Published online Oct 14, 2017. doi: 10.3748/wjg.v23.i38.6931
Peer-review started: February 11, 2017
First decision: April 25, 2017
Revised: May 30, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: October 14, 2017
Gastrointestinal ultrasound is a practical, safe, cheap and reproducible diagnostic tool in inflammatory bowel disease gaining global prominence amongst clinicians. Understanding the embryological processes of the intestinal tract assists in the interpretation of abnormal sonographic findings. In general terms, the examination principally comprises interrogation of the colon, mesentery and small intestine using both low-frequency and high-frequency probes. Interpretation of findings on GIUS includes assessment of bowel wall thickness, symmetry of this thickness, evidence of transmural changes, assessment of vascularity using Doppler imaging and assessment of other specific features including lymph nodes, mesentery and luminal motility. In addition to B-mode imaging, transperineal ultrasonography, elastography and contrast-enhanced ultrasonography are useful adjuncts. This supplement expands upon these features in more depth.
Core tip: In general terms, gastrointestinal ultrasound examination principally comprises interrogation of the colon, mesentery and small intestine using both low-frequency and high-frequency probes. In addition to B-mode imaging, transperineal ultrasonography, elastography and contrast-enhanced ultrasonography are useful adjuncts.
- Citation: Atkinson NSS, Bryant RV, Dong Y, Maaser C, Kucharzik T, Maconi G, Asthana AK, Blaivas M, Goudie A, Gilja OH, Nuernberg D, Schreiber-Dietrich D, Dietrich CF. How to perform gastrointestinal ultrasound: Anatomy and normal findings. World J Gastroenterol 2017; 23(38): 6931-6941
- URL: https://www.wjgnet.com/1007-9327/full/v23/i38/6931.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i38.6931
Gastrointestinal ultrasound (GIUS) is an accurate diagnostic imaging tool for inflammatory bowel disease[1-3]. Utilisation has steadily increased in different global regions including Asia-Pacific[4]. To correctly interpret GIUS findings, it is necessary to have a firm grounding in intestinal anatomy, the fundamentals of ultrasonography, as well as the examination techniques and approach. The indications for intestinal ultrasound are wide-ranging including inflammatory bowel disease, assessment of functional aspects and general gastroenterological conditions such as diverticular disease. Various educational theories are relevant in the process of learning intestinal ultrasonography including the learning process itself, using formative assessments such as DOPS (direct observation of procedural skills), adopting the apprenticeship or core competencies model and formulation of a GIUS curriculum. These principles and a process of learning GIUS have been recently proposed[1].
The purpose of this paper is to review our knowledge of intestinal embryology relevant to GIUS. Understanding the origins and evolution of abdominal structures during the embryological process can assist in highlighting the reasoning behind abnormalities found on GIUS. We then expand upon examination techniques relevant to different segments of and structures around the intestine, including an overview of transperineal ultrasonography. We describe specific intestinal luminal parameters to be assessed in GIUS including special techniques such as contrast-enhanced ultrasound.
In the 6th and 8th week of intrauterine life, the primitive mid-gut intestinal tube elongates on the mesentery around the superior mesenteric artery (SMA), herniating into the umbilical cord. As the gut grows and returns into the peritoneal cavity, it eventually rotates 270 degrees counter-clockwise, such that the duodenum rests behind the SMA[1]. The caecum, initially in the upper abdomen, descends to the right lower quadrant. Thus the mesentery attachment of the small bowel takes an oblique course from the duodeno-jejunal junction at the level of the left L2 process, over the 3rd part of the duodenum, down to the level of the right sacroiliac joint[5]. Though the mesentery attachment is only 15-20 cm long, it supplies a length of small intestine approximately 40 times its length, a feat achieved through progressive fan-like ruffles. Seen with traditional barium enterography, each curve of the intestine has a concave and convex aspect, the concave generally pointing towards the mesentery whilst the convex aspect representing the anti-mesenteric border[6]. These can be viewed in real time with GIUS.
Towards the end of the first trimester, the peritoneum of the newly forming ascending colon and the hind-gut derived left colonic segments, begin to fuse with the posterior abdominal wall. Although traditionally described as retroperitoneal structures, modern post-mortem studies have found that two thirds of the ascending and a third of the descending colonic segments have mobile portions of elongated mesentery[7]. Nonetheless, peritoneal attachments have significance for the flow of free fluid within the abdomen as fluid tends to flow caudally, medial to the ascending colon towards the ileocaecal junction and thus metastatic deposits may become lodged in the mesenteric ruffles en route.
Relative thickenings of the mesocolon provide ligamental support to the colonic flexures; the nephrocolic ligament runs from the inferior aspect of the right kidney to the hepatic flexure which then becomes intimately related to the descending duodenum before the transverse mesocolon begins; the splenic flexure is suspended by (1) the phrenicocolic ligament which runs from the diaphragm and also supports the spleen; (2) the splenocolic; and (3) the pancreaticocolic ligaments which are essentially extensions of the transverse mesocolon[5,7]. These attachments provide fixed points for ultrasound evaluation of the colon, which can at times, be highly mobile within the abdomen.
The taenia coli, thickenings of the longitudinal muscle layer grow from diffuse sheets at the caudal end of the bowel, become more defined in the proximal colon until they encase the caecum[6]. The taeniae are one sixth shorter than the colon[5], forming the haustrae. The muscle fibres in the longitudinal layer end by turning at right angles to merge with the circular fibres and thereby acting as fixed linkage points for contraction. Contractions can occur asymmetrically obliterating some haustrae, giving the false impression of small-bowel-like semi-circular folds and bowel wall thickening.
Vascular supply of the colon flows from the mesenteric border, vessels spreading around the colon. The vasa recta penetrate through oblique connective tissue clefts in the bowel wall, the site of diverticular protrusion, but importantly enter these clefts on the anti-mesenteric aspect. In practice, diverticula are rare on the anti-mesenteric border between the taenia omonetalis and taenia libera. A vasa recta vessel runs over the long aspect of each diverticulum before entering the submucosa at the antimesenteric border[6].
An optimal environment for United States is within a dedicated space or consulting room, offering indirect low light sources and facilitating patient comfort. Prior cross-sectional imaging and endoscopy reports should be available to inform of post-surgical and anatomical variants.
In most scenarios patient preparation is not required but specific measures can be used. Fasting for 4-6 h decreases bowel motility whilst two cups of water can be used to improve visualisation of the duodenum[8]. Negative oral contrast may improve detection of jejunal and proximal ileal stenosis, particularly where examination findings are negative; 250-800 mL of polyethylene glycol solution generally reaches the terminal ileum after an average duration of 30 min[9]. Once contrast is seen to flow into the caecum, retrograde examination of the small bowel can be performed.
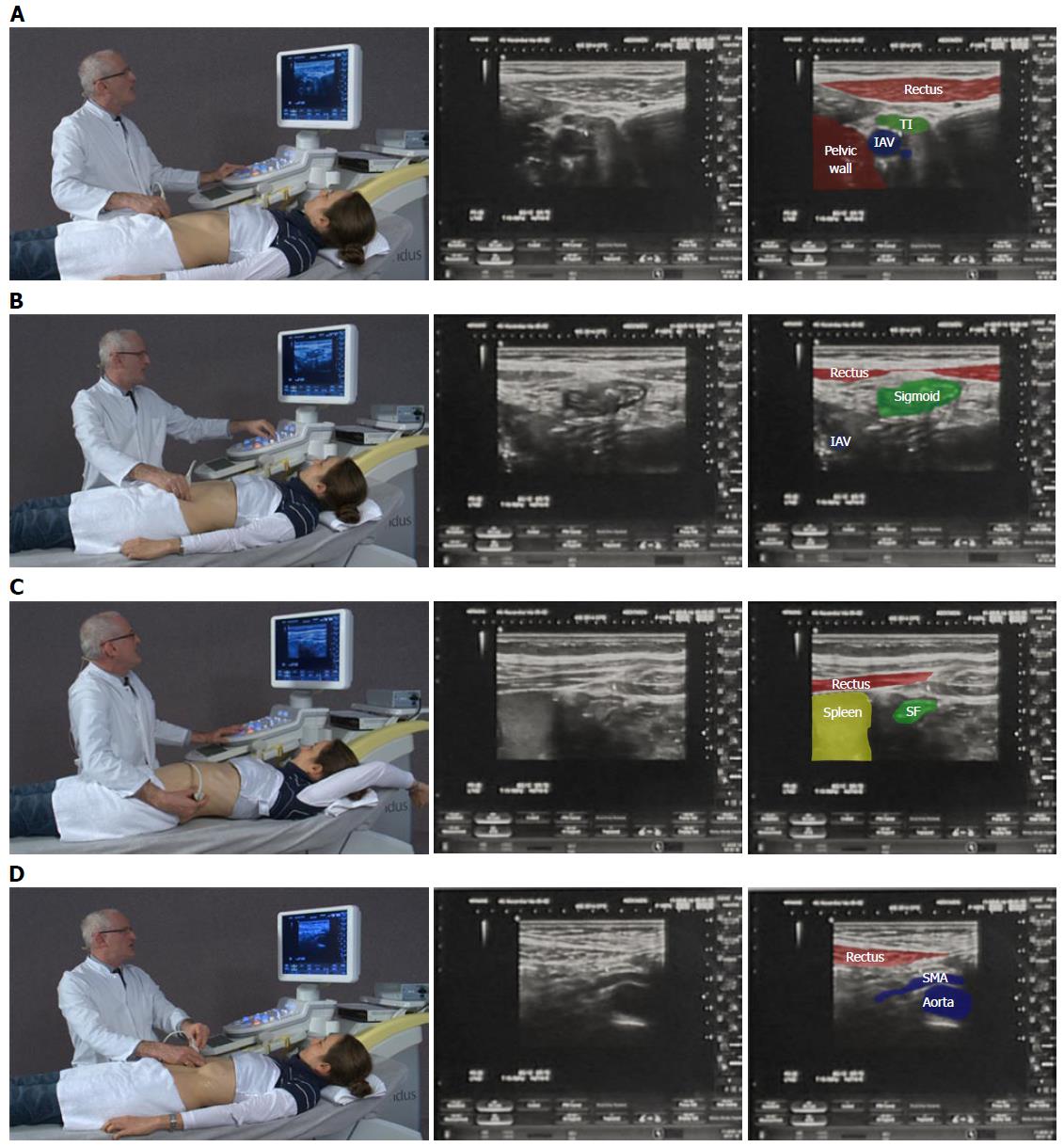
Examination of the intestinal tract begins with a comfortable patient, relaxed in a supine position so as not to tense the abdominal wall. The transducer is held maintaining contact with the patient’s skin to gauge pressure, whilst the left hand is free to optimize image characteristics on the machine. A systematic approach in examining the whole intestine is encouraged (Figure 1). Firstly, the low frequency 3-8 MHz (multifrequency) curvilinear probe initially allows orientation to the anatomy and detection of gross changes, whilst high frequency linear probes (7-17 MHz) are preferable for interrogating specific regions of interest in depth.
Beginning at the right anterior superior iliac crest and moving medially to the edge of the rectus muscles in a sagittal plane, the common iliac (iliacal) vessels are identified. Rotating anticlockwise to a transverse plane and moving cranially, the first bowel loop crossing from medial to lateral is identified as the terminal ileum. This is followed to the ileocaecal (Bauhin’s) valve and caecum. The base of the appendix can be identified at the deep margin of the caecum where the colonic taenia meet before the ascending colon is followed up towards the hepatic flexure. The rest of the colon can be followed via the transverse segment distally towards the rectum. Alternatively, the same technique can be used on the left side identifying the sigmoid colon as the first loop of bowel crossing the left iliac vessels, which can be followed to the descending colon and towards the spleen as far as the rib margin allows. The iliopsoas muscle can be used as an alternative landmark for identification of the terminal ileum and sigmoid colonic segments in the right and left iliac fossae respectively. Intercostal imaging may be required to visualise the left or splenic flexure where it has attachment to the spleen; elevating the left arm and rotating to a partial right decubitus position with a straight left leg can spread the ribs and improve image acquisition (Figure 1C). Placing the probe in the epigastric region in sagittal orientation demonstrates the liver and stomach; one can then follow the gastrocolic ligament to the transverse colon. Although the transverse colon can be followed on ultrasound, it may not be reliably viewed in its entirety. Be aware that the rectum and distal parts of the colon cannot always be displayed satisfactorily by transabdominal United States. Transperineal imaging, in such cases, can be useful to evaluate the distal rectum and perianal tissues.
Mesenteric fat is evident sonographically and is considered to be abnormal if it extends over more than half the circumference of the bowel loop, if it is thickened beyond 5-6 mm or consistently greater than the normal bowel wall thickness[10].
Examination of the mesentery begins in the epigastrium at the duodenojejunal flexure which then runs obliquely towards the right iliac fossa. To aid visualization, the patient is asked to breath in deeply and as they exhale, pressure is applied to the transducer following which excellent views of the small bowel mesentery sheets and abnormalities can be achieved. A systematic scanning of the small bowel may start in the right iliac fossa by defining the terminal ileum and following its course in a proximal direction as far as possible. Finally, a systematic overlapping “Lawn Mowing” strategy is used, sweeping up and down the abdomen to provide an overview of the small bowel. This is performed with the probe in horizontal, sagittal and oblique (parallel to the mesenteric attachment) orientations in order to allow one’s eye to follow structures and detect abnormalities. A full video explanation of abdominal and intestinal ultrasound examination technique is freely available on the EFSUMB website[11] (http://www.efsumb.org/education/cfd-videos001.asp).
TPUS allows visualisation of the perianal tissues, anal canal, sphincters, the distal 5-7 cm section of the rectum, vagina and a part of urinary bladder. A point for orientation is the symphysis. Knowledge of the pelvic anatomy is essential[12,13] (EFSUMB Case of the Month). No specific patient preparation is required. The patient is placed in the left lateral position as for a digital rectal examination. The probe is covered in either a sterile cover or an examination glove with ultrasound gel between the layers.
Examination begins in the midline just above the anus with the probe in a sagittal plane. The ultrasound probe can be moved laterally, however angulated views have reduced sensitivity for identification of pathology. Fistulous tracts can be followed by first placing the probe over the external opening. If necessary, the probe can be placed in a coronal angle, although this is usually less comfortable for the patient. It is useful to start with an abdominal convex probe (lower frequency) for the deeper structures and then continue with a higher frequency probe (7-15 MHz). Examination is also possible after rectal amputation (e.g., Quénu-Operation).
The anal canal, sphincter complexes, hemorrhoidal plexus (Figure 2), recto-vaginal plus ano-vaginal septums, the walls of the vagina and distal rectum can be defined. Fistulae should be classified as per Parks’ classification[14] although TPUS has reduced sensitivities for sphincteric relationships and therefore the American Gastroenterological Association (AGA)[15] distinction of “simple” from “complex” is of more clinical utility; the former including low fistulae (superficial, intersphincteric or intrasphincteric) below the dentate line, with a single external opening and without perianal complications or active proctitis[16]. Fistula and abscesses visibility can be improved with ultrasound contrast agents (UCA) using contrast enhanced ultrasound techniques[17,18]. Colour Doppler improves the differentiation of inflammatory reactions.
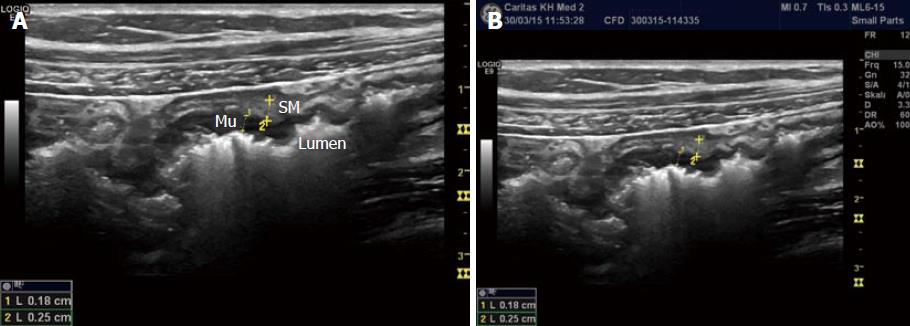
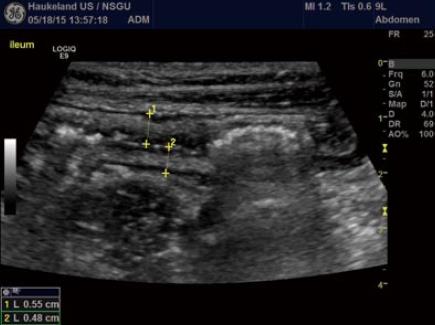
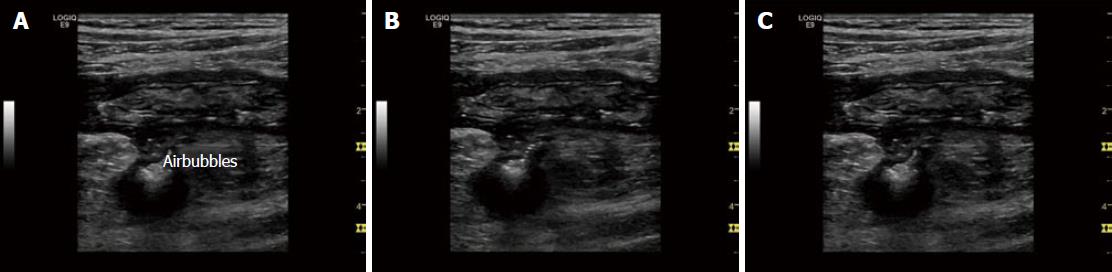
Bowel wall thickness (BWT) is the measure most consistently reported in diagnostic and activity trials. Wall thickness of the alimentary tract differs by region and depends on the degree of distension and contraction[19,20]. The overall thickness should be measured under mild compression from just above an air-mucosal interface to the outside of the outer muscularis propria layer border, including the whole bowel wall[21]. Under these standardised conditions, the stomach wall thickness measures 3-6 mm; terminal ileum 1-3 mm; and colon 0.5-2 mm. In fact, the normal range is likely to be even lower than this[22]. Values in children can be reliably obtained without the need for sedation[22] but bear in mind that values do increase over childhood[23] whilst still remaining < 2 mm. The optimal threshold for abnormal thickness is debatable, as specificity improves with increasing wall thickness at the cost of sensitivity (Figures 3 and 4).
The GI wall has five layers that usually can be visualized with ultrasound. The sonographic layers are a combination of interface echoes and the echo characteristics of the histological layers[24-26]. When imaged in the anterior wall of a bowel loop starting from the lumen the hyperechoic layer 1 corresponds to the interface between the mucosa and the lumen and is not a part of the actual GI wall. The hypoechoic layer 2 corresponds to the mucosa without the interface between the submucosa and mucosa, the hyperechoic layer 3 to the submucosa including this interface echo, the hypoechoic layer 4 to most of the proper muscle and layer 5 to the hyperechoic interface echo between the proper muscle and the serosa.
Interface echoes are always hyperechoic and located distally to the actual tissue interface. Therefore, the correspondence between histology and sonographic layers differ slightly in the dorsal wall. Specifically, the interface between lumen and mucosa (layer 1) is a part of the actual mucosa and layer 2 represents the rest of the mucosa without muscularis mucosae, which normally is covered by an interface echo and add thickness to layer 3. Moreover, the interface between submucosa and the proper muscle adds thickness to layer 3 and reduces the thickness of layer 4. Finally, the interface between the proper muscle and serosa (layer 5) extends beyond the actual serosa[27,28].
The interface from the serosa is hard to delineate. Accordingly, the measurement should be made from the start of the hypoechoic layer of the proper muscle to the end of the hypoechoic layer of the mucosa. Transducer-compression of the bowel wall will reduce thickness and can make it challenging to distinguish wall layers[29,30]. However, some operators practice mild compression suggesting that this improves reproducibility of measurements[21,22,31-33]. The examiner should also be aware of interpretation difficulties due to mucosal folds and haustrations and to keep the probe angle perpendicular to the bowel wall to avoid tangential measurements. In conclusion, dosed compression is a prerequisite for a reproducible examination for some authors whereas others use it with caution.
The layered wall structure changes with disease[34]. In severe disease the stratification may disappear due to deep mural ulcers, increasing inflammatory infiltrate and neovascularisation. In chronic inactive disease, accentuated wall layers are more common. The distinctions are less apparent with milder disease phenotypes.
The symmetrical nature of changes is relevant. Asymmetry has been assumed to correspond to endoscopic signs of focal ulceration or polypoid mucosal changes, whilst diffuse thickening is evident with ulcerative colitis (UC) or infectious colitis. The differential diagnoses for chronic inflammatory bowel findings, with or without asymmetry are listed in Tables 1 and 2.
| Crohn’s disease |
| Actinomycoses |
| Mycobacteria tuberculosis |
| Lymphoma |
| Neoplasia |
| NSAID enteropathy |
| Inflammatory bowel disease |
| Mycobacterium tuberculosis |
| Sarcoidosis |
| Diverticulitis |
| Neoplasia |
| Lymphoma |
| Ischemia |
The small and large bowel can usually be distinguished by scanning the haustrae of the colon and/or the circular folds of Kerckring in the small intestine. In unclear cases, scanning of the intestine during various stages of filling may be helpful. Changes in Kerckring’s folds and luminal fluid quantity can be associated with disease[35]. The small bowel diameter varies widely depending on recent meals and activity, but dilatation beyond 25 mm should be regarded as abnormal, particularly when motility is reduced[36]. Assessment of peristaltic activity and lumen compressibility are two advantages of ultrasound over other imaging modalities. Strictures may be identified by the co-existence of thickened and stiffened bowel wall with narrowing of the intestinal lumen, particularly if less than 10 mm[9]. The presence of proximal loop dilatation with fluid or echogenic content is not required for the diagnosis[36] but may carry clinical significance.
Dilatation of the proximal small bowel loops with hypo- or hyperperistalsis can be caused not only by chronic fibrotic strictures but also by acute inflammatory stenosis or passenger invagination. Functional ultrasonography is helpful in differentiation[37-40].
Evaluating the length and extent of involved bowel segments is performed by estimated longitudinal measurement and taking note of skip lesions. Bowel wall thickening, luminal diameter for stenosis or dilatation > 25 mm, and motility should be noted in each region. Chronic inflammation tends to produce an isolated, fixed segment without peristalsis and abnormal angulation due to the fibrofatty proliferation of the mesentery[41,42].
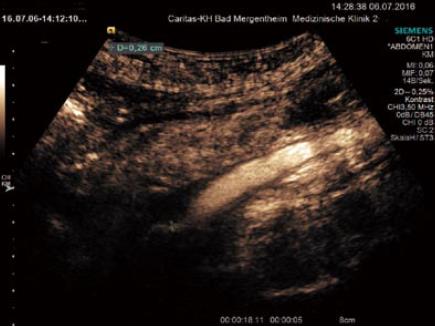
Transmural inflammation and fibrosis may result in an asymmetrically thickened, stratified bowel wall, which are the typical findings of Crohn’s disease (CD). A hypoechoic extension through the normal bowel wall stratification correlates with cellular and oedematous tissue infiltration. Even though the extent of the fibro-fatty proliferate correlates with the degree of intestinal inflammation in CD, there is no standardized method to date to quantify the mesenteric fat by using GIUS. Note should be made if the reaction extends beyond the muscularis propria layer, and whether it does so into the mesenteric or anti-mesenteric border. Fistulae are identified as hypoechoic tracts extending through the bowel wall, often with reverberations (circumscribed bright air echoes) within them (Figure 5). Rounded hypoechoic areas (non-contrast imaging) within the mesentry are suspicious for abscesses or inflammatory phlegmons often with an irregular wall or internal echoes (Figure 6). The occurrence of free peritoneal fluid is important to note, though clinical data corroborating its significance are scant[21].
Two prospective studies suggest a sensitivity and specificity for the GIUS detection of fistulae of 72%-87% and 90%-96%, respectively[43]. This performance is equivalent to CT/MR studies in meta-analysis[44], whilst small intestine contrast ultrasound (SICUS) may have a sensitivity as high as 96%. Estimates of the sensitivity and specificity for detecting abscesses have been reported in a somewhat higher range; 71%-100% and 77%-94% respectively[21,45-49]. The direct application of contrast agents into the orifice of the fistula may be helpful in determining the route and connection(s)[12,13,50].
Lymph node enlargement is a frequent sonographic finding in CD[10], however their interpretation and clinical implications remain to be further clarified in the literature. It has been suggested that they may represent a very early manifestation of CD in children for example[22]. They are correlated with duration of disease and the presence of fistulae but more importantly, for the ultrasound learner, they provide a marker of procedural competence and interpretation. B mode characteristics of lymph nodes to consider include their length and particularly for those < 15 mm; their short axis dimension should be less than half their longitudinal diameter. Furthermore, the normal lymph node architecture and hilum should be preserved in normal or inflammatory nodes[51].
The supporting structures of the intestine run within the sheets of mesentery, seen as layers of mixed echogenicity with hyperechoic serosal layers on either side, which does not have peristaltic movement and appears similar in both transverse and sagittal planes. Fat wrapping has long been recognised by surgeons as a common and specific feature of CD. So-called (creeping) fat, extending from the mesenteric attachment to partially cover the small or large intestine resulting in loss of bowel mesentery angle, is seen as an early event in the disease course and plausibly plays a role in the inflammatory milieu[52]. In practical terms the serosal planes on either side of the mesentery may be detected and should cover less than half of the bowel circumference. It is also the most common cause of bowel loop separation[41]. A subjective impression of increased thickening and echogenicity has been applied in the literature[10], correlating with clinical severity and primary luminal findings; although in long standing disease it can become more heterogeneous and hypoechoic[41].
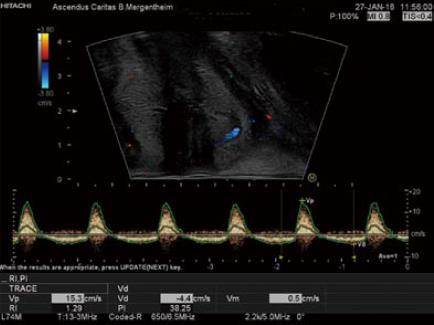
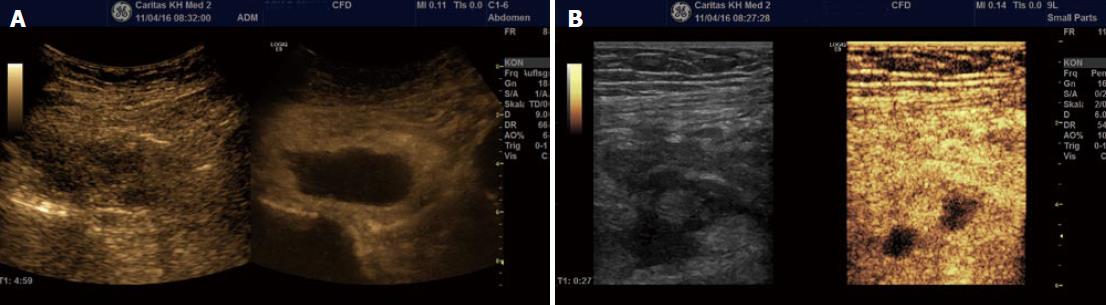
Colour Doppler imaging of the bowel wall is part of standard assessment of the intestine and mesenterial vessels (Figure 7). Hyperaemia is associated with inflammation, usually seen in the submucosal layer and the penetrating vessels of the muscularis propria. Use of Doppler evaluation increases the sensitivity of US for evaluating disease activity[33,39,53-59]. The degree of vascularity can be graded by the Limberg scale, a semi-quantitative assessment[60] that lacks routine practical relevance. Other more complex quantitative measurements of Doppler parameters have been proposed, however a standardised protocol to compensate for confounders has not yet become widely used. Power Doppler assessment of the arterial inflow, in particular the inferior mesenteric artery (IMA) for left sided colonic disease and SMA for proximal colon and small bowel activity, can be assessed in the majority of patients and correlates with other ultrasound markers of disease activity. It should therefore be interpreted in the overall context of ultrasound findings[61]. A prognostic role for Doppler parameters was previously proposed[62] but awaits further study and validation.
Second generation contrast agents such as SonoVue®, produce harmonic frequencies from micro-bubbles approximately the size of a single red blood cell, and are stable within the circulation[17,63]. Imaging systems thereafter allow visualisation of individual blood vessels through a tissue and thereby improve the accuracy of Doppler US in evaluating bowel wall vascularity. This technique has been shown to be useful in the assessment of disease activity in CD, in particular differentiating inflammatory masses from abscesses and may help to distinguish inflammatory from fibrotic strictures in certain situations[64-66]. Use of Contrast-Enhanced US (CEUS) during GIUS has been standardized, does not requires specific expertise, and its use in IBD presently is increasing[67].
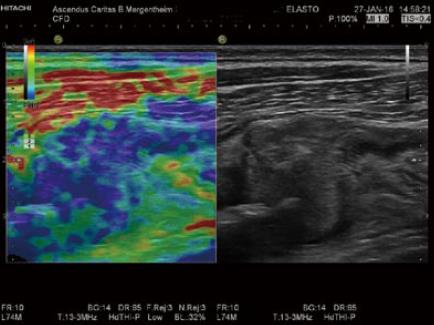
Similar to palpation, the elastic properties of a tissue can be evaluated by assessing the speed of a sheer wave through tissue or the amount of deformity created by the sheer stress (strain imaging). Various sonographic approaches to generating and measuring these parameters are available[68], which may compliment standard B-mode assessment of a lesion. Fibrotic lesions may appear stiff and inflammatory lesions soft using elastography, which can help to characterise intestinal lesions and has been correlated with endoscopic findings (Figure 8)[69,70].
Understanding the anatomy and embryology of the intestinal tract is highly relevant in identifying sonographic abnormalities relevant to GIUS. The general principles of examination involve specific interrogation of the colon, small intestine and mesentery. Further work is required to validate and understand the significance of certain sonographic parameters where understanding is limited; this includes further evaluation of abnormal wall thicknesses and quantifying this to a higher degree of accuracy, understanding the significance of peritoneal fluid present as a reflection of transmural reactions, and the accurate interpretation and implications of lymph nodes. Despite the presence of semi-quantitative measures, such as the Limberg score, they lack practical relevance and so there is a need for further multi-centre prospective studies.
Various sonographic abnormalities can be detected and interpreted currently but a standardized scoring system for GIUS in inflammatory bowel disease, akin to validated endoscopic scores (such as the Ulcerative Colitis Endoscopic Index of Severity) is lacking. Ultimately, formulating a reproducible and validated scoring system integrating different sonographic parameters to reflect severity will be highly relevant; this will require agreement amongst GIUS experts and validation in multi-centre prospective studies. Finally, a standardized method of documentation, including how to capture images, needs to be developed.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Capasso R S- Editor: Ma YJ L- Editor: A
E- Editor: Li D
| 1. | Atkinson NS, Bryant RV, Dong Y, Maaser C, Kucharzik T, Maconi G, Asthana AK, Blaivas M, Goudie A, Gilja OH. WFUMB Position Paper. Learning Gastrointestinal Ultrasound: Theory and Practice. Ultrasound Med Biol. 2016;42:2732-2742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Nylund K, Maconi G, Hollerweger A, Ripolles T, Pallotta N, Higginson A, Serra C, Dietrich CF, Sporea I, Saftoiu A. EFSUMB Recommendations and Guidelines for Gastrointestinal Ultrasound. Ultraschall Med. 2017;38:e1-e15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Nylund K, Maconi G, Hollerweger A, Ripolles T, Pallotta N, Higginson A, Serra C, Dietrich CF, Sporea I, Saftoiu A. EFSUMB Recommendations and Guidelines for Gastrointestinal Ultrasound. Ultraschall Med. 2017;38:273-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Asthana AK, Friedman AB, Maconi G, Maaser C, Kucharzik T, Watanabe M, Gibson PR. Failure of gastroenterologists to apply intestinal ultrasound in inflammatory bowel disease in the Asia-Pacific: a need for action. J Gastroenterol Hepatol. 2015;30:446-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Jorge JMN, Habr Gama A. Anatomy and Embryology of the Colon, Rectum, and Anus. The ASCRS Textbook of Colon and Rectal Surgery. New York: Springer 2007; 1-22. [Cited in This Article: ] |
| 6. | Meyers MA. Dynamic Radiology of the Abdomen. New York: Springer 2005; . [Cited in This Article: ] |
| 7. | Phillips M, Patel A, Meredith P, Will O, Brassett C. Segmental colonic length and mobility. Ann R Coll Surg Engl. 2015;97:439-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Taylor S, Mallett S, Bhatnagar G, Bloom S, Gupta A, Halligan S, Hamlin J, Hart A, Higginson A, Jacobs I. METRIC (MREnterography or ulTRasound in Crohn‘s disease): a study protocol for a multicentre, non-randomised, single-arm, prospective comparison study of magnetic resonance enterography and small bowel ultrasound compared to a reference standard in those aged 16 and over. BMC Gastroenterol. 2014;14:142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Parente F, Greco S, Molteni M, Anderloni A, Sampietro GM, Danelli PG, Bianco R, Gallus S, Bianchi Porro G. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn‘s disease. A prospective comparison with conventional ultrasound, x ray studies, and ileocolonoscopy. Gut. 2004;53:1652-1657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Maconi G, Greco S, Duca P, Ardizzone S, Massari A, Cassinotti A, Radice E, Porro GB. Prevalence and clinical significance of sonographic evidence of mesenteric fat alterations in Crohn‘s disease. Inflamm Bowel Dis. 2008;14:1555-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Dietrich CF. EFSUMB Course Book on Ultrasound. Examination technique (videos). London: European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) 2016; . [Cited in This Article: ] |
| 12. | Dietrich CF, Barreiros AP, Nuernberg D, Schreiber-Dietrich DG, Ignee A. [Perianal ultrasound]. Z Gastroenterol. 2008;46:625-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Maconi G, Tonolini M, Monteleone M, Bezzio C, Furfaro F, Villa C, Campari A, DellʼEra A, Sampietro G, Ardizzone S. Transperineal perineal ultrasound versus magnetic resonance imaging in the assessment of perianal Crohn‘s disease. Inflamm Bowel Dis. 2013;19:2737-2743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1-12. [PubMed] [Cited in This Article: ] |
| 15. | Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB. AGA technical review on perianal Crohn‘s disease. Gastroenterology. 2003;125:1508-1530. [PubMed] [Cited in This Article: ] |
| 16. | Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB, American Gastroenterological Association Clinical Practice Committee. AGA technical review on perianal Crohn‘s disease. Gastroenterology. 2003;125:1508-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 374] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 17. | Ignee A, Jenssen C, Cui XW, Schuessler G, Dietrich CF. Intracavitary contrast-enhanced ultrasound in abscess drainage--feasibility and clinical value. Scand J Gastroenterol. 2016;51:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Ignee A, Cui X, Schuessler G, Dietrich CF. Percutaneous transhepatic cholangiography and drainage using extravascular contrast enhanced ultrasound. Z Gastroenterol. 2015;53:385-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Nylund K, Hausken T, Odegaard S, Eide GE, Gilja OH. Gastrointestinal wall thickness measured with transabdominal ultrasonography and its relationship to demographic factors in healthy subjects. Ultraschall Med. 2012;33:E225-E232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Cantisani V, Dietrich CF, Badea R, Dudea S, Prosch H, Cerezo E, Nuernberg D, Serra AL, Sidhu PS, Radzina M. EFSUMB statement on medical student education in ultrasound [short version]. Ultraschall Med. 2016;37:100-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Hirche TO, Russler J, Schroder O, Schuessler G, Kappeser P, Caspary WF, Dietrich CF. The value of routinely performed ultrasonography in patients with Crohn disease. ScandJ Gastroenterol. 2002;37:1178-1183. [Cited in This Article: ] |
| 22. | Chiorean L, Schreiber-Dietrich D, Braden B, Cui X, Dietrich CF. Transabdominal ultrasound for standardized measurement of bowel wall thickness in normal children and those with Crohn‘s disease. Med Ultrason. 2014;16:319-324. [PubMed] [Cited in This Article: ] |
| 23. | Haber HP, Stern M. Intestinal ultrasonography in children and young adults: bowel wall thickness is age dependent. J Ultrasound Med. 2000;19:315-321. [PubMed] [Cited in This Article: ] |
| 24. | Folvik G, Bjerke-Larssen T, Odegaard S, Hausken T, Gilja OH, Berstad A. Hydrosonography of the small intestine: comparison with radiologic barium study. Scand J Gastroenterol. 1999;34:1247-1252. [PubMed] [Cited in This Article: ] |
| 25. | Carroll PJ, Gibson D, El-Faedy O, Dunne C, Coffey C, Hannigan A, Walsh SR. Surgeon-performed ultrasound at the bedside for the detection of appendicitis and gallstones: systematic review and meta-analysis. Am J Surg. 2013;205:102-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Pallotta N, Civitelli F, Di Nardo G, Vincoli G, Aloi M, Viola F, Capocaccia P, Corazziari E, Cucchiara S. Small intestine contrast ultrasonography in pediatric Crohn‘s disease. J Pediatr. 2013;163:778-84.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Aibe T, Fuji T, Okita K, Takemoto T. A fundamental study of normal layer structure of the gastrointestinal wall visualized by endoscopic ultrasonography. Scand J Gastroenterol Suppl. 1986;123:6-15. [PubMed] [Cited in This Article: ] |
| 28. | Boscaini M, Moscini PL, Montori A. Transrectal ultrasonography: interpretation of normal intestinal wall structure for the preoperative staging of rectal cancer. Scand J Gastroenterol Suppl. 1986;123:87-98. [PubMed] [Cited in This Article: ] |
| 29. | ter Haar G. Ultrasound bio-effects and safety considerations. Front Neurol Neurosci. 2015;36:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369-1375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 525] [Cited by in F6Publishing: 490] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 31. | Meckler U, Caspary WF, Clement T, Herzog P, Lembcke B, Limberg B, el Mouaaouy A, Nippel G, Reuss P, Schwerk WB. [Sonography in Crohn disease--the conclusions of an experts‘ group]. Z Gastroenterol. 1991;29:355-359. [PubMed] [Cited in This Article: ] |
| 32. | Schwerk WB, Schwarz S, Rothmund M. Sonography in acute colonic diverticulitis. A prospective study. Dis Colon Rectum. 1992;35:1077-1084. [PubMed] [Cited in This Article: ] |
| 33. | Schreiber-Dietrich D, Chiorean L, Cui XW, Braden B, Kucharzik T, Jüngert J, Kosiak W, Stenzel M, Dietrich CF. Particularities of Crohn‘s disease in pediatric patients: current status and perspectives regarding imaging modalities. Expert Rev Gastroenterol Hepatol. 2015;9:1313-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Meckler U, Caspary WF, Clement T, Herzog P, Lembcke B, Limberg B, el Mouaaouy A, Nippel G, Reuss P, Schwerk WB. Sonography in Crohn disease--the conclusions of an experts‘ group. Z Gastroenterol. 1991;29:355-359. [Cited in This Article: ] |
| 35. | Dietrich CF, Lembcke B, Jenssen C, Hocke M, Ignee A, Hollerweger A. Intestinal ultrasound in rare gastrointestinal diseases, update, part 1. Ultraschall Med. 2014;35:400-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Zorzi F, Stasi E, Bevivino G, Scarozza P, Biancone L, Zuzzi S, Rossi C, Pallone F, Calabrese E. A sonographic lesion index for Crohn‘s disease helps monitor changes in transmural boweldamage during therapy. Clin Gastroenterol Hepatol. 2014;12:2071-2077. [Cited in This Article: ] |
| 37. | Dietrich CF, Braden B. Sonographic assessments of gastrointestinal and biliary functions. Best Pract Res Clin Gastroenterol. 2009;23:353-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Nuernberg D, Ignee A, Dietrich CF. [Current status of ultrasound in gastroenterology--bowel and upper gastrointestinal tract--part 1]. Z Gastroenterol. 2007;45:629-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Nuernberg D, Ignee A, Dietrich CF. [Current status of ultrasound in gastroenterology--bowel and upper gastrointestinal tract--part 2]. ZGastroenterol. 2008;46:355-366. [Cited in This Article: ] |
| 40. | Nuernberg D, Braden B, Ignee A, Schreiber-Dietrich DG, Dietrich CF. [Functional ultrasound in gastroenterology]. Z Gastroenterol. 2008;46:883-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Alison M, Kheniche A, Azoulay R, Roche S, Sebag G, Belarbi N. Ultrasonography of Crohn disease in children. Pediatr Radiol. 2007;37:1071-1082. [Cited in This Article: ] |
| 42. | Strobel D, Goertz RS, Bernatik T. Diagnostics in inflammatory bowel disease: ultrasound. World J Gastroenterol. 2011;17:3192-3197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 36] [Reference Citation Analysis (0)] |
| 43. | Calabrese E, Zorzi F, Pallone F. Ultrasound of the small bowel in Crohn‘s disease. Int J Inflam. 2012;2012:964720. [Cited in This Article: ] |
| 44. | Panés J, Bouzas R, Chaparro M, García-Sánchez V, Gisbert JP, Martínez de Guereñu B, Mendoza JL, Paredes JM, Quiroga S, Ripollés T. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn‘s disease. Aliment Pharmacol Ther. 2011;34:125-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 45. | Maconi G, Bollani S, Bianchi Porro G. Ultrasonographic detection of intestinal complications in Crohn‘s disease. Dig Dis Sci. 1996;41:1643-1648. [PubMed] [Cited in This Article: ] |
| 46. | Maconi G, Sampietro GM, Russo A, Bollani S, Cristaldi M, Parente F, Dottorini F, Bianchi Porro G. The vascularity of internal fistulae in Crohn‘s disease: an in vivo power Doppler ultrasonography assessment. Gut. 2002;50:496-500. [PubMed] [Cited in This Article: ] |
| 47. | Pera A, Cammarota T, Comino E, Caldera D, Ponti V, Astegiano M, Barletti C, Rocca R, Cosimato M, Bertolusso L. Ultrasonography in the detection of Crohn‘s disease and in the differential diagnosis of inflammatory bowel disease. Digestion. 1988;41:180-184. [PubMed] [Cited in This Article: ] |
| 48. | 3 Seitz K, Reuss J. [Sonographic detection of fistulas in Crohn disease]. Ultraschall Med. 1986;7:281-283. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Orsoni P, Barthet M, Portier F, Panuel M, Desjeux A, Grimaud JC. Prospective comparison of endosonography, magnetic resonance imaging and surgical findings in anorectal fistula and abscess complicating Crohn‘s disease. Br J Surg. 1999;86:360-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Barreiros AP, Hirche TO, Ignee A, Nürnberg D, Dietrich CF. Indications and limitations of perineal ultrasound examination. Scand J Gastroenterol. 2010;45:764-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Cui XW, Hocke M, Jenssen C, Ignee A, Klein S, Schreiber-Dietrich D, Dietrich CF. Conventional ultrasound for lymph node evaluation, update 2013. Z Gastroenterol. 2014;2014:212-221. [Cited in This Article: ] |
| 52. | Bryant RV, Trott MJ, Bartholomeusz FD, Andrews JM. Systematic review: body composition in adults with inflammatory bowel disease. Alimentary. Pharmacology and Therapeutics. 2013;38:213-225. [Cited in This Article: ] |
| 53. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkuhn T, Orchard T, Rogler G, Louis E. The second European evidence-based Consensus on the diagnosis and management of Crohn‘s disease: Definitions and diagnosis. J CrohnsColitis. 2010;4:7-27. [Cited in This Article: ] |
| 54. | Scholbach T, Herrero I, Scholbach J. Dynamic color Doppler sonography of intestinal wall in patients with Crohn disease compared with healthy subjects. J PediatrGastroenterol Nutr. 2004;39:524-528. [Cited in This Article: ] |
| 55. | Martinez MJ, Ripolles T, Paredes JM, Blanc E, Marti-Bonmati L. Assessment of the extension and the inflammatory activity in Crohn‘s disease: comparison of ultrasound and MRI. AbdomImaging. 2009;34:141-148. [Cited in This Article: ] |
| 56. | Spalinger J, Patriquin H, Miron MC, Marx G, Herzog D, Dubois J, Dubinsky M, Seidman EG. Doppler US in patients with crohn disease: vessel density in the diseased bowel reflects disease activity. Radiology. 2000;217:787-791. [Cited in This Article: ] |
| 57. | Rapaccini GL, Pompili M, Orefice R, Covino M, Riccardi L, Cedrone A, Gasbarrini G. Contrast-enhanced power doppler of the intestinal wall in the evaluation of patients with Crohn disease. ScandJ Gastroenterol. 2004;39:188-194. [Cited in This Article: ] |
| 58. | Fraquelli M, Colli A, Casazza G, Paggi S, Colucci A, Massironi S, Duca P, Conte D. Role of US in detection of Crohn disease: meta-analysis. Radiology. 2005;236:95-101. [Cited in This Article: ] |
| 59. | Dietrich CF, Ignee A, Seitz KH, Caspary WF. [Duplex sonography of visceral arteries]. Ultraschall Med. 2001;22:247-257. [Cited in This Article: ] |
| 60. | Limberg B. [Colon sonography--a new method in the diagnosis of Crohn disease and ulcerative colitis]. Monatsschr Kinderheilkd. 1990;138:422-426. [PubMed] [Cited in This Article: ] |
| 61. | Dietrich CF, Jedrzejczyk M, Ignee A. Sonographic assessment of splanchnic arteries and the bowel wall. Eur J Radiol. 2007;64:202-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Ludwig D, Wiener S, Brüning A, Schwarting K, Jantschek G, Fellermann K, Stahl M, Stange EF. Mesenteric blood flow is related to disease activity and risk of relapse in ulcerative colitis: a prospective follow up study. Gut. 1999;45:546-552. [Cited in This Article: ] |
| 63. | Ignee A, Boerner N, Bruening A, Dirks K, von Herbay A, Jenssen C, Kubale R, Sattler H, Schuler A, Weiss H. Duplex sonography of the mesenteric vessels--a critical evaluation of inter-observer variability. Z Gastroenterol. 2016;54:304-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Braden B, Ignee A, Hocke M, Palmer RM, Dietrich C. Diagnostic value and clinical utility of contrast enhanced ultrasound in intestinal diseases. Dig Liver Dis. 2010;42:667-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 663] [Cited by in F6Publishing: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 66. | Ripollés T, Rausell N, Paredes JM, Grau E, Martínez MJ, Vizuete J. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn‘s disease: a comparison with surgical histopathology analysis. J Crohn‘s & colitis. 2013;7:120-128. [Cited in This Article: ] |
| 67. | Allgayer H, Ignee A, Dietrich CF. Endosonographic elastography of the anal sphincter in patients with fecal incontinence. Scand J Gastroenterol. 2010;45:30-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D‘Onofrio M, Drakonaki EE. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 756] [Cited by in F6Publishing: 690] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 69. | Ishikawa D, Ando T, Watanabe O, Ishiguro K, Maeda O, Miyake N, Nakamura M, Miyahara R, Ohmiya N, Hirooka Y. Images of colonic real-time tissue sonoelastography correlate with those of colonoscopy and may predict response to therapy in patients with ulcerative colitis. BMC Gastroenterol. 2011;31:29. [Cited in This Article: ] |
| 70. | Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 621] [Cited by in F6Publishing: 461] [Article Influence: 41.9] [Reference Citation Analysis (0)] |