Published online Nov 14, 2017. doi: 10.3748/wjg.v23.i42.7541
Peer-review started: June 30, 2017
First decision: July 27, 2017
Revised: September 8, 2017
Accepted: September 19, 2017
Article in press: September 19, 2017
Published online: November 14, 2017
To investigate the significance of heat shock protein 110 (HSP110) in gastric cancer (GC) patients with peritoneal metastasis undergoing hyperthermo-chemotherapy.
Primary GC patients (n = 14) with peritoneal metastasis or positive peritoneal lavage cytology who underwent distal or total gastrectomy between April 2000 and December 2011 were enrolled in this study. The patients underwent postoperative intraperitoneal hyperthermo-chemotherapy using a Thermotron RF-8 heating device two weeks after surgery. We analyzed nuclear HSP110 expression in surgically resected tumors using immunohistochemistry. Additionally, the effect of HSP110 suppression on hyptherthermo-chemosensitivity was assessed in vitro in the MKN45 GC cell line using the HSP inhibitor KNK437.
HSP110 immnohistochemical staining in 14 GC patients showed that five (35.7%) samples belonged to the low expression group, and nine (64.3%) samples belonged to the high expression group. Progression-free survival was significantly shorter in the HSP110 high-expression group than in the low-expression group (P = 0.0313). However, no significant relationships were identified between HSP110 expression and the clinicopathological characteristics of patients. Furthermore, high HSP110 expression was not an independent prognostic factor in GC patients with peritoneal metastasis (P = 0.0625). HSP110 expression in MKN45 cells was suppressed by KNK437 at the hyperthermic temperature of 43 °C in vitro. Comparison of MKN45 cell proliferation in the presence and absence of KNK437 at 43 °C, revealed that proliferation was significantly decreased when HSP110 was inhibited by KNK437. Additionally, HSP110 suppression via HSP inhibitor treatment increased cellular sensitivity to hyperthermo-chemotherapy in vitro.
The expression of nuclear HSP110 in GC patients might be a new marker of chemosensitivity and a therapeutic target for patients who are tolerant to existing hyperthermo-chemotherapies.
Core tip: Gastric cancer remains one of the most common cancers worldwide. Peritoneal dissemination is the most common reason behind gastric cancer (GC) recurrence, and the median survival duration for patients with metastatic and recurrent GC is only 13-16 mo. In our department, we have used intraperitoneal hyperthermo-chemotherapy in patients with advanced rectal cancer and GC with peritoneal metastasis. In this study, we evaluated the significance of heat shock protein 110 (HSP110) expression in GC patients with peritoneal metastasis and assessed the effects of HSP110 suppression on hyperthermo-chemotherapy sensitivity in vitro.
- Citation: Kimura A, Ogata K, Altan B, Yokobori T, Mochiki E, Yanai M, Kogure N, Yanoma T, Suzuki M, Bai T, Kuwano H. Nuclear heat shock protein 110 expression is associated with poor prognosis and hyperthermo-chemotherapy resistance in gastric cancer patients with peritoneal metastasis. World J Gastroenterol 2017; 23(42): 7541-7550
- URL: https://www.wjgnet.com/1007-9327/full/v23/i42/7541.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i42.7541
Despite progress in early diagnosis and improvements in treatment, gastric cancer (GC) is a central cause of cancer-related deaths worldwide. Furthermore, a particularly high GC mortality rate has been reported in Asia[1]. Peritoneal dissemination is the most common reason behind GC recurrence, and the first line of treatment for this disease is chemotherapy. New chemotherapies for metastatic and recurrent GC are being developed. However, the median survival duration for patients with metastatic and recurrent GC is only 13-16 mo[2-7].
Intraperitoneal hyperthermo-chemotherapy is an effective alternative treatment to standard chemotherapy in GC patients with peritoneal dissemination[8-12]. Indeed, hyperthermic intraperitoneal perfusion chemotherapy (HIPEC) with cisplatin combined with an intravenous chemotherapy regimen with paclitaxel, 5-fluorouracil, and leucovorin has improved survival rate and decreased the postoperative recurrence of locally advanced GC[12]. Furthermore, it has been suggested that hyperthermia and 5-fluorouracil act synergistically to promote apoptosis and enhance thermotolerance in the SGC-7901 GC cell line[9]. In our department, we have used intraperitoneal hyperthermo-chemotherapy in patients with advanced rectal cancer and GC with peritoneal metastasis[13-16]. A preliminary non-random study in a small number of patients revealed that patients in the postoperative intraperitoneal hyperthermo-chemotherapy (PIHC) group had a higher survival rate and better prognosis than did the patients in the control group[15].
Heat shock proteins (HSPs) have been characterized as molecular chaperones that prevent the formation of misfolded protein structures[17-19]. HSPs are induced by exposure to the stress condition, including fever, irradiation and chemicals. HSPs in cancer maintain several oncoproteins homeostasis and promote cancer cell survival by inhibiting apoptosis induction[17,20]. It was reported that overexpression of HSPs might be correlated with poor prognosis in several types of human carcinomas[21-26]. Additionally, it was reported that high levels of various HSP family members are associated with increased chemoresistance in several malignancies[27-29]. Previously, we found that high expression of nuclear HSP110 is associated with cancer progression and poor prognosis in GC patients and that HSP110 suppression increases chemosensitivity in human GC cell lines[17].
However, the clinicopathological significance of HSP110 expression and localization and their relationship with hyperthermo-chemotherapy sensitivity in GC patients are still unclear. Therefore, the purposes of current study were to determine the significance of HSP110 expression in GC patients with peritoneal metastasis and to evaluate the impact of HSP110 inhibition on hyperthermo-chemotherapy sensitivity in vitro.
The Institutional Review Board of Gunma University Hospital approved this study, and written informed consent was obtained from all patients. From April 2000 to December 2011, 14 GC patients with peritoneal dissemination underwent distal or total gastrectomy for cytoreduction at the Department of General Surgical Science, Gunma University. All patients were diagnosed with peritoneal metastasis (P1) or positive peritoneal lavage cytology (CY1) during surgery. Gastric cancer staging was performed according to the Classification of Gastric Carcinoma (third edition) of the Japanese Gastric Cancer Association[30]. All patients underwent PIHC.
Treatment regimens for PIHC were administered as previously described[15]. Patients underwent distal or total gastrectomy with lymph node dissection, and a 19-Fr silicon drain was inserted in the left subphrenic lesion. PIHC was administered to patients diagnosed with P1 or CY1 during surgery. Two weeks after surgery, hyperthermia was induced using 8-MHz radiofrequency capacitive heating equipment (Thermotron RF-8, Yamamoto Vinyter, Osaka, Japan). Physiologic saline (1 L) containing 80 mg/m2 cisplatin was warmed to 37 °C in a dry incubator and introduced as quickly as possible into the peritoneal cavity via a catheter. After PIHC, the catheter was clamped for six hours. A minimum temperature of higher than 40 °C was achieved in the abdominal cavity and maintained for 60 min. The treatment was repeated every two weeks for a maximum of four cycles. After completing all PIHC courses, patients took S-1 (Taiho Pharmaceutical Company, Tokyo, Japan) for one year from the date of surgery.
Resected surgical specimens were fixed in formalin, embedded in paraffin, cut into 4-μm thick sections, and mounted on glass slides. Immnohistochemical staining was performed as previously reported[17,31]. All sections were deparaffinized in xylene. Sections were dehydrated in alcohol and soaked with 0.3% hydrogen peroxide in 100% methanol for 30 min at room temperature to inhibit endogenous peroxidase. The sections were soaked in boiling water, and immersed in Immunosaver (Nishin EM, Tokyo, Japan) at 98 °C for 90 min. Non-specific binding sites were blocked by incubating the sections with Serum-Free Protein Block (DAKO, Carpinteria, CA, United States) for 30 min at room temperature. The sections were incubated with a 1:100 dilution of a rabbit monoclonal antibody against HSP110 (GeneTex, CA, United States) for 24 h at 4 °C. The signal from the primary antibody was visualized using the Histofine Simple Stain MAX-PO (MULTI) (Nichirei, Tokyo, Japan) according to the manufacturer’s instructions. The chromogen 3,3-diaminobenzidine tetrahydrochloride (DAB) was applied as a 0.02% solution containing 0.005% hydrogen peroxide in 50 mmol/L ammonium acetate-citrate acid buffer (pH 6.0). All sections were counterstained with Mayer’s hematoxylin solution.
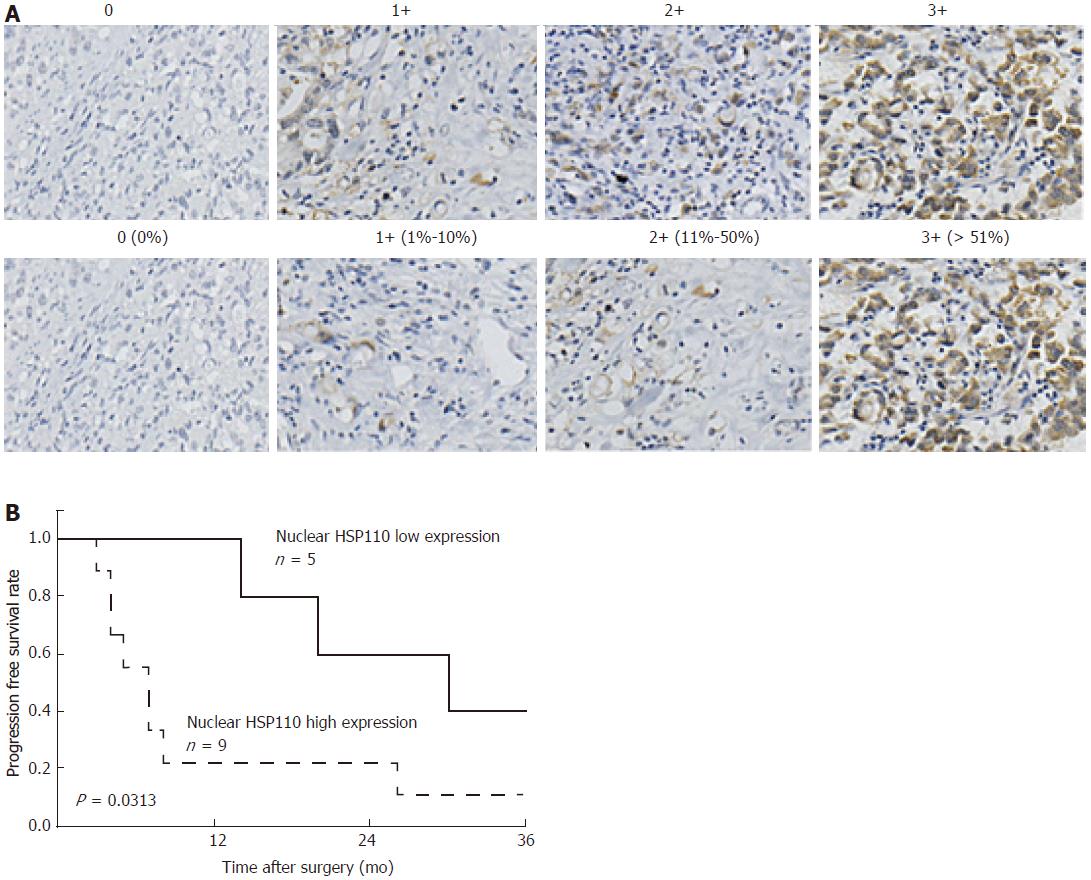
The immunohistochemically stained samples were analyzed by two researchers blinded to the patient information. Staining for HSP110 was assessed using the immunoreactive score (IRS) to evaluate the proportion of cells expressing HSP110 and their relative staining intensity as previously described[31,32]. The intensity of nuclear HSP110 staining was graded as follows: 0, no staining; 1+, weak staining; 2+, moderate staining; and 3+, strong staining (Figure 1A). The percentage of nuclear HSP110-expressing GC cells was calculated based on at least 1000 cancer cells in total from five representative areas. The percentage of nuclear HSP110 staining was scored as follows: 0, no staining; 1+, 1%-10%; 2+, 11%-50%; and 3+, 51%-100% (Figure 1A). The IRS was calculated by multiplying the intensity and expression scores to arrive at values of 0, 1+, 2+, 3+, 4+, 6+, or 9+. IRS values of 0, 1+, 2+ and 3+ represent low HSP110 expression, while IRS values of 4+, 6+, and 9+ represent high HSP110 expression.
The human GC cell line, MKN45, was purchased from RIKEN BRC through the National Bio-Resource Project of MEXT, Tokyo, Japan. MKN45 cells were maintained in RPMI 1640 medium (Wako, Osaka, Japan) containing 10% fetal bovine serum and supplemented with 100 units/mL penicillin and streptomycin sulfate (Invitrogen, Carlsbad, CA, United States).
KNK437 (benzylidene lactam compound; Merck Millipore, Darmstadt, Germany) was used as a heat shock protein inhibitor. KNK437 was dissolved in dimethyl sulfoxide (DMSO) before being added to the culture medium as described[33]. The final concentration of DMSO in the culture medium for each treatment was 0.25% (v/v). The same concentration of DMSO was used as a control. Cells were incubated at 43 °C for 3 h during heat treatment.
Western blotting was used to evaluate HSP110 and β-actin expression in MKN45 cells. MKN45 cells were treated with KNK437 at 43 °C for 3 h. Then, total proteins were extracted using the PRO-PREP Protein Extraction Solution Kit (iNtRON Biotechnology, Sungnam, Kyungki-Do, South Korea). Proteins were separated on a 10% polyacrylamide gel and transferred to nitrocellulose membranes using a wet transfer protocol. The membranes were incubated overnight at 4 °C with rabbit monoclonal antibody against HSP110 (1:1000; GeneTex) and β-actin (1:1000; Sigma, St Louis, MO, United States). The membranes were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibodies, and each proteins were evaluated using the ECL Prime Western Blotting Detection System (GE Healthcare, Tokyo, Japan) using Image Quant LAS4000 (GE Healthcare Life Sciences, United Kingdom).
Cell proliferation was measured with the Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan). MKN45 were seeded (3000 cells/well) into 96-well plates in 100 μL medium with KNK437 (50 or 100 nmol/L). To generate heat shock, cells were incubated at 43 °C for 3 h before KNK437 (50 or 100 nmol/L) was added. Cell proliferation was assessed at 0, 12, 24, and 36 h. To assess cell proliferation, 10 μL of Cell Counting Kit-8 reagent was added to each well and incubated at 37 °C for 2 h. Next, the absorbance of each well was detected at 450 nm using an xMark Microplate Absorbance Spectrophotometer (Bio Rad, Hercules, CA, United States).
Cell Counting Kit-8 was used to evaluate the sensitivity of MKN45 cells to hyperthermo-chemotherapy with cisplatin. MKN45 cells were plated (approximately 10000 cells per well) into 96-well plates in 100 μL of medium with KNK437 (50 or 100 nmol/L) before cisplatin exposure. The cells were subjected to heat shock at 43 °C for 3 h; then, 10 μL of Cell Counting Kit-8 reagent was added, and the cells were incubated for 2 h at 37 °C. The absorbance of each well was evaluated at 450 nm using an xMark Microplate Absorbance Spectrophotometer. Then, the cells were treated with various concentrations of cisplatin (0, 0.1, 1 or 10 μmol/L) for 48 h. As control, the sensitivity of the cells to chemotherapy with cisplatin in the absence of heat treatment was evaluated in the same manner.
Statistically significant differences were analyzed with Student’s t-test for continuous variables and the chi-square test for categorical variables. The data for continuous variables are expressed as the mean ± SE of the mean. Survival curves were calculated according to the Kaplan-Meier method and analyzed with the log-rank test. Univariate and multivariate analysis by the Cox proportional hazards model was used to identify prognostic factors. P < 0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed with JMP software (version 12; SAS Institute Inc., Cary, NC, United States).
We evaluated the expression of nuclear HSP110 in 14 GC patients by immunohistochemical staining. Five (35.7%) samples belonged to the low-expression group, and nine (64.3%) samples belonged to the high-expression group. The relationships between nuclear HSP110 expression with the clinicopathological features of 14 GC patients are shown in Table 1. There were no significant relationships between nuclear HSP110 expression and clinicopathological features of the GC patients. However, the three-year progression-free survival rate was significantly lower in the high HSP110 expression group than that in the low-expression group (P = 0.0313; Figure 1B). The results of univariate analyses of clinicopathological factors affecting progression-free survival rates after surgery are shown in Table 2. The relative risk for all factors including HSP110 expression was greater than 1. However, none of the results were statistically significant. Univariate regression analysis revealed that high HSP110 expression was not an independent prognostic factor in GC patients (P = 0.0625; Table 2).
| Factors | HSP110 expression in gastric cancer patients (n = 14) | ||
| Low (n = 5) | High (n = 9) | P value | |
| Age (mean ± SE) | 55.2 ± 3.8 | 59.3 ± 2.8 | 0.4025 |
| Sex, | |||
| Male | 2 (22.2) | 7 (77.8) | 0.1575 |
| Female | 3 (60.0) | 2 (40.0) | |
| Histology | |||
| Well, Moderate | 1 (33.3) | 2 (66.7) | 0.9227 |
| Muc, Poor, Signet | 4 (36.4) | 7 (63.6) | |
| Depth | |||
| sm, mp, ss | 0 (0.0) | 0 (0.0) | |
| se, si | 5 (35.7) | 9 (64.3) | |
| Lymph node metastasis | |||
| Absent | 0 (0.0) | 2 (100.0) | 0.2549 |
| Present | 5 (41.7) | 7 (58.3) | |
| Lymphatic invasion | |||
| Absent | 1 (33.3) | 3 (66.7) | 0.4797 |
| Present | 4 (40.0) | 6 (60.0) | |
| Venous invasion | |||
| Absent | 4 (40.0) | 6 (60.0) | 0.5967 |
| Present | 1 (25.0) | 3 (75.0) | |
| Peritoneal lavage cytology | |||
| Negative | 1 (25.0) | 3 (75.0) | 0.5967 |
| Positive | 4 (40.0) | 6 (60.0) | |
| Peritoneal metastasis | |||
| Absent | 2 (50.0) | 2 (50.0) | 0.4805 |
| Present | 3 (30.0) | 7 (70.0) | |
| Clinicopathological variables | Univariate analysis | ||
| RR | 95%CI | P value | |
| Age (< 65 yr/≥ 65 yr) | 2.17 | 0.46-8.33 | 0.2988 |
| Sex (male/female) | 1.62 | 0.48-6.22 | 0.4412 |
| Histology (differentiated/undifferentiated) | 2.18 | 0.46-8.33 | 0.2988 |
| Lymph node metastasis (absent/present) | 1.25 | 0.19-5.02 | 0.7822 |
| Lymphatic invasion (absent/present) | 1.29 | 0.33-4.29 | 0.6904 |
| Venous invasion (absent/present) | 1.18 | 0.26-4.13 | 0.8057 |
| Peritoneal lavage cytology (negative/positive) | 1.32 | 0.38-6.10 | 0.6766 |
| Peritoneal metastasis (absent/present) | 2.28 | 0.58-15.08 | 0.2568 |
| HSP110 expression (low/high) | 3.40 | 0.94-16.01 | 0.0625 |
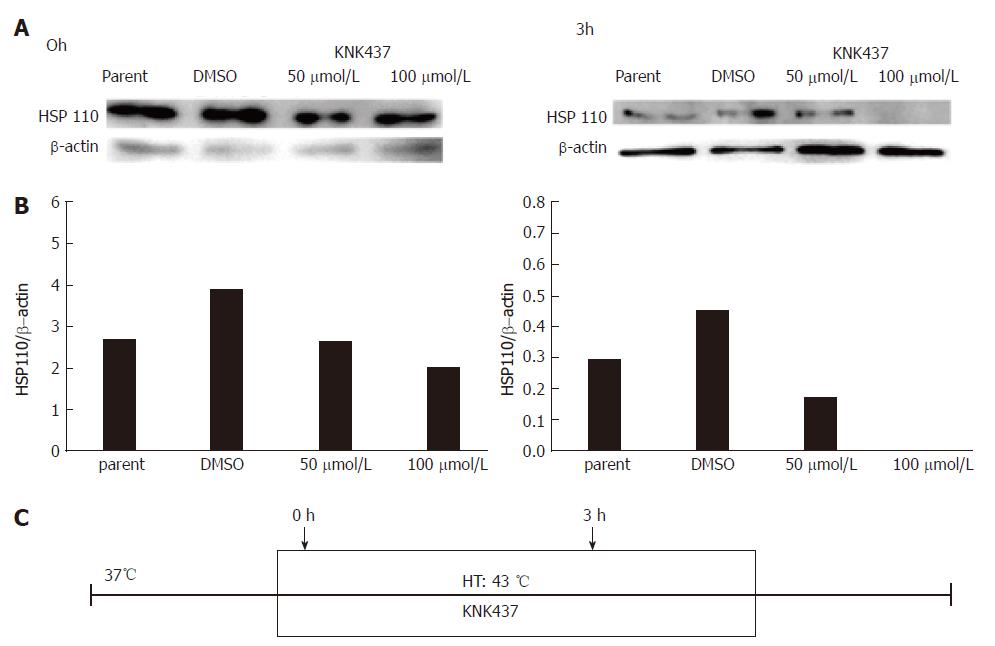
We previously detected HSP110 expression in MKN7, MKN45, and MKN74 human GC cell lines[17]. In this study, we used the MKN45, which is a poorly differentiated GC cell line, for further analysis. HSP110 expression in MKN45 cells was suppressed by KNK437 at the hyperthermic temperature of 43 °C(Figure 2).
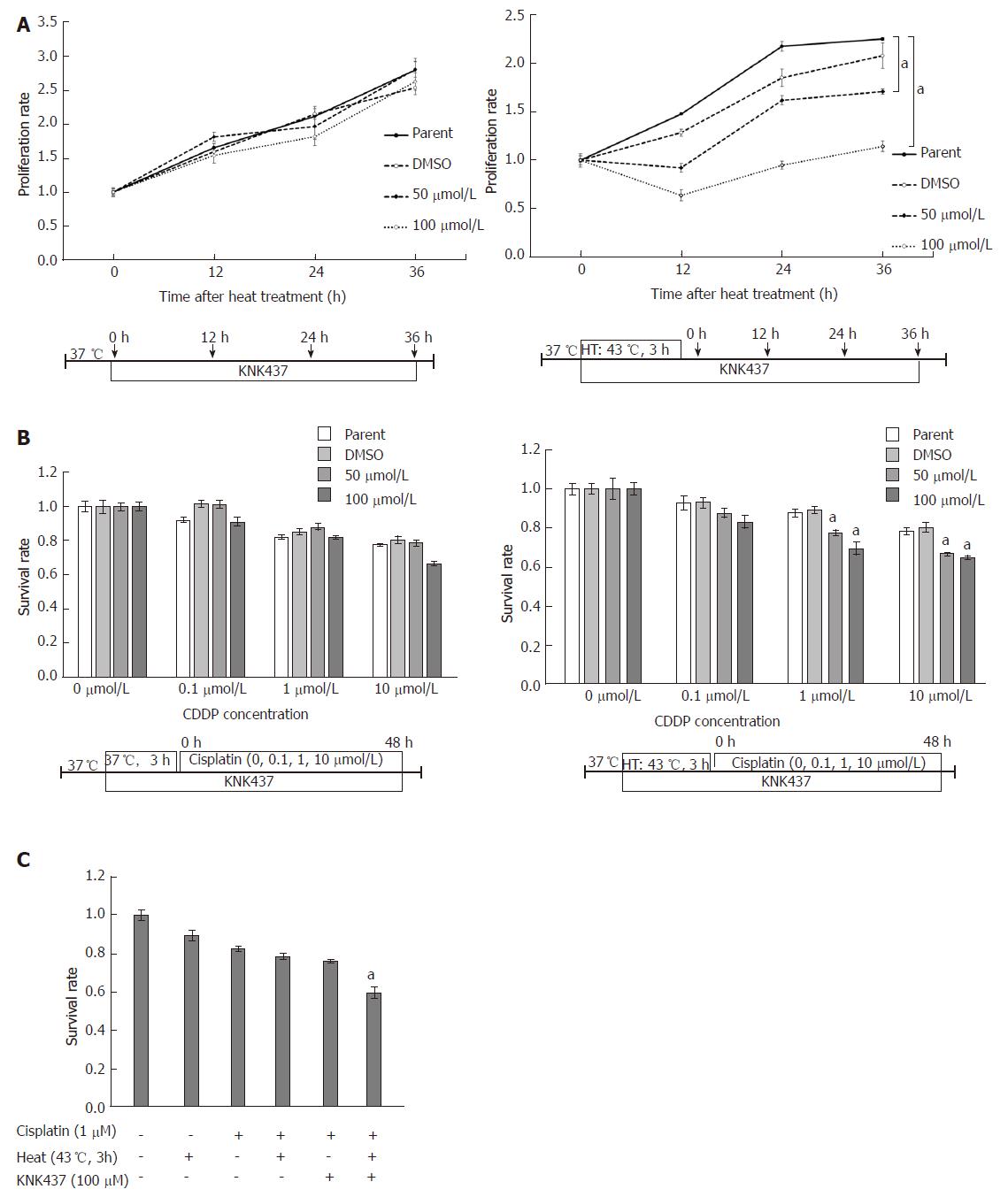
Compared to the untreated cells, the proliferation of MKN45 cells at 43 °C was significantly reduced when HSP110 was inhibited by KNK437 treatment. However, the same analysis performed at 37 °C revealed no significant difference in the proliferation of MKN45 cells grown in the presence or absence of KNK437 (Figure 3A).
At 43 °C, the cisplatin sensitivity of MKN45 cells was significantly higher in the KNK437-mediated HSP110 inhibition group than that in the parental or control cell groups. However, when the MKN45 cells were grown at 37 °C, there were no significant differences in cisplatin sensitivity among cells treated with KNK437 and parental and control cells (Figure 3B). Furthermore, the therapeutic sensitivity of MKN45 cells treated with cisplatin and KNK437 under hyperthermic conditions was higher than that observed with the other therapeutic combinations (Figure 3C).
In this study, we found that high nuclear HSP110 expression in GC patients with peritoneal metastasis undergoing hyperthermo-chemotherapy was associated with poor prognosis and poor progression-free survival. Our studies with MKN45 cells showed that the KNK437-mediated inhibition of HSP110 increased the hyperthermo-chemosensitivity of GC cells in vitro. However, there were no significant relationships between nuclear HSP110 expression and the clinicopathological features of the GC patients. Previously, we reported that nuclear HSP110 expression was associated with venous invasion in 210 GC patients[17]. However, we were unable to identify a significant association between HSP110 expression and venous invasion due to the small number of patients included in this study. It is possible that high nuclear HSP110 expression was also associated with venous invasion in the cases in the present study, which may have influenced prognosis. Additionally, our univariate regression analysis showed that high HSP110 expression was not an independent prognostic factor in GC. These results may also be attributed to the small number of patients in the current study.
In this study, we used the HSP inhibitor KNK437 to suppress HSP110 expression in MKN45 GC cells. However, the mechanism by which KNK437 inhibits HSPs is not fully understood. In COLO 320DM (human colon carcinoma) cells, KNK437 was shown to inhibit the acquisition of thermotolerance and the induction of various HSPs including HSP105, HSP70, and HSP40 in a dose-dependent manner[33]. Another study reported that thermotolerance is suppressed by KNK437 through the inhibition of heat-induced accumulation of HSP27 and HSP72 and the induction of p53-independent apoptosis[34]. Moreover, it has been reported that in SCC VII cells, the inhibition of thermotolerance by KNK437 can improve the efficacy of clinical fractionated hyperthermia[35]. Previous reports have linked certain protein expression with hyperthermo-chemosensitivity. Upregulation of miR-218 has been observed in GC patients after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy, and this was shown to increase chemosensitivity to cisplatin[36]. Consistent with this report, our results show that the alteration of various protein levels can affect hyperthermo-chemosensitivity in MKN45 cells in vitro. Specifically, this study showed that hyperthermo-chemosensitivity to the intraperitoneal infusion of cisplatin was enhanced by HSP110 suppression. Combination therapy with hyperthermo-chemotherapy and HSP110 inhibitors might be a new treatment strategy for GC patients with peritoneal metastasis.
This study has several limitations. First, the sample size was very small because hyperthermochemotherapy is not a common therapy for GC patients with peritoneal metastasis and because only a few institutions perform this therapy. Second, the KNK437 HSP inhibitor used in this study is not specific to HSP110 alone. Hence, further analysis are needed with the specific suppression of HSP110. Third, we assessed only resistance to cisplatin in this study. However, resistance to S-1 might also affect the progression-free survival of GC patients. Finally, we administered cisplatin via intraperitoneal infusion. Recently, the effectiveness of the hydrophobic drug paclitaxel has been demonstrated via intraperitoneal chemotherapy. In the future, we need to validate the results of combination therapy with an HSP110-specific inhibitor and hyperthermo-chemotherapy with paclitaxel.
In conclusion, nuclear HSP110 expression is associated with poor prognosis in GC patients with peritoneal metastasis who are treated via intraperitoneal hyperthermo-chemotherapy. Therefore, the IRS values related to HSP110 expression might be used as effective biomarkers for the prognoses of GC patients with peritoneal metastasis. Furthermore, HSP110 suppression in the MKN45 GC cell line increased their hyperthermo-chemosensitivity against cisplatin. Taken together, our results show that nuclear HSP110 expression in GC patients with peritoneal metastasis might be a clinically useful biomarker of prognosis and a therapeutic target for patients who are tolerant to existing chemotherapies or hyperthermia.
Peritoneal metastasis is the most common reason behind gastric cancer (GC) recurrence. Previously, the authors reported the significance of postoperative intraperitoneal hyperthermo-chemotherapy for GC with peritoneal metastasis. The expression of heat shock protein (HSPs) is induced by exposure to stress, including heat. In cancer, HSPs promote the survival of malignant cells by inhibiting the induction of apoptosis. However, the clinicopathological significance of heat shock protein 110 (HSP110) expression, localization, and association with hyperthermo-chemotherapy resistance in GC has not been fully elucidated. Here, the authors evaluated the significance of HSP110 expression in GC patients with peritoneal metastasis who underwent hyperthermo-chemotherapy.
High levels of HSPs might be correlated with poor prognosis in several types of cancer. Additionally, high levels of various HSP family members have been reported to be associated with increased chemoresistance in several malignancies.
In this study, the authors found that high nuclear HSP110 expression in GC patients with peritoneal metastasis who treated using hyperthermo-chemotherapy was associated with poor prognosis and poor progression-free survival. The KNK437-mediated inhibition of HSP110 increased hyperthermo-chemosensitivity of MKN45 GC cells in vitro. Therefore, nuclear HSP110 expression in GC patients with peritoneal metastasis might be a new marker of chemosensitivity and a therapeutic target in patients who are tolerant to existing hyperthermo-chemotherapies.
This study indicated that nuclear HSP110 expression in GC patients with peritoneal metastasis might be a clinically useful biomarker of prognosis and a therapeutic target for patients who are tolerant to existing chemotherapies or hyperthermia.
HSPs have been characterized as molecular chaperones that prevent the formation of misfolded protein structures. HSPs are induced by exposure to the stress condition, including fever, irradiation and chemicals. HSPs in cancer maintain several oncoproteins homeostasis and promote cancer cell survival by inhibiting apoptosis induction.
Interesting and well written paper describing the role of HSP110 in GC with peritoneal metastasis.
The authors thank Ms. Yukie Saito, Ms. Tomoko Yano, Ms. Tomoko Ubukata, Ms. Yuka Matsui, and Atsuko Sato for their excellent assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Aurello P, Caboclo JLF, Limpakan S, Smith SM S- Editor: Ma YJ L- Editor: A E- Editor: Ma YJ
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 2. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4615] [Cited by in F6Publishing: 4839] [Article Influence: 345.6] [Reference Citation Analysis (1)] |
| 3. | Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014;140:319-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1320] [Cited by in F6Publishing: 1365] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 5. | Tanabe K, Suzuki T, Tokumoto N, Yamamoto H, Yoshida K, Ohdan H. Combination therapy with docetaxel and S-1 as a first-line treatment in patients with advanced or recurrent gastric cancer: a retrospective analysis. World J Surg Oncol. 2010;8:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Yoshida K, Ninomiya M, Takakura N, Hirabayashi N, Takiyama W, Sato Y, Todo S, Terashima M, Gotoh M, Sakamoto J. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res. 2006;12:3402-3407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19:329-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 8. | Braam HJ, Schellens JH, Boot H, van Sandick JW, Knibbe CA, Boerma D, van Ramshorst B. Selection of chemotherapy for hyperthermic intraperitoneal use in gastric cancer. Crit Rev Oncol Hematol. 2015;95:282-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Liu T, Ye YW, Zhu AL, Yang Z, Fu Y, Wei CQ, Liu Q, Zhao CL, Wang GJ, Zhang XF. Hyperthermia combined with 5-fluorouracil promoted apoptosis and enhanced thermotolerance in human gastric cancer cell line SGC-7901. Onco Targets Ther. 2015;8:1265-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Polom K, Marano L, Roviello G, Petrioli R, Piagnerelli R, de Franco L, Marrelli D, Roviello F. Evolution and emerging future of cytoreducxtive surgery and hyperthermic intraperitoneal chemoperfusion in gastric cancer: From treating the incurable to preventing recurrence. Int J Hyperthermia. 2016;32:173-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Rudloff U, Langan RC, Mullinax JE, Beane JD, Steinberg SM, Beresnev T, Webb CC, Walker M, Toomey MA, Schrump D. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol. 2014;110:275-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Wu Z, Ma S, Jing S, Deng Q, Zheng Z, Wu K, Li J, Chen S, Tang R, Li X. Effect of Hyperthermic Intraperitoneal Perfusion Chemotherapy in Combination with Intravenous Chemotherapy as Postoperative Adjuvant Therapy for Advanced Gastric Cancer. Hepatogastroenterology. 2014;61:972-977. [PubMed] [Cited in This Article: ] |
| 13. | Asao T, Sakurai H, Harashima K, Yamaguchi S, Tsutsumi S, Nonaka T, Shioya M, Nakano T, Kuwano H. The synchronization of chemotherapy to circadian rhythms and irradiation in pre-operative chemoradiation therapy with hyperthermia for local advanced rectal cancer. Int J Hyperthermia. 2006;22:399-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kato T, Fujii T, Ide M, Takada T, Sutoh T, Morita H, Yajima R, Yamaguchi S, Tsutsumi S, Asao T. Effect of long interval between hyperthermochemoradiation therapy and surgery for rectal cancer on apoptosis, proliferation and tumor response. Anticancer Res. 2014;34:3141-3146. [PubMed] [Cited in This Article: ] |
| 15. | Mochiki E, Shioya M, Sakurai H, Andoh H, Ohno T, Aihara R, Asao T, Kuwano H. Feasibility study of postoperative intraperitoneal hyperthermochemotherapy by radiofrequency capacitive heating system for advanced gastric cancer with peritoneal seeding. Int J Hyperthermia. 2007;23:493-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Tsutsumi S, Tabe Y, Fujii T, Yamaguchi S, Suto T, Yajima R, Morita H, Kato T, Shioya M, Saito J. Tumor response and negative distal resection margins of rectal cancer after hyperthermochemoradiation therapy. Anticancer Res. 2011;31:3963-3967. [PubMed] [Cited in This Article: ] |
| 17. | Kimura A, Ogata K, Altan B, Yokobori T, Ide M, Mochiki E, Toyomasu Y, Kogure N, Yanoma T, Suzuki M. Nuclear heat shock protein 110 expression is associated with poor prognosis and chemotherapy resistance in gastric cancer. Oncotarget. 2016;7:18415-18423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3762] [Cited by in F6Publishing: 3571] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 19. | Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 866] [Cited by in F6Publishing: 827] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 20. | Rappa F, Farina F, Zummo G, David S, Campanella C, Carini F, Tomasello G, Damiani P, Cappello F, DE Macario EC. HSP-molecular chaperones in cancer biogenesis and tumor therapy: an overview. Anticancer Res. 2012;32:5139-5150. [PubMed] [Cited in This Article: ] |
| 21. | Langdon SP, Rabiasz GJ, Hirst GL, King RJ, Hawkins RA, Smyth JF, Miller WR. Expression of the heat shock protein HSP27 in human ovarian cancer. Clin Cancer Res. 1995;1:1603-1609. [PubMed] [Cited in This Article: ] |
| 22. | Nanbu K, Konishi I, Komatsu T, Mandai M, Yamamoto S, Kuroda H, Koshiyama M, Mori T. Expression of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Correlation with clinicopathology, sex steroid receptor status, and p53 protein expression. Cancer. 1996;77:330-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 23. | Lazaris ACh, Chatzigianni EB, Panoussopoulos D, Tzimas GN, Davaris PS, Golematis BCh. Proliferating cell nuclear antigen and heat shock protein 70 immunolocalization in invasive ductal breast cancer not otherwise specified. Breast Cancer Res Treat. 1997;43:43-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Cappello F, Rappa F, David S, Anzalone R, Zummo G. Immunohistochemical evaluation of PCNA, p53, HSP60, HSP10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Res. 2003;23:1325-1331. [PubMed] [Cited in This Article: ] |
| 25. | Cappello F, David S, Rappa F, Bucchieri F, Marasà L, Bartolotta TE, Farina F, Zummo G. The expression of HSP60 and HSP10 in large bowel carcinomas with lymph node metastase. BMC Cancer. 2005;5:139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, Kluger HM. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007;67:2932-2937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | He LF, Guan KP, Yan Z, Ye HY, Xu KX, Ren L, Hou SK. Enhanced sensitivity to mitomycin C by abating heat shock protein 70 expression in human bladder cancer cell line of BIU-87. Chin Med J (Engl). 2005;118:1965-1972. [PubMed] [Cited in This Article: ] |
| 28. | Fang X, Jiang Y, Feng L, Chen H, Zhen C, Ding M, Wang X. Blockade of PI3K/AKT pathway enhances sensitivity of Raji cells to chemotherapy through down-regulation of HSP70. Cancer Cell Int. 2013;13:48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Lai CH, Park KS, Lee DH, Alberobello AT, Raffeld M, Pierobon M, Pin E, Petricoin Iii EF, Wang Y, Giaccone G. HSP-90 inhibitor ganetespib is synergistic with doxorubicin in small cell lung cancer. Oncogene. 2014;33:4867-4876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 31. | Altan B, Yokobori T, Mochiki E, Ohno T, Ogata K, Ogawa A, Yanai M, Kobayashi T, Luvsandagva B, Asao T. Nuclear karyopherin-α2 expression in primary lesions and metastatic lymph nodes was associated with poor prognosis and progression in gastric cancer. Carcinogenesis. 2013;34:2314-2321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Watanabe A, Suzuki H, Yokobori T, Tsukagoshi M, Altan B, Kubo N, Suzuki S, Araki K, Wada S, Kashiwabara K. Stathmin1 regulates p27 expression, proliferation and drug resistance, resulting in poor clinical prognosis in cholangiocarcinoma. Cancer Sci. 2014;105:690-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Yokota S, Kitahara M, Nagata K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res. 2000;60:2942-2948. [PubMed] [Cited in This Article: ] |
| 34. | Ohnishi K, Takahashi A, Yokota S, Ohnishi T. Effects of a heat shock protein inhibitor KNK437 on heat sensitivity and heat tolerance in human squamous cell carcinoma cell lines differing in p53 status. Int J Radiat Biol. 2004;80:607-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Koishi M, Yokota S, Mae T, Nishimura Y, Kanamori S, Horii N, Shibuya K, Sasai K, Hiraoka M. The effects of KNK437, a novel inhibitor of heat shock protein synthesis, on the acquisition of thermotolerance in a murine transplantable tumor in vivo. Clin Cancer Res. 2001;7:215-219. [PubMed] [Cited in This Article: ] |
| 36. | Zhang XL, Shi HJ, Wang JP, Tang HS, Wu YB, Fang ZY, Cui SZ, Wang LT. MicroRNA-218 is upregulated in gastric cancer after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and increases chemosensitivity to cisplatin. World J Gastroenterol. 2014;20:11347-11355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 37] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |