Published online Sep 28, 2017. doi: 10.3748/wjg.v23.i36.6593
Peer-review started: February 8, 2017
First decision: March 16, 2017
Revised: April 15, 2017
Accepted: July 4, 2017
Article in press: July 4, 2017
Published online: September 28, 2017
Irritable bowel syndrome (IBS) is a chronic, recurring, and remitting functional disorder of the gastrointestinal tract characterized by abdominal pain, distention, and changes in bowel habits. Although there are several drugs for IBS, effective and approved treatments for one or more of the symptoms for various IBS subtypes are needed. Improved understanding of pathophysiological mechanisms such as the role of impaired bile acid metabolism, neurohormonal regulation, immune dysfunction, the epithelial barrier and the secretory properties of the gut has led to advancements in the treatment of IBS. With regards to therapies for restoring intestinal permeability, multiple studies with prebiotics and probiotics are ongoing, even if to date their efficacy has been limited. In parallel, much progress has been made in targeting low-grade inflammation, especially through the introduction of drugs such as mesalazine and rifaximin, even if a better knowledge of the mechanisms underlying the low-grade inflammation in IBS may allow the design of clinical trials that test the efficacy and safety of such drugs. This literature review aims to summarize the findings related to new and investigational therapeutic agents for IBS, most recently developed in preclinical as well as Phase 1 and Phase 2 clinical studies.
Core tip: Irritable bowel syndrome (IBS) is a chronic, recurring, and remitting functional disorder of the gastrointestinal tract characterized by abdominal pain, distention, and changes in bowel habits. Despite there are several drugs for IBS, effective and approved treatments for one or more of the symptoms for various IBS subtypes are needed. The understanding of pathophysiological mechanisms such as the role of impaired bile acid metabolism, neurohormonal regulation, immune dysfunction, the epithelial barrier and secretory properties of the gut has led to advancements in the treatment of IBS. This literature review aims to summarize the findings relating the new and investigational therapeutic agents for IBS, most recently developed in preclinical as well as Phase 1 and Phase 2 clinical studies.
- Citation: Sinagra E, Morreale GC, Mohammadian G, Fusco G, Guarnotta V, Tomasello G, Cappello F, Rossi F, Amvrosiadis G, Raimondo D. New therapeutic perspectives in irritable bowel syndrome: Targeting low-grade inflammation, immuno-neuroendocrine axis, motility, secretion and beyond. World J Gastroenterol 2017; 23(36): 6593-6627
- URL: https://www.wjgnet.com/1007-9327/full/v23/i36/6593.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i36.6593
Irritable bowel syndrome (IBS) is a chronic, recurring, and remitting functional disorder of the gastrointestinal (GI) tract characterized by abdominal pain, distention, and changes in bowel habits that do not have a known structural or anatomical explanation[1].
IBS is a global problem, with anywhere from 5% to 15% of the general population showing symptoms that would satisfy a definition of IBS[2-4]. IBS considerably affects quality of life and imposes a profound burden on patients, physicians and the health-care system[5]. For example, the IBIS-C study recently assessed the socio-economic burden of moderate-to-severe IBS with constipation in six European countries (France, Germany, Italy, Spain, Sweden and the United Kingdom), showing that IBS represents a main cause of absenteeism in the workplace[6].
Regarding the sex-related prevalence of IBS, in Western countries, the prevalence of IBS in women outnumbers that in men by 2:1[7,8], and within the patient population who have consultations with primary care physicians, women outnumber men by 3:1[7,9]. Finally, in tertiary care settings, the number of women with IBS is 4 to 5 times higher than the number of men[7-10].
According to Rome III, IBS is defined based on the presence of: recurrent abdominal pain or discomfort at least 3 d/mo in the past 3 mo associated with two or more of the following: (1) improvement with defecation; (2) onset associated with a change in frequency of stool; and (3) onset associated with a change in form (appearance) of stool.
These criteria should be fulfilled for the past 3 mo with symptom onset at least 6 mo before diagnosis[11]. Recently, the Rome IV criteria implemented the knowledge accumulated since Rome III was published almost ten years ago.
According to Rome IV, IBS is defined on the basis of the presence of: Recurrent abdominal pain, on average, at least 1 d per week in the last 3 mo, associated with 2 or more of the following criteria: (1) related to defecation; (2) associated with a change in frequency of stool; and (3) associated with a change in form (appearance) of stool. These criteria should be fulfilled for the last 3 mo with symptom onset at least 6 mo before diagnosis[12].
In contrast to the Rome III criteria, the term discomfort has been deleted from the last definition and from subsequent diagnostic criteria because not all languages have the term “discomfort”. This word has different meanings in different languages, which can result in ambiguity with patients[12]. Furthermore, the last definition implies a change in the frequency of abdominal pain, highlighting that patients should have symptoms of abdominal pain at least 1 d per week during the past 3 mo[12]. Finally, the sentence “improvement with defecation” was substituted in the current definition by “related to defecation”, as a large subset of IBS patients do not have an improvement in abdominal pain with defecation but instead complain of worsening[12].
According to the Rome IV criteria, IBS is subtyped according to the predominant bowel habit as follows: IBS with constipation (IBS-C), IBS with diarrhoea (IBS-D), mixed type (IBS-M), and unclassified (IBS-U)[12]. The definition of bowel habit type is based on the patient’s description of the stool form by referring to the Bristol Stool Scale[13]. Furthermore, IBS patients can be grouped into sporadic (nonspecific) and post-infectious (PI-IBS)/inflammatory bowel disease (IBD)-associated (IBD-IBS)[14,15].
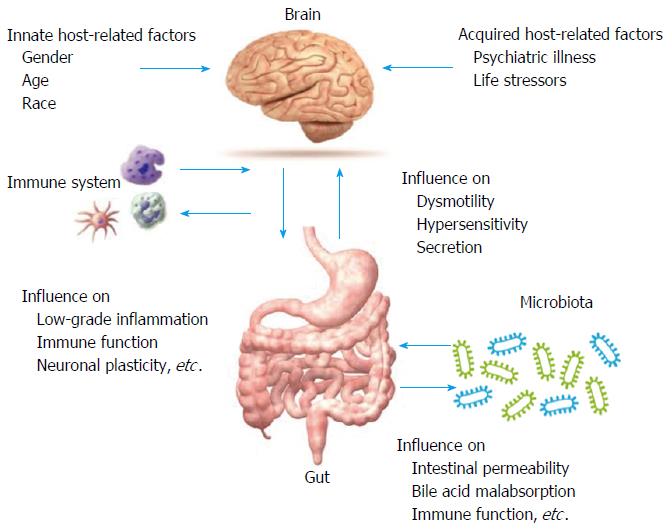
Although there are several drugs for IBS in the pipeline, there is a continuous need for effective and approved treatments for one or more of the symptoms of IBS subtypes[16-18]. The understanding of pathophysiological mechanisms such as the role of altered bile acid metabolism, neurohormonal regulation, immune dysfunction, the epithelial barrier and secretory properties of the gut has led to progress in the treatment options of IBS (Figure 1)[18,19].
This literature review aims to summarize the findings relating the new and investigational therapeutic agents for IBS most recently developed in preclinical as well as Phase 1 and Phase 2 clinical studies.
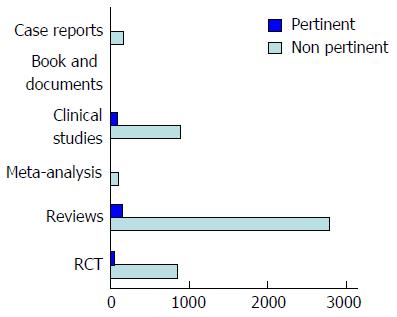
We carried out a bibliographic search in MEDLINE for the period January 1966 to December 2016 and focused on identifying publications describing the new therapeutic pharmacological approaches in IBS. Information was also obtained from abstracts and the latest results found in the Clinicaltrial.gov database. The keywords used were: irritable bowel syndrome, inflammation, immunoendocrine axis, intestinal permeability, IBS-C, IBS-D, therapy. The inclusion criteria to select articles were based on design (systematic reviews, meta-analysis, clinical trials, and experimental studies on animals) and population (adult patients > 18 years of age). We excluded articles not relevant for this topic.
According to the abovementioned criteria, 5127 studies were found and 4810 studies were excluded because they were not relevant for this topic (Figure 2).
Recently, the scientific community has focused its attention on the pivotal role of low-grade mucosal inflammation in IBS, considering evidence showing that some patients with IBS have an increased number of inflammatory cells in the colonic and ileal mucosa, with regard to control patients[20].
In fact, the intestinal mucosa harbours a florid immune system that can be regarded as “physiologically inflamed”[20,21]. Thus, low-grade inflammation, which likely plays a multifactorial role in IBS pathophysiology, can only be evaluated using quantitative assessments[20-22].
The available data[23,24] on low-grade inflammation in IBS patients is often expressed as average numbers and are mainly focused on IBS-D. Thus, it is unclear whether this event occurs only in selected subsets of IBS patients[25].
Therefore, IBS could be considered a micro-organic disease, where there is an increased number of mucosal immunocytes (i.e., mast cells, eosinophils, and T cells) in adult and paediatric patients. Several precipitating factors have been claimed, including food allergy, abnormal microbiota, bile acid malabsorption, and increased intestinal permeability[26]. The magnitude of the inflammatory response is several-fold less than that seen in acute inflammation in inflammatory bowel disease. The above-reported evidence provides a rationale to evaluate the efficacy of intestinal anti-inflammatory therapies in patients with IBS that we will touch upon in the next section.
In the study performed by Dunlop et al[24], twenty-nine patients with post-infectious irritable bowel syndrome underwent a randomized, double-blind, placebo-controlled trial of 3 wk of oral prednisolone, 30 mg/d. Mucosal enterochromaffin cells, T lymphocytes and mast cells were evaluated in rectal biopsies before and after treatment, and bowel symptoms were reported in a daily diary. In this study, enterochromaffin cell counts did not change significantly after either prednisolone or placebo. Although lamina propria T-lymphocyte counts decreased significantly after prednisolone, but not after placebo, this was not linked with any significant treatment-related improvement in abdominal pain, diarrhoea, frequency or urgency[24].
Rifaximin is a rifamycin derivative that acts by inhibiting bacterial ribonucleic acid (RNA) synthesis. It is virtually unabsorbed after oral administration, so it is used mainly to treat local dysfunctions within the gastrointestinal tract[27].
The Food and Drug Administration (FDA) initially approved rifaximin to treat traveller’s diarrhoea caused by Escherichia coli and to prevent the recurrence of hepatic encephalopathy.
Successively, the FDA approved rifaximin in IBS-D “naive” patients at a dose of 550 mg three times a day for 14 d as well as in patients experiencing a recurrence of symptoms.
Rifaximin improves IBS symptoms through a variety of mechanisms directed at the gastrointestinal tract. In fact, much evidence from animal experiments shows that rifaximin either improves or maintains microbiota diversity and bacterial composition in IBS, reduces intestinal cytokine inflammation, provides gut-barrier protection preventing attachment and internalization of coliforms and pathogens with reduced epithelial cell inflammation and pathogen-induced inflammatory response, and reduces visceral hyperalgesia[28].
In a combined analysis of two separate Phase 3 trials (TARGET 1 and 2), a 14-d course of rifaximin 550 mg three times daily in IBS-D patients significantly increased the percentage of relief of global IBS symptoms and improved IBS-related distention and abdominal pain, discomfort, and loose or watery stools compared with placebo for up to 10 wk post-treatment[29,30].
Successively, TARGET 3 was performed to test the safety and efficacy of a repeated treatment with rifaximin in patients experiencing a recurrence of IBS symptoms. In this study, the percentage of responders during the 18-wk follow-up (in terms of pain and stool consistency improvements) to randomized repeat treatment was significantly greater with rifaximin vs placebo[31]. The safety profile of rifaximin in patients with IBS-D was generally similar to that observed with placebo[30].
In fact, constipation was only reported in 1 (0.3%) patient in the rifaximin group and 3 (1.0%) patients in the placebo group. Only one patient in each treatment group suspended the drug. One case of Clostridium difficile infection occurred (in a patient who had been off of rifaximin for several weeks but was receiving a concomitant systemic antibiotic)[30,31].
In conclusion, these trials show that a 2-wk course of rifaximin could improve IBS-D-related symptoms, and in the case of persistence of symptoms, retreatment may ameliorate abdominal pain and stool consistency with possible improvements in bloating and stool urgency in some patients. While patients were retreated within an 18-wk period of follow-up in the study, it is still unclear as to when and how often treatment should be given. In addition, the identification of those patients who might likely respond to rifaximin remains to be investigated.
Recently, Ghoshal et al[32] evaluated symptom resolution among IBS patients with or without small intestinal bacterial overgrowth (SIBO) on norfloxacin treatment and its efficacy in obtaining negative SIBO test results as compared with placebo. In this study, 80 IBS patients (Rome III) were evaluated for SIBO by gut aspirate culture. Patients with a colony count ≥ 10 CFU/mL and those without SIBO were separately randomized to 800 mg/d norfloxacin for 10 d or placebo. The global symptom score (blind), Rome III criteria, aspirate culture, and glucose hydrogen breath test were assessed before and 1 mo after treatment, and patients were followed up for 6 mo. Although norfloxacin was more effective at decreasing the symptom score at 1 mo among patients with compared with those without SIBO but not placebo, the scores were comparable at 6 mo. Symptoms more often resolved to turn Rome III negative in SIBO patients treated with norfloxacin compared with placebo at 1 mo. Patients without SIBO and a colony count of 10 CFU/mL responded more than those with a colony count less than 10 CFU/mL[32].
Since mast cell activation was thought to be involved in visceral hypersensitivity, a study was undertaken by Klooker et al[33] to evaluate the effect of ketotifen, a mast cell stabilizer, on rectal sensitivity and symptoms in patients with IBS. In this case-control study, 60 patients with IBS underwent a barostat study to assess rectal sensitivity before and after 8 wk of treatment. After the initial barostat, patients were randomised to receive ketotifen or placebo. Ketotifen increased the threshold for discomfort in patients with IBS and visceral hypersensitivity but not placebo. This effect was not observed in normosensitive patients with IBS. Ketotifen significantly reduced abdominal pain and other IBS symptoms and improved quality of life. However, whether this effect was secondary to the mast cell stabilising properties of ketotifen or H1 receptor antagonism remains a topic of future research[33].
Successively, Lobo et al[34] showed a clinical Benefit of Disodium Cromoglycate (DSCG) in IBS in a double-blind, placebo-controlled clinical assay with prolonged (6 mo) oral administration of DSCG (DSCG), since it induces mast cell-mediated recovery of the healthy-like innate immunity gene expression profile in the jejunal mucosa[34].
Finally, since histamine sensitizes the nociceptor transient reporter potential channel V1 (TRPV1) and has been observed to play role in visceral hypersensitivity in animals, Wouters et al[35,36] investigated the role of ebastine, an antagonist of histamine receptor H1 (HRH1), in reducing symptoms of patients in a randomized placebo-controlled trial. After a 2-wk run-in period, subjects were enrolled randomly to groups given either the HRH1 antagonist ebastine or placebo for 12 wk. Rectal biopsy specimens were collected, barostat studies were performed, and symptoms were recorded (using the validated gastrointestinal symptom rating scale) before and after the 12-wk period. Patients were followed up for a further 2 wk. The primary end point of the study was the evaluation of ebastine efficacy on the symptom score evoked by rectal distension. Compared with the placebo group, patients treated with ebastine had reduced visceral hypersensitivity, increased symptom relief, and reduced abdominal pain scores[35,36].
The therapeutic potential of aminosalicylates, whose benefits in chronic inflammatory bowel diseases are well known, has been focused on as a potential cure for IBS[37,38].
The largest studies on mesalazine in IBS have been conducted by Barbara and Lam. Barbara et al[39] conducted a phase 3, multicentre, tertiary setting, randomised, double-blind placebo-controlled trial in patients with Rome III-confirmed IBS. Patients were randomly assigned to either 800 mg mesalazine or placebo three times daily for 12 wk and were followed for an additional 12 wk. The primary efficacy endpoint was satisfactory relief of abdominal pain/discomfort for at least half of the weeks of the treatment period. The secondary endpoint was satisfactory relief of overall IBS symptoms. The responder patients were 68.6% in the mesalazine group vs 67.4% in the placebo group. However, with the 75% rule or > 75% rule, there was a higher percentage of responders in the mesalazine group than placebo of 11.6% and 5.9%, respectively, although these differences were not significant. For the key secondary endpoint, in the mesalazine group, overall symptom improvement was observed and a significant difference of 15.1% vs placebo with the > 75% rule was reached. The authors concluded that mesalazine treatment was not superior to placebo on the study primary endpoint, but a subgroup of patients with IBS had a sustained therapy response and benefits from mesalazine therapy[39].
On the other hand, Lam et al[40] conducted a double-blind, randomised placebo-controlled trial of 2 g mesalazine twice daily compared with placebo for 3 mo in Rome III criteria patients with IBS-D. The authors compared the mesalazine and placebo effects on stool frequency as the primary endpoint and secondarily assessed the effect of mesalazine on abdominal pain, stool consistency, urgency and satisfactory relief of IBS symptoms. In total, 136 IBS-D patients (82 female, 54 male) were enrolled; 10 patients withdrew from each group. The intention to treat analysis showed that the mean daily stool frequency during weeks 11 and 12 was 2.8 (SD 1.2) in the mesalazine group and 2.7 (SD 1.9) in the placebo group, with a group difference of 0.1. The authors concluded that mesalazine did not ameliorate abdominal pain, stool consistency or percentage with satisfactory relief compared with placebo during the last 2 weeks’ follow-up. However a post hoc analysis in 13 post-infectious patients with IBS tended to show benefit, even though this finding needs to be confirmed in larger studies[40].
A point of weakness of these studies is that the use of endpoints for response may be easily met by patients in the placebo arm, resulting in placebo response rates of almost 70% for satisfactory relief of abdominal pain or discomfort and > 60% for satisfactory relief of overall IBS symptoms in the trial performed by Barbara et al[39] and in > 40% for satisfactory relief of IBS symptoms in the trial performed by Min et al[41].
It may have therefore have been preferable to use a once daily dosing schedule in both trials in order to reduce the placebo response rates, thus increasing the likelihood of detecting a statistically significant difference between mesalazine and placebo.
Based on this evidence, it is necessary that further studies prove the efficacy of mesalazine for IBS. Studies aimed at evaluating the role of aminosalicylates and other potential anti-inflammatory treatment options, including probiotics, non-absorbable antibiotics, histamine receptor antagonists and protease inhibitors on IBS symptoms or pathophysiology are now warranted[39].
Table 1 sums up the literature findings about anti-inflammatory therapies in irritable bowel syndrome.
| Drug | Ref. | No. of patients | Study design | Outcome |
| Corticosteroids (prednisolone) | Dunlop et al[24] | 29 patients with post-infectious irritable bowel syndrome | Randomized, double-blind, placebo-controlled trial of 3 wk of oral prednisolone, 30 mg/d | Not associated with any significant treatment-related improvement in abdominal pain, diarrhoea, frequency or urgency |
| Antibiotics (Rifaximin) | Pimentel et al[29] | 623 IBS patients in TARGET 1 and 637 IBS in TARGET 2 | Phase 3 trials, 14 d with rifaximin 550 mg 3 times daily | Significantly increased the percentage of relief of global IBS symptoms and improved IBS-related bloating and abdominal pain, discomfort, and loose or watery stools, with regard to placebo for up to 10 wk post-treatment |
| Target 1 e 2 | ||||
| Antibiotics (norfloxacin) | Ghoshal et al[32] | 80 IBS patients evaluate for SIBO | Randomized, double-blind, placebo-controlled trial; patients were randomized to 800 mg/d norfloxacin for 10 d or placebo | Although norfloxacin was more effective at reducing the symptom score at 1 mo among patients with compared with those without SIBO but not placebo, the scores were comparable at 6 mo. Symptoms more often resolved to turn Rome III negative in SIBO patients treated with norfloxacin compared with placebo at 1 mo |
| Mast cell stabilizers (Ketotifen) | Klooker et al[33] | 60 IBS patiens | Case Control study; abarostat study to assess rectal sensitivity before and after 8 wk of treatment and, after the initial barostat, patients were randomised to receive ketotifen or placebo | Ketotifen but not placebo increased the threshold for discomfort in patients with IBS with visceral hypersensitivity, but this effect was not observed in normosensitive patients with IBS. Ketotifen significantly decreased abdominal pain and other IBS symptoms and improved quality of life |
| Mast cells stabilizers (DSCG) | Lobo et al[34] | Randomized, double-blind, placebo-controlled trial; with prolonged (6 mo) oral administration of DSCG | Induces Mast Cell-Mediated Recovery of Healthy-Like Innate Immunity Genes Expression Profile in the Jejunal Mucosa | |
| Mast cells stabilizers (ebastin) | Wouters et al[35] | 65 IBS patients | Double-blind placebo-controlled trial, after 2-wk run-in period, subjects were assigned randomly to groups ebastine (20 mg/d; n = 28) or placebo (n = 27) for 12 wk | Compared with subjects given placebo, those given ebastine had reduced visceral hypersensitivity, increased symptom relief, and reduced abdominal pain scores |
| Mesalazine | Barbara et al[39] | 185 patients with IBS | A phase 3, multicentre, tertiary setting, randomised, double-blind, placebo-controlled trial in patients with Rome III confirmed IBS. Patients were randomly assigned to either mesalazine, 800 mg, or placebo, three times daily for 12 wk, and were followed for additional 12 wk | Mesalazine treatment was not superior than placebo on the study primary endpoint, but a subgroup of patients with IBS showed a sustained therapy response and benefits from a mesalazine therapy |
| Lam et al[40] | 136 patients with IBS-D | A double-blind, randomised placebo-controlled trial of 2 g mesalazine twice daily compared with placebo for 3 mo | The authors concluded that mesalazine did not improve abdominal pain, stool consistency or percentage with satisfactory relief compared with placebo during the last 2 weeks’ follow-up, however a post hoc analysis in 13 post-infectious patients with IBS appeared to show benefit but this needs confirmation in a larger group[40] |
An increase in intestinal permeability can be seen in many conditions, such as infectious gastroenteritis and irritable bowel disease[42]. The intestinal barrier has long been a focus of gastroenterological research[43] and its role in IBS has been discussed in many studies. Most studies show an increase in intestinal permeability of patients with IBS-D and post-infectious IBS (PI-IBS)[43-46].
Among the first to describe intestinal permeability in patients with PI-IBS were Spiller et al[47], who detected an increased lactulose/mannitol ratio in the urine of IBS patients compared to healthy controls.
Marshall et al[48] also described an increase in permeability of patients with IBS after an outbreak of bacterial gastroenteritis but could not show a difference in permeability between PI-IBS and non PI-IBS.
There are genetic risk factors for developing PI-IBS and CDH1, which codes for E-cadherin, a tight junction (TJ) protein that is involved in the epithelial barrier function of the gut[49], hence suggesting the pathophysiological mechanism through which some patients experience increased permeability.
The mechanism of increased permeability in patients with IBS is suggested to involve tight junction dysfunction or involvement of the adherence proteins[44]. Among factors that could influence permeability is stress. Male soldiers were evaluated in a prospective study during and after combat training with an increase in physiological and psychological stress. Their training induced an increase in gastrointestinal symptoms and alteration in the permeability of the gut barrier[50]. Since stress has been suggested to be one of the pathophysiological factors involved in developing IBS, this mechanism could explain the reason for the gastrointestinal symptoms[51].
Another factor that has been evaluated is the intraluminal content of patients with IBS, where faecal supernatants from patients have increased the colonic permeability in mice[52,53].
Both intracellular [zonula occludens (ZO)-1, ZO-2, and ZO-3, and cingulin] and surface-membrane proteins [occludin, claudins, and junctional adhesion molecules (JAM)] are the main components of TJ[26,54]. Adherens junctions are mainly made up of e-cadherin, catenin, and actin filaments[26,55].
Inflammation has also been described to be a factor in increasing intestinal permeability, not only in inflammatory bowel disease[43] but also in IBS, where the increase in mast cells and the mediators increased the effects on the intercellular junctions[44].
Finally, other factors, such as hormonal and neuro-hormonal pathways, nutritional factors, ethanol consumption and several drugs (nonsteroidal anti-inflammatory drugs, methotrexate, tacrolimus, protonic pump inhibitors), could affect the intestinal barrier, a factor that needs to be further evaluated[26]. The knowledge of affection of the intestinal permeability in IBS patients will help in the development of new therapies in order to restore the gut barrier, a topic we will touch upon in the next section.
Table 2 sums up the literature findings about therapies restoring intestinal permeability in irritable bowel syndrome.
| Drug | Ref. | No. of patients | Study design | Outcome |
| Probiotics | Ford et al[61] | Forty-three RCTs were eligible for inclusion | Metanalysis | Probiotics had beneficial effects on global IBS, abdominal pain, bloating, and flatulence scores. Data for prebiotics and synbiotics in IBS were sparse. Probiotics appeared to have beneficial effects in CIC (mean increase in number of stools per week = 1.49; 95%CI: 1.02-1.96), but there were only two RCTs. Synbiotics also appeared beneficial (RR of failure to respond to therapy = 0.78; 95%CI: 0.67-0.92). Again, trials for prebiotics were few in number, and no definite conclusions could be drawn |
| Mazurak et al[67] | Fifty-six papers | Metanalysis | The heterogeneity of the studies of probiotics in IBS questions the value of meta-analyses and the use of different bacterial strains and different mixtures of these strains, as well as different dosages, are the main contributors to this heterogeneity | |
| Glutamine | Akobeng et al[73] | Two randomized trial | Cochrane analysis | Not significant difference in the permeability and no effect in the clinical remission |
| Larazotide acetate | Leffler et al[78] | 342 adults with celiac disease who had been on a gluten free diet (GFD) for 12 mo or longer and maintained their current GFD during the study | Randomized, double-blind, placebo-controlled study assessed larazotide acetate 0.5, 1, or 2 mg 3 times daily | Reduce signs and symptoms in celiac disease patients on a GFD better than a GFD alone |
The human intestinal microbiota represents one of the densest, biodiverse, and rapidly evolving bacterial ecosystems. The intestinal microbiome, that is, its collective genome, is an adaptive entity that varies with diet, lifestyle and environment, providing a further metabolic flexibility to the human super organism and functional traits that humans have not evolved on their own[56]. Therefore, the potential of manipulating the gut microbiota in these disorders is assessed[57].
The mechanisms through which probiotics alter the intestinal microbial flora could be direct, changing the bacterial macroenvironment of the lumen, or indirect, through the stimulation of the immune system and the improvement of mucosal function, for example, by modulating the invasion and adherence of the epithelial cells of the gut by pathogenic bacteria, thus normalizing gut permeability[58-60]. The use of probiotics in patients with IBS seems to be effective in achieving improvement in the global IBS symptoms[61,62], but how it affects the intestinal permeability is less evaluated in humans[46]. Most studies have shown that altering the intraluminal content affects the barrier functions of the gut, and studies on rodent models of IBS have shown different data[63].
Despite the growing interest of the scientific community in research in the field of probiotics, the interpretation of the scientific literature on the value of these preparations’ results is difficult due to the wide variability in the species, strains and doses employed in the preparations as well as the low methodological quality of the available trials, often due to the poor design and the small sample size.
Several meta-analysis have been published on this topic[64-66], all concluding that probiotics might be efficacious in IBS, but the actual benefit and the most effective species and strains are uncertain.
In the meta-analysis performed by Ford et al[61], including forty-three randomized controlled trials, probiotics showed beneficial effects on global IBS, abdominal pain, bloating, and flatulence scores[61]. Probiotics appeared to be successful in chronic idiopathic constipation (CIC), but there were only two randomized controlled trials, and again, since trials for probiotics are few in number, no specific conclusions could be obtained[61].
In the last updated meta-analysis performed by Mazurak et al[67], including fifty-six papers (twenty-seven studies using multi-species bacterial preparations and twenty-nine using single-strain probiotics), they analysed the efficacy of probiotics regarding patients included, treatment duration, probiotic dosage, and outcome measures. According to the authors, the heterogeneity of the studies of probiotics in IBS impairs the value of meta-analyses. The use of different bacterial strains and different mixtures of these strains, as well as different dosages, may be the main factors contributing to this heterogeneity[67]. Currently, there is limited evidence for the efficacy of a small number of single-strain probiotics in IBS (mostly bifidobacteria), and this evidence leads to the performance of trials with inclusion and exclusion criteria closely following the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) guidelines for clinical trials in IBS[68,69], including the definition of minimal severity for inclusion, global primary endpoints, and adequate secondary end-points (pain, bloating, and a clinically meaningful responder definition). Such trials should include at least 8 wk of therapy, an adequate follow-up period and restriction to one of the different IBS subtypes[67].
Glutamine is one of the compounds that has been investigated as a treatment of conditions with leaky gut. It has been shown to regulate the protein turnover in enterocytes of pig[70], reduce intestinal permeability in intestinal cell cultures and maintain transepithelial resistance[71]. Glutamine has also been shown to maintain the integrity of the intestinal barrier in critically ill patients by reducing the incidence of infections[72]. Glutamine treatment in patients with Crohn’s disease was recently reviewed in a Cochrane analysis[73]. In this review, only two randomized controls were included, and neither showed a significant difference in the permeability and neither had any effect on clinical remission.
Glutamine treatment in patients with IBS is less examined. Glutamine synthetase expression is lower in the small bowel and colonic mucosa of patients with IBS-D with increased intestinal permeability[74]. Therefore, one recent pilot study on IBS-D showed that with a higher glutamine concentration, Claudin-1 expression increases, thus improving the permeability[75]. However, further studies are needed for using glutamine as a supplement treatment for IBS.
Larazotide acetate (LA) is a tight-junction regulator peptide preventing the opening of intestinal epithelial TJ[76]. The safety, tolerance and pharmacokinetics of LA were studied in a randomized double-blind placebo-controlled study conducted on celiac disease subjects challenged with gluten[76,77].
Recently, in a multicentre, randomized, double-blind placebo-controlled study, LA at doses of 0.5, 1, or 2 mg 3 times daily was evaluated to relieve ongoing symptoms in 342 adults with celiac disease who had been on a gluten-free diet (GFD) for 12 mo or longer and maintained their current GFD during the study. A 0.5 mg dose of Larazotide acetate appeared to reduce signs and symptoms in celiac disease patients on a GFD better than a GFD alone. Although the results were mixed, this study resulted in the successful use of a novel therapeutic agent targeting tight junction regulation in those patients with CeD who are symptomatic despite a GFD[78].
Therefore, the modulation of the tight junction could represent a paradigm shift in the treatment of immune mediated and inflammatory diseases (Celiac Disease, IBD, IBS, etc).
Constipation-predominant irritable bowel syndrome (IBS-C) is a frequent disorder and represents one of the main causes of ambulatory visits. Abdominal pain and discomfort characterize IBS-C, making it different from chronic idiopathic constipation[79].
It is now well known that treatment focusing only on bowel transit does not provide complete relief to patients with IBS-C. A global evaluation of the pathophysiology of IBS-C has led to the use of sensory end points like complete spontaneous bowel movements and the FDA combined end point (abdominal pain and complete spontaneous bowel movements) in clinical trials[79].
For example, new information on the mechanisms underlying pain sensation in chronic visceral hypersensitivity as well as insights into the mechanism of action of new drugs targeting abdominal pain in IBS have recently been obtained by preclinical experiments in rodent models[80]. A number of drugs that we will touch upon in the next section are actually in development.
Linaclotide (MD-1100 acetate) is a novel orally active 14-amino acid peptide of the guanylin family of cyclic guanosine monophosphate (cGMP)-regulating guanylate cyclase-C (GC-C) agonists. It has been approved by the FDA and by the EMA for the treatment of moderate to severe IBS-C in adults. Its action is focused on the increase of fluid secretion, favouring gastrointestinal transit, and has GC-C-mediated analgesic effects[81].
It is recommended at a dose of 290 μg orally once a day before meals. Linaclotide is converted to an active metabolite (MM-419447) that has the same pharmacodynamics and pharmacokinetics as the parent drug.
In 2007, Andresen et al[82] investigated the effect of 5 d of linaclotide on transit and bowel function in 36 women with IBS-C according to Rome II criteria randomized in a 1:1:1 fashion for placebo, linaclotide 100 μg, and linaclotide 1000 μg.
Patients with slow colonic transit or slower transit than the mean for healthy controls were studied for 5 d at baseline and 5 d during the treatment. Patients collected all the information regarding gastric, small bowel, and colonic transit by scintigraphy and bowel function using stool diaries, which included Bristol Stool Form Scale (BSFS) scores for stool consistency, ease of stool passage scores, and completeness of evacuation.
Linaclotide did not show any effect on gastric emptying or colonic filling. It did show a significant effect on ascending colon emptying t½ times (P = 0.015) and on overall total colonic transit times at 48 h (P = 0.02) at the 1000 μg dose (P = 0.004), but not at the 100 μg dose, as well as on increased stool frequency, decreased stool consistency, improved ease of passage, and acceleration of time to first bowel movement (P < 0.001)[82].
In 2010, Johnston et al[83] investigated the efficacy and safety of 12 wk of linaclotide at a daily dose range of 75-600 μg in a phase IIb randomized double-blind parallel-group multicentre placebo-controlled trial conducted on 420 patients with IBS-C (female patients = 92%). Patients had to meet Rome II criteria, with fewer than three spontaneous bowel movements (SBMs) per week, and straining, lumpy/hard stools, or sensation of incomplete evacuation more than 25% of the time for at least 12 wk in the 12 mo preceding study entry. The primary endpoint was a change in the number of complete spontaneous bowel movements (CSBMs). Secondary endpoints were the effect on individual symptoms, quality of life (QOL), the number of patients who were CSBM responders (at least three CSBMs/wk and an increase of one CSBM from baseline for 75% of the study duration), and global relief responders (symptoms being somewhat, considerably, or completely relieved for 100% of the study duration or completely relieved for 50% of the study duration).
For the 75, 150, 300 and 600 μg linaclotide doses, the mean change in CSBMs per week was 2.90, 2.49, 3.61 and 2.68, respectively (P < 0.01), and the percentage of patients who were CSBM responders was 25%, 19.5%, 32% and 24%, respectively. Patients treated with linaclotide showed an adequate relief response (33%-51% vs 22%) and a global relief response (44%-55% vs 29%) compared to placebo. All doses of linaclotide significantly improved bowel habits, including frequency of short bowel movements (SBMs) (P ≤ 0.001) and CSBMs (P ≤ 0.01), severity of straining (P ≤ 0.001), stool consistency (P ≤ 0.001), and abdominal pain scores (P ≤ 0.05), than placebo. Abdominal discomfort, bloating, and global IBS-C measures were also improved for all doses except for the 75 μg (abdominal discomfort) and 150 μg (bloating) doses. The linaclotide effect was observed at the first week and lasted throughout the 12 wk of treatment.
The approval of linaclotide for IBS-C was based on two randomized double-blind placebo-controlled phase III trials similar in study design, end points, and patient demographics[84-86].
Primary end points included both the FDA-recommended combined primary end point and a more rigorous combined primary end point that required even more CSBM responses for 9 of 12 wk. Secondary end points included patient-reported abdominal pain, discomfort, and bloating; straining severity; and weekly SBM and CSBM frequency and stool consistency. The first phase III trial included 804 adults with IBS-C who were randomized 1:1 to receive linaclotide 290 lg or placebo daily for 26 wk, with change-from-baseline end points measured at 12 and 26 wk[84]. Attrition rates were 18.5% at 12 wk and 25.6% at 26 wk. At 26 wk, the majority of patients withdrew from the study due to adverse events in the linaclotide arm (10.2%) and perceived lack of efficacy in the placebo arm (8.2%). Over 12 wk, the FDA combined primary end point was achieved by 33.7% of patients receiving linaclotide compared with 13.9% of patients receiving placebo (P < 0.0001).
Linaclotide was also superior to placebo in the more rigorous investigator-defined combined primary end point that was reached by 12.7% of linaclotide-treated patients vs 3.0% of placebo-treated patients (P < 0.0001). At 26 wk, 32.4% of patients receiving linaclotide and 13.2% of patients receiving placebo (P < 0.0001) reached the FDA combined primary end point.
Improvements in all secondary end points occurred in the linaclotide group at weeks 12 and 26. The second phase III trial of linaclotide was composed by a 12-wk treatment phase followed by a 4-wk randomized withdrawal phase[86]. A total of 803 adults with IBS-C were randomized to receive linaclotide 290 lg or placebo once/d for 12 wk. Approximately 78% of patients completed the entire 16-wk study, and most of the patients who suspended the study did so due to adverse events in the linaclotide arm (7.9%).
In the 12-wk active treatment phase, linaclotide demonstrated statistically significant improvements in all primary and secondary efficacy end points compared with placebo. Approximately one-third (33.6%) of patients receiving linaclotide fulfilled both components of the FDA end point compared with 21% of patients receiving placebo (P < 0.0001). Statistically significant improvements were observed also in abdominal pain, discomfort, and bloating in linaclotide-treated patients, with a mean reduction of about 2 points from baseline (on an 11-point scale) compared with reductions of 1.1 with placebo (P < 0.0001 for each measure).
In the linaclotide arm, an improvement in severity of straining, constipation, and stool consistency was observed compared with the placebo arm (all P < 0.0001).
Linaclotide caused diarrhoea, abdominal pain, flatulence, headache, viral gastroenteritis, and abdominal distension as adverse events. Diarrhoea, the most common, occurred in less the 20% of patients, probably due to increased fluid secretion and accelerated colonic transit[84,86].
In Johnston et al[83] phase IIb dose-ranging trial, diarrhoea of mild to moderate severity was the primary dose-dependent adverse effect observed. It occurred in 11.4%, 12.2%, 16.5% and 18.0% of patients in the 75, 150, 300 and 600 μg linaclotide dose groups, respectively, compared with 1.2% in the placebo group. Dehydration or electrolyte disturbances were not found, although one instance of faecal impaction occurred[83]. In the studies by Rao et al[86] and Chey et al[84], 4.5%-5.7% of the linaclotide-treated patients and 0.2%-0.3% of the placebo group discontinued the study due to diarrhoea.
In a phase III clinical trial in IBS-C, patients experienced adverse events more in the linaclotide 290-lg group (65.4%) than in the placebo (56.6%, P < 0.05) group[87]. In another IBS-C phase III trial, adverse effects in the linaclotide group were reported at a similar rate to placebo (56.2% vs 53.0%, P = 0.39)[88]. Adverse events were reported by 60.5% of patients receiving linaclotide 145 lg, 55.7% of patients receiving linaclotide 290 lg, and 52.1% of patients receiving placebo[89].
In phase III clinical trials in patients with IBSC, diarrhoea was the most frequently reported adverse event, occurring in 19.5%-19.7% of patients in the linaclotide groups compared with 2.5%-3.5% of patients receiving placebo (P < 0.0001).
In randomized trials, linaclotide at 145 μg/d was best tolerated with improvement in CSBM/Wand symptoms in patients with CIC. Patients with IBS-C best responded to the 290-μg daily dose[84,85]. Linaclotide appeared to be very well tolerated.
Linaclotide is approved for the treatment of IBS-C in both male and female adults at a dosage of 290 lg once/d and for the treatment of CIC at a dosage of 145 lg once/d. The medication should be taken 30 min prior to breakfast. Renal or hepatic impairment is unlikely to affect the metabolism or clearance of linaclotide or its metabolite due to its low systemic exposure.
In conclusion, linaclotide can represent a targeted approach that addresses the complexity of symptoms associated with the syndrome. Linaclotide has been reported to safely improve IBS-C abdominal pain severity, bowel movement quality, and bowel movement frequency as well as key symptoms of abdominal fullness, bloating, and discomfort, with associated improvements in QOL. Based on the United States FDA and the EMA, linaclotide fulfils the recommended endpoints with a number needed to treat (NNT) ranging from 4.39 to 7.9. It is effective and can be associated with diarrhoea as the most common adverse effect leading to suspension of the medication in approximately 5% of patients. According to recent clinical evidence, linaclotide should be considered for patients with IBS-C due to its effect on abdominal pain and bowel symptom improvement.
Plecanatide is a 16-amino acid GC-C agonist currently used in phase III clinical trials for CIC and phase II trials for IBS-C[90]. Plecanatide mimics the endogenous agonist of the GC-C receptor in the intestinal tract. Like that of uroguanylin, plecanatide’s actions are pH-dependent, with the most favorable efficacy in the acidic environment of the duodenum. Similar to linaclotide, plecanatide luminally activates the GC-C receptor on gastrointestinal mucosal epithelial cells, leading to intracellular secretory and extracellular anti-nociceptive effects via a cGMP-mediated second messenger pathway[91]. A phase III randomized double-blind trial in 951 patients with CIC treated with 0.3, 1 or 3 mg plecanatide or placebo once/d for 12 wk was conducted[92]. The primary end points were weekly (more than three CSBMs/wk and an increase of more than one CSBM/wk from baseline) or an overall study response (weekly response for 9 of 12 wk, including 3 of the last 4 wk to ensure durability of response). The percentage of overall responders was significantly higher in the plecanatide 3 mg group compared with placebo (19% vs 10.7%, P = 0.009). Weekly responder rates were also significantly higher in plecanatide 3 mg than placebo for weeks 1-12. Patients treated with 3 mg showed an improvement in stool frequency, consistency, straining, and quality of life compared with placebo. Data for other plecanatide doses were not shown.
Plecanatide potentially has low risk of adverse cardiovascular effects, as its systemic absorption is very low. According to the phase I study for evaluation of the safety and tolerability of plecanatide in humans[93], no measurable systemic absorption was observed at any doses of oral plecanatide. Plecanatide was safe and well tolerated up to the highest dose. Diarrhoea was the most prevalent side effect, but its frequency did not statistically significantly differ between placebo and plecanatide, and appeared not to be dose-related in the plecanatide-treated subjects. Other gastrointestinal events were nausea, abdominal discomfort and pain, and vomiting. In a Phase II dose escalation trial involving a total of 84 chronic constipation patients recruited with modified Rome III criteria, 14 d of plecanatide therapy improved stool frequency, stool consistency, straining and overall relief of chronic constipation symptoms. To confirm the safety and efficacy of plecanatide, two Phase III trials (NCT01982240andSP304203-00) have been planned. In the United States and Canada, the Phase III trial NCT01982240 was initiated in November 2013 with adult chronic constipation patients and was expected to be completed in February 2015[94].
Prucalopride is authorized in several countries (not in the United States) for women with CIC unresponsive to laxatives[95]. As a very highly selective 5-HT4 agonist, prucalopride has no measurable affinity for other receptors. In safety evaluation tests, prucalopride showed no h ERG (humanether-à-go-go-related gene) channel inhibitory activity. It is not arrhythmogenic, and it promotes colonic motility[96].
At dosages of 2 mg and 4 mg per day, this drug produced a low incidence of QT interval prolongation. Even up to 20 mg per day (10-fold higher than the recommended dosage), prucalopride displayed no clinically relevant effects on cardiovascular parameters in healthy volunteers. Prucalopride improved stool frequency and consistency, and it dose-dependently enhanced colonic transit in healthy controls or chronic constipation patients with no negative impact on gastric emptying or small bowel transit[97]. The patients’ quality of life was significantly improved by prucalopride treatment.
In three pivotal trials, prucalopride showed a good efficacy in increasing CSBMs per week and in improving perceived disease severity and quality of life in patients with CC. A study conducted on 620 patients with CC treated with 2 or 4 mg of prucalopride for 12 wk showed that it increased one or more CSBMs per week compared to the control group[98-100]. In another trial conducted on 713 patients with CC, 2 or 4 mg of prucalopride increased the frequency to three or more CSBMs per week and improved evacuation completeness, perceived disease severity, and quality of life[101]. In another study conducted on patients 65 years or older with CIC, prucalopride at a dose of 1 mg for 4 wk did not cause any changes in an electrocardiogram or corrected QT (QTc) interval, showing its safety for the treatment of CIC in the elderly[97,102]. A study conducted on Asian subjects with CIC reported similar efficacy and safety as that observed in Western populations[103]. In a pooled analysis of the study with Asian subjects and the three pivotal trials, increased stool frequency of approximately three or more CSBMs per week was observed in Asian (34% vs 11%, P < 0.001) and non-Asian (24.6% vs 10.6%, P < 0.001) women. Prucalopride was shown to be safe and well tolerated[104], improving CIC abdominal symptoms such as abdominal discomfort, bloating, straining, and painful bowel movements[105]. Another study conducted on a small number of patients showed the efficacy of prucalopride not only in the treatment of slow transit constipation but also of obstructed defecation and IBS-C[106].
In a recent analysis, Camilleri et al[106] evaluated the efficacy of prucalopride using the data from six phase 3 and 4 multicentre double-blind randomized placebo-controlled parallel-group trials performed across three continents.
Over the 12-wk treatment period, prucalopride-treated patients consistently achieved a mean of 3 SCBMs/wk compared to placebo with the treatment response observed in the individual trials[98-100,102]. On the other hand, the SPD555-401 trial was the only trial that failed to demonstrate a statistically significant effect of prucalopride on this primary endpoint after both 12 and 24 wk of treatment, without any plausible explanation of this lack of efficacy[107,108]. In the current study, no differences were found between men and women, although over time, a difference in the response rate has been reported. This could be related to differences in demographics (other than gender) and disease characteristics at baseline or to intrinsic differences in responsiveness to prucalopride between men and women. Furthermore, prucalopride was significantly more effective than placebo, as demonstrated by many secondary endpoints, including improvements in PAC-SYM (Patient Assessment of Constipation Symptoms) and PAC-QOL (Patient Assessment of Constipation Quality of Life) scores and rescue medication use. An exploratory efficacy analysis showed that prucalopride treatment was effective even in patients with very severe CIC and those with no SBMs at baseline.
In the current integrated analyses, the NNT with prucalopride used to achieve the primary efficacy endpoint in one patient was 8.8 (95%CI: 7.1-11.6). In a meta-analysis of data from three trials of linaclotide in patients with CIC, the NNT for the primary endpoint of these trials (3 SCBMs/wk and an increase of 1 SCBM/wk, for 75 % of weeks) was 7 (95%CI: 5-8)[109]. Prucalopride has a favorable safety and tolerability profile[110]. Notably, no cardiovascular safety signals were observed. Indeed, the mean QT interval corrected according to Bazett’s formula (QTcB) and the mean QT interval corrected according to Fridericia’s formula (QTcF) were both/470 ms. A potential limitation of this integrated analysis is moderate heterogeneity (I2 = 56%) due to a deviation of the results of one of the six trials compared to the others.
Prucalopride was well absorbed from the gastrointestinal tract, with an absolute oral bioavailability of more than 90%. Its main elimination route was via theurine (60%-70% excreted unchanged in the urine). Because prucalopride has a low level of metabolism by liver, its pharmacokinetics is unlikely to be altered by hepatic impairment, and no CYP3A4 drug interactions are anticipated. In Europe, 2 mg of prucalopride has been approved for the treatment of chronic constipation in women who have no adequate response to laxatives[111].
Headache (in 25%-30%), nausea (12%-25%), abdominal pain (16%-23%), and diarrhoea (12%-19%) were observed as adverse events.
Recently, a randomized trial compared prucalopride with Macrogol/PEG 3350 plus electrolytes in patients with CIC. Prucalopride showed a non-inferiority for the primary outcome, even though PEG showed a superiority in improving gastrointestinal transit, stool frequency, and number of spontaneous bowel movements[112]. Although no studies have yet evaluated the efficacy of prucalopride in IBS-C, it is expected that it may also be efficacious for the disease symptoms. However, the worsening of abdominal pain may limit its use in clinical practice.
YKP10811 is a novel substituted benzamide derivative, small molecule with high binding affinity to the 5-HT4 receptor[113]. In cellular functional assays conducted with the 5-HT4 receptor, YKP10811 showed weak agonist activity that was dose dependent and reproducible. These results indicated that YKP10811 acts as a partial agonist of the 5-HT4 receptor. YKP10811 did not show any significant off-target binding to any other receptors, enzymes, or serotonin-receptor subtypes at 1 mmol/L, except for binding to the 5-HT2A receptor and the 5-HT2B receptor. Thus, YKP10811 has 120-fold and 6-fold lower affinity, respectively, for 5-HT2A and 5-HT2B receptors than for 5-HT4. In cellular functional assays, YKP10811 showed antagonist activity at the 5-HT2B receptor with a median inhibitory concentration. In rats, YKP10811 accelerated colonic transit by 37% at a dose as low as 1 mg/kg. In dogs, 0.3 mg/kg YKP10811 accelerated colonic transit by 45.5% at 2 h after dosing. The accelerated colonic transit in dogs was associated with significantly increased colon contractions and defecation. YKP10811 significantly reduced visceral hypersensitivity in multiple pain models in rats. In a phase I double-blind randomized 9-d placebo-controlled multiple-ascending dose study in healthy volunteers at doses of 5, 15, 30 and 45 mg once daily, YKP10811 was well tolerated with minimal side effects. In a single-center randomized parallel-group double-blind placebo-controlled study[114] in patients with functional constipation, YKP10811 enhanced gastrointestinal and colonic transit and improved bowel function during an 8-d treatment trial. The effect of YKP10811 on colonic transit was mirrored by improvements in softer stool consistency and faster time to first bowel movement, suggesting that YKP10811 has encouraging effects on these clinical end points. In addition to pharmacodynamic effects in patients with functional constipation, improvements in bowel functions are validated and measurable end points recommended for the treatment of functional constipation[115]. These findings suggest that YKP10811 may be a potential new medication for the treatment of functional constipation. YKP10811 had a robust effect on accelerating, by 30% to 40%, colonic emptying when compared with placebo. Ascending colon emptying has been reported to have the greatest contribution to overall colonic transit[116] because the ascending and transverse colon constitute the “reservoir” or storage regions of the human colon[117]. Among the other 5-HT4-receptor agonists previously studied with the same method, 4 mg prucalopride and 30 and 50 mg velusetrag[118] also accelerated AC emptying. Emptying of the proximal colon correlates linearly with faecal weight[119], which largely reflects stool water content, and as expected based on prior studies, the overall colonic transit was correlated linearly with stool consistency, with less significant association with the number of bowel movements per day. The results also showed the dual action (agonist/antagonist) of YKP10811 seen in in vitro studies. YKP10811 facilitated the electrical field stimulation-induced neurogenic twitch of guinea pig ileum at lower concentrations. This type of dual action (agonist/antagonist) of YKP10811 under the same assay conditions was also shown in the peristaltic reflex test, with an EC50 of 0.5 mmol/L and an IC50 of 21 mmol/L. There is a significant gap in concentration ranges (> 40-fold difference) for stimulatory vs inhibitory effects of YKP10811 in vitro (unpublished data; SK Life Science, Inc). Two participants, 1 receiving placebo and 1 receiving 20 mg YKP10811, had prolonged QTc (> 470 ms). Both participants discontinued the study on the advice of the investigators, even though the QTc prolongation was minimal (functions in patients with functional constipation). Thus, YKP10811 is likely to be of benefit to patients with functional constipation without rectal evacuation disorders. The safety and efficacy of this novel agent should be studied in larger multicentre clinical trials. With further studies, the current data suggest that YKP10811 would expand the therapeutic options beyond the recently approved secretagogue medications for the treatment of functional constipation, lubiprostone and linaclotide[120,121].
YKP10811 was reported to be safe and tolerable in healthy volunteers. Except for a Phase II clinical trial in C-IBS patients (NCT02082457)[122], there were only two registered Phase II trials that evaluated the efficacy and safety of YKP10811 in comparison with placebo in subjects with CIC (NCT015 23184, NCT01989234)[123,124]. Collectively, 420 eligible subjects were enrolled to be treated with different doses of YKP10811 or placebo once daily for 8 d and 12 wk in two trials. The results have not been completed for reporting yet. This drug is pending to pass Phases II and III of clinical trials, expected in 2016.
Renzapride (a novel benzamide substitute) is a full agonist for the 5HT4 receptor and an antagonist to 5HT2b and 5HT3 receptors. It can accelerate the gastrointestinal tract transit and motility stimulating the 5HT4 and 5HT2b receptors[125], and it appears to be a promising therapeutic agent for constipation, which is predominant in IBS patients. It is safe and has only a few adverse effects[126,127]. Several clinical trials have been performed to evaluate its potential efficacy in IBS patients, confirming that renzapride does not cause cardiac arrhythmias in clinical doses, unlike cisapride[126,127]. It is excreted renally and is not metabolized by cytochrome P450 enzymes. Thus, no drug interactions via affecting cytochrome P450 enzymes have been reported[125,127]. Renzapride stimulates colonic transit and reduces transit time and pain in IBS patients due to its prokinetic property, providing a benefit in those patients with constipation[128]. In addition, a dose-dependent efficacy of this drug has been demonstrated[126]. In a phase II study of 46 women with IBS-C, renzapride at a dose of 4 mg q.d. favoured colonic transit and increased ascending colon emptying compared to placebo[129]. A large multicentre European trial confirmed the effects of 4 mg renzapride q.d. in the improvement of frequency of bowel movements and stool consistency in IBS-C[130]. Much pharmacodynamic data support renzapride’s prokinetic effects. As for the prior European study, statistically significant differences in the frequency of bowel movements and stool consistency in favour of renzapride 4 mg q.d. were relatively small. In addition, renzapride did not improve the feelings of completeness of bowel movements or the amount of straining. Several systematic reviews have shown the efficacy of 5HT receptor modulators in IBS patients. In 2009, Ford et al[131] conducted a meta-analysis by reviewing placebo-controlled clinical trials up to 2008 on the efficacy of known 5-HT3 antagonists and 5-HT4 agonists in IBS. They observed that renzapride and cisapride were not more effective than placebo in IBS patients.
Other investigators also evaluated the efficacy of combined 5HT3 antagonists/5HT4 agonists (cisapride and renzapride) in IBS patients[132] and observed that 1 and 2 mg of renzapride was ineffective in relieving IBS symptoms, supporting the results obtained by Ford. However, these authors showed that 4 mg of renzapride was significantly more effective than placebo.
Recently, a meta-analysis[133] from randomized placebo-controlled clinical trials, including 2528 C-IBS, non-C-IBS, and non-D-IBS patients according to the Rome criteria, was performed. The study confirmed that renzapride had no significant effects in relieving symptoms in IBS patients compared to placebo. To reach a convincing conclusion on the effectiveness of renzapride, a clinical trial compared with placebo was performed. Renzapride at a dose of 4 mg was compared to placebo for 5 wk or less and more than 5 wk. Although the differences were not statistically significant, the results were clinically important and significant for both treatment durations. Therefore, these results could be considered for renzapride 4 mg, while more trials are necessary to determine the effectiveness of this novel drug more precisely. As regards adverse effects, no statistically significant differences between renzapride and placebo were found, except for diarrhoea occurrence, which was higher in patients treated with renzapride. In addition, renzapride caused more withdrawals due to adverse effects and/or low efficacy in patients. One of the limitations of this meta-analysis was the evaluation of trials with different patient inclusion criteria (age, sex, lifestyle and compliance). In addition, the trials evaluated had different durations of treatment and endpoints. The treatment durations ranged from 2 wk[134] to 12 wk[135]. To avoid heterogeneity, patients were divided into two groups according to treatment duration and time of reporting the results (5 wk or less and more than 5 wk), although there were few data in each group. The safety data from these phase III studies indicated that renzapride was generally well tolerated, even though ischaemic colitis was reported in the long-term study in 3 patients. However, evaluating the total of patients treated with renzapride during the study, the overall rate of ischaemic colitis appeared comparable with that reported for other 5-HT3 receptor antagonists[136]. In conclusion, renzapride is not only superior to placebo in relieving IBS symptoms (abdominal pain and discomfort), but it also causes increased diarrhoea occurrence compared with placebo and appears to be associated with many drop-outs. Therefore, this drug might be a cost burden to patients, without any advantages in efficacy. Indeed, during the trial, no improvements in frequency of bowel movements, straining, or completeness of evacuation were observed in patients treated with renzapride. Taken together, these data suggest that renzapride is unlikely to provide clinically meaningful improvement in IBS symptoms.
Velusetrag is an orally administrated available 5-HT4 agonist developed by Theravance. The binding affinity of this drug for the 5-HT4 receptor is more than 500-fold that of other 5-HT receptor subtypes[137]. The major metabolite detected in plasma after oral velusetrag is THRX-830449, which is a full agonist and is approximately equipotent to velusetrag. Metabolism occurs through the CYP3A4 system. In healthy subjects[138], at steady a state, the THRX-830449 to velusetrag AUC ratio is approximately 0.5 following once-daily dosing of velusetrag (15 mg).
Increased smooth muscle contractility of the antrum, fundus, duodenum and jejunum was observed in velusetrag-treated dogs[139]. Velusetrag increased guinea-pig colonic transit and produced dose-dependent relaxation of the rat esophagus[140]. Relief of constipation using velusetrag was also confirmed in chronic constipation patients[141].
Velusetrag was approximately 6- or 86-fold more potent than cisapride or mosapride after intravenous dosing and 9- or 18-fold more potent than tegaserod or cisapride, respectively, after intraduodenal administration[141].
Its low risk for cardiovascular events has been confirmed in an in vitro investigation demonstrating no effect on hERG channel conductance[140]. In a preclinical study that compared in vivo activity of velusetrag vs tegaserod in guinea pig, the subcutaneous administration of velusetrag increased colonic transit more than tegaserod did. Velusetrag was more potent than tegaserod when orally administered in a dog GI smooth muscle contractility model[142]. Velusetrag exhibited an acceptable oral bioavailability in rats and dogs[140], while the systemic effect of the drug was increased by an increase in the administered dose in healthy volunteers[143-145]. Both single (up to 70 mg) and multiple (up to 50 mg, for 2 wk) dosing of velusetrag in healthy subjects showed a dose-dependent effect on GI motility[145].
There have been two Phase II clinical trials to evaluate the clinical efficacy of this drug. In one of these two studies[138], 60 healthy volunteers were randomly assigned, in double-blind fashion, to placebo or 5, 15, 30 or 50 mg velusetrag, with transit measurements after single and 6-d dosing.
The GI transit was evaluated in a randomized double-blind placebo-controlled study conducted on 60 healthy subjects randomly assigned to receive velusetrag at a dose of 5, 15, 30 or 50 mg or placebo either as a single dose or for 6 d[137]. Velusetrag at single dose (30 and 50 mg) favoured colonic transit, evaluated by colonic filling at 6 h and geometric center at 24 h, while this effect was not observed in patients treated with placebo. Similarly, velusetrag at multiple doses (15-50 mg doses) favoured gastric emptying compared with placebo (P = 0.002). In this study by Manini et al[115], an improvement of stool frequency and consistency by velusetrag in a subset of 11 patients with chronic constipation was also reported. Pharmacokinetic evaluations demonstrated a similar profile in healthy and CIC subjects[143]. Velusetrag was well tolerated in the Phase I study when administered in single and repeated doses in healthy subjects. In the Phase I clinical trial, the most commonly reported adverse event was diarrhoea, which is expected because of velusetrag’s mechanism of action[141,143]. In a Phase II randomized, double-blind, placebo-controlled trial, the efficacy and safety of velusetrag were compared with placebo in 401 subjects with CC. SBM frequency, CSBM and other associated symptoms with CIC were significantly improved compared with placebo in patients who received velusetrag for 4 wk. The most effective dose was 15 mg once daily. Most of the adverse events, such as diarrhoea, headache, nausea and vomiting, were mild to moderate. These adverse events were common in the first days of treatment with the dose of 50 mg once daily. The number of withdrawals due to adverse events was 18 vs 1 for the velusetrag- and placebo-treated subjects, respectively. The number of withdrawals were 4, 3 and 11 in the 15-, 30- and 50-mg treated groups, respectively. However, the medicine was well tolerated with no cardiac complications[146]. Another Phase II study of velusetrag in 401 patients with chronic constipation treated for 4 wk showed that there were significant treatment effects on the average daily number of bowel movements compared with placebo[147].
The most common adverse effects of velusetrag were those frequently associated with 5-HT4 agonists, including diarrhoea, headache and nausea. These dose-dependent adverse effects were mild to moderate and usually occurred within the initial days of dosing. Clinically relevant doses of velusetrag in animals or humans did not generate severe side effects on blood pressure, heart rate or electrocardiogram. In isolated porcine or canine coronary arteries, velusetrag showed no contractile activity[148]. In the randomized, double-blind, placebo-controlled study in 60 healthy subjects, there was no significant treatment effect on heart rate recorded by ECG after treatment for the prior 5-6 d. In this study, there were also no serious adverse events, and predictable GI effects such as diarrhoea and altered bowel movement were the main adverse events recorded[140]. These results suggest that velusetrag appears to be well tolerated. Further careful clinical studies will be required to further evaluate the safety and tolerability of this drug.
Naronapride (ATI-7505)[149] is a benzamide 5-HT4 receptor agonist that activates 5-HT4 receptors but has almost no actions on the other 5-HT subtypes. The design of ATI-7505 was based on the prototypical agent, cisapride. However, unlike cisapride, which is a mixture of (3R, 4S) and (3S, 4R) isomers of substituted piperidine-based scaffolds, ATI-7505 is the pure (3S, 4R) isomer. ATI-7505, with its (R)-quinuclidinyl moiety, is metabolized by ubiquitous carboxyl esterases to a single metabolite, ATI-7500.
This potent and selective 5-HT4 receptor agonist showed different pharmacodynamic and pharmacokinetic properties from previous nonselective 5-HT4 agonists. Hydrolytic esterase metabolism, unlike oxidative CYP450 metabolism, is a large-capacity metabolic system that can easily handle therapeutic amounts of xenobiotics. This large-capacity system implies that other drugs metabolized by esterases are not expected to induce drug-drug interactions of ATI-7505 with other drugs. There is also no interaction with drugs metabolized by a different enzymatic system, such as CYP450[150]. Naronapride is not metabolized by CYP450 enzymes, and thus, less drug-drug interaction occurs.
A thorough QT study showed that naronapride had no obvious effect on cardiac repolarization at either therapeutic or supratherapeutic doses. The structure of naronapride is similar to that of cisapride, but it is more selective than cisapride and thus interacts minimally with hERG channels and 5-HT3 receptors[150]. ATI-7500, the main metabolite of naronapride, is 100-fold less active than the parent drug. Unlike prucalopride and velusetrag, neither naronapride nor ATI-7500 can pass the blood-brain barrier, therefore reducing the incidence of side effects. This new benzamide exhibited GI prokinetic effects, stimulated colonic transit and reduced stool consistency in healthy male and female subjects[151]. One Phase II randomized double-blind placebo-controlled dose definition study evaluated several doses of orally administered naronapride (20, 40, 80 and 120 mg twice a day) in 210 patients with CC. This study evaluated the clinical effects of 9 days’ treatment with three doses of ATI-7505 at 3, 10 and 20 mg on GI and colonic transit using a validated scintigraphic method. There were borderline effects on gastric emptying at half-time. However, ATI-7505 stimulated colonic transit at 24 h and ascending colonic emptying. There was looser stool consistency as measured by the Bristol stool form scale with the 10- and 20-mg t.i.d. doses. This finding suggests that ATI-7505 appears to have prokinetic properties in both stomach and colon in healthy subjects and, particularly, in the colon. Further clinical trials of larger numbers of patients with functional gastrointestinal disorders, such as patients with CIC, are required to evaluate clinical efficacy.
The inhibition of the delayed rectifier K+ current in response to ATI-7505 in patch-clamped HEK293 (human embryo kidney) cells transfected with the human IKr channel is very weak, suggesting that there would be an adequate safety window between activity in the GI tract and potential cardiac toxicity. In addition, the primary metabolite ATI-7500 is 100-fold less active than the parent drug at the 5-HT4 receptor and, as with ATI-7505, has no detectable HERG channel inhibitory activity at concentrations up to 100 μmol/L. Preliminary data on intensive cardiac safety monitoring suggest that ATI-7505 is safe as regards the cardiac profile[149].
The most common drug-related adverse events were headache, diarrhoea, nausea and vomiting. Headache and abdominal pain were reported more frequently by the maximum dose of naronapride[152].
Chenodeoxycholic acid (CDCA) is a bile acid that can induce colonic electrolyte secretion by acting on the membrane-bound bile acid GPBA receptor (TGR5) on enterocytes, subsequently leading to the stimulation of cAMP generation and electrogenic chloride secretion. Supplementation with specific bile acid analogues or by using drugs that inhibit ileal bile acid reabsorption may benefit constipation patients.
Oral chenodeoxycholic acid at doses of 750-1000 mg/d can increase bowel movements, decrease stool consistency, and reduce the time to defecation in IBS-C[153].
They were previously used for the dissolution of gallstones, and they are known to favour diarrhoea at high doses in healthy controls and constipation patients[154]. The effects of CDCA on gastrointestinal and colonic function have been evaluated in healthy volunteers and patients with irritable bowel syndrome with constipation. In a randomized controlled trial, 500 mg and 1000 mg CDCA given to 60 healthy volunteers for 4 d led to dose-dependent acceleration of colonic transit. In addition, significant increases in stool frequency, decreases in stool consistency, and improvements in ease of stool passage were reported with CDCA[155]. In a double-blind placebo-controlled study, Rao et al[153] demonstrated that sodium chenodeoxycholate[156] stimulated colonic transit and improved bowel function in 36 women with irritable bowel syndrome with constipation. Increased stool frequency, greater ease of stool passage and looser stool consistency were observed in patients treated with sodium chenodeoxycholate 500 mg or 1000 mg for 4 d as compared with controls. Unfortunately, over 40% of sodium chenodeoxycholate-treated patients had light abdominal cramping or pain. Whether these side effects could be mitigated at a lower dose remains to be determined.
Elobixibat is an orally administrated available potent inhibitor of ileal bile acid transporter with minimal systemic exposure[157]. Elobixibat (A3309) reduces bile acid enterohepatic recirculation and upregulates bile acid synthesis as measured by serum C4 levels. It also depletes liver cholesterol and reduces serum LDL[158], thus increasing the delivery of bile acids to the proximal colon, which in turn increased fluid secretion, colonic motility and stool frequency, and it improved stool consistency and relieved constipation-related symptoms in chronic idiopathic constipation patients[159,160].
In a phase I trial, elobixibat stimulated colonic transit in a dose-dependent way. In a randomized phase II trial, elobixibat at doses of 15 and 20 mg/d showed an improvement of stool consistency and of stool passage, increased the number of SBMs and reduced straining in female patients with CIC[161]. In a dose-finding randomized trial, elobixibat increased C4, reduced LDL cholesterol, increased colonic transit from 3 to 1.9 d and increased the number of SBM and CSBM/wk in patients with CIC compared to placebo. The treatment with elobixibat also resulted in an improvement of bloating severity, but no effects on abdominal pain or discomfort were reported[157]. The well-tolerated doses were 5-10 mg, with a discontinuation rate during the phase IIb trial of 13%, rising to 23% for the 15 mg group. Fifty-four percent of patients developed adverse events, such as abdominal cramps, relieved by defecation, and diarrhoea. However, the side effects were not different from those of the placebo group[162]. In a large randomized trial conducted on patients with CIC, the 10- and 15-mg doses increased SBMs and reduced the time to SBM (12 h with the 10-mg dose, 7 h with the 15-mg dose and 24 h with the placebo). In patients treated with elobixibat, an increased spontaneous laxation within 24 h was observed compared with placebo (75 % on 15 mg/d and 45 % on placebo).
The side effects of elobixibat are mainly gastrointestinal tract-related. Although higher dosages of elobixibat caused abdominal pain and diarrhoea more frequently, no severe adverse effects occurred in the Phase I and Phase II clinical trials. The Phase III clinical trials are ongoing to determine the best tolerated dose and to examine the effects of long-term administration.
Complete spontaneous bowel movements per week increased in a dose-dependent way. An improvement of stool consistency and bloating was observed at the 10- and 15-mg doses. Side effects such as abdominal pain and diarrhoea were also dose-dependent, notably for the 15-mg dose[157].
Elobixibat is a promising anti-constipation drug. However, there are no studies in cancer or in OIC (opioid induced constipation) patients. Due to its prokinetic activity, elobixibat is not recommended in patients with mechanical bowel obstruction.
According to the results of Phase II trials in chronic idiopathic constipation patients, elobixibat was safe and generally well to lerated, even at a dose up to 20 mg per day.
As illustrated by elobixibat, the advantages of IBAT inhibitors may be especially attractive, which may boost research on other IBAT inhibitors, such as SC-435, S-8921 and S-0960[163-165].
Lubiprostone, a first-in-class drug for the treatment of chronic idiopathic constipation and irritable bowel syndrome in adult women with constipation is believed to be a highly selective locally acting activator of ClC-2 channels[166]. Lubiprostone can tautomerize between the inactive form I and the active form II[167]. Lubiprostone acts mainly by activating specific type-2 chloride channels (ClC-2) on the apical membrane of the enterocytes[166] that are involved in ion and fluid transport across the epithelial membrane. Once channels are opened, chloride enters the enterocyte in the basal membrane through the action of Na-K-2Cl active cotransporters. This mechanism results in an electrochemical gradient favouring chloride secretion. It leads to an overall concentration-dependent raise in intestinal fluid secretion without any impairment on serum sodium and potassium levels. These mechanisms explain how lubiprostone increases the number of colonic spontaneous bowel movements per week. However, lubiprostone efficacy on the abdominal pain score is only partially known and needs further investigation. Lubiprostone also activates a prostaglandin receptor (EP4), which in turn activates cystic fibrosis conductance regulators (CFTR)[168]. The activation of EP4 receptors favours colonic smooth muscle and gastric longitudinal muscle via vagal nerve endings[169]. Lubiprostone changes mucin, which improves the gut microbiome, creating an anti-inflammatory environment[170]. Unlike linaclotide, lubiprostone does not increase pain thresholds[171].
Lubiprostone pharmacokinetics is not impaired by renal failure. However, great adverse events with a standard dose of lubiprostone can result in cases of mild-to-moderate hepatic impairment (Child-Pugh class A and B), which increases the lubiprostone metabolite M3. Thus, in cases of liver impairment, a reduction of lubiprostone starting doses is required. Lubiprostone metabolism does not involve cytochromes. The catabolism is mediated by carbonyl reductase in the stomach and jejunum[172]. Lubiprostone is unlikely to have major drug interactions.
In healthy subjects, a reduction in gastric emptying, an increase in gastric fasting volume, a reduction in maximum tolerated gastric volumes and a stimulation of small bowel and colon transit was observed with lubiprostone at a dose of 24 μg twice daily[173]. However, the effects on gastric motility may mask the nausea side effect.
Two 12-wk double-blind randomized multicentre placebo-controlled phase III clinical trials[174] and one 36-wk open-label extension study[175] contributed to the FDA’s approval of lubiprostone for the treatment of IBSC in women. A total of 1171 patients were randomized 2:1 to receive either 8 μg lubiprostone or placebo twice/d. The primary end point of each study was the evaluation of response rate, measured by patient-reported improvements from baseline in IBS-C symptoms. As secondary end points, monthly responder rates, changes from baseline in SBM frequency, stool consistency, straining, distention, abdominal pain/discomfort (each measured on a 5-point Likert scale) and change in health-related quality of life were evaluated.
The discontinuation rate in both studies was 24%, firstly due to withdrawal of consent and secondly to adverse events and perceived lack of efficacy. Lubiprostone was superior in the primary end point compared to placebo (17.9% vs 10.1%, P = 0.001). Patients treated with lubiprostone reported more improvements in all secondary end points than placebo. Lubiprostone was associated with a more significant improvement in abdominal pain/discomfort than placebo from baseline to month 2 (0.43 vs 0.35, P = 0.039) and month 3 (0.45 vs 0.36, P = 0.028).
Lubiprostone significantly changed the mean SBM frequency from baseline to month 1 compared with placebo, even though the numerical data were not included.
Two 4-wk phase III randomized double-blind placebo-controlled multicentre clinical trials were conducted on a total of 479 patients to evaluate the short-term efficacy and safety of lubiprostone in patients with CIC with identical study designs and primary end points. After a 2-wk baseline period, eligible subjects received 24 lg lubiprostone or placebo twice/d.
The number of patient-reported SBMs, defined as any BM occurring 24 h or longer after the use of an alternative drug used to relieve constipation (rescue medication), during the first week of treatment was the primary end point of each study[176,177]. Lubiprostone was associated with a statistically higher frequency of SBMs during the first week of treatment than placebo.
Improvements in other secondary end points, such as stool consistency, straining, and constipation severity were also observed in patients treated with lubiprostone compared with placebo in all 4 wk in both studies. However, significant improvement in abdominal distention and discomfort compared with placebo was not observed in either study.
As regards side effects, a similar percentage of patients reporting at least one treatment-related adverse event for IBS-C was observed in the lubiprostone (50%) and placebo (51%) groups. The most common side effects were gastrointestinal (19% with lubiprostone vs 14% with placebo). Serious adverse events were similar between the two groups (1%). Nausea was the most frequent treatment-related event[11,12], although it may be reduced by administering lubiprostone with meals.
A unique adverse effect occurred with the initial dose. In rare cases, acute transit dyspnea and ischemic colitis were observed[176,178].
Caution in the use of lubiprostone should be used for infants of breastfeeding mothers due to the risk of diarrhoea[179]. Limited data are available on the lubiprostone effects in paediatric patients, and further, larger studies are required. In an open-label 4-wk clinical trial conducted on paediatric patients with CIC (mean age 10.2 years)[179], lubiprostone was efficacious and well tolerated at daily doses of 12-48 lg. The recommended dose of lubiprostone for the treatment of CIC in both adult men and women is 24 lg twice/d, while for the treatment of IBSC in adult women, it is 8 lg twice/d. Due to its minimal systemic absorption and its metabolism through a cytochrome P450-independent pathway, lubiprostone-drug interactions are unlikely, even though in vitro studies have suggested that methadone may decrease the efficacy of lubiprostone by reducing chloride channel type 2 activation[179].
In patients with moderate or severe hepatic impairment (Child-Pugh class B or C), a dose reduction might be suggested, while in patients with renal failure, no dosage adjustment is recommended.
A small percentage (8%-13%) of patients over 65 years were included in clinical trials with lubiprostone. The safety profile was similar in elderly and younger patients, even though, due to the limited the number of patients over 65 years, no differences in clinical response were observed[179], and further studies are needed.
Like linaclotide, lubiprostone is contraindicated in mechanical bowel obstruction. To confirm the indications in the treatment of IBS-C in adults, more and larger trials are required. Due to the chronic nature of IBS-C and CIC, post-marketing studies are necessary to confirm the long-term efficacy and safety of lubiprostone. All randomized clinical trials were of limited duration (12-26 wk). However, in open-label extension studies, a safety over 52 wk was demonstrated.
Despite the efficacy, the side effects (e.g., nausea, abdominal pain) and the high cost may limit the use of lubiprostone.
Tenapanor, also known as AZD1722 or RDX5791, is a first-in-class orally available inhibitor of NHE3 that is minimally absorbed in the gastrointestinal tract-this constitutes a significant therapeutic benefit, as it may act on the drug target[180,181]. Consequently, tenapanor increases intestinal Na+ contents, which leads to an increase in intestinal fluid volume and accelerates the whole GI transit, as shown in rats. Moreover, tenapanor inhibits the absorption of phosphorus, which is independent of typical phosphorus transporters in the intestines, namely, sodium-dependent phosphate transport protein 2B (NaPi2b) and Na (+)-dependent phosphate transporter (PiT1). Tenapanor is stable at room temperature and is formulated into tablets ranging from 1 to 50 mg. Absorption, distribution, metabolism and excretion (ADME) studies have revealed that tenapanor is minimally absorbed and metabolized. For example, experiments in rats showed 92.2% ± 1.6% recovery of tenapanor in faeces upon oral administration[182]. In humans, the inactive metabolites of tenapanor were found in plasma, but they were only approximately 9% of the parent compound. In pharmacokinetic studies, tenapanor was observed at relatively low concentrations in plasma (average < 3 ng/mL) of rats and dogs, but only sporadically (29/76 and 0/92, respectively).
Oral administration of tenapanor (at doses of 0.1 and 3 mg/kg) produced a dose-dependent increase in faecal water content and stool consistency in rats. The effect of tenapanor at a dose of 50 mg/kg twice daily on stool form was assessed in cynomolgus monkeys. The animals were observed for 4 d before treatment. Soft or watery stools were observed in monkeys on tenapanor treatment, and stool consistency was normalized on day 6 of the experiment. Under physiological conditions, tenapanor given orally at doses of 3, 10, 30 and 50 mg/kg did not affect visceral sensitivity or the changes in intestinal volumes induced by colorectal distension in comparison with the control and tegaserod-treated (5 mg/kg administered per os) groups[183]. However, tenapanor (30 and 50 mg/kg) had a dose-dependent antinociceptive effect in the acute restraint stress-induced intestinal hypersensitivity to colorectal distension. The antinociceptive potential of tenapanor was comparable with that of the tegaserod-treated group.
The safety and tolerance of tenapanor were assessed in a randomized, double-blind, placebo-controlled study[184,185]. Eighty healthy volunteers were included in the study (male and female). Tenapanor was given orally at the doses ranging from 10 to 900 mg (as a single administration) and for 7 consecutive days at doses ranging from 3 to 100 mg to assess the safety of tenapanor administration. Tenapanor was also beneficial for the percent of days with a spontaneous bowel movement. Finally, no serious side effects were observed, and there were very few adverse events[186]. Phase IIa In a IIa double-blind randomized placebo-controlled study on 181 patients with IBS-C[187], tenapanor was given orally at doses of 10, 30 and 100 mg once daily for 4 consecutive weeks with 2 wk follow-up. The primary end point (change in complete spontaneous bowel movements from baseline to week 4) was not met in this study, and the incidence of diarrhoea was comparable with that of the placebo group. However, an improvement in bloating and abdominal pain was noted in IBS-C patients. In Phase IIb In a IIb randomized double-blind placebo-controlled multicentre study, 371 IBS-C patients were divided into four groups: placebo and tenapanor (5, 20 and 50 mg) treated twice daily for 12 wk with 4 wk follow-up. The primary efficacy end point was met in 60.7% of the tenapanor-treated group (at a dose of 50 mg) vs 33.7% of the placebo-treated group. The overall responder was met in 50.0% of the tenapanor-treated group (50 mg) vs 23.6% for placebo (after 12 wk). After 12 wk, adequate relief in IBS-C symptoms was observed in 63.1% of the tenapanor-treated group (50 mg twice daily) vs 39.3% in placebo. The effectiveness of tenapanor therapy was maintained during entire time of the clinical study. The treatment satisfaction patient scale questionnaire showed that tenapanor-treated (50 mg) IBS-C patients were quite or very satisfied (65% vs 38% for the placebo-treated group). The drug was well tolerated in all groups, and no serious adverse effects were noted. The most common adverse effect was diarrhoea in the tenapanor-treated group (50 mg twice daily), reported in 11.2% of IBS-C patients vs 0% in placebo. Safety and tolerability In the preclinical studies in rats, tenapanor did not influence gastric emptying[187].
Melatonin is engaged in the regulation of gastrointestinal motility and sensation. When administered orally in pharmacological doses, it has shown beneficial effects on abdominal pain in IBS patients without any effects on sleep disturbances[188]. It was also shown that oral melatonin significantly stimulated colonic transit time in healthy subjects, and it may be a promising option for future research on the agents modulating bowel motility[189]. Melatonin synthesized in the enteroendocrine cells of the intestinal mucosa reaches the liver via the portal vein[190]. Melatonin is a potent accelerator of duodenal mucosal bicarbonate secretion, which neutralizes the acid content of the stomach in the duodenum, and it seems to be engaged in the acid-induced stimulation of the secretion[191]. Melatonin protects the gastrointestinal mucosa due to an antioxidant action, a decrease in secretion of hydrochloric acid, stimulation of the immune system, promotion of epithelial regeneration, and increased microcirculation[192,193].
Recently, it was shown that patients with IBS had significantly lower 6-SMLT (6-sulphatoxymelatonin)/creatinine level compared with healthy controls[194]. The lack of statistical difference in 6-SMLT/creatinine levels between the constipation and diarrhoea groups is difficult to explain. In some patients, the symptoms could be recurrent, or there could be some subjects with mixed (IBS-M) or unsubtyped (IBS-U) IBS. This study’s results agree with those obtained by Bultman[195] and Lu et al[196] who performed the study on female patients with IBS and found decreased salivary melatonin and urine 6-SMLT level compared to non-IBS volunteers. Low melatonin levels were observed in women with eating disorders. Low melatonin concentrations have been associated with increased depressive symptoms, such as sadness, bodily discomfort, inner tension, difficulties in attention concentration and pain.
Serotonin, an endogenous amine and the precursor of melatonin, synthesized and released from entero-endocrine cells of the gastrointestinal mucosa is thought to play an important role in the pathogenesis of IBS[197]. Antagonists of the serotonin 5-HT3 receptor are beneficial in patients with IBS-D, whereas the partial agonist of the serotonin 5-HT4 receptor (tegaserod) alleviates symptoms of IBS with constipation, especially in females. The role of melatonin as the regulator of circadian and seasonal rhythmicity has been established[198,199]. Patients with functional disorders of the gastrointestinal tract also had sleep disorders, and some of them suffered from increased neural excitability and anxiety[199,200]. There were speculations concerning a possible role of melatonin in functional dyspepsia (FD), particularly ulcer-like dyspepsia. In two types of FD, one with epigastric pain and another with postprandial distress syndrome, the melatonin level is varied, and different dyspeptic symptoms may be related to differences in melatonin secretion. Sleep disturbances are common in patients with IBS and are among the most important extraintestinal symptoms, markedly affecting quality of life and psychosocial well-being[201]. In a double-blind placebo-controlled study, Camilleri et al[202] showed that melatonin improves abdominal pain in IBS patients with sleep disturbances. Currently, conventional treatment for irritable bowel syndrome is quite unsatisfactory. Despite multiple therapeutic interventions, no long-term effect has been achieved. On the other hand, up to 80% of patients with IBS treated with hypnotherapy showed an improvement of their symptoms[203]. These observations emphasize the possible role of melatonin in the pathogenesis of irritable bowel syndrome and in its therapy[204,205].
Daikenchuto (TU-100), a traditional Japanese drug (Kampo medicine), is indicated in the treatment of adhesive bowel obstruction[206,207]. TU-100 is a mixture of extract powders from dried Japanese pepper, processed ginger, ginseng radix, and maltose powder. In many trials, the TU-100 prokinetic effect has been demonstrated to be useful in treating GI hypomotility[208]. Studies conducted on postoperative patients after gastrointestinal surgery showed that TU-100 prevented postoperative ileus, but little is known about the TU-100 effects in patients who did not undergo major gastrointestinal surgery[209]. Iturrino et al[210] performed a randomized controlled trial to evaluate the effects of oral TU-100, 2.5 g t.d.s. or 5 g t.d.s. compared to placebo t.d.s. on gastrointestinal and colonic transit, rectal compliance and sensation thresholds, anal sphincter pressures and bowel function in women with functional constipation. In this study, there were no significant effects on gastrointestinal and colonic transit, rectal compliance, anal sphincter pressures, recto-anal pressure difference, or rectal sensation thresholds. The highest dose was associated with lower rectal sensation thresholds for first sensation and gas. There were no treatment effects on psychosensory symptoms, stool frequency, stool consistency or quality of life[211].
On the other hand, Manabe et al[211] reported that TU-100 provided a clinically significant promotility effect in small bowel and ascending colon transit in healthy subjects. TU-100 is quite safe and well tolerated and is a potential treatment for IBS-C and functional constipation[210].
Recently, however, Acosta et al[212] did not report any significant effects of TU-100 on rectal sensation ratings, sensation thresholds, rectal fasting or postprandial tone, rectal compliance, bowel function, abdominal pain or bloating scores, or IBS quality of life. Further randomized controlled trials in patients with IBS-C or functional constipation using both clinical and validated biomarkers are required.
DA-6886, a gastrointestinal prokinetic benzamide derivative, is a novel 5-HT4 receptor agonist. Experimental studies showed that it may represent a highly potent and selective 5-HT4 receptor agonist to stimulate colonic transit in mice, having a favorable safety profile in patients with IBS-C and chronic constipation[213]. Currently, a phase I dose block-randomized double-blind placebo-controlled single/multiple dosing dose escalation clinical trial with an open-labelled food effect is being conducted to evaluate the safety and pharmacokinetics of single-dose DA-6866 in healthy male subjects[214].
Table 3 sums up the literature findings about irritable bowel syndrome-C therapies.
| Drug | Ref. | No. of patients | Study design | Outcome |
| Linaclotide | Andresen et al[82] | 36 women with IBS-C | Phase IIa randomized, double-blind, placebo- controlled trial. Patients were randomized in a 1:1:1 fashion to placebo, linaclotide 100 μg, and linaclotide 1000 μg and was evaluated the effect of 5 d | No treatment effects were seen for gastric emptying or colonic filling with linaclotide. Significant treatment effects were found for ascending colon emptying t½ times (P = 0.015) and overall total colonic transit times at 48 h (P = 0.02), for the 1000 μg dose (P = 0.004) but not the 100 μg dose, as well as overall treatment effects on increased stool frequency, decreased stool consistency, improved ease of passage, and acceleration of time to first bowel movement (P < 0.001)[82] |
| Johnston et al[83] | 420 patients with IBS-C | Phase IIb randomized, double-blind, parallel-group, multicenter, placebo-controlled trial evaluate 12 wk of linaclotide at a daily dose range of 75-600 μg | Compared with placebo, all doses of linaclotide significantly improved bowel habits, including frequency of SBMs and CSBMs, severity of straining, stool consistency, as well as abdominal pain scores. Abdominal discomfort, bloating, and global IBS-C measures were also improved, for all doses except for the 75 μg (abdominal discomfort) and 150 μg dose (bloating). Effects were present for the first week, and sustained throughout the 12 wk of treatment | |
| Chey et al[84] | 804 adults with IBS-C | Phase III trials randomized, double-blind, placebo-controlled to receive linaclotide 290 lg or placebo daily for 26 wk, with change-from-baseline end points measuredat 12 and 26 wk | Over 12 wk, the FDA combined primary end point was achieved by 33.7% of patients receiving linaclotide compared with 13.9% of patients receiving placebo (P < 0.0001) | |
| Videlock et al[109] | 7 trials of linaclotide in patients with IBS-C or CC | A meta-analysis from MEDLINE, EMBASE, and the Cochrane central register of controlled trials were searched for randomized, placebo-controlled trials | The NNT for the primary endpoint of these trials (3 SCBMs/wk and an increase of C1 SCBM/wk, for 75% of weeks) was 7 (95%CI: 5-8) | |
| Rao et al[86] | 803 adults with IBS-C | Phase III trials randomized, double-blind, placebo-controlled to receive linaclotide 290 lg or placebo once/d for 12 wk | Linaclotide demonstrated statistically significant improvements in all primary and secondary efficacy end points compared with placebo Severity of straining, constipation, and stool consistency also improved in the linaclotide group compared with the placebo group | |
| Plecanatide | Miner et al[92] | 951 patients with CIC | Phase III, randomized, double-blind trial, received plecanatide 0.3, 1 or 3 mg, or placebo once/d for 12 wk | The proportion of overall responders was significantly greater with plecanatide 3 mg compared with placebo (19% vs 10.7%, P = 0.009); weekly responder rates were also significantly greater for plecanatide 3 mg than placebo for weeks 1-12. Improvements in stool frequency, consistency, straining, and quality of life were also noted with the 3-mg dose vs placebo. Data for other plecanatide doses were not reported |
| Prucalopride | Quigley et al[88] | 620 patients with CC | A double-blind, placebo-controlled study. Patients receiving 2 or 4 mg of prucalopride for 12 wk | Increased one or more CSBMs per week compared to patients in the control group |
| Camilleri et al[98] | 713 patients with CC | A double-blind, placebo-controlled study. Patients receiving 2 or 4 mg of prucalopride for 12 wk | Increased frequency of three or more CSBMs per week, and improved evacuation completeness, perceived disease severity, and quality of life | |
| Müller-Lissner et al[97] | Elderly patients aged 65 years and older with CC | A double-blind, placebo-controlled study | No changes in electrocardiogram or corrected QT (QTc) interval were reported, indicating its safety for the treatment of CC in the elderly | |
| Patients receiving 2 or 4 mg of prucalopride for 12 wk | ||||
| Ke et al[102] | 4 Randomized, Placebo-controlled Studies | A Pooled Analysis | Safe and well-tolerated It was also effective in improving the abdominal symptoms of CC such as abdominal discomfort, bloating, straining, and painful bowel movements | |
| YKP10811 | Shin et al[114] | 55 patients | A single-center, randomized, parallel-group, double-blinded, placebo-controlled study were assigned randomly to groups given YKP10811 10 mg (n = 15), 20 mg (n = 16), 30 mg (n = 15), or placebo (n = 11) daily for 8 d | Enhanced gastrointestinal and colonic transit and improved bowel function during an 8-d treatment trial. In general, the 10-mg and 20-mg doses were the most effective in accelerating colonic transit. No serious adverse events were observed |
| Renzapride | Camilleri et al[101] | 46 women with IBS-C | In a phase II study | Renzapride 4 mg q.d. accelerated colonic transit and increased ascending colon emptying vs placebo |
| George et al[130] | 510 patients | Multicentre, randomized, placebo-controlled, double-blind study men and women were randomized to placebo or renzapride (1, 2 or 4 mg/d) for 12 wk | 4 mg renzapride q.d. in terms of improving frequency of bowel movements and stool consistency | |
| Ford et al[131] | 29 RCTs were eligible for inclusion | Meta-analysis of placebo-controlled clinical | Renzapride and cisapride were not more effective than placebo in IBS patients | |
| Mozaffari et al[133] | 2528 C-IBS and non C-, non D-IBS patients | Meta-analysis from randomized placebo-controlled clinical trials | Renzapride has no significant advantage over placebo in relieving symptoms in IBS patients | |
| Velusetrag | Manini et al[115] | 60 healthy volunteers | Phase II clinical trials, pt were randomly assigned, in double-blind fashion, to placebo, 5, 15, 30 or 50 mg velusetrag, with transit measurements after single and 6-d dosing | Single doses of velusetrag (30 and 50 mg), but not placebo, accelerated colonic transit, as measured by colonic filling at 6 h and geometric center at 24 h |
| Goldberg et al[140] | 401 subjects with CC | In a Phase II randomized, double-blind, placebo-controlled trial | Short bowel movement (SBM) frequency, complete SBM and other associated symptoms with CC were significantly improved in comparison with placebo in patients who received velusetrag for 4 wk | |
| Naronapride | Dennis et al[149] | 210 patients with CC. | Phase II, randomized, double-blind, placebo-controlled, dose definition study (orally 20, 40, 80 and 120 mg twice a day) to evaluate the clinical effects of 9 days’ | There were borderline effects on gastric emptying at half-time; however, ATI-7505 accelerated colonic transit at 24 h and ascending colonic emptying |
| Chenodeoxycolic acid | Odunsi-Shiyanbade et al[155] | 60 healthy volunteers | Randomized controlled trial, CDCA 500 mg and 1000 mg given for 4 d | Significant increases in stool frequency, decreases in stool consistency, and improvements in ease of stool passage were reported with CDCA |
| Rao et al[153] | 36 female patients | Double-blind placebo-controlled study | Accelerated colonic transit and improved bowel function | |
| Elobixibat | Simrén et al[162] | 30 patients | Dose-finding randomized trial five dose-levels (range: 0.1-10 mg/d) or to placebo | Increased C4, reduced LDL cholesterol and increased colonic transit from 3 to 1.9 d, and increased the number of SBM and CSBM/W in patients with CIC |
| Chey et al[157] | 190 patients | Were randomized to 5, 10, or 15 mg A3309 or placebo once daily. 8-wk, multicenter, randomized, double-blind, placebo-controlled, parallel group, phase IIb study, | A3309 increased stool frequency and improved constipation-related symptoms in CIC; effects were maintained over 8 wk of treatment | |
| Lubiprostone | Drossman et al[173] | 1171 patients in total | Two double-blind, randomized, multicenter, placebo-controlled phase III clinical trials,, a 12-wk randomized 2:1 to receive either lubiprostone 8 lg or matching placebo twice/d with food; a 36-wk open-label extension study | Lubiprostone was superior to placebo in the primary end point of overall responders, greater improvements in all secondary outcome measures compared with placebo |
| Chey et al[174] | ||||
| Johanson et al[175] | 479 patients in total | Two 4-wk phase III, randomized, double-blind, placebo-controlled, multicenter clinical trials | Patients treated with lubiprostone had a statistical higher frequency of SBMs during the first week of treatment compared with placebo | |
| Barish et al[82] | ||||
| Hyman et al[176] | 127 pediatric patients with CIC (mean age 10.2 yr) | An open-label 4-wk clinical trial | Demonstrated that lubiprostone was efficacious and well tolerated at total daily doses of 12-48 lg | |
| AZD1722 | Rosenbaum et al[185] | 181 patients with IBS-C | Phase IIa In a IIa double-blind, randomized placebo-controlled study, Tenapanor was given orally at the doses of 10, 30 and 100 mg once daily for 4 consecutive weeks with 2 wk follow-up | The primary end point [change in complete spontaneous bowel movements (CSBM) from baseline to week 4] was not met in this study and the incidence of diarrhea was comparable with placebo group. However, improvement in bloating and abdominal pain was noted in IBS-C patients |
| Rosenbaum et al[186] | 371 IBS-C patients | Phase IIb In a IIb randomized, double-blind, placebo-controlled, multicenter study | The overall responder was met in 50.0% of tenapanor-treated group (50 mg) vs 23.6% for placebo (after 12 wk). After 12 wk, adequate relief in IBS-C symptoms was observed in 63.1% of tenapanor-treated group (50 mg twice daily) vs 39.3% in placebo | |
| Rosenbaum et al[187] | 356 patients | A double-blind, placebocontrolled, randomized phase 2b trial 12-wk dose-ranging study evaluating tenapanor 5 mg, 20 mg or 50 mg b.i.d. vs placebo (1/2) | Tenapanor 50 mg b.i.d. significantly improved CSBM responder rate (primary endpoint) compared with placebo in patients with IBS-C. Tenapanor 50 mg b.i.d. also improved key secondary endpoints compared with placebo, including overall responder rate, abdominal pain responder rate and stool frequency. In addition, improvements were observed in several exploratory endpoints addressing a range of symptoms in patients with IBS-C. Tenapanor was generally well tolerated and had minimal systemic availability. Tenapanor shows promise as a future treatment option for patients with IBS-C | |
| Neu p11 (piromalatine) | Camilleri et al[202] | 40 IBS patients | In double blind placebo controlled study were randomly assigned to receive either melatonin 3 mg (n = 20) or matching placebo (n = 20) at bedtime for two weeks | Melatonin 3 mg at bedtime for two weeks significantly attenuated abdominal pain and reduced rectal pain sensitivity without improvements in sleep disturbance or psychological distress. The findings suggest that the beneficial effects of melatonin On abdominal pain in IBS patients with sleep disturbances are independent of its action on sleep disturbances or psychological profiles |
To date, the treatment options for IBS-D are limited and frequently unsuccessful. However, the incidence of IBS-D is currently increasing, thus causing a heavy economic burden both for patients and health care systems worldwide. As for IBS-C, a complete understanding of IBS-C pathophysiology has favoured the use of sensory end points such as complete spontaneous bowel movements and the FDA combined end point (abdominal pain and complete spontaneous bowel movements) in clinical trials[79].
Furthermore, also in the setting of IBS-D, preclinical studies in rodents have recently improved the understanding of the mechanisms underlying the alterations in gastrointestinal motility, sensitivity and secretion. A number of drugs that we will touch upon in the next section are actually in development.
Ramosetron is a potent and selective 5-HT3 receptor antagonist. 5-HT3 receptors can be widely found in the central and peripheral nervous system[215]. Intraluminal stimuli favour the release of 5-HT from enterochromaffin cells located in the mucosa[215]. When secreted, 5-HT can activate 5-HT3 receptors located on intrinsic primary afferent neurons with submucosal terminals. Thus, the peristaltic reflex and intestinal secretion can occur[215,216]. 5-HT also activates 5-HT3 and 5-HT4 receptors located on primary afferent neurons of both splanchnic and vagal fibres, which are involved in sensory and motor responses[217].
In experimental studies, corticotrophin-releasing hormone (CRH) exogenously administered or released from the central nervous system by stress peripherally activates the release of 5-HT, which in turn promotes defecation through the 5-HT3 receptor. Ramosetron decreased defecation by CRH in a dose-dependent way[218-219].
The first 5-HT3 receptor antagonist to be introduced was Alosetron, which has been demonstrated to be effective in the treatment of female patients with IBS-D[220]. However, due to serious gastrointestinal events (ischemic colitis and severe constipation), it is still only available in the United States and is indicated for women with severe D-IBS refractory to conventional therapy.
Ramosetron was first tested by Lee et al[221] in a multicentre randomized open-label trial on 343 men with IBS-D. Patients were randomized to a 4-wk treatment of ramosetron 5 mg once daily or mebeverine 135 mg three times daily. An improvement in abdominal pain/discomfort and bowel habits in the ramosetron and mebeverine groups was observed during the treatment period. A significant reduction in abdominal pain/discomfort and urgency, stool form score, and stool frequency severity scores in both treatment arms was reported compared with the baseline.
Adverse events were observed in 7% and 4% of patients treated with ramosetron and mebeverine, respectively, even though no statistical significant differences were reported. Additionally, all the side effects were mild or moderate[221].
Successively, Fukudo et al[222] performed a randomized double-blind placebo-controlled trial to determine whether ramosetron reduces diarrhoea in 296 male outpatients with IBS-D. Patients were treated with 5 mg of oral ramosetron (n = 147) or placebo (n = 149) once daily for 12 wk after a 1-wk baseline period. The primary end point was increased stool consistency in the first month. Secondary end points were the relief of overall IBS symptoms and the improvement of IBS-related quality of life. In the first month, patients on ramosetron treatment (74, 50.3%) showed an improvement of stool consistency compared to placebo (29, 19.6%) (P < 0.001). In patients treated with ramosetron, the monthly relief of overall IBS symptoms and IBS-related quality of life was demonstrated compared with placebo. Safety was evaluated in all 296 patients, with side effects occurring in 46.9% and 51.7% of ramosetron and placebo patients, respectively. All constipation and hard stools experienced in the ramosetron group that were related to the pharmacologic actions of ramosetron were classified as mild and resolved early without using rescue drugs[222].
In another randomized double-blind placebo-controlled trial performed by Fukudo et al[223] on 576 female outpatients with IBS-D, patients were given either 2.5 μg ramosetron or placebo once daily for 12 wk. Patients treated with ramosetron reported global improvement, increased stool consistency, a significant decrease in abdominal pain and discomfort and significant improvement in QOL compared with placebo. Of the patients tested with ramosetron, 11.0% complained of constipation[223]. Successively, in a phase III open-label uncontrolled long-term safety trial, the long-term safety and efficacy of 2.5 and 5 μg of ramosetron treatment in women with IBS-D was reported. However, the authors of the study concluded that “clinicians should be aware that one-fifth of women with IBS-D receiving ramosetron may suffer from constipation during treatment”[224].
Finally, a recent systematic review and meta-analysis performed to explore of the safety and efficacy of ramosetron both in male and female patients with IBS-D concluded that ramosetron is efficacious in both male and female patients with diarrhoea-predominant IBS, even if large-scale studies are needed to assess its effects on different ethnicities[225].
LX-1031 is a tryptophan hydroxylase (TPH) inhibitor that reduces peripheral serotonin production. It is indicated for conditions characterized by excess 5-HT expression such as IBS-D and, possibly, carcinoid diarrhoea. The blocking of excess 5-HT effects is well established[226]. Previously, pharmacological attempts aimed at inhibiting 5-HT synthesis, such as para-chlorophenylalanine, but the central adverse effects due to the inhibition of brain 5-HT synthesis with consequent affective disorders blocked their use[227].
LX-1031 does not cross the blood-brain barrier and, thus, does not pose risk of depression[228].
In a phase II multicentre randomized double-blind placebo-controlled proof-of-concept study[229] performed on 155 patients, the subjects were assigned randomly in a double-blind fashion to 1 of 2 doses of LX1031 (250 mg 4 times/d or 1000 mg 4 times/d) or placebo taken daily during the 28-d treatment period[229]. Patients treated with LX1031 at the dose of 1000 mg significantly improved the primary efficacy end point, namely, the relief of IBS pain and discomfort, compared with placebo at week 1. No significant improvements were observed at weeks 2, 3 or 4. Adverse effects reported were generally mild, self-limited, and evenly distributed across the placebo and both LX1031 treatment arms[229]. The relationship between clinical improvement and reduction in serotonin synthesis shown in this study supports LX1031’s proposed mechanism of action in IBS and thus supports serotonin synthesis inhibition in the GI tract as a novel therapeutic strategy for the treatment of IBS-D[229].
Activation of the Bombesin-2 receptor may be involved in the regulation of gastrointestinal motility and intestinal secretion. ASP7147 is a novel small molecule Bombesin-2 receptor antagonist that reduces motility and intestinal secretions. Indeed, ASP 7147-mediated inhibition of this receptor may improve symptoms in patients with IBS-D. In the RCT performed by Lembo et al[230] on 64 patients during a 4-wk study, ASP7147 showed promise as a safe and effective new therapy for both men and women with IBS-D, demonstrating improvement in multiple symptoms of IBS-D. The persistence of treatment effect suggests the possibility of retained efficacy with less-frequent dosing in follow-on trials[230].
JNJ-27018966 is a dual μ-opioid agonist and δ-opioid receptor antagonist that has been shown to have benefits in patients with IBS-D[231]. A randomized controlled double-blind study compared JNJ-27018966, at doses of 25, 100 and 200 mg twice daily, to placebo in 807 patients with IBS-D. Diarrhoea and pain were significantly improved in patients treated with JNJ-27018966 at the doses of 25 and 200 mg twice-daily compared to placebo (12, 13.8 and 5.7%, respectively, P < 0.05 for both comparisons to placebo)[232].
ROSE-010 is a glucagon-like peptide 1 analogue that decreases gastric emptying and motility[1]. Hellström et al[233] conducted a randomized crossover placebo-controlled trial on 160 patients with IBS and associated abdominal pain treated with ROSE-010 100 μg once daily, 300 μg once daily or placebo. ROSE-010 was associated with a twofold greater response to abdominal pain compared to placebo (P < 0.05 for all comparisons) and significantly higher patient-reported satisfaction (P < 0.05). The most frequent treatment-related side effect was nausea, which was experienced by 19, 37 and 0% of ROSE-010 100 μg, ROSE-010 300 μg and placebo, respectively[233-234].
AST-120, also known as kremezin, is an orally administered intestinal sorbent that has been reported to slow chronic kidney disease (CKD) progression and to delay the initiation of dialysis by reducing the levels of renal toxins or their precursors in the gastrointestinal (GI) tract[235,236].
It has been shown that AST 120 exerts its properties in IBS by acting on intestinal permeability, reduction of visceral sensitivity and alteration of gut motility[236]. In a randomized double-blind controlled study conducted on 115 non-constipation-related IBS patients, AST-120 at a dose of 2 g three times daily significantly improved the percentage of patients with at least a 50% decrease in the number of days with abdominal pain compared to placebo (26.8% vs 10.2%, respectively). Additionally, AST-120 significantly improved bloating and stool consistency compared to placebo. The safety profile of AST-120 was similar to that of placebo[237].
Antagonists of NK2 receptors have been suggested to modulate gastrointestinal chemical-induced impaired motility and stress-induced impaired bowel habits in humans, as recent phase 2 clinical trials have reported[238]. In a randomized double-blind controlled trial conducted on 559 IBS-D patients, ibodutant, a neurokinin-2 receptor antagonist, significantly improved abdominal pain, overall symptoms and quality of life compared to placebo. Ibodutant at doses of 1, 3 or 10 mg once daily showed superiority over placebo, with the 10 mg once daily dose being the most effective and women showing a better response than men[239,240]. Considering the limited number of effective available therapeutic options for IBS-D, ibodutant may become an important and safe treatment option, depending on whether ongoing phase 3 studies will confirm the efficacy observed in phase 2 studies[238].
Asimadoline, a kappa-opioid receptor agonist, acts peripherally, inducing analgesic and antidiarrheal effects[241]. Action in the central nervous system is not required for asimadoline efficacy in the treatment of IBS. Asimadoline reduces sensation in response to colonic distension at subnoxious pressures in healthy subjects and in IBS patients without impairment of colonic compliance. Asimadoline decreased the appetite and enhanced the postprandial gastric volume (in healthy women). However, there were no significant effects on gastrointestinal transit, colonic compliance, fasting or postprandial colonic tone. In a clinical trial conducted on 40 patients with functional dyspepsia (according to Rome II criteria), asimadoline did not significantly impair appetite or symptoms over 8 wk. However, asimadoline at a dose of 0.5 mg significantly reduced the appetite in patients, with higher postprandial fullness scores and daily postprandial fullness severity (over 8 wk). Patients treated with asimadoline at a dose of 1.0 mg showed borderline significant effects. In a clinical trial conducted on patients with IBS, the average pain 2 h post-on-demand treatment with asimadoline was not significantly decreased. At the post hoc analyses, asimadoline was demonstrated to be effective in mixed IBS[242]. Successively, in a randomized controlled double-blind trial conducted on 596 patients with IBS-D, asimadoline at doses of 0.15, 0.5 and 1 mg twice daily was compared to placebo. Patients treated with asimadoline at a dose of 0.5 mg twice daily had a twofold significant improvement in the total number of months with adequate relief of IBS pain, pain scores, urgency and frequency[243,244].
Bile acids have several physiologic functions and are actively reabsorbed (up to 95%) in the terminal ileum[245,246]. Disruption of the enterohepatic circulation of bile acids due to ileal disease (inflammatory bowel diseases) or idiopathic bile acid malabsorption is responsible for chronic diarrhoea[246]. Faecal concentrations of bile acids in IBS-D or functional diarrhoea are unknown. While earlier studies suggested up-regulation of the ileal active transporter[247] as a result of chronic loss of bile acids (which may reduce the bile acids reaching the colon), other data suggest increased delivery to the colon may occur if the ileal reabsorptive capacity for bile acids is exceeded[246].
Odunsi-Shiyanbade et al[155] showed in 12 IBS-D patients that colesevelam modestly affected overall colonic transit (in patients treated with colesevelam, the emptying of the ascending colon was approximately 4 h longer compared to placebo). Furthermore, colesevelam favoured stool passage and somewhat firmer stool consistency. No effects on mucosal permeability or safety were found[246]. Successively, Camilleri et al[248] performed a 10-d single-center unblinded single-dose trial on the effects of colesevelam in 12 IBS-D patients. They demonstrated that colesevelam accelerates the delivery of BAs to stool, while improving stool consistency. It also stimulates hepatic BA synthesis, avoiding steatorrhea in patients with IBS-D. The overall effects are due to luminal BA sequestration by colesevelam[248]. All the abovementioned studies suggest that there is an opportunity to diagnose and specifically treat the cause of symptoms in IBS-D.
Solifenacin is a muscarinic type 3 receptor antagonist recommended in the treatment of overactive bladder (OAB) in adults[249]. Since 1967, M3 receptor antagonists such as mepenzolate bromide have been used in Japan as modulating agents of gastrointestinal motility. However, no clinical trials had been designed to evaluate the efficacy for IBS defined under the modern Rome criteria. Given the high rate of comorbidity between IBS and OAB[250], and considering that solifenacin acts on bowel dysfunction similarly to darifenacin, a selective M3 receptor antagonist with equivalent potencies, solifenacin was evaluated on symptomatic relief in 20 IBS-D patients in an open-label trial[249]. After a 2-wk observation period, solifenacin was administered for 6 wk. Later, solifenacin was suspended, and ramosetron, a serotonin 3 receptor antagonist, was given for 4 wk. Solifenacin was not inferior to ramosetron in the treatment of IBS with diarrhoea[249].
The results of this study suggested the potential therapeutic application of solifenacin in the treatment of IBS-D. However, the possible placebo effect could not be excluded. Therefore, further placebo-controlled parallel group studies are required to confirm the efficacy of solifenacin[249].
Tiropramide, a derivative of tyrosine, has a spasmolytic effect on the intestine, decreasing Ca2+ release into intestinal smooth muscle[251-253]. In a double-blind placebo-controlled randomized trial performed by Lee et al[251], tiropamide was associated with an improvement of total symptom scores for 4 wk compared with 3 wk in the placebo group. In addition, only patients treated with tiropramide improved abdominal pain at week 4[254].
Lee et al[251] successively performed a multicentre, randomized, non-inferiority trial involving 287 patients with IBS randomly assigned to either tiropramide 100 mg or octylonium 20 mg t.i.d. (means 3 times a day) for 4 wk[1]. The visual analogue scale (VAS) scores of abdominal pain at week 4 were significantly reduced in both tiropramide and octylonium groups, even though the change from baseline was similar in the 2 groups[251]. In both groups, abdominal pain and discomfort assessed using VAS scores, diaries and IBS-QoL were improved, and no differences in the changes from baseline were observed. Side effects were similar in both groups. No severe side effects involving either drug were observed[251].
Despite the useful results of the abovementioned study, further studies are required to elucidate tiropramide’s pharmacodynamic and pharmacokinetic properties and its mechanism of action on the intestine[251].
Eluxadoline is a μ- and κ-opioid receptor agonist and δ-opioid receptor antagonist. Its action is directed to the enteric nervous system, with slight side effects in the central nervous system. Its use was approved by the United States Food and Drug Administration on May 2015[255-257].
Patients with IBS-D receiving eluxadoline (100 mg twice daily) in a phase II dose-ranging study had greater efficacy compared with patients receiving placebo after 12 wk[258]. Eluxadoline improved the number of daily bowel movements and decreased the episodes of urgency and incontinence experienced by patients during the 3-mo treatment period[258]. Eluxadoline had an overall favorable safety profile, with nausea, abdominal pain, vomiting, and constipation the most commonly reported AEs[258].
Subsequently, in two large Phase 3 trials (IBS-3001 and IBS-3002), the efficacy of eluxadoline in patients with IBS-D was shown[259].
Finally, Cash et al[260] reported pooled safety and tolerability data from Phase 2 and 3 clinical studies for the approved doses of eluxadoline: 75 and 100 mg. The authors demonstrated that constipation and nausea were the most common adverse events[260]. Consistent with the known adverse effects of opioid agonists, clinically apparent sphincter of Oddi spasm events were observed in eluxadoline-treated patients without a gallbladder. The majority of these cases were observed in patients on the higher dose of eluxadoline, thus suggesting a possible association[260].
Table 4 sums up the literature findings about irritable bowel syndrome-D therapies.
| Drug | Ref. | No. of pt | Study design | Outcomes |
| Ramosetron | Lee et al[213] | 343 male pt | A multicenter, randomized, open-label trial male patients with IBS-D; pt were randomized to either a 4-wk treatment of ramosetron 5 mg once daily ,or a 4-wk treatment of mebeverine 135 mg three times daily | Global IBS symptoms, abdominal pain/discomfort and abnormal bowel habits in the ramosetron and mebeverine groups significantly increased during the treatment period. The severity scores of abdominal pain/discomfort and urgency, the stool form score, and the stool frequency in both treatment arms were significantly reduced, compared with the baselines |
| Fukudo et al[222] | 296 male pt | A randomized, double-blind, placebo-controlled trial in male patients with IBS-D Patients were given 5 mg oral ramosetron (n = 147) or placebo (n = 149) once daily for 12 wk after a 1-wk baseline period | Improving stool consistency in the first month. The ramosetron group had significantly higher monthly rates of relief of overall IBS symptoms and IBS-related quality of life than the placebo group. Adverse events occurring in 46.9% and 51.7% of ramosetron and placebo patients, respectively | |
| Fukudo et al[223] | 576 female pt | A randomized, double-blind, placebo-controlled trial. The subjects received either 2.5 μg ramosetron or placebo once daily for 12 wk. | Global improvement, an increased stool consistency a significant reductions in abdominal pain and discomfort and greater improvement in QOL compared with placebo | |
| Lx1031 | Brown et al[229] | 155 patients | A phase-II multicenter, randomized, double-blind, placebo-controlled, the subjects were assigned randomly in a double-blind fashion to 1 of 2 doses of LX1031 (250 mg 4 times/d or 1000 mg 4 times/d) or placebo, taken daily during the 28-d treatment period | Improved significantly in patients given 1000 mg LX1031 compared with those given placebo, at week 1, together with nonsignificant improvements at weeks 2, 3 and 4. Adverse Effects reported were generally mild, self-limited, and evenly distributed across the placebo and both LX1031 treatment arms |
| ASP-7147 | Lembo et al[230] | 64 patients | RCT performed on during a 4-wk | Demonstrating improvement in multiple symptoms of IBS-D. The persistence of treatment effect suggests the possibility of retained efficacy with less frequent dosing in follow-on trials |
| JNJ-27018966 | [232] | 807 patients | A randomized, controlled, double-blind study, 25, 100, and 200 mg twice daily to placebo | The composite of diarrhea and pain was significantly improved in the JNJ-27018966 25 and 200 mg twice-daily groups compared to placebo |
| ROSE-010 | Hellström et al[233] | 160 patients | A randomized placebo-controlled trial. Patients were randomized to ROSE-010 100 μg once daily, 300 μg once daily or placebo | Treatment with ROSE-010 resulted in a two fold greater response to abdominal pain compared to placebo and significantly greater patient-reported satisfaction with ROSE-010. The most common treatment-related adverse effect was nausea |
| AST-120 | Tack et al[95] | 115 non-constipation-related IBS patients | A randomized, double-blind, controlled study | AST-120 2 g three times daily significantly improved the proportion of patients with at least a 50% reduction in the number of days with abdominal pain compared to placebo. AST-120 resulted in significantly improved bloating and numerically improved stool consistency compared to placebo. The safety profile AST-120 was similar to placebo |
| Ibodutant | Trinkley et al[231] | 559 IBS-D patients | A randomized, double-blind, controlled trial | Improved abdominal pain, satisfactory relief of overall symptoms, and quality of life compared to placebo. All three doses of ibodutant (1, 3, 10 mg once daily) were superior to placebo, but 10 mg once daily was most effective and females responded better than males |
| Asimadoline | Trinkley et al[231] | 596 IBS-D patients | A randomized, controlled, double-blind trial compared asimadoline 0.15, 0.5 and 1 mg twice daily to placebo | Asimadoline 0.5 mg twice daily significantly improved by two fold the total number of months with adequate relief of IBS pain, pain scores, urgency and frequency |
| Colesevelam | Odunsi-Shiyanbade et al[155] | 12 IBS-D patients | Single center trial | Colesevelam modestly affected overall colonic transit (emptying of the ascending colon took an average 4 h longer in patients given colesevelam compared to placebo). Furthermore, colesevelam was associated with greater ease of stool passage and somewhat firmer stool consistency. No effects on mucosal permeability or safety were identified |
| Camilleri et al[240] | 12 IBS-D patients | A 10-d single-center, unblinded, single-dose trial | Colesevelam increases delivery of BAs to stool while improving stool consistency, and increases hepatic BA synthesis, avoiding steatorrhea in patients with IBS-D | |
| Solinefacin | Fukushima et al[249] | 20 IBS-D patients | An open-label trial. After a 2-wk observation period, all participants received solifenacin for 6 wk. Subsequently, the administration of solifenacin was discontinued and ramosetron, a serotonin 3 receptor antagonist, was administered for 4 wk | The efficacy of solifenacin in the treatment of IBS with diarrhea was not inferior to that of ramosetron |
| Tiropamide | Lee et al[251] | 287 patients | A multicenter, randomized, non-inferiority Patients randomly allocated to either tiropramide 100 mg or octylonium 20 mg t.i.d (means 3 times a day) for 4 wk | Tiropamide led to symptom improvement in terms of total symptom scores for 4 wk, compared with 3 wk in the placebo group; in addition, at week 4 abdominal pain was only improved in the tiropramide group. The incidence of adverse events was similar in the 2 groups, and no severe adverse events involving either drug were observed |
IBS currently remains a field of intense therapeutic research, in which most of the aforementioned studies focus on stool-pattern-specific subcategories of patients with this condition. Multiple further drugs are also under evaluation. Among these, alpha galactosidase (AG) was shown to reduce meteorism associated with black bean ingestion, even though it is unknown whether it may have a benefit on IBS[261]. However, in a subsequent study performed by Hillilä et al[262], no evidence to support the use of AG routinely in IBS patients was found.
With regards to therapies restoring intestinal permeability, multiple studies with prebiotics and probiotics[263] are ongoing, even if to date their efficacy has been limited. In parallel, much progress has been made in targeting low-grade inflammation, especially through the introduction of drugs such as mesalazine and rifaximin, even if a better knowledge of the mechanisms underlying the low-grade inflammation in IBS may support the design of clinical trials aimed at evaluating the efficacy and safety of such drugs.
On the other hand, the non-pharmacological treatment of IBS is often viewed as attractive. Faecal microbiota transfer, dietary interventions, holistic and integrative medicine approaches currently represent possible future therapeutic alternatives in this setting.
In conclusion, long-term studies and comparative studies with pharmacotherapy, as well as elucidation of the underlying mechanisms of action, are still needed to find the correct algorithm to manage IBS patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kamiya T, Lakatos PL, Woo SY S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Sinagra E, Romano C, Cottone M. Psychopharmacological treatment and psychological interventions in irritable bowel syndrome. Gastroenterol Res Pract. 2012;2012:486067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Lovell RM, Ford AC. Global prevalence of, and risk factors for, irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721. [Cited in This Article: ] |
| 3. | Quigley EM, Abdel-Hamid H, Barbara G, Bhatia SJ, Boeckxstaens G, De Giorgio R, Delvaux M, Drossman DA, Foxx-Orenstein AE, Guarner F. A global perspective on irritable bowel syndrome: a consensus statement of the World Gastroenterology Organisation Summit Task Force on Irritable Bowel Syndrome. J Clin Gastroenterol. 2012;46:356-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Quigley EM; Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109 Suppl 1:S2-S26; quiz S27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 376] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 5. | Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 559] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 6. | Layer P, Andresen V, Diemert S, Mackinnon J, Bertsch J, Fortea J, Tack J. Economic Burden and Quality of Life of Moderate-To-Severe Irritable Bowel Syndrome With Constipation (Ibs-C) In Germany: Results From The Ibis-C Study. Value Health. 2015;18:A624. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Mulak A, Taché Y. Sex difference in irritable bowel syndrome: do gonadal hormones play a role? Gastroenterol Pol. 2010;17:89-97. [PubMed] [Cited in This Article: ] |
| 8. | Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biol Res Nurs. 2003;5:56-65. [PubMed] [Cited in This Article: ] |
| 9. | Longstreth GF, Wolde-Tsadik G. Irritable bowel-type symptoms in HMO examinees. Prevalence, demographics, and clinical correlates. Dig Dis Sci. 1993;38:1581-1589. [PubMed] [Cited in This Article: ] |
| 10. | Toner BB, Akman D. Gender role and irritable bowel syndrome: literature review and hypothesis. Am J Gastroenterol. 2000;95:11-16. [PubMed] [Cited in This Article: ] |
| 11. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [PubMed] [Cited in This Article: ] |
| 12. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1781] [Cited by in F6Publishing: 1563] [Article Influence: 195.4] [Reference Citation Analysis (2)] |
| 13. | Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924. [PubMed] [Cited in This Article: ] |
| 14. | El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: diagnosis, pathogenesis and treatment options. New York: Nova Science Publishers, Inc 2012; . [Cited in This Article: ] |
| 15. | El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20:384-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 71] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Dupont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther. 2014;39:1033-1042. [DOI] [Cited in This Article: ] |
| 17. | Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979-88. [DOI] [Cited in This Article: ] |
| 18. | Matricon J, Meleine M, Gelot A, Piche T, Dapoigny M, Muller E, Ardid D. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 134] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 19. | Wadhwa A, Camilleri M, Grover M. New and Investigational Agents for Irritable Bowel Syndrome. Curr Gastroenterol Rep. 2015;17:46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51 Suppl 1:i41-44 [PMID 12077063]. [Cited in This Article: ] |
| 21. | Lee E, Schiller LR, Fordtran JS. Quantification of colonic lamina propria cells by means of a morphometric point-counting method. Gastroenterology. 1988;94:409-418. [PubMed] [Cited in This Article: ] |
| 22. | Salzmann JL, Peltier-Koch F, Bloch F, Petite JP, Camilleri JP. Morphometric study of colonic biopsies: a new method of estimating inflammatory diseases. Lab Invest. 1989;60:847-851. [PubMed] [Cited in This Article: ] |
| 23. | Barbara G, Cremon C, Carini G, Bellacosa L, Zecchi L, De Giorgio R, Corinaldesi R, Stanghellini V. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Dunlop SP, Jenkins D, Neal KR, Naesdal J, Borgaonker M, Collins SM, Spiller RC. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:77-84. [PubMed] [Cited in This Article: ] |
| 25. | Sinagra E, Pompei G, Tomasello G, Cappello F, Morreale GC, Amvrosiadis G, Rossi F, Lo Monte AI, Rizzo AG, Raimondo D. Inflammation in irritable bowel syndrome: Myth or new treatment target? World J Gastroenterol. 2016;22:2242-2255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 65] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Barbara G, Feinle-Bisset C, Ghoshal UC, Quigley EM, Santos J, Vanner S, Vergnolle N, Zoetendal EG. The Intestinal Microenvironment and Functional Gastrointestinal Disorders. Gastroenterology. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 27. | Gillis JC, Brogden RN. Rifaximin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential in conditions mediated by gastrointestinal bacteria. Drugs. 1995;49:467-484. [PubMed] [Cited in This Article: ] |
| 28. | DuPont HL. Review article: the antimicrobial effects of rifaximin on the gut microbiota. Aliment Pharmacol Ther. 2016;43 Suppl 1:3-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP; TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 651] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 30. | Lacy BE. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med. 2016;9:7-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Lembo A, Pimentel M, Rao SS, Schoenfeld P, Cash B, Weinstock LB, Golden PL, Paterson C, Bortey E, Forbes WP. Efficacy and safety of repeat treatment with rifaximin for diarrhea-predominant irritable bowel syndrome (IBS-D): results of the TARGET 3 study. Presented at: American College of Gastroenterology (ACG) 2014; Annual Scientific Meeting; October 17-22; 2014; Philadelphia, PA. [Cited in This Article: ] |
| 32. | Ghoshal UC, Srivastava D, Misra A, Ghoshal U. A proof-of-concept study showing antibiotics to be more effective in irritable bowel syndrome with than without small-intestinal bacterial overgrowth: a randomized, double-blind, placebo-controlled trial. Eur J Gastroenterol Hepatol. 2016;28:281-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213-1221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 34. | Lobo B, Pigrau M, Martinez C, González-Castro AM, Guilarte M, de torres I, Salvo-Romero E, Rodiño-Janeiro BK, Fortea M, Cotoner CA. Clinical Benefit and Intestinal Mucosal Transcriptome Modulation After Long-Term Mast Cell Stabilization With Oral Disodium Cromoglycate in Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D) Patients. Gastroenterology. 2015;148:S-494. [DOI] [Cited in This Article: ] |
| 35. | Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA, Valdez-Morales EE, Nasser Y, Van Veldhoven PP, Vanbrabant W. Histamine Receptor H1-Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology. 2016;150:875-877.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 36. | Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 37. | Dorofeyev AE, Kiriyan EA, Vasilenko IV, Rassokhina OA, Elin AF. Clinical, endoscopical and morphological efficacy of mesalazine in patients with irritable bowel syndrome. Clin Exp Gastroenterol. 2011;4:141-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Barbara G, Stanghellini V, Cremon C, De Giorgio R, Fronzoni L, Serra M, Corinaldesi R. Aminosalicylates and other anti-inflammatory compounds for irritable bowel syndrome. Dig Dis. 2009;27 Suppl 1:115-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Barbara G, Cremon C, Annese V, Basilisco G, Bazzoli F, Bellini M, Benedetti A, Benini L, Bossa F, Buldrini P. Randomised controlled trial of mesalazine in IBS. Gut. 2016;65:82-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 40. | Lam C, Tan W, Leighton M, Hastings M, Lingaya M, Falcone Y, Zhou X, Xu L, Whorwell P, Walls AF. Efficacy and mode of action of mesalazine in the treatment of diarrhoea-predominant irritable bowel syndrome (IBS-D): a multicentre, parallel-group, randomised placebo-controlled trial. Southampton (UK): NIHR Journals Library; 2015; . [Cited in This Article: ] |
| 41. | Min T, Ford AC. Efficacy of mesalazine in IBS. Gut. 2016;65:187-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883-885. [PubMed] [Cited in This Article: ] |
| 43. | Scaldaferri F, Pizzoferrato M, Gerardi V, Lopetuso L, Gasbarrini A. The gut barrier: new acquisitions and therapeutic approaches. J Clin Gastroenterol. 2012;46 Suppl:S12-S17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Öhman L, Törnblom H, Simrén M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol. 2015;12:36-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 45. | Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:545-552. [PubMed] [Cited in This Article: ] |
| 46. | Barbara G, Zecchi L, Barbaro R, Cremon C, Bellacosa L, Marcellini M, De Giorgio R, Corinaldesi R, Stanghellini V. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46 Suppl:S52-S55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. [PubMed] [Cited in This Article: ] |
| 48. | Marshall JK, Thabane M, Garg AX, Clark W, Meddings J, Collins SM; WEL Investigators. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20:1317-1322. [PubMed] [Cited in This Article: ] |
| 49. | Villani AC, Lemire M, Thabane M, Belisle A, Geneau G, Garg AX, Clark WF, Moayyedi P, Collins SM, Franchimont D. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology. 2010;138:1502-1513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 50. | Phua LC, Wilder-Smith CH, Tan YM, Gopalakrishnan T, Wong RK, Li X, Kan ME, Lu J, Keshavarzian A, Chan EC. Gastrointestinal Symptoms and Altered Intestinal Permeability Induced by Combat Training Are Associated with Distinct Metabotypic Changes. J Proteome Res. 2015;14:4734-4742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Moloney RD, Johnson AC, O’Mahony SM, Dinan TG, Greenwood-Van Meerveld B, Cryan JF. Stress and the Microbiota-Gut-Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci Ther. 2016;22:102-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 52. | Annaházi A, Ferrier L, Bézirard V, Lévêque M, Eutamène H, Ait-Belgnaoui A, Coëffier M, Ducrotté P, Róka R, Inczefi O. Luminal cysteine-proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation-predominant IBS. Am J Gastroenterol. 2013;108:1322-1331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Gecse K, Róka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztóczy A, Izbéki F, Fioramonti J. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 54. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2121] [Cited by in F6Publishing: 2385] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 55. | Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 995] [Cited by in F6Publishing: 909] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 56. | Ash C, Mueller K. Manipulating the Microbiota. Science. 2016;352:530-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1341] [Cited by in F6Publishing: 1355] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 58. | Borowiec AM, Fedorak RN. The role of probiotics in management of irritable bowel syndrome. Curr Gastroenterol Rep. 2007;9:393-400. [PubMed] [Cited in This Article: ] |
| 59. | Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580-591. [PubMed] [Cited in This Article: ] |
| 60. | Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 61. | Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561; quiz 1546, 1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 447] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 62. | Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J Gastroenterol. 2016;22:2219-2241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 210] [Cited by in F6Publishing: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 63. | Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010;298:G807-G819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 461] [Cited by in F6Publishing: 457] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 64. | Nikfar S, Rahimi R, Rahimi F, Derakhshani S, Abdollahi M. Efficacy of probiotics in irritable bowel syndrome: a meta-analysis of randomized, controlled trials. Dis Colon Rectum. 2008;51:1775-1780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 65. | Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 66. | Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 470] [Cited by in F6Publishing: 432] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 67. | Mazurak N, Broelz E, Storr M, Enck P. Probiotic Therapy of the Irritable Bowel Syndrome: Why Is the Evidence Still Poor and What Can Be Done About It? J Neurogastroenterol Motil. 2015;21:471-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Guidance for Industry. Irritable bowel syndrome-clinical evalua- tion of drugs for treatment: Food and Drug Administration (FDA), Center for Drug Evaluation and Research, May 2012. . [Cited in This Article: ] |
| 69. | Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome. CPMP/EWP/785/97 Rev. 1: European Medicines Agency (EMA), Committee for Medicinal Products for Human use, Sept 2014; . [Cited in This Article: ] |
| 70. | Xi P, Jiang Z, Dai Z, Li X, Yao K, Zheng C, Lin Y, Wang J, Wu G. Regulation of protein turnover by L-glutamine in porcine intestinal epithelial cells. J Nutr Biochem. 2012;23:1012-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Rapin JR, Wiernsperger N. Possible links between intestinal permeability and food processing: A potential therapeutic niche for glutamine. Clinics (Sao Paulo). 2010;65:635-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med. 2005;33:1125-1135. [PubMed] [Cited in This Article: ] |
| 73. | Akobeng AK, Elawad M, Gordon M. Glutamine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2016;2:CD007348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 75. | Bertrand J, Ghouzali I, Guérin C, Bôle-Feysot C, Gouteux M, Déchelotte P, Ducrotté P, Coëffier M. Glutamine Restores Tight Junction Protein Claudin-1 Expression in Colonic Mucosa of Patients With Diarrhea-Predominant Irritable Bowel Syndrome. JPEN J Parenter Enteral Nutr. 2016;40:1170-1176. [PubMed] [Cited in This Article: ] |
| 76. | Gayathri D, Rashmi BS. Development of Celiac Disease; Pathogenesis and Strategies to Control: A Molecular Approach. J Nutr Food Sci. 2014;4:310. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757-766. [PubMed] [Cited in This Article: ] |
| 78. | Leffler DA, Kelly CP, Green PH, Fedorak RN, DiMarino A, Perrow W, Rasmussen H, Wang C, Bercik P, Bachir NM. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology. 2015;148:1311-1319.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 79. | Nusrat S, Miner PB Jr. New pharmacological treatment options for irritable bowel syndrome with constipation. Expert Opin Emerg Drugs. 2015;20:625-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Blackshaw LA, Brierley SM. Emerging receptor target in the pharmacotherapy of irritable bowel syndrome with constipation. Expert Rev Gastroenterol Hepatol. 2013;7:15-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Sood R, Ford AC. Linaclotide: new mechanisms and new promise for treatment in constipation and irritable bowel syndrome. Ther Adv Chronic Dis. 2013;4:268-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Andresen V, Camilleri M, Busciglio IA, Grudell A, Burton D, McKinzie S, Foxx-Orenstein A, Kurtz CB, Sharma V, Johnston JM. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761-768. [PubMed] [Cited in This Article: ] |
| 83. | Johnston JM, Kurtz CB, Macdougall JE, Lavins BJ, Currie MG, Fitch DA, O’Dea C, Baird M, Lembo AJ. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology. 2010;139:1877-1886.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 84. | Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, MacDougall JE, Jia XD, Shao JZ, Fitch DA. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702-1712. [PubMed] [Cited in This Article: ] |
| 85. | Chang L, Lembo AJ, Lavins BJ, Shiff SJ, Hao X, Chickering JG, Jia XD, Currie MG, Kurtz CB, Johnston JM. The impact of abdominal pain on global measures in patients with chronic idiopathic constipation, before and after treatment with linaclotide: a pooled analysis of two randomised, double-blind, placebo-controlled, phase 3 trials. Aliment Pharmacol Ther. 2014;40:1302-1312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Rao S, Lembo AJ, Shiff SJ, Lavins BJ, Currie MG, Jia XD, Shi K, MacDougall JE, Shao JZ, Eng P. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107:1714-1724; quiz p.1725. [PubMed] [Cited in This Article: ] |
| 87. | Busby RW, Bryant AP, Bartolini WP, Cordero EA, Hannig G, Kessler MM, Mahajan-Miklos S, Pierce CM, Solinga RM, Sun LJ. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol. 2010;649:328-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 88. | Quigley EM, Tack J, Chey WD, Rao SS, Fortea J, Falques M, Diaz C, Shiff SJ, Currie MG, Johnston JM. Randomised clinical trials: linaclotide phase 3 studies in IBS-C - a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther. 2013;37:49-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 89. | Lembo AJ, Schneier HA, Shiff SJ, Kurtz CB, MacDougall JE, Jia XD, Shao JZ, Lavins BJ, Currie MG, Fitch DA. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011;365:527-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 244] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 90. | ClinicalTrials. gov Registry, 2014. Available from: http:// www.clinicaltrials.gov. [Cited in This Article: ] |
| 91. | Shailubhai K. Therapeutic applications of guanylate cyclase-C receptor agonists. Curr Opin Drug Discov Devel. 2002;5:261-268. [PubMed] [Cited in This Article: ] |
| 92. | Miner P, Surowitz R, Fogel R, Koltun W, Drossman DA, Camilleri M, Mangel A, Barrow L, Jacob GS, Shailubhai K. Plecanatide, a novel guanylate cyclase-C (GC-C) receptor agonist, is efficacious and safe in patients with chronic idiopathic constipation (CIC): results from a 951 patient, 12-week, multi-center trial[abstract]. Gastroenterology. 2013;144:S163. [Cited in This Article: ] |
| 93. | Shailubhai K, Comiskey S, Foss JA, Feng R, Barrow L, Comer GM, Jacob GS. Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig Dis Sci. 2013;58:2580-2586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 94. | Shailubhai K, Barrow L, Talluto C, Comiskey S, Foss J, Feng R. Plecanatide, a guanylate cyclase C agonist improves bowel habits and symptoms associated with chronic constipation in aphase II a clinical study. Am J Gastroenterol. 2011;106:S502. [Cited in This Article: ] |
| 95. | Tack J, Quigley E, Camilleri M, Vandeplassche L, Kerstens R. Efficacy and safety of oral prucalopride in women with chronic constipation in whom laxatives have failed: an integrated analysis. United European Gastroenterol J. 2013;1:48-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Keating GM. Prucalopride: a review of its use in the management of chronic constipation. Drugs. 2013;73:1935-1950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Müller-Lissner S, Rykx A, Kerstens R, Vandeplassche L. A double-blind, placebo-controlled study of prucalopride in elderly patients with chronic constipation. Neurogastroenterol Motil. 2010;22:991-998, e255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 98. | Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344-2354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 436] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 99. | Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2009;58:357-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 100. | Quigley EM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation--a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009;29:315-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 101. | Camilleri M, Beyens G, Kerstens R, Robinson P, Vandeplassche L. Safety assessment of prucalopride in elderly patients with constipation: a double-blind, placebo-controlled study. Neurogastroenterol Motil. 2009;21:1256-e117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 102. | Ke M, Zou D, Yuan Y, Li Y, Lin L, Hao J, Hou X, Kim HJ. Prucalopride in the treatment of chronic constipation in patients from the Asia-Pacific region: a randomized, double-blind, placebo-controlled study. Neurogastroenterol Motil. 2012;24:999-e541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 103. | Ke M, Tack J, Quigley EM, Zou D, Choi SC, Leelakusolvong S, Liu A, Kim J. Effect of Prucalopride in the Treatment of Chronic Constipation in Asian and Non-Asian Women: A Pooled Analysis of 4 Randomized, Placebo-controlled Studies. J Neurogastroenterol Motil. 2014;20:458-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Tack J, Stanghellini V, Dubois D, Joseph A, Vandeplassche L, Kerstens R. Effect of prucalopride on symptoms of chronic constipation. Neurogastroenterol Motil. 2014;26:21-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 105. | Jadav AM, McMullin CM, Smith J, Chapple K, Brown SR. The association between prucalopride efficacy and constipation type. Tech Coloproctol. 2013;17:555-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | Camilleri M, Piessevaux H, Yiannakou Y, Tack J, Kerstens R, Quigley EM, Ke M, Da Silva S, Levine A. Efficacy and Safety of Prucalopride in Chronic Constipation: An Integrated Analysis of Six Randomized, Controlled Clinical Trials. Dig Dis Sci. 2016;61:2357-2372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 107. | Piessevaux H, Corazziari E, Rey E, Simren M, Wiechowska-Kozlowska A, Kerstens R, Cools M, Barrett K, Levine A. A randomized, double-blind, placebo-controlled trial to evaluate the efficacy, safety, and tolerability of long-term treatment with prucalopride. Neurogastroenterol Motil. 2015;27:805-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 108. | Yiannakou Y, Piessevaux H, Bouchoucha M, Schiefke I, Filip R, Gabalec L, Dina I, Stephenson D, Kerstens R, Etherson K. A randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the efficacy, safety, and tolerability of prucalopride in men with chronic constipation. Am J Gastroenterol. 2015;110:741-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 109. | Videlock EJ, Cheng V, Cremonini F. Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1084-1092.e3; quiz e68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 110. | Mendzelevski B, Ausma J, Chanter DO, Robinson P, Kerstens R, Vandeplassche L, Camm J. Assessment of the cardiac safety of prucalopride in healthy volunteers: a randomized, double-blind, placebo- and positive-controlled thorough QT study. Br J Clin Pharmacol. 2012;73:203-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 111. | Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, Müller-Lissner S, Quigley EM, Schuurkes J, De Maeyer JH. Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012;35:745-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 112. | Cinca R, Chera D, Gruss HJ, Halphen M. Randomised clinical trial: macrogol/PEG 3350+electrolytes versus prucalopride in the treatment of chronic constipation -- a comparison in a controlled environment. Aliment Pharmacol Ther. 2013;37:876-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 113. | SK biopharmaceuticals company. YKP10811 (chronic constipations). BIO_USA-Poster_11_YKP_1081. . [Cited in This Article: ] |
| 114. | Shin A, Acosta A, Camilleri M, Boldingh A, Burton D, Ryks M, Rhoten D, Zinsmeister AR. A randomized trial of 5-hydroxytryptamine4-receptor agonist, YKP10811, on colonic transit and bowel function in functional constipation. Clin Gastroenterol Hepatol. 2015;13:701-708.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 115. | Manini ML, Camilleri M, Goldberg M, Sweetser S, McKinzie S, Burton D, Wong S, Kitt MM, Li YP, Zinsmeister AR. Effects of Velusetrag (TD-5108) on gastrointestinal transit and bowel function in health and pharmacokinetics in health and constipation. Neurogastroenterol Motil. 2010;22:42-49, e7-e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 116. | CDER. US Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry. Irritable bowel syndrome-clinical evaluation of drugs for treatment. Accessed April 1, 2014. . [Cited in This Article: ] |
| 117. | Manabe N, Cremonini F, Camilleri M, Sandborn WJ, Burton DD. Effects of bisacodyl on ascending colon emptying and overall colonic transit in healthy volunteers. Aliment Pharmacol Ther. 2009;30:930-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 118. | Bouras EP, Camilleri M, Burton DD, Thomforde G, McKinzie S, Zinsmeister AR. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354-360. [PubMed] [Cited in This Article: ] |
| 119. | Vassallo M, Camilleri M, Phillips SF, Brown ML, Chapman NJ, Thomforde GM. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992;102:102-108. [PubMed] [Cited in This Article: ] |
| 120. | Mozaffari S, Didari T, Nikfar S, Abdollahi M. Phase II drugs under clinical investigation for the treatment of chronic constipation. Expert Opin Investig Drugs. 2014;23:1485-1497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 121. | Rao SS, Quigley EM, Shiff SJ, Lavins BJ, Kurtz CB, MacDougall JE, Currie MG, Johnston JM. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12:616-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 122. | SK Chemicals Co., Ltd. Efficacy and safety of ykp10811 in subjects with irritable bowel syndrome with constipation. 2014. . [Cited in This Article: ] |
| 123. | SK Life Science. A Phase 2 study to evaluate pharmacodynamics of ykp10811 in patients with chronic or functional constipation. 2012. . [Cited in This Article: ] |
| 124. | SK Life Science. A multicenter, doubleblind, randomized, placebo-controlled, 12-week, dose-range-finding trial of ykp10811 capsules administered once daily to subjects with chronic idiopathic constipation. 2013. . [Cited in This Article: ] |
| 125. | Meyers NL, Hickling RI. Pharmacology and metabolism of renzapride: a novel therapeutic agent for the potential treatment of irritable bowel syndrome. Drugs R D. 2008;9:37-63. [PubMed] [Cited in This Article: ] |
| 126. | Meyers NL, Hickling RI. The cardiovascular safety profile of renzapride, a novel treatment for irritable bowel syndrome. J Int Med Res. 2007;35:848-866. [PubMed] [Cited in This Article: ] |
| 127. | Tack J, Middleton SJ, Horne MC, Piessevaux H, Bloor JS, Meyers NL, Palmer RM. Pilot study of the efficacy of renzapride on gastrointestinal motility and symptoms in patients with constipation-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1655-1665. [PubMed] [Cited in This Article: ] |
| 128. | Scarpellini E, Tack J. Renzapride: a new drug for the treatment of constipation in the irritable bowel syndrome. Expert Opin Investig Drugs. 2008;17:1663-1670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 129. | Camilleri M, McKinzie S, Fox J, Foxx-Orenstein A, Burton D, Thomforde G, Baxter K, Zinsmeister AR. Effect of renzapride on transit in constipation-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2004;2:895-904. [PubMed] [Cited in This Article: ] |
| 130. | George AM, Meyers NL, Hickling RI. Clinical trial: renzapride therapy for constipation-predominant irritable bowel syndrome--multicentre, randomized, placebo-controlled, double-blind study in primary healthcare setting. Aliment Pharmacol Ther. 2008;27:830-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 131. | Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2009;104:1831-1843; quiz 1844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 132. | Ervin CM, Mangel AW. Clinical trials in irritable bowel syndrome: a review. Rev Recent Clin Trials. 2013;8:9-22. [PubMed] [Cited in This Article: ] |
| 133. | Mozaffari S, Nikfar S, Abdollahi M. Efficacy and tolerability of renzapride in irritable bowel syndrome: a meta-analysis of randomized, controlled clinical trials including 2528 patients. Arch Med Sci. 2014;10:10-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 134. | He WR, Zhang FC, Liang LX. Mixed 5-HT3 antagonists/5-HT4 agonists for irritable bowel syndrome: a systematic review. World Chin J Digestol. 2011;19:3277-3283. [Cited in This Article: ] |
| 135. | Lembo AJ, Cremonini F, Meyers N, Hickling R. Clinical trial: renzapride treatment of women with irritable bowel syndrome and constipation - a double-blind, randomized, placebo-controlled, study. Aliment Pharmacol Ther. 2010;31:979-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 136. | Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol. 2006;101:1069-1079. [PubMed] [Cited in This Article: ] |
| 137. | Jiang C, Xu Q, Wen X, Sun H. Current developments in pharmacological therapeutics for chronic constipation. Acta Pharm Sin B. 2015;5:300-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 138. | Buchwald P, Bodor N. Recent advances in the design and development of soft drugs. Pharmazie. 2014;69:403-413. [PubMed] [Cited in This Article: ] |
| 139. | Smith JA, Beattie DT, Marquess D, Shaw JP, Vickery RG, Humphrey PP. The in vitro pharmacological profile of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:125-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 140. | Goldberg M, Li YP, Johanson JF, Mangel AW, Kitt M, Beattie DT, Kersey K, Daniels O. Clinical trial: the efficacy and tolerability of velusetrag, a selective 5-HT4 agonist with high intrinsic activity, in chronic idiopathic constipation - a 4-week, randomized, double-blind, placebo-controlled, dose-response study. Aliment Pharmacol Ther. 2010;32:1102-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 141. | Beattie DT, Armstrong SR, Shaw JP, Marquess D, Sandlund C, Smith JA, Taylor JA, Humphrey PP. The in vivo gastrointestinal activity of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:139-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 142. | Beattie DT, Higgins DL, Ero MP, Amagasu SM, Vickery RG, Kersey K, Hopkins A, Smith JA. An in vitro investigation of the cardiovascular effects of the 5-HT(4) receptor selective agonists, velusetrag and TD-8954. Vascul Pharmacol. 2013;58:150-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 143. | Shaw JP, Beattie D, Cheong SK. Preclinical Pharmacokinetics of TD- 5108, a selective, high intrinsic activity and orally bioavailable 5-HT4 receptor agonist. AAPS Ann Meet Expos. Presented at the annual meeting of the American Association of Pharmaceutical Scientists 2007; 9: 2422. . [Cited in This Article: ] |
| 144. | Wong SL, Goldberg MR, Shaw J, Lanni C, Ganju J, Ballow CH, Kittal MM. In healthy subjects, TD-5108, a selective high intrinsic activity 5-HT4 receptor agonist, shows dose-proportional pharmacokinetics and exhibits a profile consistent with once-daily dosing. Gastroenterology. 2007;132:A374. [Cited in This Article: ] |
| 145. | Goldberg MR, Wong SL, Ganju J, Li YP, Ballow CH, Kitt MM. TD-5108, a selective 5- HT4 agonist with high intrinsic activity, shows immediate and sustained prokinetic activity in healthy subjects. Gastroenterology. 2007;132:A60. [Cited in This Article: ] |
| 146. | Nee J, Feuerstein JD. Review: Prucalopride, velusetrag, bisacodyl, and sodium picosulfate improve chronic idiopathic constipation. Ann Intern Med. 2016;165:JC41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 147. | Goldberg MR, Li YP, Pitzer K, Johanson JF, Mangel AW, Kitt MM. TD-5108, a selective 5-HT4 agonist, is consistently better than placebo regardless of response definition in patients with chronic constipation. Gastroenterology. 2008;134:A545. [Cited in This Article: ] |
| 148. | Beattie DT, Zamora F, Armstrong SR, Pulido-Rios T, Humphrey PP. Tegaserod, but not TD-5108, has effects in porcine and canine isolated coronary arteries. Proceedings of the British Pharmacological Society 2007, Dec; Brighton, UK. org/abstracts/Vol5Issue2abst138P.pdfS. . [Cited in This Article: ] |
| 149. | Dennis D, Palme M, IrwinI , Druzgala P, Teichman S. ATI-7505isa novel, selective5HT(4) receptor agonist that causes gastrointestinal prokinetic activity in dogs. Gastroenterology. 2004;126 Suppl2:A641. [Cited in This Article: ] |
| 150. | Camilleri M, Vazquez-Roque MI, Burton D, Ford T, McKinzie S, Zinsmeister AR, Druzgala P. Pharmacodynamic effects of a novel prokinetic 5-HT receptor agonist, ATI-7505, in humans. Neurogastroenterol Motil. 2007;19:30-38. [PubMed] [Cited in This Article: ] |
| 151. | Shin A, Camilleri M, Kolar G, Erwin P, West CP, Murad MH. Systematic review with meta-analysis: highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment Pharmacol Ther. 2014;39:239-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 152. | Palme M, Milner PG, Ellis DJ, Marmon T, Canafax DM. A novel gastrointestinal prokinetic, ATI- 7505, increased spontaneous bowel movements (Sbms) in a phase II, randomized, placebo-controlled study of patients with chronic idiopathic constipation (CIC). Gastroenterology. 2010;138:S128-S129. [Cited in This Article: ] |
| 153. | Rao AS, Wong BS, Camilleri M, Odunsi-Shiyanbade ST, McKinzie S, Ryks M, Burton D, Carlson P, Lamsam J, Singh R. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549-1558, 1558.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 154. | Bazzoli F, Malavolti M, Petronelli A, Barbara L, Roda E. Treatment of constipation with chenodeoxycholic acid. J Int Med Res. 1983;11:120-123. [PubMed] [Cited in This Article: ] |
| 155. | Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, Lamsam J, Singh R, Zinsmeister AR. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 156. | Bellini M, Gambaccini D, Salvadori S, Tosetti C, Urbano MT, Costa F, Monicelli P, Mumolo MG, Ricchiuti A, De Bortoli N. Management of chronic constipation in general practice. Tech Coloproctol. 2014;18:543-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 157. | Chey WD, Camilleri M, Chang L, Rikner L, Graffner H. A randomized placebo-controlled phase IIb trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am J Gastroenterol. 2011;106:1803-1812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 158. | Acosta A, Camilleri M. Elobixibat and its potential role in chronic idiopathic constipation. Therap Adv Gastroenterol. 2014;7:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 159. | Wong BS, Camilleri M. Elobixibat for the treatment of constipation. Expert Opin Investig Drugs. 2013;22:277-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 160. | Maneerattanaporn M, Chey WD. Targeting bile acids in the treatment of constipation. Expert Rev Gastroenterol Hepatol. 2011;5:657-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 161. | Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol. 2011;106:2154-2164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 162. | Simrén M, Bajor A, Gillberg PG, Rudling M, Abrahamsson H. Randomised clinical trial: The ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation--a double-blind study. Aliment Pharmacol Ther. 2011;34:41-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 163. | Sakamoto S, Kusuhara H, Miyata K, Shimaoka H, Kanazu T, Matsuo Y, Nomura K, Okamura N, Hara S, Horie K. Glucuronidation converting methyl 1-(3,4-dimethoxyphenyl)-3-(3-ethylvaleryl)-4-hydroxy-6,7,8-trimethoxy-2-naphthoate (S-8921) to a potent apical sodium-dependent bile acid transporter inhibitor, resulting in a hypocholesterolemic action. J Pharmacol Exp Ther. 2007;322:610-618. [PubMed] [Cited in This Article: ] |
| 164. | Li H, Xu G, Shang Q, Pan L, Shefer S, Batta AK, Bollineni J, Tint GS, Keller BT, Salen G. Inhibition of ileal bile acid transport lowers plasma cholesterol levels by inactivating hepatic farnesoid X receptor and stimulating cholesterol 7 alpha-hydroxylase. Metabolism. 2004;53:927-932. [PubMed] [Cited in This Article: ] |
| 165. | West KL, Zern TL, Butteiger DN, Keller BT, Fernandez ML. SC-435, an ileal apical sodium co-dependent bile acid transporter (ASBT) inhibitor lowers plasma cholesterol and reduces atherosclerosis in guinea pigs. Atherosclerosis. 2003;171:201-210. [PubMed] [Cited in This Article: ] |
| 166. | Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol. 2004;287:C1173-C1183. [PubMed] [Cited in This Article: ] |
| 167. | Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol. 2007;41:345-351. [PubMed] [Cited in This Article: ] |
| 168. | Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl- secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci. 2011;56:339-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 169. | Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangiah R, Sung EZ, Lee K, Sanger GJ. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol. 2008;154:126-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 170. | Musch MW, Wang Y, Claud EC, Chang EB. Lubiprostone decreases mouse colonic inner mucus layer thickness and alters intestinal microbiota. Dig Dis Sci. 2013;58:668-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 171. | Whitehead WE, Palsson OS, Gangarosa L, Turner M, Tucker J. Lubiprostone does not influence visceral pain thresholds in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2011;23:944-e400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 172. | Raschi E, De Ponti F. Lubiprostone: pharmacokinetic, pharmacodynamic, safety and regulatory aspects in the treatment of constipation-predominant irritable bowel syndrome. Expert Opin Drug Metab Toxicol. 2014;10:293-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 173. | Drossman DA, Chey WD, Johanson JF, Fass R, Scott C, Panas R, Ueno R. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome--results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 249] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 174. | Chey WD, Drossman DA, Johanson JF, Scott C, Panas RM, Ueno R. Safety and patient outcomes with lubiprostone for up to 52 weeks in patients with irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2012;35:587-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 175. | Johanson JF, Morton D, Geenen J, Ueno R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol. 2008;103:170-177. [PubMed] [Cited in This Article: ] |
| 176. | Barish CF, Drossman D, Johanson JF, Ueno R. Efficacy and safety of lubiprostone in patients with chronic constipation. Dig Dis Sci. 2010;55:1090-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 122] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 177. | Spierings EL, Rauck R, Brewer R, Marcuard S, Vallejo R. Long-Term Safety and Efficacy of Lubiprostone in Opioid-induced Constipation in Patients with Chronic Noncancer Pain. Pain Pract. 2015; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 178. | Takeda. Amitiza (lubiprostone) package insert. Deerfield, IL; 2013. . [Cited in This Article: ] |
| 179. | Hyman PE, Di Lorenzo C, Prestridge LL, Youssef NN, Ueno R. Lubiprostone for the treatment of functional constipation in children. J Pediatr Gastroenterol Nutr. 2014;58:283-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 180. | Bell N, Carreras C, Charmot D, Chen T, Leadbetter M, Jacobs J, Lewis J. Compounds and Methods for Inhibiting NHE-Mediated Antiport in the treatment of Disorders Associated with Fluid Retention or Salt Overload and Gastrointestinal Tract Disorders. World Intellectual Property Organization; WO2014029984; 2014. . [Cited in This Article: ] |
| 181. | Charmot D. Non-systemic drugs: a critical review. Curr Pharm Des. 2012;18:1434-1445. [PubMed] [Cited in This Article: ] |
| 182. | Spencer AG, Jacobs JW, Leadbetter MR, Carreras CW, Du X, Bell N, Koo-McCoy S, Kohler JN, Labonté E, Rosenbaum DP. RDX5791, a First-in-Class Minimally Systemic NHE3 Inhibitor in Clinical Development for CIC and IBSC, Increases Intestinal Sodium Leading to Enhanced Intestinal Fluid Volume and Transit. Proceedings of the Drug Disease Week 2011, Chicago. Gastroenterology. 2011;140:S-99. [Cited in This Article: ] |
| 183. | Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med. 2014;6:227ra36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 184. | Eutamene E, Charmot D, Navre M, Bueno L. Visceral Antinociceptive Effects of RDX5791, a First-in-Class Minimally Systemic NHE3 Inhibitor on StressInduced Colorectal Hypersensitivity to Distension in Rats. Proceedings of the DDW meeting 2011, Chicago IL. Gastroenterology;. 2015;140:S-57-8. [Cited in This Article: ] |
| 185. | Rosenbaum DP, Spencer AG, Jacobs J, Charmot D. The safety, tolerability, systemic exposure, and effect on bowel habits of single and multiple doses of the intestinal sodium re-uptake inhibitor RDX5791 in Normal Healthy Volunteers. Proceeding of the ACG meeting 2011, Washington DC. Am J Gastroenterol. 2011;106:S504. [Cited in This Article: ] |
| 186. | Rosenbaum DP. Safety, tolerability, pharmacokinetics and pharmacodynamic of AZD1722 in healthy male and female Japanese subjects. ClinicalTrials. gov Identifier: NCT02176252. 2011 2015; . [Cited in This Article: ] |
| 187. | Rosenbaum DP. The efficacy of AZD1722 in Constipation Predominant Irritable Bowel Syndrome (IBS-C). ClinicalTrials. gov Identifier: NCT01923428 2014; . [Cited in This Article: ] |
| 188. | Song GH, Leng PH, Gwee KA, Moochhala SM, Ho KY. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. 2005;54:1402-1407. [PubMed] [Cited in This Article: ] |
| 189. | Lu WZ, Song GH, Gwee KA, Ho KY. The effects of melatonin on colonic transit time in normal controls and IBS patients. Dig Dis Sci. 2009;54:1087-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 190. | Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360-1368. [PubMed] [Cited in This Article: ] |
| 191. | Isolauri E, Kalliomäki M, Laitinen K, Salminen S. Modulation of the maturing gut barrier and microbiota: a novel target in allergic disease. Curr Pharm Des. 2008;14:1368-1375. [PubMed] [Cited in This Article: ] |
| 192. | Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Bielanski W, Brzozowska I, Stachura J, Hahn EG. The role of melatonin and L-tryptophan in prevention of acute gastric lesions induced by stress, ethanol, ischemia, and aspirin. J Pineal Res. 1997;23:79-89. [PubMed] [Cited in This Article: ] |
| 193. | Guerrero JM, Reiter RJ. Melatonin-immune system relationships. Curr Top Med Chem. 2002;2:167-179. [PubMed] [Cited in This Article: ] |
| 194. | Radwan P, Skrzydlo-Radomanska B, Radwan-Kwiatek K, Burak-Czapiuk B, Strzemecka J. Is melatonin involved in the irritable bowel syndrome? J Physiol Pharmacol. 2009;60 Suppl 3:67-70. [PubMed] [Cited in This Article: ] |
| 195. | Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2014;35:249-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 196. | Lu WZ, Ho KY. Irritable bowel syndrome patients have decreased salivary melatonin and urine 6- hydroxymelatonin levels compared with healthy controls. Gut. 2003;52:821. [Cited in This Article: ] |
| 197. | Sun SX, Dibonaventura M, Purayidathil FW, Wagner JS, Dabbous O, Mody R. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sci. 2011;56:2688-2695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 198. | Chey WD. Tegaserod and other serotonergic agents: what is the evidence? Rev Gastroenterol Disord. 2003;3 Suppl 2:S35-S40. [PubMed] [Cited in This Article: ] |
| 199. | Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43-II47. [PubMed] [Cited in This Article: ] |
| 200. | Song KH. Practical Methods to Assess Chronic Constipation. J Neurogastroenterol Motil. 2015;21:307-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 201. | Houghton LA, Heyman DJ, Whorwell PJ. Symptomatology, quality of life and economic features of irritable bowel syndrome--the effect of hypnotherapy. Aliment Pharmacol Ther. 1996;10:91-95. [PubMed] [Cited in This Article: ] |
| 202. | Camilleri M, Drossman DA, Becker G, Webster LR, Davies AN, Mawe GM. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil. 2014;26:1386-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 203. | Nelson AD, Camilleri M. Chronic opioid induced constipation in patients with nonmalignant pain: challenges and opportunities. Therap Adv Gastroenterol. 2015;8:206-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 204. | Thor PJ, Krolczyk G, Gil K, Zurowski D, Nowak L. Melatonin and serotonin effects on gastrointestinal motility. J Physiol Pharmacol. 2007;58 Suppl 6:97-103. [PubMed] [Cited in This Article: ] |
| 205. | Bubenik GA. Thirty four years since the discovery of gastrointestinal melatonin. J Physiol Pharmacol. 2008;59 Suppl 2:33-51. [PubMed] [Cited in This Article: ] |
| 206. | Furukawa Y, Shiga Y, Hanyu N, Hashimoto Y, Mukai H, Nishikawa K. [Effect of Chinese herbal medicine on gastrointestinal motility and bowel obstruction]. Jpn J Gastroenterol Surg. 1995;28:956-960. [Cited in This Article: ] |
| 207. | Itoh T, Yamakawa J, Mai M, Yamaguchi N, Kanda T. The effect of the herbal medicine dai-kenchu-to on post-operative ileus. J Int Med Res. 2002;30:428-432. [PubMed] [Cited in This Article: ] |
| 208. | Takeda T, Kamiura S, Kimura T. Effectiveness of the herbal medicine daikenchuto for radiation-induced enteritis. J Altern Complement Med. 2008;14:753-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 209. | Endo S, Nishida T, Nishikawa K, Nakajima K, Hasegawa J, Kitagawa T, Ito T, Matsuda H. Dai-kenchu-to, a Chinese herbal medicine, improves stasis of patients with total gastrectomy and jejunal pouch interposition. Am J Surg. 2006;192:9-13. [PubMed] [Cited in This Article: ] |
| 210. | Iturrino J, Camilleri M, Wong BS, Linker Nord SJ, Burton D, Zinsmeister AR. Randomised clinical trial: the effects of daikenchuto, TU-100, on gastrointestinal and colonic transit, anorectal and bowel function in female patients with functional constipation. Aliment Pharmacol Ther. 2013;37:776-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 211. | Manabe N, Camilleri M, Rao A, Wong BS, Burton D, Busciglio I, Zinsmeister AR, Haruma K. Effect of daikenchuto (TU-100) on gastrointestinal and colonic transit in humans. Am J Physiol Gastrointest Liver Physiol. 2010;298:G970-G975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 212. | Acosta A, Camilleri M, Linker-Nord S, Busciglio I, Iturrino J, Szarka LA, Zinsmeister AR. A Pilot Study of the Effect of Daikenchuto on Rectal Sensation in Patients with Irritable Bowel Syndrome. J Neurogastroenterol Motil. 2016;22:69-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 213. | Lee MJ, Cho KH, Park HM, Sung HJ, Choi S, Im W. Pharmacological profile of DA-6886, a novel 5-HT4 receptor agonist to accelerate colonic motor activity in mice. Eur J Pharmacol. 2014;735:115-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 214. | Available from: https://clinicaltrials.gov/ct2/show/NCT01633723. [Cited in This Article: ] |
| 215. | Gershon MD. The enteric nervous system: a second brain. Hosp Pract (1995). 1999;34:31-32, 35-38, 41-2 passim. [PubMed] [Cited in This Article: ] |
| 216. | Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci. 2000;915:77-80. [PubMed] [Cited in This Article: ] |
| 217. | Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1285-1293. [PubMed] [Cited in This Article: ] |
| 218. | Miyata K, Ito H, Fukudo S. Involvement of the 5-HT3 receptor in CRH-induce defecation in rats. Am J Physiol. 1998;274:G827-G831. [PubMed] [Cited in This Article: ] |
| 219. | Funatsu T, Takeuchi A, Hirata T, Keto Y, Akuzawa S, Sasamata M. Effect of ramosetron on conditioned emotional stress-induced colonic dysfunction as a model of irritable bowel syndrome in rats. Eur J Pharmacol. 2007;573:190-195. [PubMed] [Cited in This Article: ] |
| 220. | Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035-1040. [PubMed] [Cited in This Article: ] |
| 221. | Lee KJ, Kim NY, Kwon JK, Huh KC, Lee OY, Lee JS, Choi SC, Sohn CI, Myung SJ, Park HJ. Efficacy of ramosetron in the treatment of male patients with irritable bowel syndrome with diarrhea: a multicenter, randomized clinical trial, compared with mebeverine. Neurogastroenterol Motil. 2011;23:1098-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 222. | Fukudo S, Ida M, Akiho H, Nakashima Y, Matsueda K. Effect of ramosetron on stool consistency in male patients with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2014;12:953-959.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 223. | Fukudo S, Kinoshita Y, Okumura T, Ida M, Akiho H, Nakashima Y, Nishida A, Haruma K. Ramosetron Reduces Symptoms of Irritable Bowel Syndrome With Diarrhea and Improves Quality of Life in Women. Gastroenterology. 2016;150:358-366.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 224. | Fukudo S, Kinoshita Y, Okumura T, Ida M, Hayashi K, Akiho H, Nakashima Y, Haruma K. Effect of ramosetron in female patients with irritable bowel syndrome with diarrhea: a phase III long-term study. J Gastroenterol. 2016;51:874-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 225. | Gupta N, Garg SK, Gupta R, Mahajan S, Sule S. Safety and Efficacy of Ramosetron in Men and Women With IBS-D: Systematic Review and Meta-Analysis. Gastroenterology. 2016;150:Pages S1-S1271. [Cited in This Article: ] |
| 226. | Serotonin now: clinical implications of inhibiting its synthesis with para-chlorophenylalanine. Ann Intern Med. 1970;73:607-630. [PubMed] [Cited in This Article: ] |
| 227. | Camilleri M, Bueno L, Andresen V, De Ponti F, Choi MG, Lembo A. Pharmacological, Pharmacokinetic, and Pharmacogenomic Aspects of Functional Gastrointestinal Disorders. Gastroenterology. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 228. | Camilleri M. LX-1031, a tryptophan 5-hydroxylase inhibitor, and its potential in chronic diarrhea associated with increased serotonin. Neurogastroenterol Motil. 2011;23:193-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 229. | Brown PM, Drossman DA, Wood AJ, Cline GA, Frazier KS, Jackson JI, Bronner J, Freiman J, Zambrowicz B, Sands A. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology. 2011;141:507-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 230. | Lembo A, Huber J, Schinagl RM, Waters SJ, Harris MS. Gastroenterology. 2015;148:S-69. [Cited in This Article: ] |
| 231. | Trinkley KE, Nahata MC. Medication management of irritable bowel syndrome. Digestion. 2014;89:253-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 232. | Lazard Capital Markets Annual Healthcare Conference. Furiex Pharmaceuticals. Available from: http: //files.shareholder.com/downloads/ABEA-4H9PM3/0x0x550458/a546b8d0-d614-4136-9514-3c960c74649f/Lazard Capital Markets Annual Healthcare Conference Presentation.Accessed November 11, 2013.. [Cited in This Article: ] |
| 233. | Hellström PM, Hein J, Bytzer P, Björnssön E, Kristensen J, Schambye H. Clinical trial: the glucagon-like peptide-1 analogue ROSE-010 for management of acute pain in patients with irritable bowel syndrome: a randomized, placebo-controlled, double-blind study. Aliment Pharmacol Ther. 2009;29:198-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 234. | Li ZY, Zhang N, Wen S, Zhang J, Sun XL, Fan XM, Sun YH. Decreased glucagon-like peptide-1 correlates with abdominal pain in patients with constipation-predominant irritable bowel syndrome. Clin Res Hepatol Gastroenterol. 2017; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 235. | Konishi K, Nakano S, Tsuda S, Nakagawa A, Kigoshi T, Koya D. AST-120 (Kremezin) initiated in early stage chronic kidney disease stunts the progression of renal dysfunction in type 2 diabetic subjects. Diabetes Res Clin Pract. 2008;81:310-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 236. | Mosińska P, Storr M, Fichna J. The role of AST-120 and protein-bound uremic toxins in irritable bowel syndrome: a therapeutic perspective. Therap Adv Gastroenterol. 2015;8:278-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 237. | Tack JF, Miner PB Jr, Fischer L, Harris MS. Randomised clinical trial: the safety and efficacy of AST-120 in non-constipating irritable bowel syndrome - a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;34:868-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 238. | Corsetti M, Akyuz F, Tack J. Targeting tachykinin receptors for the treatment of functional gastrointestinal disorders with a focus on irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:1354-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 239. | Available from: https://clinicaltrials.gov/ct2/show/NCT00761007. [Cited in This Article: ] |
| 240. | Camilleri M, Boeckxstaens G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut. 2017;66:966-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 241. | Mangel AW, Hicks GA. Asimadoline and its potential for the treatment of diarrhea-predominant irritable bowel syndrome: a review. Clin Exp Gastroenterol. 2012;5:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 242. | Camilleri M. Novel pharmacology: asimadoline, a kappa-opioid agonist, and visceral sensation. Neurogastroenterol Motil. 2008;20:971-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 243. | Mangel AW, Bornstein JD, Hamm LR, Buda J, Wang J, Irish W, Urso D. Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:239-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 244. | Foxx-Orenstein AE. New and emerging therapies for the treatment of irritable bowel syndrome: an update for gastroenterologists. Therap Adv Gastroenterol. 2016;9:354-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 245. | Mottacki N, Simrén M, Bajor A. Review article: bile acid diarrhoea - pathogenesis, diagnosis and management. Aliment Pharmacol Ther. 2016;43:884-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 246. | Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647-2658. [PubMed] [Cited in This Article: ] |
| 247. | van Tilburg AJ, de Rooij FW, van Blankenstein M, van den Berg JW, Bosman-Jacobs EP. Na dependent bile acid transport in the ileum: the balance between diarrhea and constipation. Gastroenterology. 1990;98:25-32. [PubMed] [Cited in This Article: ] |
| 248. | Camilleri M, Acosta A, Busciglio I, Boldingh A, Dyer RB, Zinsmeister AR, Lueke A, Gray A, Donato LJ. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:438-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 249. | Fukushima Y, Suzuki H, Matsuzaki J, Kiyosue A, Hibi T. Efficacy of Solifenacin on Irritable Bowel Syndrome With Diarrhea: Open-label Prospective Pilot Trial. J Neurogastroenterol Motil. 2012;18:317-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 250. | Matsuzaki J, Suzuki H, Fukushima Y, Hirata K, Fukuhara S, Okada S, Hibi T. High frequency of overlap between functional dyspepsia and overactive bladder. Neurogastroenterol Motil. 2012;24:821-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 251. | Lee KN, Lee OY, Choi MG, Sohn CI, Huh KC, Park KS, Kwon JG, Kim N, Rhee PL, Myung SJ. Efficacy and Safety of Tiropramide in the Treatment of Patients With Irritable Bowel Syndrome: A Multicenter, Randomized, Double-blind, Non-inferiority Trial, Compared With Octylonium. J Neurogastroenterol Motil. 2014;20:113-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 252. | Takayanagi I, Hisayama T, Iwase M, Sakuma N, Nagai H. Pharmacological properties of tiropramide, an antispasmodic drug. Gen Pharmacol. 1989;20:335-339. [PubMed] [Cited in This Article: ] |
| 253. | Uruno T, Shirane M, Wada K, Tsunematsu R, Nagahamaya K, Matsuoka Y, Sunagane N, Kubota K. Possible mechanisms of action of the antispasmodic agent tiropramide in the isolated detrusor from rats. Jpn J Pharmacol. 1992;60:275-280. [PubMed] [Cited in This Article: ] |
| 254. | Park SH, Jang CH, Han JY, Choi MG, Choi GY, Chung IS, Chung KW, Sun HS, Kim BS. Double blind clinical trial ofter- opramide in irritable bowel syndrome. Korean J Gastroenterol. 1993;25:877-883. [Cited in This Article: ] |
| 255. | Sobolewska-Włodarczyk A, Włodarczyk M, Storr M, Fichna J. Clinical potential of eluxadoline in the treatment of diarrhea-predominant irritable bowel syndrome. Ther Clin Risk Manag. 2016;12:771-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 256. | FDA approves two therapies to treat IBS-D. Available from: http: //www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm448328.htm.. [Cited in This Article: ] |
| 257. | Actavis announces FDA acceptance for filing of NDA for eluxadoline. Available from: http: //www.prnewswire.com/news-releases/actavis-announces-fda-acceptance-for-filing-of-nda-for-eluxadoline-273557591.html.. [Cited in This Article: ] |
| 258. | Dove LS, Lembo A, Randall CW, Fogel R, Andrae D, Davenport JM, McIntyre G, Almenoff JS, Covington PS. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology. 2013;145:329-338.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 259. | Lembo AJ, Lacy BE, Zuckerman MJ, Schey R, Dove LS, Andrae DA, Davenport JM, McIntyre G, Lopez R, Turner L. Eluxadoline for Irritable Bowel Syndrome with Diarrhea. N Engl J Med. 2016;374:242-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 260. | Cash BD, Lacy BE, Schoenfeld PS, Dove LS, Covington PS. Safety of Eluxadoline in Patients with Irritable Bowel Syndrome with Diarrhea. Am J Gastroenterol. 2017;112:365-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 261. | Ganiats TG, Norcross WA, Halverson AL, Burford PA, Palinkas LA. Does Beano prevent gas? A double-blind crossover study of oral alpha-galactosidase to treat dietary oligosaccharide intolerance. J Fam Pract. 1994;39:441-445. [PubMed] [Cited in This Article: ] |
| 262. | Hillilä M, Färkkilä MA, Sipponen T, Rajala J, Koskenpato J. Does oral α-galactosidase relieve irritable bowel symptoms? Scand J Gastroenterol. 2016;51:16-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 263. | Sinagra E, Tomasello G, Cappello F, Leone A, Cottone M, Bellavia M, Rossi F, Facella T, Damiani P, Zeenny MN. Probiotics, prebiotics and symbiotics in inflammatory bowel diseases: state-of-the-art and new insights. J Biol Regul Homeost Agents. 2013;27:919-933. [PubMed] [Cited in This Article: ] |