrast. On the other hand, CT can be associated with radiation hazard and contrast linked nephropathy. It is also limited by low sensitivity hepatic mapping and is expensive[62]. Magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) provide the highest precision (sensitivity and specificity) in quantifying steatosis and liver fat mapping[63] and may become the test of choice in management of NAFLD[64,65]. Hepatic stiffness measurement with MRE is superior to MRI for the non-invasive diagnosis of significant liver fibrosis and cirrhosis[66], but the role of transient elastography may be limited in subjects with high body mass indices[67]. Further, MRE has the advantage of identifying individuals with steatohepatitis, even before the onset of significant fibrosis[68]. NAFLD with inflammation but without fibrosis demonstrates greater hepatic stiffness than simple steatosis and lower mean stiffness than NAFLD with fibrosis[68]. Despite this, abdominal imaging studies are currently unable to accurately diagnose NASH.
Liver biopsy with key histologic features is essential for confirmation of NASH. However, due to its invasive nature experts recommend selective use in NAFLD patients who have a higher probability of progressing to NASH. An individualized assessment is needed with discussion of risks and benefits of a diagnostic liver biopsy. Early diagnosis of NASH has crucial management implications and these patients can benefit from newly approved medications, off-label therapy with promising agents and treatment in the setting of a clinical trial in an attempt to retard the progression of liver disease[69-74]. Steatosis may be absent in the setting of advanced fibrosis or cirrhosis[58,69]. Inter-observer variability among experienced pathologists can occur during the histologic evaluation of hepatic balloon degeneration on a liver biopsy sample[58,59,75,76]. Poor inter-observer agreement among pathologists regarding sampling error or identification of hepatic ballooning may have resulted in a lower number of patients meeting the entry criteria in clinical trials[69]. Therefore, liver biopsy although considered as a gold standard for diagnosis of NASH may have several limitations. Patients with isolated hepatic steatosis with any degree of necro-inflammation on an index liver biopsy are at risk for progressive histologic damage[77,78]. In addition, patients with metabolic syndrome or those with individual components of metabolic syndrome coupled with isolated hepatic steatosis on liver biopsy may be at risk for more rapidly worsening histologic damage[77,78]. Figure 2 organizes the predictors of histologic evidence of NASH on an index liver biopsy in patients with NAFLD. Liver biopsy is indicated in NAFLD patients who have persistently elevated ALT and/or AST levels with abdominal imaging consistent with fatty liver age 65 years or older, suspicion of other coexisting liver disease, suspicion that another liver disease has been misdiagnosed as NAFLD and those with metabolic syndrome or its components[1,79-84].
Due to high prevalence of NAFLD along with limitations of liver biopsy and clinical predictors of NASH, there has been a need to develop next generation of noninvasive biomarkers for early diagnosis of NASH[85]. These noninvasive markers may be able to differentiate lack of fibrosis or mild fibrosis from advanced bridging fibrosis or cirrhosis[85,86]. However, they are limited in their ability to consistently detect intermediate grade and stage of hepatic fibrosis[85,86]. Further, abdominal ultrasound have low sensitivity to diagnose NAFLD with less than 30% steatosis[87]. Keratin 8/18 immunostaining and other next generation noninvasive biomarkers may become available in the near future[88]. Based on preliminary data, levels of cytokeratin 18 are associated with the presence of NASH, but lacks sensitivity and the histologic details provided by a liver biopsy[89,90]. Several panels have been developed and studied to predict the presence of advanced fibrosis in patients with NASH[91] NAFLD fibrosis score[92] and FIB-4 are derived from readily available clinical markers for the assessment of advanced fibrosis[93] The Enhanced Liver Fibrosis panel utilizes an extracellular matrix marker panel to predict the stage of fibrosis in patients with chronic liver disease[94].
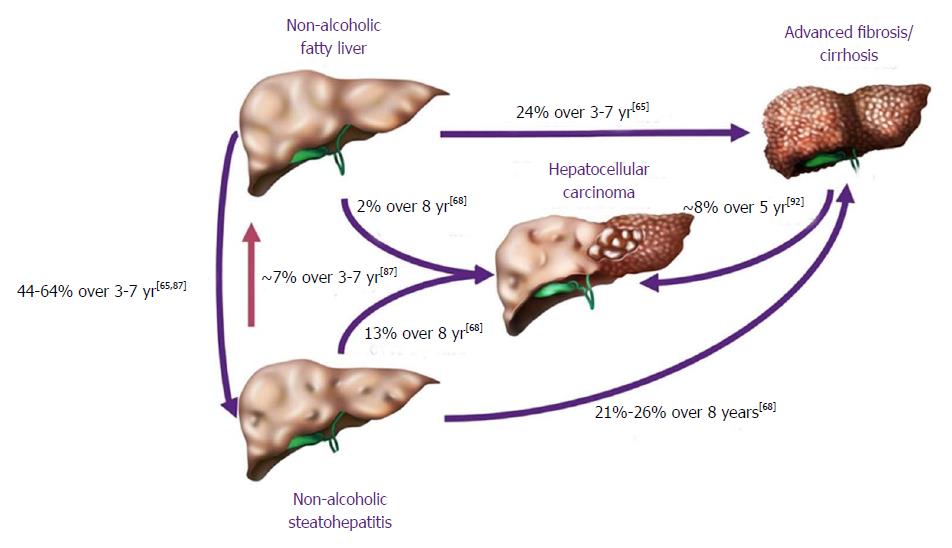
In terms of progression of NAFLD, the cohort of patients falls in two broad categories, NASH and NAFL (Figure 4). They are primarily divided by the likelihood of progression; NAFL which represents simple steatosis and steatosis with non-specific inflammatory changes, following a more indolent course of progression, while NASH may progress more rapidly to end-stage liver disease.
NAFLD activity score (NAS) has gained popularity in defining NASH, yet histology is still the gold standard. As NASH advances to cirrhosis, it loses its characteristic histologic features, including inflammation and steatosis. Thus, it is increasingly being recognized as “cryptogenic cirrhosis” which essentially means cirrhosis of unclear etiology. Cryptogenic cirrhosis is referred to as ‘burnt out’ NASH by experts in the medical literature[8,95]. Patients with cryptogenic cirrhosis have clinical manifestations commonly observed in patients with NASH, such as obesity, dyslipidemia, insulin resistance, T2DM and metabolic syndrome.
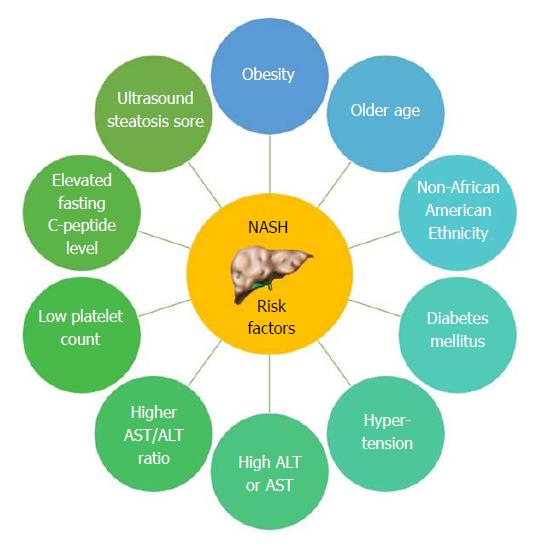
NAFL is more readily reversible if lifestyle modifications are implemented in a timely fashion. The benign progression of NAFL and rapid progression of NASH has also been supported by earlier cohort studies from United Kingdom[96] and Denmark[97]. In one of the earliest histology-based studies, biopsy-proven NAFLD was divided into 4 types with type 3 (fatty liver and ballooning degeneration) and type 4 (fatty liver, ballooning degeneration, and either Mallory bodies or fibrosis) representing the modern-day definition of NASH[80]. Over follow up periods of 8 years, 21% to 26% of patients with histological type 3 and type 4 developed cirrhosis compared to only 3% of patients with type 1 (fatty liver alone) and type 2 (fatty liver and lobular inflammation)[80]. Recent studies are challenging the widespread belief that non-NASH (simple steatosis) has a benign course. Based on histological diagnosis and follow up biopsies of 52 patients, NAFL advanced to NASH in 23% of cases over a period of 3 years[98]. The evolution into NASH can be as high as 44%-64% and progression of simple steatosis into advanced fibrosis was reported in up to 24% of the patients with NAFL[77,99] (Figure 4). Risk factors causing increasing NASH likelihood include obesity, older age, female sex, non-African American race/ethnicity, diabetes mellitus, and hypertension (Figure 5)[100]. With fibrosis staging and its progression from one stage to another being an important marker of mortality, recent studies reported around 9% to 25% of the patients developed NASH[101].
The risk of progression of NASH into cirrhosis has been delineated in previous studies, and is estimated to be between 21% and 26% in 8 years[80,102]. Although development of cirrhosis further increases the risk of progression to HCC and/or hepatic decompensation, the stage of fibrosis is also an excellent predictor of outcome.
The incidence of HCC has been increasing in parallel with the rise in NAFLD and its subsets. HCC incidence has grown four-fold from 1973 to 2011[103]. Advanced fibrosis is a reliable risk factor for HCC with 8% 5-year cumulative incidence rate of developing HCC in patients with advanced fibrosis[104]. The annual incidence of NAFLD-related HCC (0.44 per 1000 person-years) is rare at this moment and 15-35 times lower than the incidence of HCC in chronic hepatitis B[3]. In comparison, the annual incidence rate of NASH-related HCC was a significant 5.29 cases per 1000 person-years[3]. This highlights the increased need of preventative measures that should be adopted; as the prevalence of NAFLD increases so will the incidence of NASH-related HCC. Younossi et al[105] described a 9% annual increase of HCC cases related to NAFLD over a period of six years from 2004 to 2009. While previous studies have described progression of advanced fibrosis and cirrhosis as a major link between NAFLD and HCC, the latest studies are describing 35% to 50% of HCC without cirrhosis[106,107]. Understanding of underlying pathogenetic pathways remains unclear at best. A few potential mechanisms to explain the link between NAFLD and HCC include hyperinsulinemia or metabolic syndrome, functioning of hepatic progenitor cells activated by hepatocyte damage, activation of CD8+/CD4+ T lymphocyte and natural killer cells activation causing self-damage and PNPLA3-related pathways[108].
NASH is characterized by histologic evidence of progressive hepatocellular injury (ballooning) which can progress to cirrhosis and its complications including HCC with eventual need for liver transplant[1,109,110]. During last decade, NASH-related LT increased from 1.2% in 2001 to 9.7% in 2009 to become the third most common indication for LT in the United States[110]. A 2013 population cohort study based on data from the United Network for Organ Sharing/Organ Procurement Transplant Network revealed that NASH has become the second leading etiology of liver disease among adults awaiting LT in the United States and is predicted to become the leading indication in the near future[110,111]. In addition, NASH is also the second leading etiology for HCC in adults requiring LT in the United States[112].
A retrospective longitudinal study during 12.6 years showed that increasing fibrosis stage from 1 (HR = 1.88) to stage 4 (HR = 10.49) increased mortality, liver-related events and need for LT[113]. Over a 8 years follow-up period, liver-related mortality increased in NASH and NASH-related cirrhosis compared to NAFL (11% vs 2%)[80]. A more recent study using follow-up data from the same cohort reported 18% liver-related mortality in NASH patients compared to 3% in non-NASH patients during 18.5 years[81].
Previous studies comparing NAFLD to the general population have consistently shown increased mortality in NAFLD. However, these studies did not adjust for metabolic confounders in the setting of NAFLD. Data from NHANES III revealed no significant difference in the overall survival of ultrasonography-diagnosed subjects with NAFLD compared with the non-NAFLD population after adjusting for multiple metabolic factors[17]. These results suggest that NASH and/or fibrosis may be the major driver contributing to significant long-term outcomes[17].
NAFLD is associated with increased overall mortality, with ranges for the standardized mortality ratio (SMR) of 1.34-2.6 compared to the general population[114]. An early landmark study by Adams et al[82] documented that patients with NAFLD (n = 435) from Olmsted County, diagnosed histologically or by ultrasonography demonstrated a significantly higher risk of mortality during 7.6 years of follow-up (SMR = 1.34, 95%CI: 1.00-1.76). In this study, liver-related mortality was the third most common cause of death, after malignancy and cardiovascular disease[82]. This is in contrast to the general population where liver-related mortality is reported 12th most common cause of death[115]. NASH cirrhosis has been compared to hepatitis C-related cirrhosis in multiple studies with majority of the studies showing decreased or comparable mortality and lower or similar cirrhosis-related complications and/or HCC[101,114]. However, the cardiovascular mortality was higher in NASH cirrhosis[100]. The increased risk for cardiovascular mortality can be explained by the decreased morbidity when compared to chronic hepatitis C-related cirrhosis. Thus, most patients may outlive their liver disease but develop fatal complications from cardiovascular disease and malignancies.
NAFLD is a term for a host of histological findings stemming from hepatic steatosis and remains the most common liver disease globally with increasing prevalence. The vast variation in disease presentation complicates diagnosis, leading to an underestimate of actual disease occurrence. NAFLD is associated with many metabolic comorbidities, including obesity, type II diabetes, dyslipidemia, and metabolic syndrome. Its potential to develop into more severe liver conditions, such as NASH, advanced fibrosis, cirrhosis and HCC, can lead to a state in which LT is the only treatment option available. The population at risk of developing progressive liver disease creates a challenge to the healthcare system in terms of screening for this evolving epidemic of liver disease. Further research must be conducted to understand NAFLD pathophysiology and its treatment, as well as, define accurate incidence, current disease burden, and socioeconomic effects of this disease.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Blanco JR, Inzaugarat E, Trovato GMM S- Editor: Chen K L- Editor: A E- Editor: Huang Y
| 1. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterologyh. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1226] [Cited by in F6Publishing: 1253] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8406] [Cited by in F6Publishing: 8901] [Article Influence: 741.8] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5322] [Cited by in F6Publishing: 6281] [Article Influence: 785.1] [Reference Citation Analysis (0)] |
| 4. | Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1127] [Cited by in F6Publishing: 1221] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 5. | Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722-728. [PubMed] [Cited in This Article: ] |
| 6. | Suzuki A, Angulo P, Lymp J, St Sauver J, Muto A, Okada T, Lindor K. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology. 2005;41:64-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Whalley S, Puvanachandra P, Desai A, Kennedy H. Hepatology outpatient service provision in secondary care: a study of liver disease incidence and resource costs. Clin Med (Lond). 2007;7:119-124. [PubMed] [Cited in This Article: ] |
| 8. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2065] [Cited by in F6Publishing: 2143] [Article Influence: 164.8] [Reference Citation Analysis (0)] |
| 9. | Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 939] [Cited by in F6Publishing: 918] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 10. | Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 864] [Cited by in F6Publishing: 974] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 11. | Amarapurkar D, Kamani P, Patel N, Gupte P, Kumar P, Agal S, Baijal R, Lala S, Chaudhary D, Deshpande A. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161-163. [PubMed] [Cited in This Article: ] |
| 12. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1522] [Cited by in F6Publishing: 1520] [Article Influence: 116.9] [Reference Citation Analysis (1)] |
| 13. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 2593] [Article Influence: 129.7] [Reference Citation Analysis (3)] |
| 14. | Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462-E468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1119] [Cited by in F6Publishing: 1113] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 15. | Farrell GC, Wong VW, Chitturi S. NAFLD in Asia--as common and important as in the West. Nat Rev Gastroenterol Hepatol. 2013;10:307-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 327] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 16. | Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524-530.e1; quiz e60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 748] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 17. | Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 571] [Cited by in F6Publishing: 564] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 18. | Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577-1586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 783] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 19. | Martini EM, Garrett N, Lindquist T, Isham GJ. The boomers are coming: a total cost of care model of the impact of population aging on health care costs in the United States by Major Practice Category. Health Serv Res. 2007;42:201-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Younossi ZM, Henry L. Economic and Quality-of-Life Implications of Non-Alcoholic Fatty Liver Disease. Pharmacoeconomics. 2015;33:1245-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 21. | Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, Yokoo T, Chavez A, Middleton MS. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585-1592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 319] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 22. | Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883-1894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 673] [Article Influence: 51.8] [Reference Citation Analysis (1)] |
| 23. | Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ; NASH CRN. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 355] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 24. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2233] [Cited by in F6Publishing: 2311] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 25. | Speliotes EK, Butler JL, Palmer CD, Voight BF; GIANT Consortium; MIGen Consortium; NASH CRN, Hirschhorn JN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 278] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 26. | Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, Nobili V, Mozzi E, Roviaro G, Vanni E. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 497] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 27. | Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, Vogt TF, Hobbs HH, Cohen JC. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 724] [Cited by in F6Publishing: 817] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 28. | Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JB, Allison ME, Alexander GJ, Piguet AC, Anty R. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 399] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 29. | Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, Suzuki A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 30. | Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr. 2013;162:496-500.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 333] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 31. | Doycheva I, Watt KD, Rifai G, Abou Mrad R, Lopez R, Zein NN, Carey WD, Alkhouri N. Increasing Burden of Chronic Liver Disease Among Adolescents and Young Adults in the USA: A Silent Epidemic. Dig Dis Sci. 2017;62:1373-1380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 32. | Choi SY, Kim D, Kim HJ, Kang JH, Chung SJ, Park MJ, Kim YS, Kim CH, Choi SH, Kim W. The relation between non-alcoholic fatty liver disease and the risk of coronary heart disease in Koreans. Am J Gastroenterol. 2009;104:1953-1960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, Clark JM. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 619] [Cited by in F6Publishing: 591] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 34. | Kagansky N, Levy S, Keter D, Rimon E, Taiba Z, Fridman Z, Berger D, Knobler H, Malnick S. Non-alcoholic fatty liver disease--a common and benign finding in octogenarian patients. Liver Int. 2004;24:588-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:837-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 36. | Saab S, Manne V, Nieto J, Schwimmer JB, Chalasani NP. Nonalcoholic Fatty Liver Disease in Latinos. Clin Gastroenterol Hepatol. 2016;14:5-12; quiz e9-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Farrell GC. The liver and the waistline: Fifty years of growth. J Gastroenterol Hepatol. 2009;24 Suppl 3:S105-S118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Kim D, Chung GE, Kwak MS, Seo HB, Kang JH, Kim W, Kim YJ, Yoon JH, Lee HS, Kim CY. Body Fat Distribution and Risk of Incident and Regressed Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:132-138.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 39. | Yu SJ, Kim W, Kim D, Yoon JH, Lee K, Kim JH, Cho EJ, Lee JH, Kim HY, Kim YJ. Visceral Obesity Predicts Significant Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Medicine (Baltimore). 2015;94:e2159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Zelber-Sagi S, Lotan R, Shlomai A, Webb M, Harrari G, Buch A, Nitzan Kaluski D, Halpern Z, Oren R. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol. 2012;56:1145-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 41. | Chang Y, Ryu S, Sung E, Woo HY, Cho SI, Yoo SH, Ahn HY, Choi NK. Weight gain within the normal weight range predicts ultrasonographically detected fatty liver in healthy Korean men. Gut. 2009;58:1419-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Fan JG, Zhou Q, Wo QH. [Effect of body weight mass and its change on the incidence of nonalcoholic fatty liver disease]. Zhonghua Gan Zang Bing Za Zhi. 2010;18:676-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 43. | Kim HK, Park JY, Lee KU, Lee GE, Jeon SH, Kim JH, Kim CH. Effect of body weight and lifestyle changes on long-term course of nonalcoholic fatty liver disease in Koreans. Am J Med Sci. 2009;337:98-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Bray GA, Popkin BM. Calorie-sweetened beverages and fructose: what have we learned 10 years later. Pediatr Obes. 2013;8:242-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 45. | Cusi K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2009;16:141-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 46. | Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, Subbarayan S, Webb A, Hecht J, Cusi K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab. 2015;100:2231-2238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 343] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 47. | Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59:1410-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 48. | Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 418] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 49. | Hsieh SD, Muto T, Murase T, Tsuji H, Arase Y. Association of short sleep duration with obesity, diabetes, fatty liver and behavioral factors in Japanese men. Intern Med. 2011;50:2499-2502. [PubMed] [Cited in This Article: ] |
| 50. | Trovato FM, Martines GF, Brischetto D, Catalano D, Musumeci G, Trovato GM. Fatty liver disease and lifestyle in youngsters: diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016;36:427-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 51. | Trovato FM, Martines GF, Brischetto D, Trovato G, Catalano D. Neglected features of lifestyle: Their relevance in non-alcoholic fatty liver disease. World J Hepatol. 2016;8:1459-1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Kim CW, Yun KE, Jung HS, Chang Y, Choi ES, Kwon MJ, Lee EH, Woo EJ, Kim NH, Shin H. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J Hepatol. 2013;59:351-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 53. | Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, Fang Y, Elariny H, Goodman Z, Chandhoke V. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 54. | Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372-1379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 422] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 55. | Langin D, Arner P. Importance of TNFalpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metab. 2006;17:314-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787-3794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 438] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 57. | Targher G, Bertolini L, Rodella S, Zoppini G, Zenari L, Falezza G. Associations between liver histology and cortisol secretion in subjects with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf). 2006;64:337-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6807] [Cited by in F6Publishing: 7396] [Article Influence: 389.3] [Reference Citation Analysis (5)] |
| 59. | Juluri R, Vuppalanchi R, Olson J, Unalp A, Van Natta ML, Cummings OW, Tonascia J, Chalasani N. Generalizability of the nonalcoholic steatohepatitis Clinical Research Network histologic scoring system for nonalcoholic fatty liver disease. J Clin Gastroenterol. 2011;45:55-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Kaswala DH, Lai M, Afdhal NH. Fibrosis Assessment in Nonalcoholic Fatty Liver Disease (NAFLD) in 2016. Dig Dis Sci. 2016;61:1356-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 61. | Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626-637.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 514] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 62. | Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 360] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 63. | Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 520] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 64. | Le Y, Kroeker R, Kipfer HD, Lin C. Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. J Magn Reson Imaging. 2012;36:483-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, Bettencourt R, Changchien C, Brenner DA, Sirlin C. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930-1940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 387] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 66. | Venkatesh SK, Yin M, Takahashi N, Glockner JF, Talwalkar JA, Ehman RL. Non-invasive detection of liver fibrosis: MR imaging features vs. MR elastography. Abdom Imaging. 2015;40:766-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 67. | Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, Soaft L, Hooker J, Kono Y, Bhatt A, Hernandez L, Nguyen P, Noureddin M, Haufe W, Hooker C, Yin M, Ehman R, Lin GY, Valasek MA, Brenner DA, Richards L; San Diego Integrated NAFLD Research Consortium (SINC). Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology. 2015;61:1239-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 270] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 68. | Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 319] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 69. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2215] [Cited by in F6Publishing: 2196] [Article Influence: 156.9] [Reference Citation Analysis (1)] |
| 70. | Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659-1668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 765] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 71. | Lutchman G, Modi A, Kleiner DE, Promrat K, Heller T, Ghany M, Borg B, Loomba R, Liang TJ, Premkumar A. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46:424-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 72. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1569] [Cited by in F6Publishing: 1606] [Article Influence: 178.4] [Reference Citation Analysis (0)] |
| 73. | Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, Nagabhyru P, Sumner AE, Liang TJ. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 74. | Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, Roberts LR, Chaiteerakij R. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60:2008-2016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 75. | Younossi ZM, Gramlich T, Liu YC, Matteoni C, Petrelli M, Goldblum J, Rybicki L, McCullough AJ. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol. 1998;11:560-565. [PubMed] [Cited in This Article: ] |
| 76. | Gawrieh S, Knoedler DM, Saeian K, Wallace JR, Komorowski RA. Effects of interventions on intra- and interobserver agreement on interpretation of nonalcoholic fatty liver disease histology. Ann Diagn Pathol. 2011;15:19-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 77. | Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, Ratziu V; LIDO Study Group. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 339] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 78. | Pais R, Pascale A, Fedchuck L, Charlotte F, Poynard T, Ratziu V. Progression from isolated steatosis to steatohepatitis and fibrosis in nonalcoholic fatty liver disease. Clin Res Hepatol Gastroenterol. 2011;35:23-28. [PubMed] [Cited in This Article: ] |
| 79. | Noureddin M, Yates KP, Vaughn IA, Neuschwander-Tetri BA, Sanyal AJ, McCullough A, Merriman R, Hameed B, Doo E, Kleiner DE. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58:1644-1654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 80. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] [Cited in This Article: ] |
| 81. | Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 526] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 82. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [PubMed] [Cited in This Article: ] |
| 83. | Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping JH, Bugianesi E. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 84. | Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 528] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 85. | Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L; American Association for the Study of Liver Diseases; United States Food and Drug Administration. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 254] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 86. | Sanal MG. Biomarkers in nonalcoholic fatty liver disease-the emperor has no clothes? World J Gastroenterol. 2015;21:3223-3231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 87. | Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, Lonardo A. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 261] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 88. | Lackner C, Gogg-Kamerer M, Zatloukal K, Stumptner C, Brunt EM, Denk H. Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. J Hepatol. 2008;48:821-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 89. | Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 468] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 90. | Cusi K, Chang Z, Harrison S, Lomonaco R, Bril F, Orsak B, Ortiz-Lopez C, Hecht J, Feldstein AE, Webb A. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60:167-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 91. | Festi D, Schiumerini R, Scaioli E, Colecchia A. Letter: FibroTest for staging fibrosis in non-alcoholic fatty liver disease - authors’ reply. Aliment Pharmacol Ther. 2013;37:656-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 92. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1917] [Cited by in F6Publishing: 2020] [Article Influence: 118.8] [Reference Citation Analysis (1)] |
| 93. | McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 587] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 94. | Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 95. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [PubMed] [Cited in This Article: ] |
| 96. | Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714-1719. [PubMed] [Cited in This Article: ] |
| 97. | Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sørensen TI, Becker U, Bendtsen F. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750-755. [PubMed] [Cited in This Article: ] |
| 98. | Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, Chim AM, Yu J, Sung JJ, Chan HL. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 471] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 99. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 687] [Cited by in F6Publishing: 693] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 100. | Friedman LS. Liver, Biliary Tract Pancreatic disorders: Non-Alcoholic fatty liver disease. New York: Academic 2015; . [Cited in This Article: ] |
| 101. | Goh GB, McCullough AJ. Natural History of Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2016;61:1226-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 102. | Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol. 2015;13:2062-2070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 207] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 103. | Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 402] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 104. | Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Tokushige K, Shiratori K. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44 Suppl 19:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 105. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 532] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 106. | Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124-131.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 415] [Article Influence: 51.9] [Reference Citation Analysis (1)] |
| 107. | Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1508] [Cited by in F6Publishing: 1574] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 108. | Wong CR, Nguyen MH, Lim JK. Hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:8294-8303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 70] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 109. | White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342-1359.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 527] [Article Influence: 43.9] [Reference Citation Analysis (2)] |
| 110. | Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 835] [Cited by in F6Publishing: 821] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 111. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1211] [Cited by in F6Publishing: 1259] [Article Influence: 139.9] [Reference Citation Analysis (1)] |
| 112. | Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188-2195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 520] [Cited by in F6Publishing: 528] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 113. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-397.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1701] [Cited by in F6Publishing: 1920] [Article Influence: 213.3] [Reference Citation Analysis (0)] |
| 114. | Kwak MS, Kim D. Long-Term Outcomes of Nonalcoholic Fatty Liver Disease. Curr Hepatol Rep. 2015;14:69-76. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 115. | Heron M. Deaths: Leading Causes for 2014. Natl Vital Stat Rep. 2016;65:1-96. [PubMed] [Cited in This Article: ] |